NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Qayyum R, Wilson LM, Bolen S, et al. Comparative Effectiveness, Safety, and Indications of Insulin Analogues in Premixed Formulations for Adults With Type 2 Diabetes [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008 Sep. (Comparative Effectiveness Reviews, No. 14.)
This publication is provided for historical reference only and the information may be out of date.

Comparative Effectiveness, Safety, and Indications of Insulin Analogues in Premixed Formulations for Adults With Type 2 Diabetes [Internet].
Show detailsSearch Results
A summary of the search results for the primary literature review is presented in Figure 4. From the search, we retrieved 2,202 unique citations. After a review of the titles and abstracts, 135 were deemed eligible for further review, and the full articles were retrieved. A total of 50 articles were included in this review. Upon further inspection, we realized that Raskin 2005,39 Raskin 2007,40 and Brod 2007;41 Roach 200142 and Tirgoviste 2003;43 and Malone 200044 and Malone 200014 were conducted in the same study populations. These articles were abstracted together. Boehm 200445 and Boehm 20029 had the same study population of type 2 diabetes, but Boehm 200445 had the longer followup. We report only the results from Boehm 2004.45 We used data from Boehm 20029 when data were not reported in the second publication of the trial. Therefore, a total of 45 studies, represented in 50 articles, were included.
The bulk of the evidence was for insulin aspart 70/309, 11, 13, 15, 32, 39–41, 45–61 and insulin lispro 75/25,10, 12, 14, 42–44, 55, 58, 62–75 which were evaluated in 22 and 20 studies, respectively. Nine studies evaluated insulin lispro 50/50.10, 62, 64, 69, 76–80
For insulin aspart 70/30, there were seven studies comparing it to a premixed human insulin,9, 11, 15, 45, 48, 53, 56, 58 six to oral antidiabetic agents,46, 51, 54, 57, 59, 60 four to a long-acting insulin analogue,32, 39–41, 47, 50 two to an intermediate-acting human insulin,13, 15 and one each to a rapid-acting insulin analogue,32 a rapid-acting insulin analogue with a long-acting insulin analogue,52 a rapid-acting insulin analogue with an intermediate-acting human insulin,61 and exenatide.49 For insulin lispro 75/25, there were ten studies comparing it to a premixed human insulin,10, 12, 14, 44, 58, 62, 67, 70, 71, 73, 75 six to a long-acting insulin analogue,63–66, 74, 75 and three to an oral antidiabetic agent.42, 43, 68, 72 We did not identify any studies comparing insulin lispro 75/25 to a rapid-acting insulin analogue, a rapid-acting insulin analogue with a long-acting insulin analogue, an intermediate-acting human insulin, or exenatide. For insulin lispro 50/50, there were four studies comparing it to a premixed human insulin,10, 62, 77, 78 three studies comparing it with a long-acting insulin,64, 76, 79 one study comparing it to a combination of long-acting insulin analogue and rapid-acting insulin analogue,80 and one study comparing it to a rapid-acting insulin analogue.76 We did not indentify any studies comparing insulin lispro 50/50 to an intermediate-acting human insulin or exenatide. Furthermore, we did not identify any studies comparing any of the premixed insulin analogues to regular human insulin or to placebo.
There were four studies with a head-to-head comparison of premixed insulin analogues. Two studies compared insulin aspart 70/30 to insulin lispro 75/25.55, 58 Another two studies compared insulin lispro 75/25 to insulin lispro 50/50.62, 69
Table 3 shows the number of studies included that evaluated each treatment comparison for each outcome.
Table 3
Number of included studies evaluating each treatment comparison for each outcome.
Key Question 1
In adults (age ≥ 18 years) with type 2 diabetes, what is the effectiveness of premixed insulin analogues (insulin aspart 70/30, insulin lispro 75/25, insulin lispro 50/50) in achieving optimal glycemic control, as compared to insulin regimens including, but not necessarily limited to, the following preparations?
- f.
Premixed human insulin preparations (NPH/regular 70/30, NPH/regular 50/50)
- g.
Long-acting insulin analogues (insulin detemir, insulin glargine) administered alone
- h.
Intermediate-acting human insulin (NPH insulin) administered alone
- i.
Short-acting human insulin (regular insulin) administered prandially
- j.
Rapid-acting insulin analogues (insulin aspart, insulin glulisine, insulin lispro) administered separately (prandially) with a long-acting insulin analogue (insulin detemir, insulin glargine)
Key Question 2
For adults with type 2 diabetes, do premixed insulin analogues differ from other commonly used insulin preparations with regard to safety, adverse effects, or adherence? The adverse effects of interest include, but are not limited to, hypoglycemia (nocturnal and daytime), weight gain, and interactions with other medications.
Most of the studies that addressed Key Question 1 also addressed Key Question 2. We report both these questions together in one section to avoid repetition. In addition, most studies that reported intermediate outcomes also reported safety and adverse events. To prevent repetition, we discuss intermediate outcomes and adverse events together in one section.
Intermediate Outcomes and Adverse Events
Key Messages
Fasting glucose
- Premixed insulin analogues may be less effective than long-acting insulin analogues alone in lowering fasting glucose.
- Insulin aspart 70/30 was similar to long-acting insulin analogues in lowering fasting glucose (pooled mean difference = 6.4 mg/dL; 95 percent CI: -1.5 to 14.2 mg/dL; p = 0.11).
- Insulin lispro 75/25 was less effective than long-acting insulin analogues in lowering fasting glucose (pooled mean difference = 8.5 mg/dL; 95 percent CI: 3.6 to 13.3 mg/dL; p = 0.001).
- Insulin lispro 50/50 was less effective than long-acting insulin analogues in lowering fasting glucose (mean difference in 2 studies: (1) -28 mg/dL and (2) -30.6 mg/dL; p < 0.001 in both studies).
- Premixed insulin analogues were similarly effective as premixed human insulin preparations in lowering fasting glucose.
- Insulin aspart 70/30 was less effective than premixed human insulin preparations in lowering fasting glucose (pooled mean difference = 8.3 mg/dL; 95 percent CI: 0.16 to 16.5 mg/dL; p = 0.04).
- Insulin lispro 75/25 was similar to premixed human insulin preparations in lowering fasting glucose (pooled mean difference = 0.12 mg/dL; 95 percent CI: -6.05 to 6.29 mg/dL; p = 0.97).
- Insulin lispro 50/50 may be less effective than premixed human insulin preparations in lowering fasting glucose (effect size in two studies: (1) mean difference = 30.3 mg/dL; p < 0.001 and (2) mean difference = 23 mg/dL; p = nonsignificant).
- Premixed insulin analogues were more effective than noninsulin antidiabetic agents in lowering fasting glucose.
- Insulin aspart 70/30 and insulin lispro 75/25 were more effective than oral antidiabetic agents in lowering fasting glucose (insulin aspart 70/30: pooled mean difference = -13.9 mg/dL; 95 percent CI: -24.4 to -3.4 mg/dL; p = 0.009; insulin lispro 75/25: pooled mean difference = -31.4 mg/dL; 95 percent CI: -45.7 to -17.1 mg/dL; p < 0.001).
- A lack of evidence limited our ability to compare insulin lispro 50/50 to oral antidiabetic agents.
- Limited evidence suggested that insulin aspart 70/30 was similar to exenatide in lowering fasting glucose.
- A lack of evidence limited our ability to compare insulin lispro 75/25 and 50/50 to exenatide.
- Premixed insulin analogues were less effective than a combination of long-acting insulin analogue and rapid-acting insulin analogue in lowering fasting glucose.
- Insulin aspart 70/30 may be less effective than a combination of long-acting insulin analogue and rapid-acting insulin analogue in lowering fasting glucose (mean difference in one study = 7.9 mg/dL; p > 0.05).
- A lack of evidence limited our ability to compare insulin lispro 75/25 to a combination of long-acting insulin analogue and rapid-acting insulin analogue in lowering fasting glucose.
- Insulin lispro 50/50 was less effective than a combination of long-acting insulin analogue and rapid-acting insulin analogue in lowering fasting glucose (mean difference in one study = 12mg/dL; p = 0.013).
- Premixed insulin analogues may be at least as effective as rapid-acting insulin analogues in lowering fasting glucose.
- Insulin aspart 70/30 is more effective than rapid-acting insulin analogues in lowering fasting glucose (mean difference in one study = -22.0 mg/dL; p < 0.001).
- Lack of evidence limits our ability to compare insulin lispro 75/25 to rapid-acting insulin analogues in lowering fasting glucose.
- Insulin lispro 50/50 may be similar to rapid-acting insulin analogues in lowering fasting glucose (mean difference in one study = 0 mg/dL; p > 0.05).
- A lack of evidence limited our ability to compare premixed insulin analogues to intermediate-acting human insulin preparations or to a combination of intermediate-acting human insulin plus rapid-acting insulin analogues in lowering fasting glucose.
- The superiority of one premixed insulin analogue over the other in lowering fasting glucose could not be determined because of the paucity of evidence.
Postprandial glucose
- Premixed insulin analogues were more effective than long-acting insulin analogues alone in lowering postprandial glucose.
- Insulin aspart 70/30 was more effective than long-acting insulin analogues in lowering postprandial glucose (pooled mean difference = -22.6 mg/dL; 95 percent CI: -32.1 to -13.2 mg/dL; p < 0.001).
- Insulin lispro 75/25 was more effective than long-acting insulin analogues in lowering postprandial glucose (pooled mean difference = -23.6 mg/dL; 95 percent CI: -30.9 to -16.4 mg/dL; p < 0.001).
- Insulin lispro 50/50 was more effective than long-acting insulin analogues in lowering postprandial glucose (pooled mean difference = -32.7 mg/dL; 95 percent CI: -48.2 to -17.1 mg/dL; p < 0.001).
- Premixed insulin analogues were more effective than premixed human insulin preparations in lowering postprandial glucose.
- Insulin aspart 70/30 was more effective than premixed human insulin preparations in lowering postprandial glucose (pooled mean difference = -18.6 mg/dL; 95 percent CI: -31.1 to -6.0 mg/dL; p = 0.004).
- Insulin lispro 75/25 was more effective than premixed human insulin preparations in lowering postprandial glucose (pooled mean difference = -17.8 mg/dL; 95 percent CI: -27.0 to -8.6 mg/dL; p < 0.001).
- Insulin lispro 50/50 was more effective than premixed human insulin preparations in lowering postprandial glucose (pooled mean difference = -30.3 mg/dL; 95 percent CI: -55.6 to -5.0 mg/dL; p = 0.02).
- Two of the three premixed insulin analogues were much more effective than oral antidiabetic agents in lowering postprandial glucose.
- Insulin aspart 70/30 was more effective than oral antidiabetic agents in lowering postprandial glucose (pooled mean difference = -32.8 mg/dL; 95 percent CI: -62.5 to -3.1 mg/dL; p = 0.03).
- Insulin lispro 75/25 was more effective than oral antidiabetic agents in lowering postprandial glucose (pooled mean difference = -47.3 mg/dL; 95 percent CI: -63.5 to -31.0 mg/dL; p < 0.001).
- A lack of evidence limited our ability to compare insulin lispro 50/50 to oral antidiabetic agents with regard to lowering postprandial glucose.
- Not enough evidence exists to allow us to conclusively compare premixed insulin analogues to a combination of rapid-acting insulin and long-acting insulin analogues in lowering postprandial glucose.
- Insulin aspart 70/30 may be better than a combination of rapid-acting insulin and long-acting insulin analogues in lowering postprandial glucose (mean difference in one study = -11.8 mg/dL; p > 0.05).
- A lack of evidence limited our ability to compare insulin lispro 75/25 to a combination of rapid-acting insulin and long-acting insulin analogues with regard to lowering postprandial glucose.
- Insulin lispro 50/50 was less effective than a combination of rapid-acting insulin and long-acting insulin analogues in lowering postprandial glucose (final postprandial glucose values from one study: 174 versus 155 mg/dL; p = 0.002).
- Not enough evidence exists to allow us to conclusively compare the new incretin mimetic agent, exenatide, to premixed insulin analogues with regard to lowering postprandial glucose.
- A lack of evidence limited our ability to compare rapid-acting insulin analogues to premixed insulin analogues with regard to lowering postprandial glucose.
- Insulin aspart 70/30 was less effective than rapid-acting insulin analogues in lowering postprandial glucose (mean difference in one study = 15 mg/dL; p < 0.001).
- A lack of evidence limited our ability to compare insulin lispro 75/25 to rapid-acting insulin analogues with regard to lowering postprandial glucose.
- Limited evidence suggested that insulin lispro 50/50 was similar to rapid-acting insulin analogues with regard to lowering postprandial glucose (mean difference in one study = 3.6 mg/dL; p > 0.05).
- There was very little evidence to allow us to compare premixed insulin analogues to intermediate-acting insulin preparations or to a combination of intermediate-acting human insulin plus rapid-acting insulin analogue.
- The superiority of one premixed insulin analogue over the other could not be determined because of the paucity of evidence.
Hemoglobin A1c
- Premixed insulin analogues were more effective than long-acting insulin analogues in lowering A1c.
- Insulin aspart 70/30 was more effective than long-acting insulin analogues in lowering A1c (pooled mean absolute difference = -0.48 percent; 95 percent CI: -0.61 to -0.34 percent; p < 0.001).
- Insulin lispro 75/25 was more effective than long-acting insulin analogues in lowering A1c (pooled mean absolute difference = -0.33 percent; 95 percent CI: -0.48 to -0.17 percent; p < 0.001).
- Insulin lispro 50/50 was more effective than long-acting insulin analogues in lowering A1c (pooled mean absolute difference = -0.40 percent; 95 percent CI: -0.65 to -0.15 percent; p = 0.001).
- Premixed insulin analogues were as effective as premixed human insulin in lowering A1c.
- Insulin aspart 70/30 was similar to premixed human insulin preparations in terms of lowering A1c (pooled mean absolute difference = 0.06 percent; 95 percent CI: -0.04 to 0.16 percent; p = 0.22).
- Insulin lispro 75/25 was similar to premixed human insulin preparations in terms of lowering A1c (absolute mean differences reported in three studies ranged from -0.12 to 0.2 percent; p was not significant in all three studies).
- Insulin lispro 50/50 was more effective than premixed human insulin preparations in terms of lowering A1c (effect size in two studies: (1) absolute mean difference = -0.5 percent; p = 0.01 and (2) absolute mean difference = -0.31 percent; p < 0.05).
- Premixed insulin analogues are better than oral antidiabetic agents in lowering A1c.
- Insulin aspart 70/30 was more effective than oral antidiabetic agents in terms of lowering A1c (pooled mean absolute difference = -0.52 percent; 95 percent CI: -1.0 to -0.04 percent; p = 0.034).
- Insulin lispro 75/25 may be more effective than oral antidiabetic agents in terms of lowering A1c (pooled mean absolute difference = -0.42 percent; 95 percent CI: -1.0 to 0.16 percent; p = 0.15).
- Lack of evidence limited our ability to compare insulin lispro 50/50 to oral antidiabetic agents in terms of lowering A1c.
- Not enough evidence exists to allow us to conclusively compare premixed insulin analogues to a combination of rapid-acting insulin and long-acting insulin analogues in terms of lowering A1c.
- Insulin aspart 70/30 may be more effective than a combination of rapid-acting insulin and long-acting insulin analogues in lowering A1c, but the quality of the evidence was low (mean difference in one study = -0.42 percent; p < 0.05).
- A lack of evidence limited our ability to compare insulin lispro 75/25 to a combination of rapid-acting insulin and long-acting insulin analogues in terms of lowering A1c.
- Insulin lispro 50/50 was less effective than a combination of rapid-acting insulin and long-acting insulin analogues in lowering A1c (final A1c values from one study: 6.9 versus 6.8 percent; p = 0.02).
- Not enough evidence exists to allow us to conclusively compare exenatide to premixed insulin analogues.
- A lack of evidence limited our ability to compare intermediate-acting human insulin, rapid-acting insulin analogues, or the combination of the two to premixed insulin analogues.
- The superiority of one premixed insulin analogue over the other could not be reliably evaluated because of the paucity of evidence.
Hypoglycemia
- Many of the comparisons were represented by too few studies to allow us to draw any conclusions regarding this adverse effect.
- The effect of premixed insulin analogues on the incidence of serious hypoglycemia could not be conclusively addressed because only very few serious hypoglycemic events were reported in the studies.
- Premixed insulin analogues were associated with more hypoglycemic events than were oral antidiabetic agents, but the only study that compared premixed insulin analogues to exenatide found no difference in the incidence of hypoglycemic events.
- Insulin aspart 70/30 was more likely to be associated with a higher incidence of hypoglycemia than were oral antidiabetic agents (OR for minor hypoglycemia = 3.8; 95 percent CI: 1.7 to 8.5; p = 0.001 and OR for symptom-only hypoglycemia = 3.9; 95 percent CI: 1.2 to 12.4; p = 0.02).
- Limited evidence suggested that insulin lispro 75/25 may be associated with a higher incidence of overall hypoglycemia than were oral antidiabetic agents (rate ratio = 4.86; 95 percent CI: 0.5 to 49.5; p = 0.18).
- A lack of evidence limited our ability to compare insulin lispro 50/50 with oral antidiabetic agents in terms of the incidence of hypoglycemia.
- Premixed insulin analogues were more likely to be associated with hypoglycemia than were the long-acting insulin analogues.
- Insulin aspart 70/30 was more likely to be associated with hypoglycemia than were long-acting insulin analogues (odds ratio (OR) for minor hypoglycemia = 2.8; 95 percent CI: 1.4 to 5.4; p = 0.003).
- Insulin lispro 75/25 was more likely to be associated with hypoglycemia than were long-acting insulin analogues (p-values for overall hypoglycemia ranged from 0.4 to 0.01).
- Insulin lispro 50/50 was more likely to be associated with hypoglycemia than were long-acting insulin analogues (p-value for overall hypoglycemia ranged from 0.02 to 0.01).
- Premixed insulin analogues (insulin aspart 70/30, insulin lispro 75/25, and insulin lispro 50/50) were similar to premixed human insulin preparations in terms of the incidence of hypoglycemia.
- There were not enough data to allow us to compare premixed insulin analogues to a combination of intermediate-acting human insulin plus a rapid-acting insulin analogue in terms of the incidence of hypoglycemia.
- There were not enough data to allow us to conclusively compare one premixed insulin analogue with the other in terms of the incidence of hypoglycemia.
Weight change
- Premixed insulin analogues were associated with weight gain, when compared to oral antidiabetic agents as a group.
- Insulin aspart 70/30 was associated with weight gain when compared to oral antidiabetic agents (pooled mean difference = 2.8 kg; 95 percent CI: 0.6 to 5.0 kg; p = 0.01).
- Insulin lispro 75/25 was associated with weight gain when compared to oral antidiabetic agents (pooled mean difference = 1.88 kg; 95 percent CI: 1.35 to 2.41 kg; p < 0.001).
- A lack of evidence limited our ability to compare insulin lispro 50/50 to oral antidiabetic agents in terms of weight gain.
- The evidence was conflicting about whether premixed insulin analogues may cause less weight gain than do rapid-acting insulin analogues.
- Insulin aspart 70/30 was associated with less weight gain than were rapid-acting insulin analogues (mean difference in one study = -1.0 kg; p = 0.005).
- A lack of evidence limited our ability to compare insulin lispro 75/25 to rapid-acting insulin analogues in terms of weight gain.
- Insulin lispro 50/50 may be associated with more weight gain than are rapid-acting insulin analogues, although the evidence was weak (mean difference in BMI in one study = 0.3 kg/m2; p = 0.048).
- Premixed insulin analogues may cause more weight gain than do long-acting insulin analogues.
- Insulin aspart 70/30 was associated with more weight gain than were long-acting insulin analogues (pooled mean difference = 2.5 kg; 95 percent CI: 1.6 to 3.4 kg; p < 0.001).
- A lack of evidence limited our ability to compare insulin lispro 75/25 and insulin lispro 50/50 to long-acting insulin analogues in terms of weight gain.
- There was not enough evidence to allow us to conclusively compare the weight change after treatment with premixed insulin analogues to that produced by other antidiabetic drugs, except as noted above.
