NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Viswanathan M, Kahwati LC, Golin CE, et al. Medication Therapy Management Interventions in Outpatient Settings [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Nov. (Comparative Effectiveness Reviews, No. 138.)
Introduction
This section of this comparative effectiveness review (CER) on medication therapy management (MTM) first presents the results of the literature searches. We then document the results for each Key Question (KQ). KQ 1 describes MTM intervention characteristics. KQ 2 presents evidence on the effectiveness of MTM interventions, focusing on intermediate outcomes, then patient-centered (health) outcomes, and then use of health care resources or costs. The presentation of KQ 3 summarizes the evidence by intervention components and implementation features; KQ 4 summarizes evidence by patient characteristics. KQ 5 examines the evidence on harms of MTM programs. Appendix E has two parts pertaining to these KQs: the first part has the lengthy descriptions of the design of all included studies (for KQ 1); the second presents the evidence tables, organized by outcome, for the remaining KQs.
Generally, for KQs 2 through 5, the text gives key points and the related strength of evidence grades, followed by a detailed synthesis of the relevant studies. We also present pairs of tables for each outcome. One gives basic summary information about the results of included studies, indicating whether the quantitative data had been what the investigators reported or were calculated by us. The other table in these sets documents the strength of evidence grades for major outcomes (showing the ratings for required domains and, in a small number of cases, any ratings for optional domains). Appendix F contains the tables documenting how we arrived at risk-of-bias assessments for individual studies.
Most data can be found in tables and are not repeated in text. As noted in Methods, we focus on studies of low or medium risk of bias; when we need to summarize information for studies of high risk of bias, we note the principal problems leading to that rating.
Finally, our inclusion criteria for study designs were expansive and included randomized controlled trials (RCTs) and a variety of observational studies (nonrandomized controlled trials, cohort studies and the like). We use “studies” to refer to all types of investigations; we specify RCTs (or non-RCTs) as appropriate.
Results of Literature Searches
Figure 2 presents our literature search results. Literature searches through January 9, 2014, for the final report, identified 2,516 unduplicated citations. Appendix A provides a list of all search terms used and the results of each literature search. In addition, we identified 233 publications through grey literature searches, suggestions from technical experts or public comments received during topic refinement, or hand searches of included studies, or Scientific Information Packets (SIPs). After applying our eligibility and exclusion criteria to titles and abstracts of all 2,749 identified citations, we obtained full-text copies of 419 published articles. We reapplied our inclusion criteria and excluded 358 of these articles from further review before doing the risk-of-bias assessment. Appendix C provides a list of excluded studies and reasons for exclusion at the full-text stage. The 61 articles included after full-text review represent 44 studies. Evidence tables for these 44 studies are provided in Appendix D.
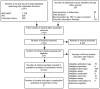
Figure 2
Disposition of articles on medication therapy management (PRISMA figure). Abbreviations: IPA = International Pharmaceutical Abstracts; PICOTS = populations, interventions, comparators, outcomes, timing, settings; TEP = technical expert panel.
The Agency for Healthcare Research and Quality (AHRQ) Effective Health Care Program Scientific Resource Center placed the request for scientific information packets (SIPs) in the Federal Register on September 16, 2013; it posted them for 30 days. We included relevant data from responses to this request.
Table 6 summarizes study characteristics of included studies. Overall, 28 (77.8 percent) of included studies were conducted in the United States, and 16 (44.4 percent) were conducted prior to the 2003 Medicare Modernization Act, which established the framework for Medicare Part D MTM programs. Just over half of included studies used an RCT design (either parallel or cluster group), 3 (8.3 percent) used a nonrandomized controlled trial design, and the remaining studies (38.9 percent) used a cohort study design. Only 3 studies used an active treatment comparison group. Intermediate outcomes were the most commonly reported outcomes. Of the 36 studies, 1 was considered low risk of bias (2.3 percent), 19 were considered medium risk of bias (43.2 percent), 16 (36.4 percent) were considered high risk of bias, and 8 (18.2 percent) had mixed risk of bias ratings, depending on outcome.
Table 6
Characteristics of included studies.
Key Question 1. Components and Implementation Features of MTM Interventions
KQ 1 was designed to synthesize descriptive findings regarding MTM intervention components and implementation features, which have been identified as important factors related to effectiveness of these interventions. Because this report is a CER, our study inclusion criteria included a requirement for a control or comparison arm. For that reason, our synthesis of descriptive findings related to MTM components and implementation features is limited to investigations that comparatively evaluated MTM; that is, it does not include all studies of MTM interventions, many of which we had excluded because of the lack of a comparison arm. Thus, our findings represent a somewhat circumscribed lens for the descriptive part of this review.
Synthesizing intervention components and implementation features across this body of evidence was challenging. Mainly, studies did not consistently describe the intervention characteristics or implementation features in sufficient detail to allow us to determine the extent to which certain components were used, at which intervals, and at what intensity. Even studies published after the 2003 Medicare Modernization Act, which formalized some aspects of pharmaceutical care, lacked sufficient reporting detail in many cases.
Overall Descriptors of Study Interventions
Table 7 specifies the components and implementation features from our analytic framework (Figure 1 in Introduction). It also gives our assessment of the suitability or feasibility of synthesis, based on information available in the included studies across the entire evidence base.
Table 7
Characteristics of medication therapy management interventions.
In the best case, we can summarize data in tables and synthesize the information with actual counts across the body of evidence. This is true for mode of delivery, type of professional giving the services, details about followup, settings, modes of reimbursement, and characteristics of health systems. Somewhat less can be done with methods for enrolling patients and level of integrating MTM with usual care, so information is just included in study-level summary tables (but not synthesized with actual counts across the body of evidence). Finally, information on specific MTM components, fidelity of implementation, and MTM goals was so inconsistent or sparse that we could not either synthesize or include information in summary tables.
Table 8 summarizes the intervention characteristics and features that were reported consistently enough to be synthesized with counts and frequencies—namely, those in Table 7 with an X in the first column. It also notes whether the investigators used the phrase “pharmaceutical care” or the phrase “medication therapy management” to refer to the program tested. For details about intervention frequency and interval of followup, the information in Table 8 is “as designed” (i.e., however, the investigators described their initial intentions).
Table 8
Characteristics of medication therapy management studies by type of patient population (broad focus or narrow focus on conditions or diagnoses).
During our abstraction process, we identified two distinct categories of interventions. One category, of 34 studies, used a broad pharmaceutical care approach or MTM intervention in serving their patient populations; that is, they were not designed to focus specifically on any one disease or clinical condition as part of the intervention. We refer to these studies in the review and Table 8 as “broadly focused.” Many of the studies in this category used retrospective designs of existing MTM programs. In these studies, the focus of the MTM intervention may have been broad, but the study may have restricted the evaluation of the MTM program to a patient population with a specific condition or disease. The other category, with 10 studies, involved interventions evaluated in the context of a single chronic condition (e.g., chronic heart failure, diabetes) or provided in a highly specialized setting (e.g., specialized HIV/AIDS community pharmacies). In these studies, the investigators implemented a pharmaceutical care approach or MTM intervention that attended to the patient's complete drug therapy regimen, but the focus of component interventions (e.g., education, counseling, care coordination) and outcomes measured may have been specific to certain diseases or conditions. We refer to these studies as “narrowly focused.”
In many cases, to distinguish narrowly focused MTM studies from case- or disease-management interventions, we had to contact study authors to clarify that their intervention included a comprehensive drug therapy assessment and drug therapy intervention beyond the single target condition of interest. The distinction between these broad-focus and narrow-focus categories may be important for interpretation of the effectiveness of these types of interventions.
Studies included in this review used “medication therapy management” to describe the intervention (Table 8) in only 18 of the 44 studies. With respect to mode of delivery (Table 8), 9 broadly focused studies used only telephone contact;37-48 by contrast, no narrowly focused studies used only telephone contact. Eleven studies (10 broad, 1 narrow) used a mixture of face-to-face and telephone contact.49-62 The studies using a mixture of modes often used face-to-face delivery for the initial consultation and did followup contacts by telephone. Except for the 2 studies that did not report mode of delivery,63-65 the remaining studies used only face-to-face delivery in pharmacies, clinics, or homes.
All included studies used a pharmacist as the interventionist (Table 8). In some studies, however, the interventionist was described as a community pharmacy resident or ambulatory care pharmacy resident, and in a few studies nonpharmacist staff performed initial intervention components, such as interviewing patient or reviewing records to compile drug history for the pharmacist.
Table 8 also summarizes the intervention frequency and interval of followup as designed, not as may have actually occurred, and these features also differed across studies. Of the 44 included studies, however, 13 (30 percent) did not report the designed frequency of contact and interval of followup. Only 5 studies reported on the actual frequency and interval of followup.49,51-54,58,66-69 Studies evaluating real-world experience with these types of interventions often included a minimum contact threshold for inclusion of patients in the data analysis, but the intervention duration and interval of followup was open-ended and determined by clinical need, as is typical in real-world practice.
Included studies provided interventions in a variety of clinical settings including community pharmacies, centralized pharmacies or call centers, outpatient medical clinics, and some used home visits (Table 8). Half of the narrowly focused interventions were delivered exclusively in an outpatient medical clinic.59,70-75
Concerning reimbursement, of the 44 studies in the evidence base, 26 (59 percent) did not report on reimbursement for MTM services at all. Of the remaining studies, 15 reported that pharmaceutical care or MTM was a covered benefit to patients; pharmacist services were reimbursed through an existing mechanism (e.g., Medicare Part D or other health care benefit).37-44,46-48,51-54,57,62,76-80 Three studies clearly indicated that pharmacist services were reimbursed through pilot, grant, health system, or study-related funding.49,58,81
Finally, the context of the MTM services also varied in terms of features of the health system or organization in which they were provided. Academic medical centers, integrated health care delivery systems, health plans, and single payer health care systems outside the United States were all represented in this evidence base.
Study-Level Descriptors of Interventions
In Appendix D, we have provided study-level summaries to describe the included interventions. Those tables (Table D-3 and D-4) document: interventions and the amount of integration with usual practice; method of identifying patients for receipt of MTM services; setting, mode of delivery, frequency and interval of followup; and health care system and reimbursement context. Table D-3 describes the 34 broad-focus studies; Table D-4 describes the remaining 10 narrow-focus studies (and additionally specifies the particular focus). We summarize the main elements in text below.
Of the 10 narrow-focus studies, 2 addressed chronic heart failure and 3 addressed hypertension or hypertension and diabetes. The remaining studies focused on patients with: depression and anxiety, diabetes alone, glucocorticoid-induced osteoporosis, HIV/AIDS, and end-stage renal disease on hemodialysis.
The 14 studies described as pharmaceutical care were generally based on the pharmaceutical care model as initially described by Strand and associates and further refined by the profession of pharmacy practice.51-54,63-65,70-74,79,82-89 Interventions termed medication therapy management (i.e., MTM) were often based on criteria defined for the Medicare Part D program, which includes elements of the pharmaceutical care model.37-49,57,58,60,62,66-69,76,77,80,81,90 The remaining interventions included elements of pharmaceutical care or MTM but did not specifically label the intervention as either one or the other.50,55,56,59,61,75,78,91-93 These studies were often described as “clinical pharmacist interventions.”
We defined the level with which pharmaceutical care or MTM services were integrated with usual care as having two main elements: (1) the degree of access that the interventionist had to clinical information in the patient's medical record, such as laboratory results, diagnoses, and progress notes and (2) the ease of access and method and process of communication between the interventionist and prescribers. Providing MTM services within an outpatient medical clinic, presumably where the patient is also receiving medical care, is one such marker of integration, particularly when the study indicated that the pharmacist was part of a multidisciplinary care team. Some studies, however, described the pharmacy or pharmacist simply as co-located in a medical clinic. In these instances, we do not know whether the level of integration with medical care would be any higher than if the pharmacist had been located in a community pharmacy. Thus, we could not rely solely on clinical setting as a marker of integration with usual care.
