NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Glacy J, Putnam K, Godfrey S, et al. Treatments for Seasonal Allergic Rhinitis [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Jul. (Comparative Effectiveness Reviews, No. 120.)
This publication is provided for historical reference only and the information may be out of date.
Results of Literature Searches
Of the 4,513 records identified through the literature search, 4,458 were excluded during screening. Four records were identified through grey literature and hand searching of bibliographies. One unpublished trial listed on ClinicalTrials.gov satisfied our inclusion criteria (NCT00960141). However, this trial was not included because quality assessment was not possible without the published report. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)49 diagram shown in Figure 4 depicts the flow of screening and study selection. A total of 59 unique trials were included. Several of these were three arm trials that addressed more than one comparison. Occasionally, more than one trial was reported in a single publication. Although search strategies were designed with the appropriate methodological filters to identify randomized controlled trials (RCTs), observational studies, systematic reviews and meta-analyses, the results did not yield studies for all comparisons of interest. For Key Question (KQ) 1 and KQ2, 56 RCTs, and one quasi-RCT, that addressed 13 of 22 comparisons of interest were found. For KQ3, no studies that addressed any of 17 comparisons of interest were found, and for KQ4, two RCTs that addressed one of 21 comparisons of interest were found. No observational studies, systematic reviews, or meta-analyses that met our inclusion criteria.

Figure 4
PRISMA diagram for identified trials.
The list of excluded studies with reasons for exclusion is presented in Appendix B.
Overview
Of 22 comparisons of interest for adults and adolescents (KQ1 and KQ2), we found studies that addressed 13 (Table 9). Of 21 comparisons of interest for children younger than 12 years of age (KQ4), we found studies that addressed one, oral selective antihistamine versus oral nonselective antihistamine. No studies were identified for pregnant women (KQ3). An overview of included studies is presented in Table 10. A summary of drugs studied in included trials is shown in Table 11.
Table 9
Results of literature searches for Key Question 1 and Key Question 2 comparisons of interest.
Table 10
Overview of included randomized controlled trials.
Table 11
Drugs studied in included trials.
The number of studies for each comparison ranged from two to 13. This variability was due in part to our inclusion requirement of Food and Drug Administration (FDA)-approved drugs only, which impacted particularly the comparison of oral selective antihistamine to oral nonselective antihistamine. The majority of these trials used terfenadine or astemizole as the selective antihistamine comparator, neither of which is currently FDA-approved due to postmarketing safety concerns. As a result, only three trials were included for this comparison.
Trial sizes ranged from 27 to 1343 patients (13 to 672 patients per treatment arm). Fourteen percent of trials had fewer than 25 patients per treatment arm, 10 percent had 25 to 50, and 32 percent had more than 100. The proportion of good and poor quality trials varied across comparisons, from 100 percent good quality trials for the comparisons of combination intranasal corticosteroid plus nasal antihistamine both to intranasal corticosteroid and to nasal antihistamine, to 100 percent poor quality trials for the comparison of intranasal corticosteroid to nasal cromolyn. Overall, approximately half of trials were rated poor quality using United States Preventive Services Task Force (USPSTF) criteria, and one quarter was rated good quality.
Detailed descriptions of trials and patient characteristics are shown in abstraction tables located in Appendix C. Eighty-one percent of trials were double-blinded. Seventy-one percent included a run-in period, a period before the start of a clinical trial used to establish baseline characteristics and to assess compliance and stability of enrolled patients; either no treatment or placebo treatment (favored by the FDA50) was given. Half of trials reported pollen counts. Most (88 percent) confirmed seasonal allergic rhinitis (SAR) diagnosis by either skin prick test or intradermal skin test. For inclusion, most trials required either a minimum duration of SAR symptoms (17 percent), or minimum severity (14 percent), or both (63 percent). Exclusions included infection (15 percent), anatomical deformity including nasal polyps (15 percent), or both (50 percent). Forty-eight trials (81 percent) restricted the use of SAR medications before trial entry. Of these, approximately half reported using FDA-recommended washout periods. Five trials excluded patients with a past or recent history of immunotherapy. Others admitted patients receiving immunotherapy provided treatments were stable before and during the trial. Seventy-one percent of trials explicitly excluded pregnant women.
For pharmacologic classes that have more than one drug, no comparison had 100 percent representation (that is, included all drugs in class). As shown in Table 11, representation of drug classes varied across comparisons. Collectively across all comparisons, oral and nasal antihistamine and intranasal corticosteroid were well represented. However, the level of representation varied across individual comparisons. Three of five oral selective antihistamines (60 percent) and five of eight intranasal corticosteroids (62.5 percent) were included in direct comparison with each other. Oral selective antihistamine also was well represented (by at least three of five drugs [60 percent]) in comparisons to nasal antihistamine, oral decongestant (alone and in combination), and oral leukotriene receptor antagonist (montelukast). In contrast, for the comparisons of combination intranasal corticosteroid and nasal antihistamine to each component, only one of eight intranasal corticosteroids (fluticasone propionate; 12.5 percent) was studied. Fluticasone propionate was the most studied intranasal corticosteroid and appeared in every comparison involving intranasal corticosteroids. The intranasal corticosteroid ciclesonide was not studied in any identified trial. No trials of nasal anticholinergic (ipratropium) or nasal decongestant were identified. One of two oral decongestants (pseudoephedrine) was studied. Only three of eleven oral nonselective antihistamines (27 percent) were represented in two comparisons, one in adolescents and adults (KQ1), and one in children (KQ4). Conclusions based on comparisons of pharmacologic classes that were poorly represented are limited to the specific drugs studied. How well such conclusions generalize to other drugs in the same class is uncertain.
Half of trials reported eye outcomes. Only two108, 127 reported asthma outcomes. Only one trial96 assessed as-needed (prn) dosing. All others used continuous daily dosing. We were therefore unable to compare intermittent to continuous treatment, a subquestion to each of our KQs. Most trials (86 percent) were 2 or 4 weeks in duration. Six trials88, 120, 123, 128, 131, 132 were 6 to 8 weeks in duration. These trials reported on five different treatment comparisons. For the remaining eight comparisons, we were unable to compare short-term to longer-term use.
The reporting of efficacy outcomes varied across trials. Most trials that assessed nasal symptoms assessed four individual symptoms (congestion, rhinorrhea, sneezing, and nasal itch) and/or a total nasal symptom score (TNSS) comprising the sum or average of scores for the individual symptoms. However, some trials reported only a total symptom score (TSS) comprising four nasal symptoms plus up to five additional symptoms (eye itching, tearing, and redness; itching of the ears and palate). Trials comparing oral antihistamine and oral decongestant assessed “TNSS minus congestion” (defined a priori) because of the known differential efficacy of the drugs for treatment of congestion. Similarly, for eye outcomes, three symptoms were most commonly assessed (itching, tearing, and redness) and summed or averaged to produce a total ocular symptom score (TOSS). However, some trials incorporated ocular swelling into the TOSS or did not define which eye symptoms were assessed by the TOSS. To facilitate comparisons of results across trials, individual symptom scores, the four-symptom TNSS, and three-symptom TOSS were abstracted.
For assessing nasal and eye symptom severity, most trials used a 4-point interval rating scale, from 0 for no symptoms to 3 for severe symptoms that interfere with one's daily activity. However, some used 6-point (0 to 5) or 3-point (0 to 2) scales. Five trials98, 100, 126, 127, 129 reported on the outcome of TNSS using a 0-10 or 0-100 visual analog scale (VAS). When pooling results for meta-analyses, differences in scales were accommodated by use of standardized rather than non-standardized mean differences. This was necessary in three of 28 meta-analyses conducted. Most trials could not be pooled due to a lack of reported variance for group-level treatment effects.
Most trials that assessed quality of life used the Rhinitis Quality of Life Questionnaire (RQLQ). The RQLQ is a 27-item questionnaire validated in patients with rhinoconjunctivitis. Scores range from 0 (no impairment) to 6 (severe impairment). The anchor-based minimum clinically important difference (MCID) is 0.5 points.65 Two trials in a single publication89 used the Nocturnal RQLQ to assess sleep disturbance due to nasal symptoms at 2 weeks. The Nocturnal RQLQ is a 16-item questionnaire validated in patients with nocturnal rhinoconjunctivitis. Nocturnal symptoms are scored on a 7-point Likert scale from 0 (not troubled) to 6 (extremely troubled). An MCID has not been identified.67 One trial used the mini-RQLQ to assess nasal symptoms.62 The mini-RQLQ is a 14-item questionnaire validated in patients with rhinoconjunctivitis. Each question is scored on a scale from 0 (not troubled) to 6 (extremely troubled). The global mini-RQLQ score is the mean of all question scores. The anchor-based MCID is 0.7 points.66
Some trials used a patient global assessment (PGA) scale to assess patient satisfaction with treatment. Integer rating scales were commonly used, but these varied in design (e.g., 7 or 11-point Likert scales of treatment response ranging from very much improved to very much worse, or 4-point scales of satisfaction with treatment ranging from extremely satisfied to not at all satisfied). Results were reported either categorically (proportion of patients with good or very good response to treatment) or continuously (mean PGA scores). Because of this variability, comparison across trials was not possible. Further, although the interpretation of PGA results is clearer when outcomes are aligned with other reported results, statistically significant improvements in PGA in a trial reporting nonstatistically significant improvements in SAR symptoms were difficult to interpret. Most PGA assessments were made at the end of treatment only, without comparison to baseline values, further limiting their utility. For the purposes of this report, PGA results aligned with other treatment effects were considered supportive findings that enhanced the robustness of the trial. Discrepant PGA results were noted. In either case, PGA was not incorporated into the formal strength of evidence assessment for any outcome.
Finally, treatment effects were calculated in a variety of ways. Most trials calculated mean change from baseline symptom scores by subtracting mean baseline scores from symptom scores averaged across the entire treatment duration. However, some used endpoint values rather than mean values for this calculation, and others performed no calculation, comparing endpoint values rather than change from baseline values. A third approach was to calculate change from baseline using mean scores during an interval of the treatment duration, for example, the mean of scores during the third and fourth week of treatment compared with baseline. Finally, some reported only relative results, for example, the percent reduction from baseline scores. When pooling results for meta-analysis, differences in efficacy calculations were accommodated by reporting mean differences rather than standardized mean differences.48 When meta-analysis was not possible, comparisons of treatment effects were approximated. The degree to which different methods of results reporting impacted the magnitude or statistical significance of observed treatment effects is uncertain. As above, when the result of statistical testing was reported, it became the main parameter for comparison of efficacy across trials.
For KQ2, 33 trials reported directly comparable, group level adverse event information. Of these, 17 were rated good quality and 16 were rated poor quality. Additionally, 14, 6, and 11 trials used active, intermediate, and passive surveillance, respectively. Headache, sedation and nosebleeds were the most commonly reported events across the treatment comparisons. There were no reports in any trials for nine of 24 adverse event categories (37.5 percent), including all systemic effects of corticosteroids. No adverse events met our criteria for performing meta-analysis.
Reporting of adverse events fell into one of three categories: (1) general statements such as, “All groups were similar in the percentage of patients with clinical and laboratory adverse experiences;”97 (2) accounts only of adverse events that occurred with a frequency greater than zero; and (3) accounts of adverse events in each treatment group. Adverse event data from trials in the second category were uninformative because we could not distinguish between missing adverse event reports and adverse events that occurred with a frequency of zero in other treatment groups. In the third category, trials that reported events as a proportion of reports rather than a proportion of patients were not useful for comparative purposes; these data were abstracted to assess consistency of the body of evidence. Trials that reported efficacy results at multiple time points did not report adverse events by occurrence in time. For this reason, it was not possible to compare the emergence of adverse events across varying treatment exposures.
As described in the Methods section, we assessed the strength of the body of evidence for each outcome using a system based on Grading of Recommendations Assessment, Development, and Evaluation (GRADE). In addition to the four main domains assessed (risk of bias, consistency, directness, and precision), the following additional domains were considered and deemed not relevant for the reasons listed:
- Dose-response association – Levels of exposure tended to be standard for each intervention.
- Strength of association – Effect sizes generally were small.
- Publication bias – We found no indication that relevant empirical findings were unpublished.
How This Section Is Organized
Results are organized by KQ and then by the treatment comparisons of interest for each KQ. A Description of Included Studies, Key Points, and Synthesis and Strength of Evidence are presented for each treatment comparison.
- Description of Included Studies
- For additional information, detailed abstraction tables are located in Appendix C. These include trial description, patient characteristics, USPSTF quality rating, outcomes, and harms tables.
- Key Points
- Key Points are organized by outcome. The strength of evidence was summarized in bullet points and in tabular form.
- In some cases, separate outcomes with similar strength of evidence ratings are bundled together for reporting in the bullet points.
- Synthesis and Strength of Evidence
- This section is organized by type of outcome (nasal symptoms, eye symptoms, asthma symptoms, and quality of life). For each type of outcome, individual outcomes are presented usually in two paragraphs: The first summarizes the findings for that outcome. The second describes the overall rating of the strength of evidence for that outcome.
- For outcomes that are straightforward, findings and strength of evidence assessment may be presented in a single paragraph. For outcomes or comparisons that are more complex, more than two paragraphs may be required.
- Tables of treatment effects for each type of outcome discussed follow the discussion.
- For each type of outcome, meta-analyses follow the tables. For example, a treatment effect table may summarize four nasal symptom outcomes. If meta-analyses were conducted for three of the outcomes, these would follow the treatment effect summary table for nasal outcomes.
Key Question 1. Comparative Effectiveness of SAR Treatments in Adults and Adolescents 12 Years of Age or Older
Oral Selective Antihistamine Versus Oral Nonselective Antihistamine
Description of Included Studies
Three RCTs81-83 published between 1987 and 1996 were identified (N=515). All three were 2-week, multicenter trials conducted in North America. Trial size ranged from 86 to 220 patients randomized to treatment groups of interest. Oral selective antihistamines studied were loratadine (two trials81, 83) and cetirizine (one trial82); oral nonselective antihistamines were clemastine (two trials81, 83) and chlorpheniramine (one trial82). Two trials81, 83 were double-blinded, and one82 was assessor-blinded only. One trial82 was industry-funded, and the other two did not report funding source.
Average patient ages were in the early 30s. Approximately 40 percent of patients were women. When reported, the majority of patients were white (74-93 percent). All three trials required a minimum severity of SAR symptoms and, in the one trial that reported values, baseline symptoms were in the moderate range. Although none of the trials required a minimum duration of SAR history, most patients had SAR symptoms for more than 16 years.
Information from one trial each was available for nasal symptoms,83 adverse events,81 and quality of life.82 No trial assessed eye or asthma symptoms. Nasal symptom outcomes were assessed using a 4-point (0=no symptoms, 3=severe symptoms) scale; the scores for congestion, rhinorrhea, sneezing and itching were summed for a TNSS ranging from 0 to 12.83 One trial82 used the RQLQ to measure quality of life (0=no impairment, 6=severely impaired), with 28 questions in 7 domains summed for a total score ranging from 0 to 168. The usual use of the RQLQ is to average the scores of each domain and the MCID is 0.5.65 By extrapolation, the MCID is 14 for this trial.
Two trials81, 82 were rated poor quality and one83 was rated fair.
Key Points
These results are summarized in Table 12.
Table 12
Strength of evidence: oral selective antihistamine versus oral nonselective antihistamine.
- TNSS at 2 weeks: Evidence was insufficient to support the use of one treatment over the other based on one trial 83 with medium risk of bias and imprecise results.
- Quality of life at 2 weeks: Evidence was insufficient to support the use of one treatment over the other based on one trial 82 with high risk of bias. Although a statistically significant treatment effect was reported, the magnitude of the treatment effect was less than the MCID.
- These results are based on trials of two of five oral selective antihistamines (40 percent) and two of eleven oral nonselective antihistamines (18 percent).
Synthesis and Strength of Evidence
Nasal symptom results discussed below are summarized in Table 13. Quality of life results are summarized in Table 14. Meta-analysis was not possible due to the small number of trials.
Table 13
Treatment effects: nasal symptoms–oral selective antihistamine versus oral nonselective antihistamine.
Table 14
Treatment effects: quality of life–oral selective antihistamine versus oral nonselective antihistamine.
Nasal Symptoms
Of three identified trials, one83 (N=209) reported nasal symptom outcomes (TNSS). This trial was rated fair quality, and reported a non-statistically significant treatment effect of 0.3 on a 0-12 point scale (3 percent of maximum score) favoring oral selective antihistamine. Risk of bias was considered moderate based on trial quality. Because consistency of the observed effect cannot be assessed with a single trial and because the effect was imprecise, the evidence was insufficient to support the use of one treatment over the other.
Quality of Life
Of three identified trials, one82 (N=86) reported quality of life outcomes. This trial was rated poor quality due to noncomparable groups at baseline and inappropriate analysis of results (unadjusted for baseline group differences). The treatment effect was 12.9 on a 0-168 point scale favoring oral selective antihistamine and was statistically significant. Extrapolating the anchor-based MCID (0.5) for the RQLQ 0-6 point scale yields an MCID of 14 points. Risk of bias for this outcome was considered high based on both trial quality and the use of quality of life measures in an unblinded trial population. Consistency is unknown with a single trial, and the treatment effect was imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
Oral Selective Antihistamine Versus Nasal Antihistamine
Description of Included Studies
Five double-blind, RCTs84-88 published between 1993 and 2006 were identified (N=1,052). Four84-87 were multicenter trials. Three trials84, 85, 87 were conducted in North America and two86, 88 in Europe. All trials were 2 weeks in duration. Trial size ranged from 30 to 360 patients randomized to treatment groups of interest. Oral selective antihistamines studied were cetirizine (three trials85-87), loratadine (one trial88), and desloratadine (one trial84); nasal antihistamine was azelastine in all five trials. Two older trials86, 88 used the lower of two FDA-approved doses of azelastine, equivalent to half the dose used in more recent trials. Two trials86, 87 were industry-funded. Three trials84, 85 did not report funding source.
Average patient ages ranged from 30 to 36 years. In most trials, the majority of patients were women (56-67 percent). In three trials that reported information on race, the majority was white (69-81 percent). Four trials required a minimum severity of SAR symptoms. Average TNSS at baseline were most commonly in the severe range. Patients with chronic asthma were excluded from four trials. One trial88 did not specify whether patients with chronic asthma were included. All five trials required a minimum duration of SAR history. Most patients had SAR symptoms for more than 18 years. Two trials84, 88 did not report disease duration.
Of four trials84-87 that assessed nasal symptoms, all reported 2-week outcomes. Three trials84, 85, 87 used a 4-point (0=no symptoms, 3=severe symptoms) rating scale for the assessment of four nasal symptoms (congestion, rhinorrhea, sneezing, and itch). In three trials84, 85, 87 patients assessed symptoms in both the morning and evening, yielding 6-point maximums for individual symptoms and a 24-point maximum for TNSS. One trial86 collected nasal symptom scores once daily using a 0 (no symptoms) to 100 (severe symptoms) VAS. Three trials85-87 assessed quality of life using the RQLQ. Of several outcomes reported by Gambardella (1993)88, sufficient information was provided to abstract adverse events only. No trials assessed eye or asthma symptoms.
Three trials84, 85, 87 were rated good quality and two86, 88 were rated poor.
Key Points
These results are summarized in Table 15.
Table 15
Strength of evidence: oral selective antihistamine versus nasal antihistamine.
- Individual nasal symptoms and TNSS at 2 weeks: Evidence was insufficient to support the use of one treatment over the other based on three trials (for rhinorrhea,84, 86, 87 nasal itch,84, 86, 87 and TNSS84, 85, 87) or four trials84-87 (for congestion and sneezing) with low risk of bias and consistent but imprecise results.
- Quality of life at 2 weeks: Evidence was insufficient to support the use of one treatment over the other based on two trials85, 87 with low risk of bias and consistent but imprecise results. Although statistically significant treatment effects were reported, the magnitude of effects was less than the MCID.
- These results are based on trials of three of five oral selective antihistamines (60 percent) and one of two nasal antihistamines (50 percent).
Synthesis and Strength of Evidence
Nasal symptom results discussed below are summarized in Table 16. Quality of life results are summarized in Table 17. As shown in these tables, only two trials provided variance estimates for reported outcomes. Thus, meta-analysis was not possible.
Table 16
Treatment effects: nasal symptoms–oral selective antihistamine versus nasal antihistamine.
Table 17
Treatment effects: quality of life–oral selective antihistamine versus nasal antihistamine.
Nasal Symptoms
Four trials84-87 (N=1022) assessed congestion after 2 weeks of treatment and reported greater improvement with nasal antihistamine than with oral selective antihistamine. Of three trials that reported p-values, this result was statistically significant in two.85, 86 One85 was a good quality trial of 360 patients (35 percent of patients reporting this outcome) that did not report the magnitude of the treatment effect. The other trial86 (n=136) was rated poor quality due to noncomparable groups at baseline and inappropriate analysis of results (unadjusted for baseline group differences). The magnitude of the treatment effect was not reported. Treatment effects of 0.08 and 0.17 on a 0-6 point scale (both less than 3 percent of maximum score) were reported by two trials84, 87 that were rated good quality (51 percent of patients reporting). Statistical significance of the former result was not assessed. The latter result was not statistically significant.
For the outcome of congestion at 2 weeks, the risk of bias was rated as low. Eighty-seven percent of patients assessed for this outcome were in good quality trials. All four trials were consistent in favoring nasal antihistamine, but treatment effects were imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
Three trials84, 86, 87 (N=662) assessed rhinorrhea after 2 weeks of treatment and reported greater improvement with nasal antihistamine than with oral selective antihistamine. Of two trials that reported p-values, this result was statistically significant in both.86, 87 One87 was a good quality trial of 307 patients (46 percent of patients reporting this outcome) that reported a treatment effect of 0.46 on a 0-6 point rating scale (8 percent of maximum score). The other86 was the poor quality trial identified above. The magnitude of the treatment effect was not reported. A treatment effect of 0.22 on a 0-6 point scale (4 percent of maximum score) was reported by one trial84 that was rated good quality (33 percent of patients reporting). Statistical significance was not assessed.
For the outcome of rhinorrhea at 2 weeks, the risk of bias was rated as low. Seventy-nine percent of patients assessed for this outcome were in good quality trials. All three trials were consistent in favoring nasal antihistamine, but treatment effects were imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
Four trials84-87 (N=1022) assessed sneezing after 2 weeks of treatment and reported greater improvement with nasal antihistamine than with oral selective antihistamine. Of three trials that reported p-values, this result was statistically significant in one.85 This was a good quality trial of 360 patients (35 percent of patients reporting this outcome) that did not report the magnitude of the treatment effect. A statistically nonsignificant treatment effect of 0.29 on a 0-6 point scale (5 percent of maximum score) was reported by another good quality trial87 of 307 patients (30 percent of patients reporting). The magnitude of the statistically nonsignificant treatment effect in the poor quality trial86 identified above was not reported. A treatment effect of 0.23 on a 0-6 point scale (4 percent of maximum score) was reported by another good quality trial84 of 219 patients (21 percent of patients reporting). Statistical significance was not assessed.
For the outcome of sneezing at 2 weeks, the risk of bias was rated as low. Eighty-seven percent of patients assessed for this outcome were in good quality trials. All four trials were consistent in favoring nasal antihistamine, but treatment effects were imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
Three trials84, 86, 87 (N=662) assessed nasal itch after 2 weeks of treatment and reported greater improvement with nasal antihistamine than with oral selective antihistamine. Of two trials84, 87 that reported p-values, results were not statistically significant in either. One87 was a good quality trial of 307 patients (46 percent of patients reporting this outcome) that reported a treatment effect of 0.30 on a 0-6 point scale (5 percent of maximum score). The other86 was the poor quality trial previously identified. No treatment effect was reported. A treatment effect of 0.25 on a 0-6 point scale (4 percent of maximum score) was reported by another good quality trial84 of 219 patients (33 percent of patients reporting). Statistical significance was not assessed.
For the outcome of nasal itch at 2 weeks, the risk of bias was rated as low. Seventy-nine percent of patients assessed for this outcome were in good quality trials. All three trials were consistent in favoring nasal antihistamine, but treatment effects were imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
Three trials84, 85, 87 (N=886) assessed TNSS at 2 weeks and reported greater improvement with nasal antihistamine than with oral selective antihistamine. Of three trials84, 85, 87 that reported p-values, this result was statistically significant in one trial.87 This was a good quality trial of 307 patients (35 percent of patients reporting this outcome) that reported a treatment effect of 1.24 on a 0-24 point scale (5 percent of maximum score). Treatment effects of 0.78 and 0.70 on a 0-6 point scale (13 percent and 12 percent of maximum score, respectively) were reported by two trials84, 85 that were rated good quality (65 percent of patients reporting). Statistical significance of the former result was not assessed. The latter result was not statistically significant.
For the outcome of TNSS at 2 weeks, the risk of bias was rated as low. All three trials reporting this outcome were rated as good quality. All three trials also were consistent in favoring nasal antihistamine, but treatment effects were imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
Quality of Life
Two trials85, 87 (N=667) assessed quality of life at 2 weeks using the RQLQ. Both were good quality trials that reported statistically significant reductions in RQLQ with nasal antihistamine compared to oral selective antihistamine. Treatment effects on a 0-6 scale were 0.4 points in one trial85 and 0.3 points in the other.87 One poor quality trial86 of 136 patients reported a statistically nonsignificant difference in the proportion of patients who reported an excellent or good response to treatment rather than a fair or poor response, with a treatment difference of 0.6 percent favoring nasal antihistamine.
For the outcome of quality of life as measured by the RQLQ at 2 weeks, the risk of bias was rated as low. Both trials reporting this outcome were rated good quality. Trials were consistent in favoring nasal antihistamine over oral selective antihistamine, but neither treatment effect exceeded the MCID of 0.5 and was therefore imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
Oral Selective Antihistamine Versus Intranasal Corticosteroid
Description of Included Studies
Thirteen RCTs89-100 published between 1995 and 2009 were identified (N=4403). Twelve were double-blinded, multicenter trials,89-95, 97-100 and one96 was an unblinded, single center trial. Eleven89-93, 95-99 were conducted in North America, and two94, 100 in Europe. Trial sizes ranged from 88 to 623 patients randomized to treatment groups of interest. Oral selective antihistamines studied were loratadine (10 trials90-99), fexofenadine (two trials89 in one publication), and cetirizine (one trial100); intranasal corticosteroids were fluticasone propionate (six trials91, 94-98), fluticasone furoate (two trials89 in one publication), triamcinolone (three trials92, 93, 99), mometasone (one trial90), and beclomethasone (one trial97). One trial96 assessed as-needed (prn) dosing of both the oral selective antihistamine (loratadine) and the intranasal corticosteroid (fluticasone propionate). All other trials evaluated continuous scheduled dosing of both drugs. Five trials89, 90, 97, 98 were 2 weeks in duration, one100 was 3 weeks, and seven91-96, 99 were 4 weeks. Twelve trials were industry-funded, and one90 did not report funding source.
Mean age ranged from 25 to 41 years. In most trials, the majority of patients were female (51-68 percent); no trial had less than 40 percent female patients. In nine trials89, 91-93, 96-99 that reported information on race, most patients were white (57-92 percent). Eleven trials89-94, 96-100 required a minimum severity of SAR symptoms. In nine trials89-92, 97-100 that reported baseline values, nasal symptom scores were most commonly in the moderate range; two trials89 in the same publication (N=1074) reported mean baseline scores in the severe range, and one trial97 reported mean baseline scores in the mild range. In five trials89, 91, 92, 99 that reported baseline eye symptoms, values were in the moderate/severe range in three trials89, 91 and in the mild range in two trials.92, 99 Ten trials89-93, 96-99 required a minimum duration of SAR history. In eight trials89, 90, 93, 94, 97, 99, 100 that reported SAR duration, most patients had SAR symptoms for more than 10 years. In one trial94, most patients had SAR for 2 to 5 years, and in another100, most patients had SAR for more than 8 years.
All 13 trials assessed at least one individual nasal symptom or TNSS. Most trials used a 4-point scale (0=no symptoms, 3=severe symptoms) to assess four individual nasal symptoms (congestion, rhinorrhea, sneezing, and itch), yielding a 12-point maximum for TNSS. Two trials91, 98 used a visual analog scale to rate these nasal symptoms on a scale of zero to 100, for a maximum TNSS of 400. One trial100 used the 4-point scale to assess five nasal symptoms (congestion when waking, daytime congestion, rhinorrhea, sneezing, and itch), for a 15-point maximum TNSS.
Of seven trials89-93, 99 that assessed eye symptoms, most assessed ocular itching, tearing, and redness using the 4-point scale described above. The maximum TOSS was 9. One trial91 used a VAS to rate ocular symptoms on a scale of zero to 100, for a maximum TOSS of 300. Two trials93, 99 did not identify which ocular symptoms were assessed. One trial90 assessed three ocular symptoms but reported results for tearing only.
Of eight trials89, 91, 92, 94, 96, 98, 100 that assessed quality of life, five91, 92, 94, 96, 98 used the RQLQ. Measures on a 0 (no impairment) to 6 (severe impairment) rating scale were recorded at 2 weeks in three trials92, 96, 98 and at 4 weeks in four trials.91, 92, 94, 96 Two trials89 in the same publication used the Nocturnal RQLQ to assess sleep disturbance due to nasal symptoms at 2 weeks. Nocturnal symptoms were scored on a 7-point scale from 0 (not troubled) to 6 (extremely troubled).
Two trials91, 95 were rated good quality, three were rated fair89, 98 and eight90, 92-94, 96, 97, 99, 100 were rated poor.
Key Points
These results are summarized in Table 18.
Table 18
Strength of evidence: oral selective antihistamine versus intranasal corticosteroid.
- Individual nasal symptoms (congestion, rhinorrhea, sneezing, and nasal itch) at 2 weeks: Evidence was insufficient to support the use of one treatment over the other based on one trial90 with high risk of bias and imprecise results.
- Individual nasal symptoms at 4 weeks: Evidence was insufficient to support the use of one treatment over the other based on six trials91-93, 95, 96, 99 (for congestion), five trials92, 93, 95, 96, 99 (for sneezing), and four trials (for rhinorrhea92, 95, 96, 99 and nasal itch92, 93, 95, 99) with high risk of bias and consistent but imprecise results.
- Quality of life assessed by Nocturnal RQLQ at 2 weeks: Evidence was insufficient to support the use of one treatment over the other based on two trials 89 with medium risk of bias and consistent but imprecise results.
- These results are based on trials of three of five oral selective antihistamines (60 percent) and five of eight intranasal corticosteroids (62.5 percent).
Synthesis and Strength of Evidence
Nasal symptom results discussed below are summarized in Table 19, eye symptom results in Table 20, and quality of life results in Table 21. As shown in Table 19 and Table 20, three trials91, 92, 99 provided variance estimates for a nasal outcome (congestion at 4 weeks) and an eye outcome (TOSS at 4 weeks). Thus, meta-analyses of these results were conducted.
Table 19
Treatment effects: nasal symptoms–oral selective antihistamine versus intranasal corticosteroid.
Table 20
Treatment effects: eye symptoms–oral selective antihistamine versus intranasal corticosteroid.
Table 21
Treatment effects: quality of life–oral selective antihistamine versus intranasal corticosteroid.
Nasal Symptoms
The one trial90 that reported on nasal congestion at 2 weeks (N=341) reported a treatment effect of 0.3 on a 0-6 point scale (5 percent of maximum score) favoring intranasal corticosteroid. This trial was rated poor quality, and the result was not statistically significant. Evidence for the outcome of congestion at 2 weeks is therefore insufficient to support the use of one treatment over the other. One poor quality trial90 with high risk of bias reported an imprecise treatment effect.
Six trials91-93, 95, 96, 99 assessed congestion at 4 weeks (N=1600). All six showed statistically significant improvements in congestion with intranasal corticosteroid. Two91, 95 were good quality trials of 558 patients total (35 percent of patients reporting this outcome). One95 reported results using a 0-3 point scale but did not report the magnitude of the treatment effect. The other91 reported a treatment effect of 10.3 on a 0-100 VAS (10 percent of maximum score). Four trials92, 93, 96, 99 were rated poor quality due to noncomparable groups at baseline92, 93, 96 and inappropriate analysis of results (unadjusted for baseline group differences93, 96 and not intention to treat99). Two92, 99 of these trials reported treatment effects of 0.3 and 0.46 on a 0-3 point scale (10 percent and 15 percent of maximum score, respectively).
Three trials91, 92, 99 (N=938; 59 percent of patients reporting this outcome) were pooled in a meta-analysis (Figure 5). Because trials used different symptom rating scales (0-3 and 0-100), the standardized mean difference was calculated. The pooled effect estimate was 0.45 (95 percent confidence interval (CI): 0.28 to 0.61), a statistically significant result that favored intranasal corticosteroid and was consistent with the direction of effect reported by individual trials. Effect estimates in the pooled trials were in the same direction, and their 95 percent CIs did not touch the “no effect” line. The magnitude of the pooled effect estimate could not be compared with estimates from individual trials not included in the meta-analysis because the latter were not reported.