Evidence Grades
Fasting glucose (see Appendix E; Evidence Table 1)
- The strength of evidence was graded as moderate for the following comparisons:
- Premixed insulin analogues versus long-acting insulin analogues alone
- Premixed insulin analogues versus premixed human insulin
- Premixed insulin analogues versus noninsulin antidiabetic agents.
- The strength of evidence was graded as low for the following comparisons:
- Premixed insulin analogues versus rapid-acting insulin analogues
- Premixed insulin analogues versus a combination of long-acting and rapid-acting insulin analogues
- Premixed insulin analogues versus intermediate-acting human insulin.
- There was no evidence for the following comparisons:
- Premixed insulin analogues versus a combination of intermediate-acting human insulin and rapid-acting insulin analogues.
Postprandial glucose (see Appendix E; Evidence Table 1)
- The strength of evidence was graded as high for the following comparisons:
- Premixed insulin analogues versus long-acting insulin analogues alone
- Premixed insulin analogues versus premixed human insulin.
- The strength of evidence was graded as moderate for the following comparison:
- Premixed insulin analogues versus noninsulin antidiabetic agents.
- The strength of evidence was graded as low for the following comparisons:
- Premixed insulin analogues versus rapid-acting insulin analogues
- Premixed insulin analogues versus intermediate-acting human insulin
- Premixed insulin analogues versus a combination of long-acting and rapid-acting insulin analogues.
- There was no evidence for the following comparisons:
- Premixed insulin analogues versus a combination of intermediate-acting human insulin and rapid-acting insulin analogue.
Hemoglobin A1c (see Appendix E; Evidence Table 1)
- The evidence was graded as high for the following comparisons:
- Premixed insulin analogues versus long-acting insulin analogues
- Premixed insulin analogues versus premixed human insulin.
- The evidence was graded as moderate for the following comparison:
- Premixed insulin analogues versus noninsulin antidiabetic agents.
- The evidence was graded as low for the following comparisons:
- Premixed insulin analogues versus intermediate-acting human insulin
- Premixed insulin analogues versus rapid-acting insulin analogues
- Premixed insulin analogues versus a combination of rapid-acting and long-acting insulin analogues
- Premixed insulin analogues versus a combination of intermediate-acting human insulin and rapid-acting insulin analogue.
Hypoglycemia (see Appendix E; Evidence Table 1)
- The strength of evidence was graded as high for the following comparisons:
- Premixed insulin analogues versus long-acting insulin analogues
- Premixed insulin analogues versus premixed human insulin
- Premixed insulin analogues versus noninsulin antidiabetic agents.
- The strength of evidence was graded as low for the following comparisons:
- Premixed insulin analogues versus intermediate-acting human insulin
- Premixed insulin analogues versus rapid-acting insulin analogues
- Premixed insulin analogues versus a combination of rapid-acting and long-acting insulin analogues
- Premixed insulin analogues versus a combination of intermediate-acting human insulin and rapid-acting insulin analogue.
Weight change (see Appendix E; Evidence Table 1)
- The strength of evidence was graded as moderate for the following comparisons:
- Premixed insulin analogues versus long-acting insulin analogues
- Premixed insulin analogues versus premixed human insulin
- Premixed insulin analogues versus noninsulin antidiabetic agents.
- The strength of evidence was graded as low for the following comparisons:
- Premixed insulin analogues versus intermediate-acting human insulin
- Premixed insulin analogues versus rapid-acting insulin analogues
- Premixed insulin analogues versus a combination of rapid-acting and long-acting insulin analogues
- Premixed insulin analogues versus a combination of intermediate-acting human insulin and rapid-acting insulin analogue.
Study Characteristics
Of the 45 studies included in this review, 43 reported on at least one of the intermediate clinical outcomes, and two did not report any intermediate clinical outcomes.11, 56 Adverse events were reported by 43 studies; two studies did not report any adverse events.56, 75 Of the 45 trials, 16 were conducted in Europe,10, 32, 43, 45, 48, 50, 51, 53, 55, 56, 58, 59, 66, 67, 71, 76 11 were conducted in North America,15, 39, 44, 60, 62–65, 74, 75, 80 six were conducted in Asia,47, 52, 57, 61, 69, 78 one was conducted in Africa;12 nine were multinational trials conducted in countries spread across different continents,13, 46, 49, 54, 68, 70, 72, 73, 79 and two trials did not report the region from which patients were enrolled (see Appendix E; Evidence Table 2).11, 77 All were RCTs except two.52, 75 In one study,52 patients were enrolled consecutively and followed prospectively, while in the other study,75 data were obtained from the medical record database of a large employer. Among the RCTs, 23 were parallel-arm,13, 15, 32, 39, 43, 45–47, 49–51, 53, 54, 57, 59–61, 68, 72, 76, 78–80 16 were crossover without a washout period,10–12, 48, 55, 63–67, 69–71, 73, 74, 77 and four were crossover trials with a washout period.44, 56, 58, 62 The median duration of followup in these trials was 16 weeks (a range of 1 day to 2 years). The longest study was by Boehm et al.,45 which first reported results after 3 months9 and then reported results of the extended followup at 2 years. There were four short studies, each of a single day's duration, in which blood glucose levels were measured after a single dose of study medications.44, 56, 58, 62
The trials showed similarities as well as differences in terms of the patient enrollment criteria. All studies except two enrolled type 2 diabetic patients.10, 45 Eight studies limited the age range of their source population to middle age and older.12, 44, 47, 59, 67, 71, 72, 79 One of these studies exclusively enrolled patients between the ages of 60 and 80 years.72 Eleven studies did not report the age range of the target populations.48, 50–53, 61, 70, 74, 75, 77, 78 Most trials excluded patients with A1c and BMI values above a certain limit (variable between the studies, but ranging from 9.5 to 14.7 percent for A1c and from 30 to 40 kg/m2 for BMI). Seven studies did not report inclusion criteria for A1c,11, 51, 52, 56, 70, 75, 77 and 13 studies did not report inclusion criteria for BMI.10, 11, 47, 51, 52, 56, 61, 63, 66, 74, 75, 77, 78
Most of these trials proposed to evaluate the comparative efficacy and safety of the study drugs. Three studies also had a stated aim of evaluating intensive blood glucose control as compared to usual control of blood glucose.63, 69, 76 Three other studies also specifically aimed to control postprandial blood glucose.53, 64, 77
These trials enrolled a total of 14,603 patients (median number per trial = 93; range: 8 to 8,166 patients). The enrolled populations in the studies had a median age of 59 years (range: 51 to 68 years), and most patients were male (median = 52 percent, range: 16 to 92 percent; see Appendix E, Evidence Table 3). The study populations had a median A1c of 8.7 percent (range: 7.3 to 10.7 percent), a median BMI of 29.4 kg/m2 (range: 24 to 37 kg/m2), and a median duration of diabetes of 11 years (range: 4 to 16 years). Eleven trials enrolled insulin-naïve patients,15, 32, 39, 43, 46, 59, 60, 64, 65, 72, 75 nine trials did not specify history of insulin treatment,47, 49–52, 54, 57, 68, 74 and the remaining 23 trials enrolled insulin-treated patients.
Source of funding for the included studies. The source of funding was reported by 33 studies; the remaining 12 studies did not clearly state the source of funding.43, 44, 47, 48, 51, 60, 65, 66, 70, 71, 78, 79 Of the studies that clearly stated the source of funding, all but two were funded by the pharmaceutical industry: One was jointly funded by the National Institutes of Health (NIH) and the pharmaceutical industry,74 while the other was funded by the Japan Diabetes Foundation.61 Of the studies that did not clearly state their source of funding, employees of the pharmaceutical industry were among the authors in six studies.48, 60, 65, 66, 70, 79 Novo Nordisk, the manufacturer of insulin aspart 70/30, funded 16 studies, all using insulin aspart 70/30 (see Table 4). One of the studies funded by Novo Nordisk also used insulin lispro 75/25 and compared it to insulin aspart 70/30.58 Eli Lilly, the manufacturer of insulin lispro 75/25 and insulin lispro 50/50, funded 16 studies, one of which was also partially funded through the NIH.74 Only one study funded by Eli Lilly used insulin aspart 70/30; it compared exenatide (manufactured by Eli Lilly) to insulin aspart 70/30.49
Table 4
List of study funding.
Reporting of Intermediate Outcomes and Adverse Events
Fasting glucose. The methods of reporting fasting or pre-meal glucose levels were not consistent among all studies. Studies either reported pre-breakfast glucose levels10, 12, 13, 15, 39, 43, 45, 46, 49–55, 57, 63, 64, 70, 72–74, 76, 77, 80 or both pre-breakfast and pre-dinner glucose levels,10, 12, 39, 43, 45, 46, 49–51, 53–55, 57, 59, 63, 64, 70, 72–74, 76, 77, 79 or they simply reported fasting glucose levels without specifying the time of the day at which the glucose was measured.32, 47, 60, 65, 66, 68, 69, 78 Ten studies did not report pre-meal glucose levels.11, 44, 48, 56, 58, 61, 62, 67, 71, 75 In six of these studies, glucose levels were reported after a single dose of the study drug with a test meal.44, 56, 58, 62, 67, 71 As pre-dinner glucose levels are unlikely to be drawn from patients in a fasting state, we evaluated pre-dinner levels separately and combined the fasting and pre-breakfast glucose levels for this review.
Postprandial glucose. In general, studies reported postprandial glucose either 90 min or 2 h after a meal. Postprandial glucose levels were measured at 90 min after meal in 10 studies13, 39, 50, 51, 53–55, 57, 59, 60 and at 2 h after a meal in 25 studies.10, 12, 15, 43, 44, 46, 49, 58, 62–74, 76, 77, 79, 80 Two studies did not specify the time of the day at which postprandial glucose was tested, or they pooled postprandial values together and reported one value.32, 52 Eight studies did not report postprandial glucose levels.11, 45, 47, 48, 56, 61, 75, 78 All studies that reported 90-min postprandial glucose levels reported results for both after breakfast and after dinner. Eighteen studies reported 2-h postprandial glucose levels after both breakfast and dinner.10, 12, 15, 43, 46, 49, 63–66, 68, 70, 72–74, 76, 77, 79 Another seven studies reported 2-h postprandial glucose levels after breakfast.44, 58, 62, 67, 69, 71, 80 Five of these seven studies evaluated postprandial glucose levels after a test meal only, and therefore they were not designed to report after-dinner values.44, 58, 62, 67, 71 Although studies reported other outcomes for postprandial glucose levels, such as glucose excursion or increment in glucose levels, we chose postprandial glucose levels for two reasons. First, postprandial glucose levels were the most frequently reported outcome measures in the trials. Second, postprandial glucose levels are frequently used in clinical practice to direct adjustments in insulin dose.
For the systematic review, we analyzed the 90-min and 2-h studies together as one group. We analyzed the breakfast postprandial glucose levels separately from the dinner postprandial levels. We analyzed the studies that reported postprandial glucose levels without specifying the time of the day together with the studies that reported dinner postprandial glucose levels.
Hemoglobin A1c. A1c levels were reported by 35 studies;10, 13, 15, 32, 39, 43, 45–55, 57, 59–61, 63–66, 68, 69, 72, 73, 75–80 however, we excluded two studies57, 69 from this review because the duration of followup was only 6 or 8 weeks, respectively.
Hypoglycemia. The definitions of severity of hypoglycemia were fairly consistent across studies. The studies defined a major hypoglycemic event as an event that required third-party help for patients who either had a blood glucose value below a pre-defined limit (usually less than 50 to 60 mg/dL) or required food, intravenous glucose, or glucagon to resolve severe central nervous system symptoms. A minor hypoglycemic event was defined as one in which a patient was able to self-treat without third-party intervention. Some studies defined a “symptom only hypoglycemic event” category if the patient felt symptoms of hypoglycemia, did not require third party assistance, and had either no blood glucose measurements or a blood glucose that was above a pre-defined lower limit. The definition of the lower limit of blood glucose varied between studies.
Studies reported hypoglycemic outcomes in different ways. Some studies chose to describe the incidence of hypoglycemia, defined as the number of patients who had at least one hypoglycemic event or the total number of episodes during the treatment period. Studies also often reported the event rate of hypoglycemic episodes, defined as the number of episodes per patients over some unit of time. Authors also commonly chose to report the incidence or event rate of hypoglycemic episodes as a function of the time of day.
Weight or BMI change. Most studies reported a change in weight as one of the adverse effects of the therapy,13, 15, 32, 39, 43, 45–47, 49–52, 57, 59, 60, 63–66, 68, 70, 72, 73, 79, 80, 81 except for three studies that reported a change in body mass index.61, 76, 78 As noted in the Methods section, we included only parallel-arm studies13, 15, 32, 39, 43, 45–47, 49–51, 54, 57, 59, 60, 61, 68, 72, 76, 78, 79, 80 in the evaluation of this outcome because, in the case of crossover studies, there was a high likelihood of a carryover effect in the second period of the study.
Insulin Aspart 70/30
Insulin aspart 70/30 versus long-acting insulin analogues. We identified four randomized parallel-arm studies that compared insulin aspart 70/30, enrolling a total of 453 patients in the insulin aspart 70/30 arm and 458 patients in the long-acting insulin analogue arm.32, 39, 47, 50 One study used insulin detemir32 as the long-acting insulin analogue; the remaining three studies used insulin glargine. All studies reported fasting glucose and A1c. One study did not report postprandial glucose levels.47
Raskin et al.39 enrolled 233 subjects in a 28-week trial. Patients received either twice-daily insulin aspart 70/30 or insulin glargine while also receiving metformin and pioglitazone. Both insulin aspart 70/30 and insulin glargine were started at between 10 and 12 units daily, depending on the fasting glucose levels, and the dose was adjusted during followup. At the end of the study, the insulin dose was significantly greater in the insulin aspart 70/30 group than in the insulin glargine group (78.5 units/day versus 51 units/day, or 0.82 units/kg/day versus 0.55 units/kg/day; p < 0.05).
Kann et al.50 enrolled 255 patients to receive either insulin aspart 70/30 in combination with metformin or to receive insulin glargine and glimepiride; these patients were followed for 26 weeks. The insulin dose was adjusted in both treatment arms during the trial, and the mean insulin doses were 0.4 units/kg/day in the insulin aspart 70/30 arm and 0.39 units/kg/day in the insulin glargine arm. The metformin and glimepiride doses were also adjusted during the study.
The study by Tamemoto et al.47 was a small study enrolling 30 patients to receive either insulin aspart 70/30 or insulin glargine. Patients were allowed to continue their oral antidiabetic agents, except that the patients in the insulin aspart 70/30 arm were not allowed to take a sulfonylurea. The starting dose was 10 to 16 units/day in the insulin aspart 70/30 arm and 6 to 8 units/day in the insulin glargine arm. The insulin dose was adjusted during the followup period. The mean insulin dose in the insulin aspart 70/30 arm was 26.7 units/day; the mean insulin glargine dose was not reported.
Holman et al.32 conducted a three-arm study in which two arms compared insulin detemir (n = 234) to insulin aspart 70/30 (n = 235). Patients were followed for 1 year while on treatment. Insulin aspart 70/30 was given twice daily, while insulin detemir was administered once daily; however, insulin detemir could be administered twice daily if the blood glucose was not under control. Patients in both arms continued metformin and sulfonylurea treatment. The insulin doses were similar in the two arms at the end of the study.
Fasting glucose (see Appendix E, Evidence Table 4). In terms of lowering fasting glucose levels, only one32 of the four studies found insulin aspart 70/30 to be less effective than long-acting insulin analogues; the other studies did not find any difference. When the results of all four studies were pooled, insulin aspart 70/30 was less effective in lowering fasting glucose than were the long-acting insulin analogues, but the difference did not reach statistical significance (mean difference = 6.4 mg/dL; 95 percent CI: -1.5 to 14.2 mg/dL; p = 0.11; see Figure 5). When we excluded the study involving insulin detemir from the pooled analysis, the results did not change (mean difference = 1.5 mg/dL; 95 percent CI: -6.0 to 9.1 mg/dL; p = 0.69; see Figure 5). Two studies reported the pre-dinner glucose levels. One study39 found insulin aspart 70/30 to be more effective than insulin glargine in lowering pre-dinner glucose levels (p < 0.05), whereas the second study50 did not find any difference between the two treatment regimens.
Postprandial glucose (see Appendix E, Evidence Table 4). All three trials found insulin aspart 70/30 to be more effective than long-acting insulin analogues in lowering postprandial glucose levels.32, 39, 50 Pooling the results of these studies showed that insulin aspart 70/30 was significantly more effective than long-acting insulin analogues in lowering postprandial glucose levels (mean difference = -22.6 mg/dL; 95 percent CI: -32.1 to -13.2 mg/dL; p < 0.001; see Figure 6). Two of the three trials also reported breakfast postprandial levels.39, 50 Insulin aspart 70/30 lowered the breakfast postprandial glucose levels in both studies, but this effect reached statistical significance in only one study.50
Hemoglobin A1c (see Appendix E, Evidence Table 4). Insulin aspart 70/30 was found to be significantly more effective than long-acting insulin analogues in lowering A1c in all four studies, except in the study by Tamemoto et al.47 Tamemoto et al. could have failed to find a difference between the two treatments because of the small sample size (n = 23), giving them a low power to detect a difference. Raskin et al.39 found that insulin aspart 70/30 lowered A1c to a larger degree in patients with poorer control of diabetes (A1c > 8.5 percent), but no comparative advantage was seen for patients with relatively better control of diabetes (A1c ≤ 8.5 percent). Holman et al.32 found that not only did patients treated with insulin aspart 70/30 have lower A1c levels (p < 0.001), but they were also more likely to reach a target A1c of 6.5 percent or lower than were those in the insulin detemir group (p = 0.001). Pooling the study results across the four trials showed that insulin aspart 70/30 was significantly better than long-acting insulin analogues in lowering A1c levels (mean difference = -0.48 percent; 95 percent CI: -0.61 to -0.34 percent; p < 0.001; see Figure 7).
Hypoglycemia (see Appendix E, Evidence Table 5). The incidence of overall hypoglycemia was reported by Holman et al.32 and was higher in the insulin aspart 70/30 group than in the insulin detemir group (216 out of 235 versus 173 out of 234 patients; p < 0.001). The incidence of minor hypoglycemia was reported by three studies.39, 47, 50 In two studies, the incidence of minor hypoglycemia events was significantly higher in the insulin aspart 70/30 group than in the comparison group.39, 50 When the results of these three studies were pooled, the incidence of minor hypoglycemia was found to be significantly higher for insulin aspart 70/30 than for insulin glargine (78 versus 34; odds ratio (OR) = 2.8; 95 percent CI: 1.4 to 5.4; p = 0.003; see Figure 8). Two studies47, 50 reported the incidence of symptoms-only hypoglycemia and did not find any difference between the two types of insulin analogues.
Weight change (see Appendix E, Evidence Table 6). In two studies,32, 39 patients treated with insulin aspart 70/30 gained significantly more weight than did those receiving a long-acting insulin analogue (p < 0.001 in both studies). In the remaining two studies, the weight change was not significant. Pooling the results of these four studies indicated that insulin aspart 70/30 was associated with a much larger weight gain than was insulin glargine (weighted mean difference = 2.5 kg; 95 percent CI: 1.6 to 3.4 kg; p < 0.001; see Figure 9).
Other serious adverse events (see Table 5 and Appendix E, Evidence Table 6). None of the included studies comparing insulin aspart 70/30 to a long-acting insulin analogue reported on injection site reactions. Table 5 shows the range of risk differences between insulin aspart 70/30 and long-acting insulin analogues in terms of total serious adverse events, other serious adverse events, and withdrawals as a result of adverse events.
Table 5
Range of risk differences between insulin aspart 70/30 and other antidiabetic agents for selected adverse events.
Insulin aspart 70/30 versus rapid-acting insulin analogues. We identified only one study on this comparison. Holman et al.32 compared twice-daily insulin aspart 70/30 plus metformin and a sulfonylurea to thrice-daily rapid-acting insulin aspart with meals plus metformin and sulfonylurea. This was a large RCT with 235 patients in the insulin aspart 70/30 arm and 239 patients in the rapid-acting insulin aspart arm. The median starting daily dose of insulin was similar in both groups, and insulin doses were adjusted based on blood glucose levels.
