NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Appel LJ, Robinson KA, Guallar E. Utility of Blood Pressure Monitoring Outside of the Clinic Setting. Rockville (MD): Agency for Healthcare Research and Quality (US); 2002 Nov. (Evidence Reports/Technology Assessments, No. 63.)
This publication is provided for historical reference only and the information may be out of date.
The utility of blood pressure monitoring outside of the clinic setting was a topic nominated to the Agency for Healthcare Research and Quality (AHRQ) by a group of experts in blood pressure measurement. In September of 2000, the AHRQ awarded a contract to the Johns Hopkins Evidence-based Practice Center (EPC) to prepare an evidence report on this topic. The Johns Hopkins EPC established a team and work plan to develop a report that would identify and synthesize the best available evidence on blood pressure monitoring. One of the first tasks was the identification of an appropriate partner.
In December 2000, the National High Blood Pressure Education Program (NHBPEP) of the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health (NIH) hosted a working meeting. The NHBPEP includes representatives from national professional and voluntary organizations as well as from federal agencies. Arising from that meeting was an agreement from the NHBPEP Coordinating Committee to partner with the Johns Hopkins EPC on this project.
The project consisted of recruiting technical experts, formulating and refining the specific questions, performing a comprehensive literature search, summarizing the state of the literature, constructing evidence tables, and submitting the report for extensive peer review.
Recruitment of Technical Experts and Peer Reviewers
Experts were sought who could provide content and/or methodological guidance. The five technical experts were chosen to cover several domains: hypertension management, SMBP, ABP, clinic BP, and evaluation of screening and diagnostic tests. Input was sought from the partner and technical experts through ad hoc correspondence as well as through more formal requests for feedback during the project. Specific requests for feedback were made for key decisions, such as selection and refinement of the questions.
Comprehensive feedback on the draft report was sought from the partner, the technical experts, and other reviewers. Reviewers included members of the NHBPEP Coordinating Committee selected through discussions with the partner. (See appendix A for list of organizations represented by reviewers from which comments were received.)
Patient Population
The search was not limited by age, gender or any other patient characteristic. However, because of the extensive volume of literature, the review did not synthesize evidence for all types of populations. For instance, it was felt that the use of blood pressure monitoring during pregnancy was a distinctive application of these technologies that was beyond the scope of this report. Likewise, articles that focused exclusively on populations of children (less than 20 years of age) were not reviewed.
Questions
The original questions provided by AHRQ included several descriptive questions that were more appropriately addressed as background text in Chapter 1. The EPC team refined the remaining questions and requested feedback from the technical experts and from the partner. When the large volume and heterogeneity of the literature became apparent, the EPC team refined the questions further. Listed below are the questions addressed in this report.
- Comparison of clinic, ambulatory, and SMBP readings:
1a. What is the distribution of the BP differences between clinic, ambulatory and SMBP readings? If there are differences, are these differences reproducible?
2a. What is the prevalence of WCH as defined by SMBP? Is this pattern reproducible?
3a. What is the prevalence of WCH as defined by ABP measurement? Is this pattern reproducible? - SMBP levels and WCH based on SMBP as related to clinical outcomes:
2a. Is SMBP more or less strongly associated with BP-related target organ damage than clinic BP measurements?
2b. Does SMBP predict subsequent clinical outcomes?
2c. What is the incremental gain in prediction of clinical outcomes from use of self-measurement devices beyond prediction from clinic BP alone?
2d. What is the effect of treatment guided by SMBP in comparison to treatment guided by clinic BP, in terms of:
- BP-related target organ damage
- symptoms
- use of anti-hypertensive drug therapy
- BP control
- ABP levels and WCH based on ABP as related to clinical outcomes:
3a. Is ambulatory blood pressure more or less strongly associated with BP-related target organ damage than clinic BP measurements?
3b. Does ambulatory blood pressure predict subsequent clinical outcomes?
3c. What is the incremental gain in prediction of clinical outcomes from use of ambulatory devices beyond prediction from clinic BP alone?
