Introduction
In recent years the experimental and clinical research has made it clear that the immune system does not stand alone, but it is profoundly affected by other organ systems, especially the central nervous and neuroendocrine systems. It is also increasingly clear that the immune system can in turn affect the functioning of these systems as well.1
The research on opioid peptides plays a fundamental role in the development of this awareness. Among the first experimental evidences suggesting the existence of a bidirectional link between brain and immune system we must include the observation by Wybran et al.2 who in 1979 showed that morphine, a drug considered to alter only neuronal functions, could affect also the responses of human B lymphocytes. This report stimulated the research in the field of neuroimmunomodulation, leading to the identification of several neuropeptide receptors on immune cells, and to the observations that many, if not all, neuropeptides, hormones and neurotransmitters can affect the immune responses.
However, although 21 years have passed and a wide literature is now available, the real significance of the role of opioids in the modulation of the immune system has not yet been ascertained.
Morphine and Endogenous Opioids
The problem of the significance of opioid-induced modulation of the immune system is complex and multifaceted. Two main conceptual problems arise: with the term opioid or opiate we indicate both endogenous opioid peptides, namely β-endorphin, Met-enkephalin and dynorphin, and opiate analgesic drugs, such as morphine. It is evident that it is very different to investigate the existence of a physiological role for opioid peptides in the complex modulatory network operating on the immune system, or to evaluate the impact of a pharmacological morphine treatment. Moreover, due to the high affinity of morphine for the opioid receptors and to its higher stability in comparison with peptides, morphine has frequently been employed in an interchangeable way as a tool for the exploration of the immune effects of the opioids. The large majority of the studies with the opioid peptides have been conducted in vitro, often evaluating the effect on a single cell population or cell line/clone. In these conditions the results have often been contradictory, depending on the doses and timing of opioid addition to cell cultures. For these series of reasons this overview will be focused mainly on “ex vivo” experiments, where the administration of opiates or the manipulation of the opioid system was achieved in vivo.
Immunomodulation by Morphine
In Vivo Studies (Table 1)
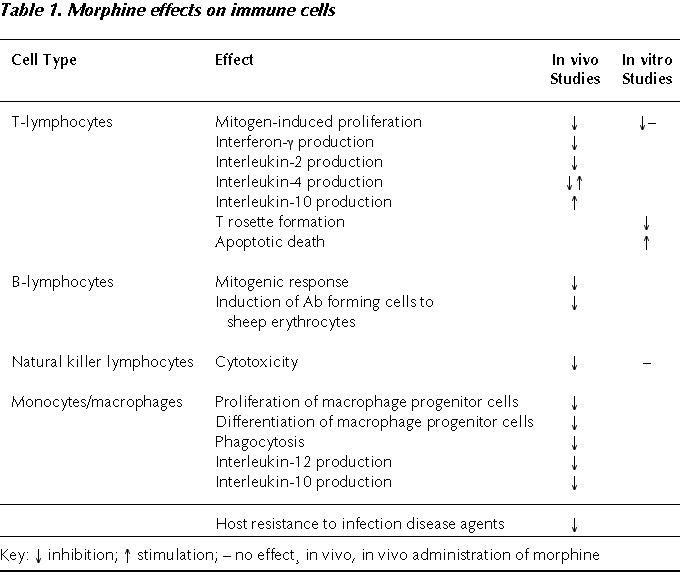
In vivo administration of morphine to rodents induces a decrease of multiple immune parameters, affecting almost all different cell types of the immune system. Natural killer cells (NK) have been shown to be very sensitive to morphine modulation in vivo. Injections of morphine have been found to lead to depressed NK in rats, mice and monkeys.3–11 There is considerable evidence that morphine given in vivo modulates T-cell functions. Independently of the stimulus utilized (polyclonal mitogens, CD3 activation, antigen-specific challenge) T-lymphocyte proliferation is decreased by both acute and chronic morphine administration.3,4,10–16 In contrast, the effects of morphine on B lymphocytes are less evident.11,17,18 Morphine at high concentrations has been shown to reduce the mitogenic response of splenic B cells4,16 and an inhibited induction of antibody-forming cells to sheep red blood cells was observed in different species.2,18 However, the formation of an antibody response to sheep red blood cells requires interaction of macrophages, T cells and B cells. It was for example shown by Weber et al. that morphine inhibited antibody responses to a T-dependent, but not to a T-independent antigen,19 suggesting that morphine did not affect B-cell function directly.
Clear inhibitory effects of in vivo morphine on monocyte/macrophage functions have been consistently described. Chronic morphine treatment inhibited proliferation and differentiation of macrophage colony stimulating factor (MCSF)-dependent macrophage progenitor cells in the bone marrow.20 Phagocytosis of different pathogens was also impaired by morphine.11,21–23 The impairment was evident with peritoneal, alveolar or splenic macrophages, indicating a general down-regulation of innate immunity.
