NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Jadad AR, Boyle M, Cunningham C, et al. Treatment of Attention-Deficit/Hyperactivity Disorder. Rockville (MD): Agency for Healthcare Research and Quality (US); 1999 Nov. (Evidence Reports/Technology Assessments, No. 11.)
This publication is provided for historical reference only and the information may be out of date.
Yield of the Literature Search
Electronic database searches, review of reference lists of review articles, and referrals from experts yielded 2,405 citations. Based on information available in the citations (titles, keywords, and abstracts), it was evident that 1,881 of these articles were not applicable to this project. Of the 524 articles regarded as potentially eligible, hard copies of 522 articles were obtained (2 are unobtainable as a result of incorrect indexing of the reference). Of these, 432 described comparative studies, and 90 described noncomparative studies. Of the 432 comparative studies, 305 were RCTs and 127 were nonrandomized controlled trials (Figure 1).
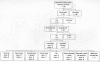
Figure
Figure 1. Yield of Literature Search.
The subset of RCTs was screened to determine the number of articles with evidence to answer the research questions of interest. The following is the number of reports found in each category (in parentheses, the number of trials described in the reports):
- Drug vs. drug 22 (22)
- Drug vs. nondrug 12 (6)
- Combination therapies (stimulants plus others) 27 (20)
- Tricyclic antidepressants vs. placebo 12 (9)
- Long-term therapies (>12 weeks) 19 (14)
- Treatment of ADHD in adults (>18 years of age) 13 (12)
- Adverse effects of interest 32 (29) (one non-RCT)
A total of 92 reports were included in this project. Numerous overlaps exist among the categories. In addition, multiple reports of the same studies were found. Information from multiple reports of the same studies was combined and considered as a single data set. Once this was accounted for, 78 different studies were included. The evidence tables in this document are based on information from these 78 studies. The remaining articles were not included because they did not provide data on any of the categories or questions of interest for this project.
The evidence identified within each of the categories of interest will be described separately (see below).
An unpublished copy was obtained of the Canadian Coordinating Officer for Health Technology Assessment (CCOHTA)-funded report on the treatment of ADHD prepared by researchers at the University of British Columbia (Miller, Lee, Raina et al., 1998). As planned, its methodological quality was assessed and information on RCTs comparing stimulants with placebo was extracted.
General Characteristics of All Studies Included
The most salient characteristics of all the studies included (Evidence Tables A1 through A34) were the following:
- Sixty-five of the 78 studies were identified in MEDLINE first. Other sources contributed little.
- Most of the studies (48 of the 78) had a crossover design.
- Most of the studies reported funding by a government agency (48 studies). The second source of funding was industry (22 studies). Charities and consumer groups funded few studies (seven studies).
- The first study identified was published in 1971 and the last in 1999. One-half of the studies (34/78) were published since 1990.
- All but six studies were published in North America.
- More than one-half the studies had 3 to 5 authors (49 studies).
- The lead author was typically a pediatrician or a psychiatrist.
- All the studies but two described a single research center.
- Seventy-five studies included men or boy patients, and 51 included women or girl patients.
- DSM-IIIR and -IV criteria were used almost as often (28 studies) as DSM-I and -III (30 studies) for the diagnosis of ADHD.
- The number of arms in the studies varied from 2 to 6; 54 studies had 2 or 3 arms.
- Thirty-two different interventions were studied. Apart from placebo (64 studies), the most frequently evaluated intervention was MPH (55 studies), followed by DEX (18 studies), and cognitive/behavioral therapy (8 studies). Intervention with pemoline was evaluated in five studies.
- No studies provided an analysis stratified by treatment setting.
- Forty-eight different outcomes were measured in the 78 studies. The most frequently measured outcomes were inattention (29 studies) and hyperactivity (30 studies). Impulsivity was measured in 13 trials. Global assessment of symptoms was provided in 22 studies and combined scores for core symptoms, in 20.
- Sixty-nine different tests were used to measure the outcomes. Conners was the most frequently used test, followed by spontaneous reports.
The reports of the studies included in the review had deficiencies that increase the likelihood of bias in their results:
- One study (MTA Cooperative Group, 1999) was given the maximum quality score with the validated scale (Jadad, Moore, Carroll et al., 1996). The average quality score was 2 out of 5 points. Only five trials were given 4 points. Only 18 of the 77 randomized trials were given scores > 3 points. As mentioned above, it has been shown consistently that trials that receive < 2 points are more likely to exaggerate treatment effects (Berman, Ezzo, Hadhazy et al., forthcoming; Khan, Daya, and Jadad, 1996; Moher, Pham, Jones et al., 1998). The main reasons for the low scores were lack of description of the method of randomization (73/77 trials), lack of description of methods to achieve double blinding (59 trials), and poor description of withdrawals and dropouts (66 trials).
- Seventy-three trials did not mention concealment of allocation to study groups.
- An association with industry was reported in 27 studies. Ciba-Geigy was associated with 16 studies.
- Most studies had one or more factors that limited their external validity or generalizability. Only one of the studies (MTA Cooperative Group, 1999) included information on all of the 20 clinically relevant elements selected a priori for extraction from the articles.
The following is a brief summary of the main problems found in the studies:
- The total duration (including the time required to administer the interventions and assess their outcomes) was 12 weeks or less in 64 studies. Results obtained in short-term studies may not apply to the long treatment periods that are often required.
- Overall, the description of the interventions evaluated in the studies, particularly nonpharmacological ones, was poor.
- Fewer than one-third of the trials mentioned ethnic groups. The most frequently considered group was white, followed by African American (35 studies).
- Twenty of the studies did not report the inclusion criteria or reported them unclearly. One-half of the trials did not report the exclusion criteria. Insufficient information on inclusion or exclusion criteria limits the ability of clinicians to compare their patients with those reported in published studies.
- The primary outcome of interest to the researchers was not described in 61 of the studies. As mentioned above, the identification of the primary outcome, especially when it is done a priori, provides the basic elements for the calculation of the sample size required by the researchers to identify clinically important differences and minimize the risk of finding false-negative results. In addition, this is likely to reduce the tendency of researchers to give more prominence to secondary outcomes when the interventions do not produce the expected effects on the primary outcome of interest.
- Most of the studies (64) included patients between the ages of 5 and 12 years.
- Forty-three studies did not report information on comorbid disorders, which are frequently present in patients with diagnosis of ADHD. The presence of comorbid disorders may increase or reduce the effectiveness of interventions targeted to ADHD. In addition, these comorbid disorders could be improved or exacerbated by the same treatments.
- Family characteristics were not mentioned in 45 studies.
- The fidelity of treatments was not described in 52 studies, and compliance was not measured in 44 of the 78 studies. Knowing the adequacy with which treatments are administered by health care providers (fidelity) or the adherence of patients to the treatments (compliance) are two essential components to determine the generalizability of the results of any study.
- Baseline measurements of ADHD symptoms were provided in 54 studies. The most frequently used test to generate baseline measurements was Conners (37 studies), followed by Weschler (23 studies). Information on baseline characteristics enables readers to compare their cases with those included in the studies.
The following seven sections provide a detailed description of the evidence and main findings within each of the categories of interest. Within each section, studies are discussed in detail when they provide information that could increase the understanding of the interventions under evaluation.
Main Findings by Category
Full references for the studies cited can be found in the References chapter. The Bibliography contains all sources that were considered, including those used and not used.
Drug vs. Drug Comparisons
Detailed information on the key characteristics of the 22 studies with drug vs. drug comparisons is included in Evidence Table B1.
Nine of the 22 studies diagnosed ADHD using DSM criteria. In two trials, the duration of the study arms was unclear (Matochik, Liebenauer, King et al., 1994; Srinivas, Hubbard, Quinn et al., 1992). The number of patients randomized to each of the study arms was not reported in eight studies. In the remaining 14 trials, a total of 650 patients were studied. The sample size of these studies ranged from 9 to 125. Of the 22 reports, only 5 were given quality scores of >3 points. Two studies described concealment of allocation (Arnold, Huestis, Smeltzer et al., 1976; Kauffman, Smith-Wright, Reese et al., 1981).
The number of clinically relevant elements present in each of the study reports ranged from 7 to 18 (Evidence Tables B2 and B3). Of the 22 reports, 6 reported the number of patients eligible for the study, 8 did not report inclusion criteria, and 15 did not report exclusion criteria. Eighteen trials did not report the primary outcome. In 19 trials, the fidelity of treatment was not reported. Only 6 trials provided measurements of compliance with treatment and 13 trials provided baseline scores.
