NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Introduction
Pain can be effectively controlled by various endogenous mechanisms. Recent research has shown that these mechanisms are not restricted to the central nervous system. Intrinsic pain inhibition can occur also in the periphery, mediated by an interaction between immune cells and sensory nerve endings. A prerequisite for the manifestation of such peripheral effects seems to be inflammation, accompanied by hyperalgesia. Opioid receptors are present on peripheral endings of sensory nerves and are upregulated during the development of inflammation. Their endogenous ligands, opioid peptides, are synthesized in circulating immune cells which, under pathological conditions, migrate to injured sites. This is orchestrated by selectins, adhesion molecules located on opioid-containing immune cells and on vascular endothelium. Under environmental stressful stimuli or in response to releasing agents (e.g., corticotropin releasing factor, CRF; cytokines) these immunocytes can secrete opioids. These activate peripheral opioid receptors and produce antinociception (analgesia) by inhibiting either the excitability of the nerves or the release of excitatory, proinflammatory neuropeptides. These effects can be abolished by opioid receptor antagonists, antibodies against opioid peptides, by immunosuppression and by blocking the selectin-dependent extravasation of opioid-containing immune cells.
Peripheral Opioid Receptors
All three opioid receptors (μ, δ and κ) are expressed within sensory neurons. They have been found on cell bodies in the dorsal root ganglia and on central and peripheral terminals of primary afferent neurons in animals and in humans. This was confirmed by in vivo experiments which showed that the peripheral analgesic effects of μ-, δ- and κ-selective agonists are abolished by pretreatment with capsaicin, a neurotoxin selective for primary afferent neurons. In inflammation, the number of opioid receptors increases on peripheral nerve terminals. In addition, preexistent, but possibly inactive neuronal opioid receptors can become active owing to the specific milieu (e.g., low pH) of inflamed tissue. Furthermore, inflammation entails a disruption of the perineurium (a normally rather impermeable barrier encasing peripheral nerve fibers) and increases the number of peripheral sensory nerve terminals in inflamed tissue (reviewed in ref. 1 and in the chapter by Stein in this volume). Activation of these opioid receptors results in potent peripherally mediated analgesia, particularly in inflamed subcutaneous tissue, viscera and joints, and confers anti-inflammatory effects (reviewed in refs. 2–6, in “Peripheral analgesic effects of immune cell-derived opioid peptides” in this chapter and in chapters by Stein and Walker in this volume).
Peripheral Opioid Peptides
Opioid peptides are the natural ligands at opioid receptors. Three families of these peptides are well characterized in the central nervous and neuroendocrine systems. Each family derives from a distinct gene and precursor protein: proopiomelanocortin, proenkephalin and prodynorphin. Appropriate processing yields their respective major representative opioid peptides β-endorphin, enkephalin and dynorphin. Each peptide exhibits different affinity and selectivity for the three receptor types μ (β-endorphin, enkephalin), δ (enkephalin, β-endorphin) and κ (dynorphin).7 Recently, two additional endogenous opioid peptides have been isolated from bovine brain: endomorphin-1 and endomorphin-2. Their precursors are not known yet. These two peptides are considered to have the highest specificity and affinity for μ-receptors of any endogenous substance so far described.8 Proopiomelanocortin and proenkephalin-derived opioid peptides have been detected in rodent and human immune cells9–12 (reviewed in the chapter by Smith in this volume).
Immune-derived opioid peptides apparently play a substantial role in the modulation of inflammatory pain.4 In inflamed rat paw mRNAs encoding proopiomelanocortin and proenkephalin and their respective opioid peptide products β-endorphin and enkephalin are found predominantly within T- and B-lymphocytes as well as in monocytes, macrophages and granulocytes.13–16 Small amounts of dynorphin are also detectable by immunocytochemistry17 (reviewed in the chapter by Mousa in this volume).
Other sources of opioids in the periphery are the adrenals and the pituitary, but these have been excluded as sources for opioid ligands at peripheral receptors. Opioid peptides have been found in sensory ganglia and in peripheral terminals of sensory nerves (reviewed in ref. 18) but direct functional evidence regarding their possible role in peripheral nociceptive transmission is to date lacking.
Interactions of Immune-Derived Opioids with Peripheral Opioid Receptors
Migration of Opioid-Containing Immune Cells to Inflamed Tissue
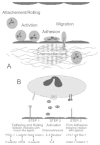
The recruitment of leukocytes into areas of inflammation begins with the binding of white blood cells to endothelium, followed by their transmigration into tissues. Although this observation has been documented for more than 150 years, only the last decade, with the identification of specific cell adhesion and chemoattractant/activator molecules (Fig. 1) uncovered the molecular mechanisms underlying leukocyte extravasation.
