NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Treadwell JR, Wu M, Tsou AY. Management of Infantile Epilepsies [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2022 Oct. (Comparative Effectiveness Review, No. 252.)
Results of Literature Searches
The electronic searches identified 11,123 citations. After title and abstract screening, 1,607 required full text review and 41 studies met our eligibility criteria for inclusion (Figure 2); see Appendix B for a list of studies excluded at full text. The most common reasons for the 1,566 full text exclusions were <80% were in the age group of interest, N of 4-29 for non-surgical interventions, and study enrolled neonates.
Only one potential dataset was submitted to the Supplementary Evidence And Data (SEAD) portal, but the data were on infantile spasms, which are outside the scope of this review.
Below we describe findings for each Key Question. Specifically, we describe included studies, list key points, and summarize treatment implementations and results.
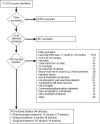
Figure 2
Study flow diagram.
Key Question 1. What are the effectiveness and comparative effectiveness of pharmacologic treatments for infantile epilepsies (infants age 1 month to <36 months)?
Description of Included Evidence
Twelve studies (published in 13 articles) met the inclusion criteria for this Key Question (Table 2). Four studies examined levetiracetam, three examined topiramate, one examined lamotrigine, one examined phenytoin, one examined vigabatrin, one example rufinamide, and one examined stiripentol. Valproate and phenobarbital were examined in separate controlled studies of levetiracetam, and we discuss their results along with levetiracetam. Carbamazepine was also examined in a non-randomized study of topiramate, and we discuss its results along with topiramate. No studies of other anti-seizure medication (ASM) met our inclusion criteria (see the full list of considered medications in Appendix A). Two studies were randomized trials, three were non-randomized comparison studies, and the other seven were pre/post studies. Four studies were conducted in the USA,29–31 one was conducted at 27 sites in Europe,32 and the other seven were conducted in seven different countries. The number of enrolled infants ranged from 36 to 204, and the length of follow-up ranged from 12 weeks to 2.5 years. Nine of 12 studies reported rates of seizure freedom, and these are plotted in a single figure in a dedicated section after evidence has been discussed. Appendix C provides study-specific details and enrollment criteria.
Table 2
Overview of included studies for Key Question 1.
Key Points
- Levetiracetam may cause seizure freedom in some infants (strength of evidence [SOE]: Low); data on quality of life are insufficient to permit conclusions (SOE: Insufficient).
- For topiramate, evidence is insufficient to permit conclusions about effectiveness, due to ancillary treatments and inconsistent results (SOE: Insufficient).
- For lamotrigine, phenytoin, vigabatrin, rufinamide, and stiripentol, evidence is insufficient to permit conclusions about effectiveness, due to the existence of only a single pre/post study for each pharmacologic treatment (SOE: Insufficient).
- Comparative effectiveness is generally unclear, but one study found the rate of freedom from monotherapy failure was higher with levetiracetam than phenobarbital.
- None of the 10 studies reported other key effectiveness outcomes such as mortality, sudden unexpected death in epilepsy (SUDEP), hospitalization, or caregiver quality of life.
Summary of Findings
We summarize the evidence separately for each ASM: levetiracetam (4 studies), topiramate (3 studies), lamotrigine (1 study), phenytoin (1 study), vigabatrin (1 study), rufinamide (1 study), and stiripentol (1 study). Valproate and phenobarbital were examined in separate controlled studies of levetiracetam, and we discuss their results in the levetiracetam section. Carbamazepine was also examined in a non-randomized study of topiramate, and we discuss its results in the topiramate section. Appendix C provides additional details about gender, race, seizure etiology, and concomitant medications. The end of the section provides our ratings of the strength of evidence pertaining to this Key Question.
Levetiracetam
Three of the four studies measured the effectiveness of levetiracetam, while the fourth study measured the comparative effectiveness of levetiracetam and phenobarbital.
Liu et al. (2020)33 measured the impact of levetiracetam by randomizing treatment-naïve infants to either valproate alone (N=50) or valproate plus levetiracetam (N=50). Valproate dosing was 40-50 mg/kg/day, and levetiracetam dosing was 20-30 mg/kg/day. The average age at treatment initiation was two years, but seizure types were not reported. Authors also did not report whether any patients received concomitant medications during follow-up. At 12 weeks, authors reported better results for infants receiving valproate plus levetiracetam for all eight outcomes: seizure freedom (32% vs 22%), ≥75% reduction in seizures (72% vs 50%), ≥50% reduction in seizures (96% vs 70%), quality of life (Quality of Life in Epilepsy 31 [QOLI-31] scores, mean 84 vs mean 60, scale range 0-100 where higher scores are better), daily living ability (Barthel index scores, mean 86 vs mean 62, scale range 0-100 where higher scores are better), and three cognitive ability scales (see data in Appendix C).
One concern with Liu et al. (2020)33 is that the authors used several outcome instruments not intended for young children (the Mini-Mental State Examination, the Weschler Memory Scale-Revised in China, QOLI-31, and the Barthel instrument). We contacted the authors asking if these instruments were modified for use in young children, but received no response. However, as we drew no conclusions about these outcomes, their inclusion does not influence our main findings.
Arzimanoglou et al. (2016)32 performed a pre/post study of 101 infants across Europe who had received levetiracetam (mean daily dose 46 mg/kg/day). The average age at treatment initiation was 6 months, 43% had focal impaired awareness seizures, 34% had focal to bilateral tonic-clinic seizures, and 25% had focal aware seizures (see other seizure types in Appendix C). At a mean of five months on levetiracetam, clinicians considered both seizure type and seizure frequency in rating each infant on a 1-7 scale where 1=marked worsening and 7=marked improvement. Improvement was marked in a third of infants (33%, 28/85), 26% (22/85) had moderate improvement, slight in 13% (11/85), and either no change or seizure worsening in the other 28% (24/85, see details in Appendix C). Clinicians also judged changes in psychomotor development using the same 1-7 scale. On this scale, improvement was marked in 19% (16/85), moderate in 13% (11/85), slight in 21% (18/85), and either no change or some worsening in the other 48% (40/85).
Arican et al. (2018)34 performed a pre/post study of 92 treatment-naïve infants who had received levetiracetam (10-60 mg/kg/day, with 52% taking 30-40 mg/kg/day). The average age at treatment initiation was six months, 58% had focal seizures, and 42% had generalized seizures. At a median of 12 months, 66% of infants were free of seizures.
For comparative effectiveness, Grinspan et al. (2018)29 retrospectively compared outcomes of 117 infants who received levetiracetam monotherapy to 38 infants who received phenobarbital monotherapy. The median target dose was 25 mg/kg/day for levetiracetam and 5 mg/kg/day for phenobarbital. The average age at treatment initiation was not reported, but all were under 1 year old. All had nonsyndromic epilepsy; seizure types were focal in 57%, generalized in 24%, and mixed/unclear in 19%. The only outcome reported was “freedom from monotherapy failure”, which was defined as no seizures during months 4-6 months after treatment initiation AND no second ASM other than pyridoxine prescribed during the six months after treatment initiation. The unadjusted rates were 40% for levetiracetam (47/117) and 16% (6/38) for phenobarbital (odds ratio 3.6, 95% confidence interval [CI] 1.5 to 10, favoring levetiracetam). Due to the non-randomized design, the authors conducted numerous additional analyses to control for selection bias, and all such analyses still favored levetiracetam over phenobarbital. The authors’ best estimate for the odds ratio was 4.2 (95% CI 1.1 to 16), which was based on a propensity score analysis.
Topiramate
Three studies addressed the effectiveness of topiramate, and one of the studies also addressed the comparative effectiveness of topiramate and carbamazepine.
Kim et al. (2009)36 performed a non-randomized comparative study that enrolled treatment-naïve infants who had received either topiramate (N=41; 3 to 9 mg/kg/day) or carbamazepine (N=105; 5 to 30 mg/kg/day). The topiramate group averaged 10 months old at treatment initiation, and seizure types were generalized seizures in 71%, partial seizures in 20%, and unclassified in 10%. The carbamazepine group averaged 8.4 months old at treatment initiation, and seizure types were generalized seizures in 47%, partial seizures in 44%, and unclassified in 10%. The study did not report whether patients received concomitant medications during follow-up. Outcomes were reported an average of 30.7 months after treatment initiation. At six months, the rates of seizure freedom were 59% for topiramate and 55% for carbamazepine. The rates of ≥50% seizure reduction were 73% for topiramate and 63% for carbamazepine. Smaller reductions, no change, or aggravations occurred in 27% of those receiving topiramate and 37% of those receiving carbamazepine.
