NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Shamliyan TA, Kane RL, Taylor FR. Migraine in Adults: Preventive Pharmacologic Treatments [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Apr. (Comparative Effectiveness Reviews, No. 103.)
This publication is provided for historical reference only and the information may be out of date.
Of 5,244 identified references, we included 245 references of RCTs and 76 nonrandomized studies (Figure 2). All excluded references are presented in Appendix C.
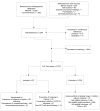
Figure 2
Study flow.
Publication Bias
By analyzing the NIH-funded and registered studies, we found that the results are available from only a small proportion of migraine prevention studies. However, we could not determine exact reasons for low availability of results based on available data. Both, posting of results and publication rates, varied by individual sponsors.
We found 18 NIH funded grants that aimed to examine migraine prevention. Six grant projects funded three RCTs. Two of those three RCTs were registered in ClinicalTrials.gov (Table 2). Overall publication rate was 44 percent (eight of 18 funded projects). The National Institute of Neurological Disorders and Stroke funded nine studies (the largest number among the agencies), and published the results from four of these projects (Table 2). We could not explain why the studies have not been published because the NIH grant database does not allow the analysis of the exact reasons for the low publication rates of the projects. Results from the NIH-funded projects were published after 1.9 to 3 years from the end dates of the projects (Table 3). Time intervals between project end dates and publication did not differ among the funding agencies.
Table 2
Registration, publication, and cost of the NIH-funded grants aimed at migraine prevention (as of May 2012).
Table 3
Years between the NIH-funded project end dates and the publication dates of the results.
Searching trial registries, we found 67 studies in ClinicalTrials.gov and 24 studies in other registries. Publication rates of study results were slightly lower for the studies registered in ClinicalTrials.gov (21 percent; 14/67) than in other registries (33 percent; 8/24). Among the studies registered in ClinicalTrials.gov most studies examined drugs (61/67). A placebo control was used by 64 percent (43/67). Most studies were completed (70 percent; 47/67) and four studies were terminated. Termination due to harms with treatments was clearly indicated in two terminated studies. The results were posted for nine studies (13 percent).
Publication rates varied depending on subjects and study characteristics (Table 4). Only 28 percent of all completed studies, 50 percent of biologics studies, and 18 percent of drug studies were published. Only 33 percent of Phase III and 50 percent of Phase IV studies were published. No terminated studies were published. Publications occurred an average of 2 years after study completion (0.5 to 6.6 years). Publication time varied among individual sponsors. Odds of publication did not reach statistical significance, probably due to the small number of studies (Table 5).
Table 4
Publication and posting of the results of migraine prevention studies registered in ClinicalTrials.gov.
Table 5
Odds of publication and posting of results in ClinicalTrials.gov among all studies registered in ClinicalTrials.gov.
The rates of the posting of the results also varied depending on subjects and study characteristics (Table 4). Half of biologics studies and 12 percent of drug studies posted the result in ClinicalTrials.gov. Only 13 percent of Phase III and 29 percent of Phase IV trials posted the results in ClinicalTrials.gov. Trials that were terminated for safety reasons did not post the results. Results were posted an average of 2.6 years after study completion dates (0.9 to 5.2 years). Biologic studies posted the results an average of 3 years after completion dates, and drug studies posted results an average of 2.6 years after completion dates. Placebo-controlled studies posted results an average of 2.5 years after completion dates, and comparative effectiveness studies 3.4 years after completion dates. Terminated studies posted the results an average of 5 years after study termination. Odds of posting the results did not reach statistical significance, probably due to the small number of studies (Table 5).
Abstracted data are available in Appendix D with evidence tables (available at https://netfiles.umn.edu/xythoswfs/webui/_xy-21041343_1-t_zdhvSpvy). Randomized trials examined 59 drugs from 14 pharmacologic drug classes (Appendix Table D1).
Most trials were funded by industry but did not disclose conflict of interest by study investigators (Appendix Table D2). Proportions of industry sponsorship and disclosed conflict of interest varied among drugs (Appendix Table D2).
Applicability
The results from eligible studies were applicable to the target population. Most RCTs were conducted in the United States and Western countries and used the International Headache Society’s definition (Appendix Table D3). Older publications used the definition of migraine developed by the Ad Hoc Committee on Classification of Headache, and about 34 RCTs did not specify a migraine definition.
Investigators recruited patients in clinics in almost half of RCTs. Half did not report this information, and eight RCTS clearly indicated community-based recruitment. RCTs enrolled an average of 210 adults, measured the outcomes at 2 to 3 months of followup, and reported about 14 percent loss of followup (Table 6 and Appendix Table D4).
Table 6
Total number randomized, weeks of followup, and loss of followup in randomized controlled clinical trials of migraine prevention in adults.
Studies enrolled mostly adults (average age, 38 years) and adolescents (Table 7). Women made up the majority of enrolled subjects (Appendix Table D5). Few trials reported a proportion of obese subjects, but many participants were overweight according to the average body mass index. Most trials included patients with and without aura (Appendix Table D5). Enrolled patients had an average of five monthly migraine attacks. Almost half of the enrolled subjects were naïve to migraine preventive drugs (Table 7). Patient age and baseline migraine characteristics were similar in most trials (Appendix Table D6).
Table 7
Reporting of patient baseline characteristics in randomized controlled clinical trials of migraine prevention drugs in adults.
Substantial variability in reporting comorbidities prevented us from using this information in quantitative synthesis of evidence. Most trials, however, excluded patients with severe medical comorbidities or psychiatric illnesses, stroke, and vascular migraine. RCTs rarely reported important patient characteristics that could modify drug effects, including family history of migraine, socioeconomic status, or response to prior preventive treatments
Risk of Bias
More than half of the RCTs had medium risk of bias and about 21 percent had low risk of bias (Table 8). Proportions of RCTs with low risk of bias varied among drugs (Appendix Table D7). Among approved drugs, the percent of low-risk-of-bias RCTs was as follows: topiramate, 45 percent; divalproex, 67 percent; and propranolol, 13 percent (Appendix Table D7). Most RCTs (86 percent) were double blind. Timolol was examined in two RCTs of medium risk of bias. We concluded unclear adequacy of allocation concealment in 94 percent of RCTs and adequacy of randomization in 51 percent of RCTs (Table 8). Planned intention to treat was reported in 24 percent of RCTs.
Table 8
Number of randomized controlled clinical trials of migraine prevention in adults that met risk of bias criteria.
Published RCTs rarely presented subject flows. Nor did RCTs report why some eligible subjects were not randomized and therefore excluded from the trials. Proportions of eligible subjects excluded from randomization varied among trials. Investigators excluded an average of 5 percent of randomized subjects from the analyses, with substantial variability among the drugs.
Key Question 1. What is the efficacy and comparative effectiveness of pharmacologic treatments for preventing migraine attacks in adults?
Results from RCTs were available in 245 references. RCTs examined four approved drugs for episodic migraine (topiramate, divalproex, propranolol, and timolol), one approved drug for chronic migraine (onabotulinumtoxin A), and various off-label preventive drugs. Most trials examined a monotherapy with one active agent compared with placebo or to another drug. RCTs rarely reported exact drugs and doses of concomitant treatments. However, we surmise there were no concomitant treatments because most trials disallowed concomitant drugs during the run-in period and after randomization. Strength of evidence was low due to medium or high risk of bias and imprecise estimates from individual or meta-analyzed RCTs.
KQ1a. How do preventive pharmacologic treatments affect patient-centered and intermediate outcomes when compared with placebo or no active treatment?
All approved drugs were better than placebo in reducing monthly migraine frequency by ≥50 percent in patients with episodic migraine and baseline <15 migraine days per month (clinical response). The relative effect of drugs was moderate: drugs resulted in clinical response in 200 to 400 patients per 1,000 treated. Clinicians need to treat three to five patients with episodic migraine to prevent half or more migraine attacks in one patient.
Strength of evidence was lowered due to medium risk of bias and imprecise estimates. Low-strength evidence from individual RCTs suggested a dose-responsive increase in migraine prevention with higher doses of onabotulinumtoxin A and topiramate (from 50–100 mg/day with no additional benefits with 200 mg/day).
Among off-label drugs, pooled analyses offered low-strength evidence that antiepileptic gabapentin, beta-blocker metoprolol, and calcium channel blocker nimodipine were better than placebo in reducing monthly migraine attacks by ≥50 percent. Individual RCTs offered low-strength evidence that off-label beta blockers, acebutolol atenolol and nadolol, were better than placebo in reducing monthly migraine attacks by ≥50 percent. Individual RCTs demonstrated that the angiotensin converting enzyme inhibitors captopril and lisinopril and the angiotensin II antagonist candesartan were better than placebo in reducing monthly migraine attacks by ≥50 percent.
We present strength of evidence for patient-centered outcomes, including complete migraine cessation (Table 9) and migraine prevention with approved (Table 10) and off-label drugs (Tables 11 and 12).
Table 9
100% reduction in monthly migraine frequency with pharmacologic preventive treatments versus placebo in adults with episodic migraine, results from randomized controlled clinical trials.
Table 10
Migraine prevention with approved pharmacologic treatments versus placebo in adults, results from randomized controlled clinical trials (pooled with random effects models).
Table 11
Migraine prevention with pharmacologic treatments versus placebo in adults with episodic migraine (pooled with random effects model results from randomized controlled clinical trials).
