NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lehmann HP, Andrews JS, Robinson KA, et al. Management of Acne. Rockville (MD): Agency for Healthcare Research and Quality (US); 2001 Sep. (Evidence Reports/Technology Assessments, No. 17.)
This publication is provided for historical reference only and the information may be out of date.
The management of acne was a topic nominated to the Agency for Healthcare Research and Quality (AHRQ, then called the Agency for Health Care Policy and Research [AHCPR]) by the American Academy of Dermatology (AAD) and the American Academy of Pediatrics (AAP). In October 1998, AHRQ awarded a contract to the Johns Hopkins Evidence-based Practice Center (EPC) to prepare an evidence report on this topic. With the assistance from the partners from AAD and AAP, the Johns Hopkins EPC established a team and work plan to develop a report that would identify and synthesize the best available evidence on the management of acne. The project consisted of recruiting technical experts, identifying the patient population, formulating and refining the specific questions, performing a comprehensive literature search, summarizing the state of the literature, constructing evidence tables, and submitting the report for extensive peer review.
Recruitment of Technical Experts
Experts were sought who could provide content and/or methodological guidance. The group of experts included representatives from the project partners AAD and AAP, as well as from the American Pharmaceutical Association (AphA), Society for Investigational Dermatology (SID), and the Skin Collaborative Review Group (CRG) from the Cochrane Collaboration. Their input was sought through ad hoc correspondence as well as through more formal requests for feedback during the project. Specific requests for feedback were made for key decisions throughout the project, such question refinement, completeness of literature search, treatment and acne severity classification, and format and layout of evidence tables and synthesis of literature.
Additional representation of AAD, AAP, American Academy of Family Physicians (AAFP), as well as from the Society for General Internal Medicine (SGIM), SID, and Society for Adolescent Medicine (SAM) joined the technical experts as peer reviewers for the report. These reviewers included dermatologists, pediatricians, and generalists (Appendix A).
Patient Population
The target population for this report is all patients with acne who do not have complicating comorbidities such as endocrinopathies. In consultation with the partners and technical experts, we decided to exclude evidence from populations with the following conditions: acne fulminans, acne necroticans, acne venenata, rosacea, and chloracne. We believed that to include endocrinopathies would necessitate an examination of underlying medical conditions that was beyond the scope of this report. The search and synthesis of evidence was not limited by age, gender, or any other patient characteristic, though synthesis includes an analysis by these characteristics, where possible.
The report sought evidence concerning all primary care and dermatology settings. In addition, self-treatment is an important modality for acne therapy. Therefore, the community-based setting in which a consumer of health care acts without the involvement of a health care provider was also considered.
Questions
Questions were identified through discussions among the team members, preliminary searches of MEDLINE®, a search of the MicroMedex pharmaceutical database, and consultation with our technical experts. We also reviewed over 14,000 consumer-generated questions posted to a major consumer health Internet Web site (InteliHealth™) for queries about acne. The questions identified through this process formed the basis of a brief questionnaire distributed to the technical experts (Appendix B). The experts were asked to rate the importance of each question, to provide a sense of the data available to address each question, and also to provide any additional comments or suggestions.
Based on the results from the questionnaire, we developed questions in factorial format to embrace the various patient treatments, outcomes, patient characteristics, practice settings, and care providers of interest (Appendix C). The factorial formatted questions, as well as the proposed exclusions, were distributed to the technical experts for feedback (Appendix D). From the factorial questions, the technical experts requested that the evidence report address the following questions:
- What treatments are effective in the management of acne?
- What is the responsiveness of acne to first-line, second-line, and third-line (referral) treatments?
- What are the side effects of treatment?
The following secondary questions were also to be addressed as the evidence allowed:
- What baseline characteristics raise the likelihood of acne?
- What care do patients provide for themselves at home?
- How effective is initial home care?
- What is the presentation of acne to physicians?
- How compliant are patients with their treatment?
- What is the residuum of untreated acne? Of treated acne?
- What is the quality of life (psychological morbidity) in untreated acne? In treated acne?
- What is the financial cost of therapy?
