NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Crandall CJ, Newberry SJ, Diamant A, et al. Treatment To Prevent Fractures in Men and Women With Low Bone Density or Osteoporosis: Update of a 2007 Report [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Mar. (Comparative Effectiveness Reviews, No. 53.)
This publication is provided for historical reference only and the information may be out of date.

Treatment To Prevent Fractures in Men and Women With Low Bone Density or Osteoporosis: Update of a 2007 Report [Internet].
Show detailsBackground
Osteoporosis is a systemic skeletal disease characterized by decreasing bone mass and microarchitectural deterioration of bone tissue, with consequent increases in bone fragility and susceptibility to fracture.1 In addition to fractures, the clinical complications of osteoporosis include disability and chronic pain. Approximately 52 million people in the United States are affected by osteoporosis or low bone density. It is especially common in postmenopausal women,2 but one in five men will experience an osteoporosis-related fracture at some point in his lifetime.3
The economic burden of osteoporosis is large and growing: the most recent estimate of US annual costs due to fractures alone have been nearly $20 billion.2A recent projection of the burden and costs of incident osteoporosis-related fractures in the United States from 2005 to 2025 estimates more than 2 million fractures in 2010 with direct medical costs of more than $18 billion (more than 25 percent attributable to men).4 Although the bulk of these costs are incurred by individuals 65 and older, direct costs and productivity loss among working women under 65 are considerable.2
Diagnosis and Risk Factors
The clinical diagnosis of osteoporosis may be based on results of bone mineral density (BMD) testing3,5,6 BMD is measured with dual energy x-ray absorptiometry (DXA). In postmenopausal women and men over 50 years, BMD is classified according to the T-score. The T-score is the number of standard deviations above or below the mean for healthy 20–29 year old adultsa, as determined by DXA. Osteoporosis is defined as a T-score of -2.5 or less. A T-score between -2.5 and -1.0 is defined as “low bone density.” A T-score of -1 or greater is considered normal. Bone density can also be classified according to the Z-score, the number of standard deviations above or below the expected BMD for the patient's age and sex. A Z-score of -2.0 or lower is defined as either “low bone mineral density for chronological age” or “below the expected range for age,” and those above -2.0 are “within the expected range for age.” Individuals who have already had minimal trauma fracture are at increased risk of future osteoporotic fracture, independent of BMD.3 Because the majority of fractures occur in patients with low bone mass rather than osteoporosis,3 risk scores that combine clinical risk factors with BMD testing results, such as FRAX, have recently been developed to refine the ability to predict fracture risk among people with low bone density.
Risk factors for osteoporotic fracture include (but are not limited to) increasing age, female sex, postmenopause for women, hypogonadism or premature ovarian failure, low body weight, history of parental hip fracture, ethnic background (whites are at higher risk than blacks), previous clinical or morphometric vertebral fracture, previous fracture due to minimal trauma (i.e. previous osteoporotic fracture), rheumatoid arthritis, current smoking, alcohol intake (3 or more drinks/day), low BMD, vitamin D deficiency, low calcium intake, hyperkyphosis, falling, and immobilization, along with chronic use of certain medications, the most commonly implicated being glucocorticoids, anticoagulants, anticonvulsants, aromatase inhibitors, cancer chemotherapeutic drugs, and gonadatropin-releasing hormone agonists.3
Several algorithms have been devised and validated for the prediction of osteoporotic fracture risk. Current National Osteoporosis Foundation guidelines as well as others endorse the use of the FRAX to select candidates for treatment.7-9 The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women.9,10 FRAX is a set of race- and nationality-specific algorithms that take into account an individual's age, sex, weight, height, previous fracture, parental history of osteoporotic fracture, smoking status, alcohol use, history of use of glucocorticoids, history of rheumatoid arthritis, secondary causes of osteoporosis, and femoral neck BMD to estimate the absolute 10-year risk of major osteoporotic fractures (i.e. clinical vertebral, hip, forearm, or proximal humerus fractures). Risk for osteoporosis may be viewed as a continuum that depends on all of these factors. A question of considerable interest is whether antifracture response to treatment is affected by (or predicted by) FRAX score.3,11
Therapy
The most recent National Osteoporosis Foundation Clinician's Guide recommends considering therapy for postmenopausal women and men age 50 and older presenting with the following: a hip or vertebral (clinical or morphometric) fracture; T-score ≤ -2.5 at the femoral neck or spine after appropriate evaluation to exclude secondary causes; Low bone mass (T-score between -1.0 and -2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture ≥ 3 percent or a 10-year probability of a major osteoporosis-related fracture ≥ 20% based on the US-adapted WHO algorithm3.