Table 12
Migraine prevention (50% or more reduction) with off-label pharmacologic treatments versus placebo in adults with episodic migraine, results from individual randomized controlled clinical trials.
Only a few RCTs examined quality of life, and they provided no consistent evidence of improvement with examined drugs. The studies rarely assess clinical importance of the changes in quality of life or disability scales. We describe those effects as well as changes in intermediate outcomes in the text and appendix tables.
Prevention of Chronic Migraine
Muscle Relaxants
Onabotulinumtoxin A
We identified 15 RCTs that examined the efficacy of botulinum toxin for migraine prevention; 13 RCTs examined onabotulinumtoxin A and two RCTs examined abobotulinumtoxin A (Appendix Table D8). The studies enrolled an average of 285 patients aged 18 to 65 years with four to 12 migraine attacks/month. Most trials included patients with 10 or more years of migraine experience. Women made up 85 percent of participants. More than half of enrolled patients had been previously treated with preventive medications for migraine. Most RCTs were industry funded and reported conflict of interest by study investigators (Appendix Table D9). All RCTs were double blind and most had low risk of bias (Appendix Table D10).
Onabotulinumtoxin A was better than placebo in reducing monthly migraine attacks by ≥50 percent (three RCTs of 459 adults, low-strength evidence) (Appendix Table D11).92–94 Onabotulinumtoxin A tended to increase the likelihood of ≥50 percent reduction in migraine frequency compared with placebo in all RCTs (Appendix Table D12). Pooled relative increase by 50 percent achieved statistical significance (pooled RR 1.5, 95% CI, 1.2 to 1.8). Pooled analyses demonstrated that 170 adults per 1,000 treated (95% CI, 82 to 258) would experience ≥50 percent reduction in migraine frequency with onabotulinumtoxin A (Table 10). No RCTs of abobotulinumtoxin A reported rates of ≥50 percent reduction in monthly migraine attacks.
A reduction in migraine days was considered as a primary outcome in the Phase III Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) RCT that reported statistically significant reduction in frequency of headache days relative to 20 headache days at baseline (−9.0 days with onabotulinumtoxin A versus −6.7 days with placebo, p < .001).137 The same trial reported a statistically significant reduction in frequency of migraine days relative to 19 migraine days at baseline (mean difference with placebo −2.4 with 95% CI −3.3 to −1.4).137 We could not pool the results from other RCTs that examined a reduction in migraine days or hours because the trials failed to report data needed for reproducible results.137–139
For intermediate outcomes, the absolute number of migraine attacks did not differ between onabotulinumtoxin A and placebo (Appendix Table D13). Improvement in migraine severity was inconsistent across four RCTs (Appendix Table D14).92,139–141 Improvement in migraine disability assessment was inconsistent across two RCTs (Appendix Table D14).93,142 A single RCT of patients who had not benefitted from previous oral prophylactic treatment demonstrated significant improvement in most domains of quality of life as assessed by the Migraine Impact Questionnaire (Appendix Table D14).142 The PREEMPT clinical program RCT demonstrated statistically significant improvement in all domains of the Migraine-Specific Quality of Life Questionnaire.143 Significant improvement was demonstrated in global assessment, severity of migraine symptoms, self-management of migraine, and ability to work and participate in recreational activities (Appendix Table D14).142
In our separate analysis of RCTs of abobotulinumtoxin A we found inconsistent effects on patient global evaluation of treatment success. The Dysport Migraine Study Group reported a statistically significant increase in patient’s global evaluation of treatment efficacy.144 Slightly or much improved migraine frequency was reported in 281 patients per 1,000 treated (95% CI 46 to 516).144 In contrast, the Dysport® In Migraine Without Aura Prophylaxis trial found no differences in patient or investigators’ perception of global assessment with the active drug versus placebo.139 Neither trial showed statistically significant reduction in absolute number of migraine attacks with abobotulinumtoxin A versus placebo.139,144
Tizanidine
Tizanidine was better than placebo in reducing migraine severity (one RCT of 136 adults) but had no effect on migraine frequency.145
Antiepileptics
The Topiramate Chronic Migraine Study Group RCT offered low-strength evidence that topiramate was better than placebo in reducing from baseline monthly migraine days, rates of 25 percent reduction in monthly migraine attacks, and frequency of associated symptoms.86,146,147 Topiramate was not better than placebo in reducing monthly migraine attacks by ≥50 percent.86 Topiramate did not decrease treatment discontinuation due to failure.146
The drug improved quality of life and migraine related disability in adult with chronic migraine. We estimated that 133 patients (95% CI, 27 to 239) per 1,000 treated would experience improvement in quality of life measured using the SGIC instrument (Subject’s Global Impression of Change). We estimated that 72 patients (95% CI, 7 to 137) per 1,000 treated experienced reduction in migraine related disability.147 Improvement in disability scale score was large and clinically important.100
Prevention of Episodic Migraine
Antiepileptics
Topiramate
Individual RCTs and two pooled analyses of individual patient data from RCTs examined efficacy of topiramate versus placebo for migraine prevention in adults (Appendix Table D15). Most trials were funded by industry (Appendix Table D16). All trials were double blind and most had low risk of bias (Appendix Table D17).
Topiramate was not better than placebo in achieving complete cessation of migraine (Table 9).84–86 Topiramate in doses of 50, 100, or 200 mg/day was better than placebo in reducing monthly migraine frequency by ≥50 percent (Table 10 and Appendix Table D18). The results were consistent across the studies and robust regardless of pooling methods (Appendix Table D19). Topiramate was also better than placebo in reducing monthly migraine days by ≥50 percent (Table 10). Topiramate tended to reduce treatment discontinuation due to lack of efficacy with borderline statistical significance in pooled analyses (pooled absolute risk difference −0.04 95% CI −0.07 to 0).84,85,96,99,146,148–150
Topiramate, 100 mg/day, decreased the absolute number of migraine days by 5 days/month in pooled analyses of RCTs (Appendix Table D20). The reduction in migraine severity scores was inconsistent across the studies (Appendix Table D21). Individual RCTs demonstrated significant improvement in quality of life as measured by scores on the Headache Impact Test,149 Migraine Specific Questionnaire,151 and Migraine Disability Assessment100 (Appendix Table D22). Topiramate was better than placebo in improving general health status in a previously published pooled analysis of individual patient data from RCTs (Appendix Table D23).152 Medical Outcome Study Short Form 36 (SF-36) scores improved by more than 200 percent for self-reported vitality and more than 100 percent for pain and general health (Appendix Table D23).152
Topiramate was better than placebo in reducing use of acute drugs (Appendix Table D24). Most individual RCTs demonstrated a small but significant reduction in the number of medications taken or in the reduction of days when drugs for acute attacks were needed (Appendix Table D24).
Divalproex
Three RCTs examined the efficacy of divalproex for migraine prevention in adults (Appendix Table D25).101–103 All three RCTs were funded by industry (Appendix Table D26) and all were double blind (Appendix Table D27).
Divalproex was better than placebo in reducing monthly migraine frequency by ≥50 percent (Table 10 and Appendix Table D28).101,102 A larger dose of divalproex (1500 mg/day) was effective in achieving a ≥50 percent reduction in migraine-related effects, including impairment of usual activities, need for symptomatic medication, and nausea, vomiting, phonophobia, or photophobia (Appendix Table D29).103 Evidence was low-strength due to imprecise treatment effects.103
Valproate
Small RCTs examined the efficacy of valproate for migraine prevention in adults (Appendix Table D25).120,153 The trials were double blind and had medium risk of bias because the investigators did not use planned intention-to-treat principles (Appendix Table D27).
Valproate was better than placebo in reducing monthly migraine frequency by ≥50 percent (Table 12).120 We estimated that 256 patients per 1,000 treated (95% CI, 77 to 435) would experience clinically important reduction in migraine attacks attributable to valproate.120 Valproate decreased the frequency of migraine attacks and severe attacks,153 duration of attacks,153 and the use of drugs for acute attacks120 (Appendix Table D30).
Beta Blockers
Propranolol
Most RCTs that examined the efficacy of propranolol versus placebo for migraine prevention in adults (Appendix Table D31) failed to report funding sources (Appendix Table D32). All trials were double blind but did not analyze the data according to planned intention-to-treat principles (Appendix Table D33).
Propranolol was better than placebo in reducing migraine monthly frequency by ≥50 percent (Table 10 and Appendix Table D34). The preventive effects of propranolol were consistent across the studies (Appendix Table D35). Propranolol caused a small but significant decrease in the absolute number of monthly migraine attacks (mean difference −1, 95% CI, −2 to −0.3).104, 105, 154,155 A single RCT demonstrated that propranolol decreased use of drugs for acute attacks, both analgesics (mean difference −0.3, 95% CI, −0.4 to −0.1 doses per patient day) and the acute drug ergotamine (mean difference −0.1, 95% CI, −0.3 to −0.1 doses per patient day).156
Timolol
Timolol was not better than placebo in achieving complete migraine cessation (Table 9).108 Timolol was better than placebo in reducing migraine monthly frequency by ≥50 percent (Table 10 and Appendix Table D36).104,107,109 Evidence was low-strength due to medium risk of bias and estimate imprecision (Appendix Table D37) Timolol also decreased absolute number of migraine attacks and severity of headaches (Appendix Table D38).
