NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lin SY, Erekosima N, Suarez-Cuervo C, et al. Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Mar. (Comparative Effectiveness Reviews, No. 111.)
This publication is provided for historical reference only and the information may be out of date.

Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review [Internet].
Show detailsBackground
Allergic rhinitis is a widespread clinical problem, estimated to affect 20 to 40 percent of the population in the United States.1–5 Inhalant allergens, such as plant pollens, characteristically cause seasonal rhinoconjunctivitis and/or asthma; whereas, cat dander, cockroaches, or dust mite allergens may induce symptoms year-round, and are associated with perennial rhinitis and/or asthma. The prevalence of asthma in the United States is approximately 9 percent, and approximately 62 percent of individuals with asthma show evidence of also having atopy (i.e., one or more positive-specific IgE levels).6,7 The medical management of patients with allergic rhinitis and asthma includes allergen avoidance, pharmacotherapy, and immunotherapy.4,5
Allergen-specific immunotherapy (SIT) is typically recommended for patients whose allergic rhinoconjunctivitis and asthma symptoms cannot be controlled by medication and environmental controls, for patients who cannot tolerate medications, or for patients who do not comply with chronic medication regimens.8,9 Currently, two forms of specific immunotherapy are used clinically in the United States. The U.S. Food and Drug Administration (FDA) has approved the use of allergen extracts for subcutaneous administration (subcutaneous immunotherapy) for the treatment of seasonal and perennial allergic rhinitis and allergic asthma. In the United States, a patient with allergies receives subcutaneous injections of an allergen-containing extract, comprised of the relevant allergens to which the patient is sensitive, in increasing doses, in an attempt to suppress or eliminate allergic symptomatology. Considerable interest has also evolved in using sublingual immunotherapy as an alternative to subcutaneous injection immunotherapy. Sublingual immunotherapy involves placement of the allergen under the tongue for local absorption to desensitize the allergic individual over a period of months to years and diminish allergic symptoms. In 1996, an Immunotherapy Task Force, assembled by the World Allergy Organization, cited the emerging clinical data on sublingual immunotherapy, recognized its potential as a viable alternative to subcutaneous therapy, and encouraged continued clinical investigation to characterize optimal techniques.10 Over the past two decades, sublingual forms of immunotherapy have gained favor in Europe; sublingual tablet immunotherapy has been approved by the European regulatory authorities. In the United States, there are currently no FDA-approved sublingual forms of immunotherapy. In the absence of FDA-approved sublingual forms of immunotherapy, some researchers and physicians in the United States are exploring the off-label use of subcutaneous aqueous allergens for sublingual desensitization. An increasing number of U.S. physicians are employing this alternate desensitization approach in the treatment of allergic respiratory conditions based on European and U.S. studies, and on the European Medicines Agency’s approval of certain oral products; however, due to differing standardization of potency in Europe and the United States, doses have been hard to translate between countries.
Scope and Key Questions
Objectives
The primary objective of this comparative effectiveness review is to evaluate the efficacy, effectiveness, and safety of SIT (including both subcutaneous and sublingual immunotherapy) that are presently available for use by clinicians and patients in the United States. We addressed the following Key Questions (KQs):
- KQ1.
What is the evidence for the efficacy and effectiveness of SIT in the treatment of allergic rhinoconjunctivitis and/or asthma?
- KQ2.
What is the evidence for safety of SIT in patients with allergic rhinoconjunctivitis and/or asthma?
- KQ3.
Is the safety and effectiveness of SIT different in distinct subpopulations with allergic rhinoconjunctivitis and/or asthma? Specifically:
- Children
- Adults
- Elderly
- Pregnant women
- Minorities
- Inner-city and rural residents
- Monosensitized individuals
- Patients with severe asthma
Analytic Framework
Our analytic framework illustrates our approach to this systematic review and displays the interventions and comparators of interest, as well as the key primary and secondary outcomes (Figure A).
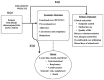
Figure A
Analytic framework for allergen-specific immunotherapy in the treatment of allergic rhinoconjunctivitis and/or asthma. KQ = Key Question; PFT-FEV = pulmonary function test- forced expiratory volume; SIT = allergen-specific immunotherapy
The analytic framework depicts the impact of treatment of allergic rhinitis and asthma. It shows the KQs within the context of the inclusion criteria described in the following sections. It depicts how allergen-specific immunotherapy in this specific population (KQ3) may improve clinical outcomes (KQ1) and functional tests or chemical biomarkers. The potential harms (KQ2) of specific immunotherapy are shown in the framework as well.
Methods
Input From Stakeholders
With the input of a key informant panel, and staff at the Agency for Healthcare Research and Quality (AHRQ) and the Scientific Resources Center, we developed the KQs. The KQs compare how the two delivery routes of immunotherapy affect intermediate outcomes, long-term clinical outcomes, and adverse events. For additional input, we recruited a panel of technical experts, which included experts on the treatment of allergies and asthma in the adult and pediatric populations and then finalized the protocol.
