NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Balk E, Chung M, Lichtenstein A, et al. Effects of Omega-3 Fatty Acids on Cardiovascular Risk Factors and Intermediate Markers of Cardiovascular Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Mar. (Evidence Reports/Technology Assessments, No. 93.)
This publication is provided for historical reference only and the information may be out of date.

Effects of Omega-3 Fatty Acids on Cardiovascular Risk Factors and Intermediate Markers of Cardiovascular Disease.
Show detailsOverview
This evidence report on omega-3 fatty acids and CVD risk factors and intermediate markers of cardiovascular disease (CVD) is based on a systematic review of the literature. To identify the specific issues central to this report, the Tufts-New England Medical Center (Tufts-NEMC) Evidence-based Practice Center (EPC) held meetings and teleconferences with technical experts, including a Technical Expert Panel (TEP) and members of the other EPCs that are reviewing topics related to omega-3 fatty acids. A comprehensive search of the medical literature was conducted to identify studies addressing the key questions. Evidence tables of study characteristics and results were compiled, and the methodological quality and applicability of the studies were appraised. Study results were summarized with qualitative reviews of the evidence, summary tables, and quantitative meta-analyses, as appropriate.
A number of individuals and groups supported the Tufts-NEMC EPC in preparing this report. The TEP served as our science partner. It engaged technical experts, representatives from the Agency for Healthcare Research and Quality (AHRQ), and institutes at the National Institutes of Health (NIH) to work with the EPC staff to refine key questions, identify important issues, and define parameters to the report. Additional domain expertise was obtained through local nutritionists who joined the EPC.
The Tufts-NEMC EPC also worked in conjunction with EPCs at the University of Ottawa and at the Southern California EPC-RAND. Together, the 3 EPCs are mandated to produce evidence reports on 10 topics related to omega-3 fatty acids over a 2-year period. The 3 EPCs coordinated activities with the goal of producing evidence reports of uniform format. Through frequent teleconferences and email contact, approaches toward data presentation, summary and evidence table layout, and study quality and applicability assessment were standardized. In addition, literature searches for all evidence reports were performed by the UO EPC, using identical search terms for studies of omega-3 fatty acids. The 3 EPCs agreed on a common definition of omega-3 fatty acids; however, some variation in definitions and study eligibility criteria were applied that reflected the different topics and key questions addressed. The studies included are described below, under Full Article Inclusion Criteria.
Accompanying reports on omega-3 fatty acids and cardiovascular outcomes, and on the animal and in vitro evidence for the effect of omega-3 fatty acids on cardiac electrogenesis, were generated using similar techniques.
Key Questions Addressed in this Report
Four key questions are addressed in this report. Questions 1 and 2 (and their sub-questions) both pertain to the effect of consumption of omega-3 fatty acids (either as treatment or in the diet) and both risk factors and intermediate outcomes. Question 3 pertains primarily to the effect of modifiers on any effects or associations. Question 4 pertains to the association between omega-3 fatty acid intake and tissue and plasma levels. The key questions and their related sub-questions are outlined in detail below.
Question 1. What is the effect of omega-3 fatty acids (eicosapentaenoic acid [EPA; 20:5 n-3], docosahexaenoic acid [DHA; 22:6 n-3], and alpha-linolenic acid [ALA, 18:3 n-3], supplements, and fish consumption) on cardiovascular risk factors and intermediate markers of cardiovascular disease?
What is their effect on CVD risk factors and intermediate markers of CVD, specifically:
- Serum lipids (total cholesterol, low density lipoprotein [LDL], high density lipoprotein [HDL], and triglycerides [Tg])
- Other CVD risk factors and intermediate markers of CVD
What is their effect on specific CVD risk factors, specifically:
- new-onset Type II diabetes mellitus (DM
- new-onset insulin resistance/metabolic syndrome
- progression of insulin resistance
- new-onset hypertension
- blood pressure among hypertensive patients
What is the relative effect of omega-3 fatty acids on different CVD risk factors and intermediate markers of CVD?
