NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lin SY, Erekosima N, Suarez-Cuervo C, et al. Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Mar. (Comparative Effectiveness Reviews, No. 111.)
This publication is provided for historical reference only and the information may be out of date.

Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review [Internet].
Show detailsThe literature search identified 7,746 citations. During the abstract review process, we excluded 5,942 citations which did not meet eligibility criteria. At the level of full-text article review, we excluded another 1,626 and included 178 articles for data abstraction. At this level we excluded 36 articles and included 142 articles for the final analysis (Figure 3).
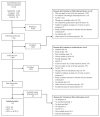
Figure 3
Literature search. RCT = randomized controlled trial; SCIT = subcutaneous immunotherapy; SLIT = sublingual immunotherapy * Total may exceed number in corresponding box, as articles were excluded by two reviewers at this level.
Summary of Findings
All studies included were randomized controlled trials. We included 74 references that investigated the efficacy and safety of subcutaneous immunotherapy (SCIT), 60 studies that investigated the efficacy and safety of sublingual immunotherapy (SLIT), and eight studies compared subcutaneous immunotherapy and sublingual immunotherapy, with only 3 of these studies reporting findings from head-to-head comparisons between both forms of SIT. Appendixes D, E, F, and G include details of all studies included; and Appendix H provides a listing of excluded articles with reasons for exclusions.
Seventy-five studies (52%) included only adults and 34 studies (24%) included only children. Thirty two studies (22%) included both adults and children (mixed population). One study in the SCIT intervention did not specify the age of the population studied32 (Figure 4).
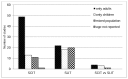
Figure 4
Count of studies including children, adults, or both. SCIT = subutaneous immunotherapy; SLIT = sublingual immunotherapy
We had no limits on study size; the number of patients randomized in the studies ranged from 15 to 511. Twenty nine studies (20%) had fewer than 30 patients and twenty-six studies (18%) had more than 100 patients. The majority of the SCIT studies (54 studies or 73%) had 50 subjects or fewer, whereas 60 percent of SLIT studies (36 studies) enrolled at least 50 subjects. (Figure 5).
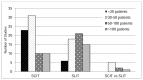
Figure 5
Count of studies by number of enrolled participants. SCIT = subutaneous immunotherapy; SLIT = sublingual immunotherapy.
We had no limitations based on duration of treatment. Only ten studies (7%) treated patients for up to 4 months (16 weeks), 50 studies (35%) treated patients for up to one year, 54 studies (38%) had a duration between 1 and 3 years, and 17 studies; 9 treating with sublingual immunotherapy and 8 treating with subcutaneous immunotherapy had a duration longer than 3 years. One study treated patients with subcutaneous immunotherapy for 4 years.33 Eleven studies (9%) were seasonal, meaning that the patients were followed only through the allergy season; 5 were studies of subcutaneous immunotherapy and 6 were sublingual immunotherapy (Figure 6).
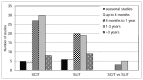
Figure 6
Count of studies by duration of treatment. SCIT = subutaneous immunotherapy; SLIT = sublingual immunotherapy
Since immunotherapy is not usually the first treatment, the number of years with disease is often a criterion for inclusion in clinical trials. However, 74 of the included studies (52%) did not report years with disease. In the rest, this was specified as an inclusion criterion. In 22 percent of the studies, patients had the disease for 1 to 5 years; in 22 percent of the studies patients had the disease for more than 5 years. In only five studies, patients had the disease for less than a year (Figure 7).
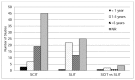
Figure 7
Count of studies by disease severity in enrolled participants. NR = not reported; SCIT = subutaneous immunotherapy; SLIT = sublingual immunotherapy
Numerous studies were designed as immunotherapy versus placebo (73% of the SCIT studies and 80% of the SLIT studies), but some of the studies comparing different immunotherapy regimens (e.g., low dose vs. high dose, coseasonal vs. continuous, cluster vs. classic) included a placebo arm, increasing the number of overall placebo controlled studies to 105 studies (74%); 54 SCIT studies, 48 SLIT studies and 3 SLIT versus SCIT studies 34–36 had a placebo arm. Very few studies were designed to compare SIT versus pharmacotherapy: only 6 SCIT studies,37–42 3 SLIT studies,43–45 and 2 SLIT versus SCIT studies37,46 included a pharmacotherapy arm (Figure 8).
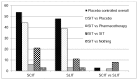
Figure 8
Count of studies by design of comparator. SCIT = subutaneous immunotherapy; SIT = allergen specific immunotherapy; SLIT = sublingual immunotherapy
The majority of the studies allowed the use of pharmacotherapy (conventional or rescue therapy) as needed; 75 percent of the SCIT studies (remaining 25% were not reported), 98 percent of the SLIT studies and 100 percent of the SLIT versus SCIT studies (see Intervention Characteristics tables in Appendixes D, E and F).
Non-English Literature
Our search identified 590 articles written in languages other than English. These articles were reviewed by two investigators, following the same procedure that all the other articles. This was done after the results of the English language articles were known. After title and abstract review, we excluded 525 references and included 65 for full article review. From these 65 articles, we excluded 44 based on language plus other criteria: did not study SIT, were review articles, used oral or nasal immunotherapy, or did not apply to our KQs. For the remaining 21 articles, we used Google’s Web-based translation services, Google Translate® (http://translate.google.com)47 to translate the article to determine if their results were comparable to those in the English language literature. The translation service did not work on eight articles. Among the remaining articles, five were not RCTs. In the nine RCTs (three Spanish, two German, two French, one Polish, one Japanese), the results were concordant with the results in the English-language literature.
Subcutaneous Immunotherapy
Study Characteristics
These 74 articles, with 4350 subjects, were published between 1967 and 2012. The publications originated from Europe (56 studies or 76%), North America (12 studies or 16%), Asia (5 studies or 7%), South America (1 study or 1%), and Australia (1 study or 1%) (Appendix D, Evidence Table D1). Thirty-five studies (50%) had at least some industry support, although 18 studies (25%) had no identified funding source (Appendix D, Evidence Table D1). Twenty one studies (28%) had a low risk of bias. Fifty-two percent (39 studies) were rated as having a medium risk of bias, and 14 studies (20%) were considered to have a high risk of bias (Appendix D, Evidence Table D4).
The primary diagnoses of the subjects were asthma in 19 studies,41,48–64 65 rhinitis in ten studies,32,66–74 rhinoconjunctivitis in 14 studies,37,75–87 asthma with rhinitis in 18 studies,33,38–40,88–101 and asthma with rhinoconjunctivitis in 13 studies42,102–113 (Appendix D, Evidence Table D1).
By design, all the studies required subjects to have positive allergy skin test results and/or positive in-vitro specific IgE test results. Forty two studies (57%) required that the subjects had not received previous immunotherapy. Eighteen (24%) focused on monosensitized individuals.41,48,53,66,77,79,84,88,90–92,95–97,99,102,103,108 The majority of studies (44 studies or 59%) evaluated seasonal allergens including trees, grasses, weeds, and seasonal molds, followed by perennial allergens in 28 studies (38%); only 2 studies (3%) included both seasonal and perennial allergens. Forty-eight studies used a single allergen, whereas the remaining 26 studies used multiple allergens. The most common allergen studied was dust mite (21 studies or 31%) (Figure 9).
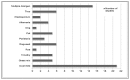
Figure 9
Subcutaneous immunotherapy studies by type of allergen.
Population Characteristics
The age range of participants in the subcutaneous immunotherapy studies was 3 to 72 years (Appendix D, Evidence Table D2). Twenty-four studies reported the mean or minimum duration of disease among the enrolled participants. Mean duration of disease ranged from 1 year to 24 years. All but twelve studies reported gender; all studies reporting gender included male and female patients. Only one study reported the race of the participants.65
Key Question 1. What is the evidence for the efficacy and effectiveness of subcutaneous immunotherapy in the treatment of allergic rhinoconjunctivitis and/or asthma?
Evidence for the Efficacy and Effectiveness of Subcutaneous Immunotherapy in the Treatment of Asthma
In this section we report findings from the 74 references that investigated the safety and efficacy of subcutaneous immunotherapy in the Treatment of Asthma
Key Points
Relative to placebo or control treatment:
- High grade evidence supports that subcutaneous immunotherapy improves asthma symptom control, based on 16 randomized controlled trials with 1178 subjects.
- Moderate grade evidence supports that subcutaneous immunotherapy improves asthma plus rhinitis/rhinoconjunctivitis symptoms, based on five randomized controlled trials with 175 subjects.
- High grade evidence supports that subcutaneous immunotherapy reduces asthma medication use, based on 12 randomized controlled trials with 1062 subjects.
- High grade evidence supports that subcutaneous immunotherapy reduces asthma plus rhinitis/rhinoconjunctivitis medication use, based on five randomized controlled trials with 203 subjects.
- Low grade evidence supports that subcutaneous immunotherapy improves asthma/rhinitis/rhinoconjunctivitis symptom control and medication use, based on six randomized controlled trials with 196 subjects.
Asthma and Asthma/Rhinoconjunctivitis Symptoms
Asthma symptom scores alone, or combined asthma with rhinitis/rhinoconjunctivitis symptom scores were reported in 20 asthma studies.39,40,48,49,52,53,56,58–61,64,65,89,95,98,101,110–112 (Appendix D, Evidence Tables D5 and D6). Eighteen studies evaluated asthma symptom scores (Appendix D, Evidence Table D6). The number of participants in each study ranged from 16 to 300. The duration of assessment ranged from 3 months to 6 years. Twelve studies compared subcutaneous immunotherapy to placebo; three studies compared subcutaneous immunotherapy to pharmacotherapy; one study compared subcutaneous immunotherapy to a control group which did not receive SIT; one study compared SCIT using a cluster schedule versus a conventional schedule; and another compared SCIT duration of 3 years versus 5 years. Various measures of asthma symptoms were used. Although the scoring system was not always described, some studies used self-reported symptoms using an ordinal scale. Other measures of asthma symptoms include time to first increase in symptoms,61 mean percentage of days and nights with asthma,40 number of asthma exacerbations per year,53 and comparison of number of subjects who were improved, unchanged, or deteriorated.64 Across studies, the immunotherapy group showed an improvement in asthma symptoms scores ranging from 17 to 84 percent greater than the comparison group.
Thirteen of sixteen studies (81%) reported statistical comparisons between subcutaneous immunotherapy and the comparison group.40,48,52,53,56,59,61,65,98,101,111,112,64 Majority of the studies used a single allergen for immunotherapy. The most common single allergen was dust mite in seven studies.52,53,56,58–60,98 Seven of the sixteen studies (44%) demonstrated significant improvement in asthma symptoms from subcutaneous immunotherapy when compared with placebo,56,61,101,112 pharmacotherapy,40,53 or another control group,52 with the absolute difference in asthma symptoms between groups ranging from 17 to 79 percent. Of note, one of these was a study of perennial allergic asthma and the investigators specifically reported data for patients only allergic to D. pteronyssinus; when patients who were sensitized to more than one perennial allergen were included in the analysis, no significant benefit was observed.52 Of the remaining six studies that compared groups, two studies demonstrated significant improvement in the subcutaneous immunotherapy group when symptom scores were compared before and after immunotherapy.65,98 In one of these studies, the placebo group also had a significant reduction in symptom scores.65
Three studies (19%) did not report statistical comparisons between the immunotherapy and the comparison groups.49,58,60 Two of these studies reported significant improvement in symptom scores for the immunotherapy group, whereas no significant changes in symptom scores were observed in the comparison groups of both studies.58,60 The third study was a 2-year study in which patients were treated with preseasonal immunotherapy only in the first year of the study.49 Symptom scores were recorded before, during, and after the pollen season for both years; however the investigators did not report a direct comparison of the symptom scores between the first and second year.
Six of 16 studies (38%) reporting asthma symptom scores were large studies with 90 to 300 participants.40,48,52,56,59,65 Among the large studies with low or moderate risk of bias, three studies investigated dust mite allergen,52,56,59 one investigated ragweed allergen,48 and one investigated multiple allergens.65 Only two of these studies, both investigating dust mites, demonstrated significant improvement in asthma symptoms, when compared with the comparison group.52,56 Of note, one of these studies reported that this significant improvement was observed exclusively in a subgroup of subjects whose only perennial allergen sensitivity was to D. pteronnysinus; there was no significant improvement in the whole study population, which included individuals with other perennial allergen sensitivity.52 Two high quality studies, including one large study, reported no significant improvement in asthma symptoms following treatment with subcutaneous immunotherapy when the immunotherapy group was compared with the placebo group.65,111 In fact, in the larger study by Adkinson et al, the placebo group had a greater reduction in symptoms than the immunotherapy group.65 Allergen doses varied across studies with no clear association between dose and symptom response.
These 16 studies reporting asthma symptom scores included 1178 participants. The overall strength of evidence is high grade to support the use of subcutaneous immunotherapy to improve asthma symptom scores (Table 2).
Table 2
Body of evidence for subcutaneous immunotherapy and asthma symptom scores.
One blinded study by Tabar et al. compared subcutaneous immunotherapy using a cluster immunotherapy schedule against a conventional schedule.38 After 1 year of immunotherapy, both groups demonstrated significant improvement in asthma symptoms scores compared with pre-treatment scores. At the end of the first year, patients were re-randomized to receive either 3 years or 5 years of subcutaneous immunotherapy; this latter study was an unblinded randomized trial.113 After 5 years, no significant difference was observed in the global asthma symptom scores between treatment groups. This study was not included in the evidence grading because both treatment groups received subcutaneous immunotherapy.
Five asthma studies reported asthma plus rhinoconjunctivitis symptom scores, each using a different allergen; these included three studies investigating pollen,39,89,101 one study investigating Alternaria,95 and one study investigating cat allergen.110 All were small studies ranging from 24 to 49 participants. Four were placebo-controlled trials with low95 or moderate risk of bias.89,101,110 Three of these demonstrated significant improvement in pooled symptom scores with subcutaneous immunotherapy when compared directly with placebo.89,95,101 One study demonstrated significant improvement in pre- versus post-treatment symptom scores in the subcutaneous immunotherapy arm.110 The single study comparing subcutaneous immunotherapy to pharmacotherapy demonstrated a significant improvement in combined symptom scores in the subcutaneous immunotherapy arm when compared with pharmacotherapy; however this study was graded as having a high risk of bias.39 The immunotherapy group showed improvement ranging from 21 to 68 percent greater than the comparison group.
These five studies reporting asthma plus rhinoconjunctivitis symptom scores included 175 participants. The overall strength of evidence is moderate to support the use of subcutaneous immunotherapy to improve combined asthma and rhinoconjunctivitis symptom scores (Table 3).
Table 3
Body of evidence for subcutaneous immunotherapy for asthma plus rhinitis/rhinoconjunctivitis symptom scores.
Asthma Medication Use and Asthma Plus Rhinitis/Rhinoconjunctivitis Medication Use
Asthma medication scores, or asthma plus rhinitis/rhinoconjunctivitis medication scores were reported in 17 asthma studies.40,42,48,49,52,53,56,59,60,65,98 39,64,89,101,111,112 (Appendix D, Evidence Tables D7 and D8). The number of participants in each study ranged from 20 to 300. The duration of assessment ranged from 4 months to 6 years. The majority of the studies used a single allergen for immunotherapy; dust mite was the most commonly used allergen. Methods of assessing medication consumption varied across studies. Some studies reported calculated scores, with different scoring scales across studies. Other measures of asthma medication consumption include number of days during which medications were used,53 proportion of subjects who did not use bronchodilators,59 comparison of number of subjects who were improved, unchanged, or deteriorated, 64 number of patients taking medications,98 amount of medication used per week,60 and sum of daily medication doses.111
Twelve studies reported medication scores for asthma alone.40,42,48,49,52,53,56,59,60,64,65,98 The most prevalent single allergen studied was dust mite in six studies.52,53,56,59,60,98 Eight studies compared subcutaneous immunotherapy to placebo,48,49,56,59,60,64,65,98 three studies compared subcutaneous immunotherapy to pharmacotherapy,40,42,53 and one study compared it to a control group which did not receive immunotherapy.52 Two placebo controlled studies; one of dust mite allergy98 and one of rye pollen allergy49 did not report results of relevant statistical analyses.
Eight studies reported results from direct comparisons between the immunotherapy group and the comparison group.40,42,48,52,53,56,64,65 Of these, 3 reported a significant difference in medication consumption in favor of the immunotherapy group when compared with pharmacotherapy40,53 or a control group.52 The allergens investigated by these studies included dust mite in all 3 studies 40,52,53 as well as parietaria and ryegrass pollen in one study.40 The remaining 5 studies found no significant difference in medication use between the immunotherapy group and the comparison groups. This included 4 placebo controlled studies investigating ragweed,48 dust mite,56Cladosporium,64 and multiple allergens,65 and one study investigating birch pollen allergy which a comparison group that was treated with nasal steroids.42 One study demonstrated significant reduction in medication use in both the immunotherapy and placebo groups after treatment, with no difference between groups.65
Only the results of post-treatment compared with pre-treatment measures were reported by 2 placebo-controlled studies; both studied dust mite immunotherapy and demonstrated significant improvement in medication consumption only in the immunotherapy groups.59,60 These 12 studies reporting asthma medication consumption included 1062 participants. The overall strength of evidence is high grade that subcutaneous immunotherapy reduces asthma medication use (Table 4).