Data Sources and Selection
We reviewed titles and then abstracts to identify randomized controlled trials (RCTs) on the effects of SIT. We included only articles published in English. Abstracts were reviewed independently by two investigators, and were excluded if both investigators agreed that the article met one or more of the exclusion criteria; disagreements were resolved by consensus. For inclusion in this review, we required that the RCTs enrolled patients with allergic rhinoconjunctivitis and/or allergic asthma due to airborne allergies, and that these diagnoses were confirmed by objective testing. The trials had to test subcutaneous immunotherapy or sublingual immunotherapy alone or in combination with usual care, which included pharmacotherapy and environmental interventions. We included trials if the comparators were placebo, other SIT regimens, or pharmacotherapy. For inclusion, the trials had to report at least one of the following: symptoms, medication use, results of provocation tests, quality of life, harms of treatment, adherence measures, convenience measures, or the long-term effects of treatment, including prevention of sequelae of allergic disease or the development of new sensitivities. Studies were excluded if they tested specific sublingual formulations that are not available in the United States, or if no similar U.S. allergen is available for off-label use. An example is our exclusion of studies of sublingual tablets. We also excluded articles in which oral immunotherapy was immediately swallowed without prolonged mucosal contact, as this type of immunotherapy is not currently in clinical use. We also excluded studies that did not clearly report the dose of allergen delivered. Differences regarding article inclusion were resolved through consensus adjudication; a third reviewer audited a random sample to ensure consistency in the reviewing process.
Data Extraction and Quality Assessment
We created standardized forms for data extraction to maximize consistency in identifying pertinent data for synthesis. Each article underwent duplicate review by study investigators for data abstraction, with the second reviewer confirming the accuracy of the first reviewer’s data abstraction. Reviewer pairs were formed to ensure clinical and methodological expertise. Reviewers were not masked to the author, institution, or journal. In most instances, data were abstracted from the published text or tables. If possible, relevant data were also abstracted from figures. Differences in opinion were resolved through consensus adjudication and by discussion during team meetings.
Reviewers extracted detailed information on study characteristics, study participants, interventions, primary and secondary outcome measures and their methods of ascertainment, and safety outcomes. For studies that recorded outcomes at multiple time points, we used the outcome data from the final time point reported. For studies which treated and assessed subjects during a single season, we extracted the outcomes at peak pollen seasons when available. All information from the article review process was entered into the DistillerSR database by the individual completing the review.
Two reviewers independently assessed the risk of bias in each article and came to consensus about the overall rating. We used a modification of the Cochrane Collaboration tool for assessing risk of bias from the “Cochrane Handbook for Systematic Reviews of Interventions.”11 We assessed six categories of potential bias: (1) lack of randomization, (2) lack of allocation concealment, (3) inadequate blinding, (4) incomplete data reporting, (5) selective reporting, and (6) other sources of bias including the funding source. Studies were categorized as having a low, moderate, or high risk of bias depending on their adequacy across the six categories.
Data Synthesis and Analysis
We distributed the studies by intervention, disease, and allergen, and addressed the KQs within each intervention and disease strata (Figure B).
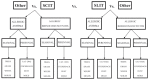
Figure B
Algorithm for the approach and classification of the studies. SCIT = subcutaneous immunotherapy; SIT = allergen specific immunotherapy; SLIT = sublingual immunotherapy
We created a set of detailed evidence tables containing information about each primary and secondary outcome that was extracted from eligible studies, and stratified the tables according to KQ. Given the substantial heterogeneity between studies and the lack of reporting of measures of variability, we did not quantitatively pool the data on efficacy. We summarized the safety of specific immunotherapy in the treatment of allergic rhinoconjunctivitis and/or asthma by extracting data on the harms or adverse events reported in the included studies. The safety data reported in this systematic review include only information from the RCTs that met the criteria for inclusion in the review. The adverse events of specific immunotherapy were divided into two categories: local reactions (reactions that occur at the site of introduction of allergen) and systemic reactions (reactions that occur distant to the site of introduction of the allergen). These data could not be pooled quantitatively, either, due to heterogeneity.
At the completion of our review, we graded the quantity, quality, and consistency of the best available evidence addressing KQs 1, 2, and 3 by adapting an evidence grading scheme recommended by the AHRQ “Methods Guide for Effectiveness and Comparative Effectiveness Reviews.”12,13 We graded the evidence for each comparison for each outcome. Our grading incorporated the risk of biases in the trials, the consistency of the direction of the effect across studies for a given comparison and outcome, the relevance of the collection of trials to the question of interest (directness), and the magnitude of the effects reported in the trials. We could not comment on the precision of the effect sizes as there were seldom measures of variability within the individual studies. The magnitude of effect in a trial was considered “weak” if there was less than a 15 percent difference in post-to-pre change comparing the SIT group and the comparator group, a 15 to 40 percent difference was considered “moderate,” and a greater than 40 percent difference was considered “strong.”
We assigned evidence grades for each outcome as follows: (1) high grade (indicating high confidence that the evidence reflects the true effect, and further research is very unlikely to change our confidence in the estimate of the effect); (2) moderate grade (indicating moderate confidence that the evidence reflects the true effect, although future research may change our confidence in the estimate of the effect and may change the estimate); (3) low grade (indicating low confidence that the evidence reflects the true effect, and further research is likely to change our confidence in the estimate of the effect and is likely to change the estimate); and (4) insufficient (evidence is unavailable). The investigator responsible for each section assigned the evidence grades, and the team reviewed the grades and came to consensus. We did not assign evidence grades for indirect outcome measures, such as pulmonary function test results and provocation tests (including nasal, conjunctival, and bronchial provocation tests).