The following were the main findings of the studies in this category:
Same-Drug Comparisons
- MPH vs. MPH (Fitzpatrick, Klorman, Brumaghim et al., 1992; Pelham, Sturges, Hoza et al., 1987; Srinivas, Hubbard, Quinn et al., 1992; Whitehouse, Shah, and Palmer, 1980): One of the four trials compared different enantiomers (Srinivas, Hubbard, Quinn et al., 1992), whereas the other three trials compared sustained-release with regular formulations. The study that evaluated different enantiomers of MPH found that drug-induced improvements in sustained attention was attributable to the d-MPH (Srinivas, Hubbard, Quinn et al., 1992). The studies evaluating different formulations found no differences between them but had small sample sizes (Evidence Table B1).
- DEX vs. levoamphetamine (Arnold, Huestis, Smeltzer et al., 1976): The main conclusion from this study is that both isomers were better than placebo but not significantly better than each other in terms of improvement of core symptoms and academic performance (Supplemental Tables B4 and B6). Only in one parent report of global symptoms was DEX better than levoamphetamine (Supplemental Table B4). Parents' ratings, but not teachers' or psychiatrists' ratings, showed significant placebo effects. This study included only 31 patients.
Stimulant vs. Stimulant
There were 13 trials in this group. Only one studied more than 100 patients and six included fewer than 30 patients. Two trials studied the effect of the interventions for 8 weeks, whereas most studied the stimulants for 3 weeks or less (Evidence Table B1).
- MPH vs. DEX (Arnold, Christopher, Huestis et al., 1978; Borcherding, Keysor, Cooper et al., 1989; Castellanos, Giedd, Elia et al., 1997; Efron, Jarman, and Barker, 1997; Elia, Borcherding, Rapoport et al., 1991; Gross, 1976; Kauffman, Smith-Wright, Reese et al., 1981; Matochik, Liebenauer, King et al., 1994; Winsberg, Press, Bialer et al., 1974). Of the nine trials, seven were placebo-controlled and three provided data on effectiveness outcomes of interest. Most of the results of these studies suggest that there are no differences between MPH and DEX. The two placebo-controlled studies with data on outcomes of interest showed that both stimulants were more effective than placebo for the improvement of core symptoms (Supplemental Table B4) (Arnold, Christopher, Huestis et al.,1978; Elia, Borcherding, Rappoport et al., 1991). Neither provided the number of patients randomized to the study groups or reached a score compatible with high quality (Evidence Table B1).
- One trial used a triple-blind crossover design and was designed to look at compliance with treatment (Kauffman, Smith-Wright, Reese et al., 1981). This study found that the mean individual patient compliance during MPH administration was 65 percent (0 to 100 percent) and during DEX administration was 60 percent (20 to 83 percent). The authors stressed that poor compliance may be an important reason for the variability and discordance among the results of the studies in this area.
- MPH vs. pemoline (Conners and Taylor, 1980; Stephens, Pelham, and Skinner, 1984). Both studies in this group were placebo-controlled, but only one reported data on effectiveness outcomes of interest (Conners and Taylor, 1980). This study received a score >3 points, provided information on 17 of the 20 clinically relevant elements, was published in 1980, and included 60 patients diagnosed as having minimal brain dysfunction. Patients were randomized to receive pemoline at 18.75 mg/d, MPH at 5 mg two times per day, or placebo for 8 weeks. Overall, the two drugs produced similar effects and were more effective than placebo (Evidence Tables B1, Supplemental Tables B4-B7).
- DEX vs. pemoline (Conners, Taylor, Meo et al., 1972): In this study, 81 children with diagnosis of minimal brain dysfunction were randomized to receive pemoline at 25 mg, DEX at 10 mg, or placebo for 8 weeks. This study found both drugs to be better than placebo but found few differences in terms of effectiveness and adverse effects between them.
- MPH vs. pemoline vs. DEX (Pelham, Greenslade, Vodde-Hamilton et al., 1990): This study compared administration of regular MPH at 10 mg two times per day, slow-release MPH at 20 mg/d, sustained-release DEX at 10 mg in the morning, and pemoline at 56.25 mg/d and a placebo-controlled arm. The main conclusion from this study was that the four medications had "generally equivalent and beneficial effects." However, the report did not provide the number of patients randomized or the actual data on formal comparisons of the outcomes produced by all drugs simultaneously. The comparisons between individual stimulants and placebo produced inconsistent results across the groups of outcomes (Supplemental Tables B4-B7). The report included information on 11 of the 20 clinically relevant elements and achieved a score compatible with low quality (Evidence Table B1).
Stimulants vs. Tricyclic Antidepressants
Only four studies, all comparing MPH with tricyclic antidepressants, were found. One trial compared MPH with desipramine (Rapport, Carlson, Kelly et al., 1993). This was a randomized, double-blind, crossover placebo-controlled study in which 16 patients were allocated to receive desipramine in titrated doses to achieve plasma levels between 125 and 225 mg/ml; MPH at 10, 15, or 20 mg; a combination of desipramine and MPH at the above levels; or placebo. The children included in this study had diagnoses of ADHD with depressive symptoms, ADHD and major depressive disorder, or major depressive disorder with prominent attentional symptoms. The main conclusions from this study were that MPH was consistently the most potent drug in affecting children's vigilance (Continuous Performance Test [CPT]) and ability to learn paired associations (Paired Associate Learning [PAL]). Additional improvements in performance were observed when desipramine was added. Desipramine alone, however, did not affect children's vigilance but did enhance paired association learning to a lesser extent than that achieved by MPH alone or in combination. The authors caution about the interpretation of their results because of the small sample size and the degree of psychopathology experienced by the children studied.
Three trials compared MPH with imipramine (Quinn and Rapoport, 1975; Spring, Yellin, and Greenberg, 1976; Werry, Aman, and Diamond, 1980). One did not provide data on effectiveness outcomes of interest (Spring, Yellin, and Greenberg, 1976). The other two trials provided inconsistent results. One showed nonsignificant differences between MPH and imipramine (Quinn and Rapoport, 1975), whereas the other showed some results in favor of imipramine (Werry, Aman, and Diamond, 1980).
In summary, the small group of studies comparing different formulations or enantiomers of the same stimulants suggests that there little difference between them, at least in the short term, but that the active medications are more effective than placebo. Similarly, despite the overall heterogeneity of the studies comparing different stimulants, they showed few, if any, differences among MPH, DEX, and pemoline. These findings agree with those of the AMA and AACAP reports. The sample size of the studies, however, is small.
Studies comparing stimulants with tricyclic antidepressants had many limitations and presented conflicting results. More rigorous studies are clearly needed to establish the relative effectiveness of stimulants and tricyclic antidepressants. Studies are required to compare the effects of stimulants with clonidine, buproprion, or selective serotonin-reuptake inhibitors.
Drug vs. Nondrug Studies
Six studies met the selection criteria described previously. Five of these studies overlapped with those studies included in the combination therapies and will be described in more detail in the following section (Brown, Wynne, and Medenis, 1985; Conrad, Dworkin, Shai et al., 1971; Firestone, Crowe, Goodman et al., 1986; Klein and Abikoff, 1997; MTA Cooperative Group, 1999; see next section), and one study compared DEX with the dietary supplement Efamol (Arnold, Kleykamp, Votolato et al., 1989). This study did not describe the number of patients randomized, had a duration of 4 weeks, and provided information on 10 of the 20 clinically relevant elements.
With the exception of the 1999 report by the MTA Cooperative Group, the studies available in this category had major limitations resulting from poor reporting of methodological characteristics and clinically relevant elements, heterogeneity in diagnostic criteria used, type of interventions evaluated and outcomes measured (Evidence Tables C1-C3, Supplemental Evidence Tables C4-C8). Four studies received scores for methodological quality <2, one received a score of 3, and none mentioned concealment of allocation. The interventions were administered for 4 to 12 weeks. One study (Conrad, Dworkin, Shai et al., 1971) did not report the duration of treatment in the study arms. None of the studies used DSM-IV as the diagnostic model. Despite their limitations, the results of these five individual studies indicate consistently that stimulants may be more effective than nonpharmacological interventions when compared head to head (Evidence Table C1). The MTA study (MTA Cooperative Group, 1999), on the other hand, used DSM-IV diagnosis criteria, received a methodological score of 3, described clearly the concealment of allocation, provided information on all 20 of the clinically relevant elements selected a priori, and had an intervention lasting 60 weeks.