The effects of in vivo morphine on T-cell and macrophage cytokines are not well clarified and relatively little explored. Morphine seems to decrease interleukin (IL)-2 and interferon (IFN)-γ production by T lymphocytes.5,10,24 Moreover, an increase of IL-4 production has been reported after morphine administration in some cases25 and a decreased IL-4 in other experiments.26 Also contradictory are the results reported of morphine modulation of IL-12 and IL-10, mostly depending on the time of cytokine measurement after administration of morphine and of the cell population analyzed. We observed a decrease of IL-10 and IL-12 production by murine peritoneal macrophages collected one hour after morphine administration (unpublished data), while an increase of the last cytokine produced by splenocyte cultures was reported 24 hours after morphine.27
In most of the results obtained with morphine a classical μ-type opiate receptor is involved, since the effects of morphine can be blocked by the antagonist naloxone.18 Moreover, the immunosuppressive effects of morphine disappear in mice knock-out (KO) for the μ-opioid receptor.28 Generally, however, the morphine doses needed in order to exert a significant immunosuppression are higher than the ones necessary to induce antinociception (analgesia). While in fact in the BALB-c mouse a relevant analgesic effect is observed at doses starting from 2.5 mg/kg, the doses generally employed in the experiments of immunosuppression start from 10 mg/kg.10 Convincing evidence of the immunosuppressive effect of morphine comes from several experiments associating opioids to microbial pathogen infections.29 It has in fact consistently been reported that acute as well as chronic administration of morphine to rodent modulates host resistance to bacteria and fungal pathogens. A single dose or repeated doses of morphine have been shown to enhance the lethality of Toxoplasma,30 Klebsiella and Candida.21 Moreover, morphine can alter pathogenesis also in virus infection models, including murine leukemia, Moloney sarcoma, herpes simplex and Friend viruses.31 It appears from all these studies that opiates act to alter host defenses against a variety of infectious disease agents. However, the outcomes of these infections are influenced by the timing of morphine administration, the state of opiate dependence, the animals species and the dose and route of infection.29 These observations are indeed particularly important in the consideration of association between AIDS epidemic and opiate abuse in the human.
In Vitro Studies (Table 1)
Although the first and pioneering study on the effects of morphine on the immune system described that morphine added in vitro to human peripheral blood lymphocytes inhibited T rosette formation,2 the subsequent studies in the experimental animal did not consistently show a direct effect on different cell populations, since in general either a decrease or no modification of the immune parameters studied were observed.
While NK activity did not seem to be affected even by high morphine concentrations in vitro,32,33 reduction as well as no effect on lymphocyte proliferation was reported, depending on the mitogen and the source of T cells.5,34,35 However, an interesting paper from Yin et al.36 demonstrated that morphine added in vitro can facilitate the triggering of apoptotic death of T lymphocytes by modulating the Fas-FasL system. These effects were mediated by opioid receptors present on the immune cells. The discrepancy between the in vitro and in vivo effects of morphine has prompted the search of the mechanism involved in the morphine-induced effects.
Mechanisms of Morphine-Induced Immunosuppression
A good agreement has been reached that opioids induce immunosuppression either interacting directly with opioid receptors on immune cells or on receptors within the central nervous system.37–41 It has been demonstrated that immune cells express μ, δ and κ receptors functionally coupled to signal transduction mechanisms.42 Moreover activation of central opioid receptors can regulate the peripheral immune system throughout the stimulation of the hypothalamus-pituitary-adrenal axis (HPA)33 and the sympathetic nervous system.15 The activation of the HPA elicits the production of adrenocorticotropin from the pituitary that in turn elicits the release of glucocorticoids that could eventually suppress the immune system.33,43 Both primary and secondary lymphoid organs (such as the spleen) are innervated by the sympathetic nervous system.44 Activation of this system by opioids elicits the release in these organs of catecholamines that have been demonstrated to suppress lymphocyte, NK cell and macrophage functions.45
Opioid Peptides
While the studies examining morphine-induced immunomodulation are quite consistent in claiming for immunosuppressive properties, the evidences collected on the role of the endogenous opioid peptides in immune responses are more complex and not definitive.