Results
Our search identified 7,746 citations. After the necessary exclusions, 142 articles were included in the review. All of the included studies were RCTs. We included 74 references that investigated the efficacy and safety of subcutaneous immunotherapy, 60 studies that investigated the efficacy and safety of sublingual immunotherapy, and 8 studies that compared subcutaneous immunotherapy and sublingual immunotherapy. Figure C shows the results of our literature search.
Study Characteristics
The primary diagnoses of the subjects in the included articles were allergic rhinoconjunctivitis and/or asthma. The majority of studies included adults only (52%), followed by studies enrolling only children (24%); studies of mixed adult and pediatric participants were least frequent. Study sizes ranged from 15 to 511 patients. Twenty-three studies (20%) had fewer than 30 patients and twenty-six studies (18%) had more than 100 patients. The majority of the subcutaneous immunotherapy studies (51 studies or 69%) had 50 subjects or fewer, whereas 60 percent of sublingual immunotherapy studies (36 studies) enrolled at least 50 subjects. The majority of studies evaluated seasonal allergens (subcutaneous immunotherapy: 59%, sublingual immunotherapy: 67%), followed by perennial allergens (subcutaneous immunotherapy: 41%, sublingual immunotherapy: 30%), while least common were mixed seasonal and perennial allergens (subcutaneous immunotherapy: 2%, sublingual immunotherapy: 3%). Nearly all studies had at least a medium risk of bias (subcutaneous immunotherapy: 80%, sublingual immunotherapy: 85%). Forty-eight percent of subcutaneous studies and 61 percent of sublingual studies had industry support in the form of either funding and/or supplies.
Population Characteristics
The age range at the time of randomization was 3 to 72 years in the subcutaneous immunotherapy studies and 4 to 74 years in the sublingual immunotherapy studies. Only one study reported race. The duration of allergic rhinoconjunctivitis and/or asthma prior to enrollment was reported in 48 percent of the studies. Twenty-two percent of the studies reported that patients had been affected for more than 5 years. In 22 percent of the studies, patients had been affected for 1 to 5 years.
Intervention Characteristics
The duration of treatment ranged from one season to 5 years; the majority of studies treated the participants for less than 3 years. Thirty-five percent of studies treated participants for less than 1 year. There was substantial heterogeneity in the doses of immunotherapy administered to participants, and the studies used a variety of units to report dosing.
Subcutaneous Immunotherapy
Key Question 1. What is the evidence for the efficacy and effectiveness of subcutaneous immunotherapy in the treatment of allergic rhinoconjunctivitis and/or asthma?
The majority of the subcutaneous immunotherapy trials used a single allergen for treatment. In the trials testing subcutaneous immunotherapy against placebo injections or usual pharmacological measures for patients with asthma, the strength of evidence is high that subcutaneous immunotherapy reduces asthma symptoms, medication use, and combined asthma plus rhinoconjunctivitis medication use. The strength of evidence is moderate that subcutaneous immunotherapy reduces asthma plus rhinitis/rhinoconjunctivitis symptoms. The strength of evidence is low that subcutaneous immunotherapy reduces asthma (with or without rhinitis) combined symptom-medication scores. Although we did not grade the evidence for indirect outcomes, we observed that subcutaneous immunotherapy consistently decreased specific bronchial reactivity to allergen challenges. No consistent benefit was observed for pulmonary-function test results and nonspecific bronchial reactivity.
Regarding the use of subcutaneous immunotherapy for control of allergic rhinoconjunctivitis, we found that the strength of evidence is high that subcutaneous immunotherapy reduces rhinitis/rhinoconjunctivitis symptoms; conjunctivitis symptoms; combined nasal, ocular, and bronchial symptoms; combined rhinoconjunctivitis plus asthma medication use; and improves disease-specific quality of life. The strength of evidence is moderate that subcutaneous immunotherapy reduces rhinitis/rhinoconjunctivitis medication use. The strength of evidence is low that subcutaneous immunotherapy reduces combined symptom-medication scores (Table A).
Table A
Subcutaneous immunotherapy: Summary of allergens, comparators, and main results per outcome.
Key Question 2. What is the evidence for safety of subcutaneous immunotherapy in patients with allergic rhinoconjunctivitis and/or asthma?
Not all of the studies reported safety data and the lack of a consistent reporting system and grading system for the adverse outcomes made it impossible to pool safety data across studies. Forty-five studies of subcutaneous immunotherapy reported safety data. Local reactions, reported in 5 percent to 58 percent of patients and 0.6 percent to 54 percent of injections, were more common than systemic reactions. Most local reactions were mild. The most common systemic reactions were respiratory reactions, occurring in up to 46 percent of patients and following 15 percent of injections. General symptoms (such as headache, fatigue, arthritis) also occurred frequently and affected up to 44 percent of patients. The majority of the systemic reactions were either mild or unspecified. Gastrointestinal reactions, reported in only one study, were the least frequent reactions. Thirteen anaphylactic reactions were reported in four trials. No deaths were reported (Table B).
Table B
Subcutaneous immunotherapy: Summary of safety per location of adverse events.
