NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Ratko TA, Belinson SE, Brown HM, et al. Hematopoietic Stem-Cell Transplantation in the Pediatric Population [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Feb. (Comparative Effectiveness Reviews, No. 48.)
This publication is provided for historical reference only and the information may be out of date.
Topic Development and Refinement
The topic of this report and preliminary Key Questions were developed through a public process involving the public, the Scientific Resource Center (available at: http://effectivehealthcare.ahrq.gov/index.cfm/who-is-involved-in-the-effective-health-care-program1/about-the-scientific-resource-center1/) for the Effective Health Care program of the Agency for Healthcare Research and Quality (AHRQ), and various stakeholder groups.
Recognizing that the scope was broad and that there were diseases for which 20 years of research had been codified into guidelines and reviews, as described in the Introduction, we took a “narrative review” approach to those diseases, reserving a systematic review approach for those indications for which the role of HSCT was not established by clinical study. This was done in consultation with a Key Informant panel and AHRQ personnel. The Key Informant panel comprised clinical experts in the various diseases covered in this report. The topic refinement process made us aware that the literature base for the systematic review was predominantly case series and case reports. This represents the circumstance that the diseases under consideration are rare diseases or in more common diseases, the subgroups of patients having poor prognosis or are refractory to therapy.
Topic refinement also outlined the frameworks and PICOTS which were also posted for public comment. In summary, the public comments addressed three main points. First, while successes have been seen with HSCT in many pediatric conditions, the measurement of comparative outcomes after HSCT is difficult due to the rarity of the conditions (e.g., retinoblastoma) and/or the number of transplants completed (e.g., autoimmune diseases). Second, comparative harms data are equally difficult to obtain, as separating out the harms associated with HSCT from the harms associated with other prior treatments or disease natural history is not possible in many cases. Third, it was suggested that we contact the Pediatric Blood and Marrow Transplant Consortium and Center for International Blood and Marrow Transplant Research (CIBMTR) to see if they could provide advice to guide the structure of the report. No major changes were made following the public comments. These points were taken into account in the CER.
Technical Expert Panel and Peer Review
With completion of the topic refinement phase, a Technical Expert Panel (TEP) was formed. The TEP included original Key Informant panel members and clinical experts not previously involved. The TEP provided consultation on the development of the protocol and evidence tables for the review. Ad hoc clinical questions were also addressed to the TEP. The draft report was reviewed by five external reviewers, including invited clinical experts and stakeholders. Revisions were made to the draft report based on reviewers' comments.
Narrative Reviews
The narrative review approach to a number of conditions presented in this report was based on recognition that there exists a substantial body of evidence from 20 years or more of transplantation research and experience that had been codified into published guidelines and reviews. Thus, systematic review of the evidence for these diseases would not be expected to offer new insights or information. By contrast, the EPC recognized there were a number of diseases for which evidence of benefits and harms was less clear or for which clinical practice was less established, so that systematic review of the literature would be more likely to provide new insight to inform the field.
The final categorization of indications for the narrative reviews was determined in an iterative process. Information sources were not identified by a systematic review of the literature. Rather, the EPC relied on recently published reviews of pediatric transplantation studies, and publicly available sources such as the National Guidelines Clearinghouse and the National Cancer Institute's Physician Data Query (PDQ) Web site, to develop an initial list of diseases for discussion with the Key Informant panel. The EPC subsequently reexamined the lists, compared them to existing evidence, in the context of the Key Informant discussions. A final list of indications for narrative reviews compiled by the EPC was posted for public comment.
Systematic Reviews
The following methods apply only to the systematic reviews presented in this report.
Literature Search
Electronic databases searched were MEDLINE®, Embase®, and the Cochrane Controlled Trials Register. Databases were initially searched without restriction on date, using the search strategy shown in Appendix A. However, during the Topic Refinement phase of this project, the Key Informants strongly recommended limiting study selection to the past 15 years to ensure we identify evidence that is comparable in terms of therapeutic regimens and management protocols. Thus, we reviewed the literature from January 1995 up to November 9, 2009. Literature searches were updated to August 17, 2011, prior to delivery of the final report to ensure the identification of new literature that potentially had an impact on the review.
All search results were compiled into an EndNote® reference manager database with exclusion of duplicates. Additional details on these materials and results of our review are provided in the Results chapter. Search strategies and results are detailed in Appendix A.
Study Selection
Inclusion and exclusion criteria are for all Key Questions.
Inclusion criteria:
- Reports on pediatric patients (age ≤21 years) who have relevant diseases (malignant solid tumors, inherited metabolic diseases, or autoimmune disease).
- Reports on an outcome of interest.
- Reported on HSCT and/or a comparator of interest.
- Intervention and comparator used contemporary regimens with respect to chemotherapy, radiation therapy and supportive care.