Kholin et al. (2014)37 performed a pre/post study of topiramate that reported subgroup data on 58 infants who were age one year or younger at treatment initiation. For this subgroup, the study did not report mean age, median age, seizure types, or doses. For the overall population (N=722 including 636 who were older than 1 year), 62% were also taking additional ASM (specific medications not reported). Patients had been receiving topiramate for an average of about one year. Among 58 infants, 19% were seizure free, and 55% had ≥50% reduction in seizure frequency (timepoint not reported). The remaining 45% either had a smaller reduction, or an increase in seizures, or the appearance of new seizure types.
Grosso et al. (2005)35 performed a pre/post study of topiramate recipients who were refractory to at least one ASM and reported subgroup data on 37 infants (median age 11 months at treatment initiation). Neither doses nor seizure types were specifically reported for these infants (although the mean dose for all 59 enrolled patients was 5.2 mg/kg/day). Specific concomitant medications were not reported for the 36 infants, but for the full population, 37% were receiving one ASM prior to starting topiramate, 41% were receiving two ASM, and 22% were receiving three ASM. At three months after starting topiramate, 8% of infants were seizure free, and 54% experienced a ≥50% reduction in seizures.
Lamotrigine
Only one included study reported effectiveness data for lamotrigine. Piña-Garza et al. (2008)30,38 performed a withdrawal randomized trial of lamotrigine vs placebo in which all infants first received lamotrigine, and then only the lamotrigine responders (those whose seizure frequencies reduced by 40% or more) were randomized to either continue lamotrigine or receive a placebo substitution. However, as the placebo comparison period was at most eight weeks, the randomized phase of the study did not meet our inclusion criteria for effectiveness data. Instead, for effectiveness, we included the long-term pre/post data reported by the long-term open label follow-on publication of the study.38 In Key Question 3, we included harms data for both the randomized portion and the open label portion of the trial.
The open label study enrolled 204 infants (mean age 15.9 months at treatment initiation) with partial seizures whose seizures had not been successfully controlled on at least one ASM. Of the 204 infants, 125 infants had already participated in the randomized portion of the trial and their parents opted for continued usage of lamotrigine. The maximum lamotrigine dosage was 5.1 mg/kg/day for those on either valproate or a non-enzyme-inducing ASM, or 15.6 mg/kg/day for those on enzyme-inducing ASM. The concomitant ASM was enzyme-inducing in 59%, not enzyme-inducing in 30%, and was valproate in 11%. Seizure types were partial only in 75%, both partial and generalized in 23%, and generalized only in 1%.
At 24+ weeks’ followup, 13% (26/204) of infants were seizure free, and 61% had ≥50% reduction in seizure frequency. The median seizure reduction (from a mean baseline of 21 seizures per week) was 74%.
Phenytoin
Only one included study reported effectiveness data for phenytoin. Sicca et al. (2000)39 performed a pre/post study of 55 infants treated with oral phenytoin. Thirty-three first received phenytoin intravenously for status epilepticus and continued receiving oral phenytoin for seizure prophylaxis, whereas the other 22 infants were only treated with oral phenytoin for prophylaxis. The mean age was 7.4 months (of note, this mean represents the average for full N=82 who had received phenytoin orally only, or intravenously then orally, or intravenously only). Doses were not reported for the N=55 infants receiving oral phenytoin, but seizure types were generalized in 51% and partial in 49%. Concomitant treatments were used by 93% of infants (percentages not reported for specific medications).
At three months, the rate of seizure freedom was 4% (2/55) and the rate of ≥50% reduction in seizure frequency was 9% (5/55).
Vigabatrin
Only one included study reported effectiveness data for vigabatrin. Jackson et al. (2017)31 reported data on 103 infants (mean age 8 months at treatment initiation) who were treated with vigabatrin (median dose 93.8 mg/kg/day at last followup which after about one year of vigabatrin treatment). Concomitant treatments included levetiracetam in 35%, topiramate in 31.1%, phenobarbital in 25.2%, and several other ASM. About 90% experienced “epileptic spasms”, 15% had focal seizures, and 10% had generalized tonic seizures, and other seizure types were at most 5% prevalence among the enrolled infants.
At an average of one year on vigabatrin, 38% of infants were seizure free (33/88 with long-term followup data), 73% of infants had ≥50% reduction in seizure frequency, and the mean percentage reduction in seizures was 97% (interquartile range [IQR] 43% to 100%; baseline seizure frequency not reported).
Rufinamide
Only one included study reported effectiveness data for rufinamide. Tanritanir et al. (2021)40 performed a pre/post study of 103 infants (median age 20 months at treatment initiation) who were treated with rufinamide (median dose was 42 mg/kg/day at last follow-up which was a median of 15 months). Concomitant treatments included levetiracetam in 69%, topiramate in 39%, clobazam in 33%, vigabatrin in 32%, clonazepam in 20%, phenobarbital in 17%, ketogenic diet in 16%, zonisamide in 14%, valproic acid in 10%, oxcarbazepine in 7%, steroid in 7%, lacosamide in 5%, lamotrigine in 5%, and other treatments in 12%. All patients had epilepsy, and seizure types were tonic in 75%, “epileptic spasms” in 64%, myoclonic in 43%, generalized tonic-clonic in 23%, focal onset in 22%, atonic in 14%, absence in 10%, and clonic in 5%.
At a median of 15 months of treatment, 19% (20/103) were seizure-free, and 50% (51/103) had experienced at least a 50% reduction in seizure frequency. The median % reduction in seizures was 54% (from ~167/month at baseline to 90/month at followup). Twenty-three percent (24/103) had discontinued rufinamide due to a lack of efficacy.
Stiripentol
Only one included study reported effectiveness data for stiripentol. Yamada et al. (2021)41 reported a subgroup analysis of 95 infants with Dravet Syndrome who had received stiripentol (age range 0-2 years at treatment initiation, average not reported). The dose for the 0-2 age group was not reported, but for the larger population of 376 patients, the median dose after one year was 32.5 mg/kg/day. Concomitant treatments were not reported specifically for the 0-2 subgroup, but for the full population, 99% were taking sodium valproate, 93% were taking clobazam, 41% were taking bromide, and 41% were taking topiramate. Seizure types were not reported, but all patients had Dravet Syndrome.
The only reported effectiveness outcome was the physician’s judgment of the degree of improvement. Specifically, physicians rated improvement on a 1-5 scale where 1=marked, 2=moderate, 3=mild, 4=no change, 5=worsened. This was based on seizure frequency, duration, intensity, and the ability to undertake activities of daily living. At two years, 54% (50/92) were rated as having either “marked” or “moderate” improvement.
Seizure Freedom Data
Figure 3 displays the seizure freedom rates reported by eight studies at various time points (Kholin et al. (2014)37 is not shown because the study did not report the length of follow-up). The rates ranged widely (4%-66%), and it is unclear the extent to which any specific medication caused any patient to experience seizure freedom. This may be due to many factors including the lack of control groups, concomitant medications or other factors.
Strength of Evidence
Table 3 below provides our SOE) ratings for Key Question 1. The evidence that levetiracetam may cause seizure freedom in some infants is supported by one randomized controlled trial (RCT) (high risk of bias) and one pre/post study (high risk of bias). The RCT was at high risk of bias due to various concerns including unclear generation of randomization sequence, unclear concealment of allocation, possible baseline imbalance, lack of blinding of staff, and possible differential ancillary treatments. The RCT found that the likelihood of seizure freedom was 32% with levetiracetam plus valproate vs only 22% with valproate alone, and the pre/post study found a relatively high rate of seizure freedom (66%) with levetiracetam. Overall, we judged the SOE as Low that levetiracetam may cause some infants to become seizure free.
As shown in the table, we judged all other outcomes as Insufficient for other treatments. For topiramate, the reasons for insufficiency involved risk of bias (either no randomization or no control groups) as well as inconsistency (wide variation in rates of seizure freedom) and imprecision (small studies). For five other pharmacologic treatments (lamotrigine, phenytoin, vigabatrin, rufinamide, and stiripentol, we identified only a single pre/post study of effectiveness, so we drew no conclusions. Stiripentol is not listed in the table because Yamada et al. (2021)41 did not report any SOE-graded effectiveness outcomes.
Table 3
Strength of evidence for Key Question 1.
Key Question 2. What are the effectiveness and comparative effectiveness of non-pharmacologic treatments for infantile epilepsies (e.g., dietary therapies, surgery, neuromodulation, gene therapy), including comparisons to other non-pharmacologic and/or pharmacologic therapies?
Description of Included Evidence
We included 24 studies for this Key Question, including 8 of dietary interventions and 16 studies of surgical interventions published in 17 articles; see Table 4. Only two were RCTs, both assessing dietary interventions. The remaining 22 studies were pre/post studies. Many surgical studies described outcomes for more than one surgical procedure used for different patients. Because indications for selecting a specific surgical procedure are typically so specific to individual patients (i.e., the location, size and distribution of seizures), we did not categorize these as comparative studies, but considered each one to be a pre/post study of multiple procedures. No studies assessed neuromodulation or gene therapy.
