NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lindegren ML, Krishnaswami S, Fonnesbeck C, et al. Adjuvant Treatment for Phenylketonuria (PKU) [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Feb. (Comparative Effectiveness Reviews, No. 56.)
This publication is provided for historical reference only and the information may be out of date.
In this section we present findings for each Key Question, beginning with an overview of the content of the phenylketonuria (PKU) literature meeting our criteria, including the range of study designs used, approaches assessed and participants included. The detailed analysis of the literature provides further discussion and analysis.
Studies also are described in more detailed summary tables in the relevant section of text. For information on studies not included in the summary tables, please see the evidence tables in Appendix C; for information on quality scores for each study, see Appendix E.
Article Selection
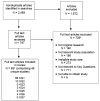
We conducted a broad search to identify any titles or abstracts that might include relevant data for the review. Of the entire group of 2,469 titles and abstracts, we reviewed the full text of 797 because they either appeared to meet criteria or didn't provide enough information to determine definitively whether they should be in included (Figure 2). Of the 797 full text articles reviewed, 69 articles (comprising 46 unique studies) met our inclusion criteria. Reasons for article exclusion are listed in Appendix G.
Key Question 1a. What is the evidence that any specific phenylalanine (Phe) levels are optimal for minimizing or avoiding cognitive impairment in individuals with PKU?
We divided the literature addressing this question into three sections: studies of the relationship of blood Phe levels and intelligence quotient (IQ) in individuals with PKU, studies of the relationship of blood Phe levels and measures of executive function in individuals with PKU, and studies offspring of mothers with PKU (maternal PKU). Children in the latter group may or may not have PKU themselves but may experience intellectual disability, low birth weight, or other impairments as a result of the mother's PKU. We considered executive function to be defined as working memory, cognitive flexibility, inhibitory control, planning, or attention domains of executive function.43
In conducting a review of reviews to identify potential systematic reviews to answer this Key Question, we identified one review that used the same inclusion and exclusion criteria and sought to answer a nearly identical question.59 However, this study was a meta-analysis of the correlation between blood Phe and IQ, and we sought to predict the probability of low IQ based on blood Phe level. Therefore, we used the existing, well-conducted review as a source of citations only.
Phe Levels and IQ Impairment in Individuals With PKU
Key Points
- Increasing blood Phe is clearly associated with decreased IQ, with a probability of having an IQ less than 85 exceeding the probability for the general population (approximately 15 percent) at a Phe level of over 400 μmol/L. This finding supports the typical target goal for Phe level in individuals with PKU (120 to 360 μmol/L).
- The probability of having an IQ of <85 does not continue to increase considerably above a blood Phe level of 2000 μmol/L.
- Historical measurements of Phe (taken more than 1 year prior to IQ testing) show a stronger correlation with the probability of having a lower IQ than do concurrent measurements. Even at the highest blood Phe measurement, observed effects differ by when the measurements are taken, both relative to IQ measurement and according to whether measurements were taken during the critical period (<6 years old).
- The best measure of blood Phe for assessing the potential impact on IQ is likely to be a historical measure of dietary control that is taken at least one year prior to the IQ test.
- The probability of low IQ (<85) increases faster with higher blood Phe measurement when historical measurements were taken during the critical period and associated with later IQ, although historical measurements taken after the critical period are also associated with risk of low IQ. Hence, control of blood Phe levels during the critical period is particularly important, but the need for dietary control continues beyond early childhood.
- The relatively modest increase in the probability of low IQ with blood Phe measurement in measurements taken concurrently during the critical period may suggest that effects are unlikely to be observed in this period, either because the IQ test is not stable for young children (under 5 years of age), or because the adverse effects take time to manifest. From a clinical perspective, this provides a basis for being cautious in interpreting measures of cognitive outcomes as they relate to blood Phe in early childhood.
Overview of the Literature
Seventeen unique studies (reported in 21 publications) met our criteria and addressed the relationship between blood Phe levels and IQ (Table 3).60-79 Age ranges and IQ levels varied widely across studies. Ten studies were conducted in Europe,62-66, 69, 70, 73-75 six in the United States,60, 71, 72, 78, 80, 81 and one in Iran.61 We rated one study67, 68 as good quality and five studies as fair quality.63, 65, 71, 74, 79, 81 The remaining studies60-62, 64, 66, 69, 70, 72, 73, 75-78 were rated as poor quality and typically did not document recruitment processes adequately, did not include all eligible participants in analyses, and/or did not assess confounding variables using valid and reliable measures.
Table 3
Overview of studies addressing Phe levels and IQ.
Overall, the number of participants in the studies was low, ranging from 10 to 57. The studies included a total of 432 individuals with PKU. Of the studies that reported on disease classification, 10 included only participants with classic PKU, and the remainder did not provide the classification or included individuals with less severe PKU. Results are therefore most clearly applicable to individuals with classic PKU.
Participant ages ranged from 2 to 34 years. A majority of studies included primarily participants under age 25 at intake,60-63, 65, 67, 70, 71, 74, 75, 78, 81 with five studies including only participants under age 15 at intake.60, 63, 71, 75, 78 Dietary control varied among the studies, with five studies reporting that all participants were adhering to a restricted diet,60, 61, 63, 72, 78 seven reporting a mix of dietary control (some participants on and some off a restricted diet),64-70, 81 and three reporting that participants had discontinued a restricted diet.62, 71, 73 Dietary status was not clearly reported in the remaining studies.74-77Table 4 outlines characteristics of study participants.
Table 4
Characteristics of participants in studies addressing Phe levels and IQ.
IQ scores ranged from 44 to 148 across studies. Five studies reported concurrent measures of Phe levels (blood Phe measurement within 6 weeks of IQ measurement),61, 62, 64, 67, 70 eight studies reported historical Phe measurements (blood Phe measurements taken more than 12 months before IQ measurement),63, 65, 66, 71, 73-75, 78 and four reported both historical and concurrent measurements.60, 69, 72, 81 Phe measurements were also taken in the critical period (blood Phe measurement before age 6) in seven studies (Table 5).60, 63, 66, 71, 72, 78, 81 The one study that included very young children used developmental quotient as the outcome measurement for the young children.60
Table 5
Summary of results of studies addressing Phe levels and IQ.
The degree to which Phe was noted to be correlated with IQ varied across the studies, with some noting a significant negative correlation and others finding little to no relationship. At the individual study level, this variation in outcomes did not appear to be related to the population or when or how the measures were taken. The observed variation is possibly due, however, to the small size of the studies, a consideration that is mitigated by the meta-analysis, below.
Meta-analysis
We developed two meta-analytic models. The first represents the relationship of blood Phe and IQ when Phe was measured “historically” (more than 12 months before IQ measurement). In the second model, Phe and IQ were measured concurrently (within 6 weeks of IQ measurement). The key model parameters for the relationship between blood Phe and IQ from both models are presented in Table 6. The baseline Phe effect denotes the slope (correlation) of the linear relationship of Phe (either historical or concurrent) and IQ, when both are measured at or after 6 years of age; the critical period effect, then, is the additive effect of using Phe measurements that were taken in the critical period (prior to 6 years of age). The magnitude of association is strongest for the historical measurement of blood Phe versus that seen when Phe and IQ are measured concurrently.
Table 6
Estimates of key parameters by model.
The implications of this relationship for a range of blood Phe levels measured at different points in life are described in Table 7. These probabilities can be used to estimate the chances of an individual's IQ being less than 85, based on blood Phe level, when Phe was measured, and the proximity of the Phe measurement to the IQ measurement. For example, Column 2 provides probabilities of the results of an IQ test showing an IQ less than 85 at different Phe levels when 1) the Phe is measured at least one year prior to the IQ, but 2) when the individual is 6 years old or greater. Note that these probabilities do not have associated levels of uncertainty, such as confidence intervals, because they were derived by integrating over the posterior distribution of the predicted IQ.
Table 7
Summary of probability (IQ<85) for various combinations of predictor variables.
