NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Coulter I, Hardy M, Shekelle P, et al. Effect of the Supplemental Use of Antioxidants Vitamin C, Vitamin E, and Coenzyme Q10 for the Prevention and Treatment of Cancer. Rockville (MD): Agency for Healthcare Research and Quality (US); 2003 Aug. (Evidence Reports/Technology Assessments, No. 75.)
This publication is provided for historical reference only and the information may be out of date.

Effect of the Supplemental Use of Antioxidants Vitamin C, Vitamin E, and Coenzyme Q10 for the Prevention and Treatment of Cancer.
Show detailsWe synthesized evidence from the scientific literature on the effectiveness of vitamin C, vitamin E, and coenzyme Q10 for the prevention and treatment of cancer, using the evidence review and synthesis methods of the Southern California Evidence-based Practice Center (SCEPC). Established by the Agency for Healthcare Research and Quality (AHRQ), the center conducts systematic reviews and technology assessments of all aspects of health care; performs research on improving the methods of synthesizing the scientific evidence, developing evidence reports, and conducting technology assessments; and provides technical assistance to other organizations in their efforts to translate evidence reports and technology assessments into guidelines, performance measures, and other quality-improvement tools.
Project staff collaborated with the National Institutes of Health's National Center for Complementary and Alternative Medicine (NCCAM), the Task Order Officer at AHRQ, and technical experts representing disciplines related to the intervention topic, conditions studied, and/or methods used.
Scope of Work
Our literature review process consisted of the following steps:
- Establish criteria for inclusion of articles in review.
- Identify sources of evidence in the scientific literature.
- Identify potential evidence with attention to controlled clinical trials using antioxidants.
- Evaluate potential evidence for methodological quality and relevance.
- Extract data from studies meeting methodological and clinical criteria.
- Synthesize the results.
- Perform further statistical analysis on selected studies.
- Perform pooled analysis where appropriate.
- Submit the results to technical experts for peer review.
- Incorporate reviewers' comments into a final report for submission to AHRQ.
Objectives
Based on a discussion with the Task Order Officer for AHRQ, the Director of NCCAM, Co-Directors of SCEPC, and project staff, we selected, as the focus for this report, the use of vitamin C, vitamin E, and coenzyme Q10 to treat and prevent cancer.
The report was guided by the following research questions:
- What kinds and numbers of study reports were available that presented research on the use of antioxidants for treating and preventing cancer?
- Were interventions used for treatment, primary or secondary prevention, or in adjunct to conventional treatment?
- Were interventions used for treatment or modification of known risk factors for cancer or pre-malignant states?
- What types of outcomes were measured for the identified condition?
- What is the methodological quality of the studies identified?
- Can statistical results from the various studies be pooled?
Literature Search Design
Technical Expert Panel
The SCEPC is advised on CAM topics by a group of technical experts regarding the search and inclusion criteria and appropriate analyses. The technical experts represent diverse disciplines including acupuncture, Ayurvedic medicine, chiropractic, dentistry, general internal medicine, gastroenterology, rheumatology, integrative medicine (the practice of combining alternative and conventional medicine), neurophysiology, pharmacology, psychiatry, psychoneuroimmunology, psychology, sociology, botanical medicine, and traditional Chinese medicine. The technical experts assisted the project in several ways. They aided us in identifying potential topics for review, appropriate sources of relevant literature, and technical experts for peer review; assessing our search strategies; and addressing specific questions in their areas of expertise. Appendix A lists members of the expert panel along with their affiliations.
Identification of Literature Sources
Potential evidence for the report came from three areas: on-line library databases, the reference lists of all relevant articles, and other sources such as identified experts and the personal libraries of project staff and their associates. The reference librarian at RAND identified traditional biomedical databases as well as databases that focus on the condition of interest and alternative and complementary medicine (Table 1).
Table
Table 1. Biomedical and other databases searched.