3d. What is the effect of treatment guided by ABP in comparison to treatment guided by clinic BP, in terms of:
- BP-related target organ damage
- symptoms
- use of anti-hypertensive drug therapy
- BP control
- Does the evidence for the above questions vary according to a patient's age, gender, income level, race/ethnicity, and clinical subgroups (e.g., hypertensive/normotensive, diabetic, renal transplant status)?
Causal Pathway
During its deliberations, the EPC team developed a conceptual framework to assist in the formulation of its research questions. (See Figure 1.) It is evident that several factors might influence the use and interpretation of BP measurements, including patient factors (age, race, gender, clinical conditions), technical factors (accuracy, reproducibility, operator, machine), other CVD risk factors, and response to treatment. Also, there are many potential outcomes of interest including clinical events (CHD, stroke, kidney disease), BP control, cost, side effects, and medication. The EPC team had sufficient resources to address several key points in this pathway (e.g., prognosis) but not all steps (e.g., assessment of device accuracy) or outcomes (e.g., cost). This pathway can also be used as a conceptual framework to identify gaps in the evidence.
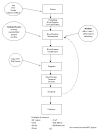
Figure
Figure 1. Conceptual Framework.
Literature Search Methods
Searching the literature included the steps of identifying reference sources, formulating a search strategy for each source, and executing and documenting each search.
Sources
A comprehensive search plan was developed that include electronic and hand searching. Several electronic databases were searched.
First searched was MEDLINE®, or MEDlars onLINE, the database of bibliographic citations and author abstracts from over 4,000 current biomedical journals published in the United States and 70 foreign countries. MEDLINE® coverage begins in the mid 1960's. MEDLINE® was accessed through PubMed®, the Internet access to MEDLINE® provided by the National Library of Medicine (NLM). Searches using PubMed were completed in January 2001 and then again, in March 2001 for newly added citations.
The Cochrane CENTRAL Register of Controlled Trials was then searched. This is a database of all clinical trials (primarily randomized controlled trials and controlled clinical trials) identified through the searching efforts of the Cochrane Collaboration. The CENTRAL database includes search results from many electronic databases, including MEDLINE® and EMBASE, as well as results from the hand searching of more than 1,000 journals, for all publication years starting in 1948.17 The CENTRAL database also includes the specialized register of controlled trials developed by the Cochrane Hypertension Collaborative Review Group (CRG). The Hypertension CRG has completed extensive searching of electronic databases and members of this CRG are hand searching a number of key hypertension journals such as American Journal of Hypertension, and the Journal of Clinical Hypertension. The CENTRAL database is made available on The Cochrane Library, which is issued quarterly. Issue 1 of the 2001 of The Cochrane Library was searched.
Internet Grateful Med®, provided as a Web-based service by the NLM, was used to access HealthSTAR. This electronic database combines the former HEALTH (Health Planning and Administration) and HSTAR (Health Service/Technology Assessment Research) databases and includes over 3.1 million citations from 1975 to present. Citations include relevant bibliographic records from MEDLINE® (1975 to present) and unique records from three sources: (1) records emphasizing health care administration selected and indexed by the American Hospital Association; (2) records emphasizing health planning from the National Health Planning Information Center; and (3) records emphasizing health services research, clinical practice guidelines, and health care technology assessment selected and indexed through NLM's National Information Center on Health Services Research and Health Care Technology. HealthSTAR was searched once in February, 2001.
Hand searching for possibly relevant citations took several forms. First, priority journals were identified through an analysis of the frequency of citations per journal in the database of search results as well as through discussions amongst the EPC team. Fifteen specialty and general journals were thus identified. (See Appendix B.) The table of contents of these journals were scanned for possibly relevant citations from January 2001 to May 31, 2001. The exception to this was the Journal of Clinical Hypertension which, in its current form, began publishing in 1999 and was not indexed in MEDLINE® during the completion of searching for this project. The hand search of this journal started with the beginning of its publication in 1999.
