NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Butler M, Urosevic S, Desai P, et al. Treatment for Bipolar Disorder in Adults: A Systematic Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Aug. (Comparative Effectiveness Review, No. 208.)
Section 1. Aripiprazole for Acute Mania
Appendix Table E1Characteristics of eligible studies: aripiprazole for acute mania
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP-I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| Kanba, 20141 RCT Multisite Asia Industry RoB High 22540407 | N = 258 Mean Age 38 Female 59% Japanese 32% Korean/Chinese 43% Other 25% BP-I 100% Inpatient | Manic/Mixed episode; YMRS ≥ 20; Current episode <4 weeks First Manic Episode Schizoaffective Neurological Disorders Other Mental Health Substance Abuse Pregnant/Nursing | Aripiprazole 24 mg/day (22.9 mg/day) | Placebo | 3 weeks | YMRS CGI-BP-S Response Adverse Events Withdrawal 47% |
| Young, 20092 RCT Multisite All Continents Industry RoB Moderate 19118324 | N = 332 Mean Age 41 Female 57% White 78% BP-I 100% Inpatient (weeks 1–2) Outpatient (weeks 3–12) | Manic/Mixed in acute relapse; YMRS ≥ 20 and MADRS ≤ 17 at baseline, < 25% decrease in YMRS score and ≤4 point MADRS score between screening and baseline visits; Current episode < 3 weeks First Manic Episode Schizoaffective Neurological Disorders Other Mental Health Taking Other Meds Substance Abuse | Aripiprazole 15–30 mg/day (22.0 mg/day) | C1: Placebo C2: Haloperidol 5–15 mg/day (7.4 mg/day) | 12 weeks for aripiprazole and haloperidol; 3 weeks for placebo | YMRS CGI-BP-S Response Adverse Events EPS Withdrawal 27% at 3 weeks |
| Sachs, 20063 RCT Multisite North America Industry RoB High 16401666 | N = 272 Mean Age 39 Female 51% White 72% BP-I 100% Inpatient (weeks 1–2) Outpatient (week 3) | Manic/Mixed episode; YMRS ≥ 20; Current episode < 4 weeks First Manic Episode Schizoaffective Substance Abuse Neurological Disorders Taking Other Meds Pregnant/Nursing Labs/Other Conditions | Aripiprazole 15–30 mg/day (27.7 mg/day) | Placebo | 3 weeks | YMRS CGI-BP-S Response Adverse Events EPS Withdrawal 47% |
| Vieta, 20054 RCT Multisite Not Disclosed Industry RoB Moderate 16135860 | N = 347 Mean Age 42 Female 28% Race NR BP-I 100% Inpatient or Outpatient | Manic/Mixed; YMRS ≥ 20; Current episode < 4 weeks Other Mental Health Taking Other Meds Substance Abuse | Aripiprazole 15–30 mg/day | C1: Placebo C2: Haloperidol 10–15 mg/day | 3 weeks (with withdrawal < 50%; 12 weeks total) | YMRS CGI-BP-S Response Adverse Events EPS Withdrawal 34% |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; BARS=Barnes Akathisia Scale; BAS=Behavioral Approach System; BMI=Body Mass Index; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=comparison; CGI= Clinical Global Impressions; CGI-I=Clinical Global Impressions-Improvement; CGI-S =CGI-Severity; CGI-BP=Clinical Global Impressions Scale-Bipolar; CGI-BP-C= Clinical Global Impressions, Bipolar, Change Scale; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; C-SSRS=Columbia Suicide Severity Rating Scale; DAI-10=Drug Attitutde Inventory, 10 question version; DIEPSS=Drug-Induced Extra-Pyramidal Symptoms Scale; DSM=Diagnostic and Statistical Manual of Mental Disorders; EPS=extrapyramidal symptoms; ER=Extended Release; ESRS=Extrapyramidal Symptom Rating Scale; GAF=General Assessment of Functioning Scale; GAS=Global Assessment Scale; HAM-A=Hamilton Scale for Anxiety; HAM-D=Hamilton Scale for Depression; HRQL=Health-related quality of life; HRQOL=Health-related quality of life; I=intervention; IDS=Inventory for Depressive Symptoms; LIFE= Longitudinal Interval Follow-up Evaluation; MADRS=Montgomery-Asberg Depression Rating Scale; MAS=Bech-Rafaelsen Mania Rating Scale; MRS=Mania Rating Scale; MSRS=Manic state rating scale; NOS=not otherwise specified; NR=not reported; PANSS=Positive and Negative Syndrome Scale; PRS=Polygenic Risk Scores; PGWB=Psychological General Well-Being Index; PMID=PubMed Identification Number; PRS=Polygenic Risk Scores; Q-LES-Q=Quality of Life Enjoyment and Satisfaction Questionnaire; RCT=randomized controlled trial; ROB=risk of bias; SADS-C= Schedule for Affective Disorders and Schizophrenia-Change version; SAE=Serious Adverse Events; SAS=Simpson Angus Scale; SF-12=12-Item Short Form Health Survey; SF-36=36-Item Short Form Health Survey; SLICE=Streamlined Longitudinal Interview Clinical Evaluation; T=Trial; YMRS = Young Mania Rating Scale
Appendix Table E2Summary risk of bias assessments: aripiprazole for acute mania
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Aripiprazole | Kanba, 20141 Industry 22540407 | High | High dropout rate (47% overall); Randomization and blinding procedures not disclosed. |
| Young, 20092 Industry 19118324 | High | Moderate dropout rate (28%); Randomization and blinding procedures not disclosed. | |
| Sachs, 20065 Industry 16401666 | High | High withdrawal rate (47%), randomization and blinding procedures not disclosed | |
| Vieta, 20056 Industry 16135860 | Moderate | Blinding not described, moderate dropout level (34%), not balanced between the groups. Groups may not be comparable at time of analysis. |
Abbreviations: ITT=Intention to Treat; PMID=PubMed Identification Number; LOCF=last observation carried forward
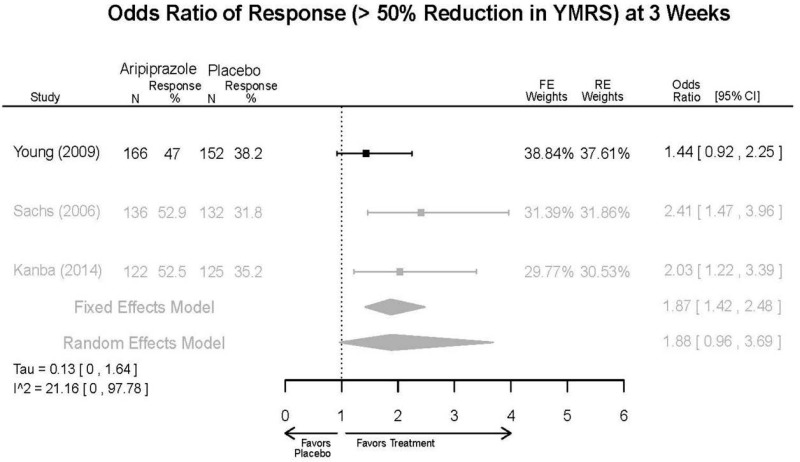
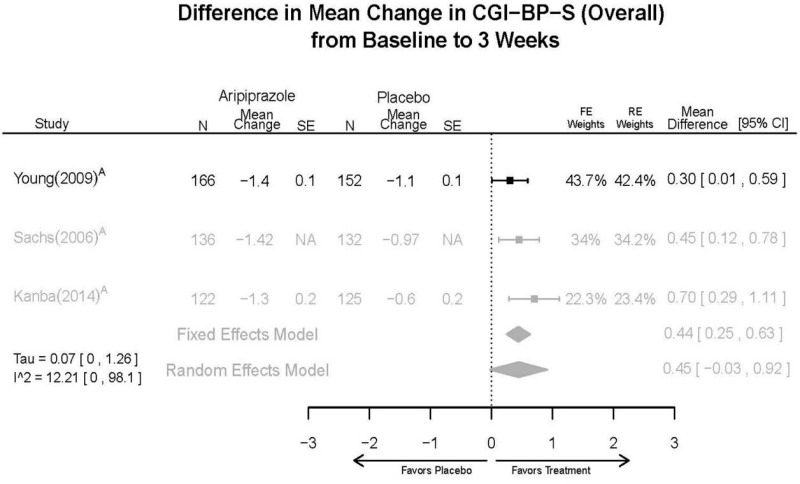
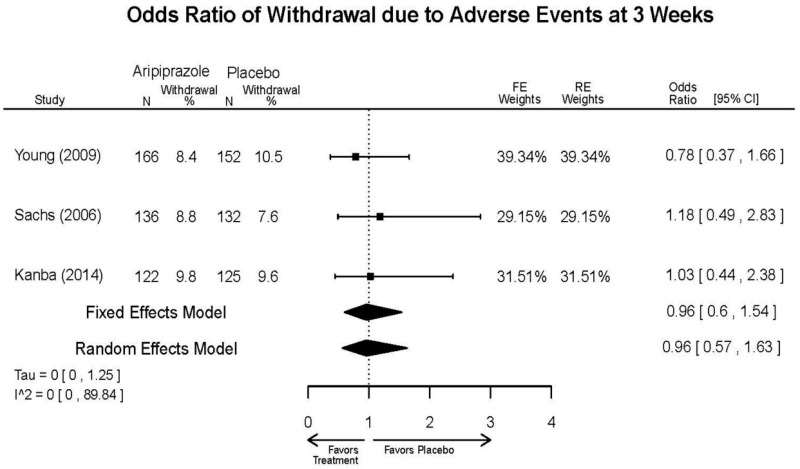
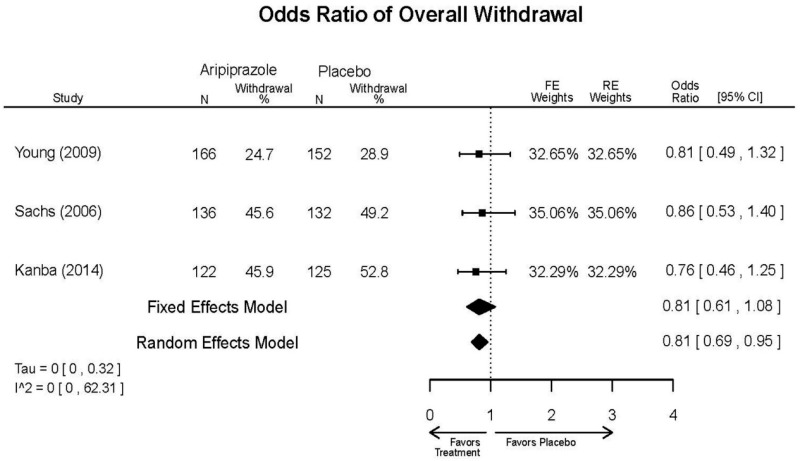
Aripirazole Forest Plots
Outcomes in studies assessed as having a high risk of bias, or low to moderate risk of bias but at least 40 percent attrition, are presented in grey tones. Both fixed-effect models and random-effects models are presented. We calculated fixed-effect models to provide a charitable estimate of the average effect among completed trials. However, we base our main conclusions on the random-effects models.
Appendix Table E3Outcomes summary: aripirazole versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
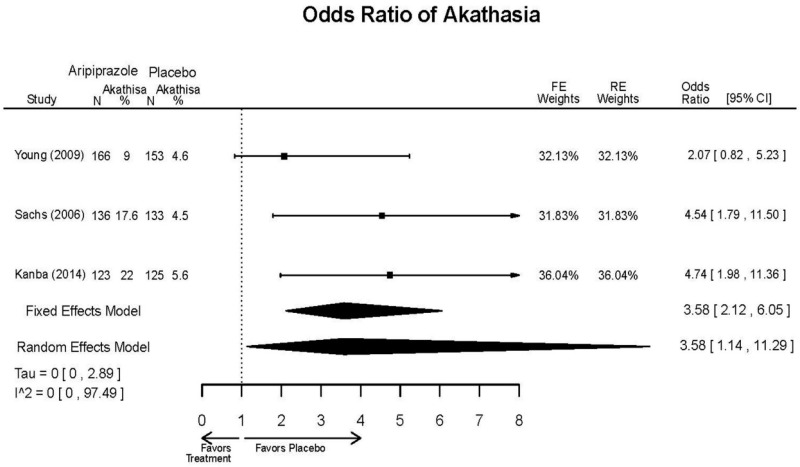
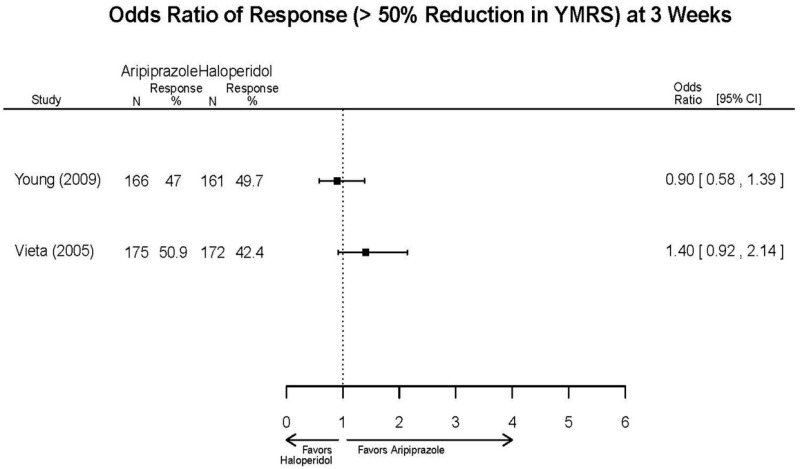
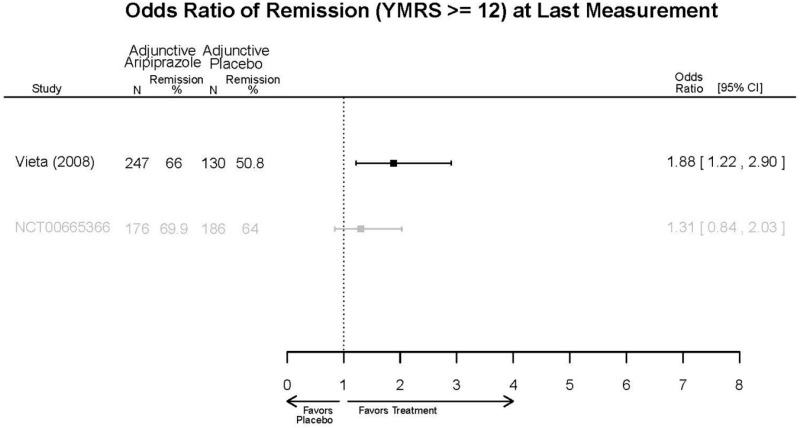
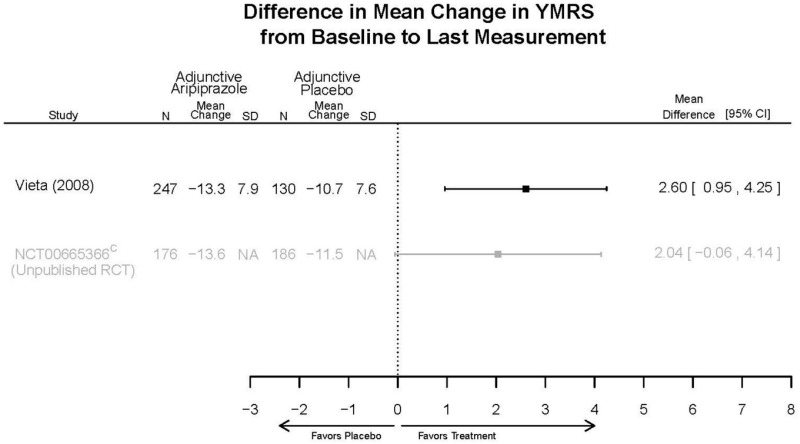
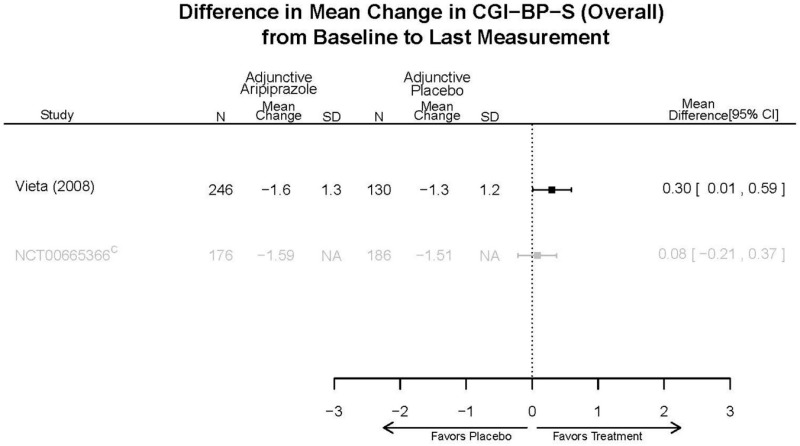
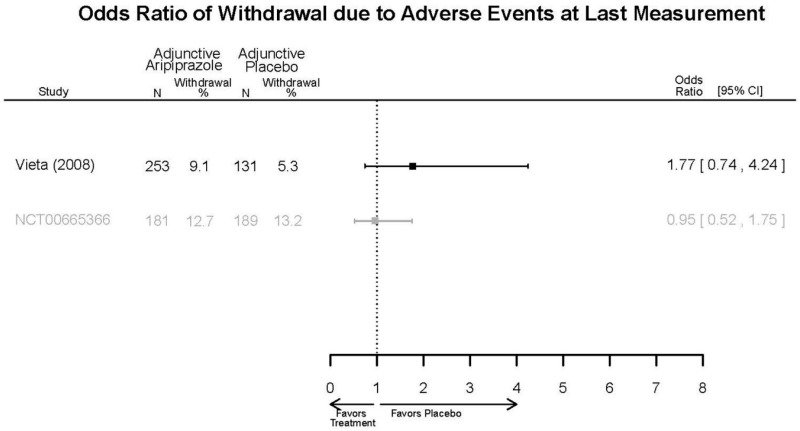
| Aripiprazole | Kanba, 20141 22540407 Moderate | See forest plot E1 above for Response. | See forest plot E2 above for YMRS. | NR | See forest plots E4, E5, E6 above for Withdrawals. | See forest plots E7, E8, E9, E10, E11 above for Adverse Effects. Very Serious AE 3 weeks 1 death during trial, unrelated to study medication |
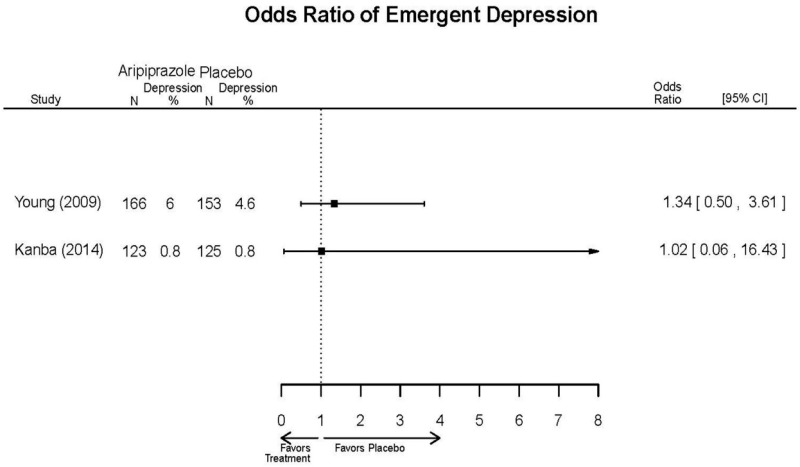
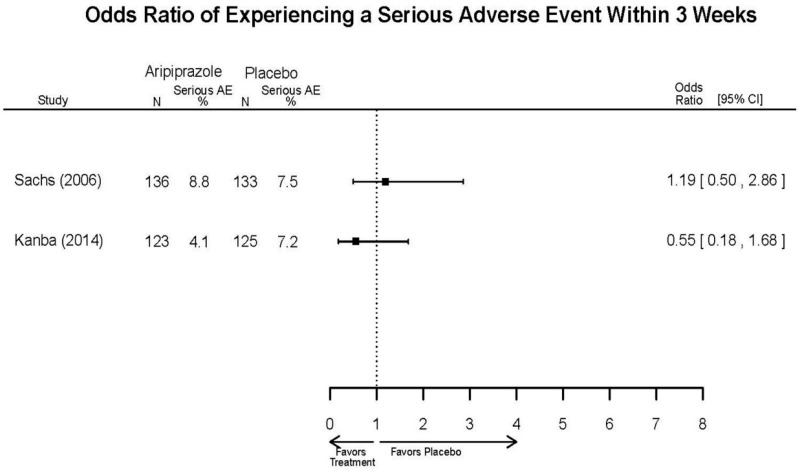
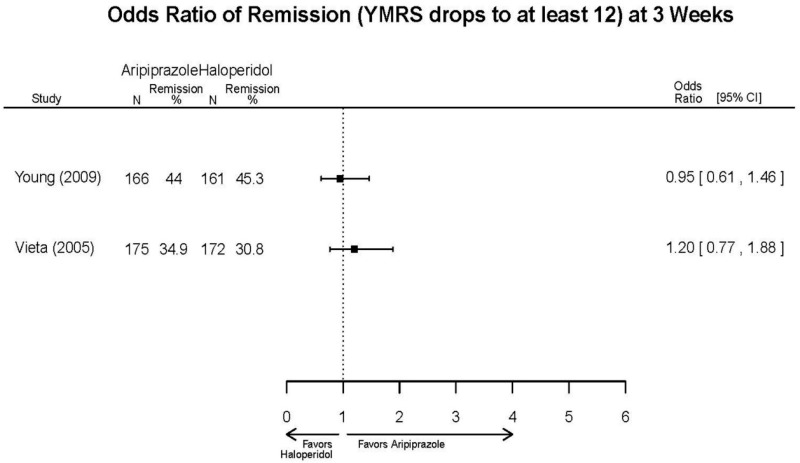
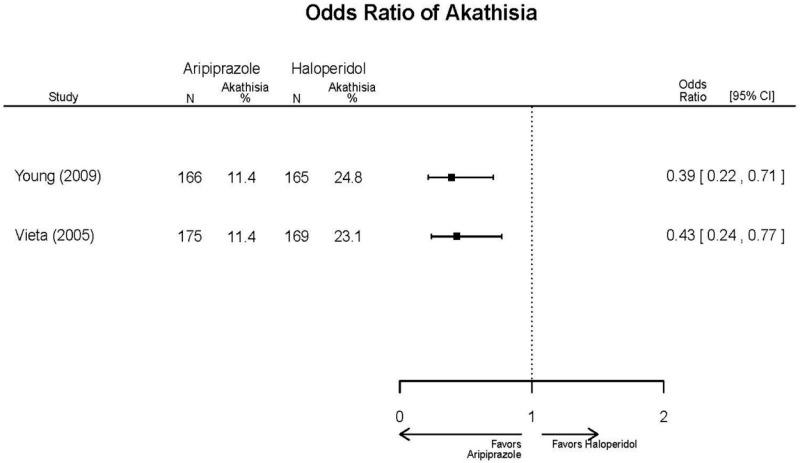
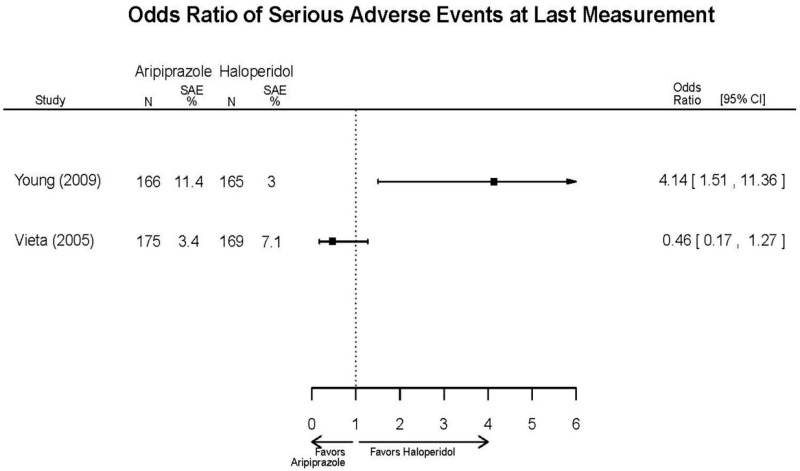
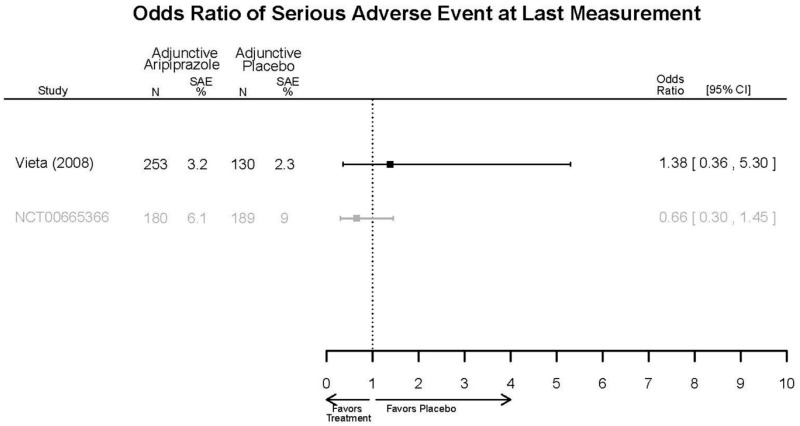
| Young, 20092 19118324 Moderate | Remission 3 weeks NS Aripiprazole = 73/166 Placebo=56/152 OR = 1.35 (95% CI 0.86, 2.11) p 0.20 | See forest plot E2 above for YMRS. | NR | See forest plots E4, E5, E6 above for Withdrawals. | See forest plots E7, E8, E9, E10, E11 above for Adverse Effects. Very Serious AE 3 weeks 1 non-fatal suicide attempt, unclear which study arm SAE 3 weeks Aripoprazole=19/166 Placebo=NR No statistical test reported Extrapyramidal Symptoms 3 weeks Aripoprazole=39/166 Placebo=NR No statistical test reported | |
| Sachs. 20065 16401666 Moderate | See forest plot E1 above for response. | See forest plot E2 above for YMRS. | NR | See forest plots E4, E5, E6 above for Withdrawals. | See forest plots E7, E8, E9, E10, E11 above for Adverse Effects. |
Abbreviations: AE=Adverse Events; ANCOVA=Analysis of Covariance; CGI=Clinical Global Impressions Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; ESRS=Extrapyrimidal Symptom Rating Scale; GAS=Global Assessment Scale; MADRS=Montgomery-Asberg Depression Rating Scale; NR=Not reported; NS=not significant; OR=Odds Ratio; PMID=PubMed Identification Number; ROB=Risk of Bias; SAE=Serious Adverse Events; YMRS = Young Mania Rating Scale
Appendix Table E4Strength of evidence assessment: aripiprazole versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Aripiprazole vs. placebo | Response 3 wks YMRS 3 wks CGI-BP-S 3 wks Overall withdrawal Withdrawal lack of efficacy Withdrawal adverse events | 3 RCT (n=823) | See forest plots | High | Consistent | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Appendix Table E5Outcomes summary: aripiprazole versus active comparator for acute mania
| Comparison | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
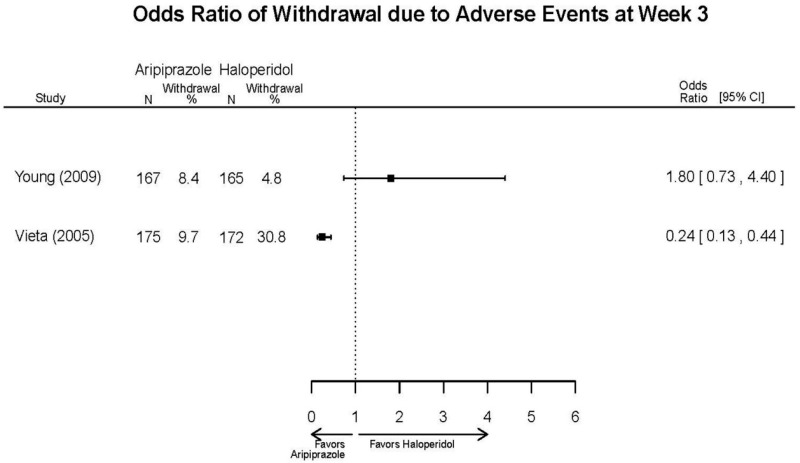
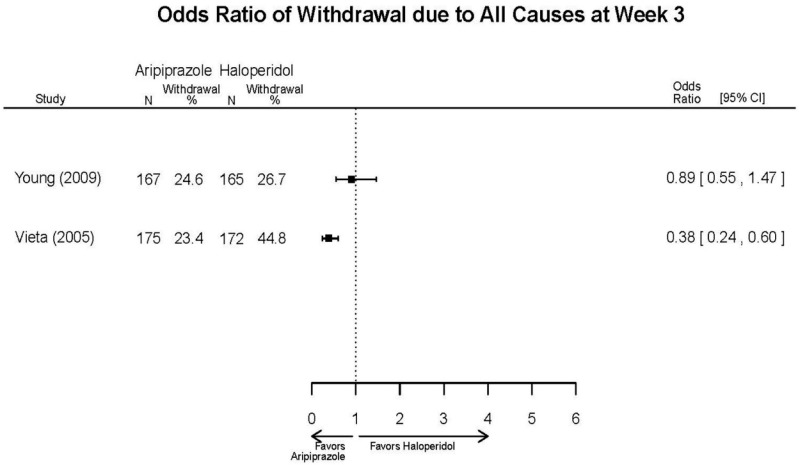
| Aripiprazole (15 or 30 mg/day) vs.. Haliperidol (5–15 mg/day) | Young, 20092 19118324 Moderate High (12 weeks) | See forest plot above. | YMRS, Mean Change 3 weeks NS Aripiprazole =−12.0 Haloperidol =−12.8 No statistical test reported 12 weeks NS Aripiprazole =−17.2 Haloperidol =−17.8 No statistical test reported CGI-BP-Sev, Mean Change 3 weeks NS Aripiprazole =−1.4 Haloperidol =−1.5 No statistical test reported 12 weeks NS Aripiprazole =−2.1 Haloperidol =−2.2 No statistical test reported | NR | Overall Withdrawal 12 weeks NS Aripiprazole=72/167 Haloperidol=70/165 OR = 1.03 (95% CI 0.67, 1.59) ; P = 0.90 Withdrawal Due to Lack of Efficacy 3 weeks NS Aripiprazole=9/167 Haloperidol=10/165 OR = 0.88 (95% CI 0.35, 2.23) p= 0.79 12 weeks NS Aripiprazole=13/167 Haloperidol=11/165 OR = 1.18 (.51, 2.72) ; P = .694 Withdrawal Due to AEs 12 weeks NS Aripiprazole=24/167 Haloperidol=18/165 OR=1.37 (.71, 2.63) ; P = 0.34 | Very Serious AEs 12 weeks 1 patient in haloperidol group suffered liver damage, potentially attributable to haloperidol Normalized Weight Change (>7% change) 12 weeks NS Aripiprazole= 5.1% Haloperidol= 5.8% OR = 0.87 No statistical test reported Cases of Depression 3 weeks Favors Comparator Aripiprazole= 10/166 Haloperidol= 3/165 OR = 3.33 (0.98, 15.83) ; P = 0.049 Cases of Manic Reaction or Relapse at Last Measurement 12 weeks Favors Comparator Aripiprazole= 8/166 Haloperidol= 1/165 OR = 7.36 (1.30, 186.76) ; P = 0.037 |
| Vieta, 20056 16135860 Moderate | See forest plot above. | YMRS 3 weeks NS Aripiprazole −15.7 Haloperidol =−15.7 No statistical test reported CGI-BP-Sev 3 weeks NS Aripiprazole −2.0 Haloperidol =−1.9 No statistical test reported | NR | See forest plot above. | See forest plot above. |
Abbreviations: AE=Adverse Events; CGI=Clinical Global Impressions Scale; CI=Confidence Interval; NS=not significant; OR=Odds Ratio; YMRS = Young Mania Rating Scale
Appendix Table E6Strength of evidence assessment: aripiprazole versus active comparator for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Aripiprazole vs. haloperidol | Response 3 wk Remission 3 wk YMRS 3 wk Withdrawal | 2 RCTs (n=674) | See table above | High | Consistent | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 2. Asenapine for Acute Mania
Appendix Table E7Characteristics of eligible studies: asenapine for acute mania
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP-I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| Landbloom, 20167 RCT Multisite 3 Continents Industry RoB Moderate 26496015 | N = 367 Mean Age 44 Female 55% Race NR BP-I 100% Inpatient (week 1) Outpatient (weeks 2–3 | Mania; Structured clinical interview (MINI). Episode began at least 1 month prior to screening. YMRS ≥ 20 First Manic Episode Schizoaffective Substance Abuse Other Mental Health Taking Other Meds Labs/Other Conditions | Asenapine T1: 5 mg BID T2: 10mg BID | Placebo | 3 weeks | Response (50% change YMRS scores) Remission (YMRS) YMRS CGI-BP-S PANSS MADRS SAEs EPS Withdrawal 28% |
| McIntyre, 20108 RCT Multisite 3 Continents Industry RoB High 20096936 | N = 488 Mean Age 39 Female 47% White 55% BP-I 100% Inpatient (week 1) Outpatient (weeks 2–3, subject to investigator discretion and successful passing of InterSePT Scale for Suicidal Thinking criteria) | Manic/Mixed; YMRS ≥ 20; Current episode ≤ 3 months First Manic Episode Neurological Disorders Substance Abuse Other Mental Health Taking Other Meds Lab/Other Conditions | Asenapine 10–20 mg/day (18.4 mg/day average) N=185 | C1: Placebo n=98 C2: Olanzapine 5–20 mg/day n=205 | 3 weeks | CGI-BP-S MADRS Remission (YMRS ≤ 12) Remission Rate (YMRS ≤ 12) Response (50% decrease in YMRS) YMRS Withdrawal 34% |
| McIntyre, 20099 RCT Multisite 3 continents Industry RoB High 19839993 | N = 489 Mean Age 39 Female 43% White 61% BP-I 100% Inpatient (week 1) Outpatient (weeks 2–3, subject to investigator discretion and successful passing of InterSePT Scale for Suicidal Thinking criteria) | Manic/Mixed; YMRS ≥ 20; Current episode ≤ 3 months First Manic Episode Neurological Disorders Substance Abuse Taking Other Meds Labs/Other Conditions | Asenapine 10–20 mg/day (18.2 mg/day) N=104 | C1: Placebo n=194 C2: Olanzapine 5–20 mg/day n=190 | 3 weeks | AIMS BARS CGI-BP-S MADRS Remission (YMRS ≤12) Response (50% decrease in YMRS) Simpson-Angus Scale (SAS) YMRS Withdrawal 31% |
| Calabrese, 201510 RCT Multisite 3 Continents Industry RoB Low 25562205 | N = 497 Mean Age 42 Female 47% White 69% BP-I 100% Inpatient (weeks 1–2) Outpatient (week 3, subject to inspector discretion) | Manic/Mixed; YMRS ≥20 AND ≥ 4 on two YMRS items; MADRS < 18 First Manic Episode Schizoaffective Substance Abuse Other Mental Health Neurological Disorders Taking Other Meds | Cariprazine I1: 3–6 mg/day I2: 6–12 mg/day | Placebo | 3 weeks | CGI-S SAS YMRS Withdrawal 11% |
| Durgam, 201511 RCT Multisite 3 Continents Industry RoB Moderate 25056368 | N = 238 Mean Age 38 Female 67% White 43% BP-I 100% Inpatient (weeks 1–2) Outpatient (week 3, subject to inspector discretion) | Manic/Mixed; YMRS ≥20 AND ≥4 on two YMRS items First Manic Episode Schizoaffective Substance Abuse Other Mental Health Neurological Disorders Taking Other Meds Pregnant/Nursing Labs/Other Conditions | Cariprazine 3–12 mg/day | Placebo | 3 weeks | YMRS MADRS CGI-S CGI-I PANSS AIMS BARS SAS Withdrawal 37% |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; BARS=Barnes Akathisia Scale; BAS=Behavioral Approach System; BMI=Body Mass Index; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=comparison; CGI= Clinical Global Impressions; CGI-I=Clinical Global Impressions-Improvement; CGI-S =CGI-Severity; CGI-BP=Clinical Global Impressions Scale-Bipolar; CGI-BP-C= Clinical Global Impressions, Bipolar, Change Scale; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; C-SSRS=Columbia Suicide Severity Rating Scale; DAI-10=Drug Attitutde Inventory, 10 question version; DIEPSS=Drug-Induced Extra-Pyramidal Symptoms Scale; DSM=Diagnostic and Statistical Manual of Mental Disorders; EPS=extrapyramidal symptoms; ER=Extended Release; ESRS=Extrapyramidal Symptom Rating Scale; GAF=General Assessment of Functioning Scale; GAS=Global Assessment Scale; HAM-A=Hamilton Scale for Anxiety; HAM-D=Hamilton Scale for Depression; HRQL=Health-related quality of life; HRQOL=Health-related quality of life; I=intervention; IDS=Inventory for Depressive Symptoms; LIFE= Longitudinal Interval Follow-up Evaluation; MADRS=Montgomery-Asberg Depression Rating Scale; MAS=Bech-Rafaelsen Mania Rating Scale; MRS=Mania Rating Scale; MSRS=Manic state rating scale; NOS=not otherwise specified; NR=not reported; PANSS=Positive and Negative Syndrome Scale; PRS=Polygenic Risk Scores; PGWB=Psychological General Well-Being Index; PMID=PubMed Identification Number; PRS=Polygenic Risk Scores; Q-LES-Q=Quality of Life Enjoyment and Satisfaction Questionnaire; RCT=randomized controlled trial; ROB=risk of bias; SADS-C= Schedule for Affective Disorders and Schizophrenia-Change version; SAE=Serious Adverse Events; SAS=Simpson Angus Scale; SF-12=12-Item Short Form Health Survey; SF-36=36-Item Short Form Health Survey; SLICE=Streamlined Longitudinal Interview Clinical Evaluation; T=Trial; YMRS = Young Mania Rating Scale
Appendix Table E8Summary risk of bias assessments: asenapine for acute mania
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Asenapine | McIntyre, 201012 Industry 20096936 | High | Randomization and blinding procedures not described. Withdrawal 34%. |
| McIntyre, 20099 Industry 19839993 | High | Randomization and blinding procedures not described. Patients discharged from inpatient care at differing times and study doesn’t include this as a point of analysis as a possible confounder. Withdrawal 31%. | |
| Landbloom, 20167 Industry 26496015 | Low | No sources of bias identified. |
Abbreviations: ITT=Intention to Treat; PMID=PubMed Identification Number; LOCF=last observation carried forward
Asenapine Forest Plots
Outcomes in studies assessed as having a high risk of bias, or low to moderate risk of bias but at least 40 percent attrition, are presented in grey tones. Both fixed-effect models and random-effects models are presented. We calculated fixed-effect models to provide a charitable estimate of the average effect among completed trials. However, we base our main conclusions on the random-effects models.
