NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Wang Z, Pianosi P, Keogh K, et al. The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017 Dec. (Comparative Effectiveness Reviews, No. 197.)

The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management [Internet].
Show detailsWe conducted a systematic review with meta-analyses to assess the diagnostic accuracy and clinical utility of FeNO testing in the management of asthma. We found that FeNO has moderate diagnostic accuracy for asthma with diagnostic odds ratios (DORs) that range from 5.85 to 16.95 across various cutoff points (in comparison, a test with 0.80 sensitivity and 0.80 specificity would have a DOR of 16). As expected, with increasing cutoff values, FeNO had gradual decrease in sensitivity and improved specificity (for cutoffs <20, 20–30, 30–40, ≥40 ppb; respectively, FeNO testing has sensitivities of 0.78, 0.63, 0.56 and 0.41; and specificities of 0.71, 0.81, 0.84, and 0.94). Therefore, knowing the cutoffs used for test interpretation is critical for interpretation by clinicians. Inferences from several preplanned subgroup analyses were limited due to limited number of studies and heterogeneity of population, intervention, and outcome; particularly regarding the impacts of reference test, the presence of atopy, and current use of ICS on FeNO diagnostic performance.
In terms of the role of FeNO in monitoring asthma activity, a large body of observational and heterogeneous literature suggests that FeNO has a weak association with the risk of subsequent and prior exacerbations and a weak association with asthma control tests (ACQ and ACT). Such associations may be higher among patients with atopy (i.e., asthma associated with either positive skin test or specific IgE to aeroallergens), consistent with these patients being more likely to have eosinophilic inflammation. Such findings underscore the need to consider atopic predisposition in patients with asthma, because FeNO may be elevated owing to atopy alone, even in absence of asthma symptoms or diagnosis. Levels of FeNO were significantly lower in frequently exacerbating patients receiving higher doses of maintenance ICS. This finding is potentially important, inasmuch as it suggests higher ICS dose may not help and direct clinician to seek co-morbidity, or choose alternative medications. In addition, in atopic adults with persistent asthma on ICS, higher FeNO levels were significantly correlated with more short acting beta agonists dispensing and oral steroids courses in the past year, and lower FEV1 percent predicted levels; suggesting that perhaps treatment adherence should be scrutinized for such patients.
FeNO is unlikely to be helpful during acute exacerbations. This can be attributed to the presence of multiple factors that can cause or contribute to exacerbations, many of which are not associated with increased lower airway eosinophilic inflammation (even if this inflammation co-existed). We also found that FeNO has the potential to detect adherence to ICS, although the available data merely demonstrated an association of FeNO level with adherence assessed using dose counters or parent report. Studies did not describe a pragmatic adherence monitoring program with interventions to improve adherence; which would have provided more compelling evidence for the utility of using FeNO to evaluate adherence. Greater utility of FeNO as an aid in detecting adherence is expected in children (who can perform test satisfactorily) because most childhood asthma is atopic, unlike the situation in adults.
In terms of the clinical utility of FeNO to guide asthma management (select treatments, monitor response, step up and step down therapy, change therapies), we found moderate SOE from multiple RCTs suggesting that such an approach can lower the risk of exacerbations and the need for systemic steroids. The strength of evidence on hospitalization and quality of life was either low or insufficient. The reduction in exacerbations was demonstrated in both adults and children.
A large body of empirical observational evidence suggested that FeNO changes with the administration of inhaled and oral corticosteroids, leukotriene receptor antagonists, and omalizumab, but not long-acting beta agonists. This is consistent with pharmacologic evidence based on the mechanism of these drugs and can have implication for monitoring the effect of, or adherence to such drugs. We also found that FeNO may also help in selecting patients who may respond to ICS as an initial therapy, and it may be used for predicting exacerbations after ICS withdrawal or reduction, but its response is heterogeneous and its prediction can be enhanced by clinical measures such as ACT.
FeNO testing in early childhood (0–4 years of age) strongly correlates with API; which is not surprising given the relation between atopy and FeNO and the fact that this index is heavily predicated on atopic constitution. FeNO levels are higher in current wheezers and persistent wheezers (compared with non-wheezers and transient wheezers, respectively). This latter evidence can be quite relevant to clinical practice because most transient wheezers outgrow this symptomatic response by 3 years of age. Therefore, toddlers who continue wheezing after that age are more likely to develop asthma in future. However, only three studies ascertained whether these associations translate into subsequent development of a diagnosis of asthma after the age of 5. Two of the studies suggested that FeNO can predict such future diagnosis; one study did not. Therefore, such evidence is of low strength due to these heterogeneous findings, and it should be considered as merely preliminary. This association between FeNO in early childhood and future development of asthma was noted more in infants with atopy or eczema than in those without.
