NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
O’Connor E, Patnode CD, Burda BU, et al. Breathing Exercises and/or Retraining Techniques in the Treatment of Asthma: Comparative Effectiveness [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Sep. (Comparative Effectiveness Reviews, No. 71.)
This publication is provided for historical reference only and the information may be out of date.

Breathing Exercises and/or Retraining Techniques in the Treatment of Asthma: Comparative Effectiveness [Internet].
Show detailsLiterature Search
Our literature search yielded 2,415 citations. From these, we provisionally accepted 106 articles for review based on abstracts and titles (Figure 2). After screening their full texts, 22 studies,50–71 published in 42 articles,50–91 were judged to have met the inclusion criteria (Appendix D). All of these studies were RCTs except one, which was a randomized crossover trial.51 The remaining 64 full-text articles were excluded (Appendix E). The primary reasons for exclusion included not studying breathing techniques, not providing primary data, not using one of the specified study designs, and being rated as poor quality. The eight publications excluded for quality concerns represented six unique studies. Most were excluded because they failed to describe multiple important areas of their methods (e.g., randomization methods and followup rates and inclusion/exclusion rules and assessment methods were all missing) and the remaining were very small trials (n=12 and 17) that either lacked comparability between groups at baseline92–94 or did not report acceptable measurement or analysis methods.95
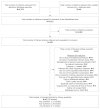
Figure 2
Literature flow diagram.
All trials were conducted with people with symptomatic asthma. Most were limited to those with stable asthma (e.g., stable dose of asthma medication, no recent use of oral steroids, and/or no recent hospitalization for asthma). Some trials were limited to people with a certain level of beta2-agonist use (e.g., twice daily,50,69 twelve times per week,56 four times per week,51,58 or twice weekly52), suggesting their asthma was not well controlled. Most trials confirmed reversibility of respiratory symptoms through pulmonary function testing. Trials primarily included adults; only one trial of IMT targeted children (ages 8 to 12 years),65 and only four other trials included people younger than 16 years of age.50,53,56,58 Trials used a variety of breathing retraining techniques (Table 3), including interventions that targeted hyperventilation reduction (e.g., Buteyko breathing technique, Papworth method),50–59,71 yoga breathing techniques,60–64 IMT,65–69 and other controlled breathing approaches using prolonged exhalation or abdominal breathing.52,70,71 Four of the trials of hyperventilation reduction used alternate breathing techniques for comparison50,52,53,58 and seven used some kind of usual care, placebo, wait list, or attention control group.51,52,54–57,59 One trial had two study arms with different treatments in addition to a placebo-control group. Comparisons from this study will be discussed in multiple sections of this report.52
Table 3
Included breathing retraining Interventions and comparisons.
Thirty-two percent of the trials described allocation as concealed. Asthma symptoms, medication use, and quality of life were usually based on self-report, and only 41 percent of the trials reported that outcomes assessments were conducted blindly. Lack of blinding may be especially problematic for pulmonary function testing, which is effort-dependent and involves coaching to get an optimal performance. Lack of blinding may also be problematic for self-reported outcomes, where social desirability could introduce bias. Most trials were small, with 68 percent including only 30 or fewer participants per treatment arm. Only a single trial included more than 100 participants per treatment arm.56 Trials were also inconsistent the degree to which they ensured the sample was limited to asthmatics: 42 percent did not report the use of pulmonary function testing to confirm an asthma diagnosis and 39 percent did not describe limiting excluding participants with other respiratory disorders or people at high risk for other respiratory disorders (e.g., smokers).
Results of Included Studies
We discuss results for the five different types of comparisons separately: hyperventilation reduction breathing techniques compared with control groups (Table 4) or with other nonhyperventilation reduction breathing techniques (Table 5); yoga breathing compared with control groups (Table 6); IMT compared with control groups (Table 7); and other nonhyperventilation reduction breathing techniques compared with control groups (Table 8). Table 9 briefly describes the instruments, including directionality, to aid in the interpretation of standardized scales (see end of chapter for all tables).
Table 4
Overview of results: hyperventilation reduction breathing techniques versus control.
Table 5
Overview of results: hyperventilation reduction breathing techniques versus nonhyperventilation reduction breathing techniques.
Table 6
Overview of results: yoga breathing techniques versus control.
Table 7
Overview of results: inspiratory muscle training versus control.
Table 8
Overview of results: nonhyperventilation reduction breathing techniques versus control.
Table 9
Instruments used for measuring asthma symptoms, control, quality of life, or related outcomes.
Key Question 1. In adults and children 5 years of age and older with asthma, does the use of breathing exercises and/or retraining techniques improve health outcomes, including: symptoms (e.g., cough, wheezing, dyspnea); health-related quality of life (general and/or asthma-specific); acute asthma exacerbations; reduced use of quick-relief medications or reduced use of long-term control medications, when compared with usual care and/or other breathing techniques alone or in combination with other intervention strategies?
Hyperventilation Reduction Breathing Techniques Versus Control Group
Key Points:
- We found moderate evidence that hyperventilation reduction breathing technique interventions with 5 or more hours of direct instruction may reduce asthma symptoms and the use of reliever medication in adults, though evidence was limited to a fairly small number of trials, most of which were at moderate risk of bias due to factors such as small sample sizes, high or differential attrition, and lack of appropriate blinding.
- Evidence is low or insufficient that hyperventilation reduction training affects controlled medication use, quality of life, or functioning in adults and children.
Eight trials (n=1,088) compared breathing retraining targeting hyperventilation reduction with a control group. Seven were RCTs52,54–57,59,71 and one was a randomized crossover trial51 (Table 4; Appendix D, Evidence Table 1a). The 4-week cross-over trial focused only on one component of the Buteyko breathing technique, specifically mouth-taping,51 while the remaining provided broader instruction in modification of breathing (Appendix D, Evidence Table 1b). Interventions generally involved controlled, shallow breathing, and encouraging diaphragmatic breathing over chest breathing, with a breath-hold at the end of the exhalation. They advocated breathing through the nose at all times, and in some trials participants were encouraged to use a porous tape to hold the mouth closed at night while sleeping. They trained users to limit what they term “overbreathing” with sighs, yawns, and gasps. They encouraged clients to use breathing techniques when they experienced asthma symptoms for 5 to 10 minutes before using bronchodilators.
Four of these trials involved fairly extensive interventions, reporting 6 to 12 months of followup.52,54–56 These trials provided 5 to 13 hours of contact with instructors, encouraged daily practice at home, and two of these included additional lifestyle components beyond breathing retraining, of dietary and sleep advice52 and stress management.52,55 Only one of these four trials included a control group with matching treatment intensity,56 the others compared hyperventilation reduction breathing training to usual care,54,55 or a sham breathing training device.52 One trial was limited to people with dysfunctional breathing, according to the Nijmegen questionnaire.71 This instrument was designed to identify patients with chronic or habitual breathing patterns that induce hyperventilation, and assesses symptoms purported to identify hyperventilation (some of which may also be related to asthma symptoms) such as accelerated or deepened breathing, being unable to breathe deeply, palpitations, tightness around the mouth, tingling fingers, and dizzy spells.