Intermediate outcomes (see Appendix E, Evidence Table 4). Holman et al.32 found that insulin aspart 70/30 was more effective than rapid-acting insulin aspart in decreasing fasting glucose levels (mean difference = -22.0 mg/dL; p < 0.001). On the other hand, rapid-acting insulin aspart was more effective in lowering postprandial blood glucose than was insulin aspart 70/30 (mean difference = -15 mg/dL; p < 0.001). Rapid-acting insulin aspart was slightly more effective in lowering A1c than was insulin aspart 70/30, although this difference was not statistically significant (mean difference = -0.1 percent; p = 0.08). The percentage of patients who achieved a target A1c of less than or equal to 7.0 percent or 6.5 percent was higher for those receiving rapid-acting insulin aspart than for those receiving insulin aspart 70/30, but this difference was not statistically significant (mean difference = 7.0 percent and 6.9 percent respectively; p = 0.08 for both A1c targets).
Adverse events (see Appendix E, Evidence Tables 5 and 6). The incidence of hypoglycemia was not significantly different between the two groups (216 out of 235 patients versus 229 out of 238 patients; p = 0.08).32 Treatment with insulin aspart 70/30 was associated with less weight gain than was treatment with rapid-acting insulin aspart (mean difference = -1.0 kg; p = 0.005). This study did not report on injection site reactions. Table 5 shows the risk differences between insulin aspart 70/30 and rapid-acting insulin analogues in terms of total serious adverse events, other serious adverse events, and withdrawals due to adverse events.
Insulin aspart 70/30 versus a combination of long-acting and rapid-acting insulin analogues. We identified one nonrandomized prospective trial by Joshi et al. that compared insulin aspart 70/30 to a combination of mealtime insulin aspart and bedtime insulin glargine.52 Doses of all insulin analogues were adjusted throughout the trial on the basis of glucose levels. At the end of the trial, the total insulin dose was lower in the insulin aspart 70/30 group (40.2 versus 52.8 units/day).
Intermediate outcomes (see Appendix E, Evidence Table 4). After 12 weeks of followup, both treatments were effective in lowering fasting glucose, postprandial glucose, and A1c from baseline levels. When Joshi et al. compared the two treatments, they found insulin aspart 70/30 to be less effective than a combination of mealtime insulin aspart and bedtime insulin glargine in lowering fasting glucose levels, although this difference was not statistically significant (mean difference = 7.9 mg/dL; p > 0.05).52 On the other hand, insulin aspart 70/30 was more effective than the combination in lowering postprandial glucose levels, although this effect was also not statistically significant (mean difference = -11.8 mg/dL; p > 0.05). Insulin aspart 70/30 was significantly more effective than the combination in lowering A1c (mean difference = -0.42 percent; p < 0.05). Moreover, a larger percentage of patients achieved an A1c of less than 7 percent with insulin aspart 70/30 than with the combination at the end of 12 weeks of followup (risk difference = -14 percent; p-value was not reported).
Adverse events (see Appendix E, Evidence Tables 5 and 6). There were no major hypoglycemic events in this study.52 Fewer patients in the insulin aspart 70/30 arm had minor hypoglycemic events, as compared to the group that received a combination of insulin glargine and rapid-acting insulin aspart (16.7 versus 58 percent; p < 0.05). There was no significant difference in the body weight in the two groups. This study did not report on injection site reactions. Table 5 shows the risk differences between insulin aspart 70/30 and rapid-acting insulin analogues for total serious adverse events and withdrawals due to adverse events.
Insulin aspart 70/30 versus premixed human insulin. Our search found three parallel-arm trials15, 45, 53 and three crossover trials11, 48, 58 that compared insulin aspart 70/30 with a premixed human insulin, NPH/regular 70/30.
McNally et al.48 compared insulin aspart 70/30 with NPH/regular 70/30 (both injected immediately before meals) in a crossover trial enrolling 160 subjects. After a 6-week run-in period, subjects were followed for two treatment periods of 16 weeks each. The insulin dose was adjusted during the followup in order to achieve glucose targets, and the mean insulin dose was similar in both groups.
In a 24-week parallel-arm study by Abrahamian et al.,53 thrice-daily insulin aspart 70/30 (given with meals) was compared to twice-daily NPH/regular 70/30 (given 30 min before meals). The mean insulin dose was slightly larger in the insulin aspart 70/30 arm than in the premixed human insulin arm (0.61 units/kg/day versus 0.59 units/kg/day, p = nonsignificant).
Boehm at al.9, 45 compared twice-daily insulin aspart 70/30 (given with meals) to twice-daily NPH/regular 70/30 (given 30 min before meals). This was a 3-month trial with an extended followup of 2 years. The initial 3-month period of the trial included both type 1 and type 2 diabetics. The extended followup was limited to type 2 diabetics only. Insulin doses were adjusted throughout the trial. After 2 years, the premixed human insulin group was receiving a significantly larger total insulin dose than the insulin aspart 70/30 group (mean difference = 0.09 units/kg/day; p < 0.001).
Kilo et al.15 conducted a three parallel-arm trial that compared insulin aspart 70/30 (given 10 min before dinner) to NPH/regular 70/30 (given 30 min before dinner) in two of its arms. Metformin was given to patients in both arms. The insulin dose was adjusted throughout the 12-week followup period. The mean insulin dose was slightly higher in the NPH/regular 70/30 group than in the insulin aspart 70/30 group (29 versus 26 units/day).
McSorely et al.11 compared twice-daily insulin aspart 70/30 with twice-daily NPH/regular 70/30 (both given immediately before meals) in a crossover trial over two treatment periods of 2 weeks each. This study did not report on the insulin dose used during the study.
Hermansen et al.58 conducted a three-arm crossover trial in which study medications were given once only after a test meal in order to study the postprandial serum glucose response. Two arms compared insulin aspart 70/30 (given with the meal) with premixed human insulin 70/30 (given 15 min before the meal). Similar doses were used for both insulins and were based on the weight of the subject (0.4 units/kg).
Fasting glucose (see Appendix E, Evidence Table 4). All three parallel-arm trials reported the change in fasting glucose levels.15, 45, 53 Individually, these studies did not report an advantage for one treatment over the other. When the results of the studies were pooled, insulin aspart 70/30 was less effective than premixed human insulin in lowering fasting glucose (mean difference = 8.3 mg/dL; 95 percent CI: 0.16 to 16.5 mg/dL; p = 0.04; see Figure 10) although the difference was small.
Postprandial glucose (see Appendix E, Evidence Table 4). All three parallel-arm trials15, 45, 53 and one crossover trial58 reported changes in postprandial glucose. Boehm et al.9, 45 found a significant decrease in postprandial glucose levels 90 min after both breakfast (p < 0.05) and dinner (p < 0.02) after 12 weeks of followup. However, they combined the results from type 2 diabetics (n = 187) with those for type 1 diabetics (n = 104) in reporting changes in postprandial glucose levels, thus making it difficult to extrapolate this finding to type 2 diabetics alone. Abrahamian et al.53 found a significant decrease in glucose levels 90 minutes after dinner but not after breakfast with insulin aspart 70/30, as compared to a premixed human insulin (p < 0.002 and p > 0.05, respectively). On the other hand, Kilo et al.15 did not find a significant difference in glucose levels between insulin aspart 70/30 and premixed human insulin. The difference in the frequency of administration of insulin injection may be responsible for the disparate results in these trials. Kilo et al.15 also did not find insulin aspart 70/30 to be more effective than premixed human insulin in lowering breakfast postprandial glucose. When the results of these studies were pooled, insulin aspart 70/30 was more effective than premixed human insulin in lowering postprandial glucose (weighted mean difference = -18.6 mg/dL; 95 percent CI: -31.1 to -6.0 mg/dL; p = 0.004; see Figure 11). These results were consistent with the results of a single-dose crossover trial58 that also did not find insulin aspart 70/30 to be more effective than premixed human insulin in lowering breakfast postprandial glucose (p > 0.05).
Hemoglobin A1c (see Appendix E, Evidence Table 4). All three parallel-arm trials15, 45, 53 and one crossover trial48 compared changes in A1c levels with insulin aspart 70/30 and NPH/regular 70/30. Boehm et al.45 found that the mean A1c increased slightly in both groups after an initial decrease in the first 6 months. There was no statistically significant difference in mean A1c after 24 months (mean difference = 0.03 percent; p = 0.89). McNally et al.48 reported that patients on insulin aspart 70/30 achieved a mean A1c of 7.28 percent, as compared to 7.22 percent after NPH/regular 70/30. The treatment difference of 0.06 percent was not statistically significant (p = 0.21). Similar results were reported by Kilo et al.15 and Abrahamian et al.,53 who found no significant differences in A1c levels (mean difference = 0.2 percent; p > 0.05 and mean difference = 0.1 percent; p = 0.64, respectively). When the results of these studies were pooled, there was no indication that one premixed preparation was more effective than the other in lowering A1c (weighted mean difference = 0.06 percent; 95 percent CI: -0.04 to 0.16 percent; p = 0.22; see Figure 12).
Hypoglycemia (see Appendix E, Evidence Table 5). Two studies reported the incidence of overall hypoglycemia and found no difference between the two premixed preparations.15, 45 Five studies11, 15, 45, 48, 53 reported the incidence of minor hypoglycemia, and none of them found a significant benefit of a particular premixed insulin. Pooling of the study results also revealed no difference (144 out of 284 patients versus 151 out of 293 patients; OR = 0.98; 95 percent CI: 0.65 to 1.46; p = 0.91; see Figure 13). The incidence of major hypoglycemia was reported in four studies,45, 48, 53, 58 but there was no difference between the treatments in individual studies or when the results of these studies were pooled (9 out of 269 patients versus 16 out of 274 patients; OR = 0.55; 95 percent CI: 0.22 to 1.34; p = 0.19). Only one study reported the incidence of symptom-only hypoglycemia and found no difference between insulin aspart 70/30 and premixed human insulin (13 out of 46 patients versus 11 out of 47 patients; p = 0.59).15
Weight change (see Appendix E, Evidence Table 6). Boehm et al.45 found that premixed human insulin was associated with a higher weight gain than was insulin aspart 70/30, although this difference was not statistically significant (mean difference = -1.5 kg; p = 0.07). Similar results were reported by Kilo et al.,15 who found that patients on insulin aspart 70/30 gained an average of 0.7 kg, while patients on premixed human insulin gained 1.0 kg (p-value not reported).
Other serious adverse events (see Appendix E, Evidence Table 6). None of the studies comparing insulin aspart 70/30 to a premixed human insulin reported on injection site reactions. Table 5 shows the range of risk differences between insulin aspart 70/30 and premixed human insulins in terms of total serious adverse events, other serious adverse events, and withdrawals due to adverse events.
Insulin aspart 70/30 versus rapid-acting insulin analogues with intermediate-acting human insulin. One 6-month Japanese randomized parallel-arm trial by Hirao et al. compared insulin aspart 70/30 with three-times-daily injections of insulin aspart with or without an intermediate-acting human insulin (NPH) in insulin-naïve patients with type 2 diabetes.61
Intermediate outcomes (see Appendix E, Evidence Table 4). This study did not report changes in fasting or postprandial glucose levels. There was no statistical difference between the two groups in the change in A1c from baseline (2.6 percent in both groups).61
Adverse events (see Appendix E, Evidence Tables 5 and 6). There were no major hypoglycemic events observed with either regimen.61 No other hypoglycemia results were reported. Both arms gained weight, but those in the insulin aspart 70/30 arm gained significantly more weight (p = 0.013) than did those in the multiple daily injection group.61 The study did not report on injection site reactions.61 Table 5 shows the range of risk differences between insulin aspart 70/30 and three-times-daily injections of insulin aspart, with or without an intermediate-acting human insulin (NPH), in terms of total serious adverse events, other serious adverse events, and withdrawals due to adverse events. There was one death in the insulin aspart 70/30 arm.
Insulin aspart 70/30 versus intermediate-acting human insulin. Two randomized parallel-arm trials that compared insulin aspart 70/30 to an intermediate-acting human insulin (NPH) met our inclusion criteria.13, 15
Kilo et al.15 compared bedtime NPH insulin with insulin aspart 70/30 given 10 min before dinner, while Christiansen et al.13 compared the two agents when given twice daily (immediately before breakfast and dinner). In both studies, the insulin dose was adjusted during the treatment in order to control glucose levels. The starting insulin dose in Christiansen et al.13 was similar in the two arms, but the mean dose was not reported in the study. During the study period, oral therapy with metformin was continued with insulin preparations in Kilo et al.,15 but all oral antidiabetic treatment was discontinued in Christiansen et al.13
Fasting glucose (see Appendix E, Evidence Table 4). Both studies did not find any difference between the two treatments in terms of lowering fasting glucose levels.13, 15 Kilo et al. also did not find any advantage of one regimen over the other with regard to lowering pre-dinner glucose levels.15
Postprandial glucose (see Appendix E, Evidence Table 4). Kilo et al.15 reported that 2-h breakfast postprandial glucose levels were lower with NPH insulin, but dinner postprandial levels were lower with insulin aspart 70/30; these differences were not statistically significant (difference between studies were not reported). In contrast, Christiansen et al.13 found insulin aspart 70/30 to be more effective than NPH insulin in lowering breakfast and dinner postprandial glucose but did not mention whether this difference was statistically significant or due to chance alone (-1.8 and -3.6 mg/dL respectively; p-value not reported).
Hemoglobin A1c (seeAppendix E, Evidence Table 4). Both trials found no difference between insulin aspart 70/30 and NPH insulin in terms of lowering A1c. Christiansen et al.13 found that although A1c decreased in both groups, with reductions of 0.67 and 0.61 percent from baseline in the insulin aspart 70/30 and NPH insulin groups, respectively, there was no difference between the two treatments (p > 0.05). Similarly, Kilo et al.15 found that although A1c decreased from baseline by 1.3 percent in the insulin aspart 70/30 group and by 1.2 percent in the NPH insulin group, the difference between the two treatments was not statistically significant (p > 0.05).
Hypoglycemia (see Appendix E, Evidence Table 5). There were no major hypoglycemic events in these studies. Kilo et al.15 found fewer patients who suffered minor hypoglycemic events (6 out of 47 patients versus 11 out of 46 patients) or symptoms of hypoglycemia (10 out of 47 patients versus 13 out of 46 patients) with NPH insulin than with insulin aspart 70/30. More patients with NPH insulin had nocturnal hypoglycemia, as compared to those taking insulin aspart 70/30 (11 out of 47 patients versus 7 out of 46 patients). Christiansen et al.13 also found that more patients in the insulin aspart 70/30 group had minor hypoglycemic events than did those in the NPH insulin group (77 out of 201 patients versus 68 out of 202 patients).
Weight change (see Appendix E, Evidence Table 6). In the study by Kilo et al.,15 insulin aspart 70/30 was associated with more weight gain than was NPH insulin (0.7 kg versus 0.1 kg), although the difference was not statistically significant.
Other serious adverse events (see Appendix E, Evidence Table 6). Neither of these studies comparing insulin aspart 70/30 to an intermediate-acting human insulin reported on injection site reactions. Table 5 shows the range of risk differences between insulin aspart 70/30 and intermediate-acting human insulins in terms of total serious adverse events, other serious adverse events, and withdrawals due to adverse events.
Insulin aspart 70/30 versus noninsulin antidiabetic agents. Of the seven studies identified by our search,46, 49, 51, 54, 57, 59, 60 two compared insulin aspart 70/30 to a combination of a thiazolidinedione and glibenclamide,54, 57 one to a combination of metformin and glibenclamide,51 one to either monotherapy or any combination of a sulfonylurea, metformin, or a meglitinide,46 one to metformin, sulfonylurea, or meglitinide alone or a combination of any two of these drugs,59 one to metformin plus pioglitazone,60 and one to exenatide.49
Bebakar et al.46 compared once- or twice-daily insulin aspart 70/30 (n = 128) to oral antidiabetic agents (n = 63) in type 2 diabetics who were poorly controlled with oral antidiabetic agents. The dose of insulin was adjusted based on the basis of glucose level. The dose of oral antidiabetic agents was also adjusted, although addition or substitution of oral antidiabetic agents was not permitted during the study.
Kvapil et al.51 randomized patients to receive twice-daily insulin aspart 70/30 (n = 107), twice-daily insulin aspart 70/30 in combination with metformin (n = 108), or metformin and glibenclamide (n = 114). In this study, the metformin dose was kept constant, but the doses of insulin aspart 70/30 and glibenclamide were adjusted on the basis of the glucose response.
Raz et al.54 randomized patients to receive twice-daily insulin aspart 70/30 (n = 97), twice-daily insulin aspart 70/30 with pioglitazone (n = 93), or glibenclamide with pioglitazone (n = 91). The pioglitazone dose was kept fixed throughout the study, while the insulin aspart 70/30 and the glibenclamide doses were adjusted in response to changes in blood glucose. The insulin dose was lower in the insulin aspart 70/30 with pioglitazone group than in the insulin aspart 70/30 alone group (0.2 versus 0.3 units/kg/day).
In another study, Raz et al.57 compared the efficacy of a combination of insulin aspart 70/30 and rosiglitazone to a combination of glibenclamide and rosiglitazone. In this study, although the dose of insulin aspart 70/30 could be adjusted, doses of rosiglitazone and glibenclamide were kept fixed throughout the study.
Ushakova et al.59 compared twice-daily insulin aspart 70/30 to oral antidiabetic therapy (metformin, sulfonylurea, or meglitinide or a combination of any two of these drugs). Although the doses of all medications were optimized during the study, there was no dosing protocol.59
Raskin et al.60 enrolled insulin-naïve individuals with poor glycemic control on oral therapy. Before randomization, all participants were given pioglitazone and metformin, and the doses of these medications were optimized over 8 weeks. Participants were then randomized to the combination of twice-daily insulin aspart 70/30, pioglitazone, and metformin or to oral antidiabetic therapy (pioglitazone plus metformin). After randomization, only insulin doses could be titrated, so that the oral antidiabetic agent arm had reached its potential to affect blood glucose measurements by the time of randomization.
Nauck et al.49 compared twice-daily exenatide (an incretin mimetic) to twice-daily insulin aspart 70/30 in patients with suboptimal diabetes control, with patients being allowed to continue their metformin and sulfonylurea treatments. Insulin aspart 70/30 dose was titrated throughout the study to achieve optimal glucose control. The exenatide dose, on the other hand, was increased only once, at 4 weeks, from 5 micrograms to 10 micrograms twice daily. Since exenatide is the only noninsulin antidiabetic agent that is injectable (all others in this review are oral agents), we pooled these studies with and without the study by Nauck et al.49
Fasting glucose (see Appendix E, Evidence Table 4). In terms of lowering fasting glucose, insulin aspart 70/30 was found to be more effective than noninsulin antidiabetic agents in all studies except one,49 but the difference was statistically significant in only three.46, 49, 60 When the results of all trials were pooled, insulin aspart 70/30 was found to be more effective than oral antidiabetic agents in lowering fasting glucose (mean difference = -13.9 mg/dL; 95 percent CI: -24.4 to -3.4 mg/dL; p = 0.009; see Figure 14). The funnel plot was asymmetrical, and the trim-and-fill method showed that three studies need to be imputed to make the plot symmetrical. Addition of the imputed studies resulted in a statistically nonsignificant pooled mean difference. When the study by Nauck et al.49 that compared exenatide with insulin aspart 70/30 was excluded, insulin aspart 70/30 remained significantly different than oral antidiabetic agents in terms of lowering fasting glucose (weighted mean difference = -17.3 mg/dL; 95 percent CI: -29.3 to -5.4 mg/dL; p = 0.009; see Figure 14), and the publication bias disappeared.
All seven studies also reported changes in pre-dinner glucose levels. Insulin aspart 70/30 was significantly more effective in lowering pre-dinner glucose levels than were noninsulin antidiabetic agents in two studies.46, 54 In the study by Raz et al.,54 insulin aspart 70/30 in combination with pioglitazone, but not alone, was more effective than the combination of glibenclamide and pioglitazone in lowering pre-dinner glucose levels. When the results of all seven trials were pooled to obtain a summary estimate, insulin aspart 70/30 was found to be similar to noninsulin antidiabetic agents in lowering pre-dinner glucose levels (mean difference = -13.9 mg/dL; 95 percent CI: -30.4 to 2.7 mg/dL; p = 0.1). When we excluded the study by Nauck et al.,49 insulin aspart 70/30 was more effective than noninsulin antidiabetic agents (mean difference = -18.0 mg/dL; 95 percent CI: -33.1 to -2.7 mg/dL; p = 0.02).
