This publication is provided for historical reference only and the information may be out of date.
This chapter presents the results of our evidence review for the following four key questions (KQs): KQ 2, factors associated with colorectal cancer (CRC) screening; KQ 3, interventions that have been tested to increase CRC screening; KQ 4, current capacity in the United States to increase CRC screening; and KQ 5, methods for tracking and monitoring the use and quality of CRC screening. As noted in Chapter 2, we identified 3,029 citations from our searches (Appendix A). Figure 2 documents the disposition of articles for the review. Working from 861 articles retrieved for full text review, we included 139 for background and excluded 571 at this stage. A total of 72 studies (74 articles) qualified for inclusion for KQ 2, 21 studies (22 articles) for KQ 3, 12 studies for KQ 4 and 8 studies for KQ 5.
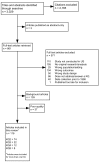
Figure 2
Quorum tree/disposition of articles.
Appendix C-1 provides the detailed evidence tables for KQs 1, 2, and 3. Appendixes C-2 and C-3 present individual quality ratings for randomized clinical trials (RCTs) and observational studies, respectively. Appendix C-3 provides detailed abstractions for KQ 4. Appendix C-4 provides detailed abstractions for KQ 5. Evidence tables for each key question are presented in alphabetical order by last name of the first author.
As noted in earlier chapters, an overall assessment of the CRC screening and related factors requires evaluation of sources of heterogeneity, including clinical context, population served, and for the randomized control trials (RCT), the type of comparator. CRC screening is conducted in a variety of clinical contexts and assessed through the completion of one or several tests (i.e., fecal occult blood test (FOBT), done at home or in the office, and/or some type of endoscopy (i.e., flexible sigmoidoscopy [FS], colonoscopy, or double contrast barium enema [DCBE]). Most studies we assessed measured the outcome of screening by completion of a FOBT at home, and included one or more of these endoscopy tests. However, since national guidelines about which tests should be used and when have been altered several times over the period of time for which we were reviewing studies (i.e., 1998-present day), the assessment of screening rates has also changed over time and is somewhat problematic to analyze. For the studies assessed under KQs 2, 3, and 4, there is a strong reliance on self-reported screening rates, with fewer studies incorporating claims data analysis in the assessment of CRC screening.
An additional source of heterogeneity is the type of studies conducted for each type of KQ and the descriptive or analytic nature of the literature. For KQ 2, we found the largest number of studies but all are based on observational data, primarily collected retrospectively through cross-sectional or cohort designs. Because of the extensive variables explored in relation to CRC screening, we present the findings for this KQ in much less detail than the other KQs, focusing on the study characteristics and overall results specific to each type of factor that may be associated with screening.
This literature is characterized by a few articles together constituting a single study. We refer to studies in the text and cite all relevant articles for each study; article and study counts, therefore, frequently do not match. Our summary tables below feature groups of studies organized by the factors that may be associated with CRC screening (KQ 2), the different types of interventions that have been tested to increase screening (KQ 3), or the types of studies that have been done to assess capacity for screening (KQ 4) and monitoring of use and quality of screening (KQ 5). We have organized the studies in each summary table such that those rated as good quality are listed first and organized alphabetically by the first author’s last name, followed by fair quality studies organized in the same way. The summary tables also provide information to identify the study (author, and date of publication), study design, population and setting, sample size, study quality, intervention (when relevant), comparators, and results.
KQ 2: What Factors Influence the Use of Colorectal Cancer Screening?
Key question (KQ) 2 focuses on the factors that are associated with the use of CRC screening. These factors can relate to either patient or provider characteristics and to the interaction between the provider and patient. Other factors that could be associated with the use of CRC screening may be system-level characteristics, such as involvement of nonclinician staff in screening, use of reminder or recall systems, having an organized referral system, or the size or type of the medical practice.
We identified a total of 72 studies (74 articles) rated good or fair quality that examined different factors that are associated with the use of CRC screening1–2,21,42,46,55–57,65–66,88,106–168 rated good or fair quality. For these studies, we categorized the factors into five topic areas: 1) patient level factors that influence CRC screening; 2) physician factors (physician characteristics, physician-patient connectedness, and physician recommendations); 3) patient and physician communication; 4) periodic health exams or annual checkups; and 5) system level factors that may be associated with screening rates. We also identified two articles that focused on patient level predictors of followup among patients who have received a positive FOBT result and present them separately under the patient factors section of this chapter.88,168
Studies for this KQ are presented somewhat differently than those for the other KQs. Because of the vast number of studies this section includes, we start by presenting findings from three nationally representative samples of respondents where the investigators present overall findings that are not stratified by some factor (e.g., race, sex). For these three studies,21,46,151 we present the absolute screening rates in order to provide benchmarks for assessing how screening rates change when other factors are presented separately in the remainder of this section. We then present the results of the four primary patient characteristics of demographics, access to care, personal health or risk factors, and psychosocial factors that are associated with CRC screening. For each of these characteristics, we then summarize the findings from the three national studies and present adjusted odds ratios (AOR) and other statistics as appropriate. After presentation of the three national ‘overview’ studies, we follow that section with all additional studies that present findings for each of the seven topic areas that may be associated with screening. In each of these sections, we provide summary tables of the key studies that examined the corresponding factor. In each table, we also present the overarching results by using the symbols of “↑” or “↓” to provide a quick assessment of each study’s findings specific to the key variables and the outcome of CRC screening (i.e., the “↑” means there is a positive association between the variable and CRC screening, and the “↓” means there is a negative association). Because this KQ includes so many studies, we think the use of these symbols helps the reader to understand what the overall results convey. Since this KQ presents descriptive findings from observational studies, we have not provided an assessment of the strength of evidence here.
The following presents the study characteristics and overview of results for each of the seven topic areas potentially associated with CRC screening.
Patient Factors: Overview
The majority of studies that have examined factors that predict the likelihood of CRC screening have focused on patient characteristics. We identified a total of 56 studies that we rated as good or fair quality that reported findings related to this topic.1–2,21,42,46,55–56,65,106–109,111–126,128‐134,136–138,141,144–147,149–151,155–158,160–163,165–166 that we rated as good or fair quality that reported findings related to this topic. We also included two studies that examined patient level factors that predict followup after a positive FOBT result.88,168
For patient factors, we categorized studies into four primary topics:
- patient demographics: studies that explore the relationship between characteristics such as age, sex, income, insurance status, race, ethnicity, and acculturation and the completion of various CRC screening tests.
- access to care: studies that explore the impact on CRC screening rates of having a regular source of care, recently visiting a provider at least once, and proximity to health care facilities.
- personal health or risk factors: studies that focus on the relationship of health factors (e.g., health status, obesity) or healthy behaviors (e.g., obtaining screenings for other cancers); or risk factors (e.g., family history of CRC, personal history of other cancers) or risky health behaviors (e.g., smoking, sedentary lifestyle, alcohol use) to the outcome of CRC screening by any test.
- psychosocial factors: studies on patient knowledge, attitudes, beliefs, and perceptions related to either CRC or screening for that type of cancer.
All the studies for KQ 2 present observational data collected either through surveys of self-reported screening rates or through analysis of claims data. These studies include those that report on national, state, regional, and local samples of respondents or patients. These studies yield a broad array of findings in a variety of populations and examine a large number of patient factors and their relationship to CRC screening; dealing with all of them simultaneously risks presenting an unnecessarily complex synthesis. For that reason, we have adopted an analytic strategy for this KQ in which we initially describe the three studies that have the most nationally representative samples and that did not stratify their results by any factors (e.g., race, ethnicity).21,46,151 In our view, these studies provide a broad overview of the issues and findings and provide a robust basis for then analyzing studies with a narrower focus.
Patient Factors: Three Nationally Representative Studies
Overview of national studies of patient characteristics. Study characteristics. Three studies examined the overall patient characteristics that seem to predict CRC screening in a national sample.21,46,151 We rated all three studies as good quality. All relied on national survey data for their analysis; specifically, all used National Health Interview Survey (NHIS) data, with two presenting findings from 200021,151 and one from 2005.46 All three presented findings for respondents ages 50 or older.21,46,151 All three explicitly excluded from their analysis people reporting a prior diagnosis of CRC.21,46,151
The studies varied slightly on how they assessed the outcome of CRC screening. Two studies used the same definition (that respondents who reported an FOBT within the past year or an endoscopy within the past 10 years were adherent with national screening guidelines).21,46 The remaining study defined adherence to screening as obtaining an FOBT in the past year, an endoscopy within the past 10 years, or both, for screening purposes. They defined this variable as “time-screening adherence” and included those who reported being screened as part of a routine physical examination, because of a specific problem, as a followup to another screening test, or because of family history of CRC.151
Overview of results. For the three studies, we present the overall findings for each of the categories of patient characteristics that may influence screening rates: demographics, access to care, personal health or risk factors, and psychosocial factors (Table 6). For each set of findings, we present only those screening rates or adjusted odds ratios (AORs, with 95 percent confidence intervals or significance levels) specific to being current with any CRC test (per the authors’ computation of their outcomes variables); we do not present findings for specific tests in this section unless the authors limited their measurement of CRC screening to only one or two tests. “Significant” in this discussion means statistically significant at least a P = 0.05 level.
Table 6
Overall findings from the three national studies for each category of patient characteristics.
Demographics. All three studies examined demographic characteristics, including age, sex, income, insurance status, race, ethnicity, acculturation, and other factors such as education and marital status. The adjusted screening rates reported for each study appear in Table 7. We then discuss the factors that one or more of the three studies reported as predictors of CRC screening rates. None of these studies adjusted for or reported findings for factors related to acculturation (i.e., English-language proficiency, foreign birth, years living in United States).
Table 7
Adjusted CRC screening rates by key patient-level demographic characteristics, for three national studies.
Age. For the three studies,21,46,151 screening rates gradually increased for each age group from the age groups from 50 to 70 years. One study using 2000 NHIS data found that older patients were more likely to be screened than younger patients. Relative to the referent group (50–54 years), respondents 55–59 years of age were slightly more likely to report being screened (AOR, 1.51; 95% CI, 1.24–1.84),151 as were those 60–64 years (AOR, 1.41; 95% CI, 1.41–2.05)151 and even more so for those 65–69 (AOR, 2.14; 95% CI, 1.75–2.62) and 70–74 (AOR, 2.20; 95% CI, 1.80–2.70).151 The other study using 2000 NHIS data reported the same trend (compared with subjects 50–59 years, AOR 1.45; 95% CI, 1.26–1.67 for those 60–69 years and AOR, 1.69; 95% CI, 1.41–2.03 for those 70–7921). The study using 2005 NHIS data showed a similar trend and presented findings as age-adjusted percentages; 42.6 percent of those 50–59 years of age (95% CI, 40.4–44.8 percent), 56.6 percent of those 60–69 years (95% CI, 54.4–58.7 percent); and 57.2 percent of those 70–79 years (95% CI, 54.5–59.9 percent) reported being screened for CRC.46
In all three studies, however, screening rates were lower for the oldest category of patients relative to the adjacent age group;21,46,151 the two 2000 NHIS studies reported an AOR of 2.08 for those 75 years of age and older (95% CI, 1.70–2.53)151 and AOR of 1.25 for those 80 years and older (95% CI, 1.01–1.56).21 The study of 2005 NHIS data showed a similar trend: 49.9 percent of respondents 80 years or more years of age reported being current with CRC screening (95% CI, 46.1–53.8 percent).
Sex. For the two studies of 2000 NHIS data, the reported screening rate was slightly lower among females than male.21,151 In one, males were more likely to report screening than females (AOR, 1.16; 95% CI, 1.03–1.31),151 and in the other females were less likely to report being screened (AOR, 0.89; 95% CI, 0.80–0.99).21 The study of 2005 NHIS data found no difference between screening rates of males (49.2 percent; 95% CI, 47.4–50.9) and females (50.4 percent; 95% CI, 48.7–52.2; P = 0.29).46 For the two studies that presented screening rates for FOBT within the past year or endoscopy within the past 10 years, one reported no difference in FOBT or endoscopy screening rates among males and female,46 and the other found similar screening rates for FOBT and only a slightly lower rate of endoscopy screening for females compared with males (AOR, 0.77; 95% CI, 0.69–0.86).21
Race. Comparisons are more challenging for the reports of CRC screening by race because the three studies reported the findings somewhat differently. All three studies reported findings for whites and blacks; all three reported adjusted rates that show no difference between blacks and whites.21,46,151 In the two studies of 2000 NHIS data, blacks had a slightly nonstatistically higher odds ratio but not a statistically significant different rate of CRC screening than whites (as the referent group for both studies).21,151 One study also reported CRC screening for the race category of “other,” which could include Asians, American Indians, and others; it found that this group was less likely to report being screened than whites (AOR, 0.67; 95% CI, 0.50–0.92).21 Another study also reported a current screening rate of 40.3 percent (95% CI, 27.7–54.4; P = 0.07) for subjects in the “other” race category.46
Ethnicity. All three studies provided CRC screening rates for Hispanics. One study reported that Hispanics were statistically less likely than whites to be screened for CRC (AOR, 0.73; 95% CI, 0.58–0.92);151 the other two studies showed that there was no statistically significant difference between Hispanics and “non-Hispanic” whites (AOR, 0.92; 95% CI, 0.75–1.1221 for Hispanics with whites as the referent group; and adjusted percentage of 45.9% for Hispanics; 95% CI, 41.7–50.2%; compared with 50.2% for non-Hispanic whites; P = 0.0646).
We have included Asians in our discussion of ethnicity throughout this chapter; in places, we present study findings specific to subgroups of Asians (Chinese, Japanese, Korean, Vietnamese).46 One study reported findings specific to Asians: the percentage reporting being screened was lower than the figure for whites (41.7 percent and 50.0 percent, respectively; P = 0.07).46
In terms of the combination of racial/ethnic differences, one study highlighted unadjusted and adjusted screening rates for Hispanics and blacks,151 whites had the highest and Hispanics had the lowest proportions of adherence to timely screening. Compared with whites, Hispanics were 50 percent (P < 0.001) less likely to be adherent, and blacks approximately 23 percent (P < 0.01) less likely to be adherent. After multivariate adjustment (for all independent variables in their analysis), the difference between blacks and whites disappeared (AOR, 1.13; 95% CI, 0.95–1.35) but remained statistically significant for Hispanics (AOR, 0.73; 95% CI, 0.58‐0.92).151 The other two studies reported similar findings in the unadjusted and adjusted rates for the different racial and ethnic groups.21,46 In one study, race was no longer a predictor of FOBT use when the rates were adjusted;21 in another, adjustment for all the other factors in their analysis weakened the association between screening and Hispanic ethnicity (45.9 percent for Hispanics and 50.2 percent for non-Hispanics; P = 0.06).46
Annual household income. Two studies reported findings based on annual household income,46,151 using slightly different income categories. Using the annual household income group of $20,000 or more as a referent, one study found that each higher income group was slightly more likely to report being screened; the group reporting an income of $65,000 or more was among those most likely to report being screened (AOR, 1.28; 95% CI, 1.04–1.58).151 In another study, screening rates differed significantly between low-income and high-income groups: 45.5 percent screening rate for those < $20,000, and 53.2 percent screening rate for those ≥ $75,000 (P = 0.006).46
Insurance status. All three studies reported findings based on whether respondents had health insurance; and all demonstrated that those with no insurance were statistically significantly less likely to report being screened than those who had any type of insurance.21,46,151 Using those without insurance as the referent group, both studies of 2000 NHIS data reported those with any insurance were more likely to report being screened than those without (AOR, 1.42; 95% CI, 1.05–1.93151 and AOR, ranges 1.66–1.93, with statistically significant 95% CIs21). The study of 2005 NHIS data demonstrated a similar finding; 31.6 percent of those without insurance versus 43.0 percent to 67.9 percent of those in other insurance categories reported screening (P < 0.0001).
Two studies reported screening by type of insurance. For 2000 among those with any insurance, those with private insurance were the least likely to be screened (AOR, 1.66; 95% CI, 1.28–2.15) and those with a combination of private insurance and Medicare or Medigap were the most likely to be screened (AOR, 1.93; 95% CI, 1.44–2.59).21 For 2005 among those with any insurance, those with Medicaid were the least likely with insurance to report being screened (43.0 percent; 95% CI, 35.7–50.6) and those from the military were the most likely to be screened (67.9 percent; 95% CI, 63.3–72.1).46
Other factors: Education level and marital status. Education level and marital status consistently reported as associated with CRC screening. All three studies reported that respondents with lower levels of education had lower levels of CRC screening than better educated groups. For 2000, both studies reported that those who finished high school or had any education beyond that level were more likely than those who did not complete high school to report being screened (AOR, range 1.27–2.08 with “less than high school” as the referent group;151 AOR, range 1.27–1.83 with “less than 12 years” as referent group21). For 2005, reported a similar trend; rates of CRC screening increased as education levels rose (ranging from 47.9 percent to 55.5 percent compared with 43.8 percent for those with less than 12 years of education; P = 0.01).46
With respect to marital status, all studies reported that being married was associated with CRC screening.21,46,151
Access to care. Access to care is a patient-level characteristic that many studies in our review examined. These three studies each reported two measures of access to care—whether an individual has a “usual (or, regular) source of care” and the frequency or recency of contact with the provider (i.e., number of visits in past year or time since the last visit).21,46,151 Table 8 provides the adjusted rates for variables related to access to care.
Table 8
Adjusted CRC screening rates by patient-level variables of access to care for three national studies.
Usual source of care. All three studies found that those respondents who reported having a usual source of care were more likely to obtain CRC screening than those who did not have a usual source of care.21,46,151 The two studies of 2000 NHIS data each reported significant differences in rates of CRC screening between those who had a usual source of care and those who did not (AOR, 1.61; 95% CI, 1.17–2.21151 and AOR 1.65; 95% CI, 1.30–2.09).21 For 2005, findings for adjusted rates were similar; 51.0 percent of those with a usual source of care (95% CI, 49.7–52.3%) and 30.5 percent of those without a usual source of care (95% CI, 26.5–34.8%; P = 0.0001) were screened.46
Frequency or recency of visits to physician. All three studies provided similar findings: those who had visited a physician more frequently in the past year or had seen a doctor more recently were more likely to report being screened for CRC.21,46,151 In one study, those with no physician visits in the past year were significantly less likely to obtain screening than those who visited a physician at least once (AOR, range 2.40–4.68).21 In another, those who had visited a physician within the past 1 to 2 years were less likely to have had a CRC screening test than those who had visited within the past 6 months (AOR, range 2.76–7.59 with less than 2 years as the referent group).151 The third study did not report adjusted rates for this variable; unadjusted rates appear in Table 8.46
Personal health factors and risk factors. Personal health factors are defined as characteristics from respondents’ family history or personal health history (e.g., prior polyp removal, screening behavior with regard to other cancers, general health status, family CRC diagnosis) that would place them at increased risk for CRC or that may be related to healthy behaviors that could influence the extent to which they obtain regular CRC screenings. Risk factors for health problems that may be related to CRC screening include smoking, sedentary lifestyle, poor eating habits, obesity, and any factor that may place a person at increased risk for developing CRC. Table 9 presents the absolute rates of these variables as reported by the three national studies, followed by a discussion of findings for each.21,46,151
Table 9
Adjusted CRC screening rates by patient-level personal health or risk factors for three national studies.
Health factors. Family history of CRC or other cancer, personal history of other non-CRC cancers, and use of mammograms or Pap tests were all found to be consistently associated with CRC screening rates.21,46,151 One study used “family cancer history” that was not specific to CRC; those who reported this as part of their history were significantly different from those who did not (AOR, 1.27; 95% CI, 1.13–1.43). Findings in another study were specific to a family history of CRC and reported a stronger association between screening rates; those with a family history were more than twice as likely to report being screened as those who had none (AOR, 2.04; 95% CI, 1.73–2.40).21 The third study did not present the adjusted rates for this variable; unadjusted rates appear in Table 9.46
Three studies assessed the relationship between personal history of other (non-CRC) cancers and CRC screening. Two studies found this variable to be strongly associated with CRC screening (AOR, 1.08; 95% CI, 0.93–1.25;151 AOR, 1.24; 95% CI, 1.12–1.37;21 adjusted percentage of 59.8 percent screening rate for those with a personal history versus 48.3 percent for those without; P < 0.000146).
Two studies reported use of mammograms and Pap tests.21,46 In one study, analyses for use of mammograms were adjusted for all variables in their analysis except sex and Pap test use, and those for use of Pap tests were adjusted for all variables except sex and mammogram use and also for hysterectomy history.21 For the association between mammography use and CRC screening, the AOR was 2.96 (95% CI, 2.50–3.50); for Pap tests the AOR was 2.41 (95% CI, 2.03–2.86). The second study did not provide adjusted rates for these variables.46 Their unadjusted rates indicate that 60.6 percent of females who had obtained a mammogram in the past 2 years versus 24.0 percent of females who had not and 56.0 percent of those who had obtained a Pap test in the past 3 years versus 33.3 percent of those who had not reported obtaining CRC screening within recommended time intervals.46
Findings specific to the association of general health status with CRC screening differed across studies. One study found little difference in CRC screening rates between respondents who considered themselves to be in excellent or good health and those in fair or poor health (AOR, 1.07; 95% CI, 0.94–1.22).21 The other two studies reported that higher levels of perceived health seemed to be associated with higher CRC screening rates (AOR, range 0.73–0.90 with “excellent” as the referent group for one study151 and 48.7 percent adjusted rates for those in “excellent/very good/good” health and 54.3 percent in “fair” or “poor” health; P < 0.000146).
