NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Chou R, Fu R, Dana T, et al. Interventional Treatments for Acute and Chronic Pain: Systematic Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 Sep. (Comparative Effectiveness Review, No. 247.)

Interventional Treatments for Acute and Chronic Pain: Systematic Review [Internet].
Show detailsResults of Literature Search
A total of 3,032 references from electronic database searches and reference lists were reviewed. From these, 336 full-text papers were evaluated for inclusion. After review of full-text papers, 288 articles were excluded. Across all interventions, 37 randomized controlled trials (RCTs) (in 48 publications) were included (Figure 1 and Appendix F). Thirteen trials addressed vertebroplasty, two kyphoplasty, four piriformis injections, three occipital nerve stimulation, three cooled radiofrequency, three pulsed radiofrequency, two methylene blue, three ozone, and one each sphenopalatine block, platelet-rich plasma, stem cells, and peripheral nerve stimulation. See Appendix G for data abstraction tables, Appendix H for quality tables, and Appendix I for additional meta-analysis results.
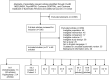
Figure 1
Literature flow diagram. Abbreviations: Int = interventional procedures and conditions *Additional sources include suggested references, reference lists, etc.
Vertebral Augmentation Procedures for Vertebral Compression Fractures
Vertebroplasty
Key Points
- Vertebroplasty for vertebral compression fracture (13 trials, N=1685, mean age 66 to 80 years) was associated with a small reduction in pain intensity versus sham vertebroplasty or usual care at 1 to 2 weeks (10 trials, N=1093), 1 to 6 months (10 trials, N=1094), 6 to 12 months (8 trials, N=993), and 12 months and longer (9 trials, N=965); and a moderate reduction at 2 to 4 weeks (8 trials, N=918) (strength of evidence [SOE]: low at 1 to 2 weeks, moderate at other time points). Restricting to sham vertebroplasty controls (5 trials, N=536) tended to decrease benefits (no difference at 1 to 2 weeks and small at other time points), but the difference between sham and usual care trials was only statistically significant at 2 to 4 weeks (p for interaction=0.01). Benefits also tended to be larger in trials of patients with more acute compared with less acute pain, but differences were not statistically significant.
- There was insufficient evidence to determine effects of vertebroplasty on function at 1 to 2 weeks (7 trials, N=743), due to marked inconsistency between sham trials (no benefit) and usual care trials (small benefit) Vertebroplasty was associated with a small improvement versus sham or usual care in function at 2 to 4 weeks (6 trials, N=708), 1 to 6 months (7 trials, N=637), 6 to 12 months (6 trials, N=690), and ≥12 months (6 trials, N=612). (SOE: insufficient for 1 to 2 weeks, moderate for 1 to 6 months and 12 months and longer, and high for 2 to 4 weeks and 6 to 12 months).
- Vertebroplasty was not associated with increased risk of incident vertebral fracture at 12 months and longer (7 trials, N=826); evidence on serious adverse events was sparse and imprecise but did not indicate increased risk (SOE: moderate for vertebral fracture, low for serious adverse events).
- Three trials that conducted within-study subgroup analyses found no interaction between duration of symptoms and effects of vertebroplasty and one trial found no interaction between sex or prior vertebral fracture and effects of vertebroplasty.
- A stratified analysis of vertebroplasty trials found no interaction between polymethyl methacrylate (PMMA) volume and effects of vertebroplasty.
Description of Included Studies
Thirteen trials (reported in 20 publications) compared vertebroplasty versus a sham procedure (5 trials)79–83 or usual care (8 trials)84–91 (Appendix Table G-1 to G-3 and Table 2). Sample sizes ranged from 34 to 400 (N=1685). Seven trials were conducted in Europe,81,82,84,87–90 two trials in Australia,79,80 two trials in China,85,91 and one trial in Iran;86 one trial83 was conducted in the United Kingdom, Australia, and the United States. The mean age of enrollees ranged from 66 to 80 years; in one trial88 that did not report the mean age the range was 56 to 82 years. One trial88 was restricted to females and in the others, the proportion female ranged from 64 to 87 percent. The trials focused on patients with osteoporotic compression fractures and excluded patients with fracture due to cancer. Two trials restricted inclusion to patients with acute pain (up to 4 to 6 weeks),88,91 five trials restricted inclusion to patients with acute or subacute pain (up to 6 to 10 weeks),80–82,87,89 three trials enrolled patients with acute, subacute, or chronic pain (up to 12 months),79,83,84 and three trials restricted inclusion to patients with subacute or chronic pain (≥3 months,85 4 weeks to 1 year,86 or 6 weeks to 5 months90). Mean or median pain duration was <4 weeks in four trials,80,88,89,91 4 to 8 weeks in three trials (including one trial that did not report average pain duration but was restricted to patients with pain for ≤8 weeks),81,82,87 and ≥8 weeks in six trials.79,83–86,90 Ten trials80–82,84–88,90,91 required participants to have magnetic resonance imaging (MRI) findings consistent with bone marrow edema at the vertebral fracture site, a marker of greater acuity. The average volume of PMMA used in vertebroplasty ranged from 2.6 to 7.5 ml; two trials80,81 reported use of greater than 5 ml and the others reported less than 5 ml or did not report the PMMA volume. The duration of followup ranged from 6 to 24 months.
Four trials were rated good quality,79–81,83 five trials were rated fair quality,82,84,85,87,90 and four trials poor quality86,88,89,91 (Appendix Table H-1). The good-quality trials utilized sham vertebroplasty for blinding; sham procedures consisted of needle insertion or pressure on the back to simulate needle insertion, tapping to simulate entry of the needle into bone, and preparation of PMMA to mimic the sounds and smells of vertebroplasty. In three of the five sham-controlled trials, patients randomized to sham received the same periosteal infiltration of local anesthetic as patients randomized to vertebroplasty.79,81,83 In one sham-controlled trial,82 local anesthetic was injected into the vertebral body and in the fifth trial,80 patients randomized to sham received subcutaneous but not periosteal local anesthetic. In the open-label trials, usual care consisted of various nonsurgical therapies (analgesics, physical therapy, graded activity, and braces or walking aids), but only one trial86 described specific medications and doses. In addition to open-label design, other limitations in the fair-quality trials (including one sham-controlled trial)82 included failure to report randomization or allocation concealment methods, baseline group differences, high attrition, or lack of intent-to-treat analysis. One poor-quality trial88 did not report efficacy outcomes in the usual care arm and another poor-quality trial86 had serious data discrepancies—implausible values for standard deviations or results (mean differences, 95% confidence intervals [CIs], and p values) inconsistent with reported data. Therefore, neither of these trials was utilized in efficacy meta-analyses, but contributed data on harms.
Table 2
Study characteristics of vertebroplasty trials.
Detailed Synthesis
Pain
At 1 to 2 weeks, the difference between vertebroplasty versus sham or usual care was small and not statistically significant (10 trials, mean difference −0.53 on a 0 to 10 scale, 95% CI, −1.36 to 0.24, I2=75%, Figure 2).79–85,87,90,91 Vertebroplasty was associated with a moderate reduction in pain intensity versus sham vertebroplasty or usual care at 2 to 4 weeks to 1 month (8 trials, mean difference −1.05 on a 0 to 10 scale, 95% CI, −1.80 to −0.32, I2=64%, Figure 3),79–83,85,87,91 with a small reduction in pain intensity at 1 to 6 months (10 trials, mean difference −0.76, 95% CI, −1.17 to −0.38, I2=5.5%, Figure 4),79–85,87,89,91 6 to 12 months (8 trials, mean difference −0.73, 95% CI, −1.33 to −0.15, I2=43%, Figure 5),79–81,83–85,87,91 and 12 months and longer (9 trials, mean difference −0.87, 95% CI, −1.43 to −0.31, I2=42%, Figure 6).79,81–85,87,89,91 At 2 to 4 weeks, the pain reduction between trials using sham control versus usual care was significantly different (p for interaction=0.01). Pain reduction in sham controlled trials was significantly lower (5 trials, mean difference −0.57, 95% CI, −1.09 to −0.05, I2=0%) than in trials of usual care (3 trials, mean difference −2.27, 95% CI, −3.20 to −0.94, I2=0%). Among the sham controlled trials, the largest effect (mean difference −1.40, 95% CI −2.44 to −0.36) was observed in the trial that enrolled patients with the most acute symptoms (<6 weeks, mean 2.6 weeks).80 At other time points, there were no statistically significant differences in pain reduction between trials using sham or usual care controls, though across time points estimates were smaller with sham than usual care and stratified estimates were imprecise (Appendix Tables I-1 and I-2). Benefits also tended to be larger in trials that enrolled patients with more acute pain. However, differences were not statistically significant, only one trial91 restricted enrollment to patients with acute pain, and only one sham-controlled trial80 reported mean pain duration of <4 weeks. Reductions in pain intensity also did not differ according to presence of bone marrow edema on MRI (required to be enrolled in trial versus not required), PMMA volume (>5 or ≤5 ml), or study quality (good, fair, or poor). However, subgroup estimates were based on small numbers of trials and were imprecise. For analyses with at least 10 trials, graphical and statistical tests did not indicate small study effects (p for Egger’s test=0.59 at 1 to 2 weeks [Appendix Figure I-1] and p=0.62 at 1 to 6 months [Appendix Figure I-2]).