Postprandial glucose (see Appendix E, Evidence Table 4). All seven studies also reported changes in postprandial glucose levels. As compared to a combination of a thiazolidinedione and glibenclamide, insulin aspart 70/30 lowered postprandial glucose levels in both studies,54, 57 but the decrease in blood glucose was significant in only one study.54 Similarly, as compared to a combination of thiazolidinedione and metformin, insulin aspart 70/30 was more effective in lowering postprandial glucose levels in another study.60 Insulin aspart 70/30 was more effective than either monotherapy or with any combination of a sulfonylurea, metformin, or a meglitinide in lowering dinner postprandial glucose levels in two studies.46, 59 On the other hand, no difference was found between insulin aspart 70/30 and a combination of metformin and glibenclamide in terms of lowering postprandial glucose levels in the study by Kvapil et al.51 Nauck et al. found that exenatide was more effective than insulin aspart 70/30 in lowering postprandial glucose levels.49 Pooling the results of these studies indicated that insulin aspart 70/30 lowered postprandial glucose levels more effectively than did the comparators (mean difference = -32.8 mg/dL; 95 percent CI: -62.5 to -3.1 mg/dL: p = 0.03; see Figure 15). The results did not change even when the study with exenatide49 was excluded from the meta-analysis (weighted mean difference = -40.7 mg/dL; 95 percent CI: -65.9 to -15.5 mg/dL; p = 0.002; see Figure 15).
Six of these seven studies also reported postprandial glucose after breakfast.46, 49, 51, 54, 59, 60 Insulin aspart 70/30 performed better than the comparator in four of these studies,46, 54, 59, 60 was equal to the comparator in one study,51 and was inferior to the comparator in the sixth study.49 Pooling the results of these studies pointed to a significant advantage of insulin aspart 70/30 in lowering postprandial glucose after breakfast (weighted mean difference = -24.5 mg/dL; 95 percent CI: -47.1 to -1.8 mg/dL; p = 0.03). When the study by Nauck et al.49 was excluded from the meta-analysis, the postprandial glucose-lowering effect of insulin aspart 70/30 remained significant (mean difference = -32.0 mg/dL; 95 percent CI: -47.0 to -17.1 mg/dL; p < 0.001).
Hemoglobin A1c (see Appendix E, Evidence Table 4). Changes in A1c in response to treatment were also reported in all seven studies. Bebakar et al.46 saw a significantly greater reduction in A1c with insulin aspart 70/30 than with oral antidiabetic agents (mean difference = -0.58 percent; p < 0.001). Similarly, Raz et al.54 reported that HbAlc was significantly lower in the combined insulin aspart 70/30 and pioglitazone group than in the glibenclamide and pioglitazone group (mean change = -0.64 percent; p = 0.005). However, insulin aspart 70/30 alone lowered A1c to a similar extent as did glibenclamide and pioglitazone in this trial (p > 0.05). In another study, Raskin et al.60 found that a combination of insulin aspart 70/30 plus metformin plus pioglitazone was more effective than metformin plus pioglitazone in lowering A1c (p < 0.001). On the other hand, Raz et al.57 reported that a combination of insulin aspart 70/30 and rosiglitazone was not more effective than a combination of rosiglitazone and glibenclamide in lowering A1c (mean change = -0.5 percent; p > 0.05); however, this study had a duration of only 6 weeks, and it is quite possible that the A1c levels had not yet reached a steady state with the study medications. Therefore, this study was excluded from the pooled analyses. Somewhat similar results were reported by Kvapil et al.51 who found that insulin aspart 70/30, whether alone or in combination with metformin, was not more effective than a combination of glibenclamide and metformin in lowering A1c (p > 0.05). Similarly, Ushakova et al.59also found that insulin aspart 70/30 alone or in combination with metformin was more effective than oral antidiabetic agents in lowering A1c levels (p < 0.001 for both comparisons). Nauck et al.49 found that insulin aspart 70/30 was slightly worse than exenatide in lowering A1c although the difference was not statistically significant (mean difference = 0.15 percent; p = 0.07). When the results of all these studies were pooled, insulin aspart 70/30 was found to be more effective than noninsulin antidiabetic agents (weighted mean difference = -0.52 percent; 95 percent CI: -1.0 to -0.04 percent; p = 0.034; see Figure 16). When the study by Nauck et al.49 was removed from the pooled analysis, the main conclusion of the meta-analysis did not change (weighted mean difference = -0.61 percent; 95 percent CI: -1.13 to - 0.1 percent; p = 0.02; see Figure 16).
Hypoglycemia (see Appendix E, Evidence Table 5). There were no major hypoglycemic events in five studies.49, 51, 54, 57, 59 Bebakar et al.46 reported one major hypoglycemic event in each arm, while Raskin et al.60 reported all four major hypoglycemic events in the insulin aspart 70/30 arm. All seven studies reported the incidence of minor hypoglycemic events. Nauck et al.49 reported the incidence of only nocturnal hypoglycemia, which was significantly lower in the exenatide group (p = 0.038). In all other studies, insulin aspart 70/30 was associated with a greater risk of minor hypoglycemia, reaching statistical significance in three studies.46, 54, 60 When the results of these studies were pooled, insulin aspart 70/30 was associated with a higher risk of minor hypoglycemia when compared to noninsulin antidiabetic agents (217 out of 762 patients versus 79 out of 672 patients; OR = 3.8; 95 percent CI: 1.7 to 8.5; p = 0.001; see Figure 17). When the study by Nauck et al.49 was excluded from the analysis, the results did not change appreciably (155 out of 539 patients versus 35 out of 473 patients; OR = 4.9; 95 percent CI: 1.8 to 13.5; p = 0.002; see Figure 17). Four studies reported the incidence of symptom-only hypoglycemia.51, 54, 57, 59 Pooling the results of these studies also indicated that insulin aspart 70/30 was associated with a higher risk of symptom-only hypoglycemia than were the noninsulin antidiabetic agents (97 out of 332 patients versus 41 out of 332 patients; OR = 3.9; 95 percent CI: 1.2 to 12.4; p = 0.02; see Figure 18).
Weight change (see Appendix E, Evidence Table 6). All seven studies reported on change in weight, but the data were not sufficiently reported in two studies to be usable in pooling the study results.54, 57 In the five other studies, insulin aspart 70/30 was found to be associated with weight gain when compared to noninsulin antidiabetic agents (weighted mean difference = 2.8 kg; 95 percent CI: 0.6 to 5.0 kg; p = 0.01; see Figure 19).
Other serious adverse events (see Appendix E, Evidence Table 6). None of these studies comparing insulin aspart 70/30 to a noninsulin antidiabetic agent reported on injection site reactions. Table 5 shows the range of risk differences between insulin aspart 70/30 and noninsulin antidiabetic agents in terms of total serious adverse events, other serious adverse events, and withdrawals due to adverse events.
Insulin aspart 70/30 versus other premixed insulin analogues. We found two crossover studies that compared insulin aspart 70/30 to insulin lispro 75/25.55, 58
Niskanen et al.55 compared twice-daily insulin aspart 70/30 to twice-daily insulin lispro 75/25 in a crossover study. This study was funded by Novo Nordisk, the manufacturer of insulin aspart 70/30. In both arms, the insulin dose was titrated to obtain optimal blood glucose control. The mean insulin dose was similar for each study drug.
Hermansen et al.58 compared a single dose of insulin aspart 70/30 to a single dose of insulin lispro 75/25 after a test meal in a crossover study. This study was also funded by Novo Nordisk. In this study, a similar dose of insulin, based on the weight of the patients, was given. As this study was a single-dose study, it was not designed to evaluate either fasting glucose or A1c. In addition, this study reported postprandial glucose excursions only.
Fasting glucose (see Appendix E, Evidence Table 4). Niskanen et al.55 did not find a significant difference between the two premixed insulin analogues in terms of lowering fasting glucose levels after breakfast (p = 0.42).
Postprandial glucose (see Appendix E, Evidence Table 4). Niskanen et al.55 also did not find a significant difference between the two premixed insulin analogues with regard to lowering 90-min breakfast or dinner postprandial glucose levels (p = 0.52 and 0.19, respectively).
Hemoglobin A1c (see Appendix E, Evidence Table 4). Although insulin aspart 70/30 appeared to be less effective than insulin lispro 75/25 in lowering A1c, the difference was not statistically significant (mean difference = 0.14 percent, p = 0.08) in the only study that reported this outcome.55
Hypoglycemia (see Appendix E, Evidence Table 5). One major hypoglycemic event occurred with each premixed insulin analogue in the trial by Niskanen et al.55 In Hermansen et al.,58 there were two major hypoglycemia episodes with insulin aspart 70/30 and five episodes with insulin lispro 75/25. Niskanen et al. reported that 57 out of 132 patients had minor hypoglycemic events with insulin aspart 70/30, as compared to 53 out of 132 patients with insulin lispro 75/25.55
Weight change (see Appendix E, Evidence Table 6). Since both studies were crossover studies, treatment-induced changes in weight were not abstracted.
Other serious adverse events (see Appendix E, Evidence Table 6). Niskanen et al.55 reported that 1 percent of those in the insulin aspart 70/30 arm and 2 percent in the insulin lispro 75/25 arm experienced injection site reactions. Table 5 shows the range of risk differences between insulin aspart 70/30 and insulin lispro 75/25 for other serious adverse events and withdrawals due to adverse events.
Insulin Lispro 75/25
Insulin lispro 75/25 versus long-acting insulin analogues. We identified five randomized crossover trials63–66, 74 and one observational study75 that compared insulin lispro 75/25 to a long-acting insulin analogue. Insulin glargine was the comparator agent in all these trials.
Roach et al.63 compared twice-daily insulin lispro 75/25 plus oral antidiabetic agents to once-daily insulin glargine plus oral antidiabetic agents in a 24-week crossover trial. The insulin dose was allowed to be titrated throughout the study to optimize glucose control. The mean daily dose of insulin lispro 75/25 was larger than the mean daily dose of insulin glargine (60 versus 44 units, respectively). The doses of the oral antidiabetic agents were not reported.
Jacober et al.64 compared insulin lispro 50/50 before breakfast and lunch plus insulin lispro 75/25 before dinner to once-daily insulin glargine in a crossover trial. The mean insulin dose at the end of therapy was greater for premixed insulin lispro than for insulin glargine (p = 0.01).
Malone et al.65 compared twice-daily insulin lispro 75/25 plus metformin to once-daily insulin glargine plus metformin in a crossover study. In this study, the metformin dose was kept fixed, but the insulin doses were adjusted to optimize glucose control. At the end of the study, the mean daily insulin dose was larger with insulin lispro 75/25, as compared to insulin glargine (0.62 versus 0.57 units/kg; p < 0.001).
With a similar study design but in a different population, Malone et al.66 compared twice-daily insulin lispro 75/25 plus metformin to once-daily insulin glargine plus metformin. The metformin dose was similar at the end of treatment; however, as in previous studies the insulin lispro 75/25 dose was significantly larger than the insulin glargine dose (0.42 versus 0.36 units/kg; p < 0.001).
Cox et al.74 compared twice-daily insulin lispro 75/25 with once-daily insulin glargine in poorly controlled type 2 diabetics. Oral antidiabetic agents were discontinued during the study. The doses of both insulin analogues were allowed to be titrated to optimize blood glucose control; however, this study did not report the insulin dose used by each treatment group.
Sun et al.75 performed a retrospective study by abstracting data on insulin naïve patients with type 2 diabetes from a U.S. national medical records database.
Fasting glucose (see Appendix E, Evidence Table 4). All five randomized trials reported fasting glucose levels. One study by Jacober et al.64 used insulin lispro 50/50 with breakfast and lunch and insulin lispro 75/25 with dinner. Because the fasting glucose level was likely to have been affected by the evening insulin injection, we included this trial in the current comparison. In all studies, insulin glargine was more effective than insulin lispro 75/25 in lowering fasting glucose, but the difference was significant in only one study.65 When these study results were pooled, insulin glargine was more effective than insulin lispro 75/25 in lowering fasting glucose levels (mean difference = 8.5 mg/dL; 95 percent CI: 3.6 to 13.3 mg/dL; p = 0.001; see Figure 20). Excluding the study by Jacober et al.64 had no effect on the results of this meta-analysis (weighted mean difference = 11.1 mg/dL; 95 percent CI: 4.6 to 17.6 mg/dL; p = 0.001; see Figure 20). We also identified an unpublished trial which found insulin glargine to be more effective than insulin lispro 75/25, but the difference was not statistically significant.82
Although all five trials reported pre-dinner glucose levels, the study by Jacober et al.64 was excluded for the reason stated above. The remaining four trials did not find any difference between insulin lispro 75/25 and insulin glargine in terms of lowering pre-dinner glucose levels.63, 65, 66, 74
Postprandial glucose (see Appendix E, Evidence Table 4). All randomized trials reported on both dinner and breakfast postprandial glucose levels.63–66, 74 However, as noted above, the trial by Jacober et al.64 used insulin lispro 50/50 with breakfast and lunch and insulin lispro 75/25 with dinner. For this trial, only dinner postprandial blood glucose values were used for the meta-analysis. Insulin lispro 75/25 was more effective than insulin glargine in lowering dinner postprandial glucose levels in all studies. In two of the four trials, insulin lispro 75/25 was also more effective in lowering breakfast postprandial levels.65, 66 Pooling of the studies identified a significant advantage for insulin lispro 75/25 in lowering dinner postprandial glucose (mean difference = -23.6 mg/dL; 95 percent CI: -30.9 to -16.4 mg/dL; p < 0.001; see Figure 21) and a nonsignificant advantage in lowering breakfast postprandial glucose (weighted mean difference = -10.0 mg/dL; 95 percent CI: -23.3 to 3.2 mg/dL; p = 0.137). We identified one unpublished trial82 that found a significant decrease in dinner postprandial glucose with insulin lispro 75/25 but no change in breakfast postprandial glucose levels (p = 0.04 and 0.97 respectively).
Hemoglobin A1c (see Appendix E, Evidence Table 4). Of the six studies that compared insulin lispro 75/25 to long-acting insulin analogues, five trials reported on the changes in A1c.63–66, 75 As noted above, Jacober et al.64 administered insulin lispro 50/50 with breakfast and lunch and insulin lispro 75/25 with dinner; therefore, the change in A1c in this study is a reflection of both strengths of insulin. This study found that the premixed insulin lispro based regimen was more effective than insulin glargine in lowering A1c (mean difference = -0.26 percent; p = 0.003). However, there was no significant difference in the percentage of patients who achieved the target A1c of less than or equal to 7 percent (44 versus 31 percent respectively; p = 0.1). On the other hand, in a study by Malone et al.65 insulin lispro 75/25 was not only able to lower A1c more effectively than was insulin glargine (mean difference = -0.4 percent; p = 0.002), but more patients achieved the target A1c of less than or equal to 7 percent (42 versus 18 percent; p < 0.001). In another study, Malone et al.66 found a combination of insulin lispro 75/25 and metformin to be more effective than a combination of insulin glargine and metformin in lowering A1c (mean difference = -0.60 percent; p < 0.001). Similar results were obtained by Roach et al.:63 They found that insulin lispro 75/25 was more effective than insulin glargine in lowering A1c (mean difference = -0.4 percent; p < 0.05). In the retrospective review of medical records, Sun et al.75 found a significantly larger reduction in A1c with insulin lispro 75/25 than with glargine (-0.10 percent, p < 0.05). When the results of the five trials were pooled, insulin lispro 75/25 was significantly more effective than insulin glargine in lowering A1c (mean difference = -0.33 percent; 95 percent CI: -0.48 to -0.17 percent; p < 0.01; see Figure 22). When the study by Jacober et al.64 was excluded, there was no change in the results of the meta-analysis (mean difference = -0.36 percent; 95 percent CI: -0.59 to -0.14 percent; p < 0.01; see Figure 22).
Hypoglycemia (see Appendix E, Evidence Table 5). The incidence of all hypoglycemia was reported for three trials.64–66 Malone et al.66 found an equal rate of all hypoglycemia with both types of insulins (0.61 versus 0.44 episodes/patient/30 days; p = 0.4). On the other hand, Jacober et al.64 and Malone et al.65 found a significantly greater incidence of overall hypoglycemia with insulin lispro than with insulin glargine (48 out of 58 versus 33 out of 54 patients and 57 out of 100 versus 40 out of 101 patients respectively). As Jacober et al.64 used two different insulin lispro preparations, it was not possible to identify the hypoglycemic events that were due solely to insulin lispro 75/25. Minor hypoglycemia was reported in two studies.63, 64 In these studies, insulin lispro was associated with a higher risk of minor hypoglycemia, but the difference was statistically significant only in the study by Jacober et al.64 Cox et al.74 only reported that there was no difference in the incidence of hypoglycemia in the two groups (data not reported).
Weight change (see Appendix E, Evidence Table 6). Since all these studies were crossover studies, treatment-induced changes in weight were not abstracted.
Other serious adverse events (see Appendix E, Evidence Table 6). None of these studies reported on injection site skin reactions. Table 6 shows the range of risk differences between insulin lispro 75/25 and long-acting insulin analogues with regard to withdrawals due to adverse events.
Table 6
Range of risk differences between insulin lispro 75/25 and other antidiabetic agents for selected adverse events.
Insulin lispro 75/25 versus rapid-acting insulin analogues. We did not find any study that had compared insulin lispro 75/25 to rapid-acting insulin analogues.
Insulin lispro 75/25 versus a combination of long-acting and rapid-acting insulin analogues. Our search did not find any study that had compared insulin lispro 75/25 to a combination of rapid-acting and long-acting insulin analogues.
Insulin lispro 75/25 versus premixed human insulin. We found nine randomized crossover studies10, 12, 44, 58, 62, 67, 70, 71, 73 and one retrospective observational study75 that compared insulin lispro 75/25 to premixed human insulin preparations. Four studies were one-dose studies and therefore had only one breakfast postprandial value after a test meal.44, 58, 62, 67 All the studies, except the study by Roach et al.,10 compared insulin lispro 75/25 to NPH/regular 70/30.
In the study by Roach et al.,10 patients were randomized to insulin lispro 50/50 before breakfast plus insulin lispro 75/25 before dinner or NPH/regular 50/50 before breakfast plus NPH/regular 70/30 before dinner. This study enrolled both type 1 and type 2 diabetics but reported results for each type of diabetes separately. The doses of all insulin preparations were adjusted to optimize glucose control, and there was no difference in the mean insulin dose at the end of the study.
Mattoo et al.70 compared twice-daily insulin lispro 75/25 to twice-daily NPH/regular 70/30 in type 2 diabetics who were fasting during the month of Ramadan. Whether the insulin doses were titrated or not was not reported.
In another study, Roach et al.73 compared twice-daily insulin lispro 75/25 to twice-daily NPH/regular 70/30 in patients with type 2 diabetes. Patients who were taking oral antidiabetic agents were excluded from the study. The insulin dose was titrated to optimize glucose control, and at the end of the study the mean daily insulin doses were similar for both treatment sequences.
Herz et al.12 compared the effect of insulin lispro 75/25 to that of NPH/regular 70/30 on the 24-h inpatient plasma glucose profile. Insulin doses were adjusted during the trial, and there was no difference in the mean daily insulin dose between the two treatments.
In another study, Herz et al.71 compared the plasma glucose response with insulin lispro 75/25 to that of a premixed human insulin before and after exercise. The insulin dose was similar in both treatment sequences.
In a three-arm crossover trial, Schwartz et al.62 compared one dose of insulin lispro 75/25 to one dose of NPH/regular 70/30 before a test meal. Patients were given an insulin dose that was comparable to their regular daily dose.
In another three-arm, crossover trial, Hermansen et al.58 compared a single dose of insulin lispro 75/25 to a single dose of NPH/regular 70/30 before a test meal. All patients received a fixed dose of insulin based on their body weight.
Coscelli et al.67 compared twice-daily insulin lispro 75/25 to NPH/regular 70/30 in diabetic patients with Italian dietary habits. However, they evaluated changes in blood glucose after a test meal. The mean daily insulin dose was similar in the two treatment groups.