Key Question 3. Is the safety and effectiveness of subcutaneous immunotherapy different in distinct subpopulations with allergic rhinoconjunctivitis and/or asthma?
Insufficient data exist to describe the strength of evidence regarding efficacy or safety of subcutaneous immunotherapy in the following subpopulations: the elderly, pregnant women, racial and ethnic minorities, inner-city residents, rural residents, and individuals with severe asthma. However, the evidence from a few studies suggests that subcutaneous immunotherapy may be more beneficial in patients with mild asthma than in those with severe asthma. There were no consistent differences in efficacy when considering only the trials enrolling mono-sensitized individuals and the trials enrolling poly-sensitized participants. The data were sufficient to comment on the pediatric subpopulation.
Efficacy of Subcutaneous Immunotherapy in the Pediatric Subpopulation
We included 13 RCTs, enrolling 920 children and comparing subcutaneous immunotherapy with placebo injections or usual pharmacological measures. As observed in the general population, the majority of studies used a single allergen for subcutaneous immunotherapy. The strength of evidence was moderate that subcutaneous immunotherapy reduces asthma symptoms. The strength of evidence was low that subcutaneous immunotherapy reduces asthma medication use, combined asthma plus rhinitis/rhinoconjunctivitis medication use, and asthma/rhinitis/rhinoconjunctivitis symptom-medication scores. We found a moderate strength of evidence to support the use of subcutaneous immunotherapy for reducing rhinitis/rhinoconjunctivitis symptoms in children. The strength of evidence was low that subcutaneous immunotherapy reduces conjunctivitis symptoms and improves quality of life in children with rhinitis/rhinoconjunctivitis (Table C).
Table C
Subcutaneous immunotherapy: Summary of allergens, comparators, and main results per outcome in the pediatric population.
Safety of Subcutaneous Immunotherapy in the Pediatric Population
Inconsistent reporting of adverse events in the pediatric subcutaneous immunotherapy articles made it impossible to pool safety data across studies. However, local reactions were the most common adverse reactions in children receiving subcutaneous immunotherapy. There were no reports of anaphylaxis or death.
Sublingual Immunotherapy
Key Question 1. What is the evidence for the efficacy and effectiveness of sublingual immunotherapy in the treatment of allergic rhinoconjunctivitis and/or asthma?
In the trials testing sublingual immunotherapy against placebo drops or usual pharmacological measures, the overall strength of evidence is moderate that sublingual immunotherapy improves allergic rhinitis and asthma outcomes. The strength of evidence is high that sublingual immunotherapy reduces asthma symptoms. The strength of evidence is moderate that sublingual immunotherapy reduces the following clinical outcomes: rhinitis/rhinoconjunctivitis symptoms, combined asthma plus rhinitis/rhinoconjunctivitis symptoms, combination medication plus symptom scores, conjunctivitis symptoms, and medication use, and improves quality of life. We observed that sublingual immunotherapy consistently improved measures of pulmonary function in the allergic asthmatic population (Table D).
Table D
Sublingual Immunotherapy: Summary of allergens, comparators, and main results per outcome.
Key Question 2. What is the evidence for safety of sublingual immunotherapy in patients with allergic rhinoconjunctivitis and/or asthma?
Forty-three studies of sublingual immunotherapy provided safety data. Local reactions were commonly reported and were described as mild. Systemic reactions were described infrequently; no life-threatening reactions, anaphylaxis, or deaths were reported in these trials. The strength of evidence is insufficient for definitive statements about the safety of sublingual immunotherapy although few serious events were reported (Table E).
Table E
Sublingual Immunotherapy: Summary of safety per location of adverse events.
Key Question 3. Is the safety and effectiveness of sublingual immunotherapy different in distinct subpopulations with allergic rhinoconjunctivitis and/or asthma?
Insufficient data exist to describe the strength of evidence regarding efficacy or safety of sublingual immunotherapy in the following subpopulations: the elderly, pregnant women, racial and ethnic minorities, inner-city residents, rural residents, and individuals with severe asthma. The data were sufficient to comment on the pediatric subpopulation.
Efficacy of Sublingual Immunotherapy in the Pediatric Subpopulation
We included 18 RCTs, enrolling 1,579 children, comparing sublingual immunotherapy with placebo drops or usual pharmacological measures. The strength of evidence is high that sublingual immunotherapy reduces asthma symptoms. The strength of evidence is moderate that sublingual immunotherapy reduces rhinitis/rhinoconjunctivitis symptoms, combined asthma plus rhinitis/rhinoconjunctivitis symptoms, conjunctivitis symptoms, and reduces medication use. The strength of evidence is low that sublingual immunotherapy reduces combined medication plus symptoms scores. There is insufficient evidence to determine the impact of sublingual immunotherapy on disease-specific quality of life. The overall strength of evidence is moderate, that sublingual immunotherapy in children and adolescents improves symptom control, when considering all domains with pertinent clinical outcomes (Table F).
Table F
Sublingual Immunotherapy: Summary of allergens, comparators, and main results per outcome in the pediatric population.
Safety of Sublingual Immunotherapy in the Pediatric Population
The inconsistent reporting of adverse events in the pediatric sublingual immunotherapy studies made it impossible to pool safety data across studies. Local reactions were common, but mild. No life-threatening reactions, anaphylaxis, or deaths were reported in these trials. The strength of evidence is insufficient for definitive statements about the safety of subcutaneous immunotherapy or sublingual immunotherapy in children, although few serious events were reported.