Off-Label Drugs
Off-Label Antiepileptic Drugs
Most RCTs that examined six off-label antiepileptic drugs: acetazolamide, gabapentin, vigabatrin, oxcarbazepine, carbamazepin, and lamotrigine (Appendix Table D39) were sponsored by industry (Appendix Table D40), and all were double blind (Appendix Table D41).
Gabapentin was not better than placebo in achieving complete cessation of migraine attacks (Table 9).87 Gabapentin was, however, better than placebo in reducing monthly migraine attacks by ≥50 percent (Table 11).87,110,111 Individual RCTs found that carbamazepin118 but not oxcarbazepine119 and acetazolamide117 were better than placebo in preventing migraine attacks (Table 11).
In addition to off-label antiepileptic drugs examined in RCTs, pregabalin was examined in one open-label uncontrolled trial.157 Pregabalin was associated with a significant decrease from baseline in headache frequency and severity and with global improvement defined as ≥50 in visual analog scale (VAS) score in 40 percent of patients.157
Beta Blockers
Most RCTs that examined the effects of off-label beta blockers versus placebo for migraine prevention in adults (Appendix Table D42) failed to report funding and conflict of interest (Appendix Table D43). All trials were double blind with medium risk of bias because the investigators did not use planned intention-to-treat principles (Appendix Table D44).
Metoprolol
Metoprolol was better than placebo in improving patient perception of marked reduction in migraine attacks (Appendix Tables D45).113,114 Pooled analysis found a significant increase in the likelihood of a clinical response (Appendix Table D46)113,114 but no effect on absolute number of migraine attacks (Appendix Table D47).113,114,158
Metoprolol reduced severity of migraine attacks in a single RCT (Appendix Table D48).114 Regarding use of drugs for acute attacks, evidence with metoprolol was mixed; one trial reported reduced use of such drugs and a second reported increased use of analgesics (Appendix Table D47).113,114
Atenolol
Atenolol was better than placebo in reducing monthly migraine attacks by ≥50 percent (Table 12 and Appendix Table D46).159 Atenolol significantly reduced use of ergotamine drugs in a single RCT (Appendix Table D46).159
Nadolol
Nadolol was better than placebo in reducing monthly migraine attacks by ≥50 percent (Table 12).124 In a single RCT, nadolol improved perceived relief in frequency, intensity, and severity of migraine attacks (Appendix Table D46).124
Alprenolol
Alprenolol was not better than placebo in achieving perceived treatment success (Table 12 and Appendix Table D46).122 Alprenolol did not reduce the absolute number of monthly migraine attacks or Headache Index scores (Appendix Table D47).122
Pindolol
Pindolol was not better than placebo in reducing headache indices by ≥50 percent (Appendix Table D48).160 Pindolol did not reduce the absolute number of monthly migraine attacks or Headache Index scores (Appendix Table D47).160
Antidepressants
Most RCTs that examined the effectiveness of off-label antidepressants for migraine prevention in adults (Appendix Table D49) were sponsored by industry (Appendix Table D50). Most trials were double blind with medium risk of bias (Appendix Table D51).
Amitriptyline
Amitriptyline was better than placebo in reducing monthly migraine attacks by ≥75 percent (Table 12).126 RCTs demonstrated inconsistent improvement in migraine days and intensity.126,161
Fluoxetine
Fluoxetine was not better than placebo in achieving an excellent self-reported clinical response.162 Improvement in pain indexes was inconsistent in RCTs.162–164
Venlafaxine
Venlafaxine in a dose of 150 but not 75 mg/day was better than placebo in achieving an excellent self-reported clinical response.165
Femoxetine
Femoxetine was not better than placebo in achieving patient satisfaction with treatment effect or migraine frequency and severity.166–168
Mianserin
Mianserin was not better than placebo in improving migraine index or reducing migraine frequency.169
Cortical Spreading Depression Inhibitor
Calcium Channel Antagonists
Most RCTs that examined calcium channel blockers for migraine prevention in adults (Appendix Table D52) were sponsored by industry and failed to disclose conflict of interest (Appendix Table D53). All trials were double blind, with medium risk of bias (Appendix Table D54).
Nimodipine
Nimodipine was better than placebo in complete cessation of migraine attacks (Table 9)89 Nimodipine was better than placebo in reducing monthly migraine attacks by ≥50 percent (Table 11).89,112
Nicardipine
Nicardipine was better than placebo in reducing migraine intensity and absolute number of migraine attacks.170
Angiotensin Converting Enzyme Inhibitors
Two RCTs examined the effects of ACE inhibitors for migraine prevention in adults (Appendix Table D55).88,128 One industry-funded RCT examined lisinopril (Appendix Table D56).128 One RCT of captopril reported neither funding source nor conflict of interest.88 Both trials were double blind with low risk of bias (Appendix Table D57).
Captopril
Captopril was examined in one small RCT that enrolled adults with comorbid hypertension and depressive symptoms for whom drugs had previously failed to prevent migraines.88 Captopril was better than placebo in achieving complete cessation of migraine (Table 9) and improvement in Headache Index scores by more than 60 percent.88 The effect was large. We estimated that 667 patients per 1,000 treated experienced no migraine (95% CI, 388 to 946).88 Captopril was also better than placebo in reducing depression symptoms.88
Lisinopril
Lisinopril was better than placebo in reducing migraine days and severity of symptoms in a single RCTs of 60 adults with episodic migraine.128 Lisinopril also reduced the absolute number of migraine days and body pain measured with SF-36 but did not decrease use of drugs for acute attacks.128
Angiotensin II Receptor Antagonists
Two RCTs examined the effects of angiotensin II receptor antagonists for migraine prevention in adults (Appendix Table D55).129,173 Both trials were funded by industry and reported conflict of interest (Appendix Table D56).129,173 Both trials were double blind (Appendix Table D57).
Antiadrenergics
Clonidine
Most RCTs that examined clonidine for its effects on migraine prevention in adults (Appendix Table D58) failed to report funding and conflict of interest (Appendix Table D59). Most trials were double blind but did not use intention-to-treat principles (Appendix Table D60).
Clonidine was better than placebo in ≥50 percent reduction in headache index174 but not in increasing the number of patients considered better according to self-reported global assessment.175 Clonidine also failed to achieve clinically noticeable reduction in migraine frequency.176 Clonidine was better than placebo in reducing migraine duration177 and use of drugs for acute attacks.178
Guanfacine
Guanfacine was better than placebo in reducing monthly migraine days and migraine days with nausea or vomiting in one small RCT.179
Ergot Alkaloids
All RCTs that examined effectiveness of ergot alkaloids for migraine prevention in adults (Appendix Table D61) failed to report funding and conflict of interest (Appendix Table D62). Most trials were double blind with medium risk of bias (Appendix Table D63).
Leukotriene Receptor Antagonists
Montelukast
Montelukast was not better than placebo in reducing monthly migraine attacks by ≥50 percent.136
Nonsteroid Anti-Inflammatory Drugs
Individual RCTs demonstrated that aspirin, flurbiprofen, rofecoxib, and tolfenamic acid were better than placebo in reducing migraine frequency by ≥50 percent (Table 12).
Antipsychotic Drugs
Published RCTs did not examine antipsychotic drugs for migraine prevention. Quetiapine was examined in one uncontrolled trial of refractory migraine, defined as migraine that was previously unresponsive to the combination of atenolol, nortriptyline, and flunarizine.180 Adult patients with <15 days of headache per month who were not overusing drugs for acute attacks were treated with quetiapine (75mg/day) for 10 weeks. Reduction in migraine frequency by ≥50 percent was achieved in 65 percent of the patients.180 Patients also experienced a significant reduction in migraine days (from 10.2 to 6.2 per month), and use of drugs for acute attacks (from 2.3 to 1.2 days/week).180
Antidementia Drugs
Published RCTs did not examine antidementia drugs. Retrospective review of case series and case reports demonstrated that with memantine treatment, 60 percent of the patients experienced ≥50 percent reduction in monthly migraine frequency, and 80 percent experienced a significant reduction in frequency of aura.181,182
KQ1b. How do preventive pharmacologic treatments affect patient-centered and intermediate outcomes when compared with active pharmacologic treatments?
Individual RCTs provided low-strength direct evidence about the comparative effectiveness of drugs and demonstrated few significant differences. Indirect adjusted analysis demonstrated no differences between approved drugs and greater odds of clinical response with the angiotensin II antagonist candesartan.
Exploratory network Bayesian meta-analyses demonstrated that approved drugs were similarly better than placebo. Among off-label drug classes; however, angiotensin inhibiting drugs demonstrated the largest significant odds of reducing monthly migraine by ≥50 percent.
Approved Drugs
Muscle Relaxants for Chronic Migraine
Onabotulinumtoxin A
Five RCTs of 350 adults examined comparative effectiveness of onabotulinumtoxin A versus other drugs for migraine prevention (Appendix Table D64). Trials enrolled an average of 70±18 patients ages 18 to 65. Subjects experienced 12 to 24 monthly migraine days. Women made up 91 percent of enrollees. Trials were funded by industry183,184 or grants,185 with most investigators disclosing conflict of interest (Appendix Table D65). All RCTs but one185 were double blind, with medium or high risk of bias due to inadequacy of randomization or unplanned intention-to-treat analyses (Appendix Table D66). The trials often concluded that both active treatments were successful based on statistically significant reduction from baseline in absolute number of migraine days or hours.
We focus on differences in outcomes at the end of the treatment with active and control drugs.
