NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-.

PDQ Cancer Information Summaries [Internet].
Show detailsThis PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood Hodgkin lymphoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
General Information About Childhood Hodgkin Lymphoma
Dramatic improvements in survival have been achieved for children and adolescents with cancer.[1] Between 1975 and 2010, childhood cancer mortality decreased by more than 50%. For Hodgkin lymphoma, the 5-year survival rate for children and adolescents has increased over the same time from 81% to more than 95%.[1]
Overview of Childhood Hodgkin Lymphoma
Childhood Hodgkin lymphoma is one of the few pediatric malignancies that shares aspects of its biology and natural history with an adult cancer. When treatment approaches for children were modeled after those used for adults, substantial morbidities resulted from unacceptably high radiation doses. Thus, new strategies using chemotherapy and lower-dose radiation were developed.
Approximately 90% to 95% of children with Hodgkin lymphoma can be cured, prompting increased attention to therapy that lessens long-term morbidity for these patients. Contemporary treatment programs use a risk-based and response-adapted approach in which patients receive multiagent chemotherapy with or without low-dose involved-field or involved-site radiation therapy. Prognostic factors used in determining chemotherapy intensity include stage, presence or absence of B symptoms (fever, weight loss, and night sweats), bulky disease, extranodal involvement, and/or erythrocyte sedimentation rate.
Epidemiology
Hodgkin lymphoma accounts for 6% of childhood cancers. In the United States, the incidence of Hodgkin lymphoma is age related and is highest among adolescents aged 15 to 19 years (29 cases per 1 million per year); children aged 10 to 14 years, 5 to 9 years, and 0 to 4 years have approximately threefold, eightfold, and 30-fold lower rates, respectively, than do adolescents.[2] In developing countries, there is a similar incidence rate in young adults but a much higher incidence rate in childhood.[3]
Hodgkin lymphoma has the following unique epidemiological features:
- Bimodal age distribution. Hodgkin lymphoma has a bimodal age distribution that differs geographically and ethnically in industrialized countries; the early peak occurs in the middle-to-late 20s and the second peak after age 50 years. In developing countries, the early peak occurs before adolescence.[4]
- Age cohorts. Hodgkin lymphoma can be segregated into the following three age cohorts because of the variation in etiologies and histological subtypes (refer to Table 1):
- -
Children:Individuals aged 14 years and younger have a higher prevalence of nodular lymphocyte-predominant disease and Epstein-Barr virus (EBV)–associated mixed-cellularity disease.
Early exposure to common infections in early childhood appears to decrease the risk of Hodgkin lymphoma, most likely by maturation of cellular immunity.[7,8]
There are more males than females affected in the younger age cohort, especially in children younger than 10 years. EBV-associated Hodgkin lymphoma increases in prevalence in association with larger family size and lower socioeconomic status.[4]
- -
Adolescents and young adults: Hodgkin lymphoma in individuals aged 15 to 34 years is associated with a higher socioeconomic status in industrialized countries, increased sibship size, and earlier birth order.[9] The lower risk of Hodgkin lymphoma observed in young adults with multiple older, but not younger, siblings, is consistent with the hypothesis that early exposure to viral infection (which the siblings bring home from school, for example) may play a role in the pathogenesis of the disease.[7]
Nodular-sclerosing Hodgkin lymphoma is the most common subtype, followed by mixed cellularity.
- -
Older adults: Hodgkin lymphoma most commonly presents in individuals aged 55 to 74 years. This group has a higher risk of lymphocyte-depleted Hodgkin lymphoma. The treatment of older adults is not discussed in this summary.
- Family history. A family history of Hodgkin lymphoma in siblings or parents has been associated with an increased risk of this disease.[10,11] In a population-based study that evaluated risk of familial classical Hodgkin lymphoma by relationship, histology, age, and sex, the cumulative risk of Hodgkin lymphoma was 0.6%, representing a 3.3-fold increased risk compared with the general population risk.[12] The risk in siblings was significantly higher than the risk in parents and/or offspring. The risk in sisters was higher than the risk in brothers or siblings of opposite sex. The lifetime risk of Hodgkin lymphoma was higher when first-degree relatives were diagnosed before age 30 years.
Table 1. Epidemiology of Hodgkin Lymphoma (HL) Across the Age Spectruma
| Variables | Childhood HL | AYA HL | Adult HL | Older Adult HL | |
|---|---|---|---|---|---|
| Age Range | ≤14 y | 15–35 y | ≥35 y | ≥55 y | |
| Prevalence of HL | 10%–12% | 50% | 35% | ||
| Gender (Male-to-Female Ratio) | 2–3 to 1 | 1 to 1–1.3 to 1 | 1.2 to 1–1 to 1.1 | ||
| Histology: | |||||
| Nodular sclerosing | 40%–45% | 65%–80% | 35%–40% | ||
| Mixed cellularity | 30%–45% | 10%–25% | 35%–50% | ||
| NLPHL | 8%–20% | 2%–8% | 7%–10% | ||
| EBV Associated | 27%–54% | 20%–25% | 34%–40% | 50%–56% | |
| Advanced Stage | 30%–35% | 40% | 55% | ||
| B Symptoms | 25% | 30%–40% | 50% | ||
| Relative Survival: Rates at 5 Years | 94% (age <20 y) | 90% (age <50 y) | 65% (age >50 y) | ||
| AYA = adolescent and young adult; EBV = Epstein-Barr virus; NLPHL = nodular lymphocyte-predominant Hodgkin lymphoma. | |||||
| aAdapted from Punnett et al.[13] | |||||
Epstein-Barr virus (EBV) and Hodgkin lymphoma
EBV has been implicated in the etiology of some cases of Hodgkin lymphoma. Patients with Hodgkin lymphoma may have high EBV titers, suggesting that a previous infection with EBV may precede the development of Hodgkin lymphoma in some patients. EBV genetic material can be detected in Reed-Sternberg cells from some patients with Hodgkin lymphoma, most commonly in those with mixed-cellularity disease.[14] In children and adolescents with intermediate-risk Hodgkin lymphoma, EBV DNA in cell-free blood correlated with the presence of EBV in the tumor; EBV DNA 8 days after the initiation of therapy predicted an inferior event-free survival (EFS).[14]
The incidence of EBV-associated Hodgkin lymphoma also shows the following distinct epidemiological features:
- Developing countries. The incidence of EBV tumor cell positivity for Hodgkin lymphoma in developed countries ranges from 15% to 25% in adolescents and young adults.[15-17] A high incidence of mixed-cellularity histology in childhood Hodgkin lymphoma is seen in developing countries, and these cases are generally EBV positive (approximately 80%).[18]
EBV serologic status is not a prognostic factor for failure-free survival in young adult patients with Hodgkin lymphoma,[15-17,19,20] but plasma EBV DNA has been associated with an inferior outcome in adults.[21] However, this is not the case in children, with better outcomes described for intermediate-risk patients with higher levels of EBV DNA at diagnosis,[14] which also correlates with better outcomes for patients with mixed-cellularity disease treated with dose-dense chemotherapy (ABVE-PC [doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide]). Patients with a previous history of serologically confirmed infectious mononucleosis have a fourfold increased risk of developing EBV-positive Hodgkin lymphoma; these patients are not at increased risk of developing EBV-negative Hodgkin lymphoma.[22]
Immunodeficiency and Hodgkin lymphoma
Among individuals with immunodeficiency, the risk of Hodgkin lymphoma is increased,[23] although the risk of non-Hodgkin lymphoma is even higher.
Characteristics of Hodgkin lymphoma presenting in the context of immunodeficiency are as follows:
- Hodgkin lymphoma usually occurs at a younger age and with histologies other than nodular sclerosing in patients with primary immunodeficiencies.[23]
- The risk of Hodgkin lymphoma increases as much as 50-fold over the general population in patients with autoimmune lymphoproliferative syndrome.[24]
- Compared with the general population, the risk of Hodgkin lymphoma is increased in recipients of solid organ transplant who are maintained on chronic immunosuppressive medications.[27]
- Hodgkin lymphoma is the second most common cancer type in children who have undergone solid organ transplant.[28]
Clinical Presentation
The following presenting features of Hodgkin lymphoma result from direct or indirect effects of nodal or extranodal involvement and/or constitutional symptoms related to cytokine release from Reed-Sternberg cells and cell signaling within the tumor microenvironment:[29]
- Approximately 80% of patients present with painless adenopathy, most commonly involving the supraclavicular or cervical area.
- Mediastinal disease is present in about 75% of adolescents and young adults and may be asymptomatic. In contrast, only about 35% of young children with Hodgkin lymphoma have mediastinal involvement, reflecting the greater prevalence of mixed-cellularity and lymphocyte-predominant histology versus nodular-sclerosing histology in this age cohort.
- Three specific constitutional symptoms (B symptoms) that have been correlated with prognosis—unexplained fever (temperature above 38.0°C orally), unexplained weight loss (10% of body weight within the 6 months preceding diagnosis), and drenching night sweats—are commonly used to assign risk in clinical trials.[32]
- Female patients with large mediastinal masses and B symptoms are most likely to present with pericardial effusions.[33][Level of evidence: 3iiC]
Fifteen percent to 20% of patients will have noncontiguous extranodal involvement (stage IV). The most common sites of extranodal involvement are the lung, liver, bones, and bone marrow.[30,31]
Prognostic Factors
As the treatment of Hodgkin lymphoma improved, factors associated with outcome became more difficult to identify. Several factors, however, continue to influence the success and choice of therapy. These factors are interrelated in the sense that disease stage, bulk, and biologic aggressiveness are frequently collinear.
Pretreatment factors
Pretreatment factors associated with an adverse outcome in one or more studies include the following:
- Presence of a pericardial effusion.[33][Level of evidence: 3iiC]
- Presence of a pleural effusion.[36][Level of evidence: 2A]
- Elevated erythrocyte sedimentation rate.[37]
- Leukocytosis (white blood cell count of 11,500/mm3 or higher).[34]
- Anemia (hemoglobin lower than 11.0 g/dL).[34]
- Hypoalbuminemia.[35]
Prognostic factors identified in selected multi-institutional studies include the following:
- In the Society for Paediatric Oncology and Haematology (Gesellschaft für Pädiatrische Onkologie und Hämatologie [GPOH]) GPOH-95 study, B symptoms, histology, and male sex were adverse prognostic factors for EFS on multivariate analysis.[31]
- In 320 children with clinically staged Hodgkin lymphoma treated in the Stanford-St. Jude-Dana Farber Cancer Institute consortium, male sex; stage IIB, IIIB, or IV disease; white blood cell count of 11,500/mm3 or higher; and hemoglobin lower than 11.0 g/dL were significant prognostic factors for inferior disease-free survival and overall survival (OS). Prognosis was also associated with the number of adverse factors.[34]
- In the CCG-5942 study, the combination of B symptoms and bulky disease was associated with an inferior outcome.[30]
- Factors associated with adverse outcome, many of which are collinear, were evaluated by multivariable analysis using the COG trial AHOD0031 (NCT00025259) for children with intermediate-risk Hodgkin lymphoma. This study enrolled 1,734 patients. The most robust predictors of outcome in this homogeneously treated cohort were stage IV disease, fever, a large mediastinal mass, and low albumin (<3.4 g/dL). The Childhood Hodgkin International Prognostic Score, highly predictive of EFS, was derived by giving a point for each adverse factor;[35] however, it requires further prospective validation.
- Pleural effusions have been shown to be an adverse prognostic finding in patients treated for low-stage Hodgkin lymphoma.[36][Level of evidence: 2A] The risk of relapse was 25% in patients with an effusion, as opposed to less than 15% in patients without an effusion. Patients with effusions were more often older (15 years vs. 14 years) and had nodular-sclerosing histology.
A single-institution study showed that African American patients had a higher relapse rate than did White patients, but OS was similar.[40] A Children’s Oncology Group (COG) analysis showed no difference in EFS by race or ethnicity. However, compared with non-Hispanic White children, Hispanic and non-Hispanic Black children had an inferior OS because of an increased postrelapse mortality rate.[41][Level of evidence: 1iiA]
Response to initial chemotherapy
The rapidity of response to initial cycles of chemotherapy also appears to be prognostically important.[38,39,42] Response evaluation in previous generations of trials relied on computed tomography and gallium uptake; more recent trials have employed positron emission tomography (PET) scanning to assess early response in pediatric Hodgkin lymphoma.[43] Fluorine F 18-fludeoxyglucose PET avidity after two cycles of chemotherapy for Hodgkin lymphoma in adults has been shown to predict treatment failure and progression-free survival.[44-46] Reduction in PET avidity after one cycle of chemotherapy was associated with a favorable EFS outcome in children with limited-stage classical Hodgkin lymphoma.[37] Additional studies in children are ongoing to assess the role of early PET-based response in modifying therapy and predicting outcome.
Prognostic factors will continue to change because of risk stratification and choice of therapy, with parameters such as disease stage, bulk, systemic symptomatology, and early response to chemotherapy used to stratify therapeutic assignment.
References
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PMC free article: PMC4136455] [PubMed: 24853691]
- Ries LAG, Harkins D, Krapcho M, et al.: SEER Cancer Statistics Review, 1975-2003. National Cancer Institute, 2006. Also available online. Last accessed June 21, 2021.
- Macfarlane GJ, Evstifeeva T, Boyle P, et al.: International patterns in the occurrence of Hodgkin's disease in children and young adult males. Int J Cancer 61 (2): 165-9, 1995. [PubMed: 7705942]
- Grufferman S, Delzell E: Epidemiology of Hodgkin's disease. Epidemiol Rev 6: 76-106, 1984. [PubMed: 6092122]
- Ries LA, Kosary CL, Hankey BF, et al., eds.: SEER Cancer Statistics Review 1973-1995. National Cancer Institute, 1998. Also available online. Last accessed June 21, 2021.
- Percy CL, Smith MA, Linet M, et al.: Lymphomas and reticuloendothelial neoplasms. In: Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649, pp 35-50. Also available online. Last accessed June 21, 2021.
- Chang ET, Montgomery SM, Richiardi L, et al.: Number of siblings and risk of Hodgkin's lymphoma. Cancer Epidemiol Biomarkers Prev 13 (7): 1236-43, 2004. [PubMed: 15247136]
- Rudant J, Orsi L, Monnereau A, et al.: Childhood Hodgkin's lymphoma, non-Hodgkin's lymphoma and factors related to the immune system: the Escale Study (SFCE). Int J Cancer 129 (9): 2236-47, 2011. [PubMed: 21170962]
- Westergaard T, Melbye M, Pedersen JB, et al.: Birth order, sibship size and risk of Hodgkin's disease in children and young adults: a population-based study of 31 million person-years. Int J Cancer 72 (6): 977-81, 1997. [PubMed: 9378561]
- Crump C, Sundquist K, Sieh W, et al.: Perinatal and family risk factors for Hodgkin lymphoma in childhood through young adulthood. Am J Epidemiol 176 (12): 1147-58, 2012. [PMC free article: PMC3571233] [PubMed: 23171883]
- Linabery AM, Erhardt EB, Richardson MR, et al.: Family history of cancer and risk of pediatric and adolescent Hodgkin lymphoma: A Children's Oncology Group study. Int J Cancer 137 (9): 2163-74, 2015. [PMC free article: PMC4833396] [PubMed: 25940226]
- Kharazmi E, Fallah M, Pukkala E, et al.: Risk of familial classical Hodgkin lymphoma by relationship, histology, age, and sex: a joint study from five Nordic countries. Blood 126 (17): 1990-5, 2015. [PubMed: 26311361]
- Punnett A, Tsang RW, Hodgson DC: Hodgkin lymphoma across the age spectrum: epidemiology, therapy, and late effects. Semin Radiat Oncol 20 (1): 30-44, 2010. [PubMed: 19959029]
- Welch JJG, Schwartz CL, Higman M, et al.: Epstein-Barr virus DNA in serum as an early prognostic marker in children and adolescents with Hodgkin lymphoma. Blood Adv 1 (11): 681-684, 2017. [PMC free article: PMC5727814] [PubMed: 29296710]
- Claviez A, Tiemann M, Lüders H, et al.: Impact of latent Epstein-Barr virus infection on outcome in children and adolescents with Hodgkin's lymphoma. J Clin Oncol 23 (18): 4048-56, 2005. [PubMed: 15961758]
- Lee JH, Kim Y, Choi JW, et al.: Prevalence and prognostic significance of Epstein-Barr virus infection in classical Hodgkin's lymphoma: a meta-analysis. Arch Med Res 45 (5): 417-31, 2014. [PubMed: 24937173]
- Jarrett RF, Stark GL, White J, et al.: Impact of tumor Epstein-Barr virus status on presenting features and outcome in age-defined subgroups of patients with classic Hodgkin lymphoma: a population-based study. Blood 106 (7): 2444-51, 2005. [PubMed: 15941916]
- Chabay PA, Barros MH, Hassan R, et al.: Pediatric Hodgkin lymphoma in 2 South American series: a distinctive epidemiologic pattern and lack of association of Epstein-Barr virus with clinical outcome. J Pediatr Hematol Oncol 30 (4): 285-91, 2008. [PubMed: 18391697]
- Armstrong AA, Alexander FE, Cartwright R, et al.: Epstein-Barr virus and Hodgkin's disease: further evidence for the three disease hypothesis. Leukemia 12 (8): 1272-6, 1998. [PubMed: 9697883]
- Herling M, Rassidakis GZ, Vassilakopoulos TP, et al.: Impact of LMP-1 expression on clinical outcome in age-defined subgroups of patients with classical Hodgkin lymphoma. Blood 107 (3): 1240; author reply 1241, 2006. [PubMed: 16434497]
- Kanakry JA, Li H, Gellert LL, et al.: Plasma Epstein-Barr virus DNA predicts outcome in advanced Hodgkin lymphoma: correlative analysis from a large North American cooperative group trial. Blood 121 (18): 3547-53, 2013. [PMC free article: PMC3643756] [PubMed: 23386127]
- Hjalgrim H, Askling J, Rostgaard K, et al.: Characteristics of Hodgkin's lymphoma after infectious mononucleosis. N Engl J Med 349 (14): 1324-32, 2003. [PubMed: 14523140]
- Robison LL, Stoker V, Frizzera G, et al.: Hodgkin's disease in pediatric patients with naturally occurring immunodeficiency. Am J Pediatr Hematol Oncol 9 (2): 189-92, 1987. [PubMed: 3592132]
- Straus SE, Jaffe ES, Puck JM, et al.: The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood 98 (1): 194-200, 2001. [PubMed: 11418480]
- Biggar RJ, Jaffe ES, Goedert JJ, et al.: Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood 108 (12): 3786-91, 2006. [PMC free article: PMC1895473] [PubMed: 16917006]
- Biggar RJ, Frisch M, Goedert JJ: Risk of cancer in children with AIDS. AIDS-Cancer Match Registry Study Group. JAMA 284 (2): 205-9, 2000. [PubMed: 10889594]
- Knight JS, Tsodikov A, Cibrik DM, et al.: Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol 27 (20): 3354-62, 2009. [PubMed: 19451438]
- Yanik EL, Smith JM, Shiels MS, et al.: Cancer Risk After Pediatric Solid Organ Transplantation. Pediatrics 139 (5): , 2017. [PMC free article: PMC5404730] [PubMed: 28557749]
- Steidl C, Connors JM, Gascoyne RD: Molecular pathogenesis of Hodgkin's lymphoma: increasing evidence of the importance of the microenvironment. J Clin Oncol 29 (14): 1812-26, 2011. [PubMed: 21483001]
- Nachman JB, Sposto R, Herzog P, et al.: Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin's disease who achieve a complete response to chemotherapy. J Clin Oncol 20 (18): 3765-71, 2002. [PubMed: 12228196]
- Rühl U, Albrecht M, Dieckmann K, et al.: Response-adapted radiotherapy in the treatment of pediatric Hodgkin's disease: an interim report at 5 years of the German GPOH-HD 95 trial. Int J Radiat Oncol Biol Phys 51 (5): 1209-18, 2001. [PubMed: 11728679]
- Gobbi PG, Cavalli C, Gendarini A, et al.: Reevaluation of prognostic significance of symptoms in Hodgkin's disease. Cancer 56 (12): 2874-80, 1985. [PubMed: 4052959]
- Marks LJ, McCarten KM, Pei Q, et al.: Pericardial effusion in Hodgkin lymphoma: a report from the Children's Oncology Group AHOD0031 protocol. Blood 132 (11): 1208-1211, 2018. [PMC free article: PMC6137557] [PubMed: 30061157]
- Smith RS, Chen Q, Hudson MM, et al.: Prognostic factors for children with Hodgkin's disease treated with combined-modality therapy. J Clin Oncol 21 (10): 2026-33, 2003. [PubMed: 12743158]
- Schwartz CL, Chen L, McCarten K, et al.: Childhood Hodgkin International Prognostic Score (CHIPS) Predicts event-free survival in Hodgkin Lymphoma: A Report from the Children's Oncology Group. Pediatr Blood Cancer 64 (4): , 2017. [PMC free article: PMC5702912] [PubMed: 27786406]
- McCarten KM, Metzger ML, Drachtman RA, et al.: Significance of pleural effusion at diagnosis in pediatric Hodgkin lymphoma: a report from Children's Oncology Group protocol AHOD0031. Pediatr Radiol 48 (12): 1736-1744, 2018. [PMC free article: PMC6208959] [PubMed: 30014200]
- Keller FG, Castellino SM, Chen L, et al.: Results of the AHOD0431 trial of response adapted therapy and a salvage strategy for limited stage, classical Hodgkin lymphoma: A report from the Children's Oncology Group. Cancer 124 (15): 3210-3219, 2018. [PMC free article: PMC6097921] [PubMed: 29738613]
- Landman-Parker J, Pacquement H, Leblanc T, et al.: Localized childhood Hodgkin's disease: response-adapted chemotherapy with etoposide, bleomycin, vinblastine, and prednisone before low-dose radiation therapy-results of the French Society of Pediatric Oncology Study MDH90. J Clin Oncol 18 (7): 1500-7, 2000. [PubMed: 10735898]
- Friedman DL, Chen L, Wolden S, et al.: Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children's Oncology Group Study AHOD0031. J Clin Oncol 32 (32): 3651-8, 2014. [PMC free article: PMC4220044] [PubMed: 25311218]
- Metzger ML, Castellino SM, Hudson MM, et al.: Effect of race on the outcome of pediatric patients with Hodgkin's lymphoma. J Clin Oncol 26 (8): 1282-8, 2008. [PubMed: 18323551]
- Kahn JM, Kelly KM, Pei Q, et al.: Survival by Race and Ethnicity in Pediatric and Adolescent Patients With Hodgkin Lymphoma: A Children's Oncology Group Study. J Clin Oncol 37 (32): 3009-3017, 2019. [PMC free article: PMC6839907] [PubMed: 31539308]
- Weiner MA, Leventhal B, Brecher ML, et al.: Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin's disease in pediatric patients: a Pediatric Oncology Group study. J Clin Oncol 15 (8): 2769-79, 1997. [PubMed: 9256118]
- Ilivitzki A, Radan L, Ben-Arush M, et al.: Early interim FDG PET/CT prediction of treatment response and prognosis in pediatric Hodgkin disease-added value of low-dose CT. Pediatr Radiol 43 (1): 86-92, 2013. [PubMed: 23151729]
- Hutchings M, Loft A, Hansen M, et al.: FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 107 (1): 52-9, 2006. [PubMed: 16150944]
- Gallamini A, Hutchings M, Rigacci L, et al.: Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. J Clin Oncol 25 (24): 3746-52, 2007. [PubMed: 17646666]
- Dann EJ, Bar-Shalom R, Tamir A, et al.: Risk-adapted BEACOPP regimen can reduce the cumulative dose of chemotherapy for standard and high-risk Hodgkin lymphoma with no impairment of outcome. Blood 109 (3): 905-9, 2007. [PubMed: 17018856]
Cellular Classification and Biologic Correlates of Childhood Hodgkin Lymphoma
Hodgkin lymphoma is characterized by a variable number of characteristic multinucleated giant cells (Reed-Sternberg cells) or large mononuclear cell variants (lymphocytic and histiocytic cells) in a background of inflammatory cells consisting of small lymphocytes, histiocytes, epithelioid histiocytes, neutrophils, eosinophils, plasma cells, and fibroblasts. The inflammatory cells are present in different proportions depending on the histologic subtype. It has been conclusively shown that Reed-Sternberg cells and/or lymphocytic and histiocytic cells represent a clonal population. Almost all cases of Hodgkin lymphoma arise from germinal center B cells.[1,2]
The histologic features and clinical symptoms of Hodgkin lymphoma have been attributed to the numerous cytokines, chemokines, and products of the tumor necrosis factor receptors (TNF-R) family secreted by the Reed-Sternberg cells and cell signaling within the tumor microenvironment.[3-5]
The hallmark of Hodgkin lymphoma is the Reed-Sternberg cell and its variants,[6] which have the following features:
- The Reed-Sternberg cell is a binucleated or multinucleated giant cell with a bilobed nucleus and two large nucleoli that give a characteristic owl's eye appearance.[6]
- The malignant Reed-Sternberg cell comprises only about 1% of the abundant reactive cellular infiltrate of lymphocytes, macrophages, granulocytes, and eosinophils in involved specimens.[6]
- In nodular lymphocyte-predominant Hodgkin lymphoma, the Reed-Sternberg cells are usually mononuclear with a markedly convoluted and lobated nucleus (popcorn cells). Also known as lymphocytic and histiocytic cells, this Reed-Sternberg–cell variant does not express CD30, but does express CD20, suggesting that it is biologically distinct from other subtypes of Hodgkin lymphoma.