Risk factors. Risk factors reported by these studies included smoking status, obesity, physical activity, and alcohol use.21,46,151 In two studies, current smokers were less likely than never or former smokers to be screened (AOR, 0.82; 95% CI, 0.70–0.9521 and adjusted percentage of 41.5 percent screening rate for smokers compared with a 53.3 percent rate for former smokers and a 45.2 percent rate for those who never smoked; P < 0.000146). One study reported no significant differences based on current or former smoking.151
None of the studies reported body mass index as a predictor of CRC screening. All three found that even some or moderate, as well as regular, respondents who reported some type of exercise had higher screening rates than those who reported no exercise.21,46,151
Alcohol use was reported in two studies.21,46 One found that those who reported 1 to 14 drinks per week were more likely to report being screened than any other group (AOR, 1.14; 95% CI, 1.03–1.26).21 The other study also reported significant differences specific to alcohol use; those who reported 1 or more drinks per week being more likely to be screened (adjusted percentages of 52.8 percent for those drinking 1 to 13 drinks/week (95% CI, 51.5–54.4%) and 51.9 percent (95% CI, 47.3–56.4%) for those drinking 14 or more drinks/week; compared with 46.5 percent (95% CI, 44.8–48.3%) for those reporting no alcohol use: P < 0.0001).46
Psychosocial factors. Two studies presented analyses based on reasons for never undergoing screening or undergoing screening beyond the recommended time intervals and include aspects of knowledge, attitudes, beliefs, or perceptions (i.e., psychosocial factors) that may be associated with CRC screening use.21,46 One study using 2000 NHIS data examined reasons for not obtaining screening compared two age groups of respondents (those 50–64 years compared with those ≥ 65 years) and reported that lack of knowledge of either the FOBT or endoscopy as a test was a common barrier to undergoing either test (52.0 percent of those 50–64 years of age and 50.7 percent of those 65 or older reported this barrier for FOBT; 49.7 percent and 50.7 percent, respectively, reported this barrier for endoscopy).21 Far fewer respondents reported any of the following reasons for not being screened, putting it off, or believing they did not need the test: expense or lack of insurance, the pain, unpleasantness, or embarrassment of having the test. Proportions ranged from 0.3 percent to 12.2 percent among those 50–64 and from 0.1 percent to 12.5 percent among those 65 or older.21
The study using 2005 NHIS data presented proportions of responses for the same items of the survey;46 they compared individuals who never had had an FOBT or endoscopy with those who had had the test before but not in the recommended time interval. Results indicated that about half of the respondents reported “never thought about it” as a reason for not being screened ever (adjusted percentage of 53.9 percent (95% CI, 52.0–55.7%) for FOBT and 51.8 percent (95% CI, 49.9–53.6%) for endoscopy) or within the time interval (adjusted percentage of 51.7 percent (95% CI, 50.0–53.4%) for FOBT and 48.7 percent (95% CI, 47.0–50.4%) for endoscopy).46 Far fewer respondents reported any of the psychosocial factors as reasons for not being screened ever or on time, such as their beliefs about testing (“did not need it”, adjusted percentage ranges of 10.3 to 12.2 percent), or their perceptions that the tests were too painful/unpleasant/embarrassing (adjusted percent ranges of 0.8 to 2.0 percent).46 Neither study commented on the extent to which any of these factors may relate to overall screening rates.
Patient Factors: Overview of Additional Studies
Here we present information from other studies that present findings from a national, regional, or local database, but that stratified their findings on one or more particular patient-level factor (e.g., age, sex, race, ethnicity). We highlight these studies in the sections specific to the variable of interest. To reduce the potential for redundancies, we only present the study characteristics and overview of results for each group of studies, and not a detailed description of all the studies included in this section.
In addition to the three overview studies, we included 53 studies1–2,42,55–56,65,106–109,111–126,128‐134,136–138,141,144–147,149–150,155–158,160–163,165–166 rated as good or fair quality. We present findings in summary tables for studies that had significant or particularly important or interesting results specific to that patient-level variable. Each table first presents studies rated as good quality listed in alphabetical order by first author’s last name, followed by studies rated as fair quality that are also listed in alphabetical order by the first author’s last name. Also, because we are reporting findings for a large number of studies, we have attempted to streamline the text such that detailed statistics (e.g., confidence intervals [CI]) are only presented in the summary tables and overall findings are presented in the text describing the studies. We also describe, just in text, other studies that provide supporting or contradicting results for each category of factors.
Although a large number of studies may have included the factors as presented in the following sections, we only present additional description in the text and include in the tables those studies that specifically aimed to explain whether the factor of interest for the section was related to CRC screening (rather than simply looked at a large number of factors). In some cases (for studies specific to both racial and ethnic differences), we include one study in more than one summary table. However, to minimize the discussion as much as possible, we generally present one study only once in a table and a few studies are not presented in summary tables at all because their findings support others presented. At the summary of each factor, we then briefly reference all of the other studies that included the factor in their final multivariate analyses and whether and how they found the factor to be associated with CRC screening.
Age. Study characteristics. All studies discussed in this section included age in their analysis of factors associated with CRC screening of their sample. Two studies, both rated fair quality, focused on the association between age and CRC screening (Table 10); both presenting results for patients 65 years or older.55,150 One study presented self-reported findings from a national database of responses to the 2003 Health Information National Trends Survey (HINTS);55 we include it here (instead of as an overview study) because the authors explored screening specifically among older people (ages 65–89 years). The second study analyzed 2002–2003 Medicare physician/supplier billings claims data from three states (Florida, Illinois, and New York).150 The HINTS study focused on the outcome of screening as defined by national guidelines (i.e., FOBT in the past year or FS/colonoscopy in the past 10 years), whereas the Medicare claims study defined CRC screening as any test (i.e., colonoscopy, FS, double-contrast barium enema, or FOBT) obtained during the study period (2002–2003).150 In terms of the “age” variable, one study focused on comparing those who were ages 65–74 years with those who were 75–89 years of age55 the other categorized the age variable into four groups (ages 65–69; 70–74; 75–79; and 80 or more years).150
Table 10
Studies of the association of age with CRC screening.
Overview of results. Age was a predictor of screening in the HINTS study such that older patients (ages 75–89) were more likely than younger patients (65–74) to be up-to-date with CRC screening (AOR, 1.92; P < 0.001).55 Age was also associated with CRC screening in the Medicare claims study, until the age of 80 year or older; these older patients were less likely to have received any CRC test than any other age groups, regardless of income (RR range, 0.84–0.90).150
These findings agree somewhat with the overview studies presented previously.21,46,151 The Medicare claims study supports those findings in that screening rates decline slightly among patients over age 80 years.150 The HINTS study found an overall increase in screening rates from the younger age range of respondents (65–74) to the older age group (75–89).55 If these investigators had defined more but shorter age ranges in their analysis, they might have found rates with respect to age similar to those in the other four studies.21,46,150–151
Of all the studies that included age as a variable in their adjusted logistic regression models, 20 reported that older patients (i.e., ages 60–75) were more likely than younger patients (i.e., 50–60 years) to be current with CRC screening and that rates among the very old age groups (i.e., 76 years or older) were lower than those for younger age groups.1–2,42,56,107–108,111,114,116,120,122,126,132–133,138,146,156–158,163 Four studies reported no differences in screening for age groups included in their analyses.106,109,130,166
Sex. Study characteristics. As with the age variable, all studies included this patient variable in their analyses of factors associated with CRC screening. Two, both rated as good quality, focused specifically on this demographic factor (Table 11).42,133 Both presented national-level findings of self-reported data with all results stratified by gender; one presented findings from the 2002–2003 HINTS133 and the second presented findings from the 2000 NHIS.42 Both studies presented findings for respondents 50 year of age or older.42,133 Both also used the same indicators to assess the outcome of screening (i.e., FOBT in the past year or endoscopy in the past 10 years).42,133
Table 11
Studies of the association of sex with CRC screening.
Overview of results. Much like the three national studies,21,46,151 gender was not consistently associated with CRC screening. One study showed that females and males were similar in their current screening rates for any test (37.0 percent and 37.1 percent, respectively),42 and the other presented findings showing the same patterns of use (66.4 percent for females and 61.8 percent for males).133 However, these studies did find differences among males and females for specific tests.42,133 In one study, females were more likely than males to report having completed an FOBT in the past year (9.3 percent compared to 5.2 percent, respectively); the groups did not differ in endoscopy screening in the past 10 years.133 The other study found no gender differences in current CRC screening rates.42
Of the studies in the patient characteristics section of this review that included this variable in their adjusted logistic regression models, 14 reported an association between gender and CRC screening. Males had higher rates of screening for all tests than females in seven studies;1,113,126,137,158,163,166 females had higher rates overall than males in three studies;56,157,169 females were more likely than males to report a recent FOBT than males in two studies;2,133,138 and males were more likely than females to have had an endoscopy (either colonoscopy or FS) in two studies.2,114,138 An additional 12 studies with sex as a variable in their final analyses found no differences in CRC screening.42,55,106–109,111,116,122,132,134,160
Race. Study characteristics. We consider six studies here because they focused specifically on the association between race and CRC screening (Table 12); we rated two as good quality1,158 and four as fair quality.114,122,129,150,165 In terms of race, we present only those findings specific to Blacks1,114,129,150,158,165 or American Indians and Alaska Natives.122 Studies specific to Asians appear below in ethnicity because we cite findings specific to subgroups of Asians.
Table 12
Studies examining the association between race and CRC screening.
Of the six studies considered here, four provided findings for non-Hispanic whites compared with non-Hispanic blacks;1,114,129,158 one presented findings for whites and all nonwhites;165 and one compared American Indians living in the Southwest United States with Alaska Natives.122 Two presented findings from a national sample of respondents;1,129 two presented findings from several states;122,150,165 and two presented locally based findings.114,158 Three studies reported self-reported findings from survey data;114,122,129 two others presented findings from medical (or Medicare) claims data;150,158,165 and the sixth presented findings from a combination of 2001–2005 Medical Expenditure Panel Survey (MEPS) and the 2000–2004 NHIS.1 Those included in the samples were 50 years of age or older,1,114,122 50–75 years of age,158 65 years of age or older,129,150 or 70–79 years.165
In terms of the screening outcome, there were several different variations of how this was operationalized:
- One study defined screening as FOBT in the past 2 years or endoscopy at any time;1
- One study defined CRC screening as FOBT in the past year, and FS or colonoscopy in the past 5 years, 165; and
- Another study only included colonoscopy or FS in the past 5 years (excluding FOBT).122
Overview of results. All but two of these studies122,165 gave both unadjusted (AOR age-adjusted only) rates for CRC test usage by various racial groups and rates from multivariable analysis that included factors that are known or thought to be associated with CRC screening. The four studies that compared CRC screening for non-Hispanic whites and either non-Hispanic blacks/Blacks1,114,129,158 reported inconsistent findings. The two studies based on nationally representative samples found no difference in adjusted screening rates and race;1,129 one study reported that non-Hispanic blacks were less likely to be screened than non-Hispanic whites114 and one study reported that Blacks were statistically significantly more likely to be screened for CRC than whites (P = 0.03).158 The study of Medicare claims data in 9 states among 70–79 year olds compared changes in screening over time for whites and blacks. In 1995, 39.4 percent of White enrollees were up-to-date with CRC screening compared with 29 percent of Blacks. In 2003, overall percentages of enrollees up-to-date increased, but disparities between racial groups persisted, with 47.3 percent of Whites up-to-date compared with 38.1 percent of Blacks. The differences were statistically significant between all groups in both 1995 and in 2003.165
Additional studies not highlighted here used race as a variable in their final multivariate analysis. One reported that non-Hispanic blacks were more likely than non-Hispanic whites to report being screened;107 six noted that non-Hispanic whites were more likely than non-Hispanic blacks (or non-whites) to report being current with screening106,120,128,138,142 or ever screened;125 and nine reported no differences by race.42,55,111,119,132,134,147,156–157 Finally, the study giving findings specific to Southwest American Indians and Alaska Natives found that Alaska Natives were more likely than those in the Southwest United States to report being screened (AOR, 3.86).122
Ethnicity - Hispanics. Study characteristics. Six studies (7 articles) had the specific aim of examining the relationship between Hispanic ethnicity and CRC screening (Table 13), Of these, we rated two as good quality;1,141,147 they used self-reported findings from a nationally representative sample collected either through the NHIS in 1998147 or a combination of the 2000–2004 NHIS and 2001–2005 MEPS.1,141 The four studies we rated as fair quality included one of 2000 NHIS findings,111 another of changes from 2000 NHIS compared to 2003 data,119 one of a trend analysis of 2000 NHIS data compared with 2005 data,113 and one of respondents living in a local county in 1998–1999 that was reported in two separate articles.116–117
Table 13
Studies of the association of Hispanic origin with CRC screening.
Studies in this section used either non-Hispanic whites or non-Hispanics (any race) as the comparison group. Three studies used all respondents 50 years of age or older in their sample;1,116,141,147 one focused on those 50–64 years,113 another included those 50–75 years,111 while one study focused on Medicare beneficiaries aged 65 or older.119
The definition of the outcomes of being up-to-date with screening varied across the studies and included the following:
- One study defined CRC screening as FOBT in the past year or endoscopy in the past 10 years;113
- One study (of the same sample) defined being screened as FOBT in the past 2 years or endoscopy in the past 5 years;116–117
- One study defined being screened as FOBT in the past year, FS in the past 5 years, or colonoscopy in past 10 years;111
- One study defined being screened as FOBT in the past year and proctoscopy in the past 5 years;147
- One study was focused on only screening by endoscopy (ever received);119
We present both unadjusted (or age-adjusted only) rates for CRC test usage by persons of Hispanic ethnicity compared with rates for some other group, as well as rates adjusted by potential confounding variables.
Overview of results. Comparisons of absolute screening rates consistently show that Hispanic ethnicity is associated with lower CRC screening test usage. Overall, adjustment for socioeconomic and health care access factors significantly attenuates, but generally does not eliminate, this disparity. Studies are mixed regarding the relative effect sizes of socioeconomic status and health care access in attenuating these differences. One study that explored the impact of changes in Medicare reimbursement on endoscopy use by different racial and ethnic groups found that, while there were increases in rates among non-Hispanic whites and blacks between 2000 and 2003 (per NHIS data), the difference between non-Hispanic whites and Hispanics in obtaining this test widened during this time period, to a statistically significant gap (P = 0.048).119 One national-level study of both MEPS (2001–2005) and NHIS (2000–2004) data included in this section stratified their analysis by Hispanic subgroup and showed that disparities for persons of Mexican and Dominican origin were greater than for persons of Cuban or Puerto Rican origin.141 Their findings indicate that respondents of Mexican or Dominican origin are less likely than non-Hispanic whites to be up-to-date with CRC screening (in a model adjusted for demographics and access to care, Mexicans had AOR, 0.70 and Dominicans had AOR, 0.44). However, no differences were found across the Hispanic subgroups once language was incorporated into the model.141
Additional studies not examined in detail here presented related findings. Four studies demonstrated that the adjusted rates for screening were lower among Hispanics than non-Hispanic whites;115,120,126,163 evidence from six other studies suggested that screening rates did not differ among Hispanics.42,55,107,112,157–158
Ethnicity - Asians. Study characteristics. Because several studies provide findings for different groups of Asians, we present these findings here (Table 14).1–2,109,118,130 We included five studies, all based on self-reported data that examine screening rates among Asians: two had data for Asians overall,1–2 and three give data for specific groups of Asians.109,118,130
Table 14
Studies of the association of Asian American origin with CRC screening.
Of the two studies of Asians overall, one rated as good quality reported findings from a combined national dataset of 2001–2005 MEPS and 2000–2004 NHIS’1 the other, rated as fair quality, presented findings from the 2001 California Health Interview Survey (CHIS).2 The three studies that present findings for specific subpopulations, all rated as fair quality for this specific variable, do not provide screening rates for the population in question compared with rates for other groups; they collected primarily convenience samples of patients from either communities in two states (California and Texas) with large samples of Vietnamese Americans,130 senior centers in New York City with relatively large groups of Chinese Americans,118 and one community health center in Seattle with a large Chinese American patient population.109 We include these only in this section because of limits in their samples. All but one of the studies defined their population as those 50 years or older; one focused on those 50–74 years.130
The outcome of screening was assessed differently across the studies as follows:
- One study defined being screened as having FOBT in the past year or endoscopy in the past 10 years2
- One study defined CRC screening as FOBT in the past 2 years and ever having had an endoscopy1
- One study defined being screened as FOBT in the past year, FS in the past 5 years, FOBT with FS in the past 5 years, or colonoscopy in the past 10 years130
- One study defined being screened as FOBT in the past year, FOBT with FS in the past 5 years, or no testing118
- One study defined CRC screening as FOBT in the past year, FS in the past 5 years, or colonoscopy in the past 10 years.109
Overview of results. Comparisons of absolute screening rates consistently show that being of Asian descent is associated with lower CRC screening test usage. Overall, adjustment for socioeconomic and health care access factors significantly attenuates, but generally does not eliminate, this disparity. For the large national study of MEPS and NHIS data, Asians were shown to be less likely than non-Hispanic whites to be current with screening, even when all adjustments were made for demographics, socioeconomic status, access to care, and language.1 In the other large study that compared different Asian subgroups on screening rates, the findings were mixed, showing that the unadjusted rates of all the Asian groups were consistently lower than those for non-Hispanic whites,2 Adjustments to the multivariate analysis eliminated these differences for all groups except for endoscopy in Filipinos (AOR, 0.62 for ever use and AOR, 0.68 for up-to-date use) and ever use of FOBT in Koreans (AOR, 0.40).2 Vietnamese were consistently more likely than non-Hispanic whites to have ever been screened and to be up-to‐date with all but FOBT (AOR, range 1.24–1.54; P < 0.05); for FOBT, Vietnamese were less likely than non-Hispanic whites to have ever received FOBT (AOR, 0.90; P < 0.05), but there were no differences for up-to-date screening by FOBT.2
We also found four studies with some related information that demonstrates the inconsistency in findings across studies of Asian use of CRC screening. One reported that Asians were less likely than non-Hispanic whites overall to have been screened;114 another reported that Asian Americans and Pacific Islanders were less likely than non-Hispanic whites to have received an FOBT,147 a third reported no difference in screening rates between Asians and non-Hispanic whites,163 and the fourth found that Vietnamese were just as likely as whites to have had a FOBT in the past year or colonoscopy in past 10 years but were significantly less likely to have received a FS in the past 5 years (P < 0.05).112
In the three studies that presented findings specific to a subgroup of Asians, one study found that 25 percent of Vietnamese were up-to-date with screening;130 another study reported that 27.9 percent of Chinese American respondents reported FOBT within the past year and 22.2 percent reported FS;118 and the third study reported that 39.7 percent of their sample of Chinese Americans were current for CRC screening.109
Acculturation. Study characteristics. We assessed nine studies in terms of the extent to which acculturation was a factor in predicting CDC screening (Table 15).1–2,118,120–122,141,147,149,161 By acculturation, we mean three possible indicators: place of birth (i.e., foreign- or US-born), years living in United States, English-language proficiency, or a combination of these. Although these factors may be important for any race or ethnicity, the studies we consider here examined them specific to non-white Hispanics,1,121,141,149,161 Asians,118 or both Hispanics and Asians;2,147 one study included all racial/ethnic groups by whether or not they were born in the United States;120 and one focused on American Indians and Alaska Natives.122 Six of the nine studies are based on nationally representative samples of respondents collected through self-reported survey data.1,120–121,141,147,149,161 One study was based on self-reported data from across several states,122 one on data from a state-based survey,2 and one on locally based claims data from three senior centers in New York City.118 All but one121 of the nine studies included those 50 years of age or older in their sample.
Table 15
Studies of the association between acculturation and CRC screening.
The operationalization of CRC screening differed across the nine studies as follows:
- One study defined screening as those who had ever had FOBT or endoscopy;120
- One study defined screening as having had either colonoscopy or FS in the past 5 years;122
- One study defined screening as a FOBT in past year, FS in past 5 years, or colonoscopy in past 10 years; 149 and,
- One study used a lack of screening as their outcome such that those who had no FOBT in the past year or no endoscopy in the past 5 years were considered to be not screened for CRC.121
Overview of results. Of the nine studies (reported in 10 articles), four reported findings specific to whether respondents were foreign- or US-born;2,118,120,147 three examined the relationship between English-language proficiency and screening;122,141,161 and three examined both place of birth and English-language proficiency.1,121,149
With respect to being foreign-born or years living in the United States, we considered three studies with findings specific to place of birth, three with findings specific to the number of years living in the United States,2,118,120 and four with information as to whether the respondent reported being born in the United States.1,120,147,149 For place of birth, two studies found that those born in the US were more likely to have been screened than those who were foreign born134 (or foreign born were less likely to be screened if living in US 10 years or less119), whereas another found no differences in screening based on place of birth once other factors were controlled. 114 Four studies also reported a negative association between number of years living in the United States and being up-to-date with CRC screening.1–2,118,120,147,149
Two studies presented findings specific to Asian Americans. In one analysis of a convenience sample of Chinese Americans, as a person’s years in the United States increased so did their odds of being screened (AOR, 0.64; P < 0.05).118 Another study based on data collected through the 2001 CHIS reported that Asian Americans living in the United States less than 15 years were less likely to be up-to-date with screening than those living here longer than 15 years (AOR, 0.58).2
Two additional studies not included here (because exploring acculturation was not a specific aim of their work) reported that the years of living in the United States made no difference in terms of CRC screening.114,163
With respect to English-language proficiency, we considered four studies.1,121–122,141,161 All but one150 found an association between this factor and CRC screening. The fourth study used a scale for acculturation that was based on English language usage (e.g., language most often spoken or read, everyday usage via TV, radio); it found no difference in screening rates once findings were adjusted for socioeconomic status and access to care variables.121 One of these four studies presented findings from American Indian and Alaska Natives across several states that demonstrated an association between CRC screening and either speaking only native languages at home (AOR, 0.50) or speaking a combination of native language and English at home (AOR, 0.65; 95% CI, 0.50–0.85).122 A similar relationship was found for any respondents of the national study of 2001–2005 MEPS and 2000–2004 NHIS data; speaking English at home was significantly associated with increased CRC screening (AOR, 1.84; 95% CI, 1.52–1.33).1 Overall, Latinos who were interviewed in Spanish were less likely to report screening than non-Hispanic whites (AOR, 0.57, or more likely to not be screened with AOR of 2.5).149,161
Income. Study characteristics. Most studies included household income as a potential confounding variable; we highlight two studies, both rated fair quality, here because a primary study aim was to examine the association between income and screening rates (Table 16).114,123 One study relied on national data from the 2002 BRFSS constructed for metropolitan or micropolitan statistical areas (MMSA) to examine the predictive value of area poverty rates on CRC screening.123 The other study presented local data collected through a Community Health Survey of New York City residents.114 Both studies relied on self-reported data from respondents 50 years of age or older.114,123 One study assessed the outcome of CRC screening by respondent reports of whether they had obtained an FOBT in the past year or a endoscopy in the past 5 years.123 The other study defined CRC screening as having received FOBT in the past year, FS in the past 5 years, or colonoscopy in the past 10 years.114
Table 16
Studies of the association of income with CRC screening.