Comparative effectiveness of onabotulinumtoxin A versus topiramate was examined in two RCTs that found no significant differences in likelihood of migraine prevention or improvement in migraine disability assessment (Appendix Table D67).183,186 Physicians found marked improvement in migraine frequency more often with topiramate than onabotulinumtoxin A (ARD 0.33, 95% CI, 0.10 to 0.57) (Appendix Table D67).183 Absolute scores on the Headache Impact Test were significantly better with topiramate than onabotulinumtoxin A;183 however, use of drugs for acute attacks did not differ between the two.183
A single RCT examined the comparative effectiveness of onabotulinumtoxin A versus divalproex sodium and found no differences in migraine prevention with two drugs (Appendix Table D68).142 Neither did the two drugs differ for absolute number of migraine days or changes in scores from baseline in the migraine Disability Assessment Scores and/or Headache Impact Test.142
A single RCT examined the comparative effectiveness of onabotulinumtoxin A versus amitriptyline and found no differences in migraine prevention with the two drugs (Appendix Table D69).185 Evidence was insufficient due to a high risk of bias in this individual RCT.185
Beta Blockers for Chronic Migraine
The National Institute of Neurological Disorders and Stroke Clinical Research Collaboration trial demonstrated no benefits from combined propranolol and topiramate treatment on migraine prevention in adults with chronic migraine for whom previous topiramate monotherapy had failed.187 Propranolol combined with the antidepressant nortriptyline was no better than propranolol alone or nortriptyline in reducing the number of days with headache by ≥50 percent.188
Antiepileptics for Episodic Migraine
Topiramate
Nine RCTs of 872 adults examined the comparative effectiveness of topiramate and other drugs for migraine prevention (Appendix Table D70). Most trials did not report funding source or conflict of interest (Appendix Table D71). All trials but one were double blind with low or medium risk of bias (Appendix Table D72).
Individual RCTs provided low-strength evidence that topiramate was more effective than amitriptyline in reducing monthly headache days by ≥50 percent with no differences in monthly migraine days (Table 13).189 Topiramate was more effective than lamotrigine in reducing monthly headache intensity by ≥50 percent.99 Differences were small (less than 20 percent absolute risk difference) but statistically significant (Appendix Table D73). Decrease in headache frequency by ≥50 percent did not differ between topiramate and zonasamide,190 valproate,191 levetiracetam,192 or lamotrigine.99 Topiramate was more effective than propranolol in reducing absolute migraine frequency, duration, and intensity.193
Table 13
Comparative effectiveness of approved and off-label drugs on migraine prevention (50% or more reduction) in adults with episodic migraine, results from individual head-to-head randomized controlled clinical trials.
Beta Blockers for Episodic Migraine
Propranolol
Most RCTs that examined the comparative effectiveness of propranolol for migraine prevention in adults (Appendix Table D74) failed to report funding (Appendix Table D75). Most trials were double blind but did not analyze the data according to planned intention-to-treat principles (Appendix Table D76). Few trials met pooling criteria (Table 14).
Table 14
Comparative effectiveness of drugs for migraine prevention in adults with episodic migraine, direct evidence from head-to-head randomized controlled clinical trials (pooled with random effects model).
Propranolol Versus Topiramate
The likelihood of ≥50 percent reduction in monthly migraine frequency did not differ between topiramate and propranolol (Table 13 and Appendix Table D35).105 Topiramate was more effective than propranolol in reducing absolute migraine frequency, duration, and intensity.193 Use of drugs for acute attacks did not differ between the two drugs.105
Propranolol Versus Timolol
The likelihood of ≥50 percent reduction in monthly migraine frequency did not differ between propranolol and timolol (Table 14).104,107
Propranolol Versus Metoprolol
The likelihood of ≥50 percent reduction of the sum of severity scores or clinically important reduction in migraine days did not differ between propranolol and metoprolol (Table 14).194,195
Propranolol Versus Nifedipine
Propranolol was more effective than nifedipine in reducing monthly migraine frequency by ≥50 percent (Table 14).195,196
Propranolol Versus Clonidine
The likelihood of ≥50 percent reduction in migraine days did not differ between propranolol and clonidine (Table 13).197
Propranolol Versus Nadolol
Nadolol, 160 mg/day, was more effective than propranolol in achieving a reduction of ≥50 percent in migraine frequency, duration, and intensity (Table 13).198 Differences between a lower dose of nadolol (80 mg/day) and propranolol (160 mg/day) were not significant.198,199
Propranolol Versus Antidepressants
The likelihood of ≥50 percent reduction in monthly migraine attacks did not differ between propranolol and amitriptyline200 nortriptyline,188 or femoxetine.201 The likelihood of ≥50 percent reduction in the number of migraine days did not differ between a combined therapy using both drugs and propranolol alone.188
Off-Label Drugs
Off-Label Beta Blockers
RCTs that examined comparative effectiveness of off-label beta blockers for migraine prevention in adults (Appendix Table D77) failed to report funding and conflict of interest (Appendix Table D78). All trials were double blind (Appendix Table D79). All RCTs examined unique drug comparisons except two RCTs that compared the effects of metoprolol and aspirin.
Metoprolol Versus Aspirin
In pooled analyses, metoprolol and aspirin resulted in similar rates of ≥50 percent reduction in monthly migraine attacks (Table 14).202,203 Individual RCTs reported that metoprolol was more effective than aspirin, 300 mg/day,202 but less effective than aspirin, 1,500 mg/day.203
Metoprolol Versus Nifedipine
Metoprolol was more effective than nifedipine in reducing monthly migraine attacks by ≥50 percent (Table 13).195
Metoprolol Versus Bisoprolol (Appendix Table D80)
Metoprolol and bisoprolol did not differ for reduction in monthly migraine attacks by ≥50 percent nor absolute number of migraine days (Appendix Table D81).204,205
Metoprolol Versus Nebivolol (Appendix Table D80)
Reduction in monthly migraine attacks by ≥50 percent did not differ between metoprolol and nebivolol.206 Neither migraine-related disability, use of drugs for acute attacks (Appendix Table D82), nor quality of life (Appendix Table D80) differed between metoprolol and nebivolol.206
Metoprolol Versus Clonidine
Reduction in monthly migraine attacks by ≥50 percent did not differ between metoprolol and clonidine.207 However, more patients noticed a reduction in migraine days with metoprolol than clonidine (Appendix Table D82).207
Antidepressants
Individual RCTs found no differences in the comparative effectiveness of antidepressants for migraine prevention. The likelihood of reducing monthly migraine attacks by ≥50 percent did not differ between femoxetine and propranolol, nor did the duration or intensity of attacks or use of acute drugs differ.201 Fluoxetine combined with amitriptyline versus amitriptyline alone resulted in similar migraine frequency and severity.208 Fluvoxamine versus amitriptyline resulted in similar migraine frequency and severity.209 Venlafaxine versus amitriptyline resulted in similar migraine frequency and severity.210
Indirect Evidence of Comparative Effectiveness of Preventive Drugs for Episodic Migraine
Among all included RCTs, 97 percent examined the outcome of clinically important reduction in migraine frequency by ≥50 percent. We found no consistent differences in baseline patient characteristics in RCTs that examined the efficacy of various drugs for migraine prevention. We conducted exploratory Bayesian network meta-analysis (Appendix Table D83) and indirect adjusted analysis of such drugs (Appendix Table D84). We found no differences among approved drugs (Table 15). Approved drugs were more effective than off-label drugs except for the angiotensin II receptor blocker candesartan, which was more effective than topiramate, divalproex, and propranolol (Table 16). Exploratory Bayesian network meta-analysis demonstrated that the approved drugs topiramate, divalproex, and propranolol, and off-label drug classes except ergot alkaloids were better than placebo (Figure 3). The strength of the association was the largest with angiotensin inhibiting drugs (Table 17).
Table 15
Indirect adjusted comparative effectiveness of clinical response in RCTs of approved drugs for the prophylaxis of episodic migraine.
Table 16
Indirect adjusted comparative effectiveness of clinical response in RCTs of approved drugs versus off-label drugs for the prophylaxis of episodic migraine.
Table 17
Odds ratio of clinical response with preventive drugs, results from exploratory Bayesian network meta-analysis.
Next, we analyzed the comparative effectiveness of nine treatments including propranolol, timolol, metoprolol, all other off-label beta blockers (atenolol, nadolol, pindolol, bisoprolol, or nebivolol), all off-label antidepressants, all approved and off-label antiepileptics, ACE inhibitors, or angiotensin II antagonists, and all other off-label drugs. This analysis clearly demonstrated that angiotensin inhibiting drugs were more effective than all other treatments. Propranolol, timolol, metoprolol, and all other off-label beta blockers resulted in significantly greater odds of migraine prevention than antiepileptics, antidepressants, and other off-label drugs.
KQ1c. How do preventive pharmacologic treatments affect patient-centered and intermediate outcomes when compared with active nonpharmacologic treatments?