Figure 5
Congestion at 4 weeks: meta-analysis of 3 trials–oral selective antihistamine versus intranasal corticosteroid.
For the outcome of congestion at 4 weeks, the risk of bias was rated as high. Sixty-five percent of patients were in poor quality trials, and 35 percent were in good quality trials. All six trials91-93, 95, 96, 99 were consistent in finding statistically significant treatment effects favoring intranasal corticosteroid, and this finding was confirmed in a meta-analysis of three of these trials.91, 92, 99 Because reported treatment effects were less than an MCID of 30 percent maximum score, and because the magnitude of effects in three trials93, 95, 96 representing 40 percent of patients reporting this outcome were not reported, the body of evidence was considered imprecise. The evidence was therefore insufficient to form a conclusion about the comparative effectiveness of oral selective antihistamine and intranasal corticosteroid for this outcome.
The one trial90 that assessed rhinorrhea at 2 weeks (N=341) reported a treatment effect of 0.3 on a 0-3 point scale (10 percent of maximum score) favoring intranasal corticosteroid. This trial was rated poor quality, and the result was not statistically significant. Five trials92, 93, 95, 96, 99 assessed rhinorrhea at 4 weeks. One poor quality trial93 reported neither the magnitude nor the direction of the treatment effect. This trial was excluded from analysis of this outcome, reducing the total number of patients assessed from 1284 to 979. The remaining four trials all favored intranasal corticosteroid over oral selective antihistamine. One trial95 was a good quality trial of 242 patients (25 percent of patients reporting this outcome) that demonstrated a statistically significant improvement in rhinorrhea with intranasal corticosteroid. The magnitude of the treatment effect was not reported. The remaining three trials92, 96, 99 were rated poor quality due to noncomparable groups at baseline and inappropriate analysis of results, as described above. Two96, 99 of these reported statistically significant treatment effects favoring intranasal corticosteroid. One99 reported a treatment effect of 0.55 on a 0-3 point scale (18 percent of maximum score). The other96 did not report the magnitude of the treatment effect. The third poor quality trial92 reported a statistically nonsignificant treatment effect of 0.2 on a 0-3 scale (7 percent of maximum score).
Evidence for the outcome of rhinorrhea at 2 weeks was insufficient to support the use of one treatment over the other. One poor quality trial90 with high risk of bias reported an imprecise treatment effect. For the outcome of rhinorrhea at 4 weeks, the risk of bias was rated as high. Seventy-five percent of patients were in poor quality trials. Effect estimates consistently favored intranasal corticosteroid. However, none exceeded an MCID of 30 percent maximum score, and the body of evidence was considered imprecise. Evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
The one trial90 that assessed sneezing at 2 weeks (N=341) reported a treatment effect of 0.1 on a 0-3 point scale (3 percent of maximum score) favoring intranasal corticosteroid. This trial was rated poor quality, and the result was not statistically significant. Five trials92, 93, 95, 96, 99 assessed sneezing at 4 weeks (N=1284). All five showed statistically significant improvements in sneezing with intranasal corticosteroid. One of these was a good quality trial95 of 242 patients (19 percent of patients reporting this outcome) that did not report the magnitude of the treatment effect. The remaining four trials92, 93, 96, 99 were rated poor quality for noncomparable groups at baseline and inappropriate analysis of results, as described above. Two of these trials92, 99 reported treatment effects of 0.3 and 0.45 on a 0-3 point scale (10 percent and 15 percent of maximum score, respectively). The other two93, 96 did not report the magnitude of the treatment effects.
Evidence for the outcome of sneezing at 2 weeks was insufficient to support the use of one treatment over the other. One poor quality trial90 with high risk of bias reported an imprecise treatment effect. For the outcome of sneezing at 4 weeks, the risk of bias was rated as high. Eighty-one percent of patients were in poor quality trials. All five trials92, 93, 95, 96, 99 were consistent in finding statistically significant treatment differences favoring intranasal corticosteroid. However, reported treatment effects were imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
The one trial90 that assessed nasal itch at 2 weeks (N=341) reported no difference in effect between oral selective antihistamine and intranasal corticosteroid. This trial was rated poor quality. Four trials92, 93, 95, 99 assessed nasal itch at 4 weeks (N=1196). All four reported statistically significant improvement with intranasal corticosteroid compared with oral selective antihistamine. One of these was a good quality trial95 of 242 patients (20 percent of patients reporting this outcome). Treatment effect was not reported. The remaining three trials92, 93, 99 were rated poor quality for noncomparable groups at baseline92, 93 and inappropriate analysis of results (unadjusted for baseline group differences93 and not intention to treat99). Two of these trials92, 99 reported treatment effects of 0.2 and 0.29 points on a 0-3 scale (7 percent and 10 percent of maximum score, respectively). The third93 did not report the magnitude of the treatment effect.
Evidence for the outcome of nasal itch at 2 weeks is insufficient to support the use of one treatment over the other. One poor quality trial90 with high risk of bias reported no difference between treatments. For the outcome of nasal itch at 4 weeks, the risk of bias was rated as high. Eighty percent of patients were in poor quality trials. All four trials92, 93, 95, 99 were consistent in finding statistically significant treatment effects favoring intranasal corticosteroid. However, the effects were imprecise. The evidence was insufficient to support the use of one treatment over the other for this outcome.
Six trials89, 90, 93, 97, 98 assessed TNSS at 2 weeks (N=2756). Three89, 98 of these showed statistically significant improvements with intranasal corticosteroid compared with oral selective antihistamine. All three were rated fair quality. Two of these trials89 reported treatment effects of 1.0 and 1.3 using a 0-12 point scale (8 percent and 11 percent of maximum score, respectively). The other three trials90, 93, 97 were rated poor quality due to noncomparable groups at baseline93 and inappropriate analysis of results (unadjusted for baseline group differences93 and not intention to treat90, 97). Two of these trials90, 93 reported statistically nonsignificant treatment effects of 0.8 and 1.0 using a 0-12 point scale (7 percent and 8 percent of maximum score, respectively). The third97 reported a treatment effect of 0.17 (1 percent of maximum score) but did not assess statistical significance.
Five trials92-94, 99, 100 assessed TNSS after 2 weeks, that is, at 3 or 4 weeks. One poor quality trial99 reported neither the magnitude nor the direction of the treatment effect at 4 weeks. This trial was excluded from analysis of this outcome, reducing the total number of patients assessed from 1306 to 1008. The four remaining trials92-94, 100 reported improvement in TNSS with intranasal corticosteroid at 3 weeks93, 100 and at 4 weeks.92-94 All four trials were rated poor quality due to noncomparable groups at baseline92-94 and inappropriate analysis of results (unadjusted for baseline group differences93 and not intention to treat100). Treatment effects at 3 weeks were 1.2 on a 0-12 point scale93 (10 percent of maximum score) and 2.17 on a 0-15 point scale100 (14 percent of maximum score). At 4 weeks, treatment effects of 0.8 on a 0-12 point scale (7 percent of maximum score) were reported by two trials.92, 93
For the outcome of TNSS at 2 weeks, the risk of bias was rated as medium. Forty-five percent of patients reporting this outcome were in poor quality trials, and 55 percent were in fair quality trials. Treatment effects consistently favored intranasal corticosteroid, although effects were imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
For TNSS at 3 to 4 weeks, the risk of bias was rated as high. All four trials92-94, 100 reporting this outcome were rated poor quality. Treatment effects consistently favored intranasal corticosteroid but were imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
Eye Symptoms
Eye symptoms were reported using a variety of measurement scales and varied definitions. Most treatment effects favored intranasal corticosteroid over oral selective antihistamine.
Four trials89, 90, 93 that assessed eye symptoms at 2 weeks reported greater improvement with intranasal corticosteroid than with oral selective antihistamine (N=1905). For TOSS, statistically significant treatment effects of 0.3 and 0.6 on a 0-9 point scale (3 percent and 7 percent of maximum score, respectively) were reported in two fair quality trials89 (N=1074). The other two trials90, 93 were rated poor quality. One90 reported a statistically significant treatment effect of unknown magnitude for the single symptom of tearing. The other93 reported a statistically nonsignificant treatment effect of unknown magnitude for undefined symptoms.
For eye symptoms at 2 weeks, the risk of bias was rated as medium. Forty-four percent of patients were in poor quality trials, and 56 percent were in fair quality trials. All four trials89, 90, 93 were consistent in favoring intranasal corticosteroid. Reported treatment effects did not exceed an MCID of 30 percent maximum score and were considered imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for eye symptoms at 2 weeks.
Four trials91-93, 99 assessed TOSS91, 92 and unspecified eye symptoms93, 99 at 4 weeks (N=1270). Three91, 93, 99 of these reported treatment effects that favored intranasal corticosteroid. One91 was a good quality trial of 316 patients (25 percent of patients reporting this outcome) that showed a statistically significant treatment effect of 16.2 on a 0-300 point scale (5 percent of maximum score). The other two trials93, 99 were rated poor quality. One93 reported a statistically significant treatment effect of unknown magnitude for undefined eye symptoms, and the other99 reported a statistically nonsignificant treatment effect of 0.11 on a 0-9 point scale (1 percent of maximum score). The fourth trial92 was rated poor quality and reported no difference in effect for TOSS. A meta-analysis of three of these trials91, 92, 99 was conducted (N=938 [74% of patients reporting this outcome]; Figure 6). Because trials used different symptom rating scales (0-9 and 0-300), the standardized mean difference was calculated. The pooled effect estimate was 0.13 (95 percent CI: -0.02 to 0.27), a statistically nonsignificant result that favored intranasal corticosteroid. Treatment effects in two91, 99 of the pooled trials favored intranasal corticosteroid, and in the third,92 showed no treatment difference. The meta-analysis excluded one trial93 that showed a statistically significant treatment effect of unknown magnitude favoring intranasal corticosteroid.
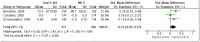
Figure 6
Eye symptoms at 4 weeks: meta-analysis of 3 trials–oral selective antihistamine versus intranasal corticosteroid.
For eye symptoms at 4 weeks, the risk of bias was rated as high. Seventy-five percent of patients were in poor quality trials.92, 93, 99 Treatment effects at 4 weeks were not consistent across individual trials, with three91, 93, 99 of four trials reporting effects in favor of intranasal corticosteroid and the fourth92 (28 percent of patients reporting this outcome) showing no treatment difference. Because all reported effects were less than an MCID of 30 percent maximum score, and because one trial93 (24 percent of patients reporting this outcome) did not report the magnitude of effect, the body of evidence was considered imprecise. The evidence was therefore insufficient to form a conclusion about the comparative effectiveness of oral selective antihistamine and intranasal corticosteroid for this outcome.
Quality of Life
All three trials92, 96, 98 that used the RQLQ to assess quality of life at 2 weeks (N=889) reported statistically significant treatment effects with intranasal corticosteroid compared to oral selective antihistamine. In two96, 98 of these trials, treatment effects of 1.0 and 0.9 on a 0-6 point scale exceeded the MCID of 0.5. The larger of these98 was a fair quality trial of 450 patients (51 percent of patients reporting this outcome), and the other96 was rated poor due to lack of blinding (n=88). The treatment effect in the third trial92 (n=351) was 0.25 on a 0-6 scale. This trial was rated poor due to noncomparable groups at baseline.
For quality of life outcomes measured using the RQLQ at 2 weeks, the risk of bias was rated as medium. Forty-nine percent of patients were in poor quality trials, and 51 percent were in the fair quality trial. All three trials92, 96, 98 were consistent in finding statistically significant treatment differences favoring intranasal corticosteroid. Treatment effects were larger than the MCID in two trials96, 98 but smaller in one trial92 that accounted for 39 percent of patients reporting. The body of evidence was therefore considered imprecise. Evidence was insufficient to support the use of one treatment over the other for this outcome.
All four trials91, 92, 94, 96 that assessed quality of life using the RQLQ at 4 weeks (N=869) reported statistically significant treatment effects favoring intranasal corticosteroid. One91 of these was a good quality trial of 316 patients (36 percent of patients reporting this outcome). The magnitude of effect was not reported. The remaining three trials were rated poor quality. Of two trials92, 96 that reported the magnitude of treatment effects, the effect in one96 (0.9) exceeded the MCID. This was a trial of 88 patients96 that was rated poor quality due to lack of blinding. The other92 reported a treatment effect of 0.25. The fourth trial94 did not report the magnitude of the treatment effect.
For quality of life outcomes measured using the RQLQ at 4 weeks, the risk of bias was rated as high. Sixty-four percent of patients were in the poor quality trials. All four trials91, 92, 94, 96 were consistent in finding statistically significant treatment differences favoring intranasal corticosteroid. However, reported treatment effects exceeded the MCID in only one trial96 representing 10 percent of patients reporting this outcome. The body of evidence was therefore considered imprecise. Evidence was insufficient to support the use of one treatment over the other for this outcome.
Two fair quality trials89 (N=1074) that used the Nocturnal RQLQ at 2 weeks reported statistically significant treatment effects of 0.5 and 0.7 on a 0-6 point scale (8 percent and 12 percent of maximum score, respectively). For quality of life outcomes measured using the Nocturnal RQLQ at 2 weeks, the risk of bias was rated as medium. Both trials89 from the same published article were rated fair quality. Both also were consistent in finding statistically significant treatment differences favoring intranasal corticosteroid. However, effect estimates were imprecise. Evidence was therefore insufficient to support one treatment over the other for this outcome.
Four trials91, 94, 98, 100 reported PGA scores at 2 weeks,98 3 weeks,100 and 4 weeks.91, 94 Results supported the quality of life findings described above (intranasal corticosteroid favored), but statistical significance of effect estimates was variable.
Oral Selective Antihistamine Versus Oral Decongestant
Description of Included Studies
Seven trials101-107 published between 1995 and 2009 were identified (N=3592). All were multicenter, double-blinded, RCTs with a primary interest in comparing a combination antihistamine/decongestant product to its component parts and/or placebo (three to four treatment arms). Trial size ranged from 398 to 749 patients randomized to treatment groups of interest, and trial durations were approximately 2 weeks (2 to 2.6 weeks). Six trials101, 102, 104-107 were conducted in North America, and one103 in Europe. Oral selective antihistamines studied were desloratadine (four trials102, 104-106), fexofenadine (one trial107), cetirizine (one trial103), and loratadine (one trial101); the decongestant was pseudoephedrine in all seven trials. Five trials101, 104-107 were industry funded, and two102, 103 did not report funding source.
Average ages of patients in the trials ranged from 30 to 37 years. Approximately 60 percent of patients were female. In four trials101, 104, 105, 107 reporting information on race, most patients were white (77-93 percent). Six101-105, 107 of seven trials required a minimum severity of SAR symptoms, and at baseline, symptom scores for congestion were moderate. Six101-106 of seven trials required a minimum duration of SAR history; the mean duration of SAR symptoms in the trial populations ranged from 8 to 19 years.
Nasal congestion was assessed in all seven trials. In six trials,101-106 four-point rating scales (0=no symptoms, 3=severe symptoms) were used. In one trial,107 a 5-point scale (0=no symptoms, 4=very severe symptoms) was used. TNSS was reported in 1 trial.101 Two trials103, 107 reported on individual nasal symptoms of rhinorrhea and sneezing, one of which also reported on nasal itch.103 Two trials103, 107 reported ocular outcomes. Grosclaude, (1997)103 (n=454) assessed ocular itching using a 0 (absent) to 3 (severe) symptom scale. Sussman (1999)107 (n=436) assessed total ocular symptoms (itching, tearing, and redness) using a 0 (absent) to 4 (very severe) symptom scale. No trial assessed asthma outcomes.
Three trials101, 103, 107 were rated good, one106 fair, and three102, 104, 105 poor quality.
Key Points
These results are summarized in Table 22.
Table 22
Strength of evidence: oral selective antihistamine versus oral decongestant.
- These results are based on trials of four of five oral selective antihistamines (80 percent) and one of two oral decongestants (50 percent).
Synthesis and Strength of Evidence
Nasal symptom results discussed below are summarized in Table 23 and eye symptom outcomes in Table 24. As shown in these tables, variance estimates for reported outcomes were not provided. Thus, meta-analysis was not possible.
Table 23
Treatment effects: nasal symptoms–oral selective antihistamine versus oral decongestant.
Table 24
Treatment effects: eye symptoms–oral selective antihistamine versus oral decongestant.
Nasal Symptoms
All seven trials101-107 assessed nasal congestion at 2 weeks, and all reported greater improvement with oral decongestant than with oral selective antihistamine (N=3592). Treatment effects of 0.1 to 0.17 on a 0-3 point scale (3 percent and 6 percent of maximum score, respectively) were reported in three101, 103, 107 good quality trials of 1327 total patients (37 percent of patients reporting this outcome). Statistical significance was not reported. Three trials102, 104, 105 were rated poor quality due to inappropriate analysis of results (not intention to treat; N=1583). Treatment effects reported by these trials and by one trial106 (n=682) rated fair quality ranged from 0.05 to 0.1 on a 0-3 point scale (all less than 3 percent of maximum score). In the two trials105, 106 that reported p-values, results were not statistically significant.
For congestion at 2 weeks, the risk of bias was rated as medium. Forty-four percent of patients were in poor quality trials, and 37 percent were in good quality trials. All seven trials were consistent in finding treatment effects that favored oral decongestant. However, none of the effects exceeded an MCID of 30 percent maximum score, and all were considered imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for the treatment of congestion.
Three101, 103, 107 good quality trials assessed other nasal symptoms at 2 weeks (rhinorrhea, sneezing, nasal itch, TNSS). Grosclaude (1997)103 and Sussman (1999)107 reported treatment effects favoring oral selective antihistamine for rhinorrhea and sneezing (N=890). Grosclaude (1997)103 reported treatment effects of 0.21 for rhinorrhea (7 percent of maximum score) and 0.32 for sneezing (11 percent of maximum score) using a 0-3 point scale. Sussman (1999)107 reported treatment effects of 0.1 for rhinorrhea (3 percent of maximum score) and 0.2 for sneezing (5 percent of maximum score) using a 0-4 point scale. Grosclaude (1997; n=454) also assessed nasal itch at 2 weeks and reported a treatment effect of 0.13 on a 0-3 point scale (4 percent of maximum score). Bronsky (1995)101 assessed TNSS at 2 weeks and reported a treatment effect of 0.1 on a 0-3 scale (3 percent of maximum score) favoring oral decongestant. Because the direct comparison of interest in these trials involved the combination treatment arm, p-values for comparative effects of the two components were not reported.
For other nasal symptoms at 2 weeks, the risk of bias is rated as low. Results come from good quality trials.101, 103, 107 For rhinorrhea and sneezing, trials103, 107 were consistent in showing treatment effects that favored oral selective antihistamine. For nasal itch and TNSS, results are from single trials,101, 103 and consistency is unknown. All effects were imprecise, and evidence was insufficient to support the use of one treatment over the other for rhinorrhea, sneezing, nasal itch, or TNSS.
Eye Symptoms
Two103, 107 of seven trials assessed ocular outcomes (N=890). Both were good quality trials. Oral selective antihistamine was favored for both ocular itching103 and total ocular symptoms (itching, tearing, and redness).107 Treatment effects were 0.08 on a 0-3 point scale103 and 0.1 on a 0-4 point scale107 (both 3 percent of maximum score). P-values were not reported.
For ocular outcomes at 2 weeks, the risk of bias was rated as low. Both trials103, 107 reporting ocular outcomes were rated good quality. Trials were consistent in showing treatment effects that favored oral selective antihistamine. However, effects did not exceed an MCID of 30 percent maximum score and were considered imprecise. Evidence was therefore insufficient to support the use of one treatment over the other for ocular outcomes at 2 weeks.
Oral Selective Antihistamine Versus Oral Leukotriene Receptor Antagonist (Montelukast)
Description of Included Studies
Nine97, 108-114 double-blinded, RCTs published between 2000 and 2009 were identified (N=4231). Eight97, 108, 110-114 were multicenter trials conducted in North America. One109 was a single center trial conducted in Europe. Trial size ranged from 187 to 950 patients randomized to treatment groups of interest. Oral selective antihistamines studied in comparison to montelukast were loratadine (seven trials97, 110-114), desloratadine (one trial108) and levocetirizine (one trial109). Six trials97, 110-113 were 2 weeks in duration, and three trials108, 109, 114 were 4 weeks. Seven trials97, 110-114 were industry funded. Two trials108, 109 did not report funding source.
Mean ages of patients ranged from 31 to 42 years. In most trials, the majority of patients were women (53-67 percent). In one trial,109 men were the majority. Seven trials108-114 reported information on race. In all of these, the majority was white (79-89 percent). Eight trials97, 109-114 required a minimum severity of SAR symptoms. Nasal symptom scores at baseline were most commonly in the moderate range. All trials required a minimum duration of SAR history. All patients had SAR symptoms for more than 14 years. One trial108 reported baseline asthma scores. Baseline asthma symptoms, as assessed by the Total Asthma Symptom Severity Score (TASS, described below) and forced expired volume in one second (FEV1), were moderate in severity.
Eight trials97, 109-114 assessed nasal symptoms, four109, 110, 113, 114 assessed eye symptoms, one trial108 assessed asthma symptoms, and six trials109-114 assessed quality of life. All trials used a 4-point scale (0=no symptoms, 3=severe symptoms) to assess four nasal symptoms (congestion, rhinorrhea, sneezing, and itch) and averaged these scores to calculate a TNSS. Maximum TNSS was three points. All four trials that reported on eye symptoms assessed ocular tearing, itching, redness, and puffiness using the 4-point scale described above. Individual scores were averaged for a maximum TOSS of 3 points. In the one trial108 that assessed asthma outcomes, the 4-point rating scale was used to assess three asthma symptoms, cough, wheezing, and difficulty breathing. Scores were summed to yield the 0-9 point TASS. An MCID was not reported. All six trials that assessed quality of life used the 27-item RQLQ. Scores ranged from 0 (no impairment) to 6 (severely impaired) with a validated MCID of 0.5 points. Measures were recorded at 2 weeks in five trials110-114 and at 4 weeks in two trials.109, 113
Three trials108, 110, 111 were rated good quality, two113, 114 were rated fair, and four97, 109, 112 were rated poor.
Key Points
These results are summarized in Table 25.
Table 25
Strength of evidence: oral selective antihistamine versus oral leukotriene receptor antagonist.
- Asthma rescue medication use at 2-4 weeks: Moderate strength evidence for superiority of oral leukotriene receptor antagonist over oral selective antihistamine based on one trial 108 with low risk of bias and precise results.
- Other asthma outcomes (individual asthma symptoms, TASS, and FEV1) at 2-4 weeks: Evidence was insufficient to support the use of one treatment over the other based on one trial108 with low risk of bias and imprecise results.
- These results are based on trials of three of five oral selective antihistamines (60 percent) in comparison to montelukast (100 percent).
Synthesis and Strength of Evidence
Nasal symptom results discussed below are summarized in Table 26, eye symptoms in Table 27, asthma outcomes in Table 28, and quality of life outcomes in Table 29. As shown in these tables, variance estimates of observed effects are provided for TNSS, TOSS, and RQLQ. Thus, meta-analyses of these results were conducted.
Table 26
Treatment effects: nasal symptoms–oral selective antihistamine versus oral leukotriene receptor antagonist.
Table 27
Treatment effects: eye symptoms–oral selective antihistamine versus leukotriene receptor antagonist.
Table 28
Treatment effects: asthma outcomes–oral selective antihistamine versus leukotriene receptor antagonist.
Table 29
Treatment effects: quality of life outcomes–oral selective antihistamine versus leukotriene receptor antagonist.
Nasal Symptoms
Individual nasal symptom scores were assessed in three trials110-112 at 2 weeks (N=1593). Two of these110, 111 were good quality trials of 643 patients total (40 percent of all patients reporting this outcome). The third trial112 (n=950) was rated poor quality due to the inappropriate analysis of results (not intention to treat). P-values were not reported for any outcome. For congestion and rhinorrhea, the good quality trials showed treatment effects ranging from 0.02 to 0.08 on a 0-3 point scale (both less than 3 percent of maximum score) favoring leukotriene receptor antagonist over oral selective antihistamine. For sneezing and itching, treatment effects in these trials favored oral selective antihistamine over leukotriene receptor antagonist and ranged from 0.02 to 0.12 (1 percent and 4 percent of maximum score, respectively). Treatment effects in the poor quality trial favored oral selective antihistamine for all four nasal symptoms and ranged from 0.10 to 0.18 (3 percent to 6 percent of maximum score).
For these outcomes at 2 weeks, the risk of bias was rated as medium. Sixty percent of patients reporting were in the poor quality trial,112 and 40 percent were in good quality trials.110, 111 Findings were not consistent across trials for congestion and rhinorrhea, but were consistent for sneezing and nasal itch. Treatment effects were imprecise. Evidence was therefore insufficient to support the use of one treatment over the other for these outcomes.
TNSS was assessed at 2 weeks in seven trials,97, 110-114 one of which also reported 4-week outcomes.114 An additional trial109 reported 4-week results only (total N=3609). Two trials110, 111 were good quality trials of 643 patients total (18 percent of patients reporting this outcome) and two113, 114 were rated fair (1321 patients total; 37 percent of patients reporting). No trial reported p-values. All but two trials110, 111 favored oral selective antihistamine over leukotriene receptor antagonist at 2 or 4 weeks, with treatment effects ranging from 0.01 to 0.17 on a 0-3 point scale (all less than 6 percent of maximum score). The two good quality trials110, 111 favored leukotriene receptor antagonist at 2 weeks with treatment effects of 0.02 and 0.04 on a 0-3 point scale (both less than 2 percent of maximum score). A meta-analysis of seven97, 109-111, 113, 114 of these eight trials was performed (total N=2648; 73 percent of patients reporting this outcome). The pooled treatment effect was 0.06 on a 0-3 point scale (95 percent CI: 0.00 to 0.12) favoring oral selective antihistamine (Figure 7). This was a statistically significant result. The larger bound of the 95 percent CI represented 4 percent of maximum score. Statistical heterogeneity was low to moderate but not statistically significant (I2=39 percent, p=0.13) and likely due to variation in treatment effect direction and precision. An eighth trial112 that was rated poor quality was not included in the meta-analysis due to lack of variance reporting (n=950). This trial reported a treatment effect of 0.1 on a 0-3 point scale (3 percent of maximum score) favoring oral selective antihistamine. A p-value was not provided. Finally, 4-week results from van Adelsberg (2003)114 were not included in the meta-analysis because 2-week results were the identified primary outcome. The treatment effect at 4 weeks was 0.07 on a 0-3 point scale (2 percent of maximum score) favoring oral selective antihistamine. A p-value was not reported.