Figure 3
Vertebroplasty versus sham or usual care, pain (continuous) at 2 to 4 weeks. Abbreviations: BME = bone marrow edema; CI = confidence interval; MRI = magnetic resonance imaging; PMMA = polymethyl methacrylate; SD = standard deviation
Few trials evaluated the association between vertebroplasty versus sham or usual care and likelihood of experiencing a pain response (defined as pain at least moderately better,79 pain <4 on a 0 to 10 numeric rating scale (NRS),80,83,84 or pain improvement ≥30%83). Results favored vertebroplasty at 2 to 4 weeks (3 trials),79,80,83 1 to 6 months (2 trials),79,80 6 to 12 months (2 trials),79,80 and 12 months and longer (2 trials),83,84 with relative risk (RR) estimates that ranged from 1.27 to 1.46 (Figure 7 and Appendix Table I-3). However, estimates were imprecise and nonstatistically significant. At 1 to 2 weeks, the estimate was very imprecise (2 trials, RR 1.05, 95% CI, 0.16 to 6.02, I2=75%).79,80

Figure 7
Vertebroplasty versus sham or usual care and likelihood of a pain response. Abbreviations: BME = bone marrow edema; CI = confidence interval; MRI = magnetic resonance imaging; PMMA = polymethyl methacrylate; SD = standard deviation
Function
Vertebroplasty was also associated with a small improvement in back-related function versus sham or usual care at 1 to 2 weeks (standard mean deviation [SMD] −0.21, 95% CI, −0.48 to 0.04, I2=49%, Figure 8).79,80,83,85,87,90,91 However, the estimate was imprecise and statistical heterogeneity was present. Vertebroplasty was associated with a small improvement in back-related function versus sham or usual care at 2 to 4 weeks (6 trials, SMD −0.27, 95% CI, −0.42 to −0.12, I2=0%, Figure 9),79,80,83,85,87,91 1 to 6 months (7 trials, SMD −0.28, 95% CI, −0.43 to −0.11, I2=0%, Figure 10),79,80,83,85,87,89,91 6 to 12 months (6 trials, SMD −0.29, 95% CI, −0.45 to −0.14, I2=0%, Figure 11),79,80,83,85,87,91 and at 12 months and longer (6 trials, SMD −0.23, 95% CI, −0.39 to −0.06, I2=0%, Figure 12).79,83,85,87,89,91 All trials except for two assessed function using the Roland-Morris Disability Questionnaire (RDQ) (scale 0 to 24) or modified RDQ (scale 0 to 23); differences on the RDQ or modified RDQ at these times points ranged from −1.64 to −1.90 points. At 1 to 2 weeks, there was marked inconsistency between the estimate from trials of sham (3 trials, SMD 0.03, 95% CI, −0.36 to 0.44, I2=34%) and usual care (3 trials, SMD −0.38, 95% CI, −0.61 to −0.18, I2=0%), though the difference was not statistically significant (p for interaction=0.10). At other time points, estimates from sham and usual care trials were similar. Effects of vertebroplasty on function did not differ based on average pain duration at enrollment or study quality (Appendix Tables I-4 and I-5). However, subgroup analyses were limited by small numbers of trials, with imprecise estimates.
Only one trial (mean pain duration at enrollment 17.8 weeks) evaluated the association between vertebroplasty versus sham or usual care and likelihood of experiencing functional improvement (defined as RDQ improved ≥30%).83 Vertebroplasty was associated with reduced likelihood of functional improvement versus sham at 2 to 4 weeks (relative risk [RR] 0.66, 95% CI, 0.45 to 0.97), but increased likelihood at 12 months and longer (RR 1.56, 95% CI, 1.12 to 2.18).
Other Outcomes
Vertebroplasty was associated with a small improvement versus sham or usual care in general quality of life as measured by the EuroQOL 5-Dimension Questionnaire (EQ-5D) at 2 to 4 weeks (4 trials, mean difference 0.05, 95% CI, 0.02 to 0.09, I2=0%, Appendix Figure I-3)79,80,83,87 and at 6 to 12 months (3 trials, mean difference 0.06, 95% CI, 0.02 to 0.11, I2=0%, Appendix Figure I-4).79,80,87 At other time points there were no differences or the difference was not statistically significant (Appendix Figures I-5 to I-7). Effects of vertebroplasty on the EQ-5D did not significantly differ according to study quality, but subgroup analyses were limited by small numbers of trials and estimates were imprecise (Appendix Tables I-6 and I-7).
Vertebroplasty was associated with no difference in Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO) scores (a vertebral fracture-specific measure of quality of life) at all time points (Appendix Figures I-8 to I-12).79,80,84,87,90,91 Differences on the QUALEFFO were very small (below the threshold for small), ranging from −1.45 to −2.98 points on a 0 to 100 scale. There was no statistically significant difference in effects of vertebroplasty on the QUALEFFO score based on control type or study quality, but subgroup analyses were limited by small numbers of trials and estimates were imprecise (Appendix Tables I-6 and I-7).
Vertebroplasty was associated with no difference versus sham or usual care in Short-Form 36 Health Survey Physical Component Score (SF-36 PCS) or Short-Form 36 Health Survey Mental Component Score (SF-36 MCS) at any time point (mean differences 1.16 points on a 0 to 100 scale favoring vertebroplasty to −3.08 points favoring controls), but findings were based on one or two trials and most estimates were imprecise (Appendix Figures I-13 and I-14, and Appendix Table I-8).82,83,89
Estimates for effects of vertebroplasty versus sham or usual care on likelihood of opioid use (defined as continued opioid use79,81,83,84 or major opioid use) were imprecise, based on one to three trials at each time point (Appendix Figure I-15 and Appendix Table I-3). One other trial found similar rates of opioid use with vertebroplasty versus sham at 12 weeks and 12 months, but data were not provided.82 Three trials found vertebroplasty associated with decreased likelihood of pain medication use (not restricted to opioids). In one trial, RR estimates ranged from 0.23 to 0.37 at 1 week to 12 months. In the other two trials, results were reported as statistically significant but risk estimates were not provided.87,88
Harms
Vertebroplasty was not associated with increased risk of incident vertebral fracture versus sham or usual care, though some imprecision was present (10 trials, RR 1.02, 95% CI, 0.66 to 1.62, I2=9.6%, Figure 13).80,81,84–91 Results were similar when the analysis was restricted to trials with ≥12 months followup (7 trials, RR 0.94, 95% CI, 0.55 to 1.49, I2=15%).81,84–87,89,91 There was no interaction between control type, pain duration, requirement for bone marrow edema on MRI for inclusion, PMMA volume, or study quality and risk of incident fracture, but stratified analyses were limited by small numbers of trials and imprecision. Estimates at 1 to 2 weeks and at 6 to 12 months were very imprecise (Appendix Table I-9).
Vertebroplasty was not associated with increased risk of mortality versus sham or usual care (7 trials, RR 0.88, 95% CI, 0.50 to 1.53, I2=0%, Appendix Figure I-16).80,81,84,86–89 Findings were similar at 6 to 12 months (3 trials, RR 0.76, 95% CI, 0.23 to 2.65, I2=0%)80,84,88 or 12 months and longer (5 trials, RR 0.98, 95% CI, 0.51 to 1.87, I2=0%),81,84,86,87,89 but estimates were more imprecise (Appendix Table I-9). Estimates for risk of serious adverse events were also imprecise at 6 to 12 months (subgroup analysis of patients with pain ≤3 weeks in the VAPOUR, vertebroplasty for acute painful osteoporotic fractures, trial,80 RR 0.67, 95% CI, 0.12 to 3.79)94 and 12 months and longer (1 trial, RR 0.95, 95% CI, 0.06 to 14.90),83 based on few events (5 and 2, respectively). One poor-quality trial found vertebroplasty associated with decreased risk of any adverse event versus usual care (16.1% vs. 35.3%, RR 0.46, 95% CI, 0.23 to 0.92).91

Figure 13
Vertebroplasty versus sham or usual care and risk of incident vertebral fracture. Abbreviations: BME = bone marrow edema; CI = confidence interval; MRI = magnetic resonance imaging; PMMA = polymethyl methacrylate; SD = standard deviation
Effects of Demographic, Clinical, and Technical Factors
Results of study-level stratified analyses based on pain duration are described above. Three trials of vertebroplasty also reported within-study subgroup analyses based on clinical or demographic factors. One trial found no interactions between duration of symptoms (<6 vs. ≥6 weeks, or as a continuous measure), sex, or prior vertebral fractures and effects of vertebroplasty on outcomes (p for interaction >0.10 for all of these factors).79 Another study found that vertebroplasty was associated with decreased pain intensity in patients with <13 weeks (mean difference −0.8 on a 0 to 10 scale, 95% CI, −2.5 to 0.8) or 14 to 26 weeks of pain (mean difference −1.3, 95% CI, −3.4 to 0.8) but not in patients with 27 to 52 weeks of pain (mean difference 0.0, 95% CI, −1.6 to 1.7).83 However, stratified estimates were imprecise, with overlapping CIs. The third trial also found no statistically significant difference between fracture duration (1 to 3 weeks vs. 4 to 6 weeks) and benefits of vertebroplasty, though benefits were larger in the more acute group (p for interaction 0.12).94
Evidence on how benefits and harms of vertebroplasty varied according to technical factors was very limited. As described above, a stratified analysis found no interaction between PMMA volume and effects on pain related to vertebral fracture.