In Vitro Studies (Table 2)
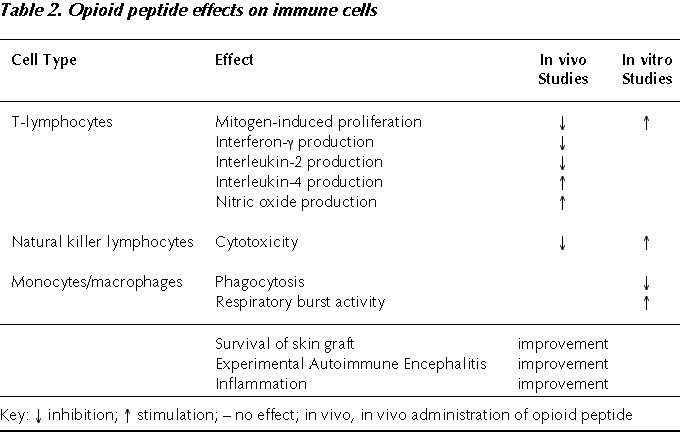
The μ and δ agonists, β-endorphin and Met-enkephalin exert surprising effects in vitro on NK activity and T lymphocyte proliferation. The majority of the studies conducted, in fact, indicate that these peptides mostly increase these two parameters.32,46–52 The stimulation was observed with splenocytes,51 lymph node cells53,54 and peripheral blood lymphocytes in different animal species.55,56 On the other hand, the entity and the duration of the stimulatory properties were often dependent on the experimental scheme, such as the timing of the peptide addition to cell cultures, i.e., before or after mitogen, and on the concentrations used. Sometimes biphasic responses were observed with low or high concentrations.57 Macrophage functions are indeed either decreased or stimulated by in vitro administration of the opioid peptides. Phagocytosis is consistently impaired by μ, δ and κ ligands,58–60 while superoxide release was shown to be increased by β-endorphin,61,62 dynorphin61,62 and Met-enkephalin.63,64 An interesting hypothesis suggested is that opioid peptides and β-endorphin in particular could be fine regulators of immune function normalizing the immune responses, enhancing them when suppressed and reducing them when stimulated.65
In Vivo Studies (Table 2)
As already reported for morphine, the data obtained with the in vivo administration of opioid peptides seem more consistent. When the opioid peptide β-endorphin was administered in vivo to rodents, either centrally in the brain or peripherally, a decrease of cellular immune functions, such as mitogen-induced lymphoproliferation and NK activity, was observed.66,67
Cell Type Effect: In Vivo/in Vitro Studies
On the contrary, the block of β-endorphin activity, achieved either with the administration of the opioid antagonists naloxone and naltrexone or of immunoglobulins that neutralize the activity of β-endorphin, induced an increase of NK activity and lymphoproliferation within minutes.66,68 The in vivo administration of the opioid antagonists also was shown to affect in a negative way nitric oxide (NO) production by splenocytes, and this effect was demonstrated to be mediated mainly by central opioid receptors.69 These observations, indicating that the removal of the opioid tone by way of an antagonist or an antibody can affect some immune responses, suggest that an endogenous opioidergic tone on some immune functions exists. The existence of this control has been further demonstrated in a model of inflammation in the rat. Indeed, administration of either β-endorphin or morphine affected the development of carrageenan-induced inflammation of rat paw. These opioid agonists in fact significantly reduced edema and cell infiltration in the inflamed paw, while naloxone or the administration of a neutralizing antibody against β-endorphin worsened the degree of edema.79
In the last years mice lacking μ, δ and κ opioid receptors have been generated and could provide tools to study the opioid impact on the immune system. Unfortunately, however, the data collected until now with these animals do not allow the drawing of final conclusions, since contradictory results have been published. Gaveriaux-Ruff et al.28 using a strain of μ-receptor KO mice did not show any difference between wild-type and mutant mice with regard to NK activity or lymphocyte number. In contrast, Tian et al.70 in different μ-opioid receptor KO mice reported the existence of an increased proliferation of granulocyte, macrophage, erythroid and multipotential progenitor cells in both bone marrow and spleen, indicating a link between hematopoiesis and the opioid system, suggesting that endogenous opioids exert a negative regulatory influence on immune functions. Many reasons such as the different background, or the fact that the absence of one receptor type could be compensated by the other remaining opioid receptors, could explain the discrepancy in the results. More studies with double or triple opioid receptor KO need to be performed.71
Modulation of Th1/Th2 Responses
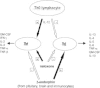
In vivo, opioid peptides have been involved in the modulation of the Th1/Th2 balance. It is well known that T helper cells are functionally polarized in two different subsets.72 The Th1 and Th2 cells produce different patterns of cytokines: Th1 cells produce mainly IL-2, IFN-γ and lymphotoxins, whereas Th2 cells produce IL-4, IL5, IL6, IL-10 and IL-13 (Fig. 1). Th1 cells are mostly involved in cell-mediated reactions, while the Th2 cytokines are commonly found in association with strong antibody and allergic responses. Moreover, the characteristic cytokine products of Th1 and Th2 cells are inhibitory for the differentiation and effector function of the opposite subset.73
The administration of the opioid antagonist naloxone profoundly affected the splenocyte production of the Th1 cytokines IFN-γ and IL-2 and of the Th2 cytokine IL-4 in different strains of mice immunized with the protein antigen keyhole limpet hemocyanin (KLH).74 In fact, acute as well chronic naloxone treatment decreased IL-4 production. On the contrary, IL-2 and IFN-γ levels were increased after naloxone administration. The administration of naloxone seems therefore to stimulate Th1 cytokines while decreasing Th2 cytokines. Given the fact that naloxone is an almost pure antagonist at the μ-opioid receptor, devoid of any intrinsic activity, the effects of the drug are likely to be due to the removal of a regulatory tone exerted by endogenous opioid peptides (Fig. 1).75
Since an imbalance of Th1/Th2 cytokines is often at the basis of immune diseases, the effect of naloxone and/or β-endorphin on immune responses can be relevant.73 Therefore in the next paragraphs we report a few examples of how both opioids and the antagonism of opioid activities can affect the onset and the development of pathologies due to an altered immune functionality.
