NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Sobieraj DM, Baker WL, Martinez BK, et al. Adverse Effects of Pharmacologic Treatments of Major Depression in Older Adults [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2019 Mar. (Comparative Effectiveness Review, No. 215.)

Adverse Effects of Pharmacologic Treatments of Major Depression in Older Adults [Internet].
Show detailsBackground
Depression is a common psychiatric disease in older adults. Prevalence of depression in adults 65 years of age and older is estimated to be 15–20 percent in the United States.1 Multiple systematic reviews have shown that antidepressant medications are better than placebo for treating depression in older patients, but with modest efficacy.2 In addition, clinicians must consider the balance of the risks and benefits of antidepressant medications, especially in comparison to other treatment options.
The American Geriatrics Society (AGS) regularly compiles the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults.3 This source identifies potentially inappropriate medications that are best avoided for most adults with specific conditions, or used with caution, at lower doses, or with careful monitoring. In 2015, this list recommended that clinicians avoid prescribing selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) in older adults with a history of falls or fractures.3 They noted that there may be situations when use of these medications may be appropriate and clinicians and patients must carefully weigh both benefits and potential harms.4 Suggested alternatives to TCAs and SSRIs include serotonin-norepinephrine reuptake inhibitors (SNRIs) and bupropion.5 However, the AGS also recommended using SSRIs and SNRIs with caution due to the potential to exacerbate or cause hyponatremia as a result of the syndrome of inappropriate antidiuretic hormone (SIADH).3
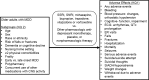
Given these concerns of potential adverse events in the older population with drugs commonly recommended to treat major depressive disorder (MDD), clinicians may be left selecting therapy based on comparative adverse effects. The objective of this review is to assess comparative adverse effects of pharmacologic antidepressants for treatment of MDD in adults 65 years of age or older (Figure A).
This review focuses on patients and drugs as classified in Table A and Figure A. The drugs selected for inclusion were therapies that were considered most likely to be used in this population, according to the expert opinion of the partner, key informants, technical expert panel and public comments received at the protocol development stage.
Table A
Included pharmacologic treatments for major depressive disorder in older adults.
Data Sources
Data sources were MEDLINE®, Embase®, Cochrane Central, and PsychINFO bibliographic databases from earliest date through May 15, 2018; hand searches of references of relevant studies; www.clinicaltrials.gov and the International Controlled Trials Registry Platform. The systematic review protocol is available in the full report.
Methods
The protocol was registered in PROSPERO (CRD42018088648) and posted on the Agency for Healthcare Research and Quality website.6 The draft report was posted for public and peer review and we revised the report based on these comments. We considered a variety of potential outcomes on which to focus, and after Technical Expert Panel input, we decided to grade strength of evidence (SOE) for the following outcomes: any adverse event, withdrawal due to adverse events, mortality, hospitalization, serious adverse events, arrhythmias, QTc prolongation, falls, fractures, cognitive impairment and SIADH. SOE was graded for the calculated effect estimates with interpretation based on statistical significance. SOE could have four grades (high (+++), moderate (++), low (+), or insufficient). We calculated number needed to treat (NNT) or harm (NNH) for graded outcomes with statistically significant findings. Outcomes that were not graded are reported in the full report.
Results
Twenty-one studies7–27 (19 randomized controlled trials [RCTs], 2 observational studies) are included in this review (Table B). RCTs enrolled patients 65 years of age and older and mostly studied moderate severity MDD and treatment of the acute phase of MDD (<12 weeks). RCTs consistently required patients to be free from uncontrolled medical comorbidities or other neuropsychological conditions and relied on spontaneous reporting of adverse events. Doses of antidepressants were low relative to suggested usual doses in older adults.28,29 Risk of bias of individual studies varied (13 studies, low; 7 studies, high; 1 study, unclear). High risk of bias was attributed to high overall or differential attrition, open-label periods in which patients were withdrawn due to adverse events prior to randomization, or exclusion of patients from continuation or maintenance phases due to adverse events during acute treatment. Evidence was overall scarce and conclusions for a given comparison and outcome are often based on a single study. None of the RCTs were powered or designed to capture adverse events and SOE was most frequently downgraded due to imprecision and suspected selective outcome reporting.
Table B
Distribution of included trials by intervention, comparator, and reported outcomes.
Key Question (KQ) 1 aimed to evaluate the adverse events and comparative adverse events of antidepressants. Results for KQ 1 are presented in Tables C and D. Although we aimed to evaluate SSRIs and SNRIs on a class basis, data for few individual drugs within the classes were identified. Thus, within Tables C and D, the representative drugs that contributed to the listed result are identified. Only outcomes with a graded SOE appear in this summary and the remaining findings are presented in the full report. Blank cells in either table indicate that we found no evidence. SOE grading is noted with the following symbols: (+)=low SOE; (++)=moderate SOE; (+++)=high SOE. Outcomes graded with insufficient evidence are listed as such.