Because many studies did not provide sufficient details regarding specific components of the intervention, whether termed pharmaceutical care, MTM, or clinical pharmacist intervention, we were unable to synthesize the use of specific intervention components beyond the components we required for study inclusion.
Only four studies used an active treatment comparator group.57,58,69,88 All other studies (regardless of focus) compared pharmaceutical care or MTM with usual medical or pharmacy care or both. This factor also impeded our assessing the effectiveness of individual intervention components. Furthermore, almost no study reported on the fidelity with which intervention components were delivered (relative to the original design or intention), including whether goals of drug therapy were established and communicated.
The methods by which patients were identified and offered pharmaceutical care or MTM services has been proposed as a moderator of effectiveness; the aim is to target patients most likely to benefit. These factors may include, for example, patients using drugs with narrow therapeutic windows, complex drug regimens, or patient characteristics such as age, cognitive status, or social situation. With respect to data sources that studies used to identify and then enroll patients for services, pharmacy prescription records (at a community pharmacy, clinic, or health plan) were the most common source. Except for the studies evaluating Medicare Part D MTM programs, few studies used the same criteria for identifying patients for enrollment. Most required either some degree of regimen complexity, such as the number of drugs taken or use of one or more drugs considered high risk for adverse events. Most studies using pharmacy data or claims mailed or telephoned eligible patients to provide information about enrollment in an MTM program. For Medicare Part D MTM programs, “opt out” is another variation of enrollment for these services. Patients meeting eligibility criteria are enrolled for services unless they specifically “opt-out.” Some studies relied solely on provider referral, patient self-referral, or routine medical record screening at time of a provider visit to identify patients for services.
Tables E-1 and E-2 also provide study-level detail on intervention setting, mode of delivery, frequency and interval of followup and health care system and reimbursement characteristics, which were summarized overall in Table 8 and in the preceding section.
Key Question 2. Effect of Medication Therapy Management Interventions on Intermediate, Patient-Centered, and Resource Utilization Outcomes
We present below key findings and a detailed synthesis of intermediate, patient-centered, and resource utilization outcomes separately. (These outcomes were specified in Table 1 of the Introduction.) When possible (a minimum of three reasonably similar studies for a given intervention or outcome), we pooled study results and document those findings below. When studies were too heterogeneous to pool, we present effect sizes for individual studies whenever possible in summary tables for each outcome that was reported in two or more studies. We also provide strength of evidence tables to support our findings.
Because in many cases the investigators did not report a full set of findings that compared changes over time between intervention and comparisons groups or other details that would permit full analysis, we calculated various statistics ourselves. In these cases, we present in the tables below only these calculated findings and related statistical levels, and we note this explicitly in the tables or text (as “calculated”). The underlying data from the study article(s) can be found in the evidence tables in Appendix D.
Key Points: Intermediate Outcomes
- Evidence was insufficient to evaluate the effect of MTM on anticoagulation after 12 months due to an imprecise, single RCT body of evidence with medium limitations.
- Evidence was insufficient to evaluate the effect of MTM on hemoglobin A1C after 6 to 12 months due to an inconsistent and imprecise body of evidence from two RCTs with medium limitations and two observational studies with high study limitations.
- Evidence was insufficient to evaluate the effect of MTM for decreasing low-density lipoprotein (LDL) cholesterol after 6 to 24 months due to an imprecise, single RCT body of evidence with medium limitations and an imprecise observational body of evidence of two studies with high limitations.
- Evidence was insufficient to evaluate the effect of MTM for reducing blood pressure (BP) after 4 to 12 months based on direct, but inconsistent and imprecise, findings from a single RCT and two cohort studies with medium limitations.
- Several studies did not report outcomes such as drug therapy problems identified and resolved for both intervention and control groups. As a result, limited evidence addresses the effectiveness of MTM compared with usual care in improving these important intermediate outcomes. Study limitations, inconsistency, and lack of precision led us to conclude that the evidence is insufficient to judge the effectiveness of MTM in improving these outcomes when compared with usual care.
- We found low strength of evidence that MTM had an effect on the percentage of people adherent to at least 80 percent of prescribed doses and on the absolute percentage of prescribed doses taken. Although these conclusions are based on inconsistent evidence with primarily nonsignificant findings of effects and high study limitations, two large cohort studies showed consistent effects of MTM on adherence although with high study limitations.
- Evidence was insufficient to evaluate the effect of MTM on medication adherence (as measured by self-report) as a result of inconsistent and imprecise evidence. The number of trials, consistency, and study limitations varied by specific adherence measure.
- MTM increases the appropriate use of medications as measured by overall scores on appropriateness indices (low strength of evidence).
- Evidence was low for benefit of MTM on medication dosing as a result of indirect, and precise evidence from one trial with medium study limitations.
Detailed Synthesis: Intermediate Outcomes
Anticoagulation
One RCT (medium risk of bias) reported on the effects of a pharmaceutical care intervention on anticoagulation among patients in family medicine clinics in a rural community after 12 months of followup.85 This intervention was conducted with 81 patients at high risk for medication-related problems; however, this outcome was reported only for the four patients in the intervention arm and the six patients in the control arm who were taking anticoagulants. The percentage of subjects who achieved a therapeutic international normalized ratio (INR) differed significantly between the intervention and control arms (100 percent versus 16.7 percent (p=0.048); calculated odds ratio [OR], 32.94; 95% confidence interval [CI], 1.06 to 1,021.35). Because of imprecision (wide confidence intervals) and unknown consistency, we graded the evidence as insufficient to evaluate the effectiveness of MTM on improving therapeutic anticoagulation (Table 9).
Table 9
Anticoagulation: Strength of evidence.
Hemoglobin A1c
Two RCTs and three cohort studies reported on outcomes related to hemoglobin A1c (HbA1c) among patients with diabetes (Table 10). One RCT (medium risk of bias) reported no significant difference in mean HbA1c between intervention (pharmaceutical care) and control patients in an Australian outpatient hospital diabetes clinic at 6 months.72 The other RCT (medium risk of bias) reported on changes in the percentage of patients with diabetes who achieved a HbA1c of less than or equal to 7.5 percent at 12 months among patients at high risk for medication-related problems seen in family medicine practices in a rural community.85 The percentage of patients at goal did not differ significantly between intervention and control arms at baseline (23.1 versus 56. 3, calculated p=0.08) but was significantly different at followup (100 versus 26.7, calculated OR, 56.455; 95% CI, 2.811 to 1,133.912. p=0.008).
Table 10
Hemoglobin A1c: Summary of results.
One medium risk of bias and one high risk of bias (because of self-selection of participants into the intervention arm and control arms) cohort study were conducted primarily by telephone within large, integrated U.S. health care systems.38,42 The remaining cohort study (medium risk of bias, described in three included publications) involved pharmacists providing MTM services to patients with diabetes within medical clinics that were part of a large, integrated U.S. health care system.66-68 This study found a larger difference in the percentage of subjects achieving HbA1C less than 7 percent between baseline and the 12-month followup for the intervention group (adjusted difference-in-difference coefficient 2.44; 95% CI, 1.22 to 4.86), but this difference was not maintained 12 months after the end of the study intervention.66-68 The telephone-based medium risk-of-bias cohort study reported no significant change in mean HbA1c or percentage of subjects achieving a HbA1C less than 7 percent at 6 months for the intervention group compared with the control group.38 The high risk-of-bias telephone-based cohort study found similar findings.
Based on direct, but inconsistent and imprecise, evidence from two RCTs and two observational studies, all with medium limitations (Table 11), we concluded that the strength of evidence is insufficient to evaluate the effectiveness of MTM interventions to improve mean HbA1c levels or increase the percent of patients achieving a goal HbA1c level.
Table 11
Hemoglobin A1c: Strength of evidence.
LDL Cholesterol
One RCT and five cohort studies reported on outcomes related to LDL cholesterol (Table 12). The RCT (medium risk of bias), reported the percentage of patients with dyslipidemia who achieved an LDL cholesterol goal based on Adult Treatment Panel III (ATPIII) criteria for lipid management among patients at high risk for medication-related problems in a rural Alabama community.85 The intervention and control groups did not differ significantly in percentage at goal at baseline (10.5 percent versus 15.8 percent, p=0.631) but differed significantly at 12 months (77.8 percent versus 5.9 percent, p=0.001; calculated OR, 50.400; 95% CI, 5.271 to 481.915). These findings are quite imprecise, largely because of a sample size of only 19 subjects in each group for this outcome.
Table 12
LDL cholesterol: Summary of results.
We also evaluated the findings from the five cohort studies. One of the medium risk-of-bias cohort studies involved pharmacists providing MTM services to patients with diabetes within medical clinics that were part of a large, integrated U.S. health care system in Minnesota.66-68 This study found no significant difference in the percentage of patients achieving an LDL cholesterol goal (defined as less than 100 mg/dl) at 12 months (adjusted difference-in-difference coefficient 1.95; 95% CI, 0.81 to 4.84; p=0.13). The other medium risk-of-bias cohort study involved a telephone-based MTM program delivered within a large, integrated U.S. health care system in California; the analysis presented was limited to patients with a diagnosis of hyperlipidemia, diabetes, or coronary artery disease.38 This study found a small but significant mean decrease in LDL cholesterol levels in the intervention group compared with controls at 6 months (calculated mean difference, -4.1; 95% CI, -6.019 to -2.181; p< 0.001) and also found a significant increase in the percentage of patients achieving an LDL goal, defined as LDL less than 100 mg/dl (calculated OR, 1.392; 95% CI, 1.160 to 1.670; p<0.001).
Two of the three high risk-of-bias (because of selection bias and baseline characteristics of groups not reported or not adjusted for) cohort studies were telephone-based MTM programs,37,42 and the remaining high risk-of-bias study was a clinic-based MTM program; all three were conducted in the United States.81 One study did not provide the information necessary to determine whether the findings reported (change in percentages reaching goal LDL) were evidence of no effect or an effect favoring the control arm.42 The other two studies reported a direction of effect similar to that reported in the RCT but at a much smaller magnitude.
Overall, we concluded that the strength of evidence is insufficient for the effectiveness of MTM interventions on lowering mean LDL-cholesterol levels or increasing the percentage of patients achieving a LDL-cholesterol goal. This body of evidence included a single RCT with medium study limitations and imprecise findings and an observational body of evidence with high study limitations consisting of two studies. Although we acknowledge the large magnitude of effect in the RCT, these findings were very imprecise, and the magnitude of effect was inconsistent with the observational study findings (Table 13).
Table 13
LDL cholesterol: Strength of evidence.
Blood Pressure
In all, we identified seven, mostly small, studies that measured blood pressure outcomes using various followup periods (Table 14). This evidence base consisted of three RCTs and four cohort studies; the outcomes involved achieving blood pressure goals or becoming normotensive, and changes in systolic or diastolic blood pressure levels (SBP; DBP) or both. Of these studies, we rated one RCT and two cohort studies as medium risk of bias; the remaining RCT and cohort studies were high risk of bias.
Table 14
Blood pressure: Summary of results.
