NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lau J, Zucker D, Engels EA, et al. Diagnosis and Treatment of Acute Bacterial Rhinosinusitis. Rockville (MD): Agency for Health Care Policy and Research (US); 1999 Mar. (Evidence Reports/Technology Assessments, No. 9.)
This publication is provided for historical reference only and the information may be out of date.
Goal of the Report
This report summarizes the scientific evidence for diagnosing and treating community-acquired, acute bacterial rhinosinusitis in children and adults. This topic was selected by the Agency for Health Care Policy and Research (AHCPR) in response to requests from both the American Academy of Otolaryngology and the American Academy of Pediatrics. It is an aid for clinical practice and, therefore, has been developed with a practical clinical focus. This report provides summaries of evidence for use by different groups, including primary care practitioners, specialists, researchers, policy decisionmakers, and insurers and other third-party payers. Recognizing the different interests and approaches of these groups, we focus the analyses on the diagnosis and treatment of acute bacterial rhinosinusitis in the primary care, clinical practice setting. We assessed the diagnostic performance of clinical criteria and other commonly used tests for identifying patients with acute bacterial rhinosinusitis and summarized evidence to assess the efficacy of common treatments for the defined patient subgroup(s). We also provide a decision analysis as a model for using the evidence in clinical decisionmaking and a cost-effectiveness analysis to guide policy decisionmaking.
Scope of the Problem
The term "sinusitis" technically refers to inflammation of the mucosa of the paranasal sinuses. Many factors underlie the development of sinusitis, including various environmental and host factors. The complexity of sinusitis and the many factors involved in its development have precluded the development of a single, standardized definition and classification system for this condition (Lanza and Kennedy, 1997). In part, the definition depends on the questions being addressed. For clinical practice -- and for this evidence report -- interest in the definition of acute sinusitis is based on the premise that identifying patients with community-acquired acute bacterial rhinosinusitis will allow for specific beneficial interventions.
Prevalence of Sinusitis
"Sinusitis" is often loosely used to denote a broad group of clinical syndromes with different causes and presentations. Estimates of disease prevalence vary, in part from differences in definitions of the disease and in part from differences in the populations in which the prevalence is assessed Sinusitis, as broadly defined and estimated through insurance reimbursement claims and population statistics (e.g., National Ambulatory Medical Care Survey [NAMCS]), is one of the most common health complaints in the United States (Kennedy, 1990). Gwaltney (1996), using estimates of the average number of acute respiratory illnesses per year (six to eight for children; two to three for adults) and data suggesting that 90 percent of patients with colds have sinusitis (viral or otherwise), estimated that there are over a billion cases of sinusitis annually.
Recent outpatient data from the 1995 National Ambulatory Medical Care Survey (NAMCS) estimated that the roughly 700 million visits to nonfederally employed physicians in office-based practices included about 3 million cases of acute sinusitis (International Classification of Diseases, 9th edition, clinically modified [ICD-9-CM]-461). The NAMCS data from 1990 to 1995 (Table 1; National Center for Health Statistics, 1990-1995) show an increasing trend in the prevalence of this diagnosis (test for trend using linear regression; r2=0.63; p=0.06). It is not clear whether this trend represents an actual increase in disease prevalence, potential changes in reporting (e.g., disease definition, billing practices), or changes in patients seeking or accessing care (Kaliner, Osguthorpe, Fireman, et al., 1997; McCaig and Hughes, 1995; Stoller, Forster, and Portugal, 1993). The National Hospital Discharge Survey documented 61,000 hospital discharges for patients with diagnoses (primary or other) of acute sinusitis (ICD-9-CM-461) for 1993; 66,000 for 1994; and 84,000 for 1995 (National Center for Health Statistics, 1993, 1994, 1995).
Table
Table 1. Estimated prevalence of acute rhinosinusitis from the National Ambulatory Medical Care Survey of visits to office-based nonfederally employed physicians1.
Although population studies agree that sinusitis is common, they use broad and different definitions of the condition that include several pathophysiologic conditions. Estimating the prevalence of various sinusitis subgroups (e.g., acute bacterial rhinosinusitis) requires the use of more specific diagnostic criteria in research studies. Even if the same definition is used, however, selection biases can lead to differing estimates for the general population, for patients in specific clinical settings, and for patients enrolled in studies where other initial selection criteria are applied. The relationship between the prevalence estimates obtained from population-based prevalence data (e.g., from insurance claims and survey data) and those from various subgroups of rhinosinusitis defined by clinical or diagnostic criteria from research studies remains unclear.