In terms of clinical implications, two scenarios commonly encountered in practice (among others) can benefit the most from FeNO testing. The first is in a patient with compatible symptoms who is clearly atopic (e.g. eczema, postivie skin tests, peripheral blood eosinophilia, positive IgE in the blood; which are routinely available and reimbursable tests). If this patient has elevated FeNO, this would imply that treatment with ICS is indicated; whereas low level (e.g. <20) implies that these compatible symptoms are not likely due to asthma. A caveat in this scenario is that low FeNO does not excluded asthma (clinical judgement and further follow up would be here warranted). The second scenario is about a patient with known asthma, who had a previously documented elevated FeNO level, but has symptoms that are not well controlled on guideline based therapy. In this patient, measuring FeNO as means to monitor adherence to treatment would be helpful.
Findings in Relation to What Is Known
The results of this systematic review are consistent with other systematic reviews that addressed diagnostic performance of FeNO testing (KQ 1.a) and clinical utility of FeNO measurements to select medication option (KQ 1.c); whereas to our knowledge, no systematic reviews have addressed clinical utility of FeNO measurements in monitoring disease activity and asthma outcomes (KQ 1.b), clinical utility of FeNO measurements to monitor response to treatment (KQ 1.d), and FeNO testing in predicting the future development of asthma (KQ 1.e). In terms of diagnostic accuracy, Li et al. reported pooled estimates of sensitivity, specificity, and DOR of 0.78, 0.74 and 11.4.191 Tang et al. evaluated the diagnosis of asthma in children and reported pooled estimates of sensitivity, specificity, and DOR of 0.79, 0.81 and 16.5.192 Guo et al reported pooled estimates of sensitivity, specificity, and DOR of 0.72, 0.78 and 15.9.193 The highest DOR (i.e. diagnostic accuracy) was observed in steroid-naive and nonsmoking patients.193 In terms of tailoring asthma management using FeNO based algorithms, two recent Cochrane systematic reviews reported that these strategies reduced exacerbations in strategies for adults and children without a significant impact on other outcomes.194, 195 Although not outcomes of interest in our systematic review, total ICS dose and final mean FeNO level were also not statistically different between the FeNO-based approach and standard management.194, 195
Limitations
For several of the key questions (KQ 1.b–e), studies were quite heterogeneous in terms of design, population, control tests, control strategies, and outcome measures; which led to narrative evidence synthesis and narrative rating of the strength of evidence. Narrative evidence synthesis is helpful for decision making; however, it does not provide a single best estimate; which is a limitation. Studies were overall small despite the fact that asthma is a very common condition. We also found limited data on baseline severity and large variations in FeNO protocols, which makes interpretation of the body of evidence challenging.
For the diagnostic accuracy question (KQ 1.a), there were several limitations. One challenge relates to the fact that there is no true gold standard of diagnosing asthma. Although we did not rate label studies as having increased risk of bias because of this issue, we recognize that it can impact diagnostic accuracy. In addition, a wide range of reference tests were reported. We categorized these reference tests as clinical diagnosis, positive bronchial challenge test, or a combination of clinical diagnosis, positive bronchial challenge, and/or bronchodilator response. However, significant heterogeneity still exist, such as to how and when these tests were administered. The studies reported a wide range of cutoffs from 0.8 ppb to 85 ppb. Although categorizations of <20, 20–30, 30–40 and >=40 ppb helped reduce heterogeneity and facilitated meta-analyses, we were not able to definitively present a best cutoff overall or within each category. We were also not able to conduct some planned subgroup analyses because of lack of data, including asthma phenotype, adequate testing procedures, body mass index (BMI) or weight, manufacturer and device model, and exhalation flow rate.
Applicability
The age of participants in the studies did not commonly conform to the definitions used in National Heart, Lung and Blood Institute prior asthma guideline (i.e. adults defined as 12 years of age or older)1. Therefore, applicability may be affected when guideline developers provide recommendations using diiferent age cutoffs. Otherwise, most studies reported on patients with asthma commonly seen in practice. FeNO measurements in the included studies were for the most part consistent with the American Thoracic Society/European Respiratory Society 2005 guidelines196 on the measurement of lower respiratory nitric oxide with the standard flow rate of 0.05L/second (body temperature [37° C] and pressure, saturated). The majority of studies did not include specific data on potential confounders including diet, use of mouthwash, and possible respiratory tract infections at the time of measurement. Such information is important for those developing institutional protocols for FeNO testing.