Subcutaneous Versus Sublingual Immunotherapy
Key Question 1. What is the evidence for the efficacy and effectiveness of subcutaneous versus sublingual immunotherapy in the treatment of allergic rhinoconjunctivitis and/or asthma?
Eight RCTs, published between 1989 and 2010, reported on the efficacy and safety of sublingual immunotherapy and subcutaneous immunotherapy when compared directly. Only three of the eight studies reported head-to-head statistical comparisons of the clinical outcomes of interest. The strength of evidence is moderate that subcutaneous immunotherapy is superior to sublingual immunotherapy for control of allergic rhinitis and conjunctivitis symptoms. The strength of evidence is low that sublingual immunotherapy is superior to subcutaneous immunotherapy for reducing medication use. There is insufficient evidence to favor either route of delivery for reducing asthma symptoms and asthma medicine use.
Key Question 2. What is the evidence for safety of subcutaneous versus sublingual immunotherapy in patients with allergic rhinoconjunctivitis and/or asthma?
The safety of sublingual immunotherapy and subcutaneous immunotherapy was assessed in all eight of the included articles. The recording and reporting of the adverse events was neither uniform nor comparable across studies. Local reactions were common and were all of mild or moderate severity. There was one report of anaphylaxis with subcutaneous immunotherapy. There were no reported deaths.
Key Question 3. Is the safety and effectiveness of subcutaneous versus sublingual immunotherapy different in distinct subpopulations with allergic rhinoconjunctivitis and/or asthma?
Insufficient data exist to describe the strength of evidence regarding efficacy or safety of sublingual versus subcutaneous immunotherapy in these subpopulations: the elderly, pregnant women, racial and ethnic minorities, inner-city residents, rural residents, and individuals with severe asthma.
Three RCTS, enrolling 135 children and adolescents, reported on the efficacy and safety of sublingual immunotherapy and subcutaneous immunotherapy when compared directly. The strength of evidence is low to support subcutaneous over sublingual immunotherapy in children and adolescents for reducing asthma symptoms, allergic rhinitis/rhinoconjunctivitis symptoms, or decreasing medication use. Local reactions were reported in both groups. No systemic reactions were reported in patients receiving sublingual immunotherapy. Among children receiving subcutaneous immunotherapy, one anaphylaxis event and three respiratory systemic reactions were reported.
Discussion
For this review of the effectiveness, efficacy, and safety of specific immunotherapy, we summarized data from 142 randomized controlled trials: 74 of subcutaneous immunotherapy, 60 of sublingual immunotherapy, and 8 comparing subcutaneous to sublingual therapy. The studies had considerable heterogeneity in the outcomes reported, scoring of outcomes, and safety data reported, which precluded quantitative pooling of the data. The majority of studies had a moderate risk of bias due to the design choices that were made.
Summary of Results
In our analysis of subcutaneous immunotherapy, key evidence was examined to determine the efficacy and effectiveness of subcutaneous immunotherapy in the treatment of allergic rhinoconjunctivitis and/or asthma. We reviewed pertinent direct clinical outcomes, such as symptoms, medication use, and quality of life. There is sufficient evidence to support the overall effectiveness and safety of both subcutaneous and sublingual immunotherapy for the treatment of allergic rhinitis and asthma.
Regarding asthma outcomes, this review provides supportive evidence subcutaneous immunotherapy improves several asthma and rhinitis/rhinoconjunctivitis outcomes. There is high-grade evidence that subcutaneous immunotherapy reduces asthma symptoms and asthma medication use. Regarding allergic rhinoconjunctivitis outcomes, we found high grade evidence that subcutaneous immunotherapy reduces rhinitis/rhinoconjunctivitis symptoms; conjunctivitis symptoms; combined nasal, ocular, and bronchial symptoms; combined asthma plus rhinitis/rhinoconjunctivitis medication use; and improves disease-specific quality of life. Overall, our findings are consistent with findings from previous systematic reviews.14–16 The majority of the studies included in this review used a single allergen for immunotherapy. In the United States, it is common practice to include multiple allergens in subcutaneous immunotherapy extracts. However, only a few trials have investigated the use of multiple allergen regimens for immunotherapy.
We note that few systematic reviews of subcutaneous immunotherapy have focused on studies in children. A systematic review by Roder et al. reviewed immunotherapy for allergic rhinoconjunctivitis in children and adolescents and identified six studies of subcutaneous immunotherapy that showed conflicting results for clinical efficacy.17 For this review, we reviewed studies in pediatric subpopulations separately. Although the evidence supports the use of subcutaneous immunotherapy to improve asthma and allergic rhinitis outcomes in children, we found fewer pediatric studies, and the strength of evidence was lower in the pediatric subpopulation than in the mixed adult and pediatric population. As observed in the mixed population, the majority of the pediatric subcutaneous immunotherapy studies used a single allergen.