Estimated Costs of Health Care: Individual and Societal
The high prevalence of sinusitis translates into high costs for individual health, work time lost, and medical expenditures. McCaig and Hughes (1995) analyzed the 1985, 1989, and 1992 NAMCS data and found an increasing trend of office visits for sinusitis. They reported sinusitis (ICD-9-CM-461 and -473, including both acute and chronic sinusitis) as the fifth most common diagnosis for antibiotic prescriptions, representing 7 percent, 9 percent, and 12 percent of all recorded prescriptions in 1985, 1989, and 1992, respectively. The use of more expensive, broad-spectrum drugs (e.g., cephalosporins) increased, and the use of less expensive, narrow-spectrum antibiotics (e.g., the penicillins) decreased during this period. These trends have important ramifications in terms of health care costs, as well as the development of resistance to antimicrobials.
In 1992, in the United States, approximately $200 million was spent on prescription cold medications for sinusitis, a $50 million increase over 1989 (Gwaltney, Jones, and Kennedy, 1995). In addition, the U.S. population spends more than $2 billion annually on over-the-counter medications for nasal and sinus disorders (Williams, Aguilar, Makela, et al., 1997). Increasing rates of antibiotic resistance have led to a recognition of the need for more prudent use of antibiotics and to limiting their use to the treatment of bacterial infections (Levy, 1998). The costs of antibiotic use need to be balanced against the limitations of diagnostic certainty and risks of nontreatment. The high prevalence of sinusitis and its associated costs highlight the need for optimizing effective therapy.
Biology of the Disease
The paranasal sinuses consist of four pairs of air-filled cavities in the skull (the frontal, maxillary, ethmoid, and sphenoid sinuses), which are lined by mucosa and connect to the nasal passages (Gwaltney, 1996; MacLeod, 1991). Normal mucous secretions contain antibodies, help collect soluble pollutants, and, together with ciliary action, work to clear particulate matter, including bacteria, from the sinuses (Gwaltney, Jones, and Kennedy, 1995). Maintaining the mucociliary flow and an intact local mucosal surface are key host defenses against infection and for maintaining sinus health (Gwaltney, 1996; Gwaltney, Jones, and Kennedy, 1995). It is widely believed that the paranasal sinuses are normally sterile, although there have been conflicting reports (Bjorkwall, 1950; Brook, 1981; Gwaltney, Scheld, Sande, et al., 1992).
Defining Acute Bacterial Rhinosinusitis
Sinusitis vs. Rhinosinusitis: Symptomatic vs. Asymptomatic
In addition to inflammation of the paranasal sinuses, most cases of sinusitis are accompanied by inflammation of the nasal passages (Gwaltney, Phillips, Miller, et al., 1994; Lund and Kennedy, 1995). As such, the clinical condition often referred to as "sinusitis" is, in fact, rhinosinusitis: inflammation of the sinuses with concomitant inflammation of the nasal passages. In some circumstances, inflammation may also include portions of the surrounding bone (Lanza and Kennedy, 1997). For the purposes of this report, this grouping of conditions will be referred to as rhinosinusitis.
Studies using computed tomography or magnetic resonance imaging (MRI) have reported sinus mucosal abnormalities in 15 percent to 49 percent of patients who have no symptoms suggesting rhinosinusitis; the clinical correlations of these findings remain unclear (Axelsson and Chidekel, 1972; Calhoun, Waggenspack, Simpson, et al., 1991; Gordts, Clement, and Destryker, 1997; Kaliner, Osguthorpe, Fireman, et al., 1997; Lloyd, Lund, and Scadding, 1991; Patel, Chavda, Violaris, et al., 1996). Although all cases of rhinosinusitis involve inflammation of the mucosal linings, in the practice setting, the focus is on those patients in whom this inflammation leads to symptoms. Table 2 lists several possible clinical presentations of rhinosinusitis, although these symptoms are not present in all patients and are not very specific (Hadley and Schaefer, 1997; Shapiro and Rachelefsky, 1992; Williams and Simel, 1993; Williams, Simel, Roberts, et al., 1992).
Table
Table 2. Symptoms associated with rhinosinusitis.
Causes of Rhinosinusitis as a Basis for Therapy
Clinical diagnosis aims to identify cases that have a similar pathophysiologic cause and presentation and that therefore would presumably benefit from similar treatment. Factors affecting inflammation of the sinuses include infectious agents, allergic conditions, anatomic abnormalities, systemic diseases (endocrine, metabolic, genetic), trauma, and noxious chemicals (Gwaltney, 1996; Gwaltney, Jones, and Kennedy, 1995; Lanza and Kennedy, 1997). In some cases, these factors cause inflammation of the mucosal linings directly (e.g., allergic, infectious); in others, host conditions predispose the mucosal linings to inflammation, infection, or both (e.g., anatomic abnormalities, neoplasms, ciliary function abnormalities). Although infectious agents can be the primary cause of sinus inflammation, they also may represent a secondary infection. In these cases, the initial inflammation predisposes the sinuses to infection, as for example, when bacterial rhinosinusitis follows viral rhinosinusitis (Berg, Carenfelt, Rystedt, et al., 1986; Gable, Jones, Floor, et al., 1994).