Kyphoplasty
Key Points
- Kyphoplasty for vertebral compression fracture (2 trials, N=434, mean age 64 and 73 years) was associated with large reductions in pain and moderate to large improvement in function versus usual care at 1 week and 1 month in patients with or without cancer. No trial compared kyphoplasty against sham (SOE: low for function at 1 week; moderate for pain and for function at 1 month).
- In one trial (N=300) of patients without cancer, effects on pain and function were small to moderate at 3 months to 2 years (SOE: low).
- Evidence on incident or worsening vertebral fracture was inconsistent and imprecise, based on two trials (N=434) (SOE: insufficient).
Description of Included Studies
Two trials (n=134 and 300) compared kyphoplasty versus usual care for vertebral compression fracture (Appendix Table G-1 to G-3 and Table 3).99–102 The population differed in the trials: one trial102 (FREE, Fracture Reduction Evaluation trial) excluded patients with vertebral fractures related to cancer (mean age 73 years, 77% female); the other trial (CAFE [Cancer Patient Fracture Evaluation]) restricted inclusion to patients with cancer (mean age 64 years, 58% female).99 Both trials were multinational; neither trial reported what type of provider performed kyphoplasty. In FREE, enrollment was restricted to patients with fracture duration of 3 months or more (mean 6 weeks) with bone marrow edema on MRI; CAFE did not specify fracture duration (median 3.5 months) or require presence of bone marrow edema on imaging (67% had edema). Baseline pain intensity was similar in both trials (~7 on a 0 to 10 scale). Neither trial reported the PMMA volume utilized and both trials described usual care as involving various nonsurgical treatments, but did not otherwise specify usual care. FREE reported followup through 2 years.100,102 Although CAFE evaluated outcomes through 6 months, crossover from usual care to kyphoplasty was high (59%) after 1 month (compared with 8% in FREE at 1 year); therefore, we focused on 1-month outcomes from CAFE. Both trials were rated fair quality, mainly due to open-label design and differential loss to followup (Appendix Table H-1).
Table 3
Study characteristics of kyphoplasty trials.
Detailed Synthesis
Pain
Both trials found kyphoplasty associated with reduced pain versus usual care. In FREE, effects were large at 1 week (analysis of variance [ANOVA] mean difference −2.2 on a 0 to 10 scale, 95% CI, −2.8 to −1.6), decreasing to small at 1 to 2 years (ANOVA mean difference −0.8 to −0.9).100,102 In CAFE, the effects on pain were large at 1 week and 1 month (mean difference in change from baseline −3.5 to −3.3 on a 0 to 10 scale).99 Neither trial evaluated pain as a dichotomous outcome.
Function
Both trials also found kyphoplasty associated with improved function versus usual care. Similar to effects on pain, FREE found that differences between kyphoplasty versus usual care on function were moderate at 1 month (ANOVA mean difference −4.0 on the 0 to 24 RDQ, 95% CI, −5.5 to −2.6) but the benefit was attenuated at 1 year (ANOVA mean difference −2.6, 95% CI, −4.1 to −2.0 at 1 year); at 2 years the effect was small (ANOVA mean difference −1.4, 95% CI, −1.4, p=0.05 [CI not reported]).100,102 In CAFE, kyphoplasty was associated with a large improvement in RDQ versus usual care at 1 month (mean difference −8.4, 95% CI, −7.6 to −9.2).99
Other Outcomes
FREE found kyphoplasty associated with a small improvement in SF-36 PCS score versus placebo at 1 month (ANOVA mean difference 5.2 on a 0 to 100 scale, 95% CI, 2.9 to 7.4); differences were attenuated and below the threshold for small at longer followup and no longer statistically significant at 1 or 2 years.100,102 Effects on the EQ-5D also favored kyphoplasty at 1 month (ANOVA mean difference 0.18 on a 0 to 1 scale, 95% CI, 0.08 to 0.28) but were attenuated at 1 or 2 years (ANOVA mean difference 0.12). Kyphoplasty was associated with decreased likelihood of any opioid use at 6 months (29.8% vs. 42.9%, p=0.4), with no difference by 1 year (28.0% vs. 33.7%, p=1.0); there was no difference in the likelihood of strong opioid use at 1 month or 1 year.
CAFE found kyphoplasty associated with moderate improvement versus usual care in SF-36 PCS score (mean difference in change from baseline 11.1, 95% CI, 10.7 to 11.5) and small improvement versus usual care in SF-36 MCS score (mean difference in change from baseline 8.4, 95% CI, 7.7 to 9.1) at 1 month.99 Kyphoplasty was also associated with reduced likelihood of analgesic use at 1 month (52.3% vs. 82.0%, RR 0.64, 95% CI, 0.49 to 0.83).
Harms
FREE found no difference between kyphoplasty versus usual care in mortality, serious adverse events, or any adverse event, though estimates were imprecise.101,102 The estimate for new of worsening fracture also was imprecise, but the proportion with this outcome was higher in the kyphoplasty arm (33.0% vs. 25.3%, absolute risk difference 7.7%, 95% CI, −4.5 to 20.0).
In CAFE, kyphoplasty was associated with increased likelihood of mortality (32.9%, vs. 18.8%, 95% CI, 0.95 to 3.23) and any adverse event (37.1% vs. 29.7%, RR 1.27, 95% CI, 0.78 to 2.06) that were not statistically significant.99 However, there were few adverse events resulting in death (2.9% [2/70] vs. 1.6% [1/64]). There were also few incident symptomatic fractures (2.9% [2/70] vs. 7.8% [5/64]). Injury or procedural complications occurred in 5.7% (4/70) of patients in the kyphoplasty arm.
Alternatives to Conventional Radiofrequency Ablation
Cooled Radiofrequency Denervation
Key Points
Versus Sham Radiofrequency Denervation for Sacroiliac Pain
- Cooled radiofrequency denervation for sacroiliac pain was associated with a moderate to large reduction in pain and small to large improvement in function versus sham radiofrequency at 1 month (2 trials, N=79); improvements in pain and function at 3 months were moderate (1 trial, N=28) (SOE: moderate for pain and function at 3 months; low for function at 1 month).
Versus Conventional Radiofrequency Denervation for Presumed Facet Joint Pain
- Cooled radiofrequency denervation for presumed facet joint pain was associated with a small, nonstatistically significant reduction in pain versus conventional radiofrequency at 6 months and no difference in function (1 trial, N=43); there were no differences at earlier (1 or 3 month) followup (SOE: low).
Across Trials of Cooled and Pulsed Radiofrequency Denervation
- Harms were not well-reported, but when recorded were usually related to temporary increase in pain. No serious complications were reported (SOE: low).
- The mean age of participants ranged from 52 to 59 years.
Cooled Radiofrequency Denervation Versus Sham Radiofrequency Denervation for Sacroiliac Pain
Description of Included Studies
Two trials (n=28 and 51) evaluated cooled radiofrequency denervation versus sham radiofrequency for sacroiliac pain. (Appendix Table G-4 to G-6 and Table 4).103,104 Both trials were conducted in the United States. Mean age was 52 and 59 years and the proportion female 61 to 72 percent. Both trials required patients to have pain in the sacroiliac area for at least 6 months and persistent pain despite standard nonoperative therapies. Patients had to have at least 75 percent pain relief with a single103 or repeat104 diagnostic sacroiliac block. Baseline pain intensity was ~6 on a 0 to 10 scale in both trials. In both trials, cooled radiofrequency denervation was performed with imaging guidance; details regarding the radiofrequency techniques are shown in Table 4. Sham radiofrequency involved needle placement as for active treatment, without radiofrequency lesioning; in one trial,103 lidocaine was administered. Both trials were rated fair quality; methodological shortcomings included unclear randomization methods and high crossover without intent-to-treat analysis (Appendix Table H-1). Crossover was high: in one trial,104 94 percent (16/17) of patients randomized to sham treatment crossed over to cooled radiofrequency denervation after 3 months and in the other,103 64 percent (9/14) crossed over after 1 month. Therefore, results focus on outcomes prior to high crossover (3 and 1 months, respectively).
Table 4
Study characteristics and results for cooled radiofrequency ablation trials.