Conversely, Column 3 provides the probabilities for individuals for whom 1) the Phe is measured at least one year prior to IQ, but 2) in the critical period (prior to 6 years of age). As expected, increasing blood Phe in all cases is associated with increasing probability of a low IQ. However, our ability to see the relationship between Phe and IQ is attenuated by when both measurements are obtained. This suggests that although a relationship between high Phe and low IQ clearly exists, the effects may not be observed during early childhood, but become apparent later in life.
The columns in Table 7 provide probabilities for low IQ for four groups of individuals whose Phe levels have been measured at distinct time periods.
- Group 1 represents probabilities for low IQ for individuals who are tested for IQ whose reported blood Phe was measured more than one year prior to their IQ test and at or after age 6.
- Group 2 represents probabilities for low IQ for individuals who are tested for IQ whose reported blood Phe was measured more than one year prior to their IQ test and before age 6.
- Group 3 represents probabilities for low IQ for individuals who are tested for IQ testing whose blood Phe and IQ measurements are occurring within 6 weeks of one another and at or after age 6.
- Group 4 represents probabilities for low IQ for individuals who are tested for IQ testing whose blood Phe and IQ measurements are occurring within 6 weeks of one another and before age 6.
Across all groups, blood Phe of 200 μmol/L is associated with a low probability of about 0.10 (10 percent) of having an IQ less than 85. As Phe increases to 400, probability of low IQ increases considerably to 0.187 (19 percent) only in Group 2 in which Phe has been measured more than one year prior to IQ and before age 6.
The association of a high blood Phe level of 1,200 μmol/L with low IQ is most clearly captured when Phe and IQ measurements take place at least one year apart. The probability of having a low IQ is 0.642 (64 percent) for those individuals who had a Phe level of 1200 before age 6 (critical period) and at least one year before IQ testing. If the Phe level was measured at or after age 6 as opposed to in the critical period, the probability of a low IQ is 0.430 (43 percent).
A less dramatic effect is observed when the blood Phe and IQ measurements are taken concurrently. In individuals whose Phe and IQ are measured concurrently and at or after age 6, if the blood Phe is 1,200 μmol/L, then there is a 0.192 (19 percent) probability of low IQ, compared with a probability of 0.163 (16 percent) when both measurements are taken concurrently in the critical period. This finding may represent tighter dietary control among individuals with frequent and concurrent measurement, or it may suggest that long-term effects of Phe on IQ cannot be seen when the two are measured too closely.
At the highest blood Phe level (3000 μmol/L), the probability of low IQ is substantially different across groups. When both are measured prior to age 6, the probability of low IQ is only 30 percent. This may be because the effects on IQ are not yet observable or because IQ measurements in this young age group are not stable. This also may reflect the fact children are more likely to be in compliance with diet when their diet is substantially controlled by the adults in their lives. Thus, of greater clinical importance is the historical effect of Phe on IQ over the longer term, as observed in Groups 1 and 2, in which earlier blood Phe measures of 3000 μmol/L are associated with approximately 80 and 90 percent probability, respectively, of low IQ measured later. However, even in Group 3, in which both measures are taken concurrently and after the critical period (i.e., in older children, adolescents and adults), very high Phe continues to be associated with low IQ, suggesting a continued effect into adulthood.
In summary, the observed influence of varying blood Phe on the probability of having an IQ <85 depends strongly on when Phe is measured relative to when IQ is tested, and whether or not the Phe measurement takes place in the critical period (before 6 years of age) (Figure 3).
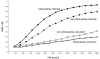
Figure 3
Probability of IQ <85 at varying blood Phe levels and Phe measurement times. IQ = intelligence quotient; Phe = phenylalanine; Pr = probability
Note that in Figure 3 the two lines depicting historical measures of blood Phe (top two lines) both demonstrate increasing probability of low IQ at higher blood Phe levels, regardless of whether IQ was measured during childhood (top line) or beyond (second line). The effect in early childhood is consistently stronger. Nonetheless, effects of Phe on IQ continue beyond early childhood. Therefore, the best measure of Phe for assessing the potential impact on IQ is likely to be a historical measure of dietary control that is taken at least one year prior to the IQ test. The probability of having an IQ <85 does not continue to increase considerably above a blood Phe level of 2,000 μmol/L.
The two lower lines in the figure describe the observed relationship of blood Phe and IQ when they are measured concurrently. The lack of strong association in measurements taken concurrently during the critical period may suggest that effects are unlikely to be observed in this period, either because the IQ test is not stable for young children (under 5 years of age), or because the adverse effects take time to manifest. From a clinical perspective, this provides a basis for being cautious in interpreting measures of cognitive outcomes as they relate to Phe in early childhood.
Phe Levels and Impairments in Executive Function in Individuals With PKU
Key Points
- Too few studies of common outcomes are available to synthesize the relationship of specific Phe levels and executive function measures.
- Among individual studies, data are inconsistent in terms of the direction and degree of association between specific Phe levels and measures of planning ability, inhibitory control and attention.
Overview of the Literature
Nineteen unique studies, reported in 26 papers,67, 68, 70, 72, 75-78, 82-99 provided data on blood Phe levels and on measures of executive function. We summarize data from these studies in Appendix H. Of the 19 studies providing Phe and executive function data, only three tests of executive function appeared in at least three studies, suggesting that we could potentially provide some synthesis on these nine studies (Table 8). The nine studies presented here include three using the Tower of London test to assess planning ability,61, 78, 84, 85 three studies using a Flanker test for inhibitory control,86-88, 90, 97 and three studies using the Color Word Interference Test as a measure of inhibitory control and attention.75-77, 91-93 After reviewing these as possible candidates for meta-analysis, clinical and statistical experts determined that a meta-analysis would not be appropriate for any component of executive function.
Table 8
Summary of studies addressing measures of executive function and Phe levels.
Overall, while blood Phe levels correlate with various assessments of executive function in some papers, the degree to which they are correlated, and the correlation on individual measures, are not conclusive. For example, in the three studies of planning skills, one study found no correlation between higher blood Phe and improved planning skills,61 one found a significant negative correlation,78and one did not measure the association.84, 85 Two out of three studies of blood Phe and inhibitory control found no association. In none of these studies can a specific Phe threshold as a target be identified to answer the Key Question. Further, these studies cannot be meaningfully aggregated since the measures of executive function relevant for individuals with PKU have not yet been established (see Future Research section also).
Phe Levels and Maternal PKU and Maternal PKU Syndrome
Key Points
- Data predominantly from one longitudinal study provide evidence for poor cognitive outcomes in the offspring of women who have high blood Phe during pregnancy.
- Several analyses of the data, including separate analyses for U.S. and German data, suggest that the time it takes for women to achieve dietary control is particularly influential on offspring outcomes, with relatively better outcomes associated with achieving control by 10 weeks postconception, but all studies recommending control as early as possible. Children of mothers with well-controlled PKU prior to pregnancy had the best outcomes.
- One complex analysis using structural equation modeling and splines was able to demonstrate that a threshold of 360 μmol/L of blood Phe is appropriate to prevent poor cognitive outcomes in offspring, and that a linear relationship exists after that threshold.
Overview of the Literature
We identified 20 papers from three unique study populations that provided some data on maternal blood Phe and cognitive outcomes in infants or children.19, 21, 24, 31-33, 92, 93, 100-111 Most of the papers in this literature come from the international Maternal PKU (MPKU) Collaborative Study, which prospectively followed women with PKU who were pregnant or planning pregnancy and their offspring from 1984 to 2002 and provides the most complete data currently available on women with PKU and their offspring. The data reported were not suitable for meta-analysis; however, we summarize key findings below and present tables outlining cognitive outcome data in Appendix I.