In many instances, a patient's symptoms are the result of several environmental and host factors working together. In clinical practice, diagnosis is undertaken with an eye toward therapeutic or preventive interventions. As such, diagnoses are developed and refined to identify specific clinical conditions for which a therapy may be effective. For treating rhinosinusitis, these therapies may be directed toward current symptoms, the underlying cause, or both (Table 3).
Table
Table 3. Some causes and potential therapies for acute rhinosinusitis.
Bacterial Rhinosinusitis
Microbiologic classification of infectious causes of rhinosinusitis includes bacteria, viruses, and fungi. Data on the prevalence of cases resulting from each of these microbiologic agents are lacking. However, viruses, as the leading cause of upper respiratory infections, are among the most common (Wald, 1996). Approximately 0.5 percent to 2 percent of adult and up to 10 percent of pediatric cases of viral rhinosinusitis develop into bacterial infections (Berg, Carenfelt, Rystedt, et al., 1986; Dingle, Badger, and Jordan, 1964; Gable, Jones, Floor, et al., 1994; Gwaltney, 1996).
The rationale for identifying patients with increased likelihood of infectious rhinosinusitis stems from the potential use of anti-infective agents. Antibiotics are widely available and are effective at eliminating specific bacteria. The ability to identify cases of bacterial rhinosinusitis (either as a primary or secondary infection) would thus identify potential candidates for antibacterial therapies (Figure 1). Adequate levels of antibiotics can eradicate bacteria from maxillary sinus fluid aspirates (Eneroth and Lundberg, 1976; Hamory, Sande, Sydnor, et al., 1979).

Figure
Figure 1. Subgroups of patients with rhinosinusitis symptoms and bacterial infection.
In this report, we focus on acute bacterial rhinosinusitis because antibiotics are widely available and should be used only for bacterial infection. Bacterial infection of the sinuses can result in chronic sinusitis, as well as in other serious complications (e.g., meningitis, brain abscess). Antibiotics can be used to prevent these developments. At the same time, concerns have increased about the inappropriate use of antibiotics, both for the individual and society. For the individual, the concerns are for potential side effects and out-of-pocket expenses. For society, the concerns are for overall costs and the development of antibiotic resistance leading to loss of the therapeutic efficacy of antibiotics (Levy, 1998).
Bacterial rhinosinusitis can refer to several physiologic conditions, all of which include the presence of bacteria concomitant with sinus inflammation. The inflammation may be a direct response to bacterial infection or to nonbacterial causes that provide a setting for a secondary bacterial infection. Given, however, that in all instances bacterial rhinosinusitis entails bacterial infection, a microbiologic definition remains the current accepted diagnostic reference standard: more than 104 colony-forming unit (CFU)/ml in sinus aspirate (Turner, Cail, Hendley, et al., 1992; Winther and Gwaltney, 1990). Lower colony concentrations could potentially represent early infection. However, studies of maxillary sinus aspirates generally yield titers above 105 CFU/ml (Winther and Gwaltney, 1990), and the cutoff choice of 104 CFU/ml of sinus aspirate (rather than complete absence of bacteria) may in part compensate for the potential contamination of the sinus aspirate during collection (Wald, 1991). Whether the sinuses are sterile under normal circumstances or whether they are routinely colonized with anaerobic and aerobic bacteria is still debated (Bjorkwall, 1950; Brook, 1981; Gwaltney, Scheld, Sande, et al., 1992).
Several studies have reported bacterial species profiles isolated from maxillary sinus aspirates and have looked at changes in the predominant species over time (Berg, Carenfelt, and Kronvall, 1988; Bjorkwall, 1950; Gwaltney, Scheld, Sande, et al., 1992; Jousimies-Somer, Savolainen, and Ylikoski, 1988; Suzuki, Nishiyama, Sugiyama, et al., 1996; Urdal and Berdal, 1949). Whereas -hemolytic streptococci and Streptococcus pneumoniae were the most frequent isolates in studies circa 1950 (Bjorkwall, 1950; Urdal and Berdal, 1949), studies in the 1970s and 1980s noted that S. pneumoniae and Hemophilus influenzae predominate (Berg, Carenfelt, and Kronvall, 1988; Gwaltney, Sydnor, and Sande, 1981; Jousimies-Somer, Savolainen, and Ylikoski, 1988; Van Cauwenberge, Verschraegen, and Van Renterghem, 1976). Followup studies in the 1990s reported that these two organisms remained the major species in adults, whereas Moraxella catarrhalis was an additional, high-prevalence species isolated from children (Benninger, Anon, and Mabry, 1997; Gwaltney, Scheld, Sande, et al., 1992; Suzuki, Nishiyama, Sugiyama, et al., 1996; Wald, Reilly, Casselbrant, et al., 1984). Other species were also cultured in many of these studies (including -hemolytic streptococci, Staphylococcus aureus, and anaerobes), but their prevalence was much lower in cases of acute bacterial rhinosinusitis (Benninger, Anon, and Mabry, 1997; Berg, Carenfelt, and Kronvall, 1988; Brook, 1996; Gwaltney, Sydnor, and Sande, 1981; Gwaltney, Scheld, Sande, et al., 1992; Jousimies-Somer, Savolainen, and Ylikoski, 1988; Wald, 1991).
