NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
ECRI Health Technology Assessment Group. Treatment of Degenerative Lumbar Spinal Stenosis. Rockville (MD): Agency for Healthcare Research and Quality (US); 2001 Jun. (Evidence Reports/Technology Assessments, No. 32.)
This publication is provided for historical reference only and the information may be out of date.
Research Questions
Focus and Refinement of Topic
In order to focus, refine, and arrive at the key questions addressed by this assessment, the research team held telephone conversations with 11 experts in the field of lumbar spinal stenosis and one patient representative. These individuals were invited to comment on a document prepared by the research team charged with preparing the present evidence report. This research team consisted of five experts in technology assessment. The document comprised a preliminary evidence model (evidence models are discussed below) and written descriptions of the specific issues depicted in this model. Conversations with the experts and the patient representative clarified that the evidence report was to cover primarily lumbar stenosis in adults and that congenital stenosis or stenosis resulting from trauma was beyond the scope of the present evidence report. Upon receipt of the comments of the experts and the patient representative, the research team met to ensure that the evidence model was refined to incorporate the comments of the experts and the patient representative.
Peer Review
Upon completion of a draft report, the document was sent to additional experts in the field of spinal stenosis. The substantive comments were addressed in a document that described the disposition of each comment, and the draft evidence report was accordingly revised.
Evidence Model
The evidence model developed for this report is a graphic that depicts the relationships between the diagnostics and treatments of spinal stenosis and the benefits and harms that result from their use. These relationships are denoted by links (represented by arrows). The important links in the model were used to generate the key questions addressed in this report. Because of the complexity of the topic, we divided the evidence model into two sections. The first section portrays the questions on the natural history of lumbar spinal stenosis, and the second section portrays the questions on the diagnosis and treatment of lumbar spinal stenosis.
Key Questions on the Natural History of Lumbar Spinal Stenosis
The evidence model for the natural history of lumbar spinal stenosis addresses three questions that relate patient characteristics to patient signs, symptoms, and conditions (see Figure 9). The three questions are:
- What is the relationship between each relevant patient characteristic and the presence and/or intensity of each of the patient signs, symptoms, and conditions of lumbar spinal stenosis?
- Which relevant patient characteristics are correlated with an increased likelihood of focal narrowing of the spinal canal?
- What is the relationship the degree of stenosis and the presence and/or intensity of each of the signs, symptoms, and patient conditions?

Figure
Figure 9. Evidence Model Depicting the Natural History of Lumbar Spinal Stenosis.
Key Questions on the Diagnosis and Treatment of Lumbar Spinal Stenosis
The evidence model for the diagnosis and treatment of lumbar spinal stenosis addresses five questions that relate to the connection between the signs and symptoms of lumbar spinal stenosis and the results of conservative treatment, the imaging examination, and the results of surgical treatment (see Figure 10).
- 4.
What is the relationship between the signs and symptoms and other features of the history and physical and the results of the imaging examination? Implicit in this question is an examination of the criteria for diagnosis of spinal stenosis.
- 5.
What is the relationship between the signs and symptoms and other features of the history and physical and results of conservative treatment and what is the relationship between the type of conservative treatment, and patient outcomes? Implicit in this question is whether any particular patient subgroup benefits from medical management of spinal stenosis.
- 6.
What is the relationship between the signs and symptoms and other features of the history and physical and the success or failure of surgical treatment? Implicit in this question is whether any particular patient subgroup benefits from surgical treatment of spinal stenosis and whether some patients might benefit more from surgery than from medical management.
- 7.
What is the relationship between the results of the imaging examination and the success or failure of surgical treatment? Implicit in this question is whether it is possible to predict that a certain patient subgroup will benefit from surgery.
- 8.
What is the relationship between the type of surgery received and patient outcomes?
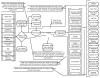
Figure
Figure 10. Evidence Model Depicting the Diagnosis and Treatment of Lumbar Spinal Stenosis.
Costs Associated With Lumbar Spinal Stenosis
The last question does not appear in our evidence models because it deals with medical costs associated with lumbar spinal stenosis.