Leukocytes are recruited from the circulation by a well-orchestrated set of events (Fig. 2). In these processes, leukocytes undergo multiple attachments to, and detachments from, the vessel endothelial cells, prior to transendothelial cell migration. This includes slowing and rolling along the endothelial cell wall that is mediated predominantly by the interaction of selectins expressed on leukocytes (L-selectin) and endothelial cells (P- and E-selectin) with their ligands expressed on endothelium or immune cells, respectively. The rolling immunocytes can then be activated by chemoattractants released from inflammatory cells and endothelium. This leads to up-regulation and increased avidity of integrins. These mediate the firm adhesion of leukocytes to endothelial cells via ligands of the immunoglobulin (Ig) superfamily. Finally, the immune cells transmigrate through the endothelial wall mediated by Ig superfamily members (e.g., platelet-endothelial adhesion molecule1; PECAM1) and are directed to the sites of inflammation to initiate a host defense (reviewed in refs. 19-21).
Recent findings suggest that these events can also be involved in endogenous control of inflammatory pain. P-selectin and PECAM1 present on endothelia are up-regulated in inflammation. The number of immunocytes expressing L-selectin is increased in inflamed subcutaneous paw tissue. Most importantly, many β-endorphin-containing cells express L-selectin in inflamed tissue22 (for details see the chapter by Mousa in this volume). Furthermore, pretreatment of rats with a selectin blocker (fucoidin) decreases the number of β-endorphin-containing immunocytes infiltrating the inflamed site.23 In consequence, this diminishes the β-endorphin content in inflamed tissue and concurrently abolishes peripheral opioid analgesia (for details see ref. 23 and “Peripheral analgesic effects of immune cell-derived opioid peptides” in this chapter). This suggests that circulating β-endorphin-producing immunocytes home to inflamed tissue where they secrete the opioid to inhibit pain. Afterwards they travel to the regional lymph nodes, depleted of the opioid peptide.14 This migratory pattern is reminiscent of memory cells. The trafficking of those cells is not random but they are specifically directed to sites of antigenic or microbial invasion (e.g., inflammatory lesions of the skin).19,20 Consistent with this notion, β-endorphin was indeed found in memory-type T-cells (the chapter by Mousa in this volume).14,15These findings suggest that local signals not only stimulate the synthesis of opioid peptides in resident inflammatory cells but also attract opioid-containing cells from the circulation to the site of tissue injury to reduce pain (for details see “Peripheral analgesic effects of immune cell-derived opioid peptides” in this chapter).
Release of Opioid Peptides from Immune Cells
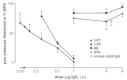
Once the immune cells reach the site of inflammation, they have to secrete the opioids to produce pain relief. Endogenous agents triggering opioid release within inflamed tissue are CRF and cytokines. CRF mRNA and immunoreactivity have been demonstrated in various lymphoid tissues such as in thymus, spleen and in T and B lymphocytes. In inflamed subcutaneous tissue CRF is detectable in immune cells, fibroblasts and vascular endothelium. Interestingly, peripheral CRF expression is enhanced in inflamed synovial and subcutaneous tissue of animals and humans. CRF and interleukin-1β (IL-1) receptors have been detected in rat synovial and subcutaneous tissue on lymphocytes and monocytes/macrophages but not on peripheral sensory nerves. The number of both receptors is greatly enhanced in inflammation. Their pharmacolgical characteristics are similar to the high-affinity CRF and IL-1 binding sites in the pituitary (reviewed in ref. 24 and in the chapters by Schäfer and Mousa in this volume). Both CRF and IL-1 can release β-endorphin from immune cell suspensions prepared from lymph nodes in vitro (Fig. 3).14,25 This release is specific to CRF and IL-1 receptors because it is reversed dose-dependently by their respective antagonists (Fig. 3), it is calcium-dependent and it is mimicked by elevated extracellular concentrations of potassium.14 This is consistent with a regulated pathway of release from secretory vesicles, as in neurons and endocrine cells. In summary, these findings indicate that CRF and IL-1 can cause secretion of opioids from immune cells. These opioid peptides subsequently activate opioid receptors on sensory nerves to inhibit pain (for details see “Peripheral analgesic effects of immune cell-derived opioid peptides” in this chapter).