Even though direct microbiologic evaluation of sinus aspirates has been the diagnostic reference standard, the puncture technique has been largely limited to sampling the maxillary sinuses. One study reported good agreement between the bacterial species obtained from the frontal sinus trephination and those obtained from the same patients' maxillary sinus (Antila, Suonpaa, and Lehtonen, 1997). However, the prevalence of bacterial species in sinuses other than the maxillary sinuses and the potential clinical significance of different patterns of the sinuses affected require further study.
Although sinus puncture and culture is the diagnostic standard for bacterial rhinosinusitis, its routine use in primary care practices is not feasible. As such, the practical identification of patients with acute bacterial rhinosinusitis remains a clinical diagnosis that relies on alternate diagnostic methods. Less invasive methods for direct sampling and microbiologic testing (i.e., nasopharyngeal swabs and middle meatal sampling by endoscopy) have been compared with sinus puncture aspirates, but the degree of agreement was weak (Axelsson and Brorson, 1972; Evans, Sydnor, Moore, et al., 1975; Gwaltney, Sydnor, and Sande, 1981; Williams, Holleman, Samsa, et al., 1995). However, modifications in the techniques for middle meatal sampling by endoscopy may improve their accuracy (Druce, 1992; Ferguson and Mabry, 1997; Klossek, Dubreuil, Richet, et al., 1996).
Further Subgrouping Based on Patterns of Disease Presentation
In patients with bacterial rhinosinusitis, further distinct subgroups may be defined that differ in pathophysiology and whose identification and distinction could further direct specific therapy. Subgrouping criteria have included patterns of disease presentation (e.g., temporal patterns) and patient characteristics (e.g., age, comorbidities).
Temporal Grouping: Acute vs. Chronic Rhinosinusitis
The use of temporal patterns as distinguishing markers has resulted from clinical experience in the response of patients to therapies and, as such, to presumed and observed differences in the likely causes and nature of the symptoms. In the clinical setting, temporal patterns can be used to define subgroups with increased likelihood of similar conditions, but these temporal distinctions lack clearly defined boundaries (Lanza and Kennedy, 1997).
The distinctions between acute rhinosinusitis, chronic rhinosinusitis, and recurrent acute rhinosinusitis are based on temporal differences in presentation and on presenting features. Acute and chronic rhinosinusitis also differ in histopathologic and bacteriologic characteristics. The most common distinction between these conditions has been made on their temporal patterns, and although somewhat arbitrary, consensus has been reached about defining these three distinct but related clinical conditions on the basis of duration of symptoms and defining clinical factors (Lanza and Kennedy, 1997).
Acute rhinosinusitis has been defined as having a sudden onset, with symptoms lasting less than 4 weeks (Lanza and Kennedy, 1997). Many cases of rhinosinusitis accompany viral infections of the upper respiratory tract. Because most common cold symptoms last 5 to 7 days and mimic those of bacterial rhinosinusitis, a minimal duration of symptoms (7 to 10 days) is generally recommended before a diagnosis of bacterial infection is made. Recurrent acute infections are defined by the presence of four or more episodes per year, each lasting more than 7 days, and by the absence of intervening signs or symptoms that would suggest an ongoing or chronic rhinosinusitis. Rhinosinusitis becomes chronic when the symptoms last longer than 12 weeks and the diagnosis is confirmed by clinical or radiographic criteria (Lanza and Kennedy, 1997). Occasional acute worsening of symptoms in individuals with chronic rhinosinusitis may suggest an acute exacerbation of the chronic condition. With treatment of the acute symptoms, these individuals return to the baseline chronic rhinosinusitis condition. Because individuals with acute rhinosinusitis have symptoms for less than 4 weeks and those with chronic rhinosinusitis have symptoms for more than 12 weeks, those who have symptoms lasting between 4 and 12 weeks are considered to have a subacute infection. Some of these cases will resolve within 12 weeks, and others will progress to chronic rhinosinusitis (Lanza and Kennedy, 1997).
Chronic rhinosinusitis is histopathologically distinct from acute rhinosinusitis. In acute rhinosinusitis, neutrophils predominate and hemorrhage, ulcerations, necrosis, and exudate are present (Coltran, Kumar, and Robbins, 1995; Lanza and Kennedy, 1997). In chronic rhinosinusitis, a proliferative process is evident and is accompanied by lymphocytes, plasma cells, and eosinophils. Fibrosis of the lamina propria is also present, and fungi and bacteria may be seen (Lanza and Kennedy, 1997). These two temporally different stages of rhinosinusitis also differ bacteriologically. S. pneumoniae and H. influenzae are the predominant organisms in acute rhinosinusitis, whereas Staphylococcus species (especially S. aureus), gram-negative bacteria (e.g., Enterobacteriaceae), and fungi also may be seen in chronic rhinosinusitis (Benninger, Anon, and Mabry, 1997).