Table 4
Body of evidence for subcutaneous immunotherapy affecting asthma medication scores.
Five studies reported asthma plus rhinoconjunctivitis medication scores, each investigating a different allergen; these included three studies that investigated pollen immunotherapy39,89,101 and two studies investigated mold immunotherapy.111,112 Studies ranged from 30 to 50 participants. The single study which compared immunotherapy with pharmacotherapy had a high risk of bias.39 All five studies demonstrated a significant reduction in asthma and rhinoconjunctivitis medication consumption in the immunotherapy group when compared with the comparison groups. The immunotherapy group experienced a 14 to 83 percent greater reduction in combined asthma and rhinoconjunctivitis medication consumption than the comparison group. These five studies reporting combined asthma plus rhinoconjunctivitis medication scores included 203 participants. The overall strength of evidence is high that subcutaneous immunotherapy reduces asthma and rhinoconjunctivitis medication consumption (Table 5).
Table 5
Body of evidence for subcutaneous immunotherapy affecting asthma plus rhinitis/rhinoconjunctivitis medication scores.
Combined Asthma Symptom and Medication Scores
In contrast to the larger number of studies reporting individual symptom scores or medication scores, only six asthma studies reported combined asthma symptom-medication scores41,50,64,95,109,112 (Appendix D, Evidence Tables D7 and D8). The number of participants in each study ranged from 23 to 50. The duration of assessment ranged from 5 months to 3 years. Five were placebo-controlled studies, and all five studies demonstrated significant improvement in the immunotherapy group compared with placebo50,64,95,109,112 These included two studies of Alternaria, one with low risk of bias95 and the other with moderate risk of bias112; one study of cat allergen with moderate risk of bias;109 and studies of Cladosporium64 and dust mite allergen50 with high risk of bias. One study, with high risk of bias, compared subcutaneous immunotherapy with dust mites to pharmacotherapy.41 After a seven-month treatment, there was more reduction of the symptom-medication scores in the immunotherapy group than the pharmacotherapy group; however, this difference was not statistically significant.41 Fifty percent of the studies did not report the magnitude of effect.
Overall, these six studies reporting asthma symptom-medication scores included 196 participants. The strength of evidence is low to support that subcutaneous immunotherapy improves asthma symptom-medication scores (Table 6).
Table 6
Body of evidence for subcutaneous immunotherapy affecting combined asthma symptom-medication scores.
Akmanlar et al. compared rush immunotherapy with conventional immunotherapy. They observed a significant reduction in symptom-medication scores in both study groups after 3 years of immunotherapy, but there was no significant difference in scores between the two groups.97 This study was not included for grading the evidence because both treatment groups received immunotherapy.
Pulmonary Function Testing
Thirteen asthma studies, including 1,024 participants, reported changes in pulmonary function test results; these included peak expiratory flow (PEF) or peak flow in 12 studies,38,42,48,56,58,59,61,65,89,91,110,111 forced expiratory volume 1 (FEV1) in 2 studies,52,56 and forced vital capacity (FVC) in 1 study56 (Appendix D, Evidence Table D10). Risk of bias was low for 2 studies65,111 and medium for 11 studies.38,42,48,52,56,58,59,61,89,91,110 Study duration ranged from 3 months to 3 years.
Nine studies (82%) compared subcutaneous immunotherapy to placebo.48,56,61,65,89,91,110,111 Only one, with a moderate risk of bias, demonstrated a statistically significant improvement in mean daily PEF in the immunotherapy group compared with the placebo group; the magnitude of this effect was small.48 Another placebo-controlled trial with low risk of bias demonstrated a small treatment effect in favor of immunotherapy (with a mean difference of 3.8% points in the predicted value of PEF), and this approached statistical significance.65 Three placebo controlled trials demonstrated significant improvement in PEF in the immunotherapy group comparing the post-treatment to pre-treatment measures.56,59,110 However, two of these also demonstrated significant improvement in the placebo group after treatment.56,110 One study compared subcutaneous immunotherapy to bronchodilators;58 treatment significantly improved PEF only in the immunotherapy group. Another study comparing subcutaneous immunotherapy to nasal steroids found no difference between the two groups after six weeks of treatment.42 Tabar et al. compared pre- and post-immunotherapy data for a group using a cluster schedule to a group using a conventional schedule; both groups demonstrated significant reduction in PEF variability after one year of immunotherapy.38
Among the studies that evaluated FEV1 and or FVC, one trial which compared subcutaneous immunotherapy with a control group that did not receive immunotherapy, observed that immunotherapy produced a 20 percent increase in FEV1 when compared with the control group.52 The other study found no significant change in FEV1 or FVC in either the immunotherapy or placebo group after treatment.56 As described in the methods, we did not grade the strength of evidence for pulmonary function test results because it is an indirect outcome measure.
Bronchial Reactivity
Twenty-five asthma studies (76%) evaluated bronchial airway reactivity. Bronchial reactivity was evaluated by two methods: specific allergen bronchial provocation tests and nonspecific chemical bronchial provocation. The majority of the studies that performed nonspecific chemical bronchial provocation tests used methacholine and/or histamine, with the exception of one study which also used adenosine 5’-monophosphate (AMP)94 (Appendix D, Evidence Table D11).
Specific allergen bronchoprovocation tests were reported in 17 studies, which included 514 participants. Of 15 studies that reported pre- versus post-treatment differences, 11 studies (73%) demonstrated significant decreases in bronchial sensitivity in favor of subcutaneous immunotherapy.41,48,50,51,60,62,89,91,100,109,111 Four trials showed no statistically significant difference between the immunotherapy group and the comparison group.54,61,63,97 Two studies reported only the pre- and post-treatment comparison.55,58 Kohno et al. demonstrated a significant decrease in bronchial sensitivity in the immunotherapy group and not the comparison group.58
Nonspecific chemical bronchoprovocation tests were reported in 16 studies, which included 750 participants.41,42,53,54,56,58,59,61,62,65,89,94,98,100,101,109 One study did not report relevant statistical comparisons.89 Of 11 studies that reported comparisons with the comparison group,41,42,53,54,56,62,65,94,98,100,101 only two demonstrated a significant decrease in bronchial sensitivity in favor of subcutaneous immunotherapy.53,101 Nine studies found no significant difference between the immunotherapy group and the comparison group.41,42,54,56,62,65,94,98,100 In the study by Hedlin et al, both groups were treated with some form of immunotherapy.100
Four studies reported only pre- versus post-treatment comparisons.58,59,61,109 Only one of these studies demonstrated a significant improvement in bronchial sensitivity in the immunotherapy group after treatment; there was no significant change in the comparison group (which received bronchodilators).58 We did not grade the strength of evidence for bronchial reactivity because it is an indirect outcome measure.
Evidence for the Efficacy and Effectiveness of Subcutaneous Immunotherapy in the Treatment of Rhinitis and Rhinoconjunctivitis
In this section we report findings from the 74 references that investigated the safety and efficacy of subcutaneous immunotherapy in the treatment of rhinitis and rhinoconjunctivitis. Figure 10 shows the distribution of allergens in the studies included.
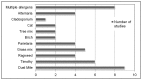
Figure 10
Subcutaneous immunotherapy studies by type of allergen in rhinitis/rhinoconjunctivitis.
Key Points
Relative to a control group:
- High grade evidence supports that subcutaneous immunotherapy improves rhinitis/rhinoconjunctivitis symptoms, based on 26 randomized controlled trials with 1764 subjects.
- High grade evidence supports that subcutaneous immunotherapy improves conjunctivitis symptoms, based on 14 randomized controlled trials with 1104 subjects.
- High grade evidence supports that subcutaneous immunotherapy improves control of combined nasal, ocular, and bronchial symptoms, based on six randomized controlled trials with 591 subjects.
- Moderate grade evidence supports that subcutaneous immunotherapy decreases rhinitis/rhinoconjunctivitis medication use, based on ten randomized controlled trials with 564 subjects
- High grade evidence supports that subcutaneous immunotherapy decreases combined medication use (rhinitis/rhinoconjunctivitis plus asthma medication use), based on 11 randomized controlled trials with 768 subjects.
- Low grade evidence supports that subcutaneous immunotherapy improves rhinitis/rhinoconjunctivitis (with or without asthma) combined symptom-medication scores, based on six randomized controlled trials with 400 subjects.
- High grade evidence supports that subcutaneous immunotherapy improves disease-specific quality of life, based on six randomized controlled trials with 889 subjects.
Rhinitis/Rhinoconjunctivitis Symptoms
Rhinitis/rhinoconjunctivitis symptom scores were reported in 30 studies. 32,37,42,48,61,66,69,70,72,73,75–77,81–86,96,98,99,101–103,106,108,111–113
Rhinitis/rhinoconjunctivitis symptom scores were included from studies that enrolled patients with rhinitis/rhinoconjunctivitis with or without asthma. Thirteen studies exclusively examined patients with a primary diagnosis of rhinitis/rhinoconjunctivitis.37,66,69,70,72,73,75,76,81–84,86 Five studies examined patients with rhinitis/rhinoconjunctivitis and asthma, although the studies did not meet criteria for inclusion with the asthma studies,77,96,102,106,108 findings from these studies are reported in this section. An additional six studies that met our criteria for inclusion with the asthma studies enrolled patients with asthma and rhinitis/rhinoconjunctivitis. 42,98,99,101,111,112 Combined outcome data from these latter six studies were previously reported with other asthma studies. Lastly, two studies of patients with asthma also described their rhinitis/rhinoconjunctivitis symptom scores48,61 (Appendix D, Evidence Table D12). Four included studies were not graded because all study groups received immunotherapy.32,85,103,113
Four studies reported combined nasal and ocular symptoms.76,82,101,114 while two studies reported unspecified nasal symptom scores69,108 The scales used to report nasal and ocular symptoms varied across studies. Two studies used visual analog scores,84,112 one examined the time to increase in nasal symptoms after allergen exposure,61 while the remainder used numeric systems to score the severity and presence or absence of nasal or nasal and ocular symptoms. The number of participants in each study ranged from 17 to 410 and the duration of follow-up ranged from 1 month to 3 years, with the majority of studies reporting symptoms at 12 months. While one study compared a group receiving subcutaneous immunotherapy to a group of patients receiving nasal steroids,42 the remainder used a placebo control group.
Nineteen studies (73%) reporting rhinitis/rhinoconjunctivitis symptom scores demonstrated statistically significant improvement in rhinitis/rhinoconjunctivitis symptoms with subcutaneous immunotherapy. Eighteen of these studies compared subcutaneous immunotherapy with placebo while one compared subcutaneous immunotherapy with patients receiving only nasal steroids.42 One of the studies81 showed a difference only with the high dose of immunotherapy, while at the lowest dose it showed no statistical difference when compared with placebo. The remaining six studies did not show significant improvement in symptoms relative to placebo treated subjects.61,70,72,83,102,114
Majority of the studies used a single allergen for immunotherapy. The most common single allergens used in the rhinitis/rhinoconjunctivitis scores were Timothy grass in four studies,70,77,81,101 and dust mite allergens in four studies.72,73,98,99 Of these studies, three (75%) evaluating Timothy Grass77,81,101 and two (50%) evaluating dust mites98,99 demonstrated significant improvement in rhinitis/rhinoconjunctivitis symptom control.
Overall, 25 RCTs reported rhinitis/rhinoconjunctivitis symptom scores in 1734 participants. The overall strength of evidence is high to support that subcutaneous immunotherapy improves rhinitis/rhinoconjunctivitis symptoms (Table 8).
Table 8
Body of evidence for subcutaneous immunotherapy affecting rhinitis/rhinoconjunctivitis symptom scores.
Conjunctivitis Symptoms
Fifteen subcutaneous immunotherapy studies reported conjunctivitis symptom scores (Appendix D, Evidence Table D13). The comparator in all studies reporting conjunctivitis scores was placebo, except for one study that was not included in grading because all study groups received immunotherapy.103 Most studies used numeric scales to quantify symptoms, except for one study,61 which evaluated the time to see an increase in ocular symptoms upon exposure to cat allergen, and two other studies, which used a visual analog score.84,112
Studies that used numeric scales were inconsistent across studies. The duration of assessment varied from 10 weeks to 5 years.
Six studies demonstrated significant improvement in conjunctivitis symptom scores when compared with placebo.77,101,112,81,84,88 The remaining studies did not show significant improvement in conjunctivitis symptom scores. Again the most commonly evaluated allergen was Timothy Grass, and three out of five studies (60%) showed significant improvement in conjunctivitis symptoms.
Fourteen subcutaneous immunotherapy trials reported conjunctivitis scores and included 1104 subjects. The majority of the studies used a single allergen for immunotherapy. The overall strength of evidence is high to support that subcutaneous immunotherapy improves allergic conjunctivitis symptoms (Table 9).
Table 9
Body of evidence for subcutaneous immunotherapy affecting conjunctivitis symptoms.
Control of Combined Symptom Scores (Nasal, Ocular, and Bronchial)
Eight rhinitis/rhinoconjunctivitis studies reported combined scores including nasal, ocular, and bronchial symptom scores (Appendix D, Evidence Table D12). Study size ranged from 28 to 410 subjects. Although many of these patients did not have an objective diagnosis of asthma, they did have bronchial symptoms at baseline. Combined symptom scores from primary asthma studies that met our criteria are reported in the subcutaneous immunotherapy asthma section. The total symptom scores used numeric scales that were not validated and varied between studies. All graded studies compared subcutaneous immunotherapy with placebo. Two studies were not graded because all study groups received immunotherapy.87,92
Three studies showed significant improvement in combined symptom scores for nasal, ocular, and bronchial symptoms when compared with placebo,81,99,104 and one in the comparison of post-treatment symptoms to pre-treatment symptoms.99
Six trials reported symptoms in 591 individuals. The strength of evidence is high to support that subcutaneous immunotherapy improves combined (nasal, ocular, bronchial) symptoms scores (Table 10).
Table 10
Body of evidence for subcutaneous immunotherapy affecting bronchial, nasal and ocular combined symptoms scores.
Medication Scores (Including Combined Medication Scores)
Rhinitis/rhinoconjunctivitis medication scores were reported in 13 of the subcutaneous immunotherapy studies as were combined medication scores (including rhinitis/rhinoconjunctivitis and asthma medications) (Appendix D, Evidence Tables D15 and D16). Three of the included studies were not graded since because all study groups received immunotherapy.32,73,82 The 10 graded studies used some type of numeric scoring scale for medication use, but these were inconsistent across studies. The duration of assessment of medication use ranged from 3 months to 3 years. Studies that reported only on rhinitis/rhinoconjunctivitis medications included oral antihistamines and intranasal corticosteroids, while those trials that described combined medication scores, included those used by patients with asthma and rhinitis/rhinoconjunctivitis, including inhaled beta agonists and oral corticosteroids.
Seven trials (70%) reporting rhinitis/rhinoconjunctivitis medication scores demonstrated significant improvement with subcutaneous immunotherapy.37,73,76,77,82,83,88 In six of these, the comparator group was placebo; one study compared treatment with immunotherapy with pharmacotherapy treatment.37 Of the two Timothy Grass allergen studies that reported medication scores,70,77 only one study showed improvement with immunotherapy.77 Similarly, of the three dust mite allergen trials,72,73,113 two demonstrated significant improvement with subcutaneous immunotherapy compared with placebo.73,113
Ten RCTs reported medication scores in 564 participants. The overall strength of evidence is moderate to support that subcutaneous immunotherapy decreases medication use for rhinitis/rhinoconjunctivitis (Table 11).
Table 11
Body of evidence for subcutaneous immunotherapy affecting medication use (rhinitis/rhinoconjunctivitis medications).
Twelve studies reported pooled asthma and rhinitis/rhinoconjunctivitis medication scores. Eleven studies were graded excluding one study where all arms received immunotherapy92 Among the graded studies that reported pooled asthma and rhinitis/rhinoconjunctivitis medication scores, ten of the eleven studies demonstrated significant improvement from subcutaneous immunotherapy when compared with placebo or when comparing medication use after treatment to a pre-treatment period66,75,77,79,81,88,96,102,105,108 (Appendix D, Evidence Table D17). Three Parietaria studies reported significant improvement in combined medication scores when compared with placebo.66,75,88 Two Timothy Grass studies also reported significant improvement in combined medication scores when compared with placebo.77,81
Thus, eleven trials reported medication scores in 768 participants. The strength of evidence is high to support that subcutaneous immunotherapy decreases combined medication use (Table 12).