Appendix C provides study characteristics, treatment details, patient characteristics, risk of bias, and results. In the first half of this section, we describe dietary interventions; in the second half, we describe surgical interventions.
Table 4
Overview of included studies for Key Question 2.
Description of Included Evidence for Dietary Interventions
Eight studies assessed effectiveness of the ketogenic diet (KD) intervention (Table 5). Two RCTs compared KD to the modified Atkins diet (MAD). In addition, one of the RCTs also included a control group (no dietary intervention). The remaining six studies were pre/post studies (three retrospective, three prospective) assessing KD (one49 enrolled a group of normal infants without seizures, and since this group is not relevant to this report, we considered it a pre/post study for our purposes). No other dietary interventions met the inclusion criteria.
Table 5
Summary of dietary interventions.
Key Points: Dietary Interventions
- The ketogenic diet may cause seizure freedom in some infants, and may reduce average seizure frequency (SOE: Low).
- The modified Atkins diet may reduce the frequency of seizures (SOE: Low), but evidence on seizure freedom was insufficient to permit conclusions (SOE: Insufficient).
- The ketogenic diet may cause greater reductions in seizure frequency than the modified Atkins diet (SOE: Low), but data on seizure freedom are inconclusive (SOE: Insufficient).
Summary of Findings: Dietary Interventions
Ketogenic Diet
Two RCTs assessed the effectiveness of KD and MAD. In the first RCT, El-Rashidy et al. (2013)42 conducted a three-arm RCT comparing KD and MAD to a control group (no-dietary-change). All enrolled patients were under 36 months of age (n=40). The KD intervention was described as the classic 4:1 KD, administered via a liquid formula (Ketocal 4:1 milk from Danone, Nutricia). In addition to the dietary intervention, all maintained the same doses of ASM (valproic acid, carbamazepine and/or clonazepam) throughout the study period.
In the second RCT, Kim et al. (2015)43 randomized patients to KD or MAD (see a discussion of the between-group comparison in the next section on KD vs MAD). Although the study enrolled patients ages 1 to 18 years, authors provided a subgroup of patients 1 to <2 years of age. The KD interventions followed the 4:1 lipid to nonlipid ratio with a nonfasting initiation protocol. While the MAD adhered to the Johns Hopkins protocol, the authors did not report if the KD used conformed to the protocol.
In the first pre/post study, Suo et al. (2012)44 prospectively enrolled 317 consecutive patients with intractable epilepsy to receive KD, of whom 147 patients were 0-2 years old and met criteria for inclusion. Infants received the Johns Hopkins Hospital protocol, with a lipid-to-non-lipid ratio of 4:1. However, there was variation in how the diet was initiated across the study. Depending on the date of KD initiation, the patient’s diet may have been self-prepared via the KD Meal Planner, KetoCal ketogenic formula, or a KD Meal Planner supplemented by various liquid milk, cookies, and set meals. Follow-ups were conducted either at an outpatient clinic or by phone call.
Wu et al. (2015)45 conducted a prospective pre/post study of KD in 87 children diagnosed with drug-resistant epilepsy. The authors did not clarify whether enrollment was selective or consecutive. The intervention used the Johns Hopkins protocol, with the addition of 24 to 48 hours pre-diet fast in the hospital. Of the 87 children in the study, only 40 (46%) met our inclusion criteria of under 36 months of age. Of the 40 infants, six were between the ages of 0 to 1 year, and 36 were between the ages of 1 to 3 years (36 months).
Kim et al. (2019)46 presented a retrospective chart review of patients with medically intractable epilepsy who started the KD. The KD protocol was determined internally with a multidisciplinary team, and was not described as being based on specific established protocols. KD was initiated at a 1:1 fat to carbohydrate + protein ratio and steadily increased to 3:1, but not all patients maintained on the 3:1 ratio. Twenty patients maintained the 3:1 ratio, 13 progressed to 3.5:1, while 59 were on 4:1 ratios or higher. Roughly one-third of patients were on solid food, liquid food, or both solid and liquid foods each. All patients were under 3 years of age, and we only extracted data on the subgroup of patients without West syndrome.
Dressler et al. (2015)47 evaluated the seizure relapse rate with a retrospective chart review of children treated with KD between March 1999 to April 2014. The intervention followed the Johns Hopkins protocol, without an initial fasting period. Of the 115 patients with complete clinical and follow-up data, 58 (50.4%) were under 1.5 years of age at the start of the KD. The outcomes the authors reported specifically for this subgroup were seizure freedom and “responders”, defined as a reduction in seizure frequency of ≥ 50%. Multiple durations of KD were also reported (3, 6, 12 months) and an additional 6 months after the KD ended.
Kang et al. (2005)48 performed a retrospective review of young patients with uncontrolled epilepsy treated with KD. The protocol used was the Johns Hopkins protocol, but not all patients had initial fasting and fluid restriction. Of the 199 patients enrolled in the study, 49 (24.6%) were < 2 years old. Follow-up was conducted up to 12 months.
Liu et al. (2021)49 enrolled 41 infants diagnosed with refractory epilepsy unresponsive to two or more anticonvulsants. Although the study’s primary goal was to examine biological and biochemical effects of the KD on infants, it did report seizure frequencies for infants treated with KD. Study authors did not specify what dietary protocol was used and some infants received different KD protocols. Infants in the KD group were further stratified into various age groups, but each individual subgroup contained too few patients to be analyzed independently according to protocol. All patients were followed for 12 months.
Seizure Freedom
Five studies (1 RCT, 4 pre/post studies) assessing KD reported the proportion of infants achieving seizure freedom. No studies reporting on seizure freedom compared KD to a control.
One RCT (Kim et al. 2015),43 compared outcomes for infants receiving KD or MAD. For KD, at both 3 and 6 months, 53% (9/17) of infants were seizure-free. (It was unclear if the same nine patients were seizure free at both time points). More discussion about the direct comparison of KD and MAD is presented in a later section.
Seizure freedom rates across the four pre/post studies ranged from 11.6% to 53%. Suo et al. (2012)44 reported seizure freedom rate of 11.6% (17/147). Notably, the study had high treatment attrition: only 33 of 147 children remained on the diet and reported on seizure freedom. Wu et al. (2015)45 reported seizure freedom rates of 25% (10/40) at 2 months, and 33% (13/40) at six months.
Dressler et al. (2015)47 was not clear and consistent in its outcome reporting for this subgroup, with ambiguous information presented in the text of the article and some conflicting information between the text and the figures. The article reported 20 of 58 patients (34%) seizure free three months into the KD. At both six months and 12 months, 19 of 58 patients (33%) were seizure free. The author also reported that 15 of 58 patients (26%) were seizure free six months after the cessation of the KD.
Kang et al. (2005)48 reported 16 of 49 patients (32%) seizure free at three months, 18 of 49 patients (36%) seizure free at six months, and 13 of 49 patients (26%) seizure free at 12 months. It is unclear at each timepoint how many patients were still on the KD, and the denominator of 49 patients represents the total number of patients that had initiated the diet. At 12 months follow-up, only 13 of the original 49 patients (27%) in the < 2 years subgroup were still receiving the KD.
Figure 4 shows the seizure freedom proportion experienced at various time points for the five studies included here. Kim et al. (2015)43 reported data for both KD and MAD, which are each displayed in the figure. Seizure freedom rates for KD ranged from 25%-53%.
Seizure Frequency
All eight studies reported seizure frequency outcomes.
In the RCT with three arms (KD, MAD, and regular diet), El-Rashidy et al. (2013)42 reported that the KD group had 58% reduction in seizures at three months (compared to a 6% increase for the normal diet group) and had 71% reduction in seizures at six months (compared to an 8% reduction for the normal diet group). For seizure severity as measured by Chalfont scores, the KD group had 32% improvement at three months (compared to only 0.45% improvement for the normal diet group), and had 36% reduction in seizures at six months (compared to only 2% improvement for the normal diet group). All of these comparisons were statistically significant (favoring KD over regular diet).
In Kim et al. (2015),43 the authors reported of the 17 patients on KD, nine experienced >90% reduction, and ten experienced >50% reduction at three months. The mean reduction in frequency was 19% of the baseline seizure frequency (i.e., a mean reduction of 79% from baseline). At six months, ten patients experienced >90% reduction, and ten patients experienced >50% reduction.
Suo et al. (2012)44 reported reduction in seizure frequency after 12 months on the diet for children age 1 to <2 years. Of the 147 children in this age group, only 33 patients remained on the diet. Of these 33 patients, 17 were seizure free, 3 experienced 90% to 99% seizure frequency reduction, 7 experienced 50% to 90% reduction, and 6 had either less than 50% reduction or no reduction.