Adverse Effects of Antidepressants
Table C
Adverse events of antidepressants versus placebo or no therapy: summary statements based on findings and statistical significance.
Comparative Adverse Effects of Antidepressants
Table D
Comparative adverse events of antidepressants versus each other: summary statements based on findings and statistical significance.
Subgroups of Interest
KQ 2 aimed to address subgroups of interest (Figure A) and their impact on adverse events and comparative adverse events of antidepressants.
- According to a single post-hoc analysis on a RCT, risk of falls on duloxetine may be associated with the presence of any cardiovascular or pulmonary disorder (low SOE).31
Discussion
Applicability of results. This review exclusively included studies that required an age of 65 years or older. The studies were consistent in excluding patients with uncontrolled/unstable comorbidities or other psychological conditions, particularly patients with high suicide risk. None of the studies were specific to nursing facility residents. Unfortunately this limits applicability of results given that older adults commonly have multiple comorbidities and are subject to taking multiple medications. Major depression was mostly diagnosed using DSM criteria. Based on scores from the Hamilton Depression Rating Scale (HAM-D) or the Montgomery-Asberg Depression Scale (MADRS) for study eligibility, the population represents those with moderate severity depression. The doses of antidepressants studied were rarely reflective of the full range cited in guideless as the usual dose range for older adults, and were more often reflective of the lower half of that range. The data in this report does not reflect higher usual antidepressant doses.
The majority of trials evaluated treatment of the acute phase of MDD which is up to 12 weeks. Although we aimed to evaluate some therapies on a class basis (SSRI and SNRI), we did not find evidence for multiple drugs within any class, limiting the ability to extrapolate results to the entire class. Concurrent pharmacologic therapies allowed, when described, were usually as-needed therapies for sleep. Importantly, consistent with inclusion criteria, studies focused on the outpatient setting and did not include hospitalized inpatient or urgent care scenarios.
Limitations of the evidence base. Several limitations pertain to the literature base of this review. Interpretations of findings were made based on statistical significance, which may miss small differences due to inadequate power. Readers should not assume a failure to find a difference means that the given interventions are similar in adverse event profiles, particularly when SOE ratings are low or for outcomes that do not have a SOE grade. None of the trials were powered to evaluate harms as they were all designed to assess efficacy. Many adverse events were not observed or reported rarely, such that there were only one or two events in the intervention arm and zero in the comparator arm. For several other adverse events, data were not reported in the peer reviewed literature at all. The issue of sparse data throughout the evidence base was further complicated by the treatment phases that studies used, as most were specific to treating the acute phase of MDD (<12 weeks), but others evaluated only the continuation (12 weeks up to 48 weeks) or maintenance (beyond 48 weeks) phases of treatment. Data beyond the acute treatment phase were very limited. Furthermore, when studies did evaluate continuation or maintenance, they were considered to have higher risk of bias because open-label acute treatment periods were used and subjects experiencing adverse events were withdrawn prior to randomization into the longer treatment period. Thus, events were less likely to occur during the randomized period.
We found no evidence for several of the specific medications and neither did evidence exist for some of the adverse events we aimed to analyze. Most data were available in comparison with placebo and little direct comparative data were found to inform comparative harms of antidepressants. Even when studies were eligible for this review, the small number of trials and smaller samples sizes posed limitations.
Most RCTs relied on spontaneous reporting of adverse events rather than active surveillance. Determining if adverse outcomes were defined or pre-specified was difficult. Commonly we suspected selective outcome reporting because studies stated that certain measurements were part of the routine clinical monitoring protocol (e.g. vitals, electrocardiogram were to be measured) although were not subsequently reported in the results. We attempted to contact authors for this information but the yield was small. Lastly, few data exist regarding subgroups that are of interest in this field and although we sought to collect and analyze such data when possible, we found only data regarding the impact of age and comorbidities.
Evidence gaps and future research needs. Important research gaps must be addressed to understand more fully the harms associated with antidepressant therapy in elderly patients with MDD. We found no evidence to assess harms for several therapies of interest including fluvoxamine, desvenlafaxine, milnacipran, levomilnacipran or vilazodone. Even within the classes of SSRIs and SNRIs, evidence for an outcome was often specific to one or two drugs within the class because others have not been studied in this age group. There were important outcomes (e.g. emergency room visits, hospitalizations) and subgroups (e.g. comorbidities, polypharmacy) that were not reported in the eligible studies despite their being important to clinicians and decision makers as identified by the key informants, technical expert panelists and partners on this project. Future studies should include these outcomes and subgroups as well as other specific populations such as nursing facility residents. Overall, additional research is needed to characterize important harms associated with therapies used to treat MDD in older patients, particularly well controlled studies powered to assess adverse events.