Detailed Synthesis
Pain
Both trials found cooled radiofrequency associated with reduced pain versus sham at 1 month; effects were moderate to large (mean difference 1.0 to 2.9 points on a 0 to 10 scale).103,104 At 3 months, one trial found cooled radiofrequency associated with a persistent moderate reduction in pain (mean change from baseline −2.4 vs. −0.8, p=0.04).103 In this trial, cooled radiofrequency was also associated with increased likelihood of pain intensity improvement of at least 50 percent at 3 months, though the difference was not statistically significant (53% vs. 29%, RR 0.81, 95% CI, 0.81 to 4.01).103
Function
Both trials found cooled radiofrequency associated with improved function versus sham at 1 month; effects were small in one trial (mean change from baseline −12 vs. −4 on the 0 to 100 Oswestry Disability Index [ODI], p=0.046)103 and large in the other trial (mean 20.9 vs. 43.6 on the ODI, p<0.05).104 One trial found cooled radiofrequency associated with a moderate improvement versus sham in ODI at 3 months (mean change from baseline −11 vs. 2, p=001).103 In this trial, patients randomized to cooled radiofrequency were also more likely to experience an ODI improvement of at least 10 points (41% vs. 5.9%, RR 7.00, 95% CI, 1.00 to 48.88).
Other Outcomes
One trial found cooled radiofrequency associated with improved SF-36 PCS versus sham at 3 months (mean change from baseline 14 vs. 3 on 0 to 100 scale, p=0.04); the difference at 1 month was small and not statistically significant.103 Cooled radiofrequency was also associated with improvement in Assessment of Quality of Life score at 3 months (mean 0.69 vs. 0.56 on 0 to 1 scale, p=0.048). The other trial found cooled radiofrequency associated with increased likelihood of a more than 20 percent reduction in opioid use or complete cessation of nonopioid analgesics at 1 month (77% vs. 8%, p<0.05).104 Both trials found cooled radiofrequency associated with increased likelihood of experiencing a composite outcome of treatment success. In one trial,104 treatment success was defined as at least a 50 percent improvement in pain, positive Global Perceived Effect, and at least a 10 point improvement in ODI or at least a 4 point improvement in ODI and reduction in medication use and assessed at 1 month (79% vs. 14.3%, RR 5.50, 95% CI, 1.48 to 20.42); in the other,103 it was defined as pain intensity improved at least 50 percent and either 10 point increase in SF-36 bodily pain or 10 point decrease in ODI and assessed at 3 months (47% vs. 12%, RR 4.00, 95% CI, 1.04 to 15.43).
Harms
One trial reported no serious complications, though some patients reported temporary worsening pain typically lasting 5 to 10 days after the procedure; one patient in the cooled radiofrequency arm reported transient nonpainful buttock paresthesias.103 Harms were not reported in the other trial.104
Cooled Versus Conventional Radiofrequency Denervation for Presumed Lumbar Facet Joint Pain
Description of Included Studies
One good-quality trial (n=43) conducted in the United States compared cooled radiofrequency versus conventional radiofrequency for presumed lumbar facet joint pain (Appendix Table G-4 to G-6, Appendix Table H-1, and Table 4).105 Patients had to have a positive response (≥75% pain relief) to one set of diagnostic medial branch nerve blocks. Mean age was 56 years and 59 percent of participants were female; the mean duration of pain was 86 months. Baseline pain intensity was approximately 7 on a 0 to 10 NRS. Cooled radiofrequency to medial branch nerve targets was performed for 165 seconds at 60 degrees C (intraregional temperature >80 degrees C). Conventional radiofrequency was performed for 90 seconds at 80 degrees C.
Detailed Synthesis
Pain
Cooled radiofrequency denervation was associated with a small, nonstatistically significant greater reduction in pain versus conventional radiofrequency at 6 months (mean change from baseline −3.8 vs. −3.0 on a 0 to 10 scale, p=0.41); there were also no statistically significant differences at 1 or 3 months.105 There was no difference in likelihood of experiencing at least a 50 percent improvement in pain at 6 months (52.3% vs. 44.4%, RR 1.18, 95% CI, 0.61 to 2.28).
Function
There was no difference between cooled versus conventional radiofrequency in improvement in ODI at 1, 3, or 6 months.105 Cooled radiofrequency was associated with increased likelihood of experiencing at least a 30 percent improvement in ODI that was not statistically significant (61.9% vs. 44.4%, RR 1.39, 95% CI, 0.75 to 2.58).
Other Outcomes
There was no difference between cooled versus conventional radiofrequency in Global Impression of Change at 6 months (mean 2 vs. 2 on a 1 to 7 scale, p=0.51).105
Harms
No serious adverse events were reported with either cooled or conventional radiofrequency.105 Self-limited post-procedural pain was reported in two patients.
Pulsed Radiofrequency Denervation
Key Points
- Evidence was insufficient to assess pulsed radiofrequency denervation for presumed facet joint pain versus sham denervation (1 trial, N=40) or continuous radiofrequency denervation (1 trial, N=40) (SOE: insufficient).
Across Trials of Cooled and Pulsed Radiofrequency Denervation
- Harms were not well-reported, but when recorded were usually related to temporary increase in pain. No serious complications were reported (SOE: low).
- The mean age of participants ranged from 52 to 59 years.
Pulsed Versus Sham Radiofrequency Denervation for Presumed Lumbar Facet Joint Pain
Description of Included Studies
One fair-quality trial106 and one poor-quality trial107 compared pulsed versus conventional radiofrequency denervation for presumed lumbar facet joint pain (Appendix Table G-4 to G-6 and Table 5). In both trials, patients had to have a positive response (>50% or complete/near complete pain relief) to one or two diagnostic medial branch blocks. The fair-quality trial (n=40) evaluated pulsed (2 Hz waves for 4 minutes [45 V] to 42 degrees C) versus electrode placement without radiofrequency current and administration of a local anesthetic.106 Mean age was 49 years, 57 percent of participants were female, and the mean duration of pain was 35 months. In the poor-quality trial (n=50),107 mean age was 57 years and the proportion female 65 percent. It compared pulsed radiofrequency (2 Hz waves for 2 minutes at 42 degrees C) of the dorsal root ganglia versus electrode placement without radiofrequency current (no local anesthetic).107 Methodological limitations in both trials included failure to report allocation concealment methods, unclear masking of care providers, and high or unclear attrition (Appendix Table H-1). In addition, the poor quality trial did not report randomization methods or baseline characteristics, did not conduct intent-to-treat analysis, and had discrepancies in reported results.
Table 5
Study characteristics and results of pulsed radiofrequency ablation trials.
Detailed Synthesis
Pain
The fair-quality trial found no difference between pulsed versus radiofrequency denervation versus sham (with local anesthetic) at 6 months (2.9 vs. 3.1 on a 0 to 10 visual analogue scale [VAS], p=0.62) or 1 year (3.5 vs. 3.9, p=0.31).106
The poor-quality trial found pulsed radiofrequency of the dorsal root ganglia associated with a large reduction in pain versus sham (without local anesthetic) at 3 months to 3 years (differences ranged from 3.3 to 7.3 points on a 0 to 10 VAS).107 Pulsed radiofrequency was also associated with an increased likelihood of experiencing greater than a 50 percent reduction in back pain (absolute differences ranged from 28% to 68%).
Function
The fair-quality trial found pulsed radiofrequency of the medial branches and sham (with local anesthetic) associated with similar function at 6 months (mean 25.4 vs. 28.9 on the 0 to 100 ODI, p=0.07), though the difference was slightly larger and favored sham at 1 year (mean 28.5 vs. 33.6, p=0.006).106
The poor-quality trial found pulsed radiofrequency of the dorsal root ganglia associated with moderate to large improvement in function versus sham (without local anesthetic) at 3 months to 3 years (differences ranged from 16.9 to 38.4 points on the 0 to 100 ODI).107
Other Outcomes
The fair-quality trial found both pulsed radiofrequency of the medial branches and sham (with local anesthetic) associated with high likelihood of analgesic use at 1 year (75% vs. 95%) and patient satisfaction reported as good or excellent (85% vs. 70%); differences were not statistically significant.106
The poor-quality trial found pulsed radiofrequency of the dorsal root ganglia associated with an increased likelihood of a composite outcome of success versus conventional radiofrequency denervation (absolute difference 30% to 72%), and greater decrease in World Health Organization (WHO) analgesic intake score (mean difference in change from baseline on a 0 to 3 scale ranged from 0.4 to 1.9 points) at 3 months to 3 years.107
Pulsed Versus Conventional Radiofrequency Denervation for Presumed Lumbar Facet Joint Pain
Description of Included Studies
One fair-quality trial106 and two poor-quality trials107,108 compared pulsed versus conventional radiofrequency denervation for presumed lumbar facet joint pain (Appendix Table G-4 to G-6 and Table 5). In all of the trials, patients had to have a positive response to one or two diagnostic medial branch blocks. The fair-quality trial (n=40) was conducted in Turkey and evaluated pulsed (2 Hz waves for 4 minutes [45 V] to 42 degrees C) versus conventional (90 seconds to 80 degrees C) radiofrequency denervation of the medial branches.106 Mean age was 49 years, 57 percent of participants were female, and the mean duration of pain was 35 months. The two poor-quality trials (n=50 and 100) were conducted in Egypt and the United States.107,108 Mean ages were 57 and 59 years and the proportion female 54 percent and 65 percent. Patients had to have chronic pain in both trials, but mean pain duration was not reported. One poor-quality trial compared pulsed radiofrequency (2 Hz waves for 2 minutes at 42 degrees C) of the dorsal root ganglia versus conventional radiofrequency (85 degrees C for 90 seconds) of the medial branches, potentially complicating interpretation because of different denervation targets.107 The other trial compared pulsed radiofrequency (2 Hz waves for 2 minutes at 42 degrees C) versus conventional radiofrequency (80 degrees C for 75 seconds) of the medial branches.108 Across trials, methodological limitations included failure to report allocation concealment methods, unclear masking of care providers, and high or unclear attrition (Appendix Table H-1). In addition, the poor-quality trials did not report randomization methods, baseline characteristics, did not conduct intent-to-treat analysis, and had discrepancies in reported results.
