This publication is provided for historical reference only and the information may be out of date.
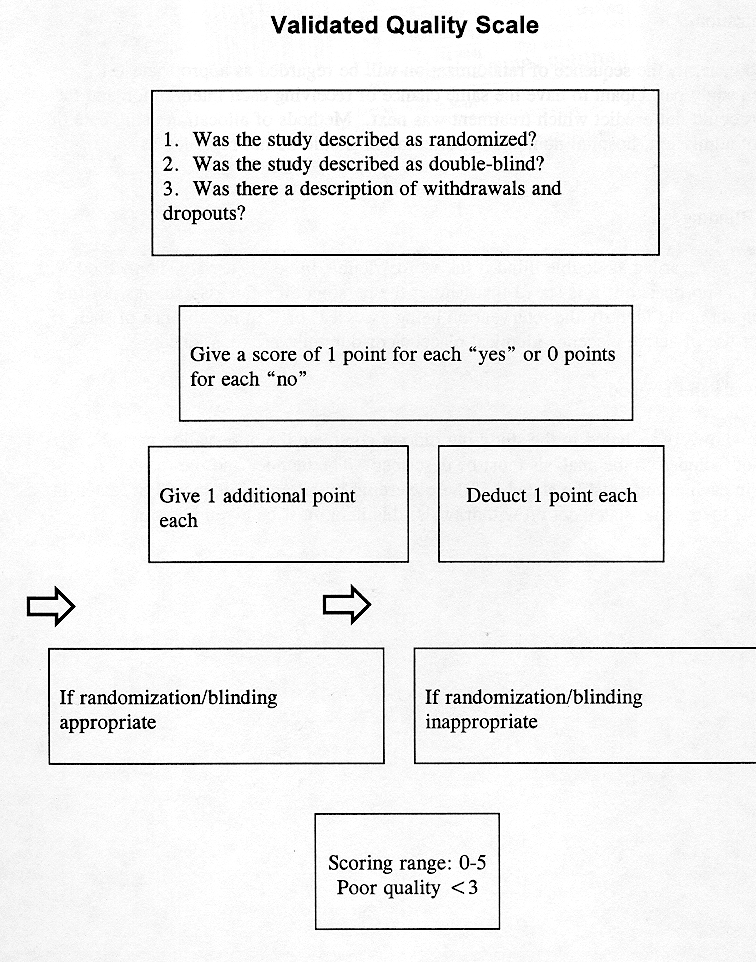
Jadad, Moore, Carroll et al. Controlled Clin Trials 1996;17:1-12.
Guidelines for Assessment
1. Randomization
A method to generate the sequence of randomization will be regarded as appropriate if it allowed each study participant to have the same chance of receiving each intervention and the investigators could not predict which treatment was next. Methods of allocation using date of birth, date of admission, hospital numbers, or alternation should be not regarded as appropriate.
2. Double Blinding
A study must be regarded as double blind if the word "double blind" is used. The method will be regarded as appropriate if it is stated that neither the person doing the assessments nor the study participant could identify the intervention being assessed, or if in the absence of such a statement for use of active placebos identical placebos or dummies are mentioned.
3. Withdrawals and Dropouts
Participants who were included in the study but did not complete the observation period or who were not included in the analysis must be described. The number and the reasons for withdrawal in each group must be stated. If there were no withdrawals, it should be stated in the article. If there is no statement on withdrawals, this item must be given no points.
Publication Details
Copyright
Publisher
Agency for Healthcare Research and Quality (US), Rockville (MD)
NLM Citation
Jadad AR, Boyle M, Cunningham C, et al. Treatment of Attention-Deficit/Hyperactivity Disorder. Rockville (MD): Agency for Healthcare Research and Quality (US); 1999 Nov. (Evidence Reports/Technology Assessments, No. 11.) Appendix F. Quality Assessment Scale.