Three lower-intensity interventions targeted breathing retraining only (i.e., included no co-interventions that were not directly targeting breathing retraining), but did attempt to provide comprehensive training rather than focusing only on a single aspect of the training. One trial used a video for both instruction and daily practice,57 and the two others, conducted by the same researcher using very similar interventions, offered 1 to 2 hours of direct instruction.59,71 Interventionists in all of these trials encouraged daily practice at home. One trial reported only 4-week outcomes,57 and the other had 26-week followup for some or all outcomes.59,71 Both trials attempted to provide attention-control comparators, one with relaxing landscape videos57 and the other with general asthma education.59
Among all of the hyperventilation reduction trials with control group comparators, three used the Buteyko method,52,56,57 three used the Papworth method or were described as similar to the Papworth method,55,59,71 and one did not identify its methods as being either Buteyko or Papworth, but the description of the intervention was consistent with Buteyko and Papworth breathing methods,54 and one addressed only a single, narrow aspect of the Buteyko method (mouth taping).51
All trials were rated as fair quality (Table 10). Two of the trials suffered from fairly high attrition52,59 and three had greater attrition in the intervention group by at least ten percentage points at one or more followups.52,55,59,71 Allocation concealment was reported in only three trials,54,56,59 and outcomes assessment was clearly blinded in only four of the trials.51,52,54,56 Only two of the RCTs randomized more than 50 participants per group,56,59 and three trials had only 1-month of followup for some or all outcomes.51,57,59
Table 10
Quality and applicability issues: hyperventilation reduction breathing techniques versus control.
Six of these trials were conducted in the United Kingdom,51,52,55,56,59,71 one in was conducted in Greece,54 and one was conducted in Australia.57 All trials were conducted in health care settings. The minimum ages of included participants ranged from 14 to 18 years, and most trials included adults up to ages 60 to 72 years. The average baseline reliever used in most trials was one to two puffs per day, generally along with 400 to 600 mcg of ICS use daily (in beclomethasone equivalent), and FEV1 between 80 percent and 89 percent. The Australian trial had somewhat higher reliever medication use than the other four, with an average of 404 mcg per day at baseline (along with an average 430 mcg of ICS daily).57
Asthma Symptoms
All eight trials reported some type of asthma symptom outcome, which was usually a standardized questionnaire (Table 4; Appendix D, Evidence Table 1c). All four of the most-intensive and comprehensive interventions reported improvements in asthma symptoms at 6- to 12- months of followup.52,54–56 Only four of the trials provided sufficient information to pool in a meta-analysis of asthma symptom scores, three of the four most intensive trials,54–56 and one lower-intensity trial comparing 2 to 2.5 hours of Buteyko training with 2 to 2.5 hours of asthma education.59 The standardized pooled effect size (or standardized mean difference [SMD]) for the four trials with sufficient data to be included in a meta-analysis was −1.39 (95% confidence interval [CI], −2.61 to −0.17, Figure 3).54–56,59 This analysis had very high statistical heterogeneity (I2=97.1) and a wide range of effect sizes. However, because the pooled effect is very similar to effect seen in two of the trials, and the other two are approximately equidistant from the pooled estimate in opposite directions, the pooled effect may be a reasonable estimate for an average effect despite the high heterogeneity.

Figure 3
Effect of hyperventilation reduction techniques on asthma symptoms at 6 to 12 months. CI: confidence interval; CG: control group; est: estimated; IG: intervention group; N: sample size; SD: standard deviation; SMD: standardized mean difference
The largest trial showed the largest effect, with SMD of −2.58 (95% CI, −2.86 to −2.29). Symptom ratings on a scale of 0 (no symptoms) to 3 (severe symptoms) dropped from an average of 2.2 at baseline for all groups to 0.7 in the Buteyko group, while the control groups slightly increased to 2.4 to 2.5.56 This was one of the relatively few trials reporting both allocation concealment and blinding of outcome assessors, although retention was somewhat lower in both control groups (82.5% and 73%) than the Buteyko group (90%).
Two other trials, both with fairly intensive interventions, reported standardized effect sizes greater than 1.2, which would generally be considered large.54,55 In the trial by Holloway and colleagues, for example, the Papworth intervention group participants showed 18- to 21-point improvements on the 100-point SGRQ symptom subscale, compared with two-point improvements in the control group at 6 and 12 month followup.55 This change is even greater than the change on the SGRQ seen in patients whose treatment was judged to be “very effective” in other research.45 Outcomes assessment was not blinded in this trial, which may have artificially increased the effect size if intervention participants were more prone to demand characteristics. On the other hand, this trial relied on an asthma registry to recruit patients and did not independently verify the asthma diagnosis with pulmonary function testing. As such, if some of the patients were misdiagnosed and actually had chronic obstructive pulmonary disease or another respiratory condition, then this would likely attenuate the intervention’s effect. The asthma registry approach likely increases the applicability to typical clinical settings.
Similarly, the trial conducted in Greece by Grammatopoulou and colleagues54 showed intervention participants moving from a score consistent with uncontrolled asthma to one in a range similar to those with completely controlled asthma at 26-week followup.96,97 The average control group score, on the other hand, remained below the average score of someone with well-controlled asthma.
The other fairly intensive trial, which was not included in the meta-analysis, reported mixed results, found differences in symptom scores from daily diaries, but no group differences in a standardized symptom scale.52 This was a fairly small trial (n=30 per group) with fairly low followup at 6 months (77% retention in the intervention group vs. 80% in the control group), using a last-observation-carried-forward (LOCF) data substitution method.
Of the remaining trials, which were all fairly low intensity, only one reported statistically significant improvements in symptoms, and only at four weeks.71 The other trials did not find improvements in asthma symptoms after 1 to 6 months.51,57,59 Other than the mouth-taping cross-over trial, these trials showed effect sizes consistent with small beneficial effects, but group differences were not statistically significant.
Medication Use
Medication use was reduced in three52,56,57 of the six trials reporting these outcomes (Table 4; Appendix D, Evidence Table 1d).51,52,56,57,59,71 However, medication use was reported in only two of the four higher-intensity trials.52,56 In the largest trial, reliever medication use dropped from 18 puffs per week at baseline in all groups to less than one puff per week in the intervention group, compared with no change in either of the control groups at 6-month followup.56 Although specific data were not shown, this trial also reported greater reductions in the use of controller medication. Although the data were self-reported, and may be subject to demand characteristics (since Buteyko participants were encouraged to delay bronchodilators), they were gathered by blind outcome assessors.