Detailed Synthesis
Pain
The fair-quality trial found a small, nonstatistically significant difference between pulsed versus radiofrequency denervation at 6 months (mean 2.9 vs. 2.3 on a 0 to 10 VAS, p=0.19), but pulsed radiofrequency denervation was associated with moderate increased pain at 1 year (mean 3.5 vs. 2.4, p=0.004).106
The two poor-quality trials reported inconsistent effects on pain intensity.107,108 The trial of pulsed versus conventional radiofrequency denervation of the medial branches found no difference in pain intensity at 3 months,108 but the trial of pulsed radiofrequency of the dorsal root ganglia versus conventional radiofrequency denervation of the medial branches found a large reduction in pain with pulsed radiofrequency at 3 months through 3 year (mean differences 3.1 to 5.6 points on a 0 to 10 scale).107 In this trial, pulsed radiofrequency was also associated with an increased likelihood of experiencing a greater than 50 percent reduction in back pain (absolute difference ranged from 20% to 60%).
Function
The fair-quality trial found no difference between pulsed versus radiofrequency denervation of the medial branches at 6 months (25.4 vs. 25.1 on the 0 to 100 ODI, p=0.92) or 1 year (28.5 vs. 28.0 on the ODI, p=0.81).106
The two poor-quality trials reported inconsistent effects on pain intensity.107,108 The trial of pulsed versus conventional radiofrequency denervation of the medial branches found no difference in function (ODI) at 3 months,108 but the trial of pulsed radiofrequency of the dorsal root ganglia versus conventional radiofrequency denervation of the medial branches found a moderate to large reduction in pain with pulsed radiofrequency at 3 months through 3 years (differences on the 0 to 100 ODI ranged from 15.6 to 32.9 point).107
Other Outcomes
The fair-quality trial found pulsed radiofrequency of the medial branches associated with increased likelihood of analgesic use versus conventional radiofrequency of the medial branches at 1 year (75% vs. 40%, RR 1.88, 95% CI, 1.04 to 3.39).106 There was no difference in likelihood of patient satisfaction rating of good or excellent (85% vs. 95%).
One poor-quality trial found pulsed radiofrequency of the dorsal root ganglia associated with increased likelihood of a composite outcome of success versus conventional radiofrequency denervation (absolute difference 22% to 58%), and greater decrease in WHO analgesic intake score (mean difference in change from baseline on a 0 to 3 scale ranged from 0.3 to 1.4 points) at 3 months to 3 years.107
Intradiscal and Facet Joint Platelet-Rich Plasma for Low Back Pain of Presumed Discogenic Origin or Into the Lumbar Facet Joint for Low Back Pain of Presumed Facet Joint Origin
Key Points
- Evidence was insufficient to assess intradiscal platelet-rich plasma injection for presumed discogenic back pain (1 trial, N=58) (SOE: insufficient).
- There were no differences between stem cell and saline injection in harms, including no serious adverse events, at up to 3 years following treatment (SOE: low).
- The mean age of participants was 42 years.
Description of Included Studies
One small (n=47) trial conducted in the United States compared intradiscal platelet-rich plasma (PRP) versus sham (contrast agent) injection for presumed discogenic low back pain (Appendix Table G-7 to G-9 and Table 6).109 Pain had to be chronic (>6 months; mean duration not reported) and patients had to have a concordant response on single provocative discography prior to undergoing the injection. The mean age of participants was 42 years; 66 percent were female. At baseline, current pain averaged 4.7 on a 0 to 10 NRS; baseline mean best pain was 2.5 and mean worst pain 7.9. Under fluoroscopic guidance, patients received 1 to 2 ml platelet-rich plasma (3 to 4 ml divided for multiple discs) or 1 to 2 ml contrast agent in the affected disc(s) and outcomes compared through 8 weeks, at which point the trial was unblinded and crossover permitted (88% [15/17] of those randomized to sham crossed over to PRP). The trial was rated fair quality, due to baseline imbalance in sex, unclear randomization method, and lack of intent-to-treat analysis (Appendix Table H-1)
No trial evaluated facet joint PRP injection versus sham or usual care.
Table 6
Study characteristics and results of platelet-rich plasma trial.
Detailed Synthesis
Pain
Intradiscal PRP was associated with small to moderate decreases in current pain versus sham at 1, 4, and 8 weeks, but differences were not statistically significant (mean differences −0.57 to −1.30 points on a 0 to 10 NRS). Results were similar for best and worst pain. There was also no difference on the SF-36 bodily pain subscale.
Function
There were no differences between intradiscal PRP versus sham in function (based on the Functional Rating Index) at 1, 4, or 8 weeks; mean differences ranged from −0.93 to 3.84 points on a 0 to 100 scale.109 There were also no difference on the SF-36 physical function subscale.
Other Outcomes
Intradiscal PRP was associated with increased likelihood versus sham of patient reporting of “satisfied” or “would undergo procedure again” versus sham (55.6% vs. 17.6%, RR 3.15, 95% CI, 1.07 to 9.28).109 However, it was unclear if assessment occurred prior to or after unblinding.
Harms
No cases of disc space infection, neurologic injury, or progressive herniation were reported.109
Intradiscal Stem Cells for Low Back Pain of Presumed Discogenic Origin
Key Points
- Evidence was insufficient to assess intradiscal stem cell injection for presumed discogenic back pain (1 trial, N=100) (SOE: insufficient).
- Mean age was 42 years.
Description of Included Studies
One trial (n=100) compared intradiscal injection of allogenic mesenchymal stem cells in hyaluronic acid delivery versus intradiscal hyaluronic acid alone or saline for chronic low back pain of presumed discogenic origin (Appendix Table G-10 and G-11, and Table 7).110 The study was conducted at 13 sites in the United States and Australia. Study participants had chronic low back pain for at least 6 months that was refractory to at least 3 months of conservative treatment. Patients were not required to undergo provocative discography, but among those who did, only those with a concordant pain response at one level were enrolled. Mean age of participants was 42 years and 47 percent were female. The mean duration of discogenic disease was 5.8 years and mean pain score was 7 on a 0 to 10 VAS. Patients were randomized to 6 million (1.0 ml at 30 million/5 ml) or 18 million (1.0 ml at 90 million/5 ml) allogenic mesenchymal stem cells (each mixed with 1% hyaluronic acid) versus 2 ml of 1 percent hyaluronic acid alone, or 2 ml saline. Although saline and hyaluronic acid injections were both considered inactive controls, hyaluronic acid could have potential therapeutic effects; therefore, results described here primarily focus on findings against saline injection. Outcomes were assessed from 1 month to 3 years after treatment. The study was rated fair quality due to presence of baseline differences (Appendix Table H-1). Specifically, the 18 million stem cell group included a higher proportion of male participants (70% versus 50%), was younger (38 years versus 44 years) and had a shorter duration of disease (3.7 years versus 5.9 years) than the saline control group.
Table 7
Study characteristics and results of intradiscal stem cell trial.
Detailed Synthesis
Pain
Intradiscal injection of 6 or 18 million stem cells was associated with a small improvement in pain versus saline injection that was not statistically significant at 1, 3, and 6 months (least squares mean differences adjusted for posttreatment interventions ranged from 0.38 to 1.05 points on a 0 to 10 VAS).110 At 1 to 3 years, stem cell injections were associated with moderate to large, statistically significant reduction in pain (differences ranged from 1.61 to 2.62 points). Both stem cell doses were associated with increased likelihood of at least a 50 percent reduction in pain at 1 year (60.0% vs. 20.0% for 6 million stem cell dose, RR 3.00, 95% CI, 1.19 to 7.56 and 53.3% vs. 20.0%, for 18 million stem cell dose, RR 2.67, 95% CI, 1.04 to 6.81) and the 6 million stem cell dose was associated with increased likelihood of at least 50 percent pain reduction at 2 years (50.0% vs. 20.0%, RR 3.33, 95% CI, 1.11 to 10.04). Although results also favored stem cell injections at 6 months and 3 years, the differences were smaller and not statistically significant. Results for stem cell injections versus hyaluronic acid alone injection also favored stem cells, but at most time points differences were smaller relative to comparisons against usual care, and few differences were statistically significant. There was no pattern to indicate that the higher stem cell dose was associated with greater effects on pain than the lower dose.