- 9.
What costs are associated with nonsurgical and surgical treatment of spinal stenosis?
Databases Searched
Our literature searches were geared towards ensuring that our retrieval of information relevant to our key questions was comprehensive. The following is a list of the databases we searched, and the dates covered by our searches:
The Cochrane Database of Systematic Reviews (through 2000 Issue 1)
The Cochrane Registry of Clinical Trials (through 2000 Issue 1)
The Cochrane Review Methodology Database (through 2000 Issue 1)
CRISP (through May 2000)
Cumulative Index to Nursing and Allied Health Literature (CINAHL)® (1988 through November 22, 1999)
Current Contents® -- Clinical Medicine (through May 2000)
The Database of Abstracts of Reviews of Effectiveness (Cochrane Library) (through 2000 Issue1)
Dissertation Abstracts (through February 29, 2000)
ECRI Library Catalog (through May 2000)
EMBASE® (Excerpta Medica) (1974 through November 19, 1999)
Health Care Financing Administration (HCFA) Web site (through May 2000) including:
Medicare Provider Analysis and Review (MEDPAR) 1999 National Physician Fee Schedule Payment Amount File
Health Devices Alerts® (1977 through May 2000)
Healthcare Standards (1975 through May 2000)
Health Devices Sourcebase® (through May 2000)
Health Services Research Projects (HSRPROJ) (through February 29, 2000)
HealthSTAR (Health Services, Technology, Administration, and Research) (1990 through May 20, 2000)
International Health Technology Assessment© (IHTA) (1990 through May 2000)
LocatorPlus (through May 2000)
MANTIS® (through February 4, 2000)
MEDLINE® (1964 through March 8, 2000)
National Guideline Clearinghouse® (NGC) (through May 2000)
NHS Economic Evaluation Database (NHS EED) (through May 2000)
PsycINFO® (1967 through January 17, 2000)
Rehabdata (through February 2000)
TARGET® (through May 2000)
Hand Searches of Journal and Nonjournal Literature
To further ensure that our searches allowed for comprehensive identification of information, we searched Current Contents© -- Clinical Medicine on a weekly basis and routinely reviewed more than 1,600 journals and supplements maintained in our collections. Nonjournal publications and conference proceedings from professional organizations, private agencies, and government agencies were also screened.
Finally, to further ensure the comprehensiveness of our identification of relevant literature, we reviewed bibliographies/reference lists from peer-reviewed and gray literature. (Gray literature includes reports and studies produced by local government agencies, private organizations, educational facilities, and corporations that do not appear in the peer-reviewed literature.)
World Wide Web Searches
Searches of the World Wide Web were also conducted using various resources and search engines, including (but not limited to) AltaVista, NorthernLight, and Google. Selected Internet resources include:
American Academy of Orthopedic Surgeons (AAOS) http://www.aaos.org/
American Back Society http://www.americanbacksoc.org/home.htm
Centre for Clinical Effectiveness http://www.med.monash.edu.au/publichealth/cce/
Development Evaluation Committee http://www.hta.nhsweb.nhs.uk/rapidhta/main.htm
GICD Online http://www.gicd.org/index.html
HCUPnet http://www.ahcpr.gov/data/hcup/hcupnet.htm
Mecqa (benchmarking data) http://www.mecqa.com/consumer/frame.htm
Medscape http://www.medscape.com
NHS Centre for Reviews and Dissemination http://www.york.ac.uk/inst/crd/welcome.htm
North American Spine Society http://www.spine.org
Spinal Disorders http://www.spinaldisorders.org/index.html
SUM Search http://sumsearch.uthscsa.edu/searchform4.htm
The Spinal Foundation http://www.spinal-foundation.org/home.htm
TRIP Database http://www.ceres.uwcm.ac.uk/section.cfm?section=trip
MESH Terms and Search Strategy
Our literature search strategies were designed to ensure broad retrieval of information. Thus, we sought to perform inclusive literature searches that had low specificity and high sensitivity. Our search strategies are described below.
