NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Balk EM, Konnyu KJ, Cao W, et al. Schedule of Visits and Televisits for Routine Antenatal Care: A Systematic Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2022 Jun. (Comparative Effectiveness Review, No. 257.)

Schedule of Visits and Televisits for Routine Antenatal Care: A Systematic Review [Internet].
Show detailsReview Approach
The Brown Evidence-based Practice Center (EPC) conducted this systematic review (SR) based on the Agency for Healthcare Research and Quality (AHRQ) Methods Guide for Effectiveness and Comparative Effectiveness Reviews (available at https://effectivehealthcare.ahrq.gov/topics/cer-methods-guide/overview). This SR also reports in accordance with the Preferred Items for Reporting in Systematic Reviews and Meta-Analyses (PRISMA),20 A Measurement Tool to Assess Systematic Reviews (AMSTAR 2),21 and relevant extension statements.
A more detailed version of the SR methodology used can be found in Appendix A.
The topic of this report and preliminary Key Questions (KQs) arose through a process soliciting input from the nominators (the American College of Obstetricians and Gynecologists [ACOG] and the Society for Maternal-Fetal Medicine [SMFM]), a panel of Key Informants (KIs), a Technical Expert Panel (TEP), the public, and AHRQ. The KIs and TEP represented ACOG, SMFM, Federal policymaking agencies (the Office of Women’s Health), research and practicing obstetricians, family medicine, obstetric nursing, midwives, doulas, pediatrics/neonatology, obstetrics/gynecology healthcare administration, disparities research, qualitative research, women’s health outcomes research, and patients. Initially, the KI panel gave input on the KQs, including the outcomes, to be examined. Based on discussions with the KIs and TEP, the EPC determined a list of prioritized outcomes. AHRQ then posted these KQs and solicited public comment through its Effective Health Care (EHC) Program website and on the Federal Register. No comments were received. The TEP provided high-level content and methodological expertise throughout development of the review protocol. The final protocol was posted on the EHC website at effectivehealthcare.ahrq.gov/products/schedule-visits-antenatal-care/protocol on August 6, 2021. We submitted the protocol for registration in PROSPERO in August 2021. On September 7, 2021, PROSPERO published the protocol with registration number CRD42021272287.
Key Questions (KQs)
- KQ 1.
What are the benefits and harms of different antenatal care schedules that vary by number or timing of visits for pregnancies requiring routine care and monitoring?
- KQ 2.
What are the benefits and harms of televisits for providing routine antenatal care during pregnancy?
- KQ 3.
What are patient, partner/family, and provider perspectives, preferences, and experiences related to (a) antenatal care visit schedules and (b) use of televisits for routine antenatal care?
Analytic Framework
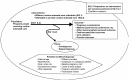
Based on discussions with KIs and TEP, we developed an analytic framework (Figure 1). The framework graphically lays out the populations, interventions, outcomes, and modifiers that pertain to each KQ.
Study Selection
Literature searches were conducted in Medline® (via PubMed®), the Cochrane Register of Clinical Trials, the Cochrane Database of Systematic Reviews, Embase®, CINAHL®, and ClinicalTrials.gov, from inception through February 12, 2022, without language restriction. Additional literature searches were conducted for qualitative studies in PsycINFO® and SocINDEX using the same set of concepts included in the search for quantitative studies, without study type filters. We also performed citation tracing and other snowballing techniques based on all relevant studies to identify studies that may have been missed in the database searches.22
For both KQ 1 (scheduled visits) and KQ 2 (televisits), we included comparative studies (both randomized and observational) of pregnant individuals receiving routine antenatal care. We did not require that study participants had low-risk pregnancies, but did require that the antenatal visits under investigation were for routine care. Studies were restricted to high-income countries, without language restriction. Appendix A (Inclusion/Exclusion Criteria Details) includes more detailed eligibility criteria.
For KQ 1, studies had to compare different strategies that scheduled fewer versus more routine antenatal care visits. We excluded studies that compared different providers or added group or home visits without changing the number of scheduled routine visits.
For KQ 2, studies had to compare use of televisits for routine antenatal care versus all in-person visits (or alternative approaches to televisits).
For both KQs, studies had to report at least one outcome listed in Appendix A pertaining to pregnancy complications; other maternal health outcomes; maternal psychosocial, preference, and related outcomes; fetal, neonatal, and infant outcomes; healthcare utilization; provider satisfaction; and harms. For KQ 2, we also included access to telemedicine and home monitoring equipment.