Blood Pressure Goal Attainment
One RCT (medium risk of bias), conducted among of a small number of patients at high risk of medication-related problems receiving pharmaceutical care through family medicine clinics in a rural Alabama community, reported a significant difference in the number of patients at blood pressure goal (SBP ≤ 140 mm Hg and DBP ≤ 90 mm Hg) at 12 months (91.7 percent versus 27.6 percent, calculated OR 28.875, 95% CI 5.486 to 151.993, p< 0.001).85 The other RCT (high risk of bias due to high attrition, lack of intention to treat analysis, and no adjustment for baseline differences) provided MTM services through community pharmacies to managed care organization enrollees with diabetes and hypertension in Oklahoma.90 This trial also reported a favorable effect of MTM on the achievement of blood pressure goals (OR 12.9, 95% CI, 1.5 to 113.8; p=0.021). The last trial (high risk of bias for unclear randomization methods, important differences in baseline with no adjustment in analysis, and other factors related to study execution) reported a direction of effect favoring the MTM group, but it was not statistically significant.89
The two medium risk-of-bias cohort studies included a telephone-based MTM program within a large, integrated U.S. health care system in California.38 The other study involved MTM provided by pharmacists in medical clinics within a large, integrated U.S. health care system in Minnesota.66-68 Both of these studies showed a directional effect favoring the control groups on the percentage of subjects who achieved blood pressure control at 6 and 12 months, but these findings were not statistically significant (calculated OR 0.953, 95% CI, 0.808 to 1.125; p=0.571, adjusted difference in difference coefficient, 0.73; 95% CI, 0.32 to 1.65; p=0.45). The two other cohort studies (both high risk of bias) reported findings that were directionally consistent with the trials, but findings were statistically significant in only one of the studies.70,71,81
Systolic and Diastolic Blood Pressure Levels
Three studies reported on systolic blood pressure outcomes, and all were rated as high risk of bias. One RCT provided MTM services through community pharmacies to managed care organization enrollees with diabetes and hypertension in Oklahoma.90 The MTM group in this trial had a mean decrease of 20.0 mmHg (95% CI, -32.7 to -7.4; p: 0.003) in systolic blood pressure compared with the control group. The other RCT provided pharmaceutical care to patients with hypertension through community pharmacies in Illinois and Wisconsin.89 This study found a mean decrease of 13.0 mmHg (95% CI, -23.739 to -2.261; p=0.018) compared with controls.89 The cohort study found directionally similar results.70,71 We found similar results for diastolic blood pressure from the two high risk-of-bias studies that reported this outcome.70,71,89 Overall, we concluded that the strength of evidence is insufficient for the effectiveness of MTM interventions to increase the percentage of patients achieving a blood pressure goal or decrease systolic blood pressure or diastolic blood pressure levels based on direct but imprecise evidence from one RCT with medium limitations and two observational studies with high limitations (Table 15). In addition, the direction of effect was not consistent between the RCT and observational evidence (Table 15).
Table 15
Improvement in blood pressure: Strength of evidence.
Drug Therapy Problems Identified
In all, 10 studies addressed the question of the effectiveness of MTM for identifying drug therapy problems. Of these, eight provided information on drug therapy problems only from the intervention arm.43,49,55,63,72-74,79,89 Thus, these studies cannot inform the question of the comparative effectiveness of MTM.
The two remaining comparative studies (one trial, one cohort study) reported findings about the effectiveness of MTM when compared with usual care (Table 16). The trial results are uninterpretable because the authors report total numbers of drug therapy problems identified in each arm without any measure of variance.91 We rated the cohort study as risk of bias for uncontrolled selection bias from the comparison of patients who refused services to patients who accepted services.44 These two studies also did not specify their expected direction of effect. We inferred that the studies expected to find fewer drug therapy problems after the completion of the intervention because the interventions were (apparently) specifically designed to identify and then resolve drug therapy problems. However, studies measuring outcomes during an MTM intervention might, instead, expect to find more drug therapy problems in the intervention arm because the intervention led to greater discovery of various problems. Consequently, we treated the evidence as indirect. Given high study limitations, unknown consistency, indirectness, and lack of precision, evidence was insufficient to draw any conclusions about the effect on MTM interventions on drug therapy problems identified (Table 17).
Table 16
Drug therapy problems identified: Summary of results.
Table 17
Drug therapy problems identified: Strength of evidence.
Drug Therapy Problems Resolved
In all, we identified nine studies that attempted to report on whether MTM programs resolved drug therapy problems that were identified. Of these, six studies provided information only from the intervention arm.42,49,51,54,73,74,81,89 Thus, as with drug therapy problems identified, they cannot inform the question of the comparative effectiveness of MTM interventions. Three other studies (two RCTs, one cohort study) provided information on the effectiveness of MTM for resolving drug therapy problems when compared with usual care (Table 18). The cohort study (medium risk of bias) found a significant effect of MTM on the difference in drug therapy problems identified between baseline and a 12-month followup; the investigators interpreted the change in number of drug therapy problems identified over time as drug therapy problems resolved between baseline and followup.39-41
Table 18
Total number of drug therapy problems resolved: Summary of results.
One RCT had a high risk of performance bias because of issues concerning site and country-specific variation, coupled with failure to control for differences at baseline and a high overall attrition.64,65 The other trial shows higher total numbers of all drug therapy problems resolved in the intervention arm, but without measures of variance that account for variation among patients, these results cannot be interpreted with confidence.91
Two studies reported on specific aspects of drug therapy problems resolved without an overall measure of total number of drug therapy problems resolved (Table 19).62,79 One medium risk-of-bias cohort study, designed to identify the impact of 2010 Part D MTM programs, compared cohorts (standalone Prescription Drug Plan or Medicare Advantage Prescription Drug Plan) receiving MTM with a comprehensive medication review with cohorts receiving usual care for congestive heart failure, chronic obstructive pulmonary disease, and diabetes, after limiting the sample to those newly eligible or enrolled for MTM and controlling for characteristics such as demographics, medical comorbidities, condition severity, and intensity of provider care.62 Only one of three measures of drug therapy outcomes showed significant differences in the MTM arm when compared with the usual care arm at 12 months (discontinuation of contraindicated medications for congestive heart failure), but the results were inconsistent, with the Medicare Part D plan outperforming usual care and Medicare Advantage Part D plan underperforming usual care. The authors note that the timing of measurement (one year after MTM enrollment for intervention arms) may have allowed prescribers to add back problematic drugs over the course of the year.
Table 19
Specific drug therapy problems resolved: Summary of results.
The other cohort study (high risk-of-bias) found significantly lower prevalence of high risk medications 9 months after becoming eligible but these result did not control for large differences in baseline prevalence (43.4 percent in the intervention arm and 35.8 percent in the control arm).79 Because the outcomes reported in these studies are included but not separately analyzed in other studies reported overall numbers of drug therapy problems resolved, we did not separately grade the evidence for these outcomes.
Together (or taking the medium risk-of-bias cohort study alone), these studies offer insufficient evidence, based on study limitations, inconsistency, and imprecision, to judge the effectiveness of MTM on resolving drug therapy problems (Table 20). Evidence from studies reporting on individual measures of drug therapy problems resolved also supports this conclusion.
Table 20
Drug therapy problems resolved: Strength of evidence.
Medication Adherence
Fourteen studies reported on the effects of MTM interventions on adherence outcomes.40,42,44,45,50,56,62,64,66-68,84-86,89,90 One cohort study reported nonadherence determined during MTM (during a mock MTM chart review for the control group);44 any adherence differences noted between the two groups were unlikely to be attributable to MTM effects. Moreover, the description of nonadherence used in that study (percentage of patients “nonadherent” per chart review) cannot be interpreted because of a lack of a clear definition. For these reasons, we excluded this study from further analysis.
Three remaining studies in the analysis are described in Table 21. Of these 13 studies, eight were RCTs44,50,56,64,84-86,89,90; five were cohort studies.40,42,45,62,66-68 Most studies assessed one of three different adherence outcomes: (1) the proportion of patients who, based on a threshold of between 75 percent and 80 percent of prescribed doses taken, were deemed to be adherent42,62,66-68,85; (2) the percentage of prescribed doses taken40,45,89,90; and (3) the scores from an adherence scale score (such as the Morisky Scale).50,64,86 Two studies assessed miscellaneous aspects of medication-taking behavior56,84 these included “remembering to take medication,” a medication-taking behavior subscore, and or determining the number of medications (not pills) for which the participant's reported manner of taking (number of pills and frequency per day) exactly matched the prescribed directions. When studies did not report statistical significance, we calculated the standard difference in means, standard errors, and 95 percent confidence intervals based on raw data.
Table 21
Medication adherence: Summary of results grouped by type of adherence outcome.
Of the 4 studies assessing the proportion of patients who achieved threshold adherence levels, one was a small RCT (medium risk of bias);85 the others were cohort studies: 1 small66-68 (medium risk of bias) 1 relatively large (high risk of bias),42 and 1 very large medium risk of bias).62 Only 1 of these studies found statistically significant positive effects of MTM on adherence62 and did so for adherence to some but not comparisons for multiple medications. Of the 4 studies that assessed MTM effects on percentage of prescribed doses taken, 2 were small RCTs (both high risk of bias);89,90 the other 2 were cohort studies, one small, one large (both medium risk of bias).40,45 Only 1 of these studies found a statistically significant positive effect of MTM on adherence. All 3 studies that assessed adherence using self-reported adherence scales were small RCTs (1 medium risk of bias86; 2 high risk of bias50,64). None found a statistically significant effect of MTM on adherence, although 1 high risk-of-bias study64 did not account for the marked baseline differences and, hence, may have missed a statistically signficant difference in change in adherence. This same study (high risk of bias) reported a statistically significant increase in the percentage of individuals who changed from nonadherent to adherent over 18 months (15.25 percent in the intervention group and 12.2 percent in the control group; p=0.028);64 however, this assessment did not take into account the percentage in each group that changed from adherent to nonadherent. Finally, the 2 RCTs (both medium risk of bias) that assessed miscellaneous aspects of adherence found no statistically significant differences between groups in adherence outcomes assessed.56,84 Hence, of the 13 studies that assessed effects of MTM on adherence, 2 large cohort studies that used an objective adherence measure found a statistically significant positive effect on some aspects of adherence to some medications but not others.
Overall, we concluded that evidence is insufficient to draw conclusions about the effectiveness of MTM for improving the proportion of patients who, based on a threshold of 80 percent of prescribed doses taken, were adherent at 6 to 12 months based on direct, imprecise evidence from one small RCT (Table 22) and direct, and precise but inconsistent (regarding effect direction and magnitude) evidence from one small and one large cohort study that together had high study limitations.
Table 22
Adherence outcome Type 1—proportion of patients adherent based on a threshold of percentage of pills taken: Strength of evidence.
Overall, we concluded that there was low strength of evidence about the effectiveness of MTM for improving the proportion of patients who, based on a threshold of 80 percent of prescribed doses taken, were adherent at 6 to 12 months based on direct, imprecise evidence from one small RCT (Table 22) and direct, and precise but inconsistent (regarding effect direction and magnitude) evidence from one small and one large cohort study that had high study limitations. Strength of evidence is also low for improving the absolute percentage of prescribed doses taken at 6 months and 12 months (mean adherence) for hypertension and dyslipidemia treatment, based on inconsistent direct, imprecise evidence from two cohort studies, one small and one large with medium study limitations (Table 23). However, we found insufficient evidence to draw conclusions about the effect of MTM on absolute percentage of prescribed doses taken for other conditions based on these same observational studies. This conclusion is consistent with findings from two small high risk-of-bias RCTs that provided direct, imprecise evidence of these effects at 4 to 9 months. Of note, these two trials had a high level of study limitations and reported opposite directions of effect on absolute percentage of prescribed doses taken, both with nonsignificant differences between groups.
Table 23
Adherence outcome Type 2—absolute measure of adherence as percentage of prescribed doses taken: Strength of evidence.
