NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lin SY, Azar A, Suarez-Cuervo C, et al. The Role of Immunotherapy in the Treatment of Asthma [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Mar. (AHRQ Comparative Effectiveness Reviews, No. 196.)
Results of the Literature Search
The search identified 2,771 citations, and we included 142 articles from the previous review. We excluded 2,163 articles during abstract screening. During article screening, we excluded an additional 512 articles (see Appendix C, List of excluded articles) that did not meet one or more of the inclusion criteria. We included 61 RCTs (reported in 68 articles) and 29 non-RCTs. (See Figure 2 for a diagram of our results.)
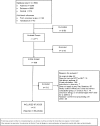
Figure 2
Search flow diagram.
Appendix C lists the studies we excluded at the full-text review stage. We excluded all studies we identified from ClinicalTrials.gov (n=105), of which 12 were ongoing, because none of them were specific to asthma.
Overall Study Characteristics
We identified 31 RCTs (35 articles) that addressed the efficacy of SCIT (KQ1), 26 RCTs (31 articles) and 18 non-RCTs that addressed the safety of SCIT (KQ2), 18 RCTs (20 articles) that addressed the efficacy of SLIT (KQ3), and 20 RCTs (23 articles) and 10 non-RCTs that addressed the safety of SLIT (KQ4). We included 43 studies of adults (12 years of age and older) only, 34 studies with mixed-age population (studies that included adults and children and studies that did not provide separate results for each population), and 12 studies that included only children (younger than 12 years of age). We provide details of studies identified per age group on Table 2.
Table 2
Number of studies included per Key Question, study design, age group, and setting.
Thirty-six studies compared immunotherapy versus placebo, 12 studies compared immunotherapy versus pharmacotherapy, 11 studies compared immunotherapy versus immunotherapy (one compared 3 vs. 5 years of treatment18 and one compared children vs. adults19), one study compared SCIT versus a desensitization vaccine (the control group received standardized glucocorticoid management and a desensitization vaccine, details not provided), 24 studies did not have a comparator and 6 studies compared SCIT versus SLIT.
All RCTs required patients to have positive allergy skin testing (via SPT) and/or in vitro specific IgE testing; however, criteria varied widely within studies (wheal diameter within 3 and 7 mm and IgE values varied in values and units) and some studies did not describe criteria for what was considered a positive test. Allergy diagnosis criteria was not reported in eight of the non-RCTs included for safety on SCIT.20-26
No consistent criteria were applied among the studies we included to establish asthma diagnosis (the criteria were not described in 37 studies; the Global Initiative for Asthma (GINA) criteria were used in 30 studies; and the remaining studies used clinical criteria, pulmonary function testing, or other definitions). We found no consistency in how asthma severity or level of asthma control was defined among studies. Asthma severity at baseline was not specified in 37 studies; 24 studies included patients with mild to moderate asthma (defined as mild and moderate or mild to moderate); and the remainder of studies included patients with mild asthma, moderate, or moderate to severe asthma. One study included all severities,27 and one study specifically excluded patients with severe asthma.28 Asthma control status was not specified in 56 studies, control status in the remainder of studies varied from grade of control (poorly controlled or controlled) to type of control (need and type of medications).
Patients were monosensitized in 44 studies (23 on SCIT, 17 on SLIT, and 4 on SLIT vs. SCIT) and polysensitized in 14 studies (8 on SCIT, 5 on SLIT and 1 on SLIT vs. SCIT). Eleven studies (5 on SCIT and 6 on SLIT) included both monosensitized and polysensitized patients, eight studies (7 on SCIT and 1 on SLIT) did not report the results of the allergy diagnosis and/or allergen identified, and 13 studies (9 on SCIT, 3 on SLIT, and 1 on SLIT vs. SCIT) did not clearly report sensitization status (patients were specifically sensitive to one allergen but authors did not specify sensitization status to other allergens). (See definitions in Appendix B.)
Patients received single-allergen immunotherapy in 69 studies (55 RCTs and 14 non-RCTS) and multiple-allergen immunotherapy in 14 studies (3 RCTs and 11 non-RCTs).
House dust mite (HDM) was the most common allergen used, with 49 HDM studies (D Pter, D far, D Pter-D far combined, or unspecified HDM). All the other allergens were used much less frequently; 14 studies used multiple allergens, 11 used grass, five used trees (4 birch and 1 cypress), two used mold (Alternaria and Cladosporium), three used animal allergens (2 cat and 1 dog) and one used ragweed.
Details of study and patient characteristics are provided in Tables 1 and 2 and Appendices E, F, G, and H.
Key Question 1. What is the evidence for the efficacy of subcutaneous immunotherapy (SCIT) in the treatment of asthma?
Key Points
- SCIT reduces the need for long-term control medication (moderate SOE).
- SCIT may improve asthma-specific quality of life, decrease use of quick-relief medications, decrease use of systemic corticosteroids, and improve FEV1 (low SOE).
- There was insufficient evidence regarding the effect of SCIT on asthma symptom control and health care utilization.
- There was insufficient evidence about any differential effect of SCIT in pediatric patients.
Overall Study Characteristics
We identified 31 RCTs (35 articles) that addressed the efficacy of SCIT. Thirteen RCTs (15 articles) included only adults, 15 RCTs (17 articles) included a mixed-age population, and 3 studies included only children. Eighteen studies compared SCIT versus placebo, nine studies compared SCIT versus pharmacotherapy, three studies compared SCIT versus SCIT (one compared 3 versus 5 years of treatment), and one study compared SCIT versus a desensitization vaccine (standardized glucocorticoid management and a desensitization vaccine, details not provided).
Patients were monosensitized in 17 studies and polysensitized in five studies.28-32 Two studies included both polysensitized and monosensitized patients,18, 33 and seven studies did not clearly report sensitization status.27, 34-39 Patients received single-allergen immunotherapy in 28 studies and multiple-allergen immunotherapy in two studies.29, 32 One study used both single- and multiple-allergen immunotherapy.28
HDM was the most common allergen used (20 studies). All the other allergens were used much less frequently: three studies used multiple allergens, two used cat, two grass, two mold (Alternaria and Cladosporium), one ragweed, and one dog.
We provided details about the studies, patient characteristics, and interventions in Appendix D and components in the assessment of risk of bias in Appendix I.
Asthma Symptoms
No studies reported on asthma symptom control using Asthma Control Test (ACT), Asthma Control Questionnaire (ACQ), or Pediatric-Asthma Control Test (P-ACT) scores.
Quality of Life
Four studies, three with HDM allergen and one with Alternaria allergen, with a total of 194 patients, examined the impact of SCIT on disease-specific quality of life using the Asthma Quality of Life Questionnaire (AQLQ).40-43 Two studies included only adults, and two studies included mixed ages. We assessed three studies as having moderate risk of bias and one study as high risk of bias (based on lack of allocation concealment and blinding).
Two studies showed statistically significant differences in quality of life compared to control 42, 43 while two showed differences that were not significant.40, 41 The two studies with significant improvement in quality of life included only adults with mild and moderate persistent asthma, treated with HDM allergen for 54 and 55 weeks.42, 43 The differences in overall AQLQ from these two studies were approximately 4 points (P=0.043) and 6 points (P=0.0025), respectively. The studies that did not show statistically significant improvements in AQLQ were in mixed-age populations with mild or moderate persistent asthma, treated with either Alternaria allergen for 12 months or HDM allergen for 8 months.40, 41
Overall, SCIT may improve quality of life as measured by the AQLQ (low SOE, with consistent but imprecise results and medium risk of bias). See Table 3. Summary of the strength of evidence for the efficacy of subcutaneous immunotherapy for details.
Table 3
Summary of the strength of evidence for the efficacy of subcutaneous immunotherapy.
No studies reported asthma-specific quality of life using the Pediatric Asthma-Specific Quality of Life Questionnaire (PAQLQ) or school or work absences.
Medication Use
We identified seven studies that reported on medication use.32, 39, 41, 42, 44-47
Quick-relief medications. One study of adults receiving HDM SCIT reported a decrease in the use of quick-relief medication [short-acting beta agonists (SABAs)]. The study reported a statistically significant reduction in medication use among those receiving SCIT (decrease from 27 to 14 puffs/week, P<0.05), and a non-significant reduction in the control group (decrease from 52 to 46 puffs/week, P NS).44 There was a substantial change, but the duration of treatment was not clear from the study report. Overall, SOE was low for the effect of SCIT on quick-relief medication use, based on one small study (n=31) with low risk of bias. See Table 3. Summary of the strength of evidence for the efficacy of subcutaneous immunotherapy for details.
Long-term control medications. We identified six studies that reported changes in the use of long-term control medications, including two in adult populations,42, 44 three in mixed-age populations,39, 41, 45 and one in children.32 All of these studies reported use of inhaled corticosteroids (ICS), though the metrics varied (e.g., dose in micrograms, rates of discontinuation, or number of weeks free of use). The approach to adjustment of ICS varied across studies and did not appear to follow strict protocols for dosage adjustment. One of these studies also compared a variety of regimens including leukotriene receptor antagonists (LTRA) and long-acting beta agonists (LABA), in addition to the use of ICS.41 Overall risk of bias was low in two studies, moderate in two, and high in one, the latter with issues of allocation concealment and blinding. The six studies included 404 patients. Five of the studies used HDM allergen, and the sixth (the pediatric study) used multiple allergens.32 Treatment ranged from 8 months to 54 weeks.
One study of adults with mild to moderate persistent asthma showed a statistically significant increase in weeks free from inhaled corticosteroids use in the SCIT group when compared to placebo (P<0.001).42 Similarly, in another study that compared SCIT alone and SCIT with co-administration of Vitamin D, the SCIT groups (analyzed together) had a higher rate of ICS discontinuation compared to the control group (28 versus 0 %, P=0.002).39 One study reported a significant reduction in ICS dose in the SCIT group during the study (38%, P <0.05) and a non-significant change in the control group,44 while another showed a significantly greater reduction in ICS dose in SCIT versus control after 3 years of treatment (P=0.027).45 In the latter study, the control group received treatment with a desensitization vaccine (standardized glucocorticoid management and a desensitization vaccine, details not provided). Finally, in the study that assessed use of multiple long-term control regimens (including ICS, LTRA, and LABA) there was a significant reduction in need for any long-term control medication in the SCIT group (decrease from 17 to 8 of 21) [P<0.046), but not in the control group (increase from 11 to 13 of 20] (P=0.158).41 The study that used multiple allergens in children found a statistically significant decrease in the number of days of ICS use in the SCIT arm but not in the placebo arm. However, there was no significant difference in the use of ICS between arms.32
Overall there was moderate strength of evidence that SCIT reduces use of long-term control medications, based on consistent and precise evidence, with medium risk of bias. See Table 3. Summary of the strength of evidence for the efficacy of subcutaneous immunotherapy for details.
Systemic corticosteroids. Two studies of SCIT, including 150 patients, reported change in systemic corticosteroid use.32, 46 The studies included a mixed-age population treated with HDM allergen for 3 years and a pediatrics study of treatment with multiple allergens for 27 months. Asthma severity was not reported in either study. In the mixed-age study, there was a significantly greater reduction in annual days of systemic corticosteroid use in the SCIT group (decrease from 22 to 1 day per year) compared to the controls (decrease from 25 to 12 days per year), (SCIT versus control, P<0.01).46 In the pediatric study, there was no significant difference in systemic corticosteroid use in SCIT versus control (-1.9 vs. -1.7 days in past 60 days, P=0.49)32 Overall there was low SOE that SCIT reduces use of systemic corticosteroids given the inconsistent results in the two studies. See Table 3. Summary of the strength of evidence for the efficacy of subcutaneous immunotherapy for details.