Wu et al. (2015)45 reported both reduction of seizure frequency after 6 months on the KD and efficacy of KD after three months. Efficacy was defined as experiencing >90% seizure reduction. After three months on the KD, the authors reported the KD effective in 13 of 40(32.5%) children under 36 months. At six months, two of 40 patients experienced >90% reduction (not counting seizure free patients), with an additional six patients experiencing 50% to 90% seizure reduction. While the article mentioned measurement of baseline seizure frequencies, baselines were not reported.
Kim et al. (2019)46 reported the response of KD, defined as experiencing a seizure reduction >50%. We only extracted data from those without West syndromes. The authors reported that 18 of 49 patients (36%) responded to KD.
Dressler et al. (2015)47 was not clear and consistent in its outcome reporting for this subgroup, with ambiguous information presented in the text of the article and some conflicting information between the text and the figures. The only outcome reported for the infants was a response to KD. Response to the diet is defined as “the absolute reduction in seizure frequency of ≥50% at follow-up, compared to baseline”. The article reported 37 (63.8%) responded to KD 3 months into the diet. At 6 months into the KD, 32 (55.2%) responded to the diet. At 12 months, 27 (84.3%) responded to KD. At 6 months after the diet ended, 21 (42.9%) were responders. The study was unclear regarding the how many patients were on the diet at those timepoints, and the percentages they reported were inconsistent.
Kang et al. (2005)48 reported rates of >50% reduction of 56% at three months (28/49), 54% at six months (27/49), and 32% at 12 months (16/49). For each time point, the authors also recorded how many patients continued the diet, but it was not clear whether that was measuring how many patients were still on the KD at the time of outcome measurement or after measuring the outcome decided to continue the diet further. Nevertheless, by 12 months only 13 of the original 49 patients (27%) in the subgroup continued the diet.
Liu et al. (2021)49 reported that >=50% seizure reduction occurred in 28 (68%), 32 (78%), and 34 (83%) of patients at 3, 6, and 12 months, respectively. They also reported that 7 (17%), 8 (20%), and 9 (22%) experienced >=90% seizure reduction at 3, 6, and 12 months, respectively.
Modified Atkins Diet
The Modified Atkins Diet (MAD) is a less restrictive dietary alternative to the ketogenic diet, with a focus on low carbohydrate intake but no restrictions on proteins, dairy, or fat. Two RCTs assessed the MAD. However, only one study, El-Rashidy et al. (2013)42 compared MAD to a control group (ASM polytherapy arm). The MAD demonstrated statistically significant reduction in seizure frequency compared to control at 6 months, but not at three months. Specifically, at six months, seizure reduction was 28% (MAD) compared to 8% (control), p<0.001. Compared to control, the MAD also demonstrated a statistically significant improvement in seizure severity (measured as Chalfont score) at both three and six months.
Ketogenic Diet Versus Other Diets
Two studies directly compared the KD with the MAD. El-Rashidy et al. (2013)42 reported that KD yielded significantly greater reduced seizure frequency at three and six months compared to the MAD. Specifically, at six months, seizure reduction was 71% (KD) vs. 28% (MAD), <0.001. However, there was no statistically difference in seizure severity (measured by Chalfont score).
Kim et al. (2015)43 reported KD demonstrated a statistically significantly higher rates of seizure freedom compared to MAD, with 9 of 17 achieving seizure freedom for KD and 4 of 40 for MAD at three months. This difference was not sustained; at six months the two groups demonstrated statistically non-significant difference. All other outcomes (i.e., >90% and >50% seizure reduction) also demonstrated no statistically significant difference between groups. The authors did find that at six months, the KD yielded lower mean and median percentage of baseline seizures than the MAD.
Strength of Evidence: Dietary Interventions
Table 6 provides SOE ratings dietary interventions. The evidence was sufficient to permit conclusions that the KD may cause seizure freedom in some infants (SOE: Low) and that seizure frequencies are generally lower after starting the KD (SOE: Low). For comparative effectiveness, two randomized trials support the conclusion that seizure frequencies are lower after the KD than the MAD. Due to the marked differences in protocol, design, and patient population between the studies, we deemed meta-analysis inappropriate.
Table 6
Strength of evidence for Key Question 2: Dietary interventions.
Description of Included Evidence for Surgery
Sixteen studies (published in 17 articles) were included for effectiveness data on surgical interventions for infants with epilepsy undergoing surgery at 1 to <36 months. We included all studies describing outcomes for a procedure of interest for ≥ 10 infants. Table 7 provides an overview of included studies, number of infants, years operations were performed, and key outcomes. Surgical interventions may be resective (hemispherectomy/hemispherotomy, nonhemispheric procedures) or palliative (e.g., corpus callosotomy, multiple subpial transections) or involve neuromodulation. No included studies assessed palliative procedures or neuromodulation.
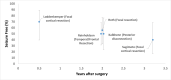
We identified studies of infants receiving hemispherectomy/hemispherotomy (12 studies, reported in 13 articles), other non-hemispheric resections (8 studies), tumor resection only (1 study). All included studies were retrospective pre/post studies which obtained data from chart reviews (16 studies) or registry data51 (1 study).
Five studies were conducted in the U.S. The remaining 11 non-US studies were conducted in Germany52,56,64 (n=3), Japan50,55 (n=2), Canada53,63 (n=2), Italy60,66 (n=2), Sweden51 (n=1) or included data from multiple countries (n=1).62 All US studies were single center studies from University of California at Los Angeles,58,59 University of Colorado,54 Cleveland Clinic,61 Boston Children’s hospital,57 and Miami Children’s hospital.65 One study (Roth et al.62) included data from 19 centers with surgical procedures performed from 1999 to 2020. Data from 6 patients cared for at 2 of 19 centers (University of California at Los Angeles and Cleveland Clinic) may also have been included in other studies58,59,61 given overlap in time periods (Figure 5 and author correspondence).
As nearly all data represent either subgroups or individual patient data, patient characteristics such as age, seizure etiology, and length of follow-up were variably reported. No studies reported on race. The number of infants meeting inclusion criteria from each study ranged from 10 to 58. Appendix C provides detailed information regarding inclusion criteria, patient characteristics, treatments, and outcomes.
Risk of bias (ROB) ratings for all studies are also provided in Appendix C. As all studies assessing surgical interventions were pre/post studies lacking a control group and retrospective, the overall ROB for all studies was high.
Table 7
Overview of surgical studies.
Studies included surgeries performed over a broad range of years (Figure 5).

Figure 5
Years when surgical procedures were performed. Note: Studies not appearing in this figure either were not included for effectiveness data, or did not report the years when surgery was performed.
Key Points: Surgery
- Hemispherectomy/hemispherotomy: Some infants with medically refractory epilepsy achieve seizure freedom after hemispherectomy/hemispherotomy (8 retrospective pre/post studies, SOE: Low).
- Hemispherectomy/hemispherotomy: Over half of infants achieved a favorable outcome (Engel I or II, ILAE I to IV, or >50% seizure reduction) at follow-up of >1 year. However, evidence is insufficient to draw a conclusion due to study limitations (9 retrospective pre/post studies, SOE: Insufficient).
- Other resections: Some infants with medically refractory epilepsy achieve seizure freedom after intralobar, multilobar, focal cortical resection, or posterior disconnection surgery (5 retrospective pre/post studies, SOE Low).
- Other resections: All studies reported that at least 50% of infants undergoing resection (intra/multilobar/focal cortical resection or posterior disconnection) achieved a favorable outcome (Engel I or II, ILAE I to IV, or >50% seizure reduction). However, due to study limitations, evidence is insufficient to draw a conclusion (6 retrospective pre/post studies, SOE: Insufficient).
- All surgical interventions: No studies described quality of life for children or caregivers, or treatment cost (SOE: Insufficient).
- All surgical interventions: Only 4 studies with key limitations reported on developmental or functional outcomes (4 pre/post studies, SOE: Insufficient).
Summary of Findings: Surgery
Below we provide a summary of evidence in three categories of surgical intervention: hemispherectomy/hemispherotomy, other resections (intralobar, lobar, multilobar, focal cortical resection, posterior disconnection), and brain tumor resection. Appendix C provides detailed information regarding gender, race, seizure etiology, concomitant treatments (i.e., other ASM) and outcomes.
Hemispherectomy/Hemispherotomy
Twelve studies (reported in 13 articles) described children 1 to 36 months old undergoing hemispherectomy/hemispherotomy. Several studies described a single center’s experience with performing different surgical procedures of which a subgroup of infants met our inclusion criteria. For instance, one pre/post study by Cook et al.58 described surgical outcomes for 55 infants with cortical dysplasia undergoing hemispherectomy/hemispherotomy over a 16 year period. Sixteen of these 55 infants had hemimegalencephaly (HME) and outcomes for this subgroup are reported in Jonas et al.59 (Authors also reported children undergoing surgery for infarction/ischemia, or Rasmussen Encephalitis; however, these groups did not meet inclusion criteria due to age). Infants underwent slightly different procedures based on year of operation: from 1986-1997 (14 anatomical hemispherectomies), from 1990-1997 (15 functional hemispherectomies), and from 1997-2002, 26 hemispherotomies).