Figure 7
Total nasal symptom score at 2 to 4 weeks: meta-analysis of 7 trials–oral selective antihistamine versus leukotriene receptor antagonist.
For TNSS at 2 to 4 weeks, the risk of bias was rated as medium. Eighteen percent of patients reporting this outcome were in good quality trials, and 45 percent were in poor quality trials. Although findings at 2 weeks were not consistent across individual trials, statistical heterogeneity of a meta-analysis that included trials with conflicting results was low to moderate. Further, the pooled treatment effect (0.06) favoring oral selective antihistamine was consistent with treatment effects reported by the one trial (Philip [2002]) not included in the meta-analysis112 and by another trial114 included in the meta-analysis that reported results at an additional time point (4 weeks). The 95 percent CI for the pooled estimate (0.00 to 0.12) fell within an interval bounded by −MCID and +MCID (-0.9 and +0.9 on the 0-3 point scale used). The Philip (2002) trial112 (26 percent of patients reporting this outcome) showed a treatment effect (0.1 on a 0-3 point scale) that was larger than the pooled effect (0.06) but smaller than the MCID (0.9). To determine the impact of this trial on the pooled estimate, we added it to the meta-analysis with an assumed standard deviation equal to half the mean change in score in each treatment group. Under this assumption, the pooled effect increased from 0.06 to 0.08 on a 0-3 point scale (95 percent CI: 0.03 to 0.12) favoring intranasal corticosteroid. The larger bound of the 95 percent CI represented 4 percent of maximum score. Based on this analysis, it is unlikely that the 95 percent confidence interval of a meta-analysis including the Philip (2002) trial112 would contain the MCID. The body of evidence supporting a conclusion of equivalence of oral selective antihistamine and leukotriene receptor antagonist for this outcome is therefore precise. The overall strength of evidence for this conclusion is rated as moderate based on these considerations.
Eye Symptoms
Four trials109, 110, 113, 114 assessed a four symptom TOSS comprising eye tearing, itching, redness, and puffiness (N=1708) at 2 or 4 weeks. One of these110 was a good quality trial of 187 patients (11 percent of patients reporting this outcome) that showed a treatment effect of 0.03 on a 0-3 point scale (1 percent of maximum score) favoring leukotriene receptor antagonist. A p-value was not reported. All other assessments favored oral selective antihistamine, including two fair quality trials113, 114 of 1321 patients (77 percent of patients reporting) and one trial109 that was rated poor quality due to noncomparable groups at baseline and inappropriate analysis of results (unadjusted for baseline group differences). Treatment effects were 0.05 and 0.12 in the fair quality trials (2 percent and 4 percent of maximum score, respectively) and 0.16 in the poor quality trial (5 percent of maximum score). All four trials were included in a meta-analysis (Figure 8). The pooled treatment effect was 0.08 on a 0-3 point scale (95 percent CI: 0.02 to 0.14) favoring oral selective antihistamine over leukotriene receptor antagonist. The larger bound of the 95 percent CI represented 5 percent of maximum score. Statistical heterogeneity was low (I2=14 percent, p=0.32). Four-week results from one trial114 were not included in the meta-analysis because 2-week results were the identified primary outcome. At 4 weeks, the treatment effect was 0.02 on a 0-3 point scale (1 percent of maximum score) favoring oral selective antihistamine. A p-value was not reported.

Figure 8
Total ocular symptom score at 2 to 4 weeks: meta-analysis of 4 trials–oral selective antihistamine versus leukotriene receptor antagonist.
For TOSS at 2 to 4 weeks, the risk of bias was rated as medium. Eleven percent of patients were in the good quality trial, and 12 percent were in the poor quality trial. Although results across individual trials were inconsistent at 2 weeks, statistical heterogeneity for the pooled treatment effect was low. The 95 percent CI for the pooled estimate fell within an interval bounded by −MCID and +MCID (-0.9 and +0.9 on the 0-3 point scale used). The body of evidence supporting a conclusion of equivalence of oral selective antihistamine and leukotriene receptor antagonist for this outcome is therefore precise. The strength of evidence for this conclusion is rated as moderate based on these considerations.
Asthma Symptoms
One good quality trial108 (N=622) reported individual and TASS scores in addition to rescue medication use and FEV1 at 2 and 4 weeks. Patients had moderate asthma symptoms at baseline. All outcomes had greater improvements with leukotriene receptor antagonist than with oral selective antihistamine, but no statistically significant differences between treatment groups were observed for any outcome during the 4 weeks of the trial. For all outcomes, the risk of bias was rated as low, and consistency could not be assessed with a single trial.
Treatment effects at 2 and 4 weeks were:
- Total asthma symptoms on a 0-9 point scale: 0.09 at 2 weeks and 0.16 at 4 weeks (1 percent and 2 percent of maximum score, respectively).
- Rescue medication use: 2.4 puffs per day at 2 weeks and 3.8 puffs per day at 4 weeks (both greater than an MCID of 1 puff per day)
Treatment effects at 4 weeks were:
- Individual asthma symptoms on a 0-3 point scale: 0.02 for cough, 0.04 for wheeze, and 0.06 for difficulty breathing (1 percent, 1 percent and 2 percent of maximum score, respectively)
- FEV1: 0.03 percent predicted (less than an MCID of 10 percent)
For rescue medication use at 2 and 4 weeks, the treatment effect is precise, and there is moderate strength evidence to support the use of oral leukotriene receptor antagonist. For all other outcomes, evidence was insufficient to support the use of one treatment over the other.
Quality of Life
All six trials109-114 that assessed quality of life at 2 and 4 weeks used the RQLQ (N=3114). Two of these were good quality trials110, 111 of 643 patients (21 percent of patients reporting this outcome), two113, 114 were fair quality trials of 1321 patients (42 percent of patients reporting), and two109, 112 were poor quality trials of 1150 patients (37 percent of patients reporting). P-values were not reported in any trial. Treatment effects exceeded the MCID of 0.5 in three trials109, 112, 113, two112, 113 at 2 weeks (0.10 and 0.08 in a poor and fair quality trial, respectively) and one109 at 4 weeks (0.13 in a poor quality trial). All three results favored oral selective antihistamine over leukotriene receptor antagonist. A meta-analysis of four trials111-114 that reported 2-week RQLQ results was performed (N=2723; Figure 9). The pooled treatment effect favored oral selective antihistamine (mean difference 0.06; 95 percent CI: -0.03 to 0.15) but was not statistically significant. Statistical heterogeneity was low (I2=0 percent, p=0.84). One110 of two trials not included in the meta-analysis represented 6 percent of patients reporting this outcome and found no treatment difference between groups at 2 weeks. The other trial,109 also representing 6 percent of patients reporting this outcome, showed a treatment effect of 0.13 favoring oral selective antihistamine at 4 weeks. One of the trials114 included in the meta-analysis reported 4-week results, which were not included because 2-week results were the identified primary outcome. In contrast to the 2-week result, the treatment effect at 4 weeks favored leukotriene receptor antagonist. The effect was 0.04, which did not exceed the MCID.

Figure 9
Rhinoconjunctivitis quality of life at 2 weeks: meta-analysis of 4 trials–oral selective antihistamine versus leukotriene receptor antagonist.
For quality of life as assessed by the RQLQ, the risk of bias was rated as medium. Twenty-one percent of patients were in good quality trials, and 37 percent were in poor quality trials. Statistical heterogeneity for the pooled effect favoring oral selective antihistamine was low, and one trial110 not included in the meta-analysis that showed a treatment difference of zero represented only 6 percent of patients reporting this outcome. The 95 percent CI for the pooled estimate (-0.03 to 0.15) fell within an interval bounded by −MCID and +MCID (-0.5 and +0.5). Of two trials not included in the meta-analysis,109, 110 one109 (6 percent of patients reporting this outcome) showed a treatment effect (0.13) favoring oral selective antihistamine that was smaller than the MCID but larger than the pooled effect (0.06). The other110 (6 percent of patients reporting this outcome) showed a treatment difference of zero that was, therefore, smaller than both the MCID and the pooled effect. If these trials were included in the meta-analysis, the pooled effect would change very little, and it is unlikely that the 95 percent confidence interval would contain the MCID. One trial,114 a large trial representing 20 percent of patients reporting this outcome, was included in the meta-analysis of results at 2 weeks and reported an additional treatment effect of 0.04 at 4 weeks favoring leukotriene receptor antagonist. If this result were included in the meta-analysis, the effect estimate favoring oral selective antihistamine would decrease and possibly cross the “no effect” line to favor leukotriene receptor antagonist, but it is unlikely that the MCID would lie within the 95 percent confidence interval. Based on these considerations, the body of evidence supporting a conclusion of equivalence of oral selective antihistamine and leukotriene receptor antagonist for this outcome is therefore considered precise. The strength of evidence for this conclusion is rated as moderate.
Intranasal Corticosteroid Versus Nasal Antihistamine
Description of Included Studies
Nine115-121 RCTs published between 1995 and 2012 were identified (N=2473). All but two116, 120 were multicenter trials. Six115, 117, 118, 121 trials were conducted in North America, two119, 120 in Europe, one116 in Asia. Six trials115, 117, 120, 121 were double-blinded, one trial116 was open-label, and two118, 119 were considered to have inadequate patient blinding. Trials included 50 to 895 patients randomized to treatment groups of interest and used either fluticasone propionate (six trials115, 117, 118, 121) or beclomethasone (three trials116, 119, 120) as the intranasal corticosteroid, and azelastine (eight trials115-117, 119-121) or olopatadine (one trial118) as the nasal antihistamine. Seven trials115, 117-119, 121 were 2 weeks in duration, one116 was 4 weeks, and one120 was six weeks. Six trials115, 117, 118, 121 were industry funded, and three116, 119, 120 did not report funding source.
The mean age of the trial populations was 36 years. Approximately 55 percent of patients were female, although men were the majority in two trials. 116, 120 In trials that reported on race, the majority of patients were white. Most patients had SAR symptoms for more than 15 years and had moderate to severe symptoms at baseline.
Trials reported outcomes after 2 to 5 weeks of treatment. Outcomes reported were nasal symptoms (nine trials115-121), eye symptoms (five trials115, 117, 118), and quality of life (two trials117, 121). All nine trials reported nasal symptom outcomes at 2 weeks, one116 at 2, 3, and 4 weeks, and one120 at 2, 3, 4 and 5 weeks. Most trials used a 4-point scale (0 = no symptoms, 3 = severe symptoms) for the assessment of nasal symptoms. Of these, the majority assessed symptoms twice daily and summed the scores for a daily TNSS ranging from 0 to 24; others assessed once daily for a TNSS of 0 to 12. Five trials115, 117, 118 assessed ocular symptoms. All but one118 assessed ocular itching, tearing, and redness using the 4-point scale (0 = no symptoms, 3 = severe symptoms) twice daily in the morning and evening (TOSS range 0 to 18); the other118 assessed once daily (TOSS range 0 to 9). Finally, two trials117, 121 assessed quality of life using the RQLQ, a validated quality of life instrument in this patient population with scores ranging from 0 (no impairment) to 6 (severely impaired); the MCID is 0.5 points.65
Five115, 117, 121 of the nine identified trials were rated good quality and four116, 118-120 were rated poor.
Key Points
These results are summarized in Table 30.
Table 30
Strength of evidence: intranasal corticosteroid versus nasal antihistamine.
- Individual nasal symptoms, TNSS, and TOSS at 2 weeks: High strength evidence to support equivalence of intranasal corticosteroid and nasal antihistamine based on eight trials98, 115-119 (for congestion, rhinorrhea, and sneezing), seven trials (for nasal itch98, 115, 117-119 and TNSS98, 115, 117, 118, 120), and five trials115, 117, 118 (for TOSS) with low risk of bias and consistent, precise results.
- Individual nasal symptoms (congestion, rhinorrhea, and sneezing) at 3-4 weeks: Evidence was insufficient to support the use of one treatment over the other based on one trial116 with high risk of bias and an imprecise result.
- TNSS at 3-5 weeks: Evidence was insufficient to support the use of one treatment over the other based on one trial120 with high risk of bias and an imprecise result.
- These results are based on trials of two of eight intranasal corticosteroids (25 percent) and both nasal antihistamines (100 percent).
Synthesis and Strength of Evidence
Trial level comparative outcome data for nasal symptoms can be found in Table 31, for ocular symptoms in Table 32, and for quality of life in Table 33. As shown in Table 31 and Table 32, variance estimates of group-level effects were provided for individual nasal symptoms, TNSS, and TOSS. Meta-analyses for these outcomes were conducted.
Table 31
Treatment effects: nasal symptoms–intranasal corticosteroid versus nasal antihistamine.
Table 32
Treatment effects: eye symptoms–intranasal corticosteroid versus nasal antihistamine.
Table 33
Treatment effects: quality of life outcomes–intranasal corticosteroid versus nasal antihistamine.
Nasal Symptoms
Eight115-119, 121 of nine trials assessed congestion after 2 weeks of treatment (N=2443 of 2473). Seven of these115-119 reported treatment effects favoring intranasal corticosteroid, although none were reported to be statistically significant. In the eighth trial,121 representing 4 percent of patients reporting this outcome, the treatment difference was zero. The USPSTF quality rating was good in five of these trials115, 117, 121 (85 percent of patients reporting this outcome) and poor in three trials.116, 118, 119 Poor USPSTF ratings were assigned for noncomparable groups at baseline,116 inadequate blinding,116, 118, 119 and inappropriate analysis of results (unadjusted for baseline group differences116 and not intention to treat119). A meta-analysis of four good quality trials115, 121 (N=1791; 73 percent of patients reporting this outcome) yielded a statistically significant pooled effect of 0.14 on a 0-6 point scale (95 percent CI: 0.02 to 0.26) favoring intranasal corticosteroid (Figure 10). The larger bound of the 95 percent CI represented 4 percent of maximum score. Statistical heterogeneity was low (I2=0 percent, p=0.54). Treatment effects for four trials116-119 not included in the meta-analysis favored intranasal corticosteroid with a range of 0.11117 on a 0-6 point scale (2 percent of maximum score) to 0.7116 on a 0-3 point scale (23 percent of maximum score).