- Most cases of classical Hodgkin lymphoma are characterized by expression of TNF-R and their ligands, as well as an unbalanced production of T helper lymphocytes type 2 (Th2) cytokines and chemokines. Activation of TNF-R results in constitutive activation of nuclear factor kappa B in Reed-Sternberg cells, which may prevent apoptosis and provide a survival advantage.[10]
- Genotyping using circulating cell-free DNA and genomic DNA from laser-captured, microdissected Reed-Sternberg cells of pediatric patients with Hodgkin lymphoma revealed single nucleotide variants, translocations, or insertions/deletions of 63 genes in 72 of the 96 patients. Variants of SOCS1 and other genes regulating the JAK/STAT pathways were the most frequently detected. The number of tumor genome equivalents was higher in patients with bulky disease.[13]
Hodgkin lymphoma can be divided into the following two broad pathologic classes:[14,15]
Classical Hodgkin Lymphoma
Classical Hodgkin lymphoma is divided into four subtypes. These subtypes are defined according to the number of Reed-Sternberg cells, characteristics of the inflammatory milieu, and the presence or absence of fibrosis.
Characteristics of the four histological subtypes of classical Hodgkin lymphoma include the following:
- Lymphocyte-rich Hodgkin lymphoma. Lymphocyte-rich classical Hodgkin lymphoma may have a nodular appearance, but immunophenotypic analysis allows distinction between this form of Hodgkin lymphoma and nodular lymphocyte-predominant Hodgkin lymphoma.[16] Lymphocyte-rich classical Hodgkin lymphoma cells express CD15 and CD30.
- Nodular-sclerosing Hodgkin lymphoma. Nodular-sclerosing Hodgkin lymphoma histology accounts for approximately 80% of Hodgkin lymphoma cases in older children and adolescents but only 55% of cases in younger children in the United States.[17]This subtype is distinguished by the presence of collagenous bands that divide the lymph node into nodules, which often contain a Reed-Sternberg cell variant called the lacunar cell. Transforming growth factor-beta may be responsible for the fibrosis in the nodular-sclerosing Hodgkin lymphoma subtype.A study of over 600 patients with nodular-sclerosing Hodgkin lymphoma from three different university hospitals in the United States showed that two haplotypes in the HLA class II region correlated with a 70% increased risk of developing nodular-sclerosing Hodgkin lymphoma.[18] Another haplotype was associated with a 60% decreased risk of developing Hodgkin lymphoma. It is hypothesized that these haplotypes are associated with atypical immune responses that predispose to Hodgkin lymphoma.
- Mixed-cellularity Hodgkin lymphoma. Mixed-cellularity Hodgkin lymphoma is more common in young children than in adolescents and adults, with mixed-cellularity Hodgkin lymphoma accounting for approximately 20% of cases in children younger than 10 years, but only approximately 9% of older children and adolescents aged 10 to 19 years in the United States.[17]Reed-Sternberg cells are frequent in a background of abundant normal reactive cells (lymphocytes, plasma cells, eosinophils, and histiocytes). Interleukin-5 may be responsible for the eosinophilia in mixed-cellularity Hodgkin lymphoma. This subtype can be difficult to distinguish from non-Hodgkin lymphoma.
- Lymphocyte-depleted Hodgkin lymphoma. Lymphocyte-depleted Hodgkin lymphoma is rare in children. It is common in adult patients with HIV.This subtype is characterized by the presence of numerous large, bizarre malignant cells, many Reed-Sternberg cells, and few lymphocytes. Diffuse fibrosis and necrosis are common. Many cases previously diagnosed as lymphocyte-depleted Hodgkin lymphoma are now recognized as diffuse large B-cell lymphoma, anaplastic large cell lymphoma, or nodular-sclerosing classical Hodgkin lymphoma with lymphocyte depletion.[19]
Nodular Lymphocyte-Predominant Hodgkin Lymphoma
The frequency of nodular lymphocyte-predominant Hodgkin lymphoma in the pediatric population ranges from 5% to 10% in different studies, with a higher frequency in children younger than 10 years than in children aged 10 to 19 years.[17] Nodular lymphocyte-predominant Hodgkin lymphoma is most common in males younger than 18 years.[20,21]
Characteristics of nodular lymphocyte-predominant Hodgkin lymphoma include the following:
- Nodular lymphocyte-predominant Hodgkin lymphoma is characterized by molecular and immunophenotypic evidence of B-lineage differentiation with the following distinctive features:
- -
Nodular lymphocyte-predominant Hodgkin lymphoma is characterized by large cells with multilobed nuclei, referred to as popcorn cells. These cells express B-cell antigens, such as CD19, CD20, CD22, and CD79A, and are negative for CD15 and may or may not express CD30.[22]
- -
The OCT2 and BOB1 oncogenes are both expressed in nodular lymphocyte-predominant Hodgkin lymphoma; they are not expressed in the cells of patients with classical Hodgkin lymphoma.[23]
- -
Reliable discrimination from non-Hodgkin lymphoma is problematic in diffuse subtypes with lymphocytic and histiocytic cells set against a diffuse background of reactive T cells.[24]
- -
Nodular lymphocyte-predominant Hodgkin lymphoma can be difficult to distinguish from progressive transformation of germinal centers and/or T-cell–rich B-cell lymphoma.[25]
- -
Histologic and immunophenotypic variants (including CD30 and immunoglobulin D expression) may impact event-free survival.[26]
- Pediatric patients (aged <20 years) have better outcomes than do adult patients, even when controlled for other prognostic factors.[21] Chemotherapy and/or radiation therapy produce excellent long-term progression-free survival and overall survival in patients with nodular lymphocyte-predominant Hodgkin lymphoma; however, radiation therapy alone should not be considered for prepubescent patients because the evidence-based doses necessary for tumor control are associated with musculoskeletal impairment. When radiation is administered with chemotherapy, lower radiation doses are effective. Late recurrences have been reported up to 10 years after initial therapy.[27,28]; [29][Level of evidence: 2A]
- Deaths observed among individuals with nodular lymphocyte-predominant Hodgkin lymphoma are more frequently related to treatment complications and/or the development of subsequent neoplasms (including non-Hodgkin lymphoma) than in refractory disease, underscoring the importance of judicious use of chemotherapy and radiation therapy at initial presentation and after recurrent disease.[27,28]
References
- Bräuninger A, Schmitz R, Bechtel D, et al.: Molecular biology of Hodgkin's and Reed/Sternberg cells in Hodgkin's lymphoma. Int J Cancer 118 (8): 1853-61, 2006. [PubMed: 16385563]
- Mathas S: The pathogenesis of classical Hodgkin's lymphoma: a model for B-cell plasticity. Hematol Oncol Clin North Am 21 (5): 787-804, 2007. [PubMed: 17908620]
- Re D, Küppers R, Diehl V: Molecular pathogenesis of Hodgkin's lymphoma. J Clin Oncol 23 (26): 6379-86, 2005. [PubMed: 16155023]
- Steidl C, Connors JM, Gascoyne RD: Molecular pathogenesis of Hodgkin's lymphoma: increasing evidence of the importance of the microenvironment. J Clin Oncol 29 (14): 1812-26, 2011. [PubMed: 21483001]
- Diefenbach C, Steidl C: New strategies in Hodgkin lymphoma: better risk profiling and novel treatments. Clin Cancer Res 19 (11): 2797-803, 2013. [PMC free article: PMC3928836] [PubMed: 23447000]
- Küppers R, Schwering I, Bräuninger A, et al.: Biology of Hodgkin's lymphoma. Ann Oncol 13 (Suppl 1): 11-8, 2002. [PubMed: 12078890]
- Portlock CS, Donnelly GB, Qin J, et al.: Adverse prognostic significance of CD20 positive Reed-Sternberg cells in classical Hodgkin's disease. Br J Haematol 125 (6): 701-8, 2004. [PubMed: 15180859]
- von Wasielewski R, Mengel M, Fischer R, et al.: Classical Hodgkin's disease. Clinical impact of the immunophenotype. Am J Pathol 151 (4): 1123-30, 1997. [PMC free article: PMC1858022] [PubMed: 9327746]
- Tzankov A, Zimpfer A, Pehrs AC, et al.: Expression of B-cell markers in classical Hodgkin lymphoma: a tissue microarray analysis of 330 cases. Mod Pathol 16 (11): 1141-7, 2003. [PubMed: 14614054]
- Skinnider BF, Mak TW: The role of cytokines in classical Hodgkin lymphoma. Blood 99 (12): 4283-97, 2002. [PubMed: 12036854]
- Steidl C, Shah SP, Woolcock BW, et al.: MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature 471 (7338): 377-81, 2011. [PMC free article: PMC3902849] [PubMed: 21368758]
- Green MR, Monti S, Rodig SJ, et al.: Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 116 (17): 3268-77, 2010. [PMC free article: PMC2995356] [PubMed: 20628145]
- Desch AK, Hartung K, Botzen A, et al.: Genotyping circulating tumor DNA of pediatric Hodgkin lymphoma. Leukemia 34 (1): 151-166, 2020. [PubMed: 31431735]
- Pileri SA, Ascani S, Leoncini L, et al.: Hodgkin's lymphoma: the pathologist's viewpoint. J Clin Pathol 55 (3): 162-76, 2002. [PMC free article: PMC1769601] [PubMed: 11896065]
- Harris NL: Hodgkin's lymphomas: classification, diagnosis, and grading. Semin Hematol 36 (3): 220-32, 1999. [PubMed: 10462322]
- Anagnostopoulos I, Hansmann ML, Franssila K, et al.: European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood 96 (5): 1889-99, 2000. [PubMed: 10961891]
- Bazzeh F, Rihani R, Howard S, et al.: Comparing adult and pediatric Hodgkin lymphoma in the Surveillance, Epidemiology and End Results Program, 1988-2005: an analysis of 21 734 cases. Leuk Lymphoma 51 (12): 2198-207, 2010. [PubMed: 21054151]
- Cozen W, Li D, Best T, et al.: A genome-wide meta-analysis of nodular sclerosing Hodgkin lymphoma identifies risk loci at 6p21.32. Blood 119 (2): 469-75, 2012. [PMC free article: PMC3257012] [PubMed: 22086417]
- Slack GW, Ferry JA, Hasserjian RP, et al.: Lymphocyte depleted Hodgkin lymphoma: an evaluation with immunophenotyping and genetic analysis. Leuk Lymphoma 50 (6): 937-43, 2009. [PubMed: 19455461]
- Hall GW, Katzilakis N, Pinkerton CR, et al.: Outcome of children with nodular lymphocyte predominant Hodgkin lymphoma - a Children's Cancer and Leukaemia Group report. Br J Haematol 138 (6): 761-8, 2007. [PubMed: 17760808]
- Gerber NK, Atoria CL, Elkin EB, et al.: Characteristics and outcomes of patients with nodular lymphocyte-predominant Hodgkin lymphoma versus those with classical Hodgkin lymphoma: a population-based analysis. Int J Radiat Oncol Biol Phys 92 (1): 76-83, 2015. [PubMed: 25863756]
- Shankar A, Daw S: Nodular lymphocyte predominant Hodgkin lymphoma in children and adolescents--a comprehensive review of biology, clinical course and treatment options. Br J Haematol 159 (3): 288-98, 2012. [PubMed: 22994199]
- Stein H, Marafioti T, Foss HD, et al.: Down-regulation of BOB.1/OBF.1 and Oct2 in classical Hodgkin disease but not in lymphocyte predominant Hodgkin disease correlates with immunoglobulin transcription. Blood 97 (2): 496-501, 2001. [PubMed: 11154228]
- Boudová L, Torlakovic E, Delabie J, et al.: Nodular lymphocyte-predominant Hodgkin lymphoma with nodules resembling T-cell/histiocyte-rich B-cell lymphoma: differential diagnosis between nodular lymphocyte-predominant Hodgkin lymphoma and T-cell/histiocyte-rich B-cell lymphoma. Blood 102 (10): 3753-8, 2003. [PubMed: 12881319]
- Kraus MD, Haley J: Lymphocyte predominance Hodgkin's disease: the use of bcl-6 and CD57 in diagnosis and differential diagnosis. Am J Surg Pathol 24 (8): 1068-78, 2000. [PubMed: 10935647]
- Untanu RV, Back J, Appel B, et al.: Variant histology, IgD and CD30 expression in low-risk pediatric nodular lymphocyte predominant Hodgkin lymphoma: A report from the Children's Oncology Group. Pediatr Blood Cancer 65 (1): , 2018. [PMC free article: PMC5699946] [PubMed: 28802087]
- Chen RC, Chin MS, Ng AK, et al.: Early-stage, lymphocyte-predominant Hodgkin's lymphoma: patient outcomes from a large, single-institution series with long follow-up. J Clin Oncol 28 (1): 136-41, 2010. [PubMed: 19933914]
- Jackson C, Sirohi B, Cunningham D, et al.: Lymphocyte-predominant Hodgkin lymphoma--clinical features and treatment outcomes from a 30-year experience. Ann Oncol 21 (10): 2061-8, 2010. [PubMed: 20332141]
- Appel BE, Chen L, Buxton AB, et al.: Minimal Treatment of Low-Risk, Pediatric Lymphocyte-Predominant Hodgkin Lymphoma: A Report From the Children's Oncology Group. J Clin Oncol 34 (20): 2372-9, 2016. [PMC free article: PMC4981978] [PubMed: 27185849]
Diagnosis and Staging Information for Childhood Hodgkin Lymphoma
Staging and evaluation of disease status is undertaken at diagnosis and performed again early in the course of chemotherapy and at the end of chemotherapy.
Diagnostic and Staging Evaluation
The diagnostic and staging evaluation is a critical determinant in the selection of treatment. Initial evaluation of the child with Hodgkin lymphoma includes the following:
- History of systemic symptoms (detailed).
- Physical examination.
- Laboratory studies, including complete blood count, chemistry panel with albumin, and erythrocyte sedimentation rate.
- Anatomical imaging, including chest x-ray and computed tomography (CT) or magnetic resonance imaging (MRI) of the neck, chest, abdomen, and pelvis. MRI and positron emission tomography (PET)–MRI provide the advantage of limiting radiation exposure.[1]
- Functional imaging, including PET scan.
Systemic symptoms
The following three specific constitutional symptoms (B symptoms) correlate with prognosis and are considered in assignment of stage:
- Unexplained fever with temperatures above 38.0°C orally.
- Unexplained weight loss of 10% within the 6 months preceding diagnosis.
- Drenching night sweats.
Additional Hodgkin-associated constitutional symptoms without prognostic significance include the following:
- Pruritus.
- Alcohol-induced nodal pain.
Physical examination
- All node-bearing areas, including the Waldeyer ring, should be assessed by careful physical examination.
- Enlarged nodes should be measured to establish a baseline for evaluation of therapy response.
Laboratory studies
- Hematological and chemical blood parameters (e.g., albumin) show nonspecific changes that may correlate with disease extent.
- Abnormalities of peripheral blood counts may include neutrophilic leukocytosis, lymphopenia, eosinophilia, and monocytosis.
- Acute-phase reactants such as the erythrocyte sedimentation rate and C-reactive protein, if abnormal at diagnosis, may be useful in follow-up evaluation.[2]
Anatomical imaging
Anatomical information from CT or MRI is complemented by PET functional imaging, which is sensitive in determining initial sites of involvement, particularly in sites too small to be considered clearly involved by CT or MRI criteria. Collaboration across international groups to harmonize definitions is ongoing.[3]
Definition of bulky disease
Historically, the presence of bulky disease, especially mediastinal bulk, predicted an increased risk of local failure and resulted in the incorporation of bulk as an important factor in treatment assignment. The definition of bulk has varied across pediatric protocols and evolved over time with advances in diagnostic imaging technology.[3]
The criteria for bulky mediastinal and nonmediastinal disease are as follows:
- Mediastinal. In North American protocols, the posteroanterior chest radiograph remains important because the criterion for bulky mediastinal lymphadenopathy is defined by the ratio of the diameter of the mediastinal lymph node mass to the maximal diameter of the rib cage on an upright chest radiograph; a ratio of 33% or higher is considered bulky. In contrast, the EuroNet-Pediatric Hodgkin Lymphoma Group defines mediastinal bulk by the volume of the largest contiguous lymph node mass being 200 mL or more on CT. These two definitions differ from the recently published consensus guidelines from the International Conference on Malignant Lymphomas Imaging Group (Lugano), where bulk is described as a mass 10 cm or larger unidimensionally on CT.[4]
- Nonmediastinal. The criteria for bulky peripheral, nonmediastinal lymphadenopathy have also varied over the years in cooperative group study protocols, and this disease characteristic has not been consistently used for treatment stratification. In contemporary U.S. protocols, bulky peripheral lymphadenopathy is defined as greater than 6 cm, with aggregates measured transversely or cranial-caudal. In EuroNet protocols, peripheral adenopathy is again defined as a volume of greater than 200 mL, which is generally larger than a 6 cm unidimensional mass.
Criteria for lymphomatous involvement by CT or MRI
Defining strict CT or MRI size criteria for lymphomatous nodal involvement is complicated by several factors, such as size overlap between what proves to be benign reactive hyperplasia versus malignant lymphadenopathy, the implication of nodal clusters, and obliquity of node orientation to the scan plane. Additional difficulties more specific to children include greater variability of normal nodal size and the frequent occurrence of reactive hyperplasia.
General concepts to consider in regard to defining lymphomatous involvement by CT or MRI include the following:
- Contiguous nodal clustering or matting is highly suggestive of lymphomatous involvement.
- Any focal mass lesion large enough to characterize in a visceral organ is considered lymphomatous involvement unless the imaging characteristics indicate an alternative etiology.
- Criteria for nodal involvement may vary by cooperative group or protocol.[3]
- -
Children's Oncology Group (COG) and EuroNet protocols consider lymph nodes abnormal if the long axis is greater than 2 cm, regardless of the short axis and PET avidity. Lymph nodes with a long axis measuring between 1 cm and 2 cm are only considered abnormal if they are part of a conglomerate of nodes and are fluorine F 18-fludeoxyglucose (18F-FDG) PET positive.
- -
In the Society for Paediatric Oncology and Haematology (Gesellschaft für Pädiatrische Onkologie und Hämatologie [GPOH]) GPOH-HD-2002 study, nodal involvement was defined as node size greater than 2 cm in largest diameter. The node was not involved if it was less than 1 cm and was considered questionable if it was between 1 cm and 2 cm. The decision on involvement was then made on the basis of additional clinical evidence.[5]
- -
In an analysis of 47,828 imaging measurements from 2,983 individual patients with adult and pediatric lymphoma enrolled in ten multicenter clinical trials, a single dimension measurement of 15 mm or more constituted involvement.[6]
Functional imaging
The recommended functional imaging procedure for initial staging is PET, using the radioactive glucose analog, 18F-FDG.[7,8] 18F-FDG PET identifies areas of tumor with increased metabolic activity, specifically anaerobic glycolysis. PET-CT, which integrates functional and anatomical tumor characteristics, is often used for staging and monitoring of pediatric patients with Hodgkin lymphoma. Residual or persistent 18F-FDG avidity has been correlated with poor prognosis and the need for additional therapy in posttreatment evaluation.[9-12]; [13][Level of evidence: 2Diii]
General concepts to consider in regard to defining lymphomatous involvement by 18F-FDG PET include the following:
- Concordance between PET and CT data is generally high for nodal regions, but may be significantly lower for extranodal sites. In one study specifically analyzing pediatric Hodgkin lymphoma patients, assessment of initial staging comparing PET and CT data demonstrated concordance of approximately 86% overall. Concordance rates were significantly lower for the spleen, lung nodules, bone/bone marrow, and pleural and pericardial effusions.[14] A meta-analysis of nine clinical studies showed that PET-CT achieved high sensitivity (96.9%) and high specificity (99.7%) in detecting bone marrow involvement in newly diagnosed patients with Hodgkin lymphoma, with focal involvement highly predictive of bone marrow involvement.[15,16]
- Staging criteria using PET and CT scan information is protocol dependent, but generally areas of PET positivity that do not correspond to an anatomical lesion by clinical examination or CT scan size criteria should be disregarded in staging, with the possible exception of focally PET-positive bone marrow findings.
- A suspected anatomical lesion that is PET negative should not be considered involved unless proven by biopsy.
18F-FDG PET has limitations in the pediatric setting. Tracer avidity may be seen in a variety of nonmalignant conditions including thymic rebound commonly observed after completion of lymphoma therapy. 18F-FDG avidity in normal tissues, such as brown fat in the neck, may confound interpretation of the presence of nodal involvement by lymphoma.[7]
Visual PET criteria are scored according to uptake involved by lymphoma from the Deauville 5-point scale, from 1 to 5, as described in Table 2. The COG and EuroNet definitions of PET response of lymph nodes or nodal masses are described in Table 3.
Table 2. Deauville Score Criteria
| Deauville Score (Visual Score) | Criteria |
|---|---|
| 1 | No uptake. |
| 2 | Uptake ≤ mediastinal blood pool. |
| 3 | Uptake > mediastinal blood pool and ≤ normal liver. |
| 4 | Moderately increased uptake > normal liver. |
| 5 | Markedly increased uptake > normal liver. |
Table 3. Children's Oncology Group and EuroNet Definition of PET Response of Lymph Node or Nodal Masses
| Timing of 18F-FDG PET | 18F-FDG PET Avidity |
|---|---|
| Baseline PET (PET 0) response visual threshold utilizes mediastinal blood pool as the reference activity: | 18F-FDG PET positive is defined as visual score 3, 4, 5. |
| 18F-FDG PET negative is defined as visual score 1, 2. | |
| Interim postcycle 2 PET (PET 2) response visual threshold uses normal liver as the reference activity: | 18F-FDG PET positive is defined as visual score 4, 5. |
| 18F-FDG PET negative is defined as visual score 1, 2, 3. | |
| End of chemotherapy PET (PET 4 or 5) response visual threshold also utilizes mediastinal blood pool as the reference activity: | 18F-FDG PET positive is defined as visual score 3, 4, 5. |
| 18F-FDG PET negative is defined as visual score 1, 2. |
18F-FDG = fluorine F 18-fludeoxyglucose; PET = positron emission tomography.
Establishing the Diagnosis of Hodgkin Lymphoma
After a careful physiologic and radiographic evaluation of the patient, the least invasive procedure should be used to establish the diagnosis of lymphoma. However, this should not be interpreted to mean that a needle biopsy is the optimal methodology. Small fragments of lymphoma tissue are often inadequate for diagnosis, resulting in the need for second procedures that delay the diagnosis.
If possible, the diagnosis should be established by biopsy of one or more peripheral lymph nodes. The likelihood of obtaining sufficient tissue should be carefully considered when selecting a biopsy procedure. Other issues to consider in choosing the diagnostic approach to lymph node biopsy include the following:
- Type of biopsy procedure.
- -
Aspiration cytology alone is not recommended because of the lack of stromal tissue, the small number of cells present in the specimen, and the difficulty of classifying Hodgkin lymphoma into one of the subtypes.
- -
An image-guided biopsy may be used to obtain diagnostic tissue from intra-thoracic or intra-abdominal lymph nodes. On the basis of the involved sites of disease, alternative procedures that may be considered include thoracoscopy, mediastinoscopy, and laparoscopy. Thoracotomy or laparotomy is rarely needed to access diagnostic tissue.
- -
Because bone marrow involvement is relatively rare in pediatric Hodgkin lymphoma patients, bilateral bone marrow biopsy should be performed only in patients with advanced disease (stage III or stage IV) and/or B symptoms.[18]
In support of this, a meta-analysis of nine clinical studies including both pediatric and adult patients showed that PET-CT achieved high sensitivity (96.9%) and high specificity (99.7%) in detecting bone marrow involvement in newly diagnosed patients with Hodgkin lymphoma.[15] (Refer to the Stage Information for Adult HL section in the PDQ summary on Adult Hodgkin Lymphoma Treatment for more information.) In a consensus statement based on these studies, this group no longer recommends bone marrow biopsy in the initial evaluation of adults with Hodgkin lymphoma, with PET-CT being used instead to identify bone marrow involvement.[4]
- Procedure-related complications.
- -
Patients with large mediastinal masses are at risk of cardiac or respiratory arrest during general anesthesia or heavy sedation.[19] After careful planning with anesthesia, peripheral lymph node biopsy or image-guided core-needle biopsy of mediastinal lymph nodes may be feasible using light sedation and local anesthesia before proceeding to more invasive procedures.
- -
If airway compromise precludes the performance of a diagnostic operative procedure, preoperative treatment with steroids or low-dose, localized radiation therapy should be considered, although that can be technically difficult if the patient cannot recline. Since preoperative treatment may affect the ability to obtain an accurate tissue diagnosis, a diagnostic biopsy should be obtained as soon as the risks associated with general anesthesia or heavy sedation are alleviated.
Ann Arbor Staging Classification for Hodgkin Lymphoma
Stage is determined by anatomical evidence of disease using CT or MRI scanning in conjunction with functional imaging. The staging classification used for Hodgkin lymphoma was adopted at the Ann Arbor Conference held in 1971 [20] and revised in 1989 (refer to Table 4).[21] Staging is independent of the imaging modality used.
Table 4. Ann Arbor Staging Classification for Hodgkin Lymphomaa
| Stage | Description |
|---|---|
| I | Involvement of a single lymphatic site (i.e., nodal region, Waldeyer's ring, thymus, or spleen) (I); or localized involvement of a single extralymphatic organ or site in the absence of any lymph node involvement (IE). |
| II | Involvement of two or more lymph node regions on the same side of the diaphragm (II); or localized involvement of a single extralymphatic organ or site in association with regional lymph node involvement with or without involvement of other lymph node regions on the same side of the diaphragm (IIE). |
| III | Involvement of lymph node regions on both sides of the diaphragm (III), which also may be accompanied by extralymphatic extension in association with adjacent lymph node involvement (IIIE) or by involvement of the spleen (IIIS) or both (IIIE,S). |
| IV | Diffuse or disseminated involvement of one or more extralymphatic organs, with or without associated lymph node involvement; or isolated extralymphatic organ involvement in the absence of adjacent regional lymph node involvement, but in conjunction with disease in distant site(s). Stage IV includes any involvement of the liver or bone marrow, lungs (other than by direct extension from another site), or cerebrospinal fluid. |
| Designations applicable to any stage | |
| A | No symptoms. |
| B | Fever (temperature >38.0ºC), drenching night sweats, unexplained loss of >10% of body weight within the preceding 6 months. |
| E | Involvement of a single extranodal site that is contiguous or proximal to the known nodal site. |
| S | Splenic involvement. |
aReprinted with permission from AJCC: Hodgkin and non-Hodgkin lymphomas. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 607-11.[22]
Extralymphatic disease resulting from direct extension of an involved lymph node region is designated E. Extralymphatic disease can cause confusion in staging. For example, the designation E is not appropriate for cases of widespread disease or diffuse extralymphatic disease (e.g., large pleural effusion that is cytologically positive for Hodgkin lymphoma), which should be considered stage IV. If pathologic proof of noncontiguous involvement of one or more extralymphatic sites has been documented, the symbol for the site of involvement, followed by a plus sign (+), is listed.
