NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Olson DWM, Bettger JP, Alexander KP, et al. Transition of Care for Acute Stroke and Myocardial Infarction Patients: From Hospitalization to Rehabilitation, Recovery, and Secondary Prevention. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 Oct. (Evidence Reports/Technology Assessments, No. 202.)
This publication is provided for historical reference only and the information may be out of date.

Transition of Care for Acute Stroke and Myocardial Infarction Patients: From Hospitalization to Rehabilitation, Recovery, and Secondary Prevention.
Show detailsLiterature Search and Screening
Searches of all sources identified a total of 5783 potentially relevant citations. Table 2 details the number of citations identified from each source.
Table 2
Sources of citations.
Figure 2 describes the flow of literature through the screening process. Of the 5783 unique citations identified by our searches, 4605 were excluded at the abstract screening stage. Of the 1178 articles that passed the initial abstract screening, 22 were not original data (e.g., editorials), which were reviewed separately and excluded from further review, and 406 articles were excluded because they were non-English publications. The remaining 750 articles went on to full-text screening. Of these, 688 were excluded, leaving a total of 62 included articles (representing 44 studies). Appendix D provides a table of studies included in this review along with their quality rating, limitations to applicability, and relevant key question s. Appendix E provides a complete list of articles excluded at the full-text screening stage, with reasons for exclusion.
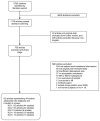
Figure 2
Literature Flow Diagram. Abbreviations: KQ = key question; MA = meta-analysis; MI = myocardial infarction; SR = systematic review
Key Question 1. For patients hospitalized with first or recurrent stroke or myocardial infarction (MI), what are the key components of transition of care services? Can these components be grouped in a taxonomy, and are they based on a particular theory?
Key Points
- Theory was rarely reported in clinical trial papers reporting study outcomes.
- Transition of care interventions for patients with MI or stroke were grouped into four categories of intervention, and each category began in a different phase of an episode of illness.
- The components of the different interventions were grouped into a taxonomy that addressed the type of transition relative to the phase of illness and care, type of intervention, recipient of the intervention, content of the intervention, facilitator of the intervention, method of recipient-to-facilitator contact, intensity and complexity, and outcomes.
Detailed Analysis
A patient experiencing an acute MI or stroke undergoes a number of transitions, from the onset of symptoms and hospital admission through the various settings of acute and subacute care before returning to the community or a final place of residence. This report focuses on the process of transitioning from an acute inpatient setting to an out -of-hospital setting. The transition may include (1) a return to home, (2) a transient stay in a setting that provides rehabilitation, or (3) relocation to a long-term care facility. As the individual moves through these various settings, a number of different health care providers may be involved in various configurations of care models to transition patients “home.”
Before the effectiveness of the various approaches to transitioning patients home can be examined, the approaches need to be categorized and the components defined. We present the transition of care interventions and models categorized as they would be delivered in an episode of care and then describe the supporting theories and the structure and process of the different types that were identified. This presentation of the structural components and processes of transition of care services supports the KQs that are focused on outcomes.
Framework. We have adapted the National Quality Forum’s episode of care framework for aligning the interventions and models identified in this literature review, modifying it only slightly to include stroke as well as MI (Figure 3). This framework, developed to guide measurement, essentially depicts the care pathway experienced by patients for an episode of illness such as an acute MI or stroke. Although the framework captures individuals prior to the acute event as they transition through the acute hospitalization and into followup care, our focus for this review is concentrated only on the transition out of the acute hospital stay. While we recognize that transitions of care as defined by Coleman et al. 1 occur even during the acute hospitalization, we focused on the transfer across settings. Thus, the acute-care hospital, and in some cases the acute hospital or the next setting for inpatient care, became our point of interest as the primary point of influence on recovery and maintenance of health for patients transitioning home. We did not identify any studies that focused solely on the transition from an alternative inpatient setting such as a skilled nursing facility to home.
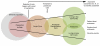
Figure 3
Framework for episodes of care. (Adapted from Patient-Focused Episodes of Care Framework, National Quality Forum 2010)
Literature identified. Four types of transition of care interventions were identified in this review of 62 articles (10 good quality, 41 fair, 11 poor)describing 44 studies:
- Hospital-initiated support for discharge to home or intermediary care units such as inpatient rehabilitation or skilled nursing facilities.
- 2a. Hospital-based patient and family education interventions2b. Community-based patient and family education interventions
- Community-based models of support interventions
- Chronic disease management models of care
Each intervention type, as tested in the studies reviewed, was intended to transition the patient back to the community and promote recovery and positive outcomes. While all studies in this review measured 3-, 6-, or 12-month outcomes, the emphasis of the intervention weighted more heavily in either the acute hospital, as with types 1 and 2a, or the community, as with 2b, 3, and 4. The following tables describe the 44 interventions organized by type, first for stroke (Table 3, 27 stroke interventions published in 41 articles) and then for MI (Table 4, 17 MI interventions published in 21 articles).
Table 3
Transition of care interventions and models for stroke.
Table 4
Transition of care interventions and models for myocardial infarction.
Intervention type 1: Hospital-initiated support for discharge to home. While the hospital-initiated models designed to prepare patients for discharge and the transition to home were generally described in detail, none of the six stroke models or the four MI models reported any theoretical foundation supporting the design of the intervention.
The components of four of the six interventions designed for stroke patients were similar.16–22,33 These “extended stroke unit services” or “hospital-supported discharge” models each included a multidisciplinary team of professionals that followed the stroke patients as they transitioned home—and provided continued support, supervision, and coordination with community-level services. The timing and length of followup postdischarge varied, but the therapeutic foci on rehabilitation and continuity of care were similar across studies. The intervention described in Grasel et al.23,24 differed in that it focused on the caregivers who provided psychoeducational education, training, and counseling prior to and after discharge from acute care for up to 3 months. The study by Sulch et al.30–32 was the only stroke -specific intervention in this category that identified a single facilitator or lead interventionist—a stroke nurse, who in this study implemented an integrated-care pathway to improve coordination and discharge planning and reduce the length of stay. Although the integrated-care pathway was developed by a multidisciplinary team, the study investigators strategically chose a single lead interventionist so that the study would not be a burden for other disciplines. Unique to this category of hospital-initiated transition of care interventions for stroke patients, the study by Sulch et al. did not provide any postdischarge followup.