Detailed Analysis
MPKU Collaborative Study
The MPKU Collaborative Study was initiated in 1984 to study the implications of maternal PKU, and specifically to assess outcomes when blood Phe is controlled in pregnant women. Initially, women were advised to maintain blood Phe levels of <600 μmol/L, but the target was changed to Phe <360 μmol/L. The study was conducted originally in the United States and Canada only. Germany, Austria, and Switzerland were added in 1992, and the study also expanded to include women with untreated mild hyperphenylalaninemia (defined as blood Phe concentrations between 240 and 599 μmol/L21), with hyperphenylalaninemia treated at different stages of pregnancy, and non-hyperphenylalaninemia controls. The study enrolled women at any time during their pregnancy and followed many of the women's offspring to test their cognition at 1, 2, 4, and 7 to 9 years. The entire study sample consisted of 572 pregnancies, 412 live births, with 416 offspring.33
Timing of maternal metabolic control, defined as the number of weeks gestation before plasma Phe levels remained consistently lower than 605 μmol/L, was associated with child cognitive scores at 4 years of age, including on the children's McCarthy General Cognitive Index and subscale scores. At four years of age, children whose mothers had not achieved dietary control by 20 weeks into their pregnancies had a mean General Cognitive Index score 2 standard deviations below the mean. Overall, children of mothers who were treated prior to pregnancy had the best outcomes, with a mean General Cognitive Index score of 99, compared with 107 in non-hyperphenylalaninemia controls, and 59 in those who had not achieved dietary control by 20 weeks.101
At 7 years of age, 228 children were evaluated using the Wechsler Intelligence Scale for Children-Revised, Peabody Individual Achievement Test-Revised, Test of Language Development-2, Visual Motor Integration Test, Stroop Color Word Test, Home Observation for Measurement of the Environment, and Child Behavior Checklist 4 to 18.100 At this point, 18 percent of the children were considered to have intellectual disability, 18 percent had borderline intellectual disability, and 64 percent were considered average in terms of intellectual ability. As at the younger ages, a decrease in children's scores for cognition, language, behavior, achievement, and visual motor skills was associated with time to maternal metabolic control.100 A separate analysis of the German data found similar results, with consistently negative correlations between start of dietary control and Bayley Mental Developmental Index (r=-0.43) and Psychomotor Development Index (r=-0.60).105
The 48 women who had mild hyperphenylalaninemia had 58 pregnancies and an average blood Phe exposure during pregnancy of 270 ± 84 μmol/L in untreated women and 269 ± 136 μmol/L in treated women.19 In the group of untreated women, 40 offspring received IQ testing; their scores were slightly below but not significantly different from mean IQ scores for controls (102 ± 15 vs. 109 ± 21).
Because they had access to the largest available dataset on maternal PKU, investigators were able to model the form of the association between maternal blood Phe levels during pregnancy and effect on offspring during childhood. They conducted a spline analysis accounting for potentially strong confounders including maternal IQ, education and socioeconomic status as maternal characteristics differed in the groups of women with and without PKU.33 The use of a spline analysis allowed for the first time confirmation that the relationship between maternal Phe and offspring cognitive outcomes is not linear, and that a blood Phe threshold of 360 μmol/L is the level at which cognition begins to be impaired. Importantly, while other factors, including maternal characteristics and infant head circumference, contribute strongly to outcomes at 1 year of age, by age 2, maternal blood Phe strongly overtakes other factors in predicting cognitive impairment.
Key Question 1b. What is the evidence that different target Phe levels are appropriate for minimizing or avoiding cognitive impairment for different age groups?
Too few studies provided data by age group to answer this question without combining the data quantitatively. Therefore, we explored the use of an age effect in the meta-analysis of the relationship between blood Phe and IQ. Any influence of age was adequately represented by whether the Phe measurements were historical or concurrent and whether they were taken in the critical period.
Key Question 2. What is the comparative effectiveness of BH4 with dietary intervention versus dietary intervention alone for affecting outcomes including measures of cognition (including executive function), quality of life, and nutritional status?
Subgroups include the following:
- Infants with PKU
- Children ages 2 to 12 years old with PKU
- Adolescents ages 13-21 years old with PKU
- Adults >21 years old with PKU
Key Points
- Two multisite RCTs and three uncontrolled open-label trials were eligible for inclusion. Studies ranged in quality from fair to good. Four of the five trials include overlapping populations.
- Studies included between 29 and 90 children and adults who were responsive to BH4 in initial loading trials that included more than 500 individuals to assess initial response.
- Between 19 and 62 percent of participants screened for inclusion in the trials demonstrated initial response to BH4 and were therefore eligible for the efficacy studies.
- In five trials (RCTs and open label), blood Phe levels were reduced by at least 30 percent (the level used in studies submitted to the U.S. Food and Drug Administration (FDA) to assess responsiveness) in almost half of treated participants (42 percent to 49 percent) at dosages of 10 to 20 mg/kg/day and for up to 22 weeks of observation, compared with small reductions in Phe in the placebo groups (9 percent).
- A subset of participants in the RCTs were ultimately followed in an uncontrolled open label trial with 2.6 years of data; most participants had achieved Phe levels in the recommended treatment range by the end of the analysis period, and harms were mild and rare.
- The strength of evidence (confidence that the current effect estimate will not change with future research) for the effects of BH4 on reducing blood Phe levels to clinically acceptable levels among BH4 responders in the short term (12 weeks or less) is moderate based on few studies.
- The strength of evidence for the effect of Phe on IQ is moderate. Therefore, the strength of evidence for the indirect relationship of BH4 on IQ is low, based on a lack of direct measurement.
- Harms were noted to be rare and mild, and the strength of evidence for this observation is moderate.
- The strength of the evidence is insufficient for the direct effect of BH4 on improving all other outcomes (Phe tolerance and the ability to liberalize the diet, Phe variability, quality of life, and cognitive and nutritional outcomes).
Overview of the Literature
Ten studies evaluated the effects of BH4 in participants with PKU (Tables 9–10).112-121 Although study populations overlap, the studies were conducted as separate studies and so are presented as such in our analysis. We note, however, those situations in which studies were conducted using the same populations. Four of the studies described in this section are linked by common participants as follows. Two are multisite placebo-controlled randomized trials that contributed to FDA approval of BH4.113, 115 One of the RCTs113 had initially screened 490 individuals to assess initial responsiveness to the drug prior to inclusion in the efficacy study. Of these individuals, 96 demonstrated an initial reduction of ≥ 30 percent and were thus included in the efficacy trial reviewed here. This comparative efficacy trial was followed by an uncontrolled open label trial114 that included 80 of the 87 completers from the comparative trial. Of the 79 completers in that extension study, 71 then were enrolled in a second open-label extension study,112 as were 40 completers from the other RCT.115
Table 9
Variation in approach to assessing responsiveness to BH4.
Table 10
Overview of studies addressing BH4.
One additional uncontrolled open label trial was conducted separately from the family of studies described above116as were one prospective cohort,121 two retrospective case series,117, 119 and two prospective case series.118, 120 We did not conduct a meta-analysis of the studies examining BH4 because the most common outcome (blood Phe level) was measured at different time points in only two RCTs and the populations were substantially heterogeneous. Furthermore, the individual RCTs had adequate power to demonstrate the effect that each noted, so combining the data would have added little to the results.
No individual study included more than 80 participants in the treatment arm, and the total number of individuals in all studies was 284, after accounting for duplication in participants across studies. There were 135 total participants in the RCTs. In the three studies explicitly providing a classification of disease, 38 individuals had classic PKU and 51 had mild, moderate, or variant PKU.116, 118, 119 All of the studies were performed in the United States, Canada, Australia, and Europe. Participants ranged in age from 3 to 58 years in the five trials and from 10 days to 34 years in the four case series. The cohort study analyzed blood samples collected from birth through roughly age 8 from individuals responsive and non-responsive to BH4.121 Most participants had demonstrated responsiveness to BH4 in a loading study; however, the approach to assessing responsiveness varied by study (Table 9) and the base populations tested for initial responsiveness were not consistent.
