NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Glacy J, Putnam K, Godfrey S, et al. Treatments for Seasonal Allergic Rhinitis [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Jul. (Comparative Effectiveness Reviews, No. 120.)
This publication is provided for historical reference only and the information may be out of date.
Background
Seasonal allergic rhinitis (SAR), also known as hay fever, is an allergic reaction in the upper airways that occurs when sensitized individuals encounter airborne allergens (typically tree, grass, and weed pollens and some molds). SAR afflicts approximately 10 percent of the U.S. population, or 30 million individuals.1,2 Although pollen seasons vary across the United States, generally, tree pollens emerge in the spring, grass pollens in the summer, and weed pollens in the fall. Outdoor molds generally are prevalent in the summer and fall. SAR is distinguished from perennial allergic rhinitis (PAR), which is triggered by continuous exposure to house dust mites, animal dander, and other allergens generally found in an individual's indoor environment. Patients may have either SAR or PAR or both (i.e., PAR with seasonal exacerbations). The four defining symptoms of allergic rhinitis are nasal congestion, nasal discharge (rhinorrhea), sneezing, and/or nasal itch. Many patients also experience eye symptoms, such as itching, tearing, and redness.3 Additional signs of rhinitis include the “allergic salute” (rubbing the hand against the nose in response to itching and rhinorrhea), “allergic shiner” (bruised appearance of the skin under one or both eyes), and “allergic crease” (a wrinkle across the bridge of the nose caused by repeated allergic salute).4-7 SAR can adversely affect quality of life,8-10 sleep,11,12 cognition,13 emotional life,14 and work or school performance.15-17 Treatment improves symptoms and quality of life.
Treatments for SAR include allergen avoidance, pharmacotherapy, and immunotherapy. Although allergen avoidance may be the preferred treatment, for SAR, total allergen avoidance may be an unrealistic approach, as it may require limiting time spent outdoors. Thus, pharmacotherapy is preferable to allergen avoidance for SAR symptom relief. Allergen-specific immunotherapy is the subject of a separate review, also sponsored by the Agency for Healthcare Research and Quality (AHRQ) and posted on the Effective Health Care Web site (www.effectivehealthcare.ahrq.gov/reports/final/cfm).
Six classes of drugs and nasal saline are used to treat SAR.
- Antihistamines used to treat allergic rhinitis bind peripheral H1 histamine receptors selectively or nonselectively. Nonselective binding to other receptor types can cause dry mouth, dry eyes, urinary retention, constipation, and tachycardia. Sedation results from the nonselective binding to central H1 receptors. In contrast, selective antihistamines may have reduced incidence of adverse effects.18 Both selective and nonselective antihistamines interact with drugs that inhibit cytochrome P450 isoenzymes,4 which may impact patient selection. Two nasal antihistamines—azelastine and olopatadine—are approved by the U.S. Food and Drug Administration (FDA) for the treatment of SAR. Adverse effects of nasal antihistamines may include a bitter aftertaste.
- Corticosteroids are potent anti-inflammatory drugs. Intranasal corticosteroids are recommended as first-line treatment for moderate/severe or persistent allergic rhinitis.5,19 However, their efficacy for the symptom of nasal congestion compared with nasal antihistamine is uncertain,20,21 particularly in patients with mild allergic rhinitis. For patients with unresponsive symptoms, it is unclear whether adding oral or nasal antihistamine provides any additional benefit. Little is known about cumulative corticosteroid effects in patients who take concomitant oral or inhaled formulations for other diseases. Intranasal corticosteroids do not appear to cause adverse events associated with systemic absorption (e.g., adrenal suppression, bone fracture among the elderly, and reduced bone growth and height in children). Adverse local effects may include increased intraocular pressure and nasal stinging, burning, bleeding, and dryness. Oral and intramuscular corticosteroids are not reviewed in this report.
- Decongestants stimulate the sympathetic nervous system to produce vasoconstriction, which results in decreased nasal swelling and decreased congestion. After several days of nasal decongestant use, rebound congestion (rhinitis medicamentosa) may occur. Other local adverse effects may include nosebleeds, stinging, burning, and dryness. Oral decongestants are used alone and in combination, often with antihistamines. Systemic adverse effects of decongestants may include hypertension, tachycardia, insomnia, headaches, and irritability.4,22 Decongestants are used with caution, if at all, in patients with diabetes mellitus, ischemic heart disease, unstable hypertension, prostatic hypertrophy, hyperthyroidism, and narrow-angle glaucoma. Oral decongestants are contraindicated with coadministered monoamine oxidase inhibitors and in patients with uncontrolled hypertension or severe coronary artery disease.23
- Ipratropium nasal spray is an anticholinergic drug approved by the FDA for treating rhinorrhea associated with SAR. Postmarketing experience suggests that there may be some systemic absorption. Cautious use is advised for patients with narrow-angle glaucoma, prostatic hypertrophy, or bladder neck obstruction, particularly if another anticholinergic is coadministered. Local adverse effects may include nosebleeds and nasal and oral dryness.24
- Nasal mast cell stabilizers are commonly administered prophylactically, before an allergic reaction is triggered, although as-needed use has been described and may be of benefit. Cromolyn is the only mast cell stabilizer approved by the FDA for the treatment of SAR. For prophylaxis, it requires a loading period during which it is applied four times daily for several weeks. Systemic absorption is minimal. Local adverse effects may include nasal irritation, sneezing, and an unpleasant taste.4,23
Nasal saline has been shown to be beneficial in treating nasal SAR symptoms.27 Because it is associated with few adverse effects, nasal saline may be particularly well suited for treating SAR symptoms during pregnancy, in children, and in those whose treatment choices are restricted due to comorbidities, such as hypertension and urinary retention.
