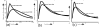NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Qayyum R, Wilson LM, Bolen S, et al. Comparative Effectiveness, Safety, and Indications of Insulin Analogues in Premixed Formulations for Adults With Type 2 Diabetes [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008 Sep. (Comparative Effectiveness Reviews, No. 14.)
This publication is provided for historical reference only and the information may be out of date.

Comparative Effectiveness, Safety, and Indications of Insulin Analogues in Premixed Formulations for Adults With Type 2 Diabetes [Internet].
Show detailsBackground
Optimal control of hyperglycemia in diabetics is of paramount importance for preventing or delaying the occurrence of diabetic complications. The United Kingdom Prospective Diabetes Study (UKPDS) found that intensive control of blood glucose in patients with type 2 diabetes mellitus (type 2 diabetes) resulted in a 10 percent risk reduction in diabetes-related mortality and a 25 percent risk reduction in microvascular complications when compared to conventional control of blood glucose.1 Although oral antidiabetic agents are used as first-line agents in patients with type 2 diabetes, insulin is required in a significant number of patients at some stage during the management of their diabetes in order to maintain optimal glycemic control. According to the National Health Interview Survey (NHIS), 28 percent of patients with type 2 diabetes are using insulin either alone (16 percent) or in combination with other oral antidiabetic agents (12 percent).2
Insulin replacement regimens can be either near-physiologic (prescribed to mimic the natural release of insulin from the beta-cells of the human pancreas) or non-physiologic (all other regimens). Physiologic insulin replacement regimens consist of a bolus of insulin administered at specific times in relation to meals in order to mimic the release of insulin from the beta-cells in response to food intake. In addition, some formulations of longer-acting insulin are prescribed to mimic the constant release of insulin that regulates hepatic gluconeogenesis and lipolysis.3, 4 Although type 2 diabetic patients are reluctant to start insulin therapy,5 insulin generally improves their quality of life.6 However, the addition of insulin to their treatment regimen may result in decreased flexibility in the timing of meals and activities, increased frequency of blood glucose monitoring, and increased risk of weight gain and hypoglycemia.4 Moreover, the need for multiple injections of short-acting (bolus insulin) and long-acting (basal insulin) agents may decrease patients' overall satisfaction with their treatment regimens.
Premixed insulin preparations offer a therapeutic alternative to multiple insulin injections that is also convenient for patients. These preparations are appropriate for patients who: (1) desire a convenient and simple insulin regimen; (2) are unwilling to administer multiple daily injections or use an insulin pump; (3) are unwilling or cannot undertake carbohydrate counting; (4) have a relatively predictable (routine) lifestyle; and (5) consume meals with approximately the same composition of calories, carbohydrates, fats, and fiber at fairly consistent and reproducible times every day.7, 8
Insulin analogues are related to human insulin and produced using recombinant DNA technology. These analogues have minor changes in their structure that impart pharmacokinetic properties that more closely mimic endogenous insulin secretion. In premixed insulin analogue preparations, insulins are mixed with their own protamine suspensions, slowing their release.
Three premixed insulin preparations are available commercially in the United States (see Figure 1). Insulin aspart 70/30, marketed by Novo Nordisk as NovoLog™ Mix 70/30, is an insulin aspart suspension containing 70 percent insulin aspart protamine crystals and 30 percent soluble insulin aspart. Insulin lispro 75/25, marketed by Eli Lilly as Humalog™ 75/25, is a mixture of 75 percent insulin lispro protamine suspension and 25 percent rapid-acting insulin lispro solution. Insulin lispro 50/50, marketed by Eli Lilly as Humalog™ 50/50, is a mixture of 50 percent insulin lispro protamine suspension and 50 percent rapid-acting insulin lispro solution (see Table 1).
Table 1
Pharmacokinetic characteristics of selected insulin preparations.
In the management of type 2 diabetes, the place of premixed insulin analogues in relation to other insulin regimens and noninsulin antidiabetic agents is unclear. They may provide a more physiologic glucose-lowering profile, thus providing better glycemic control (see Figure 2). In addition, as compared to premixed human insulin preparations (such as NPH/regular 70/30), premixed insulin analogues allow patients more flexibility in timing their meals, since these insulin preparations can be administered within 15 minutes before a meal.
Several studies have found that insulin aspart 70/30 and insulin lispro 75/25 lower postprandial glucose levels to a greater degree than does NPH/regular 70/30,9–12 although the timing of NPH/regular 70/30 may have affected the results in two studies.11, 12 However, the degree of improvement achieved in hemoglobin A1c, however, has been equivalent for the premixed insulin analogues and NPH/regular 70/30, despite improvement in postprandial glucose in a number of studies.9, 10, 13 Similarly, several studies have found that the rate of side-effects, such as hypoglycemia, is similar for premixed insulin analogues and premixed human insulins.10, 14, 15
Given the increasing prevalence of type 2 diabetes, the large number of patients who use insulin for glycemic control, and the importance of glycemic control in decreasing mortality and preventing long-term complications, it is important to establish the weight of evidence for the safety and effectiveness of these newer insulin therapies, as compared to those of traditional insulin regimens.