Initial responsiveness to the drug at screening varied by blood Phe level prior to inclusion in the efficacy studies. For example, individuals screened for participation in the Levy study and Lee follow-on trial had a baseline blood Phe level of at least 450 μmol/L, and were nonadherent to diet. Although those screened had an overall response rate of about 20 percent, more than half (54 percent) of individuals with blood Phe <600 μmol/L had a positive response, compared with 10 percent of those with blood Phe >1200 μmol/L.119
BH4 was studied in doses ranging from 5 mg/kg/day to 20 mg/kg/day (Table 10). Some participants in multiple studies (including the extension studies) were exposed to the drug for up to 2.6 years, although the follow-up period for the two RCTs was 10 weeks. One case series followed participants up to 7 years,118 although the average follow-up was 3.5 years. The mean treatment duration among participants in another case series120 was 4 years and 8 months (range= 24 to 110 months). The degree to which participants were adherent to a restricted diet varied, and one study examined a differential effect in those who maintained a restricted diet versus those who did not.116
One RCT and its follow-on uncontrolled open label trial included participants with PKU who were at least 8 years old with a mean age of 20 years, were not on a restricted diet, and had baseline blood Phe levels >450 μmol/L.113, 114 The second RCT in the “family” of studies examined the effect of 20 mg/kg/day of BH4 for 10 weeks in children ages 4 to 12 who were on a Phe-restricted diet with baseline blood Phe levels <480 μmol/L.115 The unassociated uncontrolled open label trial included both adolescents and adults both on and off a restricted diet to compare relative effectiveness across these groups.
All randomized trials and three case series evaluated the short-term outcome of reduction in blood Phe levels. Two trials and three case series reported on Phe tolerance,115-118, 120 and one cohort study121 and one case series reported on Phe variability.119 Only one case series118 assessed longer term outcomes, including cognition and nutritional status. That study used cognitive outcome measures including the Brunet-Lezine test, the Kaufman Assessment Battery, and the Wechsler Intelligence Scale for Children-Revised.118 Nutritional outcomes included brachial fat and muscular area, micronutrient levels and daily nutrient intake. No study evaluated quality of life. BioMarin, the pharmaceutical company that holds the patent for sapropterin, sponsored five of the ten studies, including two of the RCTs.
Effects of BH4 on Blood Phe Levels and Phe Tolerance
Phe levels were reduced by at least 30 percent in up to half of treated participants (32 to 50 percent) at dosages of 5 to 20 mg/kg/day and for up to 22 weeks of observation in comparative studies (Table 11). In the one RCT that compared the effect of placebo on likelihood of a 30 percent reduction in blood Phe, only 9 percent of those on placebo achieved this effect, compared with 44 percent of the treated group after 6 weeks.113 Data from the uncontrolled open label trial114 following this RCT113 suggested a sustained response for up to 22 weeks' duration, with 46 percent achieving a 30 percent reduction in blood Phe levels with most participants receiving 10 and 20 mg/kg/day doses compared with 5 mg/kg/day.
Table 11
Summary of effects of BH4 on Phe in comparative studies.
Similarly positive effects were reported at a dosage of 20 mg/kg/day in children on Phe-restricted diets. Reduction in blood Phe levels sampled at week 3 (before supplemental medical foods began) was greater among those receiving BH4.115 In the other nonrandomized clinical trial,116 BH4 (7 to 20 mg/kg/day) was associated with a reduction of blood Phe levels among participants both on and off Phe-restricted diets. Overall, participants' responses to different dosages of BH4 varied, with individualized dose adjustments needed according to target plasma Phe and dietary intake. Dosages of 10 to 20 mg/kg/day were most effective across the studies. Response also varied by different baseline Phe levels, with those with the highest baseline levels having lower response rates. As noted above, some participants from the RCTs and extension study have now been followed for up to three years; almost all participants for whom data were available achieved Phe levels within clinically recommended ranges, although specific Phe levels are not reported.112
Studies of Phe tolerance (total Phe intake an individual can tolerate without raising blood Phe to an unacceptable level) all reported improvements over time.115-118, 120 Data from the one RCT115 measuring this outcome indicate that participants in the treatment group were able to increase the supplementary Phe added in controlled amounts to a patient's usual dietary intake from 0 mg/kg at baseline to 20.9 mg/kg/day, while maintaining blood Phe levels at <360 μmol/L, compared with an increase of 2.9 mg/kg/day in the placebo group. However, response varied substantially within the treatment group, with 33 percent tolerating an increase of between 31 and 50 mg/kg/day in medical food form, but the rest of the participants tolerating lower levels of supplementary Phe. Similarly, total Phe intake (medical food plus diet) in the treatment group doubled from baseline to a mean of 43.8 mg/kg/day, response varied substantially within the treatment group.115 In the one open label trial that assessed changes in tolerance,116 participants on a Phe-restricted diet taking 10 to 20 mg/kg of BH4 per day increased their Phe tolerance by an average of 21 to 41 mg/kg/day. Participants tolerated a wide range of dietary Phe, ranging from increases of 20 to 22 mg/kg/day up to a full non-protein restricted diet. For some individuals, increasing the dose of BH4 to 20 mg/kg/day allowed further liberalization of the diet. Trials did not evaluate the impact of increasing natural protein sources on micronutrient levels, nutritional status, or quality of life.
Three case series117, 118, 120 also reported improved Phe tolerance. Among 11 children with mild or moderate PKU, participants reduced or discontinued Phe-free medical foods with 12 months of BH4 treatment. These reductions in special formula and replacement with unrestricted diet did not result in deficiencies of essential nutrients.
Although the mean blood Phe level is an important predictor of IQ, Phe variability may also be an important determinant. In one small retrospective case series (N=37), blood Phe variability as well as blood Phe levels decreased on BH4 20 mg/kg/day.119
Effect of BH4 on Longer Term Effectiveness
After nearly 3 years of following participants in the longer term extension study of BH4, most of the 90 study completers (of 111 enrolled) were reported to have reached clinical targets in Phe levels.112 Only one small prospective case series (N=11) reported on IQ and nutritional outcomes following one year of 5 mg/kg/day BH4 treatment.118 After one year of treatment, 11 participants with mild to moderate PKU discontinued use of a medical food and normalized their diet. IQ scores after 12 months on BH4 were maintained, or developmental quotients were within normal limits. Treatment was not adversely associated with anthropometric or nutritional status indicators, and all participants had normal levels of micronutrients. In another case series with 16 participants,120 treatment duration ranged from 24 to 110 months (mean=56 months), 14 individuals responded to BH4 treatment (blood Phe reduction of ≥30 percent in loading test). Among these responders, the mean blood Phe decrease was 54.6 percent, and 13 were able to maintain Phe control while increasing Phe intake or eliminating dietary restrictions. The study noted that psychomotor development was in the normal range among children between 5 and 6 years of age.
Detailed Description of Individual Studies
Given the small number of studies available for review, we have provided detailed descriptions of each study below and summary information for comparative studies and open label trials in Table 12.
Table 12
Comparative studies and open label trials of BH4 for the treatment of PKU.
Clinical Trials
The first RCT113 evaluating the efficacy of BH4 was carried out in 16 centers in North America and 14 centers in Europe. Between 2005 and 2006, 89 participants with PKU were randomized to receive either 10 mg/kg of BH4 (N=42) or placebo (N=47) once daily for 6 weeks. Eligible participants were responsive to BH4 in a previous phase I screening study, had a blood Phe of 450 μmol/L or more, were 8 years of age and older, and had relaxed or abandoned a strict low phenylalanine diet. The primary outcome was the change in blood Phe from baseline to week 6. Participants' mean age was 21.5 years in the treatment group and 19.5 years in the placebo group. Adherence to treatment was high, with 82 percent of participants taking all doses correctly during the 6 week period.