The optimal treatment of SAR during pregnancy is unknown. Drugs effective before pregnancy may be effective during pregnancy, but their use may be restricted because of concerns about maternal and fetal safety. Preferred treatments are Pregnancy Category B drugs (nasal cromolyn, budesonide, and ipratropium; several oral selective and nonselective antihistamines; and the oral leukotriene receptor antagonist montelukast) commencing in the second trimester, after organogenesis.
Objectives
Although there are multiple guidelines for the treatment of allergic rhinitis,5,20,28-31 the guidelines are not consistently based on systematic reviews of the literature and often do not address the treatment of SAR in children and pregnant women. Guidelines generally support the use of intranasal corticosteroids as first-line treatment of moderate/severe SAR. However, agreement is lacking about four other issues of importance to patients and clinicians:
- First-line treatment for mild SAR
- The comparative effectiveness and safety of SAR treatments used in combination with each other for both mild and moderate/severe SAR
- The comparative effectiveness of as-needed use compared with daily dosing
- The comparative effectiveness and harms of SAR treatments for eye symptoms and asthma symptoms that often co-occur with SAR
This review addresses the four issues above. The scope of this review is comparisons across pharmacologic classes. With input from the Technical Expert Panel (TEP), we chose to focus on across-class comparisons because this is the first question that patients, clinicians, and other decisionmakers face. Although there may be differences among drugs within the same class, previous comparative effectiveness reviews in allergic rhinitis5,20,29,32-38 have found insufficient evidence to support superior effectiveness of any single drug within a drug class. A direct consequence of the decision to conduct across-class comparisons is the inability to compare individual drugs across studies. Additionally, limited conclusions can be drawn about drug classes that are poorly represented by the drugs studied. To our knowledge, methodological approaches for meta-analysis of class comparisons based on studies of single within-class treatment comparisons have not been published.
Key Questions
Key Question 1. What is the comparative effectiveness of pharmacologic treatments, alone or in combination with each other, for adults and adolescents (≥12 years of age) with mild or with moderate/severe SAR?
- How does effectiveness vary with long-term (months) or short-term (weeks) use?
- How does effectiveness vary with intermittent or continuous use?
- For those with symptoms of allergic conjunctivitis, does pharmacologic treatment of SAR provide relief of eye symptoms (itching, tearing)?
- For those codiagnosed with asthma, does pharmacologic treatment of SAR provide asthma symptom relief?
Key Question 2. What are the comparative adverse effects of pharmacologic treatments for SAR for adults and adolescents (≥12 years of age)?
- How do adverse effects vary with long-term (months) and short-term (weeks) use?
- How do adverse effects vary with intermittent or continuous use?
Key Question 3. For the subpopulation of pregnant women, what are the comparative effectiveness and comparative adverse effects of pharmacologic treatments, alone or in combination with each other, for mild and for moderate/severe SAR?
- How do effectiveness and adverse effects vary with long-term (months) or short-term (weeks) use?
- How do effectiveness and adverse effects vary with intermittent or continuous use?
Key Question 4. For the subpopulation of children (<12 years of age), what are the comparative effectiveness and comparative adverse effects of pharmacologic treatments, alone or in combination with each other, for mild and for moderate/severe SAR?
- How do effectiveness and adverse effects vary with long-term (months) or short-term (weeks) use?
- How do effectiveness and adverse effects vary with intermittent or continuous use?
Analytic Framework
The analytic framework for this report is presented in Figure A. The figure depicts the Key Questions (KQs) in relation to SAR treatments, adverse effects, and outcomes. The six drug classes of SAR treatments and nasal saline may produce intermediate outcomes such as relief of rhinitis symptoms and, if present, eye and asthma symptoms. Treatments also may result in improved quality of life, the final health outcome. Adverse events may occur at any point after treatment is received and may impact quality of life directly.
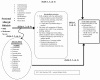
Figure A
Analytic framework.
Methods
Input From Stakeholders
We formulated the population, intervention, comparator, outcome, timing, setting (PICOTS) conceptual framework and KQs during a topic refinement stage. Key Informants were patients, providers (allergists, a pediatric pulmonologist, pharmacists, otorhinolaryngologists, and family physicians), and payers. Their input was sought to identify important clinical and methodological issues pertinent to the review. We developed a research protocol with input of a TEP. The protocol followed the methods outlined in the AHRQ “Methods Guide for Effectiveness and Comparative Effectiveness Reviews” (Methods Guide).39 The public was invited to provide comments on the KQs.
Data Sources and Selection
We developed a peer-reviewed search strategy and searched the following databases: MEDLINE® (PubMed® and Ovid), Embase® (Ovid), and the Cochrane Central Register of Controlled Trials (CENTRAL). For systematic reviews, the databases searched were the Cochrane Database of Systematic Reviews, Database of Abstracts and Reviews of Effects (DARE), and the Health Technology Assessment (HTA) databases of the United Kingdom's Centre for Reviews and Dissemination. Articles were limited to those published in the English language, based on technical expert advice that the majority of the literature on this topic is published in English. The databases were searched on July 18, 2012, with no date restrictions. We searched the FDA Web site, electronic conference abstracts of relevant professional organizations, and clinical trial registries for gray literature. Scientific information packets provided by product manufacturers were evaluated to identify unpublished trials that met inclusion criteria.
We sought expert guidance to identify the drug class comparisons most relevant for treatment decisionmaking. A total of 60 treatment comparisons were identified for all three patient populations. For all comparisons, the highest quality evidence was sought. Head-to-head randomized controlled trials (RCTs) were preferred, due to potential bias introduced in uncontrolled or noncomparative studies by the subjective reporting of both efficacy outcomes and harms in SAR research. For comparisons with sparse data from RCTs, we sought nonrandomized trials and comparative observational studies that controlled for confounders and were blinded.