Peripheral Analgesic Effects of Immune Cell-Derived Opioid Peptides
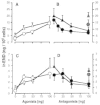
In rat unilateral hind paw inflammation induced by complete Freund's adjuvant26 endogenous opioid analgesia can be elicited by environmental stress (cold water swim; CWS).27 In this model rats swim for 1 min in water of 4°C and pain thresholds are measured afterwards in a paw pressure test (Randall-Selitto test) evaluating a response to painful mechanical stimuli.27 CWS produces analgesia in inflamed but not in noninflamed paws.27 This effect is opioid specific because it is blocked dose-dependently and stereospecifically by the opioid receptor antagonist naloxone injected into the paw.27 Using selective antagonists it was shown that μ- and δ-, but not κ- receptors play a major role (Fig. 4).27 A prominent opioid peptide involved in peripheral pain control is β-endorphin because CWS-induced analgesia is dose-dependently abolished by antibodies against β-endorphin but not against met-enkephalin or dynorphin (Fig. 5).27,28 In parallel, exogenous application of β-endorphin into the inflamed paw produces dose-dependent analgesia reversed by μ- and δ-receptor antagonists.27 The systemic (subcutaneous or intravenous) administration of opioid receptor antagonists or antibodies against opioid peptides does not change CWS-induced analgesia, demonstrating a peripheral site of action.27,28 Thus, analgesia in inflamed tissue can by induced through the activation of local opioid receptors by endogenous opioid peptides (mainly β-endorphin) released during CWS.
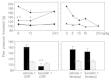
An important stimulus for peripheral β-endorphin release appears to be CRF because CWS-induced analgesia is abolished by the local injection of a CRF receptor antagonist or antibody.29 Consistently, the local application of small, systemically inactive doses of CRF produces potent CRF and opioid receptor-specific analgesic effects in inflamed, but not in noninflamed tissue.25 Although exogenous IL-1 can release opioids from immune cells14,25 (Fig. 3) and produce analgesia25 endogenous IL-1 does not seem to be involved at this stage of inflammation (4 days)29 (for details on the involvement of cytokines in peripheral opioid analgesia see the chapter by Schäfer in this volume). That immune cells are a source of opioids is demonstrated by abolishing CWS and CRF-induced analgesia by immunosuppression with cyclosporine or whole-body irradiation.13,25,28 Moreover, these effects are also extinguished by blocking the extravasation of β-endorphin-containing immune cells. Fucoidin, an L- and P-selectin blocker can dose-dependently inhibit CWS and CRF but not fentanyl (an opioid receptor agonist)-induced analgesia in inflammation (Fig. 6).23 Concurrently, the number of β-endorphin-containing cells and the total amount of β-endorphin in the tissue is significantly diminished.23 Together, these findings demonstrate that L- and P-selectins regulate the migration of β-endorphin-containing immune cells and the subsequent generation of endogenous pain control in injured tissue.
Recently, the involvement of subpopulations of opioid-containing immunocytes and their contribution to endogenous analgesia was examined in relation to the development of inflammation.16 In early (2-6 hours) inflammation a majority of opioid-producing leukocytes are granulocytes whereas at later stages (4 days) monocytes and macrophages play a dominant role. With increasing duration of inflammation the number of opioid-containing immunocytes and the β-endorphin content rise. In parallel, the CWS-induced analgesic effect increases.16 Thus, the potency of endogenous pain inhibition is proportional to the number of opioid peptide-producing cells and distinct leukocyte lineages contribute to this function at different stages of inflammation. Further details on the neural mechanisms underlying the generation of peripheral opioid analgesia are discussed in ref. 18 and in the chapter by Stein in this volume.
Clinical Implications
Peripheral opioid analgesic actions are of clinical relevance. Opioid receptors are present on peripheral terminals of nerve fibers in human synovia30 and these receptors are capable of mediating analgesia in humans.31 Opioid peptides were found in human synovial lining cells and in immune cells such as lymphocytes, macrophages and mast cells. The prevailing peptides are β-endorphin and enkephalin, while only minor amounts of dynorphin are detectable.30 These morphological findings are presented in details in the chapter by Mousa in this volume. The interaction of synovial opioids with peripheral opioid receptors was examined in patients undergoing knee surgery. Blocking intraarticular opioid receptors by the local administration of naloxone resulted in significantly increased postoperative pain.32 Taken together, these findings suggest that in a stressful (e.g., postoperative) situation, opioids are tonically released from inflamed tissue and activate peripheral opioid receptors to attenuate clinical pain.
Importantly, these endogenous opioids do not interfere with exogenous morphine, i.e., intraarticular morphine is an equally potent analgesic in patients with and without opioid-producing inflammatory synovial cells.30 This suggests that, in contrast to the rapid development of tolerance in the central nervous system, the immune cell-derived opioids do not readily produce cross-tolerance to morphine at peripheral opioid receptors.