The increasing prevalence and cost of osteoporosis have heightened interest in the effectiveness and safety of the many interventions currently available to prevent osteoporotic fracture. These interventions include pharmacologic agents, a biological agent, dietary and supplemental vitamin D and calcium, and weight-bearing exercise.
Pharmacologic agents include the bisphosphonate class of drugs, peptide hormones (parathyroid hormone and calcitonin), estrogen (in the form of menopausal hormone therapyb) for postmenopausal women, and selective estrogen receptor modulators (raloxifene for postmenopausal women). With the exception of parathyroid hormone (teriparatide), each of these agents acts to prevent bone resorption: Once-daily administration of teriparatide stimulates new bone formation on trabecular and cortical periosteal and/or endosteal bone surfaces by preferential stimulation of osteoblastic activity over osteoclastic activity. The bisphosphonates, are compounds that bind reversibly to mineralized bone surfaces and disrupt resorption by the osteoclasts. The original bisphosphonates, etidronate and clodronate, were short-chain molecules that inhibited bone resorption by disrupting the oxidative phosphorylation pathway. The second generation, which includes alendronate and pamidronate, and the third generation, which includes risedronate and zoledronic acid, contain an amino group; these molecules inhibit bone resorption by inhibiting fatty acid; they may be associated with fewer adverse effects than the first generation. A newer therapeutic agent, denosumab, was approved by the FDA in June 2010. Denosumab is a monoclonal antibody that inhibits the Receptor Activator of Nuclear factor Kappa-B Ligand (RANKL), a stimulator of osteoclast differentiation and activation. By inhibiting osteoclast formation, function, and survival, denosumab decreases bone resorption. Although denosumab is classified by the FDA as a biological, it will be considered a pharmacological agent for the purposes of this report.
Besides pharmacologic agents, dietary and supplemental calcium and vitamin D, as well as weight bearing exercise, play important roles in preserving bone mass. Lifelong calcium intake is required for the acquisition of peak bone mass and for the subsequent maintenance of bone health.3 When serum calcium levels are inadequate, bone tissue is resorbed from the skeleton to maintain serum calcium at a constant level. Adequate vitamin D levels play a key role in calcium absorption, bone health, muscle performance, balance, and fall prevention.3
The various agents used to prevent and treat osteoporosis have been linked with adverse effects, from the more common, mild effects (such as minor gastrointestinal complaints) to potentially serious issues. Some evidence suggests that these minor complaints, coupled with concerns about more serious effects, may affect the level of compliance with and persistence of treatment level of compliance with and persistence of treatment. Poor adherence and persistence may, in turn, affect the effectiveness of the treatments. These issues drove the scope of this report and its predecessor.
The FDA Approval Process
In 1979, the FDA published its first Guidance Document for the clinical evaluation of the safety and effectiveness of drugs to treat osteoporosis.12 From the outset, the FDA acknowledged certain difficulties, including quantitative assessment of skeletal bone, the inexact relationship between bone mass and fracture risk, and the study size and duration needed to detect changes in bone density and/or fracture risk. Inclusion criteria for FDA clinical trials consisted of objective evidence of participant disease (i.e., history of an osteoporosis-related fracture) or the less objective criterion of low bone mass, as determined by any one of six methods, all imperfect. In an effort to ease the process of trial implementation, the Guidance Document permitted effectiveness to be defined as improvement in bone mass during therapy if the process of new bone formation could be demonstrated to be normal, rather than requiring evidence of significant decrease in fracture risk. If new bone formation did not prove normal or if it was not possible to determine normalcy, fracture studies would be required.