Function
Intradiscal injection of 6 or 18 million stem cells was associated with no differences in ODI versus saline injection at 1, 3, and 6 months.110 At 1, 2, and 3 years, stem cell injections were associated with small to moderate reductions in function that were statistically significant (differences ranged from 8.23 to 18.15 points on the 0 to 100 ODI). Stem cell injections were also associated with increased likelihood of at least a 15-point improvement in the ODI at 2 or 3 years (at 3 years, 46.7% vs. 25.0% for 6 million stem cell dose, RR 3.11, 95% CI, 1.02 to 9.45 and 50.0% vs. 15.0% for 18 million stem cell injection, RR 3.33, 95% CI, 1.11 to 10.04). The 6 million stem cell dose was also associated at increased likelihood of ODI response at 6 months (63.3% vs. 25.0%, RR 2.53, 95% CI, 1.13 to 5.67); although the point estimate was similar at 1 year the difference was not statistically significant. Results for stem cell injections versus hyaluronic acid alone were similar to results versus saline at 1 to 6 months and indicated slightly reduced differences at 1 to 3 years. There was no pattern to indicate that the higher stem cell dose was associated with greater effects on function than the lower dose.
Other Outcomes
Stem cell injections were associated with increased likelihood of experiencing a composite measure of treatment response (≥30% pain reduction and ≥10-point ODI improvement) versus saline injection at 6 months to 3 years, but the differences were only statistically significant for the 18 million stem cell dose at 1 year (56.7% vs. 20.0%) and the 6 million stem cell injection at 2 years (46.7% vs. 15.0%).110
The 18 million stem cell injection was associated with greater improvement in SF-36 PCS versus saline (p=0.025) or hyaluronic acid (p=0.04) at 3 years (mean differences not reported). There were no statistically significant differences between injections versus controls in SF-36 MCS and PCS at earlier time points.
Harms
There were no deaths reported in the study, and no statistically significant differences between groups in the risk of serious adverse events, any adverse event, or withdrawals due to adverse events.110
Effects of Dose
As described above, there was no pattern to indicate that a higher dose (18 million) of intradiscal stem cells was associated with superior outcomes compared with a lower dose (6 million).109
Intradiscal Methylene Blue for Low Back Pain of Presumed Discogenic Origin
Key Points
- Intradiscal methylene blue for presumed discogenic back pain (1 trial, N=81) was associated with no difference versus sham at 6 weeks and 3 months. Evidence was insufficient to determine effects of intradiscal methylene blue at 6 months (2 trials, N=153, with conflicting results) and 12 months or longer (1 trial, N=72) (SOE: low for no difference at 6 weeks and 3 months; insufficient for 6, 12 and 24 months).
- Both trials of intradiscal methylene blue excluded patients older than 66 years of age (mean 41 years).
Description of Included Studies
Two trials (n=84 and 72) compared intradiscal methylene blue versus sham intradiscal therapy in patients with presumed discogenic back pain (Appendix Table G-12 to G-14 and Table 8).111,112 The trials were conducted in the Netherlands (the IMBI Study111) and China.112 Methods of the Dutch trial were intentionally similar to the earlier Chinese trial, in an effort to determine whether its results could be duplicated. The mean age was 41 years in both trials, and both trials excluded patients older than 66 years. The proportion of female patients was 72 percent in one trial111 and 43 percent in the other.112 Baseline pain intensity was similar (mean 6.6 and 7.0, on a 0 to 10 scale). Although both trials enrolled patients with chronic pain, the duration of pain symptoms was longer in the Dutch trial (9 years)111 than the Chinese trial.112 In both trials, the diagnosis of discogenic back pain was based on a positive response to provocative discography, with negative control discs. In addition, the Dutch trial excluded patients with multilevel discogenic pain or facet pain based on confirmatory facet block.111 Both trials compared an intradiscal injection of methylene blue (1 ml; 10 mg/ml) or saline (1 ml); both groups received 2 percent lidocaine (0.5 ml) injection. Duration of followup was 6 months in the Dutch trial111 and 2 years in the Chinese trial.112 The Dutch trial was rated good quality111 and the Chinese trial fair quality,112 mainly for unclear allocation concealment (Appendix Table H-1).
Table 8
Study characteristics and results of intradiscal methylene blue trials.
Detailed Synthesis
Pain
The two trials reported discordant effects of intradiscal methylene blue on pain. In the Chinese trial, intradiscal methylene blue was associated with large reduction in pain versus sham at 6, 12, and 24 months (mean differences 3.86 to 4.08 points on a 0 to 11 NRS).112 However, the Dutch trial found no differences between intradiscal methylene blue versus sham in pain at 6 weeks, 3 months, or 6 months (mean differences 0.4 to 0.5 points on a 0 to 11 NRS).111 The Dutch trial also found methylene blue and sham associated with similar likelihood of at least a 30 percent reduction in NRS (at 6 months, 35.0% [14/40] vs. 26.8% [11/41], p=0.43). The Chinese trial did not evaluate the likelihood of experiencing a pain response.
Function
Results of the two trials were also discordant regarding function. In the Chinese trial, intradiscal methylene blue was associated with large improvement in function versus sham (mean differences 32.4 to 34.8 points on the 0 to 100 ODI at 6, 12, and 24 months).112 In the Dutch trial, methylene blue was associated with a small improvement in function at 6 weeks (mean difference −6.3, 95% CI, −12.4 to −0.17 on the ODI), but differences were reduced and no longer statistically significant at 3 or 6 months (mean differences −5.2 to −2.3 points).111
Other Outcomes
As with pain and function, effects of methylene blue on medication use was also discordant. At 6 months, the Chinese trial112 found methylene blue associated with marked reduction in likelihood of regular nonsteroidal anti-inflammatory drug (NSAID) or opioid use (8.3% vs. 42.9%, p=0.002) but the Dutch trial111 found no difference in likelihood of strong opioid use (7.5% vs. 9.8%, p=1.0). The Dutch trial also found no differences between methylene blue versus sham in EQ-5D, SF-36 PCS, SF-36 MCS, or patient global impression of change “much improved” or “improved” at 6 weeks, 3 months, or 6 months; these outcomes were not evaluated in the Chinese trial.
Harms
Reporting of harms was limited. The Chinese trial reported no cases of nerve injury or disc space infection with methylene blue or sham, and no cases of back pain aggravation with methylene blue.112 The Dutch trial found no difference in risk of any adverse event (data not reported); two serious adverse events that did not appear due to the methylene blue procedure were reported (unrelated elective surgery and hospitalization for laryngitis).111
Intradiscal Ozone Injection for Radicular Low Back Pain or Nonradicular Low Back Pain of Presumed Discogenic Origin
Key Points
- Evidence was insufficient to assess intradiscal oxygen-ozone for radicular low back pain (1 trial, N=159) (SOE: insufficient).
- No trial evaluated intradiscal oxygen-ozone injection without corticosteroid or oxygen-ozone injection for presumed (nonradicular) discogenic low back pain.
- Mean age ranged from 40 to 51 years.
Description of Included Studies
No trial evaluated intradiscal ozone injection alone for radicular or nonradicular low back pain. Three trials (n=80 to 159, total N=339) evaluated intradiscal ozone (mixed with oxygen) plus corticosteroid injection for radicular back pain (Appendix Table G-15 to G-17 and Table 9).113–115 The studies were conducted in Italy,113 Egypt,114 and India.115 Mean age of study participants ranged from 40 to 51 years and 45 to 62 percent were female. The mean duration of pain was 8 and 15 weeks in two trials113,114 and 9 months in one trial.115 Baseline ODI ranged from 31 to 72; only one trial115 reported baseline pain (mean 7.1 on a 0 to 10 VAS). In all trials, patients had radicular pain with concordant herniated disc on MRI and injections were performed with CT or fluoroscopic guidance. The amount of intradiscal oxygen-ozone (concentration 28 or 40 µg/ml) administered ranged from 5 to 10 ml; in one trial, patients also received transforaminal epidural oxygen-ozone (volume 5 to 7 ml). Oxygen-ozone was administered with a corticosteroid (triamcinolone or methylprednisolone) in all trials, two113,114 of which also administered ropivacaine. The corticosteroid was intradiscal and epidural in two trials113,114 and epidural only in one trial.115 All three trials compared oxygen-ozone plus corticosteroid versus corticosteroid without oxygen-ozone; one trial114 also evaluated a local anesthetic only (without corticosteroid) control injection (site not specified). The duration of followup was 6 months.
One trial113 was rated fair quality and two trials114,115 poor quality, including the trial that compared an oxygen-ozone versus local anesthetic alone injection (Appendix Table H-1). Methodological limitations in all trials included unclear randomization and allocation methods and failure to report attrition. The poor-quality trials also did not clearly blind patients, had poor reporting of outcomes, data discrepancies, and potential selective outcomes reporting.
No trial evaluated intradiscal ozone injection for presumed (nonradicular) discogenic back pain.
Table 9
Study characteristics and results of intradiscal ozone trials.