Table 12
Body of evidence for subcutaneous immunotherapy affecting asthma and rhinitis/rhinoconjunctivitis medication use.
Combined Symptom-Medication Scores
Twelve studies reported combined rhinitis/rhinoconjunctivitis symptoms plus medication scores. The six studies where all study groups received immunotherapy were not graded.32,38,71,78,93,103 All of the studies used some type of numeric scoring scale for the combination score, but these were inconsistent across studies. The duration of assessment of medication use ranged from one pollen season up to 3 years (Appendix D, Evidence Table D17).
In five studies, nasal, ocular, and bronchial symptoms were scored in addition to medication use, specifically beta agonist, oral and nasal steroid, and antihistamine use.80,96,105,107,112 Only nasal and ocular symptoms were reported along with nasal corticosteroids and antihistamines in one study.67
Five of the six studies that reported a combination symptom plus medication score demonstrated significant improvement with subcutaneous immunotherapy. The remainder of studies compared subcutaneous immunotherapy with placebo.
Six trials reported combined symptom plus medication scores in 400 participants. The overall strength of evidence is low to support that subcutaneous immunotherapy improves combination symptoms plus medication scores (Table 13).
Table 13
Body of evidence for subcutaneous immunotherapy affecting combined rhinitis (with or without asthma) symptom-medication scores.
Quality of Life
Quality of life was reported in eight placebo-controlled trials.40,79,81,88,96,112,113 The instruments used to assess quality of life were validated, disease-specific instruments: the Rhinoconjunctivitis Quality of Life questionnaire (RQLQ, Adult, Pediatric, Adolescent, and Japanese language version) and/or the Short Form 36 questionnaire (SF-36) (Appendix D, Evidence Table D18).
Four of the six studies reported significant improvement in disease-specific quality of life when compared with placebo.79,81,96,112 The other two studies found no overall improvement.40,88
Six studies with 889 subjects included quality of life outcomes. Two studies were not graded because all study groups received immunotherapy. 93,113 The evidence is high to support that subcutaneous immunotherapy improves disease-specific quality of life among individuals with rhinitis/rhinoconjunctivitis (Table 14).
Table 14
Body of evidence for rhinitis/rhinoconjunctivitis (with or without asthma) quality-of-life scores after subcutaneous immunotherapy rhinitis.
Nasal and Ocular Allergen Challenge (Provocation)
Seventeen subcutaneous immunotherapy studies challenged subjects to specific allergens in order to quantify nasal and ocular symptoms (Appendix D, Evidence Table D19). Seven studies used nasal provocation.33,69,73,83,95,106,112 Ten studies used conjunctival provocation tests;63,85,87,89,90,98,109–111,115 for two of these studies, both treatment groups received SCIT.85,87 Four of the seven nasal challenge studies (57%) reported significant improvement in symptoms after subcutaneous immunotherapy compared with placebo or when comparing post-treatment to pre-treatment response.73,95,106,112 Six of the conjunctival provocation studies (60%) demonstrated significant improvement in symptoms after subcutaneous immunotherapy compared with placebo or with comparison of post-treatment to pre-treatment response.63,90,98,109,110,115
Secondary Outcomes
Few studies evaluated secondary outcomes such as biomarkers or asthma prevention. There is insufficient data about the effect of subcutaneous immunotherapy on these secondary outcomes. (Appendix D, Evidence Tables D20 and D21).
Key Question 2. What is the evidence for the safety of subcutaneous immunotherapy in patients with rhinitis/rhinoconjunctivitis and/or asthma?
Key Points
- Lack of a consistent reporting system and grading system for subcutaneous immunotherapy precluded pooling safety data across studies.
- Local reactions (occurring at the site of allergen administration) were most common but mild.
- Systemic reactions occurred less frequently. Of these, respiratory reactions were the most common and gastrointestinal symptoms were least frequent.
- Thirteen anaphylactic reactions were reported in four trials.
- No deaths were reported.
Figure 11 shows the distribution of adverse events by location and severity. The graph shows only adverse events reported in the Immunotherapy arms.

Figure 11
Subcutaneous immunotherapy safety data by location and severity. AE = adverse event; GI = gastrointestinal
Safety data reported in this systematic review includes only the randomized control trials that met the criteria for inclusion in the review. Not all studies reported safety data, and the lack of a consistent reporting system and grading system for the adverse outcomes made it impossible to pool safety data across studies.
Forty-five studies of subcutaneous immunotherapy reported safety data. 37,38,40,41,48,50,51,57,58,61–64,67,68,70–74,76,77,81,83,86,88,89,91,92,94–97,99–101,103,106–113 In this body of evidence, local reactions, reported in five percent to 58 percent of patients and 0.6 percent to 54 percent of injections, were more common than systemic reactions. Most local reactions were mild. The most common systemic reactions were respiratory reactions, occurring in up to 46 percent of patients and up to 15 percent of injections. General symptoms (such as headache, fatigue, arthritis, anxiety) also occurred frequently and were reported to affect up to 44 percent of patients. Majority of the systemic reactions were either mild or unspecified. Gastrointestinal reactions, reported in only one study, were the least frequent reactions. Thirteen anaphylactic reactions were reported in four trials (Executive Summary, Table B; Appendix D, Evidence Table D22).
Key Question 3. Is the safety and effectiveness of subcutaneous immunotherapy different in distinct subpopulations with rhinitis/rhinoconjunctivitis and/or asthma?
Key Points
- There is insufficient evidence to comment on safety and effectiveness in the following populations: the elderly, pregnant women, minorities, inner-city residents, rural residents, and patients with severe asthma.
- There is no consistent difference in efficacy of subcutaneous immunotherapy when comparing responses in mono-sensitized and poly-sensitized subjects.
- Although the evidence supports the use of subcutaneous immunotherapy to improve asthma and allergic rhinitis outcomes in children, we found that there are fewer pediatric studies, and as a result, the strength of evidence is weaker for the pediatric subpopulation than in the mixed adult and pediatric population.
The included articles did not present specific data on the following subgroups: the elderly, pregnant women, minorities, inner-city residents or rural residents. Insufficient data exist to comment on these subpopulations.
The majority of the studies excluded subjects with severe asthma. Few articles explicitly stated that patients with severe asthma were included, although Adkinson et al. specifically recruited children with moderate to severe perennial allergic asthma65 This was a study with low risk of bias which investigated the benefit of subcutaneous immunotherapy with injections of multiple allergens in patients already receiving appropriate medical treatment. They demonstrated, after 2 years or more of immunotherapy, continuing immunotherapy provided no additional benefit in children with moderate to severe asthma. Subgroup analysis in this study suggested that a younger age (≤8.5 years) and lower medication scores (indicating milder asthma) may be factors leading to a favorable response to subcutaneous immunotherapy.65
Seven studies were performed exclusively in monosensitized subjects.41,48,51,53,91,95,97 There was no consistent difference in the efficacy of subcutaneous immunotherapy when considering these studies of monosensitized individuals relative to studies including polysensitized individuals.
Some studies performed subgroup analyses on monosensitized individuals and select age groups. One study by Bousquet et al. demonstrated that in the subgroup of patients allergic only to D. pteronyssinus who received immunotherapy, there was a significant decrease in mean asthma symptom scores, medication scores, and a significant improvement in FEV1 in comparison to the control group that did not receive immunotherapy.52 In this study, the investigators observed that children and patients with mild asthma demonstrated the most improvement; they also observed that patients with an FEV1 less than 70 percent predicted before immunotherapy (indicating more severe asthma) did not improve after 12 months of treatment. Another study, by Wang et al., demonstrated a reduction in asthma symptom scores in both pediatric (16 years of age or younger) and adult subgroups after 1 year of immunotherapy with a dust mite extract; however when compared with placebo, no significant difference was observed in either age group.56 Similarly, there was no significant difference in treatment response in monosensitizedz or in polysensitized individuals.
Subcutaneous Immunotherapy in Pediatric Population
Thirteen articles on subcutaneous immunotherapy were eligible for inclusion in this review. Two additional articles provided long term followup outcomes. The 13 articles with 920 subjects were published between 1982 and 2011. The publications originated mostly from Europe with one each from North America and Australia. Thirty-eight percent of studies (n=5) had at least some industry support, although 7 studies had no identified funding source (Appendix G, Evidence Table G1). Four studies had a low risk of bias (31%); 4 studies were rated as having a medium risk of bias (31%), and 5 studies were considered to have a high risk of bias (38%). (Appendix G, Evidence Table G4)
The pediatric population ranged in age from 3 to 18 years. The number of participants in each study ranged from 18 to 300. The primary diagnoses of the subjects studied in the articles included asthma in 7 studies,49,50,53,55,57,63,65 rhinitis in zero studies, rhinoconjunctivitis in one study,84 asthma with rhinitis in 3 studies,40,97,100 and asthma with rhinoconjunctivitis in two studies.111,112 (Appendix G, Evidence Table G2)
Inclusion criteria required that all subjects have positive skin allergy testing and/or in vitro specific IgE allergy testing. Seven studies (54%) required that the study participants had not received prior immunotherapy. Two studies (17%) focused on monosensitized individuals only.53,97
The majority of studies evaluated perennial allergens (62%), followed by seasonal allergen (23%) and studies including both seasonal and perennial allergens (15%) (Appendix G, Evidence Table G1).
All studies allowed either conventional pharmacotherapy or rescue allergy medications during the study. The maintenance dosing interval varied from biweekly to every 6 week dosing, and the duration of treatment ranged from 4 months to 3 years. There was great heterogeneity in the reporting of the maintenance or cumulative dose delivered to the study participants, and the studies used various units to report dosing (Appendix G, Evidence Table G3).
Key Points Regarding Asthma Outcomes in the Pediatric Population
Relative to placebo or control treatment:
- Moderate evidence supports that subcutaneous immunotherapy improves asthma symptom control based on 6 randomized controlled trials with 550 subjects. Low grade evidence supports that subcutaneous immunotherapy reduces asthma medication use based on 4 randomized controlled trials with 470 subjects.
- Low grade evidence supports that subcutaneous immunotherapy reduces asthma plus rhinitis/rhinoconjunctivitis medication use based on 2 randomized controlled trials with 80 subjects.
- Low grade evidence supports that subcutaneous immunotherapy improves combined asthma/rhinitis/rhinoconjunctivitis symptom and medication scores use based on 2 randomized controlled trials with 85 subjects.
Asthma and Asthma/Rhinoconjunctivitis Symptoms
Asthma symptom scores were reported in 6 asthma studies40,49,53,65,111,112 (Appendix G, Evidence Table G5). Six (46%) of 13 studies evaluated asthma symptom scores. The number of participants in each study ranged from 20 to 300. The duration of treatment ranged from 10 months to 3 years. Four studies compared subcutaneous immunotherapy to placebo, and two studies compared subcutaneous immunotherapy to pharmacotherapy. Various measures of asthma symptoms were used. Although the scoring system was not always described, some studies used self-reported symptoms using an ordinal scale. Other measures of asthma symptoms include mean percentage of days and nights with asthma,40 and number of exacerbations per year.53 The allergens used for SCIT included dust mite, Cladosporium, ryegrass, Alternaria, and multiple allergens.
Five studies reported statistical comparisons between subcutaneous immunotherapy and the comparison group.40,53,65,111,112 Four of these studies demonstrated improvement in asthma symptoms from subcutaneous immunotherapy when compared with pharmacotherapy,40,53 or to placebo;111,112 however only three of these were reported as statistically significant.40,53,112 One study demonstrated significant improvement in the subcutaneous immunotherapy group when symptom scores were compared before and after immunotherapy, although the placebo group also had a significant reduction in symptoms scores.65
One study did not report statistical comparisons between the immunotherapy and the comparison groups.49 This study was a 2-year study in which patients were treated with preseasonal immunotherapy only in the first year of the study. Symptom scores were recorded before, during, and after the pollen season for both years; however the investigators did not report a direct comparison of the symptom scores between the first and second year.
Two of 6 studies reporting asthma symptom scores were large studies with 121 to 300 participants.40,65 One of the large studies had low risk of bias,65 and the other had a high risk of bias. Both studies investigated multiple allergens. One study showed no significant improvement.65 The other study showed a decrease in the mean percentage of days and nights with asthma symptoms in children receiving SCIT for 3 years compared with controls, but baseline data were not reported, so we were unable to determine the magnitude of effect.40 Two high quality studies, including one large study, reported no significant improvement in asthma symptoms following treatment with subcutaneous immunotherapy when the immunotherapy group was compared with the placebo group.65,111 In fact, in the larger study by Adkinson et al., the placebo group had a greater reduction in symptoms than the immunotherapy group.65 Allergen doses varied across studies with no clear association between dose and symptom response.
These 6 studies reporting asthma symptom scores include 550 participants. The overall strength of evidence is moderate that subcutaneous immunotherapy using a single allergen improves asthma symptoms. However, there is low grade evidence to support that subcutaneous immunotherapy using multiple allergens does not improve asthma symptoms. There were no studies that reported combined asthma and rhinoconjunctivitis symptom scores (Table 16).
Table 16
Body of evidence for subcutaneous immunotherapy and asthma symptom scores in children and adolescents.
Asthma Medication Use and Asthma Plus Rhinitis/Rhinoconjunctivitis Medication Use
Asthma medication scores, or asthma plus rhinitis/rhinoconjunctivitis medication scores were reported in 6 (46%) asthma studies40,49,53,65,111,112 (Appendix G, Evidence Tables G6 and G8). Methods of assessing medication consumption varied across studies. Some studies reported calculated medication scores, with scoring scales different across studies. Other measures of asthma medication consumption include number of days during which medications were used53 and sum of daily medication doses.111
Four studies reported medication scores for asthma alone.40,49,53,65 One study used dust mite as a single allergen53 while another used rye grass.49 Two studies used multiple allergens.40,65 Two studies compared subcutaneous immunotherapy to placebo,49,65 and two studies compared subcutaneous immunotherapy to pharmacotherapy.40,53 One placebo controlled study of rye pollen allergy did not report results of relevant statistical analyses.49
Three studies reported results from direct comparison between the immunotherapy group and the comparison group.40,53,65 Two of these studies reported a significant difference in medication consumption in favor of the immunotherapy group when compared with pharmacotherapy.40,53 The allergens investigated by these studies include dust mite in both studies 40,53 as well as Parietaria and ryegrass pollen in one study.40 The remaining one study found no significant difference in medication use between the immunotherapy group and the comparison groups. This placebo controlled study investigated multiple allergens65 and demonstrated significant reduction in medication use in both the immunotherapy and placebo groups after treatment, with no difference between groups.65
Overall, 4 studies reported asthma medication consumption in 470 participants. The overall strength of evidence is low grade to support the use of subcutaneous immunotherapy to improve asthma medication use (Table 17).
Table 17
Body of evidence for subcutaneous immunotherapy affecting asthma medication scores in children and adolescents.
Two studies reported combined asthma and rhinoconjunctivitis medications scores and investigated molds, Cladosporium111 and Alternaria.112 These studies included 30 to 50 participants, compared immunotherapy to placebo, and had a low risk111 and high risk112 of bias. These studies demonstrated a reduction in asthma and rhinoconjunctivitis medication consumption in the immunotherapy group when compared with the comparison groups.
The overall strength of evidence is low grade to support the use of subcutaneous immunotherapy to reduce asthma and rhinoconjunctivitis medication consumption (Table 18).
Table 18
Body of evidence for subcutaneous immunotherapy affecting asthma plus rhinitis/rhinoconjunctivitis medication scores in children and adolescents.
Combined Asthma Symptoms and Medication Scores
Two asthma studies reported combined symptom-medication scores for asthma or asthma plus rhinoconjunctivitis50,112 (Appendix G, Evidence Tables G7 and G8). These studies compared subcutaneous immunotherapy to placebo and investigated dust mite allergen50 with high risk of bias and Alternaria mold allergen112 with moderate risk of bias. Both studies demonstrated significant improvement in the immunotherapy group compared with placebo.50,112 Kuna et al. reported a 63 percent reduction in combined symptom-medication score after 3 years of treatment, compared with 17 percent reduction in the placebo group.112
Another study by Akmanlar et al. compared rush immunotherapy to conventional immunotherapy and observed significant reduction in symptom-medication scores in both study groups after immunotherapy, but there was no significant difference in scores between the two groups. This study was graded as having a high risk of bias and was not included for evidence grading because both treatment groups received SIT.97
Overall, 2 studies reporting asthma symptom-medication scores included 85 participants. The strength of evidence is low grade to support that subcutaneous immunotherapy improves asthma symptom-medication scores (Table 19).
Table 19
Body of evidence for subcutaneous immunotherapy affecting combined symptom- medication scores in children and adolescents).