The 1984 Guidance Document included several noteworthy changes. Studies were recommended that would establish an indication for the prevention of postmenopausal osteoporosis. In addition, DXA was described as providing a valid measure of spinal bone mass, and it was recommended that all participants in trials of agents for osteoporosis therapy be supplemented with calcium and vitamin D.
Operating under the initial Guidance Document—which did not require demonstration of fracture risk reduction—calcitonin was approved as an injectable drug for the treatment of osteoporosis in 1984, conditional upon the initiation and eventual completion of a trial to assess fracture risk. Calcitonin is a peptide hormone synthesized in the thyroid that participates in the physiological regulation of calcium and phosphorus; it had previously been approved for the treatment of Paget's disease (a disease characterized by abnormal bone remodeling.) Upon completion of the study, it became apparent that enrollment and retention of patients in this fracture trial was problematic, and the fracture reduction effects of calcitonin remained in doubt. In the early 1990s, the Prevent Reoccurrence of Osteoporotic Fracture (PROOF) trial tested the ability of a nasally administered form of calcitonin (100, 200, and 400 IU) to prevent fracture. Although fracture prevention was seen with 200 IU, none was seen at the higher or lower dose; this lack of dose response, combined with a lack of effect on BMD suggested either that the positive effect of the 200 IU dose was an artifact or that BMD and fracture risk are not well correlated. Nevertheless, the drug is still widely prescribed.
During the 1980s, two additional agents–sodium fluoride (NaF) and the bisphosphonate (see below) etidronate—were evaluated for the treatment of osteoporosis under the initial Guidance Document, which did not require fracture risk reduction. Although both agents increased bone density significantly when tested in large scale trials of postmenopausal women, evidence suggested that neither reduced the risk for vertebral fracture and that at least one (NaF) may have increased fracture risk. Based on this experience, the Osteoporosis Guidance Document was updated in 1994 to include the following requirements for approval of a new drug to treat postmenopausal osteoporosis: (1) demonstration that treatment resulted in preservation or improvement in bone density while retaining normal bone qualityb in preclinical studies with two laboratory animal species, including the ovariectomized rat model; (2) normal bone quality in a subset of clinical trial participants; (3) significant increase in BMD; and (4) at least a trend toward decreased fracture risk after three years (not two years) of treatment. The 1994 Guidance Document also affirmed the use of DXA and bone turnover markers for phase I and II trials and provided requirements for approval of agents for prevention of osteoporosis (in individuals at high risk but without history of osteoporotic fracture).13 Only agents that have already been approved for treatment of osteoporosis can be approved for prevention. For prevention, BMD may serve as an appropriate–and sufficient–outcome measure for effectiveness in double-blind RCTs of at least 2 years duration with multiple dosage arms (to establish a minimum effective dose). The guidance also provided recommendations for the appropriate sample population.
Based on extensive data from observational studies (of estrogen as used to treat menopausal symptoms), estrogen was approved for treatment of postmenopausal osteoporosis. Thus it was exempted from the requirement that it demonstrate effectiveness for fracture prevention, and was approved for both treatment and prevention based on BMD alone. Subsequently, however, the FDA has required evidence of fracture effectiveness or efficacy for approval of selective estrogen receptor modulators (SERMS). In 1997, the first SERM, raloxifene, was approved. The bisphosphonate alendronate was the first nonestrogenic agent to be evaluated and approved for treatment of postmenopausal osteoporosis.
In 2004, the FDA began soliciting comments on the 1994 Guidance Document in preparation for its revision. Two issues of particular interest were the continued use of placebo (as opposed to active) controls (an issue with both ethical and technical implications) and the minimum acceptable duration for treatment trials.
