NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lawrence V, Jacobs B, Dennehy C, et al. Milk Thistle: Effects on Liver Disease and Cirrhosis and Clinical Adverse Effects. Rockville (MD): Agency for Healthcare Research and Quality (US); 2000 Oct. (Evidence Reports/Technology Assessments, No. 21.)
This publication is provided for historical reference only and the information may be out of date.

Milk Thistle: Effects on Liver Disease and Cirrhosis and Clinical Adverse Effects.
Show detailsOverview of the Evidence
Sixteen prospective trials were identified. Fourteen were placebo-controlled, randomized, and single- or double-blind trials (Evidence Table 1). Two were placebo-controlled prospective studies (randomized trial and cohort) with unclear blinding (Evidence Table 2).
In addition, 16 reports of 17 trials were identified that used nonplacebo controls (Evidence Table 3). The study by Flisiak and Prokopowicz (1997) 65 is described in Evidence Table 3 as two separate trials for convenience because it compared silymarin with two different controls (misoprostol and no treatment) and because reported methods did not seem consistent for the two "substudies." Nonplacebo-controlled trials are not considered further or discussed in detail in evaluating milk thistle's efficacy because control interventions were diverse and spanned "other hepatoprotective medications," misoprostol, ursodeoxycholic acid, comparisons with different doses or formulations of silymarin, "traditional therapy and diet," multivitamins, a 12-component cocktail that included steroids, and no treatment.
Finally, two trials evaluated milk thistle as prophylaxis against hepatotoxic effects of antituberculosis drugs or tacrine (Evidence Table 4). These trials did not meet selection criteria for evaluating milk thistle's efficacy because patients did not have liver disease at baseline. However, their results are provocative, and they are discussed in this report.
The term "liver function tests," when used most precisely, refers to laboratory tests of liver function (e.g., bilirubin, albumin, and prothrombin time) and does not include aminotransferases. However, for this report, we will use the more common vernacular interpretation that includes all tests referent to liver status.
Again, interpreting available evidence and meta-analysis is compromised by poor study design and poor reporting of published studies. Across the trials there is heterogeneity in, and lack of information about, the spectrum of liver disease, etiology, severity, and chronicity; preparations and doses of milk thistle; duration of intervention; assessment of compliance; effectiveness of blinding; followups; dropouts; and statistical analyses (see Evidence Tables 1 through 4). Information is scant about screening for alcohol intake, HBV, HCV, and HIV status at baseline, as well as explicit monitoring of alcohol intake during studies. All of these factors can affect the course of liver disease and potential efficacy of milk thistle.
Information on other potential confounders (e.g., comorbidities and severity of liver disease at baseline) is also rarely reported. No information was given regarding whether oral solid dosage formulation used in the trial met any criteria for in vitro dissolution standards. The precision of the available evidence did not measure up to the precision regarding etiology, severity, and chronicity in the a priori evidence questions.
Milk Thistle Preparations and Doses
We will use the term "milk thistle" loosely for summarizing results. By "silymarin," we mean the extract of seeds, containing silybin, silychristin, and silydianin, which may or may not have been standardized to percent silymarin in the reports of published trials and for which silybin is the primary component. By Silipide ® , we mean a silybin-phosphatidylcholine complex, but again studies may not have reported standardization results. Among the 16 placebo-controlled studies, Legalon ® (Madaus) was used in 12. Of these 12 studies, the dose varied from 240 to 800 milligrams per day (mg/d) in 8 trials, duration varied from 7 days to 6 years, and neither dose nor duration of Legalon ® was given for 4 trials ( Figures 3 and 4). Of the remaining 4 of 16 placebo-controlled studies, 2 used silymarin (no further characterization regarding preparation) in doses of 210 and 800 mg, and two used Silipide ® at 240 mg/d. Treatment duration for these four trials was 7 to 446 days for three and not given for one (see Figures 3 and 4).
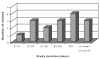
Figure
Figure 3. Distribution of durations of therapy for 16 blinded, placebo-controlled studies.
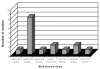
Figure
Figure 4. Distribution of doses for 16 placebo-controlled trials.
Among the 17 nonplacebo-controlled trials (Evidence Table 3), five used 420 mg/d of Legalon ® , one used 600 mg/d, and two studies used variable dosing from 210 mg/d to 420 mg/d. Preparations for the other nine studies included silymarin, silybin, Silipide ® , and Silimarol ® No further information was given, and doses ranged from 140 to 600 mg of silymarin and 10 to 360 mg of silybin; no standardized dose was given for Silimarol ® Over all 17 studies, intervention duration ranged from 14 days to 1 year for 13 and was "variable" or not given for 4 studies.