Causal Pathway
The accumulation of data alone is not sufficient for decisionmaking. The evidence needs to be organized into meaningful units, and the units must be organized to aid in making decisions. To this end, we structured a causal model, using the conventions of an influence diagram 23 (Figure 1). The diagram is an abstraction of a decision tree, and, when fully specified, can derive the flowchart of a practice guideline. For instance, rather than specifying all possible self-care options, the diagram simply shows that the choice of self-care is influenced by a patient's baseline characteristics which, in turn, influence the possibility and choice of initial medical care. It was developed through structured interviews with two technical experts and a review of the literature, and it was critiqued by the other technical experts.
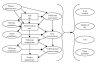
Figure
Figure 1. Influence diagram of management of acne.
Preliminary definitions and assessments were made of the nodes (states or variables) of the influence diagram:
- Patient characteristics -- These are characteristics such as race, gender, and skin type.
- Self-care -- Refers to over the counter treatments and medications, such as benzoyl peroxide. This also includes self-referral to a medical provider.
- Initial acne assessment -- Refers to the acne present at initial assessment. The arrow from baseline characteristics suggests that this acne severity is influenced by baseline characteristics, although our initial review of the evidence suggested that few investigators go beyond lesion count and type in making a severity assessment (see Chapter 3).
- Initial medical care -- Represents all possible prescription and non-prescription treatments.
- Acne reassessment -- The same as initial acne assessment, but later in time. An arrow is drawn from the initial acne assessment node, suggesting that response to treatment (from initial medical care) is influenced by the initial acne status (or baseline characteristics).
- Initial compliance -- Acknowledges that success depends on patient compliance and that initial medical choices must be made keeping compliance in mind.
- Followup medical care -- Refers to the provider's options after assessment, including continuing present therapy, changing to another treatment, or referral.
- Followup compliance -- Similar to initial compliance.
- Followup acne reassessment -- Similar to acne reassessment.
- Further medical care -- Focuses on referral if acne reassessment shows no improvement. Refers to the provider's option after assessment, including continuing present therapy, changing to another treatment, or referral. We left the full definition open, hoping to find evidence in the literature that would help us to define the therapeutic choices.
- Acne residual -- This is the long-term dermatological morbidity of acne, with or without treatment.
- Quality of life -- Outcomes such as self-esteem and social and familial relationships.
- Cost -- Includes costs related to loss of productivity in school or work, as well as medical costs.
- Other morbidity -- Other morbidity outcomes, such as the presence of lesions themselves, and the possible effects of treatment. Possible treatment effects include sun sensitivity and, of the most concern, more serious side effects such as those related to isotretinoin use.
Influence diagrams terminate in a utility node that integrates the outcomes, their probabilities, and the decisionmaker's valuation of preference for those outcomes. We plan to leave that integration to the users of our final evidence synthesis for creating their guidelines.
Literature Search Methods
Searching the literature included the steps of identifying reference sources, formulating a search strategy for each source, and executing and documenting each search.
Sources
Several literature sources were used to identify all studies potentially relevant to our study questions. The first source that we used was the CENTRAL database of the Cochrane Collaboration. This is a database of all clinical trials (primarily randomized controlled trials and controlled clinical trials) identified through the searching efforts of the Cochrane Collaboration. The CENTRAL database includes search results from many electronic databases, including MEDLINE® and EMBASE®, as well as results from the hand searching of more than 1,000 journals, for all publication years starting in 1948. The CENTRAL database also includes the specialized register of controlled trials developed by the Cochrane Skin Collaborative Review Group. The Skin CRG has completed extensive searching of electronic databases including MEDLINE®, MBASE®, Current Contents® in Dermatology, and the American Academy of Dermatology trials register. In addition, members of this CRG hand search a number of key dermatology journals such as Dermatology and Clinical and Experimental Dermatology. The CENTRAL database is made available on The Cochrane Library, which is issued quarterly. Both Issue 4 of the 1998 The Cochrane Library and Issue 1 of the 1999 The Cochrane Library were searched.
Our second source was MEDLINE®, or MEDlars onLINE, the database of bibliographic citations and author abstracts from approximately 3,900 current biomedical journals published in the United States and 70 foreign countries, which dates back to 1966. MEDLINE® was accessed through PubMed, the Internet access to MEDLINE® provided by the National Library of Medicine (NLM), which also includes the PREMEDLINE database of basic citation information and abstracts of full records before they are prepared and added to MEDLINE®. The searching of PubMed helped to confirm the completeness of the search conducted with the Cochrane Collaboration's CENTRAL database and also helped to identify new studies published while we were working on the evidence report. In addition, for the questions where there were few or no controlled clinical trials (i.e., those related to the costs of acne), PubMed was used to identify other types of studies.
