NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Butler M, Bliss D, Drekonja D, et al. Effectiveness of Early Diagnosis, Prevention, and Treatment of Clostridium difficile Infection [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 Dec. (Comparative Effectiveness Reviews, No. 31.)
This publication is provided for historical reference only and the information may be out of date.

Effectiveness of Early Diagnosis, Prevention, and Treatment of Clostridium difficile Infection [Internet].
Show detailsBackground
Clostridium difficile infection (CDI) is a serious healthcare-associated infection and a growing health care problem. C. difficile is a Gram-positive, spore-forming, anaerobic bacterium that, when ingested, can cause CDI if it is a toxigenic strain. CDI symptoms include varying levels of diarrhea severity, as well as pseudomembranous colitis and toxic megacolon. CDI incidence is estimated at 6.5 cases per 10,000 patient days in hospital.1 About 250,000 hospitalizations were associated with CDI in 2005.2 Direct attributable mortality from CDI has been reported to be as high as 6.9 percent of cases.3 Elderly people in hospitals account for the vast majority of severe morbidity and mortality.4-6 Residents of long-term care facilities are also at higher risk.7,8 Incidence rates may increase by fourfold or fivefold during outbreaks.9 In addition to institutional care environments, C. difficile is also common in the community, being easily isolated from soil and water samples.10 Community-associated CDI rates are generally much lower, accounting for 27 percent of all CDI cases in a recent prevalence study,9 but are also on the rise.11 However, the source of the C. difficile organisms responsible for cases of CDI in the community is not well understood.
In order for CDI to develop, a person must be infected with a strain of C. difficile capable of making toxin in the person's colon (Figure 1). Toxigenic strains include those that make toxin B (cytotoxin), with or without toxin A (enterotoxin). Approximately 1 to 2 percent of healthy individuals are colonized with C. difficile.12 If these people have usual, healthy colonic flora, the risk of CDI is very low. There is a small risk of CDI if the colon flora becomes disturbed, commonly through antibiotic use, while the person is colonized with a toxigenic strain. Antibiotics that disturb colon flora enough to allow CDI to develop must get into the colon, and they are associated with alterations in relative amounts of colon bacterial constituents.13,14 The immune status of the patient also contributes to the risk of developing CDI and the experienced severity.15 Other risk factors include increasing age, female gender, comorbidities, gastrointestinal procedures, and use of gastric acid suppression medications.16-25 Risk profiles for recurrent CDI are similar.21 One study, which statistically modeled CDI within the hospital setting, suggested that reducing patient susceptibility to infection is more effective in reducing CDI cases than lowering transmission rates.26

Figure 1
Pathogenesis of CDI. CDI = Clostridium difficile infection
New, more virulent strains have emerged since 2000. Characteristics associated with hypervirulent strains can include increased toxin production (due to a deletion in a toxin regulatory gene), an additional binary toxin, whose role in disease etiology is not well understood, hypersporulation, and high-level resistance to fluoroquinolone antibiotics.27 These new strains affect a wider population, often people with a lack of established risk factors for CDI based on older strains, such as previous hospitalization or antibiotic use, and include children, pregnant women, and other healthy adults.28 With hypervirulent strains, the time from symptom development to septic shock may be reduced, making quick diagnosis and proactive treatment regimens critical for positive outcomes.
The highly virulent strain associated with the epidemic of CDI described in the early 2000s may be decreasing in prevalence in limited locations.29 Recent analysis of an archived collection of C. difficile isolates revealed that predominant strains shifted from year to year among a population served at a single institution,30 suggesting that this strain shift may occur on a larger scale. However, this phenomenon potentially cuts both ways as strains drift toward lesser or higher virulence, and the possible future risks and costs of CDI remain significant.