In the two studies of prophylactic silymarin in conjunction with potentially hepatatoxic antituberculosis drugs or tacrine, an unknown formulation (unknown dose) and silymarin (not further characterized) at 420 mg/d were used. Intervention duration was 56 days and 84 days, respectively (Evidence Table 4).
Milk Thistle and Liver Disease
Milk Thistle and Alcohol-Related Liver Disease
No placebo-controlled studies clearly evaluated subjects with purely acute alcoholic hepatitis. Six randomized, blinded, placebo-controlled studies appear to have evaluated chronic alcoholic liver disease, but the spectrum of disease chronicity and severity varied among the studies or was not clear; two merely described subjects as having "alcoholic liver disease." 66 67 68 69 70 71 72 Summary Table 1 summarizes these six studies and is from Evidence Tables 1 and 2.
Across these trials, results varied and may be generally confounded by the question of abstinence from alcohol. In one, there was significant improvement in liver function with abstinence from alcohol for patients given silymarin or placebo. However, between silymarin and placebo, there was no difference in the course of liver function as measured by prothrombin time (PT), albumin, bilirubin, gammaglutamyl transpeptidase (GGTP), aspartate aminotransferase (AST), or degree of hepatitis or fibrosis on biopsy. 67 In four trials, at least one parameter of liver function improved significantly with silymarin compared with placebo, but the courses of an equal number or more parameters were not significantly different between silymarin and placebo. 66 69 70 71 One study showed no improvement in overall survival with silymarin but marginally significantly improved survival (p=0.059 by univariate analysis) in HCV-positive patients given silymarin versus placebo. 68 Qualitatively, there does not appear to be a relationship between duration of therapy and improvement in liver function.
Milk Thistle and Chronic Liver Disease of Mixed Etiology
The available evidence did not provide sufficient information to put six studies into any of our a priori etiologic categories of disease. These studies could be characterized as only addressing chronic liver disease of mixed etiologies, including both alcoholic and nonalcoholic disease and without further clarification. 73 74 75 76 77 72 Summary Table 2, extracted from Evidence Tables 1 and 2, summarizes these six studies.
Across three studies, at least one parameter showed significant improvement with silymarin compared with placebo, and there was no difference between silymarin and placebo for as many or more parameters. Two studies indicated a possible survival benefit: In one study, survival was significantly improved for patients with Child's A alcoholic liver disease with silymarin compared with placebo, and in the other study, there was a trend toward improved overall survival. 75 76 Again, there is no apparent qualitative relationship between duration of therapy and efficacy of silymarin, but duration was not given or unclear for three of the trials.
In one study, 65 subjects with "chronic persistent hepatitis, etiology unknown" were randomized to receive either Silipide ® at 240 mg/d for 3 months or placebo. 77 AST improved significantly in subjects receiving silymarin, but there were no significant differences between the two study groups in the course of alanine aminotransferase (ALT), bilirubin, albumin, and PT.
Milk Thistle and Viral Liver Disease
One study evaluated silymarin in the setting of acute viral hepatitis. Summary Table 3 summarizes this study and is extracted from Evidence Table 1.
In a blinded study, 59 patients with acute hepatitis A and B were randomized to silymarin at 420 mg/d for 25 days or placebo. 78 No further information on inclusion/exclusion criteria, acuity, or severity was given. The mean age was 37, and 22 percent of subjects were men. Compared with that seen with placebo, improvement in AST and bilirubin was significantly better with silymarin, with a nonsignificant trend toward improvement in ALT. There was no significant difference in the course of alkaline phosphatase.
Two placebo-controlled studies evaluated the efficacy of silymarin in the setting of chronic viral hepatitis (Summary Table 4). Milk thistle was given as Silipide ® at 240 mg/d and as Legalon ® at 420 mg/d; duration of treatment was 1 week for Silipide ® and 1 year for Legalon ® Summary Table 4 summarizes these studies, which are extracted from Evidence Tables 1 and 2.
One randomized, blinded, placebo-controlled trial included 20 patients with biopsy-proven chronic viral hepatitis or with AST and ALT levels greater than twice normal for more than 12 months due to HBV and/or HCV.79 Patients with portal hypertension, hepatic encephalopathy, ascites, bilirubin or alkaline phosphatase more than twice the normal limit, alcohol consumption exceeding 30 grams per day (g/d), hepatocellular carcinoma, or liver disease associated with collagen vascular disease were excluded. Subjects received placebo or Silipide ® at 240 mg/d for 7 days. AST, ALT, and GGTP significantly improved in patients receiving Silipide ® compared with placebo. There was no difference between groups in bilirubin, alkaline phosphatase, malondialdehyde (MDA), or albumin.