MEDLINE®, EMBASE®, CINAHL®, Current Contents® (presented in PubMed syntax)
Search for Lumbar Spinal Stenosis Literature
- (Spinal stenosis OR sciatica OR backache OR spinal diseases[mh] OR ischialgia OR compressive neuropathy OR spinal claudication OR neurogenic claudication OR intermittent claudication OR nerve root entrapment OR nerve root compression OR osteoarthritis OR spondylosis OR spondylolisthesis OR cauda equina OR spinal osteophytosis) AND (lumbar or lumbosacral)
- stenosis AND (lumbar OR exit zone OR nerve root canal OR foraminal)
- #1 OR #2
- #3 AND (meta-analysis[pt] OR meta-analysis OR clinical trial[pt] OR clinical trials[mh] OR random allocation[mh] OR random* OR double-blind method[mh] OR single-blind method[mh] OR placebo* OR multicenter study[pt] OR prospective* OR historical OR controls[ab])
- #4 AND (human[mh] OR preMEDLINE[sb] OR publisher[sb])
- #5 NOT (letter[pt] OR news[pt] OR editorial[pt] OR comment[pt] OR case report[mh] OR anesth* OR anaesth* OR "idiopathic scoliosis")
Search for Spinal Canal Measurement Literature
- Spinal canal/anatomy AND histology[mh] OR ((spinal canal[mh] OR spine[mh]) AND anthropometry[mh])
- Spinal (column OR canal) AND diameter AND measure*
- #1 OR #2
- #3 AND (human[mh] OR preMEDLINE[sb] OR publisher[sb])
- #4 NOT (foetal OR fetal OR embryo* OR prenatal OR neonat* OR comment[pt] OR letter[pt] OR editorial[pt] OR news[pt] OR case report[mh] OR bone density)
PsycINFO® (presented in Dialog® syntax)
- Back pain! AND chronic
- Cauda ()equina () (syndrome or compression) or sciatica or lumbago or spondylolisthesis or spinal ()osteophytosis or (spinal or lumbar or foraminal or nerve ()root ()canal) ()stenosis or intermittent ()neurogenic ()claudication
- s1 or s2
- s3 and (lumbar or lumbosacral)
- s4 and (quality of life/de or QOL or quality (2n)life or disability evaluation/de or disability)
Range of Searches for Conservative Treatments
Our literature searches were geared to seeking out articles on a broad range of conservative treatments. These included:
- Physical therapy
- Exercises: strengthening and endurance, stretching and flexibility, flexion v. extension, isometric flexion, aerobic exercise, swimming, stationary bicycle
- Application of heat or ice, ultrasound, massage, traction.
- Management of symptoms with drugs
- Nonsteroidal anti-inflammatory, muscle relaxants, analgesics, antidepressants, narcotics
- Rigid brace
- Bed rest
- Epidural injection of steroids and/or anesthetics
- Injection of calcitonin
- Acupuncture
- Trigger-point treatment: trigger points are tender nodes of degenerated muscle tissue that may cause severe pain; treatment may involve injection of anesthetics or steroids, stretch, ice massage, ultrasound, or electrical stimulation
- Electrical stimulation: transcutaneous nerve stimulation (TENS), electrical muscle stimulation
- Facet joint injections of steroids or anesthetics
- Chiropractic manipulation
- Multidisciplinary approach
Range of Searches for Surgical Treatments
We also searched for studies on a broad range of surgical treatments. The terminology for surgical procedures is inconsistent in the literature. Therefore, items in the following list of surgical procedures are general in nature. Specific modifications or refinements of these techniques appear in many publications, and these specific modifications were located by our searches.
- Total radical laminectomy (all of the facet joints are removed)
- Standard wide decompressive laminectomy
- Single or multiple levels
- Standard wide decompressive laminectomy with fusion (arthrodesis)
- Single or multiple levels
- Standard wide decompressive laminectomy with fusion and instrumentation (pedicle screw fixation)
- Single or multiple levels
- Instrumentation may be bilateral or unilateral
- Partial laminectomy, hemilaminectomy
- Laminotomy
- Single or multiple levels
- Foraminectomy
- Unilateral or bilateral
Criteria for Retrieval of Full Articles
To prevent the potential for bias in this evidence report, we adopted specific a priori criteria for determining whether we would retrieve any article identified by our literature searches. These criteria were developed by the research team charged with preparing this report and were applied by three research analysts. Separate criteria were developed for treatments of trials of conservative treatment, surgical treatment, and studies of diagnostic modalities.