Current practice is to assign a clinical stage on the basis of findings of the clinical evaluation; however, pathologic confirmation of noncontiguous extralymphatic involvement is strongly suggested for assignment to stage IV.
Risk Stratification
After the diagnostic and staging evaluation data are acquired, patients are further classified into risk groups for the purposes of treatment planning. The classification of patients into low-, intermediate-, or high-risk categories varies considerably among the different pediatric research groups, and often even between different studies conducted by the same group, as summarized in Figure 1.[23]
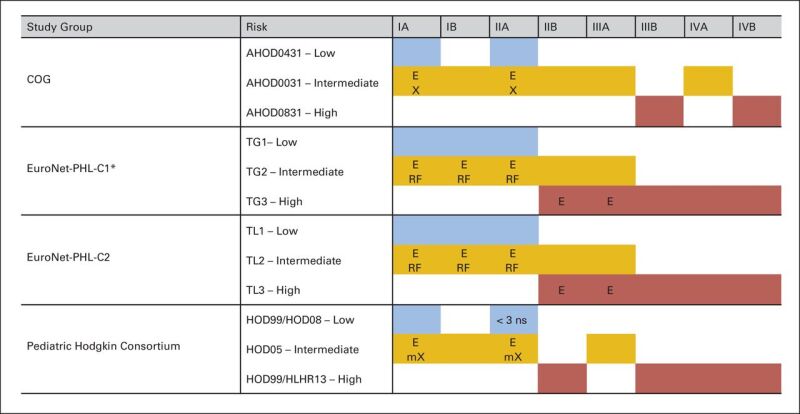
Figure 1. Variation in risk stratification across pediatric Hodgkin study groups and protocols. E, extranodal extension; X, bulky disease (peripheral >6 cm and mediastinal bulk); mX, mediastinal bulk (≥0.33 mediastinal to thoracic ratio); ns, nodal site; TG, treatment group; TL, treatment level; RF, risk factors: erythrocyte sedimentation rate (ESR) ≥30 mm/hour and/or bulk ≥200 mL. (*) EuroNet-PHL-C1 was amended in 2012: Low-risk (TG1) patients with ESR ≥30 mm/hour and/or bulk ≥200 mL were treated in TG2 (intermediate risk). Christine Mauz-Körholz, Monika L. Metzger, Kara M. Kelly, Cindy L. Schwartz, Mauricio E. Castellanos, Karin Dieckmann, Regine Kluge, and Dieter Körholz, Pediatric Hodgkin Lymphoma, Journal of Clinical Oncology, volume 33, issue 27, pages 2975–2985. Reprinted with permission. © (2015) American Society of Clinical Oncology. All rights reserved.
Although all major research groups classify patients according to clinical criteria, such as stage and presence of B symptoms, extranodal involvement, or bulky disease, comparison of outcomes across trials is further complicated because of differences in how these individual criteria are defined.[3]
Response Assessment
Further refinement of risk classification may be performed through assessment of response after initial cycles of chemotherapy or at the completion of chemotherapy.
Interim response assessment
The interim response to initial therapy, which may be assessed on the basis of volume reduction of disease, functional imaging status, or both, is an important prognostic variable in both early- and advanced-stage pediatric Hodgkin lymphoma.[24-27]; [13][Level of evidence: 2Diii]
Definitions for interim response are variable and protocol specific but can range from 2-dimensional reductions in size of greater than 50% to the achievement of a complete response with 2-dimensional reductions in size of greater than 75% or 80% or a volume reduction of greater than 95% by anatomical imaging or resolution of 18F-FDG PET avidity.[5,28,29]
The rapidity of response to early therapy has been used in risk stratification to tailor therapy in an effort to augment therapy in higher-risk patients or to reduce the late effects while maintaining efficacy.[26,27,29,30]
Results of selected trials using interim response to titrate therapy
Several studies have evaluated the use of interim response to titrate additional therapy:
- The Pediatric Oncology Group used a response-based therapy approach consisting of dose-dense ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide) for intermediate-stage and advanced-stage patients, in combination with 21 Gy involved-field radiation therapy (IFRT).[29]
- The dose-dense approach permitted reduction in chemotherapy exposure in 63% of patients who achieved a rapid early response on CT imaging after three ABVE-PC cycles.
- Five-year event-free survival (EFS) was comparable for rapid early responders (86%; treated with three cycles of ABVE-PC) and slow early responders (83%; treated with five cycles of ABVE-PC). All patients received 21 Gy of regional radiation therapy.
- The Children's Cancer Group (CCG) (CCG-59704) evaluated response-adapted therapy featuring four cycles of the dose-intensive BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) regimen followed by a sex-tailored consolidation for pediatric patients with stages IIB, IIIB with bulky disease, and IV Hodgkin lymphoma.[30]
- For rapid early responding girls, an additional four courses of COPP/ABV (cyclophosphamide, vincristine, procarbazine, prednisone/doxorubicin, bleomycin, vinblastine) (without IFRT) was given in an effort to reduce breast cancer risk.
- Rapid early responding boys received two cycles of ABVD followed by IFRT.
- Slow early responders received four additional courses of BEACOPP and IFRT.
- Rapid early response (defined by resolution of B symptoms and >70% reduction in tumor volume) was achieved by 74% of patients after four BEACOPP cycles and 5-year EFS among the cohort was 94% (median follow-up, 6.3 years).
- The COG AHOD0031 (NCT00025259), AHOD0831 (NCT01026220), and AHOD0431 (NCT00302003) trials also used interim response to titrate therapy. (Refer to the North American cooperative and consortium trial results section of this summary for more information.) The AHOD0031 trial was designed to evaluate this paradigm of care by randomly assigning patients to receive either standard or response-based therapy.
End of chemotherapy response assessment
Restaging is carried out upon the completion of all planned initial chemotherapy and may be used to determine the need for consolidative radiation therapy. Key concepts to consider include the following:
- Defining complete response. The definition of complete response may vary by cooperative group or protocol.
- -
The International Working Group (IWG) defined complete response for adults with Hodgkin lymphoma in terms of complete metabolic response as assessed by 18F-FDG PET, even when a persistent mass is present.[31] These criteria were endorsed in the Lugano Classification, with the recommendation for using a 5-point scale in assessing response.[4,32] COG protocols have adopted this approach for defining complete response.
- -
Previous studies have varied in the use of findings from clinical exam, anatomical imaging, and functional imaging to assess response. Although complete response can be defined as absence of disease by clinical examination and/or imaging studies, complete response in Hodgkin lymphoma trials is often defined by a greater than 80% reduction of disease and a change from initial positivity to negativity on functional imaging.[33] This definition is necessary in Hodgkin lymphoma because fibrotic residual disease is common, particularly in the mediastinum. In some studies, such patients are designated as having an unconfirmed complete response.
- -
The EuroNet Hodgkin lymphoma trials use a similar early response assessment definition of PET positivity, which is a Deauville score of greater than 3 after two cycles of OEPA (vincristine [Oncovin], etoposide, prednisone, doxorubicin [Adriamycin]).[34]
- -
GPOH studies use very stringent criteria for treatment group 1 (TG1) patients that includes at least 95% reduction in tumor volume or less than 2 mL residual volume on CT, as patients achieving these metrics will have radiation therapy omitted. Treatment group 2 (TG2) and treatment group 3 (TG3) patients received radiation therapy despite their potential morphologic complete response (refer to Figure 1).[5]
- -
The COG AHOD1331 (NCT02166463) high-risk Hodgkin lymphoma initial therapeutics clinical trial uses 18F-FDG PET assessment graded by a 5-point visual scale or Deauville criteria after two chemotherapy cycles to define a rapid early-responding lesion for which radiation will be omitted. A mass of any size is permitted for a complete response designation if the PET is negative. The results of using the latter criteria are not yet available; therefore, it may not be considered standard of care.
- Timing of PET scanning after completing therapy. Timing of PET scanning after completing therapy is an important issue.
- -
For patients treated with chemotherapy alone, PET scanning is ideally performed a minimum of 3 weeks after the completion of therapy, while patients whose last treatment modality was radiation therapy should not undergo PET scanning until 8 to 12 weeks postradiation.[31]
- Screening frequency and overscreening.
- -
A COG study evaluated surveillance CT and detection of relapse in intermediate-stage and advanced-stage Hodgkin lymphoma. Most relapses occurred within the first year after therapy and were detected based on symptoms, laboratory, or physical findings. The method of detection of late relapse, whether by imaging or clinical change, did not affect overall survival. Routine use of CT at the intervals used in this study did not improve outcome.[35] The concept of reduced frequency of imaging has been supported by other investigations.[36-38]
- -
Caution should be used in making the diagnosis of relapsed or refractory disease based solely on anatomical and functional imaging because false-positive results are not uncommon.[39-41] Consequently, pathologic confirmation of refractory or recurrent disease is recommended before modification of therapeutic plans.
References
- Afaq A, Fraioli F, Sidhu H, et al.: Comparison of PET/MRI With PET/CT in the Evaluation of Disease Status in Lymphoma. Clin Nucl Med 42 (1): e1-e7, 2017. [PMC free article: PMC5538384] [PubMed: 27607161]
- Haase R, Vilser C, Mauz-Körholz C, et al.: Evaluation of the prognostic meaning of C-reactive protein (CRP) in children and adolescents with classical Hodgkin's lymphoma (HL). Klin Padiatr 224 (6): 377-81, 2012. [PubMed: 23047832]
- Flerlage JE, Kelly KM, Beishuizen A, et al.: Staging Evaluation and Response Criteria Harmonization (SEARCH) for Childhood, Adolescent and Young Adult Hodgkin Lymphoma (CAYAHL): Methodology statement. Pediatr Blood Cancer 64 (7): , 2017. [PubMed: 28097818]
- Cheson BD, Fisher RI, Barrington SF, et al.: Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32 (27): 3059-68, 2014. [PMC free article: PMC4979083] [PubMed: 25113753]
- Mauz-Körholz C, Hasenclever D, Dörffel W, et al.: Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin's lymphoma: the GPOH-HD-2002 study. J Clin Oncol 28 (23): 3680-6, 2010. [PubMed: 20625128]
- Younes A, Hilden P, Coiffier B, et al.: International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol 28 (7): 1436-1447, 2017. [PMC free article: PMC5834038] [PubMed: 28379322]
- Hudson MM, Krasin MJ, Kaste SC: PET imaging in pediatric Hodgkin's lymphoma. Pediatr Radiol 34 (3): 190-8, 2004. [PubMed: 14745528]
- Hernandez-Pampaloni M, Takalkar A, Yu JQ, et al.: F-18 FDG-PET imaging and correlation with CT in staging and follow-up of pediatric lymphomas. Pediatr Radiol 36 (6): 524-31, 2006. [PubMed: 16622665]
- Naumann R, Vaic A, Beuthien-Baumann B, et al.: Prognostic value of positron emission tomography in the evaluation of post-treatment residual mass in patients with Hodgkin's disease and non-Hodgkin's lymphoma. Br J Haematol 115 (4): 793-800, 2001. [PubMed: 11843811]
- Hutchings M, Loft A, Hansen M, et al.: FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 107 (1): 52-9, 2006. [PubMed: 16150944]
- Lopci E, Burnelli R, Guerra L, et al.: Postchemotherapy PET evaluation correlates with patient outcome in paediatric Hodgkin's disease. Eur J Nucl Med Mol Imaging 38 (9): 1620-7, 2011. [PubMed: 21559977]
- Sucak GT, Özkurt ZN, Suyani E, et al.: Early post-transplantation positron emission tomography in patients with Hodgkin lymphoma is an independent prognostic factor with an impact on overall survival. Ann Hematol 90 (11): 1329-36, 2011. [PubMed: 21437590]
- Lopci E, Mascarin M, Piccardo A, et al.: FDG PET in response evaluation of bulky masses in paediatric Hodgkin's lymphoma (HL) patients enrolled in the Italian AIEOP-LH2004 trial. Eur J Nucl Med Mol Imaging 46 (1): 97-106, 2019. [PubMed: 30219963]
- Robertson VL, Anderson CS, Keller FG, et al.: Role of FDG-PET in the definition of involved-field radiation therapy and management for pediatric Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys 80 (2): 324-32, 2011. [PubMed: 20646867]
- Adams HJ, Kwee TC, de Keizer B, et al.: Systematic review and meta-analysis on the diagnostic performance of FDG-PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: is bone marrow biopsy still necessary? Ann Oncol 25 (5): 921-7, 2014. [PubMed: 24351400]
- Cistaro A, Cassalia L, Ferrara C, et al.: Italian Multicenter Study on Accuracy of 18F-FDG PET/CT in Assessing Bone Marrow Involvement in Pediatric Hodgkin Lymphoma. Clin Lymphoma Myeloma Leuk 18 (6): e267-e273, 2018. [PubMed: 29739722]
- Cheng G, Servaes S, Zhuang H: Value of (18)F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography scan versus diagnostic contrast computed tomography in initial staging of pediatric patients with lymphoma. Leuk Lymphoma 54 (4): 737-42, 2013. [PubMed: 22957898]
- Simpson CD, Gao J, Fernandez CV, et al.: Routine bone marrow examination in the initial evaluation of paediatric Hodgkin lymphoma: the Canadian perspective. Br J Haematol 141 (6): 820-6, 2008. [PubMed: 18422997]
- Anghelescu DL, Burgoyne LL, Liu T, et al.: Clinical and diagnostic imaging findings predict anesthetic complications in children presenting with malignant mediastinal masses. Paediatr Anaesth 17 (11): 1090-8, 2007. [PMC free article: PMC4400730] [PubMed: 17897276]
- Carbone PP, Kaplan HS, Musshoff K, et al.: Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 31 (11): 1860-1, 1971. [PubMed: 5121694]
- Lister TA, Crowther D, Sutcliffe SB, et al.: Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 7 (11): 1630-6, 1989. [PubMed: 2809679]
- Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. Springer, 2010.
- Mauz-Körholz C, Metzger ML, Kelly KM, et al.: Pediatric Hodgkin Lymphoma. J Clin Oncol 33 (27): 2975-85, 2015. [PubMed: 26304892]
- Kung FH, Schwartz CL, Ferree CR, et al.: POG 8625: a randomized trial comparing chemotherapy with chemoradiotherapy for children and adolescents with Stages I, IIA, IIIA1 Hodgkin Disease: a report from the Children's Oncology Group. J Pediatr Hematol Oncol 28 (6): 362-8, 2006. [PubMed: 16794504]
- Weiner MA, Leventhal B, Brecher ML, et al.: Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin's disease in pediatric patients: a Pediatric Oncology Group study. J Clin Oncol 15 (8): 2769-79, 1997. [PubMed: 9256118]
- Keller FG, Castellino SM, Chen L, et al.: Results of the AHOD0431 trial of response adapted therapy and a salvage strategy for limited stage, classical Hodgkin lymphoma: A report from the Children's Oncology Group. Cancer 124 (15): 3210-3219, 2018. [PMC free article: PMC6097921] [PubMed: 29738613]
- Friedman DL, Chen L, Wolden S, et al.: Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children's Oncology Group Study AHOD0031. J Clin Oncol 32 (32): 3651-8, 2014. [PMC free article: PMC4220044] [PubMed: 25311218]
- Keller FG, Nachman J, Constine L: A phase III study for the treatment of children and adolescents with newly diagnosed low risk Hodgkin lymphoma (HL). [Abstract] Blood 116 (21): A-767, 2010.
- Schwartz CL, Constine LS, Villaluna D, et al.: A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood 114 (10): 2051-9, 2009. [PMC free article: PMC2744567] [PubMed: 19584400]
- Kelly KM, Sposto R, Hutchinson R, et al.: BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: a report from the Children's Oncology Group. Blood 117 (9): 2596-603, 2011. [PMC free article: PMC3062352] [PubMed: 21079154]
- Cheson BD, Pfistner B, Juweid ME, et al.: Revised response criteria for malignant lymphoma. J Clin Oncol 25 (5): 579-86, 2007. [PubMed: 17242396]
- Barrington SF, Mikhaeel NG, Kostakoglu L, et al.: Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32 (27): 3048-58, 2014. [PMC free article: PMC5015423] [PubMed: 25113771]
- Molnar Z, Simon Z, Borbenyi Z, et al.: Prognostic value of FDG-PET in Hodgkin lymphoma for posttreatment evaluation. Long term follow-up results. Neoplasma 57 (4): 349-54, 2010. [PubMed: 20429626]
- Hasenclever D, Kurch L, Mauz-Körholz C, et al.: qPET - a quantitative extension of the Deauville scale to assess response in interim FDG-PET scans in lymphoma. Eur J Nucl Med Mol Imaging 41 (7): 1301-8, 2014. [PubMed: 24604592]
- Voss SD, Chen L, Constine LS, et al.: Surveillance computed tomography imaging and detection of relapse in intermediate- and advanced-stage pediatric Hodgkin's lymphoma: a report from the Children's Oncology Group. J Clin Oncol 30 (21): 2635-40, 2012. [PMC free article: PMC4559601] [PubMed: 22689804]
- Rathore N, Eissa HM, Margolin JF, et al.: Pediatric Hodgkin lymphoma: are we over-scanning our patients? Pediatr Hematol Oncol 29 (5): 415-23, 2012. [PMC free article: PMC3685486] [PubMed: 22632168]
- Hartridge-Lambert SK, Schöder H, Lim RC, et al.: ABVD alone and a PET scan complete remission negates the need for radiologic surveillance in early-stage, nonbulky Hodgkin lymphoma. Cancer 119 (6): 1203-9, 2013. [PubMed: 23132361]
- Friedmann AM, Wolfson JA, Hudson MM, et al.: Relapse after treatment of pediatric Hodgkin lymphoma: outcome and role of surveillance after end of therapy. Pediatr Blood Cancer 60 (9): 1458-63, 2013. [PMC free article: PMC4313350] [PubMed: 23677874]
- Nasr A, Stulberg J, Weitzman S, et al.: Assessment of residual posttreatment masses in Hodgkin's disease and the need for biopsy in children. J Pediatr Surg 41 (5): 972-4, 2006. [PubMed: 16677895]
- Meany HJ, Gidvani VK, Minniti CP: Utility of PET scans to predict disease relapse in pediatric patients with Hodgkin lymphoma. Pediatr Blood Cancer 48 (4): 399-402, 2007. [PubMed: 16514616]
- Picardi M, De Renzo A, Pane F, et al.: Randomized comparison of consolidation radiation versus observation in bulky Hodgkin's lymphoma with post-chemotherapy negative positron emission tomography scans. Leuk Lymphoma 48 (9): 1721-7, 2007. [PubMed: 17786707]
Treatment of Newly Diagnosed Children and Adolescents With Hodgkin Lymphoma
Historical Overview of Treatment for Hodgkin Lymphoma
Long-term survival has been achieved in children and adolescents with Hodgkin lymphoma using radiation, multiagent chemotherapy, and combined-modality therapy. In selected cases of localized lymphocyte-predominant Hodgkin lymphoma, complete surgical resection may be curative and obviate the need for cytotoxic therapy.
Treatment options for children and adolescents with Hodgkin lymphoma include the following:
- Radiation therapy as a single modality.
- Recognition of the excess adverse effects of high-dose radiation therapy on musculoskeletal development in children motivated investigations of multiagent chemotherapy alone or with lower radiation doses (15–25.5 Gy) and reduced treatment volumes (involved-fields). It also led to the abandonment of the use of radiation as a single modality in skeletally immature children.[1-3]
- Radiation therapy alone may rarely be considered for adolescents and young adults with nodular lymphocyte-predominant Hodgkin lymphoma.
- Multiagent chemotherapy as a single modality.
- The establishment of the noncross-resistant combinations of MOPP (mechlorethamine, vincristine [Oncovin], procarbazine, and prednisone) developed in the 1960s and ABVD (doxorubicin [Adriamycin], bleomycin, vinblastine, dacarbazine) developed in the 1970s made long-term survival possible for patients with advanced and unfavorable (e.g., bulky, symptomatic) Hodgkin lymphoma.[6,7]MOPP-related sequelae include a dose-related risk of infertility and subsequent myelodysplasia and leukemia.[2,8] The use of MOPP-derivative regimens substituting less leukemogenic and gonadotoxic alkylating agents (e.g., cyclophosphamide) for mechlorethamine or restricting cumulative alkylating agent dose exposure reduces this risk.[9]
- Etoposide has been incorporated into treatment regimens as an effective alternative to alkylating agents in an effort to reduce gonadal toxicity and enhance antineoplastic activity.[15]Etoposide-related sequelae include an increased risk of subsequent myelodysplasia and leukemia that appears to be rare when etoposide is used in restricted doses in pediatric Hodgkin lymphoma regimens.[16]
- All of the agents in original MOPP and ABVD regimens continue to be used in contemporary pediatric treatment regimens. COPP (substituting cyclophosphamide for mechlorethamine) has almost uniformly replaced MOPP as the preferred alkylator regimen in most frontline trials. Contemporary trials have utilized procarbazine-free standard backbone regimens, such as ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide) in North America [17,18] and OEPA (vincristine, etoposide, prednisone, doxorubicin)-COPDAC (cyclophosphamide, vincristine, prednisone, dacarbazine) in Europe.[19] Both of these regimens represent dose-dense regimens that use six drugs to maximize intensity without exceeding thresholds of toxicity.
- Radiation therapy and multiagent chemotherapy as a combined-modality therapy. Considerations for the use of multiagent chemotherapy alone versus combined-modality therapy include the following:
- Treatment with noncross-resistant chemotherapy alone offers advantages for children managed in centers in developing countries lacking radiation facilities and trained personnel, as well as diagnostic imaging modalities needed for clinical staging. This treatment option also avoids the potential long-term growth inhibition, organ dysfunction, and solid tumor induction associated with radiation.
- Chemotherapy-alone treatment protocols usually prescribe higher cumulative doses of alkylating agent and anthracycline chemotherapy, which may produce acute- and late-treatment morbidity from myelosuppression, cardiac toxic effects, gonadal injury, and subsequent leukemia. However, more recent trials are designed to significantly reduce these risks, especially in those with chemotherapy-responsive disease.[17]
- In general, the use of combined chemotherapy and low-dose involved-site radiation therapy (LD-ISRT) broadens the spectrum of potential toxicities, while reducing the severity of individual drug-related or radiation-related toxicities. The results of prospective and controlled randomized trials indicate that combined-modality therapy, compared with chemotherapy alone, produces a superior event-free survival (EFS). However, because of effective second-line therapy, overall survival (OS) has not differed among the groups studied.[20,21]
Contemporary Approaches to Treatment of Hodgkin Lymphoma
Contemporary treatment for pediatric Hodgkin lymphoma uses a risk-adapted and response-based paradigm that assigns the length and intensity of therapy based on disease-related factors such as stage, number of involved nodal regions, tumor bulk, the presence of B symptoms, and early response to chemotherapy by functional and anatomical imaging. Age, sex, and histological subtype may also be considered in treatment planning.
Treatment options for childhood Hodgkin lymphoma include the following:
Risk designation
Risk designation depends on favorable and unfavorable clinical features, as follows:
- Favorable clinical features. These features include localized nodal involvement in the absence of B symptoms and bulky disease. Risk factors considered in other studies include the number of involved nodal regions, the presence of hilar adenopathy, the size of peripheral lymphadenopathy, and extranodal extension.[22]
- Unfavorable clinical features. These features include the presence of B symptoms, bulky mediastinal or peripheral lymphadenopathy, extranodal extension of disease, and advanced (stages IIIB–IV) disease.[22] In most clinical trials, bulky mediastinal lymphadenopathy is designated when the ratio of the maximum measurement of mediastinal lymphadenopathy to intrathoracic cavity on an upright chest radiograph equals or exceeds 33%.Pleural effusions have been shown to be an adverse prognostic finding in patients treated for low-stage Hodgkin lymphoma.[23][Level of evidence: 2A] The risk of relapse was 25% in patients with an effusion, as opposed to less than 15% in patients without an effusion. Patients with effusions were more often older (15 years vs. 14 years) and had nodular-sclerosing histology.Localized disease (stages I, II, and IIIA) with unfavorable features may be treated similarly to advanced-stage disease in some treatment protocols or treated with therapy of intermediate intensity.[22]
Inconsistency in risk categorization across studies often makes comparison of study outcomes challenging.
Risk-adapted treatment paradigms
No single treatment approach is ideal for all pediatric and young adult patients because of the differences in age-related developmental status and sex-related sensitivity to chemotherapy toxicity.
- The general treatment strategy that is used to treat children and adolescents with Hodgkin lymphoma is chemotherapy for all patients, with or without radiation.
- -
The number of cycles and intensity of chemotherapy may be determined by the rapidity and degree of response, as is the radiation dose and volume. The primary exception to this strategy is in patients with nodular lymphocyte-predominant Hodgkin lymphoma when surgical resection has been advocated for stage I disease with a single resectable node in the United States [24] and for any resectable disease in Europe.[25]
- -
Sex-based regimens consider that male patients are more vulnerable to gonadal toxicity from alkylating-agent chemotherapy and that female patients have a substantial risk of breast cancer after chest irradiation. However, the cardiovascular risk to males after chest irradiation suggests that limiting radiation exposure is also desirable in males.[26]
Ongoing trials for patients with favorable disease presentations are evaluating the effectiveness of treatment with fewer cycles of combination chemotherapy alone that limit doses of anthracyclines, alkylating agents, and radiation therapy. Contemporary trials for patients with intermediate/unfavorable disease presentations are testing whether chemotherapy and radiation therapy can be limited in patients who achieve a rapid early response to dose-intensive chemotherapy regimens; trials are also testing the efficacy of regimens integrating novel, potentially less-toxic agents such as brentuximab vedotin.