Each of the four MI models was distinctly different, but each shared at least one similarity with one of the hospital-initiated stroke interventions. Like Sulch et al., the study by Eagle et al. and Rogers et al.58,59 tested a hospital-initiated program that in part aimed to improve care coordination and discharge planning but did not provide any posthospital followup as part of the intervention. Like the intervention in Grasel et al., the Petrie et al.62 study was a psychological intervention, but it focused on patients’ perceptions rather than on caregivers’ perceptions. Interventions described by Ho et al.60 and Young et al. 63 both straddled inpatient and outpatient or community-level care, but neither intervention was designed to support the handoff to community-based physicians or services. Also dissimilar from the stroke interventions in this category was the absence of a lead facilitator or specified team of professionals for any of the MI interventions. These components are depicted in Figure 4 as part of the taxonomy of MI and stroke transition of care interventions.
Intervention type 2: Patient and family education interventions, both hospital-based and community-based. Seven patient and family education interventions were identified, with five for stroke patients and two for MI patients. Of these seven, three were based in the hospital (one MI, two stroke) and four were based in the community (one MI and three stroke). While the distinction was made between home-based and community-based to support the possibility of translation to practice for interventions found effective, it seemed less important to separate the two MI interventions from the five stroke interventions because the foundation of each was similar. Furthermore, the content of education included a myriad of topics but usually concentrated more heavily on either condition-specific information or psychosocial adaptation.
Collectively, the seven interventions can be stratified into three levels of complexity. At the most basic level, both Hoffman et al.34 and Mayou et al. 64 tested structured education programs individualized for the patient and delivered by nurses. While Clark et al.,35 Johnston et al.,36 Sahebalzamani et al.,39 and Lacey et al.65 also tested education programs, each also included patient followup by a nurse,36,39 social worker, 35, or a “facilitator” that was not identified by discipline or training.65 The study by Mant et al.37,38 was the only intervention in this category that centered on information, support, and followup by a “family support organizer” and also extended the responsibilities of this role to serve as a liaison with other services. Very little detail was available on this intervention, and thus it could not be considered as a more advanced model of community-based support (intervention type 3).
Although the theoretical underpinnings of education programs are much more advanced and more commonly referred to than the theories supporting transition of care interventions, only two studies reported a theory guiding the intervention. Johnston et al.36 reported a cognitive behavioral theory that applied more to the followup provided by nurses for guidance on coping skills and self-management. Clark et al.35 described the applicability of family systems theory and how the family is a system both influenced by and influencing society and the individuals within or around the family unit. This theory seemed relevant for other interventions focused on the caregivers or the patient-caregiver dyad, but no other studies in this category of patient and family education reported its use.
Intervention type 3: Community-based models of support. Of the 4 types of transition of care interventions, community-based models were most common (10 MI models and 13 stroke models). However, the variation among community-based models resulted in the creation of three subcategories: (1) provider-driven interventions, (2) psychosocial-or behavioral-focused interventions, and(3) technical support structures. Only 3 of 23 studies reported any theoretical or conceptual foundation.40,41,49,50,71,72 Allen’ s advanced-practice nurse (APN) model was designed using Wagner’s model of chronic illness care.42 Family systems and cognitive behavioral theories were reported to support the stroke psychosocial intervention,49,50 but no citation was provided for either theory. The nurse-led MI intervention by Hanssen et al.71 used the theory on stress, appraisal, and coping.73
For conditions other than MI and stroke, provider-driven community-based models were most common and had the strongest evidence base.10 They w ere also most common in this review of the literature, with half of the MI community-based models and 10 of 13 stroke models. These models generally identified one provider to serve as the care manager or coordinator facilitating a multifaceted intervention to support holistic health—targeting both physical and psychosocial needs and the transition in health status and physical location of care delivery. Followup or contact with the provider facilitating the intervention was, in some cases, structured and predetermined or was conducted as needed. Followup usually extended over only the short term, such as 1 to 3 months posthospitalization. Access to a larger multidisciplinary team was not uncommon, but purposeful coordination between acute and community-level care was not always a core component of the intervention.
Ten provider-driven structures were tested for stroke patients. Allen et al.40,41 tested an APN model, and Mayo et al.52 tested a nurse-led model. Boter et al.46 also tested a nurse model but targeted both patients and their caregivers. Three models described in the article by Geddes et al.51 were each led by a nurse; one was led by a physical therapist and an occupational therapist; and one was coordinated by both a nurse and a physical therapist. Torres-Arreola et al.54 described a model that, instead of including nurses and physical therapists, included nurses trained to provide physical therapy. A team effort of nurses and physical therapists was used in the multidisciplinary early supported discharge model tested in Canada by Teng et al.29
The European early supported discharge models more commonly used a team approach without a designated lead coordinator or facilitator. The study by Holmqvist et al.25 was an example of this team-led model. The hospital-at-home model by Ricauda et al.53 also included a multidisciplinary team of professionals, but ultimately the intervention was led and managed by a physician, nurse, and physical therapist. Andersen et al.43,44 tested a physician model and a less intense and rehabilitation-focused physiotherapist model. Donnelly et al.48 also tested a simpler model focused on assistive equipment and goal setting facilitated by a team of professionals. The five MI provider-driven models varied little from the stroke models. Costa e Silva et al.67 used a multidisciplinary team but followed patients for up to 6 months. Kotowycz et al.61 used a more traditional APN model. Other studies by Hall et al.,69 Kovoor et al.,70 Robertson et al.,76,77 and Sinclair et al.78 used a nurse coordinator. The foci and intervention components were similar to the stroke models.
The psychosocial- and behavioral-focused community-based models were also provider driven. Of the two stroke studies, Ertel et al.49 and Glass et al.50 used a team approach to mobilize the patient’s social network to facilitate adaptation after stroke, and Claiborne et al.47 used a social worker–led model to facilitate both coordination of services and biopsychosocial needs. The psychosocial and behavioral interventions for MI were all single-provider driven and similar in length to the provider-driven models described above. The study by Bambauer et al.66 was led over 8 weeks by a specialist —psychiatrist, psychologist, or internist. The study by Gallagher et al.68 began in the hospital, extended 6 weeks after discharge, and was led by a cardiac nurse. The intervention in Oranta et al.75 was held over six sessions and led by a psychiatric nurse, and the Hanssen et al.71,72 study was also nurse -led but over 24 weeks if the patient needed the support.
The interviewer’s background and training were not specified for the Luszczynska et al.74 study, but the intervention was well described and was distinct and unique from the others. The behavior-change intervention was called “implementation intention” and was used to promote the adherence to guidelines for physical activity after MI. Proactive followup of study participants was not part of this specific model but instead added to evaluate change at 8 months.
Only one technical structure was included in this review. Ayana et al.45 tested a patient -held record that was ultimately the responsibility of the patient to use to improve communication across providers. No contact was made with the patient after the device was distributed through the completion of the study at 6 months, and other aspects of the transition of care were not evaluated. It is possible that other studies of this nature—testing a technical structure or a specific component of an intervention supporting the transition from hospital to home—were not identified in the literature review because of the keywords used in the literature search (see Appendix A).