Overview of results. Similar to the three national overview studies,21,46,151 both of these studies found an association between income and screening rates; persons with lower incomes were less likely to receive any CRC test, and those with higher incomes were more likely to be screened.114,123
These studies also add findings at both the neighborhood and MMSA levels by suggesting that even living in lower-income areas predicts CRC screening rates. People residing in low MMSAs were less likely than those in high-income MMSA to receive an endoscopy (AOR 1.10) or FOBT (AOR 1.19);123 respondents living in a medium-income neighborhood (versus a poor or a high-income neighborhood) were less likely to receive any test (AOR, 0.76).114
Of the other studies that included income level as a variable in their final multivariate analysis, 10 found either that persons with low income were less likely than those with high income to receive screening,1–2,42,107,120,122,126,130,150,156 or that higher-income respondents were more likely than lower-income ones to have obtained screening.120,150 One study that focused on exploring racial differences in screening found that controlling for SES (i.e., education, income) in a nationally representative sample of Medicare beneficiaries eliminated any differences in rates. 129 An additional six studies reported no difference in screening rates by income level.55,108,156–157,160,163
Insurance status. Study characteristics. We include seven studies, one rated as good quality56 and the rest rated as fair quality, in the highlighted results specific to insurance status107,113–114,124,138,160 (Table 17). Of these, four are based on national samples of respondents or patients;56,113,124,138 two are based on state-level samples;107,160 and one was based on a local sample of New York City residents.114 All but one study relied on self-reported data; the exception relied on a national sample of Medicare beneficiaries for their analysis.138 The populations of interest for three studies was those 50 years or age of older;107,114,160 two studies focused on those ages 50–64 years;56,113 and two relied on samples of people 65 years or older.124,138
Table 17
Studies of insurance status as a predictor of CRC screening.
In terms of the outcome of screening, definitions varied
- One defined being screened as having an FOBT in the past year or an endoscopy in the past 10 years;113
- One defined the outcome as having had an FOBT in the past 2 years or an endoscopy in the past 5 years;124
- One defined being screened as any CRC test in the past year;138 and
- One defined being screened as having had an FOBT in the past year.56
Overview of results. Four of the seven studies compared screening rates according to whether respondents reported having any insurance or no insurance.56,113–114,160 All four studies reported results similar to those from the three overview studies;21,46,151 those without insurance were far less likely to report being screened than those with any type of insurance.56,113–114,160 This relationship remained when data from of national samples of survey respondents in 2000 were compared with those in 2005; females showed no change from 2000 to 2005 in screening rates (AOR, −1.3), and males showed only a slight increase in screening over time (AOR, 3.0).113
Other studies also reported similar findings. Generally, for any tests, the uninsured were less likely to be up-to-date with screening than those with some insurance.2,116,128 Of the three studies that focused on the association between different types of insurance coverage and CRC screening, one focused on two groups, one comprising “dual” recipients of both Medicare and Medicaid and the other nondual Medicare recipients.138 Another explored how those with managed care coverage compared with those having other insurance coverage among a Medicare-enrolled population (i.e., 65 years or older),124 and a third examined these relationships in those 50 years or older.107 Another study (not in summary table) compared type of insurance among a sample of low-income women residing in Washington, DC and reported (based on self-reports) that those participating in Health Maintenance Organizations (HMO) were more likely than others to be current with screening (AOR, 6.39; 95% CI, 2.05, 19.9 for private HMO; P < 0.01).128
These studies reported two main results. Persons who are dual recipients were less likely than others to receive any of the CRC tests (i.e., FOBT, FS, colonoscopy);138 those in a managed care Medicare plan were more likely to be screened per guidelines than those with any other types of insurance (Medicare or otherwise).107,124
Overall, 13 additional studies with insurance status as a variable in their final adjusted logistic regressions reported that those who were uninsured were less likely than those insured to report current CRC screening.1–2,42,111,116,120,126,130,134,146,156–157,163 Three other studies that included insurance status in such analyses found no difference in CRC screening by this variable.109,132,137
Access to care. Study characteristics. Access to care is defined in most studies as having a usual (or regular) source of care and visiting that provider at least once within the past year. Most studies included these variables as control or potential confounding variables; here we present more detailed information on four studies that specifically highlighted the relationship between access-to-care variables and CRC screening (Table 18).108,133–134,163
Table 18
Studies of the association of access to care with CRC screening.
One study, rated as good quality, based results on the 2002–2003 HINTS;133 the others, all rated as fair quality, presented either regional134,170 or state-level findings.163 All four studies relied on self-reported data for their analysis.108,133–134,163 The two regional studies relied on survey data collected in 2005 from a five-county region in the Midwest134 or data collected in 2005 across a region in the state of Colorado.108 The state-based study used CHIS data.163 One study included respondents ages 50–79 years;134 the other three included those 50 years or older.108,133,163
To assess the CRC screening outcome, two studies used FOBT in the past year or endoscopy in the past 10 years,108,133 one study defined CRC screening as having obtained FOBT within the past year or endoscopy in the past 5 years,163 and the fourth defined the outcome as FOBT in the past year, FS in the past 5 years, or colonoscopy in the past 10 years.134
Overview of results. As with the three overview studies,21,46,151 usual source of care predicted CRC screening for both a study of multiple factors influencing screening in a national sample133 and a state-based study of the relationship between screening and having a usual source of care combined with insurance status.163 The national study found that not having a usual source of care was associated with not being screened among both males and females (AOR range, 0.1–0.5).133 In the state-based study, uninsured respondents with no usual source of care were less likely to be screened than any of the other groups of individuals in their sample (RR, 0.32 for 50–64; RR, 0.08 for 65+).163
Several other studies found similar results: usual source of care was consistently associated with higher rates of CRC screening.1–2,42,56,107–108,111,120,128,130,157 A recently published study of the 2004 BRFSS also reported a strong association between screening and having at least 1 personal health care provider (AOR, 3.95; 95% CI, 2.58–4.41).155 Another study reported no difference in CRC screening and having a regular provider.124
Similar to the three overview studies,21,46,151 other included studies consistently reported a strong association between the frequency of visits to a physician and CRC screening. Authors of the national study reported that one or more physician visits in the prior year was associated with endoscopic screening (5.12; 2.54–10.29 for males and 4.89; 1.79–13.37 for females; P < 0.05).133 The three other studies also found that the number of physician visits was strongly associated with CRC screening. Seven other studies in this review reported the same association.2,55,107,126,132,137,158,166
Personal health and risk factors. Study characteristics. A total of nine studies, all of which we rated as fair quality, are included in the summary table (Table 19) for personal health or risk factors associated with CRC screening.55,108,114,126,132,137,145,157,166 Personal health factors are characteristics from the person’s family history or personal health history (e.g., family CRC diagnosis, personal prior polyp removal, screening behavior with regard to other cancers, general health status) that would place them at increased risk for CRC and/or may be related to healthy behaviors that could influence the extent to which they obtain regular CRC screenings. Risk factors for health problems possibly related to CRC screening include smoking, sedentary lifestyle, poor eating habits, obesity, and any factor that may place a person at increased risk for developing CRC.
Table 19
Studies of the association of personal health/risk factors with CRC screening.
For this set of variables, we have included one study that specifically evaluated the association between CRC screening and family history of CRC,108 two studies that included other cancer screenings,137,157 one study specific to general health status,55 and five studies specific to risk factors (i.e., obesity/overweight, smoking, or physical inactivity).114,126,132,145,166 Eight studies relied on cross-sectional, retrospective data collected through a survey, including four of which were based on national samples;55,126,145,157 two were based on state samples;132,137 one reported on a regional sample;108 and one focused on a city-based or local sample.114 The remaining study presented findings from medical chart reviews of 22 primary care provider (PCP) practices in the states of New Jersey and Pennsylvania.166
All but two studies included respondents 50 years of age or older in their samples; persons 65–89 years were in one study,55 and persons 51–80 years in the other.126
Studies defined outcomes of CRC screening differently, as follows:
- One study defined it as FOBT in past year, FS or double-contrast barium enema in the past 5 years, or colonoscopy in the past 10 years.137
Overview of results. We have divided our overview of results into two groups. Presented first are health factors such as having a family history of CRC, participating in other healthy practices like being screened for other cancers, and general health status in relation to screening. This is followed by information on risk factors such as obesity, smoking, and sedentary lifestyle. With respect to family history of CRC, findings are generally consistent with those from the three overview studies:21,46,151 having a family history of CRC was associated with a higher likelihood of obtaining CRC screening. One study using regional, self-reported findings yielded data showing that that those with a family history of CRC were more likely than others to be up‐to-date with CRC screening in general (AOR, 1.74) and for colonoscopy (AOR, 2.61).108
Several other studies reported similar findings supporting a strong association between family history and screening for CRC.2,55,122,134,137,142,158 Only one study reported that subjects with a family history of CRC were not more likely to be screened for CRC (AOR, 0.85; P = 0.43).55 Five additional studies reported an association between family CRC history and screening rates in their final multivariate analysis.2,42,107,122,134,158
Two studies showed that, among both males and females, obtaining regular screening for other cancers was associated with CRC screening; this is consistent with the three overview studies.21,46,151 The 1998 study that examined screening among males and females found that other cancer screening, including mammogram or prostate specific antigen (PSA) testing, was significantly associated with CRC screening (AOR, 4.40; P < 0.001).137 Another, which used 2001 BRFSS data, reported that subjects up to date for mammograms and Pap smears were more likely than those no up to date to be screened for CRC (AOR 2.42 for mammograms and AOR 1.70 for Pap smears; P < 0.01 for both).157 Other studies included in this report support the positive association of other cancer screening behavior with CRC screening.42,108,122–123,133–134,156,158
With respect to other healthy behaviors, one study that reported an association between vitamin supplement use and CRC screening (AOR, 1.87).137
For general health status, a few studies examined the association between perceived health status and CRC screening, as did the three overview studies,21,46,151 but overall findings are inconsistent. The study based on the 2003 HINTS national survey showed no association between perceived general health and CRC screening (P = 0.11).55 Another study reported that females who perceived their health as good were less likely to adhere to CRC screening than those perceiving their health to be fair or poor (AOR, 0.79; P < 0.01).157 Another study presented related factors, including a number of chronic illnesses and Charlson scores (i.e., a weighted index of 19 selected categories of disease found to be associated with mortality and other important health outcomes, in which a higher scores equates with worse health) obtained through medical record review. This group reported that patients with more illnesses were more likely to be screened (AOR, 1.12; P < 0.0001) but that those with higher Charlson scores were less likely to be screened (AOR, 0.84; P = 0.0001).158
Six other studies not presented in this section but that included perceived health in their final multivariate analysis also found that those with more positive perceptions of their health (i.e., excellent, very good, good) were less likely to report current CRC screening than those with lower or less positive perceived health,1,107,111,120,134,157 another study reported the opposite results such that those with perceived good health were more likely to be screened.163 Another study found no association between perceived health and screening behavior.2
In terms of obesity, four studies reflected inconsistencies about the association of weight with CRC screening.126,132,145,166 One study using data from 84,284 subjects from the 2001 BRFSS classified patients into five body mass index categories and found that no association between body mass index and FOBT completion.145 For this study, the authors did find that overweight or obese males were more likely to have obtained FS within the past year compared to females (P < 0.05).145 Another study based on 1999 BRFSS data found only an association between CRC screening for morbidly obese females and no differences for any other body mass index category in comparisons with respondents of normal weight (AOR, −5.6).126 Another study used data from the Maryland Cancer Survey and found that overweight and obese people had odds of being up‐to-date with CRC screening similar to those for people of normal weight (AOR, 1.05 and AOR, 0.84, respectively).132 In the fourth study, obese patients in primary care provider practices were less likely than normal-weight patients to be screened for CRC (AOR, 0.75; P = 0.004).166 Another study (not included in summary table) explored the relationship between BMI and CRC screening in American Indian and Alaskan Native men and found no association between these two variables in a nationally representative survey conducted in 2004–2005.131 Yet another study found that persons who were categorized as overweight were slightly more likely to have received an endoscopy and/or FOBT (RR, 1.2 and 1.1, respectively, P < 0.05).111
With respect to smoking and sedentary lifestyle (i.e., physical inactivity), one study reported findings from a community survey of New York city residents;114 current smokers (AOR, 0.62) and residents who reported being physically inactive (AOR, 0.74) were less likely (than various comparison groups) to be current on CRC screening.114 Similarly, another study reported that current smokers were less likely to be screened by endoscopy (AOR, 0.13; P = 0.009).106 Both studies support the findings from the three overview studies, which found that current smokers were less likely to be screened for CRC than those who had never or were former smokers.21,46,151
Four other studies (omitted in Table 19 because their analysis focused on other patient characteristics and CRC screening) supported the three overview studies in reporting that current smokers were less likely than former or never smokers to report being screened.115,122,126,157 One study reported no difference in smoking status and CRC screening.116
Psychosocial factors. Study characteristics. Another topic addressed by several studies is the extent to which psychosocial factors (i.e., knowledge, attitudes, beliefs, or perceptions about cancer and/or screening) may predict CRC screening behavior. As previously noted, two of the overview studies21,46 presented findings related to some psychosocial factors; both found that knowledge of screening tests were predictors for screening.
Table 20 presents summary information for the five studies involving the association between these factors and CRC screening; all involved self-reported responses from survey data influencing screening.55,106,133–134,144 Two national studies were based on HINTS data collected from the fall 2002 through spring 2003.55,133 Another study collected data from a sample residing in a five-county region of the Midwest;134 another study collected data locally from patients using three neighborhood clinics;106 while the remaining study collected data from Japanese Americans residing in the Greater New York region.144 The ages of respondents in the five studies differed: two studies collected data from those 50 years or older,133,144 another study used data from those 51 years or older;106 a third presented findings on those 65 to 80 years of age;55 and the remaining study presented findings specific to those ages 50–79 years.134
Table 20
Studies of the association between psychosocial factors and CRC screening.
Three different definitions of screening were used to determine whether respondents were up-to-date: two studies used the definition that an FOBT had been obtained within the past year and FS or colonoscopy within the past 10 years;55,133 two studies defined being screened as reporting an FOBT in the past year, FS in the past 5 years, or colonoscopy in the past 10 years;134,144 and one focused on endoscopy screening (i.e., FS in the past 5 years or colonoscopy in the past 10 years).106
Overview of results. Various authors have tended to define psychosocial factors somewhat differently; we divide the discussion of these factors into the four categories of knowledge, attitudes, perceptions, and beliefs. Table 21 summarizes the items included in each survey analysis of the studies in this section. In terms of knowledge or awareness of CRC or the available tests, two studies presented findings about whether respondents reported: (1) understanding the appropriate intervals of testing,133 (2) being aware of the types or numbers of tests available,55 and (3) knowing the expense of each test.133
Table 21
Types of factors and descriptions of variables used in selected studies to examine the influence of psychosocial factors on CRC screening.
One national study using the 2002–2003 HINTS reported that both males and females were statistically more likely to be screened if they understood the appropriate time intervals of both the FOBT and endoscopy (AOR, range 4.69–5.42 for males and 3.18 and 5.25 for females; all P values < 0.05).133 The same study found that males and females who did not know whether tests were “too expensive” (for them) were less likely than those who did know to receive an endoscopy (AOR, 0.43 and 0.46, respectively; P < 0.05); this same finding was also reported for females and FOBT testing (AOR, 0.55; P < 0.05).133 In another study, respondents 65 years of age or older who lacked knowledge about the number of tests that were recommended for their age group were less likely to be screened that knew about the recommended guidelines for testing frequency (AOR, 0.28).55
With regard to attitudes about testing or CRC, one study reported findings about overall attitudes toward screening and anxieties about tests from a regional survey of residents in the Midwest.134 If respondents had a positive attitude toward screening in general, they were more likely to report being screened (AOR, 2.35); if they had anxiety about tests in general, they were less likely to be screened (AOR, 0.58).134 Being fearful of finding cancer was positively associated with CRC screening among women in one study (AOR, 1.78).133
Three studies reported findings specific to perceived barriers to screening and perceived social support for screening, as well as perceived risk to being diagnosed with cancer.106,133,144 Perceived barriers to screening by endoscopy (e.g., inconvenience and unpleasant aspects of screening are perceived to be a problem) were associated with not being screened for CRC (AOR, 0.33; P < 0.0001) in one study. Another study highlighted in another section of this chapter also reported findings that support a relationship between perceived barriers to screening and adherence.142 Perceived social support to be screened was not associated with screening in one study,106 but was both indirectly (through perceived emotional support from family) and directly (through perceived emotional support from friends) related to adherence to screening in the one included study that tested a structural equation model to examine factors influencing screening.144 Perceived risk to being diagnosed with cancer was positively associated with being screened for females (AOR, 2.53).133 Two other studies not presented in this section found that perceived risk to being diagnosed with CRC was associated with screening.108,130
Three studies included analyses of beliefs that may be associated with CRC screening. Positive associations with CRC screening were found with the following beliefs: that the tests are safe (AOR, 1.39);134 that it is irresponsible not to be tested (AOR, 2.16);134 and, for females, that tests lead to early detection (AOR, 3.03).133 Those who believed that, if they are healthy, they do not need to be tested were less likely to report being screened (AOR, 0.58)134 as were those who thought that arranging for testing would not be easy (AOR, 0.47).55
Another study not presented in this section (because psychosocial factors were not a specific aim of their research) reported no association between belief that testing detects cancer early and screening rates.108
Patient Factors: Followup after Positive FOBT
Study characteristics. We identified two studies, both rated as fair quality, that assessed factors that may be related to followup after an abnormal FOBT result (Table 22).88,168 Both studies were conducted using claims data from one Veterans Administration (VA) Hospital; one focused on patients 70 years of age or older168 and the other on patients 50 or older.88 The outcome measure of interest to both was whether a patient completed a colonoscopy or double-contrast barium enema168 or a full colon evaluation (defined as colonoscopy or double-contrast barium enema with FS)88 within 12 months of receiving the FOBT results.
Table 22
Studies of factors associated with followup after abnormal CRC screening results are received.
Overview of results. Each study examined different predictors for receipt of a follow-up test. One explored the association of comorbidity (measured through Charlson scores)168 and the investigated the differences in followup for white and Blacks.88 The study focusing on comorbidities found that patients who had higher Charlson scores (i.e., more comorbidities) were no more likely than those with low scores to receive followup after a positive FOBT.168 Follow-up rates after a positive FOBT were not associated with race; Blacks were as likely to receive a full colon evaluation as whites (AOR, 1.14).88
Physician Factors Associated with CRC Screening
This part of KQ 2 focuses on physician factors associated with CRC screening, CRC screening discussions, or the quality of CRC screening. Although we found many studies that examined the association of patient characteristics and CRC screening, we found only one study that examined physician characteristics,127 one study that examined “patient-physician connectedness,”152 and 12 studies that examined physician recommendation of CRC screening. Of these 12 studies, 7 were national studies from two databases (NHIS and HINTS)21,46,55‐57,111,159 and 5 were regional studies from four different states or areas.107,142,148,153
Physician characteristics. Study characteristics. This cross-sectional study, which we rated fair quality, used data from the 2000–2001 Community Tracking Study (CTS) Physician Survey (response rate 59 percent), a nationally representative telephone survey of nonfederal physicians in 60 randomly selected metropolitan statistical areas (Table 23).127 Among other items, the CTS asked physicians about their age, years in practice, specialty, board certification, and site of medical school graduation (US/Canada versus other). The investigators assessed information on CRC screening and patient care visits for Medicare beneficiaries ages 65 years and older from Medicare claims data. The investigators linked the databases by physician ID numbers, allowing them to identify 3,660 primary care physicians who cared for 24,581 Medicare beneficiaries in the database. They could then generate 1-year (2001) CRC screening rates for physicians with similar characteristics.
Table 23
Studies of the association between physician characteristics and CRC screening.