Conclusions
In patients 65 years of age or older with MDD, treatment of the acute phase of MDD with SNRIs (duloxetine and venlafaxine) led to a greater number of adverse events compared with placebo while adverse events were statistically similar to placebo with SSRIs (escitalopram, fluoxetine). SSRIs (citalopram, escitalopram and fluoxetine) and SNRIs (duloxetine and venlafaxine) led to a greater number of study withdrawals due to adverse events compared with placebo, and duloxetine increased the risk of falls. Further characterization of the comparative safety of antidepressants is difficult because few studies were identified, comparisons were based on statistical significance, trials were not powered to identify small difference in adverse events and observational studies may be confounded. Comparative, long-term, well-designed studies that report specific adverse events are needed to better inform decision making in this population.
References
- 1.
- CDC Promotes Public Health Approach to Address Depression among Older Adults https://www
.cdc.gov/aging /pdf/cib_mental_health.pdf. Accessed Nov 2, 2017. - 2.
- Kok RM, Reynolds CF III. Management of Depression in Older Adults A Review. JAMA. 2017;317(20):2114–22. PMID: 28535241. [PubMed: 28535241]
- 3.
- American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults by the American Geriatrics Society 2015 Beers Criteria Update Expert Panel (2015). J Am Geriatr Soc. 2016;63:2227–46. PMID: 26446832. [PubMed: 26446832]
- 4.
- Steinman MA, Beizer JL, DuBeau CE, Laird RD, Lundebjerg NE, Mulhausen P. How to Use the AGS 2015 Beers Criteria – A Guide for Patients, Clinicians, Health Systems, and Payors. J Am Geriatr Soc 2015;63(12):e1–e7. PMID: 26446776. [PMC free article: PMC5325682] [PubMed: 26446776]
- 5.
- Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatric Soc. 2015;63(12):e8–e18. PMID: 26447889. [PMC free article: PMC4890629] [PubMed: 26447889]
- 6.
- Agency for Healthcare Research and Quality. Adverse effects of first-line pharmacologic treatments of major depression in older adults: Research protocol. https:
//effectivehealthcare .ahrq.gov/topics /depression-harms/research-protocol. Accessed July 23, 2018. - 7.
- Schone W, Ludwig M. A double-blind study of paroxetine compared with fluoxetine in geriatric patients with major depression. J Clin Psychopharmacol. 1993;13(6, Suppl 2):34S–39S. PMID: 8106654. [PubMed: 8106654]
- 8.
- Kasper S, de Swart H, Andersen HF. Escitalopram in the treatment of depressed elderly patients. Am J Geriatr Psychiatry. 2005; 13(10):884–91. PMID: 16223967. [PubMed: 16223967]
- 9.
- Cassano GB, Puca FM, Scapicchio PL, et al. Paroxetine and fluoxetine effects on mood and cognitive functions in depressed nondemented elderly patients. J Clin Psychiatry. 2002;63(5):396–402. PMID: 12019663. [PubMed: 12019663]
- 10.
- Schatzberg A, Roose S. A double-blind, placebo-controlled study of venlafaxine and fluoxetine in geriatric outpatients with major depression. Am J Geriatr Psychiatry. 2006;14(4):361–70. PMID: 16582045. [PubMed: 16582045]
- 11.
- Reynolds CF, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354(11):1130–8. PMID: 14650613. [PubMed: 16540613]
- 12.
- Gorwood P, Weiller E, Lemming O, et al. Escitalopram prevents relapse in older patients with major depressive disorder. Am J Geriatr Psychiatry. 2007;15(7):581–93. PMID: 17586783. [PubMed: 17586783]
- 13.
- Klysner R, Bent-Hansen J, Hansen HL, et al. Efficacy of citalopram in the prevention of recurrent depression in elderly patients: placebo-controlled study of maintenance therapy. Br J Psychiatry. 2002;181:29–35. PMID: 12091260. [PubMed: 12091260]
- 14.
- Roose SP, Sackeim HA, Krishnan KRR, et al. Antidepressant pharmacotherapy in the treatment of depression in the very old: A randomized, placebo-controlled trial. Am J Psychiatry. 2004;161(11):2050–2059. PMID: 15514406. [PubMed: 15514406]
- 15.