Asthma Exacerbations
Two studies of SCIT reported asthma exacerbations.31, 46 The studies, enrolling 95 patients, treated mixed-age populations with HDM allergen for either 2 or 3 years. One study included patients with well-controlled asthma31 and, in the other study, asthma severity and control status were not reported.46 In the study that treated for 3 years there was a statistically significantly greater reduction in risk of asthma exacerbations in the SCIT group (decrease from 8+/-1.8 to 1+/-0.5 per year) compared with controls (decrease from 8.5 +/- 1.7 to 4.25 +/- 0.25 per year) (SCIT vs. control, P <0.01).46 In the other study, exacerbation rates were low for each group (two in the SCIT group and one in the control), but there were no reported comparisons between groups.31
Health Care Utilization
Two RCTs in children reported on health care utilization.32, 48 One RCT evaluated HDM SCIT compared with pharmacotherapy alone for 6 months in 40 children and found that patients in the SCIT arm had a significantly higher number of clinic visits in 6 months compared with controls, but the number of emergency room visits and hospitalizations were not significantly different between arms.48 The authors do not provide an explanation for the significant increase in clinic visits in the SCIT arm. The second RCT enrolled 121 children and compared multiple-allergen SCIT versus placebo for 30 months.32 This RCT reported no difference in the number of office visits, ED visits, or hospitalizations between baseline and final followup for either arm, and there were no differences between groups for any outcome. Two small RCTs with medium risk of bias found the following: inconsistent and imprecise results for clinic visits, and consistent but imprecise findings that there was no significant change in hospitalizations or ED visits. Overall, the strength of evidence was insufficient. See Table 3. Summary of the strength of evidence for the efficacy of subcutaneous immunotherapy for details.
Pulmonary Physiology
PEF. Ten studies of SCIT, including 704 patients, reported peak expiratory flow rate (PEF) as an outcome.31, 32, 34, 40, 45, 48-52 Most of these studies enrolled mixed-age populations, two enrolled adults only,34, 52 and two enrolled children only.32, 48 Most of these studies (6 of 10) employed HDM allergen. Two studies were of mold allergens (Cladosporium and Alternaria), one was of ragweed allergen, and one was of mixed allergens. Peak flow values were reported in the studies as a mean daily, morning, and/or evening value. Treatment ranged from 6 months to 2 years. Overall risk of bias was low in four studies, moderate in four, and high in one, the latter with issues of allocation concealment and blinding.
Seven of nine studies reported statistically significantly improved PEF with SCIT compared with controls.31, 32, 34, 40, 45, 49, 52 In one study of HDM allergen,50 there was a significant increase in PEF in the SCIT group during the study, but the change was not significantly different when compared with the change in the control group. This study enrolled patients with mild to moderate persistent asthma and treated for 1 year. In the study of Cladosporium allergen, there was not a significant difference in PEF between the SCIT and control groups.51 This study enrolled patients with mild and moderate persistent asthma and treated for 10 months.
Both studies in adults showed significant improvement in PEF. In one study of HDM allergen in only adults,34 morning PEF improved significantly in the SCIT group but not the controls. In this study, treatment was for 6 months and the asthma patients were controlled at baseline. In the other study of adults, ragweed allergen was used and there was a statistically significant difference in PEF between SCIT and control, when measured in the morning during the peak allergen season.52
Both studies in children showed increase in PEF. In the HDM study, PEF increased in the SCIT arm and decreased in the control arm, but the difference between arms was not statistically significant.48 The other RCT used multiple-allergen SCIT versus placebo and noted a clinically small increase in PEF in the SCIT arm compared with placebo (95% CI -7.8 to 0.1, P= 0.05).32
FEV1. There were six studies of SCIT, including 548 patients, that reported FEV1 as an outcome,28, 40, 41, 50, 53, 54 including one of the studies that also reported PEF as an outcome.50 Four studies were of HDM allergen, one of Alternaria, and one of multiple allergens. In one study, there was a significantly greater increase in FEV1 percent predicted in SCIT versus control (change from 82 to 99 percent predicted vs. 86 to 83 percent predicted, P <0.001).54 In this study, patients were treated with 7 weeks of therapy with HDM allergen. Asthma severity and control at baseline were not reported. In another study, FEV1 improved in the SCIT group (73 to 96 percent predicted, P=0.008), but the change was not compared with the change in the control group.40 This study used Alternaria allergen in patients with mild and moderate persistent asthma for 12 months. In one of the pediatric studies, the authors reported the number of patients with improvement in the study groups, with a significantly greater number improved in SCIT compared with control (P=0.0001).28
In the study that also reported significantly improved PEF,50 there was not a corresponding increase in FEV1. Another study reported significant changes in FEV1within the SCIT arm (P<0.001) but not for the placebo arm (P>0.05), without providing direct comparison between the groups.53 Another simply reported that at 8 months all patients had FEV1 > 80 percent predicted, but did not report changes from baseline.41
Overall, there was low SOE that SCIT improves FEV1. The findings were consistent and precise, but risk of bias was high. See Table 3. Summary of the strength of evidence for the efficacy of subcutaneous immunotherapy for details.
FEV1/FVC. No study of SCIT reported FEV1/FVC as an outcome.
FVC. One study reported change in FVC.50 This study randomized 132 patients with mild to moderate asthma and treated with HDM allergen for 1 year. There was no statistically significant increase in FVC in either the SCIT or placebo groups.
Airway Hyperresponsiveness (AHR)
Methacholine challenge. Seven studies reported methacholine challenges results, with two HDM studies in adults,46, 49 two HDM studies in mixed-age populations,31, 55 one Alternaria study in mixed-age populations,40 one of cat allergen in adults,35 and one of multiple allergens in children.32 The studies included 388 patients. Overall, two studies showed improvement in AHR, while five did not.
The study of Alternaria did show significant improvement in AHR when compared to pharmacotherapy (P=0.03).40 In this study, monosensitized patients with mild and moderate persistent asthma were treated for 12 months.
Of the four studies of HDM allergen, one showed significant improvement in AHR, while three did not show an improvement. In the study showing improvement in AHR, patients in the SCIT group had a significant increase in PD20 (dose of allergen required to cause a fall of 20% in FEV1) compared to control group, after 3 years of treatment. Disease severity was not reported.46 In the three studies that did not show improvement, asthma status of enrollees was mild to moderate severity, well-controlled, and not specified, with treatment durations of 3 years and 2 years and 7 months, respectively.31, 49, 55 Neither the study of cat allergen35 or multiple allergens32 showed improvement in AHR. (See Appendix D, Table D10 for details.)
Allergen challenge. There were 13 studies that reported results of allergen challenges, including eight with HDM; two with cat; and one each with dog, Cladosporium, and ragweed. Nine studies were done in adults (n=369),34-36, 42-44, 52, 54, 55 and four included mixed-age populations (n=110).27, 30, 37, 51
Overall, most studies(9 of 13) showed statistically significant improvement in AHR with SCIT compared with the control group, and one study showed significant improvement in the SCIT group but not in the control group.34 In three studies, there was not significant improvement in SCIT versus control.27, 30, 35
The eight studies of HDM allergen included six in adults and two in mixed-age populations.27, 34, 37, 42-44, 54, 55 In three studies, asthma severity was not reported; two studies included patients with mild and moderate asthma; one study included all severities; one study included patients whose asthma was controlled, and one study included patients whose asthma was poorly controlled. In six of the studies, there was significant improvement in AHR compared with control; in one study the improvement was demonstrated in the SCIT group but not in the control group; and in one study there was no significant difference in AHR with control. Treatment durations ranged from 7 weeks to 2 years. The study that did not show improvement in AHR was of 7 months duration.
Of the two studies of cat allergen, one study showed improvement in AHR.36 This study enrolled adults and asthma severity was not reported. Patients were monosensitized to cat allergen and were treated for at least 1 year. In the other study of cat allergen, there was not improvement in AHR.35 In this study of adults with controlled asthma, patients who were monosensitized to cat allergen were treated for 16 weeks.
For the study of dog allergen challenge, there was not improvement in AHR.30 This study enrolled mixed-age patients with monosensitization to dog allergen. Asthma severity was not reported and treatment was for 1 year.
The study of Cladosporium allergen showed significant improvement in AHR with allergen challenge after a duration of 10 months treatment.51 This study enrolled mixed-age patients with mild to moderate asthma that was controlled.
In the study of ragweed allergen, adults with moderate to severe, uncontrolled asthma were enrolled.52 Patients had to have had exacerbations of asthma during the fall season. Significant improvement in AHR was shown after 2 years of treatment. (See Appendix D, Table D10 for details.)
Exercise challenge. No SCIT studies reported exercise challenge outcomes.
Compliance
One study comparing multiple-allergen SCIT to placebo in 121 children reported that both arms had high levels of compliance (measured at each visit on the basis of prescribed doses and doses recorded in diaries) (92.6% vs. 93.6%) and there was no difference between arms.32
Immunological Outcomes
Allergen testing. Six RCTs reported allergen skin testing results before and after SCIT.28, 29, 45, 47, 56, 57 Five studies exclusively looked at skin test reactivity to HDM,29, 45, 47, 56, 57 and one study examined mixed reactivity to multiple allergens including HDM, mold, trees, animals, and grass.28
Only one study did not find any differences in SPT for HDM between SCIT and placebo over a 3 year period.45 Five studies reported significant improvement in allergen skin reactivity after SCIT using different skin testing parameters,28, 29, 47, 56, 57 one that used a cutaneous tolerance index reported improvement over a period of 15 weeks for HDM (95% CI 0.27; 0.11-0.56, P<0.05).47 One study on HDM found statistically significant improvement in multiple intradermal skin testing parameters over 3 years, including immediate phase (P=0.04) and late phase skin reactions (P=0.002), and skin prick titration tests to determine the estimated allergen concentration that caused histamine equivalent skin reactions (HEP)(P=0.0001).29 Another study demonstrated improved histamine equivalent skin test reactions for HDM over 54 weeks (P=0.029).56 The only study comparing SCIT with pharmacotherapy demonstrated significant improvement in HEP over 4 months.57 Lastly, the study using multiple allergens reported general improvement in skin testing parameters for mixed allergens for 1 year in SCIT patients compared with placebo (P=0.0001).28
Overall risk of bias was low in one study and moderate in five. The six studies included 525 patients and five used HDM allergen. Treatment ranged from 1 to 3 years. The administration of SCIT was associated with improvement in allergen skin reactivity, mainly with HDM.
Immunoglobulin E. Eleven RCT studies reported IgE levels: eight examined HDM,18, 29, 31, 39, 45, 47, 56, 58 one examined Alternaria,40 and two looked at mixed allergens for HDM, mold, trees, animals, and grass.28, 32 Six studies demonstrated significant reductions in IgE levels after SCIT.28, 29, 31, 40, 45, 58 Four studies demonstrated statistically significant decreases in serum specific IgE levels for HDM from 1 to 3 years in the SCIT group compared to either placebo, desensitization vaccine (not specific desensitization method), ICS, or untreated patients.29, 31, 45, 58 Three studies demonstrated significant reductions in specific IgE for Alternaria and mixed allergens, respectively, when SCIT was compared to pharmacotherapy.28, 32, 40 Four studies showed no change in total IgE after treatment.18, 39, 47, 56
Immunoglobulin G4. Five SCIT RCTs reported serum IgG4 levels specific for HDM,31, 37, 39, 47, 56 all of which demonstrated statistically significant reduction of IgG4 levels. All studies compared SCIT versus placebo: one study lasted 15 weeks, two studies for 1 year, and two studies for 2 years. One study compared SCIT to standard pharmacotherapy,39 while another examined SCIT and ICS versus ICS alone.31 One study reported a significant decrease in the HDM-specific IgE/IgG4 ratio in patients undergoing SCIT compared with placebo.56
Variation per Setting
Three studies did not specify setting.40, 45, 53 All other studies (n=28) were done in the clinical setting and no study was conducted in the home setting. There are no data to draw conclusions on any variation per setting.
Variation per Population
Adults
Asthma Symptoms. No studies in adults reported on asthma symptom outcomes using ACT, ACQ, or P-ACT scores.
Quality of Life. Two studies in adults assessed quality of life with AQLQ. Both studies showed statistically significant improvement in quality of life with SCIT compared with control.42, 43 These studies included adults with mild and moderate persistent asthma who were treated with HDM allergen for 54 and 55 weeks.42, 43 The differences in overall AQLQ were approximately 4 points (P=0.043) and 6 points (P=0.0025), respectively. Both of these studies of adults were positive, and SOE was moderate with consistent and precise results and medium risk of bias.
Medication Use.