The Th1/Th2 paradigm has been involved in immune responses to organ transplantation.76 Under certain experimental conditions in fact, graft rejection has been associated with the presence of Th1 cytokines, while Th2 cytokines have been linked to graft survival. We investigated the effect of the opioid receptor antagonist naloxone on the onset of allograft skin rejection in mice and on the production of IL-2, IFN-γ and IL-4 during the development of the rejection. The continuous administration of β-endorphin significantly prolonged the time of rejection while naloxone significantly shortened it.77 This effect could be due to the effects of β-endorphin or naloxone on the Th1/Th2 balance. In fact, naloxone treatment increased Th1 cytokine production by splenocytes of transplanted mice evaluated at the moment of rejection.77
A possible involvement of opioids in an experimental model of autoimmune disease has also been suggested. Rat experimental autoimmune encephalitis (EAE) is commonly considered a model for human multiple sclerosis. The development of EAE is associated with Th1 responses, while remission of symptoms is linked to the appearance of a Th2 protective immune response. Consistently with the role of opioids in the modulation of Th1/Th2 responses, the administration of the opioid receptor antagonist naloxone, by increasing Th1 responses and blocking Th2 cytokines, worsened the clinical signs of EAE and increased mortality.78
Involvement of Opioids in Stress-Induced Immunosuppression
Recent research has provided convincing evidence that physical and psychological stress can affect the immune function both in the human and in the experimental animal.80 During the period of stress (perceived as a threat to the homeostasis of the organism) the brain releases a number of chemical mediators, including opioid peptides, which stimulate an inhibitory effect on many body systems comprising the immune system. In experiments dating back to 1984 Shavit et al.81 showed that the experimental stress model of intermittent footshock in the rat induced immunosuppression. Interestingly, only stress modalities that were able to induce the release of opioid peptides, β-endorphin in particular, were associated with immunosuppression. Moreover, these stress-induced immune effects were blocked by the administration of the opioid antagonist naloxone. More recently, our group demonstrated that the administration of an antibody that neutralizes β-endorphin could block the immunosuppression induced by different stress models in the rat.82,83 We have also shown that the β-endorphin mediating stress-induced immunosuppression is not the only one released by the pituitary, but also the peptide produced by immune cells themselves under stressful conditions. In this way the peptide can regulate the immune response by an autocrine/paracrine pattern.83
Conclusions
Considering the large amount of data collected in the last years on the immunomodulatory properties of opiates, it is clear that this activity must be added to the many pharmacological and physiological effects exerted on the central and peripheral nervous system, such as on nociceptive pathways and behavior, and on the neuroendocrine and gastroenteric systems. While the pharmacological alterations of immune responses induced by exogenous opiates, i.e., morphine and congeners, point to a final immunosuppression, the physiological role of endogenous opioids has not been completely defined. It becomes in fact questionable to claim a unique immunosuppressive or immunostimulatory role for opioid peptides like β-endorphin. It can be suggested that the opioids might exert an inhibitory control on some cell populations (Th1, macrophages) probably through the stimulation of other cell types such as Th2 cells. Depending on the immune function evaluated (e.g., cellular vs. humoral), the preexisting Th1/Th2 balance (e.g., after previous exposure of animals to different pathogens or to genetic predisposition), and the stage of activation of immune cells, inhibition or stimulation of classical laboratory immune parameters can be achieved. If, however, we take into consideration the experiments with in vivo modulation of immune responses, it seems reasonable to suggest that the final outcome of the different inhibitory/stimulatory inputs exerted by opioids is that of a control aimed to prevent overshooting and excessive activation of the immune system.
References
- 1.
- Reichlin S. Neuroendocrineimmune interactions. N Engl J Med. 1993;329:1246–53. [PubMed: 8105378]
- 2.
- Wybran J, Appelboom T, Farmaey JP. Suggestive evidence for receptors for morphine and methionineenkephalin on normal human blood T lymphocytes. J Immunol. 1979;123:1068–1070. [PubMed: 224107]
- 3.