- Fraguas R, da Silva Telles RM, Ferraz Alves TCT, et al. A double-blind, placebo-controlled treatment trial of citalopram for major depressive disorder in older patients with heart failure: the relevance of the placebo effect and psychological symptoms. Contemp Clin Trials. 2009;30(3):205–11. PMID: 19470312. [PubMed: 19470312]
- 16.
- Finkel SI, Richter EM, Clary CM. Comparative efficacy and safety of sertraline versus nortriptyline in major depression in patients 70 and older. Int Psychogeriatr. 1999;11(1):85–99. PMID: 10189602. [PubMed: 10189602]
- 17.
- Kyle CJ, Petersen HE, Overo KF. Comparison of the tolerability and efficacy of citalopram and amitriptyline in elderly depressed patients treated in general practice. Depress Anxiety. 1998;8(4):147–53. PMID: 9871816. [PubMed: 9871816]
- 18.
- Hutchinson DR, Tong S, Moon CA, et al. Paroxetine in the treatment of elderly depressed patients in general practice: A double-blind comparison with amitriptyline. Int Clin Psychopharmacol. 1992;6:(Suppl 4):43–51. PMID: 1431010. [PubMed: 1431010]
- 19.
- Raskin J, Wiltse CG, Dinkel JJ, et al. Safety and tolerability of duloxetine at 60 mg once daily in elderly patients with major depressive disorder. J Clin Psychopharmacol. 2008;28(1):32–8. PMID: 18204338. [PubMed: 18204338]
- 20.
- Allard P, Gram L, Timdahl K, et al. Efficacy and tolerability of venlafaxine in geriatric outpatients with major depression: a double-blind, randomised 6-month comparative trial with citalopram. Int J Geriatr Psychiatry. 2004;19(12):1123–30. PMID: 15526307. [PubMed: 15526307]
- 21.
- Finkel SI, Richter EM, Clary CM, et al. Comparative efficacy of sertraline vs. fluoxetine in patients age 70 or over with major depression. Am J Geriatr Psychiatry. 1999;7(3):221–7. PMID: 10438693. [PubMed: 10438693]
- 22.
- Schatzberg AF, Kremer C, Rodrigues HE, et al. Double-blind, randomized comparison of mirtazapine and paroxetine in elderly depressed patients. Am J Geriatr Psychiatry. 2002;10(5):541–50. PMID: 12213688. [PubMed: 12213688]
- 23.
- Hewett K, Chrzanowski W, Jokinen R, et al. Double-blind, placebo-controlled evaluation of extended-release bupropion in elderly patients with major depressive disorder. J Psychopharmacol. 2010;24(4):521–529. PMID: 19164492. [PubMed: 19164492]
- 24.
- Robinson M, Oakes TM, Raskin J, et al. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry. 2014;22(1):34–45. PMID: 24314888. [PubMed: 24314888]
- 25.
- Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215–223. PMID: 22572889. [PubMed: 22572889]
- 26.
- Coupland C, Dhiman P, Morriss R, et al. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ: British Medical Journal. 2011;343(7819):1–15. PMID: 21810886. [PMC free article: PMC3149102] [PubMed: 21810886]
- 27.
- Wu E, Greenberg P, Yang E, et al. Comparison of treatment persistence, hospital utilization and costs among major depressive disorder geriatric patients treated with escitalopram versus other SSRI/SNRI antidepressants. Curr Med Res Opin. 2008;24(10):2805–13. PMID: 18755054. [PubMed: 18755054]
- 28.
- Gelenberg AJ, Freeman MP, Markowitz JC et al. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. American Psychiatric Association 2010.
- 29.
- Trintellix (vortioxetine) [package insert]. Takeda Pharmaceuticals America, Inc. Deerfield, IL. May 2018.
- 30.
- Lyketsos CG, Weiller E, Katona C, et al. Are old-old patients with major depression more likely to relapse than young-old patients during continuation treatment with escitalopram? BMC Geriatr. 2011;11:2. PMID: 21235759. [PMC free article: PMC3033830] [PubMed: 21235759]
- 31.
- Nelson JC, Oakes TM, Liu P, et al. Assessment of falls in older patients treated with duloxetine: a secondary analysis of a 24-week randomized, placebo-controlled trial. The primary care companion for CNS disorders. 2013;15(1):PCC.12m01419. PMID: 23724353. [PMC free article: PMC3661331] [PubMed: 23724353]
- Evidence Summary - Adverse Effects of Pharmacologic Treatments of Major Depressi...Evidence Summary - Adverse Effects of Pharmacologic Treatments of Major Depression in Older Adults
- Recurrent Nephrolithiasis in AdultsRecurrent Nephrolithiasis in Adults
Your browsing activity is empty.
Activity recording is turned off.
See more...