Reliever medication use was reduced at 6-month followup in the trial by Cooper and colleagues comparing a Buteyko intervention with a sham breathing retraining device.52 SABA use was reduced by a median of two puffs per day in the intervention group, compared to no change in the control group. Neither ICS use nor prednisone use differed at 6-month followup in this trial. After 6-month followup, 60 of the 69 participants completing the first phase of the trial took part in a steroid-reduction phase. Intervention participants in this phase reduced ICS use by a median of 41 percent, compared with no reduction in the control group. However, this difference was not tested directly; instead, the authors report only the results of the three-way comparison between the Buteyko group and another treatment arm (a device to control the pace of breathing), which was not statistically significant (p=0.70). This is the trial with fairly low and differential retention that used LOCF as a data substitution method. Medication use was not reported in the two other higher intensity trials that showed large positive effects on asthma symptoms.54,55
Medication outcomes were reported in the mouth-taping trial51 and the other three lower-intensity trials.57,59,71 Reductions in bronchodilator use in the trial of video-based instruction57 were similar to those seen in the trial by Cooper and colleagues.52 This was a small trial (n=36) with only 4 weeks of followup, but did have fairly high retention (89% in each group). This trial reported no group differences in ICS use. No group differences in medication use were seen in the mouth-taping trial51 or the lower-intensity Papworth-style interventions.59,71
Quality of Life and Functioning
Seven of the trials51,52,54,55,57,59,71 reported measures of asthma-related quality of life, functioning, or mental health symptomatology at 1 to 12 months post-baseline, and all but the one study51 (which focused only on mouth-taping) reported group differences in some measures (Appendix D, Evidence Table 1e).
Asthma-Related Quality of Life
Six trials51,52,55,57,59,71 reported asthma-specific quality of life using standardized measures, including only two of the higher-intensity trials (Table 4; Appendix D, Evidence Table 1e).52 Four reported statistically significant group differences at one or more time points. The trial with the higher-intensity Papworth intervention55 reported improvements on the SGRQ one year after the end of the intervention, however the differences appeared to be driven by the “symptoms” subscale, since neither the “impacts” nor the “activities” subscales showed group differences at either followup. Thus, improvements were seen in symptoms, but did not appear to affect other areas of day-to-day life. The remaining higher intensity trials either found no differences between groups52 or did not report this outcome.54,56 The three lower-intensity trials that reported group differences in a measure of quality of life up to 6 months post-baseline were the video-based treatment trial and both of the lower-intensity Papworth-style trials.57,59,71
Functioning and Mental Health
Changes in Short-Form Health Survey (SF-36) scores were reported in two trials, and although many SF-36 subscale outcomes were reported in these two trials, group differences were rarely seen (Appendix D, Evidence Table 1e).52,54 Two trials, including the Papworth intervention trial with a large effect on asthma symptoms,55 reported improvements in mental health outcomes of depression and anxiety at 26 weeks.55,59 Anxiety and depression scales scores indicated that participants were not, on average, anxious or depressed either before or after treatment. Reductions were small in magnitude (e.g., group differences of 1.6 on a 68-point rating scale). Group differences were maintained in one of these trials to 1 year.55
Hyperventilation Reduction Breathing Techniques Versus Other Breathing Techniques
Key Points:
- Hyperventilation reduction breathing techniques may be more likely to reduce reliever medication use than other breathing techniques in adults, but strength of evidence is low.
- Hyperventilation reduction training is no more likely to improve symptoms, controller medication use, or quality of life than other breathing techniques functioning in adults, but strength of evidence is low.
Four RCTs (n=285) directly compared the use of breathing techniques targeting hyperventilation reduction with another breathing technique that did not target hyperventilation reduction. Three of these trials used the Buteyko approach50,52,53 and one was modeled after the Papworth method (Table 5; Appendix D, Evidence Tables 2a and 2b).58 One of these involved a 10-hour Buteyko intervention and was described above, compared to the use of a device to modify breathing to achieve a typical yoga inspiration-to-expiration cycle of 1:2 with minimal one-on-one instruction and no components addressed other than the breathing technique.52
The three remaining trials employed comparators targeting controlled or paced breathing, but did not encourage the use of slow, nasal, shallow breathing with breath-holding or other techniques focused on reducing hyperventilation.50,53,58 All of these trials involved at least five contacts, usually face-to-face. Two trials used an approach that was initially intensive, meeting every day for 5 to 7 days for training.50,53 All three trials attempted to provide the same frequency and hours of treatment in both treatment groups. However, in one trial more than half of the Buteyko participants received additional instruction sessions and the average number of followup phone calls was seven in the Buteyko group, compared to one in the comparison group.50
In addition to some kind of breathing retraining in the nonhyperventilation reduction groups, one trial also included general asthma education and relaxation techniques,50 and another included shoulder and upper arm stretches.58
All four trials were rated as fair quality (Table 11). One trial had a number of quality-related issues, despite having followup on 95 percent of participants, including only a small number of participants randomized (n=20 or fewer per group), a very wide age range (age 12 to 70 years), no information on blinding of outcomes assessment, and reliance on self-report of variability in breathing symptoms that improve with beta2-agonist use for asthma diagnosis.50 Additionally, the Buteyko intervention was more intensive than the comparator.
Table 11
Quality and applicability issues: hyperventilation reduction breathing techniques versus nonhyperventilation reduction breathing techniques.
Another trial reported good measurement and randomization procedures, but was rated as “fair” quality because of the small number of participants (n=57 total) and retention below 90 percent.58 The remaining two failed to report either allocation concealment or blinding of outcomes assessment, and had either fairly high attrition overall52 or higher attrition in the Buteyko breathing technique group than the other intervention group,53 in addition to other minor issues.