After 6 weeks of treatment, participants in the BH4 group had a significant decrease in mean blood Phe levels of -235.9 ± 257 μmol/L from baseline (843 μmol/L) compared with a 2.9 ± 239.5 μmol/L increase in mean Phe levels from baseline (888 μmol/L) in the control group (p<0.0001). The mean blood Phe decreased in the BH4 group at 1 week and remained at that lower level until the 6 week end point, when the mean Phe level was 607 μmol/L. The estimated difference between treatment and placebo groups in the mean change in blood Phe at 6 weeks compared with baseline was -245 (p<0.0002). A significantly higher proportion of participants receiving BH4 (44 percent) had a 30 percent or greater reduction in blood Phe levels compared with controls (9 percent).
The proportion of individuals in the BH4 group who had blood Phe levels under 600 μmol/L increased significantly from 17 percent at baseline to 54 percent at 6 weeks compared with controls (baseline: 19 percent, week 6: 23 percent). Almost all participants (16 of 17) for whom genotyping was performed had at least one mutation known to be associated with residual enzymatic activity. Responsiveness was not consistently linked to specific mutations. Despite enrolling only those participants who had at least a 30 percent reduction in blood Phe while taking BH4 in a one week loading test, not all participants were responsive to BH4 in the trial.
A 22 week uncontrolled open label trial114 followed this RCT.113 This was conducted in three parts: the first period was a 6 week forced dose titration phase in which all participants received doses of 5, 20, and 10 mg/kg/day of BH4 consecutively for 2 weeks each. This phase was followed by a dose analysis phase in which all participants received 10 mg/kg/day for 4 weeks followed by a 12 week fixed dose phase in which participants received doses of 5, 10, or 20 mg/kg/day based on their plasma Phe concentrations during the dose titration at weeks 2 and 6. All participants enrolled in the previous RCT were eligible if they had taken at least 80 percent of their scheduled does in the trial and were willing to continue their current diet during the study. The primary endpoint was mean plasma Phe levels at week 22 and mean changes from week 0. Plasma Phe levels at weeks 2, 4, and 6 were used to estimate the effects of dose on plasma Phe levels.
Of 87 participants who completed the previous RCT,113 80 were enrolled in the extension trial,114 of whom 39 had previously received BH4 and 41 placebo. Participants' mean age was 20.4 years. Overall, 60 percent reported taking all doses correctly, 18 percent reported missing at least one dose and no incorrect doses, 9 percent took at least one dose incorrectly but did not miss any doses, and 14 percent took at least one dose incorrectly and missed at least one dose.
During the dose titration phase, individuals receiving 10 or 20 mg/kg/day had significantly greater mean reductions in blood Phe at week 6 compared with week 0 than those receiving 5 mg/kg/day. Additionally, those receiving 20 mg/kg/day had significantly greater reductions from week 6 to week 22 compared with week 0 than those receiving 10 mg/kg/day. By the end of the dose analysis phase with 10 weeks at 10 mg/kg/day, 46 percent of participants had a decrease in plasma Phe of at least 30 percent compared with week 0. During the fixed dose phase, most participants (92 percent) received either 10 (46 percent) or 20 mg/kg/day (46 percent). By week 22, plasma Phe was reduced by 190.5 μmol/L compared with week 0. The mean Phe level at 22 weeks for those on 5, 10, and 20 mg/kg/day was 438 μmol/L, 450 μmol/L, and 896 μmol/L, respectively.
Mean plasma Phe decreased from 844 μmol/L at baseline to 645 μmol/L at week 10 and was maintained at a mean of 652 μmol/L at week 22. At week 22, 46 percent of participants had achieved a 30 percent reduction in plasma Phe concentration compared with week 0. The corresponding reductions for those receiving 5, 10, and 20 mg/kg/day were 50 percent, 49 percent, and 42 percent respectively.
Another RCT115 of fair quality was carried out in the United States, Germany, Spain, and Poland between 2005 and 2006 and enrolled children with PKU between 4 to 12 years of age who were on a Phe-restricted diet, had maintained blood Phe control (blood Phe level <480 μmol/L) and had an estimated Phe tolerance of ≤1000 mg/d. The objective was to determine the safety and efficacy of BH4 at 20 mg/kg/day for 10 weeks in increasing Phe tolerance while maintaining blood Phe control. Investigators randomized BH4 responders in a 3:1 ratio to receive either 20 mg/kg of BH4 or placebo once daily for 10 weeks. Participants maintained a stable, Phe-restricted diet, monitored by food diaries. Starting at the third week, a medical food was added or removed every 2 weeks based on Phe levels. Children with blood Phe level of ≥1200 μmol/L in 2 consecutive weeks were withdrawn from study drug treatment and received dietary counseling.
The primary endpoint was daily Phe tolerance at week 10 compared with week 0. Phe tolerance was defined as the cumulative increase or decrease in medical food at the last visit for which blood Phe level was ≤360 μmol/L. Secondary endpoints were the difference in blood Phe levels in the BH4 group between week 0 (before dosing) and week 3 (before Phe supplementation), and the comparison of Phe tolerance between treatment and placebo groups at week 10. Thirty-three children were randomized to 20 mg/kg/day of BH4 for 10 weeks, and 12 children received a placebo. Baseline characteristics, including blood Phe levels, were similar between groups.
After 10 weeks of treatment, the total mean ± SD of medical food tolerated by participants on BH4 increased significantly from 0 mg/kg/day at baseline to 20.9 ± 15.4 mg/kg/day. In contrast, the placebo group tolerated only an increase of 2.9 mg/kg/day of medical food. The adjusted mean difference between the groups in Phe tolerance was 17.7 ± 4.5 mg/kg/day(p<0.001). Total Phe intake (dietary Phe intake plus total medical food) also increased significantly from baseline in the BH4 group, approximately doubling to 43.8 mg/kg/day at 10 weeks. The placebo group had a slight increase in total Phe intake from 16.3 mg/kg/day at baseline to 23.5 ± 12.6 mg/kg/day at 10 weeks.
The BH4 group tolerated a range of medical food supplementation over the 10 weeks: 36 percent tolerated an increase of 10 mg/kg/day or less, 30 percent tolerated an 11 to 30 mg/kg/day increase and 33 percent tolerated an increase of 31 to 50 mg/kg/day. No one in the placebo group tolerated an increase of more than 10 mg/kg/day, and 58 percent could not tolerate any medical food supplement. Mean blood Phe levels decreased significantly in the BH4 group between baseline and the beginning of supplementation in week 3 (decrease of 148.5 ±_134.2 μmol/L). Some participants in the BH4 group had transient low blood Phe levels (<26 μmol/L) corrected with increased medical food supplementation.
More recently,116 an uncontrolled open label trial of good quality conducted at one U.S. clinic from 2008 to 2009, included participants with classic or variant PKU with any Phe level or diet. Eligible subjects received 7 days of open label BH4 at 10 mg/kg/day with plasma Phe measurement on day 8 and weekly during a dietary modification period. The study defined response as a 30 percent reduction in plasma blood Phe or reduction to treatment range of <360 μmol/L after day 7. Investigators increased the dosage to 20 mg/kg/day for nonresponders and rechecked Phe levels after 8 days. Individuals who were still nonresponders continued on 20 mg/kg/day until day 30. Responders who were on a Phe-restricted diet underwent gradual liberalization of their diet to the maximum tolerated natural protein intake while still maintaining plasma levels in the range of 120 to 360 μmol/L.
Of the 36 participants (mean age 23.4) who began treatment with BH4, 29 (74 percent) completed the study. Of these 29 individuals, 59 percent were on some form of protein restricted diets and had a mean baseline blood Phe of 587 μmol/L. Forty-one percent were not following protein restricted diets and had a mean baseline blood Phe level of 1372 μmol/L. Overall, 62 percent were determined to be responders, with variable doses required for response; 14 participants required a dose of 7 to15 mg/kg/day, and four participants required a dose of 15 to 20 mg/kg/day. Four (29 percent) of the classic PKU participants (defined as off diet plasma Phe of >1200 μmol/L) were responders, and 100 percent of the variant PKU participants (>400 and <1200 μmol/L) were responders.
