NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Introduction
The immune-suppressive effects of painful experiences have been studied in both humans and animals for many years. Experimental pain has been induced by such means as electric shock and surgery in animals, and humans undergoing surgery have been studied extensively. In general, results have shown such perturbations to suppress the immune functions that are assessed.1,2 The exclusive contribution of the pain per se to these findings has only recently become a focus of study. If pain mechanisms were shown to mediate the observed immunosuppressive effects of experiences such as recovery from injury or undergoing surgery, then adequate pain management would become a vital adjunct to the overall care of such individuals.
The importance of such an avenue of study relates to the crucial role played by the immune system in maintaining health and resisting infection and disease.3 In the latter case, animal studies provide direct evidence that natural killer (NK) cells play a key role in controlling metastatic processes4,5 as well as support for the hypothesis that the suppressed NK cell activity following surgery underlies surgery-induced metastatic promotion.1,6 Findings of human studies are corroborative; low NK activity during the perioperative period is associated with higher rates of cancer recurrence and mortality in patients with breast,7 head and neck,8 lung,9 and colorectal cancers.10
Animal Studies of Pain and Immune Suppression
Aversive stressors such as footshock and tail shock have been used as means by which to provoke pain without tissue damage in rats. Intermittent inescapable footshock delivered over some minutes has been shown to suppress several immune functions, including NK cell activity,2,11 peripheral blood mononuclear or spleen cell proliferative responses to mitogens including phytohemagglutinin and Concavalin A.2,12 Similarly, intermittent tail shock has been shown to suppress mixed lymphocyte reactions in lymph node cells13 and reduce in vivo responses to a novel antigen assessed as immunoglobulin (Ig) G antibody levels.14 An important consideration in these studies is the psychological stress that likely occurs in animals subjected to some period of repeated, uncontrollable, aversive stimuli;15,16 thus, the observed immune suppression may be attributed to mechanisms other than pain per se.
Experimental surgery has been shown to suppress immune functions and to promote tumor development in rats and mice. Rats recovering from laparotomy exhibit decreases in both lymphocyte and splenocyte proliferative responses to mitogens and in NK cell activity;17–21 more invasive surgery is associated with greater decrements in immune function.18,19 Similarly, undergoing surgery promotes tumor development, and the magnitude of tumor promotion is associated with the invasiveness of surgery.6,22,23
Pain and Immune Function in Humans
In humans, surgery is well known to result in immune suppression, including lymphocyte proliferative responses to mitogens and NK cell activity,24–27 decreased cell-mediated immunity,28,29 and alterations in the balance of Th1 versus Th2 cells, compromising cellular immunity.30,31 The invasiveness of the surgery has been associated with the magnitude of immune suppression,31 and immune function is comparatively more suppressed by surgery in individuals with cancer,29 although this finding is not a consistent one.32 If pain is a mediator of such surgery-induced immune suppression, then it would seem that anesthesia and analgesia techniques that reduce perioperative pain would also impact the observed postoperative immune suppression. Indeed, several studies comparing general inhalational anesthesia versus epidural or spinal anesthesia for surgical procedures that are below the waist have shown that immune outcomes were significantly better preserved in groups treated with epidural anesthesia, including NK cell activity following laparotomy33 and hysterectomy;34 however, this benefit was not observed in patients undergoing total hip replacement.25 Given the importance of immune function in resisting infection, that epidural anesthesia was also shown to result in fewer infectious complications compared to standard inhalational anesthesia35 supports the above-mentioned suggestion.