- Lysle DT, Coussons ME, Watts VJ. et al. Morphineinduced alterations of immune status: dose dependency, compartment specificity and antagonism by naltrexone. J Pharmacol Exp Ther. 1993;265:1071–8. [PubMed: 7685383]
- 4.
- Lysle DT, Hoffman KE, Dykstra LA. Evidence for the involvement of the caudal region of the periaqueductal gray in a subset of morphineinduced alterations of immune status. J Pharmacol Exp Ther. 1996;277:1533–40. [PubMed: 8667220]
- 5.
- Fecho K, Maslonek KA, Dykstra LA. et al. Assessment of the involvement of central nervous system and peripheral opioid receptors in the immunomodulatory effects of acute morphine treatment in rats. J Pharmacol Exp Ther. 1996;276:626–36. [PubMed: 8632330]
- 6.
- Carr DJ, France CP. Immune alterations in morphinetreated Rhesus monkeys. J Pharmacol Exp Ther. 1993;267:9–15. [PubMed: 7693915]
- 7.
- Carr DJ, Gebhardt BM, Paul D. aadrenergic and IL-2 opioid receptors are involved in morphineinduced suppression of splenocyte natural killer activity. J Pharmacol Exp Ther. 1993;264:1179–86. [PubMed: 8383741]
- 8.
- Carr DJ, Mayo S, Gebhardt B. et al. Central aadrenergic involvement in morphinemediated suppression of splenic natural killer activity. J Neuroimmunol. 1994;53:53–63. [PubMed: 8051298]
- 9.
- Carr DJ, Gerak LR, France CP. Naltrexone antagonizes the analgesic and immunosuppressive effects of morphine in mice. J Pharmacol Exp Ther. 1994;269:693–698. [PubMed: 8182537]
- 10.
- Sacerdote P, Manfredi B, Mantegazza P. et al. Antinociceptive and immunosuppressive effects of opiate drugs: a structurerelated activity study. Br J Pharmacol. 1997;121:834–40. [PMC free article: PMC1564723] [PubMed: 9208156]
- 11.
- Eisenstein TK, Hillburger ME. Opioid modulation of immune responses: effects on phagocyte and lymphoyd cell populations. J Neuroimmunol. 1998;83:36–44. [PubMed: 9610671]
- 12.
- Bayer BM, Daussin S, Hernandez M. et al. Morphine inhibition of Iymphocyte activity is mediated by an opioid dependent mechanism. Neuropharmacology. 1990;29:369–74. [PubMed: 2160624]
- 13.
- Chuang LF, Killam KF, Chuang RY. Opioid dependency and Thelper cell functions in rhesus monkey. In Vivo. 1993;7:159–166. [PubMed: 8364167]
- 14.
- Flores LR, Hernandez MC, Bayer BM. Acute immunosuppressive effects of morphine: lack of involvement of pituitary and adrenal factors. J Pharmacol Exp Ther. 1994;268:1129–34. [PubMed: 8138926]
- 15.
- Flores LR, Dretchen KL, Bayer BM. Potential role of the autonomic nervous system in the immunosuppressive effects of the acute morphine administration. Eur J Pharmacol. 1996;318:437–46. [PubMed: 9016936]
- 16.
- Bryant HU, Roudebush RE. Suppressive effects of morphine pellet implants on in vivo parameters of immune function. J Pharmacol Exp Ther. 1990;255:410–414. [PubMed: 2243332]
- 17.
- Bussiere JL, Adler MW, Rogers TJ. et al. Effects of in vivo morphine treatment on antibody responses in C57BL/6 bgJ/bgJ (beige) mice. Life Sci. 1993;PL 52:43–48. [PubMed: 8421432]
- 18.
- Flores RG, Weber RJ. Opioids, Opioids receptors and the Immune system In: Plotnikoff, Faith, Murgo, Good, eds.Cytokines, Stress and Immunity CRC Press, 1999281–314.
- 19.
- Weber RJ, Ikejiri B, Rice KC. et al. Opiate receptor mediated regulation of the immune response in vivo. NIDA Res Mono. 1987;76:341–348. [PubMed: 2830526]
- 20.
- Roy S, Ramakrishnan S, Loh HH. et al. Chronic morphine treatment selectively suppresses macrophage colony formation in bone marrow. Eur J Pharmacol. 1991;195:359–63. [PubMed: 1831136]
- 21.
- Tubaro E, Borelli G, Croce C. et al. Effect of morphine on resistance to infection. J Infect Dis. 1983;148:656–66. [PubMed: 6355311]
- 22.
- Tubaro E, Santiangeli C, Belogi L. et al. Methadone vs morphine: comparison of their effect on phagocytic functions. Int J lmmunopharmacol. 1987;9:79–88. [PubMed: 3034811]
- 23.
- Rojavin M, Szabo I, Bussiere JL. et al. Morphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophages. Life Sci. 1993;53:997–1006. [PubMed: 8361330]
- 24.