For the second form of hand searching, a database of reference material, identified through an electronic search for relevant guidelines and reviews, through discussions with experts, and through the article review process, was created in the reference management software, ProCite. A listing of titles and abstracts from this database, the BP References Database, was reviewed by the principal investigator to identify key articles. The reference lists from these key articles were then examined to identify any additional articles for consideration.
Additionally, the proceedings of the following conferences were hand searched: Leuven Consensus Conference on Blood Pressure Monitoring, 1999; Annual Scientific Session of the American Heart Association Council for High Blood Pressure Research, October 2000; Annual Scientific Session of the American Heart Association, November 2000; Annual Scientific Session of American Heart Association Council on Epidemiology and Prevention, March 2001; Annual Scientific Meeting of the American Society of Hypertension, May 2001.
Search Terms and Strategies
Search strategies, specific to each database, were designed to maximize sensitivity. Initially, a core strategy for PubMed was developed based on an analysis of the Medical Subject Headings (MeSH) and text words of 47 key articles identified a priori. This strategy was then modified for use on the Cochrane CENTRAL Register of Controlled Trials and in searching HealthSTAR. (See Appendix C.)
Organization and Tracking of Literature Search
The results of the searches of electronic databases were downloaded and, using the duplication check in the bibliographic software ProCite, articles not previously retrieved were included in the Blood Pressure Citations Database. This ProCite database was used to store citations and to track the search results and sources. The results of the abstract review process were also tracked using ProCite.
Abstract Review
Specific inclusion and exclusion criteria were applied at each of three levels of review, with criteria becoming more stringent as the process moved from searching, to the review of abstracts and to the review of articles. After identifying a citation, its title and abstract were reviewed, and articles were included or excluded from the article review on this basis.
Identification of Inclusion and Exclusion Criteria
During the abstract review process, emphasis was placed on identifying all articles that may possibly have original data pertinent to the questions. As previously described, the technical experts were consulted during the development of inclusion and exclusion criteria.
In evaluating titles and abstracts, the following criteria were used, at the first level abstract review, to exclude articles from further consideration.
- article does not include ambulatory or self-measured blood pressure
- article does not include human data
- article not in English
- article contains no original data
- article included < 20 patients
- article was a meeting abstract only (no full article for review)
- article does not apply to any of the study questions
A prohibitively large number of citations were deemed eligible for full article review after the initial abstract review. Additional criteria were then applied during a second level abstract review:
- article included < 50 patients or article addresses reproducibility and included < 20 patients
- article describes cross-sectional/retrospective study, addresses only question #2 or #3, and does not include comparison with clinic measurement
- article describes cross-sectional/retrospective study with outcome other than left ventricular mass or proteinuria/albuminuria
- article addresses only prevalence of dipping versus non-dipping and no other research questions
- article describes clinical trial that does not have longitudinal analysis of clinical outcomes other than blood pressure
Abstract Review Process
For the first level abstract review, titles and abstracts for all articles retrieved by the literature search were printed on an abstract form and distributed to two reviewers. (See Appendix D.) In addition to screening for eligibility, the initial abstract review process was also used to classify the articles by topic. When reviewers agreed that a decision regarding eligibility could not be made because of insufficient information, the full article was retrieved for review.
The results of the abstract review process were entered into the Blood Pressure Citations Database developed in the bibliographic software ProCite. Citations deleted through the abstract review process were tagged with the reason for exclusion. Citations deemed eligible for full article review based on the initial abstract review, were printed onto the second level abstract form (Appendix D) and distributed to two reviewers. For this level of abstract review, when reviewers agreed that there was insufficient information to make a decision regarding eligibility these citations were considered eligible for full article review. As for the first level abstract review, results were tracked in a ProCite database and reasons for exclusion were noted for any citation deemed not eligible for review.
For both levels of abstract review, citations where the reviewers disagreed on eligibility were returned to the reviewers for adjudication.