Pulmonary Function Testing
Two studies reported changes in pulmonary function test results with peak expiratory flow rates (PEF or PEFR)65,111 (Appendix G, Evidence Table G9). Risk of bias was low for these 2 studies, comparing subcutaneous immunotherapy to placebo.65,111 One study demonstrated a small treatment effect in favor of immunotherapy (with a mean difference of 3.8 percentage points in the predicted value of PEFR) and this approached statistical significance.65 The other study found no significant difference in mean PEF between subcutaneous immunotherapy and placebo.111
Bronchial Reactivity
Eight asthma studies (67%) evaluated bronchial airway reactivity (Appendix G, Evidence Table G10). Bronchial reactivity was evaluated by two methods: specific allergen bronchial provocation tests and nonspecific chemical bronchial provocation. The majority of the studies that performed nonspecific chemical bronchial provocation tests used methacholine and/or histamine (Appendix G, Evidence Table G10).
Specific allergen bronchoprovocation studies were reported in 6 studies. Of 5 studies that reported pre- versus post-treatment differences, 3 studies (60%) demonstrated significant decreases in bronchial sensitivity in favor of subcutaneous immunotherapy.50,100,111 Two trials showed no statistically significant difference between the immunotherapy group and the comparison group.63,97 One study reported only the pre- and post-treatment comparison. 55 Nonspecific chemical bronchoprovocation tests were reported in 3 studies.53,65,100 All 3 studies reported comparisons with a comparator group, although only one demonstrated a significant decrease in bronchial sensitivity in favor of subcutaneous immunotherapy.53 Two studies demonstrated no significant difference between the immunotherapy group and the comparison group. 65,100 In the study by Hedlin et al, both groups were treated with some form of immunotherapy.100
Secondary Outcomes
Few studies evaluated secondary outcomes including quality of life, biomarkers, and prevention of asthma development. One study commented on asthma quality of life. Kuna et al. demonstrated a significant improvement of 38 percent in quality of life scores after 3 years of immunotherapy, compared with a 19 percent decrease in quality of life scores in the placebo group.112 There is insufficient evidence to comment on the strength of the evidence about the effect of subcutaneous immunotherapy on these secondary outcomes.
Key Points Regarding Rhinitis/Rhinoconjunctivitis Outcomes in the Pediatric Population
Relative to a control group:
- Moderate grade evidence supports that subcutaneous immunotherapy improves rhinitis/rhinoconjunctivitis symptoms based on 3 randomized controlled trials with 285 subjects.
- Low grade evidence supports that subcutaneous immunotherapy improves conjunctivitis symptoms based on 3 randomized controlled trials with 285 subjects.
- Low grade evidence supports that subcutaneous immunotherapy improves disease specific quality of life based on 2 randomized controlled trials with 350 subjects.
There were no pediatrics studies that reported on subcutaneous immunotherapy outcomes of combined nasal, ocular, and bronchial symptoms, rhinitis/rhinoconjunctivitis medication use, combined medication use (both asthma and rhinitis/rhinoconjunctivitis medications), or combined symptom and medication use.
Rhinitis/Rhinoconjunctivitis Symptoms
Rhinitis/Rhinoconjunctivitis symptom scores were reported in 3 studies84,111,112 (Appendix G, Evidence Table G11). Rhinitis/rhinoconjunctivitis symptom scores were included from studies that enrolled rhinitis/rhinoconjunctivitis and/or asthma patients. One study exclusively examined patients with a primary diagnosis of rhinoconjunctivitis,84 while the other two studies enrolled patients with asthma and/or rhinitis/rhinoconjunctivitis, and met our criteria for inclusion with the asthma studies.111,112
Two studies used visual analog scores to measure nasal symptoms,84,112 while the other study used an unspecified numeric system to score the severity and presence/absence of nasal symptoms.111 Two studies reporting rhinitis/rhinoconjunctivitis symptom scores demonstrated statistically significant improvement in rhinitis/rhinoconjunctivitis symptoms with subcutaneous immunotherapy compared with placebo.84,112 These studies had medium risk of bias, included 50 to 205 participants, and investigated grass/birch allergen mix and Alternaria respectively. The third study did not show significant improvement in symptoms relative to placebo treated subjects.111 This study also had low risk of bias, included 30 patients, and investigated Cladosporium allergen.
Overall, three RCTs reported rhinitis/rhinoconjunctivitis symptom scores in 285 participants. The overall strength of evidence is moderate to support that subcutaneous immunotherapy improves rhinitis/rhinoconjunctivitis symptoms (Table 20).
Table 20
Body of evidence for subcutaneous immunotherapy affecting rhinitis/rhinoconjunctivitis symptom scores in children and adolescents.
Conjunctivitis Symptoms
Three subcutaneous immunotherapy studies reported conjunctivitis symptom scores (Appendix G, Evidence Table G12).84,111,112 The comparator in these three studies was placebo. Two studies used a visual analog score for ocular symptoms,84,112 and the other study did not describe the scale used.111 The duration of assessment varied from 10 months to 5 years.
One study, with medium risk of bias and involving 205 participants, reported significant improvement in conjunctivitis symptom scores when compared with placebo, although actual scores were not reported to determine the magnitude of effect.84 Kuna et al. also found significant improvement with a 47 percent absolute reduction in conjunctivitis symptoms after 3 years of subcutaneous immunotherapy compared with controls.112 The third study, also with low risk of bias and involving 30 participants, did not show significant improvement in conjunctivitis symptom scores compared with placebo.111
Three subcutaneous immunotherapy trials reported conjunctivitis scores and included 285 subjects. The overall strength of evidence is low to support that subcutaneous immunotherapy improves allergic ocular symptoms in children (Table 21).
Table 21
Body of evidence for subcutaneous immunotherapy affecting conjunctivitis symptoms in children and adolescents.
Quality of Life
Quality of life (QOL) was reported in 2 trials comparing subcutaneous immunotherapy to placebo with medication treatment.40,112 One 3 year study compared the mean number and percentage of limitations of quality of life per year for the two groups. This study reported significant improvement in disease-specific quality of life when compared with placebo40 (Appendix G, Evidence Tables G14 and G15). In Kuna, et al, a 38 percent increase in QOL in treated children was seen at 3 years, compared with a 18 percent decrease in QOL in the placebo group.112 Kuna et al also described a significant increase in QOL in adolescents, compared with placebo.112 A similar increase in QOL was also seen in the parents of children with symptoms.112
Overall, two studies with 350 subjects evaluated quality of life outcomes. There is low grade evidence to support that subcutaneous immunotherapy improves disease-specific quality of life among children and adolescents with rhinitis/rhinoconjunctivitis (Table 22).
Table 22
Body of evidence for rhinitis/rhinoconjunctivitis quality-of-life scores after subcutaneous immunotherapy (in children and adolescents).
Nasal and Ocular Allergen Challenge (Provocation)
Three subcutaneous immunotherapy studies challenged subjects to specific allergens in order to quantify symptoms (Appendix G, Evidence Table G11). None of the studies used nasal provocation. Three studies performed conjunctival provocation tests comparing subcutaneous immunotherapy to placebo.63,84,111 Two of the 3 conjunctival provocation studies demonstrated significant improvement in symptoms comparing subcutaneous immunotherapy to placebo after 1 or 5 years.63,84 One study demonstrated no significant difference between subcutaneous immunotherapy and placebo after 10 weeks during peak allergy season.111 This study had low risk of bias, included 30 children, and investigated Cladosporium allergen.
Secondary Outcomes
Few studies evaluated secondary outcomes such as biomarkers. In general, there is insufficient evidence about the effect of subcutaneous immunotherapy on these secondary outcomes. Moller et al conducted a medium risk of bias study investigating asthma prevention as a primary outcome; they observed that among 151 children with allergic rhinoconjunctivitis without asthma, there was a 52 percent increased odds (OR 2.52 (1.3–5.1)) of preventing the development of asthma after 3 years of SCIT compared with placebo.84,115 A 5-year followup study, by the same investigators, found a 68 percent increased odds (OR 2.68 (1.3–5.7)) of preventing the development of asthma in children receiving SCIT 2 years after stopping a 3-year course of SCIT.84,115 In a 10-year followup study (7 years after completing a 3-year course of SCIT), there was a 50 percent increased odds (OR: 2.5 (1.1–5.9)) of preventing asthma in children that had received SCIT, compared with placebo116 (Appendix G, Evidence Table G14 and G16).
Summary of Evidence for Efficacy and Effectiveness in the Pediatric Population
When considering the key evidence for the efficacy and effectiveness of subcutaneous immunotherapy in the treatment of asthma, the pertinent direct clinical outcomes include symptom scores and medication use. The strength of evidence regarding the effectiveness of subcutaneous immunotherapy is moderately supportive that this treatment improves asthma symptom scores but there is low evidence for improvement of asthma medication use and symptom medication scores (Table 23).
Table 23
Summary of studies and strength of evidence for subcutaneous immunotherapy and asthma outcomes in children and adolescents.
When considering the key evidence for the efficacy and effectiveness of subcutaneous immunotherapy in the treatment of rhinitis and rhinoconjunctivitis, the pertinent direct clinical outcomes include symptom scores, medication use, and quality of life. The strength of evidence regarding the effectiveness of subcutaneous immunotherapy is moderately supportive that this treatment improves rhinoconjunctivitis, but there is low grade evidence to support the use of subcutaneous immunotherapy to improve conjunctivitis symptoms and quality of life in children with rhinitis/rhinoconjunctivitis (Table 24).
Table 24
Summary of studies and strength of evidence for subcutaneous immunotherapy and rhinitis/rhinoconjunctivitis outcomes in children and adolescents.
Safety of Subcutaneous Immunotherapy in the Pediatric Trials
- Few studies reported adverse events.
- Lack of a consistent reporting system and grading system for subcutaneous immunotherapy precluded pooling safety data across studies.
- There were no reports of anaphylaxis or deaths
Adverse events were noted in 10 of the 13 studies for subcutaneous immunotherapy in children. The studies reported local and systemic reactions as either number of patients with reactions or the number of events per patient. Local reactions were reported in 7 studies. Four studies reported local swelling in 11 to 17 percent of patients.50,54,97,111,112 Three studies, with 10–20 patients in each arm, reported local reactions including redness and swelling, as events with a frequency of 0.25 to 21 events per patient.57,63,111 In one study there was a greater number of local reaction events per patient in the placebo group (20.9) than in the SCIT group (20.6).63 One study reported local injection edema in 1.1 percent of all injections with 11 events occurring in 4 patients.112
Eight studies reported systemic reactions in children receiving subcutaneous immunotherapy. Respiratory reactions were observed in 1 percent to 33 percent of patients in 2 studies.40,97 There were insufficient data to determine a difference in frequency of respiratory reactions between the active group and the comparator group. One study reported a respiratory reactions occurring with approximately 4 percent of all dust mite injections.57 Cutaneous reactions with urticaria were reported in two studies in 2 to 19 percent of patients.40,111 One study reported headache in 1 patient, 3 percent of 30 patients receiving subcutaneous immunotherapy, and mild facial flushing and redness in 2 patients with placebo injections.112 Unspecified mild systemic reactions were reported in 33 percent (n=5) of patients in one study,100 in 34 percent (n=21) of patients receiving SCIT and 7 percent of patients receiving placebo injections in another study,65 and as 2.8 events per patient with 45 unspecified systemic reactions occurring in 16 patients receiving SCIT.111 There were no reports of anaphylaxis (Appendix G, Evidence Table G18).
Conclusion: Summary of Evidence for Key Question 3 for Subcutaneous Immunotherapy
We did not observe any substantial difference in the efficacy of subcutaneous immunotherapy when considering monosensitized and polysensitized individuals. Little data exist about the following subpopulations: the elderly, pregnant women, minorities, inner-city residents, rural residents, and severe asthmatics, so the evidence is insufficient to comment on the effectiveness of this therapy in these subgroups. The limited available data suggest that subcutaneous immunotherapy is less beneficial in patients with severe asthma than in individuals with mild asthma. There are few studies that focused exclusively on children and adolescents. As a result, we found that the strength of evidence is weaker for the pediatric subpopulation than in the mixed adult and pediatric population. Tables 23 and 24 summarize the studies and the strength of evidence for subcutaneous immunotherapy affecting asthma and allergic rhinitis/rhinoconjunctivitis outcomes in the pediatric subpopulation.
Sublingual Immunotherapy
Study Characteristics
Sixty articles on sublingual immunotherapy were eligible for inclusion in this review. These 60 articles, with 4870 subjects, were published between 1993 and 2012. The publications originated from North America, Europe, and Asia. Sixty-one percent of studies had at least some industry support, although 8 studies had no identified funding source (Appendix E, Evidence Table E1). Twenty-two percent of the studies were rated as having a low risk of bias; 68 percent were rated as having a moderate risk of bias, and 14 percent were considered to have a high risk of bias (Appendix E, Evidence Table E4).
The primary diagnoses of the subjects studied in the articles included asthma in eight studies,117–124 rhinitis in seven studies,125–131 rhinoconjunctivitis in 14 studies,43 132–144 asthma and rhinitis in 17 studies,44,45,145–159 and asthma with rhinoconjunctivitis in 14 studies.160–172,173
Most studies enrolled adults only, although sixteen RCTs included both adults and children,122,124,129,132,140,142,145,149,153,159,167,169,170,172–174 and 18 exclusively studied children.117,120,121,130,144 131,138,141,148,152,154,157,158,160,163,164,168,171 Seven studies did not report sex 45,132,147,149,155,165,170 and the remainder enrolled both males and females (Appendix E, Evidence Table E2).
By design, all studies required subjects to have positive skin allergy testing and/or in vitro specific IgE allergy testing. Thirty-two studies (54%) required that the subjects had not received previous immunotherapy.44,45,117,120,124,126,128–130,132,133,135,138–140,145,148,149,152,154,157–159,161,162,164–166, 123,143,171,172 Eighteen studies (32%) focused on monosensitized individuals.43,44,117,120,124,130,132,138,144,146,152,153,155,158,161,162,166,171 Nine studies specifically excluded pregnant individuals43,123,126,128,129,143,149,165,169 (Appendix E, Evidence Table E1).
The majority of studies evaluated seasonal allergens (66 percent), followed by perennial allergens (31%); a small number of studies included both seasonal and perennial allergens (3%) (Appendix E, Evidence Table E1) The study allergens were grass/grass mix (in 15 studies),44,119,125,137–142,152,164–167,174 dust mite (in 14 studies),117,120,121,129–131,149,154–159,171 tree (in 13 studies),45,126–128,134–136,143,146,150,162,163,168 weeds/weed mix (7 studies),43,124,132,133,144,160,161 mixed or multiple allergens (7 studies),122,145,147,148,151,153,172 cat (2 studies),118,170 and mold (2 studies).123,169 (Figure 12). Half of the studies used only one allergen in their study protocols, while the other half used multiple allergens in their studies.
Figure 12
Sublingual immunotherapy studies by type of allergen.
The trials compared sublingual immunotherapy to placebo (71%), to another sublingual intervention without a placebo group (15%), or to a conventional treatment without placebo (pharmacotherapy or rescue medications) (14%) (Appendix E, Evidence Table E3). All studies allowed either conventional pharmacotherapy or rescue allergy medications in both the sublingual therapy arm and in the comparison arm. The maintenance dosing interval varied from daily to weekly, and the duration of treatment ranged from 3 months to 5 years. There was great heterogeneity in the reporting of the maintenance or cumulative dose delivered to the study participants, and the studies used a variety of units to report dosing.
Population Characteristics
The mean age range of subjects in the included studies was four to 74 years (Appendix E, Evidence Table E2). Forty-two percent of the studies reported the mean or minimum duration of disease among the enrolled participants. The range of mean duration of disease was one to 19 years. Race was not reported in any study.
Key Question 1. What is the evidence for the efficacy and effectiveness of sublingual immunotherapy in the treatment of allergic rhinitis/rhinoconjunctivitis and/or asthma?
Key Points
- High grade evidence supports that sublingual immunotherapy improves asthma symptoms based on 13 randomized controlled trials with 625 subjects.
- Moderate grade evidence supports that sublingual immunotherapy improves asthma or rhinitis/rhinoconjunctivitis (asthma combined scores) symptom control based on 5 randomized controlled trials with 308 subjects.
- Moderate grade evidence supports that sublingual immunotherapy improves rhinitis/rhinoconjunctivitis symptoms based on 35 randomized controlled trials with 2658 subjects.
- Moderate grade evidence supports that sublingual immunotherapy improves control of conjunctivitis symptoms based on 13 randomized controlled trials with 1074 subjects.
- Moderate grade evidence supports that sublingual immunotherapy decreases medication use based on 38 randomized controlled trials with 2724 subjects.
- Moderate grade evidence supports that sublingual immunotherapy improves allergy symptoms or decreases medication use based on 19 randomized controlled trials with 1462 subjects.
- Moderate grade evidence supports that sublingual immunotherapy improves disease-specific quality of life based on eight randomized controlled trials with 819 subjects.