In this report, we focus on acute bacterial rhinosinusitis because it is the largest subgroup and because timely, appropriate treatment may shorten the illness and prevent progression and complications.
Patient Characteristics: Age
Patient characteristics provide another criteria for subgrouping. In particular, studies have looked at the similarities and differences between children and adults. The age of a patient with acute bacterial rhinosinusitis helps predict, to some extent, the agents most likely to be causing the sinus infection. Two major risk factors for the development of acute bacterial rhinosinusitis are acute, viral, upper respiratory infections and allergic inflammation. In children, acute community-acquired viral upper respiratory infections probably underlie 80 percent of the cases of acute bacterial rhinosinusitis, and allergic rhinitis is the cause of most of the remaining cases.
The peak age group for acute bacterial rhinosinusitis is between 3 and 6 years. This range corresponds to the peak age for incidence of community-acquired upper respiratory infections. Children in this age group experience an average of six to eight respiratory infections per year (Gwaltney, 1997). An estimated 0.5 to 10 percent of viral upper respiratory infections are complicated by acute bacterial rhinosinusitis (Berg, Carenfelt, Rystedt, et al., 1986; Dingle, Badger, and Jordan, 1964). Children with "severe" symptoms (marked fever, purulent discharge, periorbital swelling) or "persistent" symptoms (nasal discharge, daytime cough worsening at night, symptoms lasting longer than 10 days and not improving by 30 days) had high rates of abnormal radiographic findings (80 to 88 percent) and positive sinus puncture cultures (70 to 75 percent of those with symptoms and a positive radiograph) (Wald, Chiponis, and Ledesma-Medina, 1986; Wald, Reilly, Casselbrant, et al., 1984). Using these criteria of protracted respiratory symptoms (nasal discharge or cough, or both, lasting more than 10 days without evidence of improvement) plus abnormal radiographs (occipitomental radiographs showing mucosal swelling, diffuse opacification, or an air-fluid level) as a common marker of acute bacterial rhinosinusitis in an office practice setting, Ueda and Yoto (1996) found that 6.7 percent of patients with upper respiratory symptoms have maxillary rhinosinusitis. Using only the clinical criteria of "severe" or "persistent" symptoms as defined by Wald, Reilly, and Casselbrant, et al. (1984), Aitkin and Taylor (1998) in a study of children in a U.S. primary care clinic estimated the prevalence of rhinosinusitis in the winter months to be 9.3 percent of all presenting clinic patients and 17.3 percent of patients who presented for treatment of a cold or cough. In a study of children cared for in a variety of day care settings, 6 to 13 percent had upper respiratory infections that lasted more than 15 days (Wald, Guerra, and Byers, 1991). Thus, symptoms lasting more than 10 days without improvement may serve as a marker for acute bacterial rhinosinusitis. Although similar, the differences in estimates for different populations could result from the differences in diagnostic criteria (clinical vs. clinical examination and radiographic findings). Seasonal variation also may affect prevalence (Gable, Jones, Floor, et al., 1994).
Approximately 15 to 20 percent of the U.S. population has atopic disease (Spector, 1997). In children, atopic disease may be manifest as dermatitis, reactive airway disease, or allergic rhinitis. The local symptoms of allergic rhinitis may include nasal discharge, nasal obstruction, pruritis, and anosmia (Meltzer, 1997; Wright, Holberg, Martinez, et al., 1994). In children with mucositis caused by viral infection or allergy, the sinus ostia will be partially or completely obstructed (mechanically or functionally). The obstruction of the sinus ostia fosters the development of a negative pressure within the sinus cavity. In turn, the negative pressure facilitates the entry of selected normal nasopharyngeal flora into the paranasal sinus cavities, causing acute bacterial rhinosinusitis (Parsons and Wald, 1996). The microbiologic agents that subsequently infect the paranasal sinuses of children include S. pneumoniae, nontypable H.influenzae, and M. catarrhalis.
Whereas the same conditions -- community-acquired viral upper respiratory infections and allergic rhinitis -- predispose adults to acute bacterial rhinosinusitis, the incidence is considerably lower. Adults usually experience two to three upper respiratory infections annually (Gwaltney, 1997). The cause of acute bacterial rhinosinusitis in adults is mostly S. pneumoniae and H.influenzae; M. catarrhalis is an unusual cause of acute bacterial rhinosinusitis in adults (Winther and Gwaltney, 1990).
In light of these similarities and differences, we collected and analyzed evidence for both the pediatric and adult populations.
Additional Risk Factors
Additional characteristics may affect a patient's likelihood of developing acute bacterial rhinosinusitis. A strong connection between acute bacterial rhinosinusitis and allergic rhinitis has been suggested, but in light of conflicting research results, the nature and extent of this connection remain unclear (Benninger, 1992; International Rhinosinusitis Advisory Board, 1997). An association of acute bacterial rhinosinusitis with asthma also has been suggested, although this may relate to the presence of allergic rhinitis (Benninger, 1992; Kaliner, Osguthorpe, Fireman, et al., 1997; Spector, 1997).