Intervention type 4: Chronic disease management models of care. Few chronic disease management models of care were identified for this review. This may be the case because chronic disease management models are more commonly designed for ambulatory conditions such as heart failure, diabetes, and depression, with the goal of managing risk factors and comorbid conditions, promoting self-management, and positively affecting change in specific laboratory values or adherence to medication regimens. However, one MI and three stroke models were included in this review because they had outcomes of interest and components of the model specifically addressed patient transitions. These models should be considered community-level interventions.
The MI model as described by Barlow et al.79 tested an established chronic disease management program called the Expert Patient Programme—a “lay-led” community-based program—and compared this to cardiac rehabilitation. The three stroke studies,55–57 on the other hand, were clinically designed to address areas similar to traditional chronic disease management models, but the structure of the intervention was more similar to a provider-driven community-based model of care. The studies of integrated care by Joubert et al.55–57 were each nurse -led and had a lot of emphasis on the components facilitating continuity and coordination across providers and over the 12-month intervention. These components are again highlighted in the taxonomy below.
Taxonomy. Donabedian’s framework of structure, process, and outcome—originally developed to examine health care quality—and other published taxonomies or frameworks for related interventions were used to guide the development of this taxonomy describing MI and stroke transition of care interventions.11,80,81 Donabedian’s framework is now more commonly used to define the characteristics of an intervention or model of care delivery (structure), the content and activities (process), and how these relate to and interact to influence outcomes. In Figure 4, we have similarly described the structure by depicting common subdomains of the type of transition, type of model, recipient of the intervention, and facilitator or lead personnel delivering the intervention. The process is described by highlighted key processes common across model types, the method of contact between the recipient and the facilitator, and the intensity and complexity of the intervention. The outcomes listed in the taxonomy are evaluated in KQs 2–5.
Key Question 2. For patients hospitalized with first or recurrent stroke or MI, do transition of care services improve functional status and quality of life and reduce hospital readmission, morbidity, and mortality (up to 1 year postevent)?
Key Points
- The summary of evidence for KQ 2 was built on results from the past 11 years of 34 articles in stroke and 19 articles in MI that involved 4146 patients with hemorrhagic or ischemic stroke and 11,070 patients with some form of cardiac disease.
- A variety of interventions considered transition of care for patients following stroke and MI; however, there was little consistency from study to study, making cross -study comparisons challenging.
- The most studied transition of care intervention was that of “early supported discharge” (intervention type 1). Early supported discharge after stroke was shown to be effective in reducing the total number of days spent in hospital while at the same time demonstrating that patient-related outcomes such as mortality, disability, and quality of life were no different than among patients treated with standard medical care. Early supported discharge after stroke was associated with increased patient and caregiver satisfaction.
- Guideline-based practice, disease management programs, and specialty followup after MI (intervention type 1) were associated with reduced length of stay, lower rehospitalization rates, and reduced mortality.
- There were no transition of care interventions that consistently improved functional outcomes after stroke.
- Early return to normal activities in low-risk post-MI patients was shown to be safe (intervention type 3).
- The most frequently cited comparator—usual care—was not well defined in the studies.
- There was much variability in the selection of outcome measures for evaluating the success of transitions.
Detailed Analysis
Literature identified. We identified 53 peer -reviewed articles (8 good quality, 36 fair, 9 poor) between 2000 and 2011 that were relevant to KQ 2. These 53 articles presented data from 40 studies that enrolled 15,216 patients: 4146 in the stroke subpopulation and 11,070 in the MI subpopulation (Table 5/5b).
Table 5
Summary of study characteristics for stroke studies.
Table 5b
Summary of study characteristics for myocardial infarction studies.
Population. The population of interest was the set of patients with vascular disease resulting in AIS, intracerebral hemorrhage(ICH), or MI. Study populations were categorized into four population cohorts (shown in Table 5/5b) as follows. The AIS-only cohort included studies in which the sample was limited to patients with ischemic stroke. The mixed-stroke cohort included studies that enrolled patients with both ischemic and hemorrhagic stroke. Studies limited to patients presenting with transient ischemic attacks (TIAs) were not included in this review as most patients with TIA are not hospitalized, and establishing a definitive diagnosis of TIA is often problematic, thus introducing potential ascertainment bias into the conclusions drawn from the study. The MI-only cohort included studies in which the sample was limited to acute MI or unstable angina. The mixed-MI cohort included studies that enrolled patients with multiple cardiac diagnoses, where at least one diagnostic category was acute MI or unstable angina.
From the original 53 articles, 13 studies18–22,30,32,35,41,47,48,53,54 included only patients with acute ischemic stroke (AIS only), 16 studies58,59,61–65,67,69–72,74,76,77,79 included only patients with MI (MI only), 21 studies16,17,23–25,27,28,33,34,36–38,40,44,46,49,50,52,55–57 included patients with AIS, ICH, and subarachnoid hemorrhageor did not define stroke as ischemic/hemorrhagic (mixed stroke), and 3 studies60,66,78 included patients with MI or ACS and other cardiac diseases (mixed MI). There were no studies that explored transition interventions for patients diagnosed only with ICH/SAH. The final sample of 15,216 patients included 1367 patients in the AIS-only cohort, 5713 patients in the MI-only cohort, 2779 patients in the mixed-stroke cohort, and 5375 patients in the mixed-MI cohort.
Six articles23,25,35–38 reporting on five distinct studies enrolled the patient and the caregiver. In these studies, there was no attempt to describe a dyadic relationship; rather, they described the effect of the intervention on separate outcomes for patient and caregiver. The caregiver population was predominately female (70.6%) and most often described as spouse or partner (89.6%) (Table 6). The patient population for each study was stroke—none of the MI studies included in this analysis enrolled caregivers.
Table 6
Relationship of caregivers to patients.