Figure 10
Congestion at 2 weeks: meta-analysis of 4 trials–intranasal corticosteroid versus nasal antihistamine.
For nasal congestion at 2 weeks, the risk of bias was rated as low. Eighty-five percent of patients were in good quality trials. Treatment effects consistently favored intranasal corticosteroid in 96 percent of patients reporting on this outcome. This finding was consistent with results of a meta-analysis of four115, 121 of these trials (73 percent of patients reporting this outcome), including the one trial121 that reported a treatment effect of zero, and statistical heterogeneity was low. The 95 percent CI for the pooled effect (0.02 to 0.26) fell within an interval bounded by −MCID and +MCID (-1.8 and +1.8 on the 0-6 point scale used). Of four trials116-119 not included in the meta-analysis, one118 reported a treatment effect favoring intranasal corticosteroid but did not report the magnitude of the effect. Because this trial represented 5 percent of patients reporting this outcome, its impact on the pooled estimate if this trial were included in the meta-analysis likely would be minimal. One trial117 (12 percent of patients reporting this outcome) showed a treatment effect (0.11 on a 0-6 point scale) that was smaller than both the pooled effect (0.14 on a 0-6 point scale) and the MCID (1.8). If this trial were included in the meta-analysis, the pooled effect estimate would decrease slightly, and it is unlikely that the 95 percent confidence interval would include the MCID. Two trials116, 119 (9 percent of patients reporting this outcome ) showed treatment effects of 0.5119 and 0.7116 on a 0-3 point scale (1.0 and 1.4 on a 0-6 point scale, respectively). These effects were substantially larger than the pooled effect (0.14 on a 0-6 point scale) but smaller than the MCID (1.8). To determine the impact of these two trials116, 119 on the pooled estimate, we added both to the meta-analysis with assumed standard deviations equal to half the mean change in score in each treatment group. Under this assumption, the pooled effect increased from 0.14 to 0.45 on a 0-6 point scale (95 percent CI: 0.06 to 0.85) favoring intranasal corticosteroid. The larger bound of the 95 percent CI represented 14 percent of maximum score. Based on this analysis, it is unlikely that the 95 percent confidence interval of a meta-analysis including these two trials116, 119 would contain the MCID. The body of evidence in support of a conclusion of equivalence of intranasal corticosteroid and nasal antihistamine for this outcome is therefore considered precise. The overall strength of evidence for this conclusion is rated as high.
Eight115-119, 121 of nine trials assessed rhinorrhea after 2 weeks of treatment (N=2443 of 2473 patients). Seven115, 117-119, 121 of eight reported treatment effects in favor of intranasal corticosteroid, although none were reported to be statistically significant. The eighth trial116 (n=50; 2 percent of patients reporting this outcome) reported a treatment difference of zero. The USPSTF quality rating was good in five trials115, 117, 121 (85 percent of patients reporting this outcome) and poor in three trials.116, 118, 119 Poor USPSTF ratings were assigned for noncomparable groups at baseline,116 inadequate blinding,116, 118, 119 and inappropriate analysis of results (unadjusted for baseline group differences116 and not intention to treat119). A meta-analysis of four trials115, 121 (N=1791; 73 percent of patients reporting this outcome) yielded a statistically significant pooled effect estimate of 0.17 on a 0-6 point scale (95 percent CI: 0.04 to 0.30) favoring intranasal corticosteroid (Figure 11). The larger bound of the 95 percent CI represented 5 percent of maximum score. Statistical heterogeneity was low (I2=0 percent, p=0.70). Of four trials116-119 not included in the meta-analysis, three117-119 reported treatment effects favoring intranasal corticosteroid. Reported effect sizes were 0.28117 on a 0-6 point scale (5 percent of maximum score) and 0.4119 on a 0-3 point scale (13 percent of maximum score, respectively). One trial118 did not report the magnitude of effect. The fourth trial116 reported no treatment difference.

Figure 11
Rhinorrhea at 2 weeks: meta-analysis of 4 trials–intranasal corticosteroid versus nasal antihistamine.
For rhinorrhea at 2 weeks, the risk of bias was rated as low. Eighty-five percent of patients were in good quality trials. Treatment effects consistently favored intranasal corticosteroid in 98 percent of patients reporting this outcome, and statistical heterogeneity in a meta-analysis of four trials115, 121 (73 percent of patients reporting this outcome) was low. The 95 percent CI for the pooled effect (0.04 to 0.30) fell within an interval bounded by −MCID and +MCID (-1.8 and +1.8 on the 0-6 point scale used). Three117-119 of four trials116-119 not included in the meta-analysis reported treatment effects favoring intranasal corticosteroid. One118 of these did not report the magnitude of effect, but this trial represented 5 percent of patients reporting this outcome. Two trials117, 119 (19 percent of patients reporting this outcome) reported treatment effects of 0.28117 on a 0-6 point scale and 0.4119 on a 0-3 point scale (0.8 on a 0-6 point scale) that were larger than the pooled effect but smaller than the MCID. To determine the impact of these two trials117, 119 on the pooled estimate, we added both to the meta-analysis with assumed standard deviations equal to half the mean change in score in each treatment group. Under this assumption, the pooled effect increased from 0.17 to 0.34 on a 0-6 point scale (95 percent CI: 0.10 to 0.57) favoring intranasal corticosteroid. The larger bound of the 95 percent CI represented 10 percent of maximum score. Based on this analysis, it is unlikely that the 95 percent confidence interval of a meta-analysis including these two trials117, 119 would contain the MCID. The fourth trial116 reported a treatment effect of zero (and therefore smaller than both the pooled estimate [0.17] and the MCID), but this trial represented 2 percent of patients reporting this outcome. The body of evidence in support of a conclusion of equivalence of intranasal corticosteroid and nasal antihistamine for this outcome is therefore considered precise. The overall strength of evidence for this conclusion is rated as high.
Eight115-119, 121 of nine trials assessed sneezing after 2 weeks of treatment (N=2443 of 2473). Six115-117, 119 of these reported treatment effects favoring intranasal corticosteroid, although none were reported to be statistically significant. One trial121(4 percent of patients reporting this outcome) showed no treatment difference, and the eighth trial118 (5 percent of patients reporting this outcome) reported a treatment effect favoring nasal antihistamine. This treatment effect was not statistically significant and its magnitude was not reported. The USPSTF quality rating was good in five trials 115, 117, 121 (85 percent of patients assessed for this outcome) and poor in three trials.116, 118, 119 Poor USPSTF ratings were assigned for noncomparable groups at baseline,116 inadequate blinding,116, 118, 119 and inappropriate analysis of results (unadjusted for baseline group differences116 and not intention to treat119). A meta-analysis of four good quality trials115, 121 (N=1791; 73 percent of patients reporting this outcome) yielded a statistically nonsignificant pooled effect estimate of 0.12 on a 0-6 point scale (95 percent CI: -0.01 to 0.25) favoring intranasal corticosteroid (Figure 12). The larger bound of the 95 percent CI represented 4 percent of maximum score. Statistical heterogeneity was low (I2=0 percent, p=0.89). Of four trials116-119 not included in the meta-analysis, treatment effects favoring intranasal corticosteroid were reported by three.116, 117, 119 Reported effect sizes were 0.1116 and 0.4119 on a 0-3 point scale (3 percent and 13 percent of maximum score, respectively), and 0.12117 on a 0-6 point scale (2 percent of maximum score). One trial118 reported a statistically nonsignificant treatment effect favoring nasal antihistamine but did not report the magnitude of effect.

Figure 12
Sneezing at 2 weeks: meta-analysis of 4 trials–intranasal corticosteroid versus nasal antihistamine.
For the outcome of sneezing at 2 weeks, the risk of bias was rated as low. Eighty-five percent of patients were in good quality trials. Statistical heterogeneity in a meta-analysis of four trials115, 121 (73 percent of patients reporting this outcome) that favored intranasal corticosteroid was low. This included one trial121 (4 percent of patients reporting this outcome) that reported a treatment difference of zero. The pooled effect was consistent with three.116, 117, 119of four trials not included in the meta-analysis. The fourth trial118 reported a treatment effect of unknown size favoring nasal antihistamine, but this trial represented 5 percent of patients reporting this outcome. The body of evidence was therefore considered consistent. The 95 percent CI for the pooled estimate (0.01 to 0.25) fell within an interval bounded by −MCID and +MCID (-1.8 and +1.8 on the 0-6 point scale used). Of four trials116-119 not included in the meta-analysis, one118 showed a treatment effect in the opposite direction (favoring nasal antihistamine) but did not report the magnitude of effect. Because this trial represented 5 percent of patients reporting this outcome, the reduction in the pooled estimate if this trial were included in the meta-analysis likely would be minimal, unless the treatment effect was unexpectedly large. One trial117 (12 percent of patients reporting this outcome) showed a treatment effect (0.12 on a 0-6 point scale) equal to the pooled effect. Change in the pooled estimate likely would be minimal if this trial were included in the meta-analysis. Two trials116, 119 reported treatment effects of 0.1116and 0.4119 on a 0-3 point scale (0.2 and 0.8 on a 0-6 point scale, respectively). These effects were larger than the pooled effect (0.12 on a 0-6 point scale) but smaller than the MCID of 1.8. To determine the impact of these two trials116, 119 on the pooled estimate, we added both to the meta-analysis with assumed standard deviations equal to half the mean change in score in each treatment group. Under this assumption, the pooled effect increased from 0.12 to 0.25 on a 0-6 point scale (95 percent CI: 0.03 to 0.54) favoring intranasal corticosteroid. The larger bound of the 95 percent CI represented 9 percent of maximum score. Based on this analysis, it is unlikely that the 95 percent confidence interval of a meta-analysis including these two trials116, 119 would contain the MCID. The body of evidence in support of a conclusion of equivalence of intranasal corticosteroid and nasal antihistamine for this outcome is therefore considered precise. The overall strength of evidence for this conclusion is rated as high.
Seven115, 117-119, 121 of nine trials assessed nasal itch after 2 weeks of treatment (N=2393 of 2473), all of which reported treatment effects favoring intranasal corticosteroid, although none were reported to be statistically significant. The USPSTF quality rating was good in five trials115, 117, 121 (88 percent of patients reporting this outcome) and poor in two trials.118, 119 Poor USPSTF ratings were assigned for inadequate blinding118, 119 and inappropriate analysis of results (not intention to treat119). A meta-analysis of four good quality trials115, 121 (N=1791; 75 percent of patients reporting this outcome) yielded a statistically significant pooled effect of 0.19 on a 0-6 point scale (95 percent CI: 0.03 to 0.34) favoring intranasal corticosteroid (Figure 13). The larger bound of the 95 percent CI represented 11 percent of maximum score. Statistical heterogeneity was low (I2=27 percent, p=0.25). Of three trials117-119 not included in the meta-analysis, two117, 119 reported treatment effects of 0.09117 on a 0-6 point scale (2 percent of maximum score) and 0.4119 on a 0-3 point scale (13 percent of maximum score). The third trial118 did not report the magnitude of the treatment effect.

Figure 13
Nasal itch at 2 weeks: meta-analysis of 4 trials–intranasal corticosteroid versus nasal antihistamine.
For nasal itch at 2 weeks, the risk of bias was rated as low. Eighty-eight percent of patients reporting this outcome were in good quality trials. Treatment effects consistently favored intranasal corticosteroid in all trials, and statistical heterogeneity of a meta-analysis of four trials115, 121 (75 percent of patients reporting this outcome) was low. The 95 percent CI for the pooled estimate (0.03 to 0.34) fell within an interval bounded by −MCID and +MCID (-1.8 and +1.8 on the 0-6 point scale used). Of three trials117-119 not included in the meta-analysis, one118 did not report the magnitude of the treatment effect, but this trial represented 5 percent of patients reporting this outcome. One trial117 (13 percent of patients reporting this outcome) showed a treatment effect (0.09) that was less than both the pooled effect (0.19) and the MCID. If this trial were included in the meta-analysis, the pooled effect would decrease, and it is unlikely that the 95 percent CI would include an MCID of 30 percent maximum score. The third trial119 (7 percent of patients reporting this outcome) showed a treatment effect of 0.4 on a 0-3 point scale (0.8 on a 0-6 point scale). This effect was larger than the pooled effect but smaller than the MCID. To determine the impact of this trial119 on the pooled estimate, we added it to the meta-analysis with an assumed standard deviation equal to half the mean change in score in each treatment group. Under this assumption, the pooled effect increased from 0.19 to 0.35 on a 0-6 point scale (95 percent CI: 0.02 to 0.68) favoring intranasal corticosteroid. The larger bound of the 95 percent CI represented 11 percent of maximum score. Based on this analysis, it is unlikely that the 95 percent confidence interval of a meta-analysis including this trial119 would contain the MCID. The body of evidence in support of a conclusion of equivalence of intranasal corticosteroid and nasal antihistamine for this outcome is therefore considered precise. The overall strength of evidence for this conclusion is rated as high.
Seven115, 117, 118, 120, 121 of nine trials assessed TNSS after 2 weeks of treatment (N=2257 of 2473). Five115, 117, 121 reported treatment effects favoring intranasal corticosteroid, and two118, 120 reported treatment effects favoring nasal antihistamine. None were reported to be statistically significant. The USPSTF quality rating was good in five trials115, 117, 121 (93 percent of patients reporting this outcome) and poor in two trials.118, 120 Poor USPSTF ratings were assigned for noncomparable groups at baseline,120 inadequate blinding,118 and inappropriate analysis of results (unadjusted for baseline group differences120). A meta-analysis of five good quality trials115, 117, 121 (N=2097; 93 percent of patients reporting this outcome) yielded a statistically significant pooled effect estimate of 0.65 on a 0-24 point scale (95 percent CI: 0.25 to 1.05) favoring intranasal corticosteroid (Figure 14). The larger bound of the 95 percent CI represented 4 percent of maximum score. Statistical heterogeneity was low (I2=0 percent, p=0.98). Two trials118, 120 not included in the meta-analysis reported treatment effects favoring nasal antihistamine. Effect sizes were not reported.

Figure 14
Total nasal symptom score at 2 weeks: meta-analysis of 5 trials–intranasal corticosteroid versus nasal antihistamine.
For TNSS at 2 weeks, the risk of bias was rated as low. Ninety-three percent of patients were in good quality trials. Treatment effects consistently favored intranasal corticosteroid in 93 percent of patients reporting this outcome. The 95 percent CI for the pooled estimate (0.25 to 1.05) fell within an interval bounded by −MCID and +MCID (-7.2 and +7.2 on the 0-24 point scale used). The two trials118, 120 not included in the meta-analysis did not report treatment effect magnitudes, but both favored nasal antihistamine. If these trials were included in the meta-analysis, the pooled effect would decrease. Because these trials represented only 7 percent of patients reporting this outcome, it is unlikely that the 95 percent confidence interval would include the MCID. The body of evidence in support of a conclusion of equivalence of intranasal corticosteroid and nasal antihistamine for this outcome is therefore considered precise. The overall strength of evidence for this conclusion is rated as high.
Individual nasal symptoms of congestion, rhinorrhea, and sneezing were assessed at 3 weeks and 4 weeks in one trial116 (N=50). For congestion, a treatment effect of 0.5 on a 0-3 point scale (17 percent of maximum score) favored nasal antihistamine at both 3 weeks and 4 weeks. For rhinorrhea and sneezing, treatment differences were zero at both 3 weeks and 4 weeks. Statistical significance was not reported for any outcome. This trial was rated poor quality due to noncomparable groups at baseline, inadequate blinding, and inappropriate analysis of results (unadjusted for baseline group differences). Consistency could not be assessed in a single trial, and no observed result at any assessment period exceeded an MCID of 0.9, representing 30 percent of maximum score. The evidence was therefore insufficient to support the use of one treatment over the other for these outcomes.
At 3, 4 and 5 weeks, one trial120 (N=30) reported improvement in TNSS with nasal antihistamine. Treatment effects ranged from 1.0 to 1.4 on a 0-12 point scale (from 8 percent to 12 percent of maximum score) and were statistically nonsignificant. This trial was rated poor quality due to noncomparable groups at baseline and inappropriate analysis of results (unadjusted for baseline group differences). Risk of bias is therefore high. Consistency could not be assessed in a single trial, and no observed result at any assessment period exceeded an MCID of 4, representing 30 percent of maximum score. The evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
Eye Symptoms
Five115, 117, 118 of nine trials (N=2128 of 2473 patients) assessed eye symptoms at 2 weeks. Four115, 117 were rated good quality (94 percent of patients reporting this outcome). Treatment effects in these trials favored nasal antihistamine and ranged from 0.2 to 0.45 on a 0-18 point scale (from 1 percent to 3 percent of maximum score). A meta-analysis of three115 of these trials (N=1697; 80 percent of patients reporting this outcome) yielded a statistically nonsignificant pooled effect estimate of 0.22 on a 0-18 point scale (95 percent CI: -0.12 to 0.57) favoring nasal antihistamine (Figure 15). The larger bound of the 95 percent CI represented 3 percent of maximum score. Statistical heterogeneity was low (I2=0 percent, p=0.97). The fourth good quality trial117 (n=305; 14 percent of patients reporting this outcome) showed a treatment effect of 0.45 on a 0-18 point scale (11 percent of maximum score). The fifth trial118 (n=130; 7 percent of patients reporting this outcome) showed a statistically nonsignificant treatment effect of 0.6 on a 0-9 point scale (7 percent of maximum score) favoring intranasal corticosteroid. This trial was rated poor quality due to inadequate blinding.
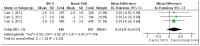
Figure 15
Total ocular symptom score at 2 weeks: meta-analysis of 4 trials–intranasal corticosteroid versus nasal antihistamine.
For the outcome of eye symptoms, the risk of bias was rated as low. Ninety-four percent of patients were in good quality trials. Treatment effects consistently favored nasal antihistamine in 94 percent of patients reporting this outcome, and statistical heterogeneity of a meta-analysis of 80 percent of patients was low. The 95 percent CI for the pooled estimate (-0.12 to 0.57) fell within an interval bounded by −MCID and +MCID (-5.4 and +5.4 on the 0-18 point scale used). Of two trials117, 118 not included in the meta-analysis, one118 reported a treatment effect of 0.06 on a 0-9 point scale (0.12 on a 0-18 point scale) favoring intranasal corticosteroid. If this trial were included in the meta-analysis, the pooled effect estimate (0.22 favoring nasal antihistamine) would decrease. Because this trial represented 7 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include the MCID of 5.4. The other trial117 (14 percent of patients reporting this outcome) showed a treatment effect of 0.45 on a 0-18 point scale favoring nasal antihistamine. If this trial were included in the meta-analysis, the pooled effect estimate would increase, but it is unlikely that the 95 percent CI would include the MCID. The body of evidence to support a conclusion of equivalence of intranasal corticosteroid and nasal antihistamine for this outcome was therefore considered precise. The overall strength of evidence supporting this conclusion is rated as high.
Quality of Life
Two trials117, 121 (N=404; 16 percent of the total patient sample for this comparison) assessed quality of life using the RQLQ instrument. Both were good quality trials, and both observed statistically nonsignificant treatment effects in favor of intranasal corticosteroid (0.26 for both).
For RQLQ at 2 weeks, the risk of bias is rated as low based in the good quality of the trials. Results consistently favored intranasal corticosteroid, but neither exceeded the MCID of 0.5 points. Evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
A meta-analysis of 2-week RQLQ outcomes from three good quality trials115 (N=1693) yielded a pooled effect estimate of 0.1 favoring intranasal corticosteroid. This result is consistent with the treatment effects reported in two trials117, 121 described above. Because the published meta-analysis lacked details about the how the analysis was conducted, this result could not be replicated and was not included in the formal evidence assessment.
Intranasal Corticosteroid Versus Nasal Cromolyn
Description of Included Studies
Four122-125 RCTs published between 1985 and 2005 were identified (N=434 patients randomized to treatment groups of interest). Two trials122, 123 were double-blinded, one124 was open-label, and one had inadequate patient blinding. 125 Three trials122-124 were conducted in Europe. Two122, 124 of these were single center trials, and one123 was a multicenter trial. One trial125 conducted in North America did not report if it was a single center or multicenter trial. Trials were 3, 4, 6 and 8 weeks in duration.122-125 Cromolyn (disodium cromoglycate) was compared with budesonide,122 mometasone,124 and fluticasone propionate123 in three separate trials, and to both flunisolide and beclomethasone in one trial.125 Three trials122, 124, 125 were industry funded and one did not identify its funding source.123
Trial participants tended to be young adults with mean ages ranging from 29 to 36 years. Most were men (approximately 55 percent). No trial reported on race. All trials required a minimum duration of SAR history, but none reported the mean duration of SAR symptoms. One trial124 required a minimum baseline severity of SAR symptoms. In the one trial122 that reported baseline TNSS, symptom severity was mild.
In three trials122-124 that assessed nasal symptoms, 4-point rating scales (from 0=no symptom to 3=severe symptom) were used. For two trials,122, 123 the identified outcome of interest was the mean change from baseline symptom scores. In Lange (2005),124 the outcome of interest was the difference between post-treatment scores at 4 weeks. Lange (2005)124 also reported mean post-treatment eye symptom scores but did not define which eye symptoms were assessed and reported only the statistical significance of treatment effects, not their magnitude. Eye symptom outcomes therefore are not reviewed here. Two trials124, 125 reported quality of life outcomes at 4 weeks124 and 8 weeks125 using PGA of treatment efficacy. Results are noted in the text.
All four trials122-125 were rated poor quality. Reasons included noncomparable groups at baseline,122, 123 lack of blinding,124, 125 and inappropriate analysis of results (unadjusted for baseline group differences123).
Key Points
These results are summarized in Table 34.
Table 34
Strength of evidence: intranasal corticosteroid versus nasal cromolyn.
- Individual nasal symptoms and TNSS at 2 weeks: Evidence was insufficient to support the use of one treatment over the other based on one trial122 with high risk of bias and imprecise results.
- These results are based on trials of five of eight intranasal corticosteroids (62.5 percent) in comparison with nasal cromolyn.
Synthesis and Strength of Evidence
Nasal symptom outcomes discussed below are summarized in Table 35. Meta-analysis was not considered for this treatment comparison due to lack of variance estimates for group-level treatment effects.
Table 35
Treatment effects: nasal symptoms–intranasal corticosteroid versus nasal cromolyn.
Nasal Symptoms
One122 of three trials that assessed nasal symptoms reported outcomes (congestion, rhinorrhea, sneezing, nasal itch, and TNSS) at 2 weeks (N=43). The trial was rated poor quality due to noncomparable groups at baseline. Statistically significant treatment effects favoring intranasal corticosteroid were reported for four nasal symptoms and for TNSS. For individual symptoms, treatment effects ranged from 0.21 for nasal itch to 0.59 for rhinorrhea on a 0-3 point scale (from 7 percent to 20 percent of maximum score). For TNSS, the treatment effect was 1.53 on a 0-12 point scale (13 percent of maximum score).
For nasal symptoms at 2 weeks, the risk of bias was considered high because the trial122 was small and of poor quality. Consistency cannot be assessed for a single trial. Effect estimates were imprecise because none exceeded an MCID of 30 percent maximum score. Evidence was insufficient to support the use of one treatment over the other for these outcomes.
Three trials (total N=344) assessed nasal symptoms beyond 2 weeks: Bjerrum (1985)122 at 3 weeks, Lange (2005)124 at 4 weeks, and Bousquet (1993)123 at 6 weeks. Trial quality ratings were poor due to noncomparable groups at baseline,122, 123 lack of blinding,124 and inappropriate analysis of results (unadjusted for baseline group differences123).
At 3 weeks,122 statistically significant treatment effects were shown for rhinorrhea, sneeze, nasal itch, and TNSS. All favored intranasal corticosteroid. Treatment effect magnitudes were comparable to those seen at 2 weeks122 and ranged from 0.15 for nasal itch to 0.49 for rhinorrhea on a 0-3 point scale (from 5 percent to 16 percent of maximum score). The treatment effect for TNSS was 1.19 on a 12-point scale (10 percent of maximum score). Nasal congestion was the only symptom for which a statistically nonsignificant treatment effect was reported (0.28 on a 0-3 point scale [9 percent of maximum score]).
At 4 weeks124 and 6 weeks123, statistically significant treatment effects favoring intranasal corticosteroid were reported for four individual nasal symptoms. At 4 weeks,124 there was a statistically significant treatment effect favoring intranasal corticosteroid for TNSS. TNSS was not assessed at 6 weeks. Magnitude of effects at 4 and 6 weeks were not reported.
For nasal symptoms at 3 to 6 weeks, the risk of bias was considered high. All three trials were rated poor quality. Treatment effects consistently favored intranasal corticosteroid. Most treatment effects were not reported. None of the reported treatment effects exceeded an MCID of 30 percent maximum score. The body of evidence was therefore imprecise, and evidence to support the use of one treatment over the other for these outcomes is insufficient.
Two trials reported PGA of treatment efficacy at 4 weeks124 and 8 weeks125 (N=173). Both trials were rated poor quality due to lack of blinding124, 125 and lack of maintenance of comparable groups.125 Both reported statistically significant results favoring intranasal corticosteroid.
Intranasal Corticosteroid Versus Oral Leukotriene Receptor Antagonist (Montelukast)
Description of Included Studies
Five97, 126-129 double-blinded, RCTs published between 2002 and 2009 were identified (N=2328 patients randomized to comparator groups of interest). All but one trial128 was a multicenter trial conducted in North America. Trial durations were 2 to 8 weeks. One small trial128 included 29 patients, and the others included 285 to 736 patients. The oral leukotriene receptor antagonist, montelukast, was compared to fluticasone propionate in four trials126-129 and to beclomethasone in one trial97. All five trials were industry funded.
Mean ages of trial participants ranged from 28 to 40 years. Approximately 60 percent of patients were women. In one trial,128 40 percent were women. In two trials97, 127 that reported on race, most patients were white (approximately 78 percent). In three trials, 97, 126, 129 patients reported SAR symptoms for an average of at least 15 years. Baseline symptom scores for the trials represented a range of severity, with patients reporting mild,128 moderate,97, 127 and severe126, 129 baseline symptoms. All five trials included patients with asthma. One trial127 included asthma outcomes and considered prior asthma treatment as a baseline characteristic in the analysis model.
All five trials assessed nasal symptoms. One trial127 assessed asthma outcomes. No trial assessed eye symptoms. All five trials included 2-week symptom assessments. One trial127 reported 4-week data, and one trial128 reported 6 to 8-week data. Nasal symptoms were assessed using several scales. In three trials,126, 127, 129 patients used a VAS to rate each nasal symptom (congestion, rhinorrhea, sneezing, and itch) on a scale of zero to 100. Scores were summed to yield a maximum TNSS of 400. In Pullerits (2002),128 patients rated each nasal symptom on a 5-point (0 to 4) scale, and scores were summed to create the TNSS (16-points maximum). Individual symptom scores were not reported. In Lu (2009),97 patients rated each nasal symptom on a 4-point (0 to 3) scale, and scores were averaged to create the TNSS (3-points maximum). Individual symptoms were not reported. To calculate the mean change from baseline, most trials subtracted baseline scores from scores averaged over the entire treatment duration. One trial128 averaged data for intervals (weeks 1 and 2, weeks 3 to 5, weeks 6 to 8) and compared the mean change during each interval to baseline. For asthma outcomes, symptom-free days, morning and evening peak expiratory flow (PEF), and albuterol-free days were assessed. Symptoms were self-evaluated using a 0-5 point Likert scale. Morning and evening peak expiratory flow were self-measured (average of three readings) with flow meters provided to patients. Albuterol use and number of nighttime awakenings due to asthma were recorded in diaries.
Three trials126, 127, 129 were rated good quality, and two97, 128 were rated poor.
Key Points
The results below are summarized in Table 36.
Table 36
Strength of evidence: intranasal corticosteroid versus oral leukotriene receptor antagonist.
- Individual nasal symptoms (congestion, rhinorrhea, sneezing, and nasal itch) and asthma outcomes (symptom-free days, albuterol-free days, morning and evening PEF, and asthma exacerbations) at 4 weeks: Evidence was insufficient to support one treatment over the other based on one trial 127 with low risk of bias and imprecise results.
- These results are based on trials using two of eight intranasal corticosteroids (25 percent) in comparison with montelukast (100 percent).
Synthesis and Strength of Evidence
Nasal symptom outcomes discussed below are summarized in Table 37. Asthma outcomes are summarized in Table 38.
Table 37
Treatment effects: nasal symptoms–intranasal corticosteroid versus oral leukotriene receptor antagonist.
Table 38
Treatment effects: asthma outcomes–intranasal corticosteroid versus oral leukotriene receptor antagonist.
As shown in Table 37, variance estimates of treatment effects were provided for nasal outcomes at 2 weeks. For these outcomes, meta-analyses were conducted. For TNSS, the analysis required use of standardized mean differences (rather than mean differences) because different rating scales were used across trials. For individual nasal symptoms, all trials used the same rating scale. The meta-analyses therefore used mean differences.
Nasal Symptoms
Three126, 127, 129 of five trials (2014 of 2328 patients, 87 percent) assessed individual nasal symptoms (congestion, rhinorrhea, sneezing, and nasal itch) at 2 weeks. All three trials were rated good quality. For each symptom, the treatment effect favored intranasal corticosteroid over oral leukotriene receptor antagonist and was statistically significant. Meta-analyses of the three trials for each symptom favored intranasal corticosteroid with statistically significant treatment effects ranging from 7.9 to 8.7 on a 100 VAS (Figure 16 through Figure 19). The larger bound of the 95 percent CIs ranged from 10.08 to 10.76 on a 100-point VAS (from 10 percent to 11 percent of maximum score, respectively). Statistical heterogeneity was low (I2=0 percent for all four analyses, p=0.33 to 0.88).