Ciliary function is important in maintaining sinus health (Gwaltney, 1996; Gwaltney, Jones, and Kennedy, 1995). Genetic disorders with mucociliary dysfunction (cystic fibrosis and Kartagener's syndrome) are associated with chronic sinusitis (Kaliner, Osguthorpe, Fireman, et al., 1997). Cigarette smoking may present an additional risk for developing rhinosinusitis. Smoking reduces the mucociliary clearance of nasal mucosa, and the extent of reduction depends on the amount and extent of smoking (Mahakit and Pumhirun, 1995). Currently, no direct evidence links smoking to acute rhinosinusitis. However, a better understanding of the relationship, if any, between smoking and acute sinusitis is needed.
Treatment Settings
The above distinctions hold in all settings, but the prevalence of acute bacterial rhinosinusitis in different clinical settings and in various patient subgroups may vary. Patients seen in related specialties (i.e., otolaryngology and infectious disease) often have been referred by primary care physicians. Therefore, patients seen in these specialty settings may have a different spectrum of rhinosinusitis than those in general medical clinics. Recognition of these setting differences is important in evaluating research results. As with study inclusion criteria, study settings may influence the underlying disease rate (prevalence) and may markedly influence study outcomes (Schmid, Lau, McIntosh, et al., 1998).
Identifying Patients with Acute Bacterial Rhinosinusitis
In clinical practice, it is necessary to identify, with reasonable certainty, patients with acute bacterial rhinosinusitis. Current tests and criteria include specific physical examination findings, sinus imaging studies (including plain-film radiography, computed tomography, and ultrasonography), and the diagnostic reference standard of bacterial isolation from a sinus puncture. The combination of tests and criteria with the highest sensitivity will identify the greatest number of patients with true acute bacterial rhinosinusitis. The most specific combination will help to identify patients without acute bacterial rhinosinusitis among those who present with upper respiratory symptoms. One goal of this report is to present the evidence for the accuracy of specific clinical and test criteria for optimal diagnosis. Appropriate application of these tests in clinical practice should help to identify those patients with a high likelihood of having acute bacterial rhinosinusitis for antibacterial therapies (Figure 2).
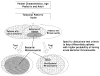
Figure
Figure 2. Strategy for using clinical characteristics and diagnostic tests to identify patients with increased likelihood of acute bacterial rhinosinusitis.
Evidence Regarding Treatment Efficacy
Choice of Therapies
This report focuses on acute bacterial rhinosinusitis, given the question of the effectiveness of antibiotics for treatment. Although antibiotic use was the a priori basis for focusing on this subgroup, we also explored evidence for other therapies for this diagnosis (Figure 3).
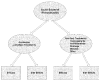
Figure
Figure 3. Options and outcomes for treatment of acute bacterial rhinosinusitis.
Antibiotic Choices and Emerging Antibiotic Resistance
As noted above, the predominant organisms isolated from cases of maxillary sinusitis include S. pneumonia, H. influenzae, and, in children, M. catarrhalis. Absent direct sampling and antibiotic-sensitivity testing, first-line antibiotic choices reflect data demonstrating effectiveness in eradicating the most likely pathogens, while also taking into account patient factors (e.g., drug-allergy history, use of other drugs that may interact), medication factors (e.g., formulation, dosing schedule) and accessibility factors (e.g., availability, cost) (Gwaltney, Jones, and Kennedy, 1995). Although studies have not reported recent significant shifts in the species and their prevalence in maxillary sinus aspirates, they have reported significant changes in the susceptibility of these organisms to various antibiotics (Gwaltney, 1996; Jorgensen, Doern, Mahar, et al., 1990; Neu, 1992).