Malone et al.44 compared a single dose of insulin lispro 75/25 to a single dose of a premixed human insulin before a test meal. Oral antidiabetic agents were discontinued for this study. All study participants received a fixed dose of insulin based on their body weight.
Fasting glucose (see Appendix E, Evidence Table 4). We identified four studies that reported on fasting blood glucose.10, 12, 70, 73 All four studies were randomized crossover trials and compared insulin lispro 75/25 to NPH/regular 70/30, except for the study by Roach et al.,10 in which subjects were given NPH/regular 50/50 before breakfast and NPH/regular 70/30 before dinner. As noted above, insulin lispro 50/50 was given before breakfast and insulin lispro 75/25 was given before dinner in this study. Since the fasting glucose levels are likely to be affected by dinner-time insulin, this study was included in this subsection. All four studies found a decrease in fasting glucose with insulin lispro 75/25, but the decrease was not statistically significant. One study12 did not report numerical values for the fasting glucose levels and therefore could not be included in meta-analysis. When the results of these studies were pooled, there was no difference between the two treatments in terms of lowering fasting glucose (weighted mean difference = 0.12 mg/dL; 95 percent CI: -6.05 to 6.29 mg/dL; p = 0.97; see Figure 23).
Three studies reported pre-dinner blood glucose levels.12, 70, 73 Of the three studies, one70 found insulin lispro 75/25 to be more effective than the premixed human insulin (mean difference = -7.2 mg/dL; p = 0.34). One study only mentioned that there was no difference in pre-dinner glucose between the two treatments during inpatient monitoring of the patients,12 and the second study reported pre-dinner glucose as a figure with a large overlap between the standard error of the mean error bars.73
Postprandial glucose (see Appendix E, Evidence Table 4). Four studies reported treatment-related changes in dinner postprandial glucose levels.10, 12, 70, 73 As noted above, we used the data from Roach et al.10 only for dinner postprandial values. Insulin lispro 75/25 lowered the dinner postprandial blood glucose in all studies, but the decrease was statistically significant in only two of the four studies.70, 73 When we pooled the study results, insulin lispro 75/25 was more effective than the premixed human insulin in lowering dinner postprandial glucose (mean difference = -17.8 mg/dL; 95 percent CI: -27.0 to -8.6 mg/dL; p < 0.001; see Figure 24). When we excluded the study by Roach et al.,10 the pooled result remained significant (weighted mean difference = -18.9 mg/dL; 95 percent CI: -28.7 to -9.2 mg/dL; p < 0.001; see Figure 24).
Breakfast postprandial glucose levels were reported in eight studies.12, 44, 58, 62, 67, 70, 71, 73 As noted above, in four of these studies, one dose of the study drugs was given with a test meal.44, 58, 62, 67 All studies found insulin lispro 75/25 to be more effective than the premixed human insulin in lowering breakfast postprandial glucose levels, but the difference was statistically significant in only three of the eight studies.67, 71, 73 In the pooled analysis, insulin lispro 75/25 was more effective than premixed human insulin in lowering breakfast postprandial glucose levels (weighted mean difference = -14.9 mg/dL; 95 percent CI: -21.4 to -8.4 mg/dL; p < 0.001).
Hemoglobin A1c (see Appendix E, Evidence Table 4). Three of nine studies reported changes in A1c levels.10, 73, 75 Roach et al.73 did not find any significant difference between insulin lispro 75/25 and the premixed human insulin in terms of decreasing A1c levels after 6 months of followup (mean difference = 0.2 percent; p = 0.41). Similar results were reported in the second study by Roach et al.,10 which used two different insulin lispro preparations (insulin lispro 75/25 and insulin lispro 50/50) and did not find any difference between the two treatment regimens in terms of lowering A1c levels (mean difference = 0.07 percent; p = 0.37). The retrospective chart review found insulin lispro 75/25 to be more effective than premixed human insulin in lowering A1c (-0.12 percent; statistical significance not reported), but it did not provide enough data for a meta-analysis.
Hypoglycemia (see Appendix E, Evidence Table 5). Schwartz et al.62 reported one episode of minor hypoglycemia in which a patient received a premixed human insulin before the test meal but received insulin lispro at lunch, making it difficult to attribute this event to a premixed formulation. Roach et al.73 did not find a difference in the incidence of all hypoglycemia between insulin lispro 75/25 and a premixed human insulin (42 versus 35 percent respectively; p = 0.4). Similarly, Herz et al.12 and Mattoo et al.70 also reported a similar hypoglycemia rate for the two treatment regimens (p = 0.59 and 0.72, respectively). Hermansen et al.58 reported fewer hypoglycemic events with a premixed human insulin than with insulin lispro 75/25 (11 versus 19 events among 44 and 42 patients, respectively), although they did not report whether the difference was significant. Malone et al.44 reported that the distribution of hypoglycemic episodes was similar for the two premixed insulin preparations. Coscelli et al.67 reported a total of 11 hypoglycemic episodes during the study, but the difference between the two treatments was not statistically significant.
Weight change (see Appendix E, Evidence Table 6). Since all these studies were crossover studies, treatment-induced changes in weight were not abstracted.
Other serious adverse events (see Appendix E, Evidence Table 6). In the study by Schwartz et al.,62 one patient (5 percent) in the insulin lispro 75/25 arm, but none in the NPH/regular 70/30 arm, experienced an injection site reaction. Table 6 shows the range of risk differences between insulin lispro 75/25 and premixed human insulins in terms of total serious adverse events, withdrawals due to adverse events, and other serious adverse events.
Insulin lispro 75/25 versus intermediate-acting human insulin. We did not find any study that compared insulin lispro 75/25 to an intermediate-acting human insulin.
Insulin lispro 75/25 versus noninsulin antidiabetic agents. We found three randomized parallel-arm studies that compared insulin lispro 75/25 to noninsulin antidiabetic agents.43, 68, 72 Tirgoviste et al.43 compared insulin lispro 75/25 to a fixed dose of glibenclamide. Malone et al.68 compared a combination of insulin lispro 75/25 and metformin to a combination of glibenclamide and metformin. Herz et al.72 compared insulin lispro 75/25 to a fixed dose of glyburide. All trials were 16 weeks in duration and reported on all intermediate outcomes. The insulin lispro 75/25 dose was titrated in order to optimize glucose control. Oral antidiabetic agents' doses were adjusted to optimize glucose control in only one study.68
Fasting glucose (see Appendix E, Evidence Table 4). In terms of fasting glucose, all three trials individually found insulin lispro 75/25 to be more effective than noninsulin antidiabetic agents in lowering fasting glucose, but this difference was not significant in one study.68 Pooling the results of all three studies indicated that insulin lispro 75/25 was more effective than noninsulin antidiabetic agents in lowering fasting glucose levels (weighted mean difference = -31.4 mg/dL; 95 percent CI: -45.7 to -17.1 mg/dL; p < 0.001; see Figure 25).
Insulin lispro 75/25 was also significantly more effective in reducing pre-dinner glucose levels in all three studies. When the results of these studies were pooled, insulin lispro 75/25 remained more effective than noninsulin antidiabetic agents in terms of lowering pre-dinner glucose levels (weighted mean difference = -25.0 mg/dL; 95 percent CI: -37.0 to -13.1 mg/dL; p < 0.001).
Postprandial glucose (see Appendix E, Evidence Table 4). With regard to postprandial glucose levels, insulin lispro 75/25 was more effective than oral antidiabetic agents in all three trials. When the results of these studies were pooled, insulin lispro 75/25 remained more effective than its comparator in lowering postprandial glucose levels after dinner (weighted mean difference = -47.3 mg/dL; 95 percent CI: -63.5 to -31.0 mg/dL; p < 0.001; see Figure 26) and after breakfast (weighted mean difference = -54.2 mg/dL; 95 percent CI: -70.7 to -37.7 mg/dL; p < 0.001).
Hemoglobin A1c (see Appendix E, Evidence Table 4). In two trials,43, 72 insulin lispro 75/25 was more effective than noninsulin antidiabetic agents in lowering A1c, but the difference was not statistically significant in the third trial.68 Pooling the study results indicated that insulin lispro 75/25 was not significantly more effective than oral antidiabetic agents in lowering A1c levels (weighted mean difference = -0.42 percent; 95 percent CI: -1.00 to 0.16 percent; p = 0.15; see Figure 27).
Hypoglycemia (see Appendix E, Evidence Table 5). In the study by Tirgoviste et al.,43 the incidence of hypoglycemia was higher in patients treated with insulin lispro 75/25 than in those treated with glibenclamide (44.7 versus 10.3 percent; p = 0.001). Similar results were reported by Herz et al.,72 who found a lower rate of hypoglycemia in patients treated with glibenclamide. In contrast, Malone et al.68 found a higher rate of overall hypoglycemia in the oral antidiabetic treatment arm, although the difference did not reach statistical significance (p = 0.07). Pooling the results of these three studies indicated no difference in the overall hypoglycemia rate (hypoglycemia incidence not reported by all trials) measured as episodes per patient per 30 days (rate ratio = 4.86; 95 percent CI: 0.48 to 49.52; p = 0.18; see Figure 28).
Weight change (see Appendix E, Evidence Table 6). Insulin lispro 75/25 was associated with weight gain in all three studies, and this effect was significant in two studies.43, 72 When the results of these studies were pooled, insulin lispro 75/25 was associated with a larger weight increase than were oral antidiabetic agents (weighted mean difference = 1.88 kg; 95 percent CI: 1.35 to 2.41 kg; p < 0.001; see Figure 29).
Other serious adverse events (see Appendix E, Evidence Table 6). Injection site reactions were not evaluated in any of these studies. Table 6 shows the range of risk differences between insulin lispro 75/25 and oral antidiabetic agents in terms of withdrawals due to adverse events and other serious adverse events.
Insulin lispro 75/25 versus other premixed insulin analogues. We identified four randomized crossover studies: two comparing insulin lispro 75/25 to insulin aspart 70/30,55, 58 one to insulin lispro 50/50,62 and one to a combination of morning insulin lispro 50/50 and dinner insulin lispro 75/25.69
Details of the comparison in two studies have been reviewed above in the insulin aspart 70/30 section.55, 58 In the third study, Schwartz et al.62 compared insulin lispro 75/25 and insulin lispro 50/50 in a single-dose, three-way crossover trial. The doses of both insulin preparations were comparable and fixed in this study. Roach et al.69 compared a regimen of insulin lispro 50/50 before breakfast plus insulin lispro 75/25 before dinner to twice-daily insulin lispro 75/25. There was no difference in the morning or evening mean insulin dose between the two treatments in this study (p = 0.85 and 0.88 respectively).
Fasting glucose (see Appendix E, Evidence Table 4). Fasting glucose levels were reported in only one study,55 which did not find a significant difference between the two premixed insulin analogues. In the second study, one dose of the study medication was given with a test meal and blood glucose levels were measured,62 and therefore fasting glucose levels from this study were not available. In the third study,69 insulin lispro 75/25 was given at dinner to both arms, and therefore fasting glucose levels were likely to reflect insulin lispro 75/25 in both arms.
Postprandial glucose (see Appendix E, Evidence Table 4). With regard to postprandial glucose, the comparison between insulin aspart 70/30 and insulin lispro 75/25 did not find a significant advantage of one premixed insulin analogue over the other in terms of lowering dinner or breakfast postprandial glucose levels.55 In the second study,62 insulin lispro 50/50 was more effective than insulin lispro 75/25 in lowering postprandial glucose levels after a test-meal (p < 0.05). In the third study,69 insulin lispro 75/25 was inferior to insulin lispro 50/50 in terms of lowering postprandial glucose levels after breakfast (p = 0.001). Dinner postprandial glucose levels were not compared in the third study, since both treatment arms received the same insulin formulation. The fourth study did not report postprandial glucose levels after 2 h or 90 min of the test meal.58
Hemoglobin A1c (see Appendix E, Evidence Table 4). Only one study reported changes in A1c levels and did not find a significant difference between insulin lispro 75/25 and insulin aspart 70/30 in terms of lowering A1c levels (mean difference = 0.14 percent, p = 0.08).69
Hypoglycemia (see Appendix E, Evidence Table 5). In one study,62 it was not possible to discern which insulin preparation was responsible for the hypoglycemic events. In the other three studies, the overall hypoglycemia event rates did not differ.55, 58, 69 When the results of these studies were combined, there was no difference between insulin lispro 75/25 and the other premixed insulin analogues (rate ratio = 1.13; 95 percent CI: 0.97 to 1.31; p = 0.12; see Figure 30).
Weight change (see Appendix E, Evidence Table 6). Because all the trials had a crossover design, we did not abstract weight change data.
Other serious adverse events (see Appendix E, Evidence Table 6). Schwartz et al.62 reported one injection site reaction in each of the premixed insulin analogue arms. Table 6 shows the range of risk differences between insulin lispro 75/25 and other premixed insulin analogues in terms of total serious adverse events and withdrawals due to adverse events.
Insulin Lispro 50/50
Insulin lispro 50/50 versus long-acting insulin analogues. Our search identified three randomized trials64, 76, 79 that compared insulin lispro 50/50 to a long-acting insulin analogue, insulin glargine in all three cases. The study by Jacober et al.64 compared a regimen of insulin lispro 50/50 before breakfast and lunch and insulin lispro 75/25 before dinner to a once-daily insulin glargine injection over a 16-week period, while the study by Kazda et al.76 was a parallel-arm trial that compared insulin lispro 50/50 to insulin glargine over a followup of 24 weeks. A third study, the parallel-arm trial,79 compared thrice-daily insulin lispro 50/50 plus metformin twice-daily to daily insulin glargine plus metformin twice-daily. The insulin dose was significantly higher in the premixed insulin analogue arm than in the insulin glargine arm in all three studies.
Fasting glucose (see Appendix E, Evidence Table 4). Fasting glucose was reported in two studies.76, 79 Kazda et al.76 reported that insulin glargine was more effective than insulin lispro 50/50 in lowering fasting glucose (mean difference = -30.6 mg/dL; p < 0.001). Similarly, Robbins et al.79 found that glargine plus metformin was more effective than insulin lispro 50/50 plus metformin in lowering fasting glucose (mean difference = -28 mg/dL; p < 0.001). Pre-dinner glucose levels were reported in three trials.64, 76, 79 Pooling the results of these trials indicated that insulin lispro 50/50 was more effective in lowering pre-dinner fasting glucose than was insulin glargine (mean difference = -10.9 mg/dL, 95 percent CI: -18.5 to -3.4 mg/dL; p = 0.004; see Figure 31).
Postprandial glucose (see Appendix E, Evidence Table 4). Postprandial glucose was reported in all three trials.64, 76, 79 Jacober et al.64 found a significant decrease in postprandial glucose levels after breakfast (p < 0.003) with insulin lispro 50/50, as compared to insulin glargine. Similarly, Robbins et al.79 found insulin lispro 50/50 to be more effective than insulin glargine in reducing 2-h postprandial glucose after breakfast (-10 mg/dL; p = 0.03) and after dinner (36 mg/dL; p < 0.001). Kazda et al.76 found insulin lispro 50/50 to be more effective than insulin glargine in lowering postprandial glucose levels after dinner but not after breakfast (p-value not reported). When the results from these trials were pooled, insulin lispro 50/50 was found to be more effective than insulin glargine in lowering postprandial glucose levels (mean difference = -32.7 mg/dL; 95 percent CI: -48.2 to -17.1 mg/dL; p < 0.001; see Figure 32).
Hemoglobin A1c (see Appendix E, Evidence Table 4). Changes in A1c in response to treatment were reported in the three studies.64, 76, 79 Jacober et al. found a greater decrease in A1c with insulin lispro 50/50 given with breakfast and lunch and insulin lispro 75/25 given with dinner than with insulin glargine (mean difference = -0.26 percent; p = 0.007). However, this result reflects combined treatment with two premixed preparations of insulin lispro and cannot be attributed to a single preparation. In the second study by Kazda et al.,76 thrice-daily insulin lispro 50/50 was more effective than once-daily insulin glargine in lowering A1c (-0.9 percent; p < 0.001). Robbins et al.79 found insulin lispro 50/50 plus metformin to be more effective than insulin glargine plus metformin in reducing A1c levels (mean difference = -0.4; p < 0.001). Pooling the results of these studies also indicated that insulin lispro 50/50 was more effective than insulin glargine in lowering A1c (mean difference = -0.4 percent; 95 percent CI: -0.65 to -0.15 percent; p = 0.002; see Figure 33).
Hypoglycemia (see Appendix E, Evidence Table 5). Insulin glargine was associated with a lower risk of hypoglycemia in the three studies.64, 76, 79 Jacober et al.64 reported that a significantly larger number of patients receiving premixed insulin analogues had at least one episode of hypoglycemia when compared with those receiving insulin glargine (48 out of 58 patients versus 33 out of 54 patients; p = 0.01). In Kazda et al.,76 44.4 percent of the patients treated with insulin lispro 50/50 had at least one episode of hypoglycemia, as compared to 32.1 percent of those treated with insulin glargine (p-value not reported). In Robbins et al.,79 both arms had a similar incidence of severe hypoglycemia and nocturnal hypoglycemia (3 versus 2 and 17 versus 19 among 157 and 158 patients respectively; p = nonsignificant for both); however, the overall hypoglycemia incidence was higher in the insulin lispro 50/50 group (45 versus 28 among 157 and 158 patients; p = 0.02).
Weight change (see Appendix E, Evidence Table 6). Robbins et al.79 reported a significant weight gain in the insulin lispro 50/50 arm as compared to the insulin glargine arm (mean difference = 1.7 kg; p < 0.001). Similarly, Kazda et al.76 reported a larger increase in BMI with insulin lispro 50/50 than with insulin glargine, although this difference did not reach statistical significance (mean difference = 0.4 kg/m2; p = 0.19).
Other serious adverse events (see Appendix E, Evidence Table 6). None of the studies reported on injection site reactions. Table 7 shows the range of risk differences between insulin lispro 50/50 and long-acting insulin analogues with regard to withdrawals due to adverse events.
Table 7
Range of risk differences between insulin lispro 50/50 and other antidiabetic agents for selected adverse events.
Insulin lispro 50/50 versus rapid-acting insulin analogues. We found only one study that compared insulin lispro 50/50 to rapid-acting prandial insulin lispro.76 In this study, Kazda et al. compared insulin lispro 50/50 thrice-daily before meals to rapid-acting insulin lispro three times before meals over a period of 24 weeks. The insulin dose was allowed to be titrated to achieve optimal glucose control. All oral antidiabetic agents were discontinued. At the end of the study, the mean daily insulin dose was lower in the rapid-acting insulin lispro group than in the insulin lispro 50/50 group (0.50 versus 0.59 units/kg; p-value not reported).
Intermediate outcomes (see Appendix E, Evidence Table 4). This study did not find any difference between insulin lispro 50/50 and rapid-acting insulin lispro in terms of lowering fasting glucose (mean difference = 0 mg/dL; p > 0.05) or postprandial glucose (mean difference = 3.6 mg/dL; p > 0.05). Similarly, there was no difference between insulin lispro 50/50 and rapid-acting insulin lispro in terms of lowering A1c levels (mean difference = -0.1 percent; p = 0.57).
Adverse events (see Appendix E, Evidence Tables 5 and 6). The incidence of hypoglycemia was greater with rapid-acting insulin lispro than with insulin lispro 50/50 (53.8 percent versus 44.4 percent; p-value not reported). Similarly, there was a larger increase in BMI with rapid-acting insulin lispro than with insulin lispro 50/50 (mean difference = 0.3 kg/m2; p = 0.048). This study did not report on injection site reactions. Table 7 shows the risk difference between insulin lispro 50/50 and rapid-acting insulin lispro in terms of withdrawals due to adverse events.
Insulin lispro 50/50 versus a combination of long-acting and rapid-acting insulin analogues. We identified one parallel-arm study by Rosenstock et al.80 that evaluated thrice-daily insulin lispro 50/50 against an insulin glargine at bedtime plus mealtime rapid-acting insulin lispro regimen. This randomized open-label trial did allow investigators to replace the evening meal insulin lispro 50/50 with insulin lispro 75/25. At the end of the study, the mean insulin dose was higher for the insulin glargine arm than for the insulin lispro 50/50 arm (147 versus 159 units, p = 0.002).