Asthma Outcomes
Asthma symptom scores alone, or asthma with rhinitis/rhinoconjunctivitis symptom scores (asthma combined scores) were reported in 24 studies.43–45,117,120,121,123,124,131,137,140,145–147,150,153,154,156,158,160,164,168,169,171 (Appendix E, Evidence Table E5). As described in the Methods, asthma scores and asthma combined symptom scores were included from studies only if objective measures of lung function were used to diagnose subjects with asthma.
Asthma symptoms scores were reported in 13 studies (22%) 44,117,120,121,123,150,154,157,158,160,168,169,171 (Appendix E, Evidence Table E6). The types of scales used to report asthma symptoms scores were not uniform. Two studies used visual analog scores,117,160 one study counted number of days with asthma,150 and the remainder used numeric systems to score presence/absence of asthma symptoms and severity. One study compared sublingual immunotherapy with inhaled corticosteroids,44 another to montelukast,45 while the remainder used a placebo control group. The number of participants across studies ranged from 15 to 110. The duration of assessment ranged from one pollen season to 5 years.
All of the studies reporting asthma symptom scores demonstrated significant improvement in asthma symptoms with sublingual immunotherapy. Ten studies with asthma symptom scores demonstrated significant improvement in asthma symptoms with sublingual immunotherapy when compared with placebo;44,117,120,121,123,131,150,154,160,168 and eight studies demonstrated significant improvement in pre- versus post-treatment asthma scores in the sublingual immunotherapy arm.44,45,117,120,123,157,158,171 The study comparing sublingual immunotherapy to inhaled corticosteroids demonstrated significant improvement from pre-treatment scores in both the sublingual and inhaled corticosteroid groups.44 However, the participants receiving immunotherapy improved significantly more than those receiving inhaled corticosteroids. One study compared sublingual immunotherapy to montelukast,45 and found a greater improvement in asthma scores in the immunotherapy group. The most common single allergen used in the asthma scores was dust mite, in seven studies.117,120,121,154,157,158,171 All dust mite studies which reported asthma scores reported significant benefit with sublingual immunotherapy.
We conclude that there is high grade evidence that sublingual immunotherapy reduces asthma symptoms (Table 25).
Table 25
Body of evidence for sublingual immunotherapy affecting asthma symptoms.
Five trials of sublingual immunotherapy, involving 308 participants, reported asthma plus rhinitis or rhinoconjunctivitis symptoms scores in comparison to placebo or control.146,147,156,168,169 Study size ranged from 31 to 98 subjects. All studies used numeric scoring systems, but the types of scales used were not validated and varied between studies. One study compared sublingual immunotherapy with pharmacotherapy,147 while the remaining studies made comparisons to a placebo group. The duration of assessment ranged from one pollen season to 4 years.
Four studies reporting asthma plus rhinitis or rhinoconjunctivitis combined symptom scores demonstrated statistically significant positive effects on combined asthma plus rhinitis/rhinoconjunctivitis symptoms with sublingual immunotherapy;146,147,168,169 one study did not.156 Three studies demonstrated significant improvement in asthma symptoms when compared with controls.140,147,168 One study found significant improvement in total symptoms when compared with pharmacotherapy.147
Several studies reporting asthma plus rhinitis/rhinoconjunctivitis symptoms made comparisons with more than one sublingual group compared with placebo or medication. One study found no improvement with either high or low dose dust mite allergen therapy when compared with placebo.156 A study comparing high dose tree allergen, low dose tree allergen, and placebo found only the high dose had a significant impact on asthma combined scores when compared with placebo.168 A study of birch allergen alone, grass allergen alone, and birch plus grass allergens delivered sublingually compared with placebo found all groups to be significantly better then placebo in asthma combined scores.147 Finally, one study identified in our search compared co-seasonal grass sublingual immunotherapy to continuous therapy, but did not include any non-immunotherapy comparators; this was not included in grading this body of evidence.164 This study found continuous sublingual immunotherapy had a greater magnitude of effect in both asthma and combined asthma scores than co-seasonal sublingual immunotherapy.
We concluded that there is moderate evidence that sublingual immunotherapy reduces asthma and/or rhinitis or rhinoconjunctivitis symptoms (Table 26).
Table 26
Body of evidence for sublingual immunotherapy affecting asthma and/or rhinitis/or rhinoconjunctivitis symptoms.
Rhinitis or Rhinoconjunctivitis Symptoms
Rhinitis or rhinitis plus conjunctivitis symptoms scores were reported in 36 of the sublingual immunotherapy articles included in this review (Appendix E, Evidence Table E5).44,45,118,124–127,129–133,137–142,144,150,151,153–155,157–160,162,163,165,167,168,171,174 The types of scales used in the studies and the scoring systems were not uniform; the articles used numeric point systems to grade symptoms. The duration of assessment ranged from one pollen season to 6 years. In the studies reporting rhinitis/rhinoconjunctivitis scores, the most common allergen was grass or grass mix, followed by dust mite and tree/tree mix (Figure 13). The comparator group was placebo in all but three studies which compared immunotherapy to medication.44,45,138

Figure 13
Allergens used in studies of rhinitis/rhinoconjunctivitis symptoms (sublingual immunotherapy).
Fifty-six percent of sublingual immunotherapy studies reporting rhinoconjunctivitis symptoms demonstrated significant improvement in allergic rhinoconjunctivitis scores with sublingual immunotherapy. Two studies compared sublingual immunotherapy to medical treatment, one to inhaled budesonide44 and one to montelukast.45 Another study compared 2 years of immunotherapy to 3 years of immunotherapy without a control group129 and was not included in the body of evidence grading. The remainder of studies reported rhinitis/rhinoconjunctivitis scores compared with a placebo group. Therefore 35 studies compared sublingual immunotherapy to either placebo or medication and were included in the grading this body of evidence (Table 27).
Table 27
Body of evidence for sublingual immunotherapy affecting rhinitis/rhinoconjunctivitis symptoms.
Nine studies reporting rhinitis/rhinoconjunctivitis scores found significant improvement in the sublingual immunotherapy study group when comparing pre-treatment to post-treatment rhinoconjunctivitis symptom scores.44,45,118,124,140,153,155,159,171 Fourteen studies found significant improvement in rhinitis/rhinoconjunctivitis scores when compared with placebo.44,45,118,125,126,132,133,139,140,144,150,157,165,168 The single study comparing 2 years to 3 years of sublingual immunotherapy found rhinitis symptoms at the 6 year evaluation to be significantly reduced in the 3-year treatment group compared with the 2-year treatment group.129
We conclude that there is moderate grade evidence that sublingual immunotherapy improves control of rhinitis or rhinoconjunctivitis symptoms, particularly with grass mix allergens (Table 27).
Conjunctivitis Symptoms
Thirteen studies of sublingual immunotherapy reported conjunctivitis symptom scores (Appendix E, Evidence Table E7).124,131,132,137,140,142,153,157,160,162,163,168,174 The comparator in all studies reporting conjunctivitis scores was placebo. All of the studies used a numeric scale when reporting the symptoms, but none of the scales appeared to be validated or consistent between studies. One study had separate scores reported for ocular redness and ocular pruritus.142 The duration of assessment ranged from one pollen season up to 2 years.
Forty-six percent of the studies demonstrated significant improvement in conjunctivitis symptom scores when compared with placebo or to pre-treatment symptom levels in the sublingual immunotherapy arm. Three studies demonstrated improvement with sublingual immunotherapy when compared with placebo during peak season or the entire pollen season.140,142,168 Two studies demonstrated significant improvement pre- versus post-treatment in the sublingual arms. 124,147
We conclude that there is moderate grade evidence that sublingual immunotherapy reduces conjunctivitis symptoms based on 13 studies (Table 28).
Table 28
Body of evidence for sublingual immunotherapy affecting conjunctivitis symptoms.
Medication Use
Medications scores were reported in 40 of the sublingual immunotherapy trials included in this review (Appendix E, Evidence Table E8).44,45,117,120,121,127,130–133,135,137–142,146,147,149,151,154,156,159–165,167,168, 123,124,143,144,153,158,169,174 However, two studies were not included in the grading of the body of evidence due to the lack of a control group not receiving sublingual immunotherapy.149,164 Therefore, 38 studies were included in grading this body of evidence (Table 29). All of the studies used some type of numeric scoring scale for medication use, but none of the scales or scoring appeared to be validated or consistent between studies. The duration of assessment of medication scores ranged from one pollen season up to 5 years. The medication use that was scored varied from study to study and included such medications as inhaled beta-agonists and corticosteroids for control of pulmonary symptoms as well as oral antihistamines and intranasal corticosteroids.
Table 29
Body of evidence for sublingual immunotherapy affecting medication use.
Forty-seven percent of the studies reporting medication scores in the body of evidence demonstrated significant improvement in this domain with sublingual immunotherapy. Fifteen of the 38 studies with medication scores reported significant improvement in medication scores when compared with controls. 44,45,117,123,133,138,140,143,146,147,158,162,165,168,176 In four of these studies the comparator group was pharmacotherapy or conventional treatment,45,138,143,147 and in the remaining 11 studies the comparator was placebo. Five studies demonstrated significant improvement in pre-treatment versus post-treatment medication scores in the sublingual immunotherapy arms.44,120,124,143,161
Grass mix was the most frequently studied allergen, with 9 studies reporting medication scores; five showed benefit from sublingual immunotherapy,44,138,140,142,165 but four studies demonstrated no improvement.139,141,167,174 Medication scores were reported in 8 studies with dust mite; of these, two studies found statistically significant improvement in medications scores,117,120 while six did not show significant benefit in medication use.121,130,131,154,156,159 Five trials of Parietaria immunotherapy studies reported medication scores; three showed significant improvement,124,161,176 while two found no improvement.144,160
The two studies that did not include a non-sublingual control group were not included in the body of evidence. One compared co-seasonal to continuous grass sublingual immunotherapy and found no significant difference in medication scores.164 The second study compared 3, 4, and 5 years of dust mite sublingual immunotherapy.149 After 20 years, the longest treatment group had a stronger magnitude of effect when compared with the shortest treatment group.
We conclude that there is moderate grade evidence that sublingual immunotherapy reduces medication use based on 38 studies with 2724 subjects (Table 29).
Combined Symptom and Medication Scores
Combined symptom plus medication scores were reported in 21 of the sublingual immunotherapy studies included in this review and involved 1312 subjects (Appendix E, Evidence Table E9).43,126–128,134,135,138,139,159,161,164,166 122–124,133,143,144,147,149,153 However, 2 studies did not include a non-sublingual comparator group and were not included in the body of evidence grading.149,164 Therefore, 19 studies were included in the body of evidence grading (Table 30).
Table 30
Body of evidence that sublingual immunotherapy affects combined medication use and symptoms.
All of the studies used some type of numeric scoring scale for the combination score, but none of the scales or scoring appeared to be validated or consistent between studies. The duration of assessment of medication scores ranged from one pollen season up to 4 years. The symptoms scored as part of the studies were combined nasal, eye, and bronchial in the majority of studies; exceptions were five studies that included only nasal symptoms.126–128,135,159 The medications scored varied from study to study and included such medications as inhaled beta-agonists and corticosteroids for control of pulmonary symptoms as well as oral antihistamines and intranasal corticosteroids.
Thirteen (68%) of the studies reporting a combination symptom plus medication score demonstrated significant improvement in scores with sublingual immunotherapy. Ten of the 13 studies with combination symptom plus medication scores reported significant improvement in medication scores when compared with controls.43,122,123,126,128,133,143,148,159,166 In three of these studies, the comparator groups was pharmacotherapy/conventional treatment,43,122,143 and in the remaining seven studies the comparator was placebo. Five studies demonstrated significant improvement in pre-treatment versus post-treatment medication scores in the sublingual immunotherapy arms.122,124,147,159,161 Three studies of Parietaria allergen reported combination symptom plus medications scores: all three found significant improvement in scores.43,124,161 Four studies of Japanese cedar allergen126–128,135 produced mixed results, as did three grass mix studies.138,139,166
The two studies not included in the body of evidence compared different sublingual groups.149,164 One compared differing lengths of dust mite sublingual immunotherapy, but p-values were not reported and magnitude of effect was unable to be determined. The second study compared co-seasonal to continuous sublingual immunotherapy and found no difference in reported medication plus symptom score.
We conclude that there is moderate grade evidence that sublingual immunotherapy reduces medication use and improves symptom control (Table 30).
Quality of Life
Quality of life was reported in eight studies involving 819 subjects.127,128,131,134,135,141,153,155 The instrument used to assess quality of life was a validated, disease-specific instrument: The Rhinoconjunctivitis Quality of Life questionnaire (Adult, Pediatric, Adolescent, and Japanese language versions). Four of the eight studies reported significant improvement in disease-specific quality of life when compared with placebo.127,128,134,135 (Appendix E, Evidence Table E11). Two studies reported significant improvement in the sublingual immunotherapy group when comparing initial to final quality of life scores.153,155 One study found no improvement in quality of life either compared with control group or with pre-treatment quality of life scores.141
We concluded that there is moderate grade evidence that sublingual immunotherapy improves disease-specific quality of life (Table 31).
Table 31
Body of evidence that sublingual immunotherapy affects disease-specific quality of life.
Pulmonary Function Testing and Chemical Bronchial Provocation
Pulmonary function testing results were reported in 14 studies involving 1375 subjects (Appendix E, Evidence Table E10). Pulmonary function results described here are from studies where subjects had a diagnosis of asthma that was objectively confirmed with methods other than clinical impression. As pulmonary function tests are not a direct clinical outcome, this evidence was not graded as a body of evidence. The studies reported measures of pulmonary function, but were heterogeneous in terms of which measures were reported: FEV1 was most commonly reported, but other measures included percent of patients with a positive methacholine challenge, peak expiratory flow rate (PEF), forced vital capacity (FVC), and PD20, the dose a substance administered by aerosol, which causes the FEV1 to fall by 20 percent.
All studies reported either significant improvement compared with controls or when considering pre- versus post-treatment pulmonary function. Six of ten studies reported a significant improvement when comparing pre-treatment to post-treatment FEV1 in groups treated with sublingual immunotherapy,120–122,147,157,171 and two reported a significant improvement in the FEV1 of the sublingual immunotherapy group when compared with controls.45,122 Two trials reported a significant decrease in the number of participants with a positive methacholine challenge in the sublingual immunotherapy group when compared with controls.145,148 Four studies reported a significant decrease in PD20 compared with controls,44,45,147,149, and three also demonstrated significant improvement when comparing post-treatment to pre-treatment scores.44,45,147 We did not grade the evidence for indirect outcomes such as pulmonary function test results. However, we observed that sublingual immunotherapy consistently improves measure of pulmonary function in the allergic asthmatic population.
Allergen Challenge (Provocation)
Ten studies of sublingual immunotherapy studies challenged subjects to specific allergen after treatment in order to quantify symptoms (Appendix E, Evidence Table E11). Six studies used nasal provocation.129,143,151,154,157,161 Three studies performed conjunctival provocation tests. 123,137,144 One study provoked cat-allergic subjects by having them remain in a “cat allergen” room.170 Seventy percent of the studies using a specific ocular or nasal allergen challenge reported a significant improvement in symptoms in the sublingual immunotherapy groups. Two studies used bronchial challenges.156,157 Both studies found significant improvement in pulmonary function testing with the dust mite bronchial challenge after sublingual immunotherapy.
Long-Term Outcomes: Disease Modification, Disease Prevention
In our review, we sought information regarding long-term outcomes in allergic rhinitis and asthma (Appendix E, Evidence Table E12). Disease modification in asthma was addressed in two studies included in this review.121,148 A study by Niu et al found that sublingual immunotherapy with dust mite in children (ages 6 to 12 years) decreased the severity of asthma over 6 months of treatment when compared with controls (p=0.043).121 Severity in this study was determined by a global assessment by physicians unfamiliar with the patient who reviewed the asthma scores, medication consumption, and pulmonary function tests. In a study of 216 children undergoing sublingual immunotherapy with dust mite, tree, and grass, Marogna found a significantly lower percentage of children with mild persistent asthma at the conclusion of the study.148
Asthma prevention was reported in one of the sublingual immunotherapy studies,138 and in one 8-year followup to a prior study. 144 Novembre et al. found that grass pollen sublingual immunotherapy in children significantly decreased the development of asthma over 3 years;138 controls in this study developed asthma 3.8 times more frequently. However, in the 8-year follow-up study, 2 years of sublingual immunotherapy had no asthma preventative effect.144
Prevention of new allergy sensitivities was discussed in three studies. Marogna found that treatment with multi-antigen sublingual immunotherapy (dust mite, birch, weeds, and grass mix) decreased the development of new skin sensitizations significantly (p=0.01);145 he reported in a second study that the proportion of children with new allergen skin sensitivities was significantly decreased after 3 years.148 However, in a different study with 8-year follow-up, there was no preventative effect on the development of new sensitivities 2 years after Parietaria sublingual immunotherapy. 144 In a 2010 study by Marogna comparing 3, 4, and 5 years of sublingual immunotherapy; in the 5 year group, 11.7 percent developed new sensitivities compared with 21.4 percent in the 3 year group.