Two reviewers screened abstracts and full-text reports, with conflicts resolved by consensus or a third reviewer. Selection criteria included: disease limited to SAR or results for patients with SAR reported separately, direct head-to-head comparison of interest of FDA-approved drugs from different drug classes, outcomes include patient-reported symptom scores and/or validated quality-of-life instruments, and minimum 2-week duration. Selective and nonselective antihistamine (based on specificity for peripheral H1 receptors) and different routes of administration (oral or nasal) were considered different classes for this purpose.
Data Abstraction and Quality Assessment
Comparative effectiveness and harms data from included studies were abstracted into an electronic database by two team members. We reconciled discrepancies during daily team discussions. Extracted information included general trial characteristics, baseline characteristics of trial participants, eligibility criteria, interventions, outcome measures and their method of ascertainment, and results of each predefined outcome.
The quality of individual RCTs was assessed using the United States Preventive Services Task Force (USPSTF)40 criteria, in accordance with the AHRQ Methods Guide.39 Two reviewers independently assigned quality ratings of good, fair, or poor. Discordant ratings were resolved with input from a third reviewer. Particular care was taken to ascertain whether patients were properly blinded to treatment because all outcomes of interest were patient reported. Open-label trials and trials in which patient blinding was deemed inadequate received a quality rating of poor.
The quality of harms reporting was assessed using the USPSTF rating, with specific attention to both patient and assessor blinding, and the McMaster Quality Assessment Scale of Harms (McHarm).41 In particular, the process of harms ascertainment was noted and characterized as either an active process if structured questionnaires were used, a passive process if only spontaneous patient reports were collected, or intermediate if active surveillance for at least one adverse event was reported. Trials using only passive harms ascertainment were considered to have a high risk of bias—specifically, underreporting or inconsistent reporting of harms.
Two reviewers independently assessed the risk of bias of relevant systematic reviews and meta-analyses using the following criteria derived from the AMSTAR tool and AHRQ guidance:42
- Details of the literature search were provided.
- Study inclusion and exclusion criteria were stated.
- The quality assessment of included studies was described and documented.
Data Synthesis and Analysis
Evidence on the comparative effectiveness and harms for each class comparison was summarized in narrative text. Quantitative pooling of results (meta-analysis) was considered if three or more clinically and methodologically similar studies reported on a given outcome. Three was an arbitrary number used as an operational criterion for meta-analyses. Only studies that reported variance estimates for group-level treatment effects could be pooled. The pooling method involved inverse variance weighting and a random-effects model. We assessed statistical heterogeneity by using Cochran's Q statistic (p = 0.10) and the I2 statistic. Meta-analysis was performed for adverse events that investigators reported as severe or that led to discontinuation of treatment. Three or more trials reporting the adverse event were required for pooling. Mean differences were calculated for continuous outcomes (effectiveness outcomes), and risk differences were calculated for dichotomous outcomes (harms). For studies that could not be quantitatively pooled, results were qualitatively combined when it was reasonable to do so (e.g., for similar studies reporting similar treatment effects).
In this review, we formed conclusions about treatment classes based on meta-analyses of studies that compared single treatments. Methodological approaches for this type of analysis have not been published. However, we proceeded with this analysis with support from the TEP. For class comparisons that were poorly represented (i.e., a small proportion of drugs in a class were assessed in included studies), we applied conclusions to the specific drugs studied; how well such conclusions generalize to other drugs in the same class is uncertain.
To assess the magnitude of treatment effects, we searched the published literature for minimal clinically important differences (MCIDs) derived from anchor-based or distribution-based methods. Anchor-based MCIDs are considered more robust and have been published for quality-of-life measures,43,44 asthma rescue medication use,45 and forced expired volume in 1 second (FEV1).45,46 Anchor-based MCIDs have not been defined for rhinitis symptom scales. One group defined a distribution-based MCID for total nasal symptom score (TNSS) as 0.52 on a 0-12 point scale.47,48 This represented one-fifth of the standard deviation of baseline TNSS scores in a trial of 27 patients. Bousquet and colleagues49 examined the responsiveness, defined as the ability of an instrument to measure change, of visual analog scale (VAS) scores to changes in TNSS scores (on an interval scale). A 2.9 cm improvement on a 10 cm VAS correlated with a 3-point improvement on a 0-12 point TNSS, defined a priori as a meaningful change. Although responsiveness and MCID are overlapping concepts, they are not identical. In allergen-specific immunotherapy trials, a minimum 30-percent greater improvement than placebo in composite symptom/rescue medication use scores is considered clinically meaningful.50 This threshold was based on an evaluation of 68 placebo-controlled double-blind trials.
In the absence of gold-standard MCIDs for symptom rating scales used in clinical rhinitis research, we sought input from our TEP, as recommended in the AHRQ Methods Guide.39 For TNSS on a 0–12 point scale, two experts considered a 4-point change meaningful and one expert considered a 2-point change meaningful.
For TNSS, potential MCIDs obtained from the sources described above are summarized in Table A. As shown, two sources (row 2 and row 4) converged around an MCID of 30-percent change in maximum TNSS score. This is supported by three TEP members who proposed a similar threshold for individual nasal symptoms (1 point on a 0–3 point scale) and two TEP members who proposed a similar threshold for total ocular symptom score (TOSS) (3 points on a 0–9 point scale). The concordance of these values increased our confidence that 30 percent of maximum score is a useful threshold for purposes of our analysis and could be applied across symptom scales. We therefore examined the strength of evidence for symptom outcomes using this MCID calculated for each scale used.
Table A
Quantified minimal clinically important differences for total nasal symptom score.