- Scott M, Carr DJ. Morphine suppresses the alloantigendriven CTL response in a dosedependent and naltrexone reversible manner. J Pharmacol Exp Ther. 1996;278:980–88. [PubMed: 8768756]
- 25.
- Roy S, Charboneau RG, Barke RA. Morphine synergizes with lipopolysaccharide in a chronic endotoxemia model. J Neuroimmunol. 1999;95:107–114. [PubMed: 10229120]
- 26.
- Casalinuovo IA, Graziano R, Di Francesco P. Cytokine pattern secretion by murine spleen cells after inactivated Candida albicans immunization. Effect of cocaine and morphine treatment. Immunopharmacol Immunotoxicol. 2000;22:35–48. [PubMed: 10737255]
- 27.
- Pacifici R, Di Carlo S, Bacosi A. et al. Pharmacokinetics and cytokine production in heroin and morphine-treated mice. Int J Immunopharmacol. 2000;22:603–614. [PubMed: 10988355]
- 28.
- GaveriauxRuff C, Matthes H W D, Peluso J. et al. Abolition of morphineimmunosuppression in mice lacking the mu opioid receptor gene. Proc Natl Acad Sci USA. 1998;95:6326–6328. [PMC free article: PMC27678] [PubMed: 9600964]
- 29.
- Risdahl JM, Khanna KV, Peterson PK. et al. Opiates and infection. J Neuroimmunol. 1998;83:4–18. [PubMed: 9610668]
- 30.
- Chao CC, Sharp BM, Pomeroy C. et al. Lethality of morphine in mice infected with Toxoplasma gondii. J Pharmacol Exp Ther. 1990;252:605–609. [PubMed: 2313590]
- 31.
- Starec M, Rouveix B, Sinet M. et al. Immune status and survival of opiate and cocaine treated mice infected with Friend virus. J Pharmacol Exp Ther. 1991;259:745–750. [PubMed: 1941622]
- 32.
- Mathews PM, Froelich CJ, Sibbit WL. et al. Enhancement of natural cytotoxicity by betaendorphin. J Immunol. 1983;130:1658–1662. [PubMed: 6300232]
- 33.
- Freier DO, Fucks BA. A mechanism of action for morphine induced immunosuppression: corticosterone mediates morphine induced suppression of NK cell activity. J Pharmacol Exp Ther. 1993;270:1127–1133. [PubMed: 7932161]
- 34.
- Palm S, Mignar T, Kuhn K. et al. Phytohemagglutinindependent T cell proliferation is not impaired by morphine. Methods Find Exp Clin Pharmacol. 1996;18:159–165. [PubMed: 8738066]
- 35.
- Bayer BM, Gastonguay MR, Hernandez MC. Distinction between the in vitro and in vivo inhibitory effects of morphine on Iymphocyte proliferation based on agonist sensitivity and naltrexone reversibility. Immunopharmacol. 1992;23:117–124. [PubMed: 1601640]
- 36.
- Yin D, Mufson RA, Wang R. et al. Fasmediated cell death promoted by opioids. Nature. 1999;397:218. [PubMed: 9930695]
- 37.
- Weber RJ, Pert A. The periaqueductal gray matter mediates opiateinduced immunosuppression. Science. 1989;245:188–190. [PubMed: 2749256]
- 38.
- Guan L, Towsend R, Eisenstein TK. et al. The cellular basis for opioidinduced immunosuppression. Adv Exp Med Biol. 1995;373:57–64. [PubMed: 7668161]
- 39.
- Carr DJ, Rogers TJ, Weber RJ. The relevance of opioid receptors on immunocompetence and immune homeostasis. Proc Soc Exp Biol Med. 1996;213:248–57. [PubMed: 8985308]
- 40.
- Shavit Y, Depaulis A, Martin FC. et al. Involvement of brain opiate receptors in the immunesuppressive effect of morphine. Proc Natl Acad Sci USA. 1986;83:7114–7. [PMC free article: PMC386663] [PubMed: 3018757]
- 41.
- Flores LR, Dretchen KL, Bayer BM. Potential role of the autonomic nervous system in the immunosuppressive effects of acute morphine administration. Eur J Pharmacol. 1996;318:437–46. [PubMed: 9016936]
- 42.
- Sharp BM, Roy S, Bidlack JM. Evidence for opioid receptors on cells involved in host defense and the immune system. J Neuroimmunol. 1998;83:45–56. [PubMed: 9610672]
- 43.
- Bryant HU, Bernton EW, Kenner JR. et al. Role of adrenal cortical activation in the immunosuppressive effects of chronic morphine treatment. Endocrinology. 1991;128:3253–3258. [PubMed: 2036988]
- 44.