Other Outcomes
Adherence
Adherence and compliance were discussed infrequently in the articles, but were discussed by Marogna.145 Adherence was determined by measuring the amount of remaining extract in returned vials compared with expected consumption as prescribed: poor adherence was less than 40 percent consumption, insufficient was less than 60 percent consumption, good was 60 to 80 percent consumption, and excellent was more than 80 percent consumption. Adherence was found to be excellent in 76 percent of subjects and good in 18 percent of subjects. In a second study by the same author, adherence was found to be excellent in 74 percent of subjects.148 Another study reported that 14 percent of subjects had poor compliance, and 48 percent of subjects forgot to take their medications from time to time. 200 In a 2010 study of 15 patients, adherence was greater than 80 percent in 10 subjects, and greater than 60 percent in five subjects.45 Another 2010 study found adherence 85–95 percent determined by the residual volume of extract in returned vials123 (Appendix E, Evidence Table E11).
Single Versus Multiple Antigen Sublingual Immunotherapy
Two sublingual studies included in this review examined single versus multi-antigen immunotherapy.147,151 The first of these articles, by Amar, compared Timothy Grass monotherapy to Timothy Grass multi-antigen therapy, consisting of Timothy Grass plus 9 other allergens.151 This study included one outcome of interest to the current review, nasal allergen challenge. While nasal challenge with Timothy Grass yielded significantly better results when comparing timothy monotherapy to placebo, there was no difference in Timothy Grass multi-antigen versus placebo. In Marogna’s paper, 3 groups were compared: sublingual birch, sublingual birch plus grass, and pharmacotherapy.147 Marogna found that the multi-antigen treatment group had significantly greater improvement in clinical symptoms when compared with the single antigen group. The data is insufficient to comment on effectiveness of single versus multiple antigen sublingual immunotherapy.
Biomarkers
During the course of the review, the number of studies reporting select biomarkers was recorded: IgG total, IgG4, and IgE. Eleven studies reported changes in specific IgG, 28 study-specific IgG4, and 32 IgE (total and/or specific IgE (Appendix E, Evidence Table E13).
Conclusion: Summary of Evidence for Key Question 1
When considering the key evidence for the efficacy and effectiveness of sublingual immunotherapy in the treatment of allergic rhinoconjunctivitis and/or asthma, the pertinent clinical outcomes include symptom scores, medication use, and quality of life. Pulmonary function testing is a useful, objective, indirect measure of asthma that can be measured by clinicians in the office.
The strength of evidence regarding the effectiveness of sublingual immunotherapy is moderately supportive that this treatment improves clinical outcomes (Table 32).
Table 32
Summary of strength of evidence regarding the effectiveness of sublingual immunotherapy.
Key Question 2. What is the evidence for the safety of sublingual immunotherapy in patients with allergic rhinitis/rhinoconjunctivitis and/or asthma?
Key Points
- Local reactions (occurring at the site of allergen administration) were common across trials
- Systemic reactions were uncommon
- No life threatening systemic reactions or anaphylaxis were reported in these trials
- No deaths were reported
Figure 14 shows the distribution of events by location and severity. The graph shows only adverse events reported in the immunotherapy arms.

Figure 14
Sublingual immunotherapy safety data by location and severity. AE = adverse event; GI = gastrointestinal
We evaluated the safety of sublingual immunotherapy in the treatment of allergic rhinoconjunctivitis and/or asthma by assessing the harms or adverse events reported in the included studies.
All 60 sublingual articles were analyzed for safety data. The studies did not uniformly report safety information, although 73 percent commented on safety.117,118,121,122,125–127,129–132,134,136–142,144–149,151–157,159,160,162,163,166–169,172–175,177 The safety data were not reported in any consistent manner between studies, as there is no standard system for grading adverse events associated with sublingual immunotherapy. Because of the lack of a standard grading system and the heterogeneous reporting systems used by the different studies, the safety outcomes are presented descriptively and we conclude that the evidence is insufficient to comment about safety.
Local reactions were much more frequent in the groups receiving sublingual immunotherapy than in the comparator groups. In those studies in which local reactions occurred and were reported by percent of patients affected, the percent of subjects receiving immunotherapy with local reactions ranged from 0.2 to 97 percent. The placebo groups in which local reactions were reported ranged from 3 to 38.5 percent (Appendix E, Evidence Table E14). The local reactions were mild or unspecified in severity
Systemic reactions were more common in the groups receiving sublingual immunotherapy than in comparator groups. The reactions ranged from ocular, rhinitis/nasal, respiratory/asthma, cutaneous, gastrointestinal and cardiovascular Overall, there were few severe systemic reactions with a small number of exceptions: in one study, severe rhinitis was reported in subjects that exceeded their maximum dose of immunotherapy; in this same study, severe asthma symptoms were reported in subjects that exceeded their maximum dose.157 These adverse events resolved when these subjects returned to a lower dose. There were no reported episodes of anaphylaxis, life threatening reactions, or death in any of the treated subjects across studies.
Key Points: Pediatric Studies
- Local reactions (occurring at the site of allergen administration) were more common across trials
- Systemic reactions were less common
- No life threatening systemic reactions or anaphylaxis were reported in these trials
- No deaths were reported
Evidence Synthesis
All eighteen articles about sublingual immunotherapy in children were analyzed for safety data. The studies did not uniformly report safety information, and 15 studies (83%) commented on safety.117,131,138,144,148,152,154,157,163,168,121,130,164,178 The safety data was not reported in any consistent manner between studies, as there is no standard system for grading adverse events associated with sublingual immunotherapy. Because of the lack of a standard grading system and the heterogeneous reporting systems used by the different studies, the safety outcomes are presented descriptively and we concluded that the evidence is insufficient to comment about safety.
Local reactions were reported in 12 studies and were more frequent in the groups receiving sublingual immunotherapy than in the comparator groups. The local reactions were mild or unspecified. Three small studies reported local adverse reactions by number of events, and the average number of episodes of local reactions per participant in the sublingual arm ranged from 25 to 40 per 100 participants.144,154,163 Local reactions were also reported in the placebo arms, ranging from seven to 19 per 100 participants (Appendix G, Evidence Table G31). Seven studies reported local reactions by percent of patients affected, and the percent of sublingual subjects with local reactions ranged from 0.7 to 50 percent.117,131,138,148,152,163,168 Three studies reported local reactions in the placebo group ranging from 14 to 25 percent131,152,168 (Appendix G, Evidence Table G31).
Overall, there were few systemic reactions reported in eight studies. The reactions ranged from (in order of greatest to least number of studies reporting event): gastrointestinal, cutaneous, respiratory/asthma, cardiovascular, and rhinitis/nasal. Eight studies compared the occurrence of reactions in the sublingual and placebo arms.117,131,138,144,152,154,163,168 In one of these studies, cutaneous systemic reactions were noted in 1.9 percent of 54 patients receiving sublingual immunotherapy, comparable to or less than the two placebo arms of 1.7 percent and 9.8 percent.138 The other comparative study described a greater number of gastrointestinal events (nausea, abdominal pain, diarrhea) and reported 95 events per 100 patients receiving sublingual immunotherapy (20 patients in SLIT arm) compared with 5 events per 100 patients in the placebo arm (21 patients in placebo arm).144 Another study had greater numbers of patients with 65 percent experiencing respiratory reactions in the placebo group compared with 57 percent of patients in the sublingual immunotherapy group.131 Three studies reported cutaneous systemic reactions (rash, urticaria, angioedema) as percentage of patients, ranging from 0.7 percent in a study with 144 patients to 10 percent of patients in a study with 30 patients138,148,157 Four studies reported gastrointestinal events as percent of patients with reactions, ranging from 0.7 to 11.4 percent. 138,148,157,168 One study reported rhinitis/nasal reactions with 0.7 events per 100 patients (1 asthma event/144 patients in SLIT arm).148 Two studies reported lower respiratory reactions as percent of patients, ranging from 7 percent in a study with 15 patients per arm to 34 percent in a study with 32 patients per arm.154,157 While few severe systemic reactions were reported, in one of these studies, severe rhinitis and severe asthma symptoms were reported in subjects that exceeded their maximum dose.157 These adverse events resolved when these subjects returned to a lower dose. There were no reported episodes of anaphylaxis, life threatening reactions, or death in any of the treated subjects across studies.
Conclusion: Summary of Evidence for Key Question 2
The lack of consistent reporting and grading systems for sublingual immunotherapy made it impossible to pool safety data across studies. Furthermore, not all studies reported safety data. However, it appears that local reactions are common but mild. Systemic reactions can occur but are infrequent; no life-threatening reactions, anaphylaxis, or deaths were reported. The evidence is insufficient to comment on the safety of sublingual immunotherapy, both in adult and pediatric studies.
Key Question 3. Is the safety and effectiveness of sublingual immunotherapy different in distinct subpopulations with allergic rhinitis/rhinoconjunctivitis and/or asthma?
Key Points
- The evidence is insufficient to comment on the effectiveness of sublingual immunotherapy in the following subpopulations: the elderly, pregnant women, minorities, inner-city, and rural residents, and severe asthmatics.
- There is low evidence to support that there is difference in the effectiveness of sublingual immunotherapy for treating mono-sensitized individuals and poly-sensitized individuals.
Our review sought information on particular subgroups of patient populations of interest, including pediatric, the elderly, pregnant, minorities, and inner-city versus rural subjects. The reviewed articles did not present specific data on the following subgroups: elderly, pregnant women, minorities, inner-city, and rural residents. The articles in general excluded subjects with severe asthma. Insufficient data exist to comment on these subpopulations. However, 32 percent of the studies were performed on mono-sensitized subjects (Table 26-General summary table SLIT). There appears to be no consistent difference in effectiveness when considering mono-sensitized compared with poly-sensitized subjects and the effect of sublingual immunotherapy. Eighteen pediatric studies of sublingual immunotherapy were reviewed as a distinct subpopulation.
Sublingual Immunotherapy in the Pediatric Population
Eighteen studies focused exclusively on children117,120,121,130,131,138,141,144,148,152,154,157,158,160,163,164,168,171 and four studies included both children and adults145,165,170,174 The subgroup analysis for the pediatric population evaluates the 18 studies that only include children 18 years of age or younger. All articles included were randomized controlled trials which reported clinical outcomes. These 18 articles with a total of 1583 subjects comprised the evidence base to answer the Key Questions regarding sublingual immunotherapy for inhalant allergens in the pediatric population. The publication dates of the included studies ranged from 1990 through 2011. The publications originated from Europe and Asia. The primary diagnoses of the subjects studied in the articles included: asthma in three studies;117,120,121 rhinitis in two studies;130,131 rhinoconjunctivitis in four studies;138,141,144,152 asthma and rhinitis in four studies;148,154,157,158 and asthma with rhinoconjunctivitis in five studies160,163,164,168,171 (Appendix G, Evidence Table G18).
Studies included perennial and/or seasonal allergens. There were nine studies each evaluating perennial and seasonal allergens for sublingual immunotherapy (Appendix G, Evidence Table G18). When considering the specific types of allergens used in the studies, these allergens were used from greatest to least frequency: dust mite (9 studies) 117,120,121,130,131,154,157,158,171 grass (4 studies),138,141,152,164 tree (2 studies),163,168 weeds (2 studies)144,160 and mixed or multiple allergens (1 study).148 (Figure 4, SLIT Studies by Allergen) The majority of the studies used multiple allergens (60%), with the remaining studies using only one allergen (40%) in their study protocols. Eleven studies (61%) required no prior history of immunotherapy.117,120,130,138,148,152,154,157,158,164,171 Eight studies (44%) focused on monosensitized individuals.117,120,130,138,144,152,158,171
The funding sources for the studies included the following, from most common to least common: industry, not stated, government, nonprofit, and other. Eleven studies (61%) had industry support, either partial or complete funding or received supplies from industry. Four studies did not identify the funding source for their study. One study was funded by academia. 152
All included sublingual immunotherapy studies had at least one comparator group. The comparator group(s) included the following (Appendix G, Evidence Table G20): placebo (15 studies), other sublingual comparator group (3 studies), conventional treatment (pharmacotherapy) or symptomatic therapy comparator group (2 studies, 20%). All studies allowed either conventional pharmacotherapy (12 studies) or only rescue allergy medications (6 studies) during the study. Maintenance dosing interval varied from daily to twice a week. Duration of treatment of the included studies ranged from 6 months to 3 years. Studies used various units to report dosing, and many studies did not include a cumulative dose. Subjects ranged from 4 to 18 years of age. All studies that reported sex included both boys and girls. The range of means for duration of disease was 1 to 5.2 years.
Key Points
- The efficacy and effectiveness of sublingual immunotherapy in the treatment of allergic rhinoconjunctivitis and/or asthma were evaluated in these categories of treatment effect: clinical endpoints, long-term outcomes, biomarker endpoints, convenience of therapy, and adherence to therapy.
- Pertinent clinical outcomes evaluated include symptom scores, medication use, and quality of life.
- High strength of evidence exists for the benefit of sublingual immunotherapy in asthma symptom control versus control groups, based on nine randomized controlled trials with 471 subjects.
- Moderate strength of evidence exists for the benefit of sublingual immunotherapy in asthma plus rhinitis/rhinoconjunctivitis (asthma combined scores) symptom control versus control groups, based on one randomized controlled trial with 98 subjects.
- Moderate strength of evidence exists for the benefit of sublingual immunotherapy in rhinitis/rhinoconjunctivitis symptom control versus control groups based on 12 randomized controlled trials with 1065 subjects.
- Moderate strength of evidence exists for the benefit of sublingual immunotherapy in control of conjunctivitis symptoms versus control groups, based on five randomized controlled trials with 513 subjects.
- Moderate strength of evidence exists for the benefit of sublingual immunotherapy versus control on decreasing medication use, based on 13 randomized controlled trials with 1078 subjects.
- Low strength of evidence exists for the benefit of sublingual immunotherapy versus control on improving allergy symptoms plus decreasing medication use based on two randomized controlled trials with 329 subjects.
- Insufficient evidence exists for the benefit of sublingual immunotherapy versus control on improving disease-specific quality of life, based on two randomized controlled trial with 461 subjects.
- The overall strength of evidence for use of sublingual immunotherapy in children and adolescents when considering all domains with pertinent clinical outcomes together is moderate.
We evaluated the efficacy and effectiveness of sublingual immunotherapy in the treatment of allergic rhinoconjunctivitis and/or asthma by using the following evaluable categories of treatment effect: clinical endpoints, long-term outcomes, biomarker endpoints, convenience of therapy, and adherence to therapy.
Asthma Symptom Control
Asthma symptom scores alone, or asthma with rhinitis/rhinoconjunctivitis symptom scores (asthma combined scores), were reported in 11 studies (61%) 117,120,121,131,154,157,158,160,164,168,171 (Appendix G, Evidence Table G22). Asthma scores and asthma combined symptom scores were included from studies only if objective measure of lung function were used to diagnose subjects with asthma; studies using clinical symptoms only for the diagnosis of asthma were not included in the asthma symptom scores analyzed.152,163
The types of scales used to report asthma symptoms scores were not validated or uniform. Two studies used visual analog scores,117,160 and the remainder used purely numeric systems to score the presence/absence of asthma symptoms and severity. 120,121,154,157,158,160,168,171,157, 164 The number of participants in each study ranged from 15 to 257. The duration of assessment ranged from one pollen season to 5 years. All of the studies used a placebo control group, except for one study that compared SLIT given continuously versus co-seasonally,164 therefore its results are not included in the evidence grading table. One study additionally reported rhinitis symptoms scores and is also categorized as asthma combined symptom scores.168 Asthma combined symptom scores include asthma plus rhinitis or rhinoconjunctivitis symptoms.
All the studies reporting asthma symptoms scores demonstrated significant improvement in asthma symptoms with sublingual immunotherapy. Six studies with asthma symptom scores demonstrated significant improvement in asthma symptoms with sublingual immunotherapy when compared with placebo;117,120,121,131,154,160 six studies demonstrated significant improvement in pre-versus post-treatment asthma scores in the sublingual immunotherapy arm.117,120,157,158,164,171
In seven studies, the most common single allergen used in the asthma scores was dust mite.117,120,121,131,154,157,158,171 All dust mite studies with asthma scores reported significant improvement in asthma scores with sublingual immunotherapy.
Nine studies fulfilling asthma diagnosis criteria reported on asthma symptom scores and included 471 participants. All included studies are randomized controlled trials. The overall strength of evidence is high to support sublingual immunotherapy use to improve asthma symptoms scores (Table 33).
Table 33
Body of evidence for sublingual immunotherapy affecting asthma symptoms in children and adolescents.