Appendix Table E9Outcomes summary: asenapine versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
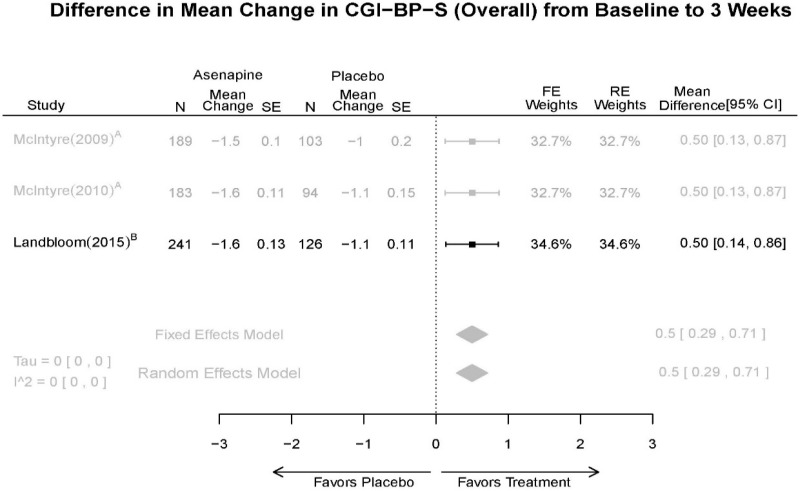
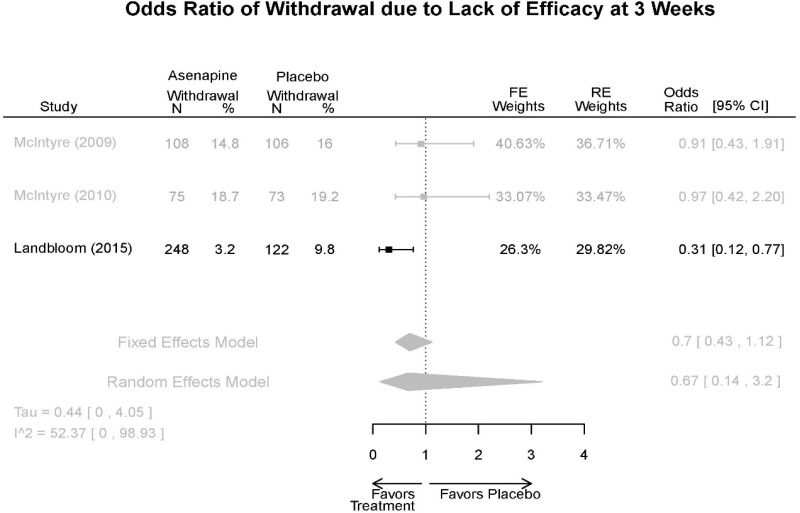
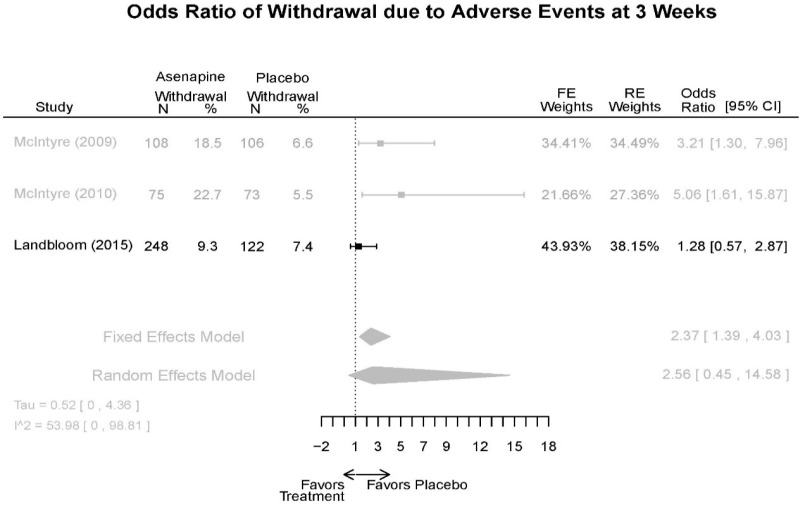
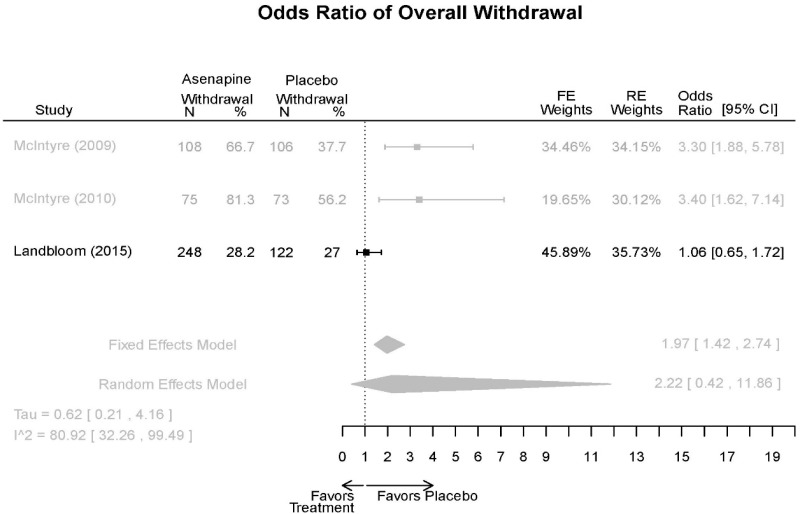
| Asenapine | McIntyre, 201012 Industry 20096936 | See forest plot E20 above for response. | See forest plot E22 above for YMRS. | See forest plot E23 above for CGI. | See forest plots E24, E25, E26 above for Withdrawals. 1 suicide in asenapine | SAE Placebo: 3.8% Asenapine: 1.5% NS EPS (<1) Placebo: 3.1% Asenapine: 10.3% p=0.03 Weight gain ≥7% Placebo: 1.2% Asenapine: 7.2% p=0.03 |
| McIntyre, 20099 Industry 19839993 | See forest plot E20 above for response. | See forest plot E22 above for YMRS. | See forest plot E32 above for CGI. | See forest plots E24, E25, E26 above for Withdrawals. | SAE Placebo: 3.8% Asenapine: 1.5% NS EPS (<1) Placebo: 3.1% Asenapine: 10.3% p=0.03 Weight gain ≥7% Placebo: 1.2% Asenapine: 7.2% p=0.03 | |
| Landbloom, 20167 Industry 26496015 | See forest plot E20 above for response. | See forest plot E22 above for YMRS. | See forest plot E32 above for CGI. | See forest plots E24, E25, E26 above for Withdrawals. Suicide Ideation Placebo: 5/126 5 mg Asenapine: 4/122 10 mg Asenapine: 1/129 NS | SAE “Most…were psychiatric disorders class” Placebo: 2/126 5 mg Asenapine: 3/122 10 mg Asenapine: 1/119 NS EPS Placebo: 6/126 5 mg Asenapine: 8/122 10 mg Asenapine: 25/129 p<0.0001 10 mg |
Abbreviations: AE=Adverse Events; ANCOVA=Analysis of Covariance; CGI=Clinical Global Impressions Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; ESRS=Extrapyrimidal Symptom Rating Scale; GAS=Global Assessment Scale; MADRS=Montgomery-Asberg Depression Rating Scale; NR=c; NS=not significant; OR=Odds Ratio; PMID=PubMed Identification Number; ROB=Risk of Bias; SAE=Serious Adverse Events; YMRS = Young Mania Rating Scale
Appendix Table E10Strength of evidence assessment: asenapine versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
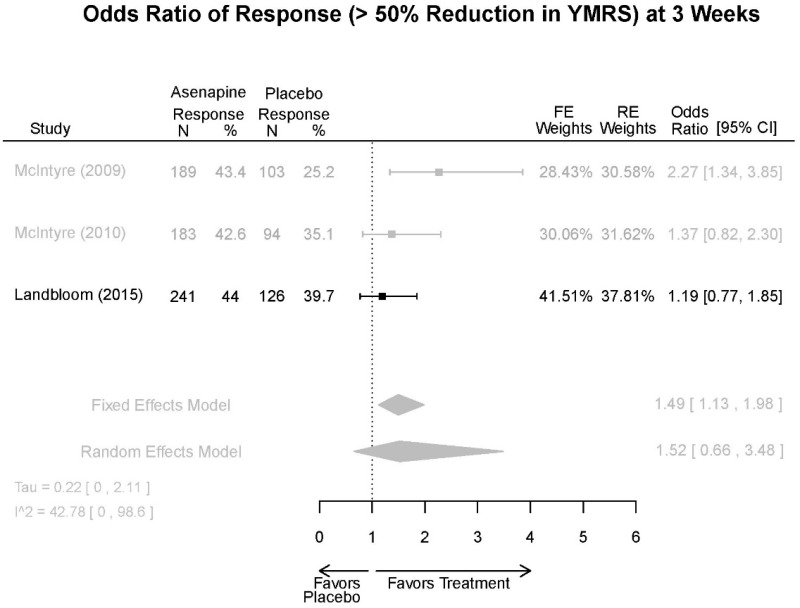
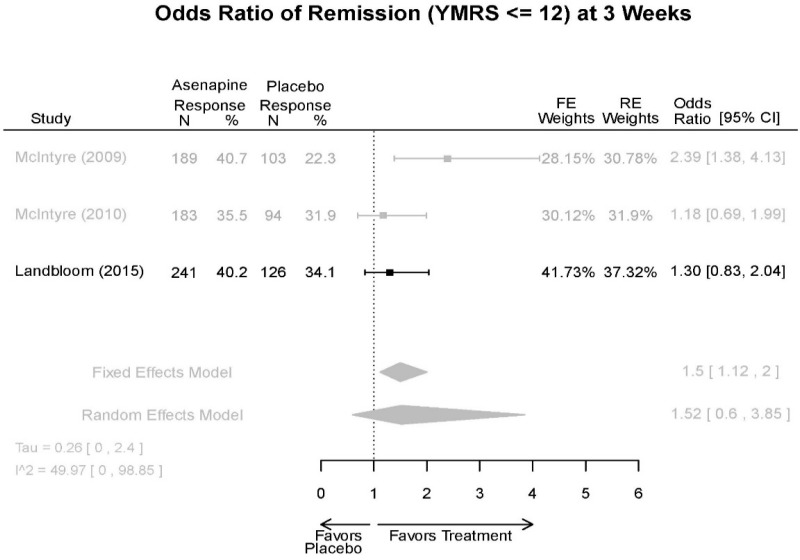
| Asenapine vs. placebo | Response Remission 3 wks | 3 RCT (n=936) | NS | Moderate | Consistent | Direct | Imprecise | Low |
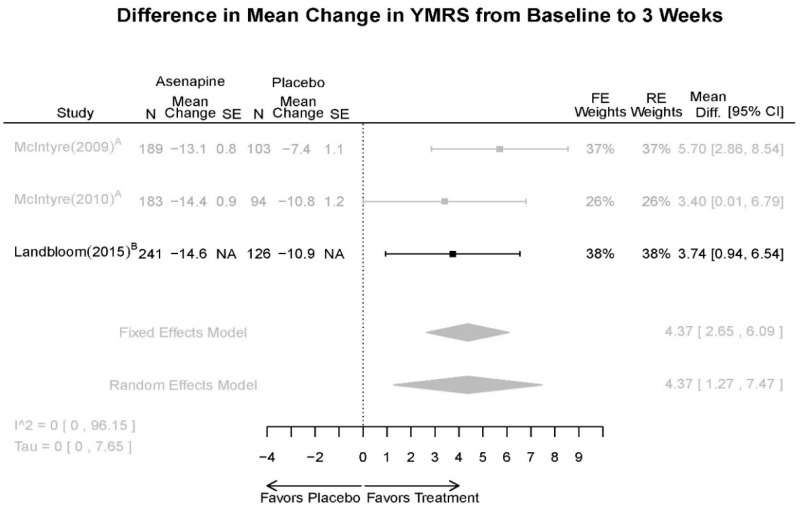
| YMRS 3 wks | 3 RCT (n=936) | Favors Asenapine. MD 4.37 (1.27, 7.47) | Moderate | Consistent | Direct | Imprecise | Low | |
| CGI-BP-S 3 wk | 3 RCT (n=936) | Favors Asenapine MD 0.5 (0.29, 0.71) | Moderate | Consistent | Direct | Imprecise | Low | |
| Withdrawal – AE, Lack of Efficacy, Overall | 3 RCT (n=936) | NS | Moderate | Consistent | Direct | Imprecise | Low |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Appendix Table E11Outcomes summary: asenapine versus active comparator for acute mania
| Comparison | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
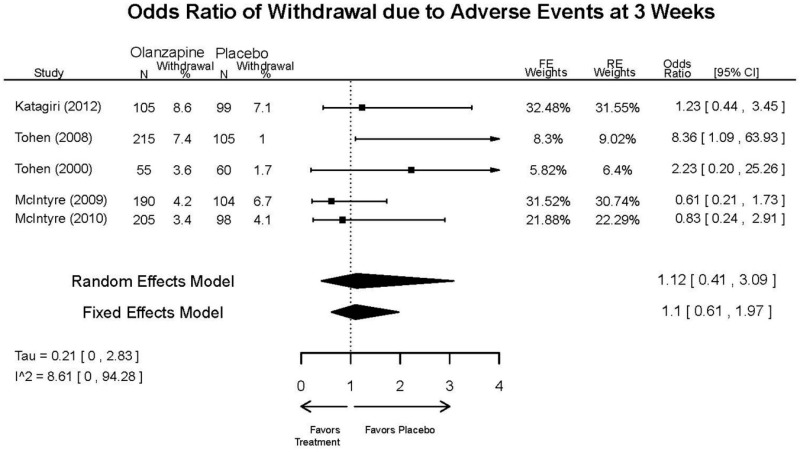
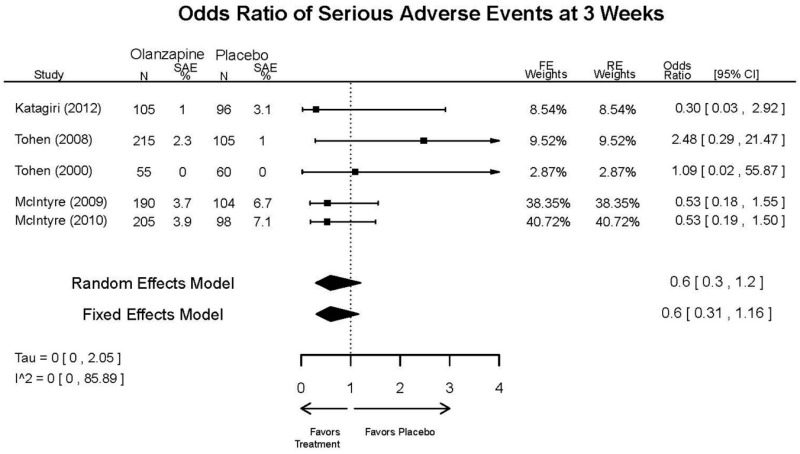
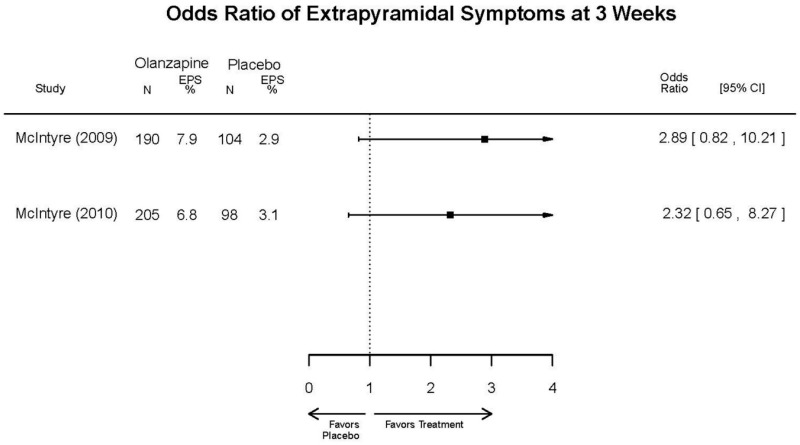
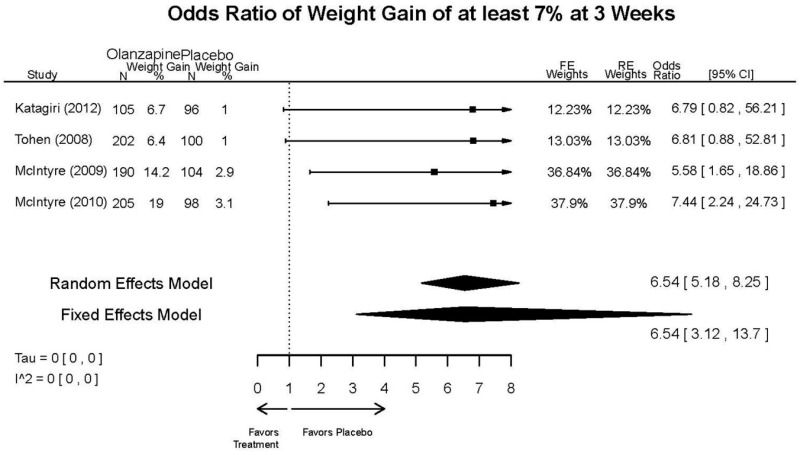
| Asenapine vs. olanzapine | McIntyre, 20099 19839993 | Response 3 weeks Asenapine 42.3% Olanzapine 50% Remission 3 weeks Asenapine 40.2% Olanzapine 39.4% NS | YMRS 3 weeks Least square mean Asenapine −10.8 SD 0.8 (effect size 0.45) Olanzapine −12.6 SD 0.8 (effect size 0.70) | CGI 3 weeks Least square mean Asenapine −1.2 SD 0.01 Olanzapine −1.4 SD 0.01 | NR Overall Withdrawal Asenapine 37.1% Olanzapine 30.9% Withdrawal Lack of Efficacy: Asenapine 8.2% Olanzapine 5.8% Withdrawal AE Asenapine 10.3% Olanzapine 4.2% | Serious Adverse Events 3 weeks 0 in all arms Deaths 3 weeks 0 in all arms EPS 3 weeks 2.9% placebo 7.2% Asenapine 7.9% Olanzapine |
| McIntyre, 201012 20096936 | Response 3 weeks Asenapine 42.6% Olanzapine 54.7% Remission 3 weeks Asenapine 35.5% Olanzapine 46.3% NS | YMRS 3 weeks Least square mean Asenapine −11.5 SD 0.8 (effect size 0.32) Olanzapine −14.6 SD 0.8 (effect size 0.63) | CGI 3 weeks Least square mean Asenapine −1.2 SD 0.10 Olanzapine −1.5 SD 0.09 | NR Overall Withdrawal Asenapine 33.0% Olanzapine 21.5% Withdrawal Lack of Efficacy: Asenapine 7.6% Olanzapine 6.3% Withdrawal AE Asenapine 9.2% Olanzapine 3.4% | Serious Adverse Events 3 weeks 1 Asenapine Deaths 3 weeks 1 Asenapine – suicide EPS 3 weeks 3.1% placebo 10.3% Asenapine 6.8% Olanzapine |
Abbreviations: CGI=Clinical Global Impressions; EPS=extrapyramidal symptoms; NR=not reported; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Appendix Table E12Strength of evidence assessment: asenapine versus active comparator for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Asenapine vs. olanzapine | Response 3 wk Remission 3 wk YMRS 3 wk CGI Withdrawal | 2 RCTs (n=763) | See table above | High | Consistent | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 3. Cariprazine for Acute Mania
Appendix Table E13Characteristics of eligible studies: cariparazine for acute mania
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP-I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| Calabrese, 201510 RCT Multisite 3 Continents Industry RoB Low 25562205 | N = 497 Mean Age 42 Female 47% White 69% BP-I 100% Inpatient (weeks 1–2) Outpatient (week 3, subject to inspector discretion) | Manic/Mixed; YMRS ≥20 AND ≥ 4 on two YMRS items; MADRS < 18 First Manic Episode Schizoaffective Substance Abuse Other Mental Health Neurological Disorders Taking Other Meds | Cariprazine I1: 3–6 mg/day I2: 6–12 mg/day | Placebo | 3 weeks | CGI-S SAS YMRS Withdrawal 11% |
| Durgam, 201511 RCT Multisite 3 Continents Industry RoB Moderate 25056368 | N = 238 Mean Age 38 Female 67% White 43% BP-I 100% Inpatient (weeks 1–2) Outpatient (week 3, subject to inspector discretion) | Manic/Mixed; YMRS ≥20 AND ≥4 on two YMRS items First Manic Episode Schizoaffective Substance Abuse Other Mental Health Neurological Disorders Taking Other Meds Pregnant/Nursing Labs/Other Conditions | Cariprazine 3–12 mg/day | Placebo | 3 weeks | YMRS MADRS CGI-S CGI-I PANSS AIMS BARS SAS Withdrawal 37% |
| Sachs, 201513 RCT Multisite 2 Continents Industry RoB Moderate 25532076 | N = 312 Mean Age 36 Female 36% White 21% BP-I 100% Inpatient (weeks 1–2) Outpatient (week 3, subject to inspector discretion) | Manic/Mixed; YMRS ≥20 AND ≥4 on two YMRS items; MADRS <18 Schizoaffective Substance Abuse Other Mental Health Neurological Disorders Taking Other Meds Pregnant/Nursing | Cariprazine 3–12 mg/day | Placebo | 3 weeks | YMRS CGI-S CGI-I MADRS PANSS C-SSRS AIMS BARS SAS Withdrawal 31% |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; BARS=Barnes Akathisia Scale; BAS=Behavioral Approach System; BMI=Body Mass Index; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=comparison; CGI= Clinical Global Impressions; CGI-I=Clinical Global Impressions-Improvement; CGI-S =CGI-Severity; CGI-BP=Clinical Global Impressions Scale-Bipolar; CGI-BP-C= Clinical Global Impressions, Bipolar, Change Scale; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; C-SSRS=Columbia Suicide Severity Rating Scale; DAI-10=Drug Attitutde Inventory, 10 question version; DIEPSS=Drug-Induced Extra-Pyramidal Symptoms Scale; DSM=Diagnostic and Statistical Manual of Mental Disorders; EPS=extrapyramidal symptoms; ER=Extended Release; ESRS=Extrapyramidal Symptom Rating Scale; GAF=General Assessment of Functioning Scale; GAS=Global Assessment Scale; HAM-A=Hamilton Scale for Anxiety; HAM-D=Hamilton Scale for Depression; HRQL=Health-related quality of life; HRQOL=Health-related quality of life; I=intervention; IDS=Inventory for Depressive Symptoms; LIFE= Longitudinal Interval Follow-up Evaluation; MADRS=Montgomery-Asberg Depression Rating Scale; MAS=Bech-Rafaelsen Mania Rating Scale; MRS=Mania Rating Scale; MSRS=Manic state rating scale; NOS=not otherwise specified; NR=not reported; PANSS=Positive and Negative Syndrome Scale; PRS=Polygenic Risk Scores; PGWB=Psychological General Well-Being Index; PMID=PubMed Identification Number; PRS=Polygenic Risk Scores; Q-LES-Q=Quality of Life Enjoyment and Satisfaction Questionnaire; RCT=randomized controlled trial; ROB=risk of bias; SADS-C= Schedule for Affective Disorders and Schizophrenia-Change version; SAE=Serious Adverse Events; SAS=Simpson Angus Scale; SF-12=12-Item Short Form Health Survey; SF-36=36-Item Short Form Health Survey; SLICE=Streamlined Longitudinal Interview Clinical Evaluation; T=Trial; YMRS = Young Mania Rating Scale
Appendix Table E14Summary risk of bias assessments: cariprazine for acute mania
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Cariprazine | Durgam, 201511 Industry 25056368 | Moderate | The large dropout rate is likely to create some bias. Lack of disclosure of methods to allocate and protect the blind also increases the risk. |
| Calabrese, 201510 Industry 25562205 | Low | Procedures for concealing allocation and blinding participants and providers are not described, but, the study appears to have been well executed, fully reported, and investigators have taken steps to ensure bias was minimized, like pattern mixture modeling. | |
| Sachs, 201513 Industry 25532076 | Moderate | A moderately high dropout rate combined with a lack of disclosure for the methods of allocation and concealment create strong conditions where bias may be present. |
Abbreviations: ITT=Intention to Treat; PMID=PubMed Identification Number; LOCF=last observation carried forward
Cariprazine Forest Plots
Outcomes in studies assessed as having a high risk of bias, or low to moderate risk of bias but at least 40 percent attrition, are presented in grey tones. Both fixed-effect models and random-effects models are presented. We calculated fixed-effect models to provide a charitable estimate of the average effect among completed trials. However, we base our main conclusions on the random-effects models.