These trials were conducted in Australia,50,58 the United Kingdom,52 and Canada.53 Average age ranged from 44 to 47, and all but one had a wide age range from 12 to 18 years up to 65 or older. Asthma severity was quite high in one trial, where participants were using an average of almost 900 mcg of reliever medication per day and 1,250 mcg of ICS (in beclomethasone equivalents).50 Baseline FEV1 was 74 percent in this trial. Participants in the remaining trials were using two to three puffs of reliever medication per day along with 650 to 850 mcg of ICS, with an average FEV1 around 80 percent.52,53,58
Asthma Symptoms
Two trials reported no group differences in asthma control, with little improvement in either group at 13 and 26 weeks (Table 5; Appendix D, Evidence Table 2c).50,53 Two reported no or minimal group differences but did report improvement in both treatment groups for either the asthma control questionnaire and physician global rating at 28 weeks58 or median change in the symptoms subscale of the Mini-AQLQ at 26 weeks.52 Within-group change in the latter was not tested statistically, but both groups showed a median improvement of more than 0.5, which is considered a clinically significant difference.98 The best quality trial in this group showed almost no group differences on five additional symptom scales; both groups improved on two of the additional symptom scales.58
Medication Use
Two50,52 of the three50,52,58 trials reporting reliever medication use found greater reductions with Buteyko breathing technique than either abdominal breathing50 or a device to train in the use of prolonged exhalation after 13 to 26 weeks (Table 5; Appendix D, Evidence Table 2d).52 The trial with the greatest baseline asthma severity (and the most quality concerns, including more intensive intervention contacts in the hyperventilation reduction group than the comparison breathing intervention) showed the greatest improvements in reliever use, reporting median reductions of 904 mcg per day in bronchodilator use at 3-month followup in the Buteyko group, compared with a 57 mcg reduction in the abdominal breathing group.50 The Buteyko group went from using approximately 9 to 10 puffs of beta2-agonist per day to approximately one puff every other day. The trial showing no group differences reported reductions in reliever medication by almost two puffs per day in both the hyperventilation-reduction group and the controlled breathing with stretching group.58
All four trials reported results for controller medication. Two trials reported little change in ICS use for either group,50,52 including the trial with the most dramatic results for beta2-agonists.50 Of the remaining trials, one reported that ICS use was reduced by 50 percent in both the Buteyko and the controlled breathing groups,58 and other trial reported greater reductions in ICS use and a greater likelihood of discontinuing long-acting beta2-agonists with hyperventilation reduction techniques than with a more typical physical therapeutic approach.53 In this trial, ICS use was reduced by an average of 317 mcg in the hyperventilation-prevention group and only 56 mcg in the physical therapy group. Two trials reported no differences between groups in prednisone use.50,52
Quality of Life and Functioning
All four trials reported an asthma-specific quality of life outcome, and none found that any group showed greater improvement than another group (Table 5; Appendix D, Evidence Table 2e). In two trials, both groups showed increases of more than 0.5 on the Juniper quality of life scales,52,53 which is the threshold for clinically significant change.45 However, the changes over time were statistically significant in only one of these two trials.53
One trial reported functioning outcomes and found that scores on the subscale role limitation due to physical problems improved by a median of 25 points on a 100-point scale at 3 months in the hyperventilation-prevention group, while the median change in the device-assisted yoga-style breathing was zero.52 Other functioning subscales showed little improvement in either group.
Yoga Breathing Versus Control
Key Points:
- Yoga breathing may improve asthma symptoms and quality of life in adults, but strength of evidence is low due to concerns about the methodological quality of the trials.
- Evidence is insufficient to determine whether yoga can reduce asthma medication use in adults and children.
Five trials (n=360) compared the yoga group with a control group (Table 6; Appendix D, Evidence Table 3a).60–64 All were fairly to highly intensive interventions and all required daily practice at home in addition to supervised sessions (Appendix D, Evidence Table 3b). Two programs conducted in India were very intensive.60,64 One included 4-hour sessions daily for 2 weeks covering yoga practice, lectures, group discussions, diet (including a study-provided breakfast), and stress management, followed by an additional 4 weeks of home practice.64 Another trial involved a 70-minute-long daily yoga session for 6 months and all patient were hospitalized initially to facilitate training.60 The duration of the inpatient stay was not specified. This trial was limited to male vegetarians aged 25 to 50 years.
A third trial in India was focused specifically on yoga breathing exercises among people with at least 6 months of prior yoga experience, compared with the use of meditation.63 Both treatment arms involved 20 minutes of practice twice daily for 12 weeks, although the number of these sessions that were supervised versus those conducted at home was not described. In this study, the authors reported that participants “had no history of regular medication and they were advised to discontinue if on any medication.” It was unclear if this is referring to all medication, or only asthma medication. No age limits were reported and the average age in this trial was 29 years.
The final two trials were conducted in the United States.61,62 One compared an eight-session yoga class with a stretching class.62 This trial was limited to participants aged 18 and over, with an average age of 51 years. The other trial involved a comprehensive naturopathic treatment program that included yoga as well as dietary restriction, nutritional supplements, and a guided journaling session.61 Participants in this trial were predominantly female and the average age was 44 years.
All trials were rated fair quality and three had substantial quality issues that limit our confidence in results (Table 12).60,61,63 Two of these trials were quite intensive and conducted in India.60,63 These trials included only 17 to 25 people per group, failed to report both allocation concealment and blinding of outcomes assessment, and provided no information on refusals or exclusions prior to randomization. In addition, one did not indicate how they divided the participants into groups and failed to report the use of pulmonary-function testing to confirm reversibility for asthma diagnosis.60 Also, the usual-care group in this trial received only bronchodilators, antibiotics, and expectorants, but not ICS. The other trial did not report the proportion of participants with followup, and it was unclear if their group assignment was truly random.63 The third trial was conducted in the United States and involved a comprehensive naturopathic intervention, which did not allow us to determine the effect of yoga breathing techniques specifically.61 Outcomes assessment in this trial was not blinded, and it was unclear whether those assigning participants to groups had access to intake assessment data. This trial also did not report the use of pulmonary function testing in the diagnosis of asthma, number of refusals or exclusions prior to randomization, nor did they describe whether they excluded people with other respiratory disorders or recent use of oral steroids from their sample.
Table 12
Quality and applicability issues: yoga breathing techniques versus control.
The Indian trial of daily 4-hour sessions also failed to report both allocation concealment and blinding of outcomes assessment, but had retention above 90 percent in both groups and good assessment procedures.64 The U.S.-based yoga class trial had the best methods of the group, but had low and somewhat differential retention (79% in the intervention group vs. 67% in the control group).62
Asthma severity was not consistently described in this subgroup of studies, but average severity would likely be considered to be moderate according to National Asthma Education and Prevention Program (NAEPP) as based on either daily reliever use62 or FEV1 in the “moderate asthma” range.
Asthma Symptoms
All but one trial62 showed greater improvement in the yoga groups on at least one measure of asthma symptoms, including all three trials conducted in India (Table 6; Appendix D, Evidence Table 3c).60,61,63,64 The U.S.-based trial of an eight-session yoga class reported no group differences in asthma symptoms.62 Although it was difficult to compare effect sizes across different measures, the largest effect size appeared to be found in one of the lower quality trials based in India, comparing yoga breathing exercises with meditation.63 This trial reported a 64 percent reduction in symptoms in the intervention group at 12 weeks, compared with a six percent reduction in symptoms in the meditation group.
Another trial with a very intensive intervention reported a very large effect size at 2- and 4-week followup, but the effect was attenuated (yet still statistically significant) after 8 weeks.64 In this trial and the U.S.-based trial of a comprehensive naturopathic intervention,61 both groups showed improvements in a Juniper symptom subscale well beyond the level of clinical significance (i.e., improvement of 0.5 points).45 Greater improvements were apparent, however, in those participating in the yoga interventions than those in the control groups.
Medication Use
Three trials reported medication use,60,62,64 including two trials conducted in India (Table 6; Appendix D, Evidence Table 3d).60,64 One trial found that 53 percent of yoga participants reduced medication required to control their dyspnea, compared with 18 percent in the control group after 26 weeks, but the specific type of medication was not reported.60 In the trial of daily 4-hour yoga sessions, as with asthma symptoms, both groups showed improvement in medication use: yoga participants reduced rescue medication use by an average of 1.5 puffs per day after 8 weeks compared with a reduction of 0.5 puffs per day among control participants.64 There were no statistically significant group differences between those taking the yoga class and those on the waiting list after 16 weeks.62
Quality of Life and Functioning
Three of the trials reported functioning or quality of life outcomes (Appendix D, Evidence Table 3e).61,62,64 The pooled standardized effect size for overall asthma-related quality of life in these three trials was 0.66 (95% CI, 0.21 to 1.10, I2=59.3%, Figure 4), consistent with improved asthma-related quality of life in yoga breathing groups compared to controls.