We initially assessed the evidence to determine whether one treatment was therapeutically superior to another and found that, for many comparisons, the evidence suggested equivalence of the treatments compared. We therefore decided post hoc to adopt an equivalence approach to evidence assessment, in accordance with the AHRQ Methods Guide,39 and assessed the body of evidence to support one of the following conclusions:
- Superiority: One treatment demonstrated greater effectiveness than the other, either for symptom improvement or harm avoidance.
- Equivalence: Treatments demonstrated comparable effectiveness, either for symptom improvement or harm avoidance.
- Insufficient evidence: The evidence supported neither a conclusion of superiority nor a conclusion of equivalence.
The strength of the body of evidence for each outcome was determined in accordance with the AHRQ Methods Guide39 and is based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.51,52 Two reviewers independently evaluated the strength of evidence, and agreement was reached through discussion and consensus when necessary. Four main domains were assessed: risk of bias, consistency, directness, and precision. The body of evidence was evaluated separately for each treatment comparison and each outcome of interest to derive a single GRADE of high, moderate, low, or insufficient evidence.
- A high GRADE indicates high confidence that the evidence reflects the true effect. Further research is very unlikely to change our confidence in the estimate of effect.
- A moderate GRADE indicates moderate confidence that the evidence reflects the true effect. Further research may change our confidence in the estimate of effect and may change the estimate.
- A low GRADE indicates low confidence that the evidence reflects the true effect. Further research is likely to change the confidence in the estimate of effect and is likely to change the estimate.
- A GRADE of insufficient indicates that evidence either is unavailable or does not permit a conclusion.
Decision rules used to assess each GRADE domain are provided in the full report.
Results
Overview
Of the 4,513 records identified through the literature search, 4,458 were excluded during screening. Four records were identified through gray literature and hand searching of bibliographies. One unpublished trial listed on ClinicalTrials.gov satisfied our inclusion criteria (NCT00960141). However, this trial was not included because quality assessment was not possible without the published report. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)53 diagram shown in Figure B depicts the flow of search screening and study selection. A total of 59 unique trials were included. For KQ 1 and KQ 2, 56 RCTs and 1 quasi-RCT that addressed 13 out of 22 comparisons of interest were found. For KQ 3, no studies that addressed any of 17 comparisons of interest were found. For KQ 4, two RCTs that addressed 1 of 21 comparisons of interest were found. No observational studies, systematic reviews, or meta-analyses that met our inclusion criteria were identified.
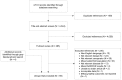
Figure B
PRISMA diagram for identified trials. PAR = perennial allergic rhinitis; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SAR = seasonal allergic rhinitis.
Key Question 1. Comparative Effectiveness of SAR Treatments in Adults and Adolescents 12 Years of Age or Older
Results for the 13 comparisons for which we found studies that satisfied our inclusion criteria are presented in Table B. For most outcomes, evidence was insufficient to form any comparative effectiveness conclusion. In five comparisons, we found evidence for comparable effectiveness (equivalence) of treatments for at least one outcome (rows 5, 6, 8, 11, and 12 in Table B). We found evidence for superior effectiveness of one treatment over another for one outcome in each of two comparisons (row 5 and row 9 in Table B). For seven comparisons, trials included only a small proportion of the drugs in each class (rows 1, 6, 8, 9, 10, 11, and 12 in Table B). Specific outcomes for the entries in Table B are detailed in the full report.
Table B
Summary of findings and strength of evidence for effectiveness in 13 treatment comparisons: Key Question 1—adults and adolescents.
Key Question 2. Comparative Adverse Effects of Treatments in Adults and Adolescents 12 Years of Age or Older
We identified two comparisons with sufficient evidence to support the use of one treatment over the other in order to avoid harm while treating SAR symptoms. These are shown in Table C. To avoid insomnia, moderate-strength evidence supported the use of oral selective antihistamine rather than either monotherapy with an oral decongestant or combination therapy with oral selective antihistamine plus oral decongestant. For all other comparisons, evidence to indicate superior harms avoidance with one treatment compared with another was insufficient or lacking. Because MCIDs for harms outcomes have not been defined, equivalence of treatments compared was not tested and cannot be assumed.
Table C
Summary of findings and strength of evidence for harms in 13 treatment comparisons: Key Question 2—adults and adolescents.
Key Question 3. Comparative Effectiveness and Adverse Effects of Treatments in Pregnant Women
For 17 comparisons of interest, no comparative trials, observational studies, meta-analyses, or systematic reviews met our inclusion criteria of directly comparing two drug classes used in pregnant women with SAR. We were unable to assess comparative effectiveness and harms of SAR treatments in pregnant women.
Key Question 4. Comparative Effectiveness and Harms of SAR Treatments in Children Younger Than 12 Years of Age
The TEP suggested 21 comparisons of interest. Two trials that compared oral selective antihistamine with oral nonselective antihistamine met our inclusion criteria. Evidence on nasal and eye symptoms and on harms was insufficient based on these trials, which had high risk of bias and reported imprecise results.
No observational studies, systematic reviews, or meta-analyses met the required inclusion criteria.
Discussion
Key Questions 1 and 2. Comparative Effectiveness and Adverse Effects of Treatments in Adults and Adolescents 12 Years of Age or Older
We did not find evidence that any single treatment demonstrated both greater effectiveness and lower risk of harms. Table D shows the four comparisons for which there was evidence to support a conclusion of superiority, either for effectiveness or for harms avoidance. Moderate-strength evidence supported the use of oral selective antihistamine to avoid insomnia associated with sympathomimetic decongestant at approximately 2 weeks (row 1 and row 4), but evidence was insufficient to draw any conclusion about comparative effectiveness between treatments. (Equivalence was not assessed in either comparison due to the inability to conduct meta-analysis.) Similarly, of two treatments shown to be comparatively superior for effectiveness (row 2 and row 3), neither was preferred for harms avoidance.