Figure 16
Congestion at 2 weeks: meta-analysis of 3 trials–intranasal corticosteroid versus oral leukotriene receptor antagonist.

Figure 17
Rhinorrhea at 2 weeks: meta-analysis of 3 trials–intranasal corticosteroid versus oral leukotriene receptor antagonist.

Figure 18
Sneezing at 2 weeks: meta-analysis of 3 trials–intranasal corticosteroid versus oral leukotriene receptor antagonist.

Figure 19
Nasal itch at 2 weeks: meta-analysis of 3 trials–intranasal corticosteroid versus oral leukotriene receptor antagonist.
For individual nasal symptoms at 2 weeks, the risk of bias was rated as low. All three trials were good quality. Treatment effects consistently favored intranasal corticosteroid in all three trials. The 95 percent CIs for the pooled estimates fell within an interval bounded by −MCID and +MCID (-30 and +30 on the 100-point VAS used). The body of evidence to support a conclusion of equivalence of intranasal corticosteroid and oral leukotriene receptor antagonist for each of these outcomes is therefore precise. The strength of evidence supporting these conclusions is high.
All five trials assessed TNSS at 2 weeks (N=2038). Three good quality trials126, 127, 129 of 2014 patients represented 87 percent of patients reporting this outcome. Thirteen percent of patients were in two trials97, 128 that were rated poor quality due to inappropriate analysis of results (not intention to treat). Treatment effects favored intranasal corticosteroid over oral leukotriene receptor antagonist and were statistically significant in all but one trial.128 All three good quality trials126, 127, 129 assessed TNSS using a 0-400 point VAS. Treatment effects were 33.6 (95 percent CI: 20.6 to 46.5);126 26.1 (95 percent CI: 9.7 to 42.5);127 and 34.4 (95 percent CI: 23.4 to 49.3)129 The larger bounds of the 95 percent CIs were 12 percent, 11 percent and 12 percent of maximum score, respectively. Of two poor quality trials reporting on this outcome using an interval rating scale, one128 (n=29) reported a statistically nonsignificant effect of 0.8 on a 0-16 point scale (5 percent of maximum score), and the other (Trial 1 in Lu [2009];97 n=285) reported a statistically significant effect of 0.34 on a 0-3 point scale (11 percent of maximum score).
A meta-analysis of four trials97, 126, 127, 129 was performed. The fifth trial128 was excluded due to lack of a variance estimate for the treatment effect. The meta-analysis yielded a statistically significant pooled effect (standardized mean difference) of 0.40 (95 percent CI: 0.27 to 0.52) favoring intranasal corticosteroid (Figure 20). Effect estimates in the pooled trials were in the same direction, and their 95 percent CIs did not touch the “no effect” line.

Figure 20
Total nasal symptom score at 2 weeks: meta-analysis of 4 trials–intranasal corticosteroid versus oral leukotriene receptor antagonist.
For TNSS at 2 weeks, the risk of bias was rated as low. Eighty-seven percent of patients were in good quality trials. Treatment effects consistently favored intranasal corticosteroid for all patients reporting this outcome. Ninety-five percent CIs of pooled treatment effects fell within an interval bounded by −MCID and +MCID (-120 and +120126, 127, 129 or -1.8 and +1.897). These effects were therefore considered precise. The one trial128 excluded from the meta-analysis did not alter the precision assessment because this trial represented 1 percent of patients reporting this outcome. The body of evidence supporting a conclusion of equivalence of intranasal corticosteroid and leukotriene receptor antagonist for this outcome is therefore considered precise. The overall strength of evidence for this conclusion is rated as high.
One good quality trial127 (N=573) assessed four individual nasal symptoms (congestion, rhinorrhea, sneezing, and nasal itch) at 4 weeks. All comparisons favored intranasal corticosteroid and were statistically significant. Using a 100-point VAS, treatment effects ranged from 6.0 for nasal itch to 8.3 for congestion (from 6 percent to 8 percent of maximum score).
The risk of bias for this outcome was rated as low based on the good quality of the trial reporting. Consistency cannot be addressed for a single trial. Results were imprecise because none exceeded an MCID of 30 percent maximum score. Evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
Two trials127, 128 assessed TNSS at time points beyond 2 weeks (N=602). One127 was a good quality trial in 573 patients (95 percent of patients reporting this outcome) that reported 4-week outcomes. A statistically significant treatment effect of 28 points on a 0-400 scale (7 percent of maximum score) favored intranasal corticosteroid. The other was a poor quality trial128 that reported outcomes (mean results during the previous 2 weeks) at 5 and 8 weeks. At 5 weeks, a statistically nonsignificant treatment effect of 1.4 on a 0-16 point scale (9 percent of maximum score) favored intranasal corticosteroid. At 8 weeks, a statistically significant treatment effect of 0.7 on a 0-16 point scale (4 percent of maximum score) favored intranasal corticosteroid.
The risk of bias for these outcomes was rated as low. Ninety-five percent of patients were in the good quality trial.127 Treatment effects consistently favored intranasal corticosteroid, but none exceeded an MCID of 30 percent maximum score. The body of evidence for these outcomes was therefore imprecise. Evidence was insufficient to support the use of one treatment over the other for this outcome.
Asthma Symptoms
One good quality trial127(N=573) assessed symptoms and objective measures of asthma over 4 weeks of treatment. There were no statistically significant differences between treatment groups in any outcome, nor were there differences when treatment groups were stratified by baseline asthma severity. For all outcomes, the risk of bias was rated as low, and consistency could not be assessed with a single trial.
Treatment effects favoring oral leukotriene receptor antagonist were:
- Proportion of symptom-free days: 1.3 percent difference between groups
- Proportion of albuterol-free days: 0.7 percent difference between groups
MCIDs for these outcomes have not been established. Because neither result was statistically significant, evidence was insufficient to support the use of one treatment over the other for these outcomes.
Treatment effects favoring intranasal corticosteroid were:
- Morning peak expiratory flow (PEF): 2.4 L/min
- Evening PEF: 1.8 L/min
- Proportion of patients experiencing an asthma exacerbation, defined as any asthma-related event that required treatment with asthma medications beyond study medications: less than 1 percent
For morning PEF, the treatment effect was less than an MCID of 25 L/min and therefore imprecise. For evening PEF, which has no well-defined MCID, the treatment effect was statistically nonsignificant and therefore imprecise. Evidence was insufficient to support the use of one treatment over the other for these outcomes.
For asthma exacerbations, any reduction in severe exacerbations may be considered clinically significant.70, 135 Because the definition of “asthma exacerbation” used in this trial is broad, the severity of exacerbations observed is unclear. Further, the outcome measure reported patients rather than number of exacerbations; it is unclear whether exacerbations were in fact reduced. The effect is therefore considered imprecise and the evidence insufficient to support the use of one treatment over the other for this outcome.
Combination Oral Selective Antihistamine Plus Intranasal Corticosteroid Versus Oral Selective Antihistamine
Description of Included Studies
Three90, 98, 130 RCTs published between 1998 and 2009 were identified (N=677). Two trials90, 98 were 2-week, double-blinded, multicenter trials in North America, and one130 was a 4-week, patient-blinded, single center trial in Europe. Oral selective antihistamines studied were loratadine (two trials90, 98) and cetirizine (one trial130); intranasal corticosteroids were mometasone (two trials90, 130) and fluticasone propionate (one trial98). Two trials90, 98 were industry funded, and one130 was funded by a national health system.
The average age of patients in the trials ranged from 25 to 42 years. Women were the majority in all trials (range 50 percent to 77 percent). In the one trial98 that reported on race, 77 percent were white, and 18 percent were Hispanic. Mean duration of SAR symptoms was 14 years in the one trial90 that reported this measure. Baseline severity of nasal symptoms was mild to moderate,130 moderate,90 and moderate to severe.98
All three trials assessed TNSS. One90 also assessed individual nasal symptoms (congestion, rhinorrhea, sneezing, and itching), two90, 130 also assessed eye symptoms, and two98, 130 also assessed quality of life. None of the trials assessed asthma outcomes. For the assessment of nasal symptoms, two trials90, 130 used an interval scale. Patients rated symptoms daily130 or twice daily90 using a 0 (no symptoms) to 3 (severe symptoms) scale. Daily scores were summed, and twice daily scores were summed then averaged, to derive a 0-12 point TNSS. One trial98 used a VAS to assess individual symptoms from 0 (no symptoms) to 100 (maximum symptoms). Scores were summed to derive a 0-400 point TNSS. For eye symptoms, patients rated each of three symptoms (itchiness, tearing, redness) on a 0 (no symptoms) to 3 (severe symptoms) scale. Scores were summed for a 0-9 point TOSS. The RQLQ was used to assess quality of life. Scores range from 0 (no impairment) to 6 (severe impairment). The minimum clinically important difference is 0.5 points.
Two trials98, 130 were rated fair quality. One90 was rated poor quality.
Key Points
The results discussed below are summarized in Table 39.
Table 39
Strength of evidence: combination oral selective antihistamine plus intranasal corticosteroid versus oral selective antihistamine.
- Individual nasal symptoms (congestion, rhinorrhea, sneezing, and nasal itch) and eye symptoms (itching, tearing, and redness) at 2 weeks: Evidence was insufficient to support one treatment over the other based on one trial90 with high risk of bias and imprecise results.
- TOSS at 2 weeks: Evidence was insufficient to support one treatment over the other based on one trial130 with medium risk of bias and an imprecise result.
- Quality of life as assessed by the RQLQ at 2 weeks: There is low strength evidence for superiority of combination oral selective antihistamine plus intranasal corticosteroid over oral selective antihistamine monotherapy based on one trial98 with medium risk of bias and a precise result.
- TNSS and TOSS at 4 weeks: Evidence was insufficient to support one treatment over the other based on one trial130 with medium risk of bias and imprecise results.
- These results are based on trials of two of five oral selective antihistamines (40 percent) and two of eight intranasal corticosteroids (25 percent).
Synthesis and Strength of Evidence
Nasal symptom outcomes discussed below are summarized in Table 40, eye symptom outcomes in Table 41, and quality of life outcomes in Table 42. These tables show that there were few trials reporting each outcome. Several trials reported on TNSS at 2 weeks, but variance estimates for observed group effects were not provided. Thus, meta-analysis was not considered for this comparison.
Table 40
Treatment effects: nasal symptoms–combination oral selective antihistamine plus intranasal corticosteroid versus oral selective antihistamine.
Table 41
Treatment effects: eye symptoms–combination oral selective antihistamine plus intranasal corticosteroid versus oral selective antihistamine.
Table 42
Treatment effects: quality of life–combination oral selective antihistamine plus intranasal corticosteroid versus oral selective antihistamine.
Nasal Symptoms
One90 of three trials90, 98, 130 (350 of 677 patients) assessed individual nasal symptoms at 2 weeks. Statistically significant improvements in all four symptoms (congestion, rhinorrhea, sneezing, and itch) with combination therapy were shown. Treatment effects on a 0-3 point scale ranged from 0.1 for nasal itch to 0.3 for congestion (from 3 percent to 10 percent of maximum score). This trial was rated poor quality due to inappropriate analysis of results (not intention to treat).
For individual nasal symptoms at 2 weeks, the risk of bias was rated as high based on the poor quality rating of the trial.90 Consistency cannot be assessed with a single trial. Estimates of treatment effects were imprecise. Evidence was insufficient to support the use of one treatment over the other for this outcome.
All three trials90, 98, 130 assessed TNSS at 2 weeks (total N=677). All showed improvements in TNSS with combination therapy. In two trials, treatment effects reached statistical significance. One98 of these was a fair quality trial of 300 patients (44 percent of total patients reporting this outcome) that showed a treatment effect of 90 using a 0-400 VAS (22 percent of maximum score). The other90 was a poor quality trial in 350 patients (52 percent of patients reporting) that reported a treatment effect of 1.1 on a 0-12 point scale (9 percent of maximum score). The third trial130 assessed TNSS at both 2 weeks and 4 weeks. This was a fair quality trial of 27 patients. Treatment effects on a 0-12 point scale were 1.2 at 2 weeks and 0.9 at 4 weeks (10 percent and 8 percent of maximum score, respectively). Both favored combination therapy, but neither was statistically significant.
For the outcome of TNSS at both 2 weeks and 4 weeks, evidence was insufficient to support the use of one treatment over the other. At 2 weeks, the risk of bias was rated as high. Fifty-two percent of patients reporting this outcome were in the poor quality trial, and neither of the other two trials were rated good quality. Results were consistent across trials but also imprecise. At 4 weeks, the risk of bias was rated as high based on the small size and fair quality rating of the trial. Consistency of results could not be assessed in a single trial, and the effect estimate was imprecise.
Eye Symptoms
Two90, 130 of three trials (377 of 677 patients) assessed eye symptoms. One trial90 reported statistically significant improvements in individual symptoms of eye itching, tearing, and redness at 2 weeks with combination therapy. This was a trial of 350 patients that was rated poor quality due to inappropriate analysis of results (not intention to treat). Treatment effects were not reported. The other trial130 assessed TOSS at 2 weeks and 4 weeks. The treatment effect at both time points was 0.2 on a 0-9 point scale (2 percent of maximum score). These were statistically nonsignificant effects that favored oral selective antihistamine monotherapy. This was a fair quality trial of 27 patients.
For individual eye symptoms at 2 weeks, the risk of bias was rated as high based on the poor quality rating of the trial.90 Consistency of results could not be assessed in a single trial. Because the magnitude of the treatment effects is unknown, they are considered imprecise. Evidence was insufficient to support the use of one treatment over the other for this outcome.
For TOSS at 2 and 4 weeks, the evidence was insufficient to support the use of one treatment over the other. The risk of bias is medium based on the fair quality rating of the trial.130 Consistency could not be assessed, and effect estimates at both time points were imprecise.
Quality of Life
One trial98 assessed quality of life at 2 weeks using the RQLQ. This was a fair quality trial in 300 patients. A statistically significant treatment effect of 1.0 favoring combination therapy was shown. This exceeded the MCID of 0.5 points for the RQLQ. This trial98 also reported a PGA of treatment response. A statistically significant treatment effect favoring combination therapy was reported (32-percentage point increase in the proportion of patients reporting moderate or significant improvement in the combination therapy arm using a 7-point Likert scale).
For quality of life at 2 weeks, the risk of bias was rated as medium based on the quality rating of the trial.98 Although the effect estimate is precise, consistency cannot be assessed. The overall strength of evidence was low.
Combination Oral Selective Antihistamine Plus Intranasal Corticosteroid Versus Intranasal Corticosteroid
Description of Included Studies
Five trials62, 90, 98, 131, 132, published between 1998 and 2004 were identified (N=1201). Four90, 98, 131, 132, (N=1136) were multicenter, double-blinded, RCTs based in Europe131, 132 or North America.90, 98 The fifth62 was a single center European crossover trial in which the unit of randomization was the order in which treatments were received. Trials were 2 to 8 weeks in duration and included between 40 and 454 patients. Oral selective antihistamines studied were loratadine (two trials90, 98), cetirizine (two trials131, 132) and levocetirizine (one trial62); intranasal corticosteroids were fluticasone propionate (four trials62, 98, 131, 132) and mometasone (one trial90). Of four trials that reported funding, two98, 131 were funded by industry, one132 by a national health system, and one62 by an academic institution.
The average age of patients in the trials ranged from 26 to 45 years. Approximately half of patients were female (range 50 percent to 57 percent). In the one trial that reported on race,98 77 percent of patients were white and 18 percent were Hispanic. Duration of SAR symptoms ranged from 2 to 4 years for the majority of patients in one trial132 to an average of 14 years for patients in another trial.90 Baseline severity of SAR symptoms ranged from mild-moderate to moderate-severe.
Three trials assessed individual nasal symptoms (congestion, rhinorrhea, sneezing, and itching), five assessed TNSS, two assessed eye symptoms, and two assessed quality of life. Three trials used an interval scale for nasal symptom severity. Patients rated symptoms daily62, 132 or twice daily90 using a 0 (no symptoms) to 3 (severe symptoms) scale. Daily scores were summed, and twice daily scores were summed then averaged, to derive a 0-12 point TNSS. One trial98 used a VAS to assess individual symptoms from 0 (no symptoms) to 100 (maximum symptoms). Scores were summed to derive a 0-400 point TNSS. For eye symptoms, Anolik (2008)90 used a 0 (no symptoms) to 3 (severe symptoms) scale to assess eye itching, tearing, and redness. Benincasa (1994)131 used a 10-point scale (0= no symptoms, 1-3 = mild symptoms, 4-6 = moderate symptoms, and 7-9 = severe symptoms). Eye symptoms were not specifically defined. To assess quality of life, the RQLQ and the mini-RQLQ were used. For both the RQLQ and the mini-RQLQ, scores range from 0 (no impairment) to 6 (severely impaired). MCID for the RQLQ is 0.5 points and for the mini-RQLQ, 0.7 points.
The largest of the five trials131 (n=454) was rated good quality. One trial98 was rated fair, and three trials62, 90, 132 were rated poor (total N=447).
Key Points
The results discussed below are summarized in Table 43.
Table 43
Strength of evidence: combination oral selective antihistamine plus intranasal corticosteroid versus intranasal corticosteroid.
- Individual eye symptoms (itching, tearing, and redness) at 2 weeks: Evidence was insufficient to support the use of one treatment over the other based on one trial90 with high risk of bias and imprecise results.
- Individual nasal symptoms (congestion, rhinorrhea, sneezing, and itch) at 6 weeks: Evidence was insufficient to support the use of one treatment over the other based on one trial132 with high risk of bias and imprecise results.
- TOSS at 8 weeks: Evidence was insufficient to support the use of one treatment over the other based on one trial131 with low risk of bias and an imprecise result.
- These results are based on trials using three of five oral selective antihistamines (60 percent) and two of eight intranasal corticosteroids (25 percent).
Synthesis and Strength of Evidence
Nasal symptom results discussed below are summarized in Table 44, eye symptom results in Table 45, and quality of life results in Table 46. As shown in these tables, few trials reported on each outcome. Although three trials assessed TNSS at 2 weeks, variance estimates of symptom improvements were not provided consistently. Thus, meta-analysis was not possible for this comparison.
Table 44
Treatment effects: nasal symptoms–combination oral selective antihistamine plus intranasal corticosteroid versus intranasal corticosteroid.
Table 45
Treatment effects: eye symptoms–combination oral selective antihistamine plus intranasal corticosteroid versus intranasal corticosteroid.
Table 46
Treatment effects: quality of life–combination oral selective antihistamine/intranasal corticosteroid versus intranasal corticosteroid.
Nasal Symptoms
Evidence for the assessment of individual nasal symptoms comes from two62, 90 of five trials (407 of 1201 patients) that assessed nasal symptoms at 2 weeks, and one trial132 that assessed nasal symptoms at 6 weeks. All three trials were rated poor quality due to noncomparable groups at baseline132 and inappropriate analysis of results (not intention to treat62, 90).
At 2 weeks, Anolik (2008)90 showed no difference between treatments for congestion (treatment effect = 0), and Barnes (2006)62 showed a statistically significant treatment effect of 0.11 on a 0-3 point scale (4 percent of maximum score) favoring intranasal corticosteroid monotherapy. At 6 weeks, Di Lorenzo (2004)132 showed a statistically nonsignificant treatment effect of 0.04 on a 0-3 point scale (1 percent of maximum score).
Evidence for the outcome of congestion at 2 weeks was insufficient to support the use of one treatment over the other. Two poor quality trials62, 90 with high risk of bias reported inconsistent and imprecise treatment effects. For the outcome of congestion at 6 weeks, evidence also is insufficient to support the use of one treatment over the other. One poor quality trial132 with a high risk of bias reported an imprecise treatment effect.
At 2 weeks, Anolik (2008)90 showed no difference between treatments for rhinorrhea (treatment effect = 0), and Barnes (2006)62 showed a statistically nonsignificant treatment effect of 0.04 on a 0-3 point scale (1 percent of maximum score) favoring combination therapy. At 6 weeks, Di Lorenzo (2004)132 also showed a statistically nonsignificant treatment effect of 0.04 on a 0-3 point scale (1 percent of maximum score), favoring intranasal corticosteroid monotherapy.
Evidence for the outcome of rhinorrhea at 2 weeks was insufficient to support the use of one treatment over the other. Two poor quality trials62, 90 with high risk of bias reported inconsistent and imprecise treatment effects. For the outcome of rhinorrhea at 6 weeks, evidence also is insufficient to support the use of one treatment over the other. One poor quality trial132 with a high risk of bias reported an imprecise treatment effect.
At 2 weeks, Anolik (2008)90 and Barnes (2006)62 both showed greater improvements in sneezing with combination therapy than with intranasal corticosteroid monotherapy. Treatment effects were 0.1 and 0.15 on a 0-3 point scale (3 percent and 5 percent of maximum score, respectively). Neither was statistically significant. At 6 weeks, Di Lorenzo (2004)132 showed a statistically nonsignificant treatment effect of 0.08 on a 0-3 point scale (3 percent of maximum score) favoring combination therapy.
Evidence for the outcome of sneezing at 2 weeks was insufficient to support the use of one treatment over the other. Two poor quality trials62, 90 with high risk of bias reported consistent but imprecise treatment effects. For the outcome of rhinorrhea at 6 weeks, evidence also is insufficient to support the use of one treatment over the other. One poor quality trial132 with a high risk of bias reported an imprecise treatment effect.
At 2 weeks, Anolik (2008)90 and Barnes (2006)62 both showed greater improvements in nasal itch with combination therapy than with intranasal corticosteroid monotherapy. Treatment effects were 0.1 and 0.03 on a 0-3 point scale (3 percent and 1 percent of maximum score, respectively). Neither was statistically significant. At 6 weeks, Di Lorenzo (2004)132 reported a statistically significant treatment effect of 0.1 on a 0-3 point scale (3 percent of maximum score) favoring combination therapy.
Evidence for the outcome of nasal itch at 2 weeks was insufficient to support the use of one treatment over the other. Two poor quality trials62, 90 with high risk of bias reported consistent but imprecise treatment effects. For the outcome of nasal itch at 6 weeks, evidence also is insufficient to support the use of one treatment over the other. One poor quality trial132 with a high risk of bias reported an imprecise treatment effect.
Three62, 90, 98 of five trials (707 of 1201 patients) assessed TNSS at 2 weeks. All three trials showed greater improvement in TNSS with combination therapy than with intranasal corticosteroid monotherapy. In two62, 98 of these, this finding was statistically significant. One98 of these was a fair quality trial of 300 patients (42 percent of patients reporting this outcome). The other 62 was rated poor quality due to inappropriate analysis of results (not intention to treat). This trial62 reported results using a 0-12 point scale and showed a treatment effect of 0.11 (1 percent of maximum score). The fair quality trial98 reported a treatment effect of 30 on a 0-400 VAS (8 percent of maximum score). The third trial90 reported a statistically nonsignificant treatment effect of 0.3 on a 0-12 point scale (3 percent of maximum score). This was a poor quality trial of 345 patients (49 percent of patients reporting this outcome).
For the outcome of TNSS at 2 weeks, the risk of bias was rated as high. Fifty-eight percent of patients were in poor quality trials, and 42 percent were in a fair quality trial. Treatment effects consistently favored combination therapy in all three trials. However, treatment effects were imprecise. The evidence was therefore insufficient to support the use of one treatment over the other for this outcome.
Two trials131, 132 assessed TNSS at time points beyond 2 weeks (N=494). The larger of these131 (92 percent of patients reporting this outcome) was rated good quality, and the smaller132 (n=40) was rated poor quality due to noncomparable groups at baseline. At 6 weeks, the latter trial132 showed a statistically nonsignificant treatment effect of 0.2 on a 0-3 point scale (7 percent of maximum score) favoring combination therapy. At 8 weeks, the larger trial131 reported a treatment difference of zero.
For the outcome of TNSS at 6 to 8 weeks, evidence also is insufficient to support the use of one treatment over the other. The risk of bias was considered low; 92 percent of patients reporting this outcome were in the good quality trial. However, reported treatment effects were inconsistent and imprecise.
Eye Symptoms
Two trials assessed eye symptoms, one90 at 2 weeks (N=345) and one131 at 8 weeks (N=454). At 2 weeks, Anolik (2008)90 reported statistically nonsignificant treatment effects favoring combination therapy for itchy eyes, watery eyes, and red eyes. Effect sizes were not reported. This trial was rated poor quality due to inappropriate analysis of results (not intention to treat). At 8 weeks, Benincasa (1994)131 reported a statistically nonsignificant treatment effect favoring combination therapy for unspecified eye symptoms. Effect size was not reported. This trial131 was rated good quality.
Evidence for the outcome of eye symptoms at 2 weeks was insufficient to support the use of one treatment over the other. One trial90 with a high risk of bias reported imprecise results. At 8 weeks, the evidence also is insufficient to support the use of one treatment over the other. One trial131 with a low risk of bias reported imprecise results.
Quality of Life
Two trials62, 98 assessed quality of life at 2 weeks using different measures. The larger trial98 (83 percent of patients reporting this outcome) was rated fair quality and showed a treatment effect of 0.1 on the 0-6 point RQLQ scale favoring combination therapy. The smaller trial62 was rated poor quality and showed a treatment effect of 0.1 on the 0-6 point mini-RQLQ scale favoring intranasal corticosteroid monotherapy. Neither result was statistically significant, and neither exceeded the MCID. The larger trial98 also assessed PGA of treatment. More patients treated with combination therapy reported moderate to significant improvement using a 7-point Likert scale (significantly worse to significantly improved) than patients treated with intranasal corticosteroid monotherapy. This result was not statistically significant.
Evidence for quality of life outcomes at 2 weeks is insufficient to support one treatment over the other. Two trials62, 98 with medium risk of bias reported inconsistent and imprecise results.
Combination Intranasal Corticosteroid Plus Nasal Antihistamine Versus Intranasal Corticosteroid
Description of Included Studies
Five115, 117, 121 multicenter, RCTs published between 2008 and 2012 were identified (total N=2102). All were 2-week, double-blinded trials based in North America. Trial size ranged from 102 to 898 patients randomized to treatment groups of interest. In all five trials, the nasal antihistamine was azelastine, and the intranasal corticosteroid was fluticasone propionate. Three trials115 from the same article used a newly approved combination product comprising both drugs, and two trials117, 121 used a separate nasal inhaler for each drug in the combination. All five trials were industry funded.
The mean age of trial participants ranged from 34 to 40 years. Most participants were female (approximately 63 percent). The majority of patients were white (minimum 64 percent). Of two trials117, 121 that reported the proportions of other races, one121 included approximately 20 percent Hispanic patients. All trials required a minimum duration and severity of SAR symptoms. Mean SAR duration ranged from 16 to 22 years. Mean baseline nasal symptoms were in the severe range.
All five trials assessed both individual and total nasal symptoms. Four115, 117 of five assessed eye symptoms, and two117, 121 assessed quality of life. No trial assessed asthma outcomes. In all five trials, patients rated symptoms twice daily. Individual nasal symptoms (congestion, rhinorrhea, sneezing, and itching) and eye symptoms (itching, tearing, and redness) were rated on a scale from 0 (no symptoms) to 3 (severe symptoms). Morning and evening scores were summed to give a maximum score of 6 for each individual symptom. TNSS ranged from 0 to 24, and TOSS ranged from 0 to 18. The RQLQ was used to assess quality of life. Scores ranged from 0 (no impairment) to 6 (severe impairment). The MCID is 0.5 points.
All five trials were rated good quality.
Key Points
These results are summarized in Table 47.
Table 47
Strength of evidence: combination intranasal corticosteroid plus nasal antihistamine versus intranasal corticosteroid.
- Individual nasal symptoms (congestion, rhinorrhea, sneezing, and itch), TNSS and TOSS at 2 weeks: High strength evidence for equivalence of combination intranasal corticosteroid plus nasal antihistamine and intranasal corticosteroid monotherapy based on five trials115, 117, 121 (for individual nasal symptoms and TNSS) and four trials115, 117 (for TOSS) with low risk of bias and consistent, precise results.
- These results are based on trials of one of eight intranasal corticosteroids (12.5 percent) and one of two nasal antihistamines (50 percent).
Synthesis and Strength of Evidence
Nasal symptom outcomes discussed below are summarized in Table 48, eye symptom outcomes in Table 49, and quality of life outcomes in Table 50. As shown in these tables and noted above, several trials reported on each outcome. Additionally, variance estimates of group-level treatment effects were provided. Thus, meta-analyses were performed for all nasal and eye outcomes.
Table 48
Treatment effects: nasal symptoms–combination intranasal corticosteroid plus nasal antihistamine versus intranasal corticosteroid.
Table 49
Treatment effects: eye symptoms–combination intranasal corticosteroid plus nasal antihistamine versus intranasal corticosteroid.
Table 50
Treatment effects: quality of life symptoms–combination intranasal corticosteroid plus nasal antihistamine versus intranasal corticosteroid.
Nasal Symptoms
All five trials115, 117, 121 assessed four individual nasal symptoms and TNSS at 2 weeks (total N=2102). Four trials115, 121 (85 percent of patients reporting this outcome) were included in meta-analyses for each nasal outcome. Variance estimates necessary for pooling were not reported by Hampel (2010),117 preventing inclusion of this trial in the meta-analyses. All five trials were rated good quality. For each outcome, results were consistent across trials.
All five trials showed greater improvement in congestion with combination therapy than with intranasal corticosteroid monotherapy. In three trials, including Hampel (2010),117 treatment effects were statistically significant and ranged from 0.3 to 0.6 on a 0-6 point scale (from 5 percent to 10 percent of maximum score). The pooled effect was 0.16 on a 0-6 point scale (95 percent CI: 0.02 to 0.30), a statistically significant result favoring combination therapy (Figure 21). The larger bound of the 95 percent CI represented 5 percent of maximum score. Statistical heterogeneity was low (I2=17 percent, p=0.31).