Penicillin has long been used against S. pneumoniae, and the use of the aminopenicillins (e.g., ampicillin, amoxicillin) broadened that activity to include many gram-negative organisms, including H. influenzae and M. catarrhalis (Chambers and Neu, 1995a; Green and Wald, 1996). However, the increasing prevalence of antibiotic resistance factors has changed and is continuing to change the susceptibility profiles of many of these species' isolates. Since the first reports of penicillin-resistant S. pneumoniae isolates in the United States in the 1970s, the prevalence of resistant strains has been increasing (Friedland and McCraken, 1994; Nelson, Mason, and Kaplan, 1994). At the same time, increasing levels of -lactamase-producing H. influenzae and M. catarrhalis (from 8 to 65 percent and up to 98 percent, respectively) are raising concerns about the choice of antibiotics for first-line treatment of acute bacterial rhinosinusitis (Green and Wald, 1996; Jorgensen, Doern, Mahar, et al., 1990; Rodriguez, Schwartz, and Thorne, 1995). Other classes of antibiotics can provide varying levels of antimicrobial activity in penicillin-resistant S. pneumoniae (e.g., clindamycin, macrolides) and in -lactamase-producing H. influenzae and M. catarrhalis strains (e.g., folate inhibitors, second-generation cephalosporins) (Friedland and McCracken, 1994; Green and Wald, 1996; Jorgensen, Doern, Mahar, et al., 1990). In addition, combinations of antibiotics with -lactamase inhibitors (e.g., clavulanate, sulbactam) provide broader spectrum activity against -lactamase-producing strains (Chambers and Neu, 1995). The finding of antibiotic-resistant strains is complicated by frequent concomitant multidrug resistance and by wide geographic variation in the prevalence of antibiotic resistance for the various bacterial species (Friedland and McCraken, 1994; Green and Wald, 1996; Jorgenson, Doern, Mahar, et al., 1990; Levy, 1993; Mason, Kaplan, Lamberth, et al., 1992). More information is needed to understand the relationships between in vitro antibiotic-susceptibility determinations and clinical responses and to understand fully the factors affecting the rise in antibiotic-resistant pathogens (Baquero, 1996; Klugman, 1996; Nelson, Mason, and Kaplan, 1994).
The increased prevalence of resistant bacterial strains has been associated with antibiotic use (Levy, Fitzgerald, and Macone, 1976), and the prevalence of resistant strains has been shown to decline when the use of the specific antibiotic is stopped (Seppala, Klaukka, Vuopio-Varkila, et al., 1997). Although the development of antibiotic resistance is a national and global concern, increased awareness of the problem and its ramifications for local clinical practice is focusing on the need for surveillance data to aid practitioners in choosing antibiotics and in heightening awareness of the need for limiting antibiotic use appropriately (Bradley, Kaplan, Klugman, et al., 1995; Green and Wald, 1996; Levy, 1993; Werk and Bauchner, 1998).
Ancillary Therapies
Several classes of medications are commonly used in the treatment of rhinosinusitis (of varying causes) to restore the normal sinus environment and function (Benninger, Anon, and Mabry, 1997; International Rhinosinusitis Advisory Board, 1997). Some of the common treatments are aimed at restoring mucociliary function by increasing mucosal moisture (e.g., saline solution sprays or irrigation) and reducing the viscosity of nasal secretions (e.g., mucolytic agents). Additionally, treatments may be directed to resolving airway blockages through reducing mucosal inflammation by vasoconstriction (e.g., decongestants) or through the effect of inflammation pathways (e.g., antihistamines, steroids). As with other medical conditions, alternative medical therapies are also being explored for treating rhinosinusitis (Davies, Lewith, Goddard, et al., 1998; Linde, Clausius, Ramirez, et al., 1997; Sezik and Yesilada, 1995; Wiesenauer, Gaus, Bohnacker, et al., 1989). These treatments are aimed at rhinosinusitis of varying causes, but we explored the evidence for their efficacy in the treatment of acute bacterial rhinosinusitis.
Outcome Measures for Assessing Efficacy
The eventual outcomes of acute rhinosinusitis, including acute bacterial rhinosinusitis, are generally excellent even without specific treatment. Short-term, placebo-controlled studies show improvement or resolution of symptoms without antibiotic treatment in approximately two-thirds of patients between 8 and 12 days after presentation to the physician. Even in the preantibiotic era, serious complications (meningitis, brain abscess, and osteomyelitis) were rare. Efficacy estimates for the treatment of acute bacterial rhinosinusitis depend on the outcomes measured and may include relief of symptoms and reduced incidence of serious complications, rates of relapse and reinfection, progression to chronic rhinosinusitis, and adverse side effects of the treatments.
Relief of Symptoms
For the large majority of patients with acute bacterial rhinosinusitis, the main effects of the illness are troublesome symptoms that may keep them out of work or school and occasionally may lead to hospitalization. Most studies of the efficacy of treatments for rhinosinusitis have evaluated the outcomes in terms of the extent of persisting symptoms at the end of treatment (typically about 10 days after the onset of treatment). Some have evaluated symptom relief at more than one interval, and a few have tracked the incidence of recurrence (relapse or reinfection) in the weeks after treatment. All of these approaches yield data in the form of percentage response (cured, improved, or failure of treatment) at one or sometimes at two or three time points. Given that rhinosinusitis usually resolves spontaneously within weeks, inferring efficacy from one or two time points ("snapshots") is insufficient to quantify the benefits of treatment. It would be better to follow the rate of resolution of symptoms (e.g., with a Kaplan-Meier plot) so that the benefits, if any, of a treatment could be expressed as a reduction in symptom-days.