The decisions of the analysts who applied the criteria were reviewed by two other research analysts, and disputes were always resolved in favor of retrieving the full article.
Once an article was retrieved, it had to meet additional criteria before we included it in our analysis. Different inclusion/exclusion criteria had to be developed for each of our key questions. These question-specific criteria are described as part of the analysis for each question.
Patient Populations Included
Few restrictions were placed on the patient populations in clinical trials of conservative or surgical treatments that were retrieved for this analysis. All patients, or a separately reported subset of patients, had to be diagnosed specifically with lumbar spinal stenosis. Groups of patients less than 20 years of age were excluded to ensure that degenerative lumbar spinal stenosis and not congenital lumbar spinal stenosis was being examined. However, several publications mixed congenital conditions with degenerative conditions. We abstracted only the data on degenerative conditions when these data were reported separately.
Years Included
For all aspects of this evidence report, publications from 1960 to the present were retrieved.
Criteria for Retrieving Articles on Conservative Treatment
We employed the following criteria for determining whether an article on conservative treatments would be retrieved:
- We retrieved any controlled trial, regardless of whether the trial was randomized or concurrently controlled.
- Only studies with at least 10 patients in each arm of the trial were retrieved.
- Studies of patients with degenerative spondylolisthesis, also called pseudospondylolisthesis, were retrieved.
Criteria for Retrieving Articles on Surgical Treatments
The following inclusion criteria were used to determine whether articles on surgical treatments were retrieved:
- Providing that the article met the two inclusion criteria listed below, we retrieved all controlled and uncontrolled trials, regardless of whether they were prospective or retrospective and, if controlled, regardless of whether the controls were historical or concurrent, and regardless of whether patients were randomly assigned to groups.
- Only studies with at least 10 patients in one arm of the trial were retrieved.
- Studies of patients with degenerative spondylolisthesis (also called pseudospondylolisthesis) were retrieved.
Criteria for Retrieving Articles on Diagnostics
Only two general criteria were applied for retrieving articles on diagnosis of spinal stenosis. First, the article had to be on a diagnostic, and second, it had to be on patients with spinal stenosis. Surgical trials included in the analysis were reviewed for image-related data.
Criteria for Retrieving Articles on the Natural History of Lumbar Spinal Stenosis
To answer questions about the natural history of lumbar spinal stenosis, we retrieved all publications that contained (or purported to contain) measurements of the spinal canal among patients with lumbar stenosis and all trials that contained (or purported to contain) measurements of the spinal canal in normal healthy patients or patients with back pain, regardless of cause. Studies reporting spinal measurements in cadavers or skeletons were retrieved, but were not used because they did not include information describing the health status of the subjects before death. To be retrieved, studies relevant to the natural history of spinal stenosis had to contain more than 10 patients, or if the study contained more than one patient group, it had to contain more than 10 patients per group.
Electronic Data Abstraction Forms
Data from all articles that met our inclusion criteria were abstracted using electronic data abstraction forms. These forms were created using Microsoft Access. Using this software, data abstraction forms were designed for entering data about basic trial design information; patient signs, symptoms, characteristics, and treatments; reporting of treatment outcomes; surgical complications; and spinal measurements. The data abstraction forms are presented in the appendix.
The abstraction form for trial information was designed to contain information on trial design, purpose, author, year of publication, general diagnosis of patient condition, a specific description of the treatment outcomes examined, inclusion and exclusion criteria, and other important information with which to judge the quality of the trial. One record containing a unique trial identification number appears for each trial entered in the database.