Similarly, the overall strength of evidence is moderate that sublingual immunotherapy improves allergic rhinitis and asthma outcomes. There is high-grade evidence that sublingual immunotherapy reduces asthma symptoms. There is moderate-grade evidence that sublingual immunotherapy reduces combined rhinitis/rhinoconjunctivitis symptoms, asthma plus rhinitis/rhinoconjunctivitis symptoms, combination medication plus symptom scores, conjunctivitis symptoms, medication use, and improves quality of life.
In the pediatric studies, the overall strength of evidence is moderate that sublingual immunotherapy improves allergic rhinitis and asthma outcomes. There is moderate-grade evidence to support that sublingual immunotherapy reduces rhinitis/rhinoconjunctivitis symptoms, combined asthma plus rhinitis/rhinoconjunctivitis symptoms, conjunctivitis symptoms, and decreases medication use. The strength of evidence is low that sublingual immunotherapy reduces combination medication use plus symptoms. The strength of evidence is insufficient to support sublingual immunotherapy use for improving disease-specific quality of life.
In studies comparing subcutaneous to sublingual immunotherapy, the evidence is insufficient to draw a conclusion about the superiority of one mode of delivery over the other.
The available safety data supports the safety of specific immunotherapy, although local reactions were commonly reported for subcutaneous and sublingual immunotherapy. Serious, life-threatening reactions were rare, and no deaths were reported. The pediatric safety data are consistent with the overall safety results reported for subcutaneous and sublingual immunotherapy. While local reactions were common, only one anaphylaxis event was reported in a child receiving subcutaneous immunotherapy in a study comparing subcutaneous and sublingual immunotherapy.
There is consistency in the observed benefits across outcomes for both sublingual and subcutaneous immunotherapy, and in the mixed and pediatric-only populations. The direction of effect largely favors immunotherapy across all outcomes.
Applicability
The results of this systematic review are applicable to patients with allergic rhinoconjunctivitis and/or asthma. We included only studies that confirmed the diagnosis of allergy, either by skin or in vitro testing. Furthermore, asthma studies were included only if the studies used objective measures to confirm asthma diagnosis. We included only studies in which the specific immunotherapy formulations used (or close substitutes) are available to clinicians in the United States, so these results should be applicable to practitioners in the United States.
The reviewed outcomes reflect important clinical outcomes for patients with environmental allergies. The majority of outcomes were direct measures of disease symptomatology, which should make the findings of our review meaningful to clinicians and to patients. Some surrogate measures, such as pulmonary function testing, were also included. While pulmonary function testing is an indirect measure of asthma outcomes, it is used frequently by clinicians in the United States.
However, the following should be considered regarding the applicability of the evidence described in this report. The majority of the included trials used a single allergen for immunotherapy; hence, it is difficult to determine the extent to which this evidence applies to U.S. practitioners using multiple allergen regimens. Based on the findings from a few studies that found subcutaneous immunotherapy to be more beneficial in patients with mild asthma than with severe asthma, the use of subcutaneous immunotherapy to treat asthma is probably most applicable to mild asthmatics. The majority of sublingual immunotherapy studies in this review included subjects with allergic rhinitis/rhinoconjunctivitis and/or mild asthma. Hence, although it may appear from this review that sublingual immunotherapy may be safer than subcutaneous immunotherapy, the safety data from these subgroups of patients must not be extrapolated to the more severely affected patients. There is little evidence supporting the use of immunotherapy in patients with severe asthma.
While a separate sub-analysis of pediatric studies was performed for this review, several studies reported outcomes on a mixed population of adults and children without stratifying the outcomes by age group, so we could not say definitively to which population the results apply. Furthermore, the dosing regimens and durations of treatment reported in these studies varied widely. Therefore, this body of evidence is insufficient for us to comment specifically on target maintenance dose or on duration of sublingual therapy. This may, however, be interpreted as supporting the effectiveness of immunotherapy across a broad range of doses.
There is no clear consensus on what is considered a clinically relevant improvement in symptoms. While some clinicians may suggest that a 15 percent change could reflect real and significant improvement in symptoms in some patients, Canonica et al reported that “the minimal clinically relevant efficacy should be at least 20 percent higher than placebo.”18 We would expect less difference in symptom improvement when comparing immunotherapy to medications. Our systematic review included both studies using placebo and other comparators, such as medications. We chose to consider a less than 15 percent difference as a weak magnitude of effect, a 15 percent to 40 percent difference as a moderate magnitude of effect, and a greater than 40 percent difference as a strong magnitude of effect. We applied this scheme to all graded outcomes in this review.
Our analysis adds to the available information about the strength of evidence for the efficacy and safety of allergen immunotherapy for the treatment of asthma and allergic rhinoconjunctivitis. These findings are relevant to clinicians who provide care for patients affected by these medical conditions. The findings are also relevant to patients making decisions regarding therapy, as they findings can help inform patients on the efficacy and safety of allergen immunotherapy. Guideline developers may also find our review useful for making recommendations about the use of allergen immunotherapy in adults and children.
Limitations
We encountered several challenges during our review process. We included only RCTs in this review; however, the studies varied substantially in their risk of bias. While all studies used randomization, several studies did not specify whether allocations schemes were concealed, or if the type of intervention was concealed from participants and outcome assessors. The majority of subcutaneous and sublingual immunotherapy studies received industry support financially or in the form of supplies. The study authors rarely reported the clear role or extent of involvement of the sponsors. For these reasons, several studies were considered to have a moderate or high risk of bias. The potential risk of bias played an important role in determining the strength of the evidence for each direct outcome.