- Can the intermediate markers and risk factors for CVD be ordered by strength of treatment effect of omega-3 fatty acids?
Is there a threshold or dose-response relationship between omega-3 fatty acids and intermediate markers and risk factors for CVD?
How does the duration of intervention or exposure affect the treatment effect of omega-3 fatty acids on intermediate markers and risk factors of CVD?
Are treatment effects of omega-3 fatty acids on CVD intermediate markers and risk factors sustained after the intervention or exposure stops?
Question 2. Effect of different omega-3 fatty acids:
What is the effect of different specific omega-3 fatty acids (EPA, DHA, ALA), and different ratios of omega-3 fatty acid components in dietary supplements, on CVD intermediate markers and risk factors?
How does the effect of omega-3 fatty acids on CVD intermediate markers and risk factors differ by source (e.g., dietary fish, dietary oils, dietary plants, fish oil supplement, flax seed supplement)?
Does the ratio of omega-6 fatty acid to omega-3 fatty acid intake affect the effect of omega-3 fatty acid intake on intermediate markers and risk factors of CVD?
Question 3. Sub-population analyses:
How does the effect of omega-3 fatty acids on intermediate markers and risk factors of CVD differ in sub-populations including men, pre-menopausal women, post-menopausal women, and different age groups?
How does baseline dietary intake of omega-3 fatty acids impact the effect of omega-3 fatty acid supplements on intermediate markers and risk factors of CVD?
What are the effects of potential confounders - such as lipid levels, body mass index (BMI), blood pressure, diabetes, aspirin use, hormone replacement therapy, and cardiovascular drugs - on associations?
Does the use of medications for CVD and CVD risk factors (including lipid lowering agents and diabetes medications) impact the effect of omega-3 fatty acids?
Question 4. Omega-3 fatty acid metabolism:
What is the association between intake levels of EPA, DHA, and ALA and blood, tissue, and cell membrane levels?
What is the efficiency of conversion from ALA to EPA/ DHA, EPA/DHA to ALA, DHA to EPA, and EPA to DHA?
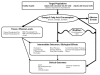
Analytic Framework
To guide our assessment of studies that examine the association between omega-3 fatty acids and cardiovascular outcomes, we developed an analytic framework that maps the specific linkages associating the populations of interest, the exposures, modifying factors, and outcomes of interest (Figure 1.2) 37. The framework graphically presents the key components of the study questions:
- Who are the participants (i.e., what is the population and setting of interest, including the diseases or conditions of interest)?
- What are the interventions?
- What are the outcomes of interest (intermediate and health outcomes)?
- What study designs are of value?
Specifically, this analytic framework depicts the chain of logic that evidence must support to link the intervention (exposure to omega-3 fatty acids) to improved health outcomes.
This report and the accompanying report, Effects of Omega-3 Fatty Acids on Cardiovascular Disease, review the evidence addressing the associations or effects in humans. Specifically, this report examines evidence addressing both the association in humans between omega-3 fatty acids and cardiovascular intermediate outcomes or risk factors and the association between omega-3 fatty acids and tissue or plasma levels of omega-3 fatty acids. The accompanying report examines evidence addressing the association between omega-3 fatty acids and clinical cardiovascular outcomes, their efficacy in improving CVD outcomes, and potential adverse effects of omega-3 fatty acid intake in humans.
In both reports, the 3 specific populations of interest are healthy adults with no known CVD or risk factors; adults at increased risk of CVD due specifically to diabetes, hypertension, or hyperlipidemia; and adults with known CVD. The exposure of interest is omega-3 fatty acids. Unlike medications, there are numerous possible sources, types, and possible dosages for omega-3 fatty acids. Thus, questions of interest include how different sources, dosages, and relative proportions of the fatty acids differ in their effects on the outcomes of interest. Included are questions addressing possible differences between the effects of supplements (e.g., fish oil capsules) and dietary sources (e.g., fatty fish), the effect of duration of intervention or exposure, and whether any effect is sustained after stopping treatment.