Antiepileptics
Topiramate Versus Exercise or Relaxation
The likelihood of ≥50 percent reduction in monthly migraine frequency did not differ between topiramate and aerobic exercise or common forms of relaxation, breathing, and stress-management techniques.213 Migraine days, pain intensity, quality of life, or acute drug use did not differ between topiramate and aerobic exercise or relaxation.213
Beta Blockers
Propranolol Versus Biofeedback
The likelihood of a reduction in monthly migraine frequency of ≥25 percent did not differ between propranolol and diaphragmatic breathing and systematic relaxation that was assisted by biofeedback and also practiced at home.214
Antidepressants
Amitriptyline Versus Spinal Manipulation
Amitriptyline was more effective than spinal manipulation for reducing monthly migraine attacks during the trial but less effective during post-treatment followup period.215 Evidence was low-strength due to risk of bias and imprecision (Appendix Table D85). Evidence from a single high-risk-of-bias RCT was insufficient to conclude the comparative effectiveness of amitriptyline versus biofeedback.216
KQ1d. How do preventive pharmacologic treatments combined with nondrug treatments affect patient-centered and intermediate outcomes when compared with pharmacologic treatments alone?
Five RCTs compared the effectiveness of drugs combined with nondrug treatments with placebo or pharmacologic treatments alone (Appendix Table D86). Most trials were funded by nonprofit grants (Appendix Table D87). Risk of bias was low in one trial, medium in two, and high in two (Appendix Table D88).
Beta Blockers
Behavioral migraine management and relaxation combined with propranolol (maximum dose 240 mg/day) or nadolol (maximum dose 120 mg/day) was more effective than placebo in reducing monthly migraine frequency by ≥50 percent (Appendix Table D89). However, effects of the combined therapy did not differ from the effects of drugs alone.217 Evidence of effectiveness and safety was low due to imprecise estimates from a single RCT (Appendix Table D90).217 We estimated that 387 adults per 1,000 treated would experience a reduction in migraine frequency by ≥50 percent (95% CI, 157 to 618) with combined therapy (Table 18).217
Table 18
Comparative effectiveness of beta blockers combined with behavioral therapy for episodic migraine prevention in adults, results from individual low risk of bias randomized controlled clinical trial.
Propranolol (240 mg/day) or nadolol (120 mg/day) combined with behavioral therapy (orientation plus relaxation training, migraine warning signs and triggers, effectively using migraine medication, reducing impact of migraines, stress management or biofeedback training, and migraine management plan) was more effective than placebo in improving self-efficacy (Appendix Table D91).218
Evidence was insufficient from a single high-risk-of-bias RCT that compared the effectiveness of propranolol combined with biofeedback and propranolol alone for migraine prevention in adults.216
Antidepressants
Amitriptyline Combined With Spinal Manipulation Versus Amitriptyline Alone or Spinal Manipulation Alone (Table 19)215
Table 19
Comparative effectiveness of antidepressant amitriptyline and spinal manipulation for episodic migraine prevention in adults, individual medium risk of bias randomized controlled clinical trial.
Spinal manipulation was more effective than combined treatment in reducing Headache Index scores.215 Combined treatment was not more effective than amitriptyline alone in improving general health status or reducing use of drugs for acute attacks (Appendix Table D92).215 Evidence from a single high-risk-of-bias RCT was insufficient to conclude comparative effectiveness between amitriptyline combined with biofeedback and the drug alone.216
KQ1e1. How might dosing regimens or duration of treatments influence the effects of the treatments on patient-centered outcomes?
Muscle Relaxants
Onabotulinumtoxin A
Dose Response Migraine Prevention With Onabotulinumtoxin A
Higher doses of onabotulinumtoxin A resulted in a greater decrease in absolute migraine frequency according to the BoNTA-024-026-036 Study Group in adults with chronic migraine.219 Higher doses of onabotulinumtoxin A resulted in less frequent use and overuse of acute pain medications at 1 and 3 months of followup according to the BoNTA-039 Study Group (Appendix Table D93).138 However, neither patients nor investigators found differences in global assessment of improvement with higher doses of onabotulinumtoxin A (Appendix Table D94).139
Higher doses of abobotulinumtoxin A did not increase the rates of positive global assessment of the treatment effect in the Dysport® In Migraine Without Aura Prophylaxis trial.139 Higher doses of abobotulinumtoxin A did not reduce migraine duration or intensity139 or depression scores.144
Antiepileptics
Topiramate
Increase in topiramate dose from 50 to 100 mg/day resulted in a higher response rate (≥50 percent reduction in monthly migraine frequency) without additional benefit from increasing the dose to 200 mg/day (Appendix Table D95). Higher topiramate doses (50 to 100 mg) resulted in significant migraine prevention of ≥50 percent in one patient for every six treated (Table 20).
Table 20
Dose response reduction in migraine attacks by ≥50% from baseline with topiramate in adults.
Divalproex
Higher doses of divalproex did not result in a greater likelihood of clinically important migraine frequency reduction (Appendix Table D96).103
Beta Blockers
Off-Label Beta Blockers
Individual RCTs examined dose response effects with pindolol,224 nadolol,225,226 and bisoprolol.227
Pindolol
Pindolol, 15 mg/day, was more effective than 7.5 mg in reducing migraine days and duration.224
Nadolol
Nadolol, 160 to 240 mg/day, was more effective than 80 mg/day in reducing migraine frequency and severity.225,226
Bisoprolol
Bisoprolol, 10 mg/day, was more effective than 5 mg/day in reducing migraine duration but not frequency.227
KQ1e2. How might approaches to drug management (such as patient-care teams, integrated care, coordinated care, patient education, drug surveillance, or interactive drug monitoring) influence results?
Six RCTs of 3,825 adults examined the effectiveness of drug management for migraine prevention in adults (Table 21 and Appendix Table D97). Most trials were sponsored by nonprofit organizations (Appendix Table D98). Half of the trials had low risk of bias, and the other half had medium risk of bias due to inadequacy of randomization (Appendix Table D99). Four RCTs examined the effectiveness of multidisciplinary migraine management programs and two examined the effectiveness of pharmacist-led drug management (Appendix Table D100).
Table 21
Prevention of migraine with drug management programs, results from individual randomized controlled clinical trials.
Multidisciplinary Intervention Versus Standard Care
The community-based multidisciplinary intervention included intake by a neurologist, physical therapist, and a psychologist, with group-supervised exercise therapy sessions, massage therapy sessions, and group lectures with a dietitian231 (Appendix Table D100). Adherence did not differ between the multidisciplinary intervention and standard medical care with the patient’s primary physician (Appendix Table D101).231 The multidisciplinary intervention was more effective in improving quality of life and reducing migraine-related disability (Appendix Table D102).231 We found no statistically significant changes in medication use or work status.231
Migraine Management Program Versus Usual Care
A multidisciplinary migraine management program was administered by a midlevel provider (e.g., nurse practitioner or physician assistant) with expertise in migraine evaluation and management.232 The program included an educational session in which patients received materials that described: migraine types and etiologies, triggers, sleep hygiene, pharmacologic treatment, and relaxation techniques.232 Patients in the control group continued with their current clinician, without access to the migraine management program. Fewer adults had migraine-related disability at 6 months of followup with the migraine management program (Appendix Table D103).232 We estimated that 196 adults per 1,000 treated (95% CI, 125 to 258) would have no migraine-related disability with the migraine management intervention.232 The program was also more effective than usual care in improving quality of life and treatment satisfaction (Appendix Table D104).232
Cognitive Behavioral Minimal Contact Program Versus Usual Care
The cognitive-behavioral minimal contact program consisted of five sessions that provided information about migraine and progressive muscle relaxation, acute and prophylactic migraine medications, and triggers for medication overuse (e.g., availability of drugs, fear of attack and loss of social functioning, and stress level in private and professional life). Participants also established individualized goals for future drug intake and improving quality of life.233 The cognitive-behavioral minimal contact program did not decrease migraine frequency or duration of migraine related disability (Appendix Table D105),233 nor did it improve engagement in social activity, self-management of pain, migraine-related anxiety, or depression.233 However, patient satisfaction with treatment was significantly greater with the cognitive-behavioral minimal contact program than with usual care.233
Headache School Versus Usual Care
Headache school involved a standardized curriculum of didactic instructions regarding migraine biogenesis and management. It consisted of classes taught by neurologists and migraine sufferers who previously had undergone intensive classroom training. Headache school classes focused mostly on acute preventive drug treatments.234 Patients in the control group received routine drug management.234 Patients who attended headache school less often overused drugs for acute attacks than patients receiving routine drug management (Appendix Table D106).234 Attending headache school also reduced migraine disability (Appendix Table D107).234
Pharmaceutical Care for Migraine Versus Standard Counseling
Pharmaceutical care intervention was defined as intensified structured counseling between patient and pharmacist and the use of drug databases. German pharmacists worked with patients individually to prioritize problems, define goals, and devise plans to work toward goals.235 Patients in the control group received standard counseling that included general information about benefits and possible adverse drug effects.235 Pharmaceutical care resulted in a statistically significant improvement from baseline in mental health and self-efficacy.236 However, the likelihood of complete migraine cessation did not differ between active and control interventions (Appendix Table D108)235 nor did the absolute number of migraine attacks or quality of life (Appendix Table D109).235
Intensive Pharmaceutical Care Campaign Versus Control Pharmacy
Danish pharmacists and pharmacy assistants provided the intervention according to the manual developed by the Danish College of Pharmacy Practice.236 The campaign targeted inappropriate use of triptans. Intervention pharmacy staff received information about migraine, detection of inappropriate triptan use and other drug-related problems, and techniques for establishing a private dialogue with patients.236 The campaign had no statistically significant impact on use of triptans (Appendix Table D110).236
Key Question 2. What are the comparative harms from pharmacologic treatments for preventing migraine attacks in adults?