Histology-based therapy
Nodular lymphocyte-predominant Hodgkin lymphoma
Histological subtype may direct therapy in patients with stage I completely resected, nodular lymphocyte-predominant Hodgkin lymphoma, whose initial treatment may be surgery alone.[24]
Evidence (surgery alone for localized nodular lymphocyte-predominant Hodgkin lymphoma):
- Although standard therapy for children with nodular lymphocyte-predominant Hodgkin lymphoma is chemotherapy plus LD-ISRT, there are reports in which patients have been treated with chemotherapy alone or with complete resection of isolated nodal disease without chemotherapy. Surgical resection of localized disease produces a prolonged disease-free survival in a substantial proportion of patients obviating the need for immediate cytotoxic therapy.[24,25,27,28]
- Results from a single-arm Children's Oncology Group (COG) trial provide data to support the strategy of observation after surgical resection and treatment with limited chemotherapy for children with favorable stage IA or IIA Hodgkin lymphoma.[24][Level of evidence: 1iiDi]
- Among 178 patients treated with surgical resection alone for single-node disease (n = 52), chemotherapy alone after complete response (CR) to three cycles of AV-PC (doxorubicin, vincristine, prednisone, and cyclophosphamide) chemotherapy (n = 115), or chemotherapy with low-dose involved-field radiation therapy (LD-IFRT) (21 Gy) after incomplete response to AV-PC chemotherapy (n = 11), the 5-year EFS was 85.5%, and the OS was 100%.
- Five-year EFS was 77% for patients observed after total resection and 88.8% for patients treated with AV-PC chemotherapy.
Advanced-stage nodular lymphocyte-predominant Hodgkin lymphoma is very rare, and there is no consensus regarding the optimal treatment, although outcomes for patients are excellent.
Evidence (chemotherapy for nodular lymphocyte-predominant Hodgkin lymphoma with unfavorable characteristics):
- In a retrospective review of 41 cases of advanced-stage nodular lymphocyte-predominant Hodgkin lymphoma, many different chemotherapy regimens were used, some included rituximab.[29][Level of evidence: 3iiiA]
- OS was 98%, with the only death resulting from a subsequent neoplasm.
- In a retrospective analysis, 97 intermediate-risk patients with lymphocyte-predominant Hodgkin lymphoma were treated on COG study AHOD0031 (NCT00025259).[30]
- These patients demonstrated a higher CR rate than did patients with classical histology; the 5-year EFS was marginally superior in patients with lymphocyte-predominant Hodgkin lymphoma (91.2%) than in patients with classical Hodgkin lymphoma (83.2%).
- Most patients treated with four cycles of the ABVE-PC regimen achieved a rapid early response with a CR status and demonstrated excellent EFS and OS without IFRT. This finding suggests that the dose-dense, response-based protocol therapy designed for patients with classical Hodgkin lymphoma may have been more intensive than necessary for patients with lymphocyte-predominant Hodgkin lymphoma.
A summary of treatment approaches for nodular lymphocyte-predominant Hodgkin lymphoma can be found in Table 9. Both children and adults treated for nodular lymphocyte-predominant Hodgkin lymphoma have a favorable outcome, particularly when the disease is localized (stage I), as it is for most patients.[24,25,27,31] Among long-term survivors of nodular lymphocyte-predominant Hodgkin lymphoma, death is more likely to result from treatment-related toxicity (both acute and long-term) than from lymphoma.[32,33]
Mixed-cellularity Hodgkin lymphoma
In addition to variable responses by histology for lymphocyte-predominant Hodgkin lymphoma, differences by mixed-cellularity histology have also been observed. COG investigators reported a 4-year EFS rate of 95.2% for children with stage I or stage II mixed-cellularity histology treated with minimal AV-PC therapy (and only rarely requiring radiation therapy), which was significantly better than the 75.8% EFS rate for patients who had nodular-sclerosing histology (P = .008).[34]
Radiation Therapy
As previously discussed, most newly diagnosed children will be treated with risk-adapted chemotherapy alone or in combination with consolidative radiation therapy. Radiation therapy volumes can have variable and protocol-specific definitions, but generally encompass lymph node regions initially involved at the time of diagnosis, without extensive inclusion of uninvolved regions. Radiation therapy field reductions are made to account for tumor regression with chemotherapy.[35]
Radiation volume
With advancements in systemic therapy, radiation therapy field definitions have evolved and become increasingly restricted. Radiation therapy is no longer needed to sterilize all disease. Advances in radiologic imaging allow more precise radiation target definition. With the use of effective chemotherapy and contemporary treatments using lower radiation doses (<21 Gy) and reduced volumes (involved-site radiation therapy [ISRT]), contralateral uninvolved sites are not irradiated.
General trends in radiation treatment volume are summarized as follows:
- Historical regional radiation therapy fields (e.g., mantle, subtotal, or total nodal) have been replaced by involved-nodal radiation therapy (INRT) or ISRT, or rarely, IFRT in select situations such as adolescents and young adults treated with radiation alone for nodular lymphocyte-predominant Hodgkin lymphoma.
- INRT defines the treatment volume using the prechemotherapy positron emission tomography (PET)–computed tomography (CT) scan that is obtained with the patient positioned in a similar manner to the position that will be used at the time of radiation therapy. This volume is later contoured onto the postchemotherapy-planning CT scan. The final treatment volume only includes the initially involved nodes with a margin, typically 2 cm.[36-38]
- ISRT, used in contemporary COG trials, is an approach to be used for patients when optimal prechemotherapy imaging (PET-CT in a position similar to what will be used at the time of radiation therapy) is not available to the radiation oncologist. Because the delineation of the area of involvement is less precise, a somewhat larger treatment volume is contoured than for INRT, typically at least 2 cm around the nodes or region where the lymphoma was located before chemotherapy was given. The exact size of this volume will depend on the individual case scenario.[35]
- Modified involved-field radiation therapy (mIFRT) is the term used in the EuroNet-PHL-C1 trial to describe treatment volumes that contain the involved lymph node(s) as seen before chemotherapy plus radiation planning margins of 1 cm to 2 cm, depending on the area of involvement. These volumes are comparable to ISRT fields, although the development preceded the widespread availability of CT-based planning. The subsequent EuroNet-PHL-C2 trial employs INRT.
Breast-sparing radiation therapy plans using proton therapy are under evaluation to determine whether there is a statistically significant reduction in dose.[39] Ongoing studies seek to determine whether doses to other critical organs, such as the heart and lungs, can be reduced with proton therapy. Long-term results are awaited.
ISRT or INRT treatment planning
Radiation therapy planning that uses CT scans obtained during the simulation procedure is a requirement for contemporary INRT or ISRT. Fusion of staging imaging (CT or PET-CT) with the planning CT dataset can facilitate delineation of the treatment volume. Radiation therapy planning scans that encompass the full extent of organs at risk (e.g., lungs) are important so that normal tissue exposures can be calculated accurately.
Definitions that are important in planning radiation therapy include the following:
- Prechemotherapy or presurgery gross tumor volume (GTV): Imaging abnormalities of nodal or non-nodal tissues at initially involved sites.
- Postchemotherapy GTV: Imaging abnormalities at initially involved sites that remain abnormal after chemotherapy.
- Postchemotherapy clinical target volume (CTV): Abnormal tissues originally involved with lymphoma, but taking into account the reduction in the axial (transverse) diameter that has occurred with chemotherapy. This delineation requires consideration of the expected routes of disease spread and the quality of pretreatment imaging.
- Internal target volume (ITV): ITV encompasses the CTV, with an added margin to account for variation in shape and motion within the patient (e.g., breathing).
- Planning target volume (PTV): This encompasses the ITV or CTV and accounts for variation in daily setup for radiation; generally 0.5 cm to 1 cm.
- Boost radiation therapy: Some protocols, such as the EuroNet-PHL-C1 protocol, give additional radiation therapy (a boost) to sites with a poor response and/or bulky residual disease after initial chemotherapy. These volumes were determined after completion of all chemotherapy. This approach is sometimes used for patients with residual areas of PET avidity after chemotherapy.
- Organ at risk (OAR) determination and dose constraints: Because of the importance of long-term tissue injury after radiation, the dose to normal tissues is kept as low as reasonably achievable while adequately treating the PTV. Some specific organ radiation dose tolerances exist to guide these decisions, and these organs are considered organs at risk.
The treatment volume for unfavorable or advanced disease is somewhat variable and often protocol-specific. Large-volume radiation therapy may compromise organ function and limit the intensity of second-line therapy if relapse occurs. In patients with intermediate or advanced disease, who often have multifocal/extranodal disease, the current standard of therapy includes postchemotherapy ISRT that limits radiation exposure to large portions of the body.[14,40]
Radiation dose
The dose of radiation is also variously defined and often protocol-specific.
General considerations regarding radiation dose include the following:
- Doses of 15 Gy to 25 Gy are typically used, with modifications based on patient age, the presence of bulky or residual (postchemotherapy) disease, and normal tissue concerns.
- Some protocols have prescribed a boost of 5 Gy to 10 Gy in regions with suboptimal response to chemotherapy.[40] This approach has not been formally evaluated to quantitate the risk-benefit relationship.
Technical considerations
Technical considerations for the use of radiation therapy to treat Hodgkin lymphoma include the following:
- A linear accelerator with a beam energy of 6 mV is desirable because of its penetration, well-defined edge, and homogeneity throughout an irregular treatment field.
- Three-dimensional conformal radiation therapy (3-D CRT) or intensity-modulated radiation therapy (IMRT) are standard techniques in the treatment of lymphoma. Appropriate CT-based image guided treatment planning and delivery is standard, preferably with fusion of staging CT and PET imaging with radiation therapy planning CT datasets to delineate the target volumes.[35]
- Data are accumulating in regard to the efficacy of IMRT and the decrease in median dose to normal surrounding tissues. Some uncertainty exists about the potential for increased late effects from IMRT, particularly subsequent neoplasms, because with IMRT, a larger area of the body receives a low dose compared with conventional techniques (although the mean dose to a volume may be decreased).
- Proton therapy is currently being investigated and may further decrease the mean dose to the surrounding normal tissue compared with IMRT or 3-D CRT, without increasing the volume of normal tissue receiving lower-dose radiation.[41]
- Individualized immobilization devices are preferable for young children to ensure accuracy and reproducibility.
- Attempts should be made to exclude or position breast tissue under the lung/axillary shielding.
- When the decision is made to include some or all of a critical organ (such as liver, kidney, or heart) in the radiation field, then normal tissue constraints are critical depending on the chemotherapy used and patient age.
Role of LD-ISRT in childhood and adolescent Hodgkin lymphoma
Because all children and adolescents with Hodgkin lymphoma receive chemotherapy, a question commanding significant attention is whether patients who achieve a rapid early response or a CR to chemotherapy require radiation therapy. Conversely, the judicious use of LD-ISRT may permit a reduction in the intensity or duration of chemotherapy below toxicity thresholds that would not be possible if single modality chemotherapy were used, thus decreasing overall acute and late toxicities.
The treatment approach for pediatric Hodgkin lymphoma should focus on maximizing disease control and minimizing risks of late toxicity associated with both radiation therapy and chemotherapy. Key points to consider in regard to the role of radiation in pediatric Hodgkin lymphoma include the following:
- The use of LD-IFRT or ISRT in pediatric Hodgkin lymphoma permits reduction in duration or intensity of chemotherapy and, thus, dose-related toxicity of anthracyclines, alkylating agents, and bleomycin that may preserve cardiopulmonary and gonadal function and reduce the risk of subsequent leukemia.
- Radiation has been used as an adjunct to multiagent chemotherapy in clinical trials for low-, intermediate-, and high-risk pediatric Hodgkin lymphoma with the goal of reducing risk of relapse in initially involved sites and preventing toxicity associated with second-line therapy.Compared with chemotherapy alone, adjuvant radiation has, in most studies, produced a superior EFS for children with intermediate-risk and high-risk Hodgkin lymphoma who achieve a CR to multiagent chemotherapy, but it does not clearly improve OS because of the success of second-line therapy.[21]However, the intermediate-risk Hodgkin lymphoma study (AHOD0031 [NCT00025259]) did not show a benefit for IFRT in patients who achieved a rapid CR to chemotherapy (defined as >60% reduction in 2-dimensional tumor burden after two cycles and metabolic remission and >80% reduction after four cycles). Four-year EFS was 87.9% for rapid responders who were randomly assigned to IFRT versus 84.3% (P = .11) for rapid responders who were not assigned to IFRT. OS was 98.8% in both groups.[17] In a subset analysis from this study of patients with anemia and bulky limited-stage disease, the EFS was 89.3% for rapid early responder or complete remission patients who received IFRT, compared with 77.9% for patients who did not receive IFRT (P = .019).[42][Level of evidence: 1iiDi]Adjuvant radiation therapy may be associated with an increased risk of late effects or mortality.[43]
- Radiation consolidation may facilitate local disease control in individuals with refractory or recurrent disease, especially in those who have limited or bulky sites of disease progression/recurrence, or persistent disease that does not completely respond to chemotherapy.[44]
- The radiation dose to the breast, heart, thyroid, and lung tissue received by patients in contemporary COG trials is 55% to 85% lower than the dose received by survivors analyzed in the Childhood Cancer Survivors Study (CCSS). This should be taken into consideration when estimating the risk of late toxicity associated with modern radiation therapy.[45]
Finally, an inherent assumption is made in a trial comparing chemotherapy alone versus chemotherapy and radiation that the effect of radiation on EFS will be uniform across all patient subgroups. However, it is not clear how histology, presence of bulky disease, presence of B symptoms, or other variables affect the efficacy of postchemotherapy radiation.
Chemotherapy
All of the agents in original MOPP and ABVD regimens continue to be used in contemporary pediatric treatment regimens. COPP (substituting cyclophosphamide for mechlorethamine) has almost uniformly replaced MOPP as the preferred alkylator regimen in most frontline trials. Etoposide has been incorporated into treatment regimens as an effective alternative to alkylating agents in an effort to reduce gonadal toxicity and enhance antineoplastic activity.
Combination chemotherapy regimens used in trials are summarized in Table 5.
Table 5. Chemotherapy Regimens for Children and Adolescents with Hodgkin Lymphoma
| Name | Drugs | Dosage | Route | Days |
|---|---|---|---|---|
| COPP [19] | Cyclophosphamide | 600 mg/m2 | IV | 1, 8 |
| Vincristine (Oncovin) | 1.4 mg/m2 | IV | 1, 8 | |
| Procarbazine | 100 mg/m2 | PO | 1–15 | |
| Prednisone | 40 mg/m2 | PO | 1–15 | |
| COPDAC [19] | Dacarbazine substituted for procarbazine in COPP | 250 mg/m2 | IV | 1–3 |
| CAPDAC [46] | Brentuximab vedotin substituted for vincristine in COPDAC | 1.2 mg/kg | IV | 1, 8 |
| OPPA [19] | Vincristine (Oncovin) | 1.5 mg/m2 | IV | 1, 8, 15 |
| Procarbazine | 100 mg/m2 | PO | 1–15 | |
| Prednisone | 60 mg/m2 | PO | 1–15 | |
| Doxorubicin (Adriamycin) | 40 mg/m2 | IV | 1, 15 | |
| OEPA [19] | Vincristine (Oncovin) | 1.5 mg/m2 | IV | 1, 8, 15 |
| Etoposide | 125 mg/m2 | IV | 3–6 | |
| Prednisone | 60 mg/m2 | PO | 1–15 | |
| Doxorubicin (Adriamycin) | 40 mg/m2 | IV | 1, 15 | |
| AEPA [46] | Brentuximab vedotin substituted for vincristine in OEPA | 1.2 mg/kg | IV | 1, 8, 15 |
| ABVD [7] | Doxorubicin (Adriamycin) | 25 mg/m2 | IV | 1, 15 |
| Bleomycin | 10 U/m2 | IV | 1, 15 | |
| Vinblastine | 6 mg/m2 | IV | 1, 15 | |
| Dacarbazine | 375 mg/m2 | IV | 1, 15 | |
| COPP/ABV [14] | Cyclophosphamide | 600 mg/m2 | IV | 0 |
| Vincristine (Oncovin) | 1.4 mg/m2 | IV | 0 | |
| Procarbazine | 100 mg/m2 | PO | 0–6 | |
| Prednisone | 40 mg/m2 | PO | 0–13 | |
| Doxorubicin (Adriamycin) | 35 mg/m2 | IV | 7 | |
| Bleomycin | 10 U/m2 | IV | 7 | |
| Vinblastine | 6 mg/m2 | IV | 7 | |
| VAMP [47] | Vinblastine | 6 mg/m2 | IV | 1, 15 |
| Doxorubicin (Adriamycin) | 25 mg/m2 | IV | 1, 15 | |
| Methotrexate | 20 mg/m2 | IV | 1, 15 | |
| Prednisone | 40 mg/m2 | PO | 1–14 | |
| DBVE [48 ,49] | Doxorubicin | 25 mg/m2 | IV | 1, 15 |
| Bleomycin | 10 U/m2 | IV | 1, 15 | |
| Vincristine (Oncovin) | 1.5 mg/m2 | IV | 1, 15 | |
| Etoposide | 100 mg/m2 | IV | 1–5 | |
| ABVE-PC a [18] | Doxorubicin (Adriamycin) | 30 mg/m2 | IV | 0, 1 |
| Bleomycin | 10 U/m2 | IV | 0, 7 | |
| Vincristine (Oncovin) | 1.4 mg/m2 | IV | 0, 7 | |
| Etoposide | 75 mg/m2 | IV | 0–4 | |
| Prednisone | 40 mg/m2 | PO | 0–9 | |
| Cyclophosphamide | 800 mg/m2 | IV | 0 | |
| BEACOPP [50] | Bleomycin | 10 U/m2 | IV | 7 |
| Etoposide | 200 mg/m2 | IV | 0–2 | |
| Doxorubicin (Adriamycin) | 35 mg/m2 | IV | 0 | |
| Cyclophosphamide | 1,200 mg/m2 | IV | 1, 8 | |
| Vincristine (Oncovin) | 2 mg/m2 | IV | 7 | |
| Prednisone | 40 mg/m2 | PO | 0–13 | |
| Procarbazine | 100 mg/m2 | PO | 0–6 | |
| CVP [51] | Cyclophosphamide | 500 mg/m2 | IV | 1 |
| Vinblastine | 6 mg/m2 | IV | 1, 8 | |
| Prednisolone | 40 mg/m2 | PO | 1–8 | |
| AV-PC [24 ,34] | Doxorubicin (Adriamycin) | 25 mg/m2 | IV | 1, 2 |
| Vincristine | 1.4 mg/m2; 2.8 mg/m2 max dose | IV | 1, 8 | |
| Prednisone | 20 mg/m2 | PO | 1–7 | |
| Cyclophosphamide | 600 mg/m2 | IV | 1, 2 |
IV = intravenous; PO = oral.
aABVE-PC modifications included reductions of doxorubicin to 25 mg/m2 in all trials and for high-risk Hodgkin lymphoma, use of cyclophosphamide at 600 mg/m2 on days 1 and 2.
North American cooperative and consortium trial results
A series of North American trials have evaluated response-based and risk-adapted therapy.
Evidence (response-based and risk-adapted therapy):
- The Pediatric Oncology Group organized two trials featuring response-based, risk-adapted therapy utilizing ABVE (doxorubicin [Adriamycin], bleomycin, vincristine, and etoposide) [49] for favorable low-stage patients and dose-dense ABVE-PC (prednisone and cyclophosphamide) for unfavorable advanced-stage patients in combination with 21 Gy IFRT.[18]
- Children and adolescents with low-risk Hodgkin lymphoma (stages I, IIA, IIIA1) treated with IFRT (25.5 Gy) after CR to two cycles of DBVE (doxorubicin, bleomycin, vincristine, and etoposide) had outcomes comparable to those treated with four cycles of DBVE and IFRT (25.5 Gy). This response-dependent approach permitted reduction in chemotherapy exposure in 45% of patients.[49]
- A dose-dense, early response–based treatment approach with ABVE-PC permitted reduction in chemotherapy exposure in 63% of patients who achieved a rapid early response after three ABVE-PC cycles.[18][Level of evidence: 1iiDi]
- Five-year EFS was comparable for rapid early responders (86%) and slow early responders (83%) treated with three and five cycles of ABVE-PC, respectively, followed by 21 Gy radiation. Patients who received dexrazoxane had more hematological and pulmonary toxicity.[18]
- A large COG study (COG-59704) evaluated response-adapted therapy featuring four cycles of the dose-intensive BEACOPP regimen followed by a sex-tailored consolidation for pediatric patients with stages IIB, IIIB with bulky disease, and IV Hodgkin lymphoma.[50][Level of evidence: 2Dii] For rapid early responding girls, an additional four courses of COPP/ABV (without IFRT) were given. Rapid early responding boys received two cycles of ABVD followed by IFRT. Slow early responders received four additional courses of BEACOPP and IFRT. Eliminating IFRT from the girls' therapy was intended to reduce the risk of breast cancer. Key findings from this trial include the following:[50]
- Rapid early response (defined by resolution of B symptoms and >70% reduction in tumor volume) was achieved by 74% of patients after four cycles of BEACOPP.
- The 5-year EFS was 94%, with a median follow-up time of 6.3 years.
- Results support that early intensification followed by less-intense response-based therapy results in high EFS.
However, infectious complications during therapy and the long-term risks of infertility and subsequent neoplasms undermine this approach as an optimal treatment, particularly in light of new and safe strategies. - The Stanford, St. Jude Children's Research Hospital, and Boston Consortium administered a series of risk-adapted trials over the last 20 years. Key findings include the following:
- The combination of vinblastine, doxorubicin, methotrexate, and prednisone (VAMP) is an effective regimen (10-year EFS, 89%) for favorable-risk (low-stage nodular lymphocyte-predominant and classical Hodgkin lymphoma without B symptoms or bulky disease) children and adolescents when used in combination with response-based LD-IFRT (15–25.5 Gy).[47]
- Patients with favorable-risk Hodgkin lymphoma treated with four cycles of VAMP chemotherapy alone who achieved an early CR had a comparable 5-year EFS to those treated with four cycles of VAMP chemotherapy plus 25.5 Gy IFRT (89% vs. 88%).[55]
- The COG AHOD0031 (NCT00025259) study enrolled 1,712 patients in a randomized controlled trial to evaluate the role of early chemotherapy response in tailoring subsequent therapy in pediatric intermediate-risk Hodgkin lymphoma. Intermediate-risk Hodgkin lymphoma was defined as Ann Arbor stages IB, IAE, IIB, IIAE, IIIA, IVA with or without bulky disease, and IA or IIA with bulky disease. All patients received two cycles of doxorubicin, bleomycin, vincristine, etoposide, cyclophosphamide, and prednisone (ABVE-PC) followed by response evaluation.[17]
- Rapid early responders (defined by CT imaging after two cycles) received two additional ABVE-PC cycles, followed by CR evaluation.
- Rapid early responders with CR at the end of chemotherapy (based on CT imaging and additionally requiring negative PET or gallium scans) were randomly assigned to receive either IFRT or no additional therapy.
- Rapid early responders with less than a CR were nonrandomly assigned to IFRT.
- Slow early responders were randomly assigned to receive two additional ABVE-PC cycles with or without two cycles of dexamethasone, etoposide, cisplatin, and cytarabine (DECA). All slow early responders were assigned to receive IFRT.
Key 4-year OS and EFS outcomes from this trial include the following:- Early response was an important prognostic factor. Overall EFS was 85.0% and significantly (P < .001) higher for rapid early responders (86.9%) than was for slow early responders (77.4%). OS was 97.8% and significantly (P < .001) higher for rapid early responders (98.5%) than was for slow early responders (95.3%).
- Approximately 45% of patients were rapid early responders and achieved CR by the end of chemotherapy. For this population, EFS did not differ significantly (P = .11) among those who were randomly assigned to IFRT (87.9%) versus no IFRT (84.3%). OS for those receiving IFRT was 98.8% (95% confidence interval [CI], 96.8%–99.5%) and OS for those receiving chemotherapy alone was 98.8% (95% CI, 96.9%–99.6%).
- Despite achieving rapid early response or CR, stage I or stage II patients with bulky mediastinal adenopathy and anemia had significantly better EFS when randomly assigned to IFRT after four cycles of ABVE-PC.[42]
- Approximately 20% of patients were slow early responders. For this population, EFS did not differ significantly (P = .11) among those who were randomly assigned to DECA (79.3%) versus no DECA (75.2%).
- Study results confirm the prognostic significance of early chemotherapy response and support the safety of avoidance of IFRT based on rapid early response with CR by the end of chemotherapy.
An analysis of patterns of failure among patients who relapsed while enrolled in the AHOD0031 (NCT00025259) study demonstrated that first relapses more commonly occurred within the previously irradiated field and within initially involved sites of disease, including both bulky and nonbulky sites.[56] - The COG AHOD0431 (NCT00302003) study utilized a response-directed treatment strategy for children and adolescents with stage I and stage IIA, nonbulky disease. Chemotherapy sensitivity was assessed by 18F-FDG PET response after three cycles of doxorubicin, vincristine, prednisone, and cyclophosphamide (AV-PC) chemotherapy. LD-IFRT (21 Gy) was administered only to patients who did not achieve a complete remission after chemotherapy. The protocol also incorporated a standardized salvage regimen (vinorelbine and ifosfamide plus dexamethasone, etoposide, cisplatin, and cytarabine) for low-risk recurrences (defined as stage I/II, nonbulky disease, regardless of time to relapse) after treatment with chemotherapy alone.[34]
- At 4 years, OS was 99.6%, with 49.0% in remission after treatment with minimal chemotherapy alone and 88.8% in remission without receiving high-dose chemotherapy with stem cell rescue or more than 21 Gy IFRT.
- Factors predicting favorable EFS after limited chemotherapy response-based approach included mixed-cellularity histology, low erythrocyte sedimentation rate, and negative 18F-FDG PET after one cycle.