Quick-relief medications. One study of adults receiving HDM SCIT for 12 months reported decrease in quick-relief medication use (SABA).44 This study included 31 patients with unspecified asthma severity or control at baseline. The study reported a statistical significant reduction in medication use among those receiving SCIT (decrease from 27 to 14 puffs/week, P<0.05) and a non-significant reduction in the control group (decrease from 52 to 46 puffs/week, P NS). There was a substantial change in the use of medications. Overall, SOE was low for the effect of SCIT on quick-relief medication use, based on one small study (n=31) (imprecise, unknown consistency) with low risk of bias.
Long-term control medications. Two studies in adults evaluated the effect of SCIT on the use of long-term control medications. One study of adults with mild asthma showed statistically significant reduction in long-term control medication use in the SCIT group when compared with placebo.42 This study reported a greater number of weeks free from ICS use in SCIT compared with placebo (P<0.001). This was a study of 64 patients with mild or moderate persistent asthma, treated with HDM allergen. Another study of adults44 reported a significant reduction in ICS dose in the SCIT group during the study (38%, P <0.05) and a non-significant change in the control group. This study enrolled 31 patients with unspecified baseline asthma severity and control. For the subgroup of adults, SCIT may reduce long-term medication use, based on consistent results from two small studies (imprecise) (low SOE).
Systemic corticosteroids. There were no studies of the effect of SCIT on systemic corticosteroids in adults.
Asthma Exacerbations. There were no studies of the effect of SCIT on asthma exacerbations in adults.
Health Care Utilization. There were no studies of the effect of SCIT on health care utilization in adults.
Pulmonary Physiology
PEF. Two studies in adults showed significant improvement in PEF. In one study of HDM allergen in 16 adults,34 morning PEF improved significantly in the SCIT group but not the controls. In this study, treatment was for 6 months and the asthma patients were controlled at baseline. In the other study of adults, 90 patients were studied who had uncontrolled asthma at baseline. Ragweed allergen was used and there was a significant difference in PEF between SCIT and control, when measured in the morning during the peak allergen season.52
FEV1. Only one study in adults assessed FEV1 and it reported significant changes within the SCIT arm but not for placebo (P<0.001 vs P >0.05); it did not directly compare the groups.53
FEV1/FVC. There were no studies of the effect of SCIT on FEV1/FVC in adults.
FVC. There were no studies of the effect of SCIT on FVC in adults.
Airway Hyperresponsiveness. There were nine studies performed in adults that assessed the effect of SCIT on allergen challenge. Of these, six used HDM allergen, two cat, and one ragweed.34, 35, 44, 52, 54, 55 Of these studies in adults, all showed improvement in AHR compared with control, except one that only showed improvement in the SCIT group but not in the control and one that showed no significant difference. Studies of SCIT in adults that examined AHR by specific allergen challenges had consistent and precise results supportive of improvement.
Compliance. There were no studies of the effect of SCIT on compliance in adults.
Children
Three studies, including 403 children, reported on the efficacy of SCIT for clinical outcomes in children 5 to 12 years of age with asthma. One study was completed in the United States,32 and two were completed in Asia.28, 48 Asthma diagnosis was per GINA criteria in two of the studies,28, 48 and physician diagnosis in the third.32 Two studies included children with moderate to severe persistent asthma,32, 48 and one study excluded patients with severe uncontrolled asthma.28 Allergy diagnosis was made by SPT and specific IgE elevation in all studies.28, 32, 48 One study enrolled patients monosensitized to HDM and used HDM SCIT;48 two studies included polysensitized patients, one of which used multi-allergen SCIT32 and the other of which used both single and multiple allergens.28 One study compared SCIT to placebo,32 and the other two studies compared SCIT to pharmacotherapy.28, 48
Asthma Symptoms. There were no studies of the effect of SCIT on asthma symptom outcomes using ACT, ACQ, or P-ACT scores in children.
Quality of Life. There were no studies of the effect of SCIT on asthma quality of life using the AQLQ, PAQLQ, or school or work absences in children.
Medication Use. One RCT that compared multiple-allergen SCIT to placebo in 121 children reported the number of days of medication use in the previous 60 days, at baseline, and at final followup.32 This study found a statistically significant decrease in the number of days of ICS use in the SCIT arm but not in the placebo arm. However, there was no significant difference in the use of ICS between arms. This study also reported that there was no significant difference within or between arms for the use of systemic steroids. There is insufficient evidence on the effect of SCIT on asthma-specific medication use in children.
Asthma Exacerbations. There were no studies of the effect of SCIT on asthma exacerbations in children.
Health Care Utilization. As noted above, two RCTs reported on health care utilization in children with allergic asthma.32, 48 Overall, the strength of evidence is insufficient.
Pulmonary Physiology
PEF. Two RCTs reported PEF in a total of 161 children.32, 48 One RCT used HDM SCIT versus pharmacotherapy alone (asthma medications per GINA guidelines) and found that the PEF increased in the SCIT arm and decreased in the control arm; however, the change both within and between arms was not statistically significant.48 The other RCT used multiple-allergen SCIT versus placebo and noted a clinically small increase in PEF in the SCIT arm compared with placebo (95% CI -7.8 to 0.1, P= 0.05).32
FEV1. One RCT that used both single- and multiple-allergen SCIT versus pharmacotherapy alone (beclomethasone inhaler 200-300 μg daily and aminophylline 100mg tablet twice daily) reported FEV1 in 242 children treated for 12 months and found that patients in the SCIT arm had significant improvement in their FEV1 compared with the pharmacotherapy arm (P= 0.0001).28 However, we were unable to draw conclusions due to insufficient evidence (unknown consistency, imprecise, medium risk of bias).
FEV1/FVC. There were no studies of the effect of SCIT on FEV1/FVC in children.
FVC. There were no studies of the effect of SCIT on FVC in children.
Airway Responsiveness. One study comparing multiple-allergen SCIT to placebo in 121 children reported methacholine challenge results.32 Both arms had a significant decrease in bronchial sensitivity to methacholine but there was no difference between arms (mean difference -0.02 (95% CI -0.66 to 0.61) P >0.99).32
Compliance. One study comparing multiple-allergen SCIT to placebo in 121 children reported that both arms had high levels of compliance (92.6 versus 93.6 percent), but the difference between arms was not reported. Compliance was measured by pill counts and the weight of metered-dose-inhaler canisters at each visit.32
Key Question 2. What is the evidence for the safety of subcutaneous immunotherapy (SCIT) in the treatment of asthma?
Key Points
- Local reactions to SCIT were frequent; however, reactions also commonly occurred with placebo injections (risk differences ranged from -0.317 to 0.4), and local reactions infrequently required a change in the SCIT dosing.
- Systemic allergic reactions to SCIT were reported frequently (risk differences ranged from 0 to 0.319). The majority of systemic allergic reactions were mild, and only a small number was consistent with anaphylaxis and required treatment with injectable epinephrine.
- There was insufficient evidence to draw conclusions regarding the effect of SCIT on anaphylaxis or death.
- Serious adverse events such as anaphylaxis and death were not reported in the included studies in the pediatric population (total of 462 patients in 4 RCTs).
- None of the studies reported providing patients SCIT in the home setting.
Overall Study Characteristics
Our search identified a total of 44 articles on 42 unique studies/populations reporting safety data on SCIT. Of the included studies, 26 were RCTs (28 articles), and 18 were either cohort, case-control, or case reports. Of all studies included (RCTs and non-RCTs), 19 included only adults, 21 included a mixed-age population, and 4 included children. The articles were published between 1984 and 2017, with 52 percent of studies originating from Europe, 21 percent from Asia, and 21 percent from the United States.
We provided details about the studies, patient characteristics, and interventions in Appendix E and components in the assessment of risk of bias in Appendix I.
Summary and Description of Characteristics in RCTs
Of the 26 RCTs (28 articles) (N=1,512), 12 studies enrolled only adults (defined as 12 years of age and older),29, 34-36, 42, 43, 47, 52-56, 59, 60 10 enrolled mixed-age populations,30, 31, 37, 39, 41, 45, 51, 57, 61, 62 and four enrolled children only.18, 28, 30, 32, 45, 48 SCIT was compared to placebo in 15 studies,29, 30, 32, 34-37, 42, 43, 47, 51-54, 56, 57, 62 to pharmacotherapy in six studies,28, 31, 39, 41, 48, 55 and to SCIT in a modified dose or duration in five studies.18, 45, 59-61
GINA criteria were used for asthma diagnosis in 10 studies (11 articles),18, 28, 29, 31, 39, 43, 48, 51, 59-61 a positive bronchial response to methacholine was used in two studies,52, 54 to histamine in one study,34 to cat allergen in one study,35 and to HDM allergen in one study.55 The diagnosis was clinical or not specified in the remaining 11 studies.(12 articles)30, 36, 37, 41, 42, 45, 47, 53, 56, 57, 62, 63
Asthma was classified as mild or moderate persistent in 14 studies(16 articles),18, 28, 29, 37, 39, 41-43, 45, 47, 51, 56, 57, 59-61 three studies included patients with severe persistent asthma,48, 52, 62 and in nine studies the severity was not classified.30-32, 34-36, 53-55 Asthma control status prior to initiation of SCIT was described in six studies: asthma was reported as controlled in four studies,34, 35, 51, 61 and uncontrolled or poorly controlled in two studies.37, 52
Documentation of allergic sensitization was made through SPT and/or serum IgE in all studies.
Patients were monosensitized in 14 studies and polysensitized in five studies.28-32 One study included both polysensitized and monosensitized patients,18 and six studies did not clearly report sensitization status.34-37, 39, 61 Patients received single-allergen immunotherapy in 23 studies and multiple-allergen immunotherapy in two studies,28, 29, 32, 59 and both multiple- or single-allergen immunotherapy in one study.28 The allergen provided included HDM in the majority (60%) of studies. Other allergens were grass, ragweed, cat, Cladosporium mold, and dog. In the three studies where multiple allergens were provided, the type of allergen was not specified. In 24 studies, SCIT was provided in the clinic setting; the location was not specified in two studies.45, 53
Adults
Of the 26 RCTs, 12 studies enrolled only adults.29, 34-36, 42, 43, 47, 52-56, 59, 60 SCIT was compared to placebo in all studies except for two studies where it was compared to pharmacotherapy,29,55 and one study where it was compared to a modified SCIT (a depigmented-glutaraldehyde polymerized extract).60
GINA criteria were used for asthma diagnosis in three studies,29, 43, 59, 60 a positive bronchial response to methacholine was used in two studies,52, 54 to histamine in one study,34 to cat allergen in one study 35 and HDM allergen in one study.55 The diagnosis was clinical or not specified in four studies.36, 42, 47, 53, 56
Asthma was classified as mild or moderate persistent in five studies,29, 42, 43, 47, 56, 59, 60 one study included patients with severe asthma,52 and in six studies the severity was not classified.34-36, 53-55Asthma control status prior to initiation of SCIT was described in three studies: asthma was reported as controlled in two studies34, 35 and uncontrolled or poorly controlled in one study.52
Documentation of allergic sensitization was made through SPT and/or serum IgE in all studies. Patients were monosensitized to a single allergen in all except for one study where patients were polysensitized.29, 59 In all studies except for one,29, 59 a single allergen was provided in SCIT. The allergen provided included HDM in 50 percent of studies. Other allergens were grass, ragweed, and cat. In the studies where multiple allergens were provided, the type of allergen was not specified.
Children
Four RCTs reported on the safety of SCIT in 466 children with asthma. Studies included children with moderate and severe persistent asthma,32, 48 mild and moderate persistent asthma,18 and one specifically excluded those with uncontrolled asthma.28 In two studies, patients had at least an allergy to HDM and HDM SCIT was used in the trial.18, 48 Two studies included polysensitized patients and used multiple-allergen SCIT.28, 32 Two studies compared SCIT to pharmacotherapy alone,28, 48 one compared SCIT to placebo,32 and one study compared 3 year to 5 year SCIT.18
Summary and Description of Characteristics in Non-RCTs
Of the 18 non-RCTs, seven studies included adults only (defined as 12 years of age and older)20, 21, 23, 64-67 and 11 studies included mixed-age populations.19, 22, 24-26, 68-71
SCIT was provided in a cluster, rush, or ultra-rush protocol in 6 of the 18 studies (33%).20, 21, 24, 25, 66, 70 Documentation of allergic sensitization was made through SPT and/or serum IgE in 10 articles,19, 64-68, 70, 71 otherwise it was not specified. Allergen identified was not reported in seven studies,20, 21, 23, 24, 26, 65, 69 four studies had monosensitized patients,25, 67, 68, 71 three polysensitized patients,22, 70, 72 three had both monosensitized and polysensitized patients,19, 64, 73 and one did not clearly report sensitization status.66 Nine studies treated with single allergen and nine with multiple allergens.