The abstraction form for patient characteristics and treatments was designed to contain information on each patient group within a trial. A separate record containing a unique patient group identification number appears for each patient group within a trial. This form contains entries for specific diagnosis and type of lumbar spinal stenosis, treatment given to the patient group, division of patient groups based on postsurgical outcomes, number of surgical levels involved, number of patients in the group, specific descriptions of patient treatment, and patient characteristics such as age, dropouts, signs, symptoms, and duration of symptoms prior to treatment. This form also has entries for extent of disease based on the degree of stenosis and reported comorbidity.
Abstraction forms with similar design were created to contain information on treatment outcomes. Separate abstraction forms were needed for ability to work, back pain, back pain relief, leg pain, leg pain relief, dependency and disability, mental status, activities of daily living and physical activities, quality of life, walking capacity, and global success of treatment. These forms have entries for the patient group identification number, number of patients reporting the outcomes, time in months when the outcome was measured (followup time after treatment), mean and standard deviation of the outcome score, statistics used and p values reported, and categorical outcomes scales (usually presented as some combination of Excellent, Good, Fair, Poor, and Very Poor categories). A separate record was entered for each patient group and each followup time for which an outcome was reported.
Special forms were designed for spinal measurements, surgical complications, and surgical outcomes.
Quality of the Literature
Our analysis of the quality of the literature emphasizes study design. In particular, we focus on the following aspects of study design:
- Whether the trial was controlled. (This does not form part of our evaluation of trials of diagnostics, inasmuch as diagnostics are rarely tested by means of controlled trials).
- If the trial was controlled, how patients were assigned to groups.
- Whether the trial contained any confounds that prevent a reader from reaching a conclusion about the efficacy of the treatment or diagnostic studied by that trial.
- Whether the study reported data required to reach a conclusion about our key questions. This includes (but is not limited to) whether the study reported data that would allow us to perform de novo statistical analyses to test hypotheses related to our key questions.
Our assessment of the quality of the literature takes the form of a systematic narrative review. This assessment was performed for each question and appears in our discussion of the literature that underlies each question. Also addressed in certain appropriate questions is an accounting of the number of articles identified, retrieved, and ultimately suitable for answering each question.
Approaches to Evaluating and Combining Evidence
Our analysis of the literature comprises questions for which we were able to combine evidence from different studies, and questions with evidence that did not permit such combination. We employed quantitative methods to answer both types of questions.
Parts of questions 1 and 2 had data that permitted us to combine evidence, and we performed meta-analyses to address them. These data came from observational studies and not randomized controlled trials (RCTs). Therefore, caution is needed in interpreting these data. In these two questions, we were interested in the difference between spinal canal sizes of patients with and without certain symptoms. To perform meta-analyses on such data, we converted the mean canal size difference between groups to the effect size, Hedges' d (Hedges and Olkin, 1985). This effect size is determined by expressing the difference between two groups in standard deviation units. A 95 percent confidence interval around the effect size indicates the range into which, if the experiment were repeated a large number of times, the effect size would be 95 percent of the time. If this interval does not include zero, the effect is considered statistically significant. In some cases, a very large effect size may be considered clinically significant even if it is not statistically significant, and vice versa. We employed a fixed effects model for all of our meta-analyses.
To check the validity of the summary statistic of these models, we used two tests for heterogeneity, the Q test and each study's standardized residual. A standardized residual of >1.96 was taken to indicate that a study's effect size was heterogeneous with respect to the other studies in the meta-analysis. Results of our meta-analyses are graphically displayed in Forrest plots. In this type of plot, each effect size (including the summary statistic) is depicted as a point, surrounded by bars illustrating the 95 percent confidence interval around each study's effect size and around the summary statistic.
To further assist in interpreting the results of our meta-analyses, we depicted the results of our meta-analyses in plots that show a pair of normal curves. One of these curves represents the distribution of nondiseased (or, where appropriate, asymptomatic) patients. The mean of this curve was arbitrarily set to zero. The second curve in each plot represents diseased (or, where appropriate, symptomatic) patients. The difference between the mean of this curve and the mean of the former curve represents d, the effect size. Perhaps more importantly, the curves depict the degree of overlap in the normalized distributions of the canal sizes of the two groups of patients that are being compared in the meta-analysis. We quantified the degree of this overlap using the ∪ statistics described by Cohen (Cohen, 1988). More specifically, we computed the ∪1 statistic, which is the percentage of nonoverlap of the two normal curves. We then subtracted this number from 100 percent to obtain the percentage of overlap between the two curves.