- An open-label, single-arm, multicenter trial evaluated two cycles of AEPA (brentuximab vedotin substituted for vincristine in the OEPA regimen) and four cycles of CAPDAC (brentuximab vedotin substituted for vincristine in the COPDAC regimen) in patients (age ≤18 years) with stage IIB, IIIB, or IV classical Hodgkin lymphoma. Residual node radiation therapy (25.5 Gy) was given at the end of all chemotherapy and only to nodal sites that did not achieve a complete response at the early-response assessment after two cycles of therapy.[46][Level of evidence: 2A]
- The 3-year EFS rate (median follow-up, 3.4 years) was 97.4%, and the OS rate was 98.7%.
- The AEPA and CAPDAC regimens were well tolerated and allowed for omission of radiation therapy in 35% of the treated patients.
- Only 4% of patients experienced grade 3 neuropathy.
- Compared with historical controls, residual node radiation volumes in patients requiring radiation were very small, sparing healthy surrounding tissue.
German multicenter trial results
In the last 30 years, German investigators have implemented a series of risk-adapted trials evaluating sex-based treatments featuring multiagent chemotherapy with vincristine, prednisone, procarbazine, and doxorubicin (OPPA)/COPP and IFRT.
Key findings from these trials include the following:
- Substitution of cyclophosphamide for mechlorethamine in the MOPP combination results in a low risk of subsequent myelodysplasia/leukemia.[9]
- Omission of procarbazine from the OPPA combination and substitution of methotrexate for procarbazine in the COPP combination (OPA/COMP) results in a substantially inferior EFS.[57]
- Substitution of etoposide for procarbazine in the OPPA combination (OEPA) in boys produces comparable EFS to that of girls treated with OPPA and is associated with hormonal parameters, suggesting lower risk of gonadal toxicity.[58]
- Omission of radiation for patients completely responding (defined as complete resolution or only minor residuals in all previously involved regions using clinical examination and anatomical imaging) to risk-based and sex-based OEPA or OPPA/COPP chemotherapy results in a significantly lower EFS in intermediate-risk and high-risk patients than in irradiated patients (79% vs. 91%), but no difference among nonirradiated and irradiated patients assigned to the favorable-risk group.[21]
- Substitution of dacarbazine for procarbazine (OEPA-COPDAC) in boys produces comparable results to standard OPPA-COPP in girls when used in combination with IFRT for intermediate-risk and high-risk patients.[19][Level of evidence: 2A]
Accepted Risk-Adapted Treatment Strategies for Newly Diagnosed Children and Adolescents with Hodgkin Lymphoma
Contemporary trials for pediatric Hodgkin lymphoma involve a risk-adapted, response-based treatment approach that titrates the length and intensity of chemotherapy and dose of radiation on the basis of disease-related factors, including stage, number of involved nodal regions, tumor bulk, the presence of B symptoms, and early response to chemotherapy as determined by functional imaging. In addition, vulnerability related to age and sex is also considered in treatment planning.
Classical Hodgkin lymphoma low-risk disease
Table 6 summarizes the results of treatment approaches used for patients with low-risk Hodgkin lymphoma.
Table 6. Treatment Approaches for Patients With Low-Risk Hodgkin Lymphoma
| Chemotherapy (No. of Cycles)a | Radiation (Gy) | Stage | No. of Patients | Event-Free Survival (No. of Years of Follow-up) | Survival (No. of Years of Follow-up) |
|---|---|---|---|---|---|
| VAMP (4)b [47] | IFRT (15–25.5) | CS I/IIc | 110 | 89% (10) | 96% (10) |
| VAMP (4)b [55] | IFRT (25.5) | CS I/IIc | 41 | 88% (5) | 100% (5) |
| None | 47 | 89% (5) | |||
| COPP/ABV (4) [14,20] | IFRT (21) | CS IA/B, IIAd | 94 | 100% (10)e | 97% (10)e |
| None | 113 | 89% (10)e | 96% (10)e | ||
| OEPA/OPPA (2) [21] | IFRT (20–35) | I, IIA | 281 | 94% (5) | N/A |
| None | 113 | 97% (5) | |||
| ABVD [59] | IFRT (21–35) | I–IV | 209 | 85% (5) | 97% (5) |
| ABVE (2-4)b [49] | IFRT (25.5) | IA, IIA, IIIA1, without bulky disease | 51 | 91% (6) | 98% (6) |
| AV-PC [34] | None | IA, IIA, without bulky disease | 278 | 79.9% (4) | 99.6% (4) |
| Response-based IFRT (21) |
CS = clinical stage; IFRT = involved-field radiation therapy; N/A = not applicable; No. = number.
aRefer to Table 5 for more information about the chemotherapy regimens.
bIncluded patients with nodular lymphocyte-predominant Hodgkin lymphoma.
cWithout bulky mediastinal (defined as one-third or more of intrathoracic ratio measured on an upright posteroanterior chest radiograph) or peripheral lymphadenopathy (defined as 6 cm or more) or B symptoms.
dWithout adverse features, defined as one or more of the following: hilar adenopathy, involvement of more than four nodal regions; mediastinal tumor with diameter equal to or larger than one-third of the chest diameter, and node or nodal aggregate with a diameter larger than 10 cm.
eResults from as-treated analysis.
Classical Hodgkin lymphoma intermediate-risk disease
Table 7 summarizes the results of treatment approaches used for patients with intermediate-risk Hodgkin lymphoma.
Table 7. Treatment Approaches for Patients With Intermediate-Risk Hodgkin Lymphoma
| Chemotherapy (No. of Cycles)a | Radiation (Gy) | Stage | No. of Patients | Event-Free Survival (No. of Years of Follow-up) | Survival (No. of Years of Follow-up) |
|---|---|---|---|---|---|
| COPP/ABV (6) [20] | IFRT (21) | CS I/IIb, CS IIB, CS III | 103 | 84% (10)c | 100% (3) |
| None | 122 | 78% (10)c | |||
| OEPA/OPPA (2) + COPP (2) [21] | IFRT (20–35) | IIEA, IIB, IIIA | 212 | 92% (5) | N/A |
| OEPA/OPPA (2) + COPDAC (2) [19] | IFRT (20–35) | IE, IIB, IIEA, IIIA | 139 | 88.3% (5) | 98.5% (5) |
| ABVE-PC (3–5) [18] | IFRT (21) | IIA/IIIA, if bulky disease | 53 | 84% (5) | 95% (5) |
| ABVE-PC: RER/CR [17] | IFRT (21) | IB, IAE, IIB, IIAE, IIA, IVA, IA, IIA + bulky disease | 380 | 87.9% (4) | 98.8% (4) |
| ABVE-PC: RER/CR [17] | None | IB, IAE, IIB, IIAE, IIA, IVA, IA, IIA + bulky disease | 382 | 84.3% (4) | 98.8% (4) |
| ABVE-PC: SER: +DECA [17] | IFRT (21) | IB, IAE, IIB, IIAE, IIA, IVA, IA, IIA + bulky disease | 153 | 79.3% (4) | 96.5% (4) |
| ABVE-PC: SER: -DECA [17] | IFRT (21) | 151 | 75.2% (4) | 94.3% (4) |
CR = complete response; CS = clinical stage; E = extralymphatic; IFRT = involved-field radiation therapy; N/A = not applicable; RER = rapid early response; SER = slow early response.
aRefer to Table 5 for more information about the chemotherapy regimens.
bWith adverse disease features, defined as one or more of the following: hilar adenopathy, involvement of more than four nodal regions; mediastinal tumor with diameter equal to or larger than one-third of the chest diameter, and node or nodal aggregate with a diameter larger than 10 cm.
cResults from as-treated analysis.
Classical Hodgkin lymphoma high-risk disease
Table 8 summarizes the results of treatment approaches used for patients with high-risk Hodgkin lymphoma.
Table 8. Treatment Approaches for Patients With High-Risk Hodgkin Lymphoma
| Chemotherapy (No. of Cycles)a | Radiation (Gy) | Stage | No. of Patients | Event-Free Survival (No. of Years of Follow-up) | Survival (No. of Years of Follow-up) |
|---|---|---|---|---|---|
| OEPA/OPPA (2) + COPP (4) [21] | IFRT (20–35) | IIEB, IIIEA/B, IIIB, IVA/B | 265 | 91% (5) | N/A |
| OEPA/OPPA (2) + COPDAC (4) [19] | IFRT (20–35) | IIEB, IIIEA/B, IIIB, IVA/B | 239 | 86.9% (5) | 94.9% (5) |
| ABVE-PC (3-5) [18,60] | IFRT (21) | IIB, IIIB, IV | 163 | 85% (5) | 95% (5) |
| BEACOPP (4); COPP/ABV (4) (RER; girls) [50] | None | IIB, IIIB, IV | 38 | 94% (5)b | 97% (5)b |
| BEACOPP (4); ABVD (2) (RER; boys) [50] | IFRT (21) | IIB, IIIB, IV | 34 | ||
| BEACOPP (8) (SER) [50] | IFRT (21) | IIB, IIIB, IV | 25 | ||
| AEPA (2); CAPDAC (4) [46] | Individual residual nodal (25.5) | IIB, IIIB, IV | 77 | 97.4% (3) | 98.7% (3) |
E = extralymphatic; IFRT = involved-field radiation therapy; N/A = not applicable; No. = number; RER = rapid early response; SER = slow early response.
aRefer to Table 5 for more information about the chemotherapy regimens.
bResults include all treatment strata.
Treatment options under clinical evaluation
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
Nodular lymphocyte-predominant Hodgkin lymphoma
The use of combination chemotherapy and/or radiation therapy can achieve excellent long-term progression-free survival and OS in patients with nodular lymphocyte-predominant Hodgkin lymphoma.[24,61,62] Late recurrences have been reported and are typically responsive to re-treatment. Because deaths observed among individuals with this histological subtype are frequently related to complications from cytotoxic therapy, risk-adapted treatment assignment is particularly important for limiting exposure to agents with established dose-related toxicities.[61,62]
Table 9 summarizes the results of treatment approaches used for nodular lymphocyte-predominant Hodgkin lymphoma, some of which feature surgery alone for completely resected disease and limited cycles of chemotherapy with or without LD-IFRT. Because of the relative rarity of this subtype, most trials are limited by small cohort numbers and nonrandom allocation of treatment.
Results from a single-arm COG trial provide data to support the strategy of observation after surgical resection and treatment with limited chemotherapy for children with favorable-stage IA or IIA Hodgkin lymphoma. Among 178 patients treated with surgical resection alone for single-node disease (n = 52), chemotherapy alone after CR to three cycles of doxorubicin, vincristine, prednisone, and cyclophosphamide (AV-PC) chemotherapy (n = 115), or chemotherapy with LD-IFRT (21 Gy) after incomplete response to AV-PC chemotherapy (n = 11), the 5-year EFS was 85.5%, and the OS was 100%. Five-year EFS was 77% for patients observed after total resection and 88.8% for patients treated with AV-PC chemotherapy.[24][Level of evidence: 1iiDi] Retrospective case series report on responses with rituximab alone [63] or in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) [64] in adults with nodular lymphocyte-predominant Hodgkin lymphoma; however, pediatric data has not been reported.
Table 9. Treatment Approaches for Patients With Nodular Lymphocyte-Predominant Hodgkin Lymphoma
| Chemotherapy (No. of Cycles)a | Radiation (Gy) | No. of Patients | Event-Free Survival (No. of Years of Follow-up) | Survival (No. of Years of Follow-up) |
|---|---|---|---|---|
| COPP/ABV (4)b [20] | None | 52 | 96% (10) | N/A |
| IFRT (21) | 35 | 96% (10) | ||
| CVP (3) [51] | None | 55 | 74% (5) | 100% (5) |
| DBVE (2–4)c [49] | None | 26 | 94% (8) | 100% (8) |
| IFRT (25.5) | ||||
| VAMP (4)c [55] | None | 26 | 89.4% (5) | N/A |
| IFRT (25) | 6 | 85.7% (5) | N/A | |
| VAMP (4) [47] | IFRT (15–25.5) | 33 | 100% (10) | 100% (10) |
| Noned [24] | Noned | 52 | 77% (5) | 100% (5) |
| AV-PC [24] | None | 124 | 85.5% (5) | 100% (5) |
| IFRT (21) | 11 | |||
| Nonee [25] | None | 51 | 67% (2) | 100% (2) |
IFRT = involved-field radiation therapy; N/A = not applicable; No. = number.
aRefer to Table 5 for more information about the chemotherapy regimens.
bAllocation to radiation therapy or no radiation therapy was based on response to therapy. These results were for the group of patients with mixed-cellularity histology and nodular lymphocyte-predominant Hodgkin lymphoma.
cAllocation was based on clinical response.
dSingle lymph node surgically resected.
eAll involved lymph nodes surgically resected.
Treatment of Adolescents and Young Adults With Hodgkin Lymphoma
The treatment approach used for adolescents and young adults with Hodgkin lymphoma may vary based on community referral patterns and age restrictions at pediatric cancer centers, and the optimal approach is debatable.
In patients with intermediate-risk or high-risk disease, the standard of care in adult oncology practices typically involves at least six cycles of ABVD chemotherapy that would deliver a cumulative anthracycline dose of 300 mg/m2.[65,66] (Refer to the PDQ summary on Adult Hodgkin Lymphoma Treatment for more information.) In late-health outcomes studies of pediatric cancer survivors, the risk of anthracycline cardiomyopathy has been shown to exponentially increase after exposure to cumulative anthracycline doses of 250 mg/m2 to 300 mg/m2.[67,68] Subsequent need for mediastinal radiation can further enhance the risk of a variety of late cardiac events.[67-69] In an effort to optimize disease control and preserve both cardiac and gonadal function, pediatric regimens for low-risk disease most often feature a restricted number of cycles of ABVD derivative combinations, whereas alkylating agents and etoposide are integrated into anthracycline-containing regimens for those with intermediate-risk and high-risk disease.
No prospective studies of efficacy or toxicity in adolescent or young adults treated with pediatric versus adult regimens have been reported; however, some secondary analyses have been conducted.[70]
- A retrospective review documented the outcomes of patients aged 17 to 22 years treated in the Eastern Cooperative Oncology Group (ECOG) trials E2496 (NCT00003389) or Stanford V versus the COG trial AHOD0031 (NCT00025259).[71][Level of evidence: 3iiiDiii]
- The 5-year failure-free survival was 68% for the ECOG trial and 81% in the COG trial, with an OS of 89% and 97%, respectively.
- Limitations of this study include differences in the study populations; more adolescents and young adults aged 17 to 22 years in the E2496 study had stage III or IV disease and B symptoms, whereas more adolescents and young adults aged 17 to 22 years in the AHOD0031 study had bulky disease and received radiation (although with smaller doses than those in E2496). Some of these differences were addressed using a propensity score analysis that confirmed inferior failure-free survival for adolescents and young adults in the E2496 trial than those in the AHOD0031 trial. The study was also not a prospective randomized trial.
- A comprehensive review of differences in outcomes between adolescent and young adult patients treated on pediatric versus adult trials was published.[72]
The optimal approach for adolescents and young adults with Hodgkin lymphoma is complicated by critical but understudied variables. Factors such as tumor biology, disease control, supportive care needs, and long-term toxicities in adolescents and young adults with Hodgkin lymphoma remain understudied.
Participation in a clinical trial should be considered for adolescent and young adult patients with Hodgkin lymphoma. Information about ongoing clinical trials is available from the NCI website.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Donaldson SS, Kaplan HS: Complications of treatment of Hodgkin's disease in children. Cancer Treat Rep 66 (4): 977-89, 1982. [PubMed: 7074658]
- Donaldson SS, Link MP: Combined modality treatment with low-dose radiation and MOPP chemotherapy for children with Hodgkin's disease. J Clin Oncol 5 (5): 742-9, 1987. [PubMed: 3572464]
- Jenkin D, Chan H, Freedman M, et al.: Hodgkin's disease in children: treatment results with MOPP and low-dose, extended-field irradiation. Cancer Treat Rep 66 (4): 949-59, 1982. [PubMed: 7042093]
- Bhatia S, Robison LL, Oberlin O, et al.: Breast cancer and other second neoplasms after childhood Hodgkin's disease. N Engl J Med 334 (12): 745-51, 1996. [PubMed: 8592547]
- Hancock SL, Donaldson SS, Hoppe RT: Cardiac disease following treatment of Hodgkin's disease in children and adolescents. J Clin Oncol 11 (7): 1208-15, 1993. [PubMed: 8315419]
- Devita VT, Serpick AA, Carbone PP: Combination chemotherapy in the treatment of advanced Hodgkin's disease. Ann Intern Med 73 (6): 881-95, 1970. [PubMed: 5525541]
- Bonadonna G, Santoro A: ABVD chemotherapy in the treatment of Hodgkin's disease. Cancer Treat Rev 9 (1): 21-35, 1982. [PubMed: 6175409]
- Ortin TT, Shostak CA, Donaldson SS: Gonadal status and reproductive function following treatment for Hodgkin's disease in childhood: the Stanford experience. Int J Radiat Oncol Biol Phys 19 (4): 873-80, 1990. [PubMed: 2211255]
- Schellong G, Riepenhausen M, Creutzig U, et al.: Low risk of secondary leukemias after chemotherapy without mechlorethamine in childhood Hodgkin's disease. German-Austrian Pediatric Hodgkin's Disease Group. J Clin Oncol 15 (6): 2247-53, 1997. [PubMed: 9196137]
- Mefferd JM, Donaldson SS, Link MP: Pediatric Hodgkin's disease: pulmonary, cardiac, and thyroid function following combined modality therapy. Int J Radiat Oncol Biol Phys 16 (3): 679-85, 1989. [PubMed: 2466027]
- Fryer CJ, Hutchinson RJ, Krailo M, et al.: Efficacy and toxicity of 12 courses of ABVD chemotherapy followed by low-dose regional radiation in advanced Hodgkin's disease in children: a report from the Children's Cancer Study Group. J Clin Oncol 8 (12): 1971-80, 1990. [PubMed: 1700080]
- Weiner MA, Leventhal BG, Marcus R, et al.: Intensive chemotherapy and low-dose radiotherapy for the treatment of advanced-stage Hodgkin's disease in pediatric patients: a Pediatric Oncology Group study. J Clin Oncol 9 (9): 1591-8, 1991. [PubMed: 1714950]
- Chow LM, Nathan PC, Hodgson DC, et al.: Survival and late effects in children with Hodgkin's lymphoma treated with MOPP/ABV and low-dose, extended-field irradiation. J Clin Oncol 24 (36): 5735-41, 2006. [PubMed: 17179107]
- Nachman JB, Sposto R, Herzog P, et al.: Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin's disease who achieve a complete response to chemotherapy. J Clin Oncol 20 (18): 3765-71, 2002. [PubMed: 12228196]
- Gerres L, Brämswig JH, Schlegel W, et al.: The effects of etoposide on testicular function in boys treated for Hodgkin's disease. Cancer 83 (10): 2217-22, 1998. [PubMed: 9827728]
- Smith MA, Rubinstein L, Anderson JR, et al.: Secondary leukemia or myelodysplastic syndrome after treatment with epipodophyllotoxins. J Clin Oncol 17 (2): 569-77, 1999. [PubMed: 10080601]
- Friedman DL, Chen L, Wolden S, et al.: Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children's Oncology Group Study AHOD0031. J Clin Oncol 32 (32): 3651-8, 2014. [PMC free article: PMC4220044] [PubMed: 25311218]
- Schwartz CL, Constine LS, Villaluna D, et al.: A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood 114 (10): 2051-9, 2009. [PMC free article: PMC2744567] [PubMed: 19584400]
- Mauz-Körholz C, Hasenclever D, Dörffel W, et al.: Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin's lymphoma: the GPOH-HD-2002 study. J Clin Oncol 28 (23): 3680-6, 2010. [PubMed: 20625128]
- Wolden SL, Chen L, Kelly KM, et al.: Long-term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin's lymphoma--a report from the Children's Oncology Group. J Clin Oncol 30 (26): 3174-80, 2012. [PMC free article: PMC3434976] [PubMed: 22649136]
- Dörffel W, Lüders H, Rühl U, et al.: Preliminary results of the multicenter trial GPOH-HD 95 for the treatment of Hodgkin's disease in children and adolescents: analysis and outlook. Klin Padiatr 215 (3): 139-45, 2003 May-Jun. [PubMed: 12838937]
- Kelly KM: Management of children with high-risk Hodgkin lymphoma. Br J Haematol 157 (1): 3-13, 2012. [PubMed: 22188115]
- McCarten KM, Metzger ML, Drachtman RA, et al.: Significance of pleural effusion at diagnosis in pediatric Hodgkin lymphoma: a report from Children's Oncology Group protocol AHOD0031. Pediatr Radiol 48 (12): 1736-1744, 2018. [PMC free article: PMC6208959] [PubMed: 30014200]
- Appel BE, Chen L, Buxton AB, et al.: Minimal Treatment of Low-Risk, Pediatric Lymphocyte-Predominant Hodgkin Lymphoma: A Report From the Children's Oncology Group. J Clin Oncol 34 (20): 2372-9, 2016. [PMC free article: PMC4981978] [PubMed: 27185849]
- Mauz-Körholz C, Gorde-Grosjean S, Hasenclever D, et al.: Resection alone in 58 children with limited stage, lymphocyte-predominant Hodgkin lymphoma-experience from the European network group on pediatric Hodgkin lymphoma. Cancer 110 (1): 179-85, 2007. [PubMed: 17526010]
- Castellino SM, Geiger AM, Mertens AC, et al.: Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood 117 (6): 1806-16, 2011. [PMC free article: PMC3056636] [PubMed: 21037086]
- Pellegrino B, Terrier-Lacombe MJ, Oberlin O, et al.: Lymphocyte-predominant Hodgkin's lymphoma in children: therapeutic abstention after initial lymph node resection--a Study of the French Society of Pediatric Oncology. J Clin Oncol 21 (15): 2948-52, 2003. [PubMed: 12885814]
- Shankar A, Daw S: Nodular lymphocyte predominant Hodgkin lymphoma in children and adolescents--a comprehensive review of biology, clinical course and treatment options. Br J Haematol 159 (3): 288-98, 2012. [PubMed: 22994199]
- Shankar AG, Roques G, Kirkwood AA, et al.: Advanced stage nodular lymphocyte predominant Hodgkin lymphoma in children and adolescents: clinical characteristics and treatment outcome - a report from the SFCE & CCLG groups. Br J Haematol 177 (1): 106-115, 2017. [PubMed: 28220934]
- Marks LJ, Pei Q, Bush R, et al.: Outcomes in intermediate-risk pediatric lymphocyte-predominant Hodgkin lymphoma: A report from the Children's Oncology Group. Pediatr Blood Cancer 65 (12): e27375, 2018. [PMC free article: PMC6192844] [PubMed: 30277639]
- Nogová L, Reineke T, Brillant C, et al.: Lymphocyte-predominant and classical Hodgkin's lymphoma: a comprehensive analysis from the German Hodgkin Study Group. J Clin Oncol 26 (3): 434-9, 2008. [PubMed: 18086799]
- Diehl V, Sextro M, Franklin J, et al.: Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin's disease and lymphocyte-rich classical Hodgkin's disease: report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin's Disease. J Clin Oncol 17 (3): 776-83, 1999. [PubMed: 10071266]
- Sandoval C, Venkateswaran L, Billups C, et al.: Lymphocyte-predominant Hodgkin disease in children. J Pediatr Hematol Oncol 24 (4): 269-73, 2002. [PubMed: 11972094]
- Keller FG, Castellino SM, Chen L, et al.: Results of the AHOD0431 trial of response adapted therapy and a salvage strategy for limited stage, classical Hodgkin lymphoma: A report from the Children's Oncology Group. Cancer 124 (15): 3210-3219, 2018. [PMC free article: PMC6097921] [PubMed: 29738613]
- Hodgson DC, Dieckmann K, Terezakis S, et al.: Implementation of contemporary radiation therapy planning concepts for pediatric Hodgkin lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Pract Radiat Oncol 5 (2): 85-92, 2015 Mar-Apr. [PubMed: 25413415]
- Girinsky T, van der Maazen R, Specht L, et al.: Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma: concepts and guidelines. Radiother Oncol 79 (3): 270-7, 2006. [PubMed: 16797755]
- Campbell BA, Voss N, Pickles T, et al.: Involved-nodal radiation therapy as a component of combination therapy for limited-stage Hodgkin's lymphoma: a question of field size. J Clin Oncol 26 (32): 5170-4, 2008. [PubMed: 18838714]
- Maraldo MV, Aznar MC, Vogelius IR, et al.: Involved node radiation therapy: an effective alternative in early-stage hodgkin lymphoma. Int J Radiat Oncol Biol Phys 85 (4): 1057-65, 2013. [PubMed: 23200817]
- Andolino DL, Hoene T, Xiao L, et al.: Dosimetric comparison of involved-field three-dimensional conformal photon radiotherapy and breast-sparing proton therapy for the treatment of Hodgkin's lymphoma in female pediatric patients. Int J Radiat Oncol Biol Phys 81 (4): e667-71, 2011. [PubMed: 21459527]
- Rühl U, Albrecht M, Dieckmann K, et al.: Response-adapted radiotherapy in the treatment of pediatric Hodgkin's disease: an interim report at 5 years of the German GPOH-HD 95 trial. Int J Radiat Oncol Biol Phys 51 (5): 1209-18, 2001. [PubMed: 11728679]
- Hoppe BS, Flampouri S, Su Z, et al.: Effective dose reduction to cardiac structures using protons compared with 3DCRT and IMRT in mediastinal Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 84 (2): 449-55, 2012. [PubMed: 22386373]
- Charpentier AM, Friedman DL, Wolden S, et al.: Predictive Factor Analysis of Response-Adapted Radiation Therapy for Chemotherapy-Sensitive Pediatric Hodgkin Lymphoma: Analysis of the Children's Oncology Group AHOD 0031 Trial. Int J Radiat Oncol Biol Phys 96 (5): 943-950, 2016. [PMC free article: PMC5584603] [PubMed: 27869096]
- Yeh JM, Diller L: Pediatric Hodgkin lymphoma: trade-offs between short- and long-term mortality risks. Blood 120 (11): 2195-202, 2012. [PMC free article: PMC3447778] [PubMed: 22826564]
- Biswas T, Culakova E, Friedberg JW, et al.: Involved field radiation therapy following high dose chemotherapy and autologous stem cell transplant benefits local control and survival in refractory or recurrent Hodgkin lymphoma. Radiother Oncol 103 (3): 367-72, 2012. [PubMed: 22398312]
- Zhou R, Ng A, Constine LS, et al.: A Comparative Evaluation of Normal Tissue Doses for Patients Receiving Radiation Therapy for Hodgkin Lymphoma on the Childhood Cancer Survivor Study and Recent Children's Oncology Group Trials. Int J Radiat Oncol Biol Phys 95 (2): 707-11, 2016. [PMC free article: PMC5001801] [PubMed: 27020112]
- Metzger ML, Link MP, Billett AL, et al.: Excellent Outcome for Pediatric Patients With High-Risk Hodgkin Lymphoma Treated With Brentuximab Vedotin and Risk-Adapted Residual Node Radiation. J Clin Oncol 39 (20): 2276-2283, 2021. [PMC free article: PMC8260923] [PubMed: 33826362]
- Donaldson SS, Link MP, Weinstein HJ, et al.: Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin's disease. J Clin Oncol 25 (3): 332-7, 2007. [PubMed: 17235049]
- Tebbi CK, Mendenhall N, London WB, et al.: Treatment of stage I, IIA, IIIA1 pediatric Hodgkin disease with doxorubicin, bleomycin, vincristine and etoposide (DBVE) and radiation: a Pediatric Oncology Group (POG) study. Pediatr Blood Cancer 46 (2): 198-202, 2006. [PubMed: 16136581]
- Tebbi CK, Mendenhall NP, London WB, et al.: Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: a report from the Children's Oncology Group. Pediatr Blood Cancer 59 (7): 1259-65, 2012. [PMC free article: PMC3468662] [PubMed: 22911615]