Thus, not all drugs currently approved for treatment of osteoporosis were required to demonstrate reduction in fracture risk (e.g., calcitonin). With the exception of estrogen products all agents approved for prevention of osteoporosis have demonstrated fracture reduction, as they were approved first for osteoporosis treatment. Further, approval of an indication for a different dose, frequency, or route of administration does not require demonstration of reduced fracture risk (however, approval for a different indication, such as glucocorticoid-induced osteoporosis, does require demonstration of reduction in fracture risk). These implications of the current guidance have heightened interest in evaluating the data on the effects of drugs approved to treat and prevent osteoporosis.
The 2007 Comparative Effectiveness Review
In December, 2007, the Evidence-based Practice Center (EPC) completed the first Comparative Effectiveness Review (CER) on the efficacy/effectiveness of these interventions in preventing osteoporosis-related fracture, their safety, and compliance with their use.14
The review found a high level of evidence suggesting that, compared with placebo, alendronate, etidronate, ibandronate, risedronate, zoledronic acid, estrogen, teriparatide, and raloxifene prevent vertebral fractures; the evidence for calcitonin compared with placebo was fair. The report also found a high level of evidence to suggest that alendronate, risedronate, and estrogen prevent hip fractures, compared with placebo; the evidence for zoledronic acid was fair. No studies were identified that assessed the effect of testosterone on fracture risk. The evidence for an effect of vitamin D on both vertebral and hip fractures varied with dose, analogue, and study population. No antifracture evidence was available for calcium or physical activity.
Further, the evidence was insufficient to determine the relative superiority of any agent or whether the agents were more effective in some populations than others.
Regarding adverse events associated with the pharmacologic agents, raloxifene, estrogen, and estrogen–progestin increased the risk for thromboembolic events, and etidronate increased the risk for esophageal ulcerations and gastrointestinal perforations, ulcerations, and bleeding. The use of menopausal hormone therapy was associated with an increased risk of breast cancer, heart disease, and stroke in the Women's Health Initiative trial. Clinical trials reported mixed findings regarding an association of zoledronic acid with the risk for atrial fibrillation. No data were found from osteoporosis trials to suggest an association between bisphosphonates or any other agents and the development of osteonecrosis: A number of case reports and case series articles reported osteonecrosis of the jaw in cancer patients taking intravenous bisphosphonates.
Although fracture trials that reported data on adherence/compliance tended to find relatively good adherence to medication use, observational studies tended to report poor adherence with osteoporotic medications, as with other chronic conditions. Poor adherence was associated with lower effectiveness.
This Report
Since the release of the original report, several of the bisphosphonates have become available in new, less frequently administered, forms, and a new biological agent (denosumab) is now available. In addition, new data have been released on adverse events associated with bisphosphonates. Thus, in 2008, the EPC was asked to conduct an assessment of the need to update the original report (as well as the other CER reports released up to that time point); that report was submitted in March, 2009.15 For that report, the EPC conducted an abbreviated search and review of the literature addressing the topics of the first review. The abbreviated search consisted of a survey of experts in the field and a MEDLINE® search (using the same search terms as the original report) of 5 of the leading medical journals and 5 leading specialty journals dating from 2006 to mid-2008. The studies identified in this search that addressed the key questions were reviewed and abstracted, and their findings qualitatively assessed using a process devised by the EPC to determine whether they confirmed, contradicted, or augmented the conclusions of the original report.
The update search identified new data on effectiveness and adverse effects. New studies were found for several agents, including denosumab, that were not included in the original report. In addition new data were found for the effects of calcium and vitamin D and for novel dosing schedules or routes of administration of the bisphosphonates, ibandronate and zoledronic acid. Based on this evidence, the assessment concluded that at least some of the conclusions of the first report regarding effectiveness may need to be updated (Key Question 1 – see below). In addition, the assessment found new evidence on the safety of some agents that might warrant an update. For example, new evidence was found on the risk of atrial fibrillation with the use of some bisphosphonates and the risk of osteosarcoma with the use of teriparatide. Also, the FDA issued a labeling revision in December 2007 regarding the possible association of the use of pamidronate with deterioration of renal function (CER Updates Assessment, 2009 - unpublished). Based on these findings, the Update Assessment suggested an updated review of the adverse effect evidence (Key Question 4).