Theoretically, the most immediate outcome related to omega-3 fatty acid intake is a change in tissue levels of the fatty acids. However, the measurement and interpretation of this effect is complicated by the variety of fatty acids, the different relative intake levels of fatty acids, metabolism of the fatty acids into other fatty acids, the different storage forms, and the wide range of cells into which the fatty acids are incorporated. The question of how omega-3 fatty acid intake relates to different measures of tissue and plasma fatty acid levels is addressed in this report. Once it is understood how to best estimate body stores of omega-3 fatty acids, it will then be of interest in future reviews to understand how levels of body stores affect cardiovascular outcomes.
Although the most important questions relating to omega-3 fatty acids pertain to their effects on clinical outcomes (and potential adverse events), collecting data on long-term cardiovascular effects is relatively difficult. As a result, the bulk of the available evidence generally pertains to the efficacy in trials of interventions on intermediate outcomes and biological effects. This evidence is summarized in this report.
The effects of omega-3 fatty acids on CVD risk factors, intermediate markers of CVD and clinical outcomes can be related to one another in two ways. First, by reducing risk factors for CVD, such as blood pressure, or putative markers of the risk factors, such as C-reactive protein, omega-3 fatty acids can directly reduce the overall risk of cardiovascular events. Second, omega-3 fatty acids can have a direct or indirect beneficial effect on specific intermediate markers of CVD, such as coronary stenosis, which would result in a lowered risk of cardiovascular events. In this report, we investigate how the effects of omega-3 fatty acids on risk factors and intermediate markers can be modified by various factors, including concomitant drugs, demographic features (e.g., sex, age), baseline diet, and subject characteristics (e.g., lipid levels, weight, blood pressure).
The analytic framework does not directly address the level of evidence that is necessary to evaluate each of the effects. Large randomized controlled trials that are adequately blinded and otherwise free of substantial bias provide the best evidence to prove causation between intervention and outcome. However, this study design is not always available (or possible). Crossover trials have the advantage of controlling fully for biases due to differences between study arms but may introduce bias due to incomplete washout of first treatment effect. In addition, they are generally small and have a narrow range of subjects. Uncontrolled trials and observational studies provide lesser degrees of evidence that are usually hypothesis-generating regarding causation. The current analysis relies as much as possible on high quality, randomized controlled trials, using evidence from other studies when data are relatively sparse.
Literature Search Strategy
We conducted a comprehensive literature search to address the key questions related to CVD and to the metabolism of omega-3 fatty acids (Appendix A.1, available electronically at http://www.ahrq.gov/clinic/epcindex.htm). Relevant studies were identified primarily through search strategies conducted in collaboration with the UO EPC. The Tufts-NEMC EPC used the Ovid search engine to conduct preliminary searches on the Medline database. The final searches used 6 databases including Medline from 1966 to week 2 of February 2003, PreMedline February 7, 2003, Embase from 1980 to week 6 of 2003, Cochrane Central Register of Controlled Trials 4th quarter of 2002, Biological Abstracts 1990 - December 2002, and Commonwealth Agricultural Bureau (CAB) Health from 1973 to December 2002. Subject headings and text words were selected so that the same set could be applied to each of the different databases with their varying attributes. Supplemental search strategies were conducted as needed. Additional publications were referred to us by the TEP and the other 2 EPCs. Details about selected terms used in the search strategy are discussed below.
Omega-3 Fatty Acids Search Strategy
A wide variety of search terms were used to capture the many potential sources of omega-3 fatty acids. Search terms used include the specific fatty acids, fish and other marine oils, and specific plant oils (flaxseed, linseed, rapeseed, canola, soy, walnut, mustard seed, butternut, and pumpkin seed). These terms were used in all search strategies.
Cardiovascular Search Strategy
The primary search strategy was designed to address both the clinical and intermediate outcomes of CVD in humans (Appendix A.1). In order to identify CVD outcomes in human studies, the search was divided into 3 categories consisting of controlled trials, other studies, and reviews. These 3 categories were further divided into English and non-English subsets.