Figure 21
Congestion at 2 weeks meta-analysis: combination intranasal corticosteroid plus nasal antihistamine versus intranasal corticosteroid.
For the outcome of congestion, the risk of bias was rated as low based on the quality of the trials. Statistical heterogeneity of a meta-analysis of four trials115, 121 was low, and the pooled effect was consistent with the effect reported in the one trial117 not included in the meta-analysis. The 95 percent CI for the pooled effect (0.02 to 0.30) fell within an interval bounded by −MCID and +MCID (-1.8 and +1.8 on the 0-6 point scale used). The Hampel (2010) trial117 reported a treatment effect of 0.38 on a 0-6 point scale (6 percent of maximum score) favoring combination therapy. If this trial were included in the meta-analysis, the pooled effect (0.16; 3 percent of maximum score) would increase. Because the trial represented only 15 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include an MCID of 30 percent maximum score. The body of evidence supporting a conclusion of equivalence of combination therapy and intranasal corticosteroid for this outcome was therefore considered precise. The overall strength of evidence for this conclusion is high.
All five trials showed greater improvement in rhinorrhea with combination therapy than with intranasal corticosteroid monotherapy. The treatment effect (0.25 on a 0-6 point scale; 4 percent of maximum score) was statistically significant in only one trial (Carr, Trial 1115). The pooled effect was 0.14 on a 0-6 point scale (95 percent CI: 0.01 to 0.28), a statistically significant result favoring combination therapy (Figure 22). The larger bound of the 95 percent CI represented 5 percent of maximum score. Statistical heterogeneity was low (I2=0 percent, p=0.74).

Figure 22
Rhinorrhea at 2 weeks meta-analysis: combination intranasal corticosteroid plus nasal antihistamine versus intranasal corticosteroid.
For the outcome of rhinorrhea, the risk of bias was rated as low based on the quality of the trials. Statistical heterogeneity of a meta-analysis of four trials115, 121 was low, and the pooled effect was consistent with the effect reported in the one trial117 not included in the meta-analysis. The 95 percent CI for the pooled effect (0.01 to 0.28) fell within an interval bounded by −MCID and +MCID (-1.8 and +1.8 on the 0-6 point scale used). The Hampel (2010) trial117 reported a treatment effect of 0.27 on a 0-6 point scale (5 percent of maximum score) favoring combination therapy. If this trial were included in the meta-analysis, the pooled effect (0.14; 2 percent of maximum score) would increase. Because the trial represented only 15 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include an MCID of 30 percent maximum score. The body of evidence supporting a conclusion of equivalence of combination therapy and intranasal corticosteroid for this outcome was therefore considered precise. The overall strength of evidence for this conclusion is high.
All five trials showed greater improvement in sneezing with combination therapy than with intranasal corticosteroid monotherapy. In four trials, including Hampel (2010),117 treatment effects were statistically significant and ranged from 0.2 to 0.6 on a 0-6 point scale (from 3 percent to 10 percent of maximum score). The pooled effect was 0.22 on a 0-6 point scale (95 percent CI: 0.07 to 0.36), a statistically significant result favoring combination therapy (Figure 23). The larger bound of the 95 percent CI represented 6 percent of maximum score. Statistical heterogeneity was low (I2=0 percent, p=0.50).

Figure 23
Sneezing at 2 weeks meta-analysis: combination intranasal corticosteroid plus nasal antihistamine versus intranasal corticosteroid.
For the outcome of sneezing, the risk of bias was rated as low based on the quality of the trials. Statistical heterogeneity of a meta-analysis of four trials115, 121 was low, and the pooled effect was consistent with the effect reported in the one trial117 not included in the meta-analysis. The 95 percent CI for the pooled effect (0.07 to 0.36) fell within an interval bounded by −MCID and +MCID (-1.8 and +1.8 on the 0-6 point scale used). The Hampel (2010) trial117 reported a treatment effect of 0.49 on a 0-6 point scale (8 percent of maximum score) favoring combination therapy. If this trial were included in the meta-analysis, the pooled effect (0.22; 4 percent of maximum score) would increase. Because the trial represented only 15 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include an MCID of 30 percent maximum score. The body of evidence supporting a conclusion of equivalence of combination therapy and intranasal corticosteroid for this outcome was therefore considered precise. The overall strength of evidence for this conclusion is high.
All five trials showed greater improvement in nasal itch with combination therapy than with intranasal corticosteroid monotherapy. In two trials, one of which was Hampel (2010),117 treatment effects of 0.31 and 0.6 on a 0-6 point scale (5 percent and 10 percent of maximum score, respectively) were statistically significant. The pooled effect was 0.10 on a 0-6 point scale (95 percent CI: -0.03 to 0.23), a statistically nonsignificant result favoring combination therapy (Figure 24). The larger bound of the 95 percent CI represented 4 percent of maximum score. Statistical heterogeneity was low (I2=0 percent; p=0.39).

Figure 24
Nasal itch at 2 weeks meta-analysis: combination intranasal corticosteroid plus nasal antihistamine versus intranasal corticosteroid.
For the outcome of nasal itch, the risk of bias was rated as low based on the quality of the trials. Statistical heterogeneity of a meta-analysis of four trials115, 121 was low, and the pooled effect was consistent with the effect reported in the one trial117 not included in the meta-analysis. The 95 percent CI for the pooled effect (-0.03 to 0.23) fell within an interval bounded by −MCID and +MCID (-1.8 and +1.8 on the 0-6 point scale used). The Hampel (2010) trial117 reported a treatment effect of 0.31 on a 0-6 point scale (5 percent of maximum score) favoring combination therapy. If this trial were included in the meta-analysis, the pooled effect (0.10; 2 percent of maximum score) would increase. Because the trial represented only 15 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include an MCID of 30 percent maximum score. The body of evidence supporting a conclusion of equivalence of combination therapy and intranasal corticosteroid for this outcome was therefore considered precise. The overall strength of evidence for this conclusion is high.
All five trials showed statistically significant improvements in TNSS with combination therapy. Treatment effects ranged from 0.6 to 2.2 on a 0-24 point scale (from 3 percent to 9 percent of maximum score). The pooled effect was 0.61 on a 0-24 point scale (95 percent CI: 0.15 to 1.08), a statistically significant result favoring combination therapy (Figure 25). The larger bound of the 95 percent CI represented 5 percent of maximum score. Statistical heterogeneity was low (I2=0 percent, p=0.46).

Figure 25
Total nasal symptom score at 2 weeks meta-analysis: combination intranasal corticosteroid plus nasal antihistamine versus intranasal corticosteroid.
For TNSS, the risk of bias was rated as low based on the quality of the trials. Effect estimates were precise. Statistical heterogeneity of a meta-analysis of four trials115, 121 was low, and the pooled effect was consistent with the effect reported in the one trial117 not included in the meta-analysis. The 95 percent CI for the pooled effect (0.15 to 1.08) fell within an interval bounded by −MCID and +MCID (-7.2 and +7.2 on the 0-24 point scale used). The Hampel (2010) trial117 reported a treatment effect of 1.47 on a 0-24 point scale (6 percent of maximum score) favoring combination therapy. If this trial were included in the meta-analysis, the pooled effect (0.61; 3 percent of maximum score) would increase. Because the trial represented only 15 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include an MCID of 30 percent maximum score. The body of evidence supporting a conclusion of equivalence of combination therapy and intranasal corticosteroid for this outcome was therefore considered precise. The overall strength of evidence for this conclusion is high.
Eye Symptoms
Four115, 117 trials that assessed eye symptoms at 2 weeks (total N=2000) showed greater improvements in TOSS with combination therapy than with intranasal corticosteroid monotherapy. In two trials, one of which was Hampel (2010),117 treatment effects of 0.45 and 0.88 on a 0-18 point scale (3 percent and 5 percent of maximum score, respectively) were statistically significant. The pooled effect from a meta-analysis of three trials115 (85 percent of patients reporting this outcome; Hampel [2010]117 excluded) was 0.48 on a 0-18 point scale (95 percent CI: 0.07 to 0.90), a statistically significant result favoring combination therapy (Figure 26). The larger bound of the 95 percent CI represented 5 percent of maximum score. Statistical heterogeneity was low (I2=21%, p=0.28).

Figure 26
Total ocular symptom score at 2 weeks meta-analysis: combination intranasal corticosteroid plus nasal antihistamine versus intranasal corticosteroid.
For TOSS, the risk of bias was rated as low based on the quality of the trials. Statistical heterogeneity of a meta-analysis of three trials115 was low, and the pooled effect was consistent with the effect reported in the one trial117 not included in the meta-analysis. The 95 percent CI for the pooled effect (0.07 to 0.90) fell within an interval bounded by −MCID and +MCID (-5.4 and +5.4 on the 0-18 point scale used). The Hampel (2010) trial117 reported a treatment effect of 0.45 on a 0-18 point scale (3 percent of maximum score) favoring combination therapy. If this trial were included in the meta-analysis, the pooled effect (0.48; 3 percent of maximum score) would decrease slightly. Because the trial represented only 15 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include an MCID of 30 percent maximum score. The body of evidence supporting a conclusion of equivalence of combination therapy and intranasal corticosteroid for this outcome was therefore considered precise. The overall strength of evidence for this conclusion is high.
Quality of Life
Both trials117, 121 that assessed quality of life (total N=408) showed greater improvement in RQLQ scores with combination therapy than with intranasal corticosteroid monotherapy. Treatment effects were 0.17 and 0.45, neither of which was statistically significant. Treatment effects did not exceed the MCID of 0.5 points.
For RQLQ, the risk of bias was rated low based on the quality of the trials. Results were consistent across trials, but effects were statistically and clinically nonsignificant, that is, imprecise. The evidence was insufficient to support the use of one treatment over the other for this outcome.
Combination Intranasal Corticosteroid Plus Nasal Antihistamine Versus Nasal Antihistamine
Description of Included Studies
Five115, 117, 121 multicenter, RCTs published between 2008 and 2012 were identified (total N=2101). All were 2-week, double-blinded trials based in North America. Trial size ranged from 101 to 893 patients randomized to treatment groups of interest. In all five trials, the nasal antihistamine was azelastine, and the intranasal corticosteroid was fluticasone propionate. Three trials115 from the same article used a newly approved combination product comprising both drugs, and two trials117, 121 used a separate nasal inhaler for each drug in the combination. All five trials were industry funded.
The mean age of trial participants ranged from 36 to 40 years. Most participants were female (approximately 62 percent). The majority of patients were white (minimum 74 percent). Of two trials117, 121 that reported the proportions of other races, one121 included approximately 15 percent Hispanic patients. All trials required a minimum duration and severity of SAR symptoms. Mean SAR duration ranged from 16 to 22 years. Mean baseline nasal symptoms were in the severe range.
All five trials assessed both individual and total nasal symptoms. Four115, 117 of five assessed eye symptoms, and two117, 121 assessed quality of life. No trial assessed asthma outcomes. In all five trials, patients rated symptoms twice daily. Individual nasal symptoms (congestion, rhinorrhea, sneezing, and itching) and eye symptoms (itching, tearing, and redness) were rated on a scale from 0 (no symptoms) to 3 (severe symptoms). Morning and evening scores were summed to give a maximum score of 6 for each individual symptom. TNSS ranged from 0 to 24, and TOSS ranged from 0 to 18. The RQLQ was used to assess quality of life. Scores range from 0 (no impairment) to 6 (severe impairment). The MCID is 0.5 points.
All five trials were rated good quality.
Key Points
These results are summarized in Table 51.
Table 51
Strength of evidence: combination intranasal corticosteroid plus nasal antihistamine versus nasal antihistamine.
- Individual nasal symptoms (congestion, rhinorrhea, sneezing, and itch), TNSS and TOSS at 2 weeks: High strength evidence for equivalence of combination intranasal corticosteroid plus nasal antihistamine and nasal antihistamine monotherapy based on five trials115, 117, 121 (for individual nasal symptoms and TNSS) and four trials115, 117 (for TOSS) with low risk of bias and consistent, precise results.
- These results are based on trials using one of eight intranasal corticosteroids (12.5 percent) and one of two nasal antihistamines (50 percent).
Synthesis and Strength of Evidence
Nasal symptom outcomes discussed below are summarized in Table 52, eye symptom outcomes in Table 53, and quality of life outcomes in Table 54.
Table 52
Treatment effects: nasal symptoms–combination intranasal corticosteroid plus nasal antihistamine versus nasal antihistamine.
Table 53
Treatment effects: eye symptoms–combination intranasal corticosteroid plus nasal antihistamine versus nasal antihistamine.
Table 54
Treatment effects: quality of life outcomes–combination intranasal corticosteroid plus nasal antihistamine versus nasal antihistamine.
As shown in these tables and noted above, several trials reported on each outcome. Additionally, variance estimates of group-level treatment effects were provided. Thus, meta-analyses were performed for all nasal and eye symptom outcomes.
Nasal Symptoms
All five trials115, 117, 121 assessed four individual nasal symptoms and TNSS at 2 weeks (total N=2101). Four trials115, 121 (85 percent of patients reporting this outcome) were included in meta-analyses for each nasal outcome. Variance estimates necessary for pooling were not reported by Hampel (2010),117 preventing inclusion of this trial in the meta-analyses. All five trials were rated good quality.
All five trials showed statistically significant improvements in congestion with combination therapy compared to nasal antihistamine monotherapy. Treatment effects ranged from 0.2 to 0.6 on a 0-6 point scale (from 3 percent to 10 percent of maximum score). The pooled effect was 0.28 on a 0-6 point scale (95 percent CI: 0.16 to 0.41), a statistically significant result favoring combination therapy (Figure 27). The larger bound of the 95 percent CI represented 7 percent of maximum score. Statistical heterogeneity was low (I2=0 percent, p=0.41).

Figure 27
Congestion at 2 weeks: meta-analysis of 4 trials–combination intranasal corticosteroid plus nasal antihistamine versus nasal antihistamine.
For the outcome of congestion, the risk of bias was rated as low based on the quality of the trials. Statistical heterogeneity of a meta-analysis of four trials115, 121 was low, and the pooled effect was consistent with the effect reported in the one trial117 not included in the meta-analysis. The 95 percent CI for the pooled effect (0.16 to 0.41) fell within an interval bounded by −MCID and +MCID (-1.8 and +1.8 on the 0-6 point scale used). The Hampel (2010) trial117 reported a treatment effect of 0.49 on a 0-6 point scale (8 percent of maximum score) favoring combination therapy. If this trial were included in the meta-analysis, the pooled effect (0.28; 5 percent of maximum score) would increase. Because the trial represented only 15 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include an MCID of 30 percent maximum score. The body of evidence supporting a conclusion of equivalence of combination therapy and nasal antihistamine for this outcome was therefore considered precise. The overall strength of evidence for this conclusion is high.
All five trials showed greater improvement in rhinorrhea with combination therapy than with nasal antihistamine monotherapy. In four trials,115, 117, 121 including Hampel (2010)117 whose results were not pooled, treatment effects were statistically significant and ranged from 0.2 to 0.6 on a 0-6 point scale (3 percent to 10 percent of maximum score). The pooled effect was 0.31 on a 0-6 point scale (95 percent CI: 0.18 to 0.45), a statistically significant result favoring combination therapy (Figure 28). The larger bound of the 95 percent CI represented 8 percent of maximum score. Statistical heterogeneity was low (I2=1 percent, p=0.39).