Overview of results. CRC screening rates did not differ between patients with male versus female physicians; neither did they differ among physicians who had been in practice for 0–10 years versus 11–20 years versus > 20 years. Patients cared for by family physicians had somewhat higher 1-year screening rates than those cared for by general internists (9.5 percent versus 7.8 percent, P < 0.001); patients cared for by board certified physicians had higher screening rates than those cared for by non-board certified physicians (9.5 percent versus 6.5 percent, P < 0.05). Patients cared for by physicians who graduated from US or Canadian medical schools had higher screening rates than those cared for by physicians who graduated from other medical schools (9.3 percent versus 7.7 percent, P < 0.05). Another study (highlighted under patient level factors) explored the relationship between race and screening among a nationally representative sample of Medicare beneficiaries and found that, controlling for other factors, patients whose usual care physician was a primary care generalist rather than another type of specialist had significantly higher odds of CRC screening (AOR, 1.31; 95% CI, 1.12–1.53).129
Physician-Patient connectedness. Study characteristics. A retrospective cohort study, which we rated good quality, examined the association between CRC screening and patient-physician connectedness (Table 24).152 Although this variable could be seen as either a system variable or a patient-physician interaction variable, we have elected to review it under physician characteristics because the study was conducted in a single large academic practice network with large variation among physicians (e.g., specialty, number of years in practice, etc.), indicating that the variable at least partially indicated physician practice style. It was conducted in the Massachusetts General Hospital adult primary care network (181 primary care physicians working in four community health centers and nine hospital-affiliated practices). Using electronic billing records, the investigators identified all patients with at least one visit to one of these practices between 2003 and 2005. Using a validated algorithm, the investigators further divided these patients into three groups: practice-connected (i.e., patient was considered a regular user of the practice but had seen a variety of physicians within the practice), physician-connected (i.e., a patient of the practice as well as seen by the same physician for most visits), or unconnected (i.e., patient could not be assigned to either a practice or a physician). Using electronic billing and laboratory data, the investigators calculated, among other indicators, CRC screening rates (colonoscopy within 10 years, FS within 5 years, or FOBT within one year) for physician-connected patients (n = 31,215) versus practice-connected patients (n = 6,453), excluding unconnected patients (who were often either just entering or leaving the practice).
Table 24
Studies of the association between physician or practice connectedness with CRC screening.
Overview of results. Physician-connected patients had higher CRC screening rates (adjusted percentage 77.1) than practice connected patients (adjusted percentage 69.5; P < 0.001).
Physician recommendation. We found 12 studies that examined the association between CRC screening and physician recommendation of CRC screening. Seven are analyses from two large national databases: NHIS and Health Information National Trends Survey (HINTS).21,46,55‐57,111,159 The other five studies are regional studies.107,136,142,148,153 We discuss the seven national papers and then assess what additional insights come from the regional studies. Agreement of results among all these studies is high.
Study characteristics of national studies. As shown in Table 25, four of the seven national studies used the NHIS 2000 cancer control module;21,57,111,159 one used the NHIS 2005 cancer control module.46 Two studies analyzed the 2002–2003 HINTS database.55–56 We rated three studies as good quality21,46,56 and the remainder as fair.
Table 25
National studies of association between physician recommendations and CRC screening.
The NHIS cancer control module for CRC did not change between 2000 and 2005; results from the analyses of these two surveys were similar. All five of the NHIS studies assessed the percentage of people who had not had a screening test within the recommended interval and who reported that they had not had a physician recommendation to be tested within the past year. One study also examined data on 1,235 primary care physicians from the Survey of Colorectal Cancer Screening Practices, a 1999–2000 nationally representative survey of primary care and specialty physicians and health plan medical directors.57
The 2002–2003 HINTS survey was a nationally representative random-digit dialing telephone survey of 6,369 noninstitutionalized civilians ages 18 and older, with over-sampling of blacks and Hispanics. Respondents who had not been screened within the recommended time interval (1 year for FOBT, 5 years for FS, and 10 years for colonoscopy) were asked an open-ended question about reasons for not being screened. The reasons were later aggregated into 12 predefined categories for analysis. Among the categories was lack of physician recommendation.55–56 One HINTS study56 primarily examined CRC screening in the uninsured (ages 50 to 64 years); the other assessed CRC screening in older respondents (ages 65 to 89 years).55
Overview of results of national studies. For people who had not had a screening test in the recommended interval but who had a physician whom they had visited within the past 12 months, three studies agreed that from 92 percent to 94.6 percent had not received a physician recommendation for screening in that year.21,111,159 The fourth NHIS 2000 study compared reasons for low screening rates given by NHIS participants and those given by primary care physicians from the 1999–2000 Survey of Colorectal Cancer Screening Practices.57 Thirty-seven percent of physicians and 20 percent of NHIS participants cited failure of physician recommendation as a primary reason for low screening rates. As in the three other studies from the NHIS 2000, this study also found that among those respondents who had not been screened and who had seen a physician within the past year, about 90 percent had not received a recommendation for screening over that year.57 The NHIS 2005 study found results almost identical to the NHIS 2000 studies.46
One HINTS study found that 75 percent to 85 percent had not been advised to be screened over this year.55 The other HINTS analysis examined barriers to CRC screening among the uninsured and found that about 91 percent of uninsured people who had received a physician recommendation for screening had in fact been screened; only 13 percent of uninsured respondents who had not received a recommendation had been screened (P < 0.001).
Study characteristics of regional studies. Of the five regional studies, all rated as fair quality (Table 26),107,136,142,148,153 four were telephone surveys of people in three different areas: two studies from Massachusetts;107,153 one from Maryland; 148 one from Iowa; 136 and one from Genessee County, Michigan.142 These studies included one that called respondents who had responded to the 1999 BRFSS,153 and findings from either a state-based,107,148 or county-based health survey.142 One other study was a project that combined patient surveys and medical record reviews from family practices in rural Iowa.136 The study from Iowa matched a 2004 mailed survey of 511 patients (53 percent response rate) with a medical record review.136
Table 26
Regional studies of the association of physician recommendation with CRC screening.
Overview of results of regional studies. All five studies found a strong association between physician recommendation and receipt of CRC screening, much like those at the national level.
Patient-Provider Communication
Study characteristics. We found five cross-sectional studies, which we rated fair quality, pertaining to the association between patient-provider communication and CRC screening (Table 27).56,135,140,154,167 Two used HINTS data to examine the association between communication and screening among the uninsured.56,135 Both used measures from the Consumer Assessment of Healthcare Providers and Systems (CAHPS®) to assess patient-provider communication; these address how well patients feel the provider listens to them, explains options, respects them, spends adequate time with them, and involves them in medical decision making. Another study used data from 8,488 survey respondents from the MEPS to examine the relationship between patient-provider communication and socioeconomic variables on the receipt of CRC screening;154 patient-provider communication was assessed by measures derived from CAHPS. Another study surveyed 397 Black church members in North Carolina;140 it assessed this factor with a “communication score” based on five patient-reported items. The final study surveyed female patients of primary care physicians in Los Angeles on their perceptions of how enthusiastically their provider recommended or discussed CRC screening with them.167
Table 27
Studies of the association of patient-provider communication and CRC screening.
All five studies measured patient-provider communication as perceived by patients. In addition, all five used patient-reported CRC screening status as their primary outcome. One study also assessed subject’s trust in cancer information from the doctor.135
Overview of results. The two national studies that used HINTS data reported no significant association between patient-provider communication measures (from CAHPS) and CRC screening status.56,135 However, the MEPS-based study demonstrated that patients who reported that their provider spent enough time with them and adequately explained information were 2.6 to 6.4 times more likely to have undergone CRC screening.154 The North Carolina study reported that better quality of patient-provider communication was significantly associated with completion of CRC screening.140 The fifth study of women of PCP physicians in Los Angeles found that even a discussion with the provider with perceived ‘low enthusiasm’ for testing was significantly more likely to result in testing than no discussion about testing (AOR, 6.246; P < 0.0001).167 One other study (presented in the patient level factors section) also supported a positive relationship between patient-provider communication and any CRC screening.144
One of the HINTS studies reported that subjects having trust in cancer information from the doctor were more likely to be up-to-date with CRC screening (OR = 2.08, 95% CI, 1.49–2.94).135 Another study, highlighted in the patient level factors section on insurance status also reported a positive relationship between adherence to FOBT and patient’s report that their provider demonstrates compassion.128
Periodic Health Examinations
Study characteristics. We found one study, which we rated as fair quality, that focused on the association between receipt of a periodic health examination (PHE) and CRC screening rates (Table 28).164 It was a retrospective cohort study of 64,288 consecutive enrollees in a Washington state health plan who had attended one or more primary care visits in 2002–2003 and had been eligible for one or more cancer screening tests (for CRC, breast cancer, or prostate cancer).164 It defined a PHE as any outpatient encounter (in 2002–2003) having either (1) an evaluation and management code indicating “initial evaluation” (codes 99386–7) or “reevaluation and management of a healthy individual” (codes 99396–7) or (2) an International Classification of Diseases, Ninth Revision, Clinical Modification, code signifying either a general medical examination (code V700 or V708-9) or a gynecologic examination (code V723). The study reported results for a combined outcome of either FOBT or invasive CRC testing (FS, colonoscopy, or barium enema).
Table 28
Studies of association between periodic health examination (PHE) and CRC screening.
Overview of results. A greater proportion of subjects who had had a PHE received CRC screening than subjects who had not had a PHE (unadjusted: 57.2 percent versus 17.2 percent, respectively).164 The incidence of CRC testing was more than three times higher in patients who received PHEs than in those who did not (adjusted relative incidence, 3.47; 95% CI, 3.34–3.59; P < 0.001).164
Results from several other studies in this review supported the finding that subjects having periodic health examinations, annual physicals,134 physicals,171 health maintenance examinations,136 or annual checkups108,153,172 are more likely to have had CRC screening than people not receiving such services. We do not describe these studies in further detail or include them in the table because they were not designed to focus on this factor (PHEs); rather, they primarily examined another factor or examined multiple factors simultaneously.
System Level Factors Associated With CRC Screening
This part of KQ 2 focuses on health care system characteristics associated with CRC screening, CRC screening discussions, or the quality of CRC screening. The issue we addressed is whether the organization of health care services influences CRC screening. Thus, we searched for studies of any research design that examined the association between system characteristics and any of our three primary outcomes (measured in a valid and reliable manner): CRC screening rates, the frequency or quality of CRC discussions, or the quality of CRC screening.
By health care system characteristics, we are referring to such variables as involvement of nonclinician staff in screening, the practice’s being part of a managed care organization, use of reminder or recall systems, having an organized endoscopy referral system, the size and/or type of the medical practice, and the degree of local autonomy over the structure of care delivery. We distinguish between these system characteristics and other factors, such as patient access to health care (including having health insurance or having a regular source of health care), characteristics of the patient-clinician interaction (including trust or having health maintenance visits), or receiving a clinician recommendation for screening.
Study characteristics. We found six fair-quality studies that provided some information about this question.66,110,127,139,143,173 Three studies used large datasets (one including 155 VA primary care clinics),110 another used Medicare claims data,139 another used Medicare claims data plus a national physician survey,127 while the sixth used survey data to compare patients who receive their regular care from county health centers versus those going to a private physician office in the New York City area.66 Two studies collected data from medical practices.143,173 One of the latter studies focused on 22 suburban primary care practices in New Jersey and Pennsylvania,143 and another examined a single primary care practice within a low-income urban New York City setting.173 Five studies were cross-sectional,66,110,127,139,143 and the sixth was a cohort study.173
The six studies examined a variety of system variables, including involvement of nonclinician staff in screening, reminder systems, endoscopy referral systems, local autonomy of the internal structure of care delivery, size or type of the practice, group versus solo practice, and degree of managed care activity in the area. All used the outcome of CRC screening (according to national screening guidelines), assessed either through administrative databases,127,139,173 through direct medical record review,110,143 or self-report via telephone surveys.66
Overview of results. Five of the six studies reported a positive association between some system characteristic and CRC screening (Table 29). The most positive associations were use of nonphysician staff (for either general counseling143 or assistance with the screening process173). In one study, a practice’s use of nonphysician staff for general lifestyle counseling was associated with a near-doubling of CRC screening (from 27.2 to 54.1 percent points);143 in another, nonphysician “patient navigators” (along with several other administrative changes) increased the number of patients receiving colonoscopy each month from 75.7 to 119.0.173
Table 29
Studies of the association between system level factors and CRC screening.
Two other studies found what appears to be moderate increases in CRC screening associated either with higher levels of managed care activity in the area139 or with a higher level of autonomy over the internal structure of the practice.110 Although both findings were statistically significant, determining the exact strength of the association in these studies is difficult because they did not provide absolute screening rates.
Use of patient reminders was associated with a higher level of CRC screening in one study (39.9 percent versus 19.6 percent).143 However, the availability (rather than the use) of computerized physician reminders was not associated with a higher screening rate after adjustment for practice size and patient covariates.127
In addition, one study found that smaller practices (within a group of large practices) were associated with higher screening rates (although absolute rates were not given).110 After adjusting for other patient and physician covariates, investigators on another study found no association between practice size among smaller practices (solo/two-person group practice versus larger group practice) and CRC screening (5.9 percent versus 5.8 percent 1-year screening rates).127 The final study found higher endoscopy screening rates among patients of private physician offices compared to those receiving care in the same geographic region through county health centers (P < 0.001).66 This study also found that patients of county health centers were more likely to cite no physician recommendation as a barrier to endoscopy when compared to patients of private physician offices (P = 0.02).66
Summary
We categorized studies examining factors associated with the use of CRC screening tests into five domains: 1) patient factors, 2) physician factors (including physician characteristics, physician-patient connectedness, and physician recommendations about screening), 3) patient-physician communication factors, 4) the periodic health examination, and 5) system level factors. We further categorized the patient factors into four groups: patient demographics, access to care, personal health or risk factors, and psychosocial factors.
All included studies focused on factors associated with underuse of CRC screening. None focused on factors associated with underuse of CRC discussions or on factors associated with overuse or misuse of CRC screening.
Several factors are consistently and significantly associated with reduced CRC screening (i.e., P < 0.05 or confidence intervals that do not overlap or include 1.0). They include:
- Low household income
- No health insurance
- Being Hispanic or Asian
- Not being acculturated into the United States
- Limited access to care (i.e., lack of a regular source of primary care and no visits in previous year to provider), and
- No physician recommendation to be screened.
Factors positively associated with CRC screening include having private insurance, being non-Hispanic white, higher education level, participating in regular screenings for other cancers, having a family history of CRC or personal history of another cancer, having regular access to care, having effective provider-patient communication, or physician recommendation. We found one study each that examined the association between screening and specific physician characteristics, physician-patient connectedness, and use of periodic health examinations. Thus, insufficient evidence exists to draw conclusions about these relationships. Studies on system level factors that might influence CRC screening did not consistently measure the same variables but seem to support counseling by nonclinicians, reminder systems, and assisting patients to keep appointments.
KQ 3: Which Strategies Are Effective In Increasing The Appropriate Use Of Colorectal Cancer Screening And Followup?
KQ 3 focuses on the evidence on effectiveness of strategies that have attempted to increase appropriate CRC screening and followup. Therefore, all included studies measured the outcome of CRC screening and/or followup rates; one also included the outcome of a discussion with a provider about screening.
We classified strategies into those that targeted the patient, the provider, the health system, and/or the community. We identified and included 15 studies that targeted the patient,85,174–187 2 that targeted the provider,186,188 and 5 (six manuscripts) that targeted the health care system.162,189–193 (Some studies had more than one focus.) We found no RCTs of either fair or good quality that tested interventions implemented within a community. Of these 21 studies in all, one focused on appropriate followup after an abnormal screening;188 the others focused only on increasing screening rates.
We present only those studies that we rated as fair or good quality. Common reasons that we rated studies as poor quality included a combination of issues. For example, the randomization process was not explained, was difficult to determine, or was not blinded to the provider; the response rate was low (< 60 percent); the investigators used nonstandard instruments or outcome measures to assess screening or followup rates; and/or the comparison samples were dissimilar on key characteristics at baseline.
Our overall summary and strength of evidence tables for studies addressing this KQ are presented at the beginning of this section. The remainder of this section provides, first, an overview of studies of patient-level interventions. We then consider the two studies of a provider-level intervention186,188 and the five studies of a system-level intervention.162,189–193
KQ 3 Overall Summary and Strength of Evidence
In the tables that follow, our overall grades of the strength of evidence appear in the far right column; grades for key domains to determine the strength of evidence are in the intermediate columns. Table 2 (Chapter 2) defined terms used to describe the strength of evidence; these definitions can also be found in the glossary for this report.
We included 21 RCTs, rated good or fair quality, of interventions designed to increase CRC screening. These included 15 studies that targeted the patient,85,174–187 2 that targeted the provider,186,188 and 5 (including two manuscripts of the same study) that targeted the health care system.162,189–193
Following categories similar to those recently used to develop recommendations by the Task Force on Community Preventive Services (TFCPS) on CRC screening,194 we divided the types of studies of interventions targeting patients into five categories: (1) patient reminders;175,182–183,186 (2) small media (with177–178,181 and without174–176,185 decision aids); (3) group education;184–185 (4) one-on-one interaction;85,179–180 and (5) reducing structural barriers.85,175,179,183,186–187 Much like recommendations for the Guide to Community Preventive Services,194 these studies include five that have more than one type of intervention.85,175,179,183,185 For each of these, we categorized them into more than one type of intervention in determining the strength of evidence and presenting the overall findings in the following sections. When possible, we attempted to evaluate the incremental contribution of each component separately. However, for most studies, the effect of all the components was evaluated collectively, such that findings were not presented by authors in a way that allowed us to assess the incremental impact of adding each component. Across these 15 RCTs focused on patient-level interventions, the range of increases in screening was 0 percent to more than 40 percentage points.85,174–187
As shown in Table 30, we found high strength of evidence that interventions that provide patient reminders lead to small to moderate increases in screening (percent increases ranged from 5.0– 15 percentage points).175,182–183,186 We also found high strength of evidence that of small media, such as delivery of education videos or brochures to patients before being seen by a physician or in the mail through a church registry list, have little to no impact on screening rates.174–176,185 Use of decision aids, delivered via small media, was less conclusive. Although we recognize that not all decision aids are equal, with some designed to be more interactive with patients than others, we found the evidence to be mixed in terms of how effective they are in increasing screening (rate change in percentage points from 3 percent [P = 1.0181] to 14.2 percent177 and 23 percent178). For this mixed evidence, with two of three studies showing benefit, we concluded that the strength of evidence is low (because of the inconsistent results) that some types of decision aids are effective for increasing screening. We identified two studies examining the impact on CRC screening rates of group education delivered either by Native Hawaiians among Native Hawaiians184 or by African Americans for their fellow church members on the need for testing.185 These studies demonstrated mixed effects; one showed a negative finding on the impact of the intervention on screening184 and another finding a borderline positive effect (P = 0.08),185 we concluded that the strength of evidence is low for this intervention type. The two remaining categories of patient level interventions (one-on-one interventions and eliminating barriers) both provided high strength of evidence that they yield an increase in screening rates. The interventions designed to provide one-on-one interactions, through either a nurse, or health educator,85,179 or on the phone,180 hold promise in their ability to increase CRC screening, with percentage point increases ranging from 14.6 percent in FOBT completion,85 20.9 percent of any CRC test through repeated telephone counseling, and 41.9 percent in FOBT completion through an intervention provided by a bi-lingual health educator.179 Those designed to eliminate barriers by providing FOBT tests to use at home or providing access to individuals who can help to address barriers were also shown to be effective in increasing screening rates (rate change from 14.6– 41.9 percentage points).85,175,179,183,187
Table 30
Effect of patient-level interventions on CRC screening rates.
We also address discussions with providers as an outcome for KQ 3 and found one study that presented findings specific to increases in this outcome (Table 31).177 These investigators reported 25.1 percent increase in discussions (Table 31), but with only one study we concluded that there is low overall strength of evidence for patient-level interventions to increase discussions with providers.
Table 31
Effect of patient-level intervention on discussions with providers.
In addition to the 15 studies of patient level interventions, we included 2 studies on provider level interventions (Table 32).186,188 One study on provider-level interventions sent reminders to physicians who had patients in need of surveillance colonoscopies;188 the other study used electronic reminders during patient office visits to increase ordering of the tests.186 The first study favored providing reminders to physicians to increase surveillance colonoscopies, but the other found no difference between CRC screening rates of patients whose providers received reminders or not (P = 0.47).186 We rated the strength of evidence as low because the included studies tended to indicate no benefit in provider reminders in increasing screening.
Table 32
Effect of provider-level interventions on CRC screening rates.
The five studies (six articles) on system-level interventions162,189–193 implemented changes to improve referral of patients for screening190–192 or identified a person such as a patient navigator189 or someone in a similar role (i.e., Prevention Care Manager or PCM)162,193 to help patients navigate the health care system (Table 33). Their findings indicated that this intervention may provide promising effects on increasing CRC screening.
Table 33
Effect of system-level interventions on CRC screening rates.
The 21 studies identified as eligible for this KQ represented a small fraction of all studies reporting on interventions designed to improve CRC screening. These other (ineligible) studies were not conducted as a RCT design, provided a limited description of the intervention, or used untested or unvalidated measures in assessing outcomes.
Patient Interventions
Study characteristics. Following categories similar to those that the TFCPS used to develop CRC screening recommendations,194 we divided the types of studies of interventions targeting patients into five categories: (1) patient reminders;175,182–183,186 (2) small media (with177–178,181 and without174–176,185 decision aids); (3) group education;184–185 (4) one-on-one interaction;85,179–180 and (5) reducing structural barriers.85,175,179,183,187 Table 34 Shows which studies employed which types of interventions; following this section and overview of results, we consider each kind of intervention in turn.
Table 34
Patient-level studies by category of intervention.
Patient reminders can be in the form of written materials such as postcards, letters, or other materials used to remind or alert patients of their need for CRC screening. Reminders can also be provided through telephone contacts with patients who are due for screenings. These reminders, when used as an intervention, are provided to patients who are due for a rescreening or who have never been screened; they are not reminders of an upcoming appointment that is already scheduled. Patient reminders are thought to be a means of effectively prompting people about their need for annual screening (or for screening related to whatever period recommended for the patient); the idea is that if patients are not scheduled to see a provider, they will initiate an appointment in order to remain current on cancer screening tests.