Adults
SCIT was provided in a cluster, rush, or ultra-rush protocol in three (43%) of seven studies.20, 21, 66 Documentation of allergic sensitization was made through SPT and/or serum IgE in four articles,64-67 otherwise it was not specified. Two studies included polysensitized patients, one monosensitized patients, one both polysensitized and monosensitized patients, and four did not specify sensitization status. In four studies patients were treated with multiple allergens. Four of the studies were case reports.21, 23, 65, 67 (See Appendix F for further details.)
Children
There were no non-RCTs assessing safety of SCIT in the pediatric population.
Local Reactions
Summary and Description of Events in RCTs
Local reactions consisting of itching, pain, paresthesia, heat, erythema, and induration at the site of injections were reported in 6.25 percent42 to 33.3 percent31 of patients. Notably, local reactions occurred with the placebo injections in zero up to 12.5 percent of patients.35, 42, 47 Calculated risk differences ranged from -0.317 to 0.4 (a range of 32 additional cases of local reactions in the placebo group to 40 additional cases per 100 people treated with SCIT). In one study, patients who received SCIT to dog allergens had 20 episodes of local swelling per patient, as compared to 21 episodes per patient in those receiving placebo injections (calculated risk difference -0.317),30, 63 compared with one study with HDM, in which eight patients who received HDM SCIT presented local swelling at injection site and none of the patients receiving placebo presented local swelling at injection (calculated risk difference 0.4).48
Adults. Local reactions, described as local erythema or induration at the site of injections, were reported in 6.25 percent42 to 22 percent35 of patients. In the latter report,35 two of nine patients (22%) had three large local reactions severe enough to require modifications of the immunotherapy schedule, while none of the placebo patients has similar reactions. Local reactions were described with placebo injections in zero to 12.5 percent of patients.35, 42, 47
Children. One study reported local, red swelling at the site of HDM SCIT injection in eight children (calculated risk difference 0.4).48
Summary and Description of Events in Non-RCTs
Local reactions, described as swelling or urticarial plaques at the site of injections, were reported in four studies and ranged from 5.6 to 27.3 percent of patients treated,20, 22, 66 and in 6.5 to 10.7 percent of SCIT doses given.20 In the study in which the size of the local swelling was reported, 10.1 percent had a small reaction (<5 cm in diameter) and 13.2 percent had a large reaction (≥ 5 cm in diameter).22
Adults. Local reactions consisting of swelling or urticarial plaques at the site of injections were reported in 5.6 to 27.3 percent of patients,20, 66 and in 6.5 to 10.7 percent of SCIT doses given.20 One patient developed multiple subcutaneous itchy nodules on the lateral aspects of both arms, at the site of previous immunotherapy injections to timothy grass pollen.23
Children. There were no non-RCTs assessing local adverse events of SCIT in the pediatric population.
Systemic Allergic Reactions
Summary and Description of Events in RCTs
Systemic allergic reactions were described in 16 studies, including 540 patients treated with SCIT compared with 182 patients treated with placebo injections and 265 patients treated with pharmacotherapy. In four studies there were specifically no systemic allergic reactions reported. The rate of systemic allergic reactions ranged from zero to 44 percent of patients (4 out of 9 patients receiving SCIT for cat);35 when reported as number of injections, the highest rate of systemic allergic reactions was 11.7 percent of total injections given (203 reactions out of 1735 total injections).45 Types of reactions included pruritus, urticaria, eczema, skin rash, rhinitis, conjunctivitis, nasal congestion, nasal obstruction, cough, asthma, bronchospasm, wheezing, dyspnea, abdominal pain, diarrhea, and hypotension. However, in several studies the types of reactions were not specified and were described as “Not specified,” “Mild systemic reaction,” “Mild-moderate systemic reaction,” “Systemic reaction,” “Systemic reaction requiring Epinephrine,” “unspecified symptoms,” and “pulmonary reactions.” The calculated risk differences based on the number of patients who developed systemic allergic reactions ranged from zero to 0.319.
Bronchoconstriction was reported in patients receiving SCIT as follows: “Bronchospasm,” “wheezing,” “asthma,” and “pulmonary reactions” were specifically reported in 15 patients receiving SCIT in seven RCTs: 1/37,52 2/18,55 2/17,39 1/15,57 3/30 (two receiving cluster and one in the conventional arm),61 4/18,62 and 2/36.18 Only one study reported pulmonary reactions in the control arm: 3/17.62
Adults. Systemic allergic reactions were described in eight studies, including 205 patients treated with SCIT compared with 152 patients treated with placebo injections and 18 patients treated with pharmacotherapy. In two studies there were specifically no systemic allergic reactions reported. The rate of systemic allergic reactions ranged from zero to 44 percent (4 out of 9 patients receiving SCIT for cat, calculated risk difference 0.319).35 Out of the patients receiving SCIT, 46 patients were receiving an accelerated SCIT protocol (rush or cluster protocol).
There were 36 patients receiving SCIT who developed systemic allergic reactions, as compared to 6 patients receiving placebo injections. Out of these 36 patients, 7 patients were receiving an accelerated protocol.54, 55 The description of the nature and severity of these systemic allergic reactions varied greatly from study to study.
Children. Three studies reported systemic allergic reactions. Two studies used multiple-allergen SCIT. One of those studies compared multiple-allergen SCIT to pharmacotherapy and reported that nine children (11%) in the SCIT arm had an immediate systemic reaction.28 Of those nine children, one had mild respiratory involvement (grade 2) and eight had a skin rash (grade 1); all reactions were successfully treated in the clinic and did not require additional observation or hospitalization. The reactions and subsequent treatment were not described in further detail.28 The other study compared multiple-allergen SCIT with placebo and reported systemic allergic reactions to injections in 21 of the 61 children in the SCIT group (34%) and in 4 of the 60 children in the placebo group (7%) (P =0.001). In this study (n=121), there were 114 total systemic allergic reactions (in 21 of the 61 children receiving SCIT and 4 of the 60 children receiving placebo), 52 of which were treated with adrenergic drugs; however, neither the severity of the reactions nor the type of adrenergic drugs was specified, and there were no dropouts due to reactions to SCIT. All 52 responded to treatment without clinical sequelae.32 In one study that compared 3 years versus 5 years of HDM SCIT, two patients with asthma in the 5-year arm had an asthma episode within 30 minutes of receiving a maintenance dose that resolved with a bronchodilator. The following dose was adjusted in both patients and the authors comment that long-term tolerance was confirmed in every patient.18 One study specifically commented that there were no systemic allergic reactions.48
Summary and description of events in non-RCTs
Systemic allergic reactions were described in 13 studies (see Appendix G), 11 were case series and two were single case reports.21, 67 The rate of systemic allergic reactions ranged from 0.6 percent of patients and 0.1 percent of injections26 to 23.9 percent of patients.19 In the latter study, 16 of 67 children (24%) receiving HDM SCIT developed “non-fatal systemic reactions.” 19 Reported systematic reactions consisted of urticaria, asthma, flushing, nasal congestion, nasal itching, wheezing, chest tightness, bronchospasm, vasculitis, and anaphylaxis. However, in several studies the types of reactions were not specified and were described as “Non-specified systemic symptoms,” “systemic reactions,” “systemic effects,” and “non-fatal systemic reactions.”
In the studies where systemic allergic reactions and numbers of patients treated were reported, 5,692 patients were treated with SCIT, 52 patients were treated with pharmacotherapy, and no patients received placebo injections. Of the patients who received SCIT, 311 were being treated with a cluster regimen,20, 21, 24 and 836 were being treated with a rush or ultra-rush regimen.25, 66, 70
Adults. Systemic allergic reactions were described in five studies of adults, two of which were single case reports.21, 67 The rate of systemic allergic reactions ranged from 1.5 percent of patients20 to 11 percent of patients;64 in the latter study, patients were treated with HDM and animal SCIT, and the highest rate of systemic reaction was in patients with asthma but without seasonal rhinitis (11%) (as compared with patients with asthma and seasonal rhinitis, where the rate of systemic allergic reactions was 3%). In the studies where systemic allergic reactions and numbers of patients treated were reported, the total number of patients treated with SCIT was 379 patients, with no patients receiving placebo injections or pharmacotherapy. Out of the patients received SCIT, 184 were being treated with a cluster regimen20, 21 and 18 were being treated with a rush or ultra-rush regimen.66
Excluding case reports, there were 20 patients receiving SCIT who were reported to have systemic allergic reactions. Six of these patients were receiving an accelerated SCIT protocol. The case reports described one patient who developed anaphylaxis treated with epinephrine, and one patient who developed leukocytoclastic vasculitis that occurred repeatedly after SCIT injections.
Children. One study that included 67 children with asthma and allergic rhinitis sensitized to HDM who received HDM SCIT for 2 years documented that systemic allergic reactions occurred in 16 of 67 (23.8%) of children with asthma (27/2045 or 1.32% of total injections). All children in this study completed the initial phase of SCIT. Not all patients had asthma in this study and the systemic allergic reactions were not described further for children with asthma, specifically.19
Anaphylaxis
Summary and description of events in RCTs
Only one RCT specifically reported anaphylaxis, reporting that there were no anaphylaxis events in 33 patients who received HDM SCIT.31 This RCT was conducted in 65 people and was considered at medium risk of bias.
Upon review of the nature of reactions in all of the SCIT RCTs, four of the remaining 25 RCTs had patients with reactions we considered consistent with anaphylaxis.39, 52, 54, 60 (See Appendix E, Table E4.A for details.) One trial compared different forms of SCIT, reporting that one out of 12 patients receiving unmodified SCIT to grass developed urticaria and bronchospasm compared to none of the 11 patients in the modified SCIT arm.60 In another trial, at high risk of bias, one patient in the placebo group (n=40) received a HDM SCIT injection by mistake, and developed bronchospasm and hypotension requiring epinephrine.52
One RCT, at high risk of bias due to lack of allocation concealment and masking of outcome assessors, reported a high rate of anaphylaxis with three of 20 patients receiving rush HDM SCIT having a reaction consistent with anaphylaxis and none of the 10 patients receiving placebo injections having such a reaction (risk difference of 0.15).54 The rush SCIT protocol was delivered over the course of 3 to 4 days, starting at 30 BU of D pter. Once maintenance was reached, patients received weekly injections of 3000 BU. Four patients experienced a “systemic reaction” during the rush protocol, and three of these patients required epinephrine injections. The underlying asthma severity in these patients was not reported. No systemic allergic reactions occurred while patients were on maintenance SCIT, and no systemic allergic reactions occurred in the placebo group.
Finally, one RCT, judged to be at low risk of bias, randomized 50 patients to receive either HDM SCIT (15 patients), HDM SCIT in addition to oral vitamin D (17 patients), or pharmacotherapy only (18 patients).39 One patient in the SCIT-alone group experienced a systemic reaction within 20 minutes after injection of vial 4 during the buildup phase and was treated with epinephrine. Two patients in the SCIT+Vitamin D group developed mild asthma attacks and were treated with inhaled beta-2 agonist. The underlying asthma severity in these patients was not described. The risk difference, comparing the SCIT groups versus placebo, is 0.03.
Overall, the reports of systemic allergic reactions consistent with anaphylaxis varied greatly (from 0 to 15 additional cases of anaphylaxis per 100 people treated with SCIT). We are unable to draw conclusions on whether SCIT increased risk of anaphylaxis, primarily because the RCTs did not directly measure or report anaphylaxis (indirectness) and were not powered to assess such effects (imprecision). See Table 4. Summary of the strength of evidence for the safety of subcutaneous immunotherapy for details.
Table 4
Summary of the strength of evidence for the safety of subcutaneous immunotherapy.