Evidence is also insufficient about improving medication adherence as measured by self-reported scales from one medium risk-of-bias trial (Table 24). Finally (Table 25), regarding miscellaneous medications taking behaviors, such as remembering to take medication, a medication-taking behavior subscore, and the proportion of medications matched with instructions, we concluded that evidence was insufficient for the effect of MTM on these outcomes, based on evidence from two RCTs that was direct but imprecise and inconsistent. Although the significant degree of heterogeneity across adherence measures precluded our ability to assess strength of evidence across all adherence studies, we note that considering the body of evidence for the effect of MTM on adherence, taken together, results from all studies were inconsistently significant with small magnitudes of effect. Across studies, the direction of effect was inconsistent, however, for the two outcomes, “proportion of adherent patients,” and “percentage of prescribed pills taken,” which in all studies were measured objectively using claims and pharmacy data, we found low strength of evidence that MTM had an effect on medication adherence, particularly for certain chronic conditions . Hence, considering the adherence studies as a whole, there appears to be low strength ofevidence regarding an effect of MTM on adherence.
Table 24
Adherence outcome Type 3—self-reported scales: Strength of evidence.
Table 25
Adherence outcome miscellaneous: Strength of evidence.
Medication Appropriateness
Five studies (four RCTs,59,63,84,85 one cohort study70) reported on the effects of MTM interventions on medication appropriateness (Table 26 and Table 27). Of these studies, three assessed medication appropriateness across a broad spectrum of regimens;70,84,85 the other two trials assessed appropriateness for specific medications.59,63 In addition, two studies evaluated single aspects of medication appropriateness, across a range of medications.62,79
Table 26
Medication appropriateness scales: Summary of results.
Table 27
Medication appropriateness for individual medications: Summary of results.
For the three broader studies, two trials used the Medication Appropriateness Index (MAI).84,85 One of these reported results for the full scale and for each item of the index (each item asks about a different aspect of medication appropriateness) individually;84 the other trial reported results only for each of the individual items.85 The cohort study of broad regimens used a panel of three pharmacists to rate the appropriateness of the various antihypertensive regimens on a visual analogue scale.70
As shown in Table 26, one RCT (low risk of bias)84 found a statistically significant improvement in the MAI Scale at 3 and 12 months' followup. The small cohort study (high risk of bias) reported no statistically significant improvement in the three appropriateness scores assessed for blood pressure regimens (appropriateness of regimens, of dosing intervals, and of dosages) although it was very underpowered.70
Of note, one84 (low risk of bias) of the two trials reporting the effect of MTM on general medication appropriateness scales, also provided descriptive data, by intervention group, regarding the proportion of inappropriate prescriptions for each of 10 items on the MAI (which address different aspects of appropriateness). These findings are reported in Appendix E. While one is unable to draw conclusions regarding the findings because they report percentages with prescriptions (rather than “per patient”) as the unit of analysis, they do suggest that some items are likely driving the improvements in MAI in the MTM group more than others. Specifically, six aspects of medication prescription appropriateness: drug indication; dosage; practicality of directions; drug-drug interactions; duplication; duration of therapy seem to show greater improvement in inappropriate prescriptions than do those for four other aspects: effective medication; correctness of directions; drug-disease interactions; expense of medication. Similarly, another study85 which did not report on the full MAI scale, also reported data regarding the effect of MTM on individual MAI items (Appendix E). The ability to interpret these descriptive findings is not only, like the other study,84 hampered by the use of prescriptions rather than patients as the unit of analysis, but also is limited by the marked baseline differences that existed between intervention groups.
Two RCTs (both medium risk of bias) assessed the appropriateness of regimens for specific medications for specific conditions (Table 27). One assessed, among patients at risk for glucocorticoid-induced osteoporosis, the percentages of patients receiving each of three indicated regimens;63 the investigators found, at 9-month followup, a statistically significant improvement in the percentage appropriately prescribed calcium supplements among MTM recipients compared with controls but not for bisphosphonate or estrogen drug therapy. The other trial assessed the use of angiotensin-conversion enzyme (ACE) inhibitors among heart failure patients.59 The pharmaceutical care program had a significant effect on the mean percentage of target dose achieved and on the proportion receiving an appropriate alternative medicine among the subsample; such services did not produce a significant effect on the percentage of patients who received an ACE inhibitor.
Overall, we concluded that the strength of evidence is low for the effect of MTM on medication appropriateness (measured by continuous scores on index) at 3 and 12 months based on indirect, precise evidence from one small RCT (Table 28). The findings are consistent with the direction of effect (indirect, imprecise evidence) from a small cohort study with high study limitations and with studies of individual aspects of medication appropriateness.
Table 28
Medication appropriateness scales: Strength of evidence.
Strength of evidence is insufficient for the efficacy of MTM for improving the appropriateness of medication prescriptions for specific medications (Table 29) based on findings from two small RCTs that provided indirect, imprecise evidence of these effects at 6 or 9 months. This evidence based had medium study limitations, but the trials reported opposite directions of effect based on medication type.
Table 29
Medication appropriateness for individual medications: Strength of evidence.
Medication Dosing
One RCTs (medium risk of bias) assessed the effect of MTM on medication dosing (Table 30).50 A second study assessed dose adjustment, but we excluded it from this analysis because dosing was assessed only at baseline.44 The single included trial assessed changes in the number of doses that primary care patients received per day at the end of 6 months; patients in the MTM arm received 1.6 fewer doses than at baseline, whereas control patients received 0.6 more doses per day than at baseline (p=0.007).
Table 30
Medication dosing: Strength of evidence.
Overall, evidence was low for benefit of MTM on medication dosing (Table 30) based on findings from one small RCT with medium study limitations, and indirect, but precise results.
Key Points: Patient-Centered Outcomes
- Evidence was insufficient to draw conclusions about the effect of MTM on adverse drug events based on inconsistent and imprecise findings from two RCTs: one with low study limitations and one with medium limitations.
- Evidence was insufficient to draw conclusions about the effect of MTM on cognitive and physical function based on direct but imprecise findings from one RCT with medium study limitations and on affective function based on direct but inconsistent and imprecise findings from two RCTs, both with medium study limitations.
- Evidence was insufficient to draw conclusions about the effect of MTM on gastrointestinal bleeding based on direct but imprecise findings from one observational study with high study limitations.
- Evidence was insufficient to draw conclusions about the effect of MTM on all-cause mortality based on one RCT with medium study limitations and two observational studies, with high study limitations.
- With two exceptions, MTM interventions had no benefit on SF-36 measures (low strength of evidence of no benefit); evidence was insufficient for the SF-36 domain of vitality or emotional role functioning because of imprecision.
- Evidence was insufficient to determine whether MTM interventions improved patient-reported measures for patients with diabetes (one imprecise medium risk-of-bias trial).
- MTM interventions did not improve measures of patient satisfaction (low strength of evidence of no benefit).
Detailed Synthesis: Patient-Centered Outcomes
Adverse Drug Events
Four RCTs50,69,84,85and one nonrandomized trial83 reported on prevalence of adverse drug events (ADEs) following MTM or pharmaceutical care interventions (Table 31). The methods for measuring adverse events differed substantially among included studies. Further, although we assumed that the beneficial direction of effect would be for MTM to decrease ADEs, the nonrandomized trial suggested that MTM services may heighten awareness of potential adverse outcomes by patients and, thus, increase reporting of ADEs by those receiving the intervention.83 For this outcome, we rated the risk of bias for some studies50,83,85 as higher than the overall risk of bias because of measurement and detection bias with respect to the measures and methods used to ascertain this outcome.
Table 31
Adverse events: Summary of results.
One RCT (low risk of bias) compared usual care with the provision of basic MTM services designed to mimic conditions similar to a community pharmacy with another study arm that included an enhanced intervention that provided clinical information about the patient to the pharmacist.69 It reported on outcomes for the period between 0 and 3 months and for the period between 3 and 6 months. The enhanced MTM intervention was superior to the basic MTM intervention at 3 months in the percentage of subjects reporting an ADE; however, the enhanced intervention and usual care at 3 months and three study arms at 6 months did not differ significantly. In addition, the mean number of ADEs per patient was not statistically different between 0 and 3 months across study arms, but both the enhanced MTM and usual care study arms had significantly fewer ADEs per patient than the basic MTM study arm between 3 and 6 months. This RCT found no statistical difference in mean ADEs per patient between the enhanced MTM study arm and usual care between 3 and 6 months. Another RCT (medium risk of bias) compared clinical pharmacy care within a VA general medicine clinic to usual care.84 This study found that intervention group subjects were less likely to have one or more ADE over 12 months, but this finding spanned the null effect (calculated OR, 0.649; 95% CI, 0.366 to 1.152, p=0.14).
The other two RCTs and the NRCT were considered high risk of bias for the ADE outcome. One RCT provided pharmaceutical care to patients at high risk for medication-related problems seen in family medicine practices in a rural Alabama community;85 the intervention and control arms did not have significantly different findings with respect to “medication misadventures” at 12 months. We rated this trial as high risk of bias because it used a nonstandard measure (medication misadventure was not defined) and because the control event rates differed by a factor of 10 relative to the low and medium risk-of-bias RCTs. The other RCT compared MTM intervention provided by a pharmacist within a family health center setting in Michigan with usual medical care and reported no significant difference between change in medication side effect scores using a scale that the study authors had developed for use in the study, but no validity or reliability data for this scale were provided.50 The nonrandomized trial (high risk of bias for this outcome)compared participants who agreed to participate in a pharmaceutical care program at one of six participating community pharmacies with a group of control patients who received medications at pharmacies that did not provide pharmaceutical care services;83 study participants were significantly more likely (OR, 1.81; 95% CI, 1.16 to 2.83) to report experiencing symptoms or problems related to prescription medication than control participants, an effect the authors attributed to increased awareness of medication side effects in the intervention group. Without a clear understanding of the hypothesized mechanism of action in each study for influencing ADEs and the lack of study methods for minimized detection bias, we cannot interpret the conflicting results presented by the nonrandomized trial relative to the findings from the RCTs.
Overall, we concluded that evidence is insufficient to draw conclusions about the efficacy of MTM for reducing adverse drug events based on direct, but inconsistent and imprecise, evidence from one low and one medium risk of bias RCT (Table 32).
Table 32
Adverse drug events: Strength of evidence.
Cognitive, Affective, and Physical Function
Two RCTs (both medium risk of bias) reported on changes in cognitive, affective, or physical function at 6 weeks and at 3 months.75,92 In one RCT, the intervention was provided in a general medicine outpatient clinic in North Carolina to simplify medication regimens among cognitively intact patients ages 65 or older at high risk for medication-related adverse events (Table 33). The investigators measured cognitive function using three different tests. They measured affective function using the Center for Epidemiological Studies Depression Scale and the Self-Rating Anxiety Scale and physical functioning using three different tests. Patients in the intervention arm experienced no significant changes in any of these measures when compared with patients in the control arm.
Table 33
Cognitive, affective, and physical function: Summary of findings.
The other RCT evaluated the use of the Dader method for pharmaceutical care among women receiving treatment for anxiety or depression in specialty clinics at a university hospital in Brazil. This study found significant mean changes in both the Beck Depression Inventory and the Beck Anxiety Inventory compared with the control arm. In addition, the percentage of patients achieving a depression remission was higher in the intervention arm, but these findings were not significant (calculated OR, 2.406; 95% CI, 0.601 to 9.632; p=0.215). Overall, we conclude that the strength of evidence is insufficient for the effect of MTM on cognitive or physical function based on a single, imprecise RCT with medium study limitations (Table 34). We also conclude that the strength of evidence is insufficient for the effect of MTM on affective function based on inconsistent and imprecise findings from two RCTs with medium study limitations (Table 35).