Appendix Table E15Outcomes summary: cariprazine versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
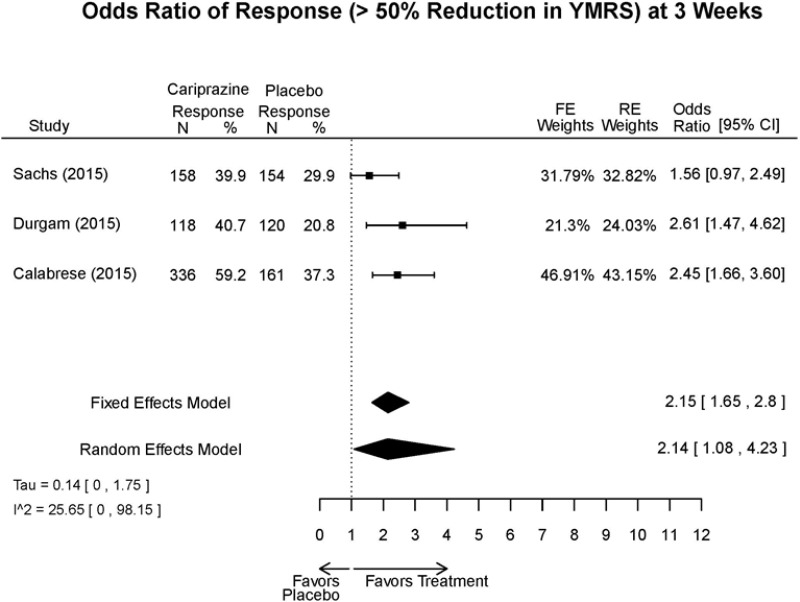
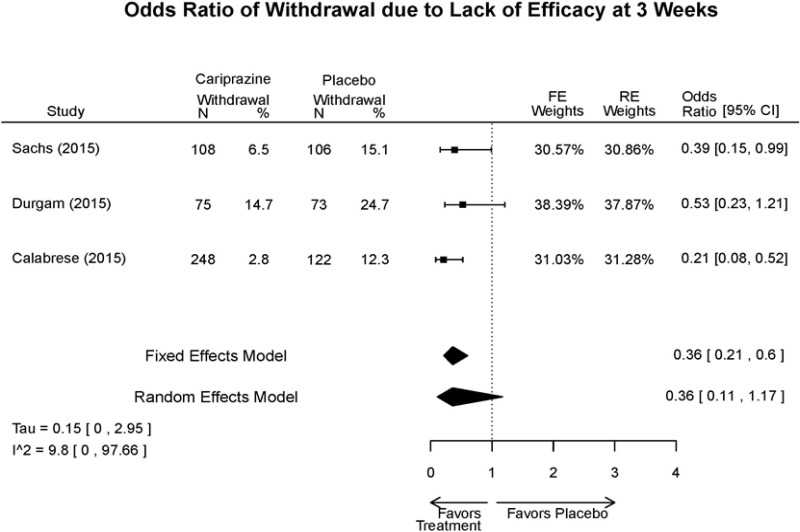
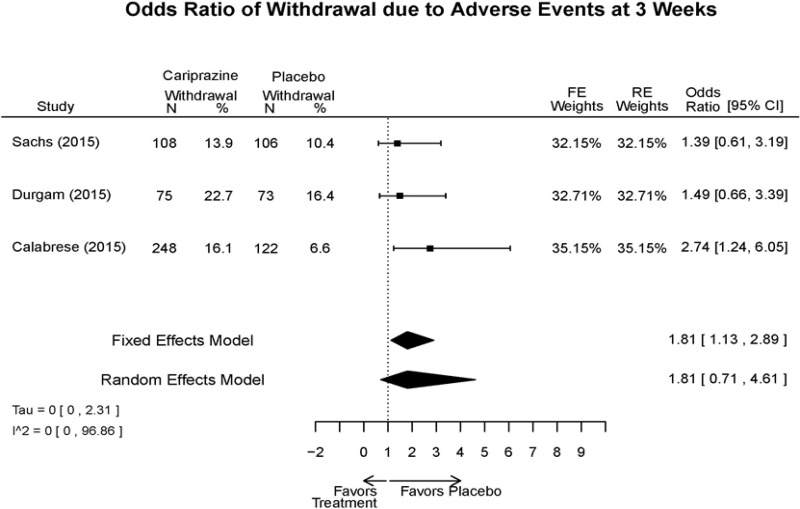
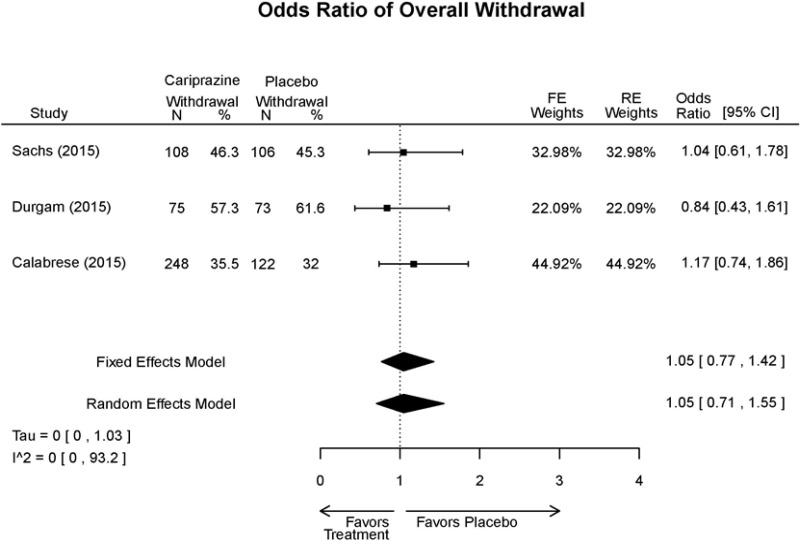
| Cariprazine | Durgam, 201511 Industry 25056368 | See forest plot E27 above for response. | See forest plot E29 above for YMRS. | See forest plot E30 above for CGI. | See forest plots E31, E32, E33 above for Withdrawals. | SAE Placebo: 5 patients Cariprazine: 4 patients No suicide attempts No difference between groups in suicide ideation EPS Placebo: 1% Cariprazine groups: 11% or 14% Akasthesia Placebo: 4% Cariprazine groups:20% or 23% |
| Calabrese, 201510 Industry 25562205 | See forest plot E27 above for response. | See forest plot E29 above for YMRS. | See forest plot E30 above for CGI. | See forest plots E31, E32, E33 above for Withdrawals. | SAE Placebo: 0 patients Cariprazine: 4 patients No suicide attempts I placebo patients reported suicide ideation EPS Placebo: 1 patient Cariprazine: 19 patients Akasthesia Placebo: 7 patient Cariprazine: 26 patients | |
| Sachs, 201513 Industry 25532076 | See forest plot E27 above for response. | See forest plot E29 above for YMRS. | See forest plot E30 above for CGI. | See forest plots E31, E32, E33 above for Withdrawals. | SAE Placebo: 3 patients Cariprazine: 5 patients No suicide attempts No difference between groups in suicide ideation EPS Placebo: 6 patients Cariprazine: 30 patients Akasthesia Placebo: 8 patient Cariprazine: 36 patients |
Abbreviations: AE=Adverse Events; ANCOVA=Analysis of Covariance; CGI=Clinical Global Impressions Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; ESRS=Extrapyrimidal Symptom Rating Scale; GAS=Global Assessment Scale; MADRS=Montgomery-Asberg Depression Rating Scale; NR=Not reported; NS=not significant; OR=Odds Ratio; PMID=PubMed Identification Number; ROB=Risk of Bias; SAE=Serious Adverse Events; YMRS = Young Mania Rating Scale
Appendix Table E16Strength of evidence assessment: cariprazine versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Cariprazine vs. placebo | Response 3 wks | 3 RCT (n=1,047) | Favors Cariprazine OR 2.14 (95% CI 1.08, 4.23) | Moderate | Consistent | Direct | Imprecise | Low |
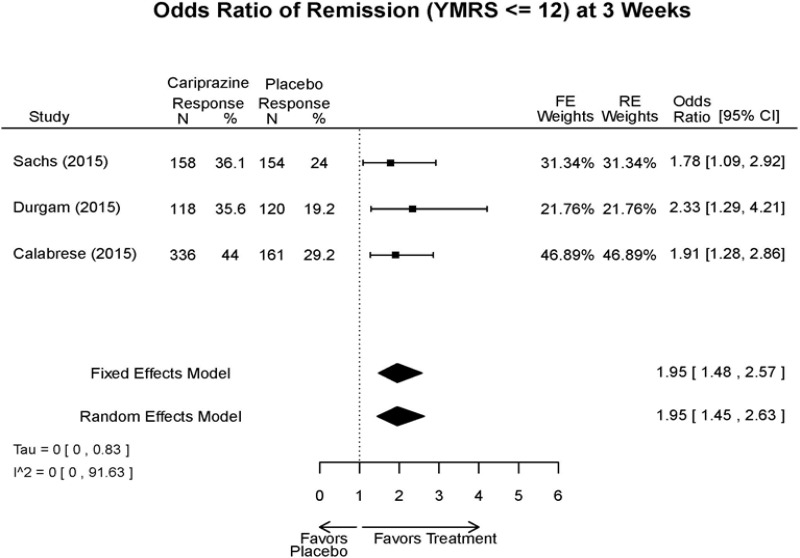
| Remission 3 wks | 3 RCT (n=1,047) | Favors Cariprazine OR 1.95 (95% CI 1.45, 2.63) | Moderate | Consistent | Direct | Imprecise | Low | |
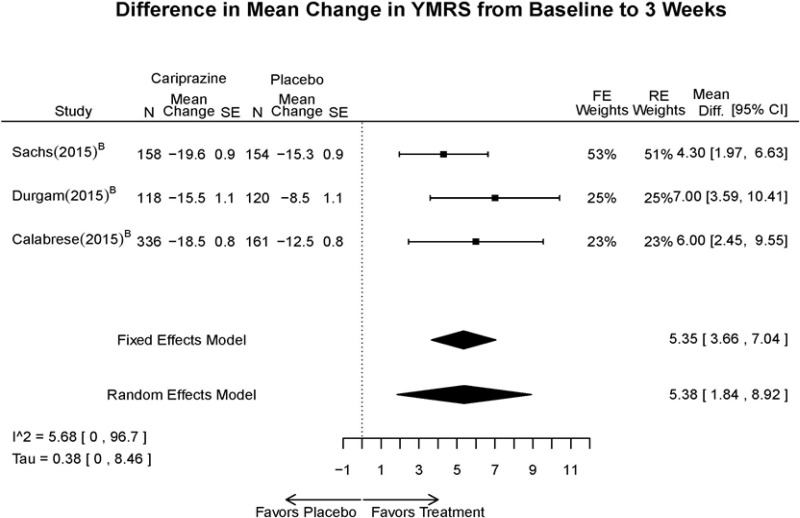
| YMRS 3 wks | 3 RCT (n=1,047) | Favors Cariprazine MD 5.38 (95% CI 1.84, 8.92) | Moderate | Consistent | Direct | Imprecise | Low | |
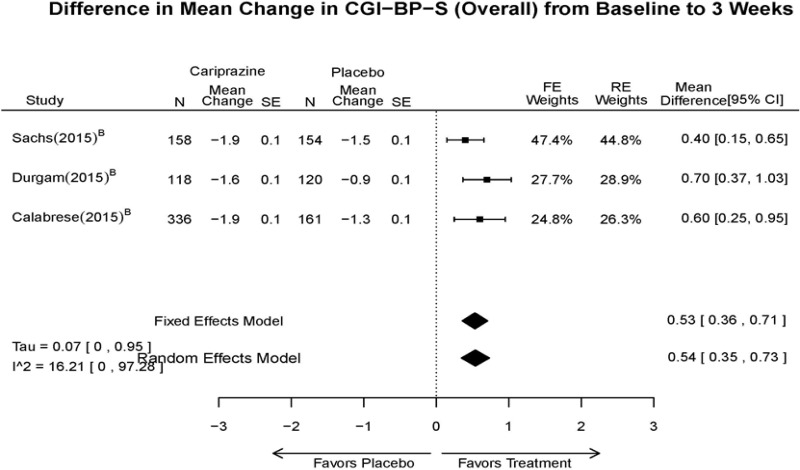
| CGI-BP-S 3 wk | 3 RCT (n=1,047) | Favors Cariprazine MD 0.54 (95% CI 0.35, 0.73) | Moderate | Consistent | Direct | Imprecise | Low | |
| Withdrawal – AE, Lack of Efficacy, Overall | 3 RCT (n=1,047) | NS | Moderate | Consistent | Direct | Imprecise | Low |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 4. Haloperidol for Acute Mania
Appendix Table E17Characteristics of eligible studies: haloperidol for acute mania
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP-I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| McIntyre, 200514 RCT Multisite 3 Continents Industry Moderate 16139175 | N = 299 Mean Age 42 Female 63% Race NR BP-I 100% Inpatient | Manic YMRS ≥ 20 CGI–BP ≥4 First Manic Episode Substance Abuse Neurological Disorders Taking Other Meds Pregnant/Nursing Labs/Other Conditions | Haloperidol Starting 2mg/day up to 8mg/day | C1: Placebo C2: Quetiapine 100mg/day increasing by 100mg up to 800 mg/day | 12 weeks | Remission Rates Adverse Events Efficacy YMRS CGI PANSS MADRS GAS Withdrawal 50% |
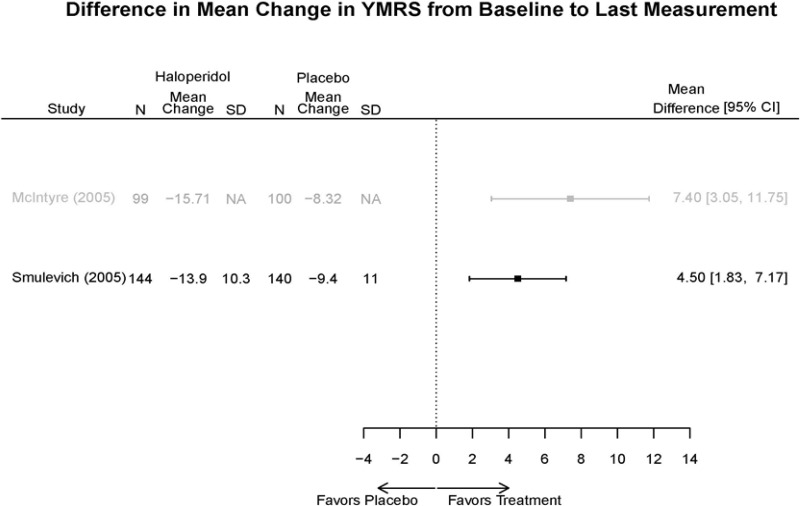
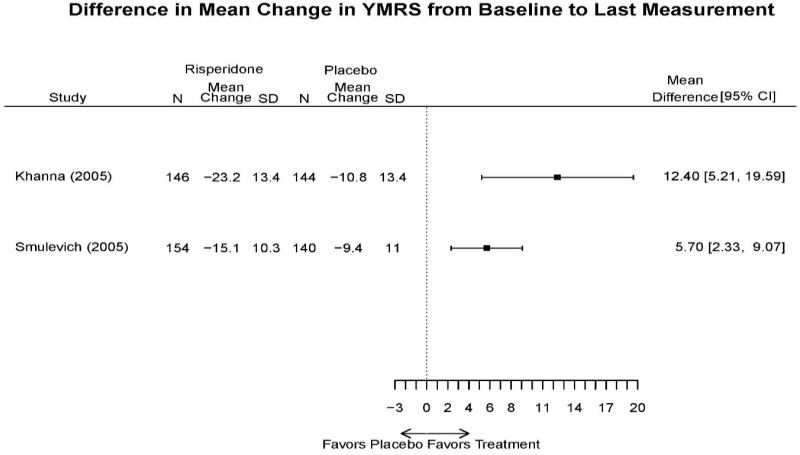
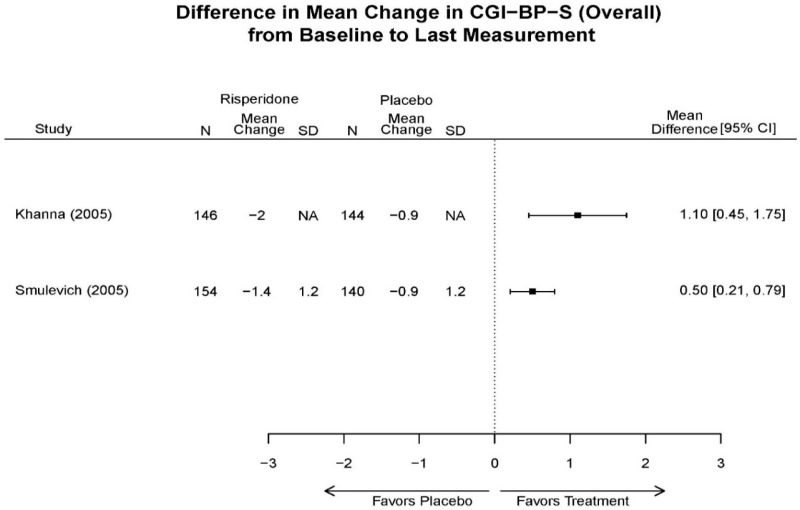
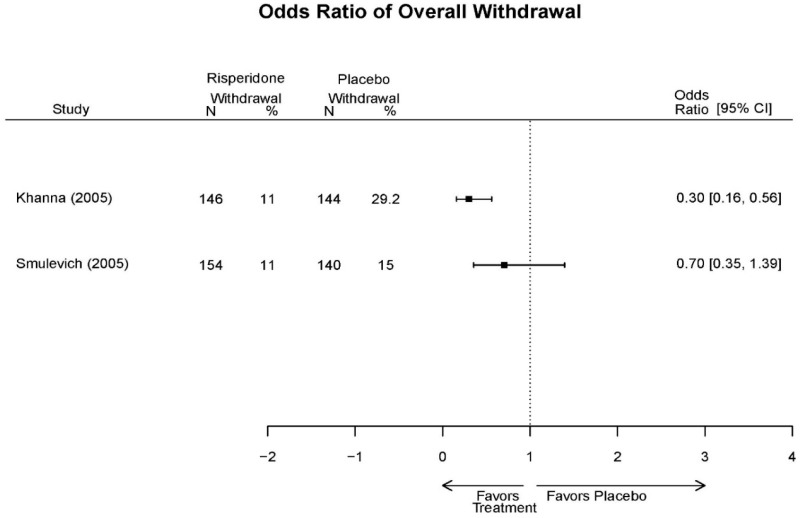
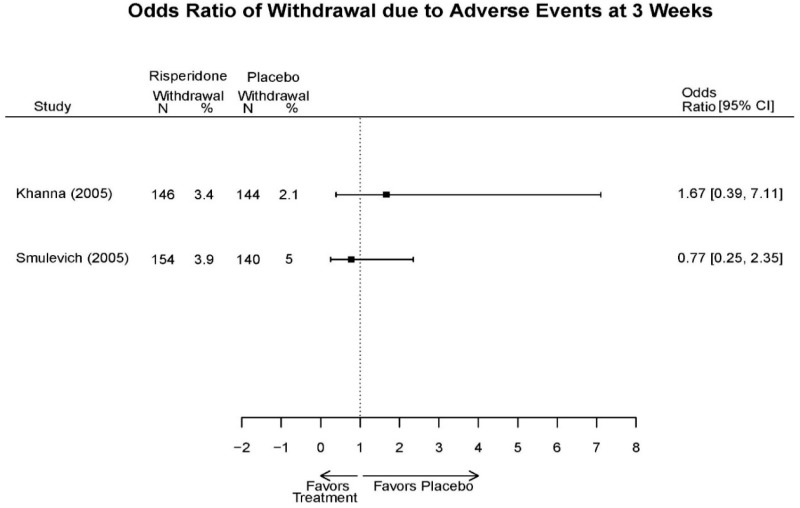
| Smulevich, 200515 RCT Multisite Location NR Industry Moderate 15572276 | N = 438 Mean Age 40 Female 47% White 65% BP-I 100% Outpatient | Manic YMRS ≥ 20 MADRS ≤ 20 First Manic Episode Schizoaffective Substance Abuse Other Mental Health Taking Other Meds | Haloperidol Initiated at 4mg/day increased by 2mg/day up to 12 mg/day | C1: Placebo C2: Risperidone Initiated at 2mg/day increased by 1mg/day up to 6mg/day | 12 weeks (12 week outcomes excluded due to attrition of Haloperidol arm) | Efficacy YMRS CGI GAS MADRS BPRS Extrapyramidal symptoms ESRS Withdrawal 48% at 12 weeks 12% at 3 weeks |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; BARS=Barnes Akathisia Scale; BAS=Behavioral Approach System; BMI=Body Mass Index; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=comparison; CGI= Clinical Global Impressions; CGI-I=Clinical Global Impressions-Improvement; CGI-S =CGI-Severity; CGI-BP=Clinical Global Impressions Scale-Bipolar; CGI-BP-C= Clinical Global Impressions, Bipolar, Change Scale; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; C-SSRS=Columbia Suicide Severity Rating Scale; DAI-10=Drug Attitutde Inventory, 10 question version; DIEPSS=Drug-Induced Extra-Pyramidal Symptoms Scale; DSM=Diagnostic and Statistical Manual of Mental Disorders; EPS=extrapyramidal symptoms; ER=Extended Release; ESRS=Extrapyramidal Symptom Rating Scale; GAF=General Assessment of Functioning Scale; GAS=Global Assessment Scale; HAM-A=Hamilton Scale for Anxiety; HAM-D=Hamilton Scale for Depression; HRQL=Health-related quality of life; HRQOL=Health-related quality of life; I=intervention; IDS=Inventory for Depressive Symptoms; LIFE= Longitudinal Interval Follow-up Evaluation; MADRS=Montgomery-Asberg Depression Rating Scale; MAS=Bech-Rafaelsen Mania Rating Scale; MRS=Mania Rating Scale; MSRS=Manic state rating scale; NOS=not otherwise specified; NR=not reported; PANSS=Positive and Negative Syndrome Scale; PRS=Polygenic Risk Scores; PGWB=Psychological General Well-Being Index; PMID=PubMed Identification Number; PRS=Polygenic Risk Scores; Q-LES-Q=Quality of Life Enjoyment and Satisfaction Questionnaire; RCT=randomized controlled trial; ROB=risk of bias; SADS-C= Schedule for Affective Disorders and Schizophrenia-Change version; SAE=Serious Adverse Events; SAS=Simpson Angus Scale; SF-12=12-Item Short Form Health Survey; SF-36=36-Item Short Form Health Survey; SLICE=Streamlined Longitudinal Interview Clinical Evaluation; T=Trial; YMRS = Young Mania Rating Scale
Appendix Table E18Summary risk of bias assessments: haloperidol for acute mania
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Haloperidol | McIntyre, 200516 Industry 16139175 | High | High dropout rates (46% overall) create a likelihood of bias, lacks some core information on how allocation was concealed and blinding of treatment staff and raters was maintained. |
| Smulevich, 200515 Industry 15572276 | Moderate | Open drugs given to some participants. Pools results for blinded and unblinded without establishing similarity of groups | |
| Sachs, 200217 Industry 12091192 | High | Lacks randomization and blinding procedures. High dropout rates across all arms (46% overall) | |
| Vieta, 201018 Industry 20565430 | High | Large dropout rate among all study arms, across all time periods; raters may not be blinded |
Abbreviations: ITT=Intention to Treat; PMID=PubMed Identification Number; LOCF=last observation carried forward
Haloperidol Forest Plots
Outcomes in studies assessed as having a high risk of bias, or low to moderate risk of bias but at least 40 percent attrition, are presented in grey tones. Both fixed-effect models and random-effects models are presented. We calculated fixed-effect models to provide a charitable estimate of the average effect among completed trials. However, we base our main conclusions on the random-effects models.
