NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Fink HA, Wilt TJ, Eidman KE, et al. Recurrent Nephrolithiasis in Adults: Comparative Effectiveness of Preventive Medical Strategies [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Jul. (Comparative Effectiveness Reviews, No. 61.)
This publication is provided for historical reference only and the information may be out of date.

Recurrent Nephrolithiasis in Adults: Comparative Effectiveness of Preventive Medical Strategies [Internet].
Show detailsDefinition of Nephrolithiasis
Nephrolithiasis is a condition in which hard masses (kidney stones) form within the urinary tract. These stones form from crystals that separate out of the urine. Formation may occur when the urinary concentration of crystal-forming substances (e.g., calcium, oxalate, uric acid) is high and/or that of substances that inhibit stone formation (e.g., citrate) is low.
Epidemiology of Nephrolithiasis
Although kidney stones may present at any age, onset is more common in young and middle-aged adults. Lifetime prevalence is 13 percent for men and 7 percent for women.1,2 Reports conflict regarding whether incidence is rising overall, but consistently report rising incidence in women and a falling male-to-female ratio.3–5 Following an initial stone event, the 5-year recurrence rate in the absence of specific treatment is 35 to 50 percent.7
Approximately 80 percent of adults with kidney stones have stones consisting predominately of calcium oxalate and/or calcium phosphate. Struvite stones and uric acid stones each account for 5 to 10 percent of stones, and cystine stones are rare.9 Given that kidney stones are more likely to form when conditions favor separation of crystals out of the urine, it is not surprising that many patients with kidney stones, in addition to having low urine volume, have one or more biochemical abnormalities in the urine or blood. Hypercalciuria is most common, while other abnormalities may include hypercalcemia, hyperuricemia, hyperuricosuria, hyperoxaluria, hypocitraturia, and either low or high urine pH.10,11
In many patients, both stones and biochemical abnormalities are caused by an interaction between genetic inheritance and environmental exposure.12 Genetic factors are thought to account for about half the risk of developing kidney stones.12,18–21 With respect to environmental factors, large observational studies have shown that low fluid intake, low calcium intake, and high fructose intake increase stone risk, while evidence is mixed for increased animal protein, increased sodium, increased sucrose, and low magnesium.13–17 Risk of kidney stones also appears to vary by beverage type,67,68 and may be increased by medical conditions such as primary hyperparathyroidism,18 obesity,19 diabetes,20 gout,21 and intestinal malabsorption,22 and by anatomic abnormalities such as medullary sponge kidney and horseshoe kidney.
Clinical Presentation of Nephrolithiasis
Kidney stones are often incidentally identified when patients undergo plain radiographs or computed tomographic imaging for another indication.6 Stones may become symptomatic when they pass out of the renal pelvis into the ureter, with potential symptoms including renal colic with abdominal and flank pain; nausea and vomiting; urinary urgency and/or bleeding; urinary tract obstruction; infection; and acute though generally transient impairment in kidney function. While even stones as small as 1 mm in diameter may cause symptoms,69 90 percent of stones smaller than 5 mm pass through the urinary system without requiring intervention to aid expulsion. By comparison, approximately 50 percent of stones 5 to 10 mm in diameter require intervention to aid expulsion.70 Large stones (e.g., struvite) also may remain in the renal pelvis and not cause pain. Studies have suggested that kidney stones may increase the risk of chronic kidney disease;71,72 they may also may lead to hospitalizations and procedure-related morbidity. Direct medical expenditures associated with kidney stones may exceed $4.5 billion annually in the United States.1,8
Laboratory Evaluation of Nephrolithiasis
Clinical guidelines recommend laboratory evaluation of patients who experience a kidney stone. Testing may include analysis of stone composition and biochemical evaluations of blood (e.g., calcium, albumin, creatinine, uric acid, potassium, bicarbonate, parathyroid hormone) and urine (e.g., pH, volume, calcium, creatinine, uric acid, oxalate, citrate, sodium, phosphate, sulfate, magnesium).30 Clinicians may use laboratory evaluations to guide initial treatment selection, to assess treatment adherence or effectiveness, and to adjust pharmacological treatment dosing. It is not clear, however, whether pretreatment laboratory test results predict effectiveness of treatment on stone recurrence or other clinical health outcomes, or whether treatment tailored to pretreatment laboratory results is associated with reduced stone recurrence risk and better clinical health outcomes than empiric therapy. Neither do we know whether follow-up biochemical test results are valid surrogates for predicting stone recurrence. Current practice varies in the use of both initial and followup biochemical testing, particularly in patients who present as first-time stone formers.
Prevention of Recurrent Stone Disease
Many randomized controlled trials (RCTs) have examined dietary or pharmacological interventions to reduce risk of recurrent kidney stones. Yet, few of these RCTs are referenced by large clinical guidelines on the management of kidney stones, despite the fact that these guidelines include recommendations to modify various dietary components and to consider selected pharmacological therapies.30,73
Dietary Therapy for Prevention of Recurrent Stone Disease
Dietary interventions are designed to alter the concentration of one or more crystal-forming and/or crystal-inhibiting substance in the urine. Increasing water intake should increase urine volume and lower the urinary concentration of all crystal-forming substances. More narrowly targeted dietary interventions include reducing dietary oxalate to lower urinary oxalate and risk of calcium oxalate stones; reducing dietary animal protein and other purines to lower urinary uric acid and risk of uric acid stones; and maintaining normal dietary calcium to bind intestinal oxalate and thereby lower urinary oxalate and risk of calcium oxalate stones. Some demographic characteristics and comorbidities predict recurrent stone outcomes, but it is unclear how these factors affect relative effectiveness of treatments. It also is unclear how patient biochemical and stone characteristics affect treatment outcomes; nonetheless they are sometimes used to justify tailored dietary interventions.
