NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Wang Z, Pianosi P, Keogh K, et al. The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017 Dec. (Comparative Effectiveness Reviews, No. 197.)

The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management [Internet].
Show detailsSearch Results
The electronic searches identified 3,884 citations. Additional 61 citations were identified through gray literature search and cross referencing. After title and abstract screening, 955 required full text review and 175 studies met eligibility criteria for inclusion in this review (Figure 2). Studies addressed the key questions as follows:
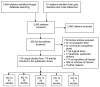
Figure 2
Literature flow diagram.
- 43 studies addressed KQ 1.a about diagnostic accuracy of FeNO measurement.
- 58 studies addressed KQ 1.b about clinical utility of FeNO measurements in monitoring disease activity.
- 24 studies addressed KQ 1.c about clinical utility of FeNO measurements to select medication options, including 14 RCTs, that tested algorithms based on FeNO to guide drug therapy and monitoring.
- 41 studies addressed KQ 1.d about clinical utility of FeNO measurements to monitor response to treatment.
- 9 studies addressed KQ 1.e about the predictive ability of FeNO measures in children less than 5 years of age on the development of asthma in children older than 5 years.
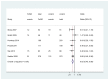
Table 2 summarizes the number of studies included per KQ by study design and age group. A list of the studies excluded at the full-text review stage is in Appendix B. We did not include three studies that were not published in English (one in Spanish, one in Turkish, and one in Japanese). A search of ClinicalTrials.gov identified 93 ongoing studies.
Table 2
Number of studies included per Key Questions, study design, and age group.
Analysis Results
KQ 1.a. What is the diagnostic accuracy of FeNO measurement(s) for making the diagnosis of asthma in individuals ages 5 and older?
Key Points
- The diagnostic accuracy of FeNO for the diagnosis of asthma varies with the FeNO level used for diagnosis. Sensitivity and specificity per cutoff were: <20 ppb (0.79, 0.72), 20–30 ppb (0.64, 0.81), 30–40 ppb (0.53, 0.84), ≥40 ppb (0.41, 0.94). (SOE: Moderate).
- Depending on the FeNO cutoff, the posttest odds of having asthma given a positive FeNO test result increased by 2.80 to 7.00 fold. (SOE: Moderate).
- In steroid-naïve asthmatics, FeNO had the highest accuracy at cutoffs of <20 ppb compared to all patients included in the main analysis (sensitivity 0.79, specificity 0.77 and diagnostic odds ratio (DOR) 12.25).
- Diagnostic accuracy is higher in nonsmokers (compared to smokers) and in children (compared to adults).
Forty-three studies with a total of 13,747 patients were included for analysis. The characteristics of these studies are in Appendix Table C.1. The majority of the studies (33 studies) included only adults >18 years old; 6 studies had children with average age 4–12 years and 4 included patients with average age 13–18 years. 19 studies were nonrandomized longitudinal studies, 23 cross sectional studies, and 1 case-control study. The studies were conducted in the United States (n=2), Canada (n=2), Europe (n=26), and other countries (n=13).
FeNO was measured online in 10 studies, offline in 3, and 1 used both methods. In terms of reference test used to compare with FeNO, 12 studies used clinical diagnosis, 13 used positive bronchial challenge test, and 20 combined tests (clinical diagnosis, positive bronchial challenge, and/or bronchodilator response). The majority of the studies had low or medium risk of bias. High risk of bias was noted primarily in the areas of cohort selection, including representativeness of the study population (whether patients were consecutive and represented the total eligible patients in a particular institution) and whether studies enrolled patients with diagnostic uncertainty (i.e., with symptoms suggestive of asthma). The details of risk of bias assessment are presented in Appendix Table G.1 and summarized in Figure 3. The overall risk of bias was low in 47% of the studies. Since the risk of bias was unclear or high in about half of the studies, the SOE was rated down to moderate.
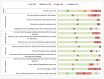
Figure 3
Risk of bias assessment for diagnostic accuracy studies using QUADAS-2 (n= 43, KQ 1.a).
Using Deeks’ funnel plot asymmetry tests and visual inspection of funnel plots, we found potential publication bias for cutoffs<20, and no indication of publication bias for cutoffs 20–30 (Appendix Figures D.10–11). We were not able to evaluate potential publication bias for other cutoffs. Overall there was no strong evidence of publication bias.
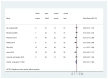
For cutoffs of <20, 20–30, 30–40, and ≥40 parts per billion (ppb); respectively, FeNO testing has sensitivities of 0.79, 0.64, 0.53, and 0.41; and specificities of 0.72, 0.81, 0.84, and 0.94. Overall DORs ranged from approximately 5.85 to 16.95 (Appendix Figure D.1–4). The strength of evidence assessment is summarized in Table 3. Detailed assessment of SOE is available in Appendix Table H.1.
Table 3
Strength of evidence (SOE) for KQ 1.a.