Scope and Key Questions
In July 2009, the EPC was asked by AHRQ to conduct a full update of the original CER. Key question 1 has been modified to include medications that were not approved for the treatment of osteoporosis prior to the release of the original report but have since been approved, including zoledronic acid (IV) (Reclast®; Novartis; once-a-year infusion) and the monoclonal antibody, denosumab (Prolia®; Amgen; every-six-months injection) and agents for which no or few data were available for inclusion in the original report, such as injectable ibandronate sodium (Boniva®; Roche Laboratories/Hoffman laRoche; once every three months). We also omitted several agents—etidronate, pamidronate, tamoxifen, and testosterone—based on their not being indicated or used for osteoporosis treatment, and also modified the question to include consideration of the sequential or combined use of different agents. Although new evidence was found for strontium ranelate, it is not likely to be considered for FDA approval in the near future, so it was not included.
Key Question 2 originally assessed the evidence for effectiveness among particular subpopulations of clinical interest. The subpopulations to be considered in the evidence review update were also augmented to include racial/ethnic differences based on evidence of differences in BMD and potential risk for osteoporosis. The subject matter experts also recommended considering the comparative utility of existing risk assessment algorithms for predicting antifracture effects of osteoporosis pharmacotherapy, i.e., whether differences in antifracture effects would be found among groups with different FRAX (or other) risk assessment cutoffs.
Key Question 3, which addresses compliance and adherence, remains as it was originally.
Key Question 4, which assesses adverse effects of the pharmacologic agents, was modified in keeping with the scope to exclude uses of the agents for any condition other than osteoporosis/low bone density.
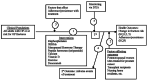
The subject matter experts also recommended that an additional question be added. Because the optimal duration for therapy (and the role of monitoring in determining how long to treat) remains unknown, a question was added to address therapy duration and efficacy and effectiveness monitoring. Key Question 5 has two parts. The first part aims to assess the evidence that antifracture effects are predicted by DXA monitoring of BMD. The second part which is really a sub-question to Key Question 1 aims to assess the evidence for comparative efficacy and effectiveness of long-term therapy (defined by the consensus of the technical expert panel as therapy of 5 years or more). Thus the following questions guided the current report (Figure 1 shows the analytic framework).
Key Question 1. What are the comparative benefits in fracture risk reduction among the following therapeutic modalities for low bone density:
- Bisphosphonate medications, specifically:
- Alendronate (Fosamax®, oral)
- Risedronate (Actonel®; oral once-a-week)
- Ibandronate (Boniva®)
- Zoledronic acid (Reclast®, Zometa®, oral and IV).
- Denosumab (Prolia®)
- Menopausal Estrogen therapy for women (numerous brands and routes of administration)
- Parathyroid hormone (PTH)
- 1-34 (teriparatide) (Forteo®)
- Selective estrogen receptor modulators (SERMs), specifically
- Raloxifene (Evista®)
- Calcium
- Vitamin D
- Combinations or sequential use of above
- Exercise in comparison to above agents
Key Question 2. How does fracture risk reduction resulting from treatments vary between individuals with different risks for fracture as determined by the following factors:
- Bone mineral density
- FRAX or other risk assessment score.
- Prior fractures (prevention vs. treatment).
- Age
- Sex
- Race/ethnicity
- Glucocorticoid use
- Other factors (e.g., community dwelling vs. institutionalized, vitamin D deficient vs. not)
Key Question 3: Regarding treatment adherence and persistence,c
- a. What are the levels of adherence and persistence with medications for the treatment and prevention of osteoporosis?