Diagnosis
Effective prevention of transmission and treatment of CDI depends on swift and accurate diagnosis. None of the risk factors or clinical signs and symptoms alone or in combination, except possibly a documented presence of pseudo membranous colitis, is sufficient to surmise with a high degree of clinical certainty that a patient does or does not have CDI. Culturing C. difficile organisms in stool specimens followed by testing grown colonies for toxins (toxigenic culture) and cultured cell cytotoxicity assay of the stool specimens are historically held as the standard reference tests; however, results can take up to 48 hours, and these diagnostic methods require a level of expertise and equipment that are not widely available. A number of faster, less demanding diagnostic tests have been developed to detect the presence of toxins produced by most disease-causing C. difficile organisms, toxins A and/or B, or the genes involved in the production or regulation of toxins A and/or B. These tests have a variety of sensitivities, specificities, biotechnologies, costs, and time-to-results. The sensitivities and specificities of the newer tests have been studied mostly using toxigenic culture or a cultured cell cytotoxicity assay as the reference test, but the estimates vary substantially, making it difficult to determine whether there are clinically significant differences between tests.98,99 Some of the variation is due to differences in the accuracy of the reference tests that are not 100 percent sensitive or specific. Toxigenic culture can be more sensitive than cytotoxicity assays that can be more specific. When a new test is evaluated using a more sensitive reference test, the estimate of its sensitivity may be lower. Greater than 90 percent of labs in the United States use one of the commercially available immunoassays to detect toxins in stool samples or because they are fast, inexpensive, and technically easier to perform.108 However, the use of toxin gene detection tests has increased in recent years. A more detailed discussion of types of diagnostic tests for C. difficile is provided in a supplemental section at the end of this chapter.
When evaluating laboratory tests for the presence of toxigenic C. difficile in patients, it is important to consider how patients were selected and the consistency of the stool specimens being tested. Testing for C. difficile infection is recommended for a person with diarrhea (generally three or more loose or unformed stools for 1 to 2 days) and one or more risk factors for CDI.109,110 However, these recommendations may not always be followed in practice. Several multivariable prediction models built on established risk factors have been published in an effort to optimize diagnostic testing for C. difficile infection.111-113 The extent of their use in clinical practice is not known.
Identifying the most accurate diagnostics tests in clinical practice could be very important. Diagnostic tests with greater sensitivity (fewer false negatives) would reduce the number of patients who do not receive appropriate treatment and isolation. Tests with higher specificity (fewer false positives) could reduce the number of unnecessary and potentially detrimental interventions, such as withholding antibiotics for other medical conditions, or initiating treatment for CDI. Swift diagnosis leading to infection prevention precautions, faster treatment, and quicker resolution of diarrhea may reduce the amount of organisms or spores in the environment that can infect other patients.
Treatment
There are a number of algorithms available to guide treatment of CDI.11,114-116 The only antimicrobial currently approved for the treatment of CDI by the U.S. Food and Drug Administration is oral vancomycin, and consensus appears to exist for treatment of severe initial incident CDI with vancomycin. However, there also appears to be clinical consensus to treat mild to moderate CDI with metronidazole, in part because of the concern that overuse of vancomycin may contribute to increasing pathogen resistance117 and cost considerations. Pepin116 suggests that both vancomycin and metronidazole are implicated in increased frequency of vancomycin-resistant enterococci (VRE). Enterococci are part of the normal gastrointestinal (GI) flora, and VRE are a major problem. Whether the increased use of vancomycin for CDI will affect the rates of VRE is unclear, especially as increased density of VRE in stool has been demonstrated in subjects receiving antimicrobials active against anaerobes (the main colonic flora), including both oral vancomycin and metronidazole.118 Surgical treatment with colectomy can be life saving in patients with fulminant, or acute severe, colitis.119
Nonstandard interventions for the treatment and prevention of CDI have been sought for several reasons. Treatment with standard antibiotics, such as vancomycin and metronidazole, is ineffective in 8 to 36 percent of patients with CDI,72,73 no antibiotic kills C. difficile spores, and rates of infection are increasing. Treatment for relapsed or recurrent CDI is much more problematic. CDI recurs in about 20 percent of patients;114 a subset of recurrent patients spiral into several subsequent recurrences.120 Clinicians have chosen from a number of antibiotics and dosing protocols and adjunctive treatments, such as the use of antimicrobials, probiotics, fecal transplant, toxin-binding agents, and immune system-enhancing agents.121-123