Intermediate outcomes (see Appendix E, Evidence Table 4). In this study, insulin glargine plus rapid-acting insulin lispro combination was more effective than insulin lispro 50/50 in lowering fasting glucose (147 versus 159 mg/dL; p = 0.013) and 2-h postbreakfast glucose (155 versus 174 mg/dL; p = 0.002).80 Similarly, the insulin glargine plus rapid-acting insulin lispro combination was more effective in lowering A1c (6.8 versus 6.9 percent; p = 0.02) and in increasing the number of patients who achieved the target A1c of less than 7.0 percent (69 versus 54 percent, p = 0.009).
Adverse events (seeAppendix E, Evidence Table 5 and 6). The incidence of overall, nocturnal, and severe hypoglycemia was similar between the two arms (169 out of 187 patients versus 166 out of 187 patients, p = 0.7; 109 versus 110, p = 1.0; 6 versus 4, p = 0.7 respectively). Similarly, there was no difference in the amount of weight gain (4.0 kg vs. 4.5 kg; p = 0.2). This study reported a total of 22 serious adverse events, nine of which were in the premixed insulin lispro group and 13 in the insulin glargine plus rapid-acting group. Table 7 shows the range of risk differences between insulin lispro 50/50 and long-acting insulin analogues plus a rapid-acting insulin analogue in terms of withdrawals due to adverse events.
Insulin lispro 50/50 versus premixed human insulin. We identified three randomized crossover studies10, 62, 77 and one parallel-arm study78 that compared insulin lispro 50/50 to premixed human insulin preparations. In the first crossover study,62 only one fixed dose of the study medication was given with a test meal, and glucose levels were measured. In the second study,10 insulin lispro 50/50 with the morning meal and insulin lispro 75/25 with evening meal was compared to NPH/regular 50/50 with breakfast and NPH/regular 70/30 with dinner. Both these studies have been discussed in the preceding sections.
Schernthaner et al.77 compared thrice-daily insulin lispro 50/50 before each meal to twice-daily NPH/regular 70/30 in type 2 diabetic patients who had previously been treated with other insulin preparations. The insulin dose was adjusted throughout the study to optimize glucose control. There was no difference in the total daily dose at the end of the study between the two treatments.
Yamada et al.78 compared twice-daily insulin lispro 50/50 with twice-daily premixed human insulin preparations. The insulin dose was adjusted during the study in order to optimize glucose control, and there was no difference in the total daily insulin dose between the two arms at the end of the study (p > 0.05).
Fasting glucose (see Appendix E, Evidence Table 4). Of the four studies, two evaluated changes in fasting glucose levels. In one trial, there was no difference between insulin lispro 50/50 and a premixed human insulin in terms of lowering fasting glucose.78 In the second trial,77 the premixed human insulin was more effective than insulin lispro 50/50 in lowering fasting glucose (p < 0.001).
Changes in pre-dinner glucose levels were evaluated in two studies.10, 77 Roach et al.10 found insulin lispro 50/50 to be less effective than NPH/regular 50/50 in lowering pre-dinner glucose levels (mean difference = 4.32 mg/dL; p = 0.01). On the other hand, Schernthaner et al.77 found insulin lispro 50/50 to be more effective than NPH/regular 70/30 in lowering pre-dinner glucose levels, although this difference was not statistically significant (mean difference = -13.0 mg/dL, p = 0.06).
Postprandial glucose (see Appendix E, Evidence Table 4). Dinner and breakfast postprandial glucose levels were reported by two and three studies, respectively. Insulin lispro 50/50 was more effective than premixed human insulin in lowering dinner postprandial glucose in one of the two studies77 and in lowering breakfast postprandial glucose levels in two of the three studies.10, 62 In a pooled analysis, insulin lispro 50/50 was more effective in lowering breakfast postprandial glucose levels (weighted mean difference = -30.3 mg/dL; 95 percent CI: -55.6 to -5.0 mg/dL; p = 0.02).
Hemoglobin A1c (see Appendix E, Evidence Table 4). Two77, 78 of the four trials reported treatment-related changes in A1c. Schernthaner et al.77 found that although A1c decreased from baseline in both treatment arms, the decrease was significantly greater with insulin lispro 50/50 than with NPH/regular 70/30 (mean difference = -0.5 percent; p = 0.01). Similarly, Yamada et al.78 also found insulin lispro 50/50 to be more effective than the premixed human insulin preparations in lowering A1c (mean difference = -0.31 percent; p < 0.05).
Hypoglycemia (see Appendix E, Evidence Table 5). In one study,62 the insulin preparation that was responsible for the hypoglycemia was not clear. In the other three studies, there was no difference between the insulin lispro 50/50 and the premixed human insulin in terms of the incidence or rate of hypoglycemia.10, 77, 78
Weight change (seeAppendix E, Evidence Table 7). The only parallel-arm study did not report on the weight change during the trial with study drugs.
Other serious adverse events (see Appendix E, Evidence Table 6). One study reported one (4 percent) injection site reaction in the insulin lispro 50/50 arm and no reactions in the premixed human insulin arm.62 Table 6 shows the range of risk differences between insulin lispro 50/50 and premixed human insulins in terms of withdrawals due to adverse events.
Insulin lispro 50/50 versus intermediate-acting human insulin. We could not identify any study that had performed this comparison.
Insulin lispro 50/50 versus noninsulin antidiabetic agents. Our search did not find any study that had performed this comparison.
Insulin lispro 50/50 versus other premixed insulin analogues. We found one study comparing insulin lispro 50/50 to insulin lispro 75/25,62 and one study comparing a regimen of insulin lispro 50/50 in the morning and insulin lispro 75/25 in the evening to twice-daily insulin lispro 75/25.69 Both these studies have been noted in the preceding sections.
Intermediate outcomes (see Appendix E, Evidence Table 4). These studies did not report changes in either fasting glucose levels or pre-dinner glucose levels after treatment with insulin lispro 50/50. Both studies reported postprandial glucose levels after breakfast and found insulin lispro 50/50 to be more effective than insulin lispro 75/25 in lowering glucose levels (p < 0.05 in both studies). Although A1c was reported in one crossover study,69 the duration of followup was only 8 weeks in each study period, with no washout period, raising the possibility of a carryover effect from the previous treatment period.
Adverse events (see Appendix E, Evidence Tables 5 and 6). Roach et al.69 found a higher incidence of overall hypoglycemia with insulin lispro 50/50 than with insulin lispro 75/25, although this difference was not statistically significant (32.4 percent versus 26.1 percent; p = 0.08).
Publication Bias
For the body of literature on fasting glucose values comparing for insulin aspart 70/30 with noninsulin antidiabetic agents, we found strong evidence of publication bias. As mentioned earlier, the funnel plot was asymmetrical and the trim-and-fill method showed that three studies need to be imputed to make the plot symmetrical. We did not find strong evidence of publication bias for all other comparisons for intermediate outcomes.
Study Quality Assessment
The overall quality of the included studies was fair to good (see Appendix E, Evidence Table 7). All the trials except two52, 75 were RCTs. Randomization methods were described in 17 studies32, 39, 43, 45, 47–51, 55, 57–59, 65, 78–80 and were adequate in all these studies except one.47 The remaining RCTs did not describe their randomization methods is sufficient detail.
Only seven trials used blinding of the treatment at some stage of the trial. Five trials used blinding for patients and providers,13, 44, 48, 62, 69 while the remaining two trials used blinding for outcome assessors.32, 45 It is difficult to achieve blinding of patients and providers because premixed insulin analogues need to be given with meals, while the other insulin preparations are generally given at other times, with different frequency, or with a different route of administration.
For outcomes based on blood tests, the outcome assessment was unlikely to have been biased by the lack of blinding. However, the assessment of other outcomes could have been biased. Several studies asked patients to maintain a diary of their blood glucose levels. This self-monitoring of blood glucose may have affected the accurate and precise measurement of fasting and postprandial blood glucose.
In two studies, followup was inadequate to fully assess the effectiveness of premixed insulin analogues in lowering A1c levels.57, 69 Four studies had complete followup, with no study participant lost during followup,10, 43, 52, 68 while eight studies lost more than 10 percent of the enrolled participants during followup.12, 54, 61, 63, 64, 74, 75, 80 In all the remaining studies, the percentage of patients who were lost to followup was less than 10 percent. Of the studies in which some patients withdrew, six studies12, 53, 67, 70, 74, 75 either did not provide a description of withdrawals or the withdrawals were described inadequately; the remaining studies had an adequate description of withdrawals.
As mentioned earlier, the source of funding was the pharmaceutical industry in the case of all trials except two; one was funded jointly by the NIH and Eli Lilly,74 and the other was funded by the Japan Diabetes Foundation.61 Six trials did not report their source of funding.43, 44, 60, 65, 66, 71 A statement regarding conflict of interest was not included in 27 studies.10–12, 15, 43, 44, 46, 47, 51, 55–61, 64, 65, 67–71, 74, 77, 78, 83
The study conclusions were reflective of the study results in most cases, except for five studies in which the conclusions were only partially supported by the results.13, 52, 53, 64, 72
Applicability Assessment
Generalization of these results to the U.S. diabetic population and current clinical practice was limited by the fact that all the identified studies were efficacy trials and not effectiveness trials (see Appendix E, Evidence Table 8). Participants were recruited from outpatient clinics in three trials,47, 49, 57 from subspecialty clinics in three additional trials,62, 67, 72 from both outpatient and subspecialty clinics in one trial,52 from clinics and hospitals in one trial,61 and from clinical centers (without further details being specified) in one trial.32 The remaining trials did not mention the population source from which the participants were enrolled. The ratio of enrolled patients to the screened population was not reported in 22 studies;10–13, 39, 43, 44, 47, 50, 56, 57, 61, 63, 66, 67, 69–71, 73, 75, 77, 78 in all the other studies, this ratio was higher than 50 percent. In four trials, more than 10 percent of the participants were excluded during the lead-in period.39, 46, 60, 66
Most studies enrolled patients similar in age to the general U.S. diabetic population, except for the case of five studies.47, 48, 62, 72, 77 Women were underrepresented in five trials,46, 52, 57, 62, 78 and in two trials there were more women than men.39, 59 One study did not report on the sex ratio.74 In five trials, the proportion of enrolled racial and ethnic groups was reflective of the general population in the U.S.15, 39, 60, 75, 80 Twenty-one trials did not report on the race/ethnicity admixture of the enrolled population,10–12, 43–45, 48–50, 54–56, 58, 59, 65, 66, 70–73, 77 and the remaining trials did not have a racial and ethnicity admixture reflective of the U.S. population.
In most trials, the spectrum of diabetic complications and comorbidities among the enrolled participants was limited. Some trials excluded insulin-naïve patients, while other trials excluded all insulin-treated patients. All trials either excluded patients with cardiac, renal, or hepatic disease or did not report whether or not such patients were included, thus limiting our ability to generalize the results to these subpopulations.
The dose of insulin, route of administration, and schedule of administration were reflective of clinical practice or were easy to replicate in clinical practice in all trials except in one,70 in which patients who wished to fast were given insulin during the month of Ramadan. The monitoring of the treatment was reflective of general clinical practice in most studies except seven.12, 13, 15, 43, 44, 53, 71 In one trial,43 adjustments to the insulin dose were made every few days, and the patients had frequent visits during the study period. In another study, investigators telephoned patients at least once weekly,12 while in three studies monitoring of blood glucose was too frequent to be implemented in clinical practice.13, 15, 53 In two studies, patients were hospitalized or closely monitored throughout the duration of the study, a situation that cannot be applied in clinical practice.44, 71 Premixed insulin analogues were compared with adequate dosing and schedule of medicines in all but six studies.43, 49, 57, 59, 60, 72 In these studies, the dose of the comparator was held constant, while the premixed insulin dose was increased on the basis of glucose levels. Holding the dose of comparator may have biased the results of these studies in favor of premixed insulin analogues.
Overall, the applicability of the studies to the diabetic population of the U.S. in terms of fasting glucose outcomes was fair. In most studies, self-monitoring of fasting blood glucose was reported, reflecting current clinical practice in which a patient maintains a blood glucose diary and the physician adjusts the insulin dose to optimize glucose control. When premixed insulin analogues were compared to or used in combination with oral antidiabetic agents, the dose of these antidiabetic agents was held constant in most studies. This situation is in contrast to usual clinical practice, in which the dose of oral antidiabetic agents is either titrated to reflect glucose control, or the antidiabetic agent is changed to a different class or discontinued all together.
The overall applicability of the studies to the diabetic population of the U.S. with regard to postprandial glucose levels was also graded as fair. In terms of fasting glucose, most studies used self-monitoring of glucose by patients, an approach that is reflective of clinical practice. Four one-dose studies44, 56, 58, 62 were not reflective of clinical practice, and because the insulin preparations had not achieved a steady state in plasma, it is possible that the results from these trials might not be reproducible in practice. Calculation of the insulin dose and a test meal also cannot be reproduced in clinical practice.
The overall applicability of the studies to the U.S. diabetic population for A1c was graded as fair and was limited because of the aforementioned reasons related to fasting and postprandial glucose.
The overall applicability of the studies to the diabetic population of U.S. in terms of the adverse event of hypoglycemia was also fair. Since most studies excluded patients with significant comorbidity, severe obesity, and very poorly controlled diabetes, it is not possible to generalize the results of these studies to the patient population in clinical practice. In some studies, the monitoring of the patient population as conducted would not be feasible in usual clinical practice.
The overall applicability of the studies to the U.S. diabetic population with regard to the adverse outcome of weight gain was graded as fair. Most studies were of relatively short duration except for two studies32, 45 that followed patients for 1 year and 2 years, respectively. Because weight gain is a relatively slow process, it is difficult to draw relevant conclusions from short-term studies. In addition, many studies using premixed insulin lispro preparations were crossover studies that were excluded from the analysis of weight gain, thus limiting the amount of evidence from which conclusions could be drawn.
Clinical Outcomes
Key Messages
- No statistically significant differences in all-cause mortality (OR = 2.93; 95 percent CI: 0.95 to 9.05), cardiovascular mortality (OR = 6.80; 95 percent CI: 0.87 to 53.12), cardiovascular morbidity (OR = 0.86; 95 percent CI: 0.49 to 1.52), or the combined outcome of all-cause mortality and cardiovascular morbidity (OR = 2.10; 95 percent CI: 0.87 to 5.10) between premixed insulin analogues and other diabetes medications were reported in these mainly short-duration RCTs.
- The low absolute number of events in these short duration trials in which clinical events were not the primary outcomes made it difficult to draw any firm conclusions regarding any of the clinical outcomes.
- Insufficient evidence existed with regard to nephropathy outcomes to allow us to draw any conclusions.
- No studies evaluated other clinical outcomes, such as retinopathy and neuropathy.
- Many of the studies in this overall review did not report on any of the clinically relevant outcomes (some did not even state whether any deaths or events occurred), introducing the possibility of publication bias.
Evidence Grades (see Appendix E, Evidence Table 1)
- The quantity, quality, and consistency of the body of evidence was graded as low for all-cause mortality and cardiovascular mortality for the following comparisons:
- Premixed insulin analogues versus other insulin or noninsulin antidiabetic agents.
- The quantity, quality, and consistency of the evidence was graded as low for cardiovascular morbidity for the following comparisons:
- Premixed insulin analogues versus other insulin or noninsulin antidiabetic agents.
- The quantity, quality, and consistency of the body of evidence was graded as low for nephropathy for the following comparisons:
- Premixed insulin analogues versus other insulin or noninsulin antidiabetic agents.
- There was no evidence to assess the quantity, quality, and consistency for all other clinical outcomes.
Study Characteristics
Out of 45 included studies, only 16 studies reported on a clinical outcome.32, 45, 49–51, 54, 55, 57, 58, 60, 61, 65, 66, 68, 77, 79 Nine studies reported on all-cause mortality,32, 45, 49, 51, 55, 61, 66, 68, 77 four reported on cardiovascular mortality,32, 45, 51, 66 eight reported on cardiovascular morbidity,45, 49, 50, 54, 57, 58, 60, 65 and three reported on nephropathy.32, 60, 79 All the studies except one45were short-duration RCTs lasting 1 year or less, in which the clinical outcome (such as mortality) was not the primary outcome (see Appendix E, Evidence Table 2). Most studies (n = 11) were parallel-arm RCTs,32, 45, 49–51, 54, 57, 60, 61, 68, 79 and the remaining studies were crossover RCTs. Most parallel-arm RCTs were moderately-sized (range: 160 to 708 subjects),32, 49–51, 54, 60, 61, 68, 79 except for two smaller studies (125 and 49 subjects).45, 57 The crossover studies were mainly small-sized (range: 35 to 133 subjects).55, 58, 65, 66, 77 Half of the studies were multicenter trials occurring in multiple countries,45, 49–51, 54, 55, 68, 79 while the other half of the studies occurred in one or two countries in Europe,32, 58, 66 the United States,60, 65 the Middle East,57 or Japan,61 or the geographic location was not reported.77 Half of the studies (n = 8) reported excluding subjects with significant comorbidity, such as a history of cardiovascular disease or complications from type 2 diabetes,32, 51, 54, 55, 57, 58, 77, 79 and most studies (n = 12) excluded moderate to severely obese subjects with BMI greater than 35 or 40 kg/m2.32, 45, 49, 50, 54, 55, 57, 58, 60, 65, 68, 77
Subjects were mainly middle-aged to older, overweight to mildly obese adults with moderate glycemia (range in mean A1c from 7.8 to 10.7 absolute percentage points; see Appendix E, Evidence Table 3). The average or median duration of diabetes for these subjects was moderate ranging from 7 to 16 years. The studies had a diverse gender ratio, yet they were less diverse in terms of racial mix. Of the five studies reporting race, three reported greater than 80 percent Caucasian subjects,32, 57, 68 while two studies reported a more diverse racial mix.60, 79 Half of the studies45, 49, 55, 58, 61, 66, 77, 79 included insulin-treated patients, and the other half included insulin-naïve patients.32, 45, 55, 58, 60, 65, 66, 77
Reporting of Clinical Outcomes
Death from any cause and cardiovascular death was abstracted from all articles. Cardiovascular morbidity could be any cardiovascular event, including myocardial infarction or stroke. If the article specified no clinical outcomes such as death, we recorded it as having zero events in each arm. If an article did not report that no events such as deaths occurred, we did not include that article in this section, since we did not want to assume that no events had occurred.
Premixed Insulin Analogues (Insulin Aspart 70/30, Insulin Lispro 50/50, and Insulin Lispro 75/25) Versus Any Other Antidiabetic Agent
All-cause and cardiovascular disease mortality
Qualitative assessment. Nine RCTs of short duration reported few absolute deaths from any cause in each study arm, with zero to three deaths for the premixed insulin analogues, versus zero to two deaths for the other diabetes agents (less than 5 percent for each arm; see Table 8 and Appendix E, Evidence Table 9).32, 45, 49, 51, 55, 61, 66, 68, 77 Four RCTs reported one to two cardiovascular deaths in the premixed insulin analogue arms and no cardiovascular deaths in the other arms (see Table 9).32, 45, 51, 66 Even the 2-year parallel-arm RCT, which did not exclude subjects with a prior history of cardiovascular disease and whose primary objective was to evaluate safety, had very few events, making it difficult to determine differences between groups.45 We further describe these trials qualitatively below.
Table 8
Summary of all-cause mortality events in studies comparing a premixed insulin analogue to another antidiabetic agent.
Table 9
Summary of cardiovascular disease mortality events in studies comparing a premixed insulin analogue to another antidiabetic agent.