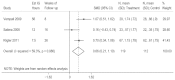
Figure 4
Effect of yoga breathing techniques on quality of life at 2 to 6 months. CI: confidence interval; est: estimated; IG: intervention group; N: sample size; SD: standard deviation; SMD: standardized mean difference
The eight-session yoga class did not lead to greater improvement in overall asthma-related quality of life than being on a waiting list after 16 weeks.62 Participants in both the comprehensive naturopathic intervention61 and the daily 4-hour sessions64 showed greater improvement overall asthma-related quality of life (again exceeding the threshold for clinically significant improvement) as well as the “activities” and “emotions” subscales than the usual care groups after 864 and 26 weeks.61 As before, however, the usual-care participants also showed clinically and statistically significant improvement in both of these trials. There were also group differences on the SF-36 subscales of physical and social functioning, role limitations due to physical limits, and both of the summary component scores (physical and mental) in the trial involving a comprehensive naturopathic treatment program.61
Inspiratory Muscle Training Versus Control
Key Points:
- Evidence is insufficient to draw conclusions about the effect of IMT on asthma symptoms, medication use, or quality of life in adults and children.
Five small trials (n=169) examined the effect of IMT on asthma (Table 7; Appendix D, Evidence Tables 4a and 4b) after 8 to 26 weeks.65–69 Three of these trials, all conducted by the same researcher in Israel, compared the use of a training device that controlled the level of resistance associated with inhalation with a sham device that provided no resistance.67–69 Level of resistance was gradually increased over the course of training with the active device, but the sham device provided no resistance at any setting. Participants’ average age ranged from 34 to 40 years, and no age limitations were listed for any of these trials. One trial was limited to women categorized as being in the mild-persistent to moderate range of asthma. Participants used an average of 3.2 puffs per day of reliever medication and had a baseline FEV1 of 83 percent.68 The second trial was limited to those using two or more puffs of SABA daily, with an average use of 2.7 puffs of reliever per day and a baseline FEV1 of 91 percent.69 The third trial was limited to people with severe asthma. Participants in this trial used an average of six puffs of reliever medication per day and had an average baseline FEV1 of 59 percent, the lowest of all included trials.67
The fourth trial was conducted among children in Brazil who had previously received no treatment for asthma and whose asthma was poorly controlled.65 Baseline FEV1 was not reported in this trial. The trial compared a 14-session program that included one-on-one instruction as well as IMT with the use of a breathing training device that built up inspiratory muscles through gradually increasing the resistance required for inspiration, plus medication (rescue and preventive) and three monthly medical visits for medication monitoring and general asthma education. This was compared with asthma education and medication alone.
The final trial was conducted in South Africa among inactive nonsmokers with moderate-persistent asthma and an average age of 22 years.66 This trial instructed participants in diaphragmatic breathing. Participants were told to hold a weight on their abdomen while breathing through a 1 centimeter wide tube. Control group participants received no breathing training.
All trials were rated fair quality, and all but one67 had fairly substantial quality issues (Table 13).65,66,68,69 The trials conducted in Israel included 15 or fewer participants per treatment group in all cases,67–69 although followup rates where high in two of the three trials.67,69 None of these trials reported whether allocation was concealed or whether they excluded participants with other respiratory disorders. None of these trials provided detailed inclusion/exclusion criteria and two them also failed to report information on baseline comparability of the treatment groups.68,69
Table 13
Quality and applicability issues: inspiratory muscle training versus control.
The trials in South Africa66 and Brazil65 were also fairly small including 22 to 25 participants per treatment arm with 100 percent followup. Neither trial, however, reported allocation concealment or blinding of outcomes assessment. In addition, the Brazilian trial did not appear to use pulmonary testing to confirm asthma diagnosis, provided little detail on their outcomes assessment methods, and they did not report whether IMT trainers were in contact with the larger asthma treatment team (and perhaps providing advice or support for general asthma management and medication use such as encouraging patients to use controllers consistently) as part of the fourteen IMT-focused sessions.65 In addition, children receiving only asthma education and medication showed little improvement, which suggests these treatments were suboptimal. The South African trial did report the use of pulmonary testing to confirm asthma diagnosis, but provided no description of refusals and exclusions prior to randomization.66 They also reported no information on changes in asthma symptoms, medication use, or quality of life, but only reported pulmonary function outcomes.
Asthma Symptoms
Only two of the trials reported asthma symptoms at followup (Table 7; Appendix D, Evidence Table 4c).65,67 The Brazilian trial reported that all of the children in the control group regularly experienced daytime symptoms after 3 months, compared with none of the children receiving IMT.65 Similarly, 22 of the 25 control group children experienced frequent asthma attacks, compared with only two of the 25 IMT participants.65 Large group differences were also found for nighttime symptoms. The fact that the children receiving only medication management and asthma education were still experiencing high levels of asthma symptoms suggests that their treatment was not effective and may not have been comparable to treatment in the United States.
The Israeli trial with the fewest quality concerns reported greater improvement in morning chest tightness, cough, daytime asthma symptoms, and nighttime asthma symptoms after 6 months in IMT participants as recorded in daily diaries, compared with those using a sham device.67
Medication Use
Four trials reported some kind of group difference in change in bronchodilator use (Table 7; Appendix D, Evidence Table 4d).65,67–69 As with asthma symptoms, medication effects were large in the Brazilian trial: at 3-month followup 16 percent of the children in the IMT group were using bronchodilators compared with 84 percent of the control group children.65 They did not report on controller medication use, which is unfortunate since the children in both groups were previously untreated, initiating both rescue and controller medication in this trial, and we cannot tell if the level of recommended controller medication use was comparable between groups.
All three Israeli trials reported statistically significant reduction in beta2-agonist use at final followup in those using the active training device, but no such change in those who used the sham device after 13 to 26 weeks.67–69 Groups were not statistically compared directly with each other in two cases,68,69 however, and in one of these that provided sufficient data to calculate a standardized effect size, the effect was not statistically different from zero.69
Quality of Life
Two trials reported functioning outcomes (Appendix D, Evidence Table 4e).65,67 The Brazilian trial reported that none of the children undergoing IMT had difficulty with activities of daily living at 3-months followup, but all of the control children did.65 One of the Israeli trials reported an average decline of 1.7 days of missed work in the prior three months, compared with almost no change in the control group participants.67
Other Nonhyperventilation Reduction Breathing Techniques Versus Control
Key Points:
- Evidence is insufficient to draw conclusions about the effect of other nonhyperventilation reduction breathing techniques on asthma symptoms, medication use, or quality of life in adults and children.