Adults. As described above, one RCT reported three out of 20 patients receiving rush HDM SCIT were treated with epinephrine due to reactions consistent with anaphylaxis.54 One out of 12 patients receiving SCIT to grass developed urticaria and bronchospasm.60
Children. There were no RCTs of SCIT assessing or reporting anaphylaxis in the pediatric population.
Summary and description of events in non-RCTs
A case series with a total of 658 patients, reported no cases of anaphylaxis in 339 patients (2712 doses) receiving cluster SCIT and no cases of anaphylaxis in 319 patients (2552 doses) receiving conventional dosing SCIT with multiple allergens.20
One case series reported specifically on the incidence of anaphylaxis in patients with mixed-age groups.69 In this study, anaphylaxis was classified as “mild, moderate, or severe” based on symptoms. Reactions were classified as uniphasic (symptoms occurred within 5-30 minutes and resolved gradually) or biphasic (initial symptoms resolved then the re-emerged within several hours). There was a total of 453 patients receiving SCIT for allergic rhinitis, asthma, or venom allergy; 133 patients had asthma. A total of 21,022 injections were given and 131 anaphylactic reactions were recorded in 76 out of the 453 patients (120 uniphasic and 11 biphasic); 65 of these reactions were treated with epinephrine. The total incidence of anaphylaxis was calculated as 1.3%. Out of these 131 reactions, 63 (48%) occurred in patients who had asthma; however, the severity of systemic allergic reactions in patients with underlying asthma was not described. Following WHO criteria for assessing case reports, we determined that it was likely that SCIT caused the anaphylaxis reactions reported in this case series (causality).
Bronchoconstriction was reported in patients receiving SCIT as follows. One case series reported one participant out of 18 presenting “Bronchospasm grade 2” after receiving treatment with HDM SCIT.66 Another study reported one case of shortness of breath and hypotension during buildup, out of 144 patients who received SCIT.72
Adults. A case series with a total of 658 patients (5264 doses with multiple allergens) (cluster vs. conventional) reported no cases of anaphylaxis.20 One case report described a patient receiving cluster grass SCIT, who presented chest tightness with wheezing, requiring epinephrine.21
Children. There were no non-RCTs of SCIT assessing anaphylaxis in the pediatric population.
Deaths
Summary and description of events in RCTs
No deaths were reported in the RCTs.
Summary and description of events in non-RCTs
There was one case report 65 of death occurring in a 17-year-old female with moderate persistent asthma who had received SCIT in childhood for 4 years and stopped due to a skin reaction. The authors report that, 12 hours after initiation of new regimen, she complained of abdominal pain, vomiting, and diarrhea without fever. Two days later, she developed an acute respiratory failure and was referred to the ICU. She had markedly elevated CPK, elevated troponin, leukopenia, thrombocytopenia, and bilateral interstitial markings on chest X-ray. On day four, she developed hypoxic coma leading to intubation and mechanical ventilation, followed by shock and acute renal impairment. By day five, she developed multi-organ failure and died. The authors considered immunological mechanism secondary to manipulation or the way the dose was escalated and considered causality probable. Following WHO criteria for assessing case reports, we also determined that the likelihood of SCIT causing this death (causality) was possible, as the event was related to intervention but was not dose-related.
Variation per setting
Of the 26 RCTs, SCIT was provided in the clinic setting in 24 studies, and two studies did not specify the location. There were no studies reporting administration of SCIT at home. Therefore, in all the studies where location was mentioned, SCIT was provided in the clinic setting. There is insufficient evidence to analyze any variation in adverse effects of SCIT by the clinic or home setting.
Key Question 3. What is the evidence for the efficacy of sublingual immunotherapy (SLIT), in tablet and aqueous form, for the treatment of asthma?
Key Points
- SLIT improves asthma symptoms, as measured by validated instruments (high SOE).
- SLIT improves disease-specific quality of life and decreases use of long-term control medications (specifically, ICS), and improves FEV1 (moderate SOE).
- SLIT may decrease quick-relief medication use (short-acting bronchodilators) and may improve disease-specific quality of life (low SOE).
- There is insufficient evidence on the effect of SLIT on systemic corticosteroid use or health care utilization.
- There is insufficient evidence about the efficacy of SLIT in children.
Overall Study Characteristics
We identified 18 RCTs regarding the efficacy of SLIT for asthma. The articles were published between 2001 and 2016, with 75 percent of the articles originating from Europe. Eleven studies included only adults (12 years of age and older),74-84 four studies included mixed adult/children populations,85-88 and three studies included only children.89-91 Patients were monosensitized in 12 studies, polysensitized in one study,78 and one study did not clearly report sensitization status.84 Four studies included both polysensitized and monosensitized patients.74-76, 85 The majority of studies treated HDM allergy; the next most commonly treated allergies in these studies were birch and grass. No study used multiple allergens.
We provided details about the studies, patient characteristics, and interventions in Appendix F and components in the assessment of risk of bias in Appendix I.
Asthma Symptoms
Asthma symptom control outcomes were reported in four SLIT RCTs,74, 75, 77, 78 which included a total of 1,193 patients, with all studies including adult patients. Clinically and statistically significant improvement in scores was found in three of four studies.75, 77, 78 Three studies were low risk of bias, and the fourth had medium risk of bias.
Three studies used HDM in comparison to placebo and utilized the ACQ to evaluate asthma symptoms.74, 75, 78 The treatment duration for all three HDM studies was 1 year, with daily maintenance dosing ranging from 1 SQ-HDM to 12 SQ-HDM or 300IR for the daily dose. Two studies used tablets,74, 75 and one used aqueous drops.78 One of the three HDM studies was performed in patients with mild to moderate persistent asthma and demonstrated statistically significant improvement in asthma symptoms with SLIT with a daily maintenance dose of 300 IR drops.78 This study compared the percentage of patients with an ACQ score of <0.75 at the end of the study based on treatment versus placebo; raw data were not reported by the authors, so whether they achieved the minimal clinically important difference (MCID) could not be determined.92 They found statistically significant improvement in their subgroup analysis of 180 moderate persistent asthmatics (percentage improvement 56% vs. 40%, P<0.039); this effect was not found in the mild asthmatics.78 The second RCT found a trend for a non-statistically significant improvement in asthma symptoms with a decrease of 0.41 in ACQ score in the 6 SQ-HDM treatment group, compared with no change in score in the control group.75 The decrease in ACQ did not meet the MCID. The third HDM study was performed in patients with moderate to severe asthma and did not demonstrate statistically significant improvement (P=0.22).74
The fourth study of asthma symptoms used birch allergen with a maintenance dose of 100 AU tablet 5 days per week for 3 years plus daily inhaled budesonide 400 μg daily and the ACT to assess asthma symptoms.77 The comparator group was treated with inhaled budesonide (800 μg daily, 1600 μg daily, or 400 μg inhaled budesonide plus montelukast 10 mg daily). Treatment with birch allergen for 3 years, in this study, resulted in a statistically significant improvement of ACT scores (mean post value 24 in SLIT arm, vs. 18 in other arms, P<0.05); the improvement exceeded the MCID for the ACT.92
There is high strength of evidence that SLIT improves asthma symptoms, based on a body of evidence that is consistent in the direction of change, precise, direct, and with an overall low risk of bias. See Table 5. Summary of the strength of evidence for the efficacy of sublingual immunotherapy for details.
Table 5
Summary of the strength of evidence for the efficacy of sublingual immunotherapy.
Quality of Life
Three RCTs, all of HDM allergen with a total of 1,120 patients, examined the impact of SLIT on disease-specific quality of life using the AQLQ.74, 75, 78 Two studies were low risk of bias, and one study was medium risk of bias. All three studies included only adult patients and each compared SLIT with placebo.
The three RCTs did not demonstrate statistically significant improvement (P =0.89, P reported as “not significant” for 2 of the studies). The largest study (n=877) reported that scores in both SLIT groups and the placebo group improved, but there was no statistically significant difference between SLIT and placebo.74 Two studies included mild to moderate asthmatics, and one study included moderate to severe asthmatics. Two of the three RCTs used tablets,74, 75 and one used aqueous drops.78 All studies treated for 1 year, with daily maintenance dosing ranging from 1 SQ-HDM to 12 SQ-HDM or 300IR for the daily dose. The RCT that reported statistically significant changes in AQLQ in the treatment group pre- versus post-treatment used a 6 SQ-HDM tablet, but no significant differences were reported when the treatment group was compared to controls.75
Heterogeneity in the study populations and how quality of life was measured prevents further synthesis. Each study reported improvement in AQLQ in both the SLIT and placebo groups. The use of SLIT may improve disease-specific quality of life with asthma, based on a body of evidence that is consistent in the direction of change, precise, direct, and with an overall low risk of bias (low SOE). See Table 5. Summary of the strength of evidence for the efficacy of sublingual immunotherapy for details.
Medication Use
Quick-relief medications. Five studies of SLIT included data on quick-relief medication (SABA) outcomes.77, 82-84, 90 Four studies reported quick-relief medication outcomes in doses of SABA over 3 months, with three studies demonstrating statistically significant decrease in the need for SABA.77, 82, 83 The fifth study reported the reduction in doses of SABA used over a 6-month period.84 The studies were performed in patients with mild to moderate asthma and included a total of 298 patients. The risk of bias was low for one study, medium for two studies, and high for the remaining study. The high risk of bias was due to lack of allocation concealment and blinding.83 Two studies were performed in adults with birch allergy, with 5 years of continuous treatment (5 drops of 10,000 AU maintenance dose 3 times per week; cumulative annual dose for 100 micrograms of Bet v 1) or 3 years of pre/co-seasonal treatment (1000 AU tablet maintenance dose 5 days per week).77, 82 The first birch SLIT study measured SABA use in doses during 3-month pollen seasons per year over 5 years; it found that the SLIT group decreased SABA intake on average by 16.1 doses, compared with the control group treated with montelukast, which had a decrease on average of 3.6 doses (P=0.019).82 The second birch SLIT study measured SABA use over 3-month pollen seasons per year for 3 years; it found that the SLIT group decreased SABA intake on average by 10.1 doses, compared with the control groups treated with inhaled budesonide (800 or 1600 μg, or inhaled budesonide 400 μg daily plus montelukast 10 mg daily), which had decreases of 0.7, 2.9, or 4.5 doses on average, respectively (P<0.001).77 One study was performed with grass mix for 5 years (maintenance dose 3 times per week, 5 drops of 10,000 RU/ml; cumulative annual does of 70 micrograms of Phl p 1). The third study was grass mix study which measured doses of SABA over 3-month pollen seasons per year for 5 years and found an average decrease of 17.9 doses in the SLIT group, compared with an average decrease of 9.4 doses in the control group treated with 800 micrograms daily of inhaled budesonide (P=0.01).83
The fourth study was performed in children with HDM (20 drops of 300 IR/ml maintenance dose) and measured puffs of SABA per day; it did not find a significant change comparing SLIT to the placebo group after treatment (P=0.951).90 The fifth study was performed in adults (maintenance dose 710 UBE/ml 3 times/week) and measured the reduction in SABA doses. The study found a 50 percent reduction in the treatment group, compared to a 21 percent reduction in the placebo group (P<0.03).84
Overall, we found low SOE that SLIT may decrease the use of quick-relief medications, based on a body of evidence that is consistent, imprecise, direct, and with an overall medium risk of bias. See Table 5. Summary of the strength of evidence for the efficacy of sublingual immunotherapy for details.
Long-term control medications. Four studies of SLIT reported long-term control medication use and included a total of 1,308 patients. All studies treated mild to moderate persistent asthmatics with HDM and evaluated the use of ICS compared to placebo.75, 78, 86, 90 Two studies were low risk of bias and two were medium risk of bias. Two studies were performed in adults,75, 78 one in mixed-age populations,86 and one in children.90 Treatment duration ranged from 6 to 24 months, with dosing ranging from 1 SQ HDM to 12 SQ HDM, 100 IR, or 300 IR. The two studies performed in adults demonstrated significant decreases in the used of ICS with treatment using a daily maintenance dose of 300 IR drops or 6 SQ-HDM tablets.75, 78 In the first of these two studies, the authors measured absolute decrease in daily inhaled budesonide dose in micrograms, with the SLIT group decreasing by 218.5 micrograms on average, compared with the placebo group, which decreased by 126.5 micrograms on average (P=0.004).78 The second study reported the difference between placebo and SLIT in change from baseline in daily ICS use in micrograms as 327 (P<0.0001).75 The third study that included mixed-age populations used a maintenance dose of 300 IR tablet, reported no statistically significant differences between SLIT and control.86 The fourth study found no significant improvement in ICS use measured in puffs per day when comparing SLIT to placebo (P=0.215).90
Four large studies with low to medium risk of bias demonstrated statistically significant improvement comparing SLIT to controls. We found moderate strength of evidence that SLIT decreases the use of long-term control medications (inhaled corticosteroids). The strength of evidence was based on a body of evidence that is consistent in the direction of change, precise, direct, and with an overall medium risk of bias. See Table 5. Summary of the strength of evidence for the efficacy of sublingual immunotherapy for details.