Figure 28
Rhinorrhea at 2 weeks: meta-analysis of 4 trials–combination intranasal corticosteroid plus nasal antihistamine versus nasal antihistamine.
For the outcome of rhinorrhea, the risk of bias was rated as low based on the quality of the trials. Statistical heterogeneity of a meta-analysis of four trials115, 121 was low, and the pooled effect was consistent with the effect reported in the one trial117 not included in the meta-analysis. The 95 percent CI for the pooled effect (0.18 to 0.45) fell within an interval bounded by −MCID and +MCID (-1.8 and +1.8 on the 0-6 point scale used). The Hampel (2010) trial117 reported a treatment effect of 0.55 on a 0-6 point scale (9 percent of maximum score) favoring combination therapy. If this trial were included in the meta-analysis, the pooled effect (0.31; 5 percent of maximum score) would increase. Because the trial represented only 15 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include an MCID of 30 percent maximum score. The body of evidence supporting a conclusion of equivalence of combination therapy and nasal antihistamine for this outcome was therefore considered precise. The overall strength of evidence for this conclusion is high.
All five trials showed greater improvement in sneezing with combination therapy than with nasal antihistamine monotherapy. In four trials, including Hampel (2010),117 treatment effects were statistically significant and ranged from 0.2 to 0.61 on a 0-6 point scale (from 3 percent to 10 percent of maximum score). The pooled effect was 0.34 on a 0-6 point scale (95 percent CI: 0.20 to 0.48), a statistically significant result favoring combination therapy (Figure 29). The larger bound of the 95 percent CI represented 6 percent of maximum score. Statistical heterogeneity was low (I2=0 percent, p=0.74).

Figure 29
Sneezing at 2 weeks: meta-analysis of 4 trials–combination intranasal corticosteroid plus nasal antihistamine versus nasal antihistamine.
For the outcome of sneezing, the risk of bias was rated as low based on the quality of the trials. Statistical heterogeneity of a meta-analysis of four trials115, 121 was low, and the pooled effect was consistent with the effect reported in the one trial117 not included in the meta-analysis. The 95 percent CI for the pooled effect (0.20 to 0.48) fell within an interval bounded by −MCID and +MCID (-1.8 and +1.8 on the 0-6 point scale used). The Hampel (2010) trial117 reported a treatment effect of 0.61 on a 0-6 point scale (10 percent of maximum score) favoring combination therapy. If this trial were included in the meta-analysis, the pooled effect (0.34; 6 percent of maximum score) would increase. Because the trial represented only 15 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include an MCID of 30 percent maximum score. The body of evidence supporting a conclusion of equivalence of combination therapy and nasal antihistamine for this outcome was therefore considered precise. The overall strength of evidence for this conclusion is high.
All five trials showed greater improvement in nasal itch with combination therapy than with nasal antihistamine monotherapy. In three trials, including Hampel (2010),117 treatment effects were statistically significant and ranged from 0.3 to 0.8 on a 0-6 point scale (from 5 percent to 13 percent of maximum score). The pooled effect was 0.30 on a 0-6 point scale (95 percent CI: 0.12 to 0.48), a statistically significant result favoring combination therapy (Figure 30). The larger bound of the 95 percent CI represented 8 percent of maximum score. Statistical heterogeneity was low to moderate (I2=34%) but not statistically significant (p=0.21). The 95 percent CI of one of the trials (21 percent of the pooled sample) included zero (-0.48 to 0.08).

Figure 30
Nasal itch at 2 weeks: meta-analysis of 4 trials–combination intranasal corticosteroid plus nasal antihistamine versus nasal antihistamine.
For the outcome of nasal itch, the risk of bias was rated as low based on the quality of the trials. Statistical heterogeneity of a meta-analysis of four trials115, 121 was low to moderate, and the pooled effect was consistent with the effect reported in the one trial117 not included in the meta-analysis. The 95 percent CI for the pooled effect (0.12 to 0.48) fell within an interval bounded by −MCID and +MCID (-1.8 and +1.8 on the 0-6 point scale used). The Hampel (2010) trial117 reported a treatment effect of 0.40 on a 0-6 point scale (7 percent of maximum score) favoring combination therapy. If this trial were included in the meta-analysis, the pooled effect (0.30; 5 percent of maximum score) would increase. Because the trial represented only 15 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include an MCID of 30 percent maximum score. The body of evidence supporting a conclusion of equivalence of combination therapy and nasal antihistamine for this outcome was therefore considered precise. The overall strength of evidence for this conclusion is high.
All five trials showed statistically significant improvements in TNSS with combination therapy. Treatment effects ranged from 0.7 to 2.6 on a 0-24 point scale (from 3 percent to 11 percent of maximum score). The pooled effect was 1.28 on a 0-24 point scale (95 percent CI: 0.82 to 1.74), a statistically significant result favoring combination therapy (Figure 31). The larger bound of the 95 percent CI represented 7 percent of maximum score. Statistical heterogeneity was low (I2=0 percent, p=0.54).

Figure 31
Total nasal symptom score at 2 weeks: meta-analysis of 4 trials–combination intranasal corticosteroid plus nasal antihistamine versus nasal antihistamine.
For TNSS, the risk of bias was rated as low based on the quality of the trials. Statistical heterogeneity of a meta-analysis of four trials115, 121 was low, and the pooled effect was consistent with the effect reported in the one trial117 not included in the meta-analysis. The 95 percent CI for the pooled effect (0.82 to 1.74) fell within an interval bounded by −MCID and +MCID (-7.2 and +7.2 on the 0-24 point scale used). The Hampel (2010) trial117 reported a treatment effect of 2.06 on a 0-24 point scale (9 percent of maximum score) favoring combination therapy. If this trial were included in the meta-analysis, the pooled effect (1.28; 5 percent of maximum score) would increase. Because the trial represented only 15 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include an MCID of 30 percent maximum score. The body of evidence supporting a conclusion of equivalence of combination therapy and nasal antihistamine for this outcome was therefore considered precise. The overall strength of evidence for this conclusion is high.
Eye Symptoms
Four115, 117 trials that assessed eye symptoms at 2 weeks (total N=2000) showed greater improvements in TOSS with combination therapy than with nasal antihistamine monotherapy. Treatment effects ranged from 0.03 to 0.71 on a 0-18 point scale (from less than 1 percent to 4 percent of maximum score), but effects were either statistically nonsignificant or statistical significance was not reported. The pooled effect from a meta-analysis of three trials (85 percent of patients reporting this outcome; Hampel [2010]117 excluded) was 0.25 on a 0-18 point scale (95 percent CI: -0.12 to 0.61), a statistically nonsignificant result favoring combination therapy (Figure 32). The larger bound of the 95 percent CI represented 3 percent of maximum score. Statistical heterogeneity was low (I2=0 percent, p=0.37).