Article Review
The purpose of the article review was to confirm relevance of each article to the research questions, to determine methodological characteristics pertaining to study quality, and to collect evidence that addressed the research questions. Where articles described more than one study, reviewers were instructed to complete the eligibility assessment (i.e., comparison to inclusion and exclusion criteria), quality assessment and data abstraction for each study separately. For each question, publications of the same information from the same study were also excluded. These apparent duplicate publications were reviewed on a per case basis. Multiple publications were kept if they reported on different results (i.e., different outcomes). Otherwise, the article with a more comprehensive reporting of the data reviewed.
Because of the large number of citations that remained eligible for full article review even after the second level abstract review, additional exclusion criteria were applied at the article review level. The final full list of exclusion criteria differed by question.
Exclusion criteria applied to all articles during article review:
- does not include human data
- not in English
- no original data
- meeting abstract (no full article for review)
- article does not apply to any of the research questions
- article does not include ambulatory or self-measured blood pressure
- article included <50 patients OR addressed reproducibility and included <20 patients
- device evaluation was the primary purpose of the study
- study population is exclusively pregnant women
- study population is exclusively children (<20 years of age)
- article addresses research question, but does not present data in an abstractable format
- article addresses only the prevalence of dipping versus non-dipping and no other research questions
Additional exclusion criteria for articles addressing question #1:
- article provided data for clinic blood pressure AND ambulatory blood pressure, or clinic blood pressure AND self-measured blood pressure but did not include a formal within-person comparison of measurements (e.g., no p-value, standard error, standard deviation, confidence intervals or only correlation coefficient(s) provided)
- clinic blood pressure measurement used in analyses was completed on one day only
The criterion of more than one day of measurement for clinic blood pressure was added because an average clinic blood pressure based on just one day of measurements (typically just one to three readings) is extremely imprecise and could lead to a biased comparison with ambulatory or self-measured blood pressure. This criterion was not applied to articles addressing questions 2-4.
For articles addressing questions #2a and #3a, the following specific exclusion criteria were applied:
- article described cross-sectional/retrospective study and did not include comparison with clinic measurement
- article described cross-sectional study but outcome was not left ventricular mass (by echocardiography) or proteinuria/albuminuria
Several endpoints were considered to compare the ability of clinic, self-measured, and ABP monitoring to assess target organ damage caused by hypertension. Left ventricular mass and protein/albumin excretion were included in the report because they are frequently used in the clinic setting to assess the severity and prognosis of hypertension, they are frequently used in hypertension research studies, and there are standard methods available that may allow for some comparability across studies. Other echocardiographic indices of left ventricular enlargement, such as septal thickness or posterior wall thickness, are not consistently reported, and were not considered in this report. Other markers of target organ damage, such as other echocardiographic determinations of left ventricular function, retinopathy, brain MRI findings, carotid intima-media thickness, were not considered in this report.
Because a relatively small number of articles were expected and the abstraction would be quite different, prospective studies (questions #2b or #3b), studies of reproducibility (question #1 a, b, c) and trials examining the impact of treatment guided by clinic versus that guided by ambulatory (question #3d) or self-measurement (question #2d), were tagged during the initial article review. A separate review was then completed for each of these questions including the following additional or modified exclusion criteria.
For articles addressing reproducibility (#1 a, b, c) the additional or modified exclusion criteria were:
- article included < 20 patients
- article does not include reproducibility of white-coat hypertension.
An initial review of articles did not identify any articles addressing reproducibility of the differences between clinic, ambulatory and/or self blood pressure measurements (question #1a). A separate review form for this question was, therefore, not developed. However, the review form used for articles addressing reproducibility was designed to identify articles addressing reproducibility of differences for future consideration.
Additional exclusion criteria for prospective or longitudinal studies (question #2b or #3b) was outcome not of interest.
For articles concerning effect of treatment guided by ambulatory or self measured blood pressure (question #2d or #3d), the additional criterion applied was non-random allocation of participants.
Quality Assessment and Data Abstraction
Forms were developed to confirm eligibility for full article review, assess study characteristics and to abstract the relevant data to address the study questions. The forms were developed through an iterative process including the review of forms used for previous EPC projects, discussions among team members and experts, and through pilot testing. This process was complex and time consuming due to the heterogeneity of the literature and the diverse questions being addressed.