Diabetes
Because specific terms referring to diabetes had been omitted from the primary search strategy, a supplemental search strategy was conducted on March 29, 2003. The diabetes supplemental search strategy included relevant search terms for diabetes. This search strategy resulted in an additional 410 citations for screening (Appendix A.2).
Supplemental Searches
Because some studies evaluated the effect of nuts on CVD outcomes without specifying in the abstract the type of nuts used in the study, we performed a supplemental Medline search on July 30, 2003 using the term “nut” as a text word for studies of CVD (Appendix A.3). Furthermore, upon noting that a number of relevant articles were missing from our search strategy, we performed a supplemental search on July 1, 2003. This search included terms specific to the CVD risk factor and intermediate markers outcomes of interest (Appendix A.4).
Overall
The number of citations for the final results of the database searches is approximate. Because the 5 main databases used in the search employ different citation formats, duplicate publications were encountered. The UO EPC eliminated most of the duplicate publications, however, because of many different permutations it was impossible to identify all of them. We eliminated duplicate publications as we encountered them.
Ongoing automatic updates of Medline searches were conducted using the CVD search strategy. The last automatic update was on April 19, 2003. The UO EPC conducted a final update search of the other databases on April 10, 2003.
Study Selection
Abstract Screening
All abstracts identified through the literature search were screened manually. At this stage, eligibility criteria were loosely defined to include all English language primary experimental or observational studies that evaluated any potential source of omega-3 fatty acids in at least 5 human subjects, irrespective of the study outcomes reported in the abstract. We excluded abstracts that clearly included only subjects who had a non-CVD-related condition (such as cancer, schizophrenia, or organ transplant), letters and abstracts.
Full Article Inclusion Criteria
Articles that passed the abstract screening process were retrieved and the full articles were screened for eligibility. Articles were rejected during this round based on the following criteria: review articles, inappropriate human population, pediatric studies and those conducted on subjects less than 19 years old, no mention of omega-3 fatty acid dietary supplements or fish consumption, daily dose of omega-3 fatty acid greater than 6 g, fewer than 5 subjects in omega-3 fatty acid arm(s), prospective interventional studies of less than 4 weeks duration, crossover studies with less than 4 week washout between treatments, and no appropriate outcome of interest reported. Studies that reported only the tissue level of omega-3 fatty acid without explicitly reporting the amount of omega-3 fatty acid consumed were also excluded. Studies that reported only lipid data among the outcomes of potential interest with fewer than 20 subjects were excluded during screening because of the large number of such studies and limited resources. In addition, with the exception of studies of Mediterranean diets and studies that reported fish servings, studies were excluded if no specific data were reported about omega-3 fatty acid consumption. Specific sources of omega-3 fatty acids considered acceptable included fish oils, dietary fish, canola (rapeseed) oil, soybean oil, flaxseed or linseed oil, walnuts or walnut oil, and mustard seed oil. Other sources were eligible if omega-3 fatty acid levels were reported to be greater than control. For each study that was rejected, the reason(s) for rejection was noted.
The exclusion criterion of more than 6 g per day for non-adverse event clinical outcomes was based on discussions with the TEP, in which it was agreed that omega-3 fatty acid intake above this amount is impractical and has little relevance on health care recommendations. Therefore, the inclusion criterion for the maximum daily intake was set at 6 g per day. The definition of dose of omega-3 fatty acids varied greatly across studies. Thus, the maximal allowable dose may have applied to total daily omega-3 fatty acid, total EPA plus DHA, or a total of other combinations of omega-3 fatty acids. The total did not refer to total fish oil. Short duration studies (less than 4 weeks) and crossover studies with washout periods less than 4 weeks were excluded since, it was agreed, a metabolic steady-state of omega-3 fatty acids is likely not achieved for about 4 weeks.
Sometimes there were multiple publications of the same study reporting interim results or different outcomes. We identified and grouped articles belonging to the same overall study and used data from the latest publication, supplemented by data from earlier publications, as appropriate.