Incidence of Serious Complications
Complications of acute bacterial rhinosinusitis are rare. The main serious complications of bacterial rhinosinusitis are local extensions of the infection (osteitis of the sinus bones, intracranial cavity infection, and orbital cellulitis) and metastatic spread to the central nervous system (meningitis, brain abscess, and infection of the intracranial venous sinuses, including the cavernous sinus). These complications are exceedingly rare, and reliable data for their frequency as a result of acute rhinosinusitis are not available. An estimated 1 in 10,000 general hospital admissions is for brain abscess, and in different geographic locations, there is wide variation in the estimated percentage of hospitalizations (0.5 percent in China and 15 to 25 percent in Northern Europe) that are secondary to rhinosinusitis (Wispelwey and Scheld, 1995). In 1995, the number of U.S. hospital discharges for brain abscesses (ICD-9 code 324.0) was 5,000 (National Center for Health Statistics, 1990-1995). Using the highest estimated percentage of brain abscess secondary to rhinosinusitis (25 percent), about 1,250 cases were likely from among 118,255,000 discharges recorded for that year (approximately 1 out of 95,000 admissions).
Relapse and Reinfection
It is well known among medical practitioners that even when patients with allergic rhinosinusitis are excluded, some patients have frequent recurrences of presumed acute bacterial rhinosinusitis. Among these patients, it is difficult to distinguish a relapse of acute bacterial rhinosinusitis (from incomplete treatment) from multiple isolated episodes of acute bacterial rhinosinusitis (reinfection). There are no standardized criteria to assess "full recovery." The current definition of recurrent acute bacterial rhinosinusitis, based on total number of recurrences (fewer than four per year) and symptom-free intervals (at least 8 weeks), is arbitrary and not related to pathophysiologic features (International Rhinosinusitis Advisory Board, 1997). The effect of antibiotic or other treatment for a given episode of rhinosinusitis on the subsequent recurrences is unknown. Although the evidence is largely circumstantial, factors relating to structural interference of sinus passage have been postulated as risk factors in the development of chronic rhinosinusitis (see below). The relationship of these factors to the development of recurrent rhinosinusitis is unclear (International Rhinosinusitis Advisory Board, 1997).
Progression to Chronic Rhinosinusitis
A small proportion of patients with acute rhinosinusitis, especially those with multiple episodes of acute rhinosinusitis, experience chronic rhinosinusitis. Although strong evidence is lacking, several risk factors have been implicated in the development of chronic rhinosinusitis. These risk factors include abnormalities in the normal flow of mucus and air through the sinus and nasal passages as a result of obstruction (ostial obstruction, allergic reaction, direct injury) or functional (ciliary abnormalities, abnormalities in mucus secretion) conditions, or as a result of increased susceptibility to infection (immunodeficiency, or secondary to aforementioned sinus or nasal blockage). The development of chronic rhinosinusitis remains poorly understood.
Side Effects of Treatments
The side effects of treatment range from the risks of the diagnostic procedure to the potential side effects of various therapies. The reference standard for diagnosis of acute bacterial rhinosinusitis has been the sinus puncture. The complication rates of a sinus puncture depend on the skill of the operator. In skilled hands, epistaxis as a minor complication is common and, for the most part, unimportant. Serious complications are rare and include damage to the orbit with possible blindness. There are no good estimates of the rate of this complication.
Major side effects from antibiotics are uncommon. Minor rashes and gastrointestinal complaints (e.g., nausea and diarrhea) are most common and usually respond to withdrawing the antibiotic. Rarely, blood dyscrasias, hepatitis, or renal impairment may occur. With some agents, such as macrolides, quinolones, and tetracyclines, drug interactions with other medications may be a problem. A few agents may be photosensitizing (e.g., sulfonamides and doxycycline). There is no evidence of an interaction between the treatment of a particular infection and the incidence of side effects. As such, there is no reason to suspect that the nature and incidence of side effects from antibiotics in the treatment of rhinosinusitis would differ from the profiles of those drugs for the treatment of other infections if the dosage and duration of treatment are similar.
Similarly, for other treatments (decongestants, steroids, and so on), other than reports of drug interactions (e.g., terfenidine and erythromycin), we found no data that support differential side-effect profiles of these medications in the treatment of acute bacterial rhinosinusitis as compared with the treatment of nonbacterial rhinosinusitis or infections at other anatomic locations.
Putting it Together
Given our current understanding of the causes and pathophysiology of acute bacterial rhinosinusitis and the premise that appropriate antibiotic use should be reserved for the treatment of bacterial infections, we summarize in this report the evidence relating to the diagnosis and treatment of acute bacterial rhinosinusitis. We performed meta-analyses to summarize evidence on several diagnostic modalities that have been used for this condition. We also performed meta-analyses to summarize the evidence on the effects of antibiotic treatments, and we sought evidence for the use of symptomatic (ancillary) treatments. We performed decision and cost-effectiveness analyses using the meta-analysis results to provide guidance on the use of the evidence.
- Introduction - Diagnosis and Treatment of Acute Bacterial RhinosinusitisIntroduction - Diagnosis and Treatment of Acute Bacterial Rhinosinusitis
Your browsing activity is empty.
Activity recording is turned off.
See more...