Despite their limitations, the individual studies available suggest that stimulants may be more effective than nonpharmacological interventions when compared head to head.
Combination Therapy
Combined interventions included stimulants vs. a combination of two or more drugs, stimulants vs. stimulants plus nondrug interventions, and nondrug vs. stimulant plus nondrug interventions. For nondrug interventions, it was difficult to determine the exact nature of the behavioral interventions administered because of the limited amount of descriptive information available in the reports. (Refer to Evidence Tables D1--D3 and Supplemental Evidence Tables D4-D8).
The search yielded 20 studies that examined combination therapies and met the above selection criteria.
The duration of interventions in 14 of these studies ranged from 1 to 12 weeks; one study treatment period ran for 14 months. In five trials, the duration of the interventions was unclear or not reported. Eleven studies had 30 or fewer patients (Evidence Table D1). One study reported the method used to generate the random sequences, only four described the method used to achieve double-blinding, and three also described withdrawals and dropouts appropriately. Two studies described concealment of allocation.
The number of clinically relevant elements found in the report of the studies varied from 10 to 20. Fifteen trials reported inclusion criteria, 9 reported the exclusion criteria, and only 4 reported the primary outcome of interest (Evidence Tables D2 and D3). Eighteen studies included MPH, 2 studies included DEX, and none assessed pemoline.
Three categories of comparisons were made by the 20 studies are described below.
Stimulant vs. a Combination of Two or More Drugs (Question: Is a Combination of Drugs More Effective Than Stimulant Alone?)
- Garfinkel, Webster, and Sloman, 1981: This study compared two doses of caffeine, combination of caffeine with MPH, MPH alone, and placebo. The sample consisted of six boys between 6 and 10 years of age attending a day treatment program at a psychiatric institution who had been referred because they were not progressing academically or socially while in a regular or special education class. The researchers found that the strongest effect was noted when caffeine was combined with MPH and that caffeine at a low dose was superior to caffeine at a high dose. This study is difficult to interpret because of the small sample size, sample selection, lack of measurement of compliance or adherence to regime, and use of teacher report only as an outcome measure.
- Gittelman-Klein, Klein, Katz et al., 1976: This study evaluated the effect of thioridazine (THIOR), MPH, and combination therapy. This study had the largest sample size in this group and obtained a high-quality score (3). It also included information on 16 of the 20 clinically relevant elements. The study used large doses of MPH (average dose of 50 mg, 1.78 mg/kg). A significant improvement was seen in global assessments of core symptoms of ADHD with MPH alone or in combination with THIOR compared with THIOR alone. Improvements in individual core symptoms, academic performance, and symptoms associated with conduct or oppositional disorders were reported by teachers but not by parents. There were no differences on the symptoms of depression (Evidence Table D1).
- Levy and Hobbes, 1996: This study examined the use of haloperidol in combination with MPH to determine whether haloperidol blocks the effect of MPH if given before MPH. The sample included 10 boys between 5 and 12 years of age who met the criteria for ADHD when DSM-III criteria were used but who also had comorbid ODD. Measure of outcome relied only on the Continuous Performance Test, and the result is difficult to interpret. MPH improves reaction time on the CPT, and this effect is eliminated by use of haloperidol before administration of MPH.
- Rapport, Carlson, Kelly et al., 1993: This study compared MPH combined with desipramine, MPH alone, desipramine alone, and placebo. The sample included 16 boys who were hospitalized with ADHD and mood disorder. The report obtained a quality score of 3 with the validated scale and included information on 17 of the clinically relevant elements (Evidence Table D1). This study found that MPH was potent in affecting children's vigilance (CPT) and their ability to learn paired associations and do the Matching Familiar Figures Test (MFFT). Desipramine had no effect on vigilance but improved learning paired associations (not as much as MPH) and was equally effective on the MFFT. Combination therapy led to some blocking of the vigilance but improved MFFT and the Stimulus Equivalence Paradigm (SEP), which suggests some additive effect on behavioral inhibition and complex problemsolving abilities. Some of the limitations of this study are the study sample that consisted of hospitalized patients who had significant coexisting mood disorder, lack of individual outcome data, and the clinical significance of outcome measures.
- Schechter and Timmons, 1985: This study used a crossover design to compare DEX at 1.6 mg and 5.0 mg, DEX at 1.6 mg combined with caffeine at 300 mg, and caffeine at 300 mg alone. The sample included 10 patients between 6 and 10 years of age who were diagnosed as having hyperkinetic syndrome using criteria other than DSM. After 2 weeks, all treatments led to significant increase in reaction time, significant decrease in errors of commission, and decreased total scores on the Conners Abbreviated Parent Rating Scale (ACRS). The report did not mention whether there was significant difference between treatments. This study is limited not only by its poor rating of quality but also because of small sample size, short duration of intervention, lack of measurement of compliance or adherence to regime, fixed doses of DEX, and use of dependent measures that are of questionable clinical significance.
Stimulant vs. Stimulant Plus Nondrug Intervention (Question: How Much Do Nondrug Interventions Add to the Effect of Stimulants?)
Of the five studies in this group, four evaluated MPH and one DEX. Four studies (Brown, Wynne, and Medenis, 1985; Conrad, Dworkin, Shai et al., 1971; Firestone, Crowe, Goodman et al., 1986; Klein and Abikoff, 1997) allowed direct head-to-head comparison of stimulant vs. nondrug intervention. The fifth study is Long, Richert, and Ashcraft, 1993. The studies are sorted alphabetically by author for ease of reading. The types of nondrug interventions vary widely and are highlighted in the summary.
- Brown, Wynne, and Medenis, 1985 (stimulant vs. stimulant plus cognitive behavioral therapy): This study compared the effect of cognitive behavioral therapy (CBT), MPH alone, and combination therapy (MPH plus CBT). Thirty hyperactive boys with academic difficulties between 6 and 11 years of age were randomized. Outcome measures were very broad, focusing on core symptoms of ADHD and academic measures. The CBT program consisted of 24 1-hour individual sessions 2 times per week over a 3-month period and consultation sessions for both parents and teachers. The results showed that only those children in the two medication treatment conditions demonstrated improvement in both attentional and behavioral ratings and that the combination therapy was not any more effective than MPH alone.
- Conrad, Dworkin, Shai et al., 1971 (stimulant vs. stimulant plus child training): This study examined the effect of DEX alone or with twice weekly "prescriptive perceptual-cognitive tutoring" (child training [CT]). The sample consisted of 68 children from a low socioeconomic class; age was not specified. The diagnosis of ADHD was based on the Schenectady Hyperkinetic Scale. The children were randomly assigned to placebo alone, DEX alone, DEX plus CT, or placebo plus CT. The duration of the interventions was not clearly reported. Neither experimental condition significantly influenced academic achievement as measured by the Wide Range Achievement Test.
- Firestone, Crowe, Goodman et al., 1986 (stimulant vs. stimulant plus parent training): In this study, the use of parent training (PT) in the treatment of ADHD was evaluated in 73 children between 5 and 9 years of age. Children were assigned to PT plus MPH, PT plus placebo, or MPH alone. Parents were asked to read a book on child management and were then asked to join a parents' discussion group. The results indicate that at 3 months there were significant differences between those in the two medication groups in terms of hyperactivity index, conduct problem, and reaction time but not the verbal grade level. Parent training added very little. At the first- and second-year followup, there were no significant differences among the groups. Some of the limitations of this study include substantial attrition over time, no report of the number of patients randomized, low-quality score, lack of measurement of compliance with treatment, and little information as to the extent of parent participation in the program.
- Klein and Abikoff, 1997 (stimulant vs. stimulant plus cognitive behavioral therapy): This study compared the effects of CBT, MPH alone, and CBT plus MPH together. The sample consisted of 89 children 6 to 12 years of age whose diagnosis of ADHD was based on the Conners Teacher Rating Scale. Children with conduct disorder were systematically excluded. Behavior therapy for home and school utilized operant conditioning principles individualized for each child. Dose titration for MPH was carried out up to a maximum of 60 mg/d for optimal efficacy. The results suggested that MPH alone and in combination with CBT were significantly better than CBT with placebo on most outcomes. Some differences between MPH alone and combination therapy include less attention-seeking behavior, better impulse control, and more cooperative behavior. Teachers and psychiatrists also rated the children more improved on the Clinical Global Improvement (CGI).