Small media interventions focus on providing respondents with educational materials; they can include videos and printed materials such as letters, brochures, and newsletters that are provided to patients explicitly to educate them about the disease under study (i.e., colorectal cancer), their risks for being diagnosed with the disease, and screening tests that are available. These materials are termed “small media” because they rely on mail, telephone, or distribution of education materials. They are not educational media campaigns that would be provided through television advertisements and public service announcements (PSAs); neither are they national media campaigns such as the one conducted by the Centers for Disease Control and Prevention (CDC) called “Screen for Life,” which uses PSAs on national TV stations to educate people about the need for cancer screening tests. Both the small media method of educating the public, as well as the large media campaigns, can be used to inform and motivate people to be screened for cancer and can be tailored to specific individuals or target general audiences.
We included in this category the three studies that tested aids in helping patients to make informed decisions (i.e., decision aids). Decision aids are mechanisms or interventions that have been developed to improve communication between health professionals and patients; their goal is to help involve patients in making decisions regarding their health care. Decision aids can include brochures, videotapes, or interactive computer programs.
Group education interventions are those conducted within a specified group setting and deliver information or motivation to encourage screening. Although these interventions often include handing out information or materials, we categorized studies that included this intervention as group education because they also provided a setting in which an individual was present to interact with the audience.
One-on-one education includes studies in which a provider (e.g., physician, nurse, health educator) works individually with patients to educate them about CRC screening and/or aid them in making decisions about which tests to complete and when to receive screening. These interventions tend to include some concentrated time with a patient to answer questions, address concerns, and help facilitate completion of screening tests. Studies included in this category provided this one-on-one education either by telephone180 or in person.85,179
The final category includes studies that address reducing or eliminating structural barriers to screening. Many problems can make it difficult for people to seek screening for cancer. Barriers can include distance from screening location, limited hours of operation, no day care for children, limited access to screening tests, and language and cultural factors. These types of interventions seek to increase screening by removing structural barriers. In this category, we included studies that tested the provision of FOBT tests through the mail, either alone183 or in combination with an intervention that also addressed language and cultural barriers that may be barriers to screening among Japanese Americans.179
Overview of results. A total of 15 articles examined the impact of various interventions targeting the patient in an attempt to increase CRC screening.85,174–187 All 15 focused on screening, not followup. All studies also partially addressed the “appropriate” use of screening by using the criteria for screening guidelines as “inclusion” or “exclusion” criteria (e.g., no FOBT in prior 12 months, no prior CRC diagnosis) in the sample. Seven studies174–179,187 had an upper age limit (from 70 to 79 years) for their studies; eight did not.85,180–186 This feature raises the issue of potentially inappropriate screening for older people. Three studies relied only on self-reported frequency of CRC screening,178,183,185 which has been shown to overestimate screening rates.
Among these studies, four presented findings of an intervention to provide patient reminders, seven focused on the use of small media (e.g., video, letters) to educate patients about the need for screening and/or types of tests available or to help their decisionmaking process, two presented findings from group education interventions, and three focused on interventions that provided one-on-one interactions either by phone on in person to increase screening.
Five studies addressed barriers to screening by providing FOBTs to patients (i.e., by mail or in health clinics) who were due for screening. One of these implemented an intervention that also addressed cultural and language barriers.179
The impact of these interventions on CRC screening rates ranged from 0 percent to 41.9 percent when the intervention groups were compared with the control groups. Studies that examined the use of educational materials presented via small media174–176,185 had no impact on screening rates (increase of 0 – 15.1 percentage point change [P = 0.08 for study with highest percentage change]); those that provided means for eliminating structural barriers, such as access to CRC screening tests or language barriers,85,175,179,183,187 demonstrated the highest impact on screening rates overall (14.6 to 41.9 percentage point change). Those that used decision aids delivered to patients through small media had mixed results; two studies demonstrated an overall increase in CRC screening (14.2 to 23 percentage point change)177–178 and the other demonstrated only a 3 percentage point increase in CRC screening.181 Interventions that provided patient reminders in the mail or over the telephone had an impact on screening using any CRC test ranging from 5.4 percent to 11.7 percent and 15 percent.175,182–183,186 Two studies tested an education intervention in a group setting and found no difference in screening rates among their samples.184–185 Only one study measured increases in discussions between the patient and providers as an outcome of their intervention, reporting a 25.1 percent increase in discussions among patients in the intervention group compared with those in the control group.177
Patient reminders. Study characteristics. Four RCTs, one rated as good quality186 and three rated as fair quality, 175,182–183 focused on testing reminders mailed to patients due for screening (Table 35). One study used usual care as a comparator; it involved a mailed reminder (brochure) sent to patients who had been referred for a screening colonoscopy after an appointment at a primary care practice.182 Another study randomized subjects into one of four groups:175 one group received a mailed standard intervention (Group 1: SI) that included an informational booklet and FOBT kit; a second group received a tailored intervention (Group 2: TI) that included the SI package plus tailored “message pages” of brief messages that addressed personal barriers to screening; a third group (Group 3: TIP) included the TI package plus a reminder telephone call; and the fourth was a control group. All three intervention groups received either a letter or a telephone call as a reminder to complete the FOBT. The intervention groups then varied on the type of additional education materials they received or the type of contact that was made, such that G3 is the only group that received phone calls. These three groups were compared with a sample of patients who received usual care.
Table 35
Studies of patient reminders on increasing colorectal cancer screening rates.
Another study mailed packages of a letter, pamphlet, and FOBT kit to patients of an ambulatory health care center who were due for CRC screening and compared these patients with others who received usual care.186 The fourth study randomized residents in a local community to mailed FOBT kits without reminders (G1), mailed FOBT kits with telephone reminders (G2), or a questionnaire about CRC (G3: control group).183 Because both G1 and G2 of this study provided mailed FOBT kits to a random sample of local residents, the differences between these two groups reflects the impact from telephone reminders to complete the FOBT.
Three studies focused on patients 50 years of age or older; one study limited its intervention to persons 50 to 80 years.186 The times for followup varied: within 4 months of the initial referral for colonoscopy screening;182 6 months of the original mailing and 1 year to measure completion rates;183 15 months after the initial mailing;186 or 24 months after the initial visit to their provider.175 The three studies focusing on populations recruited through a provider setting measured their outcomes through medical chart review;175,182,186 the fourth study with a randomized sample of residents relied on self-reported screening for their outcomes.183
Overview of results. All four studies found statistically significant increases in CRC screening rates, with absolute increases in screening from about 5.9 percentage points to about 15 percentage points. The colonoscopy study found an increase in completed colonoscopy from 59.0 percent in the control group to 70.7 percent in the intervention group (difference: 11.7 percentage points; 95% CI, 5.1 –18.4 percentage points).182 The tailored intervention study found an increase in any CRC screening for all three intervention groups compared with controls (33 percent control versus 46 percent, 44 percent, and 48 percent; OR, 1.7; 95% CI, 1.3–2.5; OR, 1.6; 95% CI, 1.2–2.1; OR, 1.9; 95% CI, 1.4–2.6), but no difference among the interventions.175 In the TIP group, 28 percent did not receive a telephone call. The study providing FOBT kits to patients of ambulatory care centers found that those who received mailings were more likely to obtain screening than those who did not (44.0 percent versus 38.1 percent, respectively, P < 0.001).186 The population-based study that mailed FOBT kits to the intervention groups and followed them either with or without reminders reported an increase in completion of any CRC screening of 7.8 percent (95% CI, 3.2–12.0 percent) for the control group, 13.2 percent (8.4–18.2 percent) for the FOBT without reminders group, and 14.1 percent (9.1–19.1 percent) for the FOBT with reminders group.183 The difference between FOBT with reminders and controls was statistically significant. Overall baseline adherence to any CRC screening was 55.8 percent; the final adherence rate for any CRC screening was above 60 percent for the intervention groups, although these rates were self-reported. The primary care and population-based studies showed little increase in screening with increased intensity of intervention.175,183
Detailed results. The one study using only mailed patient reminders provided a brochure about CRC tests for patients who had been referred for a screening colonoscopy.182 Patients in the intervention group were mailed the information brochure within 10 days of referral for the colonoscopy. Each brochure included the primary care physician’s name, encouraged patients to schedule the procedure, and explained CRC and polyps, the risks of being diagnosed with CRC, the nature of bowel preparation, alternative screening tests, and the complication risks of colonoscopy. Those patients assigned to the control group had been referred for screening colonoscopy as well but received no reminder. The findings indicated that patients receiving the reminders were more likely to complete the test (within the 4-month follow-up period) than those who did not (11.7 percentage point difference; 95% CI, 5.1–18.4 percentage points; P = 0.001).
The second study examined three different types of interventions, all with varying intensity, and compared them with a control group that received usual care and none of the study intervention contacts.175 In the standard intervention (G1), patients were mailed a package that included a CRC screening invitation letter, information booklet, FOBT kit, and reminder letter. The package also included instructions for completing a home FOBT and on arranging for a flexible sigmoidoscopy (FS). The TI patients (G2) received the SI package and two tailored message pages, which addressed personal barriers to FOBT and FS that were identified through analysis of baseline survey data collection. TIP patients (G3) received the SI and TI information and a telephone reminder to conduct the FOBT. During these telephone calls, a trained health educator reviewed the mailed materials and encouraged participants to consider screening. Although the investigators did find that groups that received some form of reminder were more likely to complete screening than those who received usual care (P = 0.001 or 0.002), they did not find differences among the intervention groups to indicate whether patients who received mail or telephone reminders (SI: AOR, 1.7; 95% CI, 1.3–2.5; TI: AOR, 1.6; 95% CI, 1.2–2.1), or a combination of the two (TIP: OR, 1.9; 95% CI, 1.4–2.6) were any more likely than any other to be screened.
The third study conducted in a clinic setting identified patients through their medical record system who were overdue for CRC screening (N = 21,860).186 Patients ages 50 to 80 years were randomly selected to receive a package that included (1) a letter from the chief medical officer explaining that the patient is overdue for screening; (2) an educational pamphlet explaining the screening test options; (3) FOBT kit with instructions; and (4) a telephone line dedicated to having patients call to make endoscopy appointments. A second mailing was sent to nonrespondents at 6 months. Patients who received the mailing were more likely than the control group to complete a FOBT (25.4 percent versus 20.4 percent, respectively; P < 0.001) or any CRC test (44.0 percent versus 39.1 percent, respectively; P < 0.001).
The fourth study tested receipt of reminders versus no reminders and compared both with a control group.183 The study identified a random sample of residents in Wright County, Minnesota, who were determined to be 50 years of age or older based on records from the Minnesota State Driver’s License and Identification Card database. The sample was divided into three groups: the control group (Group 3) and two intervention groups that both received informational packages but differed in terms of whether they received telephone reminders for testing (Group 2) or not (Group 1). All three groups were mailed an initial survey on CRC and then either received no additional information until the follow-up survey in 1 year (Group 3) or received a package of information approximately 2 months after the questionnaire that included an FOBT kit with instructions and educational material about CRC and screening test. Group 2 individual who did not return FOBT kits were mailed reminder letters 1 month later that included another FOBT kit and then, if they had still not returned an FOBT, they received a telephone call 1 month later. Of those in the “no reminder” group (Group 1), 49.6 percent of the participants accidentally received the first reminder letter with no further contact. They did not receive any of the subsequent reminders (i.e., two more mailings and telephone calls). The authors did not report the number of respondents in Group 2 who had been called Although the study did report self-reported completion of any CRC test, the findings specific to reminders demonstrated an overall increase of 6.3 percent in completion of FOBT for Group 2 received telephone reminders (received reminders: 23.2 percent FOBT completion rate; 95% CI, 17.2–29.3 percent) and Group 2 (no reminders: 16.9 percent FOBT completion rate; 95% CI, 11.5–22.3 percent).
Small media interventions. Seven RCTs were patient-directed small media interventions; that is, these studies that used various tools such as print materials or telephone calls to provide education to a targeted sample. We divided this set of studies into two categories: (1) four studies that focused on small media interventions that were not decision aids174–176,185 and (2) three studies of decision aids.177–178,181
Small media: educational materials and messages. Study characteristics. As shown in Table 36, one study in this category was rated as good quality174 and three were rated as fair quality.175–176,185 The populations targeted for all four studies were at average risk for CRC and met recommendations for screening tests. All four studies focused on those 50 years or older.174–176,185 The populations in three studies were recruited from primary care practices, and patients in each of the control groups were receiving usual care. Because these were patients already receiving care from a physician, “usual care” was defined as people who received none of the interventions.175–176 The fourth study recruited church members from predominantly African-American churches located in rural areas of one state and compared their intervention with those in churches whose members received education on unrelated health topics.185 Participants of two of the studies were predominantly non-Hispanic white;174,176 the third primary care study included 39 percent multiracial (race unspecified) participants from an urban center,175 and the fourth study included only African Americans.185 Two interventions focused on mailing educational materials, followed by telephone contact;175–176 the third intervention consisted of a mailed 15-minute videotape,174 and the fourth intervention included a combination of print and video materials that were mailed at 2-month intervals over the 9-month intervention period.185 The timing for measuring outcomes ranged from 6 months174 to 24 months.175 Three studies measured their outcome of receiving any CRC test by reviewing medical charts;174–176 the fourth measured CRC screening through self-reported responses.185
Table 36
Studies of small media: Educational materials and messages on increasing colorectal cancer screening rates.
Overview of results. The four studies that did not deal with decision aids demonstrated consistent findings with regard to education materials and information provided to patients via small media: such interventions had no influence on CRC screening rates that was found to be statistically significant (0 percent to 15.1 percentage point differences in rates among intervention and control groups across studies).
For one study, determining the increase in screening specific to the educational materials was somewhat challenging.175 It included multiple strategies; the primary difference between the control group and the three intervention groups was that all the intervention groups received both an FOBT kit and some type of reminder to complete the test (either by letter or by letter and telephone). The differences between the three intervention groups are in the type of small media used to influence completion of CRC testing. These three groups did not differ in terms of completion of CRC screening for patients who received print material only compared with those who got print materials plus telephone reminders (2 percentage point difference in screening rates).175
The other two studies also had no effect on screening rates (0–1 percentage point difference in control and intervention groups).174,176
Detailed results. One good-quality study explored the effect of mailing an educational video to patients’ homes just before they had an appointment for a physical examination.174 The research team mailed a letter to potential participants who were 50 to 74 years of age and had an appointment with their primary care provider in the next 3 weeks. After conducting a baseline interview with all interested patients, they randomly assigned patients to receive an educational video through the mail or to usual care (i.e., no video). The 15-minute video included information to encourage discussion with their provider about CRC screening and increase the use of screening, particularly by FS. The mailed package with the video also included a letter encouraging the patient to view the video. After the video was mailed, the patients were interviewed by telephone between 4 and 6 months after the primary care appointment to determine whether they had obtained screening. This study reported no difference in screening rates among the intervention and control group participants (55 percent for both groups).
Another study involved rural churches in North Carolina with high proportions of African-American members.185 The study aims included improving nutrition and physical activity, but those participating who were age 50 years or older were also encouraged to obtain CRC screening. The intervention included two components: a tailored communication to select church members randomly and another that involved group education provided by a lay health advisor. We categorized this study into both small media and group education interventions.
For the small media component of the intervention, individual computerized materials were developed based on information obtained through a baseline survey administered to all participants. The tailored package of information included newsletters along with four targeted videotapes mailed to participants’ homes. These packages were mailed bi-monthly during months 2, 4, and 6 of the intervention; the fourth mailing occurred during month 9. The videotapes included testimonials from community members and pastors on each of the targeted behaviors of the intervention. Participants in this group were compared with those attending churches in the control group, each of which were offered health education sessions and speakers on topics of their choice not directly related to the study objectives. The second component of group education by lay health educators is described under that intervention type. Members of control churches were no more likely to obtain CRC screening than those who received tailored education (P = 0.08).
A fair-quality study used telephone counseling to attempt to increase CRC screening; the investigators initiated contact with patients by first mailing a baseline survey to potential participants who were active patients of primary care practices (i.e., documented visit in the prior 2 years).176 Upon receiving the baseline survey from patients, the investigators randomized respondents to the control group to receive usual care or to the intervention group. For the intervention group, the researchers mailed a print brochure 2 months after receipt of the baseline survey; it provided basic CRC information and screening. Three months after receiving their brochure, participants received tailored computer-assisted telephone counseling; for this, a computer generated an interview protocol based on patients’ initial responses about their knowledge of CRC and screening tests. Trained interviewers administered the protocol to provide basic education (approximately 4 minutes) and motivational counseling (approximately 6 minutes) to obtain screening. Approximately 17 to 24 months after receipt of the telephone counseling, the investigators reviewed participants’ charts to determine whether CRC tests had been completed. This study found no difference in overall screening rates between the intervention and control groups (25 percent versus 24 percent; P = 0.68).
Although the third study, described previously under patient reminders, found that groups that received some form of reminder were more likely to complete screening than those who received usual care (P = 0.001 or 0.002), the researchers did not find differences among the intervention groups to indicate whether patients who receive various types of small media interventions (i.e., print or telephone) were more (or less) likely to complete CRC screening.
Small media: decision aids. Study characteristics. As shown in Table 37, three RCTs, one rated as good quality178 and the other two as fair,177,181 used decision aids to help patients make informed decisions about CRC testing and the type of test to request. All three studies focused on patients 50 years of age or older; they either were attending appointments at an internal medicine practice181 or a primary care practice177 or were selected through a random sample of local residents living in urban, suburban, or rural communities.178
Table 37
Studies of small media: Decision aids on increasing colorectal cancer screening rates.
One primary care study compared an intervention group viewing an 11-minute CRC screening video decision aid followed by a brochure for the patient and a colored chart marker for the physician with a control group viewing an automobile safety video with no colored chart marker placed in the record.177 The other primary care study randomized participants to either an interviewer-administered printed decision aid (modeled on the analytic hierarchy process) or to printed CRC screening educational materials.181 The third study, which included only participants familiar with computers, compared a computerized, interactive decision aid with a standard informational, noninteractive website concerning CRC.178
All three studies had control groups comprising patients who got some type of exposure to a CRC-related website,178 to an unrelated topic,177 or to basic information about CRC.181 Two of the three studies had comparison or control groups that received some form of CRC education.178,181 Time to followup among the studies ranged from 2 to 3 months177,181 to 24 weeks.178 Two studies assessed the outcome of completed screening of any test through medical chart review;177,181 the third used follow-up telephone interviews.178
Overview of results. Results from the three decision aids studies are mixed. One study demonstrated a statistically significant increase in CRC test completion178 (23 percent difference; OR, 3.23; 95% CI, 2.73–3.50; P = 0.035). Another showed an increase in completion of CRC testing in the intervention group compared with the control group (14.2 percentage point difference; 95% CI, 3.0–25.4 percentage points).177 This same study demonstrated that a higher proportion of patients in the intervention group than the control group reported discussing CRC screening with their provider during their appointment (68.5 percent and 43.4 percent, respectively; 25.1 percentage point difference; 95% CI, 12.7–37.6 percentage points). By contrast, the third study reported no significant difference in CRC test completion between the intervention and control groups.181
Detailed results. The good-quality study obtained a random sample of residents 50 years of age or older who live in urban, suburban, or rural communities in Michigan.178 The investigators first contacted potential participants by telephone and screened for their computer knowledge to ensure that participants could adequately search the websites in the study and meet other eligibility criteria. Participants were then scheduled for an appointment at a local community site for review of the websites. Participants were randomly assigned to view and explore one of two sites: (1) an interactive password-protected website, Colorectal Web (http://colorectalweb.org), which was designed to aid in their decision of types of CRC tests to obtain or (2) a standard, noninteractive informational website (control group) with similar content as the intervention website. During the computer sessions, participants were asked to review as much of the website as they desired. At the end of the session, participants completed a questionnaire specific to their preference for testing and decision phase of choosing to get screening. All participants were interviewed by telephone 2, 8, and 24 weeks after review of the websites to determine their intention to get screened and whether they had received any CRC test. Participants in the intervention group were more likely to have completed any CRC test within 24 weeks (56 percent) than the control group (33 percent) (AOR, 3.23; 95% CI, 2.73–3.50; P = 0.035).
The remaining two studies, rated fair quality, also tested different types of small media in aiding patient decisionmaking.177,181 One study provided written materials to patients assigned to the intervention arm who were at an appointment at an internal medicine practice in New York.181 Within a few days before a scheduled appointment with a provider, the consenting patients in the intervention group received short descriptions of CRC and the five types of screening tests available to them and completed a baseline survey. Trained interviewers also guided this group through an analytic hierarchy process specifically designed to help them make decisions that require integration of quantitative data with less tangible, qualitative considerations such as values and preferences. The control group was first interviewed face-to‐face at the time of their appointment where the interviewer provided them with a brief description of CRC and asked them to complete the same survey as the intervention group. All patients were then urged to discuss CRC screening with their provider. After their visit, all patients were asked whether they discussed the screening with their provider and whether a decision had been made. A majority of all patients (88 total or 93 percent) indicated that they had discussed CRC screening with their provider, but the intervention group was no more likely than the control group to have completed any CRC test.