Internet GratefulMed, provided as a Web-based service by the NLM, was used to access OLDMEDLINE. OLDMEDLINE contains citations published in the 1960 through 1965 Cumulated Index Medicus and covers the fields of medicine, preclinical sciences, and allied health sciences. We felt it important to include OLDMEDLINE as some of the key articles concerning treatment effectiveness, for instance of benzoyl peroxide, may have been published prior to the MEDLINE® start date of 1966. Internet GratefulMed was also used to access HealthSTAR. This electronic database combines the former HEALTH (Health Planning and Administration) and HSTAR (Health Service/Technology Assessment Research) databases and includes citations from 1975 to present.
Using the access provided by the Johns Hopkins Welch Medical Library, the PsycINFO® and CINAHL® databases were also searched. PsycINFO® covers the professional and academic literature in psychology and related disciplines, including medicine, psychiatry, nursing, sociology, education, pharmacology, physiology, linguistics, and other areas (including literature) from 1887 to present. CINAHL® is a multidisciplinary database covering the nursing, allied health, biomedicine, and consumer health literature from 1982 to present.
Two final steps were taken to ensure a comprehensive literature search. A database of reference material, identified through the electronic searching, through discussions with experts, and by the article review process, was created in the reference management software, ProCite®. A listing of titles and abstracts from this database, the Acne References Database, was reviewed by the investigators to identify key articles. The reference lists from these key articles were then examined to identify any additional articles for inclusion in the Acne Citations Database. Finally, a listing of all identified controlled trials was distributed to our technical experts, and they were asked to identify any additional articles of which they were aware that should be considered for inclusion in the review process.
Search Terms and Strategies
The search strategies were designed to maximize sensitivity and were developed in consultation with team members. A core search strategy for identifying evidence from the CENTRAL database was developed. PubMed was searched by combining the core search strategy with phases 1 and 2 of a search strategy developed for the optimal identification of controlled clinical trials from PubMed. 24 Search strategies were also modified, as needed, based on the source being accessed, with separate strategies developed for OLDMEDLINE, CINAHL®, and PsycINFO®. Because results were limited on this topic, a supplemental search was conducted on PubMed for the retrieval of cost-related articles. (See Appendix E for search strategies.)
Organization and Tracking of Literature Search
The results of the searches of electronic databases were downloaded and, using the duplication check in the bibliographic software ProCite®, articles not previously retrieved were included in the Acne Citations Database. This ProCite® database was used to store citations and to aid in the tracking of search strategies and sources. The use of this software allowed citations and abstracts to be directly imported into a database as well as catalogued with important attributes. The results of the abstract review process were also tracked using ProCite®.
Abstract Review
Specific inclusion and exclusion criteria were applied at each of three levels of review, with criteria becoming more stringent as the process moved from use of specific search terms to the review of abstracts to the review of articles. After identifying a citation, its title and abstract were reviewed, and articles were included or excluded from the article review on this basis.
Identification of Inclusion and Exclusion
During the abstract review process, the emphasis was placed on identifying all articles that may possibly have original data pertinent to the questions. As previously described, the technical experts were consulted during the development of inclusion and exclusion criteria.
In evaluating titles and abstracts, the following criteria were used to exclude articles from further consideration:
- Article does not address management of acne.
- Article does not include human data.
- Article addresses a topic excluded from this evidence report:
- chloracne
- rosacea
- acne venanta
- acne fulminans
- acne necroticans - Article contains no original data.
- Article is not in English.
Articles excluded with criterion "no original data" included those that were duplicate publications of the same trial. In those cases, the article with a more comprehensive reporting of the data was kept (if otherwise eligible).
It is recognized that the decision to exclude non-English language reports of trials is a limitation of this report because it means the possible exclusion of potentially high quality and highly relevant reports of trials. Time and other resources, however, prevented this project from searching, retrieving, translating, and then reviewing non-English reports of trials.
The abstract review process was also used to classify the articles by topic and to identify the study design. For those articles without an abstract, the abstract review process was performed using the entire article.
Abstract Review Process
Titles and abstracts for all articles retrieved by the literature search were printed on an abstract form and distributed to two reviewers (Appendix F). When reviewers agreed that, because of insufficient information, a decision regarding eligibility could not be made, the full article was retrieved and then reviewed. The principal investigators reviewed abstracts when abstract reviewers disagreed regarding eligibility of the article.