- Long, Rickert, and Ashcraft, 1993 (stimulant vs. stimulant plus bibliotherapy): This study examined the use of bibliotherapy as an adjunct to stimulant medication. Bibliotherapy refers to written behavioral management protocol. The sample consisted of 32 children between the ages of 6 and 11 who were recruited concurrently from outpatient pediatric clinics. Children were assigned to MPH alone or with bibliotherapy. Results revealed that the intensity of behavior lessened for the bibliotherapy group even though the problems remained. One of the major limitations of this study is that there was no surveillance of medication compliance during the study which could have influenced the results.
Nondrug vs. Stimulant Plus Nondrug Intervention (Question: How Much Do Stimulants Add to the Effect of Nondrug Interventions?)
There were 14 studies in this category. In total, 13 studies evaluated the effect of MPH and one the effect of DEX (Conrad, Dworkin, Shai et al., 1971). Five of these studies have been discussed in the section above (Brown, Wynne, and Medenis, 1985; Conrad, Dworkin, Shai et al., 1971; Firestone, Crowe, Goodman et al., 1986; Klein and Abikoff, 1997; Long, Rickert, and Ashcraft, 1993). The remaining nine are described below.
- Borden and Brown, 1989 (cognitive behavior therapy vs. stimulant plus cognitive behavioral therapy): This study was designed to determine whether those treated with combination of medication and CBT would tend to make external attributions for problem solutions or whether more internal attributions would be observed with CBT alone. Thus, this study did not focus on the effects of interventions on the core symptoms or associated behavior/academic difficulties in children with ADHD.
- Brown, Borden, Wynne et al., 1986 (cognitive behavior therapy vs. stimulant plus cognitive behavioral therapy): This study examined the effect of combined CBT and MPH. The study achieved a high-quality score and provided information on 14 of the 20 clinically important elements. The study included 40 patients diagnosed with ADHD using DSM-III criteria between 5 and 13 years of age and of average intellectual functioning. Both CBT and attention control (AC) involved 22 1-hour sessions over 3 months, but the difference was in the inclusion of specific problemsolving strategies that were taught in the CBT group. MPH was given at 0.3 mg/kg two times per day and was discontinued during pre- or postmeasurements. The results showed that no significant main treatment effects or interactions among treatments occurred. They also noted that the effects of stimulant medication were noticeable while children were receiving it. However, these effects dissipated rapidly upon discontinuation of the medication.
- Brown, Borden, Wynne et al., 1988: This study was designed to determine the extent to which compliance with treatments occur in treatment groups rather than to determine the effects of interventions on the core symptoms or associated behavior/academic difficulties in children with ADHD.
- Carlson, Pelham, Milich et al., 1992 (behavior therapy plus stimulant vs. regular classroom plus stimulant): Twenty-four boys between 6 and 12 years of age participating in an 8-week summer treatment program for ADHD were randomized to 2 classrooms (behavior management classroom [behavioral contingencies/modification, or BC/Mod] vs. regular classroom [AC]) and to placebo or MPH at a low dose or high dose. Children showed significantly higher rates of on-task behavior, lower rates of disruptive behavior, and higher rates of following rules when receiving MPH than when receiving placebo. The medication effects on on-task and disruptive behavior interacted with classroom setting such that in regular classroom settings, increasing the dose of MPH had a linear effect of increasing dosage. The study seems to suggest that low dosages of MPH are sufficient to maximally improve children's behavior when behavioral classroom techniques are employed.
- Christensen, 1975 (behavior therapy vs. behavior therapy plus stimulants): This study examined the effect of behavior therapy alone (BC/Mod) or in combination with MPH. The subjects were 16 "hyperactive, institutionalized retarded" children between 9 and 16 years of age who were randomly assigned to 2 groups: MPH plus BC/Mod (main element of the program was a token reinforcement system) or placebo plus BC/Mod. The results showed that the BC/Mod procedures in the presence of the placebo were just as effective as the same procedures in the presence of MPH, suggesting no added effect of MPH. However, it is important to note that MPH was given one time per day and the dose was not individually titrated.
- Hinshaw, Henker, and Whalen, 1984 (cognitive self-evaluation vs. cognitive self-evaluation plus stimulant): This study evaluated the cognitive self-evaluation procedures and MPH on social behavior in a playground setting. Twenty-four hyperactive boys between the ages of 8 and 13 who were already receiving treatment with MPH were randomized to "reinforcement alone" with MPH, "reinforcement alone" with placebo, "reinforced self-evaluation" (RSE) with MPH, or RSE with placebo. No attempts were made to standardize the medication dose, each boy remaining at his customary dosage. The results show that RSE in conjunction with MPH is superior to medication alone or RSE alone as judged by observations of social behavior on playground.
- Hinshaw, Buhrmester, and Heller, 1989 (cognitive behavioral therapy vs. stimulant plus cognitive behavioral therapy): This study examined the effect of MPH on anger control in 24 boys between the ages of 6 and 12 who were participating in a 5-week summer research program. Children received CBT using "specific curriculum" three times per week to enhance anger control during verbal provocation and were also randomized to MPH. The results indicated that MPH exerts significant effects on the global rating of self-control and physical retaliation when the child was provoked, but the data were difficult to extract. Furthermore, the outcome measures were of questionable clinical significance.
- Pelham, Carlson, Sams et al., 1993 (behavioral therapy plus stimulant vs. regular classroom plus stimulant): Thirty-one ADHD boys ranging in age from 5 to 10 years participated in an 8-week day treatment program. The boys alternated, on a weekly basis, between classrooms with behavior modification and no behavior modification and received in random order placebo or MPH at a low dose or high dose. Results revealed significant effects of both interventions with the mean effect size of medication being more than twice as great as that of the behavior modification. The addition of either dose of MPH resulted in improvement beyond the effects of behavior modification alone.
- Solanto, Wender, and Bartell, 1997 (behavioral contingencies/modification vs. behavioral contingencies/modification plus stimulants): This study evaluated the effect of MPH in combination with BC/Mod on a measure of sustained attention (CPT) in 22 children between 6 and 10 years of age who met DSM-III-R criteria for ADHD. The results indicated that MPH improved sustained attention whereas behavioral intervention alone did not. Adding behavioral intervention to MPH did not lead to further improvement. Unfortunately, this study did not explore whether children with better CPT performance had improvements in other core symptoms of ADHD.
The largest and most comprehensive study conducted to date was designed to determine the effectiveness of different treatment strategies, involving careful titration and systematic testing of drugs other than MPH if needed to achieve an adequate response.
- MTA Cooperative Group, 1999: Five-hundred-seventy-nine children, between the ages of 7 and 9, were randomly assigned to one of four 14-month-long conditions: (1) medication management (MedMgt) with monthly visits and supportive care; (2) intensive behavioral treatment (Beh) (including home- and school-based behavior management, direct behavioral management therapy for the child delivered through a summer camp program); (3) both treatments combined (Comb); or (4) assessment and referral back to community service providers (CC). Children's outcomes in clinical and functional domains were assessed throughout the study, using rating scales and direct observational measures. Comb and MedMgt treatments were clinically and statistically superior to intensive Beh and routine CC in reducing core ADHD and oppositional-aggressive symptoms. Moreover, the Comb and MedMgt approaches did not differ statistically or clinically from each other for ADHD or oppostional-aggressive symptoms. In several non-ADHD-symptom areas (parent-rated internalizing symptoms, social skills, consumer satisfaction, and reading), Comb provided modest incremental benefits over single-treatment approaches. MedMgt was superior to medication management provided in the community despite the fact that two-thirds of the CC subjects were treated with medication during the study period.
In summary, there is a lack of evidence supporting the superiority of combination therapy over stimulant alone or superiority of combination therapy over nondrug intervention alone. More research is needed to determine the added value of including nondrug interventions to the treatment scheme of patients who are already receiving stimulants, as well as the added value of treatment with stimulants when nondrug interventions fail to achieve the desired outcomes.
Tricyclic Antidepressants vs. Placebo
Detailed information on the general characteristics of the nine studies included with comparisons of tricyclic antidepressants with placebo appears in Evidence Table E1 and Supplemental Tables E4-E8.
Five of these reports studied desipramine and three the effects of imipramine. One of these trials studied desipramine and clonidine (Singer, Brown, Quaskey et al., 1995). None of the studies evaluated the effects of amitriptyline.