Interleukin (IL)-6, a cytokine produced by a host of immune cells including monocytes, macrophages and lymphocytes, is involved in a broad array of actions including inflammation and the regulation of endocrine and metabolic functions, notably the hypothalamic-pituitary-adrenal axis and catecholamines.36 Undergoing surgery results in increased IL-6 levels in plasma37 as well as cerebrospinal fluid.38 Several direct comparisons have shown that more invasive surgeries are associated with greater levels of plasma IL-6, including laparoscope-assisted vaginal versus abdominal hysterectomy,39 and cholecystectomy using a laparoscope versus an open abdominal approach.40,41 Kristiansson et al.41 also showed the laparoscopic approach resulted in reduced pain scores. Given that prostaglandins promote inflammatory changes and hyperalgesia,42 and that prostaglandins have been associated with NK suppression,43,44 interventions that reduce inflammation would be expected to both reduce pain and its immunosuppressive effects. In animals, prostaglandin E2 antagonism via cyclooxygenase inhibition or a monoclonal antibody reduced paw swelling, and both paw and serum IL-6 levels resulting from adjuvant arthritis or carrageenan injection,42,45 and significantly reduced thermal hyperalgesia.42 In humans undergoing cholecystectomy, perioperative ibuprofen administration resulted in significantly lower levels of plasma IL-6 levels compared to placebo controls.37
Animal Studies of Pain, Metastasis and Immune Suppression
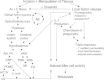
Fig. 1 offers selected possible interactions among nociceptive processes, local metabolic processes, neuroendocrine activation and immunity that are altered as a result of the cutting, tearing, and manipulation of tissues in the conduct of surgery. With particular relevance to the studies to be discussed are hypothalamic activation by ascending nociceptive (painful) impulses, and the resulting increases in sympathetic nervous system activity with epinephrine release and corticosteroid release from the adrenals, both of which have been shown to suppress NK cytotoxic activity.46–48 The release of local factors from the injury site further facilitate central pain processing by sensitizing peripheral fibers, thereby reducing the threshold for nociceptive impulse transmission and the self-perpetuating hyperresponsiveness to mechanical and thermal stimuli at the surgical site.49 Taken together, both central and peripheral changes contribute to the suppression of NK cytotoxicity that is observed postoperatively.
To attribute some measure of biologic significance to NK suppression, we have used an NK-sensitive mammary adenocarcinoma cell line, MADB106, syngeneic to the inbred Fischer 344 rats. MADB106 tumor cells seed and colonize only in the lungs following intravenous injection, and the NK sensitivity of these processes has been shown to be limited to the first 24 hours after injection in a time-dependent and decremental manner.5,50 Thus, both the colonization of MADB106 cells in the lungs assessed at 3 weeks after tumor injection and the lung retention of radiolabeled MADB106 cells assessed at 18-24 hours after tumor injection provide an indication of host susceptibility to metastasis as well as in vivo levels of NK activity throughout the early hours following tumor injection.
The overall goal of our work is to investigate possible mediation of specific pain mechanisms in surgery-induced decreases in NK activity and host resistance against metastasis. To explore the possibility that pain per se mediates these negative consequences of surgery, pharmacologic interventions have been employed with the rationale that if the drug significantly attenuates surgery-induced reductions in both host resistance against metastasis and exploratory behavior, then pain relief might be suggested as a biologically significant and beneficial treatment.
Animals
Mature Fischer 344 rats were used in all studies. Animals were maintained for 4 weeks following shipment to allow for acclimatization to the vivarium and the 12-hour dark/light schedule. Animals had unlimited access to food and water until 8 hours prior to surgery, when only water was available. All surgeries were performed within the first 5 hours of the dark phase. All protocols were approved by the institutional committee for the care and use of animal subjects.
Surgery
Animals were anesthetized with halothane throughout the preparation, including skin preparation and antibiotics injection, and conduct of the surgery. The experimental laparotomy consisted of a 4-cm midline incision with externalization of 10 cm of the small intestine, followed by gentle rubbing of the intestine between two pieces of gauze at 4 different locations. The intestine remained exteriorized for a total of 4 minutes under saline-soaked gauze and was then returned to the abdominal cavity, irrigated, and the muscle and skin layers sutured with monofilament wire. “Anesthesia only animals” were anesthetized at the same time and in the same dose as the surgery animals.
The Beneficial Effects of Morphine on Surgery-Induced Decreases in Host Resistance Against Metastasis
The first studies investigating the possibility that providing an analgesic dose of morphine would reduce the metastatic-enhancing effects of surgery used a simple 2×2 experimental design: Surgery versus Anesthesia by Morphine versus Vehicle. Pre- and postoperative morphine administration was accomplished in two different preparations: in saline intraperitoneally 30 minutes before surgery to insure that surgery was conducted at the time of peak analgesic effect, and in a slow-release suspension (SRS) subcutaneously, an oil emulsion that releases the drug over some hours, for drug administrations immediately following and at 5 hours postoperative. As for all studies discussed herein, syngeneic NK-sensitive MADB106 tumor cells were injected at 5 hours after surgery in an effort to capture the stressful nature of the postoperative experience rather than responses to intraoperative events.