We conducted four searches specifically on the interventions of interest. The full search strategies are displayed in Appendix B. We utilized the National Library of Medicine's controlled vocabulary thesaurus called Medical Subject Headings or “MeSH terms.” Limiting the output to human studies, we searched using the terms coenzyme Q10, vitamin E, vitamin C, and their many pharmacological synonyms (Table 2); the condition of interest (cancer); and study design or article type (randomized controlled trials, clinical controlled trials, meta-analyses, and systematic reviews). Because this report is focused on efficacy, clinical trials are preferred since they provide control groups which account for confounding factors. These searches yielded a total of 4595 titles, many of which were duplicates, because one article would appear repeatedly as each new search was added.
Table
Table 2. Additional search items for antioxidants studied.
Two reviewers (a physician and a PhD) independently evaluated deduplicated lists of 1079 titles that the on-line database searches generated as well as 258 additional titles from other sources, such as professional libraries and reference mining. The reviewers read the lists of titles and accepted articles that:
- focused on vitamin C, vitamin E, or coenzyme Q10 for treatment or prevention of cancer, or the modification of a known risk factor for cancer or improvement in a pre-malignant state;
- focused on controlled trials on humans;
- presented a meta-analysis or systematic review of the interventions and condition;
- presented historical or descriptive background information about antioxidants and their use.
Articles that either reviewer classified as meeting these criteria were accepted. Articles were rejected that both reviewers considered:
- focused on a disease state that was not the topic of interest;
- contained animal or in vitro data unless human clinical trial information or significant background information was also included.
Language was not considered a barrier to inclusion.
From this stage of the screening process, the reviewers requested a total of 1337 articles, of which we were able to obtain 1125. Selected articles were further evaluated to see if they met the inclusion criteria. Based on this evaluation, we selected 432 that went on to further screening.
Using Microsoft Access database software, we tracked requests for articles. We used Pro-Cite as a link to read the citations into the Access database as well as to manage our reference list. We also used the database to produce and store our data collection instruments. Table 3 summarizes the search strategy shown in Appendix B. The details of the screening process are discussed in the next section.
Table
Table 3. Summary of search strategy.
Evaluation of Evidence
Two physicians, each trained in the critical analysis of scientific literature, independently reviewed each article, abstracted data, and resolved disagreement by consensus. From the 432 articles accepted after the initial title screening, they accepted 36 articles for further study, based on the data collected using the screening form. These 36 articles were therefore included in the synthesis of evidence because they:
- focused on the antioxidants vitamin C, vitamin E or coenzyme Q10 and cancer;
- presented research on human subjects;
- reported the results of a clinical trial.
To be clear about our terminology: a “trial” refers to a controlled clinical trial; a “study” refers to a presentation of a specific portion of a trial's results, e.g., focused on one outcome or at a particular follow-up time; and an “article” refers to a published document. Some articles may contain more than one study, particularly if they contain results from more than one trial. Some trials, especially large ones, have many associated studies and articles.
Two articles11, 12described two different trials each, so a total of 38 unique studies were referred for detailed review. Many of these studies reported on three large trials—the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Trial, the Linxian General Population, and the Linxian Dysplasia Group Trials—which are discussed at length in the results section and displayed in the evidence table. We created a one-page data collection instrument that served as a screening form for this process. Appendix C contains a copy of this screening instrument.
Extraction of Data
Detailed information from each of the 38 studies was collected on a specialized data collection instrument (the Quality Review Form) designed for this purpose. This Quality Review Form (Appendix D) was developed in consultation with our technical experts. We included questions about the trial design; the quality of the trial; the number and characteristics of the patients; patient recruitment information; details on the intervention, such as the dose, route of administration, frequency, and duration; the types of outcome measures; and the time between intervention and outcome measurement. Two trained reviewers, working independently, extracted data in duplicate and resolved disagreements by consensus. A senior physician researcher on the project staff resolved any disagreements not resolved by consensus.