Appendix Table E19Outcomes summary table: haloperidol versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
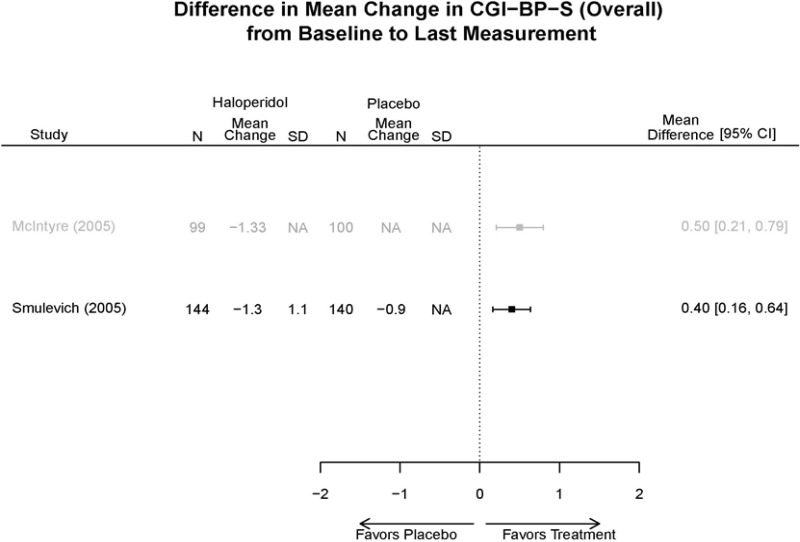
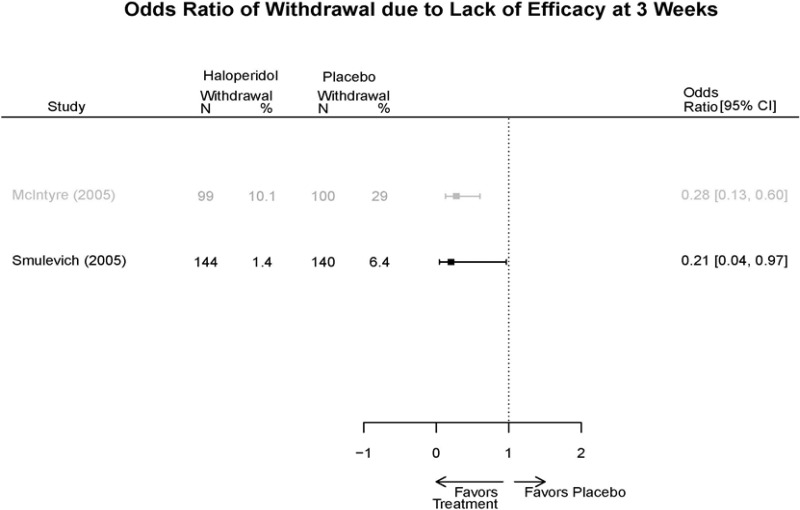
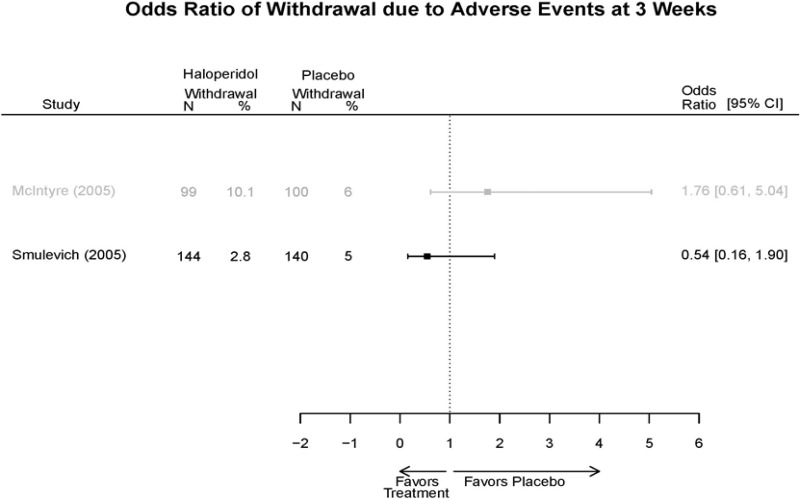
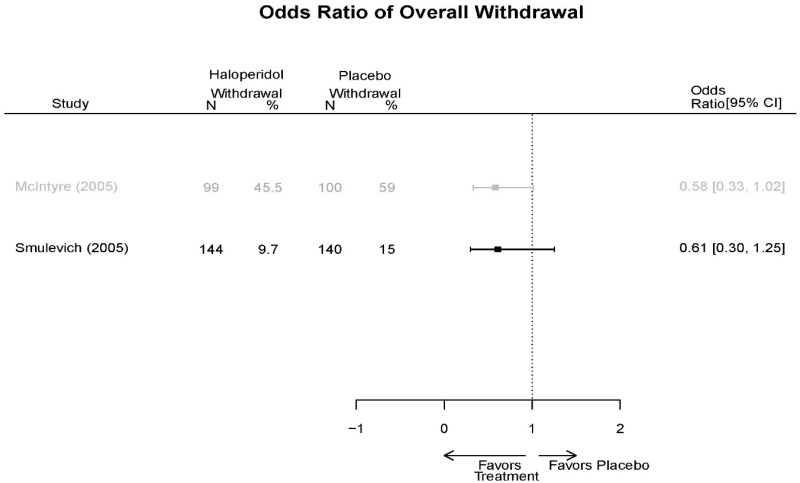
| Haloperidol | McIntyre, 200514 16139175 High | Response See forest plot E34 above for response Remission NS | See forest plot E35 above for YMRS | See forest plot E36 for CGI | See forest plot E37, E38, E39 above for Withdrawals | SAE No reported serious events EPS Haloperidol 35.4% Placebo 5.9% P<0.001 Weight gain 7% NS |
| Smulevich, 200515 15572276 Moderate | Response See forest plot E34 above for response Remission | See forest plot E35 above for YMRS | See forest plot E36 for CGI GAS 3 week Placebo −10.3(1.7) Haloperidol −13.9(10.3) No Statistical Tests reported GAS 12 week Placebo Haloperidol No Statistical Tests reported | See forest plot E37, E38, E39 above for Withdrawals | No reported SAE |
Abbreviations: AE=Adverse Events; ANCOVA=Analysis of Covariance; CGI=Clinical Global Impressions Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; ESRS=Extrapyrimidal Symptom Rating Scale; GAS=Global Assessment Scale; MADRS=Montgomery-Asberg Depression Rating Scale; NR=Not reported; NS=not significant; OR=Odds Ratio; PMID=PubMed Identification Number; ROB=Risk of Bias; SAE=Serious Adverse Events; YMRS = Young Mania Rating Scale
Appendix Table E20Strength of evidence assessment: Haloperidol versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Haloperidol vs. placebo | Relapse 3 wks YMRS CGI-BP-S Withdrawals | 2 RCTs (n=483) | See forest plots | High | Consistent | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 5. Olanzapine for Acute Mania
Appendix Table E21Characteristics of eligible studies: olanzapine for acute mania
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP-I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| Xu, 201519 RCT Single-site Government RoB Low 26060401 | N = 120 Mean Age 31 Female 52% Race NR BP-I 100% Setting NR | First manic; YMRS ≥ 17 Substance Abuse Neurological Disorders Taking Other Meds Pregnant/Nursing | Olanzapine 10 mg/day Flexible dosing 5–20 mg/day | C1: Olanzapine 10 mg/day + Valproate 600 mg/day C2: Valproate 600 mg/day alone | 4 weeks | Efficacy YMRS CGI-BP Adverse events Extrapyramidal symptoms SAS Withdrawal 5% |
| Katagiri, 201220 RCT Single Site Japan Industry RoB Moderate 22134043 | N = 221 Mean Age 45 Female 55% Race NR BP-I 100% Outpatient | Manic or Mixed Episode YMRS ≥ 20 Other Mental Health Taking Other Meds Labs/Other Conditions | Olanzapine Initiated at 10mg/day (5–20mg/day) | Placebo (Haloperidol arm not used <10 per arm completed) | 3 weeks (6 week not abstracted due to attrition) | Adverse Events Extrapyramidal symptoms DIEPSS Efficacy YMRS HAM-D CGI Withdrawal 41% at 3 weeks 52% at 6 weeks |
| Vieta, 201221 RCT Multisite 4 Continents Industry RoB High 22503488 | N = 560 Mean Age 37 Female 52% White 41% BP-I 100% Outpatient | Manic or Mixed Episode; Acute (YMRS ≥ 20 and CGI-S ≥ 4) or non-acute (mood episodes with YMRS <12 and CGI-S ≤3) | Olanzapine 10 mg/day | C1: Placebo C2: Risperidone long-acting injectable (25–50 mg) | 12 weeks (18 month extension for participants without mood recurrence) | Response (YMRS ≤ 19) Time to first recurrence of mood symptoms Efficacy YMRS CGI-S MADRS Adverse events Extrapyramidal symptoms ESRS Withdrawal 29% |
| McIntyre, 20108 RCT Multisite 3 Continents Industry RoB High 20096936 | N = 488 Mean Age 39 Female 47% White 55% BP-I 100% Inpatient and Outpatient | Manic or Mixed Episode; YMRS ≥ 20 First Manic Episode Schizoaffective Substance Abuse Other Mental Health Neurological Disorders Taking Other Meds Pregnant/Nursing Labs/Other Conditions | Olanzapine 5–20 mg/day | C1: Placebo C2: Asenapine 5–10 mg/2 times daily | 3 weeks | Response (≥ 50% YMRS reduction) Time to response (days from baseline to ≥50% YMRS reduction) Remission (≤ 12 YMRS) Efficacy YMRS CGI (BP and mania subscales) MADRS Adverse events Extrapyramidal symptoms SAS BAS AIMS Withdrawal 34% |
| Shafti, 2010 RCT Iran Funding NR RoB Moderate 19740546 | N = 40 Age NR Female 100%; Race NR BP-I 100% Inpatient | Manic (not described) Schizoaffective Substance Abuse Other Mental Health Neurological Disorders Taking Other Meds Labs/Other Conditions | Olanzapine 5 mg/day | Lithium 300 mg/day | 12 weeks | MSRS CGI-S Adverse events Extrapyramidal symptoms Withdrawal 13% |
| McIntyre, 2009 RCT Multisite 3 Continents Industry RoB High 19839993 | N = 489 Mean Age 40 Female 43% White 61% BP-I 100% Inpatient and Outpatient | Manic or Mixed Episode; YMRS ≥ 20 Schizoaffective Substance Abuse Other Mental Health Neurological Disorders Taking Other Meds Pregnant/Nursing Labs/Other Conditions | Olanzapine 5–20 mg/day | C1: Placebo C2: Asenapine 5–10 mg/2 times daily | 3 weeks | Response (YMRS, cutoff NR) Remission (YMRS, cutoff NR) Efficacy YMRS CGI (multiple subscales) MADRS Adverse events Extrapyramidal symptoms SAS BAS AIMS Withdrawal 31% |
| Niufan, 200822 RCT Multisite China Industry RoB Low 17531327 | N = 140 Mean Age 33 Female 74% Race NR Diagnosis NR Outpatient | Manic or Mixed Episode; YMRS ≥ 20 NR | Olanzapine 15 mg/day Flexible dosing 5–20 mg/day | Lithium Carbonate 300–600 mg/day Flexible dosing 600–1800 mg/day divided dose | 4 weeks | Efficacy YMRS CGI BPRS MADRS Extrapyramidal symptoms SAS Withdrawal 15% |
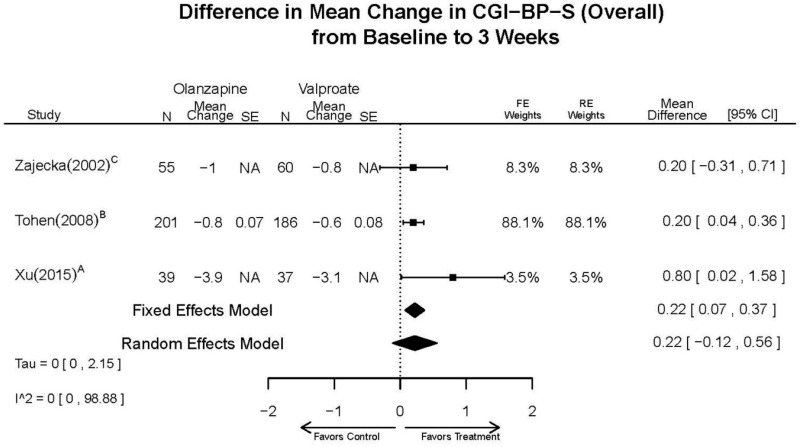
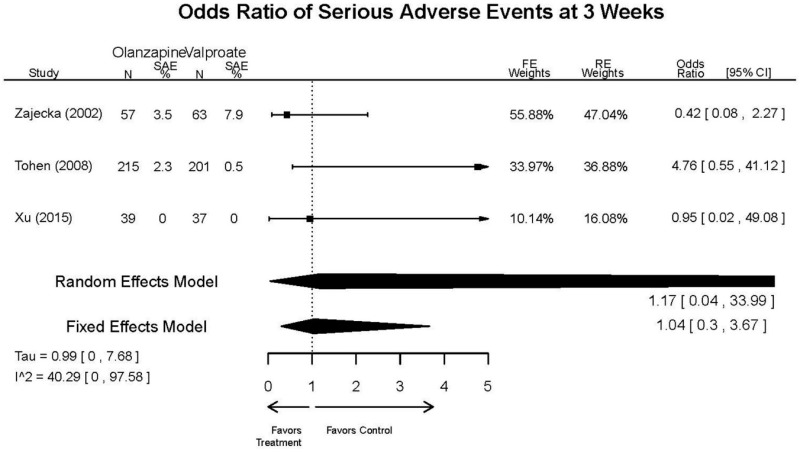
| Tohen, 2008b23 RCT 3 Continents Industry RoB Low 19014751 | N = 521 Mean Age 40 Female 49% Race NR Diagnosis NR Inpatient and Outpatient | Manic or Mixed Episode; YMRS 20–30 CGI-BP mania 3–4 Schizoaffective Other Mental Health Pregnant/Nursing | Olanzapine 5–20 mg/day | C1: Placebo C2: Divalproex 500–2500 mg/2–3 times daily | 12 weeks | Response (≥ 50% YMRS reduction) Time to response (days from baseline to ≥ 50% YMRS reduction) Remission (≤ 12 YMRS) Efficacy YMRS CGI (multiple subscales) MADRS Adverse events Extrapyramidal symptoms SAS BAS AIMS Withdrawal 26% |
| Perlis, 200624 RCT Multisite US Industry RoB High 17196055 | N = 329 Mean Age 38 Female 55% White 74% BP-I 100% Outpatient | Manic; YMRS ≥ 20 Substance Abuse Labs/Other Conditions | Olanzapine 5–20 mg/day | Risperidone 1–6 mg/day | 3 weeks | Efficacy YMRS MADRS CGI HAM-D DAI-10 PGWB SF-12 Extrapyramidal symptoms BAS Simpson-Angus Withdrawal 27% |
| Revicki, 200325 RCT Multisite US Industry RoB Moderate 12716270 Secondary analysis of Zajecka 2002 [PMID 12523875] | N = 52 Mean Age 38 Female 56% White 81% BP-I 100% Inpatient and outpatient | Manic; YMRS ≥ 25 | Olanzapine 20 mg/day | Divalproex 20 mg/kg/day up to an additional 1000 mg/day | 12 weeks (6 and 12 week outcomes excluded due to attrition) | Efficacy YMRS Quality of Life HRQL Q-LES-Q Medical Resource Use Cost Withdrawal (base study Zajecka, 2002 reports withdrawal of 69% at 12 weeks, Revicki reports 35% withdrawal at 3 weeks) |
| Tohen, 200326 RCT Multisite 4 Continents Industry RoB Moderate 14662554 1217758527 | N = 453 Mean Age 40 Female 60% Race NR BP-I 100% Inpatient or outpatient | Manic; YMRS ≥ 20 Substance Abuse Labs/Other Conditions | Olanzapine 15 mg/day flex dosing 5, 10,15, or 20 mg/day | Haloperidol 10 mg/day flex dosing 3, 5, 10, or 15 mg/day | 12 weeks | Illness Severity YMRS HAM-D Quality of Life SF-36 Extrapyramidal Symptoms AIMS BAS Remission Adverse Events Withdrawal 43% |
| Tohen, 2002b28 RCT Multisite US Industry RoB High 12042191 | N = 251 Mean Age 41 Female 57% White 81% BP-I 100% Outpatient | Mania; YMRS ≥ 20 Substance Abuse Other Mental Health Neurological Disorders Taking Other Meds Pregnant/Nursing Labs/Other Conditions | Olanzapine 15 mg/day with flexible dosing from 5–20 mg/day | Divalproex 750mg/day with flexible dosing from 500–2500 mg/day | 3 weeks | Efficacy YMRS HAM-D Remission Adverse Events Extrapyramidal symptom Withdrawal 34% |
| Tohen, 200029 RTC Multisite US Industry RoB High 10986547 | N = 115 Mean Age 39 Female 50% White 80% Diagnosis NR Inpatient (week 1) Outpatient (after week 1 if CGI-BP ≤3) | Mixed Episode; YMRS score ≥ 20 Substance Abuse; Other Mental Health; Neurological Disorders; Taking Other Meds; Labs/Other Conditions | Olanzapine 15 mg/day (adjusted between 5–20 mg/day) | Placebo | 4 weeks | Symptom Severity YMRS HAM-D CGI PANSS Safety EPS Adverse Events Withdrawal 49% |
| Berk, 199930 RCT Single-Site South Africa University RoB High 10565800 | N = 30 Mean Age 31 Sex NR Race NR Diagnosis NR Inpatient | Mania (no other inclusion criteria provided) Other Mental Health; Pregnant/Nursing; Labs/Other Conditions | Olanzapine 10 mg/day | Lithium 400mg twice /day | 4 weeks | Psychiatric Condition PRS CGI MAS Functioning GAF Side Effects SAS Withdrawal NR |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; BARS=Barnes Akathisia Scale; BAS=Behavioral Approach System; BMI=Body Mass Index; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=comparison; CGI= Clinical Global Impressions; CGI-I=Clinical Global Impressions-Improvement; CGI-S =CGI-Severity; CGI-BP=Clinical Global Impressions Scale-Bipolar; CGI-BP-C= Clinical Global Impressions, Bipolar, Change Scale; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; C-SSRS=Columbia Suicide Severity Rating Scale; DAI-10=Drug Attitutde Inventory, 10 question version; DIEPSS=Drug-Induced Extra-Pyramidal Symptoms Scale; DSM=Diagnostic and Statistical Manual of Mental Disorders; EPS=extrapyramidal symptoms; ER=Extended Release; ESRS=Extrapyramidal Symptom Rating Scale; GAF=General Assessment of Functioning Scale; GAS=Global Assessment Scale; HAM-A=Hamilton Scale for Anxiety; HAM-D=Hamilton Scale for Depression; HRQL=Health-related quality of life; HRQOL=Health-related quality of life; I=intervention; IDS=Inventory for Depressive Symptoms; LIFE= Longitudinal Interval Follow-up Evaluation; MADRS=Montgomery-Asberg Depression Rating Scale; MAS=Bech-Rafaelsen Mania Rating Scale; MRS=Mania Rating Scale; MSRS=Manic state rating scale; NOS=not otherwise specified; NR=not reported; PANSS=Positive and Negative Syndrome Scale; PRS=Polygenic Risk Scores; PGWB=Psychological General Well-Being Index; PMID=PubMed Identification Number; PRS=Polygenic Risk Scores; Q-LES-Q=Quality of Life Enjoyment and Satisfaction Questionnaire; RCT=randomized controlled trial; ROB=risk of bias; SADS-C= Schedule for Affective Disorders and Schizophrenia-Change version; SAE=Serious Adverse Events; SAS=Simpson Angus Scale; SF-12=12-Item Short Form Health Survey; SF-36=36-Item Short Form Health Survey; SLICE=Streamlined Longitudinal Interview Clinical Evaluation; T=Trial; YMRS = Young Mania Rating Scale
Appendix Table E22Summary risk of bias assessments: olanzapine for acute mania
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Olanzapine | Xu, 201519 Government 26060401 | Low | Well-constructed, described, and reported study. 5% dropout. |
| Katagiri, 201231 Industry 22134043 | Moderate | Appears to be completely reported and generally well executed, however, dropout rates and LOCF create substantial possibility for bias. | |
| Vieta, 201232 Industry 22503488 | High | High - blinding and randomization procedures not well described. Period II results are biased by the drug assignment being open label. Period three efficacy scores are likely to be biased by the large non-completer rate. | |
| McIntyre, 201012 Industry 20096936 | High | Randomization and blinding procedures not described. 34% dropout. | |
| Shafti, 201033 Funding NR 19740546 | Moderate | Randomization and blinding procedures not described. 13% dropout | |
| McIntyre, 20099 Industry 19839993 | High | Randomization and blinding procedures not described. Patients discharged from hospital at differing times and doesn’t account for this as a possible confounder. 31% overall dropout with high differential dropout between olanzapine and other groups. | |
| Niufan, 200822 Industry 17531327 | Low | Randomization procedure not described. Medication and rater blinded. 16% dropout. | |
| Tohen, 2008b23 Industry 19014751 | Low | Well-constructed and described study. No obvious sources of bias present. 26% dropout. | |
| Perlis, 200634 Industry 17196055 | High | Randomization and blinding procedure not described. 27% dropout. | |
| Revicki, 200325 Industry 12716270 | Moderate | Randomization and blinding procedures not described. 35% before 3 weeks, 52/120 complete 12 week study. Study notes consistency in traits between dropouts and those who complete, which may be an indication that outcomes may be less biased. | |
| Tohen, 200326 Industry 14662554 | Moderate | Randomization procedure not described. 43% dropout. | |
| Shi, 200227 Industry 12177585 | High | Randomization procedure not described, although does note “randomization codes”. States that 166 olanz and 141 halo complete 6 weeks and 140 olanz and 116 halo complete 12 weeks. The counts of patients who complete the follow-up assessments do not match these numbers, in some cases quite substantially. Missing patients not accounted for. Described in the methods that only patients who completed the questionnaire and provided data about the change from baseline to endpoint were included - not ITT. | |
| Tohen, 2002b28 Industry 12042191 | High | Randomization and blinding procedures not described. 33% dropout. | |
| Tohen, 200029 Industry 10986547 | High | Blinding procedures not described. Responders allowed to leave hospital, non-responders were not. 49% Dropout. | |
| Berk, 199930 University 10565800 | High | Randomization and blinding procedures not described. Does not describe handling of participants who drop out. Notes that the groups were matched at baseline by education, marital status, ethnicity, employment status but offers no details on these demographic rates. |
Abbreviations: ITT=Intention to Treat; PMID=PubMed Identification Number; LOCF=last observation carried forward
Olanzapine Forest Plots
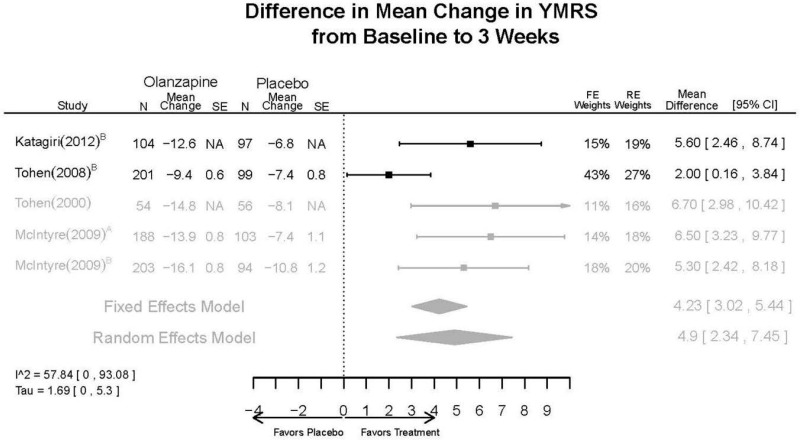
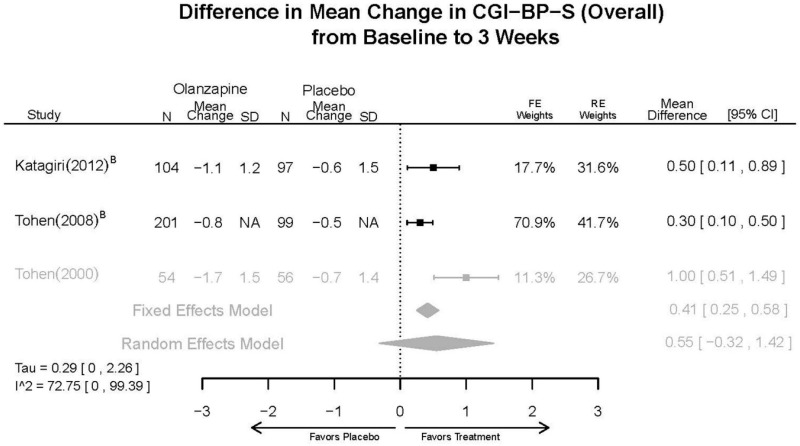
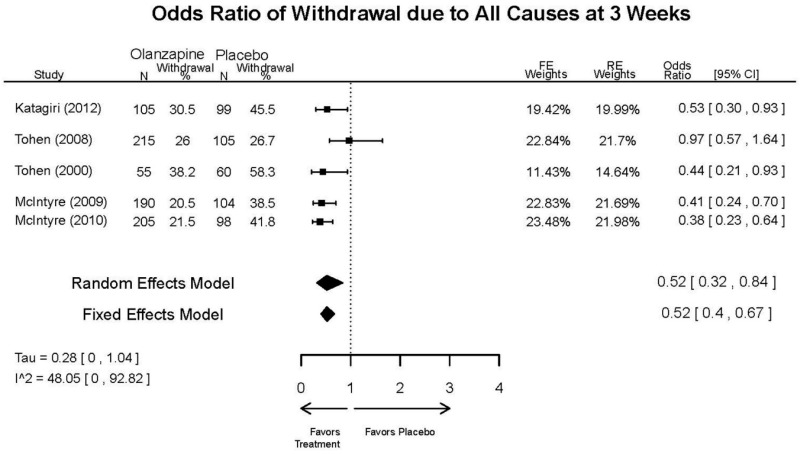
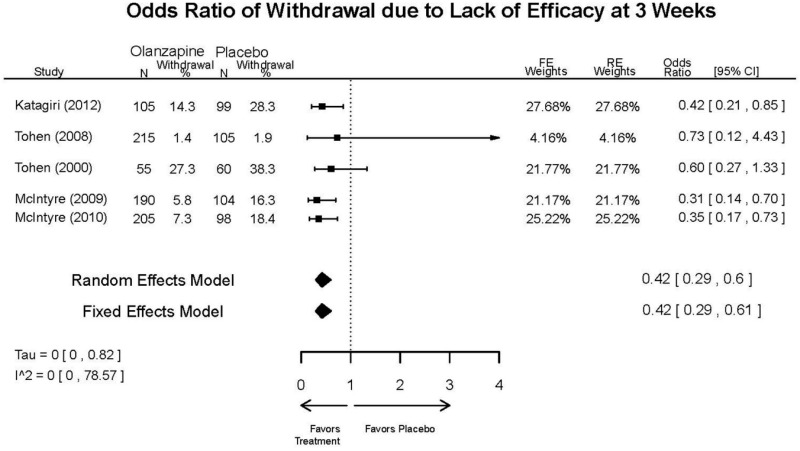
Outcomes in studies assessed as having a high risk of bias, or low to moderate risk of bias but at least 40 percent attrition, are presented in grey tones. Both fixed-effect models and random-effects models are presented. We calculated fixed-effect models to provide a charitable estimate of the average effect among completed trials. However, we base our main conclusions on the random-effects models.