Detailed Synthesis
Pain
Only one poor-quality trial reported effects on pain.115 It found no difference between intradiscal oxygen-ozone plus epidural methylprednisolone injection versus methylprednisolone alone at 1 (mean difference 0.25 on a 0 to 10 scale, 95% CI, −0.23 to 0.73) or 2 weeks (mean differences −0.21, 95% CI, −0.57 to 0.15). However, oxygen-ozone was associated with a moderate decrease in pain versus corticosteroid alone at 3 (mean difference −1.30, 95% CI, −1.72 to −0.88) and 6 months (mean difference −1.38, 95% CI, −1.75 to −1.01).
Function
The fair-quality trial found no differences between oxygen-ozone, corticosteroid, and local anesthetic versus corticosteroid plus local anesthetic without oxygen-ozone in likelihood of ODI score less than 20 (0 to 100 scale) at 2 or 3 weeks.113 Oxygen-ozone was associated with an increased likelihood of achieving an ODI score less than 20 at 6 months (74% vs. 47%, RR 1.59, 95% CI, 1.21 to 2.08)
The two poor-quality trials found little difference between oxygen-ozone and corticosteroid (with or without local anesthetic) versus corticosteroid without oxygen-ozone in the ODI at 1 or 2 weeks.114,115 Differences on the ODI were larger and statistically significant at 3 and 6 months (mean differences −10.7 to −4.8 points). One poor-quality trial found oxygen-ozone, corticosteroid and local anesthetic injection associated with moderate improvement in the ODI versus local anesthetic alone (without steroid) at 2 weeks and 3 months (mean differences 17 points).114
Other Outcomes
The trials did not evaluate outcomes other than pain or function.
Harms
No serious adverse events were reported in any trial. The risk of any adverse event was higher in the oxygen-ozone group when compared with lidocaine alone (RR 3.17, 95% CI, 1.06 to 9.45) in one poor-quality trial.114
Sphenopalatine Block for Trigeminal Neuralgia and Headache
Key Points
- Evidence was insufficient to assess sphenopalatine block versus sham for headache (1 trial, N=41) (SOE: insufficient).
- The mean age of patients in the trial of sphenopalatine block was 41 years.
Description of Included Studies
One trial (n=41) reported in two publications compared 0.5 percent intranasal bupivacaine versus saline sphenopalatine block via the intranasal approach using a flexible device (Tx360, Tian Medical Inc., Lombard, IL) in patients with chronic migraine headache (Appendix Table G-18 and G-19, and Table 10).116,117 Blocks were administered 12 times over a 6-week period (12 total treatments). Study participants had a mean age of 41 years and were predominately female (76%). Study inclusion criteria required at least a 3-month history of migraine; mean duration since migraine diagnosis was 8.6 years duration. Patients using schedule II opioids for migraine were excluded. On average, patients reported 15 days of migraine days per month, 22 headache days per month, and had a baseline mean pain score (0 to 10 NRS) of 3.37. The study was rated poor quality; limitations included high overall (27%) and differential attrition (43% in sham arm) at 1 month post-treatment followup, as well as lack of intent-to-treat analysis (Appendix Table H-1). In addition, the trial did not report the scales used to assess a number of outcomes (quality of life [general activity, work, and mood], analgesic use, and global impression of change).
No trial or controlled observational study evaluated sphenopalatine block for trigeminal neuralgia.
Table 10
Study characteristics and results of sphenopalatine block trial.
Detailed Synthesis
Pain
At 24 hours following treatments (data pooled for all 12 treatment sessions), sphenopalatine block was associated with moderate decrease in pain intensity versus sham (mean 2.85 vs. 4.20 on a 0 to 10 NRS, ANOVA p<0.001).116,117 However, differences were small and not statistically significant 1 and 6 months after completing the course of treatments (3.36 vs. 3.91 at 1 month and 2.86 vs. 4.00 at 6 months), though sphenopalatine block was associated with fewer number of headaches days per month at 1 month (17.44 vs. 22.82, ANOVA p>0.05).
Function
Effects of sphenopalatine block versus sham on the Headache Impact Test (HIT-6) were small and not statistically significant at 24 hours following treatments or at 1 to 6 months after completing the course of treatment (differences ~3 points on a 36 to 78 scale). There were also no differences in general activity or normal work at 1 or 6 months, but the scales used to measure these outcomes were not reported.
Occipital Nerve Stimulation for Headache
Key Points
- Evidence was insufficient to assess occipital nerve stimulation versus sham stimulation for headache (1 trial, N=157) (SOE: insufficient).
- For headache, occipital nerve stimulation with adjustable parameters versus usual care at 3 months was associated with a small, nonstatistically significant reduction in pain intensity, moderate decrease in headache related disability, and decrease in headache days (1 trial, N=67) (SOE: low for headache related disability and headache days; insufficient for pain).
- Lead migration occurred in 14 to 24 percent of patients (2 trials, N=224), serious device-related complications requiring hospitalization occurred in 5.9 percent of patients (1 trial, N=67), and persistent pain/numbness at implantation site in 13 percent of patients (1 trial, N=157) (SOE: low).
- One trial (N=67) found occipital nerve stimulation with adjustable parameters associated with superior outcomes compared with stimulation using preset parameters.
- The mean age of patients ranged from 43 to 46 years.
Description of Included Studies
Three trials (number randomized 30, 67, and 157, total=254) evaluated occipital nerve stimulation for chronic headache (Appendix Table G-20 to G-22 and Table 11).118–120 Two trials118,120 were multicenter studies; one additional publication121 reported results from a single participating center (n=20) in one120 of the trials. The trials were conducted in the United States, Italy, and Europe. The mean age ranged from 43 to 46 years and the proportion female ranged from 76 to 80 percent. Two trials118,120 restricted inclusion to patients who met criteria for chronic migraine headaches and one trial119 included patients with chronic migraine or medication overuse headache; all trials required patients to have unsuccessfully tried at least two prior treatments. Two trials119,120 required patients to experience at least 50 percent pain relief with trial stimulation and one trial118 required at least 50 percent response to a diagnostic occipital nerve block. Electrodes were placed subcutaneously and occipital nerve stimulation was compared against sham stimulation (electrodes placed but no current applied) (two trials)119,120 or usual care (one trial).118 One trial permitted some adjustment of stimulation parameters,119 one trial randomized patients to adjustable or preset stimulation parameters,118 and one trial120 did not report stimulation parameters (Table 11). One crossover trial was rated poor quality due to failure to report randomization or allocation concealment methods, unclear blinding, and potential selective outcomes reporting; in addition, the analysis did not account for use of crossover design (Appendix Table H-1).119 The other two trials were rated fair quality.
Table 11
Study characteristics and results of occipital nerve stimulation trials.
Detailed Synthesis
Pain
One fair-quality trial found occipital nerve stimulation associated with similar likelihood of the primary study outcome of at least a 50 percent reduction in headache pain intensity versus sham stimulation at 12 weeks (17.1% vs. 13.5%, RR 1.27, 95% CI, 0.57 to 2.86).120 However, occipital nerve stimulation was associated with increased likelihood of at least a 30 percent reduction in headache pain intensity that just met the threshold for statistical significance (33.3% vs. 17.3%, RR 1.93, 95% CI, 1.00 to 3.70). Average effects on pain intensity were not reported in the main publication reporting full multicenter results, but a report from a single center (n=20) participating in the trial found occipital nerve stimulation associated with a large decrease in pain intensity at 4 weeks (mean change from baseline −2.16 vs. 0.34 on a 0 to 10 scale, p<0.001) and 12 weeks (mean change from baseline −2.30 vs. 0.79, p<0.001). The poor-quality crossover trial also found occipital nerve stimulation associated with a large decrease in pain intensity versus sham stimulation at the end of the initial 1-month (prior to crossover) period (median 5 vs. 7.5, p<0.001).119
The other fair-quality trial found occipital nerve stimulation with adjustable parameters associated with greater reduction in pain versus usual care at 3 months, but the difference was small and not statistically significant (mean change from baseline −1.5 vs. −0.6 on a 0 to 10 scale).118 There was no difference between stimulation using preset parameters versus usual care in pain.
Function
One fair-quality trial found occipital nerve stimulation associated with greater improvement in headache related disability versus sham stimulation at 12 weeks (mean change from baseline −64.6 vs. −20.4 on the Migraine Disability Test (MIDAS) score [>20=severe disability], mean difference −44.2, 95% CI, −65.3 to −22.8).120 The poor-quality crossover trial of occipital nerve stimulation versus sham did not report effects on headache related disability by treatment group.119
The other fair-quality trial found occipital nerve stimulation with adjustable parameters associated with larger decrease in headache disability category versus usual care at 3 months (mean change in MIDAS severity category −1.3, 95% CI, −2.25 to −0.35); one category level refers to the MIDAS score decreasing from the “severe” to “moderate” or “moderate” to “mild” category.118 There was no difference between stimulation using preset parameters versus usual care in headache related disability.