Table 34
Cognitive and physical function: Strength of evidence.
Table 35
Affective function: Strength of evidence.
Mortality
One RCT59 and two cohort studies44,48 reported all-cause mortality outcomes following MTM interventions at 6 months to 4 years (Table 36). The RCT (medium risk of bias) conducted in a university general cardiology clinic compared a study arm that included a clinical pharmacist intervention for heart failure patients with usual medical care59 and found decreased mortality within 6 months, but this finding spanned the null effect (OR, 0.59; 95% CI, 0.12 to 2.49, p=0.48).
Table 36
All-cause mortality: Summary of results.
Both cohort studies (Table 34) (both medium risk of bias) measured mortality outcomes for beneficiaries who met MTM program eligibility and opted in to a telephone-based MTM program provided through an integrated health care system compared with eligible beneficiaries who opted out of the MTM program.44,48 One study reported a statistically significant reduction in all-cause mortality at 6 months in the intervention arm, when adjusted for age, sex, and baseline disease, and health care utilization levels (adjusted OR, 0.5; 95% CI, 0.3 to 0.9; p=0.044). The other study reported a similar direction of effect (adjusted HR, 0.92; 95% CI, 0.87 to 0.96; p< 0.001). The RCT reporting mortality outcome also reported a composite measure that combined all-cause mortality with nonfatal heart failure events and found the intervention arm experienced a significant benefit from the program (OR, 0.22l; 95% CI, 0.07 to 0.65; p=0.005).59
Overall, we concluded that evidence is insufficient for the efficacy of MTM for reducing all-cause mortality at 6 months to 4 years based on direct evidence from a single, imprecise RCT with medium study limitations and two inconsistent observational studies with high study limitations (Table 37). We relied more heavily on the RCT evidence for our overall SOE rating because of the inability of observational study designs to adequately mitigate for selection bias relative to a mortality outcome compared with RCT designs.
Table 37
All-cause mortality: Strength of evidence.
Gastrointestinal Bleeding Events
One cohort study (high risk of bias because of selection bias) reported the relative risk reduction in gastrointestinal bleeding events among patients with a diagnosis of arthritis enrolled in a telephone-based MTM program within a large U.S. integrated health care system.42 The investigators compared the number of gastrointestinal bleeds after 6 months between patients with arthritis who did and did not enroll in the MTM program. The specific N analyzed was not included. Enrolled patients had a 60 percent relative reduction in gastrointestinal bleeds; the nonenrolled patients had no change in gastrointestinal bleeds (p=0.001 for between-group difference in change in gastrointestinal bleeds).
Overall, we concluded that evidence is insufficient for the efficacy of MTM for reducing gastrointestinal bleeding events based on direct but imprecise evidence from one cohort study with high study limitations (Table 38).
Table 38
Gastrointestinal bleeding events: Strength of evidence.
Self-Reported Health Status: SF-36 Measures
SF-36 Measures: Overview
Eight RCTs54,55,64,84-86,89,91 and one cohort study70 reported health status outcomes using the Medical Outcomes Study Short-Form questionnaire (SF-36) (Table 39). The eight SF-36 domains, which combine into two components, are as follows—physical health: physical functioning, physical role functioning, bodily pain, and general health perceptions; and mental health: vitality, emotional role functioning, social role functioning, and mental health. Seven trials54,55,64,84,85,89,91 and the cohort study70 reported scores for all eight domains. One trial reported only its two component scores (i.e., physical health; mental health).86 Finally, one trial reported both component and domain scores.55 The trials differed by overall risk of bias (one, low; four, medium, and three, high); the cohort study was high risk of bias.
Table 39
Scores on SF-36 measures: Summary of effects from meta-analyses.
One trial (medium risk of bias) focused on patients at high risk of experiencing a drug-related problem.54 This trial compared an intervention arm that included a clinical pharmacist intervention delivered in an ambulatory care clinic with usual medical care. It reported between-group differences with p-values less than 0.05 for four of the eight SF-36 domains (namely, bodily pain, general health perceptions, vitality, and mental health) and for a question that assessed change in health status. All these differences favored the intervention group. However, to control for multiple comparisons, the investigators set alpha at 0.01 when evaluating statistical significance. Using this more conservative alpha level, they investigators reported that only the bodily pain domain and the item assessing change in health status were statistically significant.
Of the eight remaining studies reporting results for SF-36 domains), four trials (one low risk of bias;84 three medium risk of bias55,85,91) reported no statistically significant between-group differences on any SF-36 score. Two trials64,89 and the cohort study70 (all high risk of bias) reported one statistically significant (p<0.05) between-group difference, favoring the intervention group—specifically for vitality89—among the total of 24 comparisons examined across the three studies. Finally, for one trial (medium risk of bias overall), we rated risk of bias for the SF-36 outcomes as high because of numerous errors in the table reporting these findings (e.g., group mean not contained within 95% CI, group mean not centered within 95% CI);55 it reported no statistically significant between-group differences on any SF-36 elements.
SF-36 Measures: Meta-Analyses
Our analysis focuses on the three trials rated either low or medium risk of bias that provided sufficient data to calculate mean differences for the eight SF-36 domain scores.1-3 We also conducted sensitivity analyses that included the two high risk-of-bias trials in addition.55,89 We omitted one trial from the meta-analyses altogether because it reported only that none of the SF-36 domains differed significantly but did not give any precise values.91 Similarly, we excluded one trial64 and the cohort study70 in the meta-analyses because they did not report standard deviations, standard errors, or exact p-values for any of the between-group comparisons; both studies reported that MTM did not produce any significant differences in anySF-36 domain. Finally, we omitted one trial from the domain-specific meta-analyses because it reported only component scores.86 To correct for the potential inflation of Type I error attributable to multiple comparisons, we used a threshold of α/number of tests (i.e., domains; 0.05/8=0.006) when evaluating statistical significance. Below, we describe our findings for each SF-36 domain, focusing on the meta-analyses of just the low to medium risk-of-bias trials (i.e., the smaller meta-analysis). We did not conduct a meta-analysis for the SF-36 component scores because only one trial was rated as low to medium risk of bias for these outcomes.
SF-36 Domain Scores
Physical functioning. Results from the low and medium risk-of-bias analysis showed no benefit for the MTM interventions (mean difference: 1.17; 95% CI, -3.87 to 6.21; p=0.65; I2=48.36). Adding the two high risk-of-bias studies did not alter this conclusion (mean difference:-0.44; 95% CI, -2.64 to 1.77; p=0.70; I2=10.67) (Appendix F-1).
Physical role functioning. Results from the low and medium risk-of-bias analysis showed no benefit for the MTM interventions (mean difference: 3.39; 95% CI, -0.79 to 7.43; p=0.11; I2=27.66). Adding the two other studies did not alter this conclusion (mean difference: 0.73; 95% CI, -3.43 to 4.90; p=0.73; I2=44.73) (Appendix F-2).
Bodily pain. Results from the low and medium risk-of-bias analysis showed no benefit for the MTM interventions (mean difference: 3.32; 95% CI, -1.22 to 8.01, p=0.15; I2=0). Adding the two other studies did not alter this conclusion (mean difference: 1.46; 95% CI, -2.79 to 5.71; p=0.50; I2=81.01) (Appendix F-3).
General health perceptions. Results from the low and medium risk-of-bias analysis showed no benefit for the MTM interventions (mean difference: 1.92; 95% CI, -0.02 to 3.84, p=0.051; I2=0). With the additional studies, however, results suggested a beneficial effect of MTM interventions on general health perceptions (mean difference: 2.48; 95% CI, 2.12 to 2.83, p<0.001; I2=0) (Appendix F-4).
Vitality. Results of the smaller meta-analysis showed no benefit for the MTM interventions, after correcting for multiple comparisons (mean difference: 2.80; 95% CI, 0.65 to 4.94; p=0.01; I2=0). If we had set alpha at the conventional 0.05 level, our findings would demonstrate a beneficial effect of MTM interventions. Adding the two other studies to the analysis did not change the no-benefit results for the MTM interventions, even at the more conventional alpha level (mean difference: 1.30; 95% CI, -0.31 to 2.90; p=0.11; I2=15.79) (Appendix F-5).
Emotional role functioning. Results from the smaller meta-analysis showed no benefit for the MTM interventions (mean difference: 5.39; 95% CI, -7.24 to 18.02; p=0.40; I2=74.34). Adding the other two studies did not alter this conclusion (mean difference: 3.44; 95% CI, -4.00 to 10.88; p=0.37; I2=78.66). However, the high I2 statistic for both these meta-analyses suggested considerable heterogeneity among the studies for this particular domain (Appendix F-6).
Social role functioning. Results from the low and medium risk-of-bias analysis showed no benefits from MTM interventions (mean difference: 2.93; 95% CI, -0.09 to 5.95; p=0.057; I2=0). With the additional studies, however, results suggested a beneficial effect of MTM interventions (mean difference: 0.63; 95% CI, 0.29 to 0.97; p<0.001; I2=0) (Appendix F-7).
Mental health. Results from the smaller meta-analysis showed no benefit for the MTM interventions (mean difference: 1.62; 95% CI, -0.36 to 3.59; p=0.11; I2=0). Adding the two other studies did not alter this conclusion, after correcting for multiple comparisons (mean difference: 1.11; 95% CI, 0.28 to 1.94, p=0.009; I2=0) (Appendix F-8).
Two RCTs provided data for the SF-36 physical and mental component scores.55,86 Although we rated both trials as medium risk of bias overall, we rated one of them55 as high risk of bias for the SF-36 outcomes because of errors in the table presenting these findings. None of the between-group differences examined in either study were statistically significant with alpha set at 0.05.
SF-36 Strength of Evidence Grades
Based on the evidence from low- and medium risk-of-bias trials (3 trials; 1,343 randomized, 1,169 analyzed) with medium study limitations, precise, and direct evidence, we graded the strength of evidence for the effect of MTM interventions on six of the eight SF-36 domains and the overall physical and mental component scores as low for no benefit. For the remaining two domains–vitality and emotional role functioning, we judged the evidence as imprecise and rated the evidence as insufficient (Table 40).
Table 40
SF-36: Strength of evidence.
Condition-Specific Quality of Life
Two small RCTs72,74 reported condition-specific quality-of-life outcomes (Table 41). One RCT (medium risk of bias) of just patients with diabetes compared patients in a study arm that included a clinical pharmacist intervention delivered in an ambulatory care clinic with those receiving usual medical care.72 The investigators reported no significant difference in diabetes-specific quality-of-life between the intervention and control arms at the end of 6 months. The other RCT73,74 (high risk of bias) of patients with renal disease reported a significant difference at 1 year favoring the pharmaceutical care program We graded the strength of evidence, using only the medium risk-of-bias trial, as insufficient (single study, direct, but imprecise) (Table 42).
Table 41
Condition-specific quality of life: Summary of results.
Table 42
Condition-specific quality of life: Strength of evidence.
Patient Satisfaction
Five studies reported on various patient satisfaction measures and outcomes; four were trials (including two cluster randomized trials)51,64,84,86 and one was a cohort study.70 All compared patient satisfaction outcomes for patients receiving some form of MTM intervention and patients receiving some type of usual care (Table 43). Of these studies, we rated two RCTs low or medium risk of bias, two cluster randomized trials as medium or high risk of bias; and the cohort study as high risk of bias.
Table 43
Patient satisfaction: Summary of results.
One RCT (low risk of bias) focused on patients age 65 and older who were taking five or more regularly scheduled medications.84 This study compared patients who receiving clinical pharmacist intervention delivered in an ambulatory care clinic with those receiving usual outpatient care. The study reported non-significant between-group differences for two satisfaction measures (i.e., satisfaction with general health care and satisfaction with pharmacy-related care).