In the other trial, 24 subjects with chronic viral hepatitis for more than 6 months were randomized to silymarin at 420 mg/d or placebo for 1 year. 80 Exclusion criteria were prior treatment with silymarin, steroids or other toxic drugs, alcohol consumption of more than 80 g/d, or other comorbidities requiring medication. There was a trend toward histologic improvement by liver biopsy in those receiving silymarin compared with placebo.
Milk Thistle and Cirrhosis
Five randomized and placebo-controlled trials included patients with cirrhosis along with patients with a mixed spectrum of types and severity of liver disease. Four are discussed in the section on chronic alcoholic liver disease, 66 67 70 72 and one is discussed in the section on chronic liver disease of mixed etiologies. 74 Across three trials, at least one parameter of liver function improved in those taking silymarin compared with placebo. 66 70 72 Most parameters, however, did not improve. Specifically, none improved in one study, 74 results were not separately presented for cirrhotic patients in a third study, and in another study, liver function improved equally in placebo and milk thistle arms with abstinence from alcohol. 67
Two randomized, blinded, placebo-controlled trials studied patients with alcoholic or nonalcoholic cirrhosis. 75 76 Summary Table 5, extracted from Evidence Tables 1 and 2, summarizes these studies. In both studies, silymarin dose was 420 mg/d, but duration of therapy was variable or unclear. The two studies are nearly identical in sample size (n=172, n=170) and relatively similar in exclusion criteria. One showed a nonsignificant trend toward improved survival overall with silymarin, and the other found significantly improved survival in the subgroups with alcoholic liver disease and in patients initially rated as Child's score A in severity. 75 76
Only two randomized, placebo-controlled, blinded trials specifically studied patients with alcoholic cirrhosis. 68 71 These studies are summarized in Summary Table 6, which is extracted from Evidence Tables 1 and 2. In these studies, the dose of silymarin was similar (450 mg/d of Legalon ® and 420 mg/d of Legalon ® ); duration of therapy was unclear in one study and 30 days in the other. One reported nonsignificant trends favoring silymarin, compared with placebo, in rates of encephalopathy and upper gastrointestinal bleeding. There was a trend (p=0.059) toward improved survival, compared with placebo, in the subgroup of patients who also had HCV. However, HCV status was determined retrospectively on frozen sera from a convenience sample of patients. There was no apparent benefit of silymarin in aminotransferases, PT, bilirubin, alkaline phosphatase, survival, or rates of other clinical syndromes (i.e., hepatomegaly, splenomegaly, jaundice, and ascites). 68 Again, duration of treatment with silymarin was unclear. In the other study, when silymarin was given for 30 days, there was a significant improvement in aminotransferases for patients taking silymarin compared with placebo, but no improvement in bilirubin. 71
Milk Thistle and Toxin-Induced Liver Disease
Only one study met criteria for evaluating milk thistle's efficacy in the setting of nonalcohol toxin-induced liver disease. 81 Summary Table 7 summarizes this study and is extracted from Evidence Table 1. In randomized, placebo-controlled, blinded trial with factorial design, 60 women were studied who were 40 to 60 years old, had been receiving phenothiazines and/or butyrophenones for 5 or more years, and had increased aminotransferases to more than twice the normal limit. Exclusion criteria included current therapy with other potentially hepatotoxic drugs or other etiologies of liver disease, compromised renal function (blood urea nitrogen exceeding 60 mg/dL or creatinine exceeding 2.5 mg/dL), "cardiac or circulatory insufficiency," diabetes mellitus, "other important extrahepatic disease," pregnancy, and alcohol (more than 30 g/d) or opiate abuse. Subjects were treated for 90 days after randomization to the following: (1) placebo and continued psychotropic drugs; (2) silymarin at 800 mg/d and continued psychotropic drugs; (3) silymarin at 800 mg/d and psychotropic drugs discontinued; or (4) placebo and psychotropic drugs discontinued. MDA significantly improved in patients on silymarin and psychotropic drugs compared with women on placebo and psychotropic drugs. However, no difference was found in the course of liver function tests for AST, ALT, or MDA between patients taken off psychotropic drugs and given silymarin or placebo.