Appendix Table E23Outcomes summary table: olanzapine versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
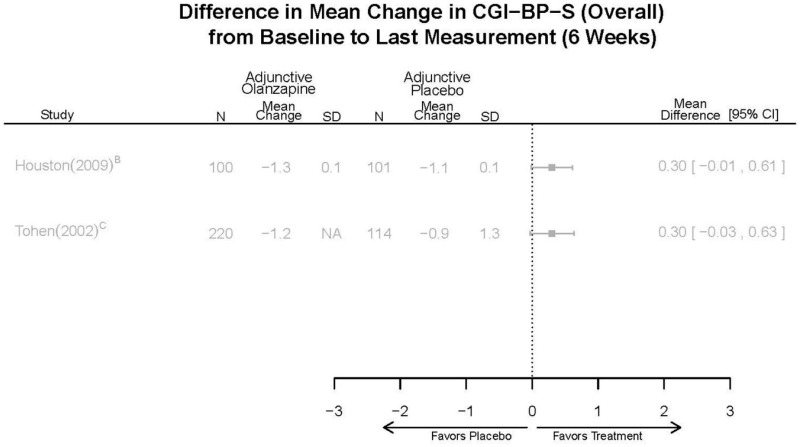
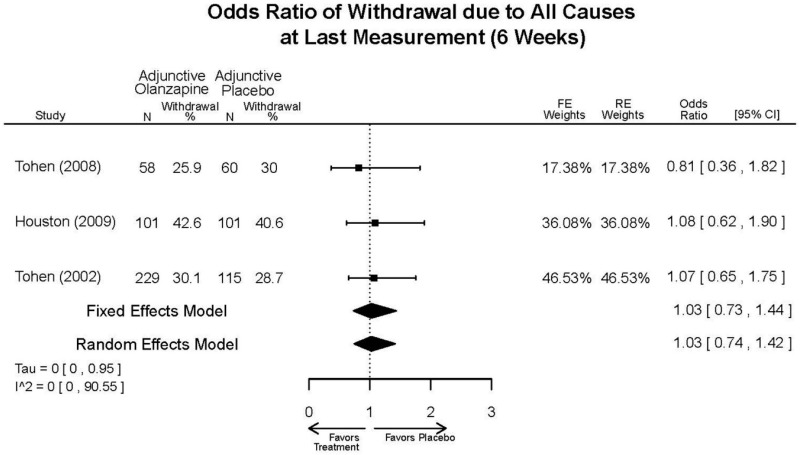
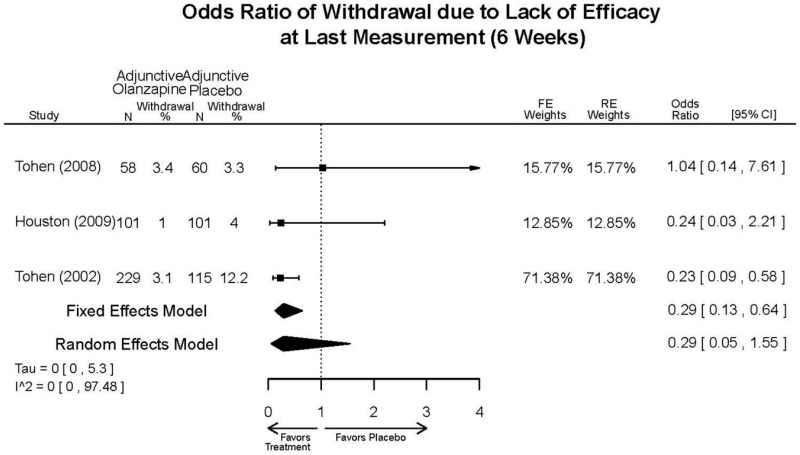
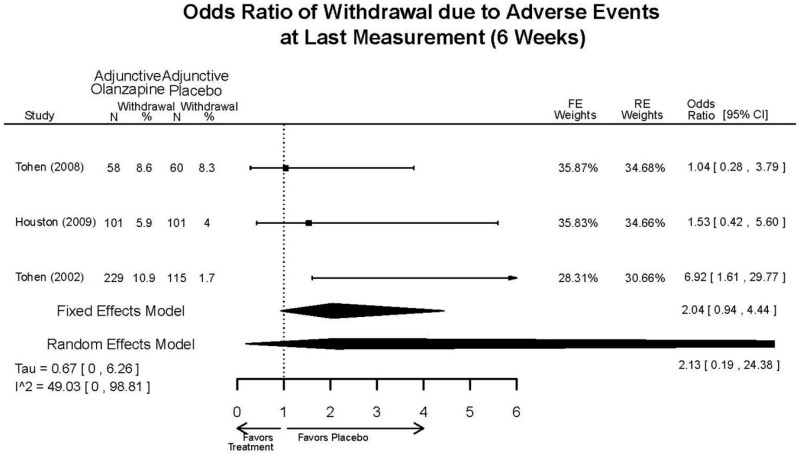
| Olanzapine | Katagiri, 201220 22134043 Moderate | See forest plot E40 above for response. | See forest plot E42 above for YMRS. | See forest plot E43 above for CGI. | See forest plot E44, E45, E46 above for Withdrawals. | See forest plots E47, E48, E49, E50 above for Adeverse Effects. |
| McIntyre, 20108 20096936 High | See forest plot E40 above for response. | See forest plot E42 above for YMRS. | See forest plot E43 above for CGI. | See forest plot E44, E45, E46 above for Withdrawals. | See forest plots E41, E42, E43, E44 above for Adeverse Effects. | |
| McIntyre, 20099 19839993 High | See forest plot E40 above for response. | See forest plot E42 above for YMRS. | See forest plot E43 above for CGI. | See forest plot E44, E45, E46 above for Withdrawals. | See forest plots E47, E48, E49, E50 above for Adeverse Effects. | |
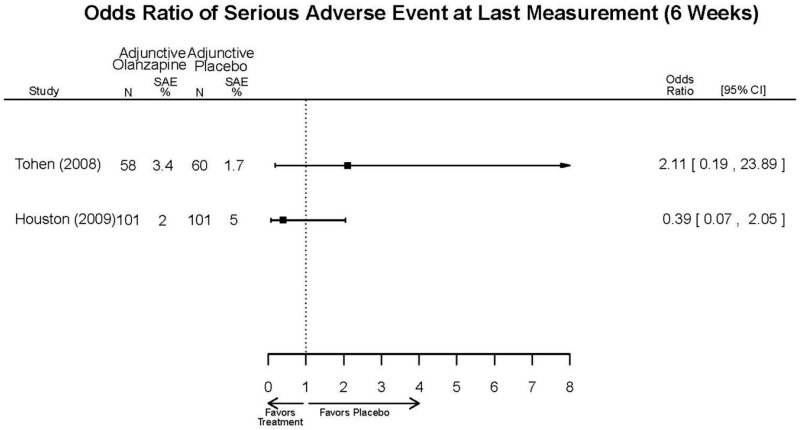
| Tohen, 2008b28 19014751 | See forest plot E40 above for response. | See forest plot E42 above for YMRS. | See forest plot E43 above for CGI. | See forest plot E44, E45, E46 above for Withdrawals. | See forest plots E47, E48, E49, E50 above for Adeverse Effects. Suicidal Ideation Olanzapine: 1 case Placebo: 0 cases | |
| Tohen, 200029 10986547 High | See forest plot E40 above for response. | See forest plot E42 above for YMRS. | See forest plot E43 above for CGI. | See forest plot E44, E45, E46 above for Withdrawals. | See forest plots E47, E48, E49, E50 above for Adeverse Effects. |
Abbreviations: AE=Adverse Events; ANCOVA=Analysis of Covariance; CGI=Clinical Global Impressions Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; ESRS=Extrapyrimidal Symptom Rating Scale; GAS=Global Assessment Scale; MADRS=Montgomery-Asberg Depression Rating Scale; NR=Not reported; NS=not significant; OR=Odds Ratio; PMID=PubMed Identification Number; ROB=Risk of Bias; SAE=Serious Adverse Events; YMRS = Young Mania Rating Scale
Appendix Table E24Strength of evidence assessment: olanzapine versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Olanzapine vs. placebo | Response 3 wks | 5 RCTs (n=1199) | Favors Olanzapine OR 1.99 (95% CI 1.29, 3.08) | Moderate | Consistent | Direct | Imprecise | Low |
| Remission 3 wks | 5 RCTs (n=1199) | Favors Olanzapine OR 1.75 (95% CI 1.19, 2.58) | Moderate | Consistent | Direct | Imprecise | Low | |
| YMRS 3 wks | 5 RCTs (n=1199) | Favors Olanzapine MD 4.9 (95% CI 2.34, 7.45) | Moderate | Consistent | Direct | Imprecise | Low | |
| CGI-BP-S 3 wks | 3 RCTs (n=611) | NS | Moderate | Consistent | Direct | Imprecise | Low | |
| Withdrawal – Lack of Efficacy, Overall | 5 RCTs (n=1199) | Favors Olanzapine | Moderate | Consistent | Direct | Imprecise | Low | |
| Withdrawal – AE | 5 RCTs (n=1199) | NS | Moderate | Consistent | Indirect | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Appendix Table E25Outcomes summary: olanzapine versus active comparator for acute mania
| Comparison | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Olanzapine vs. Haloperidol | Tohen, 200326 14662554 Moderate Shi, 200227 12177585 High | Response 6 weeks NS Olanzapine=169/234 Haloperidol=163/219 P=0.67 12 weeks NS Olanzapine=226/234 Haloperidol=206/219 P=0.42 Remission 6 weeks NS OR = 1.27 (95% CI 0.88, 1.84) P=0.15 12 weeks NS 1.37 (95% CI 0.94, 1.99) P=0.08 | YMRS 6 weeks Favors Haloperidol Difference in Difference=−2.2 (95% CI −4.2, −0.2) p=0.03 12 weeks NS Difference in Difference=−0.3 (95% CI −2.0, 1.4) p=0.72 | NR | Overall Withdrawal 12 weeks NS Olanzapine=94/234 Haloperidol=103/219 p=0.15 Withdrawal due to Aes 12 weeks NS Olanzapine=19/234 Haloperidol=25/219 p=0.27 Withdrawal, Lack of Efficacy 12 weeks NS Olanzapine=35/234 Haloperidol=33/219 | Normalized Weight Change 12 weeks Favors Olanzapine Olanzapine=94/229 Haloperidol=34/211 p<0.001 Emergent Depression 12 weeks NS p=0.10 Akathisia 12 weeks Favors Haloperidol p<0.001 |
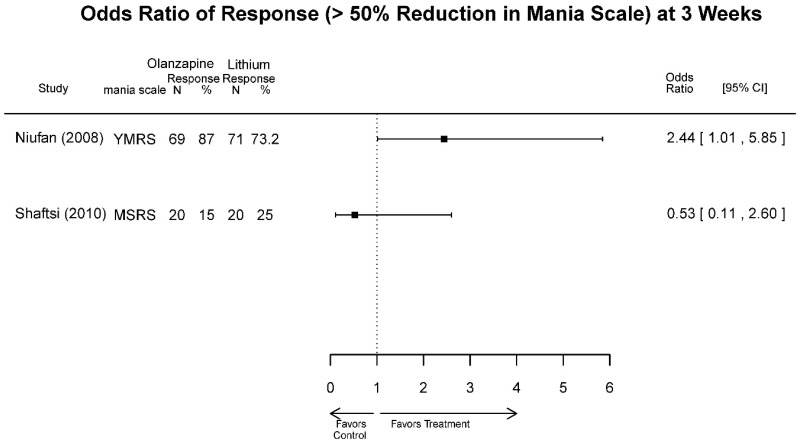
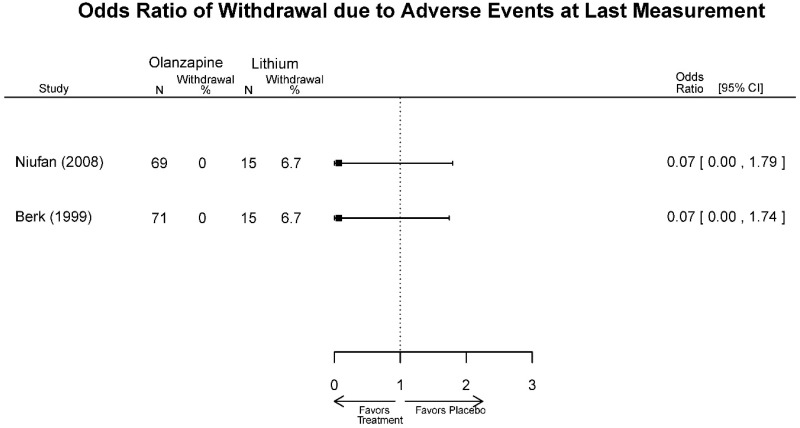
| Olanzapine vs. Lithium | Niufan, 200822 17531327 Low | See forest plot above Remission 4 weeks Olanzapine=57/69 Lithium=50/71 | YMRS 4 weeks Difference in Difference (SE)=−4.5 (1.8) p=0.013 Favors Olanzapine CGI-BP 4 weeks Difference in Difference (SE)=−0.6 (0.2) p=0.009 Favors Olanzapine MADRS 4 weeks Difference in Difference (SE)=-NS | NR | See forest plot above | Severe Harms 4 weeks NS Olanzapine=0/69 Lithium=0/71 EP Symptoms 4 weeks NS Olanzapine=1/69 Lithium=2/71 Normalized Weight Change 4 weeks NS Olanzapine=11/69 Lithium=2/71 Emergent Mood Episodes 4 weeks NS Olanzapine=0/69 Lithium=0/71 |
| Shafti, 201033 19740546 Moderate | See forest plot above | YMRS 3 weeks Favors Lithium Frequency, Difference in Difference= −12.7 (95% CI −19.0, −6.4) P<0.001 Intensity, Difference in Difference= −8.0 (95% CI −13.9, −2.1) P=0.009 CGI, Severity of Illness 3 weeks NS Difference in Difference= −0.1 | NR | See forest plot above | NR | |
| Berk, 199930 10565800 High | NR | Mania Scale 4 weeks NS Difference in Difference=3.1 P=0.32 CGI-BP 4 weeks Favors Olanzapine Difference in Difference=0.5 P=0.03 | NR | See forest plot above Withdrawal, Lack of Efficacy 4 weeks NS Olanzapine=1/15 Lithium=0/15 | NR | |
| Olanzapine vs. Risperidone | Perlis, 200624 17196055 High | Response 3 weeks NS OR = 1.12 (95% CI 0.72, 1.75) P = 0.65 Remission 3 weeks NS OR = 1.57 (95% CI 1.0, 2.51) P = 0.06 | YMRS 3 weeks NS Olanzapine=−15.0 Risperidone=−16.6 | CGI-BP 3 weeks NS Olanzapine=−1.6 Risperidone=−1.5 | Overall Withdrawal 3 weeks Favors Risperidone Olanzapine=35/165 Risperidone=54/164 OR = 0.55 (95% CI 0.33, 0.90) P = 0.019 Withdrawal due to Aes 3 weeks NS Olanzapine=9/165 Risperidone=14/164 OR = 0.62 (95% CI 0.25, 1.48) P = 0.29 Withdrawal, Lack of Efficacy 3 weeks NS Olanzapine=7/165 Risperidone=7/164 | EP Symptoms 3 weeks NS Olanzapine=23/165 Risperidone=37/164 P=0.06 Emergent Depression 3 weeks Olanzapine=2/165 Akathisia 3 weeks NS Olanzapine=13/165 Risperidone=17/164 P=0.45 Suicidality 3 weeks 3 patients in the Risperidone arm were discontinued for suicidality |
| Olanzapine vs. Divalproex/Valproate | Xu, 201519 26060401 Low | NR | YMRS % decrease 4 weeks Olanzapine 75.2 (15.08) Valproate 55.11 (5.72) Favors Olanzapine p<0.01 | NR | See forest plot above | See forest plot above |
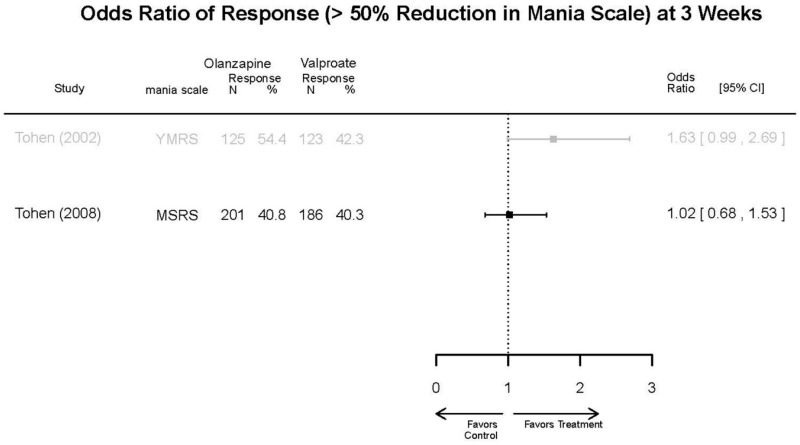
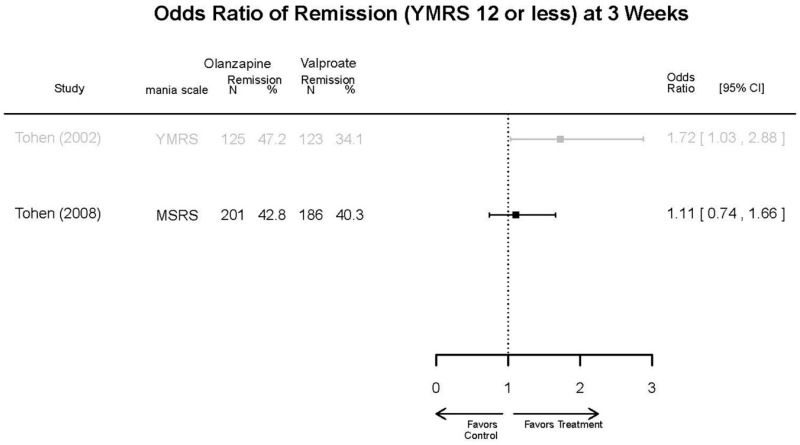
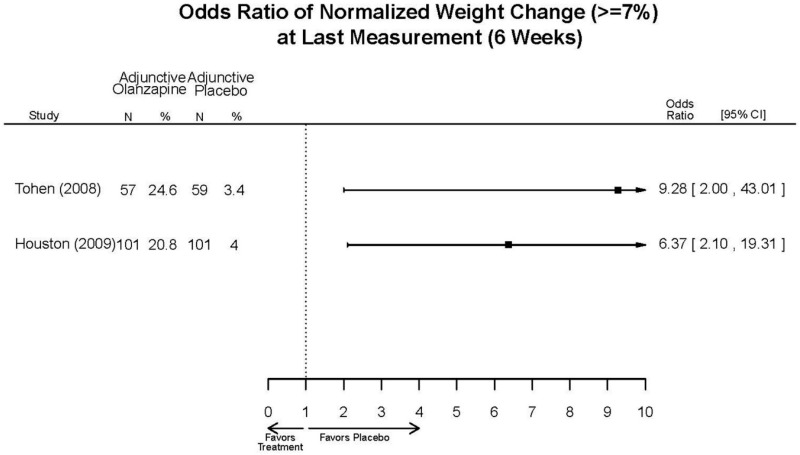
| Tohen, 200823 19014751 Low | See forest plot above | See forest plot above | NR | See forest plot above | Normalized Weight Change 4 weeks Olanzapine=13/202 Divalproex=5/188 Suicide Ideation 3 weeks NS Olanzapine=1/215 Divalproex=0/201 12 weeks NS Olanzapine=2/215 Divalproex=1/201 Weight gain >7% Favored Divalproex p=0.002 | |
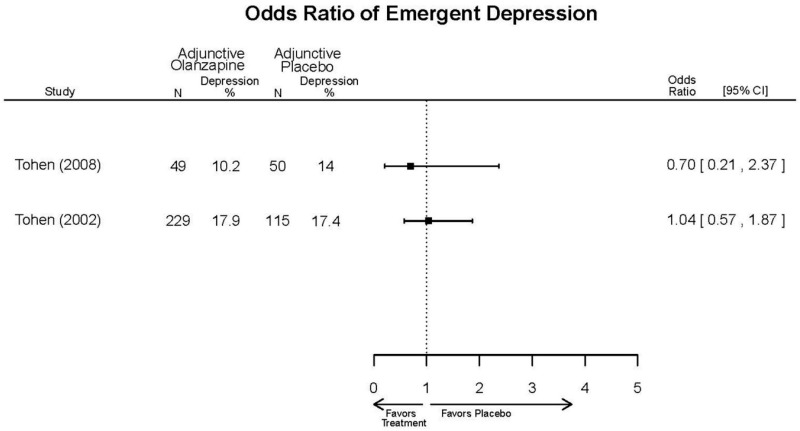
| Zajecka, 200235 12523875 Revicki, 200325 12716270 Moderate | NR | See forest plot above YMRS 4 weeks Favors Olanzapine % Reduction (SD) Olanzapine: 75.2% (15.1%) Divalproex: 55.2% (5.7%) P<0.001 | NR | See forest plot above | See forest plot above Emergent Depression 4 weeks NS Olanzapine: 1/57 Divalproex: 1/63 Deaths 4 weeks 1 Olanzapine-treated patient died from diabetic ketoacidosis | |
| Tohen, 200228 12042191 High | See forest plot above | See forest plot above | NR | See forest plot above | See forest plot above EPS No difference between groups |
Abbreviations: AE=Adverse Events; ANCOVA=Analysis of Covariance; CGI=Clinical Global Impressions Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; ESRS=Extrapyrimidal Symptom Rating Scale; GAS=Global Assessment Scale; MADRS=Montgomery-Asberg Depression Rating Scale; NR=Not reported; NS=not significant; OR=Odds Ratio; PMID=PubMed Identification Number; ROB=Risk of Bias; SAE=Serious Adverse Events; YMRS = Young Mania Rating Scale
Appendix Table E26Strength of evidence assessment: olanzapine versus active comparator for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Olanzapine vs. haloperidol | Response 6,12 wks Remission 6,12 wks YMRS 6, 12 wks Withdrawals | 1 RCT (n=453) | See table above | Moderate | Unknown | Direct | Imprecise | Insufficient |
| Olanzapine vs. Lithium | Response 4 wks | 2 RCTs (n=180) | See forest plot above | Moderate | Inconsistent | Direct | Imprecise | Insufficient |
| YMRS 4 wks CGI 4 wks Withdrawal | 3 RCT (n=210) | See table above | Moderate | Inconsistent | Direct | Imprecise | Insufficient | |
| Olanzapine vs. Risperidone | Response 3 wk Remission 3 wk YMRS 3 wk CGI Withdrawals | 1 RCT (n=329) | See table above | High | Unknown | Direct | Imprecise | Insufficient |
| Olanzapine vs. Divalproex/Valproate | Response Remission | 2 RCTs (n=635) | NS | Moderate | Consistent | Direct | Imprecise | Low |
| YMRS | 3 RCTs (n=750) | NS | Moderate | Consistent | Direct | Imprecise | Low | |
| CGI | 3 RCTs (n=578) | NS | Moderate | Consistent | Direct | Imprecise | Low | |
| Withdrawals | 4 RCTs (n=867) | NS | Moderate | Consistent | Direct | Imprecise | Low |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 6. Quetiapine for Acute Mania
Appendix Table E27Characteristics of eligible studies: quetiapine for acute mania
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP-I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
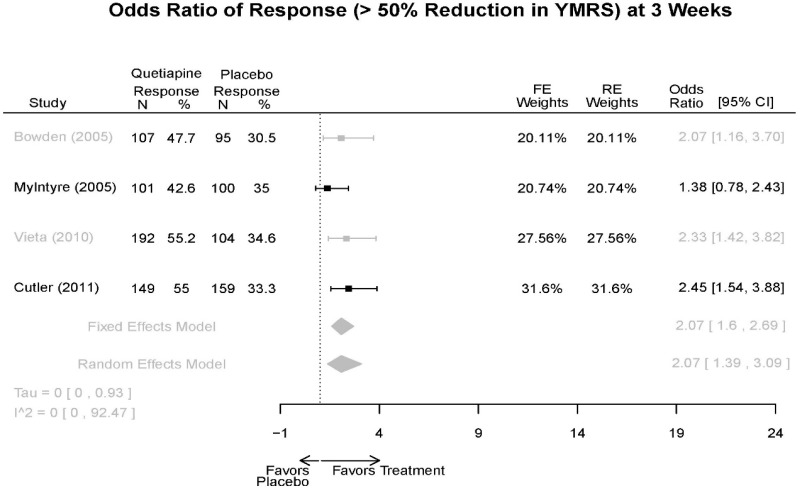
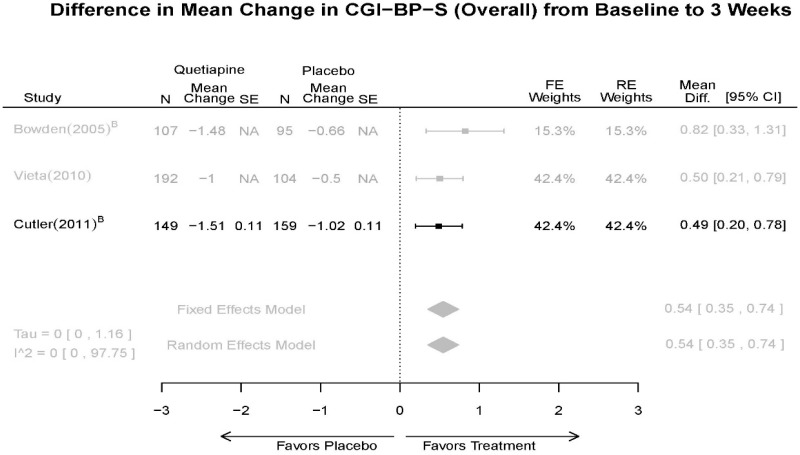
| Cutler, 201136 RCT Multisite US Industry RoB Low 22054797 | N = 316 Mean Age 41 Female 40% White 47% BP-I 100% Inpatient (days 1–4, minimum) Outpatient (at inspectors discretion) | Mania; YMRS ≥ 20 overall, YMRS ≥ 4 on at least 2 of 4 specifed mania domains, and CGI-BP-S ≥ 4 First Manic Episode Schizoaffective Substance Abuse Other Mental Health Labs/Other Conditions | Quetiapine ER 300–800 mg/day (603.8 mg/day mean) | Placebo | 3 Weeks | CGI-BP-S CGI-BP-C MADRS Remission (YMRS≤12) Response (YMRS 50% decrease) YMRS Withdrawal 29% |
| McElroy, 201037 Singlesite US Industry RoB Low 19963274 | N = 41 Mean Age 35 Female 51% White 69% BP-I 74% BP-II 21% BP-NOS 5% Outpatient | Mild to moderate hypomania or mild mania; CGI-BP ≥3 AND <5 Substance Abuse Other Mental Health Neurological Disorders Pregnant Nursing Labs/Other Conditions | Quetiapine 50–800 mg/day (232 mg/day mean) | Placebo | 8 weeks | CGI-BP-S GAF HAM-A IDS Remission (YMRS ≤7 at week 8, CGI-BP Overall ≤2 at week 8, Improvement or no change in IDS score from baseline to week 8) Response (YMRS 50% decrease) YMRS Withdrawal 36% |
| Vieta, 201018 RCT Multisite 3 continents Industry RoB High 20565430 | N = 493 Mean Age 39 Female 42% Race NR BP-I 100% Inpatient (1 week) Outpatient (weeks 2–3, subject to inspector discretion) | Mania; YMRS ≥ 20 First Manic Episode Schizoaffective Substance Abuse Neurological Disorders | Quetiapine 400–800 mg/day (600 mg/day mean) | Placebo (Palidperidone arm discussed in Other Drugs section) | 3 weeks | Duration of Episode YMRS GAF PANSS CGI-BP-S SAS AIMS MADRS Withdrawal 28% |
| Li, 200838 RCT Multisite China Industry RoB Moderate 18028587 | N = 154 Mean Age 33 Female 53% Race NR Diagnosis NR Inpatient (weeks 1–2) Outpatient (week 2–4, subject to inspector discretion) | Mania; YMRS ≥ 20 Substance Abuse Taking Other Meds Pregnant/Nursing Labs/Other Conditions | Quetiapine 100–800 mg/day (648.2 mg/day mean) | Lithium 250–2000 mg/day (target serum level 0.6–1.2 mmol/L) (0.80 mmol/L average) | 4 weeks | MADRS PANSS Remission (various definitions) Response (YMRS 50% decrease) Weight YMRS Withdrawal 12% |
| Bowden, 200539 RCT Multisite 2 Continents Industry RoB High 15669897 | N = 302 Mean Age 39 Female 24% Race NR BP-I 100% Inpatient | Mania; YMRS ≥ 20 including score of at least 4 on 2 of the 4 double-weighted items (irritability, speech, content, and disruptive/aggressive behavior), CGI ≥4 First Manic Episode Substance Abuse Taking Other Meds Pregnant/Nursing Labs/Other Conditions | Quetiapine 100–800mg/day | Placebo Lithium 0.6–1.4 mEq/L (mean 0.80 mEq/L) | 12 weeks | CGI-BP-S Overall Global Assessment Scale (GAS) MADRS PANSS (positive) Remission (YMRS≤12) Response (50% decrease in YMRS) YMRS Withdrawal 48% |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; BARS=Barnes Akathisia Scale; BAS=Behavioral Approach System; BMI=Body Mass Index; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=comparison; CGI= Clinical Global Impressions; CGI-I=Clinical Global Impressions-Improvement; CGI-S =CGI-Severity; CGI-BP=Clinical Global Impressions Scale-Bipolar; CGI-BP-C= Clinical Global Impressions, Bipolar, Change Scale; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; C-SSRS=Columbia Suicide Severity Rating Scale; DAI-10=Drug Attitutde Inventory, 10 question version; DIEPSS=Drug-Induced Extra-Pyramidal Symptoms Scale; DSM=Diagnostic and Statistical Manual of Mental Disorders; EPS=extrapyramidal symptoms; ER=Extended Release; ESRS=Extrapyramidal Symptom Rating Scale; GAF=General Assessment of Functioning Scale; GAS=Global Assessment Scale; HAM-A=Hamilton Scale for Anxiety; HAM-D=Hamilton Scale for Depression; HRQL=Health-related quality of life; HRQOL=Health-related quality of life; I=intervention; IDS=Inventory for Depressive Symptoms; LIFE= Longitudinal Interval Follow-up Evaluation; MADRS=Montgomery-Asberg Depression Rating Scale; MAS=Bech-Rafaelsen Mania Rating Scale; MRS=Mania Rating Scale; MSRS=Manic state rating scale; NOS=not otherwise specified; NR=not reported; PANSS=Positive and Negative Syndrome Scale; PRS=Polygenic Risk Scores; PGWB=Psychological General Well-Being Index; PMID=PubMed Identification Number; PRS=Polygenic Risk Scores; Q-LES-Q=Quality of Life Enjoyment and Satisfaction Questionnaire; RCT=randomized controlled trial; ROB=risk of bias; SADS-C= Schedule for Affective Disorders and Schizophrenia-Change version; SAE=Serious Adverse Events; SAS=Simpson Angus Scale; SF-12=12-Item Short Form Health Survey; SF-36=36-Item Short Form Health Survey; SLICE=Streamlined Longitudinal Interview Clinical Evaluation; T=Trial; YMRS = Young Mania Rating Scale
Appendix Table E28Summary risk of bias assessments: quetiapine for acute mania
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Quetiapine | McElroy, 201037 Industry 19963274 | Moderate | No specific sources of bias identified. Attrition rate 36% |
| Cutler, 201136 Industry 22054797 | Low | Blinding not described. No other sources of bias identified. | |
| Vieta, 201018 Industry 20565430 | High | Blinding not described; large dropout in placebo group (41%). | |
| Li, 200838 Industry 18028587 | Moderate | Randomization and blinding procedure not described. | |
| Bowden, 200539 Industry 15669897 | High | Randomization and blinding procedure not described; >50% dropout in placebo group at day 84; 33% at 3 weeks | |
| McIntyre, 200514 Industry 16139175 | Moderate | Dropout rate for quetiapine and placebo in 30%–40% range, lacks some core information on how allocation was concealed and blinding of treatment staff and raters was maintained. Author notes may be underpowered for quetiapine vs. haloperidol comparison. |
Abbreviations: ITT=Intention to Treat; PMID=PubMed Identification Number; LOCF=last observation carried forward
Quetiapine Forest Plots
Outcomes in studies assessed as having a high risk of bias, or low to moderate risk of bias but at least 40 percent attrition, are presented in grey tones. Both fixed-effect models and random-effects models are presented. We calculated fixed-effect models to provide a charitable estimate of the average effect among completed trials. However, we base our main conclusions on the random-effects models.