Only one studied an adult population (Wilens, Biederman, Prince et al., 1996). The interventions in the trials were administered for 2 to 18 weeks (in 6 trials, it was 6 weeks or fewer). All the trials but one described the inclusion criteria and three described the exclusion criteria. None described the primary outcome measure (Evidence Tables E1-E3).
Following are descriptions of the main findings of these studies.
Studies of Desipramine
Six studies provided data on effectiveness outcomes of interest. All six of these trials showed results in favor of desipramine.
- Biederman, Baldessarini, Wright et al., 1989a: This study was described in a group of three reports (Biederman, Baldessarini, Wright et al., 1989b; Biederman, Baldessarini, Wright et al., 1993). This study, which did not report the number of patients randomized, found significant improvements in parent and teacher ratings of primary symptoms and in depression in favor of desipramine.
- Donnelly, Zametkin, Rapoport et al., 1986: In this study, Conners teacher ratings of primary symptoms improved and classroom activity level declined significantly, although scores on continuous performance and memory tasks were not significantly different. Significant increases in blood pressure and heart rate were noted in the desipramine group.
- Gualtieri, Keenan, and Chandler, 1991: In this study, desipramine at three doses (low, medium, and high) was evaluated against placebo in 12 patients. Desipramine at the medium dose resulted in the best scores on "clinical significance," global rating, and teachers' reports of inpatient behavior. Of all individual school behavior, only "fidgety" behavior showed a meaningful linear trend. In addition, this study showed that patients were least "cheerful" on medication at high doses and that motor performance was adversely affected by the drug.
- Rapport, Carlson, Kelly et al., 1993: This study compared MPH combined with desipramine, MPH alone, desipramine alone, and placebo. The sample included 16 boys who were hospitalized with ADHD and mood disorder. The report obtained a quality score of 3 with the validated scale and included information on 17 of the clinically relevant elements (Evidence Table E1). This study found that MPH was potent in affecting children's vigilance (CPT) and their ability to learn paired associations and do the MFFT. Desipramine had no effect on vigilance but improved learning paired associations (not as much as MPH) and was equally effective on the MFFT. Combination therapy led to some blocking of the vigilance but improved MFFT and SEP, which suggests some additive effect on behavioral inhibition and complex problemsolving abilities. Some of the limitations of this study are the study sample that consisted of hospitalized patients who had significant coexisting mood disorder, lack of individual outcome data, and the clinical significance of outcome measures.
- Singer, Brown, Quaskey et al., 1995: In this study, the effects of clonidine, desipramine, and placebo were compared in children with Tourette's syndrome and ADHD. On a global linear analog scale, parents rated children as significantly better on desipramine than on clonidine or placebo. Parent ratings on the Child Behavior Checklist hyperactivity subscale for boys on desipramine were significantly lower than for those on clonidine. Teacher ratings on the nervous overactive subscale were lower for boys during treatment with clonidine and desipramine than placebo.
- Wilens, Biederman, Prince et al., 1996: In this study, 68 percent (13/19) of the participants showed a positive response to desipramine (very much or much improved plus at least a 30 percent reduction in rating scale symptoms). No participants showed a favorable response to placebo. Two participants receiving desipramine dropped out, and 10 had doses lowered as a result of adverse effects. Favorable response was not related to the presence of lifetime comorbidity of anxiety or depressive disorders.
Studies of Imipramine
- Gualtieri and Evans, 1988: In this study, imipramine showed a dose-response relationship with increased scores on a selective attention task but reduced motor performance (finger-tapping speed) in nine children.
- Werry, Aman, and Diamond, 1980: Compared with placebo, imipramine reduced impulsive responses and seat movement significantly, but it did not reduce omission errors or have an impact on self-esteem. Psychiatrists' evaluation of improvement using Clinical Global Improvement showed significantly better results with imipramine compared with those with MPH and placebo. Parent ratings using the Conners Parent Questionnaire showed inconsistent results. There were no differences among the three groups when teacher ratings were used.
- Winsberg, Kupietz, Yepes et al., 1980: This study included 11 children who had failed to respond to MPH. No significant benefit was obtained either on parent and teacher ratings of the child's hyperactivity or on the patient's performance in a laboratory measure of sustained attention, except for improvements in attention measured by parents using Conners.
In summary, the studies on desipramine, regardless of their heterogeneous designs, small sample sizes, and variable quality, suggest that desipramine is more effective than placebo. The studies evaluating imipramine, however, show inconsistent results.
Long-Term Therapy for ADHD
The search yielded 14 studies. The interventions in these studies were administered for periods of time that varied from 12 to 60 weeks. In 1 trial, the exact duration of the study arms was not reported, but a mean duration of 20 weeks was described (Conrad, Dworkin, Shai et al., 1971). The duration of treatments in 6 trials was 12 weeks; in 4, it was between 13 and 26 weeks; and in 3, it was 52 weeks or more (Evidence Table F1).
Eight studies included MPH, three studies included DEX, and none looked at pemoline. The limited number of studies yielded very few comparisons of any one treatment with any other (Evidence Table F1, Supplemental Tables F4-F8). Five trials compared MPH with placebo, three compared MPH with another treatment (one each imipramine, THIOR, and behavior therapy), three compared DEX with placebo, and none compared DEX with MPH. One trial each compared nonstimulants with supportive therapy, tricyclic antidepressants with MPH, no treatment with electroencephalogram (EEG) biofeedback, and MPH with CBT. The MTA study looked mostly at MPH but took a "medication management approach" to drug therapy, involving careful initial titration, a manualized algorithm guiding dosage adjustments in response to monthly clinical assessments.
Only four studies achieved high-quality scores with the validated scale (Gittelman-Klein, Klein, Katz et al., 1976; Kupietz, Winsberg, Richardson et al., 1988; MTA Cooperative Group, 1999; Schachar, Tannock, Cunningham et al., 1997). The number of clinically relevant elements found in the articles varied from 7 (Quinn and Rapoport, 1975) to 20 (MTA Cooperative Group, 1999). One of the studies identified the primary outcome of interest to the investigators, and just over one-half the studies measured patient compliance with treatment (Evidence Table F2). Core symptoms were the most commonly reported outcomes. The most frequently used outcome measure was the Conners Parent (nine trials) or Teacher Rating Scale (seven trials). All other outcome measures were used in one study each. Few studies included academic measures as outcomes of their interventions, and even fewer included objective measures of academic performance that would distinguish behavioral from academic improvement (Evidence Table F1, Supplemental Table F4).
Only 4 of the 14 studies reported the adverse effects of long-term therapy. In general, clarity was lacking on medication status at the time of posttesting, the patients were selected by atypical criteria (only two of these studies used DSM-III-R and -IV criteria), the duration of the studies was not long compared with the time patients receive treatments in the clinical setting, the sample sizes were small, and patients were included whose comorbid conditions are not typical of the general population of ADHD children (e.g., all patients in Kupietz, Winsberg, Richardson, et al., 1988, had a comorbid reading disability; many patients in Gillberg, Melander, von Knorring et al., 1997, had comorbid autism). In addition, these studies used differing measures of academic performance or cognitive function.
The following is a brief description of the main findings of the studies in each category.
Studies Evaluating MPH vs. Placebo
- Brown, Borden, Wynne et al., 1986: This study, which included 40 children and adolescents, obtained a high-quality score and included information on 14 of the 20 clinically relevant elements selected for extraction. It showed no advantage of cognitive training over no training in terms of improvement of core symptoms, academic performance, and conduct/oppositional disorders.
- Brown, Borden, Wynne et al., 1988: This study evaluated compliance with medication. Subjects were randomly assigned to a 3-month protocol of MPH at 0.3 mg/kg two times per day or inert placebo two times per day and then were also assigned randomly to a cognitive therapy or AC training program. There was a trend for parents of children receiving placebo to report that more doses of medication and more therapy sessions were missed.
- Firestone, Crowe, Goodman et al., 1986: This study included children from 5 to 9 years of age referred to various outpatient clinics who met criteria for ADHD both at home and at school. The diagnostic procedure is not specified. Patients were randomly assigned to one of three groups: parent training plus placebo, MPH plus PT, or MPH only. A real asset of this study is the careful attention to enrollment, attrition, and dropout rates. Patients given MPH and the combined PT plus MPH showed equivalent improvement from pre- to posttest (3 months), and both of these arms showed greater improvement than the PT plus placebo arm in behavior and attention, but not in reading. No differences were detected after 12 and 24 months, perhaps as a result of numerous crossovers and dropouts. Subjects who switched to MPH showed improvement following the switch.