Probiotics are a very active area of discussion for CDI.124 Probiotics are live microorganisms, including bacteria or yeast, which, when administered in adequate amounts, confer a health benefit on the host.125 Probiotics are believed to replenish nonpathogenic microorganisms to GI flora that has become altered by antibiotic therapy. It is important that the effectiveness of probiotics and related substances are evaluated specifically for their effect on CDI and not rely on the more broadly defined antibiotic-associated disease, which includes a much broader set of potential disease etiology. Fecal flora reconstitution is another intervention currently under investigation. This approach instills donor feces into the patient with CDI to normalize the intestinal flora. The procedure has been variously termed in the literature, including fecal bacteriotherapy,96,124,126,127 fecal transplantation,127-129 and donated stool.97,130
Prevention
Prevention of CDI takes two general forms, breaking routes of transmission and improving a patient's resistance to disease should colonization occur. Preventing the spread of C. difficile by breaking routes of transmission within institutional settings depends on staff compliance with national guidelines and standards131 and locally determined hygiene protocols. C. difficile is common in the environment of people with CDI,101 most of whom have diarrhea, and many of whom have incontinence and often other medical problems that tend to diminish personal hygiene. C. difficile is found on the hands of hospital workers46,101 and is more likely to be found on hands of people who have been working in a heavily contaminated room.101 Thus, C. difficile acquired in hospital settings may be spread directly or indirectly from patient to patient.103
Complicated recommendations are difficult to remember and implement, and protocols for different targeted hospital acquired infections are not always congruent. For example, the availability of alcohol hand rubs improved physician compliance and reduced methicillin-resistant Staphylococcus aureus (MRSA) infections,51 yet C. difficile produces spores that can withstand hostile environments and are resistant to alcohol hand rubs and other routine antiseptics.132 One concern has been that health care workers will use alcohol-based rubs or gels in circumstances where handwashing is preferred. Other institutional prevention strategies may be required as C. difficile transmission knowledge develops. For example, a recent study isolated C. difficile spores from air samples in a hospital in the United Kingdom 4 to 7 weeks after the last confirmed CDI case in the ward, and successfully cultured bacterium from the spores.133
Interventions to improve a patient's resistance to CDI or CDI recurrence include probiotics, a nonpathogenic strain of C. difficile, prebiotics, immune whey, C. difficile vaccine, and intravenous immunoglobulin. Probiotics, a nonpathogenic strain of C. difficile, and prebiotics aim to modify the patient's intestinal microbioecology to better resist CDI. Probiotics and a nonpathogenic strain of C. difficile deliver nonpathogenic microorganisms thought to compete with or inhibit C. difficile, while prebiotics aim to promote the growth of beneficial organisms. Immune whey, a C. difficile vaccine, and intravenous immunoglobulin confer passive immunity against C. difficile or its toxin.
Scope of the Review
The purpose of this systematic review was to provide an overarching assessment of the evidence for comparing the accuracy of diagnostic tests and the effectiveness of prevention and treatment interventions on initial and recurrent CDI related patient outcomes in adult patients. This purpose was developed during the project's topic refinement stage. There was consensus among key informants that this systematic review's single greatest contribution to the field could be to provide a comprehensive review by an independent organization that covered the major concerns of the field. CDI is an active topic in the literature as well as a vital clinical concern. The consensus opinion included the idea that clinicians and researchers both would be well served by a reaffirmation of what is and is not supported by evidence in the literature and at what level of evidence, to balance against this activity level.
The major impetus of this review is the presence of clinical disease, not asymptomatic carriage of the C. difficile organism. While we were interested in how treatment of CDI varies by organism strain, molecular epidemiology studies whose main purpose was to identify the strains of C. difficile present in the population are also outside the scope of this review. The review focuses on adult patients because adults, and particularly elderly adults, carry the large majority of the morbidity and mortality burden.
Key Questions
The following key questions form the basis for this review:
- Key Question 1. How do different methods for detection of toxigenic C. difficile to assist with diagnosis of CDI compare in their sensitivity and specificity?
- Do the differences in performance measures vary with sample characteristics?
- Key Question 2. What are effective prevention strategies?
- What is the effectiveness of current prevention strategies?
- What are the harms associated with prevention strategies?
- How sustainable are prevention practices in health care (outpatient, hospital inpatient, extended care) and community settings?
- Key Question 3. What are the comparative effectiveness and harms of different antibiotic treatments?
- Does effectiveness vary by disease severity or strain?
- Does effectiveness vary by patient characteristics: age, gender, comorbidity, hospital versus community-acquired setting?
- How do prevention and treatment of CDI affect resistance of other pathogens?
- Key Question 4. What are the effectiveness and harms of nonstandard adjunctive interventions?
- In patients with relapse/recurrent CDI?