Table D
Comparison of efficacy and harms findings for four treatment comparisons.
Additional findings for comparative effectiveness in adults and adolescents were as follows.
- High-strength evidence for comparable effectiveness (equivalence) of:
- Combination intranasal corticosteroid plus nasal antihistamine, intranasal corticosteroid monotherapy, and nasal antihistamine monotherapy for nasal and eye symptoms at 2 weeks
- Intranasal corticosteroid and oral leukotriene receptor antagonist (montelukast) for nasal symptoms at 2 weeks
- Moderate strength evidence for comparable effectiveness of oral selective antihistamine and oral leukotriene receptor antagonist for nasal and eye symptoms and for improved quality of life at 2-4 weeks
Key Question 3. Comparative Effectiveness and Adverse Effects of Treatments in Pregnant Women
For this KQ, we considered only Pregnancy Category B drugs, for which teratogenic effects have not been identified in animal studies or replicated in human studies. Evidence for the assessment of this KQ was lacking. No RCTs, observational studies, systematic reviews, or meta-analyses met the inclusion criteria.
Drugs used for the treatment of SAR have wide therapeutic windows—that is, across the range of doses at which efficacy is seen, severe adverse events are not expected. Therefore, the choice of SAR treatment in pregnant women may be cautiously informed by comparative effectiveness evidence from the nonpregnant patient population. Because physiologic changes of pregnancy alter drug disposition, generalization of findings from nonpregnant populations to pregnant women requires knowledge of the magnitude and direction of these changes. However, for SAR treatments, this knowledge is currently limited.54 The minimum effective dose is generally preferred during pregnancy.
Key Question 4. Comparative Effectiveness and Harms of SAR Treatments in Children Younger Than 12 Years of Age
Of 17 treatment comparisons of interest among children, studies that met our inclusion criteria were identified for 1, selective versus nonselective oral antihistamine. No observational studies, systematic reviews, or meta-analyses met the required inclusion criteria.
The evidence for effectiveness and for harms was insufficient to form any conclusion about oral selective and oral nonselective antihistamine for the treatment of nasal or eye symptoms in children younger than 12 years of age (mean age, 9 years; range, 4 to 12 years). This finding was based on studies of 20 percent of oral selective antihistamines and 9 percent of oral nonselective antihistamines used to treat children. As with harms outcomes, a finding of insufficient evidence to support a conclusion of superiority of one treatment over the other does not imply equivalence of the treatments. The evidence for benefit is truly insufficient; equivalence was not assessed.
Findings in Relationship to What Is Already Known
The three systematic reviews listed below provided current information about the pharmacologic treatment of allergic rhinitis, variably defined as SAR, perennial allergic rhinitis (PAR), and intermittent or persistent allergic rhinitis (IAR and PER). Each provided a description of the literature search, inclusion and exclusion criteria for identified trials, and quality assessments of included trials. Thus, the risk of bias was considered low for each.
- Guidelines from the international Allergic Rhinitis and its Impact on Asthma (ARIA) Working Group, updated in 201020
- A 2009 systematic review of treatments for hay fever55
- A 2008 Practice Parameter from the Joint Task Force on Practice Parameters, representing the American Academy of Allergy, Asthma & Immunology (AAAAI), the American College of Allergy, Asthma and Immunology (ACAAI), and the Joint Council of Allergy, Asthma and Immunology (JCAAI)5
Of 13 comparisons for which we found studies, 3 were not addressed by the systematic reviews. In 2 of the remaining 10 comparisons, our conclusions agreed with at least 1 of the systematic reviews (ARIA guidelines20 in both instances). For the remaining eight comparisons, our results differed from those in the guidelines. In all cases, discordant conclusions could be attributed to differences in inclusion criteria for trials reviewed. For five of eight discordant conclusions, other systematic reviews formed conclusions about comparative effectiveness or harms and we found insufficient evidence to do so. The other three discordant conclusions involved intranasal corticosteroid alone (vs. nasal antihistamine and vs. oral leukotriene receptor antagonist) or in combination with nasal antihistamine (vs. nasal antihistamine). We concluded that there was comparable effectiveness (equivalence) of the treatments compared, and other systematic reviews concluded that there was comparative superiority of intranasal corticosteroid.
Limitations of Current Review and Evidence Base
To narrow the scope of this project to a manageable size, we made several decisions at the start that had downstream consequences. Examples follow.
- We restricted diagnosis to SAR. Given the current state of transition between classification schemes for allergic rhinitis, use of the original scheme may have excluded some trials. However, it is acknowledged that SAR and intermittent allergic rhinitis define different patient populations.5 We decided to pick one disease to study and then find studies similar enough to compare results. Introducing studies of allergic rhinitis classified according to the newer scheme may have added to the variability of included studies.
- We did not examine every possible treatment comparison. Rather, guided by input from Key Informants and the TEP, we prioritized comparisons that reflect treatment decisions encountered in the clinical setting. It is hoped that we selected and found evidence to assess comparisons that are meaningful to users of this report.
- We excluded trials of one drug versus a placebo and focused on direct comparisons only. This decision was based on feasibility concerns, given the large scope of the project and time constraints. Harms assessment was limited by the absence of placebo groups, which can inform adverse event reporting particularly.
- We included FDA-approved drugs only. For the comparison of oral selective antihistamine with oral nonselective antihistamine, in particular, this significantly reduced the number of included trials. The majority of trials excluded for this reason used terfenadine or astemizole as the selective antihistamine comparator, neither of which is currently FDA approved. As a result, only three trials were included for this comparison.