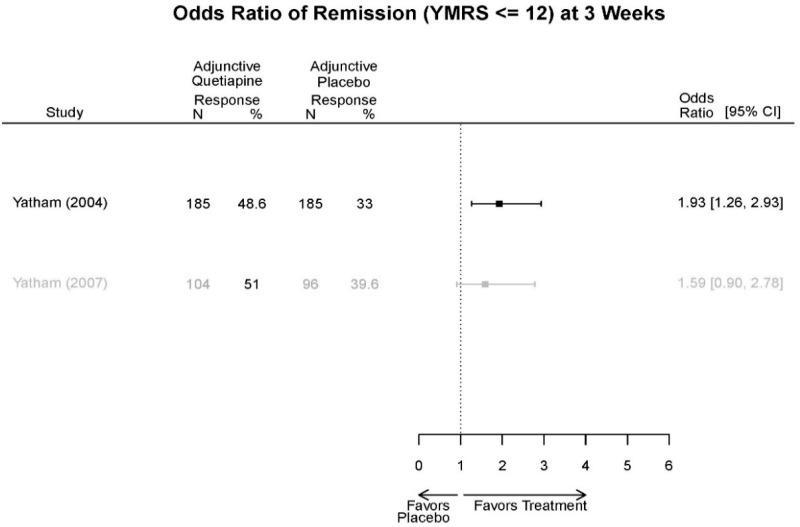
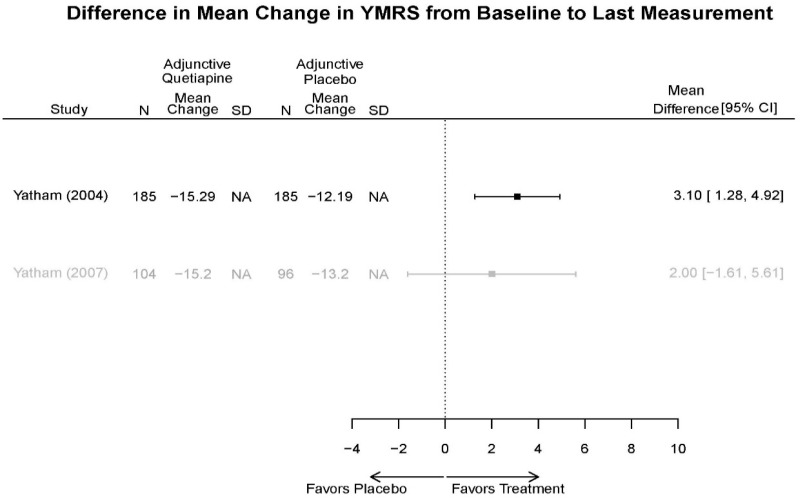
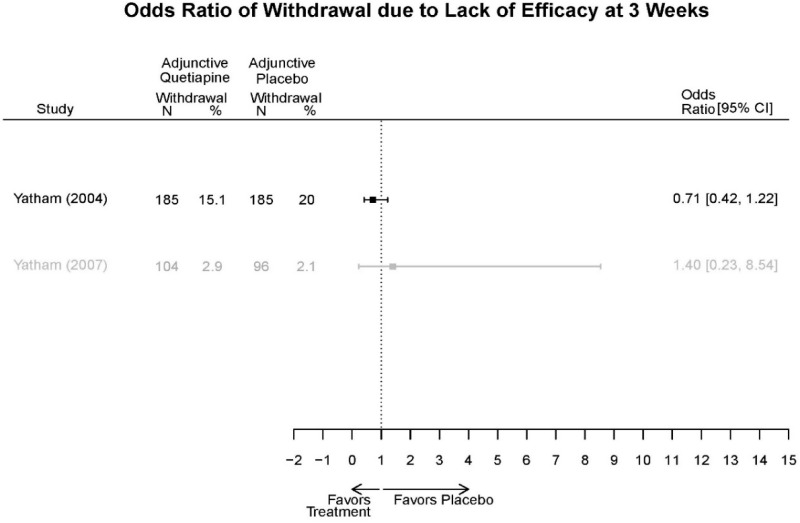
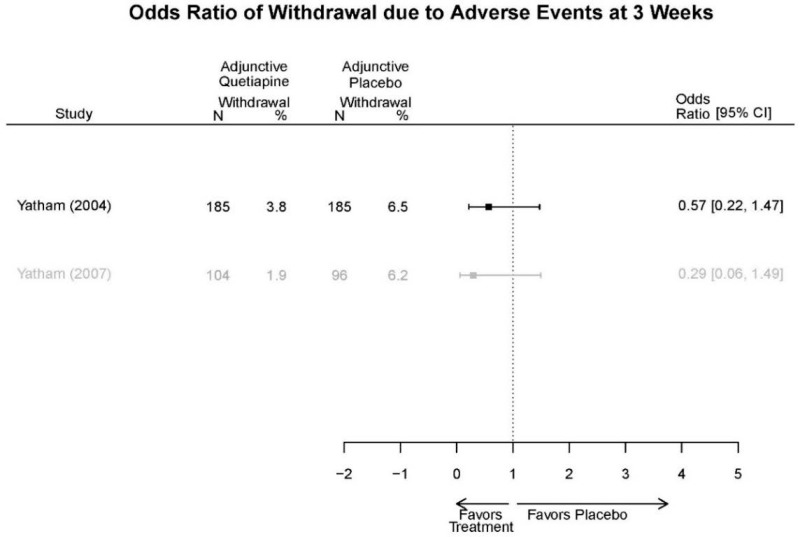
Appendix Table E29Outcomes summary: quetiapine versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Quetiapine | McElroy, 201037 19963274 Moderate | Response 8 weeks Completers only reported Remission 8 weeks Completers only reported | YMRS 8 weeks Regression model NS (p=0.06) | CGI 8 weeks Regression model Favors Quetiapine (p<0.001) GAF 8 weeks Regression model NS | Overall Withdrawal Quetiapine 6/21 Placebo 8/20 Withdrawal Lack of efficacy Quetiapine 0/21 Placebo 2/20 Withdrawal AE Quetiapine 2/21 Placebo 1/20 | Serious Adverse Events 3 weeks 3 placebo – 2 suicide, 1 death, 1 quetiapine – suicide attempt Deaths 3 weeks 1 placebo EPS 3 weeks 3.8% placebo 6.6% Quetiapine |
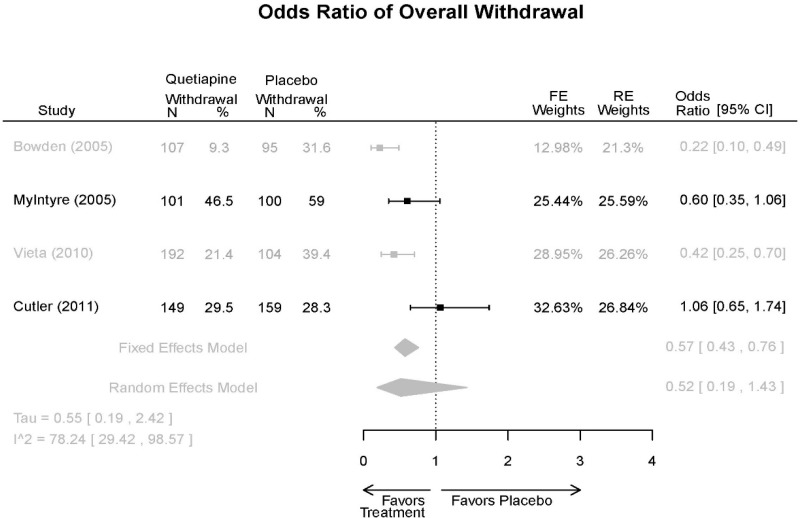
| Cutler, 201136 22054797 Low | See forest plot E62 above for Response. | See forest plot E64 above for YMRS. | See forest plot E65 above for CGI | See forest plots E66, E67, E68 above for withdrawals | SAE Quetiapine 4.0% Placebo 8.1% Deaths 3 placebo – 2 suicide, 1 death, 1 quetiapine – suicide attempt EPS 3 weeks 3.8% placebo 6.6% Quetiapine | |
| Vieta, 201018 20565430 High | See forest plot E562 above for Response. | See forest plot E64 above for YMRS. | See forest plot E65 above for CGI | See forest plots E66, E67, E68 above for withdrawals | SAE Reported no difference 1 suicide reported Akathisia Quetiapine 6 (3%) Placebo 3 (3%) | |
| Bowden, 200539 15669897 High | See forest plot E62 above for Response. | See forest plot E64 above for YMRS. | See forest plot E65 above for CGI | See forest plots E66, E67, E68 above for withdrawals | SAE None reported EPS Reported no difference Weight gain >7% Quetiapine more frequent (p=0.008) | |
| McIntyre, 200514 16139175 Moderate | See forest plot E62 above for Response. | YMRS 3 weeks ANCOVA model Favors Quetiapine p=.01 Results sustained at 12 weeks | CGI 3 weeks ANCOVA model Favors Quetiapine (p<0.05) Results sustained at 12 weeks | See forest plots E66, E67, E68 above for withdrawals | SAE None reported EPS Quetiapine 13 (12.7%) Placebo 16 (15.8%) NS Weight gain >7% Quetiapine 12.8% Placebo 4% NS |
Abbreviations: AE=Adverse Events; ANCOVA=Analysis of Covariance; CGI=Clinical Global Impressions Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; ESRS=Extrapyrimidal Symptom Rating Scale; GAS=Global Assessment Scale; MADRS=Montgomery-Asberg Depression Rating Scale; NR=Not reported; NS=not significant; OR=Odds Ratio; PMID=PubMed Identification Number; ROB=Risk of Bias; SAE=Serious Adverse Events; YMRS = Young Mania Rating Scale
Appendix Table E30Strength of evidence assessment: quetiapine versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
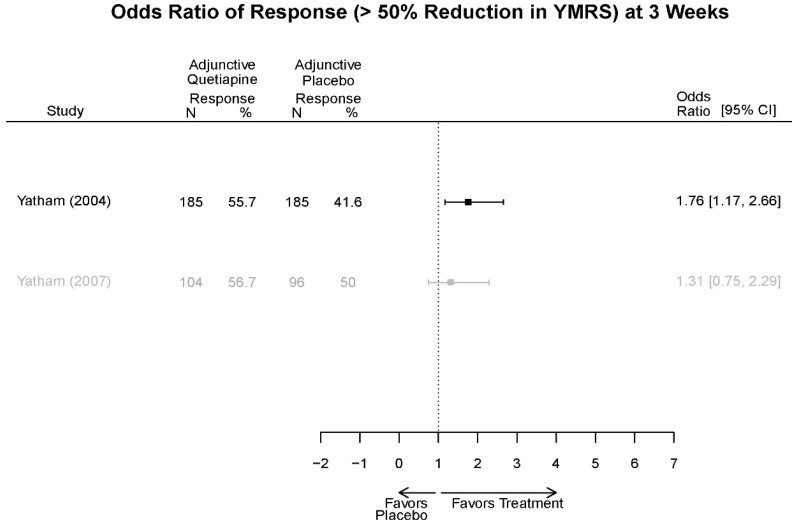
| Quetiapine vs. placebo | Response 3 wks | 4 RCT (n=1,007) | Favors Quetiapine OR 2.07 (95% CI 1.39, 3.09) | Moderate | Consistent | Direct | Imprecise | Low |
| Remission 3 wks | 3 RCT (n=699) | NS | High | Consistent | Direct | Imprecise | Insufficient | |
| YRMS 3 wks | 5 RCT (n=699 forest plot, 1439 total) | Favors Quetiapine MD 4.92 (95% CI 0.31, 9.53) | Moderate | Inconsistent | Direct | Imprecise | Low | |
| CGI-BP-S 3 wk | 5 RCT (n=806 forest plot, 1439 total) | Favors Quetiapine Mean Difference 0.54 (95% CI 0.35, 0.74) | Moderate | Consistent | Direct | Imprecise | Low | |
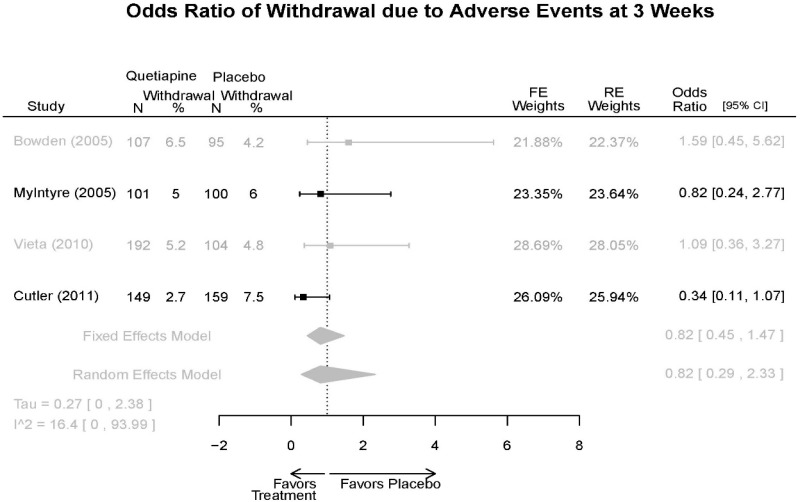
| Withdrawal – AE, Overall | 4 RCT (n=1,007) | NS | Moderate | Consistent | Indirect | Imprecise | Insufficient | |
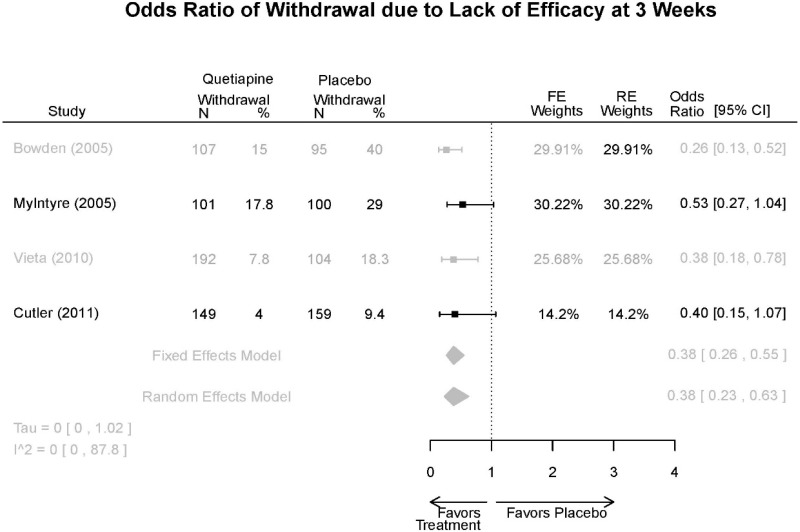
| Withdrawal –Lack of Efficacy | 4 RCT (n=1,007) | Favors Quetiapine Mean Difference 0.38 (95% CI 0.23, 0.63) | Moderate | Consistent | Direct | Imprecise | Low |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Appendix Table E31Outcomes summary: quetiapine versus active comparator for acute mania
| Comparison | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Quetiapine vs. haloperidol | McIntyre, 200516 16139175 Moderate | Response 3 weeks Quetiapine 42.6% Haloperidol 56.1% NS Remission 3 weeks Quetiapine 27.7% Haloperidol 36.7% NS | YMRS Change 3 week Quetiapine −12.29 Haloperidol −15.71 No Statistical Tests reported | CGI-BP-S Change 3 week Quetiapine −1.02 Haloperidol −1.33 No Statistical Tests reported GAS 12 weeks Favors quetiapine against placebo p < .001 12 weeks Favors haloperidol against placebo p < .001 | NR Overall Withdrawal Quetiapine 55/102 Haloperidol 45/99 Withdrawal Lack of Efficacy Quetiapine 18/102 Haloperidol 10/99 Withdrawal AE Quetiapine 5/102 Haloperidol 10/99 | Serious Adverse Events 12 weeks 0 in both arms Deaths 12 weeks 0 in both arms EPS 12 weeks 12.7% Quetiapine 59.6% Haloperidol |
| Quetiapine vs. lithium | Bowden, 2005 15669897 | Response 12 weeks Quetiapine 72.0% Lithium 75.5% No Statistical Tests reported Remission 12 weeks Quetiapine 69.2% Lithium 72.4% No Statistical Tests reported | YMRS Change 12 weeks Quetiapine −20.28 Lithium −20.76 NS | CGI-BP-S Change 12 weeks Quetiapine −2.20 Lithium −2.18 No Statistical Tests reported GAS Change 12 weeks Quetiapine 26.35 Lithium NR No Statistical Tests reported | NR | Serious Adverse Events 12 weeks 0 in both arms Deaths 12 weeks 0 in both arms EPS 12 weeks 9.3% placebo 13.1% Quetiapine NR Lithium |
| Li, 2008 18028587 Moderate | Response 4 weeks Favors quetiapine Quetiapine 77.9% Lithium 59.7% p=0.013 Remission 4 weeks Favors quetiapine Quetiapine 70.1% Lithium 48.1% p=0.007 | YMRS Change 4 weeks Quetiapine −18.2 (10.4) Lithium −15.9 (12.2) NS | NR | NR | Serious Adverse Events 4 weeks 0 in both arms Deaths 4 weeks 0 in both arms EPS 4 weeks 5.1% Quetiapine 6.5% lithium |
Abbreviations: AE=Adverse Events; ANCOVA=Analysis of Covariance; CGI=Clinical Global Impressions Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; ESRS=Extrapyrimidal Symptom Rating Scale; GAS=Global Assessment Scale; MADRS=Montgomery-Asberg Depression Rating Scale; NR=Not reported; NS=not significant; OR=Odds Ratio; PMID=PubMed Identification Number; ROB=Risk of Bias; SAE=Serious Adverse Events; YMRS = Young Mania Rating Scale
Appendix Table E32Strength of evidence assessment: quetiapine versus active comparator for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Quetiapine vs. haloperidol | Response Remission YMRS CGI Withdrawals | 1 RCT (n=199) | See table above | Moderate | Unknown | Direct | Imprecise | Insufficient |
| Quetiapine vs. lihtium | Response Remission YMRS Withdrawals | 2 RCTs (n=456) | See table | High | Inconsistent | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 7. Risperidone for Acute Mania
Appendix Table E33Characteristics of eligible studies: Risperidone for acute mania
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP-I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| Smulevich, 200515 RCT Multisite 2 Continents Industry RoB Moderate 15572276 | N = 438 Mean Age 40 Female 47% White 65% BP-I 100% Inpatient | Mania; YMRS ≥ 20 and MADRS ≤ 20 First Manic Episode Schizoaffective Substance Abuse Other Mental Health Taking Other Meds | Risperidone 1–6 mg/day (mean 4.2 mg/day) | C1: Placebo C2: Haloperidol 2–12 mg/day (mean 8.0 mg/day) | 12 weeks (12 week outcomes excluded due to attrition | BPRS CGI-S GAS MADRS YMRS Withdrawal 48% at 12 weeks 12% at 3 weeks |
| Khanna, 200540 RCT Multisite India Industry RoB Moderate 16135859 | N = 290 Mean Age 35 Female 38% Race NR BP-I 100% Inpatient | Mania; YMRS ≥ 20 Schizoaffective Substance Abuse Other Mental Health Taking Other Meds | Risperidone 1–6 mg/day | Placebo | 3 weeks | CGI-S MADRS PANSS YMRS Withdrawal 20% |
| Segal, 199841 RCT Singlesite South Africa Industry/University RoB Moderate 9617509 | N = 45 Mean Age 34 Female 78% Race NR BP-I 100% Inpatient | Mania; DSM-IV criteria for Bipolar Manic Phase Substance Abuse Taking Other Meds Pregnant/Nursing Labs/Other Conditions | Risperidone 6 mg/day | C1: Placebo C2: Haloperidol 10 mg/day C3: Lithium 0.6–1.2 mmol/L | 4 weeks | BPRS CGI Unknown Scale - Not reported whether global improvement or severity scale is being reported GAF MRS Seclusion - Hours of eclusion - Proportion of patients needing SAS Withdrawal NR |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; BARS=Barnes Akathisia Scale; BAS=Behavioral Approach System; BMI=Body Mass Index; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=comparison; CGI= Clinical Global Impressions; CGI-I=Clinical Global Impressions-Improvement; CGI-S =CGI-Severity; CGI-BP=Clinical Global Impressions Scale-Bipolar; CGI-BP-C= Clinical Global Impressions, Bipolar, Change Scale; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; C-SSRS=Columbia Suicide Severity Rating Scale; DAI-10=Drug Attitutde Inventory, 10 question version; DIEPSS=Drug-Induced Extra-Pyramidal Symptoms Scale; DSM=Diagnostic and Statistical Manual of Mental Disorders; EPS=extrapyramidal symptoms; ER=Extended Release; ESRS=Extrapyramidal Symptom Rating Scale; GAF=General Assessment of Functioning Scale; GAS=Global Assessment Scale; HAM-A=Hamilton Scale for Anxiety; HAM-D=Hamilton Scale for Depression; HRQL=Health-related quality of life; HRQOL=Health-related quality of life; I=intervention; IDS=Inventory for Depressive Symptoms; LIFE= Longitudinal Interval Follow-up Evaluation; MADRS=Montgomery-Asberg Depression Rating Scale; MAS=Bech-Rafaelsen Mania Rating Scale; MRS=Mania Rating Scale; MSRS=Manic state rating scale; NOS=not otherwise specified; NR=not reported; PANSS=Positive and Negative Syndrome Scale; PRS=Polygenic Risk Scores; PGWB=Psychological General Well-Being Index; PMID=PubMed Identification Number; PRS=Polygenic Risk Scores; Q-LES-Q=Quality of Life Enjoyment and Satisfaction Questionnaire; RCT=randomized controlled trial; ROB=risk of bias; SADS-C= Schedule for Affective Disorders and Schizophrenia-Change version; SAE=Serious Adverse Events; SAS=Simpson Angus Scale; SF-12=12-Item Short Form Health Survey; SF-36=36-Item Short Form Health Survey; SLICE=Streamlined Longitudinal Interview Clinical Evaluation; T=Trial; YMRS = Young Mania Rating Scale
Appendix Table E34Summary risk of bias assessments: risperidone for acute mania
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Risperidone | Smulevich, 200515 Industry 15572276 | Moderate | Randomization and blinding procedures not well-described. Some participants treated in open-label fashion. 12% dropout. |
| Khanna, 200540 Industry 16135859 | Moderate | Randomization and blinding procedures not described. Handling of data from missing persons not described. 20% dropout. | |
| Segal, 199841 Industry/University 9617509 | Moderate | Randomization not described, patients assigned consecutively which infers both a lack of randomization and a likelihood of a lack of allocation concealment. |
Abbreviations: ITT=Intention to Treat; PMID=PubMed Identification Number; LOCF=last observation carried forward
Risperidone Forest Plots
Outcomes in studies assessed as having a high risk of bias, or low to moderate risk of bias but at least 40 percent attrition, are presented in grey tones. Both fixed-effect models and random-effects models are presented. We calculated fixed-effect models to provide a charitable estimate of the average effect among completed trials. However, we base our main conclusions on the random-effects models.
Appendix Table E35Outcomes summary: risperidone versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Risperidone | Khanna, 200540 16135859 Moderate | See forest plot E69 above for Response | See forest plot E70 above for YMRS MADRS 3 weeks Mean change Risperidone: −3.2 (0.4) Placebo: −2.5, (0.34) P<0.001) | See forest plot E70 above for CGI | See forest plot E72, E73 above for Withdrawal | Serious Adverse Events 3 weeks 0 in both arms Deaths 3 weeks 0 in both arms EPS 3 weeks 6.0% placebo 36.0% Risperidone |
| Smulevich, 200515 15572276 Moderate | See forest plot E69 above for Response | See forest plot E70 above for YMRS | See forest plot E70 above for CGI GAS 3 week Risperidone −17.1(1.8) Placebo −10.3(1.7) No Statistical Tests reported | See forest plot E72, E73 above for Withdrawal | No reported SAE EPS Mean ESRS score increases greater for haloperidol than risperidone (p<0.001) |
Abbreviations: AE=Adverse Events; ANCOVA=Analysis of Covariance; CGI=Clinical Global Impressions Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; ESRS=Extrapyrimidal Symptom Rating Scale; GAS=Global Assessment Scale; MADRS=Montgomery-Asberg Depression Rating Scale; NR=Not reported; NS=not significant; OR=Odds Ratio; PMID=PubMed Identification Number; ROB=Risk of Bias; SAE=Serious Adverse Events; YMRS = Young Mania Rating Scale
Appendix Table E36Strength of evidence assessment: risperidone versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Risperidone vs. placebo | Response 3 wks YMRS 3 wks CGI 3 wks | 2 RCTs (n=584) | Favors Risperidone | Moderate | Consistent | Direct | Imprecise | Low |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Appendix Table E37Outcomes summary: risperidone versus active comparator for acute mania
| Drub | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Risperidone vs. haloperidol | Smulevich, 200515 15572276 Moderate | Response 3 weeks Risperidone 48% Haloperidol 47% NS | YMRS Change 3 week Risperidone −15.1(10.3) Haloperidol −13.9(10.3) NS | CGI-S Change 3 week Risperidone −1.4(1.2) Haloperidol −1.3(1.1) NS GAS 3 week Risperidone −17.1(1.8) Haloperidol −13.9(10.3) NS | Withdrawal lack of efficacy Risperidone 4% Haloperidol 3% Withdrawal adverse events Risperidone 3% Haloperidol 1% | Serious Adverse Events 12 weeks 0 in both arms Deaths 12 weeks 0 in both arms EPS NR |
| Segal, 199841 9617509 Moderate | NR | MRS 4 weeks NS | CGI 4 weeks NS GAF 4 weeks NS | NR | Serious Adverse Events 4 weeks 0 in both arms Deaths 4 weeks 0 in both arms EPS NR |
Abbreviations: AE=Adverse Events; CI=Confindence Interval; CGI=Clinical Global Impressions Scale; CGI-BP=Clinical Global Impressions Scale, Bipolar; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-S=Clinical Global Impressions; Severity Scale; EPS=extrapyramidal symptoms; GAF=Global Assessment of Functioning Scale; GAS=Global Assessment Scale; MADRS=Montgomery-Asberg Syndrome Scale; MRS=Mania Rating Scale; NR=not reported; NS=not significant; OR=Odds Ratio; PMID=PubMed Identification Number; RoB=Risk of Bias; SD=Standard Deviation; SE=standard error; YMRS = Young Mania Rating Scale
Appendix Table E38Strength of evidence assessment: risperidone versus active comparator for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Risperidone vs. haloperidol | Response 3 wks YMRS 3 wks CGI-S 3 wks | 2 RCTs (n=438) | See table above | Moderate | Consistent | Direct | Imprecise | Insufficient |
| Risperidone vs. lithium | YMRS 4 wks CGI 4 wks GAF 4 wks | 1 RCT (n=45) | See table above | Moderate | Unknown | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 8. Ziprasidone for Acute Mania
Appendix Table E39Characteristics of eligible studies: ziprasidone drug treatments for acute mania by year then first author
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP-I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| Potkin, 200542 RCT Multisite 2 Continents Industry RoB Low 16012271 | N = 206 Mean Age 40 Female 49% White 62% BP-I 100% Inpatient | Mania; Mania Rating Scale (SADS-C interview derived; Spitzer, 1978) ≥14 with score ≥2 on four items at screening and admission Schizoaffective Substance Abuse Other Mental Health Taking Other Meds Pregnant/Nursing | Ziprasidone 80-160 mg/day (112 mg/day mean) | Placebo | 3 weeks | BMI or Weight CGI-I CGI-S GAF HAM-D MADRS MRS PANSS Withdrawal 41% |
| Keck, 200343 RCT Multisite 2 Continents Industry RoB Moderate 12668364 | N = 210 Mean Age 38 Female 46% Race NR BP-I 100% Inpatient | Mania; Mania Rating Scale (SADS-C interview derived; Spitzer, 1978) ≥14 with score ≥2 on four items at screening and admission Schizoaffective Substance Abuse Other Mental Health Taking Other Meds Pregnant/Nursing Labs/Other Conditions | Ziprasidone 40-80 mg bid (mean ≈130 mg/day) | Placebo | 3 weeks | MRS CGI-S CGI-I PANSS GAF Adverse Events AIMS Withdrawal 50% |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; BARS=Barnes Akathisia Scale; BAS=Behavioral Approach System; BMI=Body Mass Index; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=comparison; CGI= Clinical Global Impressions; CGI-I=Clinical Global Impressions-Improvement; CGI-S =CGI-Severity; CGI-BP=Clinical Global Impressions Scale-Bipolar; CGI-BP-C= Clinical Global Impressions, Bipolar, Change Scale; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; C-SSRS=Columbia Suicide Severity Rating Scale; DAI-10=Drug Attitutde Inventory, 10 question version; DIEPSS=Drug-Induced Extra-Pyramidal Symptoms Scale; DSM=Diagnostic and Statistical Manual of Mental Disorders; EPS=extrapyramidal symptoms; ER=Extended Release; ESRS=Extrapyramidal Symptom Rating Scale; GAF=General Assessment of Functioning Scale; GAS=Global Assessment Scale; HAM-A=Hamilton Scale for Anxiety; HAM-D=Hamilton Scale for Depression; HRQL=Health-related quality of life; HRQOL=Health-related quality of life; I=intervention; IDS=Inventory for Depressive Symptoms; LIFE= Longitudinal Interval Follow-up Evaluation; MADRS=Montgomery-Asberg Depression Rating Scale; MAS=Bech-Rafaelsen Mania Rating Scale; MRS=Mania Rating Scale; MSRS=Manic state rating scale; NOS=not otherwise specified; NR=not reported; PANSS=Positive and Negative Syndrome Scale; PRS=Polygenic Risk Scores; PGWB=Psychological General Well-Being Index; PMID=PubMed Identification Number; PRS=Polygenic Risk Scores; Q-LES-Q=Quality of Life Enjoyment and Satisfaction Questionnaire; RCT=randomized controlled trial; ROB=risk of bias; SADS-C= Schedule for Affective Disorders and Schizophrenia-Change version; SAE=Serious Adverse Events; SAS=Simpson Angus Scale; SF-12=12-Item Short Form Health Survey; SF-36=36-Item Short Form Health Survey; SLICE=Streamlined Longitudinal Interview Clinical Evaluation; T=Trial; YMRS = Young Mania Rating Scale
Appendix Table E40Summary risk of bias assessments: ziprasidone for acute mania by year then first author
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Ziprasidone | Keck, 200343 Industry 12668364 | Moderate | All core sources of bias appear to have been addressed, however, almost 50% dropout. |
| Potkin, 200542 Industry 16012271 | Low | No sources of bias identified. Well disclosed and reported study. |
Abbreviations: ITT=Intention to Treat; PMID=PubMed Identification Number; LOCF=last observation carried forward
Ziprasidone Forest Plots
Outcomes in studies assessed as having a high risk of bias, or low to moderate risk of bias but at least 40 percent attrition, are presented in grey tones. Both fixed-effect models and random-effects models are presented. We calculated fixed-effect models to provide a charitable estimate of the average effect among completed trials. However, we base our main conclusions on the random-effects models.
Appendix Table E41Outcomes summary: ziprasidone versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Ziprasidone | Keck, 200343 12668364 Moderate | See forest plot E74 above for Response | See forest plot above | See forest plot E75 above for CGI GAF 3 weeks F=10.35, df=1, 156, p<0.005 Favors intervention | See forest plots 76, 77, and 78 above | Serious Adverse Events 3 weeks 0 in both arms Deaths 3 weeks 0 in both arms EPS NR |
| Potkin, 200542 16012271 Moderate | See forest plot E74 above for Response | See forest plot above | See forest plot E75 above for CGI GAF 3 weeks Favors intervention p ≤.001 No statistical test reported | See plots 76, 77, and 78 above | Serious Adverse Events 3 weeks 1 Ziprasidone Deaths 3 weeks 1 Ziprasidone – Suicide EPS 3 weeks 1.5% placebo 10.8% Ziprasidone |
Abbreviations: AE=Adverse Events; ANCOVA=Analysis of Covariance; CGI=Clinical Global Impressions Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; ESRS=Extrapyrimidal Symptom Rating Scale; GAS=Global Assessment Scale; MADRS=Montgomery-Asberg Depression Rating Scale; NR=Not reported; NS=not significant; OR=Odds Ratio; PMID=PubMed Identification Number; ROB=Risk of Bias; SAE=Serious Adverse Events; YMRS = Young Mania Rating Scale
Appendix Table E42Strength of evidence assessment: ziprasidone versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Ziprasidone vs. placebo | Response 3 wks YMRS 3 wks CGI 3 wks | 2 RCTs (n=402) | Favors Ziprasidone | Moderate | Consistent | Direct | Imprecise | Low |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 9. Aripiprazole Plus Mood Stabilizer
Appendix Table E43Characteristics of eligible studies: aripiprazole plus mood stabilizer drug treatments for acute mania
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| NCT00665366 2013 Unpublished RCT Multiste 3 Continents Industry RoB NA | N = 370 Mean Age 45 Female 54% White 95% BP I 100% Setting NR | Mania, manic or mixed episode, with or without psychotic features Schizoaffective Substance Abuse Other Mental Health Neurological disorders Pregnant/Nursing Labs/Other Conditions | Aripiprazole 5-15 mg/day Adjunctive to valproate or lithium | Placebo Adjunctive to valproate or lithium | 12 weeks | Response (≥50% YMRS decrease) Remission (YMRS ≤ 12) YMRS CGI-BP FAST LIFE-RIFT PGI-I Weight change Withdrawal 32% |
| Jeong, 201244 RCT Multisite South Korea Industry 22592508 RoB Low | N = 42 Mean Age 37 Female 64% Race NR BP I 100% Inpatient | Manic; DSM-IV-TR criteria YMRS ≥20 Schizoaffective Substance Abuse Other Mental Health Taking Other Meds Labs/Other Conditions | Aripiprazole Flexible dosing Mean 20 mg/day Adjunctive to valproic acid (serum level 50-125 mg/mL) | Haloperidol Flexible dosing Mean 5 mg/day Adjunctive to valproic acid (serum level 50-125 mg/mL) | 8 weeks | Response (≥50% YMRS decrease) Remission (YMRS ≤ 12) YMRS CGI-S Drug-induced Extrapyramidal Symptoms Scale Weight gain Withdrawal 7% |
| Vieta, 200845 RCT Multisite/Not Disclosed Industry RoB Moderate 18381903 | N = 384 Mean Age 42 Female 54% White 91% BP I 100% Outpatient | Manic/Mixed episode; Partial responders to Lithium or Valproate; YMRS≥16 with decrease of ≤25% after 6 weeks of stabilization treatment Substance Abuse Other Mental Health | Aripiprazole 15-30 mg/day (19.0 mg/day) Adjunctive to lithium/divalproex/valproate | Placebo Adjunctive to lithium/divalproex/valproate | 6 weeks | YMRS Response Adverse Events EPS CGI-BP Withdrawal 19% |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; ASI=Addiction Severity Index; BAS=Behavioral Approach System; BID=Twice a day; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=Comparison; CGI-BP=Clinical Global Impressions Scale, Bipolar; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-I= Clinical Global Impressions-Improvement; CGI-S== Clinical Global Impressions-Severity Scale; DSM-IV-TR= Diagnostic and statistical manual, 4th edition, Text Revision; EPS=extrapyramidal symptoms; FAST=Functional Assessment Short Test; GAF=General Assessment of Functioning Scale; GAS= Global Assessment Scale; HAM-D=Hamilton Scale for Depression; HAMD-21=Hamilton Rating Scale for Depression (21-items); HDRS-21=Hamilton Depression Rating Scale (21=items); ISST=International Suicide Prevention Trial Scale for Suicidal Thinking; LIFE-RIFT=Longitudinal Interval Follow-up Evaluation-Rating Impaired Functioning Tool; MADRS=Montgomery-Asberg Depression Rating Scale; NOS=not otherwise specified; NR=not reported; OR=Odds Ratio; PANSS=Positive and Negative Syndrome Scale; PAS=Premornid Adjustment Scale; PGI-I=Patient Global Impression Improvement; PMID=PubMed Identification Number; QLS=Quality of Life Scale; RCT=randomized controlled trial; RDQ=Readiness to Discharge Questionnaire; ROB=risk of bias; SANS=Scale for the Assessment of Negative Symptoms; SAPS=Scale for the Assessment of Positive Symptoms; SAS=Simpson Angus Scale; SF-36=36-Item Short Form Health Survey; UKU=UKU rating scale; YMRS = Young Mania Rating Scale
Appendix Table E44Summary risk of bias assessments: arirprizaole plus mood stabilizers for acute mania
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Aripiprazole | Jeong, 201244 Industry 22592508 | Low | May be underpowered and raters may not be blinded. 7% dropout. |
| Vieta 200845 Industry 18381903 | Moderate | Randomization and blinding procedures not disclosed. |
Abbreviations: ITT=intention to treat; PMID=PubMed Identification Number
Antipsychotics Plus Mood Stabilizer Forest Plots
Outcomes in studies assessed as having a high risk of bias, or low to moderate risk of bias but at least 40 percent attrition, are presented in grey tones. Both fixed-effect models and random-effects models are presented. We calculated fixed-effect models to provide a charitable estimate of the average effect among completed trials. However, we base our main conclusions on the random-effects models.