Premixed insulin analogues versus long-acting insulin analogues or rapid-acting insulin analogues. Two RCTs compared premixed insulin analogues to long-acting insulin analogues, reporting similar percentages of death in each arm (less than 1 versus 0 percent and 2 versus 2 percent).32, 66 One of these studies also compared a premixed insulin analogue to a rapid-acting insulin analogue and found similar percentages of death and cardiovascular death in both groups (1 versus less than 1 percent, respectively, for both outcomes).32 The largest 1-year parallel-arm RCT of patients already taking metformin plus a sulfonylurea (n = 708) compared three arms (insulin aspart 70/30 twice-daily, insulin aspart three times a day, and long-acting insulin detemir at bedtime or twice-daily if required).32 That study reported similar percentages of events among the groups (0.6 percent in the premixed insulin analogue arm versus 0 percent in the long-acting insulin arm), but the insulin aspart 70/30 group had a higher absolute number of deaths. The study reported one fatal myocardial infarction, one fatal case of ischemic heart disease, and one fatal case of congestive heart failure in the insulin aspart 70/30 twice-daily arm; one fatal myocardial infarction in the insulin aspart thrice-daily arm; and no deaths in the long-acting insulin detemir arm. The insulin detemir arm had no deaths, despite having a slightly higher A1c than the other two insulin analogue arms, although the insulin analogue arms were associated with slightly greater weight gain over 1 year. The smaller 16-week crossover study comparing insulin lispro 75/25 plus metformin to insulin glargine plus metformin also showed similar percentages of events in each arm (2 versus 2 percent, or one death from any cause in each study arm).66 The death in the premixed insulin analogue arm was from a myocardial infarction, whereas the etiology of the death in the insulin glargine arm was not described.
Premixed insulin analogues versus premixed human insulin. Two small RCTs (one parallel-arm and one crossover) compared a premixed insulin analogue to a premixed human insulin and reported similar percentages of death from any cause in each arm (4 versus 1 percent and 0 versus 3 percent).45, 77 The longest 2-year multicenter, multinational RCT that specifically set out to evaluate safety (hypoglycemia and adverse events) was one of these two studies.45 This study compared insulin aspart 70/30 to NPH/regular 70/30 in subjects previously on insulin regimens and reported three deaths (4 percent) in the premixed insulin analogue arm versus one death (1 percent) in the premixed human insulin arm. Of these deaths, one was due to a myocardial infarction in the premixed insulin analogue arm, as compared to no cardiovascular causes of death in the premixed human insulin arm. The smaller 12-week crossover study compared insulin lispro 50/50 to NPH/regular 70/30 and showed similarly low numbers of events in the two groups, none versus one death, respectively.77
Premixed insulin analogues versus a rapid-acting insulin analogue, with or without an intermediate-acting insulin. One parallel-arm 6-month RCT compared a premixed insulin analogue (insulin aspart 70/30) to a rapid-acting insulin analogue (insulin aspart), with or without an intermediate-acting insulin (NPH insulin), and reported one death of unspecified etiology in the premixed insulin analogue arm, as compared to no deaths in the other arm.61
Premixed insulin analogues versus noninsulin antidiabetic agents. Two parallel-arm moderate-size RCTs of short duration compared premixed insulin analogues to oral antidiabetic agents, reporting a small number of deaths in the premixed insulin analogue arms, versus no deaths in the oral antidiabetic agent arms (less than 1 percent versus 0 percent for each study).51, 68 The 16-week RCT compared three arms (insulin aspart 70/30 twice-daily alone, insulin aspart 70/30 twice-daily plus fixed metformin, and fixed metformin plus variably dosed glibenclamide).51 This study reported one fatal myocardial infarction in the insulin aspart 70/30 plus fixed metformin arm, as compared to no deaths in the other two arms. The other 16-week RCT compared insulin lispro 75/25 plus metformin to glibenclamide plus metformin in subjects previously taking metformin or a sulfonylurea.68 The study reported one death with no description of etiology in the premixed insulin analogue arm, as compared to no deaths in the oral antidiabetic agent arm.
A moderately-sized 1-year parallel-arm RCT of subjects already taking metformin plus a sulfonylurea (n = 501) compared the addition of insulin aspart 70/30 twice daily to the addition of exenatide twice daily. The total number of deaths in each group was not statistically different (one (0.4 percent) versus two (0.8 percent) deaths, respectively), but the etiology of these deaths was not described.49
Quantitative assessment. We found a statistically insignificant trend towards increased risk of all-cause mortality with premixed insulin analogues as compared to all other comparators combined in the six parallel-arm RCTs (OR = 2.93; 95 percent CI: 0.95 to 9.05; p = 0.06; see Figure 34). The inclusion of the two crossover studies in the meta-analysis did not markedly influence these results (OR = 2.06; 95 percent CI: 0.79 to 5.42; p = 0.14). When the study by Nauck et al. was removed from the meta-analysis,49 the results reached statistical significance (OR = 5.49; 95 percent CI: 1.25 to 24.08). Their study was the only study with the active comparator of exenatide, and they reported slightly more deaths in the exenatide arm than in the premixed insulin analogue arm.49 No other single study markedly influenced the all-cause mortality results. We also found a statistically insignificant trend towards increased cardiovascular mortality with the premixed insulin analogues as compared to all other active comparator combined (OR = 6.80; 95 percent CI: 0.87 to 53.12; p = 0.07; see Figure 35). No single parallel-arm RCT strongly influenced the cardiovascular disease mortality results, although the study by Holman et al. had the largest study population and contributed the most to the pooled estimate.32 The inclusion of the one crossover study in the meta-analysis did not markedly influence the point estimate, but it did cause the results to reach borderline statistical significance (OR = 7.19; 95 percent CI: 1.01 to 51.11; p = 0.049). While point estimates and confidence intervals varied somewhat depending on the meta-analytic technique used, the overall direction of the point estimates did not change (see Appendix E, Evidence Table 10). The pooled odds ratios suggested an increased risk of all-cause and cardiovascular disease mortality with the use of premixed insulin analogues versus other diabetes drug comparators, yet the wide 95 percent CI confirmed our conclusion regarding a lack of reliability in the point estimate because of the small number of absolute events in few studies. No statistically significant heterogeneity was found in these studies, with I-squared statistics of less than 50 percent for all analyses; therefore, no further analyses were done to evaluate sources of potential heterogeneity.
Cardiovascular disease morbidity
Qualitative assessment. Eight studies compared premixed insulin analogues to another diabetes medication, reporting cardiovascular morbidity between groups (see Table 10 and Appendix E, Evidence Table 9).45, 49, 50, 54, 57, 58, 60, 65 The articles compared premixed insulin analogues to premixed human insulin in the case of two studies,45, 58 oral antidiabetic agents in three studies,54, 57, 60 long-acting insulin analogues in two studies,50, 65 and exenatide in one study.49 The eight articles reported a diverse set of cardiovascular morbidity outcomes, including non-fatal myocardial infarction,54, 57, 65 transient ischemic attack,58 peripheral vascular disease,50 congestive heart failure,50, 65 angina,60 arrhythmia,60 coronary artery disease,60 chest pain,65 and unspecified total cardiac adverse events.45, 49
Table 10
Summary of cardiovascular disease morbidity events in studies comparing a premixed insulin analogue to another antidiabetic agent.
Only one of these studies specifically set out to evaluate safety over 2 years of followup.45 This and another 1-year study had larger numbers of cardiac adverse events, largely because of their vaguer definition of unspecified cardiac disorders, which could include less serious cardiac events such as palpitations.45, 49 Although the absolute numbers of events were greater in these two studies than in the other six studies, no major differences in cardiovascular morbidity were noted between groups (18 versus 17 percent and 2 versus 4 percent). Neither study broke these cardiac disorders down into more serious versus less serious events. The rest of the studies were mainly short-duration studies with few absolute events, which occurred mainly in the premixed insulin analogue arms (one event for the premixed insulin analogues versus none to two for the other antidiabetic agents; less than 2 percent for each arm). Two of these shorter-duration studies were crossover studies58, 65 that did not report whether the events occurred prior to the first crossover. We describe these studies qualitatively in more depth below.
Premixed insulin analogues versus long-acting insulin analogues. Two RCTs compared premixed insulin analogues to long-acting insulin analogues and reported no differences in cardiovascular morbidity between arms (less than 1 versus 0 percent and 0.8 versus 0.8 percent).50, 65 The 28-week parallel-arm RCT of 255 insulin-naïve patients compared insulin aspart 70/30 plus metformin to insulin glargine plus glimepiride.50 The oral antidiabetic agents could have been in place before the beginning of the study or could have been added after the study began. This study reported one congestive heart failure event in the insulin glargine arm and one peripheral vascular disorder event in the insulin aspart 70/30 arm. The 32-week crossover RCT (including an 8-week run-in period on NPH insulin plus metformin) compared insulin lispro 75/25 plus metformin to insulin glargine plus metformin and reported similarly low absolute events and no major differences between arms.65 The study reported one subject with congestive heart failure and one subject with chest pain in the insulin lispro 75/25 arm, a nonfatal myocardial infarction during the lead-in period when the subjects were using NPH insulin plus metformin, and no events in the insulin glargine arm.
Premixed insulin analogues versus premixed human insulin. Two studies compared premixed insulin analogues to premixed human insulin, reporting similar percentages of cardiovascular events between groups (18 versus 17 percent and less than 1 versus 0 percent).45, 58 The longest 2-year multicenter, multinational RCT that specifically evaluated safety (hypoglycemia and adverse events) was one of these two studies.45 This study compared insulin aspart 70/30 to NPH/regular 70/30 in subjects previously on insulin regimens, and reported 15 unspecified cardiac disorders (18 percent) in the premixed insulin analogue arm versus 17 unspecified cardiac disorders (17 percent) in the premixed human insulin arm. This study did not further elaborate on the severity of these cardiac disorders. Similarly, the one three-way crossover study compared insulin aspart 70/30, insulin lispro 75/25, and NPH/regular 70/30 and reported no major differences between groups. This study had a lower absolute number of events because of its higher specificity in defining the cardiac adverse event and because of its short 3-day study period. A transient ischemic attack was reported in the insulin aspart 70/30 arm, versus no events in the insulin lispro 75/25 or premixed human insulin arms.
Premixed insulin analogues versus noninsulin antidiabetic agents. Three RCTs compared premixed insulin analogues to oral antidiabetic agents, reporting few absolute cardiovascular morbidity events in the premixed insulin analogue groups (≤1 percent) compared with none or few events (0 to 2 percent) in the oral antidiabetic agent groups.54, 57, 60 Two were short duration (6-week and 18-week) parallel-arm RCTs comparing insulin aspart 70/30 plus a thiazolidinedione to a sulfonylurea plus a thiazolidinedione. One of these studies also had a third arm, insulin aspart 70/30 monotherapy.54 The thiazolidinedione was pioglitazone54 in one study and rosiglitazone in the other study.57 In both studies, one subject experienced a nonfatal myocardial infarction in the premixed insulin analogue arm (monotherapy insulin aspart 70/30 arm in one study and insulin aspart 70/30 plus rosiglitazone arm in the second study). The third short-duration 34-week parallel-arm RCT compared premixed insulin aspart 70/30 plus the combination of metformin and pioglitazone to the combination of metformin plus pioglitazone, reporting slightly lower withdrawals due to cardiac morbidity in the premixed insulin analogue plus oral antidiabetic agent arm (1 percent), as compared to oral antidiabetic agents alone (2 percent).60 These three studies were the only studies with thiazolidinediones in any of the arms. While the rosiglitazone and pioglitazone labels have warnings concerning increased congestive heart failure events in subjects who use insulin of any type in conjunction with these oral antidiabetic agents (versus insulin alone),84, 85 we did not observe any congestive heart failure events in these few studies, which reported few absolute events. In addition, rosiglitazone labels have warnings regarding increased ischemic risk in patients who use rosiglitazone with insulin, as compared to insulin alone. We had insufficient evidence to determine whether this risk might be increased in the presence of premixed insulin analogues, as compared to other insulins used alone.
A relatively large 1-year parallel-arm RCT of subjects already taking metformin plus a sulfonylurea (n = 501) compared the addition of insulin aspart 70/30 twice-daily to the addition of exenatide twice daily.49 The study reported slightly higher absolute nonspecific cardiac disorders in the exenatide arm, as compared to the insulin aspart 70/30 arm (10 (4 percent) versus 5 (2 percent) cardiac adverse events, respectively). They did not elaborate further on the proportion of serious and less serious events.
Quantitative assessment. We found no statistically significant differences in cardiovascular morbidity between the premixed insulin analogue arms and with any other active comparator in the six parallel-arm RCTs (OR = 0.86; 95 percent CI: 0.49 to 1.52; p = 0.60; see Figure 36). The inclusion of the two crossover studies in the meta-analysis did not markedly alter these results (OR = 0.92; 95 percent CI: 0.53 to 1.61). No one study strongly influenced these results. Despite their different definitions of cardiovascular morbidity, we decided to combine these trials in a meta-analysis because of the underlying shared pathophysiology of the cardiovascular disease outcomes. We conducted a separate meta-analysis using the three parallel-arm RCTs with serious cardiac events (i.e., nonfatal myocardial infarction or transient ischemic attack), and excluded the three RCTs that combined less serious and more serious cardiac disorders or reported more nonspecific cardiac morbidity such as arrhythmia.45, 49, 60 Although the conclusions of no significant difference did not change, the point estimate, which initially suggested a protective benefit of the premixed insulin analogues, now suggested potential harm (OR = 2.22; 95 percent CI: 0.26 to 19.12). No statistically significant heterogeneity was found in these studies, with an I-squared statistic less than 50 percent; therefore, no further analyses were performed to evaluate the sources of potential heterogeneity.
Combined mortality and cardiovascular disease morbidity
Quantitative and quantitative assessment. We decided to combine ten parallel-arm RCTs evaluating mortality and cardiovascular disease morbidity in one meta-analysis (since the current understanding of these outcomes proposes a shared pathophysiology), using the mortality results for the two studies that reported both outcomes.45, 49 We found no statistically significant differences between premixed insulin analogues and any other active diabetes medication comparator (OR = 2.10; 95 percent CI: 0.87 to 5.10; p = 0.10; see Figure 37). While no single study markedly changed the point estimate, removal of the Nauck et al.49 or Raskin et al.60studies individually brought the results to borderline statistical significance. There were no clear differences between these two studies and the other included studies. Given the small number of events in a small number of studies and the lack of statistically significant heterogeneity, we were unable to explore potential differences between studies.
Nephropathy
Premixed insulin analogues versus other diabetes medications. One study reported nephropathy as an outcome,32 and two studies reported it as an adverse event.60, 79 A 1-year parallel-arm RCT of patients already taking metformin plus a sulfonylurea (n = 708) had three arms (insulin aspart 70/30 twice daily, insulin aspart thrice daily, and long-acting insulin detemir at bedtime or twice daily if required) and reported nephropathy as an outcome.32 This study reported a statistically significant increase in plasma creatinine in the premixed and rapid-acting insulin analogue groups (0.05 mg/dL in both) after 1-year of followup, as compared to the long-acting insulin analogue group (0.02 mg/dL). The study also reported a decrease in the absolute albumin-to-creatinine ratio in the long-acting insulin group (-1.8), as compared to the other two groups (-0.9), although this difference was not statistically significant (p-value = 0.08 for the differences among the three groups). This potential difference in albumin-to-creatinine ratio may be responsible for the small yet statistically significant differences in plasma creatinine. The long-term clinical impact of these small absolute differences in plasma creatinine is uncertain.
Two short duration parallel-arm RCTs reported on withdrawals because of kidney problems but showed no consistent patterns. They reported a withdrawal due to increased creatinine in the premixed insulin analogue arm (insulin aspart 70/30 in combination with metformin plus pioglitazone), as compared with no events in the active comparator arm (metformin plus pioglitazone) in one study,60 and one withdrawal due to renal insufficiency in the active comparator arm (insulin glargine plus metformin), as compared with no events in the premixed insulin arm (insulin lispro 50/50 plus metformin) in the second study.79
Other clinical outcomes. We did not find any study that reported other clinical outcomes, such as neuropathy or retinopathy, while comparing premixed insulin analogues to other antidiabetic agents.
One Premixed Insulin Analogue Versus Another Premixed Insulin Analogue (Insulin Aspart 70/30 Versus Insulin Lispro 75/25)
All-cause and cardiovascular disease mortality. One small crossover RCT of 24-week duration compared insulin aspart 70/30 to insulin lispro 75/25 and reported one death from myocardial infarction in the insulin lispro 75/25 arm; this death occurred after the patient had withdrawn from the study following a diagnosis of malignant neoplasm; there were no deaths in the insulin aspart 70/30 arm.55
Other clinical outcomes. No study comparing one premixed insulin analogue to another reported any other clinical outcomes, such as cardiovascular morbidity or microvascular disease.
FDA, European Medicines Agency, and Pharmaceutical Industry Data
In the FDA medical reviews on insulin lispro 50/50 and insulin lispro 75/25 that evaluated three studies (study numbers IODK, IODM, IODN), there were no reports of death or other clinical outcomes.86 However, the FDA review mentioned 17 deaths that occurred in the related studies using insulin analogues in premixed and in non-premixed formulations. We were unable to synthesize these data, since the documentation stated the number of deaths but not the total number of subjects. Also, there was no information about whether there were comparator arms with any deaths.
The FDA medical reviews on insulin aspart 70/30 reported a few clinical events in a study with 35 percent type 1 diabetes subjects.87 They did not break down the events by type of diabetes; therefore, we did not include this study in our review.
We also evaluated documents from the EMEA for insulin aspart 70/30 and did not find any reports of the presence or absence of clinical outcomes in these documents.88
Finally, we evaluated product labels and data provided by several pharmaceutical companies (Eli Lilly and Company and Sanofi-Aventis). We identified three crossover studies that reported data on clinical outcomes. Although all three studies were included in our overall review because they were peer-reviewed publications,64–66 we found additional data in the information supplied by Eli Lilly and Company. For instance, the pharmaceutical information reported no deaths in either arm in two of the three studies,89 yet this result was not stated in the peer-reviewed publications.64, 65 Furthermore, the pharmaceutical information listed one person with chest pain, one with cardiac disorder (tachycardia and palpitations), and one with peripheral vascular disorder in the insulin glargine arm, versus no subjects with chest pain, two with cardiac disorders, and one with a peripheral vascular disorder in the premixed insulin analogue arm (insulin lispro 50/50 with breakfast/lunch and insulin lispro 75/25 with dinner).90 The peer-reviewed publication, however, did not report on these cardiovascular events.64 In the third study, the peer-reviewed publication66 reported one death from fatal myocardial infarction in the premixed insulin analogue arm and one death in the insulin glargine arm; however, the pharmaceutical information reported only the event in the premixed insulin analogue arm.91 No new information relevant to these clinical outcomes was reported in the data sent by Sanofi-Aventis or in any of the product labels.
The incorporation of these extra data into the meta-analyses that included crossover studies did not markedly change any of our conclusions (OR for all-cause mortality = 2.46; 95 percent CI: 0.89 to 6.80 and OR for cardiovascular morbidity = 0.93; 95 percent CI: 0.55 to 1.57).
Publication Bias
Overall, we did not find strong evidence for publication bias in this literature on clinical outcomes. Across all analyses, there was no statistically significant publication bias (p < 0.05), as assessed by the less-conservative Eggers test. It is important to note that in most cases the number of studies in each comparison was small and was unlikely to have had high power to detect moderate publication bias. Visual examination of the funnel plot for cardiovascular morbidity was suggestive that some small studies with protective effects of premixed insulin analogues may have been missing (see Figure 38).
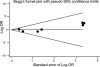
Figure
Figure 38. Begg's funnel plot for cardiovascular disease morbidity.
Study Quality Assessment
Most of the 16 studies (n = 14) were rated as being of good or fair quality.32, 45, 49, 51, 55, 57, 58, 60, 61, 66, 68, 77, 79, 83 Thirteen of these were randomized with few losses to followup, and they described dropouts and withdrawals.32, 45, 49, 51, 55, 57, 58, 60, 66, 68, 77, 79, 83 One randomized study was rated as poor quality since it excluded one-third of the subjects from the efficacy analysis due to receipt of expired drug.65 Two studies had greater than 10 percent patients who were lost during followup,54, 61 but one of these did report on reasons for withdrawals and losses to followup.54 All studies were unblinded except for one,32 and most studies (n = 12) reported receiving pharmaceutical industry funding. The source of funding has the potential to induce reporting bias, especially for outcomes such as cardiovascular morbidity, which involves more subjectivity in outcome ascertainment than does mortality.