Of the 12 participants who were not on a Phe-restricted diet, 33 percent were responders with a significantly decreased mean blood Phe of 554 μmol/L compared with baseline Phe level (1049 μmol/L). Of the 17 participants who were on a Phe-restricted diet, 82 percent were responders with significantly reduced mean blood Phe level of 226 μmol/L compared with baseline (485 μmol/L). Among individuals who were responders and on a Phe-restricted diet, the average Phe tolerance increased from 21 to 41 mg/kg/day. However, responders' Phe tolerance varied widely from an increase of 20 to 22 mg/kg/day to a non-protein restricted diet in two participants. Of the 11 who were nonresponders, three were on a Phe-restricted diet with a mean blood Phe level at the end of the trial of 978 μmol/L (baseline mean=1363 μmol/L). Eight of the 11 nonresponders were on an unrestricted diet with an end of trial mean blood Phe level of 1465 μmol/L (baseline mean=1524 μmol/L). Overall, nonresponders had an end trial blood Phe of 1333 and a baseline of 1422 μmol/L compared with responders who had significant decrease in blood Phe from a baseline of 1049 μmol/L to an end of trial level of 554 μmol/L.
Prospective Cohort Study and Case Series
One poor quality prospective cohort study121 assessed variability in blood Phe. Participants included nine children who were responsive to a 20 mg/kg BH4 loading test and 25 who were nonresponsive. Among those who were BH4 responsive, two were treated with BH4 alone and the rest also needed dietary modifications. From 2002 to 2010, there were 1384 blood samples available from BH4 responders and 4415 samples available from non-responders. Overall, there appeared to be no significant difference in mean and median blood Phe levels between the groups; however, above blood Phe levels of 600 μmol/L, confidence intervals around the mean were wider among BH4 nonresponsive participants. The authors equate these differences with variability in response.
Four poor-quality case series117-120 evaluated dosages of BH4 ranging from 5 to 26 mg/kg/day for duration of 6 months to up to 9 years among BH4-responsive participants. All reported positive outcomes in terms of reduction in blood Phe and increased Phe tolerance. As reported above, one case series118 also examined longer term functional outcomes, including IQ and developmental quotient after one year of treatment, reporting no adverse effects as participants' Phe tolerance increased and the diet was liberalized. Nutritional status was unchanged with the exception of increases in selenium. In another case series,117 12 participants were studied for up to 7 years on a dosage of 10 mg/kg twice a day. In this group, ranging in age from 2 to 16 years old, all participants eventually stopped medical food supplementation and relaxed dietary restrictions.
Another longer-term case series120 assessed Phe levels and increase in Phe tolerance (presented as the number of times Phe intake increased from baseline level for those on dietary restriction) in 16 individuals receiving BH4 for between 24 months to 9 years (mean=56 months). Of the 16 patients, 15 (94 percent) patients were initial responders. The mean blood Phe level in responders was 321 ± 236 μmol/L, and the mean decrease in blood Phe was 54.6 percent (range 28.4 to 85.6 percent). Two patients, ages 10 and 13 years at the start of treatment, were non-responders and had high fluctuations in blood Phe levels. Seven patients had stable Phe control (defined as that recommended by the 2000 NIH consensus development panel), without any dietary restriction. Of the remaining seven patients who were on dietary restrictions, six increased their Phe intake from a baseline of 200 to 300 mg/day to 800 to 1000 mg/day. Psychomotor development, (measured using the Hamburg Wechsler Intelligence test-HAWIK III) among children 5 to 6 years of age was reported to be within normal range; however, results were not presented. Finally, one case series119 provided data on Phe variability by measuring blood Phe at least six times before and after treatment initiation. Individual variability in Phe levels was lessened after treatment.
Key Question 3. What is the comparative effectiveness of BH4 with dietary intervention versus dietary intervention alone in pregnant women with PKU for affecting outcomes in their infants, including prevention of neurological impairment, microcephaly, and cardiac defects?
We did not identify any studies addressing this question.
Key Question 4. What is the comparative effectiveness of large neutral amino acids (LNAAs) with dietary intervention versus dietary intervention alone for affecting outcomes including measures of cognition (including executive function), quality of life, and nutritional status?
Subgroups include the following:
- Infants with PKU
- Children ages 2 to 12 years old with PKU
- Adolescents ages 13-21 years old with PKU
- Adults >21 years old with PKU
Key Points
- With only three very small studies, and none of good quality, the strength of evidence is insufficient to draw conclusions about the effectiveness of LNAA formulations in affecting short- or long-term outcomes, including Phe level, Phe tolerance, IQ, executive function or quality of life.
- Studies used blood Phe level as the primary outcome.
- The longest followup period was 2 weeks, and the largest study included 20 participants.
- No RCTs evaluated infants or children younger than 11 years.
Overview of the Literature
This portion of the review focused on the use of LNAA formulations for treating PKU. We did not study the use of individual large neutral amino acids. Three studies addressed the effects of LNAAs,16, 124, 125 including two RCTs124, 125 and one uncontrolled open label trial.16 The studies were very small, including a total of 47 participants, and were conducted in the United States, Brazil, Europe, and Australia. Participant numbers in the RCT treatment arms ranged from 16124 to 20125 while the uncontrolled open label trial included 11.16 Participants were between 11 and 45 years of age, and typically had classic PKU. The trials were short, with treatment between 1 and 8 weeks, and dosages ranged from 250 mg/kg/day in three divided doses to 1g/kg/day. Two of the three studies measured reductions in blood Phe levels, and one assessed cognitive outcomes (Table 13).124
Table 13
Overview of studies and populations for research on LNAA formulations.
Summary of Effects
One RCT124enrolling 16 participants reported on measures of cognition, including executive functioning. The study reported that LNAAs supplementation had a positive effect on executive functioning, specifically improving verbal generativity, cognitive flexibility, and self-monitoring. Despite improvements in some aspects of executive functioning with LNAAs supplementation, studies reported considerable individual variation. In all three studies, blood Phe decreased after one week of treatment, but remained above clinically acceptable levels. The one trial that measured correlation between blood and brain Phe found no association.124 Overall, participants who were using a Phe-free formula did not experience a decrease in blood Phe, although those not adhering to diet or not using Phe-free formula did. This finding suggests that LNAAs may be helpful in lowering blood Phe in participants unable to adhere to medical treatment, but current research suggests a lack of clinical impact.16, 124, 125Table 14 summarizes key outcomes of comparative studies.
Table 14
Comparative studies of LNAAs for the treatment of PKU.
Detailed Description of Individual Studies
Clinical Trials
One fair quality, double blind, randomized, crossover study124 was carried out in one center in Australia. Sixteen participants with early treated, classic PKU (plasma Phe >1000 μmol/L) were enrolled. The objective was to evaluate the relationship between LNAAs supplementation and cognitive and affective outcomes under four different therapeutic combinations with PKU amino acid products. Participants followed their usual Phe-restricted diet and PKU Phe-free medical food. All subjects completed four phases, each lasting 14 days with a 4 week washout period between phases. Phase 1 consisted of taking their usual medical food, usual Phe-restricted diet and LNAAs at 250 mg/kg/day in three equal daily doses.
During Phase 2, participants maintained their usual medical food, usual Phe-restricted diet and placebo. In Phase 3, participants did not take their usual medical food, maintained their usual Phe-restricted diet and received LNAAs. In Phase 4, participants did not take their usual medical food, maintained their usual Phe-restricted diet and placebo. For the phases without medical food, advice was provided on energy supplements needed to replace energy intake usually obtained from a medical food. Blood Phe levels from the previous year were used to determine baseline Phe. Of the 16 participants, nine were determined to have good control (median Phe level 450 to 750 μmol/L), six participants had marginal control (median Phe level 750 to 1000 μmol/L), and two participants had poor control (median Phe level >1000 μmol/L).