Subgroup and Sensitivity Analyses
Data on the diagnostic accuracy of FeNO for asthma were insufficient to assess the impact of several factors as planned in the protocol. The feasible subgroup analyses had been based on FeNO cutoffs, the type of reference test (clinical diagnosis, positive bronchial challenge, and a combined test (clinical diagnosis, positive bronchial challenge, and/or bronchodilator response), risk of bias, tobacco use, age group (age<=18 years vs. age >18 years), and whether the control group consisted of healthy controls (vs. symptomatic individuals without a diagnosis of asthma). The findings of the subgroup analyses were summarized as follows:
- Analysis of the impact of the FeNO levels used for diagnosis of asthma showed that cutoff levels affect sensitivity and specificity, with increasing specificity and decreasing sensitivity as cutoffs increased above 20 ppb (Table 3). Cutoffs of ≥ 40 ppb had the highest accuracy but were not as sensitive.
- Assessment of the impact of the reference test (Table 3) showed that the reference test may partially explain heterogeneity in the diagnostic accuracy of FeNO (comparative data were available mostly for cutoffs < 20 ppb).
- Control group characteristics impacted the diagnostic accuracy of FeNO; the diagnostic accuracy of FeNO may be overestimated in studies that used healthy controls compared to symptomatic controls (for cutoffs <20 ppb, DOR was 16.45 for healthy controls compared to 4.42 for symptomatic controls) (Appendix Table E.1).
- Subgroup analysis based on the risk of bias showed that the risk of bias may partially explain heterogeneity in the diagnostic accuracy of FeNO with greater reported diagnostic accuracy as the risk of bias increases (DORs across cutoffs of 10.97, 8.15 and 7.29 for high, medium and low risk; respectively) (Appendix Table E.2).
- Subgroup analysis based on tobacco use showed that the diagnostic accuracy was markedly higher in studies of nonsmokers comparing to smokers. (Appendix Table E.3).
- Subgroup analysis based on age showed that diagnostic accuracy was overall higher in children (age <= 18 years) than adults (age > 18 years) (Appendix Table E.4).
In a sensitivity analysis, we were only able to analyze studies that evaluated the diagnostic accuracy of FeNO in steroid-naïve asthmatics (the remaining studies had a mix of population, steroid naïve, and steroid users). At cutoffs of <20 ppb, FeNO had the highest accuracy in this group of patients compared to patients in the main results (sensitivity 0.79, specificity 0.77 and DOR 12.25). Results in other cutoffs were different and inconsistent. In another sensitivity analysis, we analyzed only studies that evaluated the diagnostic accuracy of FeNO in asthmatic patients with atopy. The results, which included a small number of studies (n=4), showed accuracy measures that were similar to those from the main analysis (sensitivity 0.63; specificity 0.79; DOR 6.67) (Appendix Table F.1).
KQ 1.b. What is the clinical utility of FeNO measurements in monitoring disease activity and asthma outcomes in individuals with asthma ages 5 and older?
Key Points
- In adults (ages >18) and children (ages 5 –18), FeNO levels are weakly associated with asthma control (as measured by the ACQ and ACT). This associateion can be further attenuated in those who smoke, pregnant or are on ICS. (SOE: Low)
- In adults (ages >18) and children (ages 5 –18), FeNO levels have a weak association with the risk of subsequent and prior exacerbations. (SOE: Low) The association between FeNO levels and exacerbation risk is likely increased in individuals (ages>5 years) with atopy. (SOE: Low)
- In adults (ages >18) and children (ages 5 –18)with acute asthma exacerbations, FeNO levels do not correlate with exacerbation severity and were poorly reproducible. (SOE: Low)
- In children (ages 5 – 12) and adolescents (ages 13 – 18), FeNO levels were inversely associated with adherence to asthma medications (mainly ICS). (SOE: Low)
58 studies with a total of 8,999patients were included in KQ 1.b. The characteristics of these studies are in Appendix Table C.2 and C.3. 30 studies included only adults >18 years old; 24 studies had children with average age of 5–12 years and 4 included patients with average age of 13–18 years. 34 studies were nonrandomized longitudinal studies, 7 RCTs, and 17 cross sectional studies. The studies were conducted in the United States (n=9), in Canada (n=1), in Europe (n=33), and in other countries (n=15).
FeNO was measured online in 20 studies, offline in 3, and 1 used both methods. Heterogeneity in study populations, designs, and outcome types precluded meta-analysis; therefore, we presented these data in narrative form only. The detailed risk of bias assessment is presented in Appendix Table G.2 and Table G.3 and summarized in Figures 4 and 5 for randomized controlled trials and observational studies; respectively. The risk of bias was low or medium overall in most of the RCTs and observational studies.

Figure 4
Risk of bias assessment of randomized controlled trials using the Cochrane Risk of Bias tool (n=7, KQ 1.b).
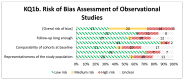
Figure 5
Risk of bias assessment of observational studies using the New-Castle Ottawa Scale (n=51, KQ 1.b).