In addition, a list of approximately 100 potential markers of CVD (e.g., coronary intima media thickness) and risk factors (e.g., hypertension, C-reactive protein) was reviewed in detail. Because of limited time and resources, 22 factors were chosen from this list for definite inclusion. A second list of factors was evaluated for possible inclusion if time and resources allowed (see Table 3.1 in Results section). Studies that reported on none of these factors were rejected.
Table
Table 3.1 Numbers of studies of omega-3 fatty acids and cardiovascular risk factors.
Because of the large number of studies available for analysis, for most outcomes of interest we decided to confine analysis to the largest randomized trials for each outcome evaluated. For outcomes with few studies, all studies were included regardless of study design or sample size (minimum of 5 subjects). We used a lower sample size threshold for crossover studies because these studies are more strongly powered for a given number of subjects than parallel studies. We generally aimed for approximately 20 to 25 studies for analysis. For studies of platelet aggregation, we used the additional inclusion criterion that platelet aggregation data must be presented in a numerical format; articles that reported platelet aggregation results only graphically were not analyzed. This additional criterion was used because of the particular difficulty in estimating data from graphs for this outcome and because of the large number of specific outcomes reported in each study. Specific criteria used are listed in Table 3.1 and described in each outcome section in Chapter 3.
Incorporation of omega-3 fatty acids into phospholipids is very commonly reported by studies, often as proof of treatment compliance. Again because of limited time and resources, we limited our review of studies examining omega-3 fatty acid incorporation (or the association between dietary omega-3 fatty acid intake and tissue levels of omega-3 fatty acids) to the larger randomized trials that met eligibility criteria for either intermediate or clinical outcomes. We based this decision on the assumption that this sample of studies should not be biased. In addition, because the primary research question concerns correlation between dietary intake and blood levels of omega-3 fatty acids, for these analyses we have included only prospective, intervention trials to avoid biases and inaccuracies inherent to retrospective or survey-based studies. We have limited measurable levels to those most commonly reported and most practically measured, including erythrocyte, platelet cell membrane, and plasma phospholipids.
Data Extraction Process
An electronic data extraction form and database were created specifically for the evaluation of studies of omega-3 fatty acids and intermediate and clinical outcomes (Appendix B, available electronically at http://www.ahrq.gov/clinic/epcindex.htm). Data were entered into the form by selecting single or multiple choice buttons or as free text, as appropriate. The form allowed direct input of data into a Microsoft Access database and further manipulation of extracted data in both Microsoft Excel and Word.
As the data extraction form was being developed, all members of the EPC were trained to use the electronic form and software. In an iterative process, in which groups of studies were extracted by all trainees, the data entry form was improved, consensus was reached on definitions, and issues specific to omega-3 fatty acid studies were addressed. After this process, each study was screened for eligibility criteria and for outcomes using the electronic form. Each eligible study was then fully extracted by a single researcher. During weekly meetings, data extraction problems were addressed. Occasional sections were re-extracted to ensure that uniform definitions were applied across extracted studies. Problems and corrections were noted through spot checks of extracted data and during the creation of summary and evidence tables. A second reviewer independently verified the data in the summary tables using the original article.
Items extracted included: study design, blinding, randomization method, allocation concealment method, country, funding source, study duration, eligibility criteria, sample characteristics (including comorbid conditions, concomitant medications, baseline diet, and demographics), number enrolled and analyzed, reasons for withdrawals, description of omega-3 fatty acid and control interventions or diets (including amount of specific fatty acids), risk factor, intermediate markers, and clinical outcomes, adverse events (which are discussed in the report, Effects of Omega-3 Fatty Acids on Cardiovascular Disease), results (including baseline value, final value, within-treatment change, or between-treatment difference, and variance, as reported), and whether each study addressed each of the key questions. In addition, each study was categorized based on applicability and study quality as described below.
Meta-Regression
To examine the association between the level of intake of omega-3 fatty acids and tissue levels, the change in omega-3 fatty acid and arachidonic acid (AA 20:4 n-6) compositions were calculated for each study arm. Data were extracted for fatty acid composition of plasma or serum phospholipids, platelet membrane phospholipids, and erythrocyte membrane phospholipids, granulocyte membrane phospholipids, and monocyte membrane phospholipids. For each tissue type, data from each treatment arm were combined in a meta-regression on the change of EPA+DHA composition compared to mean dose of EPA+DHA received in each treatment arm.38 Changes in non-omega-3-fatty-acid arms or control groups were not included in meta-regression analyses.