Interventions and comparators. There was a wide range of interventions described in these studies. By far, the most common type of intervention could be broadly described as early supported discharge (ESD) (see KQ 1 for the taxonomy of intervention programs). Key elements that emerged in the interventions included the use of inpatient or outpatient rehabilitation, patient and caregiver followup, patient and caregiver education, providing patients with specialty physician consults, and coordination of care delivery services. These elements were typically addressed in studies that defined the intervention as ESD.16,18,25,28,33,48,52 In addition to ESD, studies described intervention programs developed under umbrella titles such as extended stroke unit services (ESUS),22 and guidelines applied in practice (GAP). 58,59
Table 7 shows that—with the exception of those studies described above (each of which used a developed program)—there was a range of intervention approaches. A modification to the usual approach for rehabilitation (including inpatient, outpatient, or variations in delivery) was used as a component of eight different studies. 32,41,44,53,54,64,65,69 Some form of patient followup was utilized in 24 (60%) studies.23,28,35,36,40,41,44,46,47,49,53,55,56,60–64,66,67,71,74,76,78 Education was used in 27 (67.5%) studies.23,32,34–37,40,41,44,46,47,49,54–56,61,62,64,65,69,71,74,76,78,79 Eleven (27.5%) studies23,32,40,41,47,53,55,56,61,63,70 reported that coordinating some aspect of care delivery was part of the intervention, and five (12.5%)studies 23,40,41,55,56 reported that consulting (providing consult to) a specialist was a component of the intervention.
Table 7
Transition of care interventions.
The intervention group was most often defined as receiving some intervention in addition to receiving the usual care. In all but two studies, the comparator was defined as usual care.53,54 Table 8 shows, however, that when usual care was the default comparator, the description of usual care was generally confined to a few aspects of care. The majority of studies (52.5%)22,23,25,28,35–37,40,46,47,49,52,55,56,58–60,62,74,78,79 gave either no real description of usual care or did not addressed key elements of usual care. There were 5 studies 16,18,41,48,67 that listed some form of acute care as usual care, and 10 studies16,32,33,41,44,48,63,65,69,70 added rehabilitation. Some form of patient followup was cited as usual care in seven studies.33,44,61,63,64,71,76 Five studies 34,41,64,66,69 documented education as usual care, whereas seven studies16,32,33,41,61,63,67 included coordination of care delivery as a component of usual care.
Table 8
Elements of usual care.
Two studies53,54 that did not use the comparator of usual care were stroke studies. The study by Ricauda et al.53 explored transitioning stroke patients from the emergency department (ED) to home after 24 hours and used the “ED-to-ward” comparator. It was unclear if ED-to-ward equated with usual care given that it is not unexpected that a proportion of patients having a stroke would be discharged home directly from the ED following a 24 -hour observation period. Here, the distinction was important given that there were three major options for rehabilitation (inpatient, outpatient, and home care) and neither is considered the gold standard. Torres-Areola Ldel et al.54 explored an education interventio n in conjunction with physical therapy and compared this against an education-only cohort.
Outcomes. We identified more than 70 distinctly different outcome measures that were used to evaluate the relationship between transition of care services and impairments, functional status, quality of life, mortality, health care utilization, and family/caregiver burden. The majority of measures (n = 60) were measures that were validated or reported in prior studies or that could be classified as objective or physiological measures. Table 9 lists the reported outcome assessment measures grouped by category.
Table 9
Outcome assessment measures.
There were no universally beneficial or universally harmful transition interventions (Table 10). Most interventions had mixed effects on outcomes, with some outcomes demonstrating benefit and others showing no change or worsening. Based on the preponderance of the evidence reviewed we were able to draw a number of conclusions about the effectiveness of each intervention when applied during one of the 4 phases of transition of care as defined in our model.
Table 10
Evidence supporting specific components of transition of care interventions.
At the stage of hospital-initiated support for discharge, “early supported discharge” was shown in 8 studies18–22,24,25,27 to reduce total hospital length of stay and improve patient satisfaction while there did not appear to be any adverse effects associated with it such as altered mortality, functional disability, or quality of life.16–20,22,23,27,28,33 We were able to find only a single study that evaluated “early supported discharge” following MI,61 and that trial did not demonstrate any benefits to the patients or their caregivers. Guideline-based practice and disease management programs following MI were shown to decrease death and rehospitalization; however, this conclusion is based on only three moderate -sized studies.58,59,63
Hospital-based as well as community-based patient and family education programs were able to increase patient satisfaction,34 reduce anxiety and depression in some cases62,64,65 as well as increase patient confidence in gaining recovery;36 however, the findings were not always consistent.35,36,64,65 There was no community -based support program that consistently improved either patient or family well-being, whether that program was staffed by nurses, physical therapists, social workers or physicians (Table 10).
One program that seemed to show promise was that of allowing patients with MI and a low-risk profile to return to normal activities early while being followed by weekly phone calls, without having to go through the standard 5-week cardiac rehabilitation program.69,70 This program was not associated with any increase in mortality, reinfarction, or requirement for future coronary bypass surgery. Patients in that program were able to return to work at the same rate as the control subjects. Risk factor control was also the same between the two groups. Telephone-based supportive followup did not demonstrate consistent benefits when evaluated after MI.71,72 Guideline-based practice58,59 and specialty care followup after MI were associated with reduced mortality.60 We did not find any chronic disease transition of care interventions that produced consistent improvement in outcomes or risk factor modification.55–57,67
Of the 53 articles included in the analysis for KQ 2, six reported universal benefit to patients who received the intervention (Table 10)—one in the stroke cohort,56 and five in the MI cohort.60,62,63,65,74 The single study56 in the stroke cohort included 29 patients and concluded that integrated care reduced depressive symptoms. In total, the 5 MI cohort studies included 5410 patients. In a study of 4933 MI patients, Ho et al.60 found that a reduction in mortality was associated with transition to specialty care followup. The remaining MI cohort studies explored education interventions;62,65 followup interventions; 74 a disease -management protocol;63 and benefits to patients in reducing depression (Hospital Anxiety and Depression Scale [HADS], EuroQOL 5 Dimensions [EQ-5D]),65 improving health,62,74 and increasing satisfaction and returning to work earlier with fewer physical symptoms associated with their illness.62,63
A majority of the studies in the stroke cohort16–25,28,35,36,40,41,47,55,57,64,66,71,72 found mixed results and reported some area of benefit to patients who received the intervention but also found areas of no difference in outcomes for patients who received the intervention versus those who did not. In the stroke cohort, five articles18,64,66,71,72 from four studies reported mixed positive and null results at different time points(Table 10). Three of these studies18,64,66 initially showed a benefit to the transition intervention at 3 months after discharge that did not remain statistically significant at the 6-month or 12-month measure. However, Bambauer et al.66 found that the intervention had sustained improvement in depression (HADS) and self-rated health scores. Hanssen et al.71,72 found early benefit to a followup intervention at 6 months (improved quality -of-life and SF-36 physical component summary[PCS] scores) that did not remain significant at 12 and 18 months after discharge.