We identified 15 RCTs and six nonrandomized studies that examined the safety of onabotulinumtoxin A for chronic migraine prevention in adults. We identified 159 RCTs of 18,134 adults that examined the safety of drugs for episodic migraine prevention in adults.
Among approved drugs, onabotulinumtoxin A, topiramate, and propranolol resulted in bothersome adverse effects leading to treatment discontinuation more often than placebo.
The association was dose responsive for topiramate. Larger doses of topiramate caused higher risk of anorexia, depression, paresthesia, and difficulty in memory leading to treatment withdrawal. Larger doses of topiramate caused higher risk dry mouth, paresthesia or fatigue, mood problems, nausea, and weight loss.
Individual RCTs showed that divalproex caused adverse effects that led to treatment discontinuation, including nausea, somnolence, tremor, vomiting, and asthenia.
Among other drugs, pooled analyses demonstrated that off-label antidepressant amitriptyline caused bothersome adverse effects leading to treatment discontinuation more often than placebo.
Limited low-strength direct comparative evidence from individual head-to-head RCTs suggested that treatment discontinuation due to adverse effects was less frequent with onabotulinumtoxin A than topiramate or amitriptyline. Individual unique RCTs provided low-strength direct evidence about adverse effects with specific drugs with no consistent pattern across available drug comparisons.
Indirect adjusted analyses demonstrated no differences in treatment discontinuation due to adverse effects with approved drugs or approved versus off-label drugs. Exploratory Bayesian network meta-analyses demonstrated that topiramate and off-label antiepileptics and antidepressants resulted in bothersome adverse effects leading to treatment discontinuation more often than placebo. According to network meta-analysis, off-label angiotensin inhibiting drugs and beta blockers were the safest treatment option for adults with episodic migraine.
KQ2a. What are the harms from preventive pharmacologic treatments when compared with placebo or no active treatment?
We identified 83 RCTs that compared adverse drug effects with placebo. Most studies failed to disclose conflict of interest by trial investigators (Appendix Table D111). The results from these 83 trials that were a subset of RCTs that examined benefits with drugs for episodic migraine prevention in adults were applicable to the target population (Appendix Table D112). Women made up an average of 78 percent of all enrollees. Mean age of the enrollees varied from 29 to 49 years. Patients had an average 5.5 monthly migraine attacks. The trials followed for an average 18 weeks to assess adverse effects (Appendix Table D113). Sample size averaged 116 adults (range 12 to 818). RCTs reporting harms were not necessarily powered to detect statistically significant differences in adverse effects.
We concluded medium risk of bias in 54 RCTs and low risk of bias in 22 RCTs (Appendix Table D114). Most studies were double blind. Nonrandomized studies with high risk of bias suggested that 10 to 20 percent of patients discontinued antiepileptic drug treatments at one year or longer of followup (Appendix Table D115).
We focused on treatment discontinuation due to any and specific adverse effects from pooled analyses (Table 22).
Table 22
Treatment discontinuation due to adverse effects with migraine preventive drugs in adults, evidence from meta-analyzed randomized controlled clinical trials.
Muscle Relaxants
Onabotulinumtoxin A
Fifteen RCTs examined the safety of botulinum toxin for chronic migraine prevention in adults including 13 RCTs of onabotulinumtoxin A and two RCTs of abobotulinumtoxin A (Appendix Table D8). Onabotulinumtoxin A resulted in adverse effects and treatment discontinuation due to adverse effects more often than placebo (Table 22). Pooled analyses demonstrated that per 1,000 patients treated, 155 experienced adverse effects and 26 discontinued treatments due to bothersome adverse effects (Table 23). The results were robust and remained significant with different methods of pooling (Appendix Tables D116 and D117). Abobotulinumtoxin A RCTs did not report treatment discontinuation due to adverse effects.139,144
Table 23
Adverse effect with onabotulinumtoxin A versus placebo for chronic migraine prevention in adults (magnitude of the effect and strength of evidence from randomized controlled clinical trials).
Among individual adverse effects, neck pain and muscle weakness were the most common (Table 23). Increase in risk of adverse effects with onabotulinumtoxin A was lower in trials with higher placebo rates of adverse effects (Table 24). Increase in risk of adverse effects was dose responsive (Appendix Table D118). Patients experienced eyelid edema with 50U of onabotulinumtoxin A more often than with 25U.219 Higher doses of 150 to 225U of onabotulinumtoxin A resulted in greater risk of blepharoptosis, muscle weakness, and neck rigidity (Appendix Table D118).
Table 24
Adverse effects with onabotulinumtoxin A versus placebo in adults with chronic migraine, meta-regression by study level factors (log of relative risk in randomized controlled clinical trials).
Abobotulinumtoxin A
Abobotulinumtoxin A RCTs reported increased risk of neck weakness in 109 patients per 1,000 treated (95% CI, 22 to 196).139,144 The rates of the total adverse effects were statistically higher with the increased dose of the drug (210U versus 80U).144 The rates of specific adverse effects did not differ between the active drug and placebo.139,144
Antiepileptics
Topiramate
Most RCTs that examined safety with topiramate versus placebo for episodic migraine prevention in adults (Appendix Table D119) were funded by industry and reported conflict of interest by principal investigators (Appendix Table D120). All trials were double blind (Appendix Table D121).
Patients stopped taking topiramate more often than placebo because of intolerable adverse effects including fatigue, paresthesia, and taste perversion (Table 25). Topiramate in doses of 100 and 200 mg/day (but not 50 mg/day) resulted in treatment discontinuation due to adverse effects more often than placebo (Appendix Table D122). Compared with placebo, topiramate more often resulted in bothersome taste perversion, paresthesia, and fatigue leading to withdrawal (Appendix Table D123).
Table 25
Treatment discontinuation due to adverse effects with topiramate versus placebo in adults, pooled with random effects results from randomized controlled clinical trials.
Pooled estimates were consistent with imprecision that decreased strength of evidence. Per 1,000 treated, topiramate resulted in bothersome adverse effects leading to treatment discontinuation in 36 (with 100 mg/day) or 146 (with 200 mg/day) patients. Published pooled analysis of individual patient data demonstrated topiramate discontinuation due to anorexia, anxiety, depression, hypoesthesia, and nausea (Appendix Table D124).237 Some adverse effects leading to treatment discontinuation were reported in individual RCTs that failed to show statistically significant increase in risk of specific harms with topiramate (Appendix Table D125).
Topiramate increased risk of specific adverse effects. Individual RCTs reported small numbers of events. Pooled analyses demonstrated a statistically significant increase in risk of any adverse effect, paresthesia, cognitive difficulties, diarrhea, dry mouth, fatigue, nausea, taste alteration or perversion, and weight loss (Appendix Table D126). Topiramate caused adverse effects in one patient for every eight treated. Taste alteration, weight loss, and paresthesia were the most common adverse effects (Table 26). Individual RCTs reported increased risk of severe anorexia and mood problems (Table D127).
Table 26
Adverse effects with topiramate in adults with migraine, significant results from pooled analysis of randomized controlled clinical trials.
Risk of adverse effects was dose responsive according to the published pooled analyses of individual patient data (Appendix Table D128).237 Larger doses of topiramate increased risk of anorexia, depression, paresthesia, and difficulty in memory leading to treatment withdrawal.237 Larger doses of topiramate increased risk of dry mouth, paresthesia or fatigue, mood problems, nausea, and weight loss.237
Divalproex
Adverse effects with divalproex versus placebo were examined in three RCTs that examined efficacy of divalproex for episodic migraine prevention in adults (Appendix Table D25). All three RCTs were funded by industry (Appendix Table D26) and all were double blind (Appendix Table D27).
Treatment discontinuation due to adverse effects did not differ with divalproex versus placebo (Table 22 and Appendix Table D122).101,102 Divalproex caused alopecia, asthenia, nausea, and tremor more often than placebo (Table 27). Strength of evidence was low because of risk of bias and imprecision of the treatment effects. Larger doses of divalproex did not increase risk of bothersome adverse effects leading to treatment discontinuation (Appendix Table D129).103 Larger doses of divalproex increased risk of nausea and tremor (Appendix Table D130).103
Table 27
Treatment discontinuation due to bothersome adverse effects and adverse effects with divalproex versus placebo for episodic migraine prevention in adults, results from randomized controlled clinical trials.
Valproate
Adverse effects of valproate were examined in two small double-blind RCTs of 75 adults that examined efficacy of valproate for episodic migraine prevention in adults (Appendix Tables D25–D27).
Treatment discontinuation due to adverse effects did not differ with valproate versus placebo (Table 22).153 Rates of combined adverse effects did not differ between valproate and placebo (Appendix Table D131).120,153
Beta Blockers
Propranolol
All RCTs that examined safety with propranolol versus placebo in adults with episodic migraine (Appendix Table D31) were double blind but did not analyze the data according to planned intention-to-treat principles (Appendix Table D33). Propranolol increased risk of bothersome adverse effects leading to treatment discontinuation more often than placebo (Table 22).106,238
Propranolol resulted in adverse effects more often than placebo (Appendix Table D132). Among individual adverse effects, propranolol more often than placebo resulted in diarrhea (pooled 89 attributable events per 1,000 treated; 95% CI, 14 to 164) and nausea (pooled 43 attributable events per 1,000 treated 95% CI, 9 to 77).
Timolol
Treatment discontinuation due to bothersome adverse effects did not differ with timolol and placebo in adults with episodic migraine (low-strength evidence from individual RCT) (Table 28). Timolol increased risk of overall adverse effects but not of any specific examined adverse effects more often than placebo (Appendix Tables D133 and D134).