We performed simple linear regressions with the weighted least squares method, weighting each study arm by the square root of its sample size 39. The equation of the meta-regression line is reported for each blood marker. R2, or the goodness of fit, for the regression line is also reported. Data are presented both in summary tables and graphically in scatter plots in which the sources of the omega-3 fatty acid treatments are distinguished by different symbols.
Grading Evidence
Studies accepted in evidence reports have been designed, conducted, analyzed, and reported with various degrees of methodological rigor and completeness. Deficiencies in any of these processes may lead to biased reporting or interpretation of the results. While it is desirable to grade individual studies to inform the reader of these reports about the degree of potential bias, the grading of the quality of evidence is not straightforward. Despite many attempts, even for a single type of study design, most factors commonly used in quality assessment of randomized controlled trials have not been found to be consistently related to the direction or magnitude of the reported effect size 40. There is still no uniform approach to reliably grade published studies based on the information reported in the literature. Different EPCs have used a variety of approaches to grade study quality in past evidence reports.
Common Elements for Grading the Methodological Quality of Randomized Controlled Trials in Evidence Reports
As part of the overall omega-3 fatty acid project, the 3 collaborating EPCs agreed to use the Jadad Score and adequacy of random allocation concealment as elements to grade individual randomized controlled trials 41, 42. We also agreed that individual EPCs might add other elements to this core set, as we deemed appropriate. All EPCs agreed that studies should not be graded using a single numerical quality score, as this has been found to be unreliable and arbitrary 43.
The Jadad Score assesses the quality of randomized controlled trials using 3 criteria: adequacy of randomization, double blinding, and drop outs 41. A study that fully meets all 3 criteria gets a maximum score of 5 points. Adequacy of allocation concealment was assessed using the criteria described by Schulz et al., as adequate, inadequate, or unclear 42.
Generic Summary Quality Grade for Studies
The Jadad and Schulz scores address only some aspects of the methodological quality of randomized controlled trials. Potential biases due to reporting and analytic problems in the study are ignored. In this evidence report, we applied a 3-category grading system (A, B, C) to each randomized trial. We have used this grading system in most of our previous EPC evidence reports, as well as in several evidence based clinical practice guidelines 44. This scheme defines a generic grading system for study quality that is applicable to each type of study design (i.e., randomized controlled trial, cohort study, case-control study):
- Least bias; results are valid. A study that mostly adheres to the commonly held concepts of high quality, including the following: a formal randomized study; clear description of the population, setting, interventions and comparison groups; appropriate measurement of outcomes; appropriate statistical and analytic methods and reporting; no reporting errors; less than 20% dropout; clear reporting of dropouts; and no obvious bias.
- Susceptible to some bias, but not sufficient to invalidate the results. A study that does not meet all the criteria in category A. It has some deficiencies but none likely to cause major bias. Study may be missing information making assessment of the limitations and potential problems difficult.
- Significant bias that may invalidate the results. A study with serious errors in design, analysis, or reporting. These studies may have large amounts of missing information or discrepancies in reporting.
Studies that reported multiple results of interest to this report could receive different quality grades for different outcomes if there were reporting or methodological issues with specific outcomes but not others. We did not grade the few non-randomized studies that were analyzed.
Applicability
Applicability addresses the relevance of a given study to a population of interest. Every study applies certain eligibility criteria when selecting study subjects. Most of these criteria are explicitly stated (i.e., disease status, age, sex). Some may be implicit or due to unintentional biases, such as those related to study country, location (e.g., community vs. specialty clinic), or factors resulting in study withdrawals. The question of whether a study is applicable to a population of interest (such as Americans) is distinct from the question of the study's methodological quality. For example, due to differences in the background diets an excellent study of Japanese men may be very applicable to people in Japan, but less applicable to Japanese-American men, and even less applicable to African-American men. The applicability of a study is thus dictated by the questions and populations that are of interest to those analyzing the studies.