Including all 53 articles relevant to KQ 2,a beneficial effect from the intervention was noted in 35 of the 71 outcomes reported. While nine outcome measures—knowledge, Geriatric Depression Scale, HADS, length of stay, modified Rankin Scale, patient satisfaction, physical performance test, quality of life, and SF-36 PCS—were found by more than one study to benefit from the intervention, each of these measures was also reported as not being significantly impacted by the intervention.16,19,21,22,25,28,33–38,41,46–49,52,53,55,57,61,62,64–67,70–72,74,78,79 The most commonly reported measure was the SF-36 (or SF-36 components), which was reported in 12 studies.23,28,33,35,46–48,52,59,71,72,79 Three studies reported any level of improved SF -36 component scores,28,47,71 and no study reported an overall improvement in SF-36 scores. One study71 found that the intervention resulted in improved SF-36 mental component summary (MCS) scores at 3 months following stroke discharge. One study72 reported improved SF-36 PCS scores at 6 months after stroke but not at 12 or 18 months after stroke. Mayo et al.28 reported improved SF -36 PCS scores at 1 and 3 months after stroke but no change in SF-36 MCS scores for this period.
There were 15 studies in which the authors found neither benefit nor harm for patients assigned to receive the transition intervention—10 in the stroke cohort,27,30,33,37,38,44,46,48,49,52,54 and 5 in the MI cohort.58,59,61,70,76,77,79 The 10 stroke cohort studies explored 8 different interventions and 35 different outcome measures with no significant differences in groups (Table 10).
There were three articles from two studies30,32,34 that concluded some form of harm as a result of the intervention. In a study of 138 stroke patients, Hoffmann et al.34 found that a computer-tailored stroke education intervention resulted in worse depression (HADS) but improved patient satisfaction for the intervention group; there was no difference in Dartmouth COOP scores, knowledge of stroke, or self-efficacy. Sulch et al.30,32 also foun d that depression scores were worse for the intervention group in their study of 152 stroke patients. Patients who were treated with an integrated-care pathway were also found have lower quality-of-life and Barthel Index scores compared to their counterparts.30 There were no major negative outcomes reported in the MI cohort studies.
The impact of the intervention on the caregiver was explored in a small portion of the stroke studies and none of the MI studies. There was insufficient evidence in these studies to demonstrate an adverse response or benefit to the quality of life and functional status of the caregiver. Two studies35,37 demonstrated a relationship between the transition intervention and caregiver outcomes. Clark et al.35 found that an intervention of education and family counseling improved family functioning at 6 months but that that there was no change in SF-36 scores for the caregiver. The first study by Mant et al.37 reported a significant improvement in the instrumental activities of daily living for caregivers (reported as improved scores in Frenchay Activity Index [FAI]) but found no change in caregiver satisfaction, strain, GHQ, COOP, or SF-36 scores. In a followup study, Mant et al.38 found no change in FAI scores or in caregiver satisfaction, strain, GHQ, COOP, or SF-36 scores. In the remaining studies, the interventions had no significant impact on caregiver burden,23,30 satisfaction,30,36 symptoms (e.g., Giessen Symptom List23 and SIP 25), or depression (ZDS).23
Timing. The primary aim of this report was to explore outcomes at 3 months, 6 months, and 12 months after discharge for patients with acute stroke and MI. However, many studies reported outcomes for more than one time point. Despite a tendency for some studies to measure outcomes in weeks and others in months, it was most common for outcomes to be measured 6 months and 12 months after discharge (Table 11). The most common timing of outcome measures fell between 6 and 11 months after discharge. Using this timeframe, there were 21 articles16–18,22–25,30,32,33,35–37,41,44,46,49,50,53,54 that reported outcomes for the stroke cohort and 10 studies that reported outcomes for the MI cohort.58,65–67,69–71,74 Three studies (four articles) 4,5,23,50 measured outcomes at 1 month or 1.5 months after discharge.16,17,54,69 In each case, outcome measurements were repeated at later time intervals—either at 3 and 6 months after discharge54,69 or at 6 and 12 months after discharge.16,17,69 Approximately half of the studies evaluated outcomes only one time—6 studies34,40,47,62,78,79 at 3 months after discharge, 19 studies 22–25,30,32,35–37,41,44,46,49,50,52,53,67,71,74 at 6 months after discharge, and 11 studies19–21,27,38,48,55–57,59 evaluated outcomes at 12 months after discharge.
Table 11
Summary of study time points associated with outcomes.
Key Question 3. For patients hospitalized with first or recurrent stroke or MI, what are the associated risks, adverse events, or potential harms—both system-based and patient-based—of transition of care services?
Key Points
- Six studies reported risks, adverse events, or harms, and of those that did, none involved interventions for MI patients.
- There was insufficient evidence to determine if there were differential rates of adverse events for transition of care interventions or components of transition of care services.
- Reported rates of adverse events were similar for intervention and usual-care groups.
- The available data must be interpreted with caution because data on risks, adverse events, and harms have not been systematically collected or reported across studies.
Detailed Analysis
The definition of risks, adverse events, or harms for a transition of care intervention was any event that caused a failure to improve (i.e., complications such as death, rehospitalization, prolonged length of stay, lost to followup) or that increased burden or anxiety of the patient or caregiver. While some of these events may also be viewed as an outcome of interest for KQ 2, they also represent an adverse event for KQ 3 and thus are included in both sections.
Literature identified. Only 8 articles (2 good quality, 5 fair, 1 poor )16,27,28,40,41,45,46 representing 6 studies reported data relevant to KQ 3 (Table 12), and all were studies of stroke patients transitioning from hospital to home. With one exception,45 all were randomized controlled trials with four single-site and three multisite studies. Sample size ranged from 83 to 535 patients, and the comparator in every case was usual care. The interventions included a diverse collection of providers and strategies.
Table 12
Measurement of risks, adverse events, and potential harms by study.
Rather than being provider-focused, all interventions were patient-centered, increasing the potential risk for patient or caregiver burden or anxiety. While only Boter et al.46 reported descriptive data on the presence of a support system, two studies16,46 reported caregiver burden as measured by the Caregiver Strain Index. Neither found a difference between the intervention and control groups. Caregivers in the Boter et al. study46 were also assessed with the Sense of Competence Questionnaire as well as on the discrepancies in social support with the Social Support List-Discrepancies measure, but no differences were found between groups at 6 months. von Koch 2000, 2001 reported living arrangements after discharge (alone or with others), but no measures of burden are reported. Ayana et al.45 tested the use of a patient -held record and reported that the patients felt the intervention was burdensome. Almost half of the stroke patients discharged with a patient-held record reported that they never received one, 15 percent lost the record before the 6-month assessment, and 23 percent never read or referred to it during the 6-month intervention period. The majority of patients had difficulty engaging providers to add information to the record, and only 15 percent felt the record kept them informed about the treatment over time. There was insufficient evidence to a make a conclusion on patient or caregiver burden related to transition of care interventions for stroke patients.