Seizure Freedom
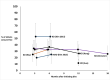
Eight retrospective pre/post studies50–52,56–59,61 (reported in 9 articles50,51,56–62) reported on seizure freedom over a follow up period of 6 months to mean 4.3 years after surgery. Rates are presented in Figure 6. Studies included a combined 188 infants. One study (Otsuki et al.)50 did not report follow up interval for subgroup of included patients. Three studies58,60,61 reported seizure freedom at 6 months. Cook et al. reported a subgroup of 55 infants with cortical dysplasia undergoing anatomical hemispherectomy or functional hemispherectomy/hemispherotomy depending on the year the surgery was performed. At 6 months, 80% were seizure free. (Jonas et al. described a subset of 16 infants with HME; at 6 months 15 of 16 (93%) of these infants were seizure free). Two additional studies reported 9 of 14 (64%)61 and 7 of 10 (70%)60 of infants were seizure free at 6 months.
Overall, seizure free rates at 1 year or longer ranged from 7% to 76%. Six studies51,56–58,60,62 described outcomes at 1 year or longer: Cook et al. reported 75% of infants were seizure free; other small studies reported seizure free rates of 50% (n=10)60 and 76%56 (n=21)56 at one year and 58% (n=12)51 at two years. A larger study, Roth et al.52,62 reported at follow up median 4.3 years, 70% of patients (30 of 43) were seizure free. In contrast to these higher proportions, Pinto et al.57 reported only 7% (1 of 15) infants were seizure free at follow up of at least 1 year after surgery.
We used stringent definition of seizure freedom which required studies using the Engel classification to report Engel Ia. However, if we had considered Engel I as seizure freedom, we note that seizure freedom from the Pinto et al. study would increase to 66% (10 of 15); also, 4 other studies52–55 reporting rates of 55% to 81% (consistent with the range of seizure freedom rates we already identified) would have been included.
Although pre/post studies have significant limitations due to lack of control group, in these studies most infants underwent surgery for medically refractory epilepsy. Their pre-surgical status, therefore, suggests strongly that none of them would have experienced seizure freedom if they had not undergone surgery. Also, while accurately capturing seizure counts using retrospective data from charts (i.e., not captured in the context of the trial) was felt to be a key study limitation, we felt seizure freedom would be much less subject to recall bias or other types of bias. The precise rates of seizure freedom varied greatly among studies. Thus, we conclude that some infants with medically refractory epilepsy achieve seizure freedom after hemispherectomy, and we rated the strength of the evidence as Low.
Seizure Frequency
Nine retrospective pre/post studies including a combined 186 infants reported on seizure frequency. Study follow up times were variably reported with some studies including a minimum follow up time (e.g., <1 year) while for other studies, follow up was determined using individual patient data often with wide ranges. All studies reported that over half of infants achieved a favorable outcome, defined as Engel I or II, ILAE I to IV, or >50% seizure reduction. Overall, the proportion of infants achieving favorable outcome ranged from 67% to 100%, with most studies reporting follow up of at least 1 year. Specifically, studies reported the following proportion of infants had favorable outcomes at follow up: 67% (10/15),57 72% (13/18),50 73% (35/48),53 72% (31/43),62 80% (8/10),55 88% (14/16),54 92% (11/12),51 93% (13/14)61 and 100% (10/10).60 However, given the retrospective study design the outcome of seizure frequency was assessed as high risk of bias, and thus, evidence was insufficient to draw a conclusion.
Eight studies54,55,60,61,63–65 reported individual patient data for pathology or etiology, surgical intervention, and outcomes. Surgeries reported in these studies were performed over nearly four decades (from 1979 to 2017). Unfortunately, many factors precluded formal meta-analysis, including heterogeneity across patients and interventions, and inconsistent data reporting. However, as favorable outcome after epilepsy surgery could depend on a number of factors, including underlying seizure etiology, we summarize data from 65 infants undergoing hemispherectomy or hemispherotomy. Seizure etiology or pathology was reported as HME (58%), focal cortical dysplasia (FCD) or malformation of cortical development (MCD) without HME (20%) or other (22%) (pathology or etiologies in the “other” category include stroke, subpial gliosis, Sturge Weber syndrome, and polymicrogyria). Our analysis found the proportion of infants achieving favorable outcomes was similar across these three groups: specifically, favorable outcomes were 89% (34/38) for HME, 92% (12/13) for FCD/MCD without HME, and 93% (13/14) for other pathology.
Other Resective Surgical Procedures
Eight retrospective pre/post studies reported outcomes for infants undergoing non-hemispheric procedures. Five studies reported on seizure freedom, and 7 studies on seizure frequency.
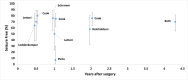
Seizure Freedom
Five pre/post studies including a combined 70 infants reported on seizure freedom. Specifically, infants underwent focal cortical resections61–63 (n=3), frontal or temporal lobe resection51 (n=1),52 and posterior disconnection64 (n=1). Rates of seizure freedom ranged from 40% (Sugimoto et al.) to 70% (Loddenkemper et al.). Figure 7 presents seizure freedom rates and follow up durations. Specifically, after focal resection studies reported seizure freedom of 70% (7/10)61, 56% (9/16)62, and 40% (4/10)63, at median 6 months, 24 months, and mean 3.2 years, respectively. Kalbhenn et al.64 reported 50% (5/10) of patients were seizure free after posterior disconnection surgery at 2 years after surgery. Reinholdson et al. reported 50% (12/24)51 seizure freedom for infants undergoing52 frontal or temporal lobe resection515251 at 2 years after surgery.
Seizure Frequency
Seven retrospective pre/post studies including a combined 148 infants reported on seizure frequency. Six of seven studies (combined n=131) reported seizure frequency data that allowed for determination of favorable outcome.51,53,61–63,65 All studies found that ≥ 50% of infants achieved a favorable outcome, defined as Engel I or II, ILAE I to IV, or >50% seizure reduction. Specifically, the proportion of infants achieving favorable outcomes was 50% (5/10),63 62 83% (20/24),51 85% (11/13),65 and 90% (52/58),53 94% (15/16)62, 100% (10/10)61. Mean follow up was at least 1 year after surgery for all studies.
A seventh study (Kadish et al.)52 reported that of 17 infants for whom the extent of final resection was intralobar, 76% (13/17) were Engel I, while 24% (4/17) were Engel II to IV.
As previously noted, 8 studies included individual patient information regarding seizure etiology/pathology, surgical procedure, and outcomes; these studies included 43 infants undergoing multilobar, lobar, or focal resection. Seizure etiology or pathology was FCD/MCD without HME (56%) or other (44%), which included encephalitis, ganglioma, astrocytoma, tuberous sclerosis, and white matter gliosis. Our analysis found the proportion of infants with favorable outcome rates was 67% (16/24) for FCD / MCD without HME and 74% (14/19) for other pathologies.
Tumor Resection
We identified 1 study Gaggero et al. 200966 focused exclusively on infants with epilepsy due to brain tumors. Authors performed a retrospective chart review of infants < 3 years referred to a single center (G. Gaslini Children’s hospital, Genoa Italy) for primary supratentorial brain tumors. Tumor location was cortical in 16, non-cortical in 4. Histologic tumor types included WHO Grade I (n=5) Grade II (n=4), Grade III (n=7), and Grade IV (n=5). Twenty children had epilepsy as a clinical manifestation with focal (n=12), generalized (n=8) and history of convulsive status epilepticus (n=5). Pre-surgery, ASM use was as follows: 1 ASM (n=9), 2 ASM (n=7), and 3 ASM (n=4). Eight had received chemotherapy, and 3 had received radiotherapy. Mean time from tumor diagnosis to surgery was 0.86 (standard deviation [SD] 0.63) months.
Post-surgical follow-up ranged from 4 to 10 years (mean 7.6, SD (3.74). We report seizure freedom rates described in the study here; however, because study authors did not appear to define seizure freedom as Engel IA, data from this study were not included in analysis of seizure freedom rates).
At 1 year after surgery, 9 of 20 patients (45%) were seizure free (of which 5 were off ASM). Seven had >90% improvement in seizure frequency (Engel II), two had >50% but <90% improvement (Engel III), and 2 had no change (Engel IV). At 4 years after surgery, 11 patients were seizure free (7 off ASM), 5 had only rare seizures (Engel II), 2 had worthwhile improvement (Engel III), and 2 had no change (Engel IV). Malignancy grade (but not histological diagnosis) was associated with seizure outcomes: specifically, children with low grade tumors were more likely to have a good outcome (Engel I or Engel II), p<.001, t=2.84). Three patients died from tumor recurrence within 4 to 4.5 years (2 choroid plexus carcinomas, 1 glioblastoma multiforme). Of 17 patients with follow-up at 8 years post-operative, 9 were seizure free (Engel I), 4 (Engel II), 2 (Engel III), and 2 (Engel IV).