Using FeNO To Monitor Asthma Control and Predict Exacerbations
Adults (ages >18 years)
Five studies assessed the correlation between FeNO measurements and ACQ scores, a measure of asthma control. Overall, the correlation was weak, and FeNO did not reliably differentiate patients who were well-controlled versus borderline controlled versus not well-controlled.73–77 In a cross sectional study, a single measurement of FeNO had lower area under the curve (AUC) (0.59) for identifying uncontrolled asthmatics (defined using ACQ-7) than sputum eosinophils (0.72) or methacholine responsiveness (0.72)73. In a prospective study, adults with not well controlled persistent asthma and a positive bronchodilator test had maintenance treatment adjusted at the beginning of the study and were reevaluated after 4 weeks using ACQ-7 versus ACQ-7+ FeNO. The combination of FeNO and ACQ-7 demosntrated 14.8% higher proportion of patients with not well controlled asthma.77
An inverse correlation between ACT scores and FeNO was noted across numerous studies with various ACT and FeNO cutoffs.78–86 The correlation (r) between FeNO and ACT in patients on ICS for 3 months was −0.31 in one study.85 In another study, mean FeNO values were significantly higher in patients with an ACT score <20 compared to those patients with an ACT score ≥20 (65.5 vs 27.4 ppb, p<0.001).78 FeNO level of >47 ppb was used to indicate inflammation and uncontrolled asthma. The best pair of sensitivity and specificity and AUC were observed at ACT cutoff of 19 (0.91, 0.81 and 0.91; respectively) whereas at ACT cutoff of 20 the sensitivity was 95.2, and the specificity was 68.8.78 In a study of steroid naïve nonsmoking asthmatics, FeNO level strongly correlated with ACT at baseline and after 6–8 weeks of ICS treatment (r= −0.74 and −0.68; respectively).87
In a study of patients with established stable asthma without recent exacerbations, FeNO had AUC of 0.79 for the identification of not well-controlled asthma (determined by ACT following GINA cutoffs).79 AUC was, however, lower in those who smoked (smokers on ICS with FeNO cutoff of > 23 ppb had AUC of 0.60; and smokers not on ICS with FeNO cutoff of > 19 pbb had AUC of 0.68).79 FeNO values >30 ppb were associated with positive predictive values > 0.85, indicating a status of not well-controlled asthma (except in smokers).79 In a study with older population (ages>65 years), FeNO values were statistically significantly higher in those with uncontrolled asthma than those with controlled/partly controlled (regardless of whether asthma control was determined using GINA control criteria or using ACT with a cutoff of 19).80
The association between asthma control and FeNO was weakened in patients on ICS as observed in four studies.79, 81–83 In addition, pregnant women who had monthly FeNO measurements showed a weak correlation between FeNO and ACT and wide variation in FeNO values. Results were the same in atopic and non atopic women. FeNO levels did not significantly differ in women before and after they lost asthma control.84 In a prospective study that followed patients who were mostly on ICS (age 10 and over) for 12 weeks, FeNO did not correlate with ACQ or with shortened ACQ (without FEV1).86
In terms of the use of FeNO to predict asthma exacerbations, several studies showed higher FeNO values in patients who had had exacerbations prior to the test (retrospective analysis) or had developed exacerbations after the test (prospective analysis).88–90 However; in one study of 267 adult asthmatics recruited from primary care clinics, FeNO values measured 12 months before and 3 months after exacerbations were significantly lower in frequently exacerbating patients receiving higher doses of maintenance ICS (compared to patients with mild disease who were corticosteroid naïve).88 In that study, measurement of FeNO was an insensitive method for identifying patients who subsequently exacerbated (sensitivity, 66.7%; specificity, 51.9% at a cutoff value of 20 ppb) suggesting that intensive ICS treatment can confound the clinical utility of FeNO.88 In another study, baseline FeNO values did not predict urgent care visits or exacerbations over the subsequent 6 months.76 In asthmatic patients on ICS, FeNO >40 ppb yielded 0.75 sensitivity and 0.90 specificity for identifying subjects with high variability in peak expiratory flow (which may suggest increased variation in airway caliber among patients with stable asthma).89 In atopic 12 to 56-year-old persistent asthmatic patients on ICS, higher FeNO levels were significantly correlated with more short-acting beta agonists dispensing and oral steroids courses in the past year, and lower FEV1 percent predicted levels.83 In another small study, 22 adults with moderate and severe persistent asthma who had an exacerbation in the previous 2 weeks had a higher mean FeNO value compared to those who did not (29.7 ppb vs. 12.9 ppb). 90 In a multivariable regression, FeNo was the only significant predictor of exacerbations (whereas patients’ assessment of their own disease, peak flow, ICS dose, and FEV1 were not).90
Summary
In adults with asthma, numerous observational studies showed that FeNO levels have weak associations both with asthma control (as measured by ACQ and ACT) and that FeNO can modestly predict exacerbations. The magnitude of association between FeNO and control tests is likely reduced in patients on ICS, smoke, or pregnant. The overall strength of this evidence is low because of the observational nature of the majority of evidence.