These findings have to be kept in mind when the immune system becomes a target for the treatment of inflammatory diseases. The blockade of immune cell extravasation by antibodies against immunocytes or against adhesion molecules has been proposed as a novel anti-inflammatory strategy.33 However, as shown above, anti-selectin treatment can cause severe impairment of opioid-mediated pain inhibition in inflammation.23 Thus, it is important that future therapeutic strategies aimed at limiting the adhesion of harmful cells in inflammatory diseases do not interfere with the migration of opioid-containing cells promoting pain control.
Conclusions
In conclusion, opioid peptides released from immune cells under stress or by CRF can activate opioid receptors on sensory nerves to produce potent peripheral, clinically relevant analgesia. This analgesia lacks central side effects such as respiratory depression, sedation, dysphoria and dependence. It appears that the immune system uses mechanisms of cell migration not only to fight pathogens but also to control pain within injured tissue. Thus, pain may be exacerbated by measures inhibiting the immigration of opioid-producing cells or, conversely, analgesia may be conveyed by adhesive interactions that recruit those cells to injured tissue. These findings provide new insights into pain associated with a compromised immune system, as in AIDS or in cancer. The activation of opioid production and release from immune cells may be a novel approach to the development of peripherally acting analgesics. Since such drugs would be targeted towards events in peripheral injured tissue, these analgesics should lack unwanted central side effects typically associated with opioids.
Acknowledgments
Supported by grants from the Deutsche Forschungsgemeinschaft and the National Institutes of Health.
References
- 1.
- Machelska H, Binder W, Stein C. Opioid receptors in the periphery In: Kalso E, McQuay H, WisenfeldHallin Z, eds. Opioid sensitivity of chronic noncancer pain Seattle: IASP Press, 199945–58.
- 2.
- Barber A, Gottschlich R. Opioid agonists and antagonists: an evaluation of their peripheral actions in inflammation. Med Res Rev. 1992;12:525–562. [PubMed: 1513187]
- 3.
- Stein C. Peripheral mechanisms of opioid analgesia. Anesth Analg. 1993;76:182–191. [PubMed: 8380316]
- 4.
- Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–1690. [PubMed: 7760870]
- 5.
- Stein C, Machelska H, Binder W. et al. Peripheral opioid analgesia. Curr Opin Pharmacol. 2001;1:62–65. [PubMed: 11712537]
- 6.
- Walker JS, Wilson JL, Binder W. et al. The anti-inflammatory effects of opioids: their possible relevance to the pathophysiology and treatment of rheumatoid arthritis. Res Alerts Rheum Arthritis. 1997;1:291–299.
- 7.
- Höllt V. Opioid peptide processing and receptor selectivity. Annu Rev Pharmacol Toxicol. 1986;26:59–77. [PubMed: 3013080]
- 8.
- Zadina JE, Hackler L, Ge LJ. et al. A potent and selective endogenous agonist for the mopiate receptor. Nature. 1997;386:499–502. [PubMed: 9087409]
- 9.
- Blalock JE. The syntax of immune-neuroendocrine communication. Immunol Today. 1994;15:504–511. [PubMed: 7802919]
- 10.
- Weisinger G. The transcriptional regulation of the preproenkephalin gene. Biochem J. 1995;307:617–629. [PMC free article: PMC1136696] [PubMed: 7741689]
- 11.
- Panerai AE, Sacerdote P. β-Endorphin in the immune system: a role at last? Immunol Today. 1997;18:317–319. [PubMed: 9238833]
- 12.
- Lyons PD, Blalock JE. Proopioimelanocortin gene expression and protein processing in rat mononuclear leukocytes. J Neuroimmunol. 1997;78:47–56. [PubMed: 9307227]
- 13.
- Przewlocki R, Hassan A H S, Lason W. et al. Gene expression and localization of opioid peptides in immune cells of inflamed tissue: functional role in antinociception. Neuroscience. 1992;48:491–500. [PubMed: 1603330]
- 14.
- Cabot PJ, Carter L, Gaiddon C. et al. Immune cell-derived β-endorphin: production, release and control of inflammatory pain in rats. J Clin Invest. 1997;100:142–148. [PMC free article: PMC508174] [PubMed: 9202066]
- 15.
- Mousa SA, Zhang Q, Sitte N. et al. β-Endorphin containing memory cells and μ-opioid receptors undergo transport into peripheral inflamed tissue. J Neuroimmunol. 2001;115:71–78. [PubMed: 11282156]
- 16.