Asthma Plus Rhinoconjunctivitis Symptom Scores
Two trials of sublingual immunotherapy, involving 98 and 80 participants, reported combined symptoms scores.164,168 In the first study by Valovirta et al, the “Asthma combined symptom score” included asthma plus rhinoconjunctivitis symptoms and used numeric scoring systems. This study, with medium risk of bias and comparing sublingual immunotherapy to placebo over the whole pollen season, demonstrated statistically significant positive effects on combined asthma plus rhinoconjunctivitis symptoms with sublingual immunotherapy. The second study by Pajno et al, was a medium risk of bias trial and compared SLIT coseasonal to SLIT continuous, with a weak magnitude of effect. Because this study does not have a placebo comparator, it was not included in the evidence grading.
We conclude that there is moderate evidence that sublingual immunotherapy reduces asthma and/or rhinitis or rhinoconjunctivitis symptoms (Table 34).
Table 34
Body of evidence for sublingual immunotherapy affecting asthma plus rhinitis/rhinoconjunctivitis symptoms in children and adolescents.
Rhinitis or Rhinoconjunctivitis Symptoms
Rhinitis or combined rhinitis plus conjunctivitis symptom scores were reported in 12 (67%) of the sublingual immunotherapy articles included in this review (Appendix G, Evidence Table G23). The types of scale used in the studies and the scoring systems were not uniform; the articles utilized numeric point systems to grade symptoms or the mean daily total of all rhinitis symptoms. The duration of assessment ranged from 6 months up to three years. In the studies reporting rhinitis/rhinoconjunctivitis scores, the most common allergen used was dust mite, used in six studies, followed by grass mix and Parietaria in two studies each, and olive or tree mix in one study each. The comparator group was placebo in all studies. One study also compared high and low dose sublingual immunotherapy.168
Overall, five of the 12 (42%) sublingual immunotherapy studies reporting rhinoconjunctivitis symptoms demonstrated significant improvement in allergic rhinoconjunctivitis scores with sublingual immunotherapy. Eleven studies compared sublingual immunotherapy to placebo, and two of these eleven studies (18%) found significant improvement in rhinitis/rhinoconjunctivitis scores with sublingual immunotherapy. 144,168 Four studies compared pretreatment to post-treatment rhinoconjunctivitis symptom scores in the sublingual immunotherapy study group,130,157,158,171 and significant improvement was found in three of the four studies.157,158,171
We conclude that there is moderate grade evidence that sublingual immunotherapy improves control of rhinitis or rhinoconjunctivitis symptoms (Table 35).
Table 35
Body of evidence for sublingual immunotherapy for rhinitis/rhinoconjunctivitis symptoms in children and adolescents.
Conjunctivitis Symptoms
Twenty-eight percent of the sublingual immunotherapy studies reported conjunctivitis symptom scores (Appendix G, Evidence Table G24). There were 5 trials involving 513 subjects.131,157,163,168 The comparator in all studies reporting conjunctivitis scores was placebo. All of the studies used a numeric scale when reporting the symptoms, but none of the scales appeared to be validated or consistent between studies. The duration of assessment ranged from one pollen season up to 18 months.
Two of the 4 studies demonstrated significant improvement in conjunctivitis symptom scores when compared with placebo or to pre-treatment symptom levels in the sublingual immunotherapy arm.
We conclude that there is moderate grade evidence that sublingual immunotherapy reduces conjunctivitis symptoms (Table 36).
Table 36
Body of evidence for sublingual immunotherapy for conjunctivitis symptoms in children and adolescents.
Medication Use
Medications scores were reported in 14 (77%) of the pediatric sublingual immunotherapy trials included in this review (Appendix G, Evidence Table G25). All of the studies used a placebo or control group, except for one study that compared SLIT given continuously versus coseasonally;164 therefore its results are not included in the evidence grading table. These 13 studies included 1078 participants. All of the studies used some type of numeric scoring scale for medication use, but none of the scales or scoring appeared to be validated or consistent between studies. The duration of assessment of medication scores ranged from 6 months or one pollen season up to three years. The medications scored varied from study to study and included such medications as inhaled beta agonists and corticosteroids for control of pulmonary symptoms as well as oral antihistamines and intranasal and oral corticosteroids.
Four of the 13 (42%) studies reporting medication scores demonstrated significant improvement in this domain with sublingual immunotherapy. Four of the 13 studies with medication scores reported significant improvement in medication scores when compared with controls.117,138,158,168 In one of these 4 studies, the comparator group was pharmacotherapy or conventional treatment;138 in the remaining studies the comparator was placebo. One study demonstrated significant improvement in pre-treatment versus post-treatment medication scores in the sublingual immunotherapy arms.120
Six studies of dust mite allergen reported medications scores: two low-medium risk of bias studies found significant improvement in medications scores117,120 while four medium-high risk of bias studies did not show significant benefit in medication use.121,130,131,154 Two trials of Parietaria immunotherapy studies reported medication scores and found no improvement.144,160 Two grass mix studies reported medication scores: one large, high risk of bias study showed a strong benefit from sublingual immunotherapy,138 and the other large, low risk of bias study demonstrated no improvement.141
We conclude that there is moderate grade evidence that sublingual immunotherapy reduces medication use (Table 37).
Table 37
Body of evidence for sublingual immunotherapy for medication scores in children and adolescents.
Combined Symptoms Plus Medication Scores
Combined symptom plus medication scores were reported in two of the sublingual immunotherapy studies included in this review and involved 329 subjects138,148 (Appendix G, Evidence Table G26). The duration of assessment of medication scores was three years for both studies, and symptom scores included nasal, eye, and bronchial symptoms. The medications scored varied from study to study. Medications in one study included nasal mast cell inhibitors, oral antihistamines, intranasal corticosteroids,148 inhaled beta agonists and corticosteroids for control of pulmonary symptoms as well as oral antihistamines and intranasal corticosteroids. Medications allowed in the other study included oral antihistamines, nasal corticosteroids, bronchodilators, and ocular corticosteroids.138
One study reporting a combination symptom plus medication score demonstrated significant improvement with sublingual immunotherapy when compared with controls.148 One study of grass mix allergen showed no significant difference between sublingual immunotherapy and conventional therapy.138
We conclude that there is low evidence that sublingual immunotherapy reduces combined medication use and symptom scores (Table 38).
Table 38
Body of evidence for sublingual immunotherapy for combined symptom plus medication scores in children and adolescents.
Quality of Life
Quality of life was reported in two studies involving 461 subjects.131,141 The instruments used to assess quality of life in both studies were validated, disease specific instrument: The Pediatric and Adolescent Rhinoconjunctivitis Quality of Life questionnaires. One study found no improvement in quality of life.141 The other study found no difference between SLIT and placebo groups in both children and adolescents after 2 years131 (Table 39). (Appendix G, Evidence Table G27).
Table 39
Body of evidence that sublingual immunotherapy affects disease-specific quality of life in children and adolescents.
We conclude that there is insufficient evidence that sublingual immunotherapy affects disease-specific quality of life in children and adolescents.
Pulmonary Function
Pulmonary function testing results were reported in five studies involving 490 subjects. (Appendix G, Evidence Table G28). Pulmonary function results described here are from studies where subjects had a diagnosis of asthma that was objectively confirmed with methods other than clinical impression. The studies reported measures of pulmonary function, but were heterogeneous in terms of which measures were reported: FEV1 was most commonly reported, but other measures included percent of patients with a positive methacholine challenge, PEF, and FVC.
All studies reported either significant improvement compared with controls or when considering pre- versus post-treatment pulmonary function. Four of five studies reported a significant improvement when comparing pre-treatment to post-treatment FEV1 in groups treated with sublingual immunotherapy.120,121,157,171 One trial reported a significant decrease in the number of participants with a positive methacholine challenge in the sublingual immunotherapy group when compared with controls.148
Allergen and Nonspecific-Chemical Challenge (Provocation)
Three of the sublingual immunotherapy studies challenged subjects to a specific allergen after treatment in order to quantify symptoms (Appendix G, Evidence Table G28). Two studies used nasal provocation.154,157 One of the nasal provocation studies found significant improvement in the sublingual immunotherapy arm before and after treatment after 1 year, although no difference was noted between the sublingual and placebo arms.154 The other nasal provocation study also found a significant improvement in the sublingual immunotherapy arm before and after treatment,157 but did not compare between the sublingual and placebo arms. One study performed conjunctival provocation tests and found significant improvement in response with sublingual immunotherapy compared with placebo.144 Two of the three studies using a specific allergen challenge reported a significant improvement in symptoms in the sublingual immunotherapy groups. One study also used bronchial challenges and found significant improvement in FEV1 with the dust mite bronchial challenge after sublingual immunotherapy.157
Long-Term Outcomes: Disease Modification, Disease Prevention
In our review, we sought information regarding long-term outcomes in allergic rhinitis and asthma (Appendix G, Evidence Table G29). Disease modification in asthma was addressed in three studies included in this review.121,148 Niu et al121 found a significant effect on the number of patients with a decrease in asthma classification from mild/moderate persistent asthma to mild intermittent asthma, after 6 months of SLIT with dust mite allergen compared with placebo. Severity in this study was determined by a global assessment by physicians who reviewed the asthma scores, medication consumption, and pulmonary function tests and were not familiar with the patient. Marogna et al found no significant difference in the percentage of children with mild intermittent asthma after 3 years of SLIT compared with placebo.148 LaRosa et al found similar reports of rhinitis symptoms during Parietaria pollen season after 8 years of followup in the SLIT and placebo groups.151
Asthma prevention was reported in two of the sublingual immunotherapy studies, and in one eight-year followup to a prior study.138,144,179 Novembre et al found that fewer children developed asthma after 3 years of grass pollen SLIT vs conventional therapy; controls in this study developed asthma 3.8 times more frequently (RR, 3.8; 95% CI,1.5–10).138 Marogna et al found a lower occurrence of the development of mild persistent asthma in SLIT patients versus pharmacotherapy group after 3 years. However, in an eight year follow-up of the LaRosa study of Parietaria, sublingual immunotherapy treatment for two years showed no asthma preventative effect.144,179
Prevention of new allergy sensitivities was discussed in one article and one followup report.148,179 Marogna found that treatment with multi-antigen sublingual immunotherapy (dust mite, birch, weeds, and grass mix) significantly decreased the development of new allergen skin sensitizations after three years (OR, 0.6; 95% CI, 0.02–0.17).148 However, in an eight year follow-up report of the LaRosa study, there was no preventative effect on the development of new sensitivities after receiving Parietaria sublingual immunotherapy for two years.144,179
Other Outcomes
Adherence and compliance were discussed infrequently in the articles. In a followup study by Marogna et al., adherence was found to be excellent in 74 percent of subjects.148 Another study reported 53 percent compliance in the SLIT arm and 67 percent compliance in the placebo arm.154
During the course of the review, the number of studies reporting select biomarkers was recorded: IgG total, IgG4, and IgE. Three studies reported changes in specific IgG, eight study-specific IgG4, and 10 IgE (total and/or specific IgE). (Appendix G, Evidence Table G31).
Conclusion: Summary of Pediatric Evidence for Key Question 1
When considering the key evidence for the efficacy and effectiveness of sublingual immunotherapy in the treatment of allergic rhinoconjunctivitis and/or asthma in children, the pertinent clinical outcomes include symptom scores, medication use, and quality of life (Table 40). The overall strength of evidence for use of sublingual immunotherapy in children and adolescents when considering all domains with pertinent clinical outcomes together is moderate.
Table 40
Summary of strength of evidence regarding the effectiveness of sublingual immunotherapy in children and adolescents.
Sublingual Versus Subcutaneous Immunotherapy
Eight studies published between 1989 and 2010 reported on the efficacy and safety of sublingual versus subcutaneous immunotherapy. Two studies originated from Italy,177,180 five from Turkey,35,36,46,181,182 and one from Denmark.34 Rhinitis was the primary diagnosis of the subjects in three studies,177,180,181 rhinoconjunctivitis in one study,34 and asthma with rhinitis in four studies.35,36,46,182 Three studies included only adults;34,35,180 two included both adults and children,177,181 and three studied children exclusively.36,46,182 All but one study required that the subjects had received no prior immunotherapy46 (Appendix F, Evidence Tables F1 and F2)
Two studies focused on tree pollen immunotherapy,34,180 and the remaining six studied dust mite immunotherapy.35,36,46,177,181,182 Each study allowed the participants to take either conventional or rescue medications during the study in addition to the immunotherapy or placebo. The maintenance dosing interval for subcutaneous immunotherapy ranged from once every three weeks to once every eight weeks. In the sublingual treatment group the maintenance dosing interval varied from daily to three times a week. The treatment duration across studies was between one and three years (Appendix F, Evidence Table F3).
Most of the studies had biases arising due to improper concealment of the allocation of interventions, unmasked interventions and incomplete reporting of missing data. Only one study was considered to be at low risk of bias34 (Appendix F, Evidence Table F4).
Key Question 1. What is the evidence for the efficacy and effectiveness of sit in the treatment of allergic rhinoconjunctivitis and/or asthma?
Key Points
- Low grade evidence favors subcutaneous immunotherapy over sublingual for allergic asthma symptom control.
- Moderate grade evidence favors subcutaneous immunotherapy over sublingual for allergic nasal and/or eye symptom control.
- Low grade evidence exists to suggest little difference between routes of therapy for reducing medication use.
- Low grade evidence exists to favor subcutaneous immunotherapy over sublingual immunotherapy for reducing symptoms and medication use in dust mite allergic patients.
Asthma Symptom Control
Four trials of dust mite allergen immunotherapy reported improvement in asthma symptom scores.35,36,46,182 Two studies reported changes in subcutaneous and sublingual immunotherapy groups compared with placebo35,36 and two compared with pharmacotherapy.46,182 Both the studies with a placebo comparison group reported significant changes in asthma symptom scores in subcutaneous treatment group after treatment relative to before treatment;35,36 one reported significant changes in the sublingual immunotherapy group after treatment.36 In the latter study, the group treated with subcutaneous immunotherapy showed a significantly greater reduction in reducing asthma symptom scores compared with the group treated with sublingual immunotherapy.36 The other two studies demonstrated the effectiveness of subcutaneous and sublingual immunotherapy groups in reducing asthma symptom scores compared with pharmacotherapy.46,182 Both studies reported that subcutaneous immunotherapy significantly reduced asthma symptoms compared with pharmacotherapy. One study reported that sublingual immunotherapy also reduced asthma symptoms significantly,182 while the other study reported that subcutaneous and sublingual treatment, when combined, reduced symptoms significantly compared with pharmacotherapy (Appendix F, Evidence Table F5).
The strength of evidence is low (4 studies, N=171) to support subcutaneous immunotherapy over sublingual immunotherapy for allergic asthma symptom control (Table 41).
Table 41
Body of evidence for sublingual immunotherapy versus subcutaneous immunotherapy affecting asthma symptoms.
Rhinitis/Rhinoconjunctivitis Symptoms
Six studies reported rhinitis or rhinoconjunctivitis symptom scores in their study participants.34–36,46,181,182 There was no uniformity in reporting of these scores and none of the scales were validated. The duration of assessment varied from one to six years. Three dust mite immunotherapy trials reported significant improvement in rhinitis/rhinoconjunctivitis symptom scores in both sublingual and subcutaneous study groups post-treatment compared with pre-treatment.35,36,181 One birch immunotherapy trial 34 and two dust mite trial36,182 demonstrated that both sublingual and subcutaneous immunotherapy reduced symptoms significantly compared with placebo or pharmacotherapy. Four studies directly compared the difference between sublingual immunotherapy and subcutaneous immunotherapy.34,36,181,182 One dust mite allergen study demonstrated that subcutaneous immunotherapy resulted in a significantly greater reduction in symptom scores compared with sublingual immunotherapy;181 two dust mite studies,36,182 and a birch study34 showed no significant difference between sublingual and subcutaneous immunotherapy for reducing rhinitis/rhinoconjunctivitis symptoms. One dust mite study reported a significant difference in rhinitis symptoms in participants receiving combined subcutaneous and sublingual immunotherapy compared with pharmacotherapy46 (Appendix F, Evidence Table F6).
These six randomized controlled trials included 412 participants with rhinitis alone or with conjunctivitis or asthma. The strength of evidence is moderate that subcutaneous immunotherapy is more effective than sublingual immunotherapy for reducing allergic nasal and/or eye symptoms (Table 42).
Table 42
Body of evidence for sublingual immunotherapy versus subcutaneous immunotherapy affecting rhinitis/rhinoconjunctivitis symptoms.
Medication Use
Medication scores were reported in five studies. Studies used various numerical scoring scales to evaluate the medications used. The duration of assessment of the scores ranged from one to two years. The medications that the study participants were allowed to use varied between studies, some allowed only rescue medications while some allowed conventional therapies including corticosteroids, beta-2 agonists and antihistamines.