Dietary analysis demonstrated that both total protein and LNAAs intake were highest in phase 1 followed by phase 2, phase 3, and lowest in phase 4, consistent with the phased study. There was no significant difference in brain Phe between the 4 phases (range 176 to 365 μmol/L). Brain Phe levels were determined by magnetic resonance spectroscopy. No participant was determined to have excellent control (median blood Phe level<450 μmol/L), and no difference in Phe reduction was observed with or without LNAAs as long as participants were on the Phe-free medical food. However, in the absence of medical food, the LNAAs arm was associated with greater reductions in Phe than placebo. However, plasma Phe levels remained high for all participants, including those taking the LNAAs formulation (958 for those not on Phe-free formula).
The second RCT125 was a crossover trial of poor quality that was carried out in 6 centers located in the United States, Italy, Denmark, Ukraine, Russia, and Brazil. Blood Phe levels dropped significantly on LNAAs from a mean level of 932.9 μmol/L at baseline to 568.4 μmol/L at one week, an average decline of 365.5 ±_233.2 (39 percent). Seven participants who adhered to PKU formula had a significant reduction in blood Phe from a baseline mean of 531.6 μmol/L to 281.5 μmol/L at one week, an average decline of 250.1 ± 173.7 (47 percent). The average decline of Phe on placebo from a baseline mean of 932.9 μmol/L to 882.66 μmol/L at one week (5.4 percent) was not significant.
Uncontrolled Open-Label Trial
The third trial16 was an uncontrolled open-label trial of poor quality that included 11 participants. Participants were not on a Phe-restricted diet and Phe intake was over 500 mg/day. Of 11 participants enrolled, eight received 0.5 mg/kg/day of LNAAs and three received 1.0 g/kg/day. Blood Phe levels decreased significantly from baseline after one week of LNAAs, an average decline of 601 μmol/L ± 370. In the eight participants taking 0.5 g/kg/day, Phe levels decreased significantly from the baseline mean of 957.4 μmol/L to 458.4 μmol/L, a decline of 52 percent. Among the three participants who took 1 g/kg/day of LNAAs, the mean blood Phe level decreased from the baseline level of 1230 μmol/L to 549.0 μmol/L, a decline of 55 percent.
Key Question 5. What is the comparative effectiveness of LNAAs with dietary intervention versus dietary intervention alone in pregnant women with PKU for affecting outcomes in their infants, including prevention of neurological impairment, microcephaly, and cardiac defects?
We did not identify any studies addressing this question.
Key Question 6. What are the harms, including adverse events, associated with the use of BH4 or LNAAs in individuals with PKU?
Key Points
- Few studies of BH4 (N=4) or LNAAs (N=1) reported harms.
- Harms commonly reported in BH4 studies included headache, throat pain, upper respiratory infection, diarrhea, abdominal pain, and nausea and vomiting.
- One study of BH4 reported that three subjects discontinued the treatment due to adverse events.
- Increased anxiety level was reported in one study of LNAAs.
- The strength of the evidence for a lack of significant harms associated with BH4 is moderate. The strength of the evidence for harms associated with LNAAs is insufficient.
Overview of the Literature
Of the ten studies examining the effectiveness of BH4 in participants with PKU, four studies with overlapping participants112-115 reported any type of harm related to the intervention drug. Three studies117, 118, 120 reported that no adverse events were observed during intervention, one study reported that BH4 was well tolerated with mild diarrhea occurring rarely,121 and there was no mention of harms in two studies.116, 119
Among the BH4 studies reporting harms, two, including one RCT and following uncontrolled open label trial, predefined harms (Table 15),113, 114 and two defined severe and serious events precisely.112, 114 Two specified the number of deaths (N=0),112, 115 and most included both active and passive collection of harms data.112-115 All studies of BH4 specified details about the investigators collecting harms data and timing of data collection,112-115
Table 15
Overview of harms reported in studies of BH4.
The studies of BH4 also reported the number of participants who withdrew or were lost to follow up in each group as well as the total number of participants affected by harms in each group.112-115
The most common side effects reported during BH4 trials were headache, throat pain, upper respiratory infection, diarrhea, abdominal pain, nausea and vomiting (Table 16).
Table 16
Harms with highest incidence in studies of BH4.
Harms probably or possibly related to study treatment (Table 17) were similar in both BH4 and placebo (23 vs. 20 percent113, 27 vs. 25 percent115).
Table 17
Harms probably/possibly related to BH4 in studies assessed.
One trial of LNAAs124 assessed neuropsychological outcomes and reported higher rates of anxiety associated with LNAA use. This study was of fair quality, very small and short term, and did not provide any details on the prespecification or collection of harms data.
Key Question 7. What is the evidence for the effectiveness of the addition of BH4 or LNAAs to dietary intervention for affecting outcomes in subgroups of patients?
The following are examples of potential defining characteristics of subgroups:
- Demographic
- Clinical
- Genotypic
- Adherence
To date, there is no evidence that predictable subgroups of individuals are likely to have a differential response to either BH4 or LNAAs. In part, the small size and research design of the studies have precluded appropriate analyses of subgroups. The following section on Grey Literature contains additional detail on current studies that may provide additional data on modifiers of effectiveness in the future.
Grey Literature
Regulatory Information
As part of the evaluation of the clinical evidence of the safety and efficacy of BH4, we examined grey literature sources to supplement the published literature. Specifically, we compared clinical trial data that were included in regulatory documents submitted to the U.S. FDA, Health Canada, and the European Medicines Agency as part of the approval process for sapropterin dihydrochloride to be marketed as Kuvan® by BioMarin. The materials obtained from the three agencies differed in content and level of detail. The material from the FDA included the following documentation: the letter granting approval for BioMarin to market sapropterin as Kuvan, administrative documents and correspondence between the FDA and BioMarin, chemistry and pharmacology reviews of BH4, clinical pharmacology and biopharmaceutics reviews, medical reviews of the efficacy clinical trials for BH4, a statistical review of company analysis of trial results, the proprietary name review, and review and approval of labeling information for consumers for BH4, as well as summary documents for the new drug application review process. The materials from the European Medicines Agency included the following documentation: a public summary of orphan designation for BH4, announcement of the drug's market approval, the report from the Committee for Medicinal Products for Human Use which provided detail about the clinical trials supporting the drug's approval, the European Public Assessment Report used to provide information to the public about BH4, and the labeling review and label information. The materials from Health Canada included only the Summary Basis of Decision report which described the evidence used to approve BH4 for the Canadian market. The information in the Committee for Medicinal Products for Human Use and Summary Basis of Decision reports mirrored information in the various documents from the FDA. Because there was significantly less detail in those reports, we decided to use only FDA documents for the grey literature analysis of the published literature.
While there is evidence of publication bias for some pharmaceuticals on the market when comparing the grey and published literatures,126, 127 there was no such discrepancy for BH4. Our review of regulatory documents found no missing studies. In order to compare the grey literature with the published literature, we extracted data from the FDA approval documents about the study design, patient characteristics, type of randomization, length of study, drug dosing protocol, pretreatment blood Phe levels, and the outcomes measured (Table 18). Information in these documents included summaries of the data submitted to the FDA as part of BioMarin's new drug application (NDA) for BH4. Next, we identified publications of those trials and compared the data submitted to the FDA with the information contained in the published literature. We examined the concordance between the published and grey literatures, looking for differences in how data were reported or the absence of grey literature data in the published literature. The published literature was essentially identical to the information on safety and efficacy provided to the FDA as part of the new drug application for its approval to the U.S. market. Further information on these studies is included in Appendix J.
Table 18
FDA documentation used for Kuvan approval process.
As part of the FDA approval process, BioMarin agreed to conduct the following postmarketing commitment studies (Table 19):
Table 19
Summary of Kuvan commitment studies.
- Assessment of the safety, efficacy, and pharmacokinetics of BH4 in children younger than 4 years old;
- Assessment of growth and neurocognitive development with long-term use of BH4 in children eight years old or younger at study entry;
- An open label extension with participants in the pivotal efficacy studies to continue the treatment period to 2 years;
- The creation of a registry of individuals treated with BH4 to collect long-term clinical status information, including a substudy of the effects of BH4 on pregnancy and lactation;
- Completion of a thorough cardiac study in healthy volunteers;
- Completion of a PAH gene mutation study to identify treatment responders and
- Assessment of the safety and efficacy of BH4 in individuals with hyperphenylalaninemia due to BH4 deficiency.