Children (ages 5 to 18)
Thirty studies evaluated the association of FeNO levels with asthma control. The definition of asthma control, however, varied among studies although commonly depended on history, clinical symptoms, and lung function. Asthmatic children (n=133, aged 5 to 14 years) who had recent symptoms (within the preceeding month of the test) compared to those without recent symptoms had higher FeNO levels (14.6 ppb vs. 6.0 ppb, p=0.004). FeNO levels also differed significantly between the controlled and uncontrolled subgroups (8.5 ppb vs. 26.4 ppb, p-0.03).91 Another cross sectional study recruited children with stable asthma (majority were on ICS, majority were allergic defined by a radio-allergosorbent test class 2 or higher or a positive skin test).92 Children with insufficient, acceptable, or good control of asthma had FeNO levels of 28 ppb, 15 ppb, 11ppb; respectively (p<0.01).93 Conversely in another study, children with allergic rhinitis and stable non severe asthma, FeNO was elevated but did not correlate with nasal or asthma symptoms.92 A prospective study also showed that FeNO values did not correlate with current disease severity in children (determined using history, clinical symptoms, and lung function). Values above normal (defined in this study as > 13 ppb) had a sensitivity of 0.67 and a specificity of 0.65 to predict a step up in therapy by providers.94 In another study, FeNO at a cutoff point of 22.9 ppb had moderate accuracy (sensitivity of 80% and specificity of 60%) to predict exacerbations in children with mild to moderate asthma who were managed using symptoms, b-agonist use, lung function, and FeNO (measured during 5 visits in 6 weeks intervals).95 In a prospective study of patients with atopic asthma (mean age 12.6, range 7–20), FeNO of 31 ppb provided optimal sensitivity (92.3%) and specificity (75.4%) to predict subsequent exacerbations.96
In a cross sectional study of children with asthma (mostly mild persistent), FeNO levels differentiated controlled, partly controlled, and uncontrolled in those not on ICS (but the trend was not statistically significant in patients on ICS).97 In another study in children on ICS, FeNO measured every 2 months did not predict exacerbations even when combined with inflammatory markers and clinical characteristics.98 In high risk children (minorities in urban areas with persistent asthma and atopy) on controller medication, FeNO measurement every 3 months was not a significant predictor of acute visits, emergency department visits, unscheduled doctor visits, or hospitalization in adjusted analysis.99 Four other studies also suggested no or weak association of FeNO and ACT in ICS users.100–103
In children with atopic asthma, FeNO was significantly elevated in those with exercise induced reduction of FEV1 (> 15%) with a negative predictive value (NPV) of 100% and a positive predictive value (PPV) of 28%. NPV and PPV for reported asthma symptoms within 2 weeks preceding the study were 96% and 26%. Thus, FeNO had good utility to exclude exercise-induced bronchoconstriction in atopic children.104 In another study in which 33 percent of the asthmatic children age 4–7 had atopic dermatitis, FeNO values correlated with asthma severity, atopic dermatitis and steroids use; and marginally with allergic rhinitis (p=0.06).105 And in a third study in patients aged 8–16 years with atopic asthma not receiving daily controller therapy and monitored bi-monthly over 2 years, loss of asthma control was predicted by the highest FeNO value of serial measurements and the percentage of sampling time points when FeNO > 21 ppb.106 Lastly, one RCT enrolled 280 children with atopic asthma and compared three management approaches: web-based monthly monitoring of ACT, versus FeNO and ACT every 4 months, versus standard care. There was no difference in terms of ACT or asthma free days. Lower ICS use was noted in the web based approach. Quality-adjusted life years (QALYs) and costs were not statistically significantly different.107, 108
Summary
In children with asthma, evidence from numerous studies suggests that FeNO levels have weak association with ACT, and risk of exacerbation. There is some evidence to suggest that the association may be attenuated in patients on ICS but increased in those with atopy. The overall strength of this evidence is low because of the observational nature of the majority of evidence.
Utility of FeNO Testing in the Acute Setting (during exacerbations)
In children with acute exacerbation of asthma, FeNO during exacerbation was not higher than median values during followup (mean followup: 434 days) but was significantly higher than personal best. FeNO during acute exacerbation did not correlate with the severity of acute exacerbation (measured using the Pulmonary Score) and could not diagnose or predict exacerbation.109
In adults seen in the ED, an increase in FeNO was observed in almost all patients with acute asthma. However; FeNO and its initial variation, within 2 hours, were not related to the severity of the attack (measured at presentation using a French instrument developed by Salmeron et al110) or the effectiveness of bronchodilator treatment.111 In a study of patients age 2–18 years seen in an urban ED for acute asthma exacerbation, measurement of FeNO was difficult for a large proportion of children and did not correlate with other measures of acute severity.112 Similar results were shown in a fourth study that combined adults and children presenting to ED.113 In this study, There was no association between FeNO values at presentation and NIH class of asthma severity, the risk of hospitalization, or relapse. Triplicate measurements of FeNO had a poor coefficient of variation suggesting poor reproducibility (12%, interquartile range: 5–15%).113
Summary
The strength of evidence supporting the utility of FeNO testing in adults and children presenting to the ED or during acute exacerbations is low. FeNO results did not correlate well with asthma severity or symptoms.
Using FeNO to Monitor Adherence to Therapy
3 studies explicitly described using FeNO to ascertain adherence to asthma medications (mainly ICS). In one RCT, FeNO concentrations in adolescents with adherence of more than 50 percent of assigned doses of mostly ICS (measured using a built-in dose counter and a structured questionnaire) was 24 ppb compared to 31ppb in those with <50 percent adherence.114 A second study in children demonstrated that FeNO values were associated with adherence to inhaled budesonide (r2 =0.59) as assessed using dose counters115. A third study also in children showed that high FeNO level (>25 ppb) was associated with lower adherence rates to any asthma medication using the parental reported Medication Adherence Report Scale (OR: 0.4; 95% CI: 0.3–0.6).100
Summary
The strength of evidence supporting the association between FeNO values and medication adherence (mainly ICS) is low. Evidence supporting a FeNO-based adherence monitoring program are unavailable (in terms of cost effectiveness, acceptability, feasibility and outcomes, of such program). The strength of evidence assessment is summarized in Table 4. Detailed assessment of SOE is available in Appendix Table H.2.