The study testing whether a video decision aid given to patients at the time of a primary care appointment in North Carolina would increase screening rates reported similar findings.177 Three primary care practices with a total of nine physicians agreed to participate in the study. For study recruitment, patients were contacted by phone before a scheduled appointment and asked to participate. The intervention group for this study was asked to watch an 11-minute video on CRC that included information about susceptibility to CRC and availability of screening tests, specifically the FOBT and FS. The video included vignettes of patients who discussed their experiences with CRC screening. At the conclusion of the video, the patients were asked about their intent to request screening and then provided one of three color-coded brochures that were designed to provide information based on a person’s intention to obtain screening. The researchers placed a laminated card with the same color as a patient’s brochure in the patient’s chart before he or she was seen by the provider. Patients in the controls watched a video of similar length on car safety and received a related brochure. No cards were attached to their charts. During the appointment, patients were asked to complete three surveys: one at baseline before seeing a video; one after viewing the video; and one after seeing the provider to assess whether a conversation about CRC occurred. The investigators completed medical record reviews within 3 months of the visits to determine whether CRC tests had been completed. The outcome reported related to discussions was whether a test was ordered. In the intervention group, 68.5 percent of patients and 43.4 percent of control group patients reported some conversation with their provider about CRC screening (25.1 percentage point difference; 95% CI, 12.7–37.6 percentage points). Screening tests were completed by 36.8 percent in the intervention group and 22.6 percent of the control group (14.2 percentage point difference; 95% CI, 3.0–25.4 percentage points).
Group education interventions. Study characteristics. Two RCTs tested an intervention to educate Native Hawaiians who are members in local civic clubs about the importance of CRC screening184 or were trained to educate their fellow church members in rural predominantly African-American churches185 (Table 38). In one study, civic clubs were randomly selected for the intervention and either a Native Hawaiian physician and cancer survivor or a non-Native
Table 38
Studies of group education on increasing CRC screening rates.
Hawaiian nurse provided an education session on the need for screening and provided participants with FOBT kits.184 Any participants ages 50 or older were included in the intervention. The study followed participants over a 16-week period and used reports from the laboratory that received completed FOBTs to determine whether they had completed a FOBT kit. The second study targeted African-American churches in rural North Carolina with the aim of improving nutrition, exercise, and CRC screening.185 Any church members within an intervention site could participate, but only those 50 years or older were targeted for CRC screening. The group education consisted of training volunteers to serve as lay health advisors, who then agreed to conduct group education sessions within their church over the 1-year study period. Outcomes for both studies were assessed through self-reported screening rates, with both focusing on FOBT completion rates as their primary outcome.
Overview of results. One study demonstrated a negative finding: those in the control group were statistically significant more likely to have completed a FOBT over the 16-week study period than those in the intervention group (AOR, 0.36; 95% CI, 0.14–0.97).184 The second study found no difference between those who received group education and those in control churches (P = 0.08).185
Detailed results. The study based in Hawaii involved members of local civic clubs who were provided with an educational session specific to CRC screening at one of their regularly scheduled meetings.184 The control group received the education from a non-Native nurse who addressed topics specific to CRC screening and the importance of screening among Native Hawaiians. She then distributed a FOBT kit along with basic instructions on completing the test, and a phone number of local providers they could contact for assistance. Within a month of the presentation, if a completed FOBT kit had not been received from participants, one reminder call was made to each and a replacement FOBT kit was mailed upon request. The intervention group differed in that the presenter at the workshop included a physician and survivor who were both Native Hawaiian. Participants in this group were also provided a FOBT and a demonstration on how to complete the test was also presented by the physician. Between 4 and 16 weeks after the presentation, multiple telephone calls were made to those who had not completed the FOBT kit and replacement kits were provided upon request. Information on the frequency and intensity of these reminder calls is not provided by the authors so this study is not categorized as one providing patient reminders since we were unable to determine the extent to which the control and intervention groups differed on this aspect of the intervention. The outcomes for the study were determined through copies of the FOBT results received from the laboratory that tested them. Overall, the authors reported that people in the intervention group were less likely to complete a FOBT than people in the control group (33 percent compared to 40 percent, respectively).
The study based in rural churches in North Carolina included two components of interventions, one that involved small media which is described elsewhere and a second that included training church members to serve as lay health advisors and conduct group education sessions with their peers.185 Church members were asked to recommend people to serve as lay health advisors, who were then invited to attend a series of trainings. A total of 62 such advisors (47 women, 15 men) from six churches were trained through six sessions. The training included information specific to CRC screening, available tests, and a detailed training manual was provided to each participant. In addition to providing information to peers through existing social networks, the lay health advisors were expected to organize and conduct at least three church-wide activities focused on spreading information about nutrition, exercise, and/or CRC screening. Findings indicated that churches where these advisors were present were no more likely to have members who received FOBT or any CRC test than control churches. In addition, some churches included both tailored or small media education combined with lay advisors, but this combination produced no effect compared with a control group.
One-on-one interactions. Study characteristics. Three RCTs, two rated as good quality179–180 and the other as fair quality,85 tested one-on-one interactions with patients as a way to increase screening rates (Table 39). Interactions involved a nurse who conducted85 a series of telephone calls to participants of a health plan,180 and a health educator.179 Two studies were conducted within a primary care or community clinic setting and relied on medical chart review for screening outcomes;85,179 the third worked with a random sample of participants in a health benefit fund.180 Two studies included patients who had not yet agreed to screening;179–180 the other involved patients who had agreed to FOBT screening.85 In two studies populations included those 50 years of age or older determined to be in need of screening based on national guidelines;85,179 the third study focused on those 52 years of age and older who were self-reported as not current on their CRC screening (i.e., no FOBT in past 2 years, no FS in past 5 years, or no colonoscopy or barium enema in past 10 years).180 All three studies were in urban settings; one had about two-thirds African-American participants,180 another had about one-third African-American participants,85 and the third comprised almost entirely Chinese participants.179 The time periods of each study varied: the one involving nurses85 and the one targeting the health benefit fund followed patients for 6 months, 180 and the one with a culturally and linguistically sensitive health educator spanned a 14-month period from the initial interaction with patients.179 The two studies based in a clinic included control groups that received usual care,85,179 while the control group for the study of health benefit fund participants received print materials in the mail, which included a brochure about CRC and available screening tests.180 All three studies relied on medical records review for measuring the outcome of completion of CRC screening, with one study first collecting self-reported data that was then compared with claims data in the health benefit fund database.180
Table 39
Studies of one-on-one interactions on increasing colorectal cancer screening rates.
Overview of results. All studies found statistically significant positive effects of their interventions. In the study of patients who had agreed to FOBT screening, 65.9 percent of intervention patients and 51.3 percent of the usual care group (P < 0.001) returned the FOBT cards; the median time to return the cards was shorter in the intervention group (36 versus 143 days, P < 0.001).85 In the study of Chinese patients considering CRC screening, 69.5 percent of intervention versus 27.6 percent of control patients had completed FOBT screening (AOR, 6.38; 95% CI, 3.44–11.85).179 The third study, which provided intensive telephone counseling to participants of a health benefit fund, also demonstrated statistically significant differences in completion of any CRC test (rate difference = 20.9 percentage points; 95% CI, 14.34–27.46 percentage points).180
Detailed results. The good-quality study of telephone outreach compared these participants to a group of patients who received only a mailed brochure with information about CRC and available screening tests.180 The sampling frame for the study included persons 52 years of age or older who were members of a health benefit fund that included CRC screening as a benefit. Potential participants were first contacted by telephone to assess their interest in the study and then randomly assigned to receive telephone education or print education. The control group was mailed a letter along with a print brochure that included information about CRC, how it can be prevented, and descriptions of screening tests. The participants were instructed to talk with their providers to seek screening. The intervention group received tailored telephone outreach that began within 2 weeks of randomization. A series of semistructured telephone calls were then conducted with the participant to discuss CRC screening and provide positive reinforcement for obtaining a screening test. The frequency and duration of calls varied, with a median of 5 calls to each participant and a median of 23.5 minutes with each participant. The topics of these calls included establishing a trusting rapport with participants, reinforcing accurate knowledge about CRC and screening, correcting misconceptions, and bolstering motivation to obtain CRC screening. All participants were contacted 6 months after randomization by telephone to obtain information about whether they had obtained any CRC screening test (i.e., single office FOBT, home FOBT, FS, or colonoscopy). This self-reported information was verified either through medical records from each participant’s provider or through the health benefit fund’s billing system. Patients who received tailored outreach were more likely to be screened than those that received only the mailed brochure (27 percent and 6.1 percent, respectively; RR, 4.4; 95% CI, 2.6–7.7).
The study that explored the role of nurses in encouraging completion of FOBT provided patients in the intervention arm with 10-to 15-minute educational sessions conducted by a nurse specifically trained for this intervention.85 All patients had been referred by their physician to primary care nursing for education and distribution of FOBT kits. The intensive session included providing patients with a two-page informational handout on FOBT and CRC. The session also provided verbal instructions on how to perform an FOBT and explanation of the meaning of different results. The nurse answered questions and instructed the patients to return the FOBT cards within 2 weeks and/or call with any questions. Patients randomly assigned to the control group received usual care, which consisted of receipt of FOBT kit that included written instructions and no individual session with a nurse. The outcomes for the study were assessed through medical record review 6 months after the patient’s appointment to determine whether the patients had returned FOBT cards. The intervention group was more likely to return FOBT cards than the control group (65.9 percent and 51.3 percent, respectively, P < 0.001).
In another study, predominately Chinese patients who had not yet agreed to screening attended an intensive education session with health educators who provided culturally and linguistically appropriate (78 percent of participants spoke Cantonese and 21 percent spoke Mandarin) education about CRC screening, including a motivational video, printed material, and FOBT kit.179 Patients were randomly selected for participation through the electronic medical database and mailed bilingual letters signed by the medical director of the two participating clinics to invite them to participate. The health educator then tracked appointments through the clinic electronic scheduling system and met face-to-face with prospective participants during their visit. Patients who agreed to participate either received usual care (no CRC information) or were asked to meet with the health educator who distributed the educational materials. Those patients assigned to the intervention group were able to watch the video at the clinic office or take it home. The control group received usual care but the study did not specify whether usual care included provision of an FOBT kit with bilingual instructions. The investigators assessed return of FOBT cards within 6 months of randomization through electronic medical records; this outcome was increased in the intervention group when compared with the control group (69.5 percent and 27.6 percent, respectively; OR, 6.38; 95% CI, 3.44–11.85).
Eliminating structural barriers. Study characteristics. This category includes five studies (Table 40); two were described under patient reminders;85,175,179,183 two under one-on-one interactions;85,175,179,183 and is described here in detail.187 All provided FOBT kits as a means to improve access to screening tests. One study also attempted to address cultural and linguistic barriers among an Asian population of patients.179 We rated two studies as good quality179,187 and others as fair.85,175,183 All five studies included people 50 years of age or older in their samples; one study specified an upper range of 79 years for study participants.187
Table 40
Studies of interventions to eliminate structural barriers on increasing colorectal cancer screening rates.
Four of these studies took place in primary care settings or clinics.85,175,179,187 All four used control groups that received usual care, and all assessed their outcomes through medical record review. The timing of followup of these four studies in this category was 6 months,85 9 months,187 14 months,179 and 24 months.175 One good-quality study provided primarily Chinese-Americans patients from a primary care clinic with access to a health educator and culturally and linguistically appropriate education materials, including bilingual instructions for FOBT.179 Another good-quality study provided patients obtaining annual flu shots in a family health clinic with a FOBT kit.187 One fair-quality study used a nurse to provide intensive counseling to patients who had agreed to FOBT.85 The final study divided patients into three groups that received varying levels of tailored materials to encourage screening.175
The fifth study provided a random sample of residents in an urban area with a letter that included a questionnaire about CRC and screening tests.183 These investigators then gave FOBT kits to two intervention groups with through the mail; one group received reminders to complete the FOBT and the other received no reminders. The investigators assessed screening rates through self-reported information obtained on a follow-up survey mailed to all participants 1 year after the start of the study.
Overview of results. In all five studies completion of screening by FOBT rose as a result of the interventions (14.6 to 41.9 percentage point increases in screening by FOBT). The study that used a culturally and linguistic appropriate intervention demonstrated the largest increase in screening among the studies in this section (41.9 percent). Findings from four studies demonstrated that interventions to eliminate barriers were effective in increasing CRC screening by FOBT;85,179,183,187 the fifth did not present findings specific to FOBT completion but rather demonstrated an increase in overall CRC screening rates.175
Detailed results. Four of the five studies in this category were described above. One good-quality study exposed patients of Asian origin to a culturally and linguistically sensitive educator.179 Predominately Chinese patients who had not yet agreed to screening attended an intensive education session with health educators who provided culturally and linguistically appropriate education about CRC screening, along with a FOBT kit. The difference in return rates of FOBT cards between the intervention and control groups was statistically significant (AOR, 6.38; 95% CI, 3.44–11.85).
The second study compared groups getting an FOBT both with and without telephone reminders with a control group.183 FOBT-specific findings demonstrated a statistically significant difference between the control group (1.5 percent; 95% CI, −2.9–5.9 percent) and both intervention groups (respectively 23.2 percent [95% CI, 17.2–29.3 percent] and 16.9 percent [95% CI, 11.5–22.3 percent]), for an overall difference in FOBT completion rates as high as 21.7 percentage points.
The third study compared groups getting three different types of interventions of varying intensity with a usual-care control group.175 All three intervention groups received FOBT kits. The control group had a 33 percent completion rate of any CRC screening test; the three intervention groups had the completion between 44 percent and 48 percent (G1: AOR, 1.7; [95% CI, 1.2–2.5]; G2: AOR, 1.6 [95% CI, 1.2–2.1]; G3: AOR, 1.9 [95% CI, 1.4–2.6]) (P-values were not reported).
The nurse-based study provided patients in the intervention arm with 10- to 15-minute educational sessions conducted by a nurse specifically trained for this intervention.85 The percentage of individuals returning FOBT cards was higher in the intervention group than the control group (65.9 percent versus 51.3 percent; P < 0.001).
The fifth study was conducted in a family health center in San Francisco, California.187 Patients of the clinic (ages 50–79) were mailed multilingual flu shot campaign information and were given dates for obtaining flu shots. Half of the days were randomly selected in blocks of 2 or 3 for provision of flu shots only (control group) or flu shots with FOBT kits (intervention group). Before each flu shot clinic, investigators gave clinic staff a list of patients with appointments who were eligible for a FOBT. Patients were given a handout at the clinic to explain the need for regular CRC testing and then a FOBT kit after their flu shot (along with instructions in several languages). Patients were telephoned if they had not returned a completed kit at 3 weeks and again (if needed) at 6 weeks. FOBT screening rates in the control group increased by 4.4 percentage points from 52.9 percent at baseline to 57.3 percent (P = 0.07) they rose in the intervention group by 29.8 percentage points from 54.5 percent to 84.3 percent (P < 0.001); this yielded a 25.4 percentage point difference between groups (P < 0.001).
Provider-level interventions. Study characteristics. Two RCTs, both rated good quality, addressed reminder interventions targeted at provider behaviors or practices (Table 41).186,188 In one case providers were reminded during an office visit that a patient was overdue for CRC screening;186 in the other, reminder cards informed primary care physicians when a patient scheduled for an appointment (identified through medical record review) might need CRC followup.188 The outcome of obtaining CRC screening or adherence to repeat colonoscopy was assessed through electronic medical record review within 6 months of mailing the initial letter to physicians for one study or 15 months after the study was initiated.186 Both studies compared patients whose physicians received specific reminders with those who received usual care.
Table 41
Study of an intervention to target provider behavior for increasing colorectal cancer screening or followup rates.
Overview of results. One study demonstrated only minimal increase in CRC screening among patients with providers who received reminders compared with those who did not (41.9 percent versus 40.2 percent; P = 0.47).186 The other study reported a small increase in completion of colonoscopy within 6 months among patients whose physicians received the reminders (9.2 percent versus 4.5 percent; P = 0.009).188
Detailed results. One study focused on patients who may need surveillance colonoscopy and had received a prior colonoscopy with one or more adenomas detected but did not have a subsequent colonoscopy within 5 years.188 The researchers sent physicians (n = 141) in two networks letters via interoffice mail to notify them of the potential need of a surveillance colonoscopy for the patients randomized to the intervention arm (n = 358). The investigators did not report the number of physicians who mailed letters to the patients in the intervention arm; 6 months after the letters were initially sent to the physician, the researchers reviewed medical records to determine whether colonoscopies had been completed. At the same time, they also sent letters to physicians of patients in the control group to ensure that physicians were aware of the potential need for colonoscopy if clinically appropriate. Completion of colonoscopy was higher among patients whose physicians received reminders related to surveillance than among those in the control group (9.2 percent versus 4.5 percent; P = 0.009). The authors did not report whether the letter to the physicians, the follow-up letter to patients if it were mailed, or a combination of both was the factor that actually raised surveillance rates.
The second provider-level patient reminder study involved 11 ambulatory health care centers in Massachusetts and targeted patients overdue for CRC screening.186 The investigators paired physicians with similar patterns of screening rates and referrals and then randomized one to receive the intervention. Throughout the 15-month study period, physicians in the centers received electronic reminders during office visits with patients overdue for screening. Before the intervention, the investigators educated physicians in both the intervention and control group on the use of the reminder system. Physicians could view the passive alert at any point during an office visit; those who received active alerts were required to acknowledge it before making any electronic orders. These active alerts provided current information about prior CRC screening for the patients and provided a “1 click” option for ordering tests. Screening rates were similar among patients of physicians receiving the electronic reminders compared to the control group (41.9 percent versus 40.2 percent; P = 0.47).
System-level interventions. Study characteristics. Five RCTs, all rated fair quality, were classified as a system-level intervention because they explored the impact of various interventions that had been implemented within an office or health care setting with the direct aim of changing the system of care (Table 42). Three studies used a patient navigator to guide the process of obtaining a screening colonoscopy189 or a Prevention Care Manager (PCM), similar to a patient navigator) to assist patients in addressing barriers to obtain any CRC screening;162,193 two studies enhanced their systems of managing patients as they obtained other types of care.190–192 All studies focused on patients 50 years of age or older; one limited the age range of patients to those no older than 79 years.192 Three included only women.162,190–191,193 All included patients of health clinics or primary care practices. One study compared women in their intervention group to women who received an intervention to increase mammography use;162 the remaining four studies used patients receiving usual care as their control groups. Usual care included patients who were in the clinic for an office visit and did not receive exposure to the system level intervention. All but one study192 specifically included patients from low-income areas to increase CRC screening rates among populations with generally low rates. The outcome of interest in one study was whether a patient completed FOBT within the 6-month follow-up period and/or got endoscopic screening if they met national guidelines for these tests;189 another focused on whether patients received an endoscopic screening procedure during the 1-year study.192 The remaining studies assessed whether patients obtained any CRC test during the study period with the time for followup ranging from 11 months162 to 24 months.191 All outcomes were assessed through medical chart review.
Table 42
Studies of interventions to target the system level for increasing colorectal cancer screening rates.
Overview of results. All five studies found statistically significant increases in CRC screening rates for their tests of interest; absolute increases in screening ranged from about 5 percentage points to 28.2 percentage points. The screening colonoscopy study found a statistically significant increase in completed endoscopy at 6 months (23.7 percent versus 5.0 percent; P = 0.019).189 The two studies that included a PCM providing assistance in addressing barriers to screening demonstrated similar findings: women in a Medicaid managed care organization had a 14 percent increase in screening rates when the PCM worked with them compared with a 9 percent increase in the control group (P = 0.04);162 among women in community and migrant health care clinics in New York City receiving the intervention had a 13 percentage point difference in screening rates compared with the control group (AOR, 0.13; 95% CI, 0.07–0.19).193 In the study of primary care practice patients who received enhanced office and patient management practices at randomly selected practices, the investigators reported a 1.63‐fold increase in CRC screening among patients in the intervention clinics compared with those in control clinics (95% CI, 1.11–2.41; P = 0.01).192 In the study that randomized patients at the clinic level to complete cancer screening checklists placed in their medical charts at the time of an office visit, along with a sticker flagging the provider for the need for screening, demonstrated that patients in the intervention group were 2.56 times more likely to obtain an FOBT at the 12‐month followup than the control group (AOR, 2.56; 95% CI, 1.65–4.01; P < 0.0001),190 but this effect was diminished at 24 months (AOR, 1.17; 95% CI, 0.92–1.48; P = 0.19).191
Detailed results. The studies using PCM staff to help patients obtain CRC screening were each conducted in New York City clinics: one in a Medicaid managed care organization (MMCO)162 and the other in 11 community and migrant health care clinics.193 Neither study provided racial or ethnic statistics of the women in their samples. The MMCO study involved women who were receiving an intervention of patient reminders to obtain mammography screening; it randomly assigned women to receive PCM assistance in obtaining CRC screening. The PCM assistance included a detailed script read to patients to explain the importance of CRC screening and types of available tests and assistance overcoming any barriers to screening, including making appointments for patients to receive tests.162 Those in the comparison group received educational materials about CRC screening and one telephone call to recommend that they obtain CRC screening. At the 11-month followup, those in the group receiving PCM assistance were more likely than the women in the comparison group to be up to date with any CRC screening (P = 0.04). The other study (by many of the same authors) applied a similar intervention to a different setting.193 In this study, PCMs received 7 hours of training for their role, worked with patients to overcome barriers, and provided motivational counseling during the study. For two of 11 centers, the PCM could also mail FOBT kits to patients; differences related to this aspect of the intervention were not reported. Those in the comparison group received usual care, which in the participating clinics included a single call to patients to answer any questions about CRC screening and advise them about the need to be screened. Those receiving PCM assistance were more likely than the control group to obtain CRC testing (0.13 difference in screening rates; 95% CI, 0.07–0.19).