- Hall DM, Suo J, Weber RJ. Opioid mediated effects on the immune system: sympathetic nervous system involvement. J Neuroimmunol. 1998;83:29–35. [PubMed: 9610670]
- 45.
- Mellon RD, Bayer BM. Evidence for central opioid receptors in the immunomodulatory effects of morphine: review of potential mechanisms of action. J Neuroimmunol. 1998;83:19–28. [PubMed: 9610669]
- 46.
- House RV, Thomas PT, Bhargava HM. A comparative study of immunomodulation produced by in vitro exposure to delta opioid receptor agonist peptides. Peptides. 1996;17:75–81. [PubMed: 8822513]
- 47.
- Kay N, Allen J, Morley JE. Endorphins stimulate normal human peripheral blood Iymphocyte natural killer activity. Life Sci. 1984;35:53–59. [PubMed: 6204182]
- 48.
- Puente J, Maturana P, Miranda D. et al. Enhancement of human natural killer cell activity by opioid peptides: similar response to methionineenkephalin and betaendorphin. Brain Behav Immun. 1992;6:32–9. [PubMed: 1571602]
- 49.
- Oleson DR, Johnson DR. Regulation of human natural cytotoxicity by enkephalins and selective opiate agonists. Brain Behav Immun. 1988;2:171–86. [PubMed: 2907409]
- 50.
- Bajpai K, Singh VK, Agarwal SS. et al. Immunomodulatory activity of metenkephalin and its two potent analogs. Int J Immunopharmacol. 1995;17:207–212. [PubMed: 7558515]
- 51.
- Gilman SC, Schwartz JM, Milner RJ. et al. betaEndorphin enhances Iymphocyte proliferative responses. Proc Natl Acad Sci U S A. 1982;79:4226–4230. [PMC free article: PMC346611] [PubMed: 6287475]
- 52.
- Gilmore W, Weiner LP. The opioid specificity of betaendorphin enhancement of murine lymphocyte proliferation. Immunopharmacology. 1989;17:19–30. [PubMed: 2540118]
- 53.
- Hemmick LM, Bidlack JM. Betaendorphin stimulates rat T lymphocyte proliferation. J Neuroimmunol. 1990;29:239–48. [PubMed: 2170440]
- 54.
- Dubinin KV, Zakharova LA, Khegai LA. et al. Immunomodulating effect of Met-enkephalin on different stages of lymphocyte proliferation induced with Concanavalin A in vitro. Immunopharmacol lmmunotoxicol. 1994;16:463–472. [PubMed: 7798596]
- 55.
- Hucklebridge FH, Hudspith BN, Lydyard PM. et al. Stimulation of human peripheral lymphocytes by methionine enkephalin and delta-selective opioid analogues. Immunopharmacology. 1990;19:87–91. [PubMed: 2161808]
- 56.
- Hucklebridge FH, Hudspith BN, Muhamed J. et al. Methionineenkephalin stimulates in vitro proliferation of human peripheral lympho cytes via deltaopioid receptors. Brain Behav Immun. 1989;3:183–9. [PubMed: 2551418]
- 57.
- Foris G, Medgyesi GA, Hauck M. Bidirectional effects of metenkephalin on macrophage effector functions. Mol Cell Biochem. 1986;69:127–137. [PubMed: 2421152]
- 58.
- Marotti T, Burek B, Rabatic S. et al. Modulation of lipopolysaccharideinduced production of cytokines by methionineenkephalin. Immunol Lett. 1994;40:43–47. [PubMed: 7927513]
- 59.
- Szabo I, Rojavin M, Bussiere JL. et al. Suppression of peritoneal macrophage phagocytosis of Candida albicans by opioids. J Pharmacol Exp Ther. 1993;267:703–6. [PubMed: 8246144]
- 60.
- Foris G, Medgyesi GA, Gyimesi E. et al. Metenkephalin induced alterations of macrophage functions. Mol Immunol. 1984;21:747–50. [PubMed: 6087124]
- 61.
- Tosk JM, Grim JR, Kinback KM. et al. Modulation of chemiluminescence in a murine macrophage cell line by neuroendocrine hormones. Int J lmmunopharmacol. 1993;15:615–20. [PubMed: 8104166]
- 62.
- Sharp BM, Keane WF, Suh HJ. et al. Opioid peptides rapidly stimulate superoxide production by human polymoprhonuclear leukocytes and macrophages. Endocrinology. 1985;117:793–5. [PubMed: 2862014]
- 63.
- Radulovic J, Dimitrijevic M, Laban O. et al. Effect of metenkephalin and opioid antagonists on rat macrophages. Peptides. 1995;16:1209–13. [PubMed: 8545240]
- 64.
- Efanov AM, Koshkin AA, Sazanov LA. et al. Inhibition of the respiratory burst in mouse macrophages by ultralow doses of an opioid peptide is consistent with a possible adaptation mechanism. FEBS Lett. 1994;355:114–8. [PubMed: 7982481]
- 65.