Two heterogeneous trials (n=153) compared a nonhyperventilation reduction breathing technique with a control group (Table 8; Appendix D, Evidence Tables 5a and 5b).52,70 One trial, conducted in the United States with paid volunteers, examined the use of biofeedback for breathing retraining.70 The intervention group engaged in biofeedback targeting respiratory resistance, respiratory reactance, and HRV as well as training in pursed-lip abdominal breathing with prolonged exhalation. This trial had three different control groups: biofeedback targeting only HRV, placebo biofeedback involving bogus “subliminal suggestions designed to help asthma,” and waiting list. The first three groups involved weekly biofeedback sessions for 10 weeks, plus the request to practice at home 20 minutes twice daily with a home-training unit. The trial did not report baseline medication use or FEV1 values, but reported that participants’ asthma was most commonly rated as being in the moderate persistent range based on medication level according to National Health, Lung and Blood Institute (NHLBI) criteria.1
The other trial compared the use of a device to modify breathing to achieve an inspiration-to-expiration cycle of 1:2 with a sham device that did not modify breathing.52 Comparisons involving other treatment arms in this trial were included above under hyperventilation reduction techniques. Participants were expected to practice using the device at home twice a day for six months. The average age of participants for this trial was 44 years, and was limited to participants aged 18 to 70 years. Both trials were rated as fair quality (Table 14). The main concerns of the biofeedback trial included lack of information on allocation concealment, higher retention in the wait list group than all other groups (92% vs. 74% to 79%), and fairly small sample size (22 to 25 per group), although they did report blinded outcomes assessment.70 The trial examining the breathing device had fairly low and somewhat differential retention (73% in the intervention group vs. 83% in the control group), conducted many statistical comparisons for the relatively small sample, and did not clearly describe whether baseline differences were controlled for, but did report blinded outcomes assessment.52 Both trials reported pulmonary function testing to confirm asthma diagnosis.
Table 14
Quality and applicability issues: other nonhyperventilation reduction breathing techniques versus control.
The comparison between the active biofeedback groups targeting breathing in addition to HRV versus HRV-only tests the unique contribution of breathing retraining.70 No differences were found between these groups on either asthma symptoms or controller medication use at 12 weeks. Both of these two groups, however, did show greater reductions in number of asthma exacerbations and controller medication use than the placebo and waitlist groups, suggesting biofeedback targeting HRV may have contributed to improvement in asthma. This trial did not examine quality or life or functioning.70
No differences on asthma symptoms, medication use or quality of life were noted at 6-month followup in the trial comparing the device to train prolonged exhalation with a placebo device.52
Key Question 1a. Does the efficacy and/or effectiveness of breathing techniques for asthma health outcomes differ between different subgroups (e.g., adults/children; males/females; different races or ethnicities; smokers/nonsmokers; various types and severities of asthma; and/or different coexisting conditions)?
Key Points:
- Evidence is insufficient to determine whether patient characteristics influence treatment effect in adults and children.
The trials were heterogeneous on too many factors and reporting was too inconsistent to allow us to assess the impact of population characteristics such as demographic characteristics or baseline asthma severity on effect size across studies. However, three trials did report results of subgroup analyses examining differential effects of treatment by different characteristics.51,59,90 It was unclear if these analyses were planned a priori, but they do target subgroups hypothesized to gain the greatest benefit from the specific interventions of their trials, based on the physiologic models of action for their interventions or prior research. None of the trials reported conducting tests for interactions before exploring subgroup analyses. The United Kingdom trial that compared a relatively low-intensity Papworth-style intervention with an asthma education comparator of comparable intensity found that results were consistent between those who scored in the “disordered breathing” range on the Nijmegen questionnaire and those who did not.59 Similarly, the trial of nighttime mouth-taping did not find larger effect among the subgroup of people who were rated as being “mouth breathers” at baseline.51 Finally, the trial using biofeedback for breathing retraining found that there were no differences in response between those older than 40 years of age and those younger than 40 years of age.90
Key Question 1b. Does the efficacy and/or effectiveness of breathing techniques for asthma health outcomes differ according to variations in implementation (e.g., trainer experience) and/or nonbreathing components of the intervention (e.g., anxiety management)?
Key Points:
- Evidence is insufficient to determine whether certification and/or training of the provider affects effect size in hyperventilation reduction trials in adults and children.
- Exploratory analyses suggest that comprehensive approaches, especially those including additional, nonbreathing components may be more likely to show a benefit than approaches that isolate a single aspect of breathing in adults.
- Exploratory analyses suggest that intensity-matched control groups and control groups that involved either an alternate breathing approach or a technique to reduce autonomic arousal may reduce the likelihood of finding group differences in adults.
Among the 11 hyperventilation reduction trials, the expertise of the trainer may have had an impact on medication use, but not on self-reported symptoms. Four of the hyperventilation reduction trials reported using providers with specific training or certification in the Buteyko breathing technique, three trials described their intervention as Buteyko, but did not involve a practitioner, either because they used video tapes to deliver the intervention57,58 or limited the intervention to mouth-taping.51 Four trials used physical therapists without describing further certification, and did not describe their method as Buteyko.54,55,59,71 All four hyperventilation reduction trials using specially training or certified providers showed reductions in medication use.50,52,53,56 Only one other trial showed reduction in medication use and only for controller use.57 However, the effect of practitioner training was not evident for self-reported symptoms: two52,56 of the four using Buteyko practitioners reported positive or mixed findings, compared with three54,55,71 of the seven trials that did not. Also, of the two trials reporting large improvements in asthma symptoms, one used certified Buteyko practitioners and one did not. Interestingly, the only trials reporting improvements in quality of life did not involve certified Buteyko practitioners.57,59
Looking across all trials, we compared the proportion of trials reporting benefits of treatment with and without several treatment components. First, interventions that included co-interventions in addition to breathing retraining52,55,61,64 (e.g., dietary advice, relaxation training) were likely to show a benefit, and interventions that provided comprehensive training and education on breathing retraining were more likely to show a benefit than interventions that isolated one aspect of breathing retraining (e.g., prolonged exhalation,52,70 mouth taping,51 strengthening inspiratory muscles67–69), which generally showed no benefit. For example, 83 percent of trials reporting extra non-breathing components reported a positive effect on asthma symptoms and 100 percent reported reductions in reliever medication use (of those reporting these outcomes), compared with 36 and 33 percent respectively among trials that restricted their interventions to breathing training. However, as discussed next, intensity of intervention (measured in hours of contact) and comprehensiveness (measured in number of intervention components) are likely confounded.