The other RCT (medium risk of bias) focused on patients at high risk of experiencing a drug-related problem.54 This study compared patients receiving a clinical pharmacist intervention delivered in an ambulatory care clinic with those in usual medical care. The study reported a nonsignificant between-group difference on a measure assessing patient satisfaction with the primary care provider.
One cluster trial (medium risk of bias) focused on patients ages 65 or older who were taking three or more medications concurrently.86 This study evaluated a community pharmacy-based intervention and assessed nine different measures of satisfaction at baseline, at 6-7 months following baseline, and at 12 to 13 months following baseline. This study reported statistically significant between-group change in a measure labeled, Evaluation and Goal Setting. This measure included six items assessing the extent to which the pharmacist involved the patient in setting therapeutic goals. However, none of the items asked directly about patient satisfaction with the goal setting process. This study also reported a statistically significant between-group change from baseline to the 12- 13-month followup on a measure labeled, Communicates with Doctor. This measure included two items asking about whether the patient's pharmacist and doctor work together to determine the most appropriate therapy for the patient. Neither item asked directly about patient satisfaction with the level of pharmacist-doctor communication. Finally, this study reported a statistically significant between-group change in a measure labeled, Trust. At baseline, patients in the intervention group reported lower trust in their pharmacist. Over the course of the study, their level of trust improved to the level reported by patients in the control group at baseline, accounting for the between group differences reported. The study reported no statistically significant between-group changes on the remaining six satisfaction measures, including a measure that directly assessed overall satisfaction with pharmacy services.
When grading strength of evidence, we did not consider the results from the remaining cluster trial RCT64 and the cohort study70 because they were rated as high risk of bias. We also did not consider findings from three other studies (one RCT,72 one nonrandomized clinical trial,49 and one cohort study42) because they assessed only changes in satisfaction over time in the intervention arm and did not make any between-group comparisons. Overall, we concluded that the strength of evidence for MTM interventions with respect to patient satisfaction was low for no benefit (Table 44).
Table 44
Patient satisfaction: Strength of evidence.
Resource Utilization
Key Points: Resource Utilization
- Effective MTM interventions might plausibly lead to either an increase or a decrease in resource utilization, depending on the baseline status of the patient and intended goals of the intervention. When studies did not present a clear hypothesis or expected direction of effect, we were unable to interpret changes in resource utilization outcomes as either a benefit or a harm of MTM interventions.
- Evidence was insufficient to assess the effectiveness of MTM in changing numerous measures of use of health care resources. These included use of generic medications; several measures of medication costs (costs of patient copays for medication, overall outlays on medications, medications and other medical costs); outpatient visits and costs; laboratory tests and costs; emergency department visits and costs; and and length of hospital stay.
- MTM intervention reduced medication costs for health plans (3 trials, medium study limitations, consistent, indirect, imprecise).
- MTM interventions among patients with a variety of clinical conditions from trials did not demonstrate a consistent change in the number of hospitalizations when compared with usual care, but one cohort study that partially addressed confounding found evidence of reduced hospitalization in the intervention arm (high study limitations, unknown consistency, direct, precise). Together, the lack of consistency across studies suggests insufficient evidence on the number of hospitalizations.
- In one large cohort, MTM interventions appeared to reduce the risk and costs of hospitalization for patients with diabetes (high study limitations, unknown consistency, direct, precise), but results for patients with unspecified or other clinical conditions did not support these results.
- MTM interventions in the home reduce the rate of hospitalizations for patients with heart failure (one cohort, high study limitations, direct, precise, low strength of evidence of benefit).
Detailed Synthesis: Resource Utilization
Use of Generic Medications
Understanding whether a change in the number of medications taken following an MTM intervention is a measure of appropriate resource utilization requires knowledge of the goal of drug therapy. A decrease in the number of medications can represent regimen simplification and resolution of therapeutic duplication; thus, it can be interpreted as a measure of appropriate resource utilization. The converse—that is, an increase in number of medications—cannot, however, be interpreted as a measure of inappropriate resource use. An increase in number of medications can, in fact, represent appropriate use of resources when it resulted from identifying and resolving an inadequate drug regimen.
Numerous studies provided information on the number of medications at followup in intervention and control arms or on the change in number of medications between baseline and followup.37,49-51,55,57,61,62,64,73,74,82,84-86,93,94, The use of generic medications, by contrast, can be interpreted as cost-saving.
Three cohort studies examined the use of generic medications (Table 45). One cohort study, designed to identify the impact of 2010 Part D MTM programs, compared cohorts (standalone Prescription Drug Plan or Medicare Advantage Prescription Drug Plan) receiving MTM with a comprehensive medication review with cohorts receiving usual care for congestive heart failure, chronic obstructive pulmonary disease, and diabetes, after limiting the sample to those newly eligible or enrolled for MTM and controlling for characteristics such as demographics, medical comorbidities, condition severity, and intensity of provider care.62 The study found very low generic substitution ratios, likely because many patients were already on generic medications. In a small number of instances, intervention arms were statistically significant from control arms, but the direction of change was inconsistent and the total magnitude of change was small. Two studies evaluated telephone-based MTM42,57 and one also compared community pharmacy-based MTM with educational mailings.57 We assessed both studies as high risk of bias owing to lack of adjustment for potential confounding from study design (intervention refusers versus acceptors)42 or lack of capacity of pharmacists or inability to reach patients.57
Table 45
Use of generic medications: Summary of results.
Together (or taking the medium risk-of-bias cohort study alone), these studies offer insufficient evidence, based on study limitations, inconsistency, and imprecision, to judge the effectiveness of MTM on use of generic medications (Table 46).
Table 46
Use of generics for MTM versus usual care: Strength of evidence.
Medication Costs: Overview
Eighteen studies reported data on costs of prescription medications (Table 47). We use the same language as the authors in describing their measures; they varied in study design and risk of bias and used a wide range of measures that cannot all be meaningfully combined. We categorized these outcomes in four groups; patient out-of-pocket costs (copayments), health plan costs or claims, combined outlays by patients and insurers, and combinations of medications and other costs. Table 47 lists studies in order by outcome category (or outcomes in some cases) and then alphabetically by author name. Later sections offer a detailed synthesis by these four categories of costs and describe the relevant studies in more detail. We were unable to categorize one high-risk-of-bias cohort study70,71 because it did not offer sufficient information on how “charges” were calculated. We note that several studies use the term “costs” although the specific measure used may not reflect true costs if they do not account for profits or subsidies.
Table 47
Measures used in studies of costs of medications.
Medication Costs: Patient Copayments
Four studies (one medium risk-of-bias RCT,60 one nonrandomized controlled trial [NRCT] of medium risk of bias49 and two cohort studies of high risk of bias37,42) compared the copayments for patients who refused MTM with patients who accepted MTM enrollment. These studies provided inconsistent evidence that patient medication co-payments increased following MTM. Table 48 documents the main findings. We calculated mean differences between groups when the original authors did not provide those data; all currencies are rounded to two decimals (i.e., for U.S. currency, cents). The trial showed a decrease in costs of $234 in the intervention arm and $170 in the control arm, but without information on numbers in each arm, we cannot calculate variance.
Table 48
Patient copayments: Summary of results.
The NRCT compared patients in the MTM arm with controls within and outside the intervention county; the control arms had declines in copayments and the MTM had increases in copayments. The two cohort studies had inconsistent and imprecise estimates of effect; one study showed an increase in copayments for the MTM arm and a decline for the control arm,42 and the other reported a smaller increase in the MTM arm than in the control arm.37 None of these studies explained whether the increase in copayment was a result of an appropriate change in medication therapy or the desired effect of the intervention. Although the results were precise in the NRCT and suggested an increase in medication copayments following MTM, the lack of directness in interpreting this outcome as a measure of appropriate resource utilization and the absence of other low and medium risk-of-bias studies with sufficient information to assess consistency of findings suggests insufficient evidence to judge the effect of MTM interventions on patient medication co-payment (Table 49).
Table 49
Patient copayments: Strength of evidence.
Medication Costs: Expenditures by Insurers
Three RCTs (all medium risk of bias),50,55,61 the NRCT reported on above,49 and six cohort studies (four medium39-41,45,62,80 and two high risk of bias76,77,79) measured the net effect of MTM on expenditures incurred by insurers on medications (Table 50). Changes in health plan drug expenditures attributable to MTM depend on the net effect of MTM activities, which can entail adding clinically needed drugs, increasing doses or frequency, substituting therapeutically equivalent lower cost drugs, and simplifying regimens (singly or in combination). For individual patients, a net increase in expenditures may be the outcome of a more appropriate drug regimen. Included studies provided only the net effect on expenditures at the study arm level. All trials demonstrated that MTM either reduced health plan expenditures or limited the increase in expenditures over time for patients receiving the MTM intervention when compared with patients in the control or comparison arm. These results were not precise, however; confidence intervals included the null effect for all but one trial. Results from the nonrandomized studies came from very disparate studies: the smallest included 120 patients and the largest as many as 200,722 patients. The inherent heterogeneity within and across these studies likely explains the lack of consistency in direction, magnitude, and precision of effects.
Table 50
Total expenditures on medications by insurers: Summary of results.
We did not pool estimates of effect for the trials or the observational studies because of heterogeneity in outcomes, timing, and setting.
Based on the lack of precision and directness, we rated the evidence from medium risk-of-bias trials as low for benefit to evaluate the effect of MTM on expenditures by insurers; results from cohort and nonrandomized studies do not support a similar judgment, but the discrepancy in the strength of evidence from trials and nonrandomized studies is likely explained by the huge variability within and across observational studies (Table 51).
Table 51
Health plan expenditures: Strength of evidence.
Medication Costs: Total Outlays on Medications
Seven RCTs (one low risk of bias,84,94 five medium risk of bias51-55,84,91,92,94 and one high risk of bias73,74) and seven cohort studies, two medium38,48 and five high risk of bias,37,42-44,57 measured the effect of MTM on total outlays on medications. As with other data on resource utilization, we found it challenging to interpret inconsistent results when studies did not specify the expected mechanism of action and predicted direction of effect. An additional challenge relates to the wide variation in data sources and degree of clarity on how investigators calculated outlays. In some studies, the specific measure used includes the combination of expenditures incurred by insurers and patients for prescription medications (Table 52). In other studies, the measure is based on wholesale costs or full retail costs in the absence of insurance, but whether and how the cost is split between the insurer and the patient is unclear, nor is it clear how these wholesale or retail costs relate to actual incurred costs. Calculated differences in Table 52 are rounded to two decimals.
Table 52
Total outlays on medications: Summary of results.
We did not pool the five medium risk-of-bias studies because of the heterogeneity of measures.51-55,92 Two suggested an increase in outlays in the intervention arm51-55 (although estimates were imprecise and confidence intervals contained the null effect), two suggested a reduction,61,92 and one suggested no effect.91 The medium risk-of-bias cohort studies similarly demonstrated inconsistent results;38,48 in fact, the same study demonstrated a difference in outcomes based on the specific criteria for MTM enrolment by year within the program.48 The high risk-of-bias studies similar reported inconsistent results: some reported reduced outlays42,43,57 and others showed increased outlays37,44 or no effect73,74 following MTM.
Based on the lack of consistency, directness, and precision, we rated the evidence from five medium risk-of-bias trials and two cohort studies as insufficient to evaluate the effect of MTM on total outlays on medications (Table 53).
Table 53
Total outlays on medications: Strength of evidence.