A note about equivalence of units for data extraction: dosages of vitamin E, often given as alpha-tocopherol, are reported in either milligrams or international units (IU). To interconvert these units, consider 1 milligram of alpha-tocopherol approximately equal to 1.5 IU of vitamin E.
To evaluate the quality of the trials, we collected information on the study design, appropriateness of randomization, blinding, description of withdrawals and dropouts and concealment of allocation.13 A score for quality was calculated for each trial using a system developed by Jadad.14 The Jadad score rates studies on a 0 to 5 scale.14 A score is based on the answer to three questions: Was the study described as randomized? Was the study described as double-blind? Was there a description of withdrawals and dropouts? One point is awarded for each “yes” answer, and no points are given for a “no” answer. An additional point is given if the randomization method described was appropriate. A point is deducted if the method is described but is not appropriate. A point is awarded if the method of blinding is appropriate and described, and one point is deducted if the blinding method is described, but inappropriate. Empirical evidence has shown that studies scoring 2 or less report exaggerated results compared with studies scoring 3 or more.15 Thus, studies with a Jadad score of 3 or more are referred to as “high” quality, and studies scoring 2 or less are referred to as “poor” quality.
The flow of articles from the point at which they entered our database, through the article ordering, screening, quality review, and statistical analysis stages is displayed in Figure 1. All articles that went on for abstraction were examined for inclusion in the data synthesis.
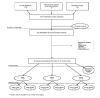
Figure
Figure 1. Literature Flow.
Selection of Studies for Meta-Analysis
Prior to the analysis, we entered all data on outcomes and treatments into the statistical program SAS.16 We analyzed this dataset to identify the clinically relevant outcomes that were reported most commonly and would therefore be appropriate for data synthesis. These outcomes were death, development of new tumors and progression of existing tumors, and development of adenomatous colonic polyps. The grouping of treatments and the appropriate comparison group, e.g., any combination of vitamins with vitamin E versus placebo, was based on clinical knowledge and was decided a priori. In addition to these three clinical outcomes, our review also examines intermediate outcomes. These are outcomes that are considered as precursors to such clinical outcomes as new tumor development and death, although they vary in the degree to which they are good predictors of those outcomes.
We defined the outcomes of interest as follows. “Death” from cancer was used as an outcome for any trial which gave survival results. “New tumors” includes the development of new tumors in a subject with no prior history of tumor as well as recurrence of tumor in a subject with a history of cancer or precancerous lesion. Adenomatous colonic polyps, or simply “colonic polyps” refers to new adenomatous polyps of the colon.
Several trials had multiple associated studies, so our first task was to discern what each study contained in terms of unique data for that trial. For example, two studies of the same trial might present data on deaths due to two different types of cancer, and therefore each contributed unique data to the analysis. Other studies contained duplicate data. Some studies did not contain sufficient data for a statistical analysis. The two primary causes of insufficient data were that only survival curves were presented graphically for death or new tumors rather than the number of outcomes at a specific follow-up time, or an outcome, e.g., number of deaths, was presented for all patients combined rather than separately by treatment group. After determining which studies could contribute to the analysis, we extracted data into the spreadsheet program Microsoft Excel17 and performed statistical and meta-analytic methods in the statistical package Stata.18
All three outcomes were dichotomous and we used a risk ratio to summarize each individual study, so we discuss this statistical approach jointly for all outcomes. For death and new tumors, the studies were too heterogeneous to pool meta-analytically. We did pool the colonic polyp risk ratios, and we discuss this meta-analytic approach below.