Figure 32
Total ocular symptom score at 2 weeks: meta-analysis of 4 trials–combination intranasal corticosteroid plus nasal antihistamine versus nasal antihistamine.
For TOSS, the risk of bias was rated as low based on the quality of the trials. Statistical heterogeneity of a meta-analysis of three trials115 was low, and the pooled effect was consistent with the effect reported in the one trial117 not included in the meta-analysis. The 95 percent CI for the pooled effect (-0.12 to 0.61) fell within an interval bounded by −MCID and +MCID (-5.4 and +5.4 on the 0-18 point scale used). The Hampel (2010) trial117 reported a treatment effect of 0.71 on a 0-18 point scale (4 percent of maximum score) favoring combination therapy. If this trial were included in the meta-analysis, the pooled effect (0.25; 1 percent of maximum score) would increase. Because the trial represented only 15 percent of patients reporting this outcome, it is unlikely that the 95 percent CI of the pooled effect would include an MCID of 30 percent maximum score. The body of evidence supporting a conclusion of equivalence of combination therapy and nasal antihistamine for this outcome was therefore considered precise. The overall strength of evidence for this conclusion is high.
Quality of Life
Both trials117, 121 that assessed quality of life showed statistically significant improvement in RQLQ scores with combination therapy. The larger trial117 (n=459; 75 percent of patients reporting this outcome) showed a treatment effect of 0.43. The smaller trial121 showed a treatment effect of 0.71. The latter result exceeds the MCID for the RQLQ of 0.5 points.
For the outcome of quality of life, the risk of bias was rated as low based on the quality of the trials. Effect estimates were consistent across trials but not precise. Evidence to support the use of one treatment over the other for this outcome is insufficient.
Combination Oral Selective Antihistamine Plus Oral Decongestant Versus Oral Selective Antihistamine
Description of Included Studies
Seven101-107 multicenter, RCTs published between 1995 and 2009 were identified (N=3575). All were double-blinded, 2-week trials. Six101, 102, 104-107 were conducted in North America, and one103 in Europe. Trial size ranged from 398 to 744 patients randomized to treatment groups of interest. Oral selective antihistamines studied were desloratadine in four trials102, 104-106 and fexofenadine,107 cetirizine,103 and loratadine101 in one trial each. Pseudoephedrine was the decongestant in all seven trials. Five trials101, 104-107 were industry funded, and two102, 103 did not report funding.
Mean ages of patients ranged from 30 to 37 years. Most patients were female (50 percent to 70 percent), and most were white (80 percent to 87 percent). The mean duration of SAR symptoms ranged from 9 to 19 years. All trials required a minimum duration and severity of SAR symptoms. Mean baseline nasal congestion scores were in the moderate to severe range.
All seven trials assessed nasal congestion. Two trials103, 107 also assessed rhinorrhea, sneezing, and eye symptoms, and one103 assessed nasal itch. In six trials,101-106 patients rated symptom severity on 0 (no symptoms) to 3 (severe symptoms) scale. In the one trial101 that reported on TNSS, individual nasal symptom scores were summed for a 0-12 point TNSS scale. One trial107 used a 5-point (0 = no symptoms, 4 = very severe symptoms) scale. Of the two trials reporting on eye symptoms, one103 assessed only ocular itching using a 4-point (0-3) symptom rating scale. The other trial107 assessed ocular itching, tearing, and redness using the 5-point (0-4) scale.
Three trials101, 103, 107 were rated good quality (37 percent of all patients), one106 was fair (19 percent), and three102, 104, 105 were poor (44 percent).
Key Points
Results discussed below are summarized in Table 55.
Table 55
Strength of evidence: combination oral selective antihistamine plus oral decongestant versus oral selective antihistamine.
- These results are based on trials using four of five oral selective antihistamines (80 percent) and one of two oral decongestants (50 percent).
Synthesis and Strength of Evidence
Nasal symptom outcomes discussed below are summarized in Table 56 and eye symptom outcomes in Table 57. Although several authors reported on the outcome of nasal congestion, none provided variance estimates of group-level treatment effects. Thus, meta-analysis was not possible.
Table 56
Treatment effects: nasal symptoms–combination oral selective antihistamine plus oral decongestant versus oral selective antihistamine.
Table 57
Treatment effects: eye symptoms–combination oral selective antihistamine plus oral decongestant versus oral selective antihistamine.
Nasal Symptoms
All seven trials101-107 assessed congestion at 2 weeks (total N=3575). All seven showed statistically significant improvements in nasal congestion with combination therapy. Three101, 103, 107 were good quality trials of 1329 patients total (37 percent of patients reporting this outcome). Two101, 103 showed treatment effects of 0.2 and 0.25 on a 0-3 point scale (7 percent and 8 percent of maximum score, respectively). One fair quality trial106 (n=676, 19 percent of patients reporting) showed a treatment effect of 0.2 on a 0-3 point scale (7 percent of maximum score). Three trials102, 104, 105 were rated poor quality due to inappropriate analysis of results (not intention to treat). Treatment effects reported by these trials ranged from 0.16 to 0.27 on a 0-3 point scale (from 5 percent to 9 percent of maximum score).
For the outcome of nasal congestion at 2 weeks, the risk of bias was assessed as medium. Forty-four percent of patients were in poor quality trials, and 37 percent were in good quality trials. Treatment effects consistently favored combination therapy in all trials. Although statistically significant, no treatment effect exceeded an MCID of 30 percent maximum score. The body of evidence was therefore considered imprecise. Evidence was insufficient to support the use of one treatment over the other for the treatment of congestion.
Two103, 107 of seven trials assessed rhinorrhea at 2 weeks (total N=891). Both trials were large (approximately 450 patients in each), and both were rated good quality. Both favored combination therapy over oral selective antihistamine monotherapy for this outcome. Treatment effects were 0.1 and 0.13 on a 0-3 point scale (3 percent and 4 percent of maximum score, respectively); the latter was statistically significant.
For the outcome of rhinorrhea at 2 weeks, the risk of bias was assessed as low based on the quality of the trials. Treatment effects were consistent but imprecise. The evidence was insufficient to support the use of one treatment over the other for this outcome.
Two103, 107 of seven trials assessed sneezing at 2 weeks (total N=891). Both trials were large (approximately 450 patients in each), and both were rated good quality. Both favored combination therapy over oral selective antihistamine monotherapy. Treatment effects were 0.08 and 0.1 on a 0-3 point scale (both 3 percent of maximum score); the former was statistically significant.
For the outcome of sneezing at 2 weeks, the risk of bias was assessed as low based on the quality of the trials. Treatment effects were consistent but imprecise. The evidence was insufficient to support the use of one treatment over the other for this outcome.
One good quality trial103 assessed nasal itch at 2 weeks (N=458). The treatment effect (0.1 on a 0-3 point scale; 3 percent of maximum score) favored combination therapy and was statistically significant.
For the outcome of nasal itch at 2 weeks, the risk of bias was rated as low based on the quality of the trial. Consistency of results could not be assessed in a single trial, and the effect estimate was imprecise. The evidence was insufficient to support the use of one treatment over the other for this outcome.
One good quality trial101 assessed TNSS at 2 weeks (N=438). The treatment effect (0.6 on a 0-3 point scale; 20 percent of maximum score) favored combination therapy and was statistically nonsignificant.
For TNSS at two weeks, the risk of bias was rated as low based on the quality of the trial. Consistency could not be assessed in a single trial, and the effect estimate was imprecise. Evidence was insufficient to support the use of one treatment over the other for this outcome.
Eye Symptoms
Two good quality trials103, 107 assessed eye symptoms at 2 weeks (total N=891). One trial103 assessed ocular itching, and the other107 assessed TOSS comprising ocular itching, tearing, and redness. The treatment effect for ocular itch103 was 0.01 on a 0-3 point scale (less than 1 percent of maximum score), a statistically nonsignificant result that favored oral selective antihistamine monotherapy. The treatment effect for TOSS107 was 0.1 on a 0-4 point scale (3 percent of maximum score), favoring combination therapy. Statistical significance was not reported.
For eye symptoms at 2 weeks, the risk of bias was low based on the quality of the trials. Treatment effect estimates were inconsistent and imprecise. Evidence was insufficient to support the use of one treatment over the other for this outcome.
Key Question 2. Comparative Adverse Effects of Treatments in Adults and Adolescents 12 Years of Age or Older
Oral Selective Antihistamine Versus Oral Nonselective Antihistamine
Key Points
- Evidence from three poor quality trials was insufficient to support the use of either oral selective or nonselective antihistamine to avoid sedation or headache.
Synthesis and Evidence Assessment
All three trials81-83 (N=515) that reported efficacy outcomes also reported adverse events. Table 58 displays the risk differences and elements for the synthesis of evidence for this comparison.
Table 58
Strength of evidence: comparative adverse events for oral selective antihistamine versus oral nonselective antihistamine.
All three trials reported sedation. In two81, 82 of these, risk differences favored selective antihistamine to avoid moderate sedation (13 percent81) and unspecified severity sedation (28.9 percent82). Both results were statistically significant. Statistically nonsignificant differences also favored selective antihistamine to avoid severe sedation81 and unspecified severity sedation.83 Risk of bias was considered high in all three trials81-83 due to poor USPSTF rating,81-83 insufficient surveillance for adverse events,83 and lack of patient blinding.82 It is unclear whether effects were reported consistently based on differences in classification schemes across trials. Risk differences were otherwise consistent but imprecise. Forty-one percent of patients were in a trial83 that reported a statistically nonsignificant result. Evidence was insufficient to conclude that either comparator is favored to avoid sedation.
In the two trials81, 83 reporting headache, risk differences favored nonselective antihistamine to avoid headache (1.6 percent and 4.5 percent). Neither result was statistically significant. The risk of bias was considered high based on poor trial quality81 and insufficient adverse event surveillance.83 Risk differences were consistent but imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid headache.
Oral Selective Antihistamine Versus Nasal Antihistamine
Key Points
- Evidence was insufficient to support using either oral or nasal antihistamine to prevent common adverse events of sedation, headache, bitter aftertaste, and nosebleed.
- For bitter aftertaste, it is unclear whether future comparative trials would observe similar effects because all of the included trials used an older formulation of the currently available product. Newer formulations were designed to mitigate this adverse effect.
Synthesis and Evidence Assessment
All four trials84-87 that reported efficacy outcomes also reported adverse events (N=886). Adverse event data also was abstracted from a fifth trial88 (n=30) for this comparison. Table 59 displays the risk differences and elements for the synthesis of evidence for this comparison.
Table 59
Strength of evidence: comparative adverse events for oral selective antihistamine versus nasal antihistamine.
Only one trial reported nasal discomfort87 (risk difference 0.3 percent, favoring oral antihistamine), insomnia87 (reported in nasal antihistamine arm only [0.7 percent]), and hypertension leading to discontinuation85 (risk difference 0.6 percent, favoring oral antihistamine). Synthesis of evidence was not conducted for these outcomes.
Sedation, described as severe or leading to discontinuation, was reported in two trials.85, 88 Risk differences were not statistically significant, but favored oral antihistamine to avoid sedation in both (0.6 percent and 6.7 percent). Unspecified sedation was reported by four trials84-87 with risk differences ranging from 1 percent in favor of oral antihistamine to 5 percent in favor of nasal antihistamine; none were statistically significant. The risk of bias was considered medium. Thirty-seven percent of the patient sample for this adverse event was in three84, 86, 88 trials with poor USPSTF rating86, 88 or inadequate surveillance for adverse events.84, 88 Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid sedation.
Headache was reported by four trials84-87 with risk differences ranging from 1.6 percent in favor of oral antihistamine to 3 percent in favor of nasal antihistamine; none were statistically significant. The risk of bias was considered medium. Thirty-five percent of the patient sample for this adverse event was in two84, 86 trials with poor USPSTF rating86 or inadequate surveillance for adverse events.84 Risk differences were small, inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid headache.
Bitter aftertaste was reported by three trials84, 85, 87 with risk differences ranging from 2.3 percent to 11 percent favoring oral antihistamine to avoid a bitter aftertaste. Risk differences were statistically significant in two of these trials.84, 85 The risk of bias was considered medium. Fifty-six percent of the patient sample for this adverse event was in good quality trials85, 87 that performed active surveillance for adverse events, and 44 percent were in a good quality trial84 that did not perform active surveillance. Risk differences were consistent but not precise. Thirty-five percent of patients were in a trial87 that reported a statistically nonsignificant difference. Evidence was insufficient to conclude that either comparator is favored to avoid a bitter aftertaste. It is important to note that all trials reporting on this outcome used an older formulation of azelastine nasal spray, which was reformulated to address this adverse effect. It is unclear whether future comparative trials would observe similar effects.
Nosebleeds were reported by two trials.85, 87 Risk differences were 0 percent in one85 and 1 percent (not statistically significant) favoring oral antihistamine in the other.87 The risk of bias was considered low. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid nosebleeds.
Oral Selective Antihistamine Versus Intranasal Corticosteroid
Key Points
- Evidence from these trials was insufficient to support the use of either oral selective antihistamine or intranasal corticosteroid to avoid headache or nosebleed.
Synthesis and Evidence Assessment
Six90-93, 95, 99 of 13 trials reporting efficacy outcomes also reported adverse events of interest (N=2038). Table 60 displays the risk differences and elements for the synthesis of evidence for this comparison.
Table 60
Strength of evidence: comparative adverse events for oral selective antihistamine versus intranasal corticosteroids.
One trial (Jordana [1996]95) presented adverse events as percentages of total reports, rather than as percentages of patients. This trial was included in the synthesis of evidence only to assess consistency of effect. This trial was the only one to perform active surveillance for local corticosteroid effects (rhinoscopy). Nasal septal atrophy and nasal candidiasis were not reported. Only one trial90 reported sedation (risk difference, 1 percent, favoring intranasal corticosteroid), nasal burning (0 percent in each group), and nosebleed (1 percent in each group). Synthesis of evidence was not conducted for these outcomes.
Five trials90-93, 99 (N=1796) reported headache. In three trials90-92 the risk difference favored intranasal corticosteroid (1-2 percent, none statistically significant) to avoid headache, and in two93, 99 the risk difference favored oral selective antihistamine (4 percent and 8 percent, neither statistically significant). All but one90 of the five trials was 4 weeks in duration. The risk difference in this 15-day trial90 was 2 percent favoring intranasal corticosteroid to avoid headache. Risk of bias was considered high because of poor USPSTF quality rating in four trials90, 92, 93, 99 and insufficient surveillance for adverse events in the fifth.91 The observed effect was not consistent across trials, even when considering only 4-week trials, and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid headache.
Oral Selective Antihistamine Versus Oral Decongestant
Key Points
- There is moderate strength evidence favoring oral antihistamine rather than oral decongestant to avoid insomnia. This evidence was from four trials,101, 103-105 each with statistically significant differences in the proportion of patients reporting insomnia. The body of evidence was consistent, precise and associated with medium risk of bias.
- Evidence was insufficient to conclude that either oral antihistamine or oral decongestant is favored to avoid sedation, headache or anxiety.
Synthesis and Evidence Assessment
All seven trials101-107 reporting efficacy outcomes also reported adverse events. Table 61 displays the risk differences and elements for the synthesis of evidence for this comparison.
Table 61
Strength of evidence: comparative adverse events for oral selective antihistamine versus oral decongestant.
Two trials, Schenkel (2002)106 and Sussman (1999),107 presented adverse events as percentages of total reports, rather than as percentages of patients. In a third trial,102 it was unclear whether the reporting unit was the patient or an incident event. These three trials were included in the synthesis of evidence only to assess consistency of effect. Only one trial105 reported palpitations (risk difference 2 percent, favoring oral antihistamine to avoid palpitations). Synthesis of evidence was not conducted for this outcome.
Sedation was reported by three trials101, 103, 105 (N=1640) with risk differences ranging from 1 percent in favor of oral antihistamine to 3 percent in favor of oral decongestant; none were statistically significant. The risk of bias was considered medium. Fifty-four percent of the patient sample was in good quality trials101, 103 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid sedation.
Headache was reported by four trials101, 103-105 (N=2038) with risk differences ranging from no difference to 4.9 percent favoring oral antihistamine to avoid headache; none were statistically significant. The risk of bias was considered medium. Fifty-six percent of the patient sample for this adverse event was in two trials104, 105 that had poor USPSTF quality ratings104, 105 or inadequate surveillance for adverse events.105 Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid headache.
Insomnia was reported by four trials101, 103-105 (N=2038) with risk differences ranging from 6 percent to 11.1 percent favoring oral antihistamine to avoid insomnia; all were statistically significant. The risk of bias was considered medium. Fifty-six percent of the patient sample for this adverse event was in two trials104, 105 that had poor USPSTF quality ratings104, 105 or inadequate surveillance for adverse events.105 Risk differences were consistent and precise. To avoid insomnia, there is moderate strength evidence favoring oral selective antihistamine rather than oral decongestant.
Anxiety was reported in three trials101, 103, 105 (N=1640) with risk differences ranging from 2 percent to 3 percent favoring oral antihistamine to avoid anxiety; one result103 was statistically significant. The risk of bias was considered medium. Fifty-four percent of the patient sample for this adverse event was in good quality trials101, 103 that actively ascertained adverse events. Risk differences were consistent but imprecise. Seventy-two percent of the patient sample for this adverse event was in trials101, 105 that reported statistically nonsignificant risk differences. Evidence was insufficient to conclude that either comparator is favored to avoid anxiety.
Oral Selective Antihistamine Versus Oral Leukotriene Receptor Antagonist (Montelukast)
Key Points
- Evidence was insufficient to support the use of either selective oral antihistamine or oral leukotriene receptor antagonist to avoid headache as an adverse outcome. Although the body of evidence included less than half of the trials identified for efficacy, the finding is indirectly supported by the assertions of four other trials97, 109, 113, 114 that adverse events were similar in frequency between trial arms.
Synthesis and Evidence Assessment
Four108, 110-112 of nine trials reporting efficacy outcomes also reported adverse events. Four other trials97, 109, 113, 114 did not report specific events, but included statements suggesting that there were no differences between groups with regard to adverse events. These eight trials were comparable with regard to baseline SAR symptoms (all trials reported baseline nasal symptom scores in the moderate range), and size. One108 was a 4-week trial, and the others were 2 weeks in duration. However, the trials that reported group level adverse events tended to have higher USPSTF quality ratings (three good, and one poor among those reporting group level outcomes, compared with three poor, and two fair among those not reporting group level outcomes). Table 62 displays the risk differences and elements for the synthesis of evidence for this comparison.
Table 62
Strength of evidence: comparative adverse events for oral selective antihistamine versus oral leukotriene receptor antagonist.
Headache was reported by four trials108, 110-112 (N=2215) with risk differences ranging from 1 percent in favor of oral selective antihistamine to 3.4 percent in favor of leukotriene receptor antagonist; none were statistically significant. The risk of bias was considered medium. Fifty-one percent of the patient sample for this adverse event was in two trials110, 112 that had poor USPSTF quality ratings 112 or inadequate surveillance for adverse events,110 and 20 percent was in a good quality trial111 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid headache. This finding is consistent with four trials97, 109, 113, 114 that did not report group level incidences of adverse events but reported no between-group differences.
Intranasal Corticosteroid Versus Nasal Antihistamine
Key Points
- Evidence was insufficient to support the use of either intranasal corticosteroid or nasal antihistamine to avoid any of the following adverse events reported in eight trials: sedation, headache, nasal discomfort, bitter aftertaste, and nosebleeds.
Synthesis and Evidence Assessment
Eight115-119, 121 of nine trials that reported efficacy outcomes also reported adverse events. Table 63 displays the risk differences and elements for the synthesis of evidence for this comparison.
Table 63
Strength of evidence: comparative adverse events for intranasal corticosteroid versus nasal antihistamine.
Two trials119, 121 presented adverse events as a percentage of total reports, rather than as a percentage of patients. These trials were included in the synthesis of evidence only to assess consistency of effect. Only one trial reported burning or dryness116 (risk differences 2 percent, favoring nasal antihistamine to avoid dryness, and 4 percent, favoring intranasal corticosteroids to avoid burning). Synthesis of evidence was not conducted for these outcomes.
Sedation was reported by three trials115, 117, 118 (N=1330) with risk differences ranging from no risk difference to 1.5 percent favoring intranasal corticosteroid to avoid sedation; none were statistically significant. The risk of bias was considered medium. Sixty-seven percent of the patient sample for this adverse event was in a good quality trial115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid sedation.
Headache was reported by four trials115, 117 (N=1998) with risk differences ranging from 0.7 percent in favor of intranasal corticosteroid to 2.6 percent in favor of nasal antihistamine; none were statistically significant. The risk of bias was considered low. Eighty-five percent of the patient sample for this adverse event was in good quality trials115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid headache.
Nasal discomfort was reported by four trials115-117 (N=1153) with risk differences ranging from 8 percent in favor of intranasal corticosteroids to 0.7 percent in favor of nasal antihistamine; none were statistically significant. The risk of bias was considered medium. Sixty-nine percent of the patient sample for this adverse event was in good quality trials115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid nasal discomfort.
Bitter aftertaste was reported by six trials115-117 (N=2178) with risk differences ranging from 2 percent to 6.7 percent favoring intranasal corticosteroid to avoid a bitter aftertaste. Effects were statistically significant in two trials in the same publication.115 The risk of bias was considered medium. Seventy-eight percent of the patient sample for this adverse event was in good quality trials115 that actively ascertained adverse events. Risk differences were consistent but imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid a bitter aftertaste.
Nosebleeds were reported by five trials.115, 117, 118 (N=2128) Risk differences ranged from 4.6 percent in favor of intranasal corticosteroid to 1.9 percent in favor of nasal antihistamine; none were statistically significant. The risk of bias was considered low. Eighty percent of the patient sample for this adverse event was in good quality trials115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid nosebleeds.
Intranasal Corticosteroid Versus Nasal Cromolyn
Key Points
- Evidence was insufficient to support the use of either intranasal corticosteroid or nasal cromolyn to avoid any of the following adverse events: headache, dryness, burning, nasal discomfort, and nosebleeds.
Synthesis and Evidence Assessment
Four trials122-125 (five direct comparisons) that reported efficacy outcomes also reported adverse events. Table 64 displays the risk differences and elements for the synthesis of evidence for this comparison.
Table 64
Strength of evidence: comparative adverse events for intranasal corticosteroid versus nasal cromolyn.
Two trials123, 124 presented adverse events as a percentage of total reports, rather than as a percentage of patients. These trials were included in the synthesis of evidence only to assess consistency of effect.
Headache was reported in two trials122, 125 (three comparisons; N=133) with risk differences ranging from 13.4 percent in favor of intranasal corticosteroid to 4.5 percent in favor of nasal cromolyn; none were statistically significant. The risk of bias was considered high; both trials122, 125 were rated poor quality and one125 had inadequate patient blinding and ascertained adverse events in a passive fashion. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid headache.
Dryness was reported in two trials122, 125 (three comparisons; N=133) with risk differences ranging from 14.5 percent in favor of intranasal corticosteroid to 3.3 percent in favor of nasal cromolyn; none were statistically significant. The risk of bias was considered high; both trials122, 125 were rated poor quality and one125 had inadequate patient blinding and ascertained adverse events in a passive fashion. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid dryness.
Burning was reported in one trial125 (two comparisons; N=90). Risk differences were 3.3 percent for both intranasal corticosteroid groups compared with nasal cromolyn and favored nasal cromolyn to avoid burning. Neither was statistically significant. The risk of bias was considered high; the trial125 was rated poor quality, had inadequate patient blinding, and ascertained adverse events in a passive fashion. Risk differences were consistent but imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid burning.
Nasal discomfort was reported in two trials122, 125 (three comparisons; N=133) with risk differences ranging from 0 percent to 14.3 percent favoring intranasal corticosteroid to avoid nasal discomfort; none were statistically significant. The risk of bias was considered high; both trials122, 125 were rated poor quality and one125 had inadequate patient blinding and ascertained adverse events in a passive fashion. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid nasal discomfort.
Nosebleed was reported in two trials122, 125 (three comparisons; N=133) with risk differences ranging from 3.3 percent in favor of intranasal corticosteroid to 4.5 percent in favor of nasal cromolyn; none were statistically significant. The risk of bias was considered high; both trials122, 125 were rated poor quality and one125 had inadequate patient blinding and ascertained adverse events in a passive fashion. Risk differences were consistent but imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid nasal discomfort.
Intranasal Corticosteroid Versus Oral Leukotriene Receptor Antagonist (Montelukast)
Key Points
Synthesis and Evidence Assessment
Three126, 127, 129 of five trials that reported efficacy outcomes also reported adverse events. The trials97, 128 that did not report adverse events were smaller and included patients with milder symptoms than those that did. Both of these trials were rated poor quality; the three that reported adverse events were rated good quality. Table 65 displays the risk differences and elements for the synthesis of evidence for this comparison.
Table 65
Strength of evidence: comparative adverse events for intranasal corticosteroid versus oral leukotriene receptor antagonist.
Headache was reported by all three trials,126, 127, 129 (N=2014) with risk differences ranging from 0.3 percent to 5 percent favoring intranasal corticosteroid to avoid headache. None of these risk differences were statistically significant. The risk of bias was considered medium. Sixty-three percent of the patient sample for this adverse event was in good quality trials127, 129 that actively ascertained adverse events. Risk differences were consistent but imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid headache.
Nosebleed was reported by all three trials,126, 127, 129 (N=2014) with risk differences ranging from 1 percent in favor of intranasal corticosteroid to 1 percent in favor of oral leukotriene receptor antagonist. None of these risk differences were statistically significant. The risk of bias was considered medium. Sixty-three percent of the patient sample for this adverse event was in good quality trials127, 129 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid nosebleed.
Combination Oral Selective Antihistamine Plus Intranasal Corticosteroid Versus Oral Selective Antihistamine
Key Points
- Adverse event reporting in trials included in the efficacy review for this comparison was inadequate to permit analysis.
Synthesis and Evidence Assessment
Of three trials90, 98, 130 that reported efficacy outcomes, adverse events were assessed in two.90, 98 However, one98 of these reported adverse events in the total trial population rather than by treatment arm. The other90 reported risk differences of 2 percent and 3 percent favoring oral antihistamine monotherapy to avoid burning and nosebleeds, respectively. A risk difference of 4 percent favored combination therapy to avoid headache, and a risk difference of zero was observed for sedation. No differences were statistically significant. The trial used some active adverse event surveillance but was rated poor quality using USPSTF criteria. This single trial provides insufficient evidence to support the use of one treatment over the other to avoid adverse events.
Combination Oral Selective Antihistamine Plus Intranasal Corticosteroid Versus Intranasal Corticosteroid
Key Points
- Adverse event reporting in trials included in the efficacy review for this comparison was inadequate to permit analysis.
Synthesis and Evidence Assessment
All five trials62, 90, 98, 131, 132 that reported efficacy outcomes reported adverse events. However, one131 of these used reports (rather than patients) as denominator, one98 reported adverse events in the total trial population rather than by treatment arm, and two reported adverse events specific to one trial arm only.62, 132 The remaining trial90 reported statistically nonsignificant risk differences of 0 percent for sedation, and 4 percent for headache, both favoring combination therapy. Risk differences of 2 percent and 3 percent for burning and nosebleeds, respectively, favored intranasal corticosteroid monotherapy, and neither was statistically significant. The trial used some active adverse event surveillance but was rated poor quality using USPSTF criteria. This single trial provides insufficient evidence to support the use of one treatment over the other to avoid adverse events.
Combination Intranasal Corticosteroid Plus Nasal Antihistamine Versus Intranasal Corticosteroid
Key Points
- Evidence was insufficient to support using either combination intranasal corticosteroid plus nasal antihistamine or intranasal corticosteroid monotherapy to prevent common adverse events including sedation, headache, nasal discomfort, bitter aftertaste, and nosebleed.
- Three115 of four trials115, 117 reporting bitter aftertaste (85 percent of the patient sample for this adverse event) used a newly approved (May 2012) formulation that includes a corticosteroid and an antihistamine in the same device. It is unlikely that the new formulation impacted observed effects.
Synthesis and Evidence Assessment
All five trials115, 117, 121 that reported efficacy outcomes also reported adverse events. Table 66 displays the risk differences and elements for the synthesis of evidence for this comparison.
Table 66
Strength of evidence: comparative adverse events for combination intranasal corticosteroid plus nasal antihistamine versus intranasal corticosteroid.
One trial121 presented adverse events as a percentage of total reports, rather than as a percentage of patients. This trial was included in the synthesis of evidence only to assess consistency of effect.
Sedation was reported by two trials.115, 117 (N=1802) there was no difference between treatments in one trial,117 and a risk difference of 1.1 percent favoring intranasal corticosteroid monotherapy to avoid sedation in the other trial.115 The 1.1 percent difference was statistically significant. Risk of bias was considered low. Seventy-five percent of the patient sample for this adverse event was in the good quality trial115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid sedation.
Headache was reported by four trials.115, 117 (N=3000) Risk differences ranged from 1.9 percent in favor of combination therapy to 0.5 percent in favor of intranasal corticosteroid monotherapy, and none were statistically significant. Risk of bias was considered low. Eighty-five percent of the patient sample for this adverse event was in good quality trials115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid headache.
Nasal discomfort was reported by three trials.115, 117 (N=1657) Risk differences ranged from no difference to 0.6 percent favoring intranasal corticosteroid monotherapy to avoid nasal discomfort, and none were statistically significant. Risk of bias was considered low. Seventy-two percent of the patient sample for this adverse event was in good quality trials115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid nasal discomfort.
Bitter aftertaste was reported by four trials.115, 117 (N=3000) Risk differences ranged from 1.4 percent to 7.2 percent favoring intranasal corticosteroid monotherapy to avoid a bitter aftertaste. Two of these estimates115, 117 were statistically significant. Risk of bias was considered low. Eighty-five percent of the patient sample for this adverse event was in good quality trials115 that actively ascertained adverse events. Risk differences were consistent but imprecise. Forty percent of the patient sample for this adverse event was in trials115 that reported statistically nonsignificant risk differences. Evidence was insufficient to conclude that either comparator is favored to avoid a bitter aftertaste. Of note, three trials,115 representing 85 percent of the patient sample for this adverse event, used a newly approved (May 2012) formulation that includes a corticosteroid and an antihistamine in the same device. It is unclear whether the new formulation impacted the observed effects.
Nosebleed was reported by four trials.115, 117 (N=3000) Risk differences ranged from 1.4 percent in favor of combination therapy to 0.7 percent in favor of intranasal corticosteroid monotherapy; none were statistically significant. Risk of bias was considered low. Eighty-five percent of the patient sample for this adverse event was in good quality trials115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid nosebleed
Combination Intranasal Corticosteroid Plus Nasal Antihistamine Versus Nasal Antihistamine
Key Points
- Evidence from four trials was insufficient to support using either combination intranasal corticosteroid plus nasal antihistamine or nasal antihistamine monotherapy to avoid common adverse events of sedation, headache, nasal discomfort, bitter aftertaste, and nosebleed.
- Three115 of four trials115, 117 reporting bitter aftertaste (85 percent of the patient sample for this adverse event) used a newly approved (May 2012) formulation that includes a corticosteroid and an antihistamine in the same device. In these three trials, an older version of nasal antihistamine rather than a newer formulation designed to mitigate bitter aftertaste was used as a comparator.
Synthesis and Evidence Assessment
All five trials115, 117, 121 that reported efficacy outcomes also reported adverse events. Table 67 displays the risk differences and elements for the synthesis of evidence for this comparison.
Table 67
Strength of evidence: comparative adverse events for combination intranasal corticosteroid plus nasal antihistamine versus nasal antihistamine.
One trial121 presented adverse events as a percentage of total reports, rather than as a percentage of patients. This trial was included in the synthesis of evidence only to assess consistency of effect.
Sedation was reported by two trials.115, 117 (N=1802) Risk differences were 0 percent and 0.7 percent favoring nasal antihistamine to avoid sedation; neither was statistically significant. Risk of bias was considered low. Seventy-five percent of the patient sample for this adverse event was in a good quality trial115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid sedation.
Headache was reported by four trials.115, 117 (N=3000) Risk differences ranged from 0.7 percent in favor of combination therapy to 1.3 percent in favor of nasal antihistamine; none were statistically significant. Risk of bias was considered low. Eighty-five percent of the patient sample for this adverse event was in good quality trials115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid headache.
Nasal discomfort was reported by three trials.115, 117 (N=1657) Risk differences ranged from 0.9 percent in favor of combination therapy to1.3 percent in favor of nasal antihistamine; none were statistically significant. Risk of bias was considered low. Seventy-two percent of the patient sample for this adverse event was in good quality trials115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid nasal discomfort.
Bitter aftertaste was reported by four trials.115, 117 (N=3000) Risk differences ranged from 5.1 percent in favor of combination therapy to 5.2 percent in favor of nasal antihistamine. Both extremes were statistically significant. Risk of bias was considered low. Eighty-five percent of the patient sample for this adverse event was in good quality trials115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Thirty-five percent of the patient sample for this adverse event was in trials115, 117 that reported imprecise risk differences. Evidence was insufficient to conclude that either comparator is favored to avoid a bitter aftertaste. Of note, three115 of four trials115, 117 reporting bitter aftertaste (85 percent of the patient sample for this adverse event) used a newly approved (May 2012) formulation that includes a corticosteroid and an antihistamine in the same device. In these three trials, an older version of nasal antihistamine rather than a newer formulation designed to mitigate bitter aftertaste was used as a comparator.
Nosebleed was reported by four trials115, 117 (N=3000). Risk differences ranged from 0.9 percent in favor of combination therapy to 1.3 percent in favor of nasal antihistamine; none were statistically significant. Risk of bias was considered low. Eighty-five percent of the patient sample for this adverse event was in good quality trials115 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid nosebleed.
Combination Oral Selective Antihistamine Plus Oral Decongestant Versus Oral Selective Antihistamine
Key Points
- There is moderate strength evidence to support the use of oral selective antihistamine rather than combination oral selective antihistamine plus oral decongestant to avoid insomnia. This evidence was from four 2-week trials,101, 103-105 each with statistically significant differences in the proportion of patients reporting insomnia. The body of evidence was consistent, precise and associated with moderate risk of bias.
- Evidence was insufficient to support using either oral antihistamine or oral decongestant to avoid sedation, headache or anxiety.
Synthesis and Evidence Assessment
All seven trials101-107 that reported efficacy outcomes also reported adverse events. Table 68 displays the risk differences and elements for the synthesis of evidence for this comparison.
Table 68
Strength of evidence: comparative adverse events for oral selective antihistamine plus oral decongestant versus oral selective antihistamine.
Two trials106, 107 presented adverse events as a percentage of total reports, rather than as a percentage of patients. In a third trial102 it was unclear if the reporting unit was the patient or an incident event. These three trials were included in the synthesis of evidence only to assess consistency of effect. Only one trial reported palpitations105 (risk difference 0 percent). One trial106 reported chest pain in 0.3 percent of reports in the combination arm only. Synthesis of evidence was not conducted for these outcomes.
Sedation was reported by three trials101, 103, 105 (N=1640) with risk differences ranging from 2 percent in favor of oral selective antihistamine monotherapy to 3 percent in favor of combination therapy; no differences were statistically significant. The risk of bias was considered medium. Fifty-five percent of the patient sample for this adverse event was in good quality trials101, 103 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid sedation.
Headache was reported by four trials101, 103-105 (N=2038) with risk differences ranging from 2 percent in favor of oral selective antihistamine monotherapy to 2.8 percent in favor of combination therapy. No estimates were statistically significant. The risk of bias was considered medium. Fifty-six percent of the patient sample for this adverse event was in poor quality trials,104, 105 one of which also had inadequate surveillance for adverse events,105 and forty-four percent was in good quality trials101, 103 that actively ascertained adverse events. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid headache.
Insomnia was reported by four trials101, 103-105 (N=2038) with risk differences ranging from 4 percent to 11.1 percent favoring oral antihistamine monotherapy to avoid insomnia; all were statistically significant. The risk of bias was considered medium. Fifty-six percent of the patient sample for this adverse event was in poor quality trials,104, 105 one of which also had inadequate surveillance for adverse events,105 and forty-four percent was in good quality trials101, 103 that actively ascertained adverse events. Risk differences were consistent and precise. To avoid insomnia, there is moderate strength evidence to support the use of oral antihistamine rather than oral decongestant.
Anxiety was reported by three trials101, 103, 105 (N=1640) with risk differences ranging from 4 percent in favor of oral selective antihistamine monotherapy to 2.2 percent in favor of combination therapy; both extremes101, 103 were statistically significant. The risk of bias was considered medium. Fifty-five percent of the patient sample for this adverse event was in good quality trials101, 103 that actively ascertained adverse events, and 45 percent was in a poor quality trial105 that ascertained adverse events in a passive fashion. Risk differences were inconsistent and imprecise. Evidence was insufficient to conclude that either comparator is favored to avoid anxiety.
Key Question 3. Comparative Effectiveness and Adverse Effects of Treatments in Pregnant Women
For the identified comparisons of interest, no comparative trials, observational studies, meta-analyses, or systematic reviews met our inclusion criteria of directly comparing two drug classes used in pregnant women with SAR. We were unable to assess comparative effectiveness and harms of SAR treatments in pregnant women.
Key Question 4. Comparative Effectiveness and Harms of SAR Treatments in Children Younger Than 12 Years of Age
Of 21 treatment comparisons of interest for children, studies that met our inclusion criteria were identified for one, oral selective antihistamine versus oral nonselective antihistamine. For all comparisons, we considered inclusion of studies that reported results for adults and children mixed together. Eight trials that met all other inclusion criteria were identified.136-143 However, none of these trials provided subgroup analysis by age. Because mixed results would not inform the answer to this Key Question, these studies were not included.
Oral Selective Antihistamine Versus Oral Nonselective Antihistamine
Description of Included Studies
Two RCTs133, 134 published in 1989 and 1996 were identified. One134 was a multicenter, 2-week trial in North America (N=126). The other133 was a 2-week trial in Europe (N=40). The selective antihistamines were cetirizine and loratadine, and the nonselective antihistamines were chlorpheniramine and dexchlorpheniramine. One trial134 was open-label, and one133 was assessor-blinded only. One trial134 was industry-funded, and the other133 did not report funding source.
The average age of patients was 8.6 years. In both trials, more than 60 percent of patients were male (63 percent to 70 percent). One trial134 reported information on race, and 82 percent were white. Neither trial required a minimum duration of SAR history; the mean duration of SAR ranged from three to six years. Although both trials required a minimum severity of SAR symptoms, no baseline symptom scores were reported.
The open-label trial134 assessed individual nasal (congestion and sneezing) and eye (itching and watering) symptoms using a four-point rating scale (0=no symptoms, 3=severe symptoms). The other trial133 reported only change in total symptom score, comprising both nasal and eye symptoms. This was not a prespecified outcome of interest and was not abstracted. Neither trial assessed asthma outcomes.
Both trials were rated poor quality.
Effectiveness: Key Points
These results are summarized in Table 69.
Table 69
Strength of evidence: oral selective antihistamine versus oral nonselective antihistamine in children.
- Nasal congestion and sneezing at 2 weeks: Evidence was insufficient to support the use of one treatment over the other based on a single trial with high risk of bias and imprecise results.
- Ocular itching and tearing: Evidence was insufficient to support the use of one treatment over the other based on a single trial with high risk of bias and imprecise results.
- These results are based on trials using one of five oral selective antihistamines (20 percent) and one of twelve oral nonselective antihistamines (eight percent).
Effectiveness: Detailed Synthesis
Nasal symptom outcomes discussed below are summarized in Table 70, and eye symptom outcomes in Table 71.
Table 70
Treatment effects: nasal symptoms–oral selective antihistamine versus oral nonselective antihistamine in children.
Table 71
Treatment effects: ocular symptoms–oral selective antihistamine versus oral nonselective antihistamine in children.
Nasal Symptoms
One134 of two trials (N=126) assessed nasal congestion and sneezing at 2 weeks. For nasal congestion, there was a statistically nonsignificant treatment effect of 0.1 on a 0-3 point scale (3 percent of maximum score) that favored nonselective antihistamine. For sneezing, no treatment difference was reported. The trial was rated poor quality due to lack of blinding; therefore, risk of bias was high. Treatment effects for both nasal symptoms were imprecise. The evidence was insufficient to support the use of one treatment over the other for either outcome.
Eye Symptoms
One134 of two trials (N=126) assessed ocular itching and tearing. For both outcomes, treatment effects were 0.1 on a 0-3 point scale (3 percent of maximum score). Both favored nonselective antihistamine, but neither was statistically significant. The trial was rated poor quality due to lack of blinding; therefore, risk of bias was high. Treatment effects for both ocular symptoms were imprecise. The evidence was insufficient to support the use of one treatment over the other for either outcome.
Harms: Key Points
Evidence from two trials133, 134 was insufficient to support using either oral selective antihistamine or nonselective antihistamine to avoid the adverse event of sedation.
Harms: Synthesis and Evidence Assessment
Both trials133, 134 reported harms (N=165). Risk differences and elements for the evidence synthesis are displayed in Table 72.
Table 72
Risk differences and strength of evidence for harms–oral selective antihistamine versus oral nonselective antihistamine in children.
Both trials reported sedation (N=165 patients assessed for harms). In one trial,133 there was a statistically significant risk difference of 21.1 percent favoring oral selective antihistamine. This trial was rated poor quality due to lack of patient blinding. Assessors also were unblinded, and harms ascertainment was only partially active. In the other trial,134 the risk difference was 4.3 percent favoring oral selective antihistamine, but this was not statistically significant. This trial was rated poor quality due to lack of blinding and inappropriate analysis of results (not intention to treat). In addition, harms ascertainment was passive.
Based on the quality of the trials, the risk of bias was considered high. Risk differences were consistent but imprecise. Evidence was insufficient to conclude that one treatment is favored to avoid sedation.
- Results of Literature Searches
- Overview
- How This Section Is Organized
- Comparative Effectiveness of SAR Treatments in Adults and Adolescents 12 Years of Age or Older
- Comparative Adverse Effects of Treatments in Adults and Adolescents 12 Years of Age or Older
- Comparative Effectiveness and Adverse Effects of Treatments in Pregnant Women
- Comparative Effectiveness and Harms of SAR Treatments in Children Younger Than 12 Years of Age
- Results - Treatments for Seasonal Allergic RhinitisResults - Treatments for Seasonal Allergic Rhinitis
- Peer Reviewers - Transitions of Care From Pediatric to Adult Services for Childr...Peer Reviewers - Transitions of Care From Pediatric to Adult Services for Children With Special Healthcare Needs
- Methods - Living Systematic Review on Cannabis and Other Plant-Based Treatments ...Methods - Living Systematic Review on Cannabis and Other Plant-Based Treatments for Chronic Pain: 2022 Update
- Results - Pressure Ulcer Risk Assessment and Prevention: Comparative Effectivene...Results - Pressure Ulcer Risk Assessment and Prevention: Comparative Effectiveness
- Preface - Treatments for Basal Cell and Squamous Cell Carcinoma of the SkinPreface - Treatments for Basal Cell and Squamous Cell Carcinoma of the Skin
Your browsing activity is empty.
Activity recording is turned off.
See more...