We found a significant interaction between the effects of surgery and morphine such that morphine attenuated the observed surgery-induced increase in both the colonization of MADB106 cells in the lungs assessed at 3 weeks after surgery51 and the retention of radiolabeled MADB106 cells in the lungs at 18 hours postoperative.52 Surgery resulted in a more than 5-fold increase in lung tumor cell retention, and morphine treatment reduced this effect by more than 50%.52 Morphine administration exerted no significant effects in the “anesthesia only animals” for both tumor outcomes.51,52
Exploratory behavior studies have been an important aspect of our work. The rationale for such observations relates to the clinical behavior of humans following major abdominal surgery, the characteristic splinting of the abdomen and hunching posture. It was noted that following abdominal surgery, rats also exhibit such a posture and are hesitant to rear (lift both forepaws off of the cage floor), thus stretching the incision in a way not unlike the human assuming an erect posture. Therefore, for exploratory behavior studies, the number of rears exhibited by each animal during the latter 30 minutes of each of the first 4 postoperative hours was enumerated to serve as an indicator of abdominal discomfort. Surgery resulted in a profound suppression of rearing behavior, and morphine administration restored rearing incidences to the levels observed in the unoperated rats.51
To further investigate the possibility that it was the pain-relieving effects of morphine that were responsible for its beneficial effect on the NK-suppressive and metastatic-enhancing consequences of surgery, we designed a subsequent study to assess the relative importance of the pre- versus postoperative administration of morphine in this paradigm. Either morphine or its vehicle was administered to all animals at the same three above-mentioned time points: (1) 30 minutes before surgery, (2) at the completion of surgery in SRS, and (3) at the time of radiolabeled MADB106 tumor cell injection at 5 hours after surgery in SRS. Surgery groups received either the vehicle or morphine in one of four different regimens: before surgery only, at all three times, after surgery only at times 2 and 3, and after surgery total at times 2 and 3 with the preoperative dose added at time 2. Two anesthesia control groups received either morphine or vehicle at all three times. Surgery resulted in a two-fold increase in lung tumor retention, which was significantly attenuated by all four morphine treatment regimens. Furthermore, the two surgery groups that were treated with morphine preoperatively appeared to derive greater benefit, suggesting that the preoperative administration of an opioid is key in optimizing its beneficial effects on surgery-induced increases in metastatic susceptibility. With regard to exploratory behavior, morphine treatment similarly restored surgery-induced activity suppression in all animals except those that received both the pre- and postoperative morphine doses at the completion of surgery. These animals were laying on their bellies, virtually unmoving until the third postoperative hour, likely indicating overmedication.53
Pre- and Postoperative Fentanyl Attenuates Surgery-Induced Increases in Metastatic Susceptibility
The more selective agonist, fentanyl, was used to further explore the possibility that the pain per se is a mediator of surgery-induced increases in metastatic susceptibility. Additionally, female rats were used in this and the subsequent studies described herein. Using the same 2×2 design of surgery versus anesthesia by drug versus vehicle, fentanyl citrate was administered subcutaneously 30 minutes preoperatively, and in the SRS immediately after the completion of surgery. Surgery resulted in a more than four-fold increase in the lung tumor retention of radiolabeled MADB106 cells, which was significantly ameliorated by fentanyl administration; fentanyl exerted no effects in the “anesthesia only animals”. Importantly, females derived similar benefits from drug treatment.