Medication Costs: Combined Medication and Other Costs
Three trials (one low,84,94 one medium,55 and one high risk of bias64,65), one NRCT (medium risk of bias82), and three cohort studies (two medium45,80 and one high risk of bias76,77) provided inconsistent evidence of change in combined medication and other costs (variably defined in each study) (Table 54). Studies did not report their results in sufficient detail to allow pooling. Based on available information, we judged the evidence to be insufficient to evaluate the effect of MTM on combined medication and other costs (Table 55).
Table 54
Medication and other costs: Summary of results.
Table 55
Medication and other costs: Strength of evidence.
Number of Outpatient Visits
Eleven studies examined the effect of MTM interventions, when compared with usual care, on outpatient visits. These studies varied in geographic setting (seven Western European countries,64,65 the United States,45,51-54,56,69-71,79,82,84,94 the United Kingdom,91 Canada55), period of evaluation (3 months to 36 months), specific outcome measure (ranging from a focus on visits with physicians to total ambulatory care visits or contacts with physicians and nurses), and risk of bias. They are described in Table 56. No study indicated whether the intervention was specifically designed to increase or to decrease outpatient visits; as a result, the directionality of the results cannot be interpreted as a benefit or a harm.
Table 56
Number of outpatient visits: Summary of results.
Three RCTs (all low or medium risk of bias) provided sufficient data on outpatient visits within the first year to pool results.51-55,69 A meta-analysis of these studies, including results for the basic MTM arm for Touchette et al. (rather than the “enhanced MTM” arm),69 across outcomes from 5 to 12 months yielded an estimated standardized mean difference of 0.049 (95% CI, -0.034 to 0.133, p=0.247; I2=0) (Appendix F-9). Including the results of the “enhanced MTM” arm instead of the basic MTM arm did not change the direction or precision or results (standardized mean difference: 0.041; 95% CI -0.042 to 0.125, p=0.331, I2=0). Likewise, adding one trial with high risk of bias (stemming primarily from attrition bias64,65) to the meta-analysis did not alter the direction or precision of the estimate of effect (standardized difference in means: 0.032; 95% CI, -0.032 to 0.095, p=0.326, I2=0). Two studies (one low and one medium risk of bias) found fewer outpatient visits in the intervention arm, but confidence intervals spanned the null56 or the authors reported that the results were not statistically significant at the p=0.05 level.84,94 A seventh medium risk-of-bias RCT noted “no differences in hospital clinic attendance, use of social services or contacts with district nurses and health visitors before and after the pharmacist review” but did not indicate whether this observation extended to the control arm and offered no statistics.91 The single nonrandomized controlled study found no differences between study arms in an intention-to-treat analysis.82
One medium risk-of-bias cohort study reported a greater increase in physician visits in the intervention arm compared with the control arm.45Two high risk-of-bias cohort studies,70,71,79 reported no statistically significant differences between study arms in the number of outpatient facility claims but offered no additional information.
Based on the lack of consistency, we graded the body of evidence of medium risk-of-bias trials and cohort study as insufficient to evaluate the effect of MTM interventions on outpatient resource utilization (Table 57).
Table 57
Number of outpatient visits: Strength of evidence.
Cost of Outpatient Visits
Five studies examined the effect of MTM interventions, when compared with usual care, on the costs of outpatient visits (Table 58). These studies included four set in the United States51-54,70,71,76,77,79 and one set in Canada.55 The period of evaluation ranged from 5 months to 3 years. As with studies on the number of outpatient visits, no study indicated that the intervention was designed specifically to raise or lower the costs of outpatient visits; as a result, the directionality of the results cannot be interpreted as a benefit or a harm. As with other costs analyses, the data are in U.S. dollars unless otherwise specified and rounded to nearest two decimals.
Table 58
Costs of outpatient visits: Summary of results.
One low risk-of-bias and two medium risk-of-bias trials offered inconsistent evidence on the effect of MTM interventions on outpatient costs. Two U.S.-based VA studies51-54,84,94 found results favoring the intervention group, but the results were not statistically significant in one study.84,94 The Canadian study found no significant differences by study arm.55 Two cohort studies (high risk of bias) found significantly higher costs for the intervention arm than the usual care arm.70,71,76,77 Another U.S.-based cohort study of the Medicaid program in Iowa (high risk of bias) found no statistically significant differences in cost of outpatient visits by intervention arm but did not report details to determine direction of effect.79
Based on the lack of consistency and precision, we graded the body of evidence from the two trials as being insufficient to evaluate the effect of MTM interventions on the costs of outpatient visits (Table 59).
Table 59
Costs of outpatient resource utilization: Strength of evidence.
Number of Laboratory and Diagnostic Tests
Understanding whether a change in the number and costs of laboratory tests as a result of an MTM intervention measures appropriate resource use requires knowledge of the goals of drug therapy. MTM could raise numbers and costs of laboratory and diagnostic tests by identifying patients who should be receiving more frequent laboratory monitoring or by starting patients on new drugs that require laboratory monitoring based on their clinical situation. However, MTM could also lower numbers and costs of laboratory and diagnostic tests if it produces better coordination of care and prevents duplicative testing. Included studies did not specify the expected direction of effect from MTM on the number and costs of laboratory and diagnostic tests.
Two trials (both medium risk of bias; one set in the United States51-54 the other in Canada55) reported on the number of laboratory tests following MTM interventions (Table 60). The Canadian study included the number and costs of imaging procedures over a 5-month period;55 the U.S.-based study did not specify the inclusion of imaging procedures and evaluated tests and costs over a 12-month period. The U.S.-based found statistically significant differences; the Canadian study failed to find any significant differences.
Table 60
Number of laboratory and diagnostic tests: Summary of results.
The small number of studies limits our ability to explore causes for the observed heterogeneity. Factors such as differences in health systems, period of evaluation, and definition of the outcome could explain differences in results. Based on lack of consistency, we graded the body of evidence from these two medium risk-of-bias trials as insufficient to evaluate either the effect of MTM interventions on the number of laboratory and diagnostic and diagnostic tests (Table 61).
Table 61
Number of laboratory and diagnostic tests: Strength of evidence.
Costs of Laboratory and Diagnostic Tests
The two medium risk-of-bias studies reporting data on number of laboratory and diagnostic tests also provided information on costs.51-55 Two other studies, one low risk of bias84,94 and one high risk of bias,76,77 provided information on costs. The challenges associated with interpreting evidence on number of laboratory and diagnostic tests apply to costs as well (Table 62). The three U.S.-based studies showed a trend of reduced costs of laboratory and diagnostic tests in the intervention arm, but these results were not consistent in magnitude of effect or precision.51-54,76,77,84,94 The Canadian study showed an increase in laboratory and imaging costs in the intervention arm.55
Table 62
Costs of laboratory tests: Summary of results.
Based on lack of consistency, we graded the body of evidence from these three medium risk-of-bias trials as insufficient to evaluate either the effect of MTM interventions on the costs of laboratory tests (Table 63).
Table 63
Costs of laboratory tests: Strength of evidence.
Emergency Department Visits
Nine studies reported changes in emergency department (ED) visits following MTM interventions: four trials (one low risk of bias,84,94 two medium risk of bias,55,69 and one high risk of bias85) and five cohort studies (four medium risk of bias44,45,62,80 and one high risk of bias46,47) (Table 64). We excluded data from one study in the analysis below because it reported total number of events by each intervention arm rather than by patients within intervention arm.80 We could not pool results from the eight studies with complete data because we did not have sufficient numbers of studies with similar designs. Across all low and medium risk-of-bias studies, the confidence intervals for the effects from the medium risk-of-bias studies spanned the null effect for the entire study44,55,84,94 or a subset of analyses.62,69 Studies with multiple comparisons found some signals of benefit. For example, the low risk-of-bias trial found a lower mean number of ED visits in the intervention arm when compared with the usual care, but this effect was statistically significant only for the basic MTM arm.69 Likewise, a medium risk-of-bias cohort study of 2010 Medicare Part D found consistently lower odds of all-cause ED visits across all clinical conditions but did not find the same consistency of direction, magnitude, or precision for condition-specific ED visits.62 One trial, rated high risk of bias for this outcome, reported a decline in ED visits in the intervention arm and no change in the control arm;85 it did not, however, provide patient-level means. As a result, we are unable to judge the variance within the sample. Another high risk-of-bias cohort study found lower odds of ED visits among patients accepting MTM when compared with patients refusing or disenrolling from MTM, but the study did not account for confounders that may have influenced MTM participation and outcomes.46,47
Table 64
Emergency department visits: Summary of results.
Given the lack of consistency and precision, evidence is insufficient to draw conclusions about the effectiveness of MTM in reducing ED visits (Table 65).
Table 65
Emergency department visits: Strength of evidence.
Emergency Department Costs
Two trials (one low risk of bias84,94 and one medium risk of bias55) and two cohort studies (one medium risk of bias62 and one high risk of bias79) reported on costs of ED visits following MTM interventions (Table 66). Despite differences in geographic setting and health care delivery systems (Canada55 and the United States62,79,84,94), period of evaluation (5 months,55 and 12 months62,79), and risk of bias, no study demonstrated an effect of any MTM-type intervention (Table 66).55,62,79,84,94 Variations within the large cohort study and across studies suggest that MTM may be associated with additional costs or costs saving. Because the evidence does not offer consistency or precision of results, we graded it as insufficient to evaluate the effect of MTM on ED costs (Table 67).
Table 66
Costs of emergency department visits: Summary of results.
Table 67
Cost of emergency department visits: Strength of evidence.
Hospitalizations
Twelve studies measured hospitalizations as an outcome following MTM interventions.44,47,48,51-55,62,64,65,69,73,74,78,80 ,84,91,94 Of these, we have excluded data from two studies in the analysis below because they reported total number of events by each intervention arm rather than by patients within intervention arm. As a result, we are unable to assess variance.80,91 We report on the mean number of hospitalizations, the risk of hospitalization, and the rates of hospitalization (Table 68).
Table 68
Hospitalizations: Mean number, risk and rates.
Five trials (one low risk-of-bias study,84,94 three medium risk-of-bias study,51-55,69 and one high risk-of-bias study74) and one medium risk-of-bias cohort study45 reported on the change in number of hospitalizations or mean number of hospitalizations following MTM interventions (Table 68). The low risk-of-bias study did not provide sufficient information on variance to allow pooling.84,94 Using a random-effects model, we pooled results for three medium risk-of-bias trials51-55,69 for all-cause hospitalizations and obtained a mean difference of 0.037, 95% CI, -0.006 to 0.080; p=0.094; I2=0 (Appendix F-10). We obtained similarly small effect sizes and wide confidence intervals spanning the null when including the enhanced arm of the Touchette et al. study (0.038, 95% CI -0.004 to 0.081; p=0.076; I2=0)69 or including the single high risk-of-bias trial (0.033, 95% CI -0.046 to 0.112; p=0.412; I2=54.484).74 One study also provided data to calculate an effect size and confidence intervals for drug-related hospitalizations that also overlapped the null effect.55 These results are consistent with the findings of the low risk-of-bias study, which reported no statistically significant differences in number of hospitalizations. The medium risk-of-bias cohort study was not consistent with the trial evidence: it found significantly lower inpatient visits among MTM acceptors compared with MTM refusers.45
Six studies (one medium-risk-of-bias RCT,69 three medium risk-of-bias cohort studies,44,48,62 one high risk-of-bias RCT,64,65 and one high risk-of-bias cohort study46,47) reported on the percentage hospitalized following MTM (Table 68) and odds or hazard ratios of hospitalization. Not all studies provided sufficient data to allow the generation of a summary estimate of effect with confidence intervals, nor we did find sufficient numbers of studies of similar design to permit pooling. The results are inconsistent; two studies (one low risk-of-bias trial and one medium risk-of-bias cohort) suggested higher hospitalizations with MTM rather than usual care,44,69 and three studies (one medium risk-of-bias cohort study,48 one high risk-of-bias trial,64,65 and one high risk-of-bias cohort study)46,47 suggested lower hospitalizations for the MTM arm (but with confidence intervals overlapping the null in one instance).64,65 The sixth study, a large medium risk-of-bias study of Medicare Part D in 2010, conducted separate analyses for cohorts by plan type (standalone Prescription Drug Plan or Medicare Advantage Prescription Drug Plan) and clinical condition (congestive heart failure, chronic obstructive pulmonary disease, and diabetes) for the odds of all-cause hospitalization and condition-specific hospitalization.62 The diabetes cohort had lower risks of hospitalization (all-cause or condition-specific) regardless of plan type. For the other cohorts in this study, the magnitude, direction, and precision of the effect varied by specific analysis. The inconsistency in results may be a consequence of the wide range of included populations and interventions.