Systemic corticosteroids. One study reported on the effects of SLIT on systemic corticosteroid use.90 This study included only children and is discussed in the pediatric section below. See Table 5. Summary of the strength of evidence for the efficacy of sublingual immunotherapy for details.
Asthma Exacerbations
Three studies reported on the effects of SLIT on asthma exacerbations using HDM in 1,498 adult patients with mild to moderate persistent asthma.74, 75, 84There were no children-only or mixed-aged population studies. One study, which used maintenance doses of 6 SQ-HDM or 12 SQ-HDM for 6 months in comparison with placebo, showed a statistically significant improvement in all of the following outcomes with the higher dose: time to asthma exacerbation, time to first asthma exacerbations with deterioration in asthma symptoms or nocturnal awakening, time to first exacerbation with deterioration in lung function, time to first asthma exacerbation and use of SABAs, and time to first severe asthma exacerbations. These were reported as hazard ratios with SLIT compared with placebo, with the placebo group as reference. The hazard ratios for the 12 SQ-HDM dose in this study are as follows: time to first asthma exacerbation, 0.69 (P=0.03); time to first asthma exacerbation with deterioration in asthma symptoms or nocturnal awakenings, 0.64 (P=0.03); time to first asthma exacerbation with deterioration in lung function, 0.52 (P=0.02); time to first exacerbation with increased use of SABA, 0.52 (P=0.03); and time to first severe asthma exacerbation, 0.69 (P=0.02). The hazard ratios for the 6 SQ-HDM dose in this study are as follows: time to first asthma exacerbation, 0.72 (P=0.45); time to first asthma exacerbation with deterioration in asthma symptoms or nocturnal awakenings, 0.72 (P=0.17); time to first asthma exacerbation with deterioration in lung function, 0.62 (P=0.03); time to first exacerbation with increased use of SABA, 0.62 (P=0.09); time to first severe asthma exacerbation, 0.72 (P=0.03).74 However, the second study, which utilized 1 SQ-HDM, 3 SQ-HDM, or 6 SQ-HDM maintenance dose for 1 year in comparison with placebo did not find a statistically significant improvement in the number of asthma exacerbations. The authors did not report the data for asthma exacerbations in this article.75 The third study, which used maintenance doses of 710 UBE/ml of HDM three times per week, reported the total number of exacerbation at the end of the study.84 The SLIT group had 71 exacerbations, compared with the placebo group, which had 123 (P<0.001).
Health Care Utilization
There were no studies of the effect of SLIT on health care utilization.
Pulmonary Physiology
PEF. PEF was reported in five studies,80, 86, 88-90 including a total of 341 patients. One study included only adults, two studies included only children, and two studies included mixed-age populations. The risk of bias was low in three studies and medium in two. All studies compared SLIT with placebo. Three studies were of HDM and two of grass pollen. While none of the studies demonstrated statistically significant improvement when compared with controls, three studies showed minimal improvement in those treated with SLIT,86, 88, 90 and one study showed improvement only in the evening measurements.89
FEV1. FEV1 was the most commonly reported outcome, reported in 11 studies.75, 77, 78, 80, 83, 84, 86, 88-91 Six of these studies included adults only,75, 77, 78, 80, 83, 84 three studies included children only,89-91 and two studies included mixed-age populations.86, 88 The total number of patients in these studies was 1,694 and all had mild to moderate asthma. Seven studies were of HDM, two of grass mix, one of birch, and one of timothy grass.
When considering seasonal allergens, three of four pollen allergen studies found statistically significant improvement in FEV1. One trial of grass mix SLIT versus control (treated with montelukast alone), at a dose of 5 drops of 10,000RU/ml 3 times per week for 5 years, reported an increase from an average of 78.5% to 96.2% of predicted FEV1 in the SLIT group, compared with a change in control group of 76.4% to 81.2% (p<0.0001).83 The second study, of birch allergen, was performed with a dose pre/co-seasonal 1000AU tablets 5 days a week for 3 years, and reported that mean FEV1 improved from 85.2 to 103.3 in the SLIT group, compared with 3 control groups treated with budesonide alone, which improved from 88.3 to 90.3, 87.0 to 92.4, and 86.2 to 96.5, respectively (p <0.05 for SLIT compared to any of the control groups).77 The third pollen study demonstrating statistically significant change was of grass mix over 6 months (maintenance dose of 43,800 IR three times per week), and demonstrated mean percent predicted FEV1 in the treatment group improved from 92.9 to 100.4, compared with the placebo group, which improved from 87.9 to 88.2 (P=0.005).88
One HDM study demonstrated statistically significant improvement in FEV1, with the treatment group improving from 2.16 to 2.86 (percentage increase after salbutamol), compared with the placebo group, which improved from 2.58 to 2.81 (P<0.03).84 The maintenance dose used in this study over 6 months was 710 UBE/ml.
The three pediatric studies noted a statistically significant improvement in FEV1 in the SLIT arm but there was no statistically significant difference between arms.89-91 Of the remaining three studies, demonstrated a non-statistically significant improvement in those treated with SLIT (numbers not reported).
The risk of bias was medium in five studies, low in five studies, and high in one study. SLIT may improve FEV1, based on evidence that is precise, direct, consistent, and with a medium overall risk of bias (moderate SOE). See Table 5. Summary of the strength of evidence for the efficacy of sublingual immunotherapy for details.
FEV1/FVC. There were no studies of the effect of SLIT on FEV1/FVC.
FVC. One study reported on the effect of HDM SLIT on FVC in children,90 and one study reported on the effect of HDM in adults.84 Neither study found any statistically significant effects on FVC.
Airway Hyperresponsiveness
Methacholine challenge. Four studies reported methacholine challenge results, including two birch studies in adults with mild asthma,77, 83 one study of grass mix in a mixed-age population,88 and one HDM study in a mixed-age population with severe asthma,85 There were no studies of children only. The studies included a total of 233 patients. Both birch studies demonstrated significant improvement in AHR after treatment with SLIT. The first birch study reported methacholine dose in micrograms causing a 20 percent fall in FEV1 from baseline (PD20), with the change in dose in the SLIT group improving by 592.9 after treatment, compared with the control group, which was treated with montelukast alone, of 190.1 (P=0.001).83 The second birch study reported methacholine dose in micrograms causing a 20 percent fall in FEV1 from baseline, with the SLIT group improving from 166.8 to 997.1 after treatment, compared with three control groups: budesonide 800 micrograms (from 226 to 520.0 μg of methacholine PD20), budesonide 1600 micrograms (from 199.8 to 644.9), and budesonide 400 micrograms plus montelukast (from 165.7 to 728.7) (SLIT vs. all treatment arms P<0.05). The grass mix study (6-month treatment with a maintenance dose of 710 UBE/ml 3 times per week) demonstrated improvement that did not reach statistical significance in the treatment group with improvement from 3.51 to 4.05 Mg/ml methacholine, compared with the placebo group improvement from 4.35 to 4.0 (P=0.058).88 The HDM study reported increases in cumulative methacholine dose in micrograms causing a reduction of 20 percent of the baseline FEV1 for the SLIT group and an improvement from 626.4 to 1277.7 after treatment (p=0.001), compared with an improvement from 616.1 to 860.3 for the control group, which was treated with non-specified pharmacotherapy (P=0.08); however, this study did not make a direct statistical comparison of SLIT to SCIT for the methacholine challenge outcome (PD20). The maintenance dosing used for the studies included the following: HDM, 1000 AU 2 times per week for 1 year; birch, 5 drops of 10,000AU/ml 3 times per week for 5 years; and birch, 1000 AU 5 days per week pre/co-seasonal 5 days per week. Two of four small studies with medium to high risk of bias demonstrated statistically significant improvement compared with controls.
Allergen challenge. There were no studies of the effect of SLIT on allergen challenge.
Exercise challenge. There were no studies of the effect of SLIT on exercise challenge.
Compliance
Three HDM studies reported on compliance in mild to moderate persistent asthmatics. The three studies involved adults only and included 1,022 patients.75, 76, 78 Compliance in these trials ranged from 90 to 99 percent. The first study reported compliance as mean compliance with study drug, the second study reported compliance as the number of non-compliant patients, and the third study reported compliance by determining the number of unused SLIT packs.
Immunological Outcomes
Skin testing. Three placebo-controlled SLIT trials reported allergen skin testing results for HDM.78, 86, 93 Two studies using HDM SLIT tablets demonstrated statistically significant reduction in skin wheal diameter when comparing SLIT baseline and post-therapy values and mean differences between SLIT and placebo groups.78, 86
Immunoglobulin E. Six SLIT aqueous or tablets versus placebo RCTs reported HDM-specific IgE levels.78, 86, 87, 89, 90, 93 Only one study reported a statistically significant effect: an increase in HDM-specific IgE levels after SLIT tablets compared to placebo (P<0.001).86
Immunoglobulin G4. Four RCTs using SLIT reported HDM-specific IgG4 levels.74, 78, 86, 89 Three studies reported statistically significant increases in specific IgG4 levels after SLIT compared with placebo.74, 78, 86, 89 One study comparing two doses of HDM SLIT tablets versus placebo along with ICS in 834 HDM allergic asthmatics measured IgG4 levels for both Der p1 and Der f. Those studies reported significant increases in both Der p1/Der f1 specific IgG4 at both doses when compared with placebo (P<0.001).74 Two other studies also reported significant increases in specific IgG4 using aqueous and tablet forms of SLIT (P<0.01 and P=0.026, respectively).86, 89
Variation per Setting
Ten studies of SLIT did not specify setting,75, 80-83, 86, 87, 89-91 four reported administration at home,76, 77, 79, 85 and two reported administration at the clinic.74, 78 The body of evidence is insufficient to draw conclusions on any variation per setting.
Variation per Population
Adults
Asthma symptoms. In the studies done on adults only, there was no variation compared with the full body of evidence in asthma symptoms. (See description above.)
Quality of life. In the studies done on adults only, there was no variation compared to the full body of evidence in quality of life. (See description above.)
Medication use. In the studies done on adults only, there was variation compared with the full body of evidence in the long-term control medication use. The two studies involving adults only demonstrated significant decrease in the use of ICS with treatment using a maintenance dose of 300 IR or 6 SQ-HDM.75, 78 This was not demonstrated in the two other studies, of children only and mixed-age populations. No studies evaluated quick-relief medications or systemic corticosteroids use in adults only.
Asthma exacerbations. In the studies done on adults only, there was no variation compared with the full body of evidence in asthma exacerbations. (See description above.)
Health care utilization. There were no studies of the effect of SLIT on health care utilization in adults
Pulmonary physiology. In the studies done on adults only, there was no variation compared with the full body of evidence in pulmonary physiology. Five studies, including 1,520 patients with mild to moderate asthma treated with HDM, reported on pulmonary physiology.75, 77, 78, 80, 83 (See results in the section above.)
Airway hyperresponsiveness. In the studies done on adults only, there was no variation compared with the full body of evidence in airway hyperresponsiveness when using methacholine challenge. (See description above.)
Compliance. Three adult-only HDM studies reported compliance outcomes in a total of 1,022 mild to moderate persistent asthmatics.75, 76, 78 Compliance in these trials ranged from 90 to 99 percent. The first reported compliance as mean compliance with study drug, the second study reported compliance as the number of non-compliant patients, and the third study reported compliance by determining the number of unused SLIT packs. Compliance was similar in the placebo arms.