Table 4
Strength of evidence (SOE) for KQ 1.b.
KQ 1.c. What is the clinical utility of FeNO measurements to select medication options (including steroids) for individuals ages 5 and older?
Key Points
- In adults (ages of >18 years) and children (ages of 5–18 years), using asthma management algorithms that incorporate FeNO testing reduced the risk of exacerbations (SOE: High), and possibly the risk of exacerbations requiring oral steroids (SOE: Moderate), but did not affect other outcomes such as hospitalization, quality of life, asthma control, or FEV1% predicted.
- Management algorithms that incorporate FeNO testing may be associated with a modest reduction in medical expenses, compared to management approaches that do not include FeNO testing.
- FeNO testing can identify patients who are more likely to respond to inhaled corticosteroids (SOE: Low).
24 studies with a total of 2,820 patients were included in KQ 1.c. The characteristics of these studies are in Appendix Tables C.4–6. The majority of the studies (15 studies) included only adults >18 years old; 8 studies had children with average age of 5–12 years and 1 included patients with average age of 13–18 years. 8 studies were nonrandomized longitudinal studies, 14 RCTs, and 2 cross sectional studies. The studies were conducted in the United States (n=3), in Europe (n=16), and in other countries (n=5). FeNO was measured online in 14 studies.
The detailed risk of bias assessment is presented in Appendix Tables G.4 and G.5 and summarized in Figures 6 and 7 for RCTs and observational studies; respectively. The overall risk of bias was low in 36% of the RCTs and 50% of the observational studies.
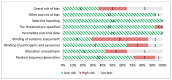
Figure 6
Risk of bias assessment of randomized controlled trials using the Cochrane Risk of Bias tool (n=14, KQ 1.c).
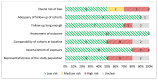
Figure 7
Risk of bias assessment of observational studies using the New-Castle Ottawa Scale (n=10, KQ 1.c).
Using FeNO to Guide Asthma Medication Selection, Monitoring and Management
Randomized Controlled Trials
14 RCTs evaluated various strategies in which FeNO was used to monitor disease activity and to change therapy (stepping up therapy vs. stepping down therapy). These trials aimed to evaluate the incremental value of adding an algorithm in which FeNO was maintained below a certain level (variable across studies) compared to standard monitoring that included spirometry and clinical parameters (which was the control intervention that varied across studies).
Trials were conducted in adults114, 127–133 (FeNO cutoffs between 15 and 35 ppb, followup 4 to 12 months), children95, 108, 134–138 (FeNO cutoffs between 20 and 30 ppb, or between 10 and 15 ppb with symptoms, followup 6–12 months), and in pregnant women139.
In adults (ages of >18 years) and children (ages of 5 to 18 years), FeNO based strategies were associated with reduction in the risk of exacerbations (Figures 8 and 9). Other outcomes did not differ signficantly in children or adults, including hospitalization from asthma, exacerbations requiring oral steroids, FEV1 % predicted, ACT, or quality of life questionnaires (Appendix Figures D.5–9). For the outcome of exacerbations requiring oral steroids, exploratory analysis that combines data from adults and children, demonstrated that the reduction was statistically significant (I2=0%), suggesting that this analysis in each subgroup analysis (adults or children) was underpowered because of small sample sizes. The strength of evidence is summarized in Table 5. The number of patients needed to treat using FeNO-based algorithms to prevent one person with exacerbation is 9 (for both, adults and children).
Table 5
Strength of evidence (SOE) for KQ 1.c.
FeNO-based algorithms varied across trials in terms of FeNO cutoffs for changing therapy and frequency of testing; the details of these algorithms are described in Appendix Table I.2. Data were insufficient to determine whether a certain approach was the most effective; however, analyses consistently suggested that the effect might be similar across these algorithms. There was no statistically significant difference on any outcome between studies at increased risk of bias and studies at decreased risk of bias. We did not identify any studies that reported on adverse effects of FeNO testing per se, or of the strategy that used FeNO testing.
Other Randomized Trials Not Included in Meta-Analysis
Three trials were not included in meta-analysis because of being a cluster trial128, focusing on oral corticosteroid tapering strategies130 and for evaluating a combination of FeNO and sputum eosinophils to guide management129.