For the study in which a patient navigator helped patients obtain screening colonoscopy, patients eligible for the study had been referred for CRC screening after an appointment with a primary care provider.189 Patients were “navigated” in an effort to improve compliance with referrals to screening colonoscopy. Patients, from a federally qualified health center in New York City serving predominantly minority and low-income patients, were 50 years of age or older and eligible for CRC screening. More than 70 percent were female, about 80 percent were Hispanic, and less than half of the participants spoke English. Those patients randomly assigned to the intervention received patient navigation; those assigned to the control group received usual care, which included placement of an FOBT card in the patient’s chart to remind his/her physician of the need for screening. The patient navigator contacted patients in the intervention group 2 to 3 weeks after the patient agreed to participate and provided education about CRC screening by telephone. The patient navigator continued to provide written reminders, further telephone calls, and scheduling assistance to the intervention group. Using completion of an endoscopic examination as a key outcome, the authors reported that patient navigation improved completion of these tests within 6 months of physician recommendation (15.8 percent compliance in the navigated group versus 5 percent in the nonnavigated group; P = 0.019).189
Another study focused on several aspects of providing enhanced office and patient management among 10 primary care practices in Pennsylvania to increase endoscopy screening.192 All patients determined to be eligible for the study were mailed letters from their physicians recommending endoscopic CRC screening and asked patients to phone for an appointment. These letters were either tailored or nontailored; findings specific to this aspect of the intervention were discussed earlier in the “small media” category. Patients in the control group had office visits in practices that had received educational workshops for their physicians and office staff on improving CRC screening and written protocols on systematically implementing screening. Clinics randomly assigned to the intervention received this information and assistance in implementing the office protocols and tracking patient acceptance of referral for endoscopic screening. The research team then conducted motivational interviewing with patients who had not obtained screening within 3 months after receiving a physician recommendation (by mail) to do so. During these interviews, staff worked with the patients to address any barriers to obtaining screening (e.g., scheduling appointments, obtaining transportation, addressing insurance needs). Medical records were reviewed within a year after the initial letter was mailed to assess screening rates. The study demonstrated a 1.63-fold increased odds of completing a colonoscopy or FS among patients in the intervention (95% CI, 1.11–2.41; P = 0.01).192
The results of the final study in this category were published in two articles; one presented 12-month findings190 and the 24-month results.191 In this study, the investigators randomized eight county-funded clinics in Florida as control or intervention sites. Patients receiving care at the control sites received usual care specific to CRC screening. Those receiving care at the intervention sites were asked to complete a cancer screening checklist at the time of an office visit. The checklist indicated the tests they had previously received and when each was obtained. Based on these responses, medical charts were flagged with stickers to indicate to the provider whether a patient was due for CRC screening. Before implementing this process, the research team also trained staff about the need for CRC screening. Throughout the 12-month study, the team also made unannounced visits to the clinics to conduct chart audits and then gave formal feedback to staff of their screening rates. They also abstracted data from medical records of independent random samples of patients at baseline and at each of the two follow-up periods to determine the extent to which patients had obtained CRC screening. At 12 months, the study demonstrated a 28.2 percentage point increase among patients receiving care at the intervention sites compared to the control sites (40.1 percent versus 11.9 percent; P< 0.0001).190 At 24 months, the difference in screening rates across sites was smaller (28.2 percent versus 12.6 percent; P = 0.19).190
KQ 4: Current and Projected Capacity to Deliver Colorectal Cancer Screening and Followup
If efforts to increase screening rates for CRC are successfully implemented, providers and health care systems must be able to handle the resultant increased demand for services, particularly for endoscopic procedures, that will be needed both for primary screening and for follow-up of abnormal screening results from noninvasive screening strategies. Note that to avoid confusion over the use of the word ‘surveillance’, we refer to monitoring of patients after receipt of abnormal results as ‘followup’. As shown in the analytic framework (Figure 1, Chapter 2), capacity to deliver CRC screening is an important variable in determining the population-level benefit from screening. This key question (KQ) addresses the current and projected capacity of the health care system to deliver CRC screening and followup for the US population.
In this section we have defined key terms as follows:
- Current capacity (or current potential volume): the sum of current volume and additional available capacity, where:
- Current volume is the estimate of the current number of FS or colonoscopy procedures conducted in the present year; and
- Additional available capacity is the number of additional FS or colonoscopy procedures that could be conducted in the current year;
- Projected capacity: future capacity to conduct FS or colonoscopy under various scenarios such as changes in workforce or changes in the number of facilities that provide procedures;
- Ability to meet projected demand: the ability of current capacity (or projected capacity if known) to meet the projected demand under various demand scenarios, such as screening the entire eligible US population with a specific test.
Although this KQ gave priority to projected capacity of FS or colonoscopy, we found no studies that examined this topic. Most common were studies that provided estimates of current volume of FS or colonoscopy and compared those estimates with a projected demand. In this section, not only do we compile the varying estimates of current capacity and projected demand across studies and evaluate the strength of evidence of these estimates, but we also compare the estimate of current capacity based on multiple studies with that of projected demand, based on multiple studies. This approach enables us to answer better than heretofore the question of the nation’s ability to meet projected demand.
In addition to the concepts defined above, we found data on current volume by provider type and geographic variation in current volume and additional available capacity.61,195–197 Because these measures are related to our outcomes of current volume and additional available capacity, we have completed summary tables and text for these and included them as Appendix G. We also found four studies that report on current volume and additional available capacity in individual states.61,195–196,198 Because results from these studies did not change our conclusions from the national data, we have included them as part of Appendix G rather than in the main text.
We present our overall summary and strength of evidence tables for studies addressing this KQ at the beginning of this section. The remainder of this section provides a more detailed assessment of the individual studies that informed our conclusions and our assessment of the strength of evidence.
KQ 4 Overall Summary and Strength of Evidence
In Table 43, our overall grades of the strength of evidence appear in the far right column; grades for key domains to determine the strength of evidence (risk of bias, consistency, directness, and precision) are in the intermediate columns. In assessing research specific to KQ 4 about capacity for increasing CRC screening, we ultimately had grades of only low strength of evidence. Low means that we have only low confidence that the evidence reflects the true effect; further research is likely to change the confidence in the estimate of effect and is likely to change the estimate.31
Table 43
Strength of evidence for the current and projected capacity to deliver CRC screening.
Overall, evidence suggests that FS current volume is not sufficient to meet projected demand if a significant proportion of the population is screened by either FS or FOBT/FS. Current volume of colonoscopy is likely to be sufficient to meet projected demand if a significant proportion of the US population is screened by FOBT or FS but not by colonoscopy. Based on one study’s estimates of additional available capacity, current capacity for FS is sufficient for a screening program by FOBT/FS or FS alone, and current capacity for colonoscopy may be sufficient for a screening program by colonoscopy alone. All these estimates represent steady-state scenarios.
If the US were to adopt a colonoscopy-only approach to CRC screening, either colonoscopy capacity would need to be substantially increased or at least 5 years would be required to do the “catch-up” screening required to screen people who have not been screened.
Overall capacity study characteristics. We found six studies (seven articles) of good or fair quality that reported national estimates of current capacity (current volume and/or available capacity), projected demand, and ability of current capacity to meet projected demand.195–196,199–203 We rated one additional study as poor quality and did not include it because it did not incorporate increased demand for surveillance colonoscopy following use of FOBT in its demand estimates.204
All six included studies reported on current volume or additional available capacity for one or more of the following screening procedures: FS, colonoscopy, or CT colonography. No study reported on these outcomes for FOBT, although FOBT screening is included in the various demand scenarios that are examined. Five of the studies (five articles) included estimates of both current and projected demand.195,199–200,202–203 One article reported only estimates of current capacity (current volume as well as additional available capacity).196 One study modeled only projected demand under different demand scenarios.201 Among the five studies that reported on both capacity and demand, a single study can have different quality ratings for these two separate parts of the study.
Of the five studies (six articles) that report on current capacity, two studies obtained the data through national surveys, either of endoscopic facilities196,202 or of endoscopic providers195; both sets of respondents reported on the number of FSs or colonoscopies they perform per week or month. These studies both reported on volume of both FS and colonoscopy, and one of the two reported on additional available capacity of FS and colonoscopy as well. Two studies,199–200 which reported only on current volume of colonoscopy, conducted secondary analyses of a database from the Clinical Outcomes Research Initiative (CORI), a voluntary consortium of 400 endoscopists at 42 sites in 22 states. The final study, which reported on current volume of CT colonography, used secondary data on CT scanners in the United States for its estimates.203
Six studies that reported projected demand used a variety of mathematical models to do so. For their modeling, investigators used various refinements of population estimates, e.g., population growth, percentage of population that are at high risk because of family history or inflammatory bowel disease, and the percentage of the population ineligible for screening because of comorbid conditions. Also, a critical assumption in the modeling of demand that varied across these studies was the percentage of persons participating in screening overall; this figure ranged from 40 percent to 100 percent. A subset of key assumptions for each study is noted in the tables.
Overall capacity study results. Table 44 provides an overview of the results for KQ 4. In each row are the types of procedures for which the outcomes of capacity and demand were available (total FS, total colonoscopy, and screening colonoscopy); the columns contain the outcomes of current capacity (current volume and additional available capacity) and projected demand under various demand scenarios. For each demand scenario, we also present an assessment of whether current capacity is able to meet projected demand.
Table 44
Overview of results of capacity studies.
Studies varied in their estimates of current volume of FS procedures (2.8 million to 4.9 million) and screening colonoscopy procedures (1.6 million to 6.6 million) (Table 44). A single study provided estimates of additional available capacity of 6.7 million FSs and 8.2 million colonoscopies.
Results of the modeling studies suggest that current volume of FS is not sufficient to meet projected demand if a significant proportion of the population is screened by FS or FOBT/FS. Current volume of colonoscopy is likely to be sufficient to meet projected demand if a significant proportion (70 percent to 75 percent) of the US population is screened by FOBT or FS but not by colonoscopy. Only one estimate of additional available capacity is available; based on this study’s results, current capacity for FS is sufficient for a screening program by FOBT/FS or FS alone. Based on this study’s estimates of additional available capacity for colonoscopy, current capacity for colonoscopy may be sufficient for a screening program by colonoscopy alone.
All these estimates represent steady-state scenarios. None of these models incorporated current estimates of the unscreened. In the single study that modeled available capacity to screen the unscreened population, using 100 percent of additional available capacity, it would take 3 years at current screening patterns, 6 years using 100 percent FS or FOBT/FS, or 5 years using 100 percent colonoscopy to screen the unscreened population.
National-Level Estimates of Current Capacity of Endoscopy Screening
This section consists of two parts. We first describe the studies that present data on current volume of endoscopy screening. We next describe studies of additional available capacity of endoscopy.
Current volume of endoscopy. Study characteristics. Four studies provided national-level estimates of current volume of endoscopy; all four provided estimates for colonoscopy195–196,199–200 and two also did so for FS (Table 45).195–196 Two studies reporting estimates for both FS and colonoscopy195–196 obtained the data through national surveys, either of endoscopic facilities196 or of endoscopic providers.195 In these studies the facility or provider reported the number of colonoscopies they perform per week or month. The remaining two studies,199–200 which reported only on current volume of colonoscopy, report analyses of a database from the Clinical Outcomes Research Initiative (CORI), a voluntary consortium of 400 endoscopists at 42 sites in 22 states. The year for which current volume was reported in these studies varied from 2000 through 2003. Current volume in each of these studies was compared with projected demand (described in the section below, “National Estimates of Ability to Meet Projected Demand for Endoscopy, by Different Demand Scenarios”).
Table 45
National estimates of current volume of endoscopy screening.
We rated one study as good quality196 and three studies as fair.195,199–200 Two of the three rated fair quality199–200 received this rating because they extrapolated data from a limited dataset of voluntary gastroenterologist physicians to estimate the number of colonoscopies performed by the entire number of gastroenterologists in the United States. The third study195 was rated fair quality because of limitations in measurement of the outcome.
Overview of results. Studies varied in their estimates of current volume of FS and colonoscopy. Differences between this study and the three fair-quality studies (giving data for, variously, 2000 to 2003) were greater for estimates of current volume of screening colonoscopy (1.6 to 6.6 million colonoscopies per year among four studies) than for FS (2.8 to 4.9 million per year in two studies). Differences may reflect differing methods of data collection or underlying issues of validity of self-report or report of volume by clinic administrators; they may also be consistent with increases in current volume of colonoscopy over a short period and concurrent decreases in current FS volume.
Detailed assessment, colonoscopy. In the good-quality study, authors from the Centers for Disease Control and Prevention (CDC) surveyed a national sample of 1,809 endoscopic facilities in the United States.196 They identified practices using lists of facilities known to have purchased or leased lower endoscopic equipment between 1996 and 2000 and then screened practices by telephone to ensure that they did in fact conduct CRC screening. A physician or clinic administrator completed this survey. The survey, which achieved a response rate of 74 percent, found that, in 2002, 6.6 million screening colonoscopies had been conducted.
Three studies rated fair quality produced similar estimates of the current volume of colonoscopy, but their results differed from those from the CDC study.195,199–200 One study, conducted by the National Cancer Institute (NCI),195 surveyed a national sample of primary care physicians, gastroenterologists, and general surgeons to estimate the current volume of colonoscopy among these providers nationally. The study did not adjust estimates of current volume for procedures by other types of providers. The NCI study estimated current colonoscopy volume in 2000 to be 4.0 million, including 1.6 million colonoscopies for screening. The two studies using data from the CORI database199–200 reported results similar to those of the NCI study. The first found that, in 2003, the estimated current volume (provided by gastroenterologists alone) was 6.47 million colonoscopies, with 1.98 million for screening; 199 the second estimated that, in 2002–2003, the current volume for screening colonoscopy, adjusted to estimate colonoscopies done by all provider types, was 1.69 million.200
Several differences in methods may account for the widely varying estimates among the four studies. Results from the two CORI-based investigations are likely based on nonrepresentative data, as the dataset includes volunteer physicians participating in this registry. Of the two studies with the stronger methods, the CDC study was conducted 3 years later than the NCI study and asked for actual numbers of procedures; the NCI study gave categories for response with ranges such as “11–20 procedures.” The highest category was “more than 20 procedures” per month, which may have set a potentially inaccurate ceiling on numbers for very active endoscopists. The CDC study also surveyed endoscopy practices, whereas the NCI study surveyed three types of providers; thus, the latter study may have missed perhaps up to 8 percent of colonoscopies (based on data from the CDC study).
Studies varied as to whether the investigators included surveillance colonoscopies in estimates of current volume. In the two CORI studies, one included such procedures200 and the other did not.199 In the NCI study, the authors could not determine whether respondents classified followup procedures as screening or diagnostic.195 (We contacted the author of the CDC study but received no response.) All four studies provided estimates of the percentage of all colonoscopies that are conducted for screening purposes: 29 percent,199 40 percent,195 and 46 percent.196,200 Two studies estimated the average number of procedures per month: 32 for gastroenterologists and 8 for colorectal surgeons195 and, in another study, 21 for gastroenterologists.200
Detailed assessment, FS. Two estimates of current volume for FS were available. The NCI study estimated the 2000 current volume of FS at 4.9 million;195 the CDC study estimated that the 2002 FS current volume was 2.8 million.196
Additional available capacity of endoscopy. Study characteristics. The CDC study reported on additional available capacity of FS and colonoscopy at the national level (Table 46).196 This study was a survey of a national sample of 1,809 endoscopic practices; they reported the number of colonoscopies they perform per week and the weekly maximum number they could perform.
Table 46
National estimates of additional available capacity of endoscopy screening.
Overview of results. The CDC article reported additional available capacity in 2002 of 6.7 million for FS (239 percent of current volume) and 8.2 million for colonoscopy (58 percent of current volume).196
Detailed assessment. The CDC study asked respondents (clinic physicians or administrators) to estimate the weekly number of FSs and colonoscopies that the practice performed per week, and the weekly potential maximum the practice could perform. Available capacity was determined by subtracting the current volume from the maximum. For national estimates, these investigators imputed missing values of these numbers and incorporated weights into their analysis to make estimates generalizable to all US health care practices that use endoscopic equipment for CRC screening.
National Estimates of Ability to Meet Projected Demand for Endoscopy, by Different Demand Scenarios
Projected demand. Study characteristics. Six studies provided estimates at the national level of ability of current volume or additional available capacity to meet projected demand for endoscopy (Table 47). Many of these studies evaluated ability to meet demand for colonoscopy, even under screening scenarios using FOBT or FS, as it is capacity for colonoscopy that is most likely restricted and is of most interest.
Table 47
National estimates of ability of current volume or additional available capacity of flexible sigmoidoscopy or colonoscopy to meet projected demand for endoscopy, by different demand scenarios.
We rated three studies as good quality195,201–202 and two studies as fair quality;199,203 for a sixth study, we rated separate parts as good and fair.200 The studies rated fair quality received this rating because of either the representativeness of the data used to estimate capacity or the assumptions made for modeling demand. We rated an additional study as poor quality and excluded it because it did not incorporate increased demand for surveillance colonoscopy following use of FOBT in its estimates.204
Two studies collected and analyzed survey data of physicians or endoscopic facilities to estimate current volume and additional available capacity.195,202 Of the four other studies that estimated capacity, two studies used secondary data from a survey of endoscopic practitioners,199–200 one study used secondary data providing the number of CT scanners,203 and the final study modeled only demand and did not have estimates of current or additional available capacity.201
All six studies used census data with specific refinements (such as omitting persons who are above average risk or who may be too “sick” for screening) as inputs into mathematical models to estimate current and projected demand. The types of refinements of population estimates, the types of models, and the assumptions regarding demand (most importantly, what percentage of the population would be included in a future screening scenario) used to construct the models varied widely among the studies. The percentage of the population included in future screening scenarios ranged from 40 percent to 100 percent.
Overview of results. Six studies provided some data on the ability of current capacity or volume to meet projected demand under various steady-state scenarios. For each scenario, we present estimates of projected demand from across all studies and compared these levels of projected demand with estimates of current capacity from across all studies.
In the first scenario, in which 75 percent of the US population is screened by FOBT alone, the projected demand for colonoscopy is 3.8 million.201 Based on estimates of current capacity from across the studies, current capacity is likely sufficient to meet the demand for colonoscopy. If a similar proportion (70 percent to 75 percent) of the US population is screened using either FS or combined FS/FOBT, an estimated 6.9 million to 10 million FSs and 2.7 million to 4.7 million colonoscopies are needed. Current volume of FS is not sufficient, but current capacity, including estimates of additional available capacity, is likely sufficient to meet projected demand; current volume of colonoscopy is sufficient. Finally, if 70 percent of the US population is screened by a colonoscopy alone, the projected demand is 4.8 million to 8.1 million colonoscopies. Current volume is not sufficient to meet the projected demand, but current capacity, including additional available capacity, may be sufficient. All of these estimates represent steady-state scenarios; none of these models incorporated current estimates of the unscreened.
In the single study that modeled the extent to which available capacity was sufficient to screen the unscreened population, the investigators determined that, using 100 percent of additional available capacity, it would take 3 years at current screening patterns, 6 years using 100 percent FS or FOBT/FS, or 5 years using 100 percent colonoscopy to screen the unscreened population.
Detailed assessment, FOBT screening scenario. One good-quality study used a Markov model to estimate endoscopic demand under various screening demand scenarios.201 It reported that if 75 percent of the US population were screened by FOBT alone, 3.8 million colonoscopies would be needed for followup of abnormal FOBTs, for post polypectomy surveillance, or for diagnosis of symptomatic CRC or followup after CRC treatment.
Detailed assessment, FS screening scenario. Two studies, one rated good and one rated fair, reported similar estimates on the number of FS needed if a large proportion of the population were screened with FS every 5 years. One study, which used a Markov model to estimate endoscopic demand under various screening demand scenarios, found that if 75 percent of the US population were screened by FS alone, 10.0 million FSs and 2.7 million colonoscopies would be needed annually.201 The NCI study also used a microsimulation model that incorporated population estimates and assumptions about test performance to estimate demand for FS.195 They found that if 70 percent of the US population were screened by FS every 5 years, the number of FS procedures required annually would be “almost 10 million,” which is approximately twice their estimate of FS current volume.
Detailed assessment, FOBT/FS screening scenario. Two studies, both rated good, reported on the number of colonoscopies needed if a proportion of the population were screened with FS every 5 years and FOBT every year. One used a Markov model to estimate endoscopic demand under various screening demand scenarios and found that if 75 percent of the US population were screened by FOBT/FS, 6.9 million FSs and 4.7 million colonoscopies would be needed annually.201 The other study, which estimated current volume using the CORI database and demand based on a Markov model, found that, assuming 70 percent adherence to a FOBT/FS screening strategy, an incremental number of 1.2 million colonoscopies would be needed above the baseline of 1.69 million per year (total of ~2.9 million screening colonoscopies).200
Detailed assessment, colonoscopy screening scenario. Three studies, two rated good and one rated fair, reported on projected demand if 70 percent to 75 percent of the US population were screened by colonoscopy alone. The study that estimated demand scenarios found that if 75 percent of the US population were screened by colonoscopy alone, 8.1 million screening colonoscopies would be needed annually.201 The study that estimated current volume using the CORI database and demand based on a Markov model, found, assuming 70 percent adherence to a colonoscopy screening strategy every 10 years, that an incremental number of 5.0 million colonoscopies would be needed above a baseline of 1.69 million per year (total of 6.69 million screening colonoscopies).200 The NCI study found that if 70 percent of the US population were screened by colonoscopy every 10 years, the number of screening colonoscopy procedures required annually would be 4.8 million (which was three times the estimated current volume in that study).195
None of these studies gave estimates of additional available capacity. Of the two studies that estimated current volume in addition to projected demand, projected demand far exceeded current volume. This pattern suggested that a colonoscopy screening strategy for a large proportion of the population could not be supported. However, if the estimates of current available capacity from Seeff and colleagues202 are taken into account (an additional 8.2 million colonoscopies per year), the current endoscopy infrastructure might possibly support a colonoscopy strategy of this sort.
Detailed assessment, screening the unscreened by various scenarios. One study was unique in that it modeled the ability of additional available capacity (rather than current volume) to screen all current average-risk unscreened persons in the US population (rather than modeling various screening strategies for the entire US population).202 This study, rated good quality, modeled the time needed to screen the current unscreened US population (41.8 million persons) by various strategies. This study found that, using 100 percent of additional available capacity, it would take 3 years at current screening patterns or 6 years using 100 percent FS or FOBT/FS to screen the average-risk unscreened population. Using 100 percent of additional available capacity, it would take 5 years to screen the unscreened population with colonoscopy.