- Kupietz, Winsberg, Richardson et al., 1988: All 58 subjects in this study met criteria for ADHD and for reading disability and therefore are not typical of ADHD children in most clinics. The study also obtained a high-quality score and included information on 16 of the clinically relevant elements extracted. Behavioral improvement was noted for patients given MPH compared with those given placebo. MPH at the higher doses yielded the greatest effect. Eleven patients dropped out because of the lack of benefit or because of adverse effects. There was no improvement in academic performance, apart from improvement in reading which was observed even though posttesting was conducted off medication. There was evidence of relapse between 3 and 6 months.
- Schachar, Tannock, Cunningham et al., 1997: In this trial, 91 school-age children were randomized to receive a titrated dose of MPH or placebo two times per day for 16 weeks. This study achieved a high-quality score and included information on 17 of the clinically relevant elements extracted from the reports. The children had been referred to a psychiatric clinic for disruptive behavior problems and met criteria for ADHD based on parent and teacher interviews. Subjects were randomized to receive MPH or placebo, and their families were randomized to attend parent training or parent support groups. Medication was titrated against behavior effects and adverse effects up to a typical dose of 0.5 mg/kg given two times per day. MPH administered two times per day resulted in improvement in ADHD and ODD symptoms at school compared with placebo, but not at home (Evidence Table F1). The behavioral effects were evident within several weeks of treatment initiation. Physiological adverse effects were also evident within weeks, but some adverse effects such as irritability were not detected until 4 months after treatment initiation. This study did not measure academic outcomes.
Studies Evaluating MPH vs. Other Interventions
- Brown, Wynne, and Medenis, 1985: In this study, CBT alone resulted in minimal improvement in attention and no improvement in behavior or academic performance. The MPH and the combined treatments resulted in improved attention and behavior but little improvement in academic performance. Combined treatment was not superior to MPH alone.
- Gittelman-Klein, Klein, Katz et al., 1976: This study has been described in previous sections. Initially, the combination MPH plus THIOR was superior to MPH or THIOR alone, although combined treatment was not superior to MPH alone after 12 weeks. Adverse effects were more common with the combined treatment group. The duration of the effect of the combined treatment was longer than that of MPH only, resulting in greater improvement in parent ratings.
- Quinn and Rapoport, 1975: Patients who had participated in two independent RCTs of MPH vs. placebo and imipramine vs. placebo were followed for 1 year. Children who had been assigned to placebo in the initial studies (6 weeks) were assigned to either MPH or imipramine. Treatments were provided by community physicians. Behavioral improvement was evident in both medicated groups but not in the unmedicated group according to teacher ratings; equivalent improvement in behavior was evident in all three groups according to parents. No improvement was evident on academic, intellectual, or cognitive measures. MPH appeared to be the more effective medication, since fewer subjects discontinued treatment as a result of adverse effects or lack of improvement.
Studies Evaluating Dextroamphetamine
- Conrad, Dworkin, Shai et al., 1971: Eighty-one subjects were selected from children in kindergarten and first and second grade attending 7 schools in a lower class neighborhood in 1969. To qualify for the treatment program, children had to be rated in the top 19 percent on the teacher hyperactivity ratings and exhibit abnormalities on at least one of various measures of perceptual-cognitive impairment. Dosage of medication (or placebo) was initiated at 5 mg/d and increased to 10 to 20 mg/d based on response. Subjects who had not received at least a 60-day supply of medication were excluded from the analysis on the grounds that they were noncompliant. The tutoring program was elaborate, covered academic and emotional issues, was lengthy (2 times per week for 20 weeks), and was well described. Fidelity of the tutoring program was not assessed. Neither experimental condition influenced academic achievement significantly.
- Gillberg, Melander, von Knorring et al., 1997: This study merits special mention because it is the only long-term trial conducted outside of North America and one of the four studies published since 1991. Several features of this study are unusual and weaken the ability to generalize to other samples of ADHD children. Reasons for withdrawal from the study suggest that subjects were either nonresponders or were having adverse effects of DEX during the initial unblinded assessment of DEX effects. This limits the validity of the results of the subsequent comparison of DEX and placebo. The description of comorbidity indicates that this sample was unlike those treated with psychostimulants in North America. Seven of 62 subjects had many autistic features, 10 had mild mental retardation, and 2 had Tourette's syndrome. Hallucinations occurred more often in medicated children than has been reported in other studies (3/61), but on the whole, adverse effects were few and relatively mild. Positive effects of the drug were present 15 months after treatment was started.
- Greenhill, Rieder, Wender et al., 1973: Subjects were 9 severely hyperactive children, 6 to 16 years of age, who were unresponsive to drugs and psychotherapy and had no evidence of psychosis or medical illness. Lithium treatment was initiated while the children were inpatients and then continued for 3 months while they were outpatients. The trial consisted of an initial, nonrandomized phase in which placebo, DEX, and lithium were each administered for 1 week in a single-blind fashion. The second phase was conducted on an outpatient basis and consisted of a modified double-blind trial of 3-week phases of DEX, lithium, and placebo. Children showed no improvement or worsening of symptoms when given lithium. DEX was associated with improvement in hyperactivity and concentration compared with placebo as reported by parents, teachers, and psychologists and by several cognitive-perceptual measures. Tutoring accounted for improvement on the Information subtest of the Weschler Intelligence Scale for Children (WISC). The combination of medication and tutoring resulted in improvement in copying, motor pattern, reduction in errors on a cognitive task, reduction in hyperactivity, and increase in full-scale intelligence quotient (IQ). The interventions did not improve academic achievement.
Studies Evaluating Other Interventions
- Fehlings, Roberts, Humphries et al., 1991: This study included 26 boys, 7 to 13 years of age, who had been referred to a child development clinic. Patients in the CBT group showed greater improvement in parent-rated behavior and child-rated self-concept than those in the supportive therapy group, and these improvements persisted from posttreatment assessment at 4 months to the 5-month followup.
- Linden, Habib, and Radojevic, 1996: Subjects were 18 children, age 5 to 15 years with a "primary" diagnosis of ADHD which was based on unspecified measures (e.g., "family interview"). Subjects were referrals to an outpatient psychology clinic. Inclusion and exclusion criteria were not clearly defined. Nine subjects were assigned to EEG biofeedback treatment over 6 months, and nine subjects to a wait-list control group. Adjunctive treatment was controlled, and subjects received no medication or treatment other than that provided in the trial throughout the study. Measures were taken before and after the 6-month treatment program. Active treatment resulted in significantly greater improvement in IQ and attentiveness than did control treatment.
- MTA Cooperative Group, 1999: Five-hundred-seventy-nine children, age 7 to 9, were randomly assigned to one of four 14-month-long conditions: medication management (MedMgt) with monthly visits and supportive care, intensive behavioral treatment (Beh) (including home- and school-based behavior management, direct behavioral management therapy for the child delivered through a summer camp program), both treatments combined (Comb), or assessment and referral back to community service providers (CC). Children's outcomes in clinical and functional domains were assessed throughout the study, using rating scales and direct observational measures. Comb and MedMgt treatments were clinically and statistically superior to intensive Beh and routine CC in reducing core ADHD and oppositional-aggressive symptoms. Moreover, Comb and MedMgt approaches did not differ statistically or clinically from each other for ADHD or oppositional-aggressive symptoms. In several non-ADHD-symptom areas (parent-rated internalizing symptoms, social skills, consumer satisfaction, and reading), Comb provided modest incremental benefits over single-treatment approaches. MedMgt was superior to medication management provided in the community despite the fact that two-thirds of the CC subjects were treated with medication during the study period.
Overall, the studies in this category show a trend to general improvement over time regardless of treatment. MPH appears to reduce behavioral disturbance in ADHD children for as long as it is taken. However, the studies lack information on the reasons that so many children discontinue medication and provide little evidence for improvement in academic performance with stimulants, even though MPH treatment does appear to produce consistent behavior improvement. Lithium does not appear to be an effective alternative in subjects who do not respond to stimulants. A limitation of this category is that all but one study was restricted to school-age children. Few studies followed children for a time period equivalent to the duration children typically remain on these treatments in the clinical setting. There is little information on situation-specific outcome measures (e.g., outcomes measured at home and at school), and adverse effects are reported infrequently.