The results of the abstract review process were entered into the Acne Citations Database. Articles deleted through the abstract review process were tagged with reason for exclusion and removed to the Acne Deletions Database. The maintenance of this database allowed for the ongoing search results to be compared to already retrieved citations (in Acne Citations and Acne Deletions Databases) for the identification of newly retrieved articles and also allowed for the efficient tracking of the abstract review results.
Article Review
The purpose of the article review was to confirm relevance of each article to the questions at hand, to determine methodological characteristics pertaining to study quality, and to collect evidence pertaining to the questions. Where articles included reports of more than one trial, reviewers were instructed to complete the relevance assessment (i.e., comparison to inclusion and exclusion criteria), quality assessment, and data abstraction for each trial separately.
Quality Assessment and Data Abstraction
Forms were developed to confirm eligibility for full article review, assess study characteristics, and abstract the relevant data to address the study questions. The forms were developed through an iterative process including the review of forms used for previous EPC projects, discussions among team members and experts, and through pilot testing.
Three forms were developed and color-coded to aid reviewers and data entry personnel (Appendix G).
Study Characteristics and Quality
The first form completed for all trials comprised three sections. The first section included the same exclusion criteria used during the abstract review phase. In this way, reviewers could confirm the eligibility of the trial before proceeding with the full article review. The second section (questions 1 to 5) contained general study characteristic questions such as the number of treatment groups, number of sites, and location of trial. A classification of study design was also included in this section. Once the decision was made to limit full article review to controlled trials, this question was added to the previous section in that a trial classified as any study design other than a controlled trial would not be reviewed. The final section (questions 6 to 26) contained questions designed to provide an assessment of study quality. The questions were designed to assess characteristics such as research design, treatment allocation, and blinding, as well as the potential for bias and generalizability. These questions allowed for the identification of methodological strengths and weaknesses used in the evidence tables and summary of evidence.
Arm Characteristics
The characteristics of each arm or group in the trial were assessed on the second form. The first section gathered information concerning the treatment or intervention. For example, treatment name and dose were noted. The following pages abstracted information concerning the patients and these 15 questions were completed for each arm and/or for the entire trial, as possible. It was in this section that baseline characteristics such as age and gender, as well as source of care and phase of care, were noted.
Outcomes
Two forms were developed for outcomes: one for discrete outcomes and one for continuous outcomes. These one-page forms gathered definitions and data for each outcome. For discrete outcomes, data collected included the categories and raw numbers for each measurement, while for continuous outcomes the means and standard deviations or standard error were collected. Where available, P-values provided by the investigators were also collected. The number of patients included in outcome assessment, the number of patients based on intention to treat and the number of patients lost to followup were also collected where possible. Finally, reviewers judged the objectivity and consistency of the assessment of each outcome. These questions, combined with those on the study characteristics form, provided the information used to identify the methodological strengths and weaknesses of each trial.
Article Review Process
Because of the large number of articles to review, a serial article review process was employed. In this process, the quality assessment and abstraction forms were completed by the primary reviewer. The secondary reviewer, after reading the article, checked each item on the forms for completeness and accuracy. The secondary reviewer also indicated the number of disagreements with the primary reviewer for those items related to study quality. The secondary reviews were completed by the principal investigators. We did not mask reviewers to author, institution, or journal.
All information from the article review process was entered in a Microsoft Access database (Acne Evidence Database) to collect, organize, display, and report the evidence.
Peer Review
Feedback was sought from the technical experts throughout the project through ad hoc and formal requests for guidance. Drafts of the report were sent to technical experts, as well as to peer reviewers identified by AAD and AAP. (See Appendix A for a list of peer reviewers and technical experts who reviewed one or more drafts of this evidence report.) We logged the dates each reviewer's forms were received and entered feedback requiring responses into a database. Revisions were made to the report, as appropriate. Responses to the comments, descriptions of the revisions and/or explanations of decisions, were sent back to reviewers and a conference call was scheduled to discuss the comments and responses. The review process was then repeated with the revised report.
- Methodology - Management of AcneMethodology - Management of Acne
- Appendixes - Management of AcneAppendixes - Management of Acne
Your browsing activity is empty.
Activity recording is turned off.
See more...