One dust mite allergen trial demonstrated significant reductions in medication scores post-treatment compared with pre-treatment in both sublingual and subcutaneous immunotherapy groups.35 Four studies compared changes in scores between the immunotherapy and placebo or pharmacotherapy groups.34,36,46,182 In a birch immunotherapy trial, both sublingual immunotherapy and subcutaneous immunotherapy demonstrated significant reductions in scores compared with placebo, but the differences between sublingual and subcutaneous treatment groups were not significant.34 In a dust mite study, only sublingual immunotherapy significantly reduced scores compared with pharmacotherapy; subcutaneous immunotherapy did not.182 In another dust mite trial, there was significant reduction in rhinitis medication use in both subcutaneous and sublingual immunotherapy groups comparing pre-treatment to post treatment, but in the same trial there was significant reduction in asthma medication use only in subcutaneous immunotherapy group. Also there was no significant difference between the sublingual and subcutaneous immunotherapy groups.36 Another dust mite trial reported changes in medication score for subcutaneous, sublingual and combined subcutaneous and sublingual immunotherapy compared with pharmacotherapy. It was demonstrated that sublingual immunotherapy significantly reduced asthma medication use compared with pharmacotherapy, while subcutaneous immunotherapy significantly reduced asthma medication, rhinitis medication and total medication scores compared with pharmacotherapy. The same was true in the combined subcutaneous-sublingual immunotherapy group.46 (Appendix F, Evidence Table F6)
The strength of evidence is low (5 studies, N= 219). Given the inconsistency of the evidence, these studies support that there may not be a difference between these routes of administration for reducing medication use (Table 43).
Table 43
Body of evidence for sublingual immunotherapy versus subcutaneous immunotherapy affecting medication use.
Combined Medication and Symptoms Scores
Two studies reported improvement in symptoms and medication scores.177,180 A dust mite trial reported significant improvement post-treatment compared with pre-treatment in the subcutaneous immunotherapy group.177 The sublingual immunotherapy group showed significant improvement during early treatment, but the effect was not sustained at two years. Another study in tree pollen allergic patients reported no significant differences in symptoms and medication scores between the sublingual and subcutaneous immunotherapy groups.180 None of the studies reported between-group differences. The evidence is low to support subcutaneous immunotherapy over sublingual immunotherapy for improving combined medication and symptom scores for dust mite allergic patients (Table 44).
Table 44
Body of evidence that sublingual immunotherapy versus subcutaneous immunotherapy affects combined medication use and symptoms.
Quality of Life
Quality of life was assessed in one study using the Danish version of SF-36 Health status Questionnaire.34 Although definitive scores at baseline and after treatment are not provided, the study reports no statistically significant differences in quality of life scores in the groups receiving sublingual immunotherapy, subcutaneous immunotherapy or placebo.
Limited data (1 study, N= 48) precludes grading of strength of evidence for quality of life assessment.
Allergen or Chemical Challenge (Provocation)
Four dust mite studies evaluated nasal symptoms after exposure to allergen after immunotherapy.36,46,181,182 All studies showed statistically significant increases in the tolerated allergen dose in the sublingual immunotherapy and subcutaneous immunotherapy groups. Two studies reported changes in bronchial symptoms to methacholine challenge.35,46 Neither the sublingual or subcutaneous immunotherapy groups showed a statistically significant reduction in the dose of methacholine required for provocation. Another dust mite study evaluated allergen induced bronchial changes.36 Significant changes in allergen dose were seen in the subcutaneous immunotherapy group only.
Biomarkers
Changes in biomarkers following immunotherapy were reported in six studies.35,36,46,177,180,182 Allergen specific IgE was described in six studies, IgG4 in five studies and IgG in one study.
Conclusion: Summary of Evidence for Key Question 1
The evidence for efficacy and effectiveness of sublingual immunotherapy versus subcutaneous in the treatment of allergic rhinoconjunctivitis and/or asthma is drawn from clinically important outcomes such as symptom scores, medication use, and quality of life. The data is inadequate to comment on reduction of medication use, symptom and medication reduction, and quality of life. The strength of evidence lowly favors subcutaneous immunotherapy for reducing asthma symptoms and for control of nasal and eye symptoms. (Table 45).
Table 45
Summary of strength of evidence regarding the effectiveness of sublingual immunotherapy versus subcutaneous immunotherapy.
Key Question 2. What is the evidence for safety of SIT in patients with allergic rhinitis/rhinoconjunctivitis and/or asthma?
The safety of sublingual immunotherapy and subcutaneous immunotherapy was assessed in all eight of the included articles. The recording and reporting of the adverse events was neither uniform nor comparable across studies. Adverse events were divided into local reactions and systemic reactions.
The local reactions consisted of oral cavity/oropharynx itching in the sublingual immunotherapy group and injection site reactions in the subcutaneous immunotherapy group. Four studies reported local reactions in sublingual immunotherapy treated patients ranging from seven to 56 percent of patients.34–36,181 One study reported 0.2 local reactions per patient in the sublingual immunotherapy group.180 In the subcutaneous immunotherapy treated group, local reaction frequency ranged from 6 to 18 per 100 patients across four studies.177,180–182 Two studies reported that 20 percent of patients developed reactions at injection site.35,36 All reactions were mild or moderate.
Systemic reactions were reported in seven of the trials.34,35,46,177,180–182 Gastrointestinal events such as nausea, pain, and diarrhea were the most frequent systemic reaction reported in sublingual immunotherapy groups. In the subcutaneous immunotherapy group, three studies, the occurrence of respiratory events such as rhinitis/asthma were reported in five patients of which two were severe reactions that required hospitalization.35,46,182
Safety in the Pediatric Population
The safety of sublingual immunotherapy versus subcutaneous immunotherapy was assessed in the three studies with a total of 135 patients.36,46,182
In the Eifan study, side effects were only reported in the subcutaneous immunotherapy group. Two patients (12.5%) receiving subcutaneous immunotherapy experienced severe systemic reactions. A grade 3 reaction occurred in a 5 year old girl who experienced severe asthma symptoms after every injection given in the induction phase. The grade 4 reaction occurred in a 10 year old girl with flushing, wheezing, and dyspnea after the ninth injection during the induction phase and required adrenaline. One local event occurred in the subcutaneous group with swelling at the injection site (0.06 events per patient). No systemic or local reactions were reported in the sublingual or pharmacotherapy groups.
In the Yukselen study, 3 patients (30%) receiving SLIT experienced local oral cavity/oropharynx itching and 2 patients (20%) receiving SCIT experienced a local injection site reaction.36 No systemic reactions were observed in either group.
In the Keles study, 2 patients (18.2%) experienced moderate respiratory reactions after receiving SCIT, while no systemic reactions were noted in the SLIT group.46 No local reactions were reported in either group.
Among these three studies with a total of 135 patients, local injection site reactions were reported in three patients receiving subcutaneous immunotherapy, and local reactions (oral itching) were reported in three patients receiving sublingual immunotherapy. No systemic reactions were reported in patients receiving sublingual immunotherapy. Among patients receiving subcutaneous immunotherapy, four experienced systemic reactions, including 1 anaphylaxis event and 3 patients with moderate – severe respiratory symptoms.
These studies suggest that sublingual immunotherapy may be safer than subcutaneous immunotherapy (Appendix G, Evidence Table G42).
Key Question 3. Is the safety and effectiveness of allergen-specific immunotherapy different in distinct subpopulations with allergic rhinitis/rhinoconjunctivitis and/or asthma?
Key Points
- The evidence is insufficient to comment on the effectiveness and safety of sublingual immunotherapy compared with subcutaneous immunotherapy in subpopulations of the elderly, pregnant women, ethnic minorities, inner-city residents, rural residents, and patients with severe asthma.
- There is no apparent difference in efficacy of sublingual immunotherapy and subcutaneous immunotherapy in mono-sensitized versus poly-sensitized subjects.
The eight included studies did not report effectiveness and safety of sublingual compared with subcutaneous immunotherapy in subpopulations of the elderly, pregnant women, ethnic minorities, inner-city residents, rural residents, or patients with severe asthma. Four studies included only mono-sensitized subjects.35,36,180,182 The results of these studies did not differ significantly from the results of the three studies that enrolled polysensitized patients.
Pediatric Population: Key Points
- Inadequate evidence exists to support sublingual immunotherapy over subcutaneous or vice versa for improvement of asthma or rhinitis symptoms or medication use.
- Low grade evidence favors subcutaneous immunotherapy over sublingual for allergic asthma symptom control, based on 3 randomized controlled trials with 135 subjects.
- Low grade evidence favors subcutaneous immunotherapy over sublingual for allergic nasal and/or eye symptom control, based on 3 randomized controlled trials with 135 subjects.
- Low grade evidence exists to suggest little difference between routes of therapy for reducing medication use, based on 3 randomized controlled trials with 135 subjects.
Only three RCTs, published in 2010 and 2011 and originating from Turkey, reported on the efficacy and safety of sublingual versus subcutaneous immunotherapy exclusively in children.36,46,182 The primary diagnosis of the subjects in all 3 studies was asthma with rhinitis. All studies focused on dust mite immunotherapy. Two of the studies required that the subjects had received no prior immunotherapy and only included monosensitized individuals.36,182 The ages of patients included in the study ranged from about 5 to 14 years of age. Two of the studies were funded by industry.36,46 (Appendix G, Evidence Tables G32 and G33)
One study allowed the participants to take conventional medications36 and two studies only allowed rescue medications during the study in addition to the immunotherapy.46,182 The maintenance dosing interval for subcutaneous immunotherapy ranged from three times a week to monthly, while in the sublingual treatment group, the maintenance dosing interval was three times a week in all 3 studies.36,46,182 The treatment duration across studies was for 1 year. Comparison groups in the study included sublingual immunotherapy, subcutaneous immunotherapy, and placebo/pharmacotherapy arms (Appendix G, Evidence Table G34). The three studies were considered to have a medium risk of bias (Appendix G, Evidence Table G35).
Asthma Symptom Control
All three trials of dust mite allergen immunotherapy reported improvement in asthma symptom scores.36,46,182 One study reported changes in subcutaneous and sublingual immunotherapy groups compared with placebo with conventional therapy,36 and two reported these changes compared with pharmacotherapy.46,182 The study with the placebo comparison group reported significant changes in asthma symptom scores in the subcutaneous and sublingual treatment groups after treatment relative to before treatment;36 The group treated with subcutaneous immunotherapy showed a significantly greater reduction asthma symptom scores compared with the group treated with sublingual immunotherapy.36 The other two studies demonstrated the effectiveness of subcutaneous and sublingual immunotherapy groups in reducing asthma symptom scores compared with pharmacotherapy.46,182 Both studies reported that subcutaneous immunotherapy significantly reduced asthma symptoms compared with pharmacotherapy. One study reported that sublingual immunotherapy also reduced asthma symptoms significantly,182 while the other study reported that subcutaneous and sublingual treatment, when combined, reduced symptoms significantly compared with pharmacotherapy. (Appendix G, Evidence Table G36)
The strength of evidence is low (3 studies, N=135) to support subcutaneous immunotherapy over sublingual immunotherapy for allergic asthma symptom control (Table 46).
Table 46
Strength of evidence for sublingual immunotherapy versus subcutaneous immunotherapy affecting asthma symptoms in children and adolescents.
Rhinitis/Rhinoconjunctivitis Symptoms
Three studies reported rhinitis or rhinoconjunctivitis symptom scores in their study participants.36,46,182 There was no uniformity in reporting of these scores and none of the scales were validated. The duration of assessment was over one year. One trial reported significant improvement in rhinitis/rhinoconjunctivitis symptom scores in both sublingual and subcutaneous study groups post-treatment compared with pre-treatment.36 Two other dust mite trials36,182 demonstrated that both sublingual and subcutaneous immunotherapy reduced symptoms significantly compared with placebo or pharmacotherapy. Two studies directly compared the difference between sublingual immunotherapy and subcutaneous immunotherapy.36,182 They showed no significant difference between sublingual and subcutaneous immunotherapy for reducing rhinitis/rhinoconjunctivitis symptoms. One dust mite study reported a significant difference in rhinitis symptoms in participants receiving combined subcutaneous and sublingual immunotherapy compared with pharmacotherapy46 (Appendix G, Evidence Table G37)
These three randomized controlled trials included 135 participants with rhinitis alone or with conjunctivitis or asthma. The strength of evidence is low that subcutaneous immunotherapy is more effective than sublingual immunotherapy for reducing allergic nasal and/or eye symptoms (Table 47).
Table 47
Body of evidence for sublingual immunotherapy versus subcutaneous immunotherapy affecting rhinitis/rhinoconjunctivitis symptoms in children and adolescents.
Medication Use
Medication scores were reported in the three studies. Studies used various numerical scoring scales to evaluate the medications used. The duration of assessment of the scores was one year. The medications that the study participants were allowed to use varied between studies, some allowed only rescue medications while some allowed conventional therapies including corticosteroids, beta-2 agonists and antihistamines.
The three studies compared changes in scores between the immunotherapy and placebo or pharmacotherapy groups.36,46,182 In one of the dust mite studies, only sublingual immunotherapy significantly reduced scores compared with pharmacotherapy; subcutaneous immunotherapy did not.182 In another dust mite trial, there was significant reduction in rhinitis medication use in both subcutaneous and sublingual immunotherapy groups comparing pre-treatment to post treatment, but in the same trial there was significant reduction in asthma medication use only in subcutaneous immunotherapy group. Also there was no significant difference between the sublingual and subcutaneous immunotherapy groups. Another dust mite trial reported changes in medication score for subcutaneous, sublingual and combined subcutaneous, and sublingual immunotherapy compared with pharmacotherapy. It was demonstrated that sublingual immunotherapy significantly reduced asthma medication use compared with pharmacotherapy, while subcutaneous immunotherapy significantly reduced asthma medication, rhinitis medication and total medication scores compared with pharmacotherapy. The same was true in the combined subcutaneous-sublingual immunotherapy group.46 (Appendix G, Evidence Table G39)
With the inconsistent direction of change and risk of bias, the strength of evidence is low (3 studies, N= 135) to support improved medication use with sublingual immunotherapy compared with subcutaneous immunotherapy (Table 48).
Table 48
Body of evidence for sublingual immunotherapy versus subcutaneous immunotherapy affecting medication use in children and adolescents.
Combined Medication and Symptoms Scores
None of the pediatric studies reported combined medication and symptom scores.
Quality of Life
None of the pediatric studies evaluated quality of life outcomes.
Allergen or Chemical Challenge (Provocation)
Three dust mite studies evaluated nasal symptoms after exposure to allergen after immunotherapy.36,46,182 All studies showed statistically significant increases in the tolerated allergen dose in the sublingual immunotherapy and subcutaneous immunotherapy groups. One study reported changes in bronchial symptoms to methacholine challenge.46 Neither the sublingual or subcutaneous immunotherapy groups showed a statistically significant reduction in the dose of methacholine required for provocation. Another dust mite study evaluated allergen induced bronchial changes.36 Significant changes in allergen dose were seen in subcutaneous immunotherapy group only. (Appendix G, Evidence Table G40)
Biomarkers
Changes in biomarkers following immunotherapy were reported in three studies.36,46,182 Allergen specific IgE was described in three studies and IgG4 in two studies.
Conclusion: Summary of Evidence for Key Question 3
The evidence for efficacy and effectiveness of sublingual immunotherapy versus subcutaneous in the treatment of allergic rhinoconjunctivitis and/or asthma in the pediatric population is drawn from clinically important outcomes such a symptom scores and medication use. The data is inadequate to comment on reduction of combined symptom and medication use and quality of life. The strength of evidence is low for favoring subcutaneous immunotherapy for reducing asthma symptoms and for control of nasal and eye symptoms (Table 49).
Table 49
Summary of strength of evidence regarding the effectiveness of sublingual immunotherapy versus subcutaneous immunotherapy in the pediatric population.
Fewer pediatric specific studies have been performed, compared with SCIT versus placebo studies in adults. The strength of evidence for almost all clinically relevant asthma outcomes have been downgraded from high strength of evidence to low strength of evidence, when evaluating only studies with participants less than or equal to 18 years of age. The strength of evidence for asthma symptom-medication scores increased from low to moderate strength of evidence.
- Results - Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinocon...Results - Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review
- Summary and Discussion - Breathing Exercises and/or Retraining Techniques in the...Summary and Discussion - Breathing Exercises and/or Retraining Techniques in the Treatment of Asthma: Comparative Effectiveness
- Results - Breathing Exercises and/or Retraining Techniques in the Treatment of A...Results - Breathing Exercises and/or Retraining Techniques in the Treatment of Asthma: Comparative Effectiveness
- Methods - Diagnosis and Treatment of Obstructive Sleep Apnea in AdultsMethods - Diagnosis and Treatment of Obstructive Sleep Apnea in Adults
- Peer Reviewers - Breast Reconstruction After Mastectomy: A Systematic Review and...Peer Reviewers - Breast Reconstruction After Mastectomy: A Systematic Review and Meta-Analysis
Your browsing activity is empty.
Activity recording is turned off.
See more...