More comprehensive programs were also more likely to offer more hours of exposure to interventionists, and data were insufficient to truly tease apart the effects of hours of contact from the effects of the content that was presented. However, we were able to compare patterns of results among the 13 trials that had the same number of contact hours in the treatment and comparator groups52,53,56–59,62,63,67–71 with the 10 trials in which intervention participants received more hours of contact than those in the comparator group.50–52,54,55,60,61,64–66 Based on the number of trials reporting positive results (and not magnitude of effects), trials that matched intensity between treatment groups were less likely to show reductions in reliever medication use (83% of trials with more intensive intervention than control groups showed reductions in reliever use, compared with 30% of those with matching intensity in the two groups). However, comparable differences were not seen for asthma symptoms or quality of life outcomes. This exploratory analysis is limited by incomplete and perhaps selective reporting of these major outcomes.
Trials that compared any breathing retraining with either another breathing technique or an intervention likely to induce relaxation or a reduced state of autonomic arousal50,52,53,57,58,62,63,70 (k=8) were less likely to show group differences on asthma symptoms and quality of life compared with trials containing control groups that did not include either of these components (k=15).51,52,54–56,59–61,64--69,71 Seventy-five percent of trials with a nonbreathing or nonrelaxation comparator showed greater improvement on a measure of asthma symptoms in the intervention than the control group, compared with 12.5 percent of those with breathing or relaxation comparators. Similar results were seen for quality of life in these trials (20% showing benefit when compared with another breathing technique and/or relaxation vs. 57% showing benefit when compared with nonbreathing/relaxation control). We saw no qualitative relationship between likelihood of effect and study quality rating or whether the treatment involved the use of a device. These data are purely exploratory and do not account for magnitude or precision of effect, and they do not consider the impact of incomplete and perhaps selective reporting. As such, these data must be interpreted cautiously.
Key Question 2. In adults and children 5 years of age and older with asthma, does the use of breathing exercises and/or retraining techniques improve pulmonary function or other similar intermediate outcomes when compared with usual care and/or other breathing techniques alone or in combination with other intervention strategies?
Hyperventilation Reduction Breathing Techniques Versus Control Group
Key Points:
- Evidence is moderate that hyperventilation reduction breathing techniques do not improve lung function in adults.
Seven of the eight hyperventilation reduction trials reported one or more pulmonary function outcomes, primarily FEV1, FVC, and PEF at 4 to 52 weeks (Table 4; Appendix D, Evidence Table 1f).51,52,54–57,59 The standardized pooled effect size of five trials that could be combined showed minimal impact of hyperventilation reduction techniques on FEV1 (SMD=0.18, 95% CI, 0.00 to 0.37, I2=18.4%, Figure 5).52,54–56,59 Absolute changes in the interventions in these groups were small, for example improvements of 20 milliliters or less in FEV1 or less than 2 percent improvement in percent predicted of FEV1. The two trials that could not be pooled were the video-based interventions with matched-intensity control video for comparison, and the mouth-taping trial. Both found no effect of the intervention on FEV1.51,57

Figure 5
Effect of breathing retraining for asthma on pulmonary function at 1 to 6 months. CG: control group; CI: confidence interval; est: estimated; IG: intervention group; N: sample size; SD: standard deviation; SMD: standardized mean difference
Group differences were only found in one trial, and only when compared to one of the two control groups.56 In this trial, percent predicted FEV1 increased from 80 to 81 percent in the Buteyko group while dropping from 75 to 74 percent in the nurse education control group. However, the lower-intensity control group of asthma education only (which was not included in the meta-analysis) did not show a drop and did not differ from the Buteyko group in change from baseline.
Three trials measured end-tidal CO2,54,55,59 which is a specific target of interventions to reduce hyperventilation. Only one trial found group differences, reported at 4, 12, and 26 weeks.54 Breathing rate was reduced in two of these trials, which suggests that participants did modify their breathing as instructed, but that modification did not always alter the CO2 levels as hypothesized by the Buteyko method proponents.54,55
Hyperventilation Reduction Breathing Techniques Versus Other Breathing Techniques
Key Points:
- Hyperventilation reduction breathing techniques do not differ from other breathing techniques in terms of effect on pulmonary function in adults, but the evidence to support this is low.
All four trials in this group reported on change in FEV1 at 13 to 28 weeks (Appendix D, Evidence Table 2f). None found group differences, and there was little change within groups in any trials. The standardized pooled effect size of the three trials that provided sufficient data for analysis was −0.02 (95% CI, −0.29 to 0.26, I2=0.0%, Figure 5).50,52,53 Only one trial reported PEF, and found no group differences.50 Other measures of pulmonary function similarly showed no group differences including end-tidal CO2,50,58 provocative dose of methacholine causing 20 percent reduction in FEV1,52 and FVC.58 One trial did find that those undergoing Buteyko breathing technique had lower minute volume, a specific target of hyperventilation-reduction approaches, than those being trained in abdominal breathing.50 Thus, participants did modify their breathing in a manner consistent with the Buteyko breathing technique approach, but this change did not alter the amount of CO2 in their exhalation, which suggests that CO2 levels may not be an important trigger for asthma as suggested by Buteyko breathing technique proponents.
Yoga Breathing Versus Control
Key Points:
- Yoga breathing techniques may improve pulmonary function in adults, but the evidence to support this is low.
Neither of the U.S.-based trials improved pulmonary function outcomes,61,62 despite the positive effects on other outcomes for the comprehensive naturopathic treatment program (Table 6; Appendix D, Evidence Table 3f).61 However, intensive yoga training in India resulted in substantial improvements in pulmonary function with a standardized pooled effect size for these three trials of 1.07 (95% CI, 0.72 to 1.43 I2=0.0%, Figure 5).60,63,64 The trial with the largest effect (and the greatest quality concerns) showed improvement in percent predicted FEV1 of 12 percentage points, compared with only two percentage points in the control group.63 The best-quality trial of the three Indian trials reported improvements of 7.7 percentage points in the intervention group on percent predicted FEV1 compared with a 2.6 percentage point reduction in the control group at eight-week followup.64 Group differences were also found on FVC,60 FEV1/vital capacity (VC) ratio,60,64 and PEF readings60,63,64 in the trials conducted in India, but not in those conducted in the United States.61,62 Only one of the trials reported that outcomes assessment was blinded.62 None of the trials described training or quality assurance measures for the spirometry technicians, and only one provided any detail about spirometry procedures beyond naming the machine that was used. The best-quality intensive India-based trial64 reported taking the best of three FEV1 readings, in accordance with ATS standards.11
Inspiratory Muscle Training Versus Control
Key Points:
- Evidence is insufficient to determine whether IMT improves pulmonary function in adults and children.
Results from IMT trials were mixed and could not be pooled due to substantial differences in population, setting, and treatment approach in the three trials reporting the same outcome. Treatment-naïve Brazilian children with previously uncontrolled asthma improved PEF readings by an average of 80 percent after 3 months of IMT training along with asthma medication management and education, compared to almost no change on average in those receiving medication and asthma education alone (Table 7; Appendix D, Evidence Table 4f).65 Lack of improvement in the control group suggests that medication management may have been suboptimal in this group. Among adults, two trials showed improvements in both FEV1 and FVC, one with the use of an IMT device,67 and the other using weights placed on the abdomen while in a semi-recumbent position.66 Another trial found no differences in FEV1.68
Other Nonhyperventilation Reduction Breathing Techniques Versus Control
Key Points:
- Evidence is insufficient to determine whether other nonhyperventilation reduction techniques improve pulmonary function in adults and children.