The body of literature reviewed has much heterogeneity. The clinical outcomes reported varied from study to study, and there were no consistent scoring or grading systems for reporting pertinent primary outcomes, such as symptoms or medication use. The study authors used varying criteria for diagnosing asthma and assessing asthma severity and control. Some of the asthma criteria may overestimate, while other criteria may underestimate, the degree of asthma control. Some studies that reported combined asthma and rhinoconjunctivitis scores demonstrated significant improvement. It is possible that a preferential effect of immunotherapy on one of these disease processes may have highly influenced the combined scores. Studies with multiple allergens presented a similar dilemma; response to one allergen may have determined the overall clinical score; therefore, the true effect of desensitization with each allergen remains unclear. The heterogeneity of the data on symptoms and medication use precluded pooling the data for further analysis.
The same issues of heterogeneity existed with the safety data reported in the studies; the adverse events were reported with different denominators from study to study. The lack of a consistent reporting and grading system made it impossible to pool data. In further regards to the safety data, although it may appear from this review that sublingual immunotherapy may be safer than subcutaneous immunotherapy, it should be noted that there are few studies of sublingual immunotherapy for treating patients with moderate or severe asthma, which may affect the incidence of more severe reactions. Furthermore, our study reports only the safety data from RCTs, and, therefore, is not a comprehensive review of the incidence of adverse events. A comprehensive review would require the review of observational studies and case reports.
There were also deficiencies in the statistical reporting in the included studies. Most of the studies had small sample sizes; so, relevant statistical information on continuous outcomes, such as scores, were frequently unavailable (i.e., standard deviation, standard error, or confidence intervals). Therefore, precision of the point estimates could not be assessed. As a result, we used the magnitude of effect in place of precision when grading the strength of evidence for each outcome. In the six studies that compared subcutaneous and sublingual immunotherapy head-to-head, only three reported direct statistical comparisons between the groups for the clinical outcomes of interest.
There are concerns that there may be publication bias in the specific immunotherapy literature, as positive outcomes are more likely to be published than negative outcomes. While our study did not formally assess this, publication bias is a concern in this body of literature. In an attempt to identify unpublished studies, we requested information from the relevant pharmaceutical companies, but we did not receive any requested information packets. Therefore, we did not report on any unpublished studies.
Future Research
Additional RCTs are needed to examine the efficacy, effectiveness, and safety of SIT. The RCTs should be conducted with attention to the design elements that reduce bias, such as clear concealment of allocation and masking of the intervention throughout the study, to allow for more definitive conclusions. Future studies will benefit from standardized methods to report symptoms and symptom scoring, adverse events, and dosing quantity, frequency, and formulation. Published guidelines for allergen immunotherapy clinical trials recommend that the combined symptom-medication score be used as the primary outcome measure;18 future studies should be encouraged to comply with these guidelines.19–21
There is a specific need for studies investigating the efficacy and safety of multiple allergen regimens, as multiple regimens are commonly used in the United States. There is increasing discussion in the scientific community about the clinical use and efficacy of single-allergen versus multiple-allergen therapy, and there are insufficient numbers of studies which compare these head-to-head. Future studies are needed to directly compare the effectiveness of single-allergen versus multiple-allergen regimens for desensitization. On the other hand, studies restricting immunotherapy to a single allergen will allow for a greater understanding of dose effect, dosing strategy effect, and effect of treatment duration on relevant clinical outcomes.
Studies including patients with asthma should clearly describe how patients are diagnosed with asthma. Restricting asthma severity in studies to mild, moderate, or severe would be helpful in assessing whether there is a subgroup of patients with asthma that may benefit from immunotherapy. Adequately powered trials with appropriate subgroups of patients and utilizing correct methodology are needed to address the efficacy and safety of allergen immunotherapy in specific subpopulations (e.g., pregnant women, monosensitized versus polysensitized patients, patients with severe asthma, urban vs. rural patients).
There is a need to document with future research whether immunotherapy has a disease-modifying activity. Especially in the pediatric population, there is a need to determine if immunotherapy can prevent or modify the atopic march in children at high risk for allergic rhinitis and asthma. Additional considerations for pediatric studies include identifying the optimal age for initiation of immunotherapy and evaluating the differential effects of immunotherapy based on the developmental stage of children and adolescents.
Although our review and others have found sublingual immunotherapy effective for improving symptoms of allergic rhinoconjunctivitis and asthma, there are several unanswered questions. The target maintenance dose, dosing strategies, and the necessary duration of treatment for sublingual immunotherapy with various allergens have not yet been fully determined.
Finally, there is a need for studies that directly compare sublingual to subcutaneous immunotherapy to strengthen the evidence base in children and adults. Future studies comparing subcutaneous to sublingual immunotherapy should use doses previously shown to be effective in earlier, high-quality studies, and direct statistical comparisons between the outcomes of the two groups would be useful for ensuring a fair comparison of the two therapies.