Two blinded studies evaluated prophylactic milk thistle in conjunction with hepatotoxic medications-drugs for treating tuberculosis and tacrine for treating Alzheimer's disease. 82 83 Summary Table 8 summarizes these studies and is extracted from Evidence Table 4.
In the study of prophylactic milk thistle during treatment for tuberculosis, 29 subjects with normal liver function tests received antituberculosis drugs plus Hepabene ® , a mixture of silymarin and Fumaria officinalis alkaloids (two capsules daily, no other information given), and 93 subjects received antituberculosis drugs alone. 82 Proportions receiving various antituberculosis drugs, in the control and silymarin groups respectively, were the following: rifampin (98 percent and 100 percent); isoniazid (99 percent and 97 percent); pyrazinamide (84 percent and 100 percent); ethambutol (50 percent and 38 percent); and streptomycin (37 percent and 14 percent). Initially, AST increased in 49 percent of control subjects and 38 percent of subjects receiving Hepabene ® Similarly, ALT increased in 48 percent of control and 31 percent of those receiving Hepabene ® These differences were statistically significant over the course of 8 weeks; AST then fell in 40 percent of subjects receiving Hepabene ® , compared with 19 percent of controls. Similarly, ALT fell in 48 percent of those receiving Hepabene ® , compared with 22 percent in controls. Again, these differences were statistically significant.
The second study of prophylatic milk thistle was a randomized, double-blind trial in which 222 patients meeting a priori criteria for Alzheimer's dementia were randomized to tacrine and silymarin or tacrine with placebo. 83 Patients with prior liver disease were excluded. Silymarin (420 mg/d) was given for 1 week, and then tacrine was added, first at 40 mg/d for 6 weeks, then at 80 mg/d for 6 additional weeks. (Note: Published report 83 states that silymarin [Legalon ® ] dose was 420 mg/d, but an internal study summary reportedly states dose was 140 mg/d from Madaus. 84 ) There were no significant differences in the courses of AST or ALT levels or the rate of side effects during the study. There was no significant difference even though rates appeared lower.
Milk Thistle and Cholestasis
No studies met criteria for evaluating efficacy of milk thistle in treating cholestatic liver disease, pregnancy-related or not.
Milk Thistle and Primary Hepatic Malignancy
No studies met criteria for evaluating milk thistle efficacy in treating or preventing primary hepatic malignancy (hepatocellular carcinoma or cholangiocarcinoma).
Effectiveness of Different Preparations of Milk Thistle
Pharmacokinetic studies indicate that one preparation of oral Silipide ® (silybin-phosphatidylcholine complex) has better absorption from the gastrointestinal tract and, as a result, is more bioavailable than a particular preparation of oral silymarin. However, because of variability in different preparations, differences in bioavailability may not be generalizable. No randomized, placebo-controlled trials directly compared a standardized silymarin extract with Silipide ® for effectiveness in treating liver disease. One Phase II, dose-response study compared different doses of Silipide ® with no treatment, and one nonrandomized study compared silymarin, Silipide ® , and "no treatment or placebo control." 85 Summary Table 9 summarizes the Phase II study of Silipide ® and is extracted from Evidence Table 3.
In the Phase II study, 60 subjects with chronic viral or alcoholic hepatitis were randomized to 14 days of Silipide ® at 160 mg/d, 240 mg/d, or 360 mg/d or no treatment. AST, GGTP, and bilirubin improved significantly more in subjects receiving 240 mg/d or 360 mg/d, compared with those receiving 160 mg/d. 85 No comparative data and data specific to the control group (no treatment) versus any dose of Silipide ® were reported.
Exploratory Meta-Analyses Results
Exploratory meta-analyses proceeded in the following sequence: (1) all 16 placebo-controlled studies (studies in Evidence Tables 1 and 2); (2) the 14 randomized, placebo-controlled trials in Evidence Table 1; and (3) subgroup analyses by etiology of liver disease and duration of followup ( <45 days or 46 to 90 days). Relevant funnel plots, Galbraith plots, and forest plots are given in Appendix B.
Summary Table 10 shows the results for pooling all 16 placebo-controlled trials. Effect size is given both as the standardized mean difference, in standard deviation units, and in converted laboratory values (see preceding Data Analysis Process section). Statistical significance is expressed as the probability that the effect of silymarin is not zero. When one outlier study was removed, there was no significant heterogeneity. Most effect sizes favored silymarin, but effect sizes were small or not statistically significant. Results were similar for pooling only the 14 higher quality trials in Evidence Table 1 (Summary Table 11).