Treatment of ADHD in Adults
The search yielded 13 reports (12 relevant studies), all of which were published in the United States. The interventions in these studies were administered for 1 to 6 weeks. In three trials, the duration of the study arms was not reported. Only one study (Spencer, Wilens, Biederman et al., 1995) achieved a high-quality score. The report of this study was also the one that included the highest number of clinically relevant elements (Evidence Table G1). All studies reported the inclusion criteria and all but one reported the exclusion criteria, but only three described the primary outcome (Evidence Tables G2 and G3). Six of the studies compared placebo with another drug, one each of pemoline, desipramine, two doses of DEX and secobarbital, phenylalanine, selegiline, and nicotine; and the 12th study compared MPH with DEX. The main findings of the studies are described below.
MPH vs. Placebo
Five RCTs compared MPH with placebo. These studies had very heterogeneous designs and incomplete reporting and provided contradictory results. The only study that was given a quality score >3 was in this group (Spencer, Wilens, Biederman et al., 1995). It was a randomized, double-blind, crossover comparison of titrated doses of MPH with placebo. The dose of MPH was titrated to approximately 1 mg/kg. This study included 25 patients out of 85 who had been referred. On MPH, 78 percent of patients showed end-of-treatment CGI scores <2 and 30 percent reduction in individual rating scale scores. Only 4 percent of the participants met these criteria on placebo. Of the four additional studies comparing the effect of MPH with placebo in adults (Evans, Vallano, and Pelham, 1994; Mattes, Boswell, and Oliver, 1984; Reimherr, Wender, Ebert et al., 1984; Wender, Reimherr, Wood et al., 1985), one had a single-patient design (n-of-1 design) and lasted for 1 week (Evans, Vallano, and Pelham, 1994); one study evaluated the interventions for 2 weeks but did not report the number of patients randomized to each arm (Wender, Reimherr, Wood et al., 1985); and another did not include any of the outcomes of interest (Reimherr, Wender, Ebert et al., 1984). The remaining study (Mattes, Boswell, and Oliver, 1984) included an evaluation of two groups of adults, one with evidence of residual attention-deficit disorder (ADD) with hyperactivity and the other without. In this study, no overall benefit from MPH was evident.
Additional information describing these studies can be found in Supplemental Evidence Tables G5-G8.
Studies Evaluating the Effect of Dextroamphetamine
- DeVeaugh-Geiss and Joseph, 1980: This was a single-subject study (a 20-year-old man) and a double-blind investigation of his response to DEX, secobarbital, and placebo. The authors noted a reduction of anxiety and motor activity while the subject was taking DEX.
- Matochik, Liebenauer, King et al., 1994: This study was primarily interested in the effects of MPH and DEX on cerebral glucose metabolism as measured by positron emission tomograph (PET) before and after treatment. Subjects were adults who met criteria for ADHD, met Utah criteria for ADHD in adulthood, had a definite childhood history of ADHD, had no psychiatric disorder, and were unmedicated prior to the study. Most had parents and/or children with ADHD. Medication dose was individually titrated and administered for 6 to 15 weeks. No differences in glucose metabolism with medication were observed. No direct comparison of MPH and DEX was conducted.
Studies Evaluating Other Interventions
- Conners, Levin, Sparrow et al., 1996: This 1-week trial found that nicotine at 7 mg delivered via transdermal patch yielded significant improvements in CGI ratings (56 percent much improved, 31 percent no change, and 12 percent unchanged or worse) and in self-ratings of vigor and concentration and improved performance on time estimation tasks, when compared with placebo.
- Ernst, Liebenauer, Jons et al., 1996: This trial found that selegiline treatment was not more effective than placebo.
- Wender, Reimherr, and Wood, 1981: This study examined the effects of pemoline vs. placebo. More than one-half the patients in the pemoline-treated group complained of moderate to severe adverse effects. Although the overall response to pemoline in the placebo and drug groups did not differ, adults with a childhood history of ADHD on parent reports showed a more favorable response than those without a childhood history of ADHD.
- Wilens, Biederman, Prince et al., 1996: This study, also described in the tricyclic antidepressants section, found that 68 percent (13/19) of the participants showed a positive response to desipramine (very much or much improved) plus at least a 30 percent reduction in rating-scale symptoms. No participants showed a favorable response to placebo. Two participants receiving desipramine dropped out, and 10 had doses lowered as a result of adverse effects. Favorable response was not related to the presence of lifetime comorbidity of anxiety or depressive disorders.
- Wood, Reimherr, and Wender, 1985: This was a crossover trial of phenylalanine that yielded a significant improvement in mood and mood ability over placebo, but the positive effects were lost within 3 months.
In conclusion, few RCTs are designed to evaluate the long-term effects of treatments for ADHD. Most of the studies available had incomplete reports, had small sample sizes, and were of short duration. The few studies evaluating MPH vs. placebo show contradictory results, but mostly in favor of MPH. The only study available with evaluation of the effect of DEX vs. placebo included few data of clinical relevance. Antidepressants may be effective in adults. Studies (one each) comparing pemoline, nicotine, or phenylalanine with placebo did not produce evidence in favor of these medications. No studies were designed to determine the proportion of adults with ADHD who will use, and benefit from, other interventions, particularly nonpharmacological.
Adverse Effects of Treatments for ADHD
The search yielded 33 reports of 28 RCTs and 1 non-RCT of pharmacological treatment for ADHD that provided data on adverse effects and met the inclusion criteria. Detailed information on the general characteristics of the 29 studies is included in Tables H1a-H1h. The earliest study was published in 1972 and the most recent, in 1997. Almost one-half the reports were published since 1993. Although the period of time during which the interventions were studied varied from 1 to 89 weeks, 19 evaluated adverse effects for 12 weeks or fewer, whereas 3 of the studies did not report the exact duration of followup (Fine and Johnston, 1993; Matochik, Liebenauer, King et al., 1994; Rapport, Carlson, Kelly et al., 1993). Unfortunately, 11 RCTs failed to indicate the number of patients randomized to 1 or more of the treatment arms, making it impossible in these studies to identify with confidence the proportion of patients adversely affected by treatment. Among the studies reporting on the number of patients randomized, substantial variation was seen in sample sizes, from 16 to 166 patients. Eight of the 17 RCTs that provided the number of patients randomized included 30 or fewer subjects. The failure to report clearly on the number of patients randomized was symptomatic of overall poor quality. The interventions evaluated were MPH in 25 RCTs, amphetamines (DEX or l-amphetamine [L-amph]) in 9 RCTs, pemoline in 2 RCTs, and antidepressants in 2 trials. The single non-RCT evaluated MPH.
The adverse effects examined most often across the studies included sleep disorders/disturbances (including nightmares), headaches, motor tics, decreased appetite/anorexia, abdominal pain, irritability, nausea, and fatigue/tiredness (Evidence Tables H1a-H1h and H2). No study evaluated the risk of addiction with stimulants, liver toxicity with pemoline, or cardiac arrhythmias with antidepressants.
Meta-analysis was deemed inappropriate in this category for the same generic reasons described in the Methodology chapter, but in addition, there was marked heterogeneity in the methods used to elicit, measure, and report adverse side effects-different concepts (differences in type as well as characterization: occurrence, frequency, severity), instruments, respondents, and method (elicitation vs. spontaneous report); very short exposure periods; little or no information on interactions between developmental stage and adverse effects; and imprecise estimates of adverse effects because of small sample sizes.
The following is a more detailed description of each of the adverse effects most frequently studied. Tables H1a-H1h provide a detailed summary of these adverse effects, according to the interventions evaluated in the studies.
- Sleep disorders (Evidence Table H1a): Twenty-three studies looked at "sleep disorders," or "disturbed sleep" in general, or insomnia. Eight of the studies did not report the statistical significance of the differences between the arms. Of the remaining studies, only four found statistically significant differences between the groups (Ahmann, Waltonen, Olson et al., 1993; Barkley, McMurray, Edelbrock et al., 1990; Elia, Borcherding, Rapoport et al., 1991; Fitzpatrick, Klorman, Brumaghim et al., 1992). In 4 studies for MPH (out of a total of 20) and in 1 for DEX (out of 8), the stimulants produced more sleep disorders than placebo. No evidence was seen of increased sleep disorders in any of the active interventions. Nightmares were addressed specifically in seven studies that are included in this table. The three studies that compared the proportion of patients with nightmares across the arms did not show statistically significant differences (Barkley, McMurray, Edelbrock et al., 1990; Buitelaar, van der Gaag, Swaab-Barneveld et al., 1996; Stein, Blondis, Schnitzler et al., 1996).