Clinicians considering FeNO as an adjunct to diagnose asthma should expect a fair number of false negatives (that is larger with higher test cutoffs) and an even a larger number of false positives (that is larger with lower test cutoff). The prevalence of asthma in the population being tested also impacts the expected positive and negative predictive values. Using several plausible asthma prevalence values in Figures 13 and 14, we simulate the number of false negative and false positive results expected in 1,000 patients tested for asthma using various FeNO test cutoffs. As the FeNO test sensitivity goes up (i.e. lower cutoff) the percentage of false negatives goes down, but the percentage of false positives goes up. Additionally as the prevalence of asthma increases in the screened population, the positive predictive value for confirmed asthma also increases.
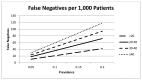
Figure 13
False negatives per 1,000 patients.

Figure 14
False positive per 1,000 patients.
Suggestions for Future Research
Studies with better stratification according to asthma phenotype are needed (eosinophilic/versus non–eosinophilic) to identify populations who may benefit from serial FeNO measurement. Blood eosinophilia and atopy are likely good surrogates for airway eosinophilia and can be used to aid stratification of patients enrolled in studies. The field also needs studies of FeNO-based adherence monitoring programs that specifically evaluate cost effectiveness, acceptability, feasibility, and outcomes of such programs. These studies should also be either group stratified as above, or focus on atopic or eosinophilic patients.
In this review, we demonstrated that FeNO can identify those who will be steroid responsive; therefore, studies of FeNO-based medication titration are needed and should focus on symptomatic patients with previously documented elevated FeNO. Studies evaluating disease activity and outcome, should use validated measures of activity and well defined outcomes.
The role of serial FeNO measurements in children ages 0–5 year who develop illness associated with wheezing remains unclear. Cohort studies of such infants with follow up into later years of childhood and adolescence are needed to establish if persistently elevated levels correlate with increased risk of ultimate asthma diagnosis. This question is of particular importance, because the best biomarker we have at this time to predict asthma in this setting is the presence of eczema, which can be subjective. In addition, some children (regardless of age) often suffer from wheezy bronchitis, also known as wheezing associated respiratory infections. These are discrete illnesses with good prognosis that are quite common in pre-school age. Despite the benign outcome, many of these children still receive oral steroids. Would point of care FeNO measurements identify the children who do not require oral steroids? Such knowledge might address a very common clinical problem and spare children and their parents the adverse effects of steroids.
This review has yeilded a very small body of evidence on geriatric asthma. It will be important to determine the clinical utility of FeNO in a population that was underrepresented in the current literature.
Future research should also address the effect of emerging treatments such as anti-IL13 and anti-IL5 drugs on FeNO levels. Knowledge of such effect may demonstrate a role of FeNO in monitoring the use and adherence to some of these treatments and not others.
A challenge we faced in this review is to define the reference test for asthma diagnosis. Future studies should be explicit in describing the reference standard and use the modern testing approach recommended in current clinical practice guidelines; which may improve accuracy of diagnosis and make evidence more relevant. Similarily, studies should attempt to be consistent with guideline recommendations in definition of variables such as age, FeNO protocols and cutoffs, and asthma control categories, to further enhance applicability. Studies should also investigate factors that may affect FeNO diagnostic accuracy, including asthma phenotype, adequate testing procedures, body mass index (BMI) or weight, manufacturer and device model, and exhalation flow. More research should be done to evaluate diagnostic utility of FeNO testing in picking up asthma in a general population not on any type of treatment.
- Discussion - The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in A...Discussion - The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management
- Glossary and List of Definitions - The Role of Immunotherapy in the Treatment of...Glossary and List of Definitions - The Role of Immunotherapy in the Treatment of Asthma
- Peer Reviewers - Impact of Healthcare Algorithms on Racial and Ethnic Disparitie...Peer Reviewers - Impact of Healthcare Algorithms on Racial and Ethnic Disparities in Health and Healthcare
- Methods - Pain Management Interventions for Hip FractureMethods - Pain Management Interventions for Hip Fracture
- Results - Local Hepatic Therapies for Metastases to the Liver From Unresectable ...Results - Local Hepatic Therapies for Metastases to the Liver From Unresectable Colorectal Cancer
Your browsing activity is empty.
Activity recording is turned off.
See more...