Appendix Figure E89Adjunctive aripiprazole vs. placebo – akasthisia
Appendix Table E45Outcomes summary: aripiprazole plus mood stabilizers versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Aripiprazole adjunctive vs. placebo | NCT00665366 2013 | See forest plots E79, E80, E81, and E82 above. | See forest plot E83 above. | See forest plot E84 above for CGI | See forest plot E85, E86 and E87 above for Withdrawals. | See forest plot E88, E89, and E90 above for adverse events. Very Serious AE 1 reported case of suicide ideation related to depression in the aripiprazole arm 1 case of acute respiratory failure in the Aripiprazole arm Cases of Severe Depression 12 weeks NS Aripiprazole = 4/180 Placebo= 2/189 OR = 2.05 (0.37, 16.72) ; P = 0.38 Cases of Manic Reaction or Relapse 12 weeks NS Aripiprazole = 2/180 Placebo= 7/189 OR = 0.31 (0.04, 1.34) ; P = 0.18 |
| Vieta, 200845 18381903 Moderate | See forest plots E79, E80, E81, and E82 above. | See forest plot E83 above. | NR | See forest plot E85, E86 and E87 above for Withdrawals. | See forest plot above. EPS 6 weeks Favors Placebo Aripiprazole =71/253 Placebo=18/130 OR = 2.41 (1.39, 4.37) ; P = 0.002 Normalized Weight Change (> 7% change) 6 weeks NS Aripiprazole = 7/253 Placebo= 5/130 OR = 0.71 (0.22, 2.50) ; P = 0.57 Cases of Psychiatric Disorder 6 weeks NS Aripiprazole = 6/253 Placebo= 3/130 OR = 1.00 (0.25, 5.11) ; P = 0.97 |
Abbreviations: AE=Adverse Events; ASI=Addiction Severity Index; BPRS=Brief Psychiatric Rating Scale; CGI-Bp=Clinical Global Impresions Scale for Bipolar Disorder; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-S=Clinical Global Impressions, Severity Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; GAF=General Assessment of Functioning Scale; HAM-D=Hamilton Scale for Depression; MADRS=Montgomery-Asberg Syndrome Scale; NR=not reported; NS=not significant; OR=Odds Ratio; PAS=Premorbid Adjustment Scale; PMID=PubMed Identification Number; QLS=Quality of Life Scale; ROB=risk of bias; SAE=Serious Adverse Events; SE=Standard Error; UKU=UKU rating scale;YMRS = Young Mania Rating Scale
Appendix Table E46Strength of evidence assessment: aripiprazole plus mood stabilizers versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Aripiprazole adjunctive vs. placebo | Response 6 wks Remission 6 wks YMRS 6 wks CGI-BP 6 wks Withdrawals | 2 RCTs (n=752) | See forest plots | High | Unknown | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Appendix Table E47Outcomes summary: aripipirazole plus mood stabilizers versus active comparison for acute mania
| Drug | Study PMID | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Aripiprazole adjunctive vs. haloperidol adjunctive | Jeong, 201244 22592508 RoB | Response 3 weeks NS Aripiprazole= 20 (71.4%) Haloperidol= 11 (78.6%) No statistical test reported 8 weeks NS Aripiprazole=24 (85.7%) Haloperidol= 13 (92.9%) No statistical test reported Remission 3 weeks NS Aripiprazole= 18 (64.3%) Haloperidol= 9 (64.3%) No statistical test reported 8 weeks NS Aripiprazole= 23 (82.1%) Haloperidol= 12 (85.7%) No statistical test reported | Change in YMRS 8 weeks NS Change (SE) Aripiprazole= −16.3 (1.6) Haloperidol= −17.5 (2.3) P=0.66 | Change in CGI-BP 8 weeks NS Change (SE) Aripiprazole= 2.0 (0.2) Haloperidol= 1.7 (0.3) P=0.41 | Overall Withdrawal 8 weeks NS Aripiprazole = 2 (7.1%) Haloperidol = 1 (7.1%) No statistical test reported Withdrawal due to Lack of Efficacy 8 weeks NS Aripiprazole =1 (3.6%) Haloperidol =0 (0%) No statistical test reported Withdrawal due to AEs 8 weeks NS Aripiprazole = 1 (3.6%) Haloperidol = 1 (7.1%) No statistical test reported | Extrapyramidal Symptoms 8 weeks Favors Aripiprazole Aripiprazole =32.1% Haloperidol =50% No statistical test reported Normalized Weight Change (>7% change) 8 weeks Favors Comparator Aripiprazole =18/28 Haloperidol =4/14 P=0.049 Emergent Mood Episode (Depression) 8 weeks Aripiprazole =2/28 Haloperidol =3/14 No statistical test reported Akathisia 8 weeks Aripiprazole =7/28 Haloperidol =2/14 No statistical test reported |
Abbreviations: AE=Adverse Events; CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; CGI-BP-S-Clinical Global Impressions, Bipolar, Severity Scale; HAMD= Hamilton Scale for Depression; NS=not significant; PMID=PubMed Identification Number; ROB=risk of bias; SAE; Serious Adverse Events; SE=standard error; YMRS = Young Mania Rating Scale
Appendix Table E48Strength of evidence assessment: aripiprazole plus mood stabilizers versus active comparison for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Aripiprazole adjunctive vs. haloperidol adjunctive | 3 weeks Response Remission YMRS CGI-BP Withdrawal – overall Withdrawal – lack of efficacy Withdrawal – Aes | 1 RCT (n=42) | See table above | Low | Unknown | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 10. Asenapine Plus Mood Stabilizer
Appendix Table E49Characteristics of eligible studies: asenapine plus mood stabilizer drug treatments for acute mania
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| Szegedi, 201246 RCT Multisite 4 Continents Industry RoB Moderate 22198448 | N = 324 Mean Age 39 Female 43% White 57% BP I 100% Outpatient | Mania; YMRS ≥ 20 Current episode ≤3 months Substance Abuse Other Mental Health Neurological Disorders Taking Other Meds Pregnant/Nursing Labs/Other Conditions First Manic Episode | Asenapine 5-10 mg BID (Mean 11.8 mg/day) Adjunctive to Lithium 0.6-1.2 mmol/L (mean 12.8 mg/day) OR Valproate 50-125 mcg/mL (mean 11.0 mg/day) | Placebo Adjunctive to Lithium 0.6-1.2 mmol/L (mean 12.8 mg/day) OR Valproate 50-125 mcg/mL (mean 11.0 mg/day) | 3 weeks (12 week >50% attrition) | YMRS MADRS CGI-BP ISST SF-36 RDQ Adverse Events Withdrawal 37% |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; ASI=Addiction Severity Index; BAS=Behavioral Approach System; BID=Twice a day; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=Comparison; CGI-BP=Clinical Global Impressions Scale, Bipolar; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-I= Clinical Global Impressions-Improvement; CGI-S== Clinical Global Impressions-Severity Scale; DSM-IV-TR= Diagnostic and statistical manual, 4th edition, Text Revision; EPS=extrapyramidal symptoms; FAST=Functional Assessment Short Test; GAF=General Assessment of Functioning Scale; GAS= Global Assessment Scale; HAM-D=Hamilton Scale for Depression; HAMD-21=Hamilton Rating Scale for Depression (21-items); HDRS-21=Hamilton Depression Rating Scale (21=items); ISST=International Suicide Prevention Trial Scale for Suicidal Thinking; LIFE-RIFT=Longitudinal Interval Follow-up Evaluation-Rating Impaired Functioning Tool; MADRS=Montgomery-Asberg Depression Rating Scale; NOS=not otherwise specified; NR=not reported; OR=Odds Ratio; PANSS=Positive and Negative Syndrome Scale; PAS=Premornid Adjustment Scale; PGI-I=Patient Global Impression Improvement; PMID=PubMed Identification Number; QLS=Quality of Life Scale; RCT=randomized controlled trial; RDQ=Readiness to Discharge Questionnaire; ROB=risk of bias; SANS=Scale for the Assessment of Negative Symptoms; SAPS=Scale for the Assessment of Positive Symptoms; SAS=Simpson Angus Scale; SF-36=36-Item Short Form Health Survey; UKU=UKU rating scale; YMRS = Young Mania Rating Scale
Appendix Table E50Summary risk of bias assessments: asenapine plus mood stabilizers for acute mania
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Asenapine | Szegedi, 201246 Industry 22198448 | Moderate (3 week outcomes) | Statistically significant difference in the number of patients who complete the trial for both arms, but ‘if patients who had recurrence were also counted as completers, then completion rates were 73.3% for olanzapine and 67.3% for lithium’ -- which are similar but dropout rates are still moderately high (30%). |
Abbreviations: ITT=intention to treat; PMID=PubMed Identification Number
Appendix Table E51Outcomes summary: asenapine plus mood stabilizers versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Asenapine adjunctive vs. placebo | Szegedi, 201246 22198448 Moderate | Response No difference between groups OR 1.14 (0.71, 1.84) Remission No difference between groups OR 1.47 90.90, 2.42) | YRMS Favors asenapine Mean difference 2.03 (0.26, 3.80) | CGI-BP-S Favors asenapine Mean difference 0.30 (0.08, 0.52) | NR at 3 weeks | Serious Adverse Events 52 weeks 1 Asenapine Deaths 52 weeks 1 Asenapine – suicide EPS 52 weeks 12% placebo 9.5% Asenapine |
Abbreviations: AE=Adverse Events; ASI=Addiction Severity Index; BPRS=Brief Psychiatric Rating Scale; CGI-Bp=Clinical Global Impresions Scale for Bipolar Disorder; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-S=Clinical Global Impressions, Severity Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; GAF=General Assessment of Functioning Scale; HAM-D=Hamilton Scale for Depression; MADRS=Montgomery-Asberg Syndrome Scale; NR=not reported; NS=not significant; OR=Odds Ratio; PAS=Premorbid Adjustment Scale; PMID=PubMed Identification Number; QLS=Quality of Life Scale; ROB=risk of bias; SAE=Serious Adverse Events; SE=Standard Error; UKU=UKU rating scale;YMRS = Young Mania Rating Scale
Appendix Table E52Strength of evidence assessment: asenapine plus mood stabilizers versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Asenapine adjunctive vs. placebo | Response 3 wks YMRS 3 wks CGI-BP 3 wks | 1 RCT (n=324) | NS | Moderate | Unknown | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 11. Olanzapine Plus Mood Stabilizer
Appendix Table E53Characteristics of eligible studies: olanzapine plus mood stabilizer drug treatments for acute mania
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| Conus, 201547 RCT Single-site Australia Industry RoB Low 26485297 | N = 83 Mean Age 22 Female 32% Race NR BP I 100% Inpatient | First manic or mixed episode; YMRS ≥20 Other Mental Health Neurological Disorders Taking Other Meds Pregnant/Nursing Labs/Other Conditions | Olanzapine 5 mg/day adjusted by 2.5 mg/day Adjunctive to lithium 500 mg/day increased by 500 mg/twice daily | Chlorpromazine 100 mg/day adjusted by 50-100 mg/day Adjunctive to lithium 500 mg/day increased by 500 mg/twice daily | 8 weeks | Response (≥ 50% YMRS reduction) Remission (YMRS ≤ 12) Symptomatic recovery (YMRS ≤ 12 and HAMD-21 < 7) Efficacy YMRS CGI-BP BPRS HAMD-21 SAPS SANS ASI PAS GAF QLS Adverse events UKU Withdrawal 14% |
| Xu, 201519 RCT Single-site China Government RoB Low 26060401 | N = 120 Mean Age 31 Female 52% Race NR BP 1 100% Setting NR | First manic; YMRS ≥17 Substance Abuse Neurological Disorders Taking Other Meds Pregnant/Nursing | Olanzapine 10 mg/day + Valproate 600 mg/day | C1: Olanzapine 10 mg/day Flexible dosing 5-20 mg/day C2: Valproate 600 mg/day alone | 4 weeks | Efficacy YMRS CGI-BP Adverse events Extrapyramidal symptoms SAS Withdrawal 5% |
| Houston, 200948 RCT US and Puerto Rico Industry RoB High 19778495 | N = 202 Mean Age 39 Female 59% White 51% BP I 100% Outpatient | Mixed Episode; YMRS ≥ 16 HDRS-21 (inadequate response to divalproex) First Manic Episode Taking Other Meds Labs/Other Conditions | Olanzapine 5-20 mg/day Adjunctive to divalproex (adjusted for blood levels between 75-125 µg/mL) | Divalproex alone (adjusted for blood levels between 75-125 µg/mL) | 6 weeks | Response (≥ 50% decrease HDRS-21 and ≥ 25% decrease YMRS) Remission (YMRS ≤ 12 and HDRS-21 ≤ 8) Efficacy HDRS-21 YMRS CGI-BP Adverse events Extrapyramidal symptoms Withdrawal 42% |
| Tohen, 2008a49 RCT Multisite 3 Continents Industry RoB Moderate 18245032 | N = 118 Mean Age 41 Female 58% Race NR BP I 100% Inpatient and Outpatient | Manic or Mixed Episode; YMRS ≥20 Labs/Other Conditions | Olanzapine 10-30 mg/day Adjunctive to carbamazepine 400-1200 mg/day | Placebo Adjunctive to carbamazepine 400-1200 mg/day | 6 weeks blinded (26 weeks open-label extension) | Efficacy YMRS CGI (multiple subscales) MADRS Adverse events Extrapyramidal symptoms SAS BAS AIMS Withdrawal 28% at 6 weeks |
| Tohen, 2002a50 RCT Multisite US and Canada Industry RoB High 11779284 1533732651 1557273752 | N = 344 Mean Age 41 Female 52% White 85% Diagnosis NR Outpatient | Manic or Mixed Episode; YMRS ≥ 16 First Manic Episode Labs/Other Conditions | Olanzapine 10 mg/day with flexible dosing from 5-20 mg/day Adjunctive to ongoing open-label valproate or lithium | Placebo Adjunctive to ongoing open-label valproate or lithium | 6 weeks | Efficacy YMRS HAM-D PANSS CGI Remission Adverse Events Extrapyramidal symptom Withdrawal 30% |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; ASI=Addiction Severity Index; BAS=Behavioral Approach System; BID=Twice a day; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=Comparison; CGI-BP=Clinical Global Impressions Scale, Bipolar; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-I= Clinical Global Impressions-Improvement; CGI-S== Clinical Global Impressions-Severity Scale; DSM-IV-TR= Diagnostic and statistical manual, 4th edition, Text Revision; EPS=extrapyramidal symptoms; FAST=Functional Assessment Short Test; GAF=General Assessment of Functioning Scale; GAS= Global Assessment Scale; HAM-D=Hamilton Scale for Depression; HAMD-21=Hamilton Rating Scale for Depression (21-items); HDRS-21=Hamilton Depression Rating Scale (21=items); ISST=International Suicide Prevention Trial Scale for Suicidal Thinking; LIFE-RIFT=Longitudinal Interval Follow-up Evaluation-Rating Impaired Functioning Tool; MADRS=Montgomery-Asberg Depression Rating Scale; NOS=not otherwise specified; NR=not reported; OR=Odds Ratio; PANSS=Positive and Negative Syndrome Scale; PAS=Premornid Adjustment Scale; PGI-I=Patient Global Impression Improvement; PMID=PubMed Identification Number; QLS=Quality of Life Scale; RCT=randomized controlled trial; RDQ=Readiness to Discharge Questionnaire; ROB=risk of bias; SANS=Scale for the Assessment of Negative Symptoms; SAPS=Scale for the Assessment of Positive Symptoms; SAS=Simpson Angus Scale; SF-36=36-Item Short Form Health Survey; UKU=UKU rating scale; YMRS = Young Mania Rating Scale
Appendix Table E54Summary risk of bias assessments: olanzapine plus mood stabilizers for acute mania
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Olanzapine | Conus, 201547 Industry 26485297 | Low | Physician and patients may not be blinded. Dropout balanced and accounted for in analysis. |
| Xu, 201519 Government 26060401 | Low | Well-constructed, described, and reported study. 5% dropout. | |
| Houston, 200948 Industry 19778495 | High | Randomization and blinding procedures not described. 42% dropout. | |
| Tohen, 2008a49 Industry 18245032 | Moderate | Blinding procedure not described. 28% dropout. | |
| Tohen, 2002a50 Industry 11779284 | High | Randomization and blinding procedures not described. 30% dropout. |
Abbreviations: ITT=intention to treat; PMID=PubMed Identification Number
Antipsychotics Plus Mood Stabilizer Forest Plots
Outcomes in studies assessed as having a high risk of bias, or low to moderate risk of bias but at least 40 percent attrition, are presented in grey tones. Both fixed-effect models and random-effects models are presented. We calculated fixed-effect models to provide a charitable estimate of the average effect among completed trials. However, we base our main conclusions on the random-effects models.
Appendix Table E55Outcomes summary: olanzapine plus mood stabilizers versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Olanzapine adjunctive vs. chlorpromazine | Conus, 201547 26485297 Low | Symptomatic Recovery 8 weeks Olanzapine=53.3% Chlorpromazine=54.5% P=0.56 Response 8 weeks Olanzapine=96.7% Chlorpromazine=84.8% P=0.12 | YMRS 8 weeks Score ≤12 Olanzapine=93.3% Chlorpromazine=72.7% P=0.03 Favors intervention HAMD-21 8 weeks Score <7 Olanzapine=53.3% Chlorpromazine=63.6% P=0.28 NS | GAF 8 weeks NS No statistical test reported QLS 8 weeks NS No statistical test reported PAS 8 weeks NS No statistical test reported CGI-BP 8 weeks NS No statistical test reported | ASI 8 weeks NS No statistical test reported Weight Gain 8 weeks NS; p=0.22 Overall Withdrawal 8 weeks Olanzapine=4/42 Chlorpromazine=5/41 NS | UKU 8 weeks NS No statistical test reported AE Leading to Trial Medication Interruption 8 weeks Olanzapine=5.3% Chlorpromazine=6.8% NS P=0.47 |
| Olanzapine adjunctive vs. placebo or no placebo | Xu, 201519 26060401 Low | NR | YMRS 3 weeks Favors Olanzapine % Reduction (SD) Olanzapine+Valporate=86.5% (8.9%) Valproate=55.2% (5.7%) P<0.01 CGI-BP 3 weeks Difference in Difference (SE) 1.4 (0.53) Favors Olanzapine p<0.01 | NR | Overall Withdrawal 3 weeks NS Olanzapine+Valporate=2/40 Valproate=3/40 Withdrawal due to AEs 3 weeks NS Olanzapine+Valporate=2/40 Valproate=0/40 Withdrawal, Lack of Efficacy 3 weeks NS Olanzapine+Valporate=0/40 Valproate=2/40 | Severe Harms 3 weeks NS Olanzapine+Valporate=1/38 Valproate=0/37 Normalized Weight Change 3 weeks Olanzapine+Valporate=31/38 Valproate=21/37 Emergent Mood Episodes 3 weeks NS Olanzapine+Valporate=0/38 Valproate=0/37 |
| Houston, 200948 19778495 High | See forest plots E91 and E92 above | YMRS See forest plot E93 above | CGI-BP See forest plot E94 above | See forest plots E95, E96 and E97 above | SAE See forest plot E98 above Deaths 1 fatality from a car accident in the Olanzapine arm Hepatic Failure 1 case of acute hepatic failure in the Olanzapine arm Weight gain See forest plot E100 above | |
| Tohen, 200849 18245032 Moderate | See forest plots E91 and E92 above | YMRS See forest plot E93 above MADRS 6 weeks Change (SE) Olanzapine −1.22 (0.96) Placebo −1.00 (0.96) NS | NR | See forest plots E95, E96 and E97 above | SAE See forest plots E98 aboveabove Emergent depression See forest plot E99 above Weight gain See forest plot E100 above | |
| Tohen, 200250 11779284 High Baker, 200452 15572737 High Namijoshi, 200451 15337326 High | See forest plots E91 and E92 above | YMRS See forest plot E93 above | CGI-BP See forest plot E94 above | See forest plots E95, E96 and E97 above | SAE See forest plot E98 above EPS NS Emergent depression See forest plot E99 above |
Abbreviations: AE=Adverse Events; ASI=Addiction Severity Index; BPRS=Brief Psychiatric Rating Scale; CGI-Bp=Clinical Global Impresions Scale for Bipolar Disorder; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-S=Clinical Global Impressions, Severity Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; GAF=General Assessment of Functioning Scale; HAM-D=Hamilton Scale for Depression; MADRS=Montgomery-Asberg Syndrome Scale; NR=not reported; NS=not significant; OR=Odds Ratio; PAS=Premorbid Adjustment Scale; PMID=PubMed Identification Number; QLS=Quality of Life Scale; ROB=risk of bias; SAE=Serious Adverse Events; SE=Standard Error; UKU=UKU rating scale;YMRS = Young Mania Rating Scale
Appendix Table E56Strength of evidence assessment: olanzapine plus mood stabilizers versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Olanzapine vs. Placebo/adjunctive to Lithium or Valproate | 6 weeks Remission Response YMRS CGI-BP Withdrawals | 3 RCTs (n=664) | See table above | High | Consistent | Direct | Imprecise | Insufficient |
| Olanzapine vs. Placebo/adjunctive to Carbamazepine | Response Remission YMRS CGI-BP Withdrawals | 1 RCT (n=118) | See table above | Moderate | Unknown | Direct | Imprecise | Insufficient |
| Olanzapine vs. No Placebo/adjunctive to Divalproex/Valproate | Response Remission YMRS CGI-BP Withdrawals | 1 RCT (n=202) | See table above | High | Unknown | Direct | Imprecise | Insufficient |
| Olanzapine vs. No Placebo/adjunctive to Valproate | YMRS 3 weeks CGI-BP 3 weeks Withdrawals | 1 RCT (n=80) | See table above | Low | Unknown | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Appendix Table E57Outcomes summary: olanzapine plus mood stabilizers versus active comparison for acute mania
| Drug | Study PMID | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Olanzapine adjunctive vs. chlorpromazine adjunctive | Conus, 201547 26485297 Low | Response 8 weeks Olanzapine 94.7% Chlorpromazine 97.2% NS Remission 8 weeks Olanzapine 89.5% Chlorpromazine 88.9% NS | YMRS 8 weeks NS (data not reported) HAMD 8 weeks NS (data not reported) | CGI-BP-S 8 weeks NS (data not reported) | Withdrawal – Aes Olanzapine 26.3% Chlorpromazine 30.6% NS | SAE 3 chlorpromazine (extrapyramidal symptoms) 2 olanzapine (neutropenia, sedation) Weight gain NS |
Abbreviations: AE=Adverse Events; CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; CGI-BP-S-Clinical Global Impressions, Bipolar, Severity Scale; HAMD= Hamilton Scale for Depression; NS=not significant; PMID=PubMed Identification Number; ROB=risk of bias; SAE; Serious Adverse Events; SE=standard error; YMRS = Young Mania Rating Scale
Appendix Table E58Strength of evidence assessment: olanzapine plus mood stabilizers versus active comparison for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Olanzapine adjunctive vs. chlorpromazine adjunctive | Response Remission YMRS HAMD Withdrawal AE | 1 RCT (n=83) | See table above | Low | Unknown | Direct | Imprecise | Iinsufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 12. Quetiapine Plus Mood Stabilizer
Appendix Table E59Characteristics of eligible studies: quetiapine plus mood stabilizer drug treatments for acute mania by year then first author
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| Yatham, 200753 RCT Multisite 4 Continents Industry RoB High 17519644 | N = 200 Mean Age 40 Female 50% Race NR BP I 100% Inpatient (1 week) Outpatient (weeks 2-3, subject to inspector discretion) | Mania; YMRS ≥ 20 AND ≥ 4 on at least 2 YMRS subscales AND CGI-BP-S ≥ 4 First Manic Episode Taking Other Meds Pregnant/Nursing | Quetiapine 100-800 mg/day (455 mg/day mean) Adjunctive to Valproate 50-100 mcg/mL target OR Lithium 0.7-1.0 mEq/L target | Placebo Adjunctive to Valproate 50-100 mcg/mL target OR Lithium 0.7-1.0 mEq/L target | 3 weeks | CGI-BP-S Remission (Various definitions) Response (YMRS 50% decrease) YMRS Withdrawal 36% |
| Yatham, 200454 RCT Multisite 4 Continents Industry RoB Moderate 15538120 | N = 402 Mean Age 40 Female 47% Race NR BP I 100% Inpatient | Mania; At least one manic or mixed episode in previous 5 years. YMRS ≥ 20, including score ≥ 4 on two core YMRS items; CGI-BP ≥ 4 First Manic Episode Substance Abuse Other Mental Health Pregnant/Nursing | Quetiapine 100-800 mg/day (492 mg/day mean) Adjunctive to Valproate 50-100 mcg/mL target OR Lithium 0.7-1.0 mEq/L target | Placebo Adjunctive to Valproate 50-100 mcg/mL target OR Lithium 0.7-1.0 mEq/L target | 6 weeks | YMRS CGI-BP PANSS MADRS GAS Adverse Events Withdrawals 38% |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; ASI=Addiction Severity Index; BAS=Behavioral Approach System; BID=Twice a day; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=Comparison; CGI-BP=Clinical Global Impressions Scale, Bipolar; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-I= Clinical Global Impressions-Improvement; CGI-S== Clinical Global Impressions-Severity Scale; DSM-IV-TR= Diagnostic and statistical manual, 4th edition, Text Revision; EPS=extrapyramidal symptoms; FAST=Functional Assessment Short Test; GAF=General Assessment of Functioning Scale; GAS= Global Assessment Scale; HAM-D=Hamilton Scale for Depression; HAMD-21=Hamilton Rating Scale for Depression (21-items); HDRS-21=Hamilton Depression Rating Scale (21=items); ISST=International Suicide Prevention Trial Scale for Suicidal Thinking; LIFE-RIFT=Longitudinal Interval Follow-up Evaluation-Rating Impaired Functioning Tool; MADRS=Montgomery-Asberg Depression Rating Scale; NOS=not otherwise specified; NR=not reported; OR=Odds Ratio; PANSS=Positive and Negative Syndrome Scale; PAS=Premornid Adjustment Scale; PGI-I=Patient Global Impression Improvement; PMID=PubMed Identification Number; QLS=Quality of Life Scale; RCT=randomized controlled trial; RDQ=Readiness to Discharge Questionnaire; ROB=risk of bias; SANS=Scale for the Assessment of Negative Symptoms; SAPS=Scale for the Assessment of Positive Symptoms; SAS=Simpson Angus Scale; SF-36=36-Item Short Form Health Survey; UKU=UKU rating scale; YMRS = Young Mania Rating Scale
Appendix Table E60Summary risk of bias assessments: quetiapine plus mood stabilizers for acute mania by year then first author
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Quetiapine | Yatham, 200753 Industry 17519644 | High | Randomization and blinding not described. Improper definition of ITT “consisted of all the patients who had received at least one dose of study drug and had undergone a baseline AND at least one postbaseline efficacy assessment"; 2 patients missing from baseline quet. Measures, 9 patients missing from baseline placebo measures. |
| Yatham, 200454 Industry 15538120 | Moderate | Blinding and randomization not described; differential dropout rates between arms may alter effectiveness of randomization |
Abbreviations: ITT=intention to treat; PMID=PubMed Identification Number
Antipsychotics Plus Mood Stabilizer Forest Plots
Outcomes in studies assessed as having a high risk of bias, or low to moderate risk of bias but at least 40 percent attrition, are presented in grey tones. Both fixed-effect models and random-effects models are presented. We calculated fixed-effect models to provide a charitable estimate of the average effect among completed trials. However, we base our main conclusions on the random-effects models.