The impact of the intervention on insurance coverage that was selected as a focus for this review was not reported for any study; however, it may not have been an appropriate focal point for the majority of studies because in this subsample, five of six studies were conducted internationally in countries with national health insurance models. Only the study by Allen et al.40,41 was conducted in the U.S. and did not report the insurance status of stroke patients. This study did, on the other hand, report a composite outcome of severe complications at 3 months40 and institutional time and death at 6 months.41 Both were measures summarizing death, rehospitalization, and nursing home admission, with the latter reporting the number of days and the former reporting a dichotomous outcome (yes/no). Although the intervention was found to produce a positive effect on severe complications at 3 months (moderate effect size of 0.43, 90% confidence interval 0.09 to 0.78), the 6-month outcome was not significantly different between groups. Von Koch et al.26,27 examined a similar negative outcome of death or dependency on the Barthel Index and had similar findings concluding no difference. There was no significant difference between groups at 6 or 12 months. These two interventions and the structure of the outcome are too dissimilar to draw any conclusions from these findings.
Finally, only Mayo et al.28 examined those patients lost to followup for whether poor performance influenced attrition. Although it was concluded that the intervention-group patients who did not complete the final evaluation could not have been predicted, persons lost to followup from the usual-care group had significantly lower performance for mobility and activities of daily living. Other studies did not report specific analyses of those lost to followup.
Key Question 4. Do transition of care services improve aspects of systems of care for patients with stroke or MI (e.g., more efficient referrals, more timely appointments, better provider communication, reduced use of urgent care, or fewer emergency room visits as a result of transition of care services)? Is there improved coordination among multiple subspecialty care providers, and are new providers added to the care plan as a result of transition of care services?
Key Points
- From a system resource perspective, the evidence for transition of care services for patients with stroke or MI was insufficient because of study designs, sample sizes, and non-U.S. populations.
- Resource use was complex; however, the use of services, particularly emergency department services, is lessened by early education regarding stroke or MI symptoms.
- The greatest impact for education efforts appeared to be early and attenuate d over time (during the first 3 months).
MI-specific key points
- Disease management programs may be more effective than remote phone calls only.
- Early return to work after an MI was safe and may be cost-effective from a societal perspective. Returning to work did not increase health care utilization, and it saved the cost of cardiac rehabilitation in patients without complications or comorbidity.
Stroke-specific key points
- Early supported discharge, particularly in patients without complications or comorbidity, was either cost-neutral or cost-effective as it substantially reduced overall hospital days. This difference in hospital days was driven by the early discharge strategy, with no difference observed in rehospitalization rates in either arm. Early supported discharge did not increase burden on family providers, and it reduced days in the hospital and outpatient physical therapy and occupational therapy visits.
- Rehabilitation in a day hospital or at home resulted in similar overall system resource use.
- Integrated-care pathways may facilitate communication transfer to other providers.
- Physician appointments or home visits by physical therapists may reduce readmission rate for stroke patients, particularly those with prolonged rehabilitation and stroke-related impairment prior to the intervention. Visits by nurses did not produce a similar effect.
- Family support and case management services reduced visits to physical therapists and specialists, the cause of which was unknown.
Detailed Analysis
In addition to improving outcomes, transition of care services can facilitate coordination and continuity of care, thereby improving quality and efficiency at a system level. For KQ 4, we addressed the data pertaining to the impact of transition of care services on systems of care for patients with stroke or MI. This question explored resource use and cost implications as well as coordination and use of important services. Less than half of the 44 studies (N = 18)identified in this report addressed the effect of transition of care services on systems of care. Also, the majority of these studies looked at rehospitalization and costs of care, rather than at communication or followup appointments. The MI population data focused on cardiac rehabilitation, post discharge support and education, and specialist care. The stroke population focused on the transition from hospitalization to home, in terms of early discharge as well as supporting services. Little was found in regard to patient referrals, timely appointments, provider communication, coordination of care, use of urgent care, and emergency department visits. Many of the studies were single center, with non-U.S. populations of fewer than 200 patients. Most studies had few event rates and small population sizes, which limited their ability to provide meaningful comparisons or conclusions.
Literature identified—MI studies. The eight MI studies60,61,63,69,70,72,76,78 all lacked sufficient numbers of patients or events to determine the impact on resource use as a result of transition of care services (Table 13). Most observations did not show statistical significance because of sample size and low occurrence of the outcomes. All studies except Ho et al.60 were outside the U.S., which limits the generalizability of practices to U.S. health care.
Table 13
Resource use and transition of care for patients with MI.
Of the eight MI studies, five61,63,72,76,78 examined post discharge support programs, two 69,70 looked at timing for return to work, and one 60 looked at specialty versus primary care in Veterans Affairs (VA) systems. The five studies looking at postdischarge support described phone or nurse visits for 6 to 12 weeks after discharge from an MI.61,63,72,76,78 These studies were following patients for quality of life, satisfaction, and emotional health as well as adverse events and resource use. The reported rehospitalization rates were not significantly different because of the rare occurrence of rehospitalization. Only the study by Young et al.,63 which utilized a disease management program with four components after an MI discharge, demonstrated lower use of emergency department visits, diagnostic and therapeutic tests, and hospitalization days in the disease management group compared to the control group.
Two studies looked at the impact of returning to work and specialty care on physician visits after discharge. The study by Ho et al.60 noted that when both inpatient and outpatient services to acute MI patients were provided by cardiologists (specialists), there was an increased likelihood of visits with primary care physicians in 90 days after discharge. The study by Kovoor et al.70 determined that early return to work after MI—defined as 2 weeks as opposed to 6 to 8 weeks—had no effect on the resulting use of primary care or specialist clinic visits. In addition, there were no additional increases in health care utilization of laboratory tests or imaging.69 This also established the safety of early return to work for patients with uncomplicated MI, yielding overall economic benefits due to saving on cardiac rehabilitation costs and returning to productive work.
Literature identified—stroke studies. The 10 stroke studies18,21,25,27,30,33,37,43,48,52 that examined resource utilization were similarly small in size, often single center, and all were non-U.S. (Table 14). This limits their statistical power and relevance to U.S. practice regarding the impact of transition of care services on cost and care coordination. However, readmission is common in stroke patients, occurring in up to one-third of all patients, and is likely due to stroke-related complications. Accordingly, data in the stroke population are better in terms of rehospitalizations as compared with the MI population. Of the 10 stroke studies, five18,21,25,27,48 studied early supported discharge, four33,37,43,52 studied transition of care services following standard discharge, and one studied an integrated-care pathway.31
Table 14
Resource use and transition of care for patients with stroke.