As a final way of facilitating interpretation of the results of our meta-analyses, we expressed their results in terms of a binomial effect size display, or BESD (Rosenthal, 1991). This display depicts the results of a meta-analysis as a 2 by 2 table in which there are 100 patients in each of the two groups being compared. The BESD also artificially dichotomizes the dependent variable and sets the number of patients in each category of this variable to 100. As such, the BESD illustrates the proportion of patients in each group who do or do not have a certain characteristic.
Some minor differences exist between the results derived from the ∪ statistic and those illustrated by the BESD. These differences have not been explored in the statistical literature, but they do make it important to display the results of both statistical procedures. We stress that these differences are minor, and one would arrive at the same conclusions using either one of these methods alone.
Our quantitative analyses of those questions with data that did not permit combination consisted of performing de novo statistical analyses of the data published in the relevant articles wherever possible. Such statistics were either computed from raw data presented in the article or from data read off of figures contained in the article. In some cases, we computed effect sizes based on the published data. For computation of effect sizes derived from dichotomous outcomes, we employed the odds ratio and natural log transformation as described by Hasselblad and Hedges (Hasselblad and Hedges, 1995). We used the method of Torgerson (1958) to analyze data from rating scales that comprised more than two categories and transformed the result of this statistic into Hedges' d. We also conducted additional de novo statistical analyses using a variety of standard tests. Because these tests are quite varied, we describe each of them in the text when used (Torgerson, 1958).
Previous Meta-analyses of Treatments for Lumbar Spinal Stenosis
Prior meta-analysis has been performed on clinical studies of treatments for lumbar spinal stenosis (Niggemeyer, Strauss, and Schulitz, 1997; Turner, Ersek, Herron et al., 1992), degenerative lumbar spondylolisthesis (Mardjetko, Connolly, and Shott, 1994), and degenerative lumbar spondylosis (Gibson, Grant, and Waddell, 1999). These meta-analyses differ in approaches to the pooling of data from across studies and in the interpretation of results. Important differences exist between our approach to the meta-analysis of lumbar spinal stenosis and these other meta-analyses. None of these meta-analyses attempted to evaluate conservative therapy for lumbar spinal stenosis or spondylolisthesis.
Turner et al. (1992) looked at the literature concerning outcomes of surgery for spinal stenosis from 1966 to 1990. Studies were included if they were original studies of patients undergoing decompressive laminectomy for lumbar spinal stenosis and appeared in English-language journals. Studies were excluded when spinal stenosis patients could not be separated from other diagnoses, outcome data were not reported, sample size was less than six, and there were no data concerning patient preoperative characteristics. Seventy-four articles met the inclusion/exclusion criteria. The authors noted the wide variation in outcome measures and created their own definitions for the outcome categories of good-to-excellent, fair, and poor. For actual analysis, the outcomes were dichotomized into good-to-excellent versus fair and poor. Thirty-one studies reported outcomes that allowed the recoding according to this good-to-excellent versus fair and poor rating scale. Studies were included regardless of study design, presence of a control group, or patient characteristics that would preclude combining data across studies. Only three studies had prospective designs. A chi-square test was used to determine whether the proportion of good-to-excellent versus fair and poor outcomes varied significantly across studies. This test was highly significant (p <0.0001), indicating significant heterogeneity of outcomes across studies. The average proportion of good-to-excellent outcomes among the studies was 64 percent, but the range was 26 percent to 100 percent. No standard deviations or confidence limits were calculated. The authors used regression analysis to examine whether specific predetermined variables could explain the large variation across studies. No significant associations between outcome and patient variables such as age, gender, neurogenic claudication, number of levels of laminectomy, or prior back surgery were found. The authors concluded that the poor scientific quality of the literature, especially major deficits in study design, analysis, and reporting, prevented the construction of a proper meta-analysis. In particular, the lack of comparative trials, especially randomized controlled trials, prohibited any attempt at addressing the question of efficacy of various therapies through meta-analysis (Turner, Ersek, Herron et al., 1992).