Spirometry results did not change over time in either the trial of prolonged exhalation using a training device52 or in any of the treatment groups in the biofeedback trial (Appendix D, Evidence Table 5f).70
Key Question 2a. Does the efficacy and/or effectiveness of breathing techniques for other asthma outcomes differ between different subgroups (e.g., adults/children; males/females; different races or ethnicities; smokers/nonsmokers; various types and severities of asthma; and/or different coexisting conditions)?
Key Points:
- Evidence is insufficient to determine whether patient characteristics influence the effect of treatment on pulmonary function in adults and children.
The best-quality trial of yoga conducted in India displaying large benefits of treatment reported that participants with exercise-sensitive asthma showed a greater improvement on FEV1 than those whose asthma was not sensitive to exercise.64 This analysis did not appear to be planned a priori, nor did the intervention particularly target factors that purported to differentially affect those with exercise-sensitive asthma. No other trials reported subgroup analyses for any pulmonary function outcomes.
Key Question 2b. Does the efficacy and/or effectiveness of breathing techniques for other asthma outcomes differ according to variations in implementation (e.g., trainer experience) and/or nonbreathing components of the intervention (e.g., anxiety management)?
Key Points:
- Evidence is insufficient to determine whether certification and/or training of the provider affects effect size in hyperventilation reduction trials.
- Exploratory analyses suggest that control groups that involved either an alternate breathing approach or a technique to reduce autonomic arousal may reduce the likelihood of finding group differences in adults.
Benefits were more likely to be seen if the control group did not involve breathing training of any kind or relaxation techniques (42% positive vs. 14% positive with breathing/relaxation comparison group). These data are preliminary, however, and only valid for hypothesis generation and did not account for effect size.
Key Question 3. What is the nature and frequency of serious adverse effects of treatment with breathing exercises and/or retraining techniques, including increased frequency of acute asthma exacerbations?
Key Points:
- Hyperventilation reduction breathing techniques do not appear to be associated with any harms in adults, other than minor annoyances associated with mouth taping at night, but the evidence to support this is low.
- Yoga breathing techniques do not appear to be associated with any harms in adults, but the evidence to support this is low.
- There was no evidence on harms associated with IMT or other non-hyperventilation reduction approaches in adults or children.
Seven trials reported on adverse events,51,53,55,57,58,61,62 five of which examined a hyperventilation reduction approach compared with either a control or another breathing retraining approach,51,53,55,57,58 and two examined yoga interventions.61,62 Three of the seven studies (including one yoga trial62) noted that there were no adverse events or harms that occurred in either the intervention or control group over 16 to 52 weeks of intervention and followup.53,55,62 One study of a Buteyko breathing technique intervention, compared to a relaxation control group, noted that one hospitalization occurred with one member of the control group.57 Another study comparing a Buteyko breathing technique intervention delivered by video with a placebo intervention involving nonspecific upper body mobility exercises reported 138 adverse events in the Buteyko breathing technique group and 121 in placebo group, none of which was considered to be related to treatment.58 The trial of comprehensive naturopathic treatment reported mild headache, fatigue, and/or nausea, which they attributed to the use of the supplements and not yoga.61 In the study focused on the effect of a nighttime mouth-taping intervention, participants reported problems related to the intervention including it being uncomfortable, causing sore lips, making breathing more difficult, feeling unnatural, decreasing sleep quality, causing a feeling a suffocation, or was embarrassing.51
Key Question 3a. Do the safety or adverse effects of treatment with breathing techniques differ between different subgroups (e.g., adults/children; males/females; different races or ethnicities; smokers/nonsmokers; various types and severities of asthma; and/or different coexisting conditions)?
Key Points:
- There was no evidence on whether patient characteristics influenced the likelihood of experience harm in adults or children from any treatment included in the review.
No trials examined harms of treatment within subgroups or compared subgroups on likelihood of harms.
- Literature Search
- Results of Included Studies
- In adults and children 5 years of age and older with asthma, does the use of breathing exercises and/or retraining techniques improve health outcomes, including: symptoms (e.g., cough, wheezing, dyspnea); health-related quality of life (general and/or asthma-specific); acute asthma exacerbations; reduced use of quick-relief medications or reduced use of long-term control medications, when compared with usual care and/or other breathing techniques alone or in combination with other intervention strategies?
- Does the efficacy and/or effectiveness of breathing techniques for asthma health outcomes differ between different subgroups (e.g., adults/children; males/females; different races or ethnicities; smokers/nonsmokers; various types and severities of asthma; and/or different coexisting conditions)?
- Does the efficacy and/or effectiveness of breathing techniques for asthma health outcomes differ according to variations in implementation (e.g., trainer experience) and/or nonbreathing components of the intervention (e.g., anxiety management)?
- In adults and children 5 years of age and older with asthma, does the use of breathing exercises and/or retraining techniques improve pulmonary function or other similar intermediate outcomes when compared with usual care and/or other breathing techniques alone or in combination with other intervention strategies?
- Does the efficacy and/or effectiveness of breathing techniques for other asthma outcomes differ between different subgroups (e.g., adults/children; males/females; different races or ethnicities; smokers/nonsmokers; various types and severities of asthma; and/or different coexisting conditions)?
- Does the efficacy and/or effectiveness of breathing techniques for other asthma outcomes differ according to variations in implementation (e.g., trainer experience) and/or nonbreathing components of the intervention (e.g., anxiety management)?
- What is the nature and frequency of serious adverse effects of treatment with breathing exercises and/or retraining techniques, including increased frequency of acute asthma exacerbations?
- Do the safety or adverse effects of treatment with breathing techniques differ between different subgroups (e.g., adults/children; males/females; different races or ethnicities; smokers/nonsmokers; various types and severities of asthma; and/or different coexisting conditions)?
- Results - Breathing Exercises and/or Retraining Techniques in the Treatment of A...Results - Breathing Exercises and/or Retraining Techniques in the Treatment of Asthma: Comparative Effectiveness
- Methods - Diagnosis and Treatment of Obstructive Sleep Apnea in AdultsMethods - Diagnosis and Treatment of Obstructive Sleep Apnea in Adults
- Peer Reviewers - Breast Reconstruction After Mastectomy: A Systematic Review and...Peer Reviewers - Breast Reconstruction After Mastectomy: A Systematic Review and Meta-Analysis
- Acknowledgments - Adverse Effects of Pharmacologic Treatments of Major Depressio...Acknowledgments - Adverse Effects of Pharmacologic Treatments of Major Depression in Older Adults
- Preface - Self-Measured Blood Pressure Monitoring: Comparative EffectivenessPreface - Self-Measured Blood Pressure Monitoring: Comparative Effectiveness
Your browsing activity is empty.
Activity recording is turned off.
See more...