- Kelly KM, Sposto R, Hutchinson R, et al.: BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: a report from the Children's Oncology Group. Blood 117 (9): 2596-603, 2011. [PMC free article: PMC3062352] [PubMed: 21079154]
- Shankar A, Hall GW, Gorde-Grosjean S, et al.: Treatment outcome after low intensity chemotherapy [CVP] in children and adolescents with early stage nodular lymphocyte predominant Hodgkin's lymphoma - an Anglo-French collaborative report. Eur J Cancer 48 (11): 1700-6, 2012. [PubMed: 22093944]
- Tebbi CK, London WB, Friedman D, et al.: Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol 25 (5): 493-500, 2007. [PubMed: 17290056]
- Friedmann AM, Hudson MM, Weinstein HJ, et al.: Treatment of unfavorable childhood Hodgkin's disease with VEPA and low-dose, involved-field radiation. J Clin Oncol 20 (14): 3088-94, 2002. [PubMed: 12118022]
- Hudson MM, Krasin M, Link MP, et al.: Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin's disease. J Clin Oncol 22 (22): 4541-50, 2004. [PubMed: 15542805]
- Metzger ML, Weinstein HJ, Hudson MM, et al.: Association between radiotherapy vs no radiotherapy based on early response to VAMP chemotherapy and survival among children with favorable-risk Hodgkin lymphoma. JAMA 307 (24): 2609-16, 2012. [PMC free article: PMC3526806] [PubMed: 22735430]
- Dharmarajan KV, Friedman DL, Schwartz CL, et al.: Patterns of relapse from a phase 3 Study of response-based therapy for intermediate-risk Hodgkin lymphoma (AHOD0031): a report from the Children's Oncology Group. Int J Radiat Oncol Biol Phys 92 (1): 60-6, 2015. [PMC free article: PMC4395527] [PubMed: 25542311]
- Schellong G: The balance between cure and late effects in childhood Hodgkin's lymphoma: the experience of the German-Austrian Study-Group since 1978. German-Austrian Pediatric Hodgkin's Disease Study Group. Ann Oncol 7 (Suppl 4): 67-72, 1996. [PubMed: 8836413]
- Schellong G, Pötter R, Brämswig J, et al.: High cure rates and reduced long-term toxicity in pediatric Hodgkin's disease: the German-Austrian multicenter trial DAL-HD-90. The German-Austrian Pediatric Hodgkin's Disease Study Group. J Clin Oncol 17 (12): 3736-44, 1999. [PubMed: 10577845]
- Marr KC, Connors JM, Savage KJ, et al.: ABVD chemotherapy with reduced radiation therapy rates in children, adolescents and young adults with all stages of Hodgkin lymphoma. Ann Oncol 28 (4): 849-854, 2017. [PMC free article: PMC5834115] [PubMed: 28327925]
- Kelly KM, Cole PD, Pei Q, et al.: Response-adapted therapy for the treatment of children with newly diagnosed high risk Hodgkin lymphoma (AHOD0831): a report from the Children's Oncology Group. Br J Haematol 187 (1): 39-48, 2019. [PMC free article: PMC6857800] [PubMed: 31180135]
- Chen RC, Chin MS, Ng AK, et al.: Early-stage, lymphocyte-predominant Hodgkin's lymphoma: patient outcomes from a large, single-institution series with long follow-up. J Clin Oncol 28 (1): 136-41, 2010. [PubMed: 19933914]
- Jackson C, Sirohi B, Cunningham D, et al.: Lymphocyte-predominant Hodgkin lymphoma--clinical features and treatment outcomes from a 30-year experience. Ann Oncol 21 (10): 2061-8, 2010. [PubMed: 20332141]
- Eichenauer DA, Plütschow A, Fuchs M, et al.: Long-Term Course of Patients With Stage IA Nodular Lymphocyte-Predominant Hodgkin Lymphoma: A Report From the German Hodgkin Study Group. J Clin Oncol 33 (26): 2857-62, 2015. [PubMed: 26240235]
- Fanale MA, Cheah CY, Rich A, et al.: Encouraging activity for R-CHOP in advanced stage nodular lymphocyte-predominant Hodgkin lymphoma. Blood 130 (4): 472-477, 2017. [PMC free article: PMC5578726] [PubMed: 28522441]
- Viviani S, Zinzani PL, Rambaldi A, et al.: ABVD versus BEACOPP for Hodgkin's lymphoma when high-dose salvage is planned. N Engl J Med 365 (3): 203-12, 2011. [PubMed: 21774708]
- Chisesi T, Bellei M, Luminari S, et al.: Long-term follow-up analysis of HD9601 trial comparing ABVD versus Stanford V versus MOPP/EBV/CAD in patients with newly diagnosed advanced-stage Hodgkin's lymphoma: a study from the Intergruppo Italiano Linfomi. J Clin Oncol 29 (32): 4227-33, 2011. [PubMed: 21990405]
- van der Pal HJ, van Dalen EC, van Delden E, et al.: High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol 30 (13): 1429-37, 2012. [PubMed: 22473161]
- Blanco JG, Sun CL, Landier W, et al.: Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children's Oncology Group. J Clin Oncol 30 (13): 1415-21, 2012. [PMC free article: PMC3383117] [PubMed: 22124095]
- Mulrooney DA, Yeazel MW, Kawashima T, et al.: Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 339: b4606, 2009. [PMC free article: PMC3266843] [PubMed: 19996459]
- Kahn JM, Kelly KM: Adolescent and young adult Hodgkin lymphoma: Raising the bar through collaborative science and multidisciplinary care. Pediatr Blood Cancer 65 (7): e27033, 2018. [PMC free article: PMC5980713] [PubMed: 29603618]
- Henderson TO, Parsons SK, Wroblewski KE, et al.: Outcomes in adolescents and young adults with Hodgkin lymphoma treated on US cooperative group protocols: An adult intergroup (E2496) and Children's Oncology Group (COG AHOD0031) comparative analysis. Cancer 124 (1): 136-144, 2018. [PMC free article: PMC5735034] [PubMed: 28902390]
- Flerlage JE, Metzger ML, Bhakta N: The management of Hodgkin lymphoma in adolescents and young adults: burden of disease or burden of choice? Blood 132 (4): 376-384, 2018. [PMC free article: PMC6071556] [PubMed: 29895665]
Treatment of Primary Refractory or Recurrent Hodgkin Lymphoma in Children and Adolescents
The excellent response to frontline therapy among children and adolescents with Hodgkin lymphoma limits opportunities to evaluate second-line (salvage) therapy. Because of the small number of patients that fail primary therapy, no uniform second-line treatment strategy exists for this patient population.
Adverse prognostic factors after relapse include the following:[1][Level of evidence: 3iiA]
- The presence of B symptoms (fever, weight loss, and night sweats) and extranodal disease.[2]
- Inadequate response to initial second-line therapy.[4]
Children with localized favorable (relapse ≥12 months after completing therapy) disease recurrences whose original therapy involved reduced cycles of risk-adapted therapy or with chemotherapy alone and/or low-dose involved-field radiation therapy (LD-IRFT) consolidation have a high likelihood of achieving long-term survival after treatment with more intensive conventional chemotherapy.[5,6]
Treatment options for children and adolescents with refractory or recurrent Hodgkin lymphoma include the following:
Chemotherapy and Targeted Therapy
Chemotherapy is the recommended second-line therapy, with the choice of specific agents, dose-intensity, and number of cycles determined by the initial therapy, disease characteristics at progression/relapse, and response to second-line therapy.
Agents used alone or in combination regimens in the treatment of refractory or recurrent pediatric Hodgkin lymphoma include the following:
- ICE (ifosfamide, carboplatin, and etoposide).[7]
- Ifosfamide and vinorelbine with or without bortezomib.[8][Level of evidence: 2Div]; [9][Level of evidence: 3iiiDiv]
- Ifosfamide, gemcitabine, and vinorelbine.[10][Level of evidence: 3iii]
- Vinorelbine and gemcitabine.[11]
- Vinorelbine, gemcitabine, and dexamethasone.[12][Level of evidence: 3iiiA]
- IEP/ABVD/COPP (ifosfamide, etoposide, prednisone/doxorubicin, bleomycin, vinblastine, dacarbazine/cyclophosphamide, vincristine, procarbazine, prednisone).[3]
- EPIC (etoposide, prednisolone, ifosfamide, and cisplatin).[13]
- APE (cytosine arabinoside, cisplatin, and etoposide).[14]
- MIED (high-dose methotrexate, ifosfamide, etoposide, and dexamethasone).[15]
- Rituximab (for patients with CD20-positive disease) alone or in combination with second-line chemotherapy.[16]
- Brentuximab vedotin. Brentuximab vedotin has been evaluated in adults with Hodgkin lymphoma. (Refer to the Recurrent Adult Classic HL Treatment section in the PDQ summary on Adult Hodgkin Lymphoma Treatment for more information.) The U.S. Food and Drug Administration (FDA) indications for brentuximab vedotin in adult patients are as follows: (1) classical Hodgkin lymphoma after failure of autologous HCT or after failure of at least two previous multiagent chemotherapy regimens in patients who are not autologous HCT candidates, and (2) classical Hodgkin lymphoma at high risk of relapse or progression, as postautologous HCT consolidation therapy.
- A phase I study in adults with CD30-positive lymphomas identified a recommended phase II dose of 1.8 mg/kg on an every 3-week schedule and showed an objective response rate of 50% (6 of 12 patients) at the recommended phase II dose.[17][Level of evidence: 2Div]
- A phase II trial in adults with Hodgkin lymphoma (N = 102) who relapsed after autologous HCT showed the following:[18-21]
- Patients who achieved a complete remission (n = 34) had a 3-year progression-free survival (PFS) rate of 58% and a 3-year overall survival (OS) rate of 73%, with only 6 of 34 patients proceeding to allogeneic HCT while in remission.
- Further follow-up demonstrated a 5-year OS rate of 41% and a PFS rate of 22%. However, patients who achieved a complete remission (38%) had a 5-year OS rate of 64% and a PFS rate of 52%.[21][Level of evidence: 2A]
- The number of pediatric patients treated with brentuximab vedotin is not sufficient to determine whether they respond differently than do adult patients. Clearance and volume of brentuximab vedotin significantly correlates with weight (P < .001), and its area under the curve and C max are lower in children than those reported in adult studies with weekly dosing.[22]
- The Children’s Oncology Group phase I/II study AHOD1221 (NCT01780662) investigated treatment with brentuximab vedotin and gemcitabine in 46 children and young adults with primary refractory Hodgkin Lymphoma or early relapse.[23]
- The recommended phase II dose of brentuximab vedotin was 1.8 mg/kg.
- Twenty-four of 42 patients (57%; 95% CI, 41%–72%) treated at this dose level experienced a complete response within the first four cycles. Four of 13 patients (31%) with partial response or stable disease had all target lesions with Deauville scores of 3 or less after cycle four. By modern response criteria, these are also complete responses, increasing the complete response rate to 28 of 42 patients (67%; 95% confidence interval [CI], 51%–80%).
- Compared with alternate second-line regimens, brentuximab vedotin with gemcitabine offers the advantage of avoiding agents associated with late treatment-related sequelae, such as anthracyclines, alkylators, or epipodophyllotoxins.
There are ongoing trials to determine the toxicity and efficacy of combining brentuximab vedotin with chemotherapy.
Checkpoint Inhibitor Therapy
Treatments that block the interaction between programmed death-1 (PD-1) and its ligands have shown high levels of activity in adults with Hodgkin lymphoma.
Evidence (nivolumab):
- The anti–PD-1 antibody nivolumab induced objective responses in 20 of 23 adult patients (87%) with relapsed Hodgkin lymphoma.[24]
- In a phase I/II study of nivolumab in children with refractory malignancies, single-agent nivolumab was found to be tolerable and showed antitumor activity. Among ten children with Hodgkin lymphoma, there was one complete response, two partial responses, and five stable diseases.[26][Level of evidence: 3iiiDiv]
Evidence (pembrolizumab):
- The anti–PD-1 antibody pembrolizumab produced an objective response rate of 65% in 31 heavily pretreated adult patients with Hodgkin lymphoma who relapsed after receiving brentuximab vedotin.[27] (Refer to the Recurrent Adult Classic HL Treatment section in the PDQ summary on Adult Hodgkin Lymphoma Treatment for more information.)
- A phase II study of 210 adult patients (median age, 35 years; range, 18–76 years) with refractory/relapsed classical Hodgkin lymphoma who were treated with pembrolizumab reported an overall response rate of 69% (95% CI, 62.3%–75.2%), with a complete response rate of 22.4% (95% CI, 6.9%–28.6%).[28][Level of evidence: 3iiiDiv]
- In a multicenter, nonrandomized, open-label, single-arm phase I/II study, 15 pediatric patients with relapsed or refractory Hodgkin lymphoma were treated with pembrolizumab at a dose of 2 mg/kg every 3 weeks. Two patients achieved complete responses and seven patients achieved partial responses, for an overall objective response rate of 60% (95% CI, 32.2%–83.7%).[29][Level of evidence: 3iii] Adverse events were documented in 97% of the 154 patients enrolled on the study and were mostly grades 1 to 2 toxicities. Grades 3 to 5 events were seen in 45% of the cases and consisted mostly of anemia and lymphopenia. Treatment interruptions were most commonly caused by transaminitis, hypertension, pleural effusion, and pneumonitis; two deaths were attributed to drug administration (one resulting from pulmonary edema, and the other because of pleural effusion and pneumonitis).
Pembrolizumab is FDA approved for use in cases of refractory disease or relapse after three or more lines of therapy.
There are ongoing trials to determine the toxicity and efficacy of combining and/or comparing brentuximab vedotin and nivolumab with chemotherapy in pediatric patients with Hodgkin lymphoma.
Chemotherapy Followed by Autologous Hematopoietic Cell Transplantation (HCT)
Myeloablative chemotherapy with autologous HCT is the recommended approach for patients who develop refractory disease during therapy or relapsed disease within 1 year after completing therapy.[7,30- 37]; [38][Level of evidence: 3iiA]; [39][Level of evidence: 3iiiA] In addition, this approach is also recommended for those who recur with extensive disease after the first year of completing therapy or for those who recur after initial therapy that included intensive (alkylating agents and anthracyclines) multiagent chemotherapy and radiation therapy.
- Autologous HCT has been preferred for patients with relapsed Hodgkin lymphoma because of the historically high transplant-related mortality (TRM) associated with allogeneic transplantation.[40] After autologous HCT, the projected survival rate is 45% to 70% and PFS is 30% to 89%.[20,38,41,42]; [43][Level of evidence: 3iiiA]
- Brentuximab vedotin as maintenance therapy given for 1 year after autologous HCT in adult patients with high risk of relapse or progression was demonstrated to improve PFS in a randomized, placebo-controlled, phase III trial.[44]
- A multicenter, open-label, dose-escalation, phase I/II study evaluated the safety, maximum tolerated dose, and pharmacokinetics of brentuximab vedotin and identified a recommended phase II dose in 36 pediatric patients with relapsed or refractory classical Hodgkin lymphoma (n = 19) and anaplastic large cell lymphoma (n = 17). Toxicity was manageable (33% of patients had transient, limited-severity peripheral neuropathy), the maximum tolerated dose was not reached, and pediatric pharmacokinetics were similar to that of adults. The recommended phase II dose of brentuximab vedotin was 1.8 mg/m2 and it was administered for up to 16 cycles (median, 10 cycles) on the phase II arm. Among Hodgkin lymphoma participants on the phase II arm, 47% of patients achieved an overall response (33% complete response, 13% partial response), which provided a bridge to HCT for some patients.[45][Level of evidence: 3iii]
- The most commonly utilized preparative regimen for peripheral blood stem cell transplant is the BEAM regimen (carmustine [BCNU], etoposide, cytarabine, melphalan) or CBV regimen (cyclophosphamide, carmustine, etoposide).[36,41-43]; [38][Level of evidence: 3iiA]; [39][Level of evidence:3iiiA] Carmustine may produce significant pulmonary toxicity.[43]
- Other noncarmustine-containing preparative regimens have been utilized, including high-dose busulfan, etoposide, and cyclophosphamide [46] and lomustine, cytarabine, cyclophosphamide, and etoposide (LACE).[47][Level of evidence: 3iii]
Adverse prognostic features for outcome after autologous HCT include extranodal disease at relapse, bulky mediastinal mass at time of transplant, advanced stage at relapse, primary refractory disease, poor response to chemotherapy, and a positive positron emission tomography scan before autologous HCT.[1,41-43,48,49]
(Refer to the Autologous HCT section in the PDQ summary on Childhood Hematopoietic Cell Transplantation for more information about transplantation.)
Chemotherapy Followed by Allogeneic HCT
For patients who fail after autologous HCT or for patients with chemoresistant disease, allogeneic HCT has been used with encouraging results.[13,40,50-52] Investigations of reduced-intensity allogeneic transplantation that typically use fludarabine or low-dose total body irradiation to provide a nontoxic immunosuppression have demonstrated acceptable rates of TRM.[53-57]
(Refer to the Allogeneic HCT section in the PDQ summary on Childhood Hematopoietic Cell Transplantation for more information about transplantation.)
Involved-site Radiation Therapy (ISRT)
ISRT to sites of recurrent disease may enhance local control if these sites have not been previously irradiated. ISRT is generally administered after high-dose chemotherapy and stem cell rescue.[58] For patients who are not responsive to salvage therapy, ISRT may be an appropriate consideration before HCT.[59,60]
Response Rates for Primary Refractory Hodgkin Lymphoma
Salvage rates for patients with primary refractory Hodgkin lymphoma are poor even with autologous HCT and radiation. However, intensification of therapy followed by HCT consolidation has been reported to achieve long-term survival in some studies.
Evidence (response to treatment of primary refractory Hodgkin lymphoma):
- In one large series, 5-year OS after primary refractory Hodgkin lymphoma was attained with aggressive second-line therapy (high-dose chemoradiation therapy) and autologous HCT in 49% of patients.[61]
- In a Gesellschaft für Pädiatrische Onkologie und Hämatologie (GPOH) study, patients with primary refractory Hodgkin lymphoma (progressive disease on therapy or relapse within 3 months from the end of therapy) had 10-year event-free survival (EFS) and OS rates of 41% and 51%, respectively.[3]
- A study of 53 adolescent patients of the same types as those who participated in the GPOH study had similar results for EFS and OS.[62] Chemosensitivity to standard-dose second-line chemotherapy predicted a better survival (66% OS), and tumors that remained refractory to chemotherapy did poorly (17% OS).[63]
- Another group has reported the PFS post-HCT for chemosensitive patients as 80%, compared with 0% for those with chemoresistant disease.[38]
Second Relapse After Initial Treatment With Autologous HCT
In a phase II study, patients (median age, 26.5 years) who had relapsed or refractory disease after autologous HCT received brentuximab vedotin, with an objective response rate of 73% and a complete remission rate of 34%. Patients who achieved a complete remission (n = 34) had a 3-year PFS rate of 58% and a 3-year OS rate of 73%, with only 6 of 34 patients proceeding to allogeneic SCT while in remission.[20][Level of evidence: 2A]
Treatment Options Under Clinical Evaluation
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
Anti-CD30 chimeric antigen receptor (CAR) T-cell therapy clinical trials
Preliminary data on CAR T cells targeting CD30 have been published. In a phase I/II trial of 41 adults with multiply relapsed or refractory Hodgkin lymphoma, CD30 CAR T cells were administered after lymphoreduction with either bendamustine alone, bendamustine and fludarabine, or cyclophosphamide and fludarabine.[64] Treated patients had a median of seven previous lines of therapy, including brentuximab, checkpoint inhibitors, and autologous and allogeneic HCTs. The overall response rate was 72% for the 32 patients with active disease who received fludarabine-based lymphodepletion. For all evaluable patients, the 1-year PFS rate was 36%, and the OS rate was 94%. The CD30 CAR T-cell therapy was well tolerated.
A number of clinical trials of anti-CD30 CAR T-cell therapy for patients with relapsed Hodgkin lymphoma are listed on ClinicalTrials.gov. The following are examples of national and/or institutional clinical trials that are currently enrolling patients younger than 18 years:
- RELY-30 (NCT02917083) (CD30 CAR T Cells With or Without Cyclophosphamide and Fludarabine in Treating Participants With Relapsed or Refractory CD30-Positive Lymphoma): Patients aged 12 years and older with relapsed or refractory Hodgkin lymphoma will receive CD30 CAR T-cell therapy after chemotherapy or autologous transplant in this phase I study.
- LCCC 1524-ATL (NCT02663297) (Autologous T Lymphocytes in Preventing Relapse After High-Dose Chemotherapy and Stem Cell Transplant in Patients with CD30-Positive Lymphoma): This phase I study will determine a safe dose of the ATLCAR.CD30 cells for patients aged 3 years and older with recurrent Hodgkin lymphoma after undergoing an autologous transplant.
Other clinical trials
The following are examples of other national and/or institutional clinical trials that are currently being conducted:
Anti–PD-1 antibodies being studied in children with Hodgkin lymphoma include the following:
- Pembrolizumab (NCT02332668).
- Nivolumab in combination with brentuximab vedotin is being studied in an international phase II trial (CheckMate 755 [NCT02927769]).
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Metzger ML, Hudson MM, Krasin MJ, et al.: Initial response to salvage therapy determines prognosis in relapsed pediatric Hodgkin lymphoma patients. Cancer 116 (18): 4376-84, 2010. [PMC free article: PMC2936658] [PubMed: 20564743]
- Moskowitz CH, Nimer SD, Zelenetz AD, et al.: A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood 97 (3): 616-23, 2001. [PubMed: 11157476]
- Schellong G, Dörffel W, Claviez A, et al.: Salvage therapy of progressive and recurrent Hodgkin's disease: results from a multicenter study of the pediatric DAL/GPOH-HD study group. J Clin Oncol 23 (25): 6181-9, 2005. [PubMed: 16135485]
- Gorde-Grosjean S, Oberlin O, Leblanc T, et al.: Outcome of children and adolescents with recurrent/refractory classical Hodgkin lymphoma, a study from the Société Française de Lutte contre le Cancer des Enfants et des Adolescents (SFCE). Br J Haematol 158 (5): 649-56, 2012. [PubMed: 22757721]
- Nachman JB, Sposto R, Herzog P, et al.: Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin's disease who achieve a complete response to chemotherapy. J Clin Oncol 20 (18): 3765-71, 2002. [PubMed: 12228196]
- Rühl U, Albrecht M, Dieckmann K, et al.: Response-adapted radiotherapy in the treatment of pediatric Hodgkin's disease: an interim report at 5 years of the German GPOH-HD 95 trial. Int J Radiat Oncol Biol Phys 51 (5): 1209-18, 2001. [PubMed: 11728679]
- Cairo MS, Shen V, Krailo MD, et al.: Prospective randomized trial between two doses of granulocyte colony-stimulating factor after ifosfamide, carboplatin, and etoposide in children with recurrent or refractory solid tumors: a children's cancer group report. J Pediatr Hematol Oncol 23 (1): 30-8, 2001. [PubMed: 11196267]
- Horton TM, Drachtman RA, Chen L, et al.: A phase 2 study of bortezomib in combination with ifosfamide/vinorelbine in paediatric patients and young adults with refractory/recurrent Hodgkin lymphoma: a Children's Oncology Group study. Br J Haematol 170 (1): 118-22, 2015. [PMC free article: PMC4478135] [PubMed: 25833390]
- Trippett TM, Schwartz CL, Guillerman RP, et al.: Ifosfamide and vinorelbine is an effective reinduction regimen in children with refractory/relapsed Hodgkin lymphoma, AHOD00P1: a children's oncology group report. Pediatr Blood Cancer 62 (1): 60-4, 2015. [PMC free article: PMC4465390] [PubMed: 25308760]
- Marr K, Ronsley R, Nadel H, et al.: Ifosfamide, gemcitabine, and vinorelbine is an effective salvage regimen with excellent stem cell mobilization in relapsed or refractory pediatric Hodgkin lymphoma. Pediatr Blood Cancer 67 (4): e28167, 2020. [PubMed: 31925920]
- Cole PD, Schwartz CL, Drachtman RA, et al.: Phase II study of weekly gemcitabine and vinorelbine for children with recurrent or refractory Hodgkin's disease: a children's oncology group report. J Clin Oncol 27 (9): 1456-61, 2009. [PMC free article: PMC2668553] [PubMed: 19224841]
- Ganesan P, Mehra N, Joel A, et al.: Gemcitabine, vinorelbine and dexamethasone: A safe and effective regimen for treatment of relapsed/refractory hodgkin's lymphoma. Leuk Res 84: 106188, 2019. [PubMed: 31325732]
- Shankar A, Hayward J, Kirkwood A, et al.: Treatment outcome in children and adolescents with relapsed Hodgkin lymphoma--results of the UK HD3 relapse treatment strategy. Br J Haematol 165 (4): 534-44, 2014. [PubMed: 24754633]
- Wimmer RS, Chauvenet AR, London WB, et al.: APE chemotherapy for children with relapsed Hodgkin disease: a Pediatric Oncology Group trial. Pediatr Blood Cancer 46 (3): 320-4, 2006. [PubMed: 16200630]
- Sandlund JT, Pui CH, Mahmoud H, et al.: Efficacy of high-dose methotrexate, ifosfamide, etoposide and dexamethasone salvage therapy for recurrent or refractory childhood malignant lymphoma. Ann Oncol 22 (2): 468-71, 2011. [PMC free article: PMC3030464] [PubMed: 20624787]
- Schulz H, Rehwald U, Morschhauser F, et al.: Rituximab in relapsed lymphocyte-predominant Hodgkin lymphoma: long-term results of a phase 2 trial by the German Hodgkin Lymphoma Study Group (GHSG). Blood 111 (1): 109-11, 2008. [PubMed: 17938252]
- Younes A, Bartlett NL, Leonard JP, et al.: Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 363 (19): 1812-21, 2010. [PubMed: 21047225]
- Seattle Genetics, Inc: ADCETRIS (Brentuximab Vedotin): Prescribing Information. Bothell, Wa: Seattle Genetics, 2012. Available online. Last accessed June 21, 2021.