Other Outcomes (All Procedures)
Only 4 pre/post studies reported on developmental outcomes (developmental quotient [DQ], language or functional status). Two studies reported DQ after hemispherectomy. Loddenkemper et al.61 included 24 infants undergoing hemispherectomy or focal resection (median age at surgery was 14 months [3 to 34]). Infants were evaluated at median 12 months (3 to 34) preoperatively, and median 24 months (10 to 53) after surgery using the Bayley scale. Although the proportion of infants with developmental delay (defined as DQ <70) decreased after surgery, this change was not statistically significant (p=0.125). However, of note, authors excluded 26 of 50 consecutive infants (due to incomplete data or use of other neuropsychological tests), limiting the generalizability of these findings. Another study (Jonas et al.)59 found that in 16 infants undergoing hemispherectomy for HME, the Vineland DQ increased by 9.1 (SD 16) at 24 months (compared to 6 months). The spoken language rank also increased from 0.33 (SD 0.5) to 1.4 (SD 1.8) after surgery.
One study (Lettori60) included 10 infants meeting inclusion criteria and undergoing hemispherectomy. Before surgery functional status of 2/10 infants was dependent (and status could not be assessed for 8/10). However, after surgery, functional status improved (6 dependent, 3 semi-independent, 1 independent). Finally, 1 study (Sugimoto et al.63) reported some infants had improvement in developmental delay after undergoing focal cortical resection (8/10 with delay pre-operatively, 6 infants with improved or good status after surgery). However, the study did not report how delay was assessed.
Key Question 3. What are the harms or comparative harms of treatments for infantile epilepsies?
Description of Included Evidence
Table 9 lists the studies included for this Key Question.
Table 9
Overview of included studies for Key Question 3.
Key Points
- Levetiracetam is rarely discontinued due to adverse effects (SOE: Low)
- Topiramate is rarely discontinued due to adverse effects, and severe events are rare (SOE: Low). However, loss of appetite and upper respiratory tract infection are risks (SOE: Moderate)
- Lamotrigine is rarely discontinued due to adverse effects, and severe events are rare (SOE: Low)
- Only four of the eight studies of dietary interventions reported whether there were harms for those age 1-36 months, and the data were too sparse to permit conclusions (SOE: Insufficient)
- Surgical mortality for functional hemispherectomy/hemispherotomy is rare (SOE: Low)
- Evidence is insufficient to draw a conclusion regarding mortality after anatomical hemispherectomy (SOE: Insufficient)
- Surgical mortality for multilobar, unilobar, or focal resection is rare (SOE: Low)
- Hydrocephalus requiring shunt placement after multilobar, unilobar, or focal resection is rare (SOE: Low)
Summary of Findings
Harms of Levetiracetam
One nonrandomized comparison study36 and two pre/post/studies32,34 reported adverse event data for levetiracetam. Please see Key Question 1 for summaries of their enrolled patients and treatment details.
We first discuss the reported data on levetiracetam discontinuation due to adverse events including “serious” events, “severe” events, or events requiring dose modification. We then summarize any adverse events that occurred in 10% or more of patients who received levetiracetam. Appendix C provides all adverse event data (including those with rates <10%).
Arzimanoglou et al. (2016)32 administered levetiracetam at a mean daily dose of 46 mg/kg/day. At least one treatment-emergent adverse event led to study discontinuation in 7% of patients (7/101). The specific events were respiratory disorder (2 patients), respiratory distress and infantile spasms (two patients), irritability (one patient), lower respiratory tract infection, psychomotor retardation (one patient), and respiratory failure (one patient). Also, 12% of patients (12/101) had a “severe” treatment-related adverse event, but authors stated that none were considered related to levetiracetam by the clinician. “Serious” events occurred in 32% (32/101), but only two of them (both convulsions) were considered levetiracetam-related (authors did not define the difference between “severe” and “serious”). Furthermore, 10% (10/101) had an adverse event that required dose modification.
Arican et al. (2018)34 reported that among 92 patients taking levetiracetam 10-60 mg/kg/day (52% on 30-40 mg/kg/day), no patients discontinued due to adverse events. The study made no statements about serious or severe events or any events requiring dose modification.
Liu et al. (2020)33 reported an RCT comparing valproate alone (40-50 mg/kg/day) to valproate + levetiracetam (20-30 mg/kg/day). The study did not explicitly report whether any infants discontinued levetiracetam due to adverse events, whether any events were severe/serious, or whether any dose modifications were necessary. Effectiveness data were reported for all enrolled patients at 12 weeks, so likely there were no <12-week discontinuations due to adverse events.
Regarding non-severe events experienced by at least 10% of patients, the only pertinent events were bronchitis (10% as reported by Arzimanoglou et al. (2016)32) and convulsion (10% as reported by Arzimanoglou et al. (2016)32).
Overall, the only small signal from these data involve possible respiratory harms of levetiracetam (as per the study discontinuations in Arzimanoglou et al. (2016)32). However, neither of the other two studies reported any respiratory events.
Harms of Topiramate
Four studies of topiramate reported data on harms: two RCTs,68,70 one nonrandomized comparative study,36 and one pre/post study.67 Both RCTs compared different doses of topiramate: Novotny et al. (2010)69,70 compared placebo to three different doses (5 or 15 or 25 mg/kg/day; 37-38 infants per group), while Manitpisitkul et al. (2013)68 compared four different doses (3 or 5 or 15 or 25 mg/kg/day; 13-15 infants per group). Dose comparisons are particularly informative, because the medication-harm causal connection is stronger if there is a clear dose-response association. Conversely, the absence of a no dose-response association reduces the likelihood that the medication causes the harm. We first discuss events causing medication discontinuation, then severe/serious events and dose modifications, and finally, events experienced by 10% or more of infants.
Reported harms in three of the four studies are summarized in Table 10 (the fourth study only reported hypohydrosis, so we discuss its results separately). For discontinuation due to adverse events as well as serious/severe events, rates were low in all three studies, with no dose-response association. For less severe events (occurring in at least 10% of patients) the two RCTs found dose-response associations for loss of appetite and upper respiratory tract infection. For three other less severe events, one RCT found a dose-response association, but the other RCT did not. For seven other less severe events, neither RCT showed a dose-response association (coughing, diarrhea, fever, viral infection, somnolence, otitis media, and rhinitis). Kim et al. (2009)36 also reported a rate of 17% (7/41) for psychomotor retardation
The pre/post study by Kim et al. (2010)67 focused only on the risk of hypohydrosis with topiramate, and authors had performed a subgroup analysis of 81 infants age 1 year or less. The rate of hypohydrosis in these infants was 48% (39/81). The only other study to mention this type of event was Kim et al. (2009),36 which reported a rate of “anhidrosis” of 2% (1/41) (far lower than the 48% reported by Kim et al. 2010).67
Overall, these adverse event data suggest topiramate is not difficult to tolerate. We did find consistent evidence for two non-severe adverse events with topiramate: loss of appetite (7%-20% with risk increasing with dose) and upper respiratory tract infection (8%-38% with risk increasing with dose).
Table 10
Summary of harms of topiramate.
Harms of Lamotrigine
Piña-Garza et al. (2008)30,38 studied lamotrigine and reported three sets of data: the initial open label phase (N=177) for at least five 5 weeks, the RCT phase comparing continued lamotrigine to replacement of lamotrigine with placebo for eight weeks (N=19 per group), and the long-term open label phase (at least 24 weeks in 92% of infants; N=204). All harms data from these sets appear in Appendix C. In this section, we discuss only the RCT phase and the long-term open label phase (due to its larger N than the initial open label phase).
During the eight-week randomized phase, none of the 38 patients had lamotrigine discontinued due to adverse events; this may be because the only infants who entered this phase had demonstrated tolerance during the initial five-week open label phase. In the long-term open label phase, 9% of patients (18/204) had lamotrigine discontinued due to adverse events. The authors reported specifics for 15 of 18 discontinuations: pneumonia (n = 4), complex partial seizures (n = 3), status epilepticus (n = 3), rash (n = 3), and fever (n = 2).
Regarding serious events, two events occurred in the randomized phase: one lamotrigine patient (5%, 1/19) had serious bronchitis, and one placebo patient (5%, 1/19) had status epilepticus. During the long-term open label phase with lamotrigine, 8% had pneumonia (16/204), 6% (12/204) had status epilepticus, 6% (12/204) had complex partial seizures, 4% (12/204) had fever, 3% (6/204) had convulsion, 3% (6/204) had dehydration, and 3% (12/204) had gastroenteritis.