Children
Three studies, including 216 children, reported on the efficacy of SLIT in children 5 to 12 years of age with asthma. All studies enrolled children with mild to moderate persistent asthma. All studies used HDM SLIT in children who were monosensitized to HDM and compared SLIT to placebo.89-91
Asthma symptoms. There were no studies of the effect of SLIT on asthma symptom outcomes using ACT, ACQ, or P-ACT scores in children.
Quality of life. There were no studies of the effect of SLIT on asthma quality of life using the AQLQ, PAQLQ, or school or work absences in children.
Medication use. One trial of HDM SLIT versus placebo in 110 children with mild to moderate persistent asthma reported on the use of asthma-specific medications after a 24-week intervention.90 This study found no difference in the use of quick-relief medication (Beta-agonists puffs per day) within or between groups. It also found no difference within or between groups for the use of long-term control medications (ICS puffs per day) or in the use of systemic corticosteroids (tablets per day). Overall strength of evidence is insufficient, based on a single small RCT with medium risk of bias.
Asthma exacerbations. There were no studies of the effect of SLIT on asthma exacerbations in children.
Health care utilization. There were no studies of the effect of SLIT on health care utilization in children.
Pulmonary physiology
PEF. Two studies reported on PEF as an outcome in children. One study included 20 patients and noted an improvement in evening, but not morning, PEF values compared with baseline in the SLIT arm.89 The second study included 110 patients and demonstrated that PEF did improve significantly at followup compared with baseline in only the SLIT group.90 Neither study noted a significant difference between arms.89, 90
FEV1. Three studies, including 216 children, reported FEV1 values.89-91 All three studies noted a statistically significant improvement in FEV1 in the SLIT arm, but there was no statistically significant difference between arms.89-91 The overall strength of evidence is low that SCIT improves FEV1 in children based on three RCTs with medium risk of bias, with consistent but imprecise results.
FEV1/FVC. There were no studies of FEV1/FVC in children only.
FVC. One study reported FVC values and found that children in the SLIT arm had significant improvement at the end of treatment, but there was no significant change in the placebo arm. There was no significant difference between arms.90
Airway hyperresponsiveness. There were no studies of the effect of SLIT on airway responsiveness in children.
Compliance. There were no studies of the effect of SLIT on compliance in children.
Key Question 4. What is the evidence for the safety of sublingual immunotherapy (SLIT) in the treatment of asthma?
Key Points
- Local reactions to SLIT were frequent (some reactions occurring in up to 80% of patients in RCTs); however, reactions also commonly occurred with placebo (risk differences ranged from -0.03 to 0.765).
- Systemic allergic reactions to SLIT were frequent (some reactions occurring in up to 22% of patients in RCTs), with only a few reports of anaphylaxis and no reports of deaths (risk differences ranged from -0.03 to 0.06).
- Although rates of anaphylaxis with SLIT compared to no treatment could not be determined (no cases reported in RCTs, insufficient evidence), three case reports suggest that rare cases may occur with SLIT treatment. Two of the three reports of anaphylaxis secondary to SLIT were in patients who received multiple-allergen therapy.
- No deaths secondary to SLIT therapy were reported (moderate SOE).
Overall Study Characteristics
Our search identified a total of 33 articles on 30 unique studies/populations reporting safety data. Of the included studies, 20 were RCTs (23 articles74-80, 84-91, 94-101), while 10 were either cohort, case-control, or case reports.102-111
We provided details about the studies, patient characteristics, and interventions in Appendix G and components in the assessment of risk of bias in Appendix I.
Summary and Description of Characteristics in RCTs
Ten RCTs enrolled adults, six enrolled mixed-age populations,85-88, 98, 99 and four enrolled children only.89-91, 100 Thirteen used GINA criteria to identify asthmatics,74-77, 79, 84, 89-91, 94, 95, 97-99, 101 while the other half used a positive methacholine challenge, bronchodilator reversibility, or did not describe the methods used. Asthma severity ranged from mild to severe persistent, with two studies specifying the recruitment of poorly-controlled patients.74, 77 Allergy was diagnosed using SPT and IgE in all studies but one in which diagnostic criteria was not specified.101 Patients were monosensitized in 13 studies77-80, 86-91, 98, 99, 101 and polysensitized in two studies.97, 100 Four studies included both polysensitized and monosensitized patients,74-76, 85 and one was unclear about monosensitization versus polysensitization.84 All studies examined single-allergen therapy, with allergens including HDM, birch, and grass. Five studies compared different doses of SLIT and included a placebo arm,74-76, 80, 94, 95, 98 while the remaining compared SLIT versus placebo, control, or standard asthma pharmacotherapy.77, 78, 85-87, 89-91, 96, 97, 99-101 Studies variably reported on treatment for adverse events or discontinuation of SLIT therapy due to adverse events, and many did not report whether adverse events were considered drug-related. Two studies took place in a combined clinic and home setting,87, 97, 98 three in the home,74, 76, 79 and the remainder did not specify setting. (See Appendix G, Table G1.A for patient characteristics and Table G3.A for SLIT dosing characteristics.)
Adults. Nine studies included adults only,74-78, 80, 84, 94-96, 101 and one reported results separately for adults.98 Seven studies used GINA criteria for asthma identification.74-77, 79, 84, 94, 95, 98 In these studies, asthma severity ranged from mild to severe persistent, and two studies specified recruitment of poorly-controlled patients.74, 77 Just over half of the studies of adults included polysensitized patients. HDM, birch, and grass allergens were represented. Five trials compared different doses of SLIT and included a placebo arm,74-76, 80, 94, 95, 98 while the remaining studies compared a SLIT versus placebo, control, or standard asthma pharmacotherapy.77-79, 84, 97 Four studies took place in the clinic,74, 78, 97, 98 three at home,76, 77, 79 and two did not specify setting.
Children. Four studies, including 270 children, reported safety data for the use of SLIT. All studies included patients with mild to moderate persistent asthma. Three studies, including 216 patients, compared HDM SLIT to placebo in patients who were monosensitized to HDM.89-91 One study evaluated ultra-rush high dose birch pollen SLIT in patients with tree pollen allergy.100
Summary and Description of Characteristics in Non-RCTs
We included 10 non-RCTs, of which five included adults only (4 case reports,102-105 1 retrospective cohort111), two included mixed-age populations,106, 107 and three included children only.108-110 Two studies described asthma diagnosis criteria: one based on American Thoracic Society criteria,108 and the other using bronchodilator reversibility for criteria.109 Asthma severity ranged from mild intermittent to moderate persistent and was not specified for five of the studies.102, 103, 105, 109, 110 Asthma control was also variably described. Eight studies used SPT for diagnosis, with five adding IgE criteria103, 104, 106, 107, 111 and two which did not specify atopic criteria.105, 110 Patients were monosensitized in three studies108, 109 118, 110 and polysensitized in three studies.102-104 Two studies included both polysensitized and monosensitized patients,107, 111 two studies did not clearly report sensitization status,105, 106 and one study did not report sensitized allergen.105 Three case reports examined administration of multiple-allergen SLIT,102, 103, 105 while the others examined single-allergen SLIT with HDM, grass, or pollen. Studies variably reported on treatment for adverse events or discontinuation of SLIT therapy due to adverse events. Three studies took place at least partially in the home,102, 105, 109 the other studies took place in clinic or hospital or were not specified. (See Appendix G, Table G1.Bfor Study characteristics and Table G3.B for Intervention characteristics.)
Adults. Four adult non-RCTs were case reports,102-105 and the fifth was a retrospective cohort.111 Four included polysensitized patients,102-104, 111 and two of those were given multiple-allergen SLIT.102, 103 Patients in one study in which allergic status was not specified also received multiple-allergen SLIT.105 Two studies occurred in the home,102, 105 one in the clinic,103 and two were not specified104, 111 (See Appendix G, Table G1.B Study characteristics.)
Children. Three studies reported safety data for the use of SLIT in children with asthma.108-110 All studies were case reports, included monosensitized patients to HDM, and had patients who received single-allergen SLIT.
Local Reactions
Summary and description of events in RCTs
Local events, including pruritus and/or swelling of the mouth, tongue, or lip, were reported in ten RCTs including roughly 2,500 patients,74, 75, 78-80, 86, 88, 90, 91, 95, 98 with risk differences between SLIT therapy and placebo ranging from -0.336 to 0.252. Throat irritation was reported in five studies including roughly 1,700 patients,74, 75, 79, 80, 95, 98 with risk differences ranging from -0.089 to 0.004. Abdominal pain, nausea, vomiting, and other gastrointestinal complaints were reported in six studies including roughly 1,500 patients,74, 78, 86, 88, 97, 98 with risk differences ranging from -0.004 to 0.384. Also reported were local rashes in three studies with roughly 750 patients.78, 97, 100 Frequency of local reactions was not consistently dose-dependent. Participants in trials reporting local reactions had mild to moderate asthma in thirteen studies, with one study including patients with moderate to severe asthma.74 Only two of the included studies took place in the home.74, 79 (See Appendix G, Table G5.A- Local reactions for further detail.)
Adults. Six of the eight RCTs reporting pruritus and/or swelling of the mouth, tongue, or lip,74, 75, 78-80, 95, 98 all of the five studies reporting throat irritation,74, 75, 79, 80, 98 four of the six studies reporting abdominal pain, nausea, vomiting, and other gastrointestinal complaints,74, 78, 97, 98 and two of the three studies reporting local rashes78, 97 were either exclusively conducted in adults or reported results separately in an adult population. The risk difference in the adult population was therefore similar to those in the overall population. (Summary above.)
Children. One study comparing birch SLIT versus placebo in 116 patients, reported local reactions, including application site itching and paresthesia. The number of reactions was not included.100 Another study comparing HDM SLIT versus placebo in 110 patients reported local reactions (tongue disorder, vomiting, abdominal pain, and circumoral paresthesia) in 5 children (10 incidences) in the SLIT group.90 One study found that there were no relevant local side effects in 86 children.91 One study did not comment on local reactions.89
Summary and description of events in non-RCTs
Local reactions were all related to gastrointestinal events, reported in three studies encompassing 79 patients. Reports included abdominal pain, nausea, vomiting, general malaise, and eosinophilic esophagitis.104, 106, 110 (See Table G4.B.4 Local reactions).
Adults. Abdominal pain, nausea, and vomiting was noted in one case report of a polysensitized adult female receiving single-allergen (HDM) therapy at home.104 No other local reactions were documented in non-RCTs.
Children. One pediatric case report documented a diagnosis of eosinophilic esophagitis related to HDM SLIT therapy.110
Systemic Allergic Reactions
Summary and description of events in RCTs
Reported systemic events included lower respiratory symptoms in eight RCTs including approximately 2,100 patients,74-76, 80, 86, 97-99 with risk differences between SLIT and placebo ranging from -0.089 to 0.002. Bronchospasm was not specifically addressed, though lower respiratory symptoms included asthma exacerbation or “aggravation” and chest tightness, which are often symptoms of bronchospasm. Mucosal irritation (other than mouth or gastrointestinal tract) was reported in five studies including approximately 1,800 patients,74, 75, 78, 97, 98 with risk differences of -0.07 to 0.035. Cutaneous systemic allergic reactions were reported by one study in 2 of 78 patients and resolved without treatment.77 This study was also the only RCT conducted in the home setting that reported systemic allergic reactions. All participants in studies reporting systemic effects had mild to moderate asthma. One study did not specify asthma severity.74 Incidence of systemic allergic reactions was not consistently associated with higher dose. (See Appendix G, Table G5.A Systemic allergic reactions).
Adults. Four of five studies documenting lower respiratory symptoms74, 80, 97, 98 demonstrated an identical range of risk difference between SLIT versus placebo to that described above for all studies. All other studies included in the systemic allergic reactions to SLIT were adult studies.
Children. No RCTs of children only reported systemic allergic reactions to SLIT. One study commented that there were no systemic allergic reactions in 86 patients treated with HDM SLIT or placebo.91
Summary and description of events in non-RCTs
Lower respiratory symptoms were reported in five studies,105-109 with asthma severity ranging from mild intermittent to moderate persistent. The symptoms included descriptions of wheezing requiring beta agonists and “worsening” of asthma, all of which may be consistent with bronchospasm, though bronchospasm was not specifically reported as an outcome. One pediatric case report documented a diagnosis of eosinophilic esophagitis related to HDM SLIT therapy.110 Two of the studies reported SLIT administered at least part of the time in the home. (See Appendix G, Table G5.B Systemic allergic reactions.)