One cohort study (medium risk of bias) reported a decreased rate of hospitalization for heart failure at any time during study. This study of home medications review was designed specifically to delay the next hospitalization among patients with heart failure in Australia.78
Based on inconsistent results from trials with medium study limitations, we rated MTM as having insufficient on the mean number of hospitalizations. One cohort study offers low strength of evidence of reduced number of inpatient visits (Table 69). The lack of consistency between the trial and cohort results and the higher risk of bias from cohort studies of acceptors and refusers suggest that the overall strength of evidence from all designs is insufficient for lack of consistency. We rated the evidence on the risk of hospitalization as insufficient based on inconsistent (or unknown consistency) and imprecise evidence (Table 70) for unspecified clinical conditions, COPD, or CHF alone. We rated the evidence as low for benefit for diabetes cohort. We draw attention to the risk of selective analysis reporting in the evidence on diabetes. Only one study elected to provide condition-specific outcomes62; further analysis on diabetes cohorts from existing studies or new studies with fewer limitations may well change the direction, magnitude, and precision of effect from available evidence. By contrast, we rated the evidence on the rate of hospitalization as low based on a precise estimate from a large cohort study (Table 71); we note that the findings from a single study of a very specific intervention (home medicines review) of heart failure patients limits its applicability to patients with other morbidities and settings. Together, the lack of consistency across these measures of hospitalization likely reflects heterogeneity in numerous factors in this evidence base.
Table 69
Mean number of hospitalizations: Strength of evidence.
Table 70
Risk of hospitalization: Strength of evidence.
Table 71
Rate of hospitalization: Strength of evidence.
Hospitalization Costs
Two trials (medium risk of bias)51-55 and two cohort studies (one medium risk of bias62 and one high risk of bias79) reported changes in costs of hospitalization following MTM interventions (Table 72). Although two studies were set in the United States, one evaluated outcomes from Veteran Affairs Medical Centers51-54and the other evaluated claims from the Iowa Medicaid program.79 The third study was set in Canada.55 The period of evaluation of outcomes ranged from 5 months55 to 12 months.51-54,79 The other cohort study, designed to identify the impact of 2010 Part D MTM programs, compared cohorts (standalone Prescription Drug Plan or Medicare Advantage Prescription Drug Plan) receiving MTM with a comprehensive medication review with cohorts receiving usual care for congestive heart failure, chronic obstructive pulmonary disease, and diabetes, after limiting the sample to those newly eligible or enrolled for MTM and controlling for characteristics such as demographics, medical comorbidities, condition severity, and intensity of provider care.62 Three were consistent in demonstrating no effect of MTM interventions on the costs of hospitalization; the large Part D evaluation demonstrated inconsistent results by clinical condition. MTM appeared to consistently reduce costs of all-cause and condition-specific hospitalization costs for the diabetes cohort only. Based on lack of consistency in direction of effect and lack of precision, we graded the body of evidence as being insufficient to evaluate the effect of MTM interventions on the cost of hospitalization overall and low for diabetes (Table 73).
Table 72
Costs of hospitalization: Summary of results.
Table 73
Cost of hospitalization: Strength of evidence.
Hospital Length of Stay
Two trials (one low84,94 and one high risk of bias73,74) reported inconsistent results on the effects of MTM interventions on length of hospital stay. Neither study reported statistically significant results, but the low risk-of-bias study found longer stays in the intervention arm and the high risk-of-bias study found shorter stays in the intervention arm (Table 74). Based on lack of precision of the results, we graded this outcome as having insufficient evidence to evaluate the effect of MTM interventions on the length of hospital visits (Table 75).
Table 74
Length of hospitalization: Summary of results.
Table 75
Length of hospital stay: Strength of evidence.
Key Question 3. Outcomes of Medication Therapy Management by Intervention Features
Key Points
- Studies do not routinely report outcomes of MTM by intervention features.
- We found evidence on five intervention features informed by a single study for each feature: access to patient data,69 intensity of care coordination and followup after comprehensive medication review,88 pharmacy intensity of adoption of the intervention,79 community pharmacy versus call-center pharmacy,57 and private versus Medicaid coverage of pharmaceutical care.58 With the exception of the investigation reporting on access to patient data, these studies had a high risk of bias.
- Evidence was insufficient on access to patient data, intensity of care coordination and followup after comprehensive medication review, pharmacy intensity of adoption of the intervention, community pharmacy versus call-center pharmacy, and private versus Medicaid coverage of pharmaceutical care for most outcomes.
- MTM programs with pharmacist access to patient records reduces the number of adverse drug events (low strength of evidence) when compared basic MTM programs.
- Community pharmacists increase the generic dispensing ratio more than call-center-based pharmacists (low strength of evidence).
Detailed Synthesis: Intervention Features
Access to Patient Records
A single trial (medium risk of bias) of 556 patients overall (373 in the two MTM arms) evaluated differences between two MTM intervention arms; one without access to patient records (denoted “basic” MTM) and one specifically with such access in the form of a two-page clinical synopsis containing basic data on a patient's medical history, laboratory values, and current medications, including over-the-counter and herbal medications (denoted “enhanced MTM”).69 Table 76 provides the effect size and strength of evidence for the seven outcomes assessed in this trial. In all instances, we rated the trial as medium for study limitations and unknown for consistency; we do not repeat these ratings in the table. With the exception of mean number of adverse drug events, which suggested benefit for enhanced MTM when compared with basic MTM (low strength of evidence), we found insufficient evidence to evaluate the comparative effectiveness of the two arms.
Table 76
Access to patient records (basic MTM versus enhanced MTM): Strength of evidence.
Intensity of Care Coordination and Followup Following Comprehensive Medication Review
One RCT (high risk of bias) of 131 patients aged 65 or older compared comprehensive drug therapy review and subsequent coordination and followup with the patient and physician with comprehensive review and subsequent referral to the usual pharmacist only (Table 77).88 For all outcomes, we rated this study as high for study limitations and unknown for consistency (not repeated in table). We found insufficient evidence to judge the effectiveness of MTM by intensity of adoption on all reported outcomes.
Table 77
Intensity of care coordination and followup following comprehensive medication review: Strength of evidence.
Pharmacy Intensity of Adoption
One cohort study (high risk of bias) of 2,211 patients evaluated eight outcomes based on pharmacy intensity of adoption of the MTM intervention (Table 78).79 Specifically, the authors categorized pharmacies that completed recommendations in at least one quarter into four groups: (1) for at least 50 percent of patients, high-intensity pharmacy (2) 25 to 49 percent as moderate intensity; (3) 1 to 24 percent as low intensity; and (4) no recommendations study as no intensity. For all outcomes, we rated this study as high for study limitations and unknown for consistency (not repeated in table). Outcomes for which we can infer a benefit or a harm from the effect are rated as direct outcomes. We found insufficient evidence to judge the effectiveness of MTM by intensity of adoption on all reported outcomes.
Table 78
Pharmacy intensity of adoption: Strength of evidence.
Community Pharmacy Versus Call Center
One large cohort study (high risk of bias) of the MirixiaPro platform (95,736 patients enrolled, 73,793 analyzed) compared patients using a community pharmacy, which included both face-to-face and telephone interactions, with patients using a call center pharmacy (Table 79).57 The investigators measured three diverse outcomes. In all instances, we rated the study as high for study limitations and unknown for consistency (not repeated in table).Outcomes for which we can infer a benefit or a harm from the effect are rated as direct outcomes. We found insufficient evidence for drug cost and drug use outcomes, which we rated as indirect evidence with high study limitations. MTM delivered by community pharmacists increases the weighted generic dispensing ratio (GDR) when compared with MTM delivered by call-center pharmacists (low strength of evidence). The study defines the weighted GDR as the number of generic 30-day equivalent claims divided by the total number of claims, and then weighted for each patient by a factor equal to the individual's total prescription volume multiplied by a constant to hold sample size unchanged.
Table 79
Community pharmacy versus call center: Strength of evidence.
Type of Payer
One cohort study (high risk of bias, N=615) compared outcomes for patients with Medicaid and patients with private insurance (Table 80).58 The investigators reported on three diverse outcomes. In all instances, we rated the study as high for study limitations and unknown for consistency (not repeated for each outcome in the table). We found insufficient evidence to judge the effectiveness of MTM by type of payer on all reported outcomes.
Table 80
Type of payer: Strength of evidence.
Key Question 4. Outcomes of MTM by Patient Characteristics
We did not identify any studies that analyzed outcomes of MTM by patient characteristics.
Key Question 5. Harms of Medication Therapy Management Interventions
Key Points
- Studies do not routinely report harms that result from MTM interventions. One study reported on inconvenience from information received through an MTM intervention. Study limitations and lack of precision of these results suggested that evidence was insufficient to evaluate the effect of MTM interventions on harms.
Detailed Synthesis: Inconvenience
A single cohort study (high risk of bias) compared pharmaceutical care with usual care (Table 81).70,71 The investigators reported that patients in the intervention arm were less likely to agree or strongly agree with the statement that they were inconvenienced by monthly appointments with the pharmacists than patients in the control arm (40 percent versus 69 percent; calculated OR, 0.278; 95% CI, 0.088 to 0.875; p=0.029). The sample size does not meet optimal information size criteria, suggesting lack of precision of the results.
Table 81
Inconvenience: Strength of evidence.
- Introduction
- Results of Literature Searches
- Components and Implementation Features of MTM Interventions
- Effect of Medication Therapy Management Interventions on Intermediate, Patient-Centered, and Resource Utilization Outcomes
- Outcomes of Medication Therapy Management by Intervention Features
- Outcomes of MTM by Patient Characteristics
- Harms of Medication Therapy Management Interventions
- Results - Medication Therapy Management Interventions in Outpatient SettingsResults - Medication Therapy Management Interventions in Outpatient Settings
- Key Informants - Testing of CYP2C19 Variants and Platelet Reactivity for Guiding...Key Informants - Testing of CYP2C19 Variants and Platelet Reactivity for Guiding Antiplatelet Treatment
- Cdc42 GTPase complex scaffold subunit Scd2 [Schizosaccharomyces pombe]Cdc42 GTPase complex scaffold subunit Scd2 [Schizosaccharomyces pombe]gi|19114656|ref|NP_593744.1|Protein
- MRSA Data Abstraction Form Elements - Screening for Methicillin-Resistant Staphy...MRSA Data Abstraction Form Elements - Screening for Methicillin-Resistant Staphylococcus Aureus (MRSA)
- Sleep Disturbance Plots - Menopausal Symptoms: Comparative Effectiveness of Ther...Sleep Disturbance Plots - Menopausal Symptoms: Comparative Effectiveness of Therapies
Your browsing activity is empty.
Activity recording is turned off.
See more...