For non-serious events, the RCT phase had five events in which one of the two groups had rates of 10% of more (cough, nasal congestion, upper respiratory tract infection, fever, and teething). The long-term open label phase had 15 events with rates of 10% of more: fever, upper respiratory tract infection, ear infection, cough, vomiting, otitis media, irritability, constipation, nasopharyngitis, teething, rash, bronchitis, pneumonia, status epilepticus, upper respiratory tract congestion. See Appendix C for the rates of these events.
Overall, the most common adverse events reported with lamotrigine are fever (45%), upper respiratory tract infection (28%), and ear infection (22%). Left untreated, some of these events may later be associated with pneumonia, as 8% had lamotrigine discontinued for that reason.
Harms of Phenytoin
The pre/post study by Sicca et al. (2000)39 enrolled 55 infants who had received oral phenytoin for seizure prophylaxis. The study reported neither phenytoin discontinuation due to adverse events nor any “serious” or “severe” harms of phenytoin. Of less severe events, only four had rates of 10% or more:
- Drowsiness: 22% (12/55)
- Gingival hyperplasia: 15% (8/55)
- Sleep troubles: 15% (8/55)
- Hyperactivity: 11% (6/55)
Although drowsiness is fairly common (22%), in general, these data are too sparse to draw clear conclusions regarding the adverse effects of oral phenytoin when administered to infants.
Harms of Vigabatrin
Jackson et al. (2017)31 was a pre/post study that administered vigabatrin to 103 infants. The authors reported a rate of vigabatrin discontinuation-due-to-adverse effects of 9% (9/103), with specific reasons being vision abnormality (n=5), fatigue (n=1), fatigue and anorexia (n=1), “possible vigabatrin toxicity” (n=1), and anemia (n=1). The study did not report rates of “severe” or “serious” adverse events.
Authors reported rates of adverse events specifically for the 71 infants who discontinued vigabatrin for any reason (e.g., the reason was successful control of seizures in 31 infants, and unsatisfactory therapeutic effect in 23 infants). None of the reported events reached 10% or more (see all data in Appendix C). Authors also specifically conducted eye exams before vigabatrin (N=49), during vigabatrin (n=62), and after vigabatrin (n=49) to evaluate for vision abnormalities possibly caused by vigabatrin. The authors reported these three time periods separately, but unfortunately did not report the number of infants whose vision status had changed between measurements (which would have more directly addressed the influence of vigabatrin).
Prior to vigabatrin administration, 69% (34/49) had vision abnormalities, which authors attributed to tuberous sclerosis complex, refractive errors, and prior medication. The study did not report which baseline medications were likely responsible for the pre-vigabatrin abnormal eye exam results. During vigabatrin, 81% (50/62) had at least one abnormal exam. After vigabatrin, 63% (31/49) had vision abnormalities.
Overall, some evidence suggests that vigabatrin may cause temporary vision abnormalities, but only a single pre/post study has addressed the issue.
Harms of Rufinamide
Tanritanir et al. (2021)40 was a pre/post study that administered rufinamide to 103 infants. Fifteen (15%) discontinued rufinamide due to adverse effects (three of these were solely due to adverse effects, and the other 12 were due to both adverse effects and lack of efficacy). Authors did not mention whether any events were serious or severe. Rates of 10% or higher were observed for somnolence (12% or 12/103) and irritability (10% or 10/103).
Harms of Stiripentol
Yamada et al. (2021)41 was a pre/post study that administered stiripentol to 95 infants age 0-2 years. Authors did not report the specific types of adverse reactions in these patients, but did report that 61% (58/95) had at least one adverse drug reaction. Physicians in charge determined that all 58 had a causal relationship to stiripentol (either “clearly” or “probably” or possibly”). One patient died due to liver damage, which physicians deemed a “possible” relationship to stiripentol, but the patient was also taking both valproate and clobazam.
Harms of Ketogenic Diet
Of 8 included studies of ketogenic diet, 4 reported data pertaining to harms for infants age 1-36 months.42,46,47,49 Three43,44,48 of the other four reported data on adverse events, but data were not specific to age 1-36 months, and the fourth study45 did not investigate whether adverse events occurred.
Two of four studies reported the rate of withdrawal due to side effects or diet intolerance. Kim et al. 201946 found a rate of 2% (2/109; one behavioral food refusal and one persistent acidosis), and El-Rashidy et al. (2013)42 found a rate of 20% (2/10; both diet intolerance).
For other reported adverse effects:
- Kim et al. (2019)46 reported 33% of patients had decreased HC03 level (36/109), 32% constipation (35/109), , and 20% vomiting/reflux (22/109); three other events were experienced by <10% of patients.
- Dressler et al. (2015)47 reported that 50% (29/58) of patients experienced “side effects”, but authors did not report specifics. Also, for 28% (16/58) there was “difficulty introducing solid foods”.
- Liu et al. (2021)49 reported z scores of body mass index (BMI)-for-age at 3-12 months after KD initiation. For the 41 patients, the baseline mean was 0.49, which changed to 0.06 at 3 months, 0.16 at 6 months, and 0.35 at 1 year; none of these changes were statistically significant. Authors also reported statistically non-significant changes over time in the percentage of patients who were underweight (defined as weight-for-age z score <-2), stunting (defined as height-for-age z score <-2), and wasting (defined as BMI-for-age z score <-2). However, the percentage of patients who were overweight/obese decreased (statistically significantly) from 17% at baseline to 2% at one year after KD initiation.
- El-Rashidy et al. (2013)42 reported low rates of vomiting, constipation, diarrhea, and dysphagia (0%, 20%, 10% and 10%, respectively).
Harms of Modified Atkins Diet
One study reported harms data for Modified Atkins diet. Specifically, El-Rashidy et al. (2013)42 reported that 13% (2/15) of patients dropped out due to diet intolerance and had experienced “significant” weight loss. Also, El-Rashidy et al. (2013)42 reported 27% vomiting (4/15), 13% constipation (2/15), 13% diarrhea (2/15), and 20% dysphagia (3/15).
Harms of Hemispherectomy/Hemispherotomy
Eleven pre/post studies50–55,57,58,60,62,71 reported harms after hemispherectomy/hemispherotomy including mortality, hydrocephalus, infection, and other adverse events. One study (Roth et al.62) included data from 19 centers with surgical procedures performed from 1999 to 2020. Seven patients cared for at 3 of 19 centers (University of California at Los Angeles, Cleveland Clinic, and Great Ormond Street Hospital) may have also been previously reported in other studies included in this report.58,61,71 (author correspondence). A second study, Iwasaki et al. (2021)72 described harms for 75 infants, of which 9 hemispherectomy patients had previously been described in Otsuki et al. (2013)50 (author correspondence). Further details are provided in Appendix C.
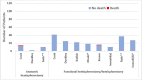
Mortality
Nine pre/post studies reported on mortality after hemispherectomy/hemispherotomy (see Figure 8). Studies described mortality after anatomical hemispherectomy (n=3), functional hemispherectomy or hemispherotomy (n=8), or across multiple procedures (lesionectomy, cortical resection, and hemispherectomy/hemispherotomy, 1 study). Only 3 retrospective chart reviews reported data for infants specifically undergoing anatomical hemispherectomy: Cook et al.58 reported that of 14 infants (operated on from 1986 to 1997 at University of California Los Angeles) there was 1 intra-operative death, an 8 month old infant with HME. Dunkley et al.71 reported no deaths for either of the 2 infants undergoing anatomical hemispherectomy over a 10 year period (year not reported). Roth et al.62 reported no deaths for 10 infants undergoing anatomical hemispherectomy. Given the sparse data, we deemed the evidence insufficient to permit conclusions.
Eight studies described surgical mortality for a combined 196 infants undergoing functional hemispherectomy/hemispherotomy. Seven of 8 studies (including 180 infants)50,52,55,58,62,71,72 reported that there were no deaths; Kumar et al.54 reported a single death of 16 hemispherotomies. The death occurred in an infant with epidermal nevus syndrome, right HME, and multiple other congenital abnormalities who developed refractory seizures after surgery and care was withdrawn.
Finally, Steinbok et al.53 reported a single death across 116 infants undergoing 151 surgical procedures which were either a hemispherectomy/hemispherotomy, lesionectomy, or cortical resections. The intraoperative death occurred in a 3.9-month child with tuberous sclerosis undergoing attempted resection of intraventricular and extraventricular lesions.
Although limited, evidence from these studies suggests peri-operative mortality after functional hemispherectomy or hemispherotomy is uncommon. However, these studies were primarily single center retrospective chart reviews including heterogenous infants (with many different seizure etiologies), and studies often failed to specify what proportion of infants did not meet criteria for inclusion based on missing data. Furthermore, it is possible that centers with higher mortality rates might choose not to publish their data. However, despite these limitations of the evidence base, we concluded that surgical mortality after functional hemispherectomy/hemispherotomy is rare (SOE: Low).