The studies of early supported discharge varied in process; however, reductions in initial hospitalization seemed feasible and potentially cost-saving. Bautz-Holtert et al.18 found that combining a multidisciplinary team with early discharge to home resulted in fewer overall hospital days (including baseline hospitalization) without adverse events; however, this was on a selected group of high-functioning stroke patients. Donnelly et al.48 also noted, on a smaller sample of stroke patients, a trend to fewer hospital days and lower costs with the strategy of early discharge and community multidisciplinary support teams. Similar findings among 320 patients were noted in another study21 of early supported discharge, with fewer overall hospital days (p = 0.012) and no difference in overall costs. In this study, a slight increase in clinic visits in the early discharge group (11.4 versus 8.9, p = 0.027) was counterbalanced by fewer overall inpatient rehabilitation days (11.1 versus 23.4, p = 0.0001). A study by Holmqvist et al.25 showed that early discharge resulted in fewer hospital days overall (15 versus 30, p < 0.0001), mostly due to the shortened initial hospitalization. The early discharge to home rehabilitation group had no significant differences in other services such as transportation, home health, or assistance from family caregivers. The costs were neutral.25 Those studies that looked at providing rehabilitation at home versus in a rehabilitation hospital following standard discharge found no significant resource differences. Further differences were an increase in day hospital attendance (p < 0.001) and outpatient visits to occupational therapy and physical therapy.27
In the four studies33,37,43,52 of transition of care services following standard discharge, Torp et al.33 studied discharge supported by an interdisciplinary stroke team versus standard aftercare and found no differences in resource use, GP visits, or health care services. Mant et al.37 found that family support group patients had a lower use of outpatient PT visits (44 versus 56%, p = 0.04), but otherwise there were no differences in use of services. Mayo et al.52 studied case management involving nursing phone calls and visits to coordinate care 6 weeks after discharge and found no differences in health care utilization between case management patients and controls. There was, however, a slight increase in specialist visits in the usual care group (3.4 versus 2.2, p < 0.01). Andersen et al.43 found that physician or physiotherapist visits to patients with stroke following discharge from an inpatient rehabilitation facility significantly reduced readmissions over and above contact with nursing services only (control) (26% and 33% versus 44%, p < 0.028). Therefore, from a cost and resource use perspective, it appears that support is most useful when used in combination with a shortening of the initial hospitalization in selected patients and when delivered in person by skilled staff.
Two studies by Sulch et al.30,31 studied t he role of integrated-care pathways. Integrated-care pathways did not shorten length of stay.30 In addition, integrated pathways were tested for their ability to improve communications with the outpatient setting. The integrated pathway tested by Sulch et al.31 did increase notification of primary physicians related to discharge and documentation of information, but there were no significant differences in the process of care between the integrated-care pathway group and the control group.
Key Question 5. For patients hospitalized with first or recurrent stroke or MI, do benefits and harms of transition of care services vary by characteristics—both patient-based and system-based—such as disease etiology and severity, comorbidities, sociodemographic factors, training of the health care providers, participants (patients, caregivers), geography (rural/urban, regional variations), and insurance status?
Key Points
- Thirteen studies of transition of care explored the benefits and harms as they varied by characteristics.
- The most commonly reported characteristics in transition of care studies were severity, age, sex and presence/absence of depressive symptoms.
- There was a lack of consistency by which characteristics are measured or reported.
Detailed Analysis
Literature identified. We identified 14 peer -reviewed articles (2 good quality, 10 fair, 2 poor) between 2000 and 2011 that were specifically relevant to KQ 5. These 14 articles presented data from 13 studies that enrolled 3420 patients.
Population. The population of interest is the set of patients with vascular disease resulting in AIS or MI who received transition of care services for which there was a difference in benefit or harm that can be related to patient characteristics (Table 15). Study populations were categorized into the followed four cohorts: (1) AIS only, (2) MI only, (3) mixed stroke, and (4) mixed MI. To be included in the sample, studies with a mixed population must also have included patients with ICH stroke, ischemic stroke, MI, or unstable angina with evidence of ischemia.
Table 15
Studies of how benefits and harms may vary by patient or system characteristics.
From these 14 original articles, three studies21,22,41 included only patients with AIS (AIS only); two studies74,79 included only patients with MI (MI only); seven studies 17,29,33,37,43,49,51 had a mixed-stroke population that included patients with stroke, and either did not define stroke as ischemic/hemorrhagic or included AIS and other stroke (ICH and/or SAH); and one study68 included a mixed-MI population defined as acute coronary syndrome or patients with MI as well as other patients (but not stroke). There were no studies that included only patients with combined stroke and MI, and no studies that explored transition interventions for patients diagnosed only with ICH.
The 14 articles described single-center and multicenter studies around the world. Single-center studies were performed in the U.S., 41 Norway, 17,21,22 U.K.,79 Australia, 68 Poland,74 Canada,29 Denmark, 33 and Sweden.27 Multicenter studies were conducted in Denmark, 43 U.S., 49 and U.K.51
Subgroups. There was a lack of consistency with regard to which characteristics were explored for their relationship to benefits and harms of transition of care interventions. Table 16 provides details about the most frequently reported subgroup characteristics in transition intervention studies.
Table 16
Subgroup characteristics.
The relationship between outcomes and severity of illness was the most frequently reported characteristic.17,21,22,29,33,41,49,51 The measures of severity that were reported included National Institutes of Health Stroke Scale (NIHSS), Scandinavian Stroke Scale (SSS), Barthel Index, and modified Rankin Scale (mRS). Other common subgroup analyses were performed for age,41,43,49,51,68,79 and sex, 41,43,49,68,79 while only one study 41 explored the relationship of race/ethnicity to outcomes. Depression was an outcome of interest in 18 articles, 32,34–38,41,46–48,52,53,55,56,64–66,79 but depression was also explored as a characteristic in four studies. 27,41,43,49 Three studies43,49,51 explored some form of patient baseline knowledge/education. The patient’s premorbid status or number of comorbidities was explored in four studies.41,49,74,79 Only two studies43,51 explored system -level characteristics by describing the relationship of health care provider to patient outcomes.
Benefits and harms. Each of the studies reported at least one significant finding attributed to one or more patient-based or system-based characteristic (Table 17). Functional status at discharge, while measured using different tools, was found to be a significant predictor of outcome in 8 studies.16,17,19–22,26,27,29,33,49,51 The outcome for these 8 studies varied from balance to depression and morbidity; there was no universal outcome associated with functional status. The most common characteristics analyzed were age and sex, which were reported in all but two studies.74,78 Only two studies 37,38,51 reported systems-based characteristics with significant findings. Geddeset al. 51 found that patients who were deceased or transferred to a nursing home 1 year after home-based rehabilitation were more likely to have been referred by a general practitioner compared to having been referred by hospital-based personnel. In a study of MI patients, Mant37,38 found that admission to a specialty rehabilitation after discharge was associated with improved functional status at 6 months.