Conclusions
In summary, we found sufficient evidence to support the effectiveness and safety of subcutaneous and sublingual immunotherapy for the treatment of allergic rhinitis and asthma, particularly using single-allergen immunotherapy regimens in adults and children. Strengthening the evidence for the effectiveness and safety of multiple allergen regimens should be high priority for future studies. There are far fewer pediatric studies than adult studies; hence, the evidence is less strong for the pediatric population. Additional pediatric studies may strengthen the evidence for the effectiveness and safety of allergen immunotherapy in the pediatric population. When comparing subcutaneous with sublingual immunotherapy, the existing evidence is insufficient and inconclusive. Additional trials are needed to establish the efficacy and safety of the interventions when directly compared in the usual care settings, given the expectation of differences in adherence.
References
- 1.
- Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007 Jan–Feb;28(1):3–9. [PubMed: 17390749]
- 2.
- Wright AL, Holberg CJ, Martinez FD, et al. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994 Dec;94(6 Pt 1):895–901. [PubMed: 7971008]
- 3.
- Min YG. The pathophysiology, diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2010 Apr;2(2):65–76. [PMC free article: PMC2846743] [PubMed: 20358020]
- 4.
- Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466–76. [PubMed: 20816182]
- 5.
- Fanta CH. Asthma. N Engl J Med. 2009 Mar 5;360(10):1002–14. [PubMed: 19264689]
- 6.
- Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007 Nov;120(5 Suppl):S94–138. [PubMed: 17983880]
- 7.
- Gergen PJ, Arbes SJ Jr, Calatroni A, et al. Total IgE levels and asthma prevalence in the US population: Results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2009 Sep;124(3):447–53. [PMC free article: PMC2758573] [PubMed: 19647861]
- 8.
- Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998 Oct;102(4 Pt 1):558–62. [PubMed: 9802362]
- 9.
- Passalacqua G, Compalati E, Canonica GW. Advances in allergen-specific immunotherapy. Curr Drug Targets. 2009;10(12):1255–62. [PubMed: 19909232]
- 10.
- Canonica GW, Bousquet J, Casale T, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009 Dec;64 Suppl 91:1–59. [PubMed: 20041860]
- 11.
- Altman D, Antes G, Gøtzsche P, et al. Cochrane Handbook for Systematic Reviews of Interventions. Assessing risk of bias in included studies Vol Version 5.0.2. London, England: The Cochrane Collaboration; 2009.
- 12.
- Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. [PMC free article: PMC428525] [PubMed: 15205295]
- 13.
- Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions--Agency for Healthcare Research and Quality and the Effective Health Care Program. J Clin Epidemiol. 2010 May;63(5):513–23. [PubMed: 19595577]
- 14.
- Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010 Aug 4;(8):CD001186. [PubMed: 20687065]
- 15.
- Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD001936. [PMC free article: PMC7017974] [PubMed: 17253469]
- 16.
- Matricardi PM, Kuna P, Panetta V, et al. Subcutaneous immunotherapy and pharmacotherapy in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011 Oct;128(4):791–799. e6. [PubMed: 21620452]
- 17.
- Roder E, Berger MY, de Groot H, et al. Immunotherapy in children and adolescents with allergic rhinoconjunctivitis: a systematic review. Pediatr Allergy Immunol. 2008;19(3):197–207. [PubMed: 18221463]
- 18.
- Canonica GW, Baena-Cagnani CE, Bousquet J, et al. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007 Mar;62(3):317–24. [PubMed: 17298350]
- 19.
- Casale TB, Canonica GW, Bousquet J, et al. Recommendations for appropriate sublingual immunotherapy clinical trials. The Journal of allergy and clinical immunology. 2009;124(4):665–670. [PubMed: 19766297]
- 20.
- Bousquet J, Schunemann HJ, Bousquet PJ, et al. How to design and evaluate randomized controlled trials in immunotherapy for allergic rhinitis: an ARIA-GA(2) LEN statement. Allergy. 2011 Jun;66(6):765–774. [PubMed: 21496059]
- 21.
- Canonica GW, Baena-Cagnani CE, Bousquet J, et al. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007 Mar;62(3):317–324. [PubMed: 17298350]
Abbreviations
- AHRQ
Agency for Healthcare Research and Quality
- FDA
Food and Drug Administration
- KQ
Key Question
- RCT
Randomized controlled trial
- SIT
Allergen-specific immunotherapy
- SCIT
Subcutaneous immunotherapy
- SLIT
Sublingual immunotherapy
- Executive Summary - Allergen-Specific Immunotherapy for the Treatment of Allergi...Executive Summary - Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review
- Executive Summary - ADHD Diagnosis and Treatment in Children and AdolescentsExecutive Summary - ADHD Diagnosis and Treatment in Children and Adolescents
- C57244 Yuji Kohara unpublished cDNA Caenorhabditis elegans cDNA clone yk301a11 3...C57244 Yuji Kohara unpublished cDNA Caenorhabditis elegans cDNA clone yk301a11 3', mRNA sequencegi|2415275|gnl|dbEST|1278236|dbj|C5 1|Nucleotide
- transcription factor AP-4 [Homo sapiens]transcription factor AP-4 [Homo sapiens]gi|4507447|ref|NP_003214.1|Protein
- List of Included Studies - Stroke Prevention in Patients With Atrial Fibrillatio...List of Included Studies - Stroke Prevention in Patients With Atrial Fibrillation: A Systematic Review Update
Your browsing activity is empty.
Activity recording is turned off.
See more...