Table 3 shows the variables for which data were available in etiologic subgroups. Tables 4, 5, and 6 show results of these subgroup analyses. Results are similar throughout: Silymarin was generally associated with small favorable, but not statistically significant, effect sizes.
Table
Table 3. Outcome measures and followup times for trials of chronic liver disease, chronic liver disease of mixed etiology, and viral liver disease.
Table
Table 4. Effect sizes and meta-analysis for chronic alcoholic liver disease (6 studies).
Table
Table 5. Effect sizes and meta-analysis for chronic liver disease of mixed etiologies (6 studies).
Table
Table 6. Effect sizes and meta-analysis for viral liver disease, acute and chronic (3 studies).
In summary, results of multiple exploratory meta-analyses show either nonsignificant effects or a few statistically significant effects that are small in magnitude. There is no obvious relationship between observed effectiveness and etiology of liver disease or duration of followup. It is possible that clinical heterogeneity in subject populations and small sample sizes may have masked statistically significant effects of milk thistle in different clinical groups. As we conducted multiple exploratory analyses, it is also possible that some of the statistically significant findings occurred by chance.
Adverse Effects of Milk Thistle
Overview of Adverse Effect Literature
The adverse effect literature regarding milk thistle is difficult to interpret for many reasons. First, searching for studies that report adverse effects is difficult and, given current indexing systems for electronic databases, probably incomplete. Many studies may mention adverse effects in passing, but they do not use adverse effects as a key index word or in their abstracts. If these studies do not otherwise meet selection criteria in a review, they will be missed. Second, information is frequently missing on whether adverse effects were elicited by voluntary self-report or standardized probes. Third, in some case reports and case series, adverse effects cannot be directly attributed to milk thistle because chance, coincidence, or confounding factors could have been responsible. Fourth, case reports and case series may miss delayed adverse reactions because such associations are more difficult to make than those that occur immediately after administration of an agent. Fifth, although case reports and case series can provide qualitative information about the nature of an adverse effect, they cannot generate estimates of incidence. 86
Based on identified reports, milk thistle appears to be safe for most people. Seventeen reports of adverse effects possibly due or attributed to milk thistle oral supplements were identified (Evidence Table 5). Data were abstracted from seven randomized clinical trials, 66 68 75 77 83 85 87 six cohort studies, 88 89 90 91 92 93 and five case reports. 94 95 96 97 98 Besides study characteristics, we attempted to abstract data regarding evidence for causality: (1) documentation of improved adverse effect after the milk thistle supplement was discontinued, (2) adverse effect recurrence when rechallenged with the milk thistle formulation, (3) careful search for other causes of the adverse effect, and (4) documentation of a dose-response relationship. 56 None of the 17 reports provided information regarding a possible dose-response relationship. Detailed data are shown in Evidence Table 5. An overall summary of reported adverse effects, by level of evidence, is shown in Summary Table 12.
Common Symptomatic Effects
Gastrointestinal side effects are the most commonly reported adverse effects, in both randomized clinical trials and prospective cohort studies. 75 77 83 85 88 89 90 91 92 93 98 Overall incidence appears relatively low, and in RCTs there is no apparent difference between treatment and control groups. 66 68 77 Skin manifestations of adverse drug effects are also fairly commonly reported, but again overall incidence appears low and no more frequent in groups receiving milk thistle than in control groups. 66 68 77 Incidence of headaches also appears low and no different between silymarin and control groups in RCTs. 66 68
Common and Uncommon Serious Adverse Effects
Serious adverse effects identified in available evidence for this systematic review were anaphylactoid reactions or anaphylaxis. Available evidence was limited to three case reports. In one case report, a variety of symptoms plus "collapse" was associated with milk thistle, improved after discontinuation of milk thistle, reappeared on rechallenge, but was also associated with potential alternative etiologies. 98 In a second case report of anaphylactic shock, no information was given about associated evidence of causality. 94 A third case report of an apparent anaphylactoid reaction improved with prednisone and discontinuing silymarin, and there were no apparent alternative possible etiologies. 95
- Results - Milk Thistle: Effects on Liver Disease and Cirrhosis and Clinical Adve...Results - Milk Thistle: Effects on Liver Disease and Cirrhosis and Clinical Adverse Effects
- Future Research - Diagnosis and Treatment of Acute Bacterial RhinosinusitisFuture Research - Diagnosis and Treatment of Acute Bacterial Rhinosinusitis
- Appendix 5. Project Personnel - Rehabilitation for Traumatic Brain InjuryAppendix 5. Project Personnel - Rehabilitation for Traumatic Brain Injury
Your browsing activity is empty.
Activity recording is turned off.
See more...