Other Outcomes
One fair-quality trial found occipital nerve stimulation associated with greater percent decrease in headache days (mean change from baseline −27.2% vs. −14.9%, p<0.05) and increased likelihood of reporting “good” or “excellent” headache relief versus sham stimulation (50% vs. 18%, RR 2.86, 95% CI, 1.53 to 5.34) at 12 weeks.120 The other fair-quality trial found occipital nerve stimulation with adjustable parameters associated with greater decrease in headaches days/month versus usual care at 3 months (mean difference −5.7 days, 95% CI, −10.9 to −0.54). Stimulation was also associated with greater improvement from baseline in Profile of Mood States score (mean difference −8.3 on a 0 to 168 scale, 95% CI, −15.2 to −1.4) and SF-36 MCS (mean difference 7.0 on a 0 to 100 scale, 95% CI, 1.7 to 12.3).118 There were no differences between occipital nerve stimulation with present parameters versus usual care in headache days or measures of psychological well-being.
Harms
The most common device-related adverse event was lead migration, which occurred in 14 to 24 percent of patients in two trials. One trial reported three cases of serious device-related adverse events requiring hospitalization (5.9% [3/51]).118 The events were implant site infection, lead migration, and postoperative nausea. In the other trial, persistent pain or numbness at the implant or lead site was reported in 13.1 percent of patients, skin erosion in 3.7 percent, and wound site complications in 2.8 percent.120
Effects of Technical Factors
As described above, one trial found occipital nerve stimulation with patient-adjustable parameters associated with superior outcomes compared with stimulation using preset (nonadjusted) parameters.118
Piriformis Injection for Piriformis Syndrome
Key Points
- One trial (N=50) found piriformis injection with corticosteroid and local anesthetic for piriformis syndrome associated with no difference versus local anesthetic alone in pain at rest at 1 week; piriformis injection was associated with a moderate reduction in pain at rest versus local anesthetic at 1 month (SOE: low for no difference at 1 week and for benefit at 1 month).
- Evidence was insufficient to assess piriformis injection with botulinum toxin.
- The mean age of participants ranged from 42 to 57 years.
Description of Included Studies
Four RCTs assessed the effectiveness of piriformis injection for piriformis syndrome (Appendix Table G-23 to G-25 and Table 12).122–125 Ten participants were randomized in one small pilot trial122 and the sample sizes in the other trials ranged from 50 to 87. In three trials, diagnosis of piriformis syndrome required a positive flexion, adduction and internal rotation (FAIR) test. The fourth trial122 based diagnosis of piriformis syndrome on history and physical examination. In three trials, mean age ranged from 42 to 57 and symptom duration ranged from 2 to 3 years.122,123,125 One trial did not report demographic or clinical characteristics.124 The comparisons varied: three trials compared botulinum toxin A (dose 100 to 300 units) versus placebo (saline) injection,122–124 one of which also evaluated a corticosteroid (triamcinolone acetonide 20 mg) plus local anesthetic (lidocaine) control injection.123 The fourth trial compared a corticosteroid (betamethasone) plus lidocaine injection versus lidocaine alone.125 Imaging or electromyographic guidance was used in all trials (Table 12).
Table 12
Study characteristics and results of piriformis injection trials.
The trial of a corticosteroid plus lidocaine versus lidocaine was rated fair quality and the other three trials were rated poor quality (Appendix Table H-1). Methodological limitations in the poor-quality trials included unclear randomization and allocation concealment, unreported baseline characteristics, unclear blinding of outcome assessors and care providers, high attrition, lack of intent-to-treat analysis, and use of unreported (potentially unvalidated) scales to measure outcomes, including pain. In addition to a very small sample size, the pilot trial did not account for crossover design in the analysis.122
Detailed Synthesis
Pain
The fair-quality trial (n=50) found no difference between piriformis injection with corticosteroid plus lidocaine versus lidocaine alone in pain at rest at 1 week (mean difference 0.40 on 0 to 10 VAS, 95% CI, −0.97 to 1.77).125 Differences were larger (mean difference 1.20) at 1 and 3 months, but only statistically significant at 1 month. A corticosteroid plus lidocaine was associated with a large reduction in pain with activity at 1 month (mean difference −2.00, 95% CI, −3.30 to −0.70); differences were smaller at 1 week and 3 months (mean differences −1.10 and −1.30 points) and not statistically significant. For pain at sleep, mean differences were less than 1 point and not statistically significant.
Three poor-quality trials (n=10, 50, and 56)122–124 found piriformis injection with botulinum toxin A associated with reduced pain versus placebo (saline) injection at 2 to 12 week followup and one poor-quality trial (n=61)123 found piriformis injection with corticosteroid plus lidocaine associated with reduced pain versus placebo, but results are difficult to interpret due to serious methodological limitations.
Function
One poor-quality trial found piriformis injection with botulinum toxin A associated with improvement in interference with daily activities versus placebo (saline) at 1 and 4 weeks, but enrolled a small sample (n=10) and did not report the instrument used to measure this outcome.122 The other trials did not report function.
Other Outcomes
One small (n=10) poor-quality trial found piriformis injection with botulinum toxin A associated with decreased distress versus placebo (saline) injection at 1 week, using an unreported instrument.122
Harms
The fair-quality trial reported similar rates of transient sciatic nerve block with piriformis injections with corticosteroid plus lidocaine and lidocaine alone (24.0% vs. 27.3%).125 Reporting of harms in the poor-quality trials was limited. The small crossover trial reported no serious adverse events122 and one trial reported similar rates of any adverse event between piriformis injection with botulinum toxin versus saline (RR 0.96, 95% CI, 0.32 to 2.94).124
Peripheral Nerve Stimulation for Ulnar, Median, and Radial Neuropathy
Key Points
- Evidence was insufficient to assess peripheral nerve stimulation for upper extremity peripheral neuropathic pain (SOE: insufficient).
Description of Included Studies
No RCT or controlled observational study compared peripheral nerve stimulation versus usual care, no stimulation, or sham for upper extremity (ulnar, median, or radial nerve) neuropathy. One RCT (n=94) compared peripheral nerve stimulation versus sham (current not applied) for patients with chronic (>3 months) upper extremity, lower extremity, or trunk peripheral neuropathic pain; some results were reported in the subgroup of patients with upper extremity pain (n=26) (Appendix Table G-26 and G-27, and Table 13).126 Specific causes of upper extremity pain were not reported, though pain had to be posttraumatic or postsurgical. The mean age of patients was 53 years and 42 percent were female. The mean duration of pain was not reported. Lead placement was performed with imaging (ultrasound or fluoroscopic guidance) and test stimulation for verification. Parameters varied with phase duration 70 to 500 µ/sec, pulse rate 1 to 200 Hz, time on ranging from 10 minutes to 12 hours (mean 6 hours per day). Typical settings were 200 µ/sec and 100 Hz, with amplitude set for paresthesia. Outcomes were assessed at 3 months. The trial was rated fair quality due to failure to report allocation concealment methods, unclear blinding of outcome assessors and care providers, and failure to report attrition (Appendix Table H-1).
Table 13
Study characteristics and results of peripheral nerve stimulation trial.
Detailed Synthesis
Pain
Among the subgroup of patients with upper extremity pain, peripheral nerve stimulation was associated with greater percent change from baseline in pain intensity at 3 months, but the estimate was imprecise and not statistically significant (mean difference −19.8%, 95% CI, −44.6 to 5.0).126 The estimate for treatment response (defined as ≥30% reduction in pain with no increase in pain medication use) also favored peripheral nerve stimulation, but was very imprecise (33% vs. 0%, RR 10.4, 95% CI, 0.6 to 175.2).
Function and Other Outcomes
The trial did not report function and other outcomes separately for the subgroup of patients with upper extremity pain.126 For all participants, peripheral nerve stimulation was associated with greater improvement in Brief Pain Inventory general activity score (mean change from baseline −2.3 vs. −0.4 on a 0 to 10 scale, p=0.001). Peripheral nerve stimulation was also associated with greater improvement in Short-Form 12 (SF-12), but the difference was very small (mean change from baseline 1.4 vs. −0.2 on a 0 to 100 scale, p=0.04).
Harms
Harms were not reported separately for the subgroup of patients with upper extremity pain. Among all participants, there was no difference between peripheral nerve stimulation versus sham in likelihood of any adverse events, device related adverse events, or nondevice related adverse events.126 There were no serious device-related serious adverse events.
- Results - Interventional Treatments for Acute and Chronic Pain: Systematic Revie...Results - Interventional Treatments for Acute and Chronic Pain: Systematic Review
- Introduction - Postpartum Care up to 1 Year After Pregnancy: A Systematic Review...Introduction - Postpartum Care up to 1 Year After Pregnancy: A Systematic Review and Meta-Analysis
- Discussion - Acute Treatments for Episodic MigraineDiscussion - Acute Treatments for Episodic Migraine
- Pectobacterium aroidearum NADP-dependent isocitrate dehydrogenase (icdA) gene, p...Pectobacterium aroidearum NADP-dependent isocitrate dehydrogenase (icdA) gene, partial cds.PopSet: 2750944160PopSet
- Neotis nuba strain:NANeotis nuba strain:NANeotis nubaBioProject
Your browsing activity is empty.
Activity recording is turned off.
See more...