Table 17
Studies showing significant findings for transition intervention.
Secondary analysis for KQ5. After exploring the data from the 14 transition articles identified above that analyzed differences in outcome for various characteristics within a single study, we sought to explore for patterns of benefit or harm between the transition studies. To accomplish this, we examined the relationships of key characteristics between studies that reported benefit or harm associated with a transition of care intervention.
From the 62 articles included in this systematic review, there were 22 articles17,19–22,25,34–36,41,47,53,55–57,60,62,65–67,74,78 that identified some benefit to a transition intervention. To fully explore for patterns in characteristics associated with patients who benefited from the intervention, the four main subgroup characteristics identified in Table 16 (severity of stroke/MI, age, sex, and depression) were abstracted for each outcome measure that reported a benefit of a transition intervention (Table 18). Across the studies that reported benefit, the mean age was between 56 and 82 years. The percentage of male patients in the stroke studies that reported benefit was 42 to 61 percent.17,19–22,25,34–36,41,47,53,55–57
Table 18
Studies that found a benefit of transition intervention.
Of studies that found a benefit to the intervention and reported sex in the MI population, one study conducted in a VA population60 reported that 97 percent of subjects were male; in the remaining MI population studies, the percentage of male subjects ranged from 46 to 70 percent.62,65–67 Severity of illness/injury was reported in 11 studies. 17,19,22,25,36,41,53,56,57,60,67 Studies that reported baseline mRS values and found benefit from the transition intervention generally reported baseline mRS of greater than 2 as a characteristic, 17,22,56,57 whereas Fjaertoft et al. 19 reported that 6.3 percent of patients who benefited from ESUS had mRS less than 3. Studies that included NIHSS scores found benefit from the transition intervention when the mean NIHSS was less than 5.36,41,53 Baseline depression was explored using CES -D,41 EQ -5D,65 GDS, 47,53 and HADS.36,65,66 With the exception that depression scores at baseline predicted later depression scores,47,65,66 there was no consistent relationship between depression and a transition intervention.
Characteristics from studies that found a benefit to the intervention. There were 17 studies (22 articles)17,19–22,25,34–36,41,47,53,55–57,60,62,65–67,74,78 that reported a positive effect from the transition intervention and included data for patient characteristics. The four most frequently reported subgroup characteristics were severity of stroke/MI, age, sex, and depression. As noted in Table 18, there was insufficient evidence to support the hypothesis that response to any given transition of care intervention varies by patient characteristics. There was only a modest variation in age and sex reported. The mean age reported varied from a low of 56 in one MI study62 of 65 patients to a high of 82 in a stroke study.53 Given the exception of a study performed in a VA population where the sample was 97 percent male,60 the percentage of male patients ranged from 42 percent in a study of 138 stroke patients34 to 70 percent in a study of 65 patients with MI. 62
There was a lack of consistency in the use of instruments for reporting illness severity. There were only 11 studies that reported severity of illness at the time of admission in the subset of studies that found a benefit to the intervention and reported characteristics associated with outcomes. Studies including patients with stroke reported severity using NIHSS,36,41,53 SSS,17 Barthel Index,17,19,22,36 CNS,53 FIM, 53 and mRS;17,19,22,56,57 and one study 25 reported CT scan results along with coping and comorbidities. For the studies including cardiac patients, only two reports60,67 included illness severity scales. Ho et al. 60 found a mean thrombolysis in myocardial infarction (TIMI)risk score of 2.2 in their study of 4922 cardiac patients, wherein the finding of lower mortality was associated with a hospital-initiated support model of transition intervention. A smaller study67 of 153 MI patients reported that STEMI versus non -STEMI as a measure of severity in a community-based support intervention was associated with improved compliance with dietary recommendations and improved rates of physician followup.
The presence and rating of depression was reported in four stroke studies36,41,47,53 and two MI studies.65,66 Six studies reported findings using four different depression rating scales: HADS,36,65,66 GDS, 47,53 EQ -5D,65 and CES -D.41 Notably, four 47,53,65,66 of the six studies that reported depression scores as a characteristic also reported that the transition intervention improved depression; hence, these studies suggest that while the transition intervention improved depression, the effect of the transition was moderated by the presence/absence of baseline depression.
Characteristics of studies for which the intervention was associated with a negative outcome. Only 2 articles32,34 of the 62 articles reported any form of harm for subjects who received a transition intervention. Sulch et al.32 used an integrated-care pathway for 76 patients in the intervention group and compared results to 76 patients who received usual care (no integrated-care pathway). Groups were similar at baseline, but depression scores at 3 months were worse for those who received the intervention compared to those who did not; mRS at discharge was independently associated with depression. Hoffman et al.34 provided 69 patients with a computer-generated educational intervention that was tailored to their needs. The intervention group, when compared to the 69 subjects who received usual education, was reported to have higher levels of anxiety. In this study, the intervention group had a significantly higher male-to-female ratio than did the control group; there was no report of independent association of anxiety scores and sex.
- Literature Search and Screening
- For patients hospitalized with first or recurrent stroke or myocardial infarction (MI), what are the key components of transition of care services? Can these components be grouped in a taxonomy, and are they based on a particular theory?
- For patients hospitalized with first or recurrent stroke or MI, do transition of care services improve functional status and quality of life and reduce hospital readmission, morbidity, and mortality (up to 1 year postevent)?
- For patients hospitalized with first or recurrent stroke or MI, what are the associated risks, adverse events, or potential harms—both system-based and patient-based—of transition of care services?
- Do transition of care services improve aspects of systems of care for patients with stroke or MI (e.g., more efficient referrals, more timely appointments, better provider communication, reduced use of urgent care, or fewer emergency room visits as a result of transition of care services)? Is there improved coordination among multiple subspecialty care providers, and are new providers added to the care plan as a result of transition of care services?
- For patients hospitalized with first or recurrent stroke or MI, do benefits and harms of transition of care services vary by characteristics—both patient-based and system-based—such as disease etiology and severity, comorbidities, sociodemographic factors, training of the health care providers, participants (patients, caregivers), geography (rural/urban, regional variations), and insurance status?
- Results - Transition of Care for Acute Stroke and Myocardial Infarction PatientsResults - Transition of Care for Acute Stroke and Myocardial Infarction Patients
Your browsing activity is empty.
Activity recording is turned off.
See more...