Honkoop et al. allocated 611 adults with asthma from primary care clinics to three treatment strategies: (1) aiming at ACQ score <1.50; (2) ACQ score <0.75; and (3) aiming at ACQ score <0.75 and FeNO value <25 ppb. During the 12-month followup, treatment was adjusted every 3 months by using an online decision support tool. The strategy that included FeNO improved asthma control compared with the ACQ <1.50 strategy (P < 0.02). There were no differences in quality of life.128
Hashimoto et al. enrolled 95 adults (ages of 18–75 years) with prednisone-dependent asthma and compared two tapering strategies over 6 months: internet-based monitoring system (home monitoring of symptoms, lung function, and FeNO weekly titrated below 10 ppb) versus conventional treatment based on GINA guidelines (conventional strategy, no FeNO testing). Changes in prednisone dose from baseline averaged −4.79 mg/day versus +1.59 mg/day, in the internet strategy group compared with the conventional treatment group, respectively (p < 0.001). Asthma control, asthma-related quality of life, FEV1, exacerbations, hospitalizations, and satisfaction with the strategy were not statistically different between groups.130
Malerba et al. enrolled 28 adults with asthma (mean age of 46 years) and compared treatment based on the combination of FeNO and sputum eosinophils to treatment based on clinical score. At 24 months, exacerbation rate and mean symptom scores were lower in the intervention than in the control group.129
Observational Studies
Observational studies also evaluated the effect of using FeNO to guide therapy. In adults, two studies showed that titration of ICS based on FeNO and sputum eosinophils in those with mild-to-moderate persistent asthma (compared with conventional management) was associated with reduction in symptom scores and ICS dosage, and fewer exacerbations.140, 141 One study in children showed that FeNO values above 13 ppb weakly correlated with the changes in asthma therapy and had a modest sensitivity of 0.67 and a specificity of 0.65 to predict a step up in therapy.94 In a mixed age population, treatment decisions made in an office visit based on a single FeNO test in 50 asthmatic patients led to change in therapy in a small proportion of patients (augmentation in 20% and reduction in 16%).142 These studies were overall at moderate to high risk of bias.
Cost and Utilization Data
Only a few studies addressed cost-effectiveness and economic evaluation of FeNO-based treatment strategies. Honkoop et al., in a cluster RCT, showed that medication costs over a year was lower for a treatment strategy that kept ACQ score <1.50, followed by keeping ACQ score <0.75 and FeNO value <25 ppb, followed by keeping ACQ score <0.75 ($452, $456, $551; P ≤ 0.04).128
Beerthuizen et al. assessed the cost-effectiveness of web-based monthly monitoring and of 4-monthly monitoring of FeNO compared with standard care (followup evaluation of RCT in 272 children with asthma, aged 4–18 years, followed for 1 year). No statistically significant differences were found in QALYs and costs between the three strategies. The web-based strategy had 77 percent chance of being most cost-effective from a health care perspective at a willingness to pay a generally accepted €40 000/QALY. The FeNO-based strategy had 83 percent chance of being most cost-effective at €40 000/QALY from a societal perspective.107
Berg et al. evaluated cost effectiveness from a German payer perspective comparing FeNO based approaches for diagnosis and management to standard guidelines in a mixed-age population with asthma. Asthma diagnosis based on FeNO measurement resulted in a cost of €38 per patient comparing to €26 for standard diagnostics. In patients with mild to severe asthma, asthma management with FeNO measurement instead of standard guidelines results in cost-savings of €30 per patient year (up to savings of €160 in a more severe population).143
In a mixed-age population, treatment decisions made in a single office visit based on a single FeNO test were estimated to reduce cost by $629 per patient per year. 142 Lastly, a cost-effectiveness analysis model evaluated adding FeNO monitoring to asthma management over a 1-year period. The results showed that adding FeNO to standard asthma care saved €62.53 per patient-year in the adult population and improved quality-adjusted life years by 0.026 per patient-year. The budget impact analysis revealed a potential net yearly saving of €129 million if FeNO monitoring had been applied across primary care settings in Spain.144
Using FeNO To Aid in Drug Type Selection
Several studies used FeNO to determine whether patients would respond to ICS. In adults, FeNO > 47 ppb predicted a positive response to ICS (defined as change in symptoms, peak flows, spirometry, or airway hyperresponsiveness to adenosine based on established guidelines and recommendations) in patients with undiagnosed respiratory symptoms.145 In another study, FeNO reliably predicted those who responded to ICS (AUC 0.89 and 0.86 at 4 and 12 weeks; respectively); FeNO levels <27ppb predicted non-response in adults with undifferentiated chronic respiratory symptoms.146 In steroid-naive adults with asthma, FeNO predicted clinical responsiveness to ICS but the combination of FeNO values and urinary bromotyrosine levels had the best prediction power.147 In children, FeNO identified ICS dependent asthma phenotype148 but this study used complex orthogonal varimax rotation to phenotype patients rather than more traditional classification. FeNO >20 ppb predicted exacerbations in another study in children with mild asthma on low-dose ICS who were switched to montelukast.149 SOE summary is available in Table 5. Detailed assessment of SOE is available in Appendix Table H.3.
KQ 1.d. What is the clinical utility of FeNO measurements to monitor response to treatment in individuals ages 5 and older?
Key Points
- FeNO levels are reduced when patients with asthma take inhaled corticosteroids, leukotriene receptor antagonists or omalizumab.
- FeNO levels are not reduced when patients with asthma take long acting beta agonists.
- FeNO predicts exacerbations in patients undergoing ICS reduction or withdrawal, but FeNO alone is likely insufficient and its ability to predict exacerbations can be substantially enhanced by clinical measures (e.g. ACT).