For the general article review completed initially (for questions #1, #2a, and #3a), three forms were developed and color-coded to aid reviewers and data entry personnel (Appendix E). As necessary, separate forms were created for the three types of studies previously described (i.e., prospective studies (questions #2b or #3b), studies of reproducibility (question #1 a, b, c), and trials examining the impact of treatment guided by clinic versus that guided by self-measured or ambulatory blood pressure measurement (question #3d or #2d)). (See Appendix F).
General Review: Quality Assessment
The first form completed comprised three sections. The first section included the exclusion criteria so that reviewers could confirm the eligibility of the article before proceeding with the full article review. The second section contained a list of each of the study questions allowing reviewers to tag articles by question addressed. This allowed for the identification of articles to be pulled and abstracted separately (e.g., those describing prospective studies). The final section contained questions designed to provide an assessment of study quality. The questions were designed to assess characteristics such as research design and blinding. These questions allowed for the identification of methodological strengths and weaknesses.
General Review: Data Abstraction Part I
The characteristics of the study and baseline information, such as the details concerning the method of BP measurement, were collected on this form.
General Review Data Abstraction: Part II
The specific population characteristics and the results were abstracted using this form. Data were abstracted separately for the whole study population and subgroups by completing multiple forms, as necessary.
Question Specific Reviews
For prospective studies, studies concerning reproducibility of white coat hypertension and trials assessing treatment guided by blood pressure measurement, separate forms were developed as necessary. For prospective studies, the same quality assessment and Part I of the data abstraction form were used. Additional results were abstracted directly into specific fields of a spreadsheet. A separate form was developed for articles addressing reproducibility. For trials, a new quality assessment form was developed, the same Part I of the data abstraction was used, and additional data was entered into a spreadsheet. (See Appendix F for separate forms developed for these articles and for the fields of the spreadsheets.)
Article Review Process
A serial article review process was employed. In this process, the quality assessment and abstraction forms were completed by the primary reviewer. The secondary reviewer, after reading the article, checked each item on the forms for completeness and accuracy. The reviewer pairs were formed to include personnel with clinical and/or methodological expertise. Reviewers were not masked to the article author, institution, or journal. In most instances, data were directly abstracted from the article. If possible, relevant data were also abstracted from figures. In some instances, data were recalculated to meet the specification of the report (e.g., calculation of relative risks from incidence rates).
During the general article review, articles were tagged as to what question(s) they addressed. This process identified those articles requiring separate review (i.e., use of the question specific review instruments).
All information from the general article review process was entered in a relational database (Blood Pressure Evidence Database) via a web-interface. Data from question specific reviews were entered into the Blood Pressure Evidence Database (where same forms completed) or directly into spreadsheets.
Peer Review
Throughout the project, feedback was sought from the technical experts through ad hoc and formal requests for guidance. A draft of the completed report was sent to the technical experts, as well as to the partner, AHRQ, and other peer reviewers. Substantive comments were entered into a database. Revisions were made to the evidence report, as warranted, and a summary of the comments and their disposition was submitted to AHRQ with the final report.
- Methodology - Utility of Blood Pressure Monitoring Outside of the Clinic SettingMethodology - Utility of Blood Pressure Monitoring Outside of the Clinic Setting
- Appendix B Journals Searched - Utility of Blood Pressure Monitoring Outside of t...Appendix B Journals Searched - Utility of Blood Pressure Monitoring Outside of the Clinic Setting
- Randomized Controlled Trials - Systems to Rate the Strength Of Scientific Eviden...Randomized Controlled Trials - Systems to Rate the Strength Of Scientific Evidence
- Methods - Systems to Rate the Strength Of Scientific EvidenceMethods - Systems to Rate the Strength Of Scientific Evidence
- Sahlingia subintegra isolate K27 (K19-4-112-2) photosystem II reaction center pr...Sahlingia subintegra isolate K27 (K19-4-112-2) photosystem II reaction center protein D1 (psbA) gene, partial cds; chloroplastgi|2560331278|gb|OP235413.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...