Pharmacological Therapy for Prevention of Recurrent Stone Disease
Previous systematic reviews of RCTs of pharmacological therapies have reported that thiazide diuretics24–26 and citrate therapy26,27 reduce stone recurrence, but that evidence was insufficient regarding the efficacy of other pharmacological treatments.24,26,28,29 These reviews did not include more recent RCTs. Nor did they evaluate evidence that compared different pharmacological treatments with each other or combinations of pharmacological treatments versus monotherapy. Previous reviews also did not account for baseline fluid and diet intake or the effect of fluid and dietary co-interventions in pharmacological treatment trials. Further, previous reviews did not address the potential impact of patient demographics, comorbidities, biochemical measures, and stone characteristics on pharmacological treatment outcomes.
Purpose of Comparative Effectiveness Review
Current practice to prevent recurrent kidney stones varies significantly. Clinical uncertainty exists regarding the effectiveness, comparative effectiveness, and adverse effects of dietary and pharmacological preventive treatments; the value of urine and blood biochemical measures for initiating and/or modifying treatment; and the potential impact of patient and stone characteristics on important treatment outcomes. Our review and meta-analysis comprehensively addresses these questions to the degree possible with available data. Our findings should inform providers and patients making treatment decisions, organizations developing clinical guidelines, policymakers making coverage decisions, and researchers designing future studies to address remaining evidence gaps.
Analytic Framework and Key Questions
During this project’s topic refinement, the topic nominator and other interested parties agreed that an independent, comprehensive review of the issues introduced above and as elaborated in the following analytic framework (Figure 1) and Key Questions would provide helpful guidance to clinicians and policymakers regarding prevention of recurrent kidney stones.
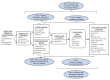
Figure 1
Analytic framework.
Key Question 1
In adults with a history of nephrolithiasis, do results of baseline stone composition and blood and urine chemistries predict the effectiveness of diet and/or pharmacological treatment on final health outcomes and intermediate stone outcomes, and reduce treatment adverse effects?
- Do effectiveness and adverse effects of treatment differ according to patient baseline stone composition and blood and urine biochemical measures?
- Does treatment tailored to the results of baseline stone composition and blood and urine chemistries improve final health outcomes and intermediate stone outcomes, and reduce adverse effects compared with empiric treatment?
Key Question 2
In adults with a history of nephrolithiasis, what is the effectiveness and comparative effectiveness of different dietary therapies on final health outcomes and intermediate stone outcomes?
- Does effectiveness of diet therapy differ according to patient baseline demographic and comorbid characteristics?
- Does effectiveness of diet therapy differ according to patient baseline diet and fluid intake?
- Does effectiveness of diet therapy differ according to characteristics of stone history?
Key Question 3
In adults with a history of nephrolithiasis, what is the evidence that dietary therapies to reduce risk of recurrent stone episodes are associated with adverse effects?
- Does the risk of adverse effects differ according to patient baseline demographic and comorbid characteristics?
- Does the risk of adverse effects differ according to patient baseline diet and fluid intake?
- Does the risk of adverse effects differ according to characteristics of stone history?
Key Question 4
In adults with a history of nephrolithiasis, what is the effectiveness and comparative effectiveness of different pharmacological therapies on final health outcomes and intermediate stone outcomes?
- Does effectiveness differ according to patient baseline demographic and comorbid characteristics?
- Does effectiveness differ according to patient baseline diet and fluid intake?
- Does effectiveness differ according to characteristics of stone history?
Key Question 5
In adults with a history of nephrolithiasis, what is the evidence that pharmacological therapies to reduce risk of recurrent stone episodes are associated with adverse effects?
- Does the risk of adverse effects differ according to patient demographic and comorbid characteristics?
- Does the risk of adverse effects differ according to patient baseline diet and fluid intake?
- Does the risk of adverse effects differ according to characteristics of stone history?
Key Question 6
In adults with a history of nephrolithiasis being treated to prevent stone recurrence, do results of followup blood and urine biochemistry measures predict final health outcomes and intermediate stone outcomes?
- Does prediction of final health outcomes and intermediate stone outcomes differ according to the frequency or duration of followup biochemistry measurements?
- Introduction - Recurrent Nephrolithiasis in AdultsIntroduction - Recurrent Nephrolithiasis in Adults
- Introduction - Nonsurgical Treatments for Urinary Incontinence in Adult Women: D...Introduction - Nonsurgical Treatments for Urinary Incontinence in Adult Women: Diagnosis and Comparative Effectiveness
- Schizosaccharomyces pombe Cdc42 GTPase complex scaffold subunit Scd2 (scd2), mRN...Schizosaccharomyces pombe Cdc42 GTPase complex scaffold subunit Scd2 (scd2), mRNAgi|2745689427|ref|NM_001019175.3|Nucleotide
- kinesin-like protein KIF24 isoform X3 [Mus musculus]kinesin-like protein KIF24 isoform X3 [Mus musculus]gi|1039766357|ref|XP_017175402.1|Protein
- Acknowledgments - Local Therapies for Unresectable Primary Hepatocellular Carcin...Acknowledgments - Local Therapies for Unresectable Primary Hepatocellular Carcinoma
Your browsing activity is empty.
Activity recording is turned off.
See more...