Children. Three studies reported safety data for the use of SLIT in children with asthma.108-110
Adults. One case was reported of a 16 year-old female with mild intermittent asthma and HDM allergy.105
Children. One case was reported of a 6-year-old male with persistent asthma and HDM allergy. Asthma symptoms were well controlled on daily fluticasone. PEF was 75 percent predicted and FEV1 was 85 percent predicted and was reversible with bronchodilator. HDM SLIT was initiated (D far;D pter=50:50, 300 IR/ml). Following the induction phase, when the patient reached maintenance dosing (8 pumps), he developed wheezing within 2 minutes of his dose; symptoms persisted for 25 minutes and resolved with beta agonist (grade 2 reaction). He continued HDM SLIT at a reduced maintenance dose (4 pumps) and completed 3 years of therapy.109 Another case reported of a 10-year-old female with asthma of unspecified severity and unspecified controlled status, who received a standardized mix of D far;D pter=50:50, at 300 IR/ml concentration, presented with reflux and vomiting 6 weeks after starting SLIT. Symptoms did not respond to treatment. Histopathology confirmed a diagnosis of eosinophilic esophagitis which resolved after discontinuation of SLIT.110 Another retrospective case series reported no significant side effects in 39 pediatric patients with mild to moderate asthma receiving 3 years of HDM SLIT.107
Anaphylaxis
Summary and description of events in RCTs
No cases of anaphylaxis were reported among RCTs. Six studies74-76, 86, 97, 100 specifically reported no episodes of anaphylaxis with HDM SLIT administered in the clinic setting or the home. Dose ranged up to 12 SQ, and included patients were either monosensitized or polysensitized with mild to severe persistent asthma (See Appendix G, Table G6.A Anaphylaxis for further detail.)
Adults. Three studies in adults74-76 specifically reported no episodes of anaphylaxis with HDM SLIT administered in the clinic setting or the home. Dose ranged up to 12 SQ, and included patients were either monosensitized or polysensitized with mild to severe persistent asthma.
Children. No RCTs with children only reported anaphylactic reactions to SLIT.
Summary and description of events in non-RCTs
Three case reports, all in adults, reported anaphylactic reactions to SLIT therapy. The first was a 16-year-old female who received multi-allergen SLIT and developed anaphylactic shock.105 The second was a polysensitized 25-year-old female who received multi-allergen SLIT and developed flushing, hoarseness, dyspnea, dizziness, and mild hypotension.103 The last was a polysensitized, 31-year-old female who received multi-allergen SLIT and developed anaphylaxis.102 Asthma severity and control were not identified in any of the cases. For one case, SLIT was discontinued; for another case, SLIT was maintained at a low dose; and, for a third case, the ultimate therapy decision was not noted. All three received aqueous SLIT: two in a home setting and one in a clinic setting. Following WHO criteria for assessing case reports, we determined that it was certain that SLIT caused these reactions of anaphylaxis (causality) in two cases102, 105 and likely caused this reaction in one case,103 with the main difference being that this reaction was not time-related. (See Appendix G, Table G6.B Anaphylaxis.)
Death
Three RCTs, including 934 patients in the SLIT arm and 489 in the placebo arm,74, 78, 99 specifically reported that no deaths occurred during the study. There is moderate strength of evidence that SLIT does not increase the risk of death compared to placebo, based on a body of evidence that is consistent in the direction of change, precise, direct, and with an overall medium risk of bias.
No deaths were reported in any of the non-RCTs evaluated.
Other
See Appendix G, table G8.B for reactions that were not otherwise classified. These included studies for which no serious reactions were reported, specific reactions were not specified, or reactions could not be categorized and it was unclear that the reaction was mechanistically related to SLIT therapy.
Conclusions
Most reported reactions were local with few systemic reactions noted. Occurrence did not differ systematically by setting of administration: home versus clinic versus other. Most studies looked at single-allergen therapy with HDM extract, which was generally well tolerated. Dose of SLIT did not demonstrate a clear association with risk of adverse events in all studies, though a subgroup of individual studies did report an association. One study comparing adult and child populations noted that adverse events tended to occur at lower doses in children than in adults.98 No episodes of anaphylaxis were reported in RCTs, and three case reports of anaphylaxis were found among those who were polysensitized and/or treated with multiple allergen extracts. RCTs did not consistently report medication use or SLIT discontinuation in response to adverse events, though several studies did one or both. Of the three case reports of anaphylaxis, only one required a definite discontinuation of therapy (one followed a modified protocol of dosing and the other was not reported). No reports of death secondary to SLIT were found. See Table 6. Summary of the strength of evidence for the safety of sublingual immunotherapy for details.
Table 6
Summary of the strength of evidence for the safety of sublingual Immunotherapy.
Subcutaneous Versus Sublingual Immunotherapy
Key Points
- There is insufficient evidence to assess the relative efficacy of SCIT versus SLIT.
- There is insufficient evidence to assess the relative safety of SCIT versus SLIT.
Overall Study Characteristics
We included six studies published between 1989 and 2016 that reported on the efficacy and safety of SCIT versus SLIT.112-118 The studies included 267 patients; all studies used SPT for allergy diagnosis, included monosensitized patients, and used HDM as allergen, except for one study that included polysensitized patients and used multiple allergens.118
We provided details on the studies, patient characteristics, and interventions in Appendix H and components in the assessment of risk of bias in Appendix I.
Asthma Symptoms
One study of SCIT versus SLIT aqueous HDM therapy reported asthma symptoms using ACT.113 The study included 90 adult and pediatric patients. Asthma severity was not specified. The study reported that both the SCIT and SLIT arms had statistically significant improvement when comparing pre- and post-treatment scores and when compared to treatment with a combination inhaled steroid and short-acting bronchodilator (pre/post improvement in scores: SCIT 5.91, SLIT 4.29, control 4.27). However, the article did not report a direct comparison of ACT score for the SCIT to SLIT treatment groups.
The strength of evidence is insufficient to draw conclusions on the efficacy of SLIT versus SCIT on asthma symptoms.
Quality of Life
No SCIT versus SLIT studies that met inclusion criteria for this review reported on quality of life.
Medication Use
No SCIT versus SLIT studies that met inclusion criteria for this review reported on medication use.
Asthma Exacerbations
No SCIT versus SLIT studies that met inclusion criteria for this review reported on asthma exacerbations.
Health Care Utilization
No SCIT versus SLIT studies that met inclusion criteria for this review reported on health care utilization.
Pulmonary Physiology
One RCT of SCIT versus SLIT for HDM in comparison to medication alone reported pulmonary physiology outcomes in 90 mixed-aged patients in the form of PEF and FEV1.113 Asthma severity was not specified. The study reported that both the SLIT and SCIT arms had statistically significant improvement when comparing pre- and post-treatment PEF and FEV1 and when compared to treatment with a combination inhaled steroid and short-acting bronchodilator. However, the study did not report a direct comparison of the SCIT to SLIT treatment groups for these pulmonary physiology measures. The strength of evidence is insufficient to draw conclusions on the efficacy of SLIT or SCIT of pulmonary function.
Airway Hyperresponsiveness
Methacholine Challenge. One HDM study including adults only reported methacholine challenge results in 90 patients treated with SCIT, SLIT aqueous immunotherapy, or placebo/pharmacotherapy.112, 115 The study did not specify asthma severity. The study reported non-statistically-significant changes in AHR after treatment with 1 year of treatment in any of the groups. The publications did not report a direct comparison of results of those treated with SCIT with those treated with SLIT, nor was the specific data on the methacholine challenge values reported.
Allergen Challenge. One HDM study of mixed-age patients with mild persistent asthma reported bronchial provocation results with HDM after 1 year of treatment with SCIT (0.2-0.8 ml of 5000 TU/ml monthly), SLIT (28 drops of 100 TU/ml 3 times per week), or placebo. The total number of patients in this study was 32. There was a statistically significant improvement pre- versus post-treatment in the SCIT group only (P=0.003). However, when comparing SCIT to SLIT patients, there was no statistically significant difference in HDM bronchial provocation.114
Exercise Challenge. No SCIT versus SLIT studies that met inclusion criteria for this review reported on exercise challenge.
Immunological Outcomes
Four studies compared HDM-specific IgE levels between patients receiving SCIT versus SLIT.112, 113, 115, 117 Two studies reported individual statistically significant decreases in HDM-specific IgE at baseline and after SCIT or SLIT compared with placebo.113, 117
Two RCTs reported HDM-specific IgG4 levels over 1 year comparing SCIT, SLIT, and placebo.114, 115 One trial found that only SCIT was associated with an increase in HDM-specific IgG4 compared with either SLIT or SCIT.114 Another RCT compared four groups: SCIT, SLIT, SCIT in addition to SLIT, and pharmacotherapy and reported HDM-specific IgG4 increases in only the SCIT and SCIT+SLIT groups when compared with pharmacotherapy alone.115
Safety of SCIT Versus SLIT
Local Reactions
Three of the five RCTs reported local reactions.112, 113, 117 In two studies the incidence of reactions at the site of AIT application were comparable for SCIT and SLIT (13% vs. 10%)112 and one out 30 patients presented grade 2 events in each arm.113 Incidence was higher for SLIT in one study (oral itching was reported in only one of 16 patients in the SLIT arm)117 and higher for SCIT in a second study (10 out 27 patients receiving SCIT presented Grade 1 events compared to 3 out of 30 receiving SLIT).113 (See Appendix H.)
Systemic Allergic Reactions
Four of five RCTs reported systemic events.112, 113, 115, 117 Respiratory symptoms were reported only for SCIT,112, 115, 117 with an incidence ranging from 6 to 18 percent (1 or 2 patients). Gastrointestinal events (mild nausea) were reported for only one patient receiving SLIT.112 One study reported events as unspecified systemic allergic reactions; events were higher for SCIT than SLIT (2 patients vs. 1 out of 30 in each arm).113 (See Appendix H.)
Anaphylaxis
One study reported a case of anaphylactic reaction to SCIT therapy. One out of 16 patients receiving SCIT presented flushing, wheezing, and dyspnea requiring adrenaline, and required treatment discontinuation. All patients receiving SLIT (n=16) and pharmacotherapy (n=16) were able to complete the study.117
Safety in Non-RCTs
We included one case series that compared SCIT versus SLIT.118 It reports on two cases of adolescents (14 years of age and 13 years of age) receiving SCIT, who presented painful local reactions at the site of injection, significant enough to discontinue therapy. The patients were started on SLIT looking for a better safety profile. However, neither of these patients tolerated treatment; they both developed respiratory reactions and asthma worsening. Both patients required treatment discontinuation. (See Appendix H.)
- Results of the Literature Search
- What is the evidence for the efficacy of subcutaneous immunotherapy (SCIT) in the treatment of asthma?
- What is the evidence for the safety of subcutaneous immunotherapy (SCIT) in the treatment of asthma?
- What is the evidence for the efficacy of sublingual immunotherapy (SLIT), in tablet and aqueous form, for the treatment of asthma?
- What is the evidence for the safety of sublingual immunotherapy (SLIT) in the treatment of asthma?
- Subcutaneous Versus Sublingual Immunotherapy
- Results - The Role of Immunotherapy in the Treatment of AsthmaResults - The Role of Immunotherapy in the Treatment of Asthma
- Methods - Interventions To Improve Cardiovascular Risk Factors in People With Se...Methods - Interventions To Improve Cardiovascular Risk Factors in People With Serious Mental Illness
- GPI ethanolamine phosphate transferase 2 [Caenorhabditis elegans]GPI ethanolamine phosphate transferase 2 [Caenorhabditis elegans]gi|1831508724|ref|NP_001367076.1|Protein
- Results - Pain Management Interventions for Hip FractureResults - Pain Management Interventions for Hip Fracture
- Technical Expert Panel - The Clinical Utility of Fractional Exhaled Nitric Oxide...Technical Expert Panel - The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management
Your browsing activity is empty.
Activity recording is turned off.
See more...