Hydrocephalus and Other Adverse Events
Twelve studies reported other adverse events (AEs) for infants undergoing hemispherectomy/hemispherotomy (see Table 11).
For anatomical hemispherectomy, studies reported that development of hydrocephalus/ventriculoperitoneal shunt placement was common (see Figure 9). Three studies (combined n=19 surgeries) reported this AE: Dunkley et al. reported that both infants (2 of 2) required a VP shunt at 12 months after surgery. Similarly, 7 of 10 infants undergoing anatomical hemispherectomy in another study (Pinto et al.57) required ventriculoperitoneal shunt placement. A third study (Lettori et al.60) reported 3 of 7 infants undergoing anatomical hemispherectomy or hemidecortication developed hydrocephalus (follow-up interval not reported).
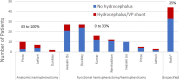
For functional hemispherectomy or hemispherotomy, 9 studies (combined n=196, plus infants from 1 additional study only reporting a percentage52 and 1 study with an unclear denominator53) reported on this AE. Generally, studies reported lower rates of hydrocephalus/shunt placement compared to anatomical hemispherectomy. 1 of 9 studies reported no infants (0 of 10) developed hydrocephalus.55 Another study reported 4 infants undergoing functional hemispherectomy developed hydrocephalus (at least 22 infants underwent functional hemispherotomy, but the total number of infants undergoing this procedure was unclear).53 The remaining 7 studies reported rates of 8.3% (1 of 12),51 11% (3 of 27),71 16% (Kadish et al.52), 20% (1 of 5),57 22% (6 of 27),72 25% (4 of 16),54 and 33% (1 of 3).60
Finally, Roth et al.62 reported hydrocephalus in 25% (11 of 44) infants undergoing either anatomical hemispherectomy or functional hemispherectomy/hemispherotomy. Notably, only 1 study71 reported when hydrocephalus occurred. Another study reported a time range (months).53 Four studies52,55,55,60 did not report when hydrocephalus occurred, and remaining studies provided a timepoint at which other outcomes were measured (eg. >1 year after surgery), but no other information regarding timing of hydrocephalus.
Other AEs reported for anatomical hemispherectomy include:
- Central nervous system (CNS) infection (2/14)58
- Deep infection (1/7)60
- Superficial infection (1/7)60
- Cranial nerve III palsy (1/14)58
- Inappropriate antidiuretic hormone (1/14)58
- Subdural fluid collection (1/7)60
- Cerebrospinal fluid leakage (1/7)60
- Transient fever (2/7)60
Other AEs reported for functional hemispherectomy/hemispherotomy include:
- CNS infection (2/41)58
- Superficial infection (1/3)60
- Dural adhesions requiring “late reoperation” (1/41)58
- Acute post-surgical seizures (23%)52
- Epidural hemorrhage requiring surgical revision (1/22)52
- Excessive bleeding (1/16)54
- Pituitary failure due to thalamic lesion(1/22)52
- Inadvertent extubation (1/16)54
- Intraoperative disseminated intravascular coagulation (1/37)62
- Cyst formation requiring surgical intervention (2/27)72
- Cerebral salt wasting syndrome (2/27)72
- Diabetes insipidus (3/27)72
- Sinus thrombosis (resulting from diabetes insipidus) (2/27)72
- Asymptomatic hemorrhagic infarction (1/27)72
Finally, two studies reported combined AEs for multiple procedure types. One study (Steinbok et al53) reported AEs for procedures including anatomical hemispherectomy, hemidecortication, functional hemispherectomies, and peri-insular hemispherotomies: 31 of 40 infants required blood transfusion, and 13 infants developed perioperative aseptic meningitis which was successfully managed with steroids.
A second study (Roth et al.62) reported AEs for a total 69 procedures which included hemispheric and non-hemispheric surgeries. However, with the exception of one AE (disseminated intravascular coagulation), remaining AEs were not reported by procedure.
Table 11
Surgical studies reporting harms.
Harms of Other Resective Surgical Procedures
Mortality
Four pre/post studies described surgical mortality for infants undergoing non-hemispheric procedures. Three studies62,71,72 described surgical mortality for a combined 82 infants undergoing multilobar, lobar, or focal resections. All 3 studies reported no deaths.
Studies described a range of procedures. Dunkley et al.71 included 15 infants undergoing either multilobar, lobar, or focal resections. Iwasaki et al. 202172 included 48 infants undergoing multilobar or unilobar surgeries. Multilobar procedures included 13 posterior quadrantic disconnections, 5 multifocal cortical resections, 1 subtotal hemispherotomy; unilobar procedures included 16 focal cortical resections or lesionectomies, 8 anterior temporal lobectomies, 5 frontal lobectomies or disconnections. A third study by Roth et al.62 included 19 infants undergoing focal resections. Finally, Steinbok et al.53 reported a single mortality across 116 infants undergoing 151 procedures which were either a hemispherectomy/hemispherotomy, lesionectomy, or cortical resections. (As previously noted, Roth et al. included 1 patient that may have been previously reported in Dunkley et al.71)
This evidence base is small with important limitations. For instance, all studies were retrospective pre/post studies and 2 studies reported experience drawn from only single centers. However, results from Roth et al. (which included data from 19 centers) were consistent in also reporting no deaths. Another consideration is that reported mortality rates may be artificially low if centers with higher mortality rates choose not to publish their data. Nevertheless, despite these limitations, we concluded that surgical mortality after multilobar, lobar, or focal resection is rare (SOE: Low).
Hydrocephalus and Other Adverse Events
Five studies reported on infants undergoing focal, intralobar, or multilobar resections. Four studies (Dunkley et al.,71 Kadish et al.,52 Roth et al.,62 Iwasaki et al.72) with a combined 108 procedures) reported that no patients developed hydrocephalus (follow up duration not reported for 3 studies,52,71,72 and the 4th reported all study outcomes at a median 24 months.62 A fifth study (Steinbok et al.53) reported 3 infants undergoing cortical resection developed hydrocephalus within a few months after surgery, but the total number of infants undergoing non-hemispheric procedures was unclear.
Two studies reported AEs other than hydrocephalus. A single German study (Kalbhenn et al. 2019)64 included 10 infants undergoing posterior disconnection for refractory posterior quadrantic epilepsy. Authors reported a single patient developed transient hemiparesis. A second Japanese study (Iwasaki et al. 202172) reported the following complications requiring surgical or medical intervention in 48 infants undergoing multilobar, unilobar, or focal resections: subdural hygroma (n=3), cyst formation (n=2), asymptomatic cerebral infarction (n=1), bacterial meningitis (n=1), and psychiatric symptoms (n=1).
Strength of Evidence
Our strength of evidence (SOE) ratings for Key Question 3 appear in Table 12. The evidence permits the following conclusions about the harms of pharmacologic treatments:
- Levetiracetam is rarely discontinued due to adverse effects (SOE: Low)
- Topiramate is rarely discontinued due to adverse effects, and severe events are rare (SOE: Low). However, loss of appetite and upper respiratory tract infection are risks (SOE: Moderate)
- Lamotrigine is rarely discontinued due to adverse effects, and severe events are rare (SOE: Low)
Other adverse effects data for pharmacologic treatments were insufficient to permit conclusions, primarily due to risk of bias in pre/post studies, inconsistency, and imprecision.
With regard to surgical interventions, we concluded the following:
- Surgical mortality is rare after functional hemispherectomy/hemispherotomy (SOE: Low)
- Surgical mortality is rare after multilobar, unilobar, or focal resections (SOE: Low).
- Evidence is insufficient to permit conclusions regarding surgical mortality for anatomic hemispherectomy, primarily due to sparse data (SOE: Insufficient)
- Hydrocephalus requiring shunt placement after multilobar, unilobar, or focal resections is uncommon (SOE: Low)
Table 12
Strength of evidence for Key Question 3.
- Results of Literature Searches
- What are the effectiveness and comparative effectiveness of pharmacologic treatments for infantile epilepsies (infants age 1 month to <36 months)?
- What are the effectiveness and comparative effectiveness of non-pharmacologic treatments for infantile epilepsies (e.g., dietary therapies, surgery, neuromodulation, gene therapy), including comparisons to other non-pharmacologic and/or pharmacologic therapies?
- What are the harms or comparative harms of treatments for infantile epilepsies?
- Results - Management of Infantile EpilepsiesResults - Management of Infantile Epilepsies
- Evidence Tables - Treatment for Restless Legs SyndromeEvidence Tables - Treatment for Restless Legs Syndrome
- Treatments for Ankyloglossia and Ankyloglossia With Concomitant Lip-TieTreatments for Ankyloglossia and Ankyloglossia With Concomitant Lip-Tie
- Radiation Therapy for Brain MetastasesRadiation Therapy for Brain Metastases
Your browsing activity is empty.
Activity recording is turned off.
See more...