- Our minimum 2-week duration excluded examination of other treatment features that may be important to patients—for example, onset of action and harms associated with shorter exposure. However, harms associated with the interventions as defined (i.e., minimum 2-week exposure) were included. Trials of less than 2 weeks' duration often did not replicate natural methods of exposure to airborne allergens (i.e., instead used environmental exposure chambers, direct application of allergen, or prolonged weekend visits to parks), and their results may be less applicable.
- As described below, reporting of efficacy outcomes in SAR research is currently nonstandard. To maximize our ability to compare outcomes across trials, we selected the most commonly used symptom measures, namely the four-symptom TNSS and the three-item TOSS. Symptoms potentially important to patients but seldom assessed (e.g., postnasal drip, and ear and palate itching) were not included in this review.
- The scope of this report is class comparisons of SAR treatments. As a consequence of this approach, individual drug comparisons were beyond the scope of this report. Also, when comparing trials that studied a small proportion of the drugs in a class, we were limited in our ability to make conclusions about entire pharmacologic classes, particularly for larger classes such as intranasal corticosteroids and oral nonselective antihistamines. The impact of this limitation may be small for certain drug classes, such as oral nonselective antihistamines, which are less commonly used, and oral decongestants, of which the more commonly used drug (pseudoephedrine) was studied.
- Limitations in the quality of trial reporting directly impacted the conclusions that could be drawn and strength-of-evidence ratings, particularly for older trials. For example, insufficient group-level data reporting prevented equivalence assessments. It is hoped that continued implementation of guidelines for trial reporting will address such difficulties.
Limitations of the evidence base included nonstandard stratification and definitions of severity for symptoms and adverse events; underrepresentation of populations of interest, especially children and pregnant women; and nonstandard definitions and collection of nasal and eye symptoms. Additionally, the lack of well-defined MCIDs for symptom scales (which would preferably be anchor based but could be distribution based) is a prime research gap. Although our selection of clinically informed MCIDs permitted us to draw clinically relevant conclusions, validation of the values used (30% maximum score) using anchor-based approaches is desirable. Without such well-defined MCIDs, at least three analytic tools important for clinical research—power calculations, noninferiority margins, and responder analyses—are compromised.
Research Gaps
The greatest need in SAR research is increased methodological rigor. Widely used symptom rating scales require standardization and validation. Lack of anchor-based MCIDs is a major deficiency. Agreed-upon reporting standards for effectiveness and harms outcomes are needed. Agreed-upon classifications of patients by age and standardized definitions of symptom and harms severity also are needed. Study designs that can more efficiently assess the effects of additive therapies are lacking. Studies in which all patients are treated with one component of a combination (e.g., oral selective antihistamine) and only those who are resistant receive the second component (e.g., intranasal corticosteroid) may more efficiently isolate the additive effect of the second component. We identified one trial with this design.56
Lack of evidence on populations of interest is a research gap. Currently, the majority of trial participants are relatively homogeneous: white and middle-aged with moderate/severe SAR symptoms. Inclusion of different races, greater proportions of patients toward both ends of the age spectrum, and patients with mild symptoms may inform our understanding not only of the comparative effectiveness and harms of SAR treatments in different groups, but also of the expression of SAR in various ethnic groups, the natural history of the disease across the lifespan, and the effect (if any) of early treatment on later symptom expression. As noted above, however, ethical considerations may limit the inclusion of vulnerable populations (e.g., children) in well-designed studies of pharmacologic interventions.
For pregnant women, pregnancy registries and rigorous studies based on the data therein can fill the gap. This presumes the use of Pregnancy Category B drugs to avoid potential known or unknown teratogenic effects of other drugs. Additionally, greater understanding of how the physiologic changes of pregnancy affect the magnitude and direction of change in drug disposition may facilitate application of effectiveness and safety findings from the nonpregnant population to pregnant women.
References
- 1.
- Skoner DP, Rachelefsky GS, Meltzer EO, et al. Detection of growth suppression in children during treatment with intranasal beclomethasone dipropionate. Pediatrics. 2000 Feb;105(2):E23. [PubMed: 10654983]
- 2.
- Meltzer EO. The prevalence and medical and economic impact of allergic rhinitis in the United States. J Allergy Clin Immunol. 1997 Jun;99(6 Pt 2):S805–28. [PubMed: 9215265]
- 3.
- Bousquet J, Knani J, Hejjaoui A, et al. Heterogeneity of atopy. I. Clinical and immunologic characteristics of patients allergic to cypress pollen. Allergy. 1993 Apr;48(3):183–8. [PubMed: 8506986]
- 4.
- Orban N, Saleh H, Durham S. Allergic and non-allergic rhinitis. In: Adkinson N, Bochner B, Busse W, et al., editors. Middleton's Allergy: Principles and Practice. 7th ed. New York: Mosby; 2008.
- 5.
- Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008 Aug;122(2 Suppl):S1–84. [PubMed: 18662584]
- 6.
- Plaut M, Valentine MD. Clinical practice. Allergic rhinitis. N Engl J Med. 2005 Nov 3;353(18):1934–44. [PubMed: 16267324]
- 7.
- Gross GN. What are the primary clinical symptoms of rhinitis and what causes them? Immunol Allergy Clin North Am. 2011 Aug;31(3):469–80. [PubMed: 21737038]
- 8.
- Laforest L, Bousquet J, Pietri G, et al. Quality of life during pollen season in patients with seasonal allergic rhinitis with or without asthma. Int Arch Allergy Immunol. 2005 Mar;136(3):281–6. [PubMed: 15722638]
- 9.
- Majani G, Baiardini I, Giardini A, et al. Health-related quality of life assessment in young adults with seasonal allergic rhinitis. Allergy. 2001 Apr;56(4):313–7. [PubMed: 11284798]
- 10.