Review Framework
The conceptual framework that guided this review is provided in Figure 2. The figure lays out the clinical path for patients with the potential to develop CDI, from diagnostic laboratory tests, through their impact on treatment decisions, to finally implications for prevention strategies, and locates the key questions of this review within the context of the framework. Diagnostic testing has two parts, the technical efficiency of the tests and diagnostic accuracy. Technical efficiency is outside the scope of this review; rather, for Key Question 1 we focus on the comparative diagnostic accuracy of commonly used rapid tests, such as immunoassays for C. difficile toxin and toxin gene detection tests, which may reduce the time lapse between the onset of symptoms and laboratory confirmation of CDI and treatment decisions. Repeat testing of selected specimens does not provide good comparative information about test accuracy and therefore is not covered in the focused review of diagnostic test accuracy. When a patient is treated for CDI, whether for an initial case, a relapse, or recurrence, the clinical outcomes of interest establish the patient treatment efficacy. Of particular interest, Key Question 3 will compare effectiveness of established treatments used for CDI, particularly vancomycin and metronidazole. For Key Question 4, the clinical question of interest is what nonstandard treatments are being utilized, and their efficacy, particularly for recurrent CDI. After diagnostic accuracy, treatment, and patient outcome efficacy concerns, prevention is a societal-level efficacy measure, as the benefits of prevention of infectious disease can extend beyond the individual patient. This is the area of focus for Key Question 2. Key Question 4 also contributes to this area to the extent that nonstandard treatments assist a patient in fending off an infection.
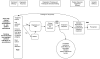
Figure 2
Analytic framework for CDI diagnostic testing, prevention, and treatment. CDI = Clostridium difficile infection; KQ = Key Question
Figure 3 expands the framework for the key question related to prevention. The illustration lays the pathway of preventive strategies and practices from the target patient population of patients at risk for CDI due to potential for exposure, through intermediate outcomes and on to health outcomes. This framework was included to highlight both the linkage and the conceptual difference between the intermediate outcomes of prevention and health outcomes of clinical significance important to the patient. Intermediate outcomes are often process measures of the uptake of a prevention strategy, or counts of vegetative C. difficile or spores remaining in the environment. Key Question 2 is mainly concerned with evidence for the direct effect of prevention on health outcomes.
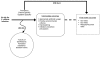
Figure 3
Supplemental prevention framework. CDI = Clostridium difficile infection; KQ = Key Question
Diagnostic Test Descriptions
Cytotoxicity Assay
The cultured cell cytotoxicity assay often has been used as a reference test for evaluating new diagnostic tests for toxigenic C. difficile. Briefly, a diluted and filtered aliquot of a stool sample is mixed with cultured test cells. The test cells are examined for toxin effects (cell rounding) that are not seen in comparator test cells where an excess amount of antitoxin is present.134 The diagnostic rounding of cultured test cells and the clinical signs and symptoms of CDI can be caused by cellular interactions with both C. difficile toxins, although toxin B is much more cytotoxic and the cytotoxicity assay is often considered to be a test for toxin B.135,136 A cytotoxicity assay requires up to 48 hours for the toxin effects to appear, especially when toxin level in the test material is low. Cytotoxicity testing is not a perfectly accurate gold standard.137 Methodological differences in the time to process and dilution of stool samples, the age and type of cultured test cells being used for the test, the antitoxins, and the interpretation of results all can cause cytotoxicity assay results to vary.138 Toxins can degrade or be inactivated depending on how long stool specimens are stored before being tested and the storage temperature. Nevertheless, the imperfect cytotoxicity assay is often used as the reference test in the evaluation of other diagnostic tests for C. difficile.
Detection of C. difficile Organisms
Culturing C. difficile by anaerobic incubation of fecal aliquots on selective cycloserine-cefoxitin, fructose agar or other media can be more sensitive than the cytotoxicity assay for detecting the presence of C. difficile organisms.138,139 However, C. difficile culture techniques also are not standardized, are susceptible to methodological variation, and require expertise, equipment, and several days to complete. Furthermore, cultured C. difficile organisms need to be tested to determine whether they can produce disease-causing toxins because many individuals may be carriers of C. difficile organisms that do not produce toxins or clinically significant CDI. Nevertheless, expert culture of C. difficile from stool samples followed by a cytotoxicity assay or another method of detecting toxins is considered the most sensitive method for detection of toxigenic C. difficile, albeit not very practical.110,140 However, the concentration of toxins produced in culture might not be the same as that present in patients.