We have therefore performed a systematic review of published studies on the comparative effectiveness and safety of all the premixed insulin analogues that are approved by the Food and Drug Administration (FDA) and available in the United States. A comparison of the intermediate outcomes and clinical outcomes of these analogues to those of other antidiabetic treatments may give clinicians a better sense of how to choose the appropriate treatment for type 2 diabetic patients. In addition, the results presented here may provide policymakers, pharmacy benefit managers, and insurers with useful insights as they consider policies relating to medication coverage.
Conceptual Model
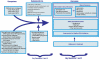
Our conceptual model (see Figure 3) summarizes the premixed insulin analogues, their main comparators, and their effects on intermediate and clinical outcomes, including potential adverse events. Premixed insulin analogues were developed to affect intermediate outcomes such as fasting glucose, postprandial glucose, and A1c. We call these intermediate outcomes and not clinical outcomes, since these are blood tests that are relevant only because of their relationship to clinical outcomes such as mortality. We visualize intermediate outcomes as being connected to metabolic derangements in the body on one hand and to clinical outcomes on the other, thus occupying an intermediate place in the development of diabetic complications.
Premixed insulin analogues and other antidiabetic agents may affect clinical outcomes directly or may do so indirectly by altering intermediate outcomes. Use of these medications may also be associated with adverse effects. These medications can also affect the patients' quality of life in the short-term because of the need for frequent injections and symptoms or because of the anxiety associated with poor glycemic control. The quality of life can also be affected in the long-term as a result of the effects on overall morbidity and mortality. Adherence to treatment is another important aspect of insulin administration that not only determines intermediate and clinical outcomes but also quality of life. Intermediate and clinical outcomes, safety and adverse events, and quality of life are affected not only by important population variables such as age and comorbid conditions but also by the intensity of intended glucose control and the target chosen for glucose control.
Because this was a comparative effectiveness review, we focused on the outcome measures that are routinely used in clinical practice and are known to help in optimizing glucose control. We did not evaluate outcomes that were not used in clinical practice, such as area under the curve or glucose excursions after premixed insulin analogue injection.
Scope and Key Questions
This systematic review was commissioned by the Agency for Healthcare Research and Quality (AHRQ) to address the following key questions:
- 5.
In adults (age ≥ 18 years) with type 2 diabetes, what is the effectiveness of premixed insulin analogues (insulin aspart 70/30, insulin lispro 75/25, insulin lispro 50/50) in achieving optimal glycemic control, as compared to insulin regimens including, but not necessarily limited to, the following preparations?
- a.
Premixed human insulin preparations (neutral protamine Hagedorn [NPH]/regular 70/30, NPH/regular 50/50)
- b.
Long-acting insulin analogues (insulin detemir, insulin glargine) administered alone
- c.
Intermediate-acting human insulin (NPH insulin) administered alone
- d.
Short-acting human insulin (regular insulin) administered prandially
- e.
Rapid-acting insulin analogues (insulin aspart, insulin glulisine, insulin lispro) administered separately (prandially) with a long-acting insulin analogue (insulin detemir, insulin glargine)
- 6.
For adults with type 2 diabetes, do premixed insulin analogues differ from other commonly used insulin preparations with regard to safety, adverse effects, or adherence? The adverse effects of interest include, but are not limited to, hypoglycemia (nocturnal and daytime), weight gain, and interactions with other medications.
- 7.
Does the effectiveness or safety of the new premixed insulin analogue regimens vary across the following subpopulations of patients with type 2 diabetes
- a.
The elderly (≥ 65 years), very elderly (≥ 85 years)
- b.
Other demographic groups (ethnic or racial groups, genders)
- c.
Individuals with comorbid medical conditions
- d.
Individuals with limited life expectancy
- e.
Individuals with disabilities
- 4.
What is the effectiveness and safety of the new premixed insulin analogue regimens in individuals on oral antidiabetic agents and individuals with different blood glucose patterns (such as fasting hyperglycemia or postprandial hyperglycemia) or types of control (such as tight control, usual control, good fasting or postprandial control)?
- Introduction - Comparative Effectiveness, Safety, and Indications of Insulin Ana...Introduction - Comparative Effectiveness, Safety, and Indications of Insulin Analogues in Premixed Formulations for Adults With Type 2 Diabetes
- KQ3- What is the evidence for the efficacy of sublingual immunotherapy (SLIT) in...KQ3- What is the evidence for the efficacy of sublingual immunotherapy (SLIT) in tablet and aqueous form, in the treatment of asthma? - The Role of Immunotherapy in the Treatment of Asthma
- helix-turn-helix domain-containing protein [Bifidobacterium breve]helix-turn-helix domain-containing protein [Bifidobacterium breve]gi|2811239287|ref|WP_376712467.1|Protein
- Methods - Self-Measured Blood Pressure Monitoring: Comparative EffectivenessMethods - Self-Measured Blood Pressure Monitoring: Comparative Effectiveness
- Preface - Pain Management Interventions for Hip FracturePreface - Pain Management Interventions for Hip Fracture
Your browsing activity is empty.
Activity recording is turned off.
See more...