- For Key Questions 3 and 4 (inherited metabolic diseases) studies reporting outcomes on the natural history of disease were included as comparators.
Exclusion criteria:
- Studies older than 15 years as they would not represent contemporary regimens except the natural history data for Key Questions 3 and 4.
- Studies where pediatric data could not be separated and abstracted from adult data.
- Duplicate studies or reports with duplicate patients were excluded except the study with the largest number of patients with the longest followup.
Abstract and study selection was performed by a single reviewer for each section of the report. If a reviewer was uncertain whether a study should be selected for inclusion, this was resolved through discussion at team meetings.
Figure 4 shows a PRISMA11 diagram of the studies included in the systematic review. A listing of excluded references with reasons for exclusions is available in Appendix B.
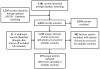
Figure 4
PRISMA diagram of articles included in the systematic review.
Data Abstraction
Data were abstracted by a single reviewer, and fact checked by another reviewer. If there were disagreements, they were resolved through discussion among the review team. The following data elements of primary studies were abstracted from the articles meeting selection criteria:
- Critical features of the study design
- Patient inclusion/exclusion criteria
- Number of participants and flow of participants through steps of study
- Patient characteristics, including:
- Age
- Sex
- Race/ethnicity
- Disease and stage
- Disease duration
- Other prognostic characteristics
- Treatment characteristics, including
- Stem-cell source
- Chemotherapy versus chemo-radiotherapy
- Immunosuppressive therapy as prophylaxis for graft versus host disease
- Supportive care
- Outcome assessment details
- Identified primary outcome
- Secondary outcomes
- Response criteria
- Use of independent outcome assessor
- Followup frequency and duration
- Data analysis details
- Statistical analyses (statistical test/estimation results)
- –
Test used
- –
Summary measures
- –
Sample variability measures
- –
Precision of estimate
- –
P values
Full data abstraction tables are available in Appendix C. Evidence tables were generated in Microsoft Excel® and Microsoft Word®.
Study Quality
Evidence consisted largely of case series and case reports; therefore we did not attempt to assess the quality of individual studies. It is well recognized in the study of rare diseases that a common challenge is the preponderance of small, uncontrolled studies.12 Therefore, because studies tended to be homogenous in design, quality assessment would be unlikely to discriminate between higher and lesser quality studies.
Data Synthesis
Data synthesis was qualitative. We attempted to identify subgroups based on prognostic factors such as tumor stage or location in solid tumors, or disease severity or rate of progression in the inborn metabolic disorders, to see if these subgroups showed patterns of treatment success or failure. The evidence base was considered insufficient and too heterogeneous to use quantitative pooling methods. Where possible we calculated confidence intervals for results and reported ranges of results for studies that addressed the same population and treatment.
Grading the Evidence for Each Key Question
The strength of the body of evidence for each indication was assessed according to the process developed by the AHRQ EPC Program13 for the EPC Methods Guide, based on a system developed by the GRADE Working Group.14 This comprised an iterative, qualitative consensus-driven process among EPC team members familiar with the summarized literature, using the 4 required domains specified in the EPC Methods Guide: risk of bias, consistency, directness, and precision. There were no head-to-head comparative studies for most diseases; in those situations, directness was based on the outcome (e.g., overall survival or other clinically important health outcomes) rather than on the comparison. For small series or a compilation of case reports in which the prognosis absent HSCT is uniformly fatal (e.g., Wolman's disease), the known natural history was considered an indirect comparator. An optional domain, strength of association (SOA, magnitude of effect) was thus ascribed to the body of evidence when there was an apparent benefit or harm, increasing the overall strength beyond what may be normally considered appropriate for such evidence. SOA was deemed not applicable for diseases where there was no clear evidence of benefit or harm with HSCT versus comparators, or if results (e.g., overall survival rates) of individual studies within a body of literature were inconsistent or conflicted. No quantitative scoring method was applied.
Table 3 displays the EPC Methods Guide definitions and applications of GRADE and describes how we applied the domains in this review.
Table 3
Elements of evidence grading for Key Questions.
The overall grade of evidence strength was classified into the following four categories:
- High: Further research is very unlikely to change our confidence in the estimate of effect
- Moderate: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate of effect
- Low: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
- Insufficient: Any estimate of effect is very uncertain
- Methods - Hematopoietic Stem-Cell Transplantation in the Pediatric PopulationMethods - Hematopoietic Stem-Cell Transplantation in the Pediatric Population
- Stage IV Uterine Corpus Endometrial Stromal Sarcoma AJCC v8Stage IV Uterine Corpus Endometrial Stromal Sarcoma AJCC v8MedGen
- Irregular epiphysis of the distal phalanx of the thumbIrregular epiphysis of the distal phalanx of the thumbMedGen
Your browsing activity is empty.
Activity recording is turned off.
See more...