- Scadding GK, Richards DH, Price MJ. Patient and physician perspectives on the impact and management of perennial and seasonal allergic rhinitis. Clin Otolaryngol Allied Sci. 2000 Dec;25(6):551–7. [PubMed: 11122298]
- 11.
- McNicholas WT, Tarlo S, Cole P, et al. Obstructive apneas during sleep in patients with seasonal allergic rhinitis. Am Rev Respir Dis. 1982 Oct;126(4):625–8. [PubMed: 7125355]
- 12.
- Stuck BA, Czajkowski J, Hagner AE, et al. Changes in daytime sleepiness, quality of life, and objective sleep patterns in seasonal allergic rhinitis: a controlled clinical trial. J Allergy Clin Immunol. 2004 Apr;113(4):663–8. [PubMed: 15100670]
- 13.
- Marshall PS, O'Hara C, Steinberg P. Effects of seasonal allergic rhinitis on selected cognitive abilities. Ann Allergy Asthma Immunol. 2000 Apr;84(4):403–10. [PubMed: 10795648]
- 14.
- Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy. 1991 Jan;21(1):77–83. [PubMed: 2021881]
- 15.
- Vuurman EF, van Veggel LM, Uiterwijk MM, et al. Seasonal allergic rhinitis and antihistamine effects on children's learning. Ann Allergy. 1993 Aug;71(2):121–6. [PubMed: 8346863]
- 16.
- Walker S, Khan-Wasti S, Fletcher M, et al. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case-control study. J Allergy Clin Immunol. 2007 Aug;120(2):381–7. [PubMed: 17560637]
- 17.
- Szeinbach SL, Seoane-Vazquez EC, Beyer A, et al. The impact of allergic rhinitis on work productivity. Prim Care Respir J. 2007 Apr;16(2):98–105. [PMC free article: PMC6634185] [PubMed: 17404676]
- 18.
- Hoyte FC, Katial RK. Antihistamine therapy in allergic rhinitis. Immunol Allergy Clin North Am. 2011 Aug;31(3):509–43. [PubMed: 21737041]
- 19.
- Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010 Sep;126(3):466–76. [PubMed: 20816182]
- 20.
- Yanez A, Rodrigo GJ. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2002 Nov;89(5):479–84. [PubMed: 12452206]
- 21.
- Kaliner MA, Berger WE, Ratner PH, et al. The efficacy of nasal antihistamines in the treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2011 Feb;106(2 Suppl):S6–S11. [PubMed: 21277531]
- 22.
- Kushnir NM. The role of decongestants, cromolyn, guafenesin, saline washes, capsaicin, leukotriene antagonists, and other treatments on rhinitis. Immunol Allergy Clin North Am. 2011 Aug;31(3):601–17. [PubMed: 21737044]
- 23.
- Institute for Clinical Systems Improvement. Health Care Guideline: Diagnosis and Treatment of Respiratory Illness in Children and Adults. 3rd ed. Jan, 2011.
- 24.
- Boehringer Ingelheim. Atrovent®(ipratropium bromide) nasal spray 0.06% prescribing information. Oct, 2011.
- 25.
- Kumar R, Kumar D, Parakh A. Fluticasone furoate: A new intranasal corticosteroid. Journal of Postgraduate Medicine. 2012 January-March;58(1):79–83. [PubMed: 22387656]
- 26.
- Mattke S, Martorell F, Hong SY, et al. Anti-inflammatory medication adherence and cost and utilization of asthma care in a commercially insured population. J Asthma. 2010 Apr;47(3):323–9. [PubMed: 20394518]
- 27.
- Harvey R, Hannan SA, Badia L, et al. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394. [PubMed: 17636843]
- 28.
- Price D, Bond C, Bouchard J, et al. International Primary Care Respiratory Group (IPCRG) Guidelines: management of allergic rhinitis. Prim Care Respir J. 2006 Feb;15(1):58–70. [PMC free article: PMC6730679] [PubMed: 16701759]
- 29.
- Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008 Jan;38(1):19–42. [PMC free article: PMC7162111] [PubMed: 18081563]
- 30.
- Van Cauwenberge P, Bachert C, Passalacqua G, et al. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy. 2000 Feb;55(2):116–34. [PubMed: 10726726]
- 31.
- Siow JK, Alshaikh NA, Balakrishnan A, et al. Ministry of Health clinical practice guidelines: Management of Rhinosinusitis and Allergic Rhinitis. Singapore Med J. 2010 Mar;51(3):190–7. [PubMed: 20428739]
- 32.
- Benninger M, Farrar JR, Blaiss M, et al. Evaluating approved medications to treat allergic rhinitis in the United States: an evidence-based review of efficacy for nasal symptoms by class. Ann Allergy Asthma Immunol. 2010 Jan;104(1):13–29. [PubMed: 20143641]
- 33.
- Nasser M, Fedorowicz Z, Aljufairi H, et al. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2010;(7):CD006989. [PMC free article: PMC7388927] [PubMed: 20614452]
- 34.
- Al Sayyad JJ, Fedorowicz Z, Alhashimi D, et al. Topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2007;(1):CD003163. [PMC free article: PMC7035883] [PubMed: 17253485]
- 35.
- Nasal Corticosteroids: Update 1 Final Report. Oregon Health & Science University, Center for Evidence-Based Policy; Oct 1, 2008. Drug Effectiveness Review Project. http://derp
.ohsu.edu /about/final-document-display.cfm. - 36.
- Newer Antihistamines: Update 2 Final Report. Oregon Health & Science University, Center for Evidence-Based Policy; May 1, 2010. Drug Effectiveness Review Project. http://derp
.ohsu.edu /about/final-document-display.cfm. - 37.