For commitment 1, the study of 61 PKU participants <4 years old is noted by the FDA as delayed (the due date has passed and no final report has been submitted). The study for commitment 2, with an estimated enrollment of 230, is in children with PKU 0 to 6 years of age and is ongoing. An open label extension of the NDA studies appears to meet the requirement of commitment 3; the study, PKU-008, is noted as “completed” by www.clinicaltrials.gov, “submitted” on the FDA Web site, and now has published results.112
BioMarin and Merck KGaA have set up U.S. (PKU Demographic, Outcomes, and Safety [PKUDOS] registry) and European (Kuvan Adult Maternal Pediatric European Registry [KAMPER]) registries, respectively, for commitment 4 that are recruiting and ongoing. The PKUDOS registry includes a substudy on pregnancy and lactation effects, including a subregistry of pregnant women with PKU (PKUMOMS); data from the PKUDOS and KAMPER registries are due for submission to the FDA in early 2025. A completed but as yet unpublished study in 56 healthy volunteers to evaluate BH4's effect on QT intervals is listed by the FDA as having fulfilled commitment 5. Commitment 6 requires analysis of blood samples for PAH gene mutation collected as part of an NDA study (PKU-001); it is listed as fulfilled, and some data are published in the Levy et al. RCT of BH4.113 PKU-007, another open label extension of NDA studies in an estimated 12 individuals, has been completed and is listed by the FDA as submitted for commitment 7.
A number of other postmarketing studies have been initiated and should provide some additional data regarding the use of BH4 in the treatment of PKU (Table 20). Two studies are looking at the efficacy of BH4 in individuals with PKU older than 4 years old. The ENDURE study, based in Denmark and Norway and sponsored by Merck KGaA / Merck Serono, is in 150 patients and is ongoing. The other study, sponsored by the University of Miami, is in 20 patients with an unknown status at the time of publication. One ongoing study sponsored by Graz Medical University in Austria includes 30 PKU participants 4 to 18 years old and uses blood Phe level to evaluate a test to identify BH4-responsive individuals. To evaluate the effect of BH4 on amino acids and fatty acid patterns, the Aragon Institute of Health Sciences in Spain has sponsored a study in 30 PKU participants that is noted as recruiting.
Table 20
Summary of additional Kuvan postmarketing studies.
Two studies sponsored by U.S.-based academic centers are evaluating BH4 on cognitive effects. The first, sponsored by Tulane University School of Medicine, is looking at executive function and behavior in 30 participants with PKU between 2 and 21 years of age and is currently recruiting. Another study, sponsored by Washington University School of Medicine in collaboration with BioMarin and University of Missouri, Columbia, is using the Wechsler Abbreviated Intelligence Scale to study cognition in 35 participants with PKU ≥ 6 years of age and enrolling by invitation only.
Three studies are evaluating behavioral effects of BH4 in PKU. One BioMarin-sponsored study is a U.S.- and Canada-based RCT recruiting 200 individuals with PKU and ≥12 years of age to evaluate attention deficit hyperactivity disorder symptoms and BH4 use. The second is studying effect on behavior in 20 six to 18 year old individuals with PKU (sponsored by Washington University School of Medicine collaborating with BioMarin and the University of Missouri, Columbia, Northwestern University, and Oregon Health and Science University, enrolling by invitation only). The third study, from the University of Southern California collaborating with BioMarin, is evaluating behavior in 13 participants with PKU taking BH4 (enrollment by invitation only).
Three studies are evaluating the effect of BH4 on the brain. One focuses on brain glucose metabolism using positron emission tomography imaging in five adults with PKU (sponsored by Children's Hospital of Philadelphia and recruiting). The second uses magnetic resonance imaging to study the drug's effect on brain connectivity in 20 individuals ≥6 years of age (sponsored by University of Missouri, Columbia collaborating with BioMarin and enrolling by invitation only). The third study, sponsored by Emory University collaborating with BioMarin and the Clinical Interaction Network of the Atlanta Clinical and Translational Science Institute, is an ongoing study of the effect on neurotransmitter concentrations in 62 individuals with PKU ≥4 years of age (Table 20).
Summary
The results of the commitment studies and the other ongoing clinical studies, especially those focusing on neurocognitive development and behavior, will be especially critical to shed light on the clinical utility of BH4 treatment for the management of PKU. It should be noted, however, that the majority of clinical studies are sponsored by BioMarin or Merck, with only 5 studies out of 24 being conducted independently of BH4 marketers. The efficacy studies for the drug used blood Phe levels as surrogate endpoints to assess the broader benefits of drug treatment. This fact indicates that additional research is needed to confirm that the drug (and any additional nutritional supplementation used in conjunction with drug therapy) has positive outcomes on the neurocognitive development of children with PKU.
Conference Abstracts
We identified 46 abstracts that appeared to address adjuvant treatment for PKU; abstracts discussing the same population may have been presented at multiple conferences. Thirty-six abstracts appeared to be unpublished at this point (10 are now represented in the published literature, and 4 of these 10 studies are discussed in this review112-114, 119). Conference abstracts are considered unpublished or ongoing studies at this time, and because there is inadequate information to fully extract the studies or to assess quality, they cannot be integrated with the results in the review. Nonetheless, preliminary results appear consistent with the published literature and as these data are published, they should provide additional information on short- and long-term efficacy, effects on behavior, and nutritional outcomes. We provide the abstracts in Appendix K as information for the reader.
- Article Selection
- What is the evidence that any specific phenylalanine (Phe) levels are optimal for minimizing or avoiding cognitive impairment in individuals with PKU?
- What is the evidence that different target Phe levels are appropriate for minimizing or avoiding cognitive impairment for different age groups?
- What is the comparative effectiveness of BH4 with dietary intervention versus dietary intervention alone for affecting outcomes including measures of cognition (including executive function), quality of life, and nutritional status?
- What is the comparative effectiveness of BH4 with dietary intervention versus dietary intervention alone in pregnant women with PKU for affecting outcomes in their infants, including prevention of neurological impairment, microcephaly, and cardiac defects?
- What is the comparative effectiveness of large neutral amino acids (LNAAs) with dietary intervention versus dietary intervention alone for affecting outcomes including measures of cognition (including executive function), quality of life, and nutritional status?
- What is the comparative effectiveness of LNAAs with dietary intervention versus dietary intervention alone in pregnant women with PKU for affecting outcomes in their infants, including prevention of neurological impairment, microcephaly, and cardiac defects?
- What are the harms, including adverse events, associated with the use of BH4 or LNAAs in individuals with PKU?
- What is the evidence for the effectiveness of the addition of BH4 or LNAAs to dietary intervention for affecting outcomes in subgroups of patients?
- Grey Literature
- Results - Adjuvant Treatment for Phenylketonuria (PKU)Results - Adjuvant Treatment for Phenylketonuria (PKU)
- Preface - Combination Therapy Versus Intensification of Statin Monotherapy: An U...Preface - Combination Therapy Versus Intensification of Statin Monotherapy: An Update
- Preface - Chronic Venous Ulcers: A Comparative Effectiveness Review of Treatment...Preface - Chronic Venous Ulcers: A Comparative Effectiveness Review of Treatment Modalities
- Detailed Search Strategies - Combination Therapy Versus Intensification of Stati...Detailed Search Strategies - Combination Therapy Versus Intensification of Statin Monotherapy: An Update
- Risk of Bias Assessment - Venous Thromboembolism Prophylaxis in Major Orthopedic...Risk of Bias Assessment - Venous Thromboembolism Prophylaxis in Major Orthopedic Surgery: Systematic Review Update
Your browsing activity is empty.
Activity recording is turned off.
See more...