- Headaches (Evidence Table H1b): Nineteen studies provided data on headaches, but 8 did not report statistical significance. Of the remaining 12 studies, only 2 showed an increase in headaches. Both studies found the increase in headaches associated with MPH compared with placebo (out of 10 studies comparing MPH with placebo) (Ahmann, Waltonen, Olson et al., 1993; Barkley, McMurray, Edelbrock et al., 1990). No statistically significant differences in studies comparing different doses of MPH, DEX with placebo, or DEX with MPH were seen. Two studies evaluating pemoline did not report values for statistical significance.
- Motor tics (Evidence Table H1c): Seven studies provided data on motor tics. Five of these studies did not report statistical significance of the differences between the groups. One study showed that patients that received MPH had more tics than those receiving placebo, as was indicated by teachers in the frequency of vocal tic index. No differences were found between different doses of MPH (three studies), MPH and DEX (one study), MPH and pindolol (one study), or amphetamine and placebo (one study).
- Decreased appetite/anorexia (Evidence Table H1d): Twenty-three studies provided data on this adverse effect. In eight studies, there was no information on the statistical significance of the differences between the arms. Of the remaining 15 studies, 8 showed that patients receiving stimulants reported decreased appetite or were associated with anorexia more frequently than those receiving placebo. MPH was associated with these adverse effects more frequently than placebo in 7 studies (out of 12 studies that reported statistical significance). The three studies that reported statistical significance for the differences between DEX and placebo showed more frequent anorexia and decreased appetite in patients receiving DEX. One study showed a difference among different doses of MPH (out of six studies). No differences were shown between MPH and DEX (a total of four studies with two reporting significance).
- Abdominal pain (Evidence Table H1e): Nineteen studies provided data on abdominal pain, but 8 did not provide data on the statistical significance of the differences among the study groups at the p=0.05 level. Of the remaining 12 studies, only 2 studies showed statistically significant increase in the frequency of abdominal pain. Both studies compared different doses of MPH with placebo (out of 10 that reported statistical differences between MPH and placebo) (Ahmann, Waltonen, Olson et al., 1993; Barkley, McMurray, Edelbrock et al., 1990). No statistically significant differences were seen between MPH and DEX (three studies, one of which provided data on significance), DEX and placebo (six studies, of which two reported significance), or among different doses of MPH (six studies). The two studies that evaluated pemoline did not report the statistical significance of the differences in abdominal pain when pemoline was compared with placebo, DEX, or MPH.
- Irritability (Evidence Table H1f): Thirteen studies provided data on irritability, but 4 did not provide data on the statistical significance of the differences among the study groups. Of the remaining nine studies, only two studies showed statistically significant increase in the frequency of irritability. Both studies compared different doses of MPH with placebo and showed the increased frequency only for the highest dose tested (Ahmann, Waltonen, Olson et al., 1993; Handen, Feldman, Gosling et al., 1991). There were no statistically significant differences between MPH and DEX (two studies, one of which provided data on significance), DEX and placebo (four studies, of which two reported significance), or among different doses of MPH (five studies). The two studies that evaluated pemoline did not report the statistical significance of the differences in abdominal pain when pemoline was compared with placebo, DEX, or MPH.
Studies that provided data on nausea, fatigue and tiredness either showed no significance among the interventions evaluated in them or did not provide data on significance (Evidence Tables H1g and H1h).
Effect of Stimulants Compared With Placebo: What Did We Learn?
As planned, information on the effects of stimulant medication compared with placebo was obtained from three sources: the individual RCTs included in this review, three of the systematic reviews included in the review of reviews (Appendix C), and the systematic review conducted at the University of British Columbia (Miller, Lee, Raina et al., 1998). This Task Order review included 35 RCTs comparing the short-term effects of stimulants and placebo and 5 RCTs comparing their long-term effects (Evidence Table I1-I3). In five studies, it was unclear how long the followup was. A brief summary of the data from these sources follows.
Studies Comparing the Short-Term Effects of Stimulants With Placebo
The short-term RCTs had duration that varied from 1 to 8 weeks and were published between 1972 and 1997 (Evidence Table I1-I3). Thirty-four of these studies evaluated the effects of MPH, 13 evaluated DEX, and 5 evaluated pemoline. Twenty-five of the studies evaluating MPH; 11 of those evaluating DEX and 4 of those evaluating pemoline had nonstatistically significant results.
Studies Comparing the Long-Term Effects of Stimulants With Placebo
Little evidence was found on the long-term effects of stimulants compared with placebo (Evidence Table I1-I3). Five RCTs evaluated the long-term effects of MPH, and two evaluated DEX. No studies evaluating the long-term effects of pemoline were found.
Overall, as described above, the studies in this category show a trend to general improvement over time regardless of treatment. The studies suggest that stimulants reduce behavioral disturbance but provide little evidence for improvement in academic performance. The studies have major methodological deficiencies which are compounded by their restriction to school-age children, relatively short followup, and few data on adverse effects.
Published Systematic Reviews
Three of the meta-analyses included in the review of reviews evaluated the effect of stimulants compared with placebo (Appendix C). Although these reviews had major methodological limitations, they showed consistently that stimulants improve behavior in children with diagnosis of ADHD in the short-term (Kavale, 1982; Ottenbacher and Cooper, 1983; Thurber and Walker, 1983).
Systematic Review Conducted at University of British Columbia
The report of the systematic review conducted at the University of British Columbia was assessed and obtained a quality score of 6 out of 7 using Oxman and Guyatt's methodological index (Oxman and Guyatt, 1991). This review was based on an extensive search of seven databases to August 1997, contact with pharmaceutical companies, and hand search of journals.
The review focused on RCTs comparing stimulants with placebo that provided data on children's general "ADHD behaviors" obtained with 11 teacher measures: the Abbreviated Conners Teacher Rating Scale; the Iowa Conners Teacher Rating Scale; the Attentional Problems Subscale of the Achenbach Child Behavior Checklist (Teacher Report Form [TRF]); the Child Attention Profile (CAP) Questionnaire; School Situations Questionnaire (SSQ); Revised Behavior Problem Checklist (RBPC); ADD-H Comprehensive Teacher Rating Scale (ACTeRS); Attention-Deficit Disorder Evaluation Scale (ADDES); Children's Attention and Adjustment Survey (CAAS); ADHD Rating Scale; and the Swanson, Nolan, and Pelham (SNAP). Nine behavior rating scales used to measure parent opinions were: Conners Parent Rating Scale; Conners, Loney, and Milich (CLAM) Questionnaire; Achenbach Child Behavior Checklist Attentional Problems score; Home Situations Questionnaire (HSQ); Revised Behavior Problem Checklist; Attention-Deficit Disorder Evaluation Scale; Children's Attention and Adjustment Survey; ADHD Rating Scale; and SNAP.
The authors standardized data that had been obtained with different tools to a common measure across the studies. Despite the fact that the authors acknowledged the limitations of the data available, they performed meta-analysis using DerSimonian and Laird's method. The review included 16 RCTs evaluating efficacy of stimulant treatment using teacher rating scales, 2 of which did not show advantage for the stimulants. Twelve RCTs included parent rating scales, one of which did not show advantage for the stimulants. When all the studies were combined, the overall results indicated that drug therapy was more effective than placebo. The results from the analysis of seven RCTs of MPH and three of DEX was possible only with the Conners Teacher Rating Scale and showed that both drugs were better than placebo. The only trial comparing pemoline vs. placebo also showed advantage for patients receiving pemoline.
In summary, the results of studies comparing stimulants with placebo indicate that stimulants offer benefit at least in the short term. The few data on long-term effects suggest that stimulants may improve behavioral disturbances. Little evidence shows long-term benefit of stimulant therapy on academic performance, and there is a paucity of data for long-term adverse effects.
- Findings - Treatment of Attention-Deficit/Hyperactivity DisorderFindings - Treatment of Attention-Deficit/Hyperactivity Disorder
- KASH domain containing 5 isoform a [Rattus norvegicus]KASH domain containing 5 isoform a [Rattus norvegicus]gi|2778574709|ref|NP_001419168.1|Protein
- zinc finger protein 197 isoform 1 [Homo sapiens]zinc finger protein 197 isoform 1 [Homo sapiens]gi|11386193|ref|NP_008922.1|Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...