41 studies with a total of 1,728 patients were included in KQ 1.d. The characteristics of these studies are in Appendix Table C.7–c.11. The majority of the studies (23 studies) included only adults aged >18 years; 16 studies had children with the average age of 5–12 years and 2 included patients with the average age of 13–18 years. 16 studies were nonrandomized longitudinal studies, 20 RCTs, and 5 cross sectional studies. The studies were conducted in the United States (n=6), in Canada (n=3), in Europe (n=16), and in other countries (n=16). FeNO was measured online in 17 studies and offline in 1 study. The details of the risk of bias assessment is presented in Appendix Tables G.6 and G.7 and summarized in Figures 10 and 11 for RCTs and observational studies respectively. The risk of bias was overall low in 35% of RCTs and 32% in observational studies.
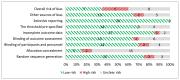
Figure 10
Risk of bias assessment of randomized controlled trials using the Cochrane Risk of Bias tool (n=20, KQ 1.d).
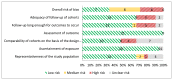
Figure 11
Risk of bias assessment of observational studies using the New-Castle Ottawa Scale (n=21, KQ 1.d).
Of the 41 included studies, 31 studies reported a change in FeNO levels after administration of an asthma drug. These 33 studies provided evidence only regarding which drugs could affect FeNO level (and thus may be theoretically monitored using FeNO). These studies had a different objective than evaluating the effectiveness of using FeNO for monitoring response to therapy. They did not test an established monitoring program that could provide evidence regarding patient important outcomes. Such evidence about the effectiveness of monitoring is better derived from the randomized trials described in KQ 1.c that evaluated FeNO-based algorithms for medication management. Eight other studies used FeNO to monitor the response to ICS when those medications were tapered or discontinued.
Studies Documenting a Change in FeNO Associated With Certain Medications
Corticosteroids
Twenty-two studies demonstrated that FeNO levels declined after the administration of ICS. Response was seen after 4 to 8 weeks of treatment, though one study150 showed reduction after 10 days without further reduction observed at 40 days. The decline in FeNO was dose-dependent and observed in both adults and children; in one study, it varied according to ICS type beyond the dose equivalents.151 FeNO correlated with airway hyperresponsiveness in steroid-naïve mild asthmatics but not in steroid using asthmatics.152 In a study of children with atopic persistent asthma, FeNO decreased significantly after 12 weeks of using either 80 or 160 mcg of inhaled ciclesonide (no difference between the two doses).153 FeNO values decreased significantly after 5 days of oral prednisone given for acute exacerbation of asthma.154
Leukotriene Receptor Antagonists
Six studies showed that leukotriene receptor antagonists (LTRA) also reduced FeNO in adults (ages >18 years) and children (ages between 5 and 18 years). Montelukast reduced FeNO in adults with mild asthma in an RCT as early as day 1 with a maximum effect on reduction noted for day 7.155 Pranlukast added to ICS plus inhaled long acting beta agonist (LABA) also reduced FeNO.156 Montelukast reduced FeNO concentrations in children with asthma, and withdrawal of this medication increased FeNO values and was associated with worsening lung function and clinical deterioration in 4 of 14 children.157 Withdrawal of montelukast led to rising FeNO in another study.158
Omalizumab
Omalizumab reduced exacerbations, and symptoms, and FeNO levels in both adults159 and in children with asthma.160
Bronchodilators
Concerns regarding potential masking of inflammation by long acting beta-agonists were examined in 4 studies. Regular use of salmeterol did not increase FeNO levels in adults or children with asthma, regardless of whether they were taking ICS or not.161–164 In a fifth study, adults (mean age 57) with symptomatic asthma on ICS and LABA were randomized to tiotropium vs continued same management. There was no difference in feNO between the two groups.165
Studies Reporting on FeNO use for ICS Reduction or Withdrawal
Eight studies described monitoring FeNO in patients undergoing ICS reduction or withdrawal (6 in adults and 2 in children).
In adults with asthma on high dose ICS that was reduced by 50 percent, FeNO values at baseline >15 ppb predicted reduction failure.166 Both single measurements and changes of FeNO (10 ppb, 15 ppb, or an increase of > 60% over baseline) had positive predictive values that ranged from 80 to 90 percent for predicting and diagnosing loss of asthma control after ICS withdrawal.167 In adult patients with moderate or severe asthma but no clinical symptoms of asthma for at least 6 months in whom ICS dose was reduced by half, FeNO was a statistically independent predictor of success.168
However, the response of FeNO in adults with moderate persistent asthma undergoing withdrawal of ICS was heterogeneous.169 In one RCT, adults with newly diagnosed asthma received budesonide/formoterol for 8 weeks and were then randomized to continue or step-down group. In both groups, pulmonary function indicators and symptoms did not change. FeNO level decreased significantly in the dosage-continued group from 50.9 ppb to 45.0 ppb, and increased significantly in the step-down group from 51.0 ppb to 65.7 ppb.170 Therefore, FeNO alone is likely insufficient to guide ICS withdrawal. In another study, adults with moderate asthma treated with either budesonide 400 μg plus salmeterol 100 μg or salmeterol/fluticasone 250 at 2 puffs, step down from medium to low dose was safely performed using a combined FeNO and ACT approach at 8 week intervals. 171
Similarly, inconsistency is noted in studies in children. One study showed that FeNO measurements 2 and 4 weeks after discontinuation of ICS predicted those who relapsed (value of 49 ppb at 4 weeks after discontinuation had the best sensitivity (71%) and specificity (93%).172 Conversely, another study showed that in children with moderate-to-severe asthma undergoing ICS reduction, FeNO measured biweekly and expressed either as a continuous variable or dichotomized, was not associated with future risk for exacerbations.173 However, despite ICS dose held constant and all 32 children remaining in good control during the 2 month run-in period (before tapering ICS dose began), FeNO at start of dose reduction still averaged 38 ppb.