- Younes A, Gopal AK, Smith SE, et al.: Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol 30 (18): 2183-9, 2012. [PMC free article: PMC3646316] [PubMed: 22454421]
- Gopal AK, Chen R, Smith SE, et al.: Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood 125 (8): 1236-43, 2015. [PMC free article: PMC4335079] [PubMed: 25533035]
- Chen R, Gopal AK, Smith SE, et al.: Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 128 (12): 1562-6, 2016. [PMC free article: PMC5034737] [PubMed: 27432875]
- Flerlage JE, Metzger ML, Wu J, et al.: Pharmacokinetics, immunogenicity, and safety of weekly dosing of brentuximab vedotin in pediatric patients with Hodgkin lymphoma. Cancer Chemother Pharmacol 78 (6): 1217-1223, 2016. [PMC free article: PMC5206991] [PubMed: 27837256]
- Cole PD, McCarten KM, Pei Q, et al.: Brentuximab vedotin with gemcitabine for paediatric and young adult patients with relapsed or refractory Hodgkin's lymphoma (AHOD1221): a Children's Oncology Group, multicentre single-arm, phase 1-2 trial. Lancet Oncol 19 (9): 1229-1238, 2018. [PMC free article: PMC6487196] [PubMed: 30122620]
- Ansell SM, Lesokhin AM, Borrello I, et al.: PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 372 (4): 311-9, 2015. [PMC free article: PMC4348009] [PubMed: 25482239]
- Younes A, Santoro A, Shipp M, et al.: Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 17 (9): 1283-94, 2016. [PMC free article: PMC5541855] [PubMed: 27451390]
- Davis KL, Fox E, Merchant MS, et al.: Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol 21 (4): 541-550, 2020. [PMC free article: PMC7255545] [PubMed: 32192573]
- Armand P, Shipp MA, Ribrag V, et al.: Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J Clin Oncol 34 (31): 3733-3739, 2016. [PMC free article: PMC5791838] [PubMed: 27354476]
- Chen R, Zinzani PL, Fanale MA, et al.: Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol 35 (19): 2125-2132, 2017. [PMC free article: PMC5791843] [PubMed: 28441111]
- Geoerger B, Kang HJ, Yalon-Oren M, et al.: Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1-2 trial. Lancet Oncol 21 (1): 121-133, 2020. [PubMed: 31812554]
- Rancea M, Monsef I, von Tresckow B, et al.: High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma. Cochrane Database Syst Rev 6: CD009411, 2013. [PubMed: 23784872]
- Aparicio J, Segura A, Garcerá S, et al.: ESHAP is an active regimen for relapsing Hodgkin's disease. Ann Oncol 10 (5): 593-5, 1999. [PubMed: 10416011]
- Kobrinsky NL, Sposto R, Shah NR, et al.: Outcomes of treatment of children and adolescents with recurrent non-Hodgkin's lymphoma and Hodgkin's disease with dexamethasone, etoposide, cisplatin, cytarabine, and l-asparaginase, maintenance chemotherapy, and transplantation: Children's Cancer Group Study CCG-5912. J Clin Oncol 19 (9): 2390-6, 2001. [PubMed: 11331317]
- Bonfante V, Viviani S, Santoro A, et al.: Ifosfamide and vinorelbine: an active regimen for patients with relapsed or refractory Hodgkin's disease. Br J Haematol 103 (2): 533-5, 1998. [PubMed: 9827930]
- Zinzani PL, Bendandi M, Stefoni V, et al.: Value of gemcitabine treatment in heavily pretreated Hodgkin's disease patients. Haematologica 85 (9): 926-9, 2000. [PubMed: 10980630]
- Santoro A, Bredenfeld H, Devizzi L, et al.: Gemcitabine in the treatment of refractory Hodgkin's disease: results of a multicenter phase II study. J Clin Oncol 18 (13): 2615-9, 2000. [PubMed: 10893294]
- Baker KS, Gordon BG, Gross TG, et al.: Autologous hematopoietic stem-cell transplantation for relapsed or refractory Hodgkin's disease in children and adolescents. J Clin Oncol 17 (3): 825-31, 1999. [PubMed: 10071273]
- Akhtar S, Rauf SM, Elhassan TA, et al.: Outcome analysis of high-dose chemotherapy and autologous stem cell transplantation in adolescent and young adults with relapsed or refractory Hodgkin lymphoma. Ann Hematol 95 (9): 1521-35, 2016. [PubMed: 27376363]
- Shafer JA, Heslop HE, Brenner MK, et al.: Outcome of hematopoietic stem cell transplant as salvage therapy for Hodgkin's lymphoma in adolescents and young adults at a single institution. Leuk Lymphoma 51 (4): 664-70, 2010. [PMC free article: PMC2932472] [PubMed: 20367182]
- Claviez A, Sureda A, Schmitz N: Haematopoietic SCT for children and adolescents with relapsed and refractory Hodgkin's lymphoma. Bone Marrow Transplant 42 (Suppl 2): S16-24, 2008. [PubMed: 18978738]
- Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al.: An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant 31 (8): 667-78, 2003. [PubMed: 12692607]
- Lieskovsky YE, Donaldson SS, Torres MA, et al.: High-dose therapy and autologous hematopoietic stem-cell transplantation for recurrent or refractory pediatric Hodgkin's disease: results and prognostic indices. J Clin Oncol 22 (22): 4532-40, 2004. [PubMed: 15542804]
- Akhtar S, Abdelsalam M, El Weshi A, et al.: High-dose chemotherapy and autologous stem cell transplantation for Hodgkin's lymphoma in the kingdom of Saudi Arabia: King Faisal specialist hospital and research center experience. Bone Marrow Transplant 42 (Suppl 1): S37-S40, 2008. [PubMed: 18724297]
- Harris RE, Termuhlen AM, Smith LM, et al.: Autologous peripheral blood stem cell transplantation in children with refractory or relapsed lymphoma: results of Children's Oncology Group study A5962. Biol Blood Marrow Transplant 17 (2): 249-58, 2011. [PMC free article: PMC3072756] [PubMed: 20637881]
- Moskowitz CH, Nademanee A, Masszi T, et al.: Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 385 (9980): 1853-62, 2015. [PubMed: 25796459]
- Locatelli F, Mauz-Koerholz C, Neville K, et al.: Brentuximab vedotin for paediatric relapsed or refractory Hodgkin's lymphoma and anaplastic large-cell lymphoma: a multicentre, open-label, phase 1/2 study. Lancet Haematol 5 (10): e450-e461, 2018. [PubMed: 30290902]
- Wadehra N, Farag S, Bolwell B, et al.: Long-term outcome of Hodgkin disease patients following high-dose busulfan, etoposide, cyclophosphamide, and autologous stem cell transplantation. Biol Blood Marrow Transplant 12 (12): 1343-9, 2006. [PubMed: 17162217]
- Gupta A, Gokarn A, Rajamanickam D, et al.: Lomustine, cytarabine, cyclophosphamide, etoposide - An effective conditioning regimen in autologous hematopoietic stem cell transplant for primary refractory or relapsed lymphoma: Analysis of toxicity, long-term outcome, and prognostic factors. J Cancer Res Ther 14 (5): 926-933, 2018 Jul-Sep. [PubMed: 30197327]
- Jabbour E, Hosing C, Ayers G, et al.: Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer 109 (12): 2481-9, 2007. [PubMed: 17497648]
- Satwani P, Ahn KW, Carreras J, et al.: A prognostic model predicting autologous transplantation outcomes in children, adolescents and young adults with Hodgkin lymphoma. Bone Marrow Transplant 50 (11): 1416-23, 2015. [PMC free article: PMC4633349] [PubMed: 26237164]
- Cooney JP, Stiff PJ, Toor AA, et al.: BEAM allogeneic transplantation for patients with Hodgkin's disease who relapse after autologous transplantation is safe and effective. Biol Blood Marrow Transplant 9 (3): 177-82, 2003. [PubMed: 12652468]
- Claviez A, Klingebiel T, Beyer J, et al.: Allogeneic peripheral blood stem cell transplantation following fludarabine-based conditioning in six children with advanced Hodgkin's disease. Ann Hematol 83 (4): 237-41, 2004. [PubMed: 14625790]
- Sureda A, Schmitz N: Role of allogeneic stem cell transplantation in relapsed or refractory Hodgkin's disease. Ann Oncol 13 (Suppl 1): 128-32, 2002. [PubMed: 12078894]
- Carella AM, Cavaliere M, Lerma E, et al.: Autografting followed by nonmyeloablative immunosuppressive chemotherapy and allogeneic peripheral-blood hematopoietic stem-cell transplantation as treatment of resistant Hodgkin's disease and non-Hodgkin's lymphoma. J Clin Oncol 18 (23): 3918-24, 2000. [PubMed: 11099321]
- Robinson SP, Goldstone AH, Mackinnon S, et al.: Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood 100 (13): 4310-6, 2002. [PubMed: 12393626]
- Devetten MP, Hari PN, Carreras J, et al.: Unrelated donor reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 15 (1): 109-17, 2009. [PMC free article: PMC2929570] [PubMed: 19135949]
- Robinson SP, Sureda A, Canals C, et al.: Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin's lymphoma: identification of prognostic factors predicting outcome. Haematologica 94 (2): 230-8, 2009. [PMC free article: PMC2635413] [PubMed: 19066328]
- Rauf MS, Maghfoor I, Elhassan TA, et al.: High-dose chemotherapy and auto-SCT for relapsed and refractory Hodgkin's lymphoma patients refractory to first-line salvage chemotherapy but responsive to second-line salvage chemotherapy. Med Oncol 32 (1): 388, 2015. [PubMed: 25429839]
- Wadhwa P, Shina DC, Schenkein D, et al.: Should involved-field radiation therapy be used as an adjunct to lymphoma autotransplantation? Bone Marrow Transplant 29 (3): 183-9, 2002. [PubMed: 11859389]
- Constine LS, Yahalom J, Ng AK, et al.: The Role of Radiation Therapy in Patients With Relapsed or Refractory Hodgkin Lymphoma: Guidelines From the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 100 (5): 1100-1118, 2018. [PubMed: 29722655]
- Tinkle CL, Williams NL, Wu H, et al.: Treatment patterns and disease outcomes for pediatric patients with refractory or recurrent Hodgkin lymphoma treated with curative-intent salvage radiotherapy. Radiother Oncol 134: 89-95, 2019. [PMC free article: PMC6478442] [PubMed: 31005229]
- Morabito F, Stelitano C, Luminari S, et al.: The role of high-dose therapy and autologous stem cell transplantation in patients with primary refractory Hodgkin's lymphoma: a report from the Gruppo Italiano per lo Studio dei Linfomi (GISL). Bone Marrow Transplant 37 (3): 283-8, 2006. [PubMed: 16327815]
- Akhtar S, El Weshi A, Rahal M, et al.: High-dose chemotherapy and autologous stem cell transplant in adolescent patients with relapsed or refractory Hodgkin's lymphoma. Bone Marrow Transplant 45 (3): 476-82, 2010. [PubMed: 19734949]
- Moskowitz CH, Kewalramani T, Nimer SD, et al.: Effectiveness of high dose chemoradiotherapy and autologous stem cell transplantation for patients with biopsy-proven primary refractory Hodgkin's disease. Br J Haematol 124 (5): 645-52, 2004. [PubMed: 14871252]
- Ramos CA, Grover NS, Beaven AW, et al.: Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J Clin Oncol 38 (32): 3794-3804, 2020. [PMC free article: PMC7655020] [PubMed: 32701411]
Special Considerations for the Treatment of Children With Cancer
Cancer in children and adolescents is rare, although the overall incidence of childhood cancer has been slowly increasing since 1975.[1] Children and adolescents with cancer should be referred to medical centers that have a multidisciplinary team of cancer specialists with experience treating the cancers that occur during childhood and adolescence. This multidisciplinary team approach incorporates the skills of the following health care professionals and others to ensure that children receive treatment, supportive care, and rehabilitation that will achieve optimal survival and quality of life:
- Primary care physicians.
- Pediatric surgeons.
- Radiation oncologists.
- Pediatric medical oncologists and hematologists.
- Rehabilitation specialists.
- Pediatric nurse specialists.
- Social workers.
- Child life professionals.
- Psychologists.
(Refer to the PDQ Supportive and Palliative Care summaries for specific information about supportive care for children and adolescents with cancer.)
Guidelines for pediatric cancer centers and their role in the treatment of pediatric patients with cancer have been outlined by the American Academy of Pediatrics.[2] At these pediatric cancer centers, clinical trials are available for most types of cancer that occur in children and adolescents, and the opportunity to participate in these trials is offered to most patients and their families. Clinical trials for children and adolescents with cancer are generally designed to compare potentially better therapy with therapy that is currently accepted as standard. Most of the progress made in identifying curative therapies for childhood cancers has been achieved through clinical trials. Information about ongoing clinical trials is available from the NCI website.
References
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PMC free article: PMC4136455] [PubMed: 24853691]
- Corrigan JJ, Feig SA; American Academy of Pediatrics: Guidelines for pediatric cancer centers. Pediatrics 113 (6): 1833-5, 2004. [PubMed: 15173520]
Late Effects From Childhood/Adolescent Hodgkin Lymphoma Therapy
Childhood and adolescent survivors of Hodgkin lymphoma may be at risk of developing numerous late complications of treatment related to radiation, specific chemotherapeutic exposures, and surgical staging.[1,2] Adverse treatment effects may impact the following:
- Reproductive system (male gonadal toxicity and female gonadal toxicity).
- Endocrine system (thyroid abnormalities).
- Oral/dental health.
- Pulmonary function.
- Musculoskeletal growth and development.
- Immune system.
In the past 30 to 40 years, pediatric Hodgkin lymphoma therapy has changed dramatically to proactively limit exposure to radiation and chemotherapeutic agents, such as anthracyclines, alkylating agents, and bleomycin. When counseling individual patients about the risk of specific treatment complications, the era of treatment should be considered.
In this regard, Childhood Cancer Survivor Study (CCSS) investigators determined the incidence of serious health conditions among 2,996 five-year survivors of pediatric Hodgkin lymphoma (mean age, 35.8 years), compared outcomes by treatment era and strategies, and estimated risks associated with contemporary therapy.[3]
- The cumulative incidence of any grade 3 to 5 conditions by age 35 years was 31.4%; females were twice as likely to experience these conditions than were males (HR, 2.1).
- The decade-specific risk of grade 3 to 5 conditions declined by 20% from the 1970s to the 1990s (Ptrend = .002).
- Compared with survivors who were treated with chest radiation therapy of 35 Gy or higher in combination with an anthracycline or alkylating agent, patients who received contemporary regimens for low- or intermediate-risk disease had an estimated 40% reduction in risk of grade 3 to 5 conditions (HR, 0.6).
- The risk of grade 3 to 5 conditions in survivors who had a recurrence or underwent hematopoietic cell transplantation was substantially elevated and similar to that of survivors treated with high-dose, extended-field radiation therapy.
Table 10 summarizes late health effects observed in Hodgkin lymphoma survivors, followed by a limited discussion of the common late effects. (Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for a full discussion of the late effects of cancer treatment in children and adolescents.)
Table 10. Treatment Complications Observed in Hodgkin Lymphoma Survivors
| Health Effects | Predisposing Therapy | Clinical Manifestations |
|---|---|---|
| Reproductive | Alkylating agent chemotherapy | Hypogonadism |
| Gonadal irradiation | Infertility | |
| Thyroid | Radiation impacting thyroid gland | Hypothyroidism |
| Hyperthyroidism | ||
| Thyroid nodules | ||
| Cardiovascular | Radiation impacting cardiovascular structures | Subclinical left ventricular dysfunction |
| Cardiomyopathy | ||
| Pericarditis | ||
| Heart valve dysfunction | ||
| Conduction disorder | ||
| Coronary, carotid, subclavian vascular disease | ||
| Myocardial infarction | ||
| Stroke | ||
| Anthracycline chemotherapy | Subclinical left ventricular dysfunction | |
| Cardiomyopathy | ||
| Congestive heart failure | ||
| Subsequent neoplasms or disease | Alkylating agent chemotherapy | Myelodysplasia/acute myeloid leukemia |
| Epipodophyllotoxins | Myelodysplasia/acute myeloid leukemia | |
| Radiation | Solid benign and malignant neoplasms | |
| Oral or dental | Any chemotherapy in a patient who has not developed permanent dentition | Dental maldevelopment (tooth or root agenesis, microdontia, root thinning and shortening, enamel dysplasia) |
| Radiation impacting oral cavity and salivary glands | Salivary gland dysfunction | |
| Xerostomia | ||
| Accelerated dental decay | ||
| Periodontal disease | ||
| Pulmonary | Radiation impacting the lungs | Subclinical pulmonary dysfunction |
| Bleomycin | Pulmonary fibrosis | |
| Musculoskeletal | Radiation of musculoskeletal tissues in any patient who is not skeletally mature | Growth impairment |
| Glucocorticosteroids | Bone mineral density deficit | |
| Multiple sclerosis | ||
| Immune | Splenectomy | Overwhelming post-splenectomy sepsis |
Male Gonadal Toxicity
Important concepts related to male gonadal toxicity include the following:
- Gonadal irradiation and alkylating agent chemotherapy may produce testicular Leydig cell or germ cell dysfunction, with risk related to cumulative dose of both modalities.
- Chemotherapy regimens that do not include alkylating agents such as ABVD (doxorubicin [Adriamycin], bleomycin, vinblastine, dacarbazine), ABVE (doxorubicin [Adriamycin], bleomycin, vincristine, etoposide), OEPA (vincristine [Oncovin], etoposide, prednisone, doxorubicin [Adriamycin]), or VAMP (vincristine, doxorubicin [Adriamycin], methotrexate, prednisone) are not associated with male infertility.
- ABVE-PC (prednisone and cyclophosphamide) and OEPA-COPDAC (cyclophosphamide, vincristine, prednisone, dacarbazine) are titrated to limit alkylating agent dose to below the usual threshold associated with male sterility. Investigations evaluating germ cell function in relation to single alkylating agent exposure suggest that the incidence of permanent azoospermia will be low if the cyclophosphamide dose is less than 7.5 g/m2.[8,11]
- Chemotherapy regimens that include more than one alkylating agent, usually procarbazine in conjunction with cyclophosphamide (i.e., COPP [cyclophosphamide, vincristine (Oncovin), prednisone, procarbazine]), chlorambucil, or nitrogen mustard (MOPP) confer a high risk of permanent azoospermia if treatment exceeds three cycles.[12,13]
(Refer to the Testis section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.)
Female Gonadal Toxicity
Ovarian hormone production is linked to the maturation of primordial follicles. Depletion of follicles by alkylating agent chemotherapy can potentially affect both fertility and ovarian hormone production. Because of their greater complement of primordial follicles, the ovaries of young and adolescent girls are less sensitive to the effects of alkylating agents than are the ovaries of older women. In general, girls maintain ovarian function at higher cumulative alkylating agent doses compared with the germ cell function maintained in boys.
Important concepts related to female gonadal toxicity include the following:
- Most females treated with contemporary risk-adapted therapy will have menarche (if prepubertal at treatment) or regain normal menses (if pubertal at treatment) unless pelvic radiation therapy is given without oophoropexy. Current regimens used in pediatric oncology are tailored to minimize the risk of ovarian failure. Data presented below related to pediatric treatment before 1987 [14,15] or adult trials in Europe (European Organization for Research and Treatment of Cancer H1–H9 trials) [16] are not likely reflective of the expected reproductive outcomes in the current era.
- Ovarian transposition to a lateral or medial region from the planned radiation volume may preserve ovarian function in young and adolescent girls who require pelvic radiation therapy for lymphoma.[17] Ovarian transposition did not appear to modify risk of premature ovarian insufficiency in a cohort of 49 long-term survivors of Hodgkin lymphoma enrolled in the St. Jude Lifetime Cohort Study treated with gonadotoxic therapy who underwent ovarian transposition before pelvic radiation therapy.[18]
- The risk of acute ovarian failure and premature menopause is substantial if treatment includes combined-modality therapy with alkylating agent chemotherapy and abdominal or pelvic radiation or dose-intensive alkylating agents for myeloablative conditioning before hematopoietic cell transplantation.[14,15] The risk of ovarian failure after treatment with contemporary regimens using lower cumulative doses of cyclophosphamide without procarbazine is anticipated to be lower.
- In the CCSS, investigators observed that Hodgkin lymphoma survivors were among the highest risk groups for acute ovarian failure and early menopause. In this cohort, the cumulative incidence of nonsurgical premature menopause among survivors treated with alkylating agents and abdominal or pelvic radiation approached 30%.[14,15] These patients were treated before 1986, usually with substantially higher doses of alkylating agents than are used in current regimens in the Children's Oncology Group, EuroNet, or other consortiums.
- A German study demonstrated that parenthood for female survivors of Hodgkin lymphoma was similar to that of the general population, although parenthood was lower for survivors who received pelvic radiation therapy.[19]
(Refer to the Ovary section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.)
Thyroid Abnormalities
Abnormalities of the thyroid gland, including hypothyroidism, hyperthyroidism, and thyroid neoplasms have been reported to occur at a higher rate among survivors of Hodgkin lymphoma than in the general population.
- Hypothyroidism. Risk factors for hypothyroidism include increasing dose of radiation, female sex, and older age at diagnosis.[20-22] CCSS investigators reported a 20-year actuarial risk of 30% of developing hypothyroidism in Hodgkin lymphoma survivors treated with 3,500 cGy to 4,499 cGy of radiation and 50% for subjects whose thyroid received 4,500 cGy or more of radiation.Hypothyroidism develops most often in the first 5 years after treatment, but new cases have been reported to emerge more than 20 years after the diagnosis.[21]
- Subsequent neoplasms. Thyroid neoplasms, both benign and malignant, have been reported with increased frequency after neck irradiation. The incidence of nodules varies substantially across studies (2%–65%) depending on the length of follow-up and detection methods used.[20-22]The relative risk (RR) of thyroid cancer is increased among Hodgkin lymphoma survivors (approximately 18-fold for the CCSS Hodgkin lymphoma cohort compared with the general population).[22] Risk factors for the development of thyroid nodules in Hodgkin lymphoma survivors reported by CCSS include time since diagnosis of more than 10 years (RR, 4.8; 95% confidence interval [CI], 3.0–7.8), female sex (RR, 4.0; 95% CI, 2.5–6.7), and radiation dose to thyroid higher than 25 Gy (RR, 2.9; 95% CI, 1.4–6.9).[22] The absolute risk of thyroid cancer is relatively low, with approximately 1% of the CCSS Hodgkin cohort developing thyroid cancer, with a median follow-up of approximately 15 years.[22]A single-institution Hodgkin lymphoma survivor cohort that included both adult and pediatric cases showed a cumulative incidence of thyroid cancer at 10 years from diagnosis of 0.26%, increasing to approximately 3% at 30 years from diagnosis. In this cohort, age younger than 20 years at Hodgkin lymphoma diagnosis and female sex were significantly associated with thyroid cancer.[24]
(Refer to the Thyroid Gland section in the PDQ summary on Late Effects of Treatment for Childhood Cancer summary for more information.)
Cardiac Toxicity
Hodgkin lymphoma survivors exposed to doxorubicin or thoracic radiation therapy are at risk of long-term cardiac toxicity. The effects of thoracic radiation therapy are difficult to separate from those of anthracyclines because few children undergo thoracic radiation therapy without the use of anthracyclines. The pathogenesis of injury differs, however, with radiation primarily affecting the fine vasculature of the heart, and anthracyclines directly damaging myocytes.[25-27]
Survivors of childhood Hodgkin lymphoma older than 50 years will experience more than two times the number of chronic cardiovascular health conditions and nearly five times the number of more severe (grades 3–5) cardiovascular conditions compared with community controls and, on average, have one severe, life-threatening, or fatal cardiovascular condition.[28]
Cardiac mortality is higher for survivors of adolescent Hodgkin lymphoma than for survivors of young adult Hodgkin lymphoma. This was demonstrated in the Teenage and Young Adult Cancer Survivor Study cohort, with standardized mortality ratios (SMR) of 10.4 (95% CI, 8.1–13.3) for those diagnosed at age 15 and 19 years, compared with an SMR of 2.8 (95% CI, 2.3–3.4) for those diagnosed at age 35 to 39 years.[29]
Radiation-associated cardiovascular toxicity
- The risks to the heart are related to the amount of radiation delivered to different depths of the heart, volume and specific areas of the heart irradiated, total and fractional irradiation dose, age at exposure, and latency period.