For both KQs, prioritized outcomes included:
- Maternal quality of life
- Maternal mental health measures or diagnosis (e.g., anxiety, depression)
- Patient satisfaction with antenatal care
- Lost work time
- Delivery timing (preterm birth, full-term delivery, gestational age at birth)
- Small for gestational age/low birth weight
- Apgar scare
- Breastfeeding
- Completion of ACOG recommended services
- Number of unplanned visits
- Neonatal intensive care unit admissions
- Delayed diagnoses (e.g., gestational diabetes)
For KQ 3, we included qualitative research studies that examined perspectives and/or preferences about the number of scheduled routine antenatal visits or use of televisits for routine antenatal care. Study participants could include pregnant or postpartum individuals or those considering pregnancy, partners and family members, and any providers of antenatal care. The qualitative studies had to conduct focus groups, interviews, ethnography, or surveys with open-ended questions. We excluded studies of surveys with only closed-ended questions that did not provide data amenable to qualitative analyses.
Risk of Bias Assessment
We evaluated each quantitative study (KQs 1 and 2) for risk of bias and methodological quality. For randomized controlled trials (RCTs), we used the items from the Cochrane Risk of Bias tool 2.0.23 For nonrandomized comparative studies (NRCSs), we used specific elements from the ROBINS-I Tool (Risk Of Bias In Non-randomized Studies – of Interventions)24 related to confounding and selection bias in addition to items from the Cochrane Risk of Bias tool that were not specific to randomized trials.
We evaluated each qualitative study (KQ 3) for risk to rigor using the Critical Appraisal Skills Programme (CASP) appraisal tool for qualitative studies, which addresses issues related to clear qualitative research aims; congruence between the research aims and methodological approach; sampling and data collection; appropriate application of methods; richness/conceptual depth of findings; appropriate interrogation of findings; and reflexivity of the researchers.25–27
Data Synthesis and Analysis
Within the main report, data are summarized either (1) in succinct tables that focus on outcome, interventions, and comparative (when applicable) results or (2) in forest plots or succinct summary tables (for most topics). When feasible and appropriate, we conducted random effects model pairwise meta-analyses. For the qualitative synthesis, we categorized extracted data (all relevant primary data from participants and secondary data from study authors) into one of 14 domains defined by the Theoretical Domains Framework (TDF), with a Best Fit Framework approach.28–30 The TDF is a well-established tool for characterizing the cognitive, affective, social, and environmental factors that may influence ones adoption of a behavior.28, 29 It is commonly used to diagnose the determinants of behavior in implementation problems (e.g., adopting a new guideline-based practice, technology, policy).31 We selected the TDF as it was recommended by a member of the TEP with qualitative expertise, was familiar to members of the review team, and was a suitably comprehensive framework to guide our planned Best Fit Framework approach. Details are provided in Appendix A. Appendix B lists the excluded studies. Appendix C contains detailed tables that describe study and participant characteristics, intervention (and comparator) details, outcomes (and definitions), and arm- and comparison-level results. Appendix C also includes tables providing study-level risk of bias or rigor assessments. Appendix D contains more detailed, study-level results for each topic.
Grading the Strength of the Body of Evidence
For KQs 1 and 2, we evaluated the strength of evidence (SoE) addressing each major analysis for each KQ. We graded the SoE as per the AHRQ Methods Guide.32, 33 We did not grade SoE for the qualitative research but instead, we assessed the confidence for the summary of qualitative findings using the GRADE-CERQual (Grading of Recommendations Assessment, Development and Evaluation - Confidence in Evidence from Reviews of Qualitative research) tool.25, 34 For each SoE assessment, we evaluated the evidence base for each prioritized outcome, accounting for the number of studies (both RCTs and NRCSs) and study participants, the study limitations (accounting for both study design and risk of bias), the directness of the evidence to the KQs, the consistency of study results, the precision of any estimates of effect, and other limitations (particularly sparseness of evidence, including rare events). Based on these assessments, we assigned a SoE rating as being either high, moderate, low, or insufficient to estimate an effect. Outcomes with highly imprecise estimates (e.g., 95% confidence interval extends beyond both 0.50 and 2.0), inconsistent findings across studies (that do not allow for an overall conclusion), or with data from only one study (unless it was a high quality, broadly generalizable, and adequately powered trial) were deemed to have insufficient evidence to allow a conclusion. For each qualitative finding, we assessed the methodological limitations of the body of data contributing to the finding, and the findings’ coherence (in addressing the complexity and variation of the data),35 adequacy (defined by richness and quantity of data addressing a finding),36 and relevance (to the context specified in the review question).37
- Methods - Schedule of Visits and Televisits for Routine Antenatal Care: A System...Methods - Schedule of Visits and Televisits for Routine Antenatal Care: A Systematic Review
- solute carrier family 35 member D3 isoform X1 [Mus musculus]solute carrier family 35 member D3 isoform X1 [Mus musculus]gi|568965788|ref|XP_006512979.1|Protein
- Management of Suspected Opioid Overdose With Naloxone by Emergency Medical Servi...Management of Suspected Opioid Overdose With Naloxone by Emergency Medical Services Personnel
- Diagnostic Errors in the Emergency Department: A Systematic ReviewDiagnostic Errors in the Emergency Department: A Systematic Review
- Acute Treatments for Episodic MigraineAcute Treatments for Episodic Migraine
Your browsing activity is empty.
Activity recording is turned off.
See more...