Other Quality Issues
Only one RCT evaluated safety outcomes for longer than 1 year.45 Followup was sufficient in the remaining RCTs for intermediate outcomes but not for assessment of clinical outcomes, which were relatively rare, especially mortality. Even the one RCT with 2-year data had extremely few mortality events.45 While four studies did not report on whether the analysis for adverse events was intention-to-treat,32, 45, 55, 68 this information would not have affected our results, since we abstracted data assuming intention-to-treat. Several studies reported on serious adverse events but did not specifically mention clinical outcomes or did not define what was meant by serious events. Two studies reported one death but did not state in which arm the event occurred,43, 69 Although the elimination of the two studies did not affect our results, elimination of many studies as a result of a lack of reporting by authors could bias these results in either direction.
Applicability Assessment
Although the evidence was insufficient to allow us to conclusively identify an effect of premixed insulin analogues on clinical outcomes, the applicability of these scanty data was fair, with some limitations as noted below. We found that the studies were mainly representative of the general population of type 2 diabetes, with the exception of race and severity/spectrum of illness. Race was not reported in most of the studies and was deemed not representative or only partially representative in the majority of studies that did report race.32, 51, 57, 68, 79 Studies had diverse inclusion and exclusion criteria that excluded selected populations in the case of most studies. Four studies excluded subjects with major complications from type 2 diabetes.54, 58, 77, 79 Others excluded insulin-naïve subjects,51, 80 subjects with suboptimal glucose control,49 and/or insulin-treated subjects.32, 50, 58, 65 Outpatient clinics were the population source in the few studies supplying this information.32, 49, 57, 61 Comparator medications were considered a good alternative in most studies,32, 45, 50, 51, 54, 55, 58, 61, 65, 66, 68, 77, 79 and the dose schedules and routes of administration of these medications were reflective of current clinical practice in all the studies. Standards of care for subjects with diabetes were similar to the U.S. standards in most studies.32, 45, 51, 54, 55, 57, 58, 60, 65, 66, 68, 77, 79 The percentage of subjects enrolled versus those screened was greater than 50 percent for most studies, although five studies did not report this information.50, 57, 61, 66, 77 All except two studies60, 66 had fewer than 10 percent of subjects excluded during run-in, or no run-in period existed.
Adherence
Key Messages
- No evidence was found in any studies regarding the outcome of patient adherence. We therefore evaluated quality of life outcomes, since these outcomes may affect patient adherence.
Quality of Life
Key Messages
- In the four studies using validated measurement tools, only one of six quality of life outcomes (psychological distress) showed a statistically significant difference, favoring the premixed insulin analogues over other antidiabetic agents.
- No firm conclusions could be drawn regarding quality of life outcomes for any comparisons because of the differences in outcome definitions, measurement techniques, populations, and comparators between studies.
Evidence Grades (see Appendix E, Evidence Table 1)
- The quantity, quality, and consistency of the body of evidence was graded as low for the following comparison:
- Premixed insulin analogues versus other antidiabetic agents.
- There was no evidence to assess the quantity, quality, and consistency of the body of evidence or the following comparisons:
- Premixed insulin analogues versus any other comparison evaluated in this report but not listed above.
Study Characteristics
Of the 45 studies, only six reported on quality of life.32, 48, 59, 72, 74, 76 Four studies were moderate-size parallel-arm RCTs (143 to 708 subjects),32, 59, 72, 76 while the other two were small- to moderate-sized crossover studies with no washout period (49 to 160 subjects).48, 74 Most studies had a short duration of followup, lasting 4 to 6 months,48, 59, 72, 74, 76 with one study lasting 1 year.32 Four studies were conducted in one or two mainly European countries32, 48, 76 or Russia,59 while the rest of the studies were conducted in the U.S.32 or multiple countries.72 Exclusion criteria varied depending on the study. Most studies (n = 5) excluded moderate to severely obese subjects with a BMI over 35 or 40 kg/m2,32, 48, 59, 72, 76 and/or subjects with severe hyperglycemia (i.e., A1c greater than or equal to 9.5, 10, or 10.5 percent).32, 48, 74, 76 Most studies reported either excluding subjects who used insulin during the past 3 to 6 months,48, 72, 76 or excluding prior insulin users.32, 59 Half of the studies excluded subjects with significant comorbidity, such as a history of cardiovascular disease or complications from type 2 diabetes.32, 59, 72
Subjects were mainly middle-aged or older, overweight to mildly obese adults with mild to moderate hyperglycemia (mean A1c range from 7.5 percent to 10.4 percent). The average or median duration of diabetes for these subjects ranged from 5 to 12 years. The studies had a diverse gender ratio but were not as diverse in terms of racial mix. One study that reported race had mainly Caucasian subjects.32
Reporting of Quality of Life
We used the definitions for the quality of life outcome that were reported by the authors; these definitions included a variety of outcomes, such as treatment satisfaction or depression.
Premixed Insulin Analogues Versus Long-Acting Insulin Analogues or Rapid-Acting Insulin Analogues
Three studies32, 74, 76 evaluated four different quality of life outcomes (quality of life, treatment satisfaction, willingness to continue treatment, and depression) and compared premixed insulin analogues to long-acting insulin analogues or rapid-acting insulin analogues (see Table 11 and Appendix E, Evidence Table 4).
Table 11
Summary of quality of life measures in studies comparing a premixed insulin analogue to another antidiabetic agent.
The largest and longest 1-year parallel-arm RCT of patients already taking metformin plus a sulfonylurea (n = 708) compared three treatments (insulin aspart 70/30 twice daily, insulin aspart thrice daily, and long-acting insulin detemir at bedtime or twice-daily if required). This study did not find a statistically significant difference between groups in terms of self-reported quality of life using the Euro-QoL validated questionnaire (winsorized means 0.76, 0.76, and 0.78, respectively).32
The second study was a crossover RCT comparing insulin lispro 75/25 to insulin glargine for 12 weeks on each treatment. It reported no statistically significant differences in depression using the Beck Depression Inventory II, which is a validated questionnaire (mean scores of 5.5 and 6.8, respectively).74
The third, and relatively lower-quality, parallel-arm RCT compared thrice-daily insulin lispro 50/50 to thrice-daily insulin lispro or once-daily insulin glargine. Study participants who were already on oral antidiabetic agents were advised to stop their medications.76 The authors reported an increased proportion of subjects with treatment satisfaction in all three arms at the end of the study, as compared with baseline, and a high willingness to continue treatment in all three arms when measured using nonvalidated questionnaires. While the long-acting insulin arm had a smaller increase in treatment satisfaction (24.5 percent) than the other two arms (44.2 percent and 44.5 percent) and had a lower proportion of subjects willing to continue treatment (77.4 percent versus 88.5 percent and 83.3 percent), the authors did not analyze whether these small differences were statistically significant. The insulin glargine comparator arm was not given with oral antidiabetic agents as is typical in clinical practice; therefore, those in the insulin glargine arm had a higher fasting glucose and lower decreases in A1c than did those in the other two arms, a result that may have caused patients to be less satisfied with their overall treatment.
Premixed Insulin Analogues Versus Premixed Human Insulin
One double-blinded crossover RCT compared treatment satisfaction after 16 weeks in subjects taking insulin aspart 70/30 to the subjects taking NPH/regular insulin 70/30 and reported no statistically significant difference between groups (mean difference = -0.46) using the validated Diabetes Treatment Satisfaction Questionnaire (DTSQ; see Table 11 and Appendix E, Evidence Table 4).48
Premixed Insulin Analogues Versus Oral Antidiabetic Agents
Two short duration parallel-arm RCTs evaluated five different quality of life outcomes (treatment satisfaction, barriers to activity, willingness to continue treatment, disinhibitory eating, or psychological distress), comparing premixed insulin analogues to oral antidiabetic agents (see Table 11 and Appendix E, Evidence Table 4).59, 72 Only one of these studies used validated questionnaires (the DTSQ for treatment satisfaction and the Diabetes Health Profile for disinhibitory eating, barriers to activity, and psychological distress).59 This 16 week parallel-arm RCT compared these quality of life measures among three arms: thrice-daily insulin aspart 70/30, thrice-daily insulin aspart 70/30 plus metformin, and continuation of oral antidiabetic agents. They reported no statistically significant differences between groups in any of the validated measures, except for a lower psychological distress score in the insulin aspart 70/30 arm than in the continued oral antidiabetic agent arm. They did not report any quantitative results beyond a p-value of 0.026; therefore, we were unable to determine the clinical relevance of these data.
Another 16-week parallel-arm RCT compared overall treatment satisfaction among subjects 60 to 80 years of age who received insulin lispro 75/25 preprandial injections, insulin lispro 75/25 postprandial injections, or glyburide. The authors reported a slightly lower overall treatment satisfaction score with glyburide than with insulin lispro 75/25 (mean score 3.98 versus 4.35, respectively, p = 0.014) using a nonvalidated questionnaire.72 The clinical importance of this minor difference in scores was unclear. They also reported a higher willingness to continue treatment in the insulin lispro arms than in the glyburide arm (92 percent versus 79 percent, respectively, p = 0.041). The glyburide active comparator arm was not the best comparator for the premixed insulin analogues, since all subjects were hyperglycemic on maximum dose sulfonylureas prior to study randomization. Therefore, the subjects may have been less satisfied with their treatment because they were not receiving optimal treatment for their hyperglycemia. As one might expect, the insulin lispro 75/25 arm showed a greater reduction in HbA1c than did glyburide in this trial.
FDA, European Medicines Agency, and Pharmaceutical Industry Data
No additional data on quality of life outcomes were identified from these sources.
Study Quality Assessment
Most of the studies (n = 5) were rated as being of good or fair quality,32, 48, 59, 74, 76 were randomized with few losses to followup,32, 48, 59, 72, 76 and described dropouts and withdrawals (see Appendix E, Evidence Table 7).32, 48, 59, 72, 76 One randomized study was rated as poor quality since the comparator, glyburide, was maintained at pretrial doses while the premixed insulin analogue dose was adjusted to obtain optimal glucose control.72 This difference could potentially have affected glycemic control outcomes and thereby affected patients' satisfaction with their treatment. Another study had a greater than 10 percent loss to followup, and the reasons for withdrawals and participant loss during followup were not reported.74 The majority of these studies were unblended, with the exception of two,32, 48 and most studies (n = 5) received funding from the pharmaceutical industry.
Applicability Assessment
We concluded that the body of evidence regarding quality of life outcomes had fair applicability to the U.S. population with type 2 diabetes (see Appendix E, Evidence Table 8). These studies were mainly representative of the general population with type 2 diabetes, with the exception of race and severity/spectrum of illness. Drug comparators were only considered the best alternatives in four32, 48, 59, 74 of the six studies. Race was not reported in half of the studies48, 59, 72 and was deemed non-representative in the ones that did report race.32, 74, 76 The studies had diverse eligibility criteria that excluded selected populations from most of the studies. They excluded subjects with a new diagnosis of type 2 diabetes72 or subjects taking insulin already.32, 59, 76 One study excluded subjects under 60 years of age or greater than 80 years of age.72 Outpatient clinics were the population source in the two studies reporting these data,32, 72 and one of these trials occurred in subspecialty clinics.72 The dose schedules and routes of administration of the comparator medications were reflective of current clinical practice in all the studies. Standards of care for subjects with diabetes were similar to those in the U.S. in all of the studies except one.59 The percent of subjects enrolled versus screened were greater than 50 percent for all the studies, and all had fewer than 10 percent of subjects excluded during the run-in, or no run-in period existed.32, 48, 59, 72, 74, 76
Key Question 3
Does the effectiveness or safety of the new premixed insulin analogue regimens differ in the following sub-populations from that of diabetes patients as a whole?
- a.
The elderly (≥ 65 years), very elderly (≥ 85 years)
- b.
Other demographic groups (ethnic or racial groups, genders)
- c.
Individuals with comorbid medical conditions
- d.
Individuals with limited life expectancy
- e.
Individuals with disabilities
We could not find any studies that had specifically explored the effect of premixed insulin analogues on specific subpopulations such as the very elderly, minorities, or patients with comorbid conditions. Only one study enrolled somewhat older patients (between 60 and 80 years of age) and compared insulin lispro 75/25 to glyburide.72 This study found a significant decrease in fasting glucose as well as pre-dinner glucose levels with insulin lispro 75/25 as compared to glyburide (mean difference = -43.9 and -32.6 mg/dL, respectively; p < 0.01 for both). In addition, there was a significant decrease in postprandial glucose after breakfast and dinner with insulin lispro 75/25 in this relatively older population (mean difference = -58.3 and -43.9 mg/dL, respectively; p < 0.01 for both). This study also found a significant decrease in A1c with insulin lispro 75/25, as compared to glyburide (mean difference = -0.78 percent; p < 0.01).
Key Question 4
Does the effectiveness or safety of the new premixed insulin analogue regimens differ in individuals on oral antidiabetic agents and with different blood glucose patterns (such as fasting hyperglycemia or postprandial hyperglycemia) or types of control (such as tight control, usual control, good fasting or postprandial control) from that of diabetes patients as a whole?
Effect of Premixed Insulin Analogues in Patients Taking Oral Antidiabetic Agents
We identified three published studies51, 54, 59 and two unpublished studies92, 93 that compared premixed insulin analogue monotherapy to a combination of oral antidiabetic agents and premixed insulin analogues. In the study by Kvapil et al., insulin aspart 70/30 was compared to a combination of insulin aspart 70/30 and metformin.51 The insulin aspart 70/30 dose was adjusted in both arms throughout the trial to optimize glucose control, while the metformin dose was fixed throughout the trial. At the end of the trial, the daily insulin dose was higher in the insulin aspart 70/30 monotherapy group than in the combination group (0.51 units/kg/day versus 0.30units/kg/day; p-value not reported).
Raz et al.54 compared insulin aspart 70/30 monotherapy to a combination of insulin aspart 70/30 and pioglitazone over 18 weeks. The insulin dose was adjusted to optimize glucose control, while the pioglitazone dose was kept fixed at 30 mg daily. At the end of the trial, the insulin dose in the monotherapy arm was significantly larger than that in the combination arm (0.4 units/kg versus 0.3 units/kg; p = 0.002).
Ushakova et al.59 compared thrice-daily insulin aspart 70/30 to twice-daily insulin aspart 70/30 plus metformin over a 16-week period. Insulin aspart and metformin doses were adjusted to optimize glycemic control. At the end of the trial, the total daily insulin dose was higher in the insulin aspart 70/30 monotherapy arm (55.5 versus 44.8 units).
In one unpublished study, insulin aspart 70/30 twice daily was compared to insulin aspart 70/30 once daily plus metformin over a 24-week period.92 In the second unpublished study, insulin aspart 70/30 twice daily was compared with aspart 70/30 twice daily plus metformin over a 24-week period.93
Fasting glucose (see Appendix E, Evidences Table 4 and 11). All studies found that the combination of insulin aspart 70/30 with oral antidiabetic agents was more effective than monotherapy with insulin aspart 70/30 in lowering fasting glucose, although the differences did not reach statistical significance in any study. Similarly, pre-dinner glucose levels were lower in two studies with the combination therapy, but the reduction did not reach statistical significance in one study,51 and statistical significance was not reported in the second study.54 In contrast, monotherapy with insulin aspart 70/30 three times daily was more effective in lowering pre-dinner glucose levels than was combination therapy in the third study, although this difference also did not reach statistical significance.59 In one unpublished study, combination therapy with insulin aspart 70/30 once daily plus metformin was significantly more effective than twice-daily monotherapy in lowering fasting glucose (-18 mg/dL, p = 0.01).92 In contrast, a second unpublished study found twice-daily monotherapy to be more effective than combination therapy with twice-daily insulin aspart 70/30 plus metformin in lowering fasting glucose levels (-18 mg/dL, p = nonsignificant).93
Postprandial glucose (see Appendix E, Evidences Table 4 and 11). All three studies reported breakfast and dinner postprandial glucose levels and found postprandial glucose levels to be lower with the combination therapy than with the monotherapy. However, this difference reached statistical difference in only one study.59 When the results of these three studies were pooled, there was no beneficial effect of either of the two therapies on breakfast (mean difference = 5.8 mg/dL, 95 percent CI: -4.1 to 15.7 mg/dL; p = 0.25) or dinner postprandial glucose levels (mean difference = 1.2 mg/dL; 95 percent CI: -1.7 to 1.0 mg/dL; p = 0.42).
Hemoglobin A1c (see Appendix E, Evidence Tables 4 and 11). In all three studies combination therapy with insulin aspart 70/30 plus an oral antidiabetic agent was more effective than monotherapy with insulin aspart 70/30 in lowering A1c levels. When the results of the three trials were pooled, the A1c-lowering effect of combination therapy remained significant (mean difference = 0.37 percent; 95 percent CI: 0.12 to 0.62 percent; p = 0.004; see Figure 39). In both unpublished studies, combination therapy was more effective than insulin aspart 70/30 monotherapy in lowering A1c levels, although the difference was statistically nonsignificant in one study,92 and the other study did not report statistical significance.93
Hypoglycemia (see Appendix E, Evidence Tables 5 and 11). There were no major hypoglycemic events in the three studies.51, 54, 59 The incidence of minor hypoglycemic events was greater in the combination arm in the two studies than in the insulin aspart 70/30 monotherapy arm,51, 59 but it was lower in the third study.54 On the other hand, all three studies found a statistically nonsignificant increase in the incidence of symptom-only hypoglycemia with insulin aspart 70/30 monotherapy. Pooling the results of these studies indicated that the incidence of minor hypoglycemic events (29 versus 33; OR = 0.84; 95 percent CI: 0.45 to 1.56; p = 0.6) and symptom-only hypoglycemia (89 versus 82; OR = 1.1; 95 percent CI: 0.77 to 1.6; p = 0.6) was similar for both therapies. One unpublished study reported a single major hypoglycemic event in the combination therapy arm,92 while there were no major events in the second unpublished study.93 In an unpublished study,92 there were more minor or symptom-only hypoglycemic events in the monotherapy arm (43 percent vs. 40 percent; p = not reported), while the second study93 had more events in the combination therapy arm (40 percent vs. 29 percent, p = not reported).
Weight change (see Appendix E, Evidence Tables 6 and 11). Patients on combination therapy with insulin aspart 70/30 plus metformin gained less weight than did patients who were on monotherapy,51, 59 although the difference was not statistically significant. On the other hand, patients on the combination therapy with insulin aspart 70/30 plus pioglitazone gained more weight than did patients on monotherapy (4.0 vs. 2.2 kg); the difference between the two groups in this trial was also not significant.54 In both unpublished studies, patients on insulin aspart 70/30 plus metformin gained less weight than did those on monotherapy.92, 93
Quality of life (See Appendix E, Evidence Tables 6 and 11). One study59 reported this outcome and found no difference between insulin aspart 70/30 monotherapy and insulin aspart 70/30 plus oral antidiabetic agents.
Adherence. No study evaluated this outcome.
Clinical outcomes (See Appendix E, Evidence Tables 6 and 11). One study reported a death due to myocardial infarction in the combination therapy arm.51 Another study reported one incidence of myocardial infarction in a patient assigned to the monotherapy group.54
Effect of Premixed Insulin Analogues in Patients With Different Intensity of Glucose Control
We did not find any trials that specifically studied the use of premixed insulin analogues in patients with different intensities of glucose control.
Effect of Premixed Insulin Analogues in Patients With Postprandial Versus Fasting Glucose Control
We did not find any study that studied premixed insulin analogues and compared targeting control of fasting glucose with targeting control of postprandial glucose.
- Results - Comparative Effectiveness, Safety, and Indications of Insulin Analogue...Results - Comparative Effectiveness, Safety, and Indications of Insulin Analogues in Premixed Formulations for Adults With Type 2 Diabetes
- Preface - Comparative Effectiveness of Therapies for Clinically Localized Prosta...Preface - Comparative Effectiveness of Therapies for Clinically Localized Prostate Cancer
- Introduction - Effectiveness of Early Diagnosis, Prevention, and Treatment of Cl...Introduction - Effectiveness of Early Diagnosis, Prevention, and Treatment of Clostridium difficile Infection
- Evidence Summary - The Role of Immunotherapy in the Treatment of AsthmaEvidence Summary - The Role of Immunotherapy in the Treatment of Asthma
- small conductance calcium-activated potassium channel protein 2 isoform X2 [Corv...small conductance calcium-activated potassium channel protein 2 isoform X2 [Corvus hawaiiensis]gi|2240567238|ref|XP_048147381.1|Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...