In this report, the focus is on the US population, as specified in the Scope of Work for this series of evidence reports. We also address specific subgroups within that population (i.e., healthy Americans, Americans with CVD, and Americans with diabetes or dyslipidemia), as specified. To capture the potential applicability of studies to the different populations of interest as defined in the scope of work we define the following target population categories:
GEN General population. Typical healthy people similar to Americans without known CVD, diabetes or dyslipidemia.
CVD Cardiovascular disease population. Subjects with a history of or currently with cardiac, peripheral vascular, or cerebrovascular disease, as defined by the author. In addition studies of hypertensive patients were included.
DM Diabetic population. Subjects with any type of diabetes, including type I (DM I), type II (DM II), insulin dependent (IDDM) and non-insulin dependent (NIDDM), as defined by the authors.
DysLip Population with dyslipidemia, either elevated total cholesterol, LDL, or Tg, or low levels of HDL, as defined by the authors.
One study was classified as CVD Risk because it included a combination of subjects with known CVD, diabetes, dyslipidemia and other potential CVD risk factors. In addition, some studies received multiple classifications (CVD/DM or DM/DysLip), when inclusion criteria included multiple conditions.
Even though a study may focus on a specific target population, limited study size, eligibility criteria and the patient recruitment process may result in a narrow population sample that is of limited applicability, even to the target population. To capture this parameter, we categorize studies within a target population into 1 of 3 levels of applicability 44:
- Sample is representative of the target population. It should be sufficiently large to cover both sexes, a wide age range, and other important features of the target population including baseline dietary intake broadly similar to that of the US population.
- Sample is representative of a relevant sub-group of the target population, but not the entire population. For example, while the Nurses Health Study is the largest such study and the results are highly applicable to women, it is nonetheless representative only of women. A fish oil study in Japan, where the background diet is very different from that of the US, would also fall into this category.
- Sample is representative of a narrow subgroup of subjects only, and not well applicable to other subgroups. For example, a study of male college students or a study of a population on a highly controlled diet.
In the summary tables, each study receives a combined applicability grade comprised of the target population (GEN, CVD, DM, and DysLip) and the 3-level grade (I, II, III).
Sample Size
The study sample size provides a quantitative measure of the weight of the evidence. In general, large studies provide more precise estimates of effect and associations. In addition, large studies are more likely to be generalizable; however, large size alone does not guarantee broad applicability.
Reporting Results
Most outcomes evaluated were continuous variables, such as lipid level or intima-media thickness. For these outcomes, summary tables report 3 sets of data: the mean (or median) baseline level in the omega-3 fatty acid arm; the net change of the outcome, and the reported P value of the difference between the omega-3 fatty acid arm and control. The net change of the outcome is the difference between the change in the omega-3 fatty acid arm and the change in the control arm, or:
Net change = (Omega 3Final - Omega 3Initial) - (ControlFinal - ControlInitial).
The great majority of articles reported these 4 values and P values. While some studies reported adjusted and unadjusted within-arm and between-arm (net) differences, to maintain consistency across studies we calculated the unadjusted net change using the above formula for all studies when the data were available. To provide a rough estimate of the effect of omega-3 fatty acids when median values were reported (as for lipoprotein (a)), we used the above formula with the median values, recognizing that the resultant net change is not mathematically valid. When data were available at multiple time points, we extracted data on only the time point at the end of omega-3 fatty acid intervention. Data from other time points are discussed in the text.
We included only the reported P values for the net differences. We did not calculate any P values, but, when necessary, used provided information on the 95% confidence interval or standard error of the net difference to determine whether the P value was less than .05. We included any reported P value less than .10. Reported P values above .10 and values reported as “non-significant” were included as NS, non-significant.