- Modern radiation techniques allow a reduction in the volume of cardiac tissue incidentally exposed to higher radiation doses. It is anticipated that this will reduce the risk of adverse cardiac events.
- Austrian-German investigators evaluated the development of cardiac disease (via patient self-report supplemented by physician report) in a cohort of 1,132 pediatric Hodgkin lymphoma survivors monitored for a median of 20 years. The 25-year cumulative incidence of heart disease increased with higher mediastinal radiation doses: 3% (unirradiated), 5% (20 Gy), 6% (25 Gy), 10% (30 Gy), and 21% (36 Gy). Valve defects were most common, followed by coronary artery disease, cardiomyopathy, rhythm disorders, and pericardial abnormalities.[34]
- In a study of adult survivors of Hodgkin lymphoma, vigorous exercise lowered the risk of cardiovascular events, independent of the treatment received.[35]
Anthracycline-related cardiac toxicity
- Late complications related to anthracycline injury may include subclinical left ventricular dysfunction, cardiomyopathy, and congestive heart failure.[27]
- Increased risk of doxorubicin-related cardiomyopathy is associated with female sex, cumulative doses higher than 200 mg/m2 to 300 mg/m2, younger age at time of exposure, and increased time from exposure.[36]
- Dexrazoxane (a bisdioxopiperazine compound that readily enters cells and is subsequently hydrolyzed to form a chelating agent) has been shown to prevent heart damage in adults and children treated with anthracyclines.[39] Studies suggest that dexrazoxane is safe and does not interfere with chemotherapeutic efficacy. Dexrazoxane has been associated with increased hematologic toxicity and typhlitis in children with Hodgkin lymphoma receiving ABVE-PC chemotherapy.[40]
- A number of trials have studied the risk of subsequent neoplasms following dexrazoxane administration and none have found a significant association with subsequent neoplasms.[41,42] However, one study found a borderline statistical increase in subsequent neoplasms in patients randomly assigned to receive dexrazoxane that was attributed to the administration of three topoisomerase inhibitors (doxorubicin, etoposide, and dexrazoxane) within 2 to 3 hours of each other.[43]
(Refer to the Late Effects of the Cardiovascular System section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.)
Subsequent Neoplasms
A number of series evaluating the incidence of subsequent neoplasms in survivors of childhood and adolescent Hodgkin lymphoma have been published.[46-55]; [56][Level of evidence: 3iii] Many of the patients included in these series received high-dose radiation therapy and high-dose alkylating agent chemotherapy regimens, which are no longer used.
- Subsequent neoplasms comprise two distinct groups:[57,58]
- -
Myelodysplasia and acute myeloid leukemia (AML) that are chemotherapy related.
Subsequent hematological malignancy (most commonly AML and myelodysplasia) is related to the use of alkylating agents, anthracycline, and etoposide and exhibit a brief latency period (<10 years from the primary cancer).[59] This excess risk is largely related to cases of myelodysplasia and subsequent AML.
A single-study experience suggests that there could be an increase in malignancies when multiple topoisomerase inhibitors are administered in close proximity.[43]
Clinical trials using dexrazoxane in childhood leukemia have not observed an excess risk of subsequent neoplasms.[43,60,61]
Chemotherapy-related myelodysplasia and AML are less prevalent following contemporary therapy because of the restriction of cumulative alkylating agent doses.[62,63]
Among 1,711 intermediate-risk Hodgkin lymphoma survivors treated with response-adapted therapy in the COG AHOD0031 (NCT00025259) trial (median follow-up, 7.3 years), the 10-year cumulative incidence of subsequent malignancy was 1.3%, and the cumulative incidence of secondary myelodysplastic syndrome or AML was 0.2%. Of the three cases of secondary AML, the median time to onset was 2 years (range, 1.8–2.7 years).[64]
- -
Solid neoplasms that are predominately radiation related.
Solid neoplasms most often involve the skin, breast, thyroid, gastrointestinal tract, lung, and head and neck, with risk increasing with radiation dose.[53,55,65]; [56][Level of evidence: 3iii] The risk of a solid subsequent neoplasm escalates with the passage of time after diagnosis of Hodgkin lymphoma, with a latency of 20 years or more. (Refer to the Thyroid Abnormalities section of this summary for more information about subsequent thyroid neoplasms.)
Breast cancer is the most common therapy-related solid subsequent neoplasm after Hodgkin lymphoma:
- High risk of breast cancer has been found to increase as early as 8 years from radiation exposure, is rare before age 25 years, and continues to increase with time from exposure. Importantly, breast cancer in female childhood cancer survivors typically develops at least 25 years earlier than that of primary breast cancer in the general population and often years before the implementation of population-based screening.[52]
- The cumulative incidence of breast cancer by age 40 to 45 years ranges from 13% to 20%, compared with a 1% risk for women in the general population.[52,66,68,69] This risk is similar to what is observed for women with a BRCA gene mutation, where, by age 40 years, the cumulative incidence of breast cancer ranges from 10% to 19%.[70]
- The risk of breast cancer in female survivors of Hodgkin lymphoma is directly related to the dose of radiation therapy received over a range from 4 Gy to 40 Gy.[71] Female patients treated with both radiation therapy and alkylating agent chemotherapy have a lower RR of developing breast cancer than women receiving radiation therapy alone.[53,72] CCSS investigators also demonstrated that breast cancer risk associated with breast irradiation was sharply reduced among women who received 5 Gy or more to the ovaries.[73] The protective effect of alkylating chemotherapy and ovarian radiation is believed to be mediated through induction of premature menopause, suggesting that hormone stimulation contributes to the development of radiation-induced breast cancer.[74]
- Hereditary syndromes, other than high-risk breast cancer syndromes, may modify the effect of radiation exposure on breast cancer risk after childhood cancer.[75]
- A study of women survivors who received chest radiation for Hodgkin lymphoma showed that one of the most important factors in obtaining breast cancer screenings per guidelines was recommendation from their treating physician.[76] Standard guidelines for routine breast screening are available. The COG guidelines recommend annual screening with magnetic resonance imaging and mammograms for women beginning 8 years after treatment or at age 25 years, whichever is later.[76]
(Refer to the Subsequent Neoplasms section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.)
References
- Ng AK: Current survivorship recommendations for patients with Hodgkin lymphoma: focus on late effects. Blood 124 (23): 3373-9, 2014. [PubMed: 25428219]
- Dörffel W, Riepenhausen M, Lüders H, et al.: Late Effects Following Treatment of Hodgkin Lymphoma During Childhood and Adolescence. Results of the Hodgkin Lymphoma Late Effects Research Project. Klin Padiatr 228 (6-07): 286-293, 2016. [PubMed: 27846658]
- Oeffinger KC, Stratton KL, Hudson MM, et al.: Impact of Risk-Adapted Therapy for Pediatric Hodgkin Lymphoma on Risk of Long-Term Morbidity: A Report From the Childhood Cancer Survivor Study. J Clin Oncol 39 (20): 2266-2275, 2021. [PMC free article: PMC8260906] [PubMed: 33630659]
- Greenfield DM, Walters SJ, Coleman RE, et al.: Prevalence and consequences of androgen deficiency in young male cancer survivors in a controlled cross-sectional study. J Clin Endocrinol Metab 92 (9): 3476-82, 2007. [PubMed: 17579201]
- Howell SJ, Radford JA, Adams JE, et al.: The impact of mild Leydig cell dysfunction following cytotoxic chemotherapy on bone mineral density (BMD) and body composition. Clin Endocrinol (Oxf) 52 (5): 609-16, 2000. [PubMed: 10792341]
- Rowley MJ, Leach DR, Warner GA, et al.: Effect of graded doses of ionizing radiation on the human testis. Radiat Res 59 (3): 665-78, 1974. [PubMed: 4428015]
- Howell SJ, Shalet SM: Effect of cancer therapy on pituitary-testicular axis. Int J Androl 25 (5): 269-76, 2002. [PubMed: 12270023]
- Kenney LB, Laufer MR, Grant FD, et al.: High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer 91 (3): 613-21, 2001. [PubMed: 11169946]
- Fitoussi, Eghbali H, Tchen N, et al.: Semen analysis and cryoconservation before treatment in Hodgkin's disease. Ann Oncol 11 (6): 679-84, 2000. [PubMed: 10942055]
- Viviani S, Ragni G, Santoro A, et al.: Testicular dysfunction in Hodgkin's disease before and after treatment. Eur J Cancer 27 (11): 1389-92, 1991. [PubMed: 1835853]
- Meistrich ML, Wilson G, Brown BW, et al.: Impact of cyclophosphamide on long-term reduction in sperm count in men treated with combination chemotherapy for Ewing and soft tissue sarcomas. Cancer 70 (11): 2703-12, 1992. [PubMed: 1423201]
- Hobbie WL, Ginsberg JP, Ogle SK, et al.: Fertility in males treated for Hodgkins disease with COPP/ABV hybrid. Pediatr Blood Cancer 44 (2): 193-6, 2005. [PubMed: 15390272]
- da Cunha MF, Meistrich ML, Fuller LM, et al.: Recovery of spermatogenesis after treatment for Hodgkin's disease: limiting dose of MOPP chemotherapy. J Clin Oncol 2 (6): 571-7, 1984. [PubMed: 6547167]
- Chemaitilly W, Mertens AC, Mitby P, et al.: Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab 91 (5): 1723-8, 2006. [PubMed: 16492690]
- Sklar CA, Mertens AC, Mitby P, et al.: Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst 98 (13): 890-6, 2006. [PubMed: 16818852]
- van der Kaaij MA, Heutte N, Meijnders P, et al.: Premature ovarian failure and fertility in long-term survivors of Hodgkin's lymphoma: a European Organisation for Research and Treatment of Cancer Lymphoma Group and Groupe d'Etude des Lymphomes de l'Adulte Cohort Study. J Clin Oncol 30 (3): 291-9, 2012. [PubMed: 22184372]
- Thibaud E, Ramirez M, Brauner R, et al.: Preservation of ovarian function by ovarian transposition performed before pelvic irradiation during childhood. J Pediatr 121 (6): 880-4, 1992. [PubMed: 1447649]
- Fernandez-Pineda I, Davidoff AM, Lu L, et al.: Impact of ovarian transposition before pelvic irradiation on ovarian function among long-term survivors of childhood Hodgkin lymphoma: A report from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer 65 (9): e27232, 2018. [PMC free article: PMC6105417] [PubMed: 29750388]
- Brämswig JH, Riepenhausen M, Schellong G: Parenthood in adult female survivors treated for Hodgkin's lymphoma during childhood and adolescence: a prospective, longitudinal study. Lancet Oncol 16 (6): 667-75, 2015. [PubMed: 25959806]
- Constine LS, Donaldson SS, McDougall IR, et al.: Thyroid dysfunction after radiotherapy in children with Hodgkin's disease. Cancer 53 (4): 878-83, 1984. [PubMed: 6692289]
- Hancock SL, Cox RS, McDougall IR: Thyroid diseases after treatment of Hodgkin's disease. N Engl J Med 325 (9): 599-605, 1991. [PubMed: 1861693]
- Sklar C, Whitton J, Mertens A, et al.: Abnormalities of the thyroid in survivors of Hodgkin's disease: data from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab 85 (9): 3227-32, 2000. [PubMed: 10999813]
- Loeffler JS, Tarbell NJ, Garber JR, et al.: The development of Graves' disease following radiation therapy in Hodgkin's disease. Int J Radiat Oncol Biol Phys 14 (1): 175-8, 1988. [PubMed: 3335451]
- Michaelson EM, Chen YH, Silver B, et al.: Thyroid malignancies in survivors of Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 88 (3): 636-41, 2014. [PubMed: 24521679]
- Fajardo LF, Eltringham JR, Steward JR: Combined cardiotoxicity of adriamycin and x-radiation. Lab Invest 34 (1): 86-96, 1976. [PubMed: 1246126]
- Adams MJ, Lipshultz SE: Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer 44 (7): 600-6, 2005. [PubMed: 15856486]
- van Nimwegen FA, Schaapveld M, Janus CP, et al.: Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med 175 (6): 1007-17, 2015. [PubMed: 25915855]
- Bhakta N, Liu Q, Yeo F, et al.: Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin's lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol 17 (9): 1325-34, 2016. [PMC free article: PMC5029267] [PubMed: 27470081]
- Henson KE, Reulen RC, Winter DL, et al.: Cardiac Mortality Among 200 000 Five-Year Survivors of Cancer Diagnosed at 15 to 39 Years of Age: The Teenage and Young Adult Cancer Survivor Study. Circulation 134 (20): 1519-1531, 2016. [PMC free article: PMC5106083] [PubMed: 27821538]
- Hancock SL, Tucker MA, Hoppe RT: Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA 270 (16): 1949-55, 1993. [PubMed: 8411552]
- King V, Constine LS, Clark D, et al.: Symptomatic coronary artery disease after mantle irradiation for Hodgkin's disease. Int J Radiat Oncol Biol Phys 36 (4): 881-9, 1996. [PubMed: 8960517]
- Adams MJ, Lipshultz SE, Schwartz C, et al.: Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol 13 (3): 346-56, 2003. [PubMed: 12903022]
- Küpeli S, Hazirolan T, Varan A, et al.: Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin's lymphoma. J Clin Oncol 28 (6): 1025-30, 2010. [PubMed: 20038721]
- Schellong G, Riepenhausen M, Bruch C, et al.: Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer 55 (6): 1145-52, 2010. [PubMed: 20734400]
- Jones LW, Liu Q, Armstrong GT, et al.: Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol 32 (32): 3643-50, 2014. [PMC free article: PMC4220043] [PubMed: 25311213]
- Trachtenberg BH, Landy DC, Franco VI, et al.: Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol 32 (3): 342-53, 2011. [PubMed: 21221562]
- van Dalen EC, van der Pal HJ, Kok WE, et al.: Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer 42 (18): 3191-8, 2006. [PubMed: 16987655]
- Krischer JP, Epstein S, Cuthbertson DD, et al.: Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol 15 (4): 1544-52, 1997. [PubMed: 9193351]
- van Dalen EC, Caron HN, Dickinson HO, et al.: Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev (2): CD003917, 2008. [PubMed: 18425895]
- Schwartz CL, Constine LS, Villaluna D, et al.: A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood 114 (10): 2051-9, 2009. [PMC free article: PMC2744567] [PubMed: 19584400]
- Vrooman LM, Neuberg DS, Stevenson KE, et al.: The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer 47 (9): 1373-9, 2011. [PMC free article: PMC3736806] [PubMed: 21514146]
- Chow EJ, Asselin BL, Schwartz CL, et al.: Late Mortality After Dexrazoxane Treatment: A Report From the Children's Oncology Group. J Clin Oncol 33 (24): 2639-45, 2015. [PMC free article: PMC4534526] [PubMed: 26014292]
- Tebbi CK, London WB, Friedman D, et al.: Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol 25 (5): 493-500, 2007. [PubMed: 17290056]
- Silber JH, Cnaan A, Clark BJ, et al.: Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol 22 (5): 820-8, 2004. [PubMed: 14990637]
- Lipshultz SE, Lipsitz SR, Sallan SE, et al.: Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol 20 (23): 4517-22, 2002. [PubMed: 12454107]
- Beaty O, Hudson MM, Greenwald C, et al.: Subsequent malignancies in children and adolescents after treatment for Hodgkin's disease. J Clin Oncol 13 (3): 603-9, 1995. [PubMed: 7884422]
- van Leeuwen FE, Klokman WJ, Veer MB, et al.: Long-term risk of second malignancy in survivors of Hodgkin's disease treated during adolescence or young adulthood. J Clin Oncol 18 (3): 487-97, 2000. [PubMed: 10653864]
- Green DM, Hyland A, Barcos MP, et al.: Second malignant neoplasms after treatment for Hodgkin's disease in childhood or adolescence. J Clin Oncol 18 (7): 1492-9, 2000. [PubMed: 10735897]
- Metayer C, Lynch CF, Clarke EA, et al.: Second cancers among long-term survivors of Hodgkin's disease diagnosed in childhood and adolescence. J Clin Oncol 18 (12): 2435-43, 2000. [PubMed: 10856104]
- Wolden SL, Lamborn KR, Cleary SF, et al.: Second cancers following pediatric Hodgkin's disease. J Clin Oncol 16 (2): 536-44, 1998. [PubMed: 9469338]
- Sankila R, Garwicz S, Olsen JH, et al.: Risk of subsequent malignant neoplasms among 1,641 Hodgkin's disease patients diagnosed in childhood and adolescence: a population-based cohort study in the five Nordic countries. Association of the Nordic Cancer Registries and the Nordic Society of Pediatric Hematology and Oncology. J Clin Oncol 14 (5): 1442-6, 1996. [PubMed: 8622057]
- Bhatia S, Yasui Y, Robison LL, et al.: High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: report from the Late Effects Study Group. J Clin Oncol 21 (23): 4386-94, 2003. [PubMed: 14645429]
- Constine LS, Tarbell N, Hudson MM, et al.: Subsequent malignancies in children treated for Hodgkin's disease: associations with gender and radiation dose. Int J Radiat Oncol Biol Phys 72 (1): 24-33, 2008. [PMC free article: PMC3531236] [PubMed: 18722263]
- Swerdlow AJ, Higgins CD, Smith P, et al.: Second cancer risk after chemotherapy for Hodgkin's lymphoma: a collaborative British cohort study. J Clin Oncol 29 (31): 4096-104, 2011. [PubMed: 21969511]
- Chowdhry AK, McHugh C, Fung C, et al.: Second primary head and neck cancer after Hodgkin lymphoma: a population-based study of 44,879 survivors of Hodgkin lymphoma. Cancer 121 (9): 1436-45, 2015. [PubMed: 25572913]
- Holmqvist AS, Chen Y, Berano Teh J, et al.: Risk of solid subsequent malignant neoplasms after childhood Hodgkin lymphoma-Identification of high-risk populations to guide surveillance: A report from the Late Effects Study Group. Cancer 125 (8): 1373-1383, 2019. [PubMed: 30556153]
- Reulen RC, Frobisher C, Winter DL, et al.: Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA 305 (22): 2311-9, 2011. [PubMed: 21642683]
- Friedman DL, Whitton J, Leisenring W, et al.: Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 102 (14): 1083-95, 2010. [PMC free article: PMC2907408] [PubMed: 20634481]
- Le Deley MC, Leblanc T, Shamsaldin A, et al.: Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: a case-control study by the Société Française d'Oncologie Pédiatrique. J Clin Oncol 21 (6): 1074-81, 2003. [PubMed: 12637473]
- Lipshultz SE, Scully RE, Lipsitz SR, et al.: Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol 11 (10): 950-61, 2010. [PMC free article: PMC3756093] [PubMed: 20850381]
- Barry EV, Vrooman LM, Dahlberg SE, et al.: Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol 26 (7): 1106-11, 2008. [PubMed: 18309945]
- Koontz MZ, Horning SJ, Balise R, et al.: Risk of therapy-related secondary leukemia in Hodgkin lymphoma: the Stanford University experience over three generations of clinical trials. J Clin Oncol 31 (5): 592-8, 2013. [PMC free article: PMC3565182] [PubMed: 23295809]
- Schellong G, Riepenhausen M, Creutzig U, et al.: Low risk of secondary leukemias after chemotherapy without mechlorethamine in childhood Hodgkin's disease. German-Austrian Pediatric Hodgkin's Disease Group. J Clin Oncol 15 (6): 2247-53, 1997. [PubMed: 9196137]
- Giulino-Roth L, Pei Q, Buxton A, et al.: Subsequent malignant neoplasms among children with Hodgkin lymphoma: a report from the Children's Oncology Group. Blood 137 (11): 1449-1456, 2021. [PMC free article: PMC7976513] [PubMed: 33512412]
- Daniëls LA, Krol AD, Schaapveld M, et al.: Long-term risk of secondary skin cancers after radiation therapy for Hodgkin's lymphoma. Radiother Oncol 109 (1): 140-5, 2013. [PubMed: 23932152]
- Kenney LB, Yasui Y, Inskip PD, et al.: Breast cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Intern Med 141 (8): 590-7, 2004. [PubMed: 15492338]
- Ng AK, Bernardo MV, Weller E, et al.: Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long-term risks and risk factors. Blood 100 (6): 1989-96, 2002. [PubMed: 12200357]
- Taylor AJ, Winter DL, Stiller CA, et al.: Risk of breast cancer in female survivors of childhood Hodgkin's disease in Britain: a population-based study. Int J Cancer 120 (2): 384-91, 2007. [PubMed: 17066449]
- Henderson TO, Amsterdam A, Bhatia S, et al.: Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med 152 (7): 444-55; W144-54, 2010. [PMC free article: PMC2857928] [PubMed: 20368650]
- Easton DF, Ford D, Bishop DT: Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet 56 (1): 265-71, 1995. [PMC free article: PMC1801337] [PubMed: 7825587]
- Alm El-Din MA, Hughes KS, Finkelstein DM, et al.: Breast cancer after treatment of Hodgkin's lymphoma: risk factors that really matter. Int J Radiat Oncol Biol Phys 73 (1): 69-74, 2009. [PubMed: 18538497]
- Travis LB, Hill DA, Dores GM, et al.: Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA 290 (4): 465-75, 2003. [PubMed: 12876089]
- Inskip PD, Robison LL, Stovall M, et al.: Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol 27 (24): 3901-7, 2009. [PMC free article: PMC2734395] [PubMed: 19620485]
- De Bruin ML, Sparidans J, van't Veer MB, et al.: Breast cancer risk in female survivors of Hodgkin's lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 27 (26): 4239-46, 2009. [PubMed: 19667275]
- Morton LM, Sampson JN, Armstrong GT, et al.: Genome-Wide Association Study to Identify Susceptibility Loci That Modify Radiation-Related Risk for Breast Cancer After Childhood Cancer. J Natl Cancer Inst 109 (11): , 2017. [PMC free article: PMC6059172] [PubMed: 29059430]
- Oeffinger KC, Ford JS, Moskowitz CS, et al.: Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA 301 (4): 404-14, 2009. [PMC free article: PMC2676434] [PubMed: 19176442]
Changes to This Summary (08/04/2021)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Treatment of Newly Diagnosed Children and Adolescents With Hodgkin Lymphoma
Revised Table 5 to add the CAPDAC and AEPA regimens (cited Metzger et al. as reference 46).
Added text about an open-label, single-arm, multicenter trial that evaluated brentuximab vedotin and risk-adapted residual node radiation therapy in patients with stage IIB, IIIB, or IV classical Hodgkin lymphoma (cited Metzger et al. as reference 46 and level of evidence 2A).
Revised Table 8 to add the results of a study that used the AEPA and CAPDAC chemotherapy regimens and residual nodal radiation therapy to treat patients with high-risk Hodgkin lymphoma.
Treatment of Primary Refractory or Recurrent Hodgkin Lymphoma in Children and Adolescents
Added Anti-CD30 chimeric antigen receptor (CAR) T-cell therapy clinical trials as a new subsection.
Late Effects From Childhood/Adolescent Hodgkin Lymphoma Therapy
Added text about the results of a Childhood Cancer Survivor Study (CCSS) study that determined the incidence of serious health conditions among 2,996 five-year survivors of pediatric Hodgkin lymphoma, compared outcomes by treatment era and strategies, and estimated risks associated with contemporary therapy (cited Oeffinger et al. as reference 3).
Added text to state that among 1,711 intermediate-risk Hodgkin lymphoma survivors treated with response-adapted therapy in the COG AHOD0031 trial, the 10-year cumulative incidence of subsequent malignancy was 1.3%, and the cumulative incidence of secondary myelodysplastic syndrome or acute myeloid leukemia (AML) was 0.2%. Of the three cases of secondary AML, the median time to onset was 2 years (cited Giulino-Roth et al. as reference 64).
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood Hodgkin lymphoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Hodgkin Lymphoma Treatment are:
- Louis S. Constine, MD (James P. Wilmot Cancer Center at University of Rochester Medical Center)
- Alan Scott Gamis, MD, MPH (Children's Mercy Hospital)
- Thomas G. Gross, MD, PhD (National Cancer Institute)
- Melissa Maria Hudson, MD (St. Jude Children's Research Hospital)
- Kenneth L. McClain, MD, PhD (Texas Children's Cancer Center and Hematology Service at Texas Children's Hospital)
- Arthur Kim Ritchey, MD (Children's Hospital of Pittsburgh of UPMC)
- Malcolm A. Smith, MD, PhD (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Hodgkin Lymphoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/lymphoma/hp/child-hodgkin-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389170]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
- General Information About Childhood Hodgkin Lymphoma
- Cellular Classification and Biologic Correlates of Childhood Hodgkin Lymphoma
- Diagnosis and Staging Information for Childhood Hodgkin Lymphoma
- Treatment of Newly Diagnosed Children and Adolescents With Hodgkin Lymphoma
- Treatment of Primary Refractory or Recurrent Hodgkin Lymphoma in Children and Adolescents
- Special Considerations for the Treatment of Children With Cancer
- Late Effects From Childhood/Adolescent Hodgkin Lymphoma Therapy
- Changes to This Summary (08/04/2021)
- About This PDQ Summary
- Review Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Childhood Non-Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Childhood Non-Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version.PDQ Pediatric Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Non-Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Non-Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Non-Hodgkin Lymphoma Treatment During Pregnancy (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Non-Hodgkin Lymphoma Treatment During Pregnancy (PDQ®): Health Professional Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review B-Cell Non-Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review B-Cell Non-Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Childhood Hodgkin Lymphoma Treatment (PDQ®) - PDQ Cancer Information SummariesChildhood Hodgkin Lymphoma Treatment (PDQ®) - PDQ Cancer Information Summaries
- Osada Taira anterior neuroectoderm (ANE) pCS105 cDNA libraryOsada Taira anterior neuroectoderm (ANE) pCS105 cDNA librarybiosample
- BioSample links for Nucleotide (Select 46036718) (1)BioSample
- Taxonomy Links for Nucleotide (Select 38333985) (1)Taxonomy
- Tssr37344 AND (alive[prop]) (0)Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...