With regard to behavioral activity, operated animals exhibited a significant reduction in rearing behavior compared to the nonoperated animals. Fentanyl treatment did not restore activity levels to those of the unoperated animals; however, among the surgery animals, fentanyl treatment resulted in a significant increase in activity levels compared to the vehicle-injected animals.54
The Beneficial Effects of Preoperative Intrathecal Bupivacaine Plus Morphine on Surgery-Induced Decreases in Host Resistance Against Metastasis
This study explored the possibility that blocking ascending impulses from the abdominal laparotomy would affect the tumor-enhancing effects of surgery. Thus, the same above-mentioned 2×2 design was employed, and drug-treated animals received an intrathecal (between L4 and L5 lumbar vertebrae) injection of bupivacaine combined with morphine immediately before the conduct of surgery. Control animals received intrathecal saline injections. Surgery resulted in a more than three-fold increase in the lung tumor retention of radiolabeled MADB106 cells, which was significantly attenuated by the intrathecal injection of bupivacaine plus morphine; intrathecal drug injections exerted no effects in the “anesthesia only animals”. Females and males derived equivalent benefits from the drug intervention.54 In a separate set of experiments, the preoperative intrathecal administration of similar doses of bupivacaine plus morphine significantly reduced surgery-induced increases in both the lung retention of radiolabeled MADB106 cells and the number of tumor colonies evident three weeks after MADB106 tumor injection.55
Postoperative Indomethacin Administration Attenuates Surgery-Induced Increases in Host Susceptibility to Metastasis
Given the contribution of prostaglandins to wound inflammatory changes and hyperalgesia, the cyclooxygenase inhibitor indomethacin was employed as the drug treatment for this study. Using the same 2×2 design, drug-treated animals received a subcutaneous injection of indomethacin at the completion of surgery. Surgery resulted in a more than two-fold increase in the lung tumor retention of radiolabeled MADB106 cells, which was significantly attenuated by indomethacin treatment in both males and females. Indomethacin administration exerted no effects in the “anesthesia only animals”. This significant interaction between the effects of surgery and drug treatment were also observed in the animals' activity levels. Specifically, indomethacin administration restored rearing incidence to normal levels in both the males and females.56
In a subsequent study, at the time of tumor injection, 5 hours postoperative, all animals underwent cardiac blood withdrawal for the assessment of plasma levels of IL-6 by quantitative sandwich enzyme immunoassay (ELISA). We found that no “anesthesia only animal” exhibited detectable plasma levels of IL-6. Among the surgery animals, indomethacin administration resulted in a significant reduction of surgery-induced increases in plasma IL-6 levels in the males, no such benefits were observed in the females (unpublished data).
Discussion and Conclusions
Taken together, the findings of these studies support the suggestion that the provision of pain relief ameliorates surgery-induced decreases in host resistance against metastasis. In particular, these studies show that a variety of morphine regimens provide protection against the tumor-enhancing effects of surgery including both pre- and postoperative administration51,52 as well as only preoperative or only postoperative treatment.53 The pre- and postoperative systemic administration of fentanyl confers similar benefits as morphine in this paradigm and is also beneficial in the females.54 The benefits conferred by the preoperative intrathecal injection of bupivacaine plus morphine54 as well as the postoperative systemic administration of indomethacin56 on the metastatic-enhancing effects of surgery further support the suggestion that pain is a mediator of this negative consequence of undergoing and recovering from surgery. That either morphine or indomethacin administration completely restored surgery-induced activity suppression to normal levels, and that fentanyl significantly improved surgery-induced activity suppression suggests that these regimens provided pain relief.
Two points support the hypothesis that it is the pain-relieving effects of these drugs that attenuated surgery-induced tumor promotion rather than direct effects on immunity, the tumor cells themselves, or other mechanisms that affect metastasis. First, the very low doses and intrathecal administration of bupivacaine plus morphine argue against possible direct peripheral effects of these drugs. Second, all of the analgesia regimens provided benefit only to the animals undergoing surgery; there were no significant drug-induced changes in metastatic susceptibility in animals not experiencing pain. Such an interaction between the effects of the analgesic agents and exposure to surgery implicates some blockade of the impact of surgery rather than an independent drug effect on tumor development.
In conclusion, these findings show that some analgesic techniques provide significant protection against the tumor-enhancing effects of undergoing and recovering from surgery. Although it remains unknown whether the alleviation of perioperative pain will provide similar benefits in humans, the current studies suggest that it will, specifically in patients with potentially metastasizing cancer. Therefore, it is suggested that the relief of perioperative pain becomes a high priority in the care of individuals with cancer.