Coronary artery restenosis studies provided rate data on a dichotomous variable (restenosis or no restenosis). For these studies, we report 3 equivalent sets of data: the control rate (the rate of restenosis in the control group, a standard measure of the underlying severity of illness in the study population), the relative risk of restenosis, and the 95% confidence interval. In addition we performed a random effects model meta-analysis 45.
All exceptions and caveats are described in footnotes.
Evidence and Summary Tables
We report the evidence in 2 complementary forms:
Evidence tables offer a detailed description of studies we analyzed that address each of the key questions. These tables provide detailed information about the study design, patient characteristics, inclusion and exclusion criteria, interventions and comparison groups evaluated, and outcomes. Baseline and follow-up data for each analyzed outcome are reported in the Results column. A study, regardless of how many interventions or outcomes were reported, appears once in the evidence tables. The studies are ordered alphabetically by the first author's last name and study year.
Summary tables succinctly report on each study using summary measures of the main outcomes. These tables were developed by condensing information from the evidence tables and are designed to facilitate comparisons and synthesis across studies. Summary tables include important concise information regarding study size, intervention and control, study population (e.g., general population or CVD), outcome measures, methodological quality and applicability. Studies are grouped by omega-3 fatty acid source (EPA/DHA oils, plant oils, fish and Mediterranean diets, and combinations - comparisons - of different sources). Then studies are ordered first by omega-3 fatty acid dose and second by omega-3 fatty acid study arm size (both largest to smallest). A study with outcomes may appear multiple times in different summary tables.
Methodological Limitations
Due to practical limitations of time and resources, many constraints were applied to the available data, as described above. In consultation with the TEP and NIH representatives, we prioritized the original list of questions to focus on those of greatest interest to the scientific and medical communities and for which data were likely to be available. Likewise, the list of specific CVD risk factors that we examined was reduced to those that members of the TEP agreed have the greatest clinical relevance and are most clearly related to CVD. Therefore, a large number of commonly evaluated markers were not included. For example, tissue plasminogen activator (TPA), plasminogen activator inhibitor (PAI), and LDL oxidation were not included because their levels are not clearly associated with clinical CVD outcomes, or the meaning of a change in their levels is not well understood, or there is much variability in how the factor is measured and interpreted, among other reasons. In addition, the TEP attempted to focus on those factors which are most relevant to clinical practice.
The decision about which specific outcomes to evaluate from the list of potential outcomes was based on an evaluation of the available evidence. CVD risk factors and intermediate markers with more limited evidence, possibly due to publication bias, or that were primarily evaluated in small or non-randomized or uncontrolled trials were generally omitted; although data on particular outcomes of interest, such as C-reactive protein and exercise tolerance testing, were included despite limited data.
Finally, because of the large number of studies, only the highest quality, larger studies were analyzed. While we attempted to find data to answer all the key questions, only those studies included in the main analyses were evaluated in thorough detail. This has implications for questions regarding populations, covariates, comparison of omega-3 fatty acid sources, and other sub-questions. However, it is unlikely that any of the missed studies were critical to our understanding of the key questions, since only the smaller, lower quality studies would have been missed.
It is also important to note that for almost all analyzed outcomes, the available data are biased toward positive results. Many articles reported that omega-3 fatty acid treatment did not affect levels of various outcomes, but did not report supporting data. These studies were not evaluated for the reported outcomes.
- Methods - Effects of Omega-3 Fatty Acids on Cardiovascular Risk Factors and Inte...Methods - Effects of Omega-3 Fatty Acids on Cardiovascular Risk Factors and Intermediate Markers of Cardiovascular Disease
- Conclusions - Garlic: Effects on Cardiovascular Risks and Disease, Protective Ef...Conclusions - Garlic: Effects on Cardiovascular Risks and Disease, Protective Effects Against Cancer, and Clinical Adverse Effects
- pruning defect 1, isoform A [Drosophila melanogaster]pruning defect 1, isoform A [Drosophila melanogaster]gi|24646090|ref|NP_650115.1|Protein
- mct-1 protein, partial [Catonephele numilia]mct-1 protein, partial [Catonephele numilia]gi|2569176621|gb|WMI16231.1|Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...