Appendix Table E61Outcomes summary: quetiapine plus mood stabilizers versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Quetiapine adjunctive vs. placebo | Yatham, 200753 17519644 High | See forest plots E101 and E102 above | See forest plot E103 above | See forest plot E104 above | See forest plots E105, E106, and E107 above | Serious Adverse Events 6 weeks 1 placebo Deaths 6 weeks 1 placebo EPS 6 weeks 29.2% placebo 17.9% Quetiapine + Lithium |
| Yatham, 200454 15538120 Moderate | See forest plots E101 and E102 above | See forest plot E103 above | See forest plot E104 above | See forest plots E105, E106, and E107 above | Serious Adverse Events 6 weeks 0 in all arms Deaths 6 weeks 0 in all arms EPS 6 weeks 19.2% placebo 21.4% Quetiapine + Lithium |
Abbreviations: AE=Adverse Events; ASI=Addiction Severity Index; BPRS=Brief Psychiatric Rating Scale; CGI-Bp=Clinical Global Impresions Scale for Bipolar Disorder; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-S=Clinical Global Impressions, Severity Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; GAF=General Assessment of Functioning Scale; HAM-D=Hamilton Scale for Depression; MADRS=Montgomery-Asberg Syndrome Scale; NR=not reported; NS=not significant; OR=Odds Ratio; PAS=Premorbid Adjustment Scale; PMID=PubMed Identification Number; QLS=Quality of Life Scale; ROB=risk of bias; SAE=Serious Adverse Events; SE=Standard Error; UKU=UKU rating scale;YMRS = Young Mania Rating Scale
Appendix Table E62Strength of evidence assessment: quetiapine plus mood stabilizers versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Quetiapine adjunctive vs. placebo | Response 3 wks Remission 3 wks YMRS 3 wks CGI-BP 3 wks Withdrawals | 2 RCTs (n=570) | See forest plots above | High | Consistent | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 13. Risperidone Plus Mood Stabilizer
Appendix Table E63Characteristics of eligible studies: risperidone plus mood stabilizer drug treatments for acute mania by year then first author
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| Yatham, 200355 RCT Multisite 4 Continents Industry RoB Low 12562742 | N = 151 Mean Age 40 Female 58% Race NR Inpatient | Mania; YMRS ≥ 20 Schizoaffective Substance Abuse Other Mental Health Pregnant/Nursing Labs/Other Conditions | Risperidone 2-6 mg/day (Mean 4.0 mg/day) Adjunctive to Lithium or Divalproex or Carbamazepine | Placebo Adjunctive to Lithium or Divalproex or Carbamazepine | 3 weeks | Response (YMRS 50% decrease) BPRS CGI HAM-D YMRS Withdrawal 44% |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; ASI=Addiction Severity Index; BAS=Behavioral Approach System; BID=Twice a day; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=Comparison; CGI-BP=Clinical Global Impressions Scale, Bipolar; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-I= Clinical Global Impressions-Improvement; CGI-S== Clinical Global Impressions-Severity Scale; DSM-IV-TR= Diagnostic and statistical manual, 4th edition, Text Revision; EPS=extrapyramidal symptoms; FAST=Functional Assessment Short Test; GAF=General Assessment of Functioning Scale; GAS= Global Assessment Scale; HAM-D=Hamilton Scale for Depression; HAMD-21=Hamilton Rating Scale for Depression (21-items); HDRS-21=Hamilton Depression Rating Scale (21=items); ISST=International Suicide Prevention Trial Scale for Suicidal Thinking; LIFE-RIFT=Longitudinal Interval Follow-up Evaluation-Rating Impaired Functioning Tool; MADRS=Montgomery-Asberg Depression Rating Scale; NOS=not otherwise specified; NR=not reported; OR=Odds Ratio; PANSS=Positive and Negative Syndrome Scale; PAS=Premornid Adjustment Scale; PGI-I=Patient Global Impression Improvement; PMID=PubMed Identification Number; QLS=Quality of Life Scale; RCT=randomized controlled trial; RDQ=Readiness to Discharge Questionnaire; ROB=risk of bias; SANS=Scale for the Assessment of Negative Symptoms; SAPS=Scale for the Assessment of Positive Symptoms; SAS=Simpson Angus Scale; SF-36=36-Item Short Form Health Survey; UKU=UKU rating scale; YMRS = Young Mania Rating Scale
Appendix Table E64Summary risk of bias assessments: risperidone plus mood stabilizers for acute mania
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Risperidone | Yatham, 200355 Industry 12562742 | Low | A well described and reported study with some minor lack of disclosure in blinding procedure and adverse events reporting. 44% dropout. |
Abbreviations: ITT=intention to treat; PMID=PubMed Identification Number
Appendix Table E65Outcomes summary: risperidone plus mood stabilizers versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Risperidone adjunctive vs. placebo | Yatham, 200355 12562742 Low | Response, Mean Difference 3 weeks 17.7% (95% CI 0.8-33.5); p<0.05 Favors intervention | YMRS, Mean Difference in Change Score 3 weeks -4.2 (95% CI −7.60, 0.53); p=0.09 NS HAM-D 3 weeks NS No statistical test reported BPRS, Mean Change 3 weeks Risperidone=-10.1 Placebo=-4.8 p=0.01 Favors intervention | CGI-BP, Mean Difference in Responders 3 weeks 17.5% (95% CI 1.1-33.9) Favors Intervention | Overall Withdrawal 3 weeks Risperidone=36% Placebo=52% Mean difference in completion rates=16% (95% CI 0.32, 31.68) Favors Intervention | One or More AEs, Between Group Difference 3 weeks 6% (95% CI −9.9, 21.9) NS EPS Symptoms 3 weeks Risperidone=16/75 Placebo=8/75 P=0.013 |
Abbreviations: AE=Adverse Events; ASI=Addiction Severity Index; BPRS=Brief Psychiatric Rating Scale; CGI-Bp=Clinical Global Impresions Scale for Bipolar Disorder; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-S=Clinical Global Impressions, Severity Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; GAF=General Assessment of Functioning Scale; HAM-D=Hamilton Scale for Depression; MADRS=Montgomery-Asberg Syndrome Scale; NR=not reported; NS=not significant; OR=Odds Ratio; PAS=Premorbid Adjustment Scale; PMID=PubMed Identification Number; QLS=Quality of Life Scale; ROB=risk of bias; SAE=Serious Adverse Events; SE=Standard Error; UKU=UKU rating scale; YMRS = Young Mania Rating Scale
Appendix Table E66Strength of evidence assessment: risperidone plus mood stabilizers versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Risperidone adjunctive vs. placebo | Response 3 wks YMRS 3 wks CGI-BP 3 wks Withdrawal – overall | 1 RCT (n=150) | See table above | Low | Unknown | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
Section 14. Ziprazidone Plus Mood Stabilizer
Appendix Table E67Characteristics of eligible studies: ziprasidone plus mood stabilizer drug treatments for acute mania
| Study, Year Design Location Funder Risk of Bias PMID | # Randomized Age (mean) Sex (% Female) Race (% White) Diagnosis (% BP I, II, NOS) Setting | Inclusions Key Exclusions | Intervention Dosage | Comparison Dosage | Follow-up Duration | Outcomes Reported Withdrawal (%) at endpoint |
|---|---|---|---|---|---|---|
| Sachs, 201256 RCT Multisite US Industry RoB High 23218157 | N = 680 Mean Age 41 Female 50% White 65% BP I 100% Outpatient | Mania; YMRS ≥ 18 with 25% improvement between screening and baseline; current episode ≤ 3 months First manic episode Schizoaffective Substance Abuse Other Mental Health Conditions Taking Other Meds Labs/Other Conditions | Low Dose Ziprasidone (40-80 mg/day) Adjunctive to Lithium/Valproate OR High Dose Ziprasidone (120-160 mg/day) Adjunctive to Lithium/Valproate | Placebo Adjunctive to Lithium/Valproate | 3 weeks | CGI-I CGI-S GAF LIFE-RIFT MADRS PANSS YMRS Withdrawal 42% |
Abbreviations: AIMS=Abnormal Involuntary Movement Scale; ASI=Addiction Severity Index; BAS=Behavioral Approach System; BID=Twice a day; BP=bipolar disorder; BPRS=Brief Psychiatric Rating Scale; C=Comparison; CGI-BP=Clinical Global Impressions Scale, Bipolar; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-I= Clinical Global Impressions-Improvement; CGI-S== Clinical Global Impressions-Severity Scale; DSM-IV-TR= Diagnostic and statistical manual, 4th edition, Text Revision; EPS=extrapyramidal symptoms; FAST=Functional Assessment Short Test; GAF=General Assessment of Functioning Scale; GAS= Global Assessment Scale; HAM-D=Hamilton Scale for Depression; HAMD-21=Hamilton Rating Scale for Depression (21-items); HDRS-21=Hamilton Depression Rating Scale (21=items); ISST=International Suicide Prevention Trial Scale for Suicidal Thinking; LIFE-RIFT=Longitudinal Interval Follow-up Evaluation-Rating Impaired Functioning Tool; MADRS=Montgomery-Asberg Depression Rating Scale; NOS=not otherwise specified; NR=not reported; OR=Odds Ratio; PANSS=Positive and Negative Syndrome Scale; PAS=Premornid Adjustment Scale; PGI-I=Patient Global Impression Improvement; PMID=PubMed Identification Number; QLS=Quality of Life Scale; RCT=randomized controlled trial; RDQ=Readiness to Discharge Questionnaire; ROB=risk of bias; SANS=Scale for the Assessment of Negative Symptoms; SAPS=Scale for the Assessment of Positive Symptoms; SAS=Simpson Angus Scale; SF-36=36-Item Short Form Health Survey; UKU=UKU rating scale; YMRS = Young Mania Rating Scale
Appendix Table E68Summary risk of bias assessments: ziprasidone plus mood stabilizers for acute mania
| Drug | Study Funding Source PMID | Overall Risk of Bias Assessment | Rationale |
|---|---|---|---|
| Ziprasidone | Sachs, 201256 Industry 23218157 | High | Randomization and blinding not disclosed. Did not address how 680 were randomized but only 656 treated and analyzed. ITT noted, but they call this a modified ITT. |
Abbreviations: ITT=intention to treat; PMID=PubMed Identification Number
Appendix Table E69Outcomes summary: ziprasidone plus mood stabilizers versus placebo for acute mania
| Drug | Study PMID RoB | Responder/Remitter | Symptom | Function | Other | AE |
|---|---|---|---|---|---|---|
| Ziprasidone adjunctive vs. placebo | Sachs, 201256 23218157 High | NR | YMRS Change 3 week Low dose NS High dose NS | CGI-S 3 week Low dose NS High dose NS GAF 3 week Low dose NS High dose NS | Overall Withdrawal Low dose 48/226 High dose 62/232 Placebo 38/222 Withdrawal Lack of efficacy Low dose 6/226 High dose 4/232 Placebo 8/222 Withdrawal Adverse event Low dose 15/226 High dose 33/232 Placebo 11/222 | SAE 11 ziprasidone groups 6 placebo EPS Low dose 1.9% High dose 4.9% Placebo 0.5% |
Abbreviations: AE=Adverse Events; ASI=Addiction Severity Index; BPRS=Brief Psychiatric Rating Scale; CGI-Bp=Clinical Global Impresions Scale for Bipolar Disorder; CGI-BP-S=Clinical Global Impressions, Bipolar, Severity Scale; CGI-S=Clinical Global Impressions, Severity Scale; CI=Confidence Interval; EPS=extrapyramidal symptoms; GAF=General Assessment of Functioning Scale; HAM-D=Hamilton Scale for Depression; MADRS=Montgomery-Asberg Syndrome Scale; NR=not reported; NS=not significant; OR=Odds Ratio; PAS=Premorbid Adjustment Scale; PMID=PubMed Identification Number; QLS=Quality of Life Scale; ROB=risk of bias; SAE=Serious Adverse Events; SE=Standard Error; UKU=UKU rating scale;YMRS = Young Mania Rating Scale
Appendix Table E70Strength of evidence assessment: ziprasidone plus mood stabilizers versus placebo for acute mania
| Comparison | Outcome | # Studies/Design (n analyzed) | Finding or Summary Statistic | Study Limitations | Consistency | Directness | Precision | Overall Grade/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Ziprasidone adjunctive vs. placebo | YMRS 3 wks CGI-BP 3 wks Withdrawal | 1 RCT (n=680) | See table above | High | Unknown | Direct | Imprecise | Insufficient |
Abbreviations: CGI-BP=Clinical Global Impressions Scale for Bipolar Disorder; NS=not significant; RCT=randomized controlled trial; YMRS = Young Mania Rating Scale
Notes:
- 1
Publication bias for antipsychotics, antidepressants, and behavioral interventions for depressive disorders is suspected.
- 2
Data were generally imprecise due to missing data from high attrition rates, which was commonly dealt with by Last Observation Carried Forward (LOCF). LOCF requires an assumption that the health status of patients who dropped out of the trial would not have changed had future observations been recorded, a strong assumption in the context of bipolar disorder research.
References for Appendix E
- 1.
- Kanba S, Kawasaki H, Ishigooka J, et al A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole for the treatment of acute manic or mixed episodes in Asian patients with bipolar I disorder (the AMAZE study). The World Journal of Biological Psychiatry. 2014 2014);15(2):113–21. doi: http://dx
.doi.org/10 .3109/15622975.2012.669047. PMID: 22540407. [PubMed: 22540407] - 2.
- Young AH, Oren DA, Lowy A, et al Aripiprazole monotherapy in acute mania: 12-week randomised placebo- and haloperidol-controlled study. British Journal of Psychiatry. 2009 Jan;194(1):40–8. doi: http://dx
.doi.org/10 .1192/bjp.bp.108.049965. PMID: 19118324. [PubMed: 19118324] - 3.
- Sachs G, Bowden C, Calabrese JR, et al Effects of lamotrigine and lithium on body weight during maintenance treatment of bipolar I disorder. Bipolar Disorders. 2006 Apr;8(2):175–81. PMID: 16542188. [PubMed: 16542188]
- 4.
- Vieta E, Mullen J, Brecher M, et al Quetiapine monotherapy for mania associated with bipolar disorder: combined analysis of two international, double-blind, randomised, placebo-controlled studies. Current Medical Research & Opinion. 2005 Jun;21(6):923–34. PMID: 15969892. [PubMed: 15969892]
- 5.
- Sachs G, Sanchez R, Marcus R, et al Aripiprazole in the treatment of acute manic or mixed episodes in patients with bipolar I disorder: a 3-week placebo-controlled study. Journal of Psychopharmacology. 2006 Jul;20(4):536–46. PMID: 16401666. [PubMed: 16401666]
- 6.
- Vieta E, Bourin M, Sanchez R, et al Effectiveness of aripiprazole v. haloperidol in acute bipolar mania: double-blind, randomised, comparative 12-week trial. British Journal of Psychiatry. 2005 Sep;187:235–42. PMID: 16135860. [PubMed: 16135860]
- 7.
- Landbloom RL, Mackle M, Wu X, et al Asenapine: Efficacy and safety of 5 and 10mg bid in a 3-week, randomized, double-blind, placebo-controlled trial in adults with a manic or mixed episode associated with bipolar I disorder. Journal of Affective Disorders. 2016 Jan 15;190:103–10. doi: http://dx
.doi.org/10 .1016/j.jad.2015.06.059. PMID: 26496015. [PubMed: 26496015] - 8.
- McIntyre RS. Aripiprazole for the maintenance treatment of bipolar I disorder: A review. Clinical Therapeutics. 2010;32 Suppl 1:S32–8. doi: http://dx
.doi.org/10 .1016/j.clinthera.2010.01.022. PMID: 20152551. [PubMed: 20152551] - 9.
- McIntyre RS, Cohen M, Zhao J, et al A 3-week, randomized, placebo-controlled trial of asenapine in the treatment of acute mania in bipolar mania and mixed states. [Erratum appears in Bipolar Disord. 2010 May;12(3):350]. Bipolar Disorders. 2009 Nov;11(7):673–86. doi: http://dx
.doi.org/10 .1111/j.1399-5618.2009.00748.x. PMID: 19839993. [PubMed: 19839993] - 10.
- Calabrese JR, Keck PE, Jr., Starace A, et al Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. Journal of Clinical Psychiatry. 2015 Mar;76(3):284–92. doi: http://dx
.doi.org/10.4088/JCP.14m09081. PMID: 25562205. [PubMed: 25562205] - 11.
- Durgam S, Starace A, Li D, et al The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: A phase II trial. Bipolar Disorders. 2015 Feb;17(1):63–75. doi: http://dx
.doi.org/10.1111/bdi.12238. PMID: 25056368 (pubmed) 2014-30788-001(Psychinfo). [PubMed: 25056368] - 12.
- McIntyre RS, Cohen M, Zhao J, et al Asenapine in the treatment of acute mania in bipolar I disorder: a randomized, double-blind, placebo-controlled trial. Journal of Affective Disorders. 2010 Apr;122(1–2):27–38. doi: http://dx
.doi.org/10 .1016/j.jad.2009.12.028. PMID: 20096936. [PubMed: 20096936] - 13.
- Sachs GS, Greenberg WM, Starace A, et al Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, phase III trial. Journal of Affective Disorders. 2015 Mar 15;174:296–302. doi: http://dx
.doi.org/10 .1016/j.jad.2014.11.018. PMID: 25532076. [PubMed: 25532076] - 14.
- McIntyre RS, Brecher M, Paulsson B, et al Quetiapine or haloperidol as monotherapy for bipolar mania-a 12-week, double-blind, randomised, parallel-group, placebo-controlled trial. European Neuropsychopharmacology. 2005 Oct;15(5):573–85. PMID: 16139175. [PubMed: 16139175]
- 15.
- Smulevich AB, Khanna S, Eerdekens M, et al Acute and continuation risperidone monotherapy in bipolar mania: a 3-week placebo-controlled trial followed by a 9-week double-blind trial of risperidone and haloperidol. European Neuropsychopharmacology. 2005 Jan;15(1):75–84. PMID: 15572276. [PubMed: 15572276]
- 16.
- McIntyre RS, Cucchiaro J, Pikalov A, et al Lurasidone in the treatment of bipolar depression with mixed (subsyndromal hypomanic) features: post hoc analysis of a randomized placebo-controlled trial. Journal of Clinical Psychiatry. 2015 Apr;76(4):398–405. doi: http://dx
.doi.org/10.4088/JCP.14m09410. PMID: 25844756. [PubMed: 25844756] - 17.
- Sachs GS, Grossman F, Ghaemi SN, et al Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of efficacy and safety. American Journal of Psychiatry. 2002 Jul;159(7):1146–54. PMID: 12091192. [PubMed: 12091192]
- 18.
- Vieta E, Nuamah IF, Lim P, et al A randomized, placebo- and active-controlled study of paliperidone extended release for the treatment of acute manic and mixed episodes of bipolar I disorder. Bipolar Disorders. 2010 May;12(3):230–43. doi: http://dx
.doi.org/10 .1111/j.1399-5618.2010.00815.x. PMID: 20565430. [PubMed: 20565430] - 19.
- Xu L, Lu Y, Yang Y, et al Olanzapine-valproate combination versus olanzapine or valproate monotherapy in the treatment of bipolar imania: A randomized controlled study in a chinese population group. Neuropsychiatric Disease and Treatment. 2015 25 May;11:1265–71. doi: http://dx
.doi.org/10.2147/NDT.S81146. PMID: 26060401 (pubmed) 2015078440 (embase). [PMC free article: PMC4450656] [PubMed: 26060401] - 20.
- Katagiri H, Takita Y, Tohen M, et al Efficacy and safety of olanzapine in the treatment of Japanese patients with bipolar I disorder in a current manic or mixed episode: a randomized, double-blind, placebo- and haloperidol-controlled study. Journal of Affective Disorders. 2012 Feb;136(3):476–84. doi: http://dx
.doi.org/10 .1016/j.jad.2011.10.045. PMID: 22134043. [PubMed: 22134043] - 21.
- Vieta E, Suppes T, Ekholm B, et al Long-term efficacy of quetiapine in combination with lithium or divalproex on mixed symptoms in bipolar I disorder. Journal of Affective Disorders. 2012 Dec 15;142(1–3):36–44. doi: http://dx
.doi.org/10 .1016/j.jad.2012.04.014. PMID: 23062763. [PubMed: 23062763] - 22.
- Niufan G, Tohen M, Qiuqing A, et al Olanzapine versus lithium in the acute treatment of bipolar mania: a double-blind, randomized, controlled trial. Journal of Affective Disorders. 2008 Jan;105(1–3):101–8. PMID: 17531327. [PubMed: 17531327]
- 23.
- Tohen M, Vieta E, Goodwin GM, et al Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. Journal of Clinical Psychiatry. 2008 Nov;69(11):1776–89. PMID: 19014751. [PubMed: 19014751]
- 24.
- Perlis RH, Welge JA, Vornik LA, et al Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. Journal of Clinical Psychiatry. 2006 Apr;67(4):509–16. PMID: 16669715. [PubMed: 16669715]
- 25.
- Revicki DA, Paramore LC, Sommerville KW, et al Divalproex sodium versus olanzapine in the treatment of acute mania in bipolar disorder: health-related quality of life and medical cost outcomes. Journal of Clinical Psychiatry. 2003 Mar;64(3):288–94. PMID: 12716270. [PubMed: 12716270]
- 26.
- Tohen M, Goldberg JF, Gonzalez-Pinto Arrillaga AM, et al A 12-week, double-blind comparison of olanzapine vs haloperidol in the treatment of acute mania. Archives of General Psychiatry. 2003 Dec;60(12):1218–26. PMID: 14662554. [PubMed: 14662554]
- 27.
- Shi L, Namjoshi MA, Zhang F, et al Olanzapine versus haloperidol in the treatment of acute mania: clinical outcomes, health-related quality of life and work status. International Clinical Psychopharmacology. 2002 Sep;17(5):227–37. PMID: 12177585. [PubMed: 12177585]
- 28.
- Tohen M, Baker RW, Altshuler LL, et al Olanzapine versus divalproex in the treatment of acute mania. [Erratum appears in Am J Psychiatry. 2005 Feb;7(1):102]. American Journal of Psychiatry. 2002 Jun;159(6):1011–7. PMID: 12042191. [PubMed: 12042191]
- 29.
- Tohen M, Jacobs TG, Grundy SL, et al Efficacy of olanzapine in acute bipolar mania: a double-blind, placebo-controlled study. The Olanzipine HGGW Study Group. [Erratum appears in Arch Gen Psychiatry 2002 Jan;59(1):91]. Archives of General Psychiatry. 2000 Sep;57(9):841–9. PMID: 10986547. [PubMed: 10986547]
- 30.
- Berk M, Ichim L, Brook S. Olanzapine compared to lithium in mania: a double-blind randomized controlled trial. International Clinical Psychopharmacology. 1999 Nov;14(6):339–43. PMID: 10565800. [PubMed: 10565800]
- 31.
- Katagiri H, Takita Y, Tohen M, et al Safety and efficacy of olanzapine monotherapy and olanzapine with a mood stabilizer in 18-week treatment of manic/mixed episodes for Japanese patients with bipolar I disorder. Current Medical Research & Opinion. 2012 May;28(5):701–13. doi: http://dx
.doi.org/10 .1185/03007995.2012.666961. PMID: 22356118. [PubMed: 22356118] - 32.
- Vieta E, Montgomery S, Sulaiman AH, et al A randomized, double-blind, placebo-controlled trial to assess prevention of mood episodes with risperidone long-acting injectable in patients with bipolar I disorder. European Neuropsychopharmacology. 2012 Nov;22(11):825–35. doi: http://dx
.doi.org/10 .1016/j.euroneuro.2012.03.004. PMID: 22503488. [PubMed: 22503488] - 33.
- Shafti SS. Olanzapine vs. lithium in management of acute mania. Journal of Affective Disorders. 2010 May;122(3):273–6. doi: http://dx
.doi.org/10 .1016/j.jad.2009.08.013. PMID: 19740546. [PubMed: 19740546] - 34.
- Perlis RH, Baker RW, Zarate CA, Jr., et al Olanzapine versus risperidone in the treatment of manic or mixed States in bipolar I disorder: a randomized, double-blind trial. Journal of Clinical Psychiatry. 2006 Nov;67(11):1747–53. PMID: 17196055. [PubMed: 17196055]
- 35.
- Zajecka JM, Weisler R, Sachs G, et al A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. Journal of Clinical Psychiatry. 2002 Dec;63(12):1148–55. PMID: 12523875. [PubMed: 12523875]
- 36.
- Cutler AJ, Datto C, Nordenhem A, et al Extended-release quetiapine as monotherapy for the treatment of adults with acute mania: a randomized, double-blind, 3-week trial. Clinical Therapeutics. 2011 Nov;33(11):1643–58. doi: http://dx
.doi.org/10 .1016/j.clinthera.2011.10.002. PMID: 22054797. [PubMed: 22054797] - 37.
- McElroy SL, Martens BE, Winstanley EL, et al Placebo-controlled study of quetiapine monotherapy in ambulatory bipolar spectrum disorder with moderate-to-severe hypomania or mild mania. Journal of Affective Disorders. 2010 Jul;124(1–2):157–63. doi: http://dx
.doi.org/10 .1016/j.jad.2009.11.014. PMID: 19963274. [PubMed: 19963274] - 38.
- Li H, Ma C, Wang G, et al Response and remission rates in Chinese patients with bipolar mania treated for 4 weeks with either quetiapine or lithium: a randomized and double-blind study. Current Medical Research & Opinion. 2008 Jan;24(1):1–10. PMID: 18028587. [PubMed: 18028587]
- 39.
- Bowden CL, Grunze H, Mullen J, et al A randomized, double-blind, placebo-controlled efficacy and safety study of quetiapine or lithium as monotherapy for mania in bipolar disorder. Journal of Clinical Psychiatry. 2005 Jan;66(1):111–21. PMID: 15669897. [PubMed: 15669897]
- 40.
- Khanna S, Vieta E, Lyons B, et al Risperidone in the treatment of acute mania. The British Journal of Psychiatry. 2005 2005);187(3):229–34. doi: http://dx
.doi.org/10.1192/bjp.187.3.229. PMID: 16135859. [PubMed: 16135859] - 41.
- Segal J, Berk M, Brook S. Risperidone compared with both lithium and haloperidol in mania: a double-blind randomized controlled trial. Clinical Neuropharmacology. 1998 May–Jun;21(3):176–80. PMID: 9617509. [PubMed: 9617509]
- 42.
- Potkin SG, Keck PE, Jr., Segal S, et al Ziprasidone in acute bipolar mania: a 21-day randomized, double-blind, placebo-controlled replication trial. Journal of Clinical Psychopharmacology. 2005 Aug;25(4):301–10. PMID: 16012271. [PubMed: 16012271]
- 43.
- Keck PE, Jr., Versiani M, Potkin S, et al Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. American Journal of Psychiatry. 2003 Apr;160(4):741–8. PMID: 12668364. [PubMed: 12668364]
- 44.
- Jeong HG, Lee MS, Ko YH, et al Combination treatment with aripiprazole and valproic acid for acute mania: an 8-week, single-blind, randomized controlled trial. Clinical Neuropharmacology. 2012 May–Jun;35(3):97–102. doi: http://dx
.doi.org/10 .1097/WNF.0b013e3182560401. PMID: 22592508. [PubMed: 22592508] - 45.
- Vieta E, T’Joen C, McQuade RD, et al Efficacy of adjunctive aripiprazole to either valproate or lithium in bipolar mania patients partially nonresponsive to valproate/lithium monotherapy: a placebo-controlled study. American Journal of Psychiatry. 2008 Oct;165(10):1316–25. doi: http://dx
.doi.org/10 .1176/appi.ajp.2008.07101560. PMID: 18381903. [PubMed: 18381903] - 46.
- Szegedi A, Calabrese JR, Stet L, et al Asenapine as adjunctive treatment for acute mania associated with bipolar disorder: results of a 12-week core study and 40-week extension. Journal of Clinical Psychopharmacology. 2012 Feb;32(1):46–55. doi: http://dx
.doi.org/10 .1097/JCP.0b013e31823f872f. PMID: 22198448. [PubMed: 22198448] - 47.
- Conus P, Berk M, Cotton SM, et al Olanzapine or chlorpromazine plus lithium in first episode psychotic mania: An 8-week randomised controlled trial. European Psychiatry: the Journal of the Association of European Psychiatrists. 2015 Nov;30(8):975–82. doi: http://dx
.doi.org/10 .1016/j.eurpsy.2015.09.009. PMID: 26485297. [PubMed: 26485297] - 48.
- Houston JP, Tohen M, Degenhardt EK, et al Olanzapine-divalproex combination versus divalproex monotherapy in the treatment of bipolar mixed episodes: a double-blind, placebo-controlled study. [Erratum appears in J Clin Psychiatry. 2011 Aug;72(8):1157], [Erratum appears in J Clin Psychiatry. 2010 Jan;71(1):93]. Journal of Clinical Psychiatry. 2009 Nov;70(11):1540–7. doi: http://dx
.doi.org/10 .4088/JCP.08m04895yel. PMID: 19778495. [PubMed: 19778495] - 49.
- Tohen M, Bowden CL, Smulevich AB, et al Olanzapine plus carbamazepine v. carbamazepine alone in treating manic episodes. British Journal of Psychiatry. 2008 Feb;192(2):135–43. doi: http://dx
.doi.org/10 .1192/bjp.bp.107.041301. PMID: 18245032. [PubMed: 18245032] - 50.
- Tohen M, Chengappa KN, Suppes T, et al Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Archives of General Psychiatry. 2002 Jan;59(1):62–9. PMID: 11779284. [PubMed: 11779284]
- 51.
- Namjoshi MA, Risser R, Shi L, et al Quality of life assessment in patients with bipolar disorder treated with olanzapine added to lithium or valproic acid. Journal of Affective Disorders. 2004;81(3):223–9. PMID: 15337326. [PubMed: 15337326]
- 52.
- Baker RW, Brown E, Akiskal HS, et al Efficacy of olanzapine combined with valproate or lithium in the treatment of dysphoric mania. British Journal of Psychiatry. 2004 Dec;185:472–8. PMID: 15572737. [PubMed: 15572737]
- 53.
- Yatham LN, Vieta E, Young AH, et al A double blind, randomized, placebo-controlled trial of quetiapine as an add-on therapy to lithium or divalproex for the treatment of bipolar mania. International Clinical Psychopharmacology. 2007 Jul;22(4):212–20. PMID: 17519644. [PubMed: 17519644]
- 54.
- Yatham LN, Paulsson B, Mullen J, et al Quetiapine versus placebo in combination with lithium or divalproex for the treatment of bipolar mania. [Erratum appears in J Clin Psychopharmacol. 2005 Apr;25(2):201], [Erratum appears in J Clin Psychopharmacol. 2005 Feb;25(1):103]. Journal of Clinical Psychopharmacology. 2004 Dec;24(6):599–606. PMID: 15538120. [PubMed: 15538120]
- 55.
- Yatham LN, Grossman F, Augustyns I, et al Mood stabilisers plus risperidone or placebo in the treatment of acute mania. International, double-blind, randomised controlled trial. [Erratum appears in Br J Psychiatry. 2003 Apr;182:369]. British Journal of Psychiatry. 2003 Feb;182:141–7. PMID: 12562742. [PubMed: 12562742]
- 56.
- Sachs GS, Vanderburg DG, Edman S, et al Adjunctive oral ziprasidone in patients with acute mania treated with lithium or divalproex, part 1: results of a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry. 2012 Nov;73(11):1412–9. doi: http://dx
.doi.org/10.4088/JCP.11m07388. PMID: 23218157. [PubMed: 23218157]
- Aripiprazole for Acute Mania
- Asenapine for Acute Mania
- Cariprazine for Acute Mania
- Haloperidol for Acute Mania
- Olanzapine for Acute Mania
- Quetiapine for Acute Mania
- Risperidone for Acute Mania
- Ziprasidone for Acute Mania
- Aripiprazole Plus Mood Stabilizer
- Asenapine Plus Mood Stabilizer
- Olanzapine Plus Mood Stabilizer
- Quetiapine Plus Mood Stabilizer
- Risperidone Plus Mood Stabilizer
- Ziprazidone Plus Mood Stabilizer
- References for Appendix E
- Antipsychotics for Mania - Treatment for Bipolar Disorder in Adults: A Systemati...Antipsychotics for Mania - Treatment for Bipolar Disorder in Adults: A Systematic Review
- Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Study an...Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Study and Other Psychosocial and Somatic Interventions - Treatment for Bipolar Disorder in Adults: A Systematic Review
- Evidence Tables - Social and Structural Determinants of Maternal Morbidity and M...Evidence Tables - Social and Structural Determinants of Maternal Morbidity and Mortality: An Evidence Map
- Results - Radiation Therapy for Brain MetastasesResults - Radiation Therapy for Brain Metastases
- Other Drugs for Acute Mania - Treatment for Bipolar Disorder in Adults: A System...Other Drugs for Acute Mania - Treatment for Bipolar Disorder in Adults: A Systematic Review
Your browsing activity is empty.
Activity recording is turned off.
See more...