There are difficulties in interpreting the Turner et al. (1992) analysis. The authors do not provide a detailed description of their methods and, in particular, do not state how (or whether) they calculated effect sizes for each study. Therefore, it is not at all clear that they used meta-analytic methods to perform their analysis. Failure to use such methods could severely compromise their findings. Consequently, we will not assess their findings.
Niggemeyer et al. (1997) conducted a meta-analysis of surgical treatments for lumbar spinal stenosis, again using their own definitions for good, fair, and poor outcomes. Studies published between 1975 and 1995 in English, French, or German were included if the studies reported cases of degenerative lumbar spinal stenosis with no prior back surgery and had a minimum of seven patients. Thirty articles met the inclusion criteria. As in the meta-analysis by Turner et al., studies were included regardless of study design, presence of a control group, or patient characteristics that would preclude combining data across studies. The authors calculated a pooled weighted proportion for each of the three outcome categories within each of three surgical approaches: decompression alone, decompression plus fusion, and decompression with fusion and instrumentation. Proportions for each outcome category were then compared across surgical approaches using a Z score and pooled variance. This resulted in the calculation of nine separate Z scores (3 categories by 3 surgical comparisons). None of the Z scores were significantly different from zero. The use of uncontrolled trials in this analysis compromises its validity. The study was further compromised by the lack of any test of heterogeneity. Therefore, one cannot determine whether the trials were, in fact, combinable. Consequently, we do not discuss this analysis further (Niggemeyer, Strauss, and Schulitz, 1997).
Mardjetko et al. (1994) performed a meta-analysis of studies examining decompression and fusion for treating degenerative lumbar spondylolisthesis. Studies were included if they enrolled patients with degenerative spondylolisthesis with radicular leg pain or neurogenic claudication, were published in English between 1970 and 1993, had at least four patients, and reported primary outcomes specifically for the spondylolisthesis patients. Twenty-five papers met the inclusion criteria, and of these, 21 were classified as retrospective, nonrandomized, and uncontrolled. Outcomes were reassigned into satisfactory or unsatisfactory categories, and pooled weighted proportions were calculated. Z scores were then calculated for comparisons of decompression without fusion to decompression with fusion and decompression with fusion and instrumentation. Significant Z scores were found in favor of fusion increasing patient satisfaction and instrumentation increasing fusion rates. The authors commented on the serious methodological flaws, such as poorly defined outcome measures, lack of blinded outcome assessment, failure to provide adequate patient demographics, and lack of stratification of outcomes by diagnosis, that led to a poor meta-analysis. The meta-analysis method allowed data from poorly designed studies to dilute the data from better-quality studies. Like the Niggemeyer et al. (1997) analysis, this analysis does not provide a test of heterogeneity. For these reasons, we do not consider the results of this analysis (Mardjetko, Connolly, and Shott, 1994).
In 1999, Cochrane Review published a discussion of the difficulties of conducting a meta-analysis of randomized controlled trials on surgery for degenerative lumbar spondylosis (Gibson, Grant, and Waddell, 1999). Their analysis addressed the questions of what evidence exists concerning the clinical effectiveness of lumbar spine surgery and what evidence exists concerning alternative forms and techniques of lumbar spine surgery. All randomized and quasirandomized controlled trials identified between 1966 and 1998 and pertaining to degenerative lumbar spondylosis and the associated pathologies or clinical syndromes of back pain, instability, spinal stenosis, and degenerative spondylolisthesis were included. Fourteen RCTs for degenerative lumbar spondylosis were retrieved. Many of the trials had major defects of design, such as including heterogeneous pathologies and clinical syndromes, and the subgroups were often too small to give meaningful results. The authors mentioned that clinical outcomes were mainly crude ratings on a three- or four-point scale. The reader should note that spondylosis is a large category of spinal pathology. Differences in the number of RCTs mentioned in the present analysis and the Cochrane Review are due to separating out the specific pathologies of lumbar spinal stenosis and spondylolisthesis, as well as not including studies that did not report outcomes separately for these conditions. The Cochrane Review found no RCTs dealing with the efficacy of surgical decompression for degenerative lumbar spondylosis or spinal stenosis or of surgical fusion for degenerative spondylosis and back pain. A meta-analysis of fusion rates in trials of instrumented fusion versus fusion alone was performed. This analysis suggested that solid fusion was more likely if instrumentation was used. However, the significant heterogeneity among the trials prevented the drawing of conclusions about the results of instrumented fusion for any particular pathologic condition or about any particular instrumentation system. The authors concluded that there is a serious lack of scientific evidence supporting surgical management for degenerative lumbar spondylosis. The authors go on to say that "there is no scientific evidence on the effectiveness of any form of surgical decompression or fusion for degenerative lumbar spondylosis compared with natural history, placebo, or conservative management."