Table 28
Treatment discontinuation due to adverse effects with propranolol or timolol for episodic migraine prevention in adults, results from randomized controlled clinical trials.
Off-Label Drugs
Antiepileptics
All RCTs that examined the safety of six off-label antiepileptic drugs for episodic migraine, including acetazolamide, gabapentin, vigabatrin, oxcarbazepine, carbamazepin, and lamotrigine (Appendix Table D39) were double blind (Appendix Table D41). Pooled analyses demonstrated no differences in treatment discontinuation due to adverse effects with gabapentin or lamotrigine versus placebo (Table 22 and Appendix Table D135) but increase in risk of the total adverse effects with gabapentin (Appendix Table D136). Antiepileptic drugs increased risk of the specific adverse effects as follows.
Acetazolamide
Acetazolamide caused paresthesia, drowsiness, memory impairment, malaise, and fasciculation more often than placebo in adults with episodic migraine (Appendix Table D137).117
Carbamazepin
Carbamazepin caused adverse effects that led to dose reductions more often than placebo in adults with episodic migraine. Specific adverse effects included vertigo and drowsiness. (Appendix Table D138).118
Gabapentin
Gabapentin caused somnolence and dizziness more often than placebo in adults with episodic migraine (Appendix Table D139)111; however, the validity of the results was questioned due to exclusion of patients from the analyses and biased tolerability conclusions.239
Lamotrigine
Treatment discontinuation due to the specific side effects, including rash, occurred more frequently with lamotrigine than placebo in adults with episodic migraine (Appendix Table D140).240 A fixed dose of 200 mg/day of lamotrigine caused skin rash more often than placebo. In contrast, a gradually escalated dose of lamotrigine starting with 25 mg/day did not cause skin rash.240
Oxcarbazepine
Oxcarbazepine caused adverse effects including fatigue, dizziness, and nausea more often than placebo in adults with episodic migraine (Appendix Table D141).119
Antidepressants
Pooled analyses demonstrated that amitriptyline but not femoxetine caused adverse effects leading to treatment discontinuation more often than placebo in adults with episodic migraine (Table 22). Amitriptyline increased the risk of dizziness, drowsiness, and constipation (Appendix Table D142). Femoxetine and fluoxetine increased the risk of any adverse effects (Appendix Table D142).
Cortical Spreading Depression Inhibitor
Individual RCTs demonstrated no differences between placebo and tonabersat in treatment discontinuation due to bothersome adverse effects in adults with episodic migraine.127
Beta Blockers
Atenolol
Treatment discontinuation due to bothersome adverse effects did not differ between atenolol and placebo in adults with episodic migraine (Appendix Table D143).123,159,241 Less than 1 percent of participants discontinued atenolol due to bothersome side effects (Appendix Table D144).123,159,241 Among all examined adverse effects, only rates of slight orthostatic dizziness during the first week of treatments were greater with atenolol than with placebo.
Bisoprolol
Treatment discontinuation due to bothersome adverse effects did not differ between bisoprolol and placebo in adults with episodic migraine.227 In fact, side effects occurred no more often from bisoprolol than from placebo (Appendix Table D144). A higher dose of bisoprolol did not result in greater rates of adverse effects or treatment discontinuation due to adverse effects.227 Bisoprolol, 10 mg/day, decreased heart rate when compared with 5 mg/day.227 Systolic and diastolic blood pressure did not differ with two doses of bisoprolol.227
Metoprolol
Treatment discontinuation due to bothersome adverse effects did not differ between metoprolol and placebo in adults with episodic migraine.114 Rates of total adverse effects were greater with metoprolol than with placebo in a single RCT.113 Metoprolol caused fatigue and sleep disturbances more often than placebo (Appendix Table D145).113
Nadolol
Treatment discontinuation due to bothersome adverse effects did not differ between nadolol and placebo in adults with episodic migraine.124 In fact, nadolol caused adverse effects no more often than placebo. An increased dose of nadolol did not result in greater rates of adverse effects.225,226
Ergot Alkaloids
In individual underpowered RCTs, treatment discontinuation due to bothersome adverse effects did not differ with placebo, lisuride, or methysergide in adults with episodic migraine.131,242
Angiotensin Converting Enzyme Inhibitors
Individual RCTs of adults with episodic migraine did not examine treatment discontinuation to bothersome adverse effects with lisinopril128 or captopril.88 Captopril caused adverse effects no more often than placebo.88 The rates of any adverse effects were greater with lisinopril than placebo; however, rates of the most common adverse effects with ACE inhibitors (coughing, fatigue, dizziness, or tendency to faint) did not differ between lisinopril and placebo.128
Angiotensin II Antagonists
Individual RCTs did not examine treatment discontinuation to bothersome adverse effects with candesartan129 or telmisartan in adults with episodic migraine.173 Neither drug caused any adverse effect more often than placebo.129,173
Calcium Channel Antagonists
Treatment discontinuation due to bothersome adverse effects did not differ between placebo and nifedipine,243 nimodipine,112,244 or verapamil in adults with episodic migraine.171
Compared with placebo, verapamil more often caused tolerable constipation that did not result in treatment discontinuation.171 Nifedipine resulted in adverse effects more often than placebo.243 Among individual adverse effects, nifedipine increased rates of headache, dizziness, and edema.243 Nimodipine increased rates of abdominal cramps but no other examined adverse effects.89
KQ2b. What are the harms from preventive pharmacologic treatments when compared with active pharmacologic treatments?
There was low-strength evidence from individual RCTs that examined comparative safety with migraine preventive drugs.
Muscle Relaxants
Onabotulinumtoxin A for Chronic Migraine
Comparative safety of onabotulinumtoxin A versus topiramate was examined in two RCTs that demonstrated better safety with onabotulinumtoxin A than topiramate (Appendix Table D146).183,186 Patients experienced depression or mood disturbance, weight loss, paresthesias, or cognitive deficits more often with topiramate (Appendix Table D146).183,186
A single RCT examined the comparative safety of onabotulinumtoxin A versus divalproex sodium and found a higher risk of ptosis with onabotulinumtoxin A (Appendix Table D147).184 In contrast, risk of fatigue, nausea, and total adverse effects was higher with divalproex (Appendix Table D147).
A single RCT examined the comparative safety of onabotulinumtoxin A versus amitriptyline and concluded better safety with onabotulinumtoxin A (Appendix Table D148).185 Patients experienced dry mouth, constipation, somnolence, and weight gain several times more often with amitriptyline than with onabotulinumtoxin A.185
Topiramate
Treatment discontinuation due to adverse effects did not differ between topiramate and amitriptyline in adults with episodic migraine (Table 22).189,247 Comparative safety of topiramate with other drugs was examined in individual RCTs. Treatment discontinuation due to any adverse effects did not differ between topiramate and zonasamide or valproate (Table 29). Treatment discontinuation due to specific adverse effects differed with topiramate and other drugs according to individual RCTs (Appendix Table D149). Somnolence or weight increase leading to withdrawal was less common with topiramate than amitriptyline (Table 29).189,247 Treatment discontinuation to treatment failure, however, did not differ between topiramate and amitriptyline or lamotrigine (Appendix Table D150).
Table 29
Treatment discontinuation due to any adverse effects with topiramate versus other drugs for episodic migraine prevention in adults.
Risk of specific adverse effects differed between topiramate and other drugs in individual RCTs in adults with episodic migraine (Appendix Table D151). Topiramate increased risk of weight loss when compared with amitriptyline,189 levetiracetam,192 and valproate248 (Appendix Table D151). Topiramate increased risk of paresthesia when compared with amitriptyline189,247 (Appendix Table D151). Risk of dry mouth and constipation was lower with topiramate than amitriptyline (Appendix Table D152).189,247 Individual RCTs demonstrated higher risk of headache with topiramate than amitriptyline (Appendix Table D153).
Comparative safety of topiramate combined with amitriptyline versus monotherapy was examined in one small RCT.247 Treatment discontinuation due to adverse effects did not differ between topiramate combined with amitriptyline and monotherapy.247 The risk of adverse effects was lower with combined therapy when compared with amitriptyline alone but not topiramate alone (Appendix Table D154).247
Beta Blockers for Episodic Migraine
Propranolol
Treatment discontinuation due to bothersome adverse effects did not differ between propranolol and aspirin (Table 28).249 Evidence of comparative safety with propranolol ergotamine intake was insufficient due to high risk of bias in individual RCT (Appendix Table D155).
Treatment discontinuation due to adverse effects did not differ between behavioral migraine management and propranolol (Appendix Table D156).217 Treatment discontinuation due to bothersome adverse effects did not differ between combined behavioral migraine management with propranolol versus propranolol alone.217 Combined therapy was more effective than propranolol alone in having self-efficacy and internal control over headache (Appendix Table D157).218
Off-Label Drugs for Episodic Migraine
Off-Label Beta Blockers
Metoprolol Versus Clonidine
Metoprolol resulted in treatment discontinuation due to bothersome adverse effects or treatment failure less often than clonidine (Table 30 and Appendix Table D158).207
Table 30
Comparative safety of beta blockers for episodic migraine prevention in adults, treatment discontinuation due to bothersome adverse effects in randomized controlled clinical trials.