Assays for glutamate dehydrogenase enzyme constitutively produced by C. difficile have been used as a faster and less demanding alternative to culturing C. difficile organisms. These tests are not entirely specific because other organisms can produce glutamate dehydrogenase or interfering substances.104,138 Like stool cultures, a positive glutamate dehydrogenase test requires a second test to detect C. difficile toxins. Because stool cultures and the cytotoxicity assay are demanding, costly, and time consuming, and most stool samples sent to clinical laboratories turn out to be negative for toxigenic C. difficile, some laboratories have proposed using a test for glutamate dehydrogenase first, and then testing only the positive specimens for toxins.141-143 In this two-stage approach, a negative test for glutamate dehydrogenase would preclude the need for a toxin test. However, the sensitivities of glutamate dehydrogenase assays need to be high enough to have an acceptably low number of false negatives.109 Furthermore, the performance of a two-stage test also will depend on the sensitivity and specificity of the second test used to detect toxins.
Immunoassays for Toxins
A variety of faster (within a few hours), less costly commercial immunoassays for C. difficile toxins have been developed and have been commercially available since the late 1980s. Initially, most immunoassays detected only toxin A. More recently it was discovered that a small but increasing number of clinically significant C. difficile strains produced only toxin B.144-147 The incidence of clinically significant toxin A-negative, B-positive organisms in the United States is not known and could vary by site and time.148 When the performance of a diagnostic test depends on the level of toxins in test specimens and most organisms produce both toxins A and B, immunoassays that detect both toxins might be more sensitive if other critical factors such as dilution of the specimens are equal.149 Therefore, experts have recommended using immunoassays that can detect both toxins A and B.109,110,137,148 A highly sensitive and specific immunoassay for these toxins may be used as a second test after either stool culture or the glutamate dehydrogenase assay.
Data from the College of American Pathology proficiency testing program for C. difficile toxin detection indicated that 90 percent of labs used an immunoassay for toxins A and B in June 2009. The most commonly used tests were the Immunocard and Premier A & B test kits manufactured by Meridian, the TechLab Tox AB II and Toxin A/B QUIK CHEK kits, and the Remel ProSpecT and Xpect Toxin A/B tests. These data are consistent with an online survey of members of the Association for Professionals in Infection Control and Epidemiology, Inc. in 2008 that indicated that an immunoassay was used in 95 percent of patients who were diagnosed with CDI in 648 responding American laboratories, and 60 percent were diagnosed using an immunoassay for toxins A and B, while only 3 percent used an immunoassay for only toxin A.9
Toxin Gene Detection Tests
Three tests of stool specimens for the presence of genes involved in the production of C. difficile toxins have recently become commercially available. These tests use the polymerase chain reaction to amplify (replicate) targeted gene fragments to detect the presence of a gene or genes involved in the production of toxins, not the actual toxins. The target of the assays can be the genes that produce toxin B and a gene C that negatively regulates the production of toxins A and B. A mutation in gene C has been detected in an increasingly common hypervirulent strain of C. difficile that produces large amounts of toxins A and B.148 One concern about using the tests based on amplification of toxin gene fragments is that very small, clinically unimportant genetic residue or specimen contamination may be detected. Clinically speaking, these would be false positives that would reduce test specificity. Therefore, some experts have recommended using this type of test only when a patient has clinical signs and symptoms suggestive of CDI.109,138
- Introduction - Effectiveness of Early Diagnosis, Prevention, and Treatment of Cl...Introduction - Effectiveness of Early Diagnosis, Prevention, and Treatment of Clostridium difficile Infection
- Evidence Summary - The Role of Immunotherapy in the Treatment of AsthmaEvidence Summary - The Role of Immunotherapy in the Treatment of Asthma
- small conductance calcium-activated potassium channel protein 2 isoform X2 [Corv...small conductance calcium-activated potassium channel protein 2 isoform X2 [Corvus hawaiiensis]gi|2240567238|ref|XP_048147381.1|Protein
- Peer Reviewers - Management of Colonic DiverticulitisPeer Reviewers - Management of Colonic Diverticulitis
- Methods - PCA3 Testing for the Diagnosis and Management of Prostate CancerMethods - PCA3 Testing for the Diagnosis and Management of Prostate Cancer
Your browsing activity is empty.
Activity recording is turned off.
See more...