References
- 1.
- Ben-Eliyahu S, Page GG, Yirmiya R. et al. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–888. [PubMed: 10074922]
- 2.
- Sacerdote P, Manfredi B, Bianchi M. et al. Intermittent but not continuous inescapable foot-shock stress affects immune responses and immunocyte beta-endorphin concentrations in the rat. Brain Behav Immun. 1994;8:251–260. [PubMed: 7865896]
- 3.
- Whiteside TL, Herberman RB. Role of human natural killer cells in health and disease. Clin Diagn Lab Immunol. 1994;1:125–133. [PMC free article: PMC368214] [PubMed: 7496932]
- 4.
- Wiltrout RH, Herberman RB, Zhang S. et al. Role of organ-associated NK cells in decreased formation of experimental metastases in lung and liver. J Immunol. 1985;134:4267–4275. [PubMed: 3989307]
- 5.
- Ben-Eliyahu S, Page GG. The in vivo assessment of natural killer cell activity in rats. Prog Neuro Endocrin Immunol. 1992;5:199–214.
- 6.
- DaCosta ML, Redmond HR, BouchierHayes DJ. The effect of laparotomy and laparoscopy on the establishment of spontaneous tumor metastasis. Surgery. 1998;124(3):516–525. [PubMed: 9736904]
- 7.
- Levy SM, Herberman RB, Maluish AM. et al. Prognostic risk assessment in primary breast cancer by behavioral and immunological parameters. Health Psychol. 1985;4:99–113. [PubMed: 4018006]
- 8.
- Schantz SP, Taylor DL, Racz T. et al. Natural killer cells and metastases from pharyngeal carcinoma. Am J Surg. 1989;158:361–366. [PubMed: 2802042]
- 9.
- Fujisawa T, Yamaguchi Y. Autologous tumor killing activity as a prognostic factor in primary resected non-small cell carcinoma of the lung. Cytopathology. 1997;79:474–481. [PubMed: 9028357]
- 10.
- Koda K, Saito N, Takiguchi N. et al. Preoperative natural killer cell activity: Correlation with distant metastases in curatively research colorectal carcinomas. Int Surg. 1997;82:190–193. [PubMed: 9331851]
- 11.
- Shavit Y, Martin FC, Yirmiya R. et al. Effects of single administration of morphine or footshock stress on natural killer cell cytotoxicity. Brain Behav Immun. 1987;1:318–328. [PubMed: 3453207]
- 12.
- Pezzone MA, Dohanics J, Rabin BS. Effects of footshock stress upon spleen and peripheral blood lymphocyte mitogenic responses in rats with lesions of the paraventricular nuclei. J Neuroimmunol. 1994;53:39–46. [PubMed: 8051296]
- 13.
- Fleshner M, Bellgrau D, Watkins LR. et al. Stressinduced reduction in the rat mixed lymphocyte reaction is due to macrophages and not to changes in T cell phenotypes. J Neuroimmunol. 1995;56:45–52. [PubMed: 7822481]
- 14.
- Laudenslager ML, Fleshner M, Hofstadter P. et al. Suppression of specific antibody production by inescapable shock: Stability under varying conditions. Brain Behav Immun. 1988;2:92–101. [PubMed: 3148338]
- 15.
- Kant GJ, Bauman RA, Anderson SM. et al. Effects of controllable vs. uncontrollable chronic stress on stress-responsive plasma hormones. Physiol Behav. 1992;51:1285–1288. [PubMed: 1322544]
- 16.
- Drugan RC, Basile AS, Ha JH. et al. Analysis of the importance of controllable versus uncontrollable stress on subsequent behavioral and physiological functioning. Brain Res Brain Res Protoc. 1997;2:69–74. [PubMed: 9438074]
- 17.
- Nelson CJ, Lysle DT. Severity, time, and beta-adrenergic receptor involvement in surgery-induced immune alterations. J Surg Res. 1998;80(2):115–122. [PubMed: 9878301]
- 18.
- Toge T, Hirai T, Takiyama W. et al. Effects of surgical stress on natural killer activity, proliferative response of spleen cells and cytostatic activity of lung macrophages in rats. Gann. 1981