A meta-analysis of the accuracy of CT, MRI, and myelography was published in 1992 (Kent, Haynor, Larson et al., 1992). The authors wished to evaluate what was known about the diagnostic accuracy of these imaging tests for the diagnosis of lumbar spinal stenosis in adults without prior surgery. Studies were included if they contained cases of canal, lateral recess, or foraminal stenosis. Cases with entirely acute-onset radiculopathy, where all cases had herniated disk, or with fewer than 10 cases of lumbar spinal stenosis were excluded. The contribution of plain film to diagnosis was not reviewed. One hundred and sixteen articles from 1986 to 1991 were read, and only 14 were retained for analysis. A series of quality criteria were developed based on the technical quality of study procedures (index test quality, reference test quality, application of reference test) and on the proper selection of patients and unbiased interpretation of information (independence of interpretations, clinical description, cohort assembly, and sample size). Based on these criteria, an overall rating from A (best) to D (worst) was assigned to each article. All studies received a C or D rating. C studies had poor clinical descriptions but had intermediate ratings in all other categories. Studies failing the C rating received a D. The authors calculated sensitivities of 0.81 to 0.97 for MRI (four studies), 0.70 to 1.0 for CT (nine studies), and 0.67 to 0.78 for myelography (four studies) and specificities of 0.72 to 1.0 for MRI (three studies), 0.80 to 0.83 for CT (four studies), and 0.7 for myelography (one study). Based on their review of the literature, the authors concluded that any estimates of sensitivity or specificity must be considered imprecise, since the estimates are based on small sample sizes and on studies with serious methodological flaws. In particular, the imaging literature for spinal stenosis failed to provide independent corroboration with a reference standard for the diagnosis. Thirty articles were excluded due to the lack of a proper reference standard. Failure to assemble a representative cohort and failure to maintain independence between image reading and reference standards were also common problems. The applicability of these cases to general practices was reduced by a selection of patients referred for surgery. These patients will tend to have the most severe disease and increase the frequency of true-positive diagnosis. The variation in study design prevented the pooling of sensitivity and specificity estimates.
Our current assessment of studies examining the diagnostic accuracy of MRI, CT, and myelography for lumbar spinal stenosis also concludes that these studies have one or more flaws in design or reporting that adversely affect the reliability or applicability of the results.
- Methodology - Treatment of Degenerative Lumbar Spinal StenosisMethodology - Treatment of Degenerative Lumbar Spinal Stenosis
- Appendix F. Statistical Reference - Diagnosis and Treatment of Parkinson's Disea...Appendix F. Statistical Reference - Diagnosis and Treatment of Parkinson's Disease
- Acknowledgments - Diagnosis and Treatment of Acute Bacterial RhinosinusitisAcknowledgments - Diagnosis and Treatment of Acute Bacterial Rhinosinusitis
- Appendix 2. Search Strings - Rehabilitation for Traumatic Brain InjuryAppendix 2. Search Strings - Rehabilitation for Traumatic Brain Injury
- Bibliography - Rehabilitation for Traumatic Brain InjuryBibliography - Rehabilitation for Traumatic Brain Injury
Your browsing activity is empty.
Activity recording is turned off.
See more...