In conclusion, FeNO predicts exacerbation after ICS withdrawal or reduction, but its response is heterogeneous and its prediction can be substantially enhanced by clinical measures such as ACT. The SOE supporting the utility of FeNO in predicting exacerbations is low due to the observational nature of the studies.
KQ 1.e. In children ages 0–4 years with recurrent wheezing, how accurate is FeNO testing in predicting the future development of asthma at age 5 and above?
Key Points
- It is unclear whether FeNO testing in children at ages 0–4 years with symptoms suggestive of asthma can predict a future asthma diagnosis (SOE: insufficient).
- The results of FeNO testing in children at ages 0–4 years correlate well with the Asthma Predictive Index and wheezing (SOE: Low).
- FeNO levels are higher in patients with current or persistent wheezing (compared to those with no or transient wheezing; respectively). This association is also observed in infants with atopy or eczema.
Nine studies with a total of 1,735 patients were included in KQ I.e. The characteristics of these studies are in Appendix Table C.12. All studies included children less than 5 years old. 6 studies were nonrandomized longitudinal studies, and 3 cross sectional studies. The studies were conducted in the United States (n=2), in Europe (n=6), and in other countries (n=1).
FeNO was measured online in 5 studies and offline in 2 studies. The details of risk of bias assessment are provided in Appendix Table G.8 and summarized in Figure 12. The risk of bias was overall low in 67% of the observational studies. We also identified 7 additional studies that evaluated the correlation between FeNO measured in early childhood and current wheezing. These studies were excluded from the systematic review because they do not directly answer KQ 1.e; they are however summarized in Appendix Table I.1.
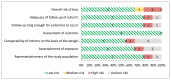
Figure 12
Risk of bias assessment of observational studies using the New-Castle Ottawa Scale (n=9, KQ 1.e).
We identified four studies in which FeNO was measured in early childhood and an outcome of asthma was subsequently diagnosed (after the age of 5). Two of the studies showed that higher FeNO predicted a diagnosis of asthma (one of them was specifically performed in infants with eczema).15, 174 A third study showed contradictory results and a non-significant association with asthma diagnosis.175 The fourth study is an ongoing prospective cohort that has reported only preliminary findings not relevant to this question; final results will be relevant because the study will attempt to develop a prediction rule based on data from demographics, history, specific IgE, FeNO and peak expiratory flow.176 Another study was only published as an abstract. In a population-based birth cohort, FeNO was measured in 234 healthy term infants aged 5 weeks during quiet tidal breathing in unsedated sleep. At the follow-up with 6 years, FeNO at infancy was not associated with asthma, atopy or positive skin prick test at the age of 6 years. Associations were not modified by sex, parental atopy, parental asthma or smoking during pregnancy. 177, 178
The four published studies overall had no major methodological limitations. This body of evidence was small (592 children in all), observational, and inconsistent; therefore, the strength of evidence supporting the outcome of asthma development is insufficient at the present time.
Five other studies examined the correlation between FeNO measured in early childhood and the Asthma Predictive Index (API).179–183 Except for one study,182 all showed good correlation between FeNO and API. In one study, FeNO was superior to API in predicting future exacerbations and persistence of wheezing at age 3 years. 180
Lastly, seven studies evaluated the correlation between FeNO measured in early childhood and current wheezing.16, 184–189 These studies were excluded from the systematic review, because they do not directly answer KQ 1.e; however, they showed that young children with wheezing had higher FeNO levels than non-wheezing children; particularly in those children with eczema, airway hyperresponsiveness, atopy, family history of atopy, and mothers who smoke.
Across these studies, the differences in FeNO values were small. It remains unclear whether FeNO values obtained in infants correlate with the FeNO levels measured with a standardized method at school age190. Therefore, though FeNO appears to reflect eosinophilic bronchial inflammation early in life, the current evidence is insufficient to state that FeNO performed in children at 0 to 4 years of age predicts a diagnosis of asthma at age 5 and above. However; future studies (one is ongoing176) may demonstrate otherwise. The strength of evidence assessment is summarized in Table 6. Detailed assessment of SOE is available in Appendix Table H.4.
Table 6
Strength of evidence (SOE) for KQ 1.e.
- Results - The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asth...Results - The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management
- Acknowledgments - Total Worker Health®Acknowledgments - Total Worker Health®
- Technical Expert Panel - Management of Colonic DiverticulitisTechnical Expert Panel - Management of Colonic Diverticulitis
- Acknowledgements - The Role of Immunotherapy in the Treatment of AsthmaAcknowledgements - The Role of Immunotherapy in the Treatment of Asthma
- Preface - The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asth...Preface - The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management
Your browsing activity is empty.
Activity recording is turned off.
See more...