Metoprolol Versus Bisoprolol
Treatment discontinuation due to adverse effects did not differ between the two drugs (Table 30 and Appendix Table D158)204 nor did rates of individual examined adverse effects differ between the drugs (Appendix Table D159).
Metoprolol Versus Nebivolol
Treatment discontinuation due to adverse effects did not differ between the two drugs (Table 30).206 Patients experienced moderate adverse effects, fatigue, and bradycarida more often with metoprolol than with nebivolol (Appendix Table D159).206
Metoprolol Versus Aspirin
Gastrointestinal side effects leading to withdrawal were more common with aspirin than metoprolol (Table 30 and Appendix Table D158).202 However, autonomic nervous system and psychiatric disorders were more common with metoprolol than aspirin (Appendix Table D159).203
Metoprolol Versus Clomipramine
Treatment discontinuation because of severe adverse reactions was more common with clomipramine than metoprolol (Table 30).250 Clomipramine caused insomnia and sweating more often than metoprolol (Appendix Table D159).250
Antidepressants
Clomipramine Versus Metoprolol
Clomipramine resulted in treatment discontinuation due to bothersome adverse effects more often than metoprolol (Table 30).250
Femoxetine Versus Propranolol
Treatment discontinuation due to bothersome adverse effects did not differ between femoxetine and propranolol.201
Amitriptyline Versus Spinal Manipulation
Treatment discontinuation due to adverse effects occurred less with spinal stimulation than with amitriptyline (Table 31).215 Strength of evidence was low due to risk of bias and imprecise estimate (Appendix Table D160).215
Table 31
Treatment adherence and discontinuation due to adverse effects with antidepressant amitriptyline and spinal manipulation for migraine prevention in adults, individual medium risk of bias randomized controlled clinical trial.
Treatment discontinuation due to adverse effects did not differ between combined treatment using spinal manipulation with amitriptyline and amitriptyline alone (Appendix Table D161).215
Ergot Alkaloids
A single RCT of 253 adults (low-strength evidence) found that treatment discontinuation due to adverse effects was less common with lisuride than with methysergide.212
Indirect Evidence of Comparative Safety of Drugs for Episodic Migraine Prevention in Adults
Bothersome adverse effects leading to treatment discontinuation were examined in 68 RCTs. Indirect adjusted analyses demonstrated no differences in treatment discontinuation due to adverse effects with approved drugs or approved versus off-label drugs (Appendix Table D162). Exploratory Bayesian network meta-analyses demonstrated that topiramate, off-label antiepileptics, and antidepressants resulted in bothersome adverse effects leading to treatment discontinuation more often than placebo (Figure 4). According to network meta-analysis, off-label angiotensin inhibiting drugs and beta blockers were the safest treatment option for adults with episodic migraine (Appendix Table D163)
KQ2c. How might approaches to drug management (such as patient-care teams, integrated care, coordinated care, patient education, drug surveillance, or interactive drug monitoring) influence results?
We found no studies that examined adverse effects with different approaches to drug management (such as patient-care teams, integrated care, coordinated care, patient education, drug surveillance, or interactive drug monitoring).
Key Question 3. Which patient characteristics predict the effectiveness and safety of pharmacologic treatments for preventing migraine attacks in adults?
Muscle Relaxants
Onabotulinumtoxin A for Chronic Migraine
Placebo Responders
Four RCTs examined the efficacy of onabotulinumtoxin A among placebo responders versus nonresponders.94,257–259 Onabotulinumtoxin A was better than placebo in preventing migraine attacks/month by ≥50 percent, regardless of placebo response, according to the BOTULINUM TOXIN CDH Study Group.94 Magnitude of the effect was slightly larger in placebo nonresponders (RR 2.2, 95% CI, 1.4 to 3.4) than in placebo responders (RR 1.6, 95% CI, 1.1 to 2.4).94 The European BoNTA Headache Study Group demonstrated no additional benefits from increasing onabotulinumtoxin A dose, regardless of placebo response.259 The number of migraine days did not differ by dose of onabotulinumtoxin A (75, 15, or 225U).259
Baseline Migraine Frequency
Onabotulinumtoxin A was more effective in patients with a higher mean baseline migraine frequency in a single RCT from the BOTULINUM TOXIN North American Episodic Migraine Study Group.257 Onabotulinumtoxin A decreased the likelihood of use of drugs for acute attacks in patients with more than 12 migraine days per month at baseline (RR 0.78, 95% CI, 0.66 to 0.92).257
Concurrent Prophylactic Medication Use
Onabotulinumtoxin A caused adverse effects more often than placebo (blepharoptosis, muscle weakness, and neck pain, regardless of concurrent prophylactic medication use) according to the BOTULINUM TOXIN CDH Study Group.258
Antiepileptics for Episodic Migraine
Topiramate
Presence of Aura
No trials directly compared drug effects in patients with and without aura. Several post hoc subgroup analysis of topiramate versus placebo trials provided conflicting evidence of the drug efficacy in respect to aura. Two publications suggested that topiramate was better than placebo in patients with aura. Post hoc subgroup analysis of one RCT found a statistically significant reduction in migraine frequency with topiramate versus placebo (−2.43 vs. −0.79 respectively, p value=0.02) only in subjects with aura.85 Post hoc subgroup analysis of the other RCTs found that in patients with aura, topiramate was better than placebo in reducing migraine frequency, number of migraine days, severity and duration of attacks, and photophobia.260 In contrast, however, post hoc analysis of the Prolonged Migraine Prevention found that topiramate efficacy was similar in patients with and without aura.261
Beta Blockers for Episodic Migraine
Propranolol
Prior Medication Use
Subgroup analysis in chronic migraine patients by prior topiramate use or overuse of the drugs for acute migraine was conducted in a single RCT.187 This study examined adding propranolol to topiramate treatment for chronic migraine subjects for whom topiramate monotherapy had failed.187 Propranolol with topiramate was no better than topiramate alone in reducing migraine frequency, regardless of patients’ prior drug histories.187 Quality of life score changes from baseline difference depend on prior topiramate use (Figure 5). Patients with prior stable topiramate use experienced worsening in quality of life with combined therapy versus improvement in quality of life with topiramate monotherapy. In contrast, patients without stable prior topiramate use experienced improvement in quality of life with combined therapy versus insignificant changes with topiramate monotherapy.187
Sex
Topiramate caused a complete cessation of migraine attacks and a reduction of monthly migraine attacks by 50 percent in women but not men according to one low-risk-of-bias RCT.84 Topiramate would cause a complete cessation of migraine attacks in 37 (95% CI, 8 to 67) and a reduction of monthly migraine attacks by 50 percent in 249 (95% CI, 178 to 320) per 1,000 treated women.84 However, both men and women experienced a reduction of monthly migraine 75 to 90 percent more often with topiramate than with placebo.84
Gabapentin for Episodic Migraine
Presence of Aura
Gabapentin reduced the frequency and intensity of migraine attacks significantly more than placebo, regardless of aura.262 Patients with aura experienced a slightly greater reduction in migraine frequency (mean difference −2.2, 95% CI, −2.7 to −1.7) than patients without aura (mean difference −1.6, 95% CI, −2.2 to −0.9). Patients with aura experienced a slightly greater reduction in migraine intensity (mean difference −0.83, 95%, CI, −1.12 to −0.54) than patients without aura (mean difference −0.42, 95% CI, −0.77 to −0.07).
Prior Medication Use
In a single, low-risk-of-bias RCT, gabapentin was not better than placebo in reducing acute drug use, regardless of prior use of triptans, opioids, or prescription or over-the-counter acute medications.87
Antidepressants for Episodic Migraine
Amitriptyline
Baseline Migraine Frequency
Amitriptyline was better than placebo in reducing monthly migraine but only in patients with baseline frequent and severe migraine (Appendix Table D164).125 Amitriptyline was better than placebo in reducing monthly migraine only in depressed patients whose baseline migraine was frequent and severe (Appendix Table D165).253
A higher dose of amitriptyline increased the odds of reducing monthly migraine by ≥50 percent in accordance with increased baseline migraine days (odds ratio 2.35, 95%, CI 1.45 to 3.8 for every additional day of baseline migraine) (Appendix Table D166).228
Selective Calcium Channel Blockers for Episodic Migraine
- Publication Bias
- Applicability
- Risk of Bias
- What is the efficacy and comparative effectiveness of pharmacologic treatments for preventing migraine attacks in adults?
- What are the comparative harms from pharmacologic treatments for preventing migraine attacks in adults?
- Which patient characteristics predict the effectiveness and safety of pharmacologic treatments for preventing migraine attacks in adults?
- Results - Migraine in Adults: Preventive Pharmacologic TreatmentsResults - Migraine in Adults: Preventive Pharmacologic Treatments
- Caenorhabditis elegans MATH domain-containing protein (math-13), partial mRNACaenorhabditis elegans MATH domain-containing protein (math-13), partial mRNAgi|1799133521|ref|NM_001377702.1|Nucleotide
- Peer Reviewers - Partial Breast Irradiation for Breast CancerPeer Reviewers - Partial Breast Irradiation for Breast Cancer
- Technical Expert Panel - Acute Treatments for Episodic MigraineTechnical Expert Panel - Acute Treatments for Episodic Migraine
- Preface - Emerging Approaches to Diagnosis and Treatment of Non–Muscle-Invasive ...Preface - Emerging Approaches to Diagnosis and Treatment of Non–Muscle-Invasive Bladder Cancer
Your browsing activity is empty.
Activity recording is turned off.
See more...