- Heijnen CJ, Kavelaars A, Ballieux RE. Betaendorphin: cytokine and neuropeptide. Immunol Rev. 1991;119:41–63. [PubMed: 2045122]
- 66.
- Panerai AE, Manfredi B, Granucci F. et al. The β-endorphin inhibition of mitogeninduced splenocytes proliferation is mediated by central and peripheral paracrine/autocrine effects of the opioid. J Neuroimmunol. 1995;58:71–76. [PubMed: 7537282]
- 67.
- Schneider GM, Lysle DT. Effects of centrally administered opioid agonists on macrophage nitric oxide production and splenic lymphocyte proliferation In: Friedman H, ed. AIDS, Drugs of Abuse and the Neuroimmune Axis New York: Plenum Press, 1996. [PubMed: 8787647]
- 68.
- Manfredi B, Sacerdote P, Bianchi M. et al. Evidence for an opioid inhibitory effect on T cell proliferation. J Neuroimmunol. 1993;44:43–48. [PubMed: 8388406]
- 69.
- Lysle DT, How T. Endogenous opioids regulate the expression of inducible nitric oxide synthase by splenocytes. J Pharmacol Exp Ther. 1999;288:502–508. [PubMed: 9918551]
- 70.
- Tian M, Broxmeyer HE, Fan Y. et al. Altered hematopoiesis, behavior and sexual function in mu opioid receptor deficient mice. J Exp Med. 1997;185:1517–1522. [PMC free article: PMC2196276] [PubMed: 9126934]
- 71.
- Kieffer BL. Opioids: first lesson from knockout mice. TiPS. 1999;20:19–25. [PubMed: 10101958]
- 72.
- Mosman TR, Sad S. The expanding universe of Tcell subsets; Th1, Th2 and more. Immunol Today. 1996;17:138–146. [PubMed: 8820272]
- 73.
- Romagnani S. Lymphokine production by human T cell in disease states. Annu Rev Immunol. 1994;12:227–230. [PubMed: 8011282]
- 74.
- Sacerdote P, Manfredi B, Gaspani L. et al. The opioid antagonist naloxone induces a shift from type 2 cytokine pattern to type 1 cytokine pattern in balb/cJ mice. Blood. 2000;95:2031–2036. [PubMed: 10706871]
- 75.
- Panerai AE, Sacerdote P. Betaendorphin in the immune system: a role at last? Immunol Today. 1997;18:317–319. [PubMed: 9238833]
- 76.
- Strom T, RoyChaundhury P, Manfro R. et al. The Th1/Th2 paradigm and the allograft response. Curr Opin Immunol. 1996;8:688–93. [PubMed: 8902395]
- 77.
- Sacerdote P, Rosso di San Secondo V E M, Sirchia G. et al. Endogenous opioids modulate allograft rejection time in mice: possible relation with Th1/Th2 cytokines. Clin Exp Immunol. 1998;113:465–469. [PMC free article: PMC1905056] [PubMed: 9737678]
- 78.
- Panerai AE, Radulovic J, Monastra G. et al. Beta-endorphin concentrations in brain areas and peritoneal macrophages in rats susceptible and resistant to experimental allergic encephalomyelitis: a possible relationship between tumor necrosis factor α and opioids in the disease. J Neuroimmunol. 1994;51:169–176. [PubMed: 7514185]
- 79.
- Sacerdote P, Bianchi M, Panerai AE. Involvement of beta-endorphin in the modulation of paw inflammatory edema in the rat. Regul Pept. 1996;63:79–83. [PubMed: 8837214]
- 80.
- Khansari DN, Murgo AJ, Faith RE. Effects of stress on the immune system. Immunol Today. 1990;11:170–5. [PubMed: 2186751]
- 81.
- Shavit Y, Lewis JW, Terman GW. et al. Opioid peptides mediate the suppressive effects of stress on natural killer cell cytotoxicity. Science. 1984;223:188–190. [PubMed: 6691146]
- 82.
- Sacerdote P, Manfredi B, Bianchi M. et al. Intermittent but not continous inescapable footshock stress affects immune responses and immunocyte beta-endorphin concentrations in the rat. Brain Behav Immun. 1994;8:251–60. [PubMed: 7865896]
- 83.
- Sacerdote P. Interleukins and immunocyte betaendorphin In: Plotnikoff, Faith, Murgo, Good, eds. Cytokines, Stress and Immunity CRC Press, 1999271–280.
Publication Details
Author Information and Affiliations
Authors
Paola Sacerdote, Elena Limiroli, and Leda Gaspani.Copyright
Publisher
Landes Bioscience, Austin (TX)
NLM Citation
Sacerdote P, Limiroli E, Gaspani L. Experimental Evidence for Immunomodulatory Effects of Opioids. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.