Detailed assessment, increasing demand for CT colonography (CTC) scenario. Three studies, one rated good and two rated fair, modeled increasing demand for CTC. Two had as outcomes the effect on demand for colonoscopy;199,201 the third asked whether projected CTC capacity is sufficient to meet projected CTC demand.203 The good-quality study using a Markov model to estimate endoscopic demand under various screening demand scenarios reported, assuming 75 percent uptake, that demand for colonoscopy would be 6.2 million if all screening was done by CTC.201 The fair-quality study, estimated capacity from data from the CORI database and modeled demand based on a mathematical model. Assuming 55 percent adherence to any kind of screening and 67 percent of screening being CTC (overall 37 percent utilization of CTC), these investigators reported that, in the initial 5-year period after implementation of CTC, demand for colonoscopy could decrease by 1.78 million. This would be partially offset by 0.34 million follow-up colonoscopies for CTC with positive findings.199 Because assumptions for utilization of CTC varied widely between the two studies, they cannot be directly compared.
The third study asked a very different question: whether projected capacity of CTC is sufficient to meet projected demand.203 The authors assumed 60 percent compliance with any kind of screening, 67 percent of screening being CTC, and a rise in the number and percentage of CT scanners performing CTC from 718 and 10 percent to 10,000 and 90 percent. Given these factors, they concluded that the nation will have sufficient capacity to screen 10 years from now in a steady-state scenario.
KQ 5: Effective Approaches for Monitoring Use and Quality of Colorectal Cancer Screening
Valid data on the use and quality of CRC screening are central to efforts to decrease morbidity and mortality from CRC in the United States. To understand the current status of CRC screening and the effects of interventions to increase the use and quality of screening, we must have both valid measures of CRC screening use and quality of those services and effective monitoring approaches to obtain data on these measures. KQ 5 examines the approaches for monitoring use and quality of CRC screening in populations and the effectiveness of these monitoring approaches.
As a starting point for defining an effective approach for monitoring use and quality of CRC screening, we identified frameworks for public health monitoring (or surveillance) systems from both the United States and Canada.47,205 To avoid confusion over the term ‘surveillance’, we have opted to use it to describe surveillance colonoscopy (colonoscopy for patients who have had a previous colonic polyp (and, usually, polypectomy)) and have replaced the term ‘surveillance’ with regard to data collection related to CRC test use to the term ‘monitoring’. Therefore, all discussions about data systems will be referred to as those that monitor use or quality. These frameworks provide complementary lists of characteristics or attributes of monitoring systems that are applicable to the design of an ideal approach to monitoring CRC use and quality. Although the notion of a monitoring system may be more common for infectious diseases than for cancer or other chronic conditions, these frameworks are intended to be applicable to both chronic and infectious diseases. Also, although monitoring systems are often thought of in terms of disease incidence and mortality (rather than health care utilization or health care quality), public health is beginning to monitor risk factors and preventive services such as CRC screening, not just diseases.
The frameworks that we identified provide a comprehensive and logical way to think about evaluating existing approaches to monitoring the use and quality of CRC screening, and they provide guidance for the design of optimal monitoring approaches. Table 48 describes characteristics or attributes of monitoring systems that the review team found applicable to CRC screening; it also gives working definitions adapted from the US and Canadian frameworks. In addition to these characteristics, the frameworks described more global system performance characteristics of usefulness, effectiveness, and/or efficiency. The items in this table are considered to contribute to overall system performance, including effectiveness; in addition to these characteristics, a critical component of effectiveness as defined in one framework is how well the system achieves its intended results.205
Table 48
Characteristics of public health monitoring systems that contribute to effectiveness.
In our literature search, all the articles identified relevant to KQ 5 pertained only to the first system characteristic, data quality. We found no articles that measured other characteristics of a monitoring system or that compared any of these characteristics between systems. Also, we found no articles that addressed the monitoring of quality of CRC screening, just monitoring of CRC screening use.
Specifically, most of the articles that we identified evaluated the accuracy of measures of CRC screening as obtained from various data sources (self-report, medical record review, or administrative data);35,39–40,206–207 these studies add to the evidence from a recent systematic review208 and other literature that appeared before our time period of included articles. We also found one study that described an attempt to solve one of the barriers in using administrative data to determine screening rates, that of distinguishing screening from diagnostic endoscopies,209 and two studies that evaluated novel means of combining more than one data source to produce hybrid measures of CRC use.35,210
Other than establishing the quality of data on CRC screening use by evaluating the accuracy of measures of CRC screening as obtained from various data sources (self-report, medical record review, or administrative data), we found no other studies that measured or compared any of the other characteristics of monitoring systems (such as acceptability or cost). Thus, although this body of literature gives indications of data quality of the various sources for monitoring CRC use, it provides little evidence to inform the larger questions of what monitoring approaches, overall, are effective.
Our overall summary and strength of evidence tables for studies addressing this KQ are presented at the beginning of this section. The remainder of this section provides a more detailed assessment of the individual studies that informed our conclusions and our assessment of the strength of evidence.
KQ 5 Overall Summary and Strength of Evidence
In Table 49, our overall grades of the strength of evidence appear in the far right column; grades for key domains to determine the strength of evidence are in the intermediate columns. In assessing research specific to KQ 5 about effectiveness of varying approaches to monitoring CRC use and quality, we found varying grades between low and high depending for different aspects of this KQ. The grade can be interpreted as the confidence that the evidence reflects the true effect. For example, a grade of low means that further research is likely to change the confidence in the estimate of effect, and is likely to change the estimate.
Table 49
Strength of evidence for approaches to monitoring effectiveness of CRC screening use and quality.
Overall, the evidence suggests that self-reported rates of CRC screening are higher than rates obtained by medical record review or administrative data (high strength of evidence). Nevertheless, rates of agreement between self-reported CRC screening and information found in medical records or administrative data are at least moderate (moderate strength of evidence), indicating that all three methods are generally appropriate for monitoring CRC screening use. The evidence suggests, although strength of evidence is low, that concordance among data sources is higher for rates of endoscopy screening than for rates of FOBT screening. Evidence was insufficient for using algorithms to determine whether a colonoscopy identified in administrative data was conducted for screening or for diagnostic purposes. The evidence also suggests that using a hybrid method (administrative data plus medical record review or self report of CRC screening) will increase the reported prevalence of screening, but whether validity is increased is not known (low strength of evidence).
Overall study characteristics. We found seven studies of good or fair quality that reported data on effectiveness of approaches to monitor use of CRC screening.35,39–40,206–207,209–210 Three studies that were specific to validation of a set of survey questions developed by the National Cancer Institute211 were not included as they did not meet our inclusion criteria.212–214 Four studies took place within a managed care setting or a health plan35,206,209–210 and three included Medicare patients seen in non-managed care settings.39–40,207 All were cross-sectional studies that compared two or more data sources on CRC screening to evaluate the accuracy of the method of interest.
Overall study results. Included studies addressed only data quality; we found no studies that described or compared other monitoring system attributes. Although none of the three data sources can be considered a gold standard, all three appear to be generally appropriate for monitoring CRC screening status. However, self-reported rates of CRC screening are consistently higher than rates obtained from either medical records or administrative data.
The included studies reported a wide range of measures of concordance (agreement and/or kappa statistic, which accounts for agreement expected by chance) comparing CRC screening measures from the three data sources.35,39–40,206–207 In most studies that report accuracy of self-report for FOBT, any endoscopy, or any testing, concordance between self-report and medical record or administrative data was at least moderate (agreement greater than 70 percent or kappa greater than 0.40). Concordance appears to be higher for endoscopy than for FOBT.
One problem with using administrative data is that distinguishing screening from diagnostic examinations is difficult; the single study reviewed was not able to use an algorithm to do so effectively.209 Two studies demonstrated that administrative claims will underreport CRC screening rates (by showing that survey or medical record review will pick up additional screenings that were false negatives in the claims data), but they do not demonstrate conclusively that measuring CRC screening rates using hybrid methods is a more valid or a more effective approach overall.35,210
The following chapter is divided into three sections, based on the purpose of the studies. The first category includes studies that compared two or three types of data sources to evaluate data validity.35,39–40,206–207 The second category includes one study, which evaluated the use of a computer algorithm to distinguish diagnostic from screening endoscopy in administrative data.209 The final category includes two studies that used combinations of administrative, medical record, and survey data to assess the accuracy of these novel ways of CRC screening measurement.35,210
As with other KQs, tables in this section list studies by quality (good followed by fair) and then alphabetically by last name of the first author of the article(s). Appendix C presents the evidence tables with the details of these studies.
Validity of Self-Report, Administrative Data, and Medical Record Review to Measure CRC Screening Status
Study characteristics. We found five studies of good or fair quality that reported data on the validity of various data sources of CRC screening rates.35,39–40,206–207 The single study rated fair did not report detail on methods to ensure valid medical record abstraction.35
Two studies took place within a managed care setting or a health plan;35,206 of these, one included all plan members 51 years or older,35 and one used slightly different age cutoffs for men and women (45 and older for men and 55 and older for women).206 Three studies evaluated measures of CRC screening in Medicare populations.39–40,207 Of these, two included Medicare patients ages 55 to 8039–40 and the third included Medicare patients 65 and older.207
All studies were cross-sectional studies that compared two or more data sources on CRC screening to evaluate the accuracy of the method of interest. One study compared self-report of CRC screening with medical record review only,206 whereas the remaining studies examined all three sources of data—self-report, medical records, and administrative data. One study used secondary data from the Medicare Current Beneficiary Survey (MCBS);207 the remaining four studies involved surveys of patients for self-reported CRC screening history.
In general, these studies asked questions about several types of screening tests, followed by questions about the time frame when the tests occurred. Two studies reported that their questions were modeled after national studies such as the NHIS and BRFSS.35,206 Studies generally compared the prevalences of CRC screening as measured by different data sources and/or reported agreement, defined as the percentage of persons for whom the two data sources agreed and a kappa statistic.
Overview of results. Results of the studies were of two main types (Tables 50 and 51). In the five studies that compared prevalence rates of CRC screening from self-report with prevalence rates from medical record data or administrative data, the rates of FOBT, FS, and colonoscopy are generally higher as measured by self-report than by medical record data or by administrative data (Table 50). In the two studies that compared medical record data with administrative data, both in Medicare patients, administrative data may have slightly higher prevalence rates than those reported by the medical records. The range of concordance among the studies that compared the three data sources (Table 51) was wide. In most studies that reported accuracy of self-report for FOBT (two studies), for endoscopy (two studies), and for any testing (one study), concordance between self-report and medical record or administrative data was at least moderate (agreement greater than 70 percent or kappa greater than 0.40). Concordance appears to be higher for endoscopy than for FOBT.
Table 50
Assessing validity of CRC screening measures: Comparing prevalence of CRC screening rates by various data sources.
Table 51
Assessing validity of CRC screening measures: Concordance among data sources for CRC screening measures.
Detailed assessment. In the first study, conducted by CDC staff and authors from three health plans (in Georgia, Minnesota, and North Carolina), participants were recruited who had been enrolled in their plan for at least 5 years and were ages 45 years and older (men) or 55 and older (women).206 The investigators stratified the sample by site and sex and oversampled African-Americans members. Participants were recruited by letter and telephone; the cooperation rate was 64.8 percent. Investigators examined participants’ medical records for the previous 5 years. The study found that a higher percentage of respondents received testing when measured by self-report than by medical record audit (Table 51). The authors calculated a kappa statistic and used a cutoff of 0.40, above which indicates at least fair agreement (by the authors’ definition of poor less than 0.40; fair to good of 0.40–0.75; and excellent of > 0.75). They concluded that agreement was fair to good for FS and colonoscopy among most groups and poor for FOBT in two or three HMOs (Table 51).
In the second study, researchers compared Medicare claims data with self-report from the MCBS of having received an FOBT, FS, or colonoscopy among white, African-American, or Hispanic enrollees who were at least 65 years, did not reside in a long-term care facility, and were not enrolled in a Medicare HMO.207 The survey was conducted in 2000; the study did not mention the time frame for administrative claims review nor distinguish screening from diagnostic procedures in either data source. Only race-specific prevalences were reported, as the goal of the study was to examine disparities in screening rates as measured by different data sources. In this study, rates of screening were as follows: white, self-report 38 percent; white, claims 30.1 percent; minority, self-report 34.8 percent; and minority, claims 20.4 percent (Table 50). The kappa score measuring agreement between self-report and claims was 0.37 for whites and 0.19 for minorities. The authors also calculated ORs for reporting a procedure in the absence of a claim, or vice versa. Minorities were more likely to report receipt of CRC screening in the absence of a claim (OR, 1.92, 95% CI, 1.32–2.79), with little change after adjustment for age, gender, income, educational level, health status, proxy response, and supplemental insurance. Having a claim for CRC testing in the absence of self-report did not differ by race or ethnicity.
The North Carolina Quality Improvement Organization (the Carolinas Center for Medical Excellence) did two studies to evaluate all three data sources, namely self-report, medical records, and administrative data, for measuring CRC screening among Medicare patients. One study evaluated these data sources for measuring endoscopy39 and the other for measuring FOBT.40 Included persons were Medicare beneficiaries who were white or African-American, between the ages of 55 and 80, with no history of CRC, and residing in 10 urban counties in North Carolina who had responded to a telephone survey in 2002 on CRC screening.
The survey provided explanations of the FOBT, FS, and colonoscopy procedures, attempting to distinguish in-office FOBT from home FOBT, and also asked respondents if the examination was part of a check up or because of a problem. For the medical record review, the investigators linked patients to a medical provider (to complete the medical record review) using a hierarchical approach. First, they asked survey respondents to name a provider; if that provider could not be located or if the response to the question was missing or unusable, they used a claims algorithm to identify a likely primary care provider. If the abstracted record from the primary care provider did not contain information about an endoscopy noted in claims data, then the claims data were used to identify the physician who had performed the procedure and the researchers then abstracted the medical record from this physician as well. Specific to the endoscopy study, medical record review captured whether the test was done for screening or diagnostic reasons. Specific to the FOBT study, data on the four most recent FOBTs were abstracted, including the reason for the test and the nature of the test (sending three samples collected at home to the laboratory, a digital rectal examination [DRE] with a FOBT performed in the office, or not specified). For the claims data, Medicare inpatient, physician, and outpatient claims for endoscopies were obtained for the 5-year period 1/1/1998 through12/31/2002. Screening and diagnostic codes were available for both FOBT and endoscopic procedures.
In the first study, self-reported FS within the past 4 years or colonoscopy in the past 5 years was compared with evidence in claims or medical record review that the procedure had been done. Prevalence of endoscopy screening was highest when measured by self-report (50.1 percent) followed by claims data (44.9 percent) and medical record review (42.3 percent); sociodemographic subgroups differed somewhat in these percentages (Table 50). The authors also found high agreement (95 percent; kappa = 0.89) between claims and medical records and good agreement (70 percent) between self-report and medical records and self-report and claims (kappa = 0.39–0.40) (Table 51). Also, all three data sources were able to distinguish the type of procedure done (FS versus colonoscopy), based on agreement between the data sources (77 percent to 93 percent), but none showed reliable levels of agreement regarding whether the test was screening or diagnostic (Table 51).
The second North Carolina study evaluated measurement of FOBT in the past year in a similar fashion.40 Overall, the level of self-report of FOBT was higher (28.7 percent) than the level measured by claims (21.2 percent) or medical record review (19.4 percent); again, subgroups differed somewhat in these rates (Table 50). Lower rates of agreement were found among the three data sources for FOBT (67 percent to 82 percent) than for endoscopy (Table 51). The authors concluded that no data source could be established as providing valid information about FOBT among Medicare enrollees.
The final study, which we rated fair quality, was a field test of a National Committee for Quality Assurance (NCQA) performance measure.35 The investigators randomly selected 200 persons age 51 or older from each of five health plans who had been enrolled continuously for at least 2 years and who lacked evidence of recent CRC screening; they conducted both a survey and medical record review. For the survey, they selected an additional 400 persons per plan were selected (for a total of 600 per plan). The response rate to the survey, which asked about CRC screening and time frames in which they occurred, was 48.1 percent. CRC screening status was ascertained from administrative data, from the survey, and from a hybrid method of administrative records plus medical record review (for overall, not test-specific, screening status). Among members in each plan, the percentages of respondents who received testing were generally higher when measured by self-report than by administrative data (Table 50). Of note, survey respondents were more likely than nonrespondents to have evidence of CRC screening (62.7 percent versus 46.5 percent; P < 0.001).
Distinguishing Screening from Diagnostic Endoscopy Using an Algorithm for Administrative Data
Study characteristics. Two studies evaluated an algorithm’s ability to distinguish between screening and diagnostic endoscopy (Table 52).64,209 We rated both studies as fair quality, the first because of the limitations in their methods used to ensure validity of the medical record review data209 and the second because of limited reporting of the outcome.64 One study took place in a sample of patients from one HMO;209 the second in VA patients from one medical center.64
Table 52
Comparison of classification of diagnostic versus screening procedure using an algorithm for administrative data.
Overview of results. Algorithms that use concomitant diagnostic codes to distinguish whether an endoscopy is screening or diagnostic have not been able accurately to distinguish the two types of endoscopies.
Detailed assessment. In the first study, using data from a large staff-model HMO, the algorithm classified an endoscopy as diagnostic if administrative data included certain conditions in the year before the examination or either specific signs or symptoms or an FOBT within 45 days before the examination. All participants in this HMO ages 50 to 70 who had been continuously enrolled for 5 years and who had completed an endoscopy during that time were eligible for the study. The investigators selected a stratified random sample of 220 participants based on the algorithm’s classification of the endoscopy (for each of FS and colonoscopy, 30 diagnostic and 80 screening). They then reviewed medical charts and classified the examination as diagnostic based on the chart review if it was a follow-up to a previous abnormality or if clear-cut conditions or signs were present, using the same list as the algorithm. The algorithm had a low sensitivity for diagnostic endoscopies (48.1 percent for FS and 23.8 percent for colonoscopy). Overall, the agreement was better for sigmoidoscopies (kappa = 0.76) than for colonoscopies (kappa = 0.44).
In the second study, national VA datasets were used to identify all FOBT, FS, DCBE, and colonoscopy procedures performed in the VA between 1998 and 2003. All FOBTs were designated screening. All FS, DCBE, and colonoscopy procedures were classified as screening, followup, or diagnostic based on an algorithm considering diagnoses in the year before the procedures. A random sample of 303 medical records from a single VA hospital was reviewed by two gastroenterologists blinded to the designated status given by the algorithm. Agreement between the reviewers was achieved in 92 percent of cases; they resolved differences by discussion. Results from the medical record review were compared with the designation by the algorithm; only sensitivity and specificity for the algorithm’s ability to identify screening colonoscopy were reported, 70.1 percent and 71.l6 percent, respectively.
Evaluating Novel Ways to Combine Data Sources for CRC Screening Measurement
Study characteristics. We found two studies that evaluated novel ways to combine data sources to improve routine measurement of CRC screening use.35,210 We rated these studies as fair quality; one lacked data to assess the outcome fully,210 and the other did not ensure valid medical record abstraction.35,209 Both studies took place within a managed care or health plan setting. One study attempted to improve measurement of CRC screening use by augmenting administrative data with survey data, and the second by augmenting with medical record data. Although one study compared the rates of CRC screening from the hybrid method with both administrative and survey data for the entire sample, 35 the second could compare its rates only with administrative data, 210 because the survey was conducted only among persons for whom no evidence of CRC screening had been found in the administrative data.
Overview of results. In both studies, reported rates of CRC screening increased when administrative data were combined with either survey data or medical record data. The investigators provided no evidence (other than reporting prevalences) of the validity of these hybrid methods.
Detailed assessment. In one study, the researchers recruited a sample of members in a single health plan (Aetna), ages 52 to 80, from 32 primary care practices in Florida and Georgia that were taking part in a randomized trial of a CRC decision aid and practice-level academic detailing.210 Participants with no evidence of screening in the claims data were surveyed about completion of any CRC tests and the time frame (within 1 year, 1 to 5 years, 5 to 10 years, or more than 10 years). The researchers excluded from their calculations persons with evidence of medical exclusions in the claims data and persons found to be at above-average risk on the survey. Insurance claims were examined for evidence of FOBT within 1 year, FS or barium enema within 5 years, or colonoscopy within 10 years. The indication for the test was not specified in the survey and the authors do not discuss using screening versus diagnostic codes in analyzing the claims data. The authors reported that the prevalence of current screening among average-risk persons by claims data was 27 percent; combining claims data and survey data and accounting for survey nonresponse, they estimated that 47 percent to 59 percent of member patients were actually up-to-date.
In the NCQA field test, described above, the investigators constructed samples in five geographically dispersed health plans of persons both with and without administrative claims evidence of CRC screening.35 Among those with such evidence, the researchers selected a sample for the survey; of those without evidence in the claims, they selected a sample for both medical record review and the survey. The hybrid method combined administrative and medical record data to provide an estimate based on both. Among members in each of the five health plans, the percentages of respondents who received testing were generally higher when measured by the hybrid method than by administrative data, but they were lower than those recorded by survey data (Table 53).
Table 53
Evaluating novel ways to combine data sources for CRC screening measurement.
Publication Details
Copyright
Publisher
Agency for Healthcare Research and Quality (US), Rockville (MD)
NLM Citation
Holden DJ, Harris R, Porterfield DS, et al. Enhancing the Use and Quality of Colorectal Cancer Screening. Rockville (MD): Agency for Healthcare Research and Quality (US); 2010 Feb. (Evidence Reports/Technology Assessments, No. 190.) 4, Results.