Risk Ratio Analysis
For each of the three outcomes (death, tumors, and colonic polyps) that a trial reported, we estimated the log risk ratio comparing the relevant treatment group to either placebo or another comparison group as appropriate. We note that occasionally death or new tumor outcomes were further subdivided, e.g., death due to different types of cancers. We note further that some studies for the same trial would present comparisons in alternative ways. For example, one study might present death data for vitamin C and placebo groups separately, while another study presented death data for a different type of cancer for the vitamin E group versus all other study groups combined. The available data thus limited our ability to evaluate different comparisons for different outcomes. We also estimated the standard error of the log risk ratio for each trial and constructed a 95% confidence interval. We conducted the analysis on the log scale to stabilize the variance. We then back-transformed the log risk ratio and its confidence interval to the risk ratio scale for interpretability.
In summary, for each trial, comparison, and outcome for which data were available, we estimated the risk ratio (RR) and its 95% confidence interval. As an example of how to interpret a risk ratio, consider the outcome of all-cancer death when comparing the treatment of beta-carotene versus placebo. A risk ratio smaller than 1 indicates that a lower risk of death is associated with beta-carotene as compared to placebo.
For the death and new tumor outcomes, the trials were considered too heterogeneous to pool meta-analytically. For these outcomes, we present trial results individually with the separate outcomes defined as they appear in specific trial reports. In particular, three large trials (ATBC and the two Linxian Trials) were significantly different from each other and from the other small trials, so that meta-analytic pooling was not advisable. The main differences were study population (primary prevention versus treatment) and length of follow-up. For these large trials, we did consider whether we could combine related outcomes within trial. We note that we distinguish pooling results meta-analytically across trials from combining outcomes within a single trial. For example, a trial may report deaths due to different types of cancers separately. If clinically appropriate, we combined these deaths across all types of cancers reported, assuming that a patient's death could not be attributed to more than one type of cancer, so that we were not double-counting deaths in the combined count. For this new combined cancer death outcome that we created, we estimated a risk ratio as described previously.
Meta-Analysis for the Colonic Polyps Outcome
The trials that examined colonic polyps as an outcome were considered clinically homogeneous enough to warrant meta-analysis. We performed meta-analysis for any subgroup of three or more trials that had similar designs and comparison groups, and that measured colonic polyps for a particular type of cancer over similar follow-up periods.
For each subgroup of trials that qualified for meta-analysis, we estimated the DerSimonian and Laird random effects19 pooled log risk ratio, and its confidence interval. We also present the chi-squared test for heterogeneity p-value.20 We back-transformed the pooled result to the risk ratio scale for interpretation, and present the pooled risk ratio, its 95% confidence interval, and associated forest plot. In this plot, each individual study risk ratio is shown with its confidence interval as a box whose area is inversely proportional to the estimated study variance. The pooled risk ratio and its confidence interval are shown as a diamond at that bottom of the plot with a dotted vertical line indicating the pooled estimate. A vertical solid line at a risk ratio of 1 indicates no treatment effect.
Publication Bias
For each subgroup of studies for which we conducted a meta-analysis, we assessed the possibility of publication bias by evaluating a funnel plot of the log risk ratios graphically for asymmetry resulting from the nonpublication of small, negative studies. Because graphical evaluation can be subjective, we also conducted an adjusted rank correlation test21 and a regression asymmetry test22 as formal statistical tests for publication bias.
- Methodology - Effect of the Supplemental Use of Antioxidants Vitamin C, Vitamin ...Methodology - Effect of the Supplemental Use of Antioxidants Vitamin C, Vitamin E, and Coenzyme Q10 for the Prevention and Treatment of Cancer
- Appendix C. Abbreviations - Endoscopic Retrograde CholangiopancreatographyAppendix C. Abbreviations - Endoscopic Retrograde Cholangiopancreatography
- Preface - Endoscopic Retrograde CholangiopancreatographyPreface - Endoscopic Retrograde Cholangiopancreatography
- Methods - Outcomes of Community Health Worker InterventionsMethods - Outcomes of Community Health Worker Interventions
- Future Research - Systematic Review of the Literature Regarding the Diagnosis of...Future Research - Systematic Review of the Literature Regarding the Diagnosis of Sleep Apnea
Your browsing activity is empty.
Activity recording is turned off.
See more...