- McCrory DC, Williams JW, Dolor RJ, et al. Management of Allergic Rhinitis in the Working-Age Population. Evidence Report/Technology Assessment No. 67. Rockville, MD: Agency for Healthcare Research and Quality; Mar, 2003. (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-17-0014.) AHRQ Publication No. 03-E015. [PMC free article: PMC4781251] [PubMed: 12674744]
- 38.
- Long A, McFadden C, DeVine D, et al. Management of Allergic and Nonallergic Rhinitis. Evidence Report/Technology Assessment No. 54. Rockville, MD: Agency for Healthcare Research and Quality; May, 2002. (Prepared by the New England Medical Center Evidence-based Practice Center under Contract No. 290-97-0019.) AHRQ Publication No, 02-E024. [PMC free article: PMC4781385] [PubMed: 12173440]
- 39.
- Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; Aug, 2011. AHRQ Publication No. 10(11)-EHC063-EF. www
.effectivehealthcare.ahrq.gov. - 40.
- Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001 Apr;20(3 Suppl):21–35. [PubMed: 11306229]
- 41.
- Santaguida PL, Raina P. McMaster Quality Assessment Scale of Harms (McHarm) for Primary Studies. Hamilton, Ontario: McMaster University Evidence-Based Practice Center; 2010. p. 11.
- 42.
- White CM, Ip S, McPheeter M, et al. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; Mar, 2011. Using existing systematic reviews to replace de novo processes in conducting Comparative Effectiveness Reviews. September 2009. Chapters available at www
.effectivehealthcare.ahrq.gov. [PubMed: 21433402] - 43.
- Mohar D, Desai S, Huang H, et al. An analysis of quality of life in subjects 18-64 years of age with seasonal allergic rhinitis following treatment with ciclesonide aqueous nasal spray. J Allergy Clin Immunol. 2011;127(2 Suppl 1):AB201.
- 44.
- Juniper EF, Thompson AK, Ferrie PJ, et al. Development and validation of the mini Rhinoconjunctivitis Quality of Life Questionnaire. Clin Exp Allergy. 2000 Jan;30(1):132–40. [PubMed: 10606940]
- 45.
- Santanello NC, Zhang J, Seidenberg B, et al. What are minimal important changes for asthma measures in a clinical trial? Eur Respir J. 1999 Jul;14(1):23–7. [PubMed: 10489824]
- 46.
- Tepper RS, Wise RS, Covar R, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012 Mar;129(3 Suppl):S65–87. [PMC free article: PMC4263032] [PubMed: 22386510]
- 47.
- Barnes ML, Ward JH, Fardon TC, et al. Effects of levocetirizine as add-on therapy to fluticasone in seasonal allergic rhinitis. Clin Exp Allergy. 2006 May;36(5):676–84. [PubMed: 16650054]
- 48.
- Barnes ML, Vaidyanathan PA, Williamson PA, et al. The minimal clinically important difference in allergic rhinitis. Clin Exp Allergy. 2010 May;36(5):676–84. [PubMed: 16650054]
- 49.
- Bousquet PJ, Combescure C, Klossek JM, et al. Change in visual analog scale score in a pragmatic randomized cluster trial of allergic rhinitis. J Allergy Clin Immunol. 2009 Jun;123(6):1349–54. [PubMed: 19368963]
- 50.
- Malling HJ. Criteria for clinical efficacy--readout and monitoring of clinical studies. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M. 2003;(94):119–23. discussion 23-5. [PubMed: 15119028]
- 51.
- Owens DK, Lohr KN, Atkins D, et al. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; Mar, 2011. Grading the strength of a body of evidence when comparing medical interventions. July 2009. Chapters available at www
.effectivehealthcare.ahrq.gov. [PubMed: 19595577] - 52.
- Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions--Agency for Healthcare Research and Quality and the effective health-care program. J Clin Epidemiol. 2010 May;63(5):513–23. [PubMed: 19595577]
- 53.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLo S Med. 2009 Jul 21;6(7):e1000097. [PMC free article: PMC2707599] [PubMed: 19621072]
- 54.
- Hodge LS, Tracy TS. Alterations in drug disposition during pregnancy: implications for drug therapy. Expert Opin Drug Metab Toxicol. 2007 Aug;3(4):557–71. [PubMed: 17696806]
- 55.
- Sheikh A, Singh Panesar S, Salvilla S, et al. Hay fever in adolescents and adults. Clin Evid (Online). 2009 Nov 18;11:509. [PMC free article: PMC2907832] [PubMed: 21726475]
- 56.
- Topuz B, Ogmen GG. Montelukast as an adjuvant to mainstay therapies in patients with seasonal allergic rhinitis. Clin Exp Allergy. 2003 Jun;33(6):823–6. [PubMed: 12801319]
- Executive Summary - Treatments for Seasonal Allergic RhinitisExecutive Summary - Treatments for Seasonal Allergic Rhinitis
- Study-Level Results - Treatments for Basal Cell and Squamous Cell Carcinoma of t...Study-Level Results - Treatments for Basal Cell and Squamous Cell Carcinoma of the Skin
- Risk of Bias Assessment - Physiologic Predictors of Severe Injury: Systematic Re...Risk of Bias Assessment - Physiologic Predictors of Severe Injury: Systematic Review
- Care Interventions for Transition - Transitions of Care From Pediatric to Adult ...Care Interventions for Transition - Transitions of Care From Pediatric to Adult Services for Children With Special Healthcare Needs
- zinc finger FYVE domain-containing protein 21 isoform X2 [Mus musculus]zinc finger FYVE domain-containing protein 21 isoform X2 [Mus musculus]gi|568980320|ref|XP_006516257.1|Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...