- Rittner HL, Brack A, Machelska H. et al. Opioid peptide expressing leukocytesidentification, recruitment and simultaneously increasing inhibition of inflammatory pain. Anesthesiology. 2001;95:500–508. [PubMed: 11506126]
- 17.
- Hassan A H S, Przewlocki R, Herz A. et al. Dynorphin, a preferential ligand for kappaopioid receptors, is present in nerve fibers and immune cells within inflamed tissue of the rat. Neurosci Lett. 1992;140:85–88. [PubMed: 1357608]
- 18.
- Stein C, Schäfer M, Cabot PJ. et al. Peripheral opioid analgesia. Pain Rev. 1997;4:171–185.
- 19.
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. [PubMed: 7507411]
- 20.
- Butcher EC, Picker LJ. Lymphocyte homing homeostasis. Science. 1996;272:60–66. [PubMed: 8600538]
- 21.
- Petruzzelli L, Takami M, Humes D. Structure and function of cell adhesion molecules. Am J Med. 1999;106:467–476. [PubMed: 10225251]
- 22.
- Mousa SA, Machelska H, Schäfer M. et al. Coexpression of β-endorphin with adhesion molecules in a rat model of inflammatory pain. J Neuroimmunol. 2000;108:160–170. [PubMed: 10900350]
- 23.
- Machelska H, Cabot PJ, Mousa SA. et al. Pain control in inflammation governed by selectins. Nat Med. 1998;4:1425–1428. [PubMed: 9846582]
- 24.
- Schäfer M, Mousa SA, Stein C. Corticotropin-releasing factor in antinociception and inflammation. Eur J Pharmacol. 1997;323:1–10. [PubMed: 9105870]
- 25.
- Schäfer M, Carter L, Stein C. Interleukin-1 beta and corticotropin-releasing-factor inhibit pain by releasing opioids from immune cells in inflamed tissue. Proc Natl Acad Sci USA. 1994;91:4219–4223. [PMC free article: PMC43756] [PubMed: 7910403]
- 26.
- Stein C, Millan MJ, Herz A. Unilateral inflammation of the hindpaw in rarts as a model of prolonged noxious stimulation: alterations in behavior and nociceptive thresholds. Pharmacol Biochem Behav. 1988;31:445–451. [PubMed: 3244721]
- 27.
- Stein C, Gramsch C, Herz A. Intrinsic mechanisms of antinociception in inflammation. Local opioid receptors and beta-endorphin. J Neurosci. 1990;10:1292–1298. [PMC free article: PMC6570200] [PubMed: 2158530]
- 28.
- Stein C, Hassan A H S, Przewlocki R. et al. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc Natl Acad Sci USA. 1990;87:5935–5939. [PMC free article: PMC54444] [PubMed: 1974052]
- 29.
- Schäfer M, Mousa SA, Zhang Q. et al. Expression of corticotropinreleasing factor in inflamed tissue is required for intrinsic peripheral opioid analgesia. Proc Natl Acad Sci USA. 1996;93:6096–6100. [PMC free article: PMC39195] [PubMed: 8650225]
- 30.
- Stein C, Pflüger M, Yassouridis A. et al. No tolerance to peripheral morphine analgesia in presence of opioid expression in inflamed synovia. J Clin Invest. 1996;98:793–799. [PMC free article: PMC507490] [PubMed: 8698872]
- 31.
- Stein C, Comisel K, Haimerl E. et al. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med. 1991;325:1123–1126. [PubMed: 1653901]
- 32.
- Stein C, Hassan A H S, Lehrberger K. et al. Local analgesic effect of endogenous opioid peptides. Lancet. 1993;342:321–324. [PubMed: 8101583]
- 33.
- Choy E H S, Panayi GS, Kingsley GH. Therapeutic monoclonal antibodies. Br J Rheumatol. 1995;34:707–715. [PubMed: 7551652]
- Functional Evidence of Pain Control by the Immune System - Madame Curie Bioscien...Functional Evidence of Pain Control by the Immune System - Madame Curie Bioscience Database
- Future Perspectives of Interstitial and Perfusional Hyperthermia - Madame Curie ...Future Perspectives of Interstitial and Perfusional Hyperthermia - Madame Curie Bioscience Database
- α1-Microglobulin - Madame Curie Bioscience Databaseα1-Microglobulin - Madame Curie Bioscience Database
- Macroautophagy in Mammalian Cells - Madame Curie Bioscience DatabaseMacroautophagy in Mammalian Cells - Madame Curie Bioscience Database
- Regulation of Phagocytosis by FcγRIIb and Phosphatases - Madame Curie Bioscience...Regulation of Phagocytosis by FcγRIIb and Phosphatases - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...