- b. What factors affect adherence and persistence?
- c. What are the effects of adherence and persistence on the risk of fractures?
Key Question 4: What are the short- and long-term harms (adverse effects) of the above therapies (when used specifically to treat or prevent low bone density/osteoporotic fracture), and do these vary by any specific subpopulations (e.g., the subpopulations identified in Key Question 2)?
Key Question 5: With regard to treatment for preventing osteoporotic fracture:
- a. How often should patients be monitored (via measurement of bone mineral density) during therapy, how does bone density monitoring predict antifracture benefits during pharmacotherapy, and does the ability of monitoring to predict antifracture effects of a particular pharmacologic agent vary among the pharmacotherapies?
- b. How does the antifracture benefit vary with long-term continued use of pharmacotherapy, and what are the comparative antifracture effects of continued long-term therapy with the various pharmacotherapies?
Table 1 describes selected characteristics of, and current indications for, the drugs evaluated in this review.
Table 1
Prescription drugs indicated for prevention and treatment of low bone density/osteoporosis.
Figure 1 shows the inter-relationships of study-level factors and outcomes addressed by the key questions. The population of interest is all adults with osteoporosis or who are at risk for osteoporosis, with the exception of those with cancer and those with other diseases of the bone. Key Question 1 addresses the effectiveness of drugs, dietary supplements (vitamin D and calcium), and exercise in preventing fractures. Key Question 2 addresses factors that might affect the effectiveness of the treatments addressed in Key Question 1 (effects of the agents in subpopulations) in terms of fracture risk. Key Question 3 addresses the specific effect of adherence to and persistence with medication on the effects of these medications as well as factors that affect adherence and persistence. Key Question 4 addresses adverse events associated with treatment. Key Question 5 addresses the effects of monitoring and treatment duration on the effects of treatment.
Footnotes
- a
Note: Authorities disagree about whether to use young males or young females as the reference group to assess T scores in men.
- b
The North American Menopause Society has established the following terminology for menopausal hormone therapy (formerly referred to as hormone replacement therapy): EPT=combined estrogen-progestogen therapy; ET=estrogen therapy; HT=therapy that encompasses both EPT and ET.
- b
The FDA recognizes that components of bone strength include bone mineral density and bone quality; some aspects of bone quality that might affect fracture risk have been identified but are difficult to measure. Nevertheless, the requirements for approval specify that drugs must not result in accretion of new bone (or preservation of existing bone) with abnormal morphology.
- c
The terms adherence and persistence are defined based on principles outlined by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).(Cramer, 2008) Adherence (or compliance) is defined as “the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen.” Although not specifically stated in the ISPOR definition, we view adherence to specific dosing instructions (which for bisphosphonates can affect both effectiveness and risk of adverse events) as an important component of adherence. Persistence is defined as “the duration of time from initiation to discontinuation of therapy.”(Cramer, 2008)
- Introduction - Treatment To Prevent Fractures in Men and Women With Low Bone Den...Introduction - Treatment To Prevent Fractures in Men and Women With Low Bone Density or Osteoporosis: Update of a 2007 Report
- Discussion - Treatment To Prevent Fractures in Men and Women With Low Bone Densi...Discussion - Treatment To Prevent Fractures in Men and Women With Low Bone Density or Osteoporosis: Update of a 2007 Report
- Caenorhabditis elegans protein of unknown function WSN domain-containing protein...Caenorhabditis elegans protein of unknown function WSN domain-containing protein (Y51A2B.6), partial mRNAgi|1845979030|ref|NM_001029086.5|Nucleotide
- Calponin-homology (CH) domain-containing protein [Caenorhabditis elegans]Calponin-homology (CH) domain-containing protein [Caenorhabditis elegans]gi|808357684|ref|NP_001294368.1|Protein
- chromatin complexes subunit BAP18 isoform 4 [Mus musculus]chromatin complexes subunit BAP18 isoform 4 [Mus musculus]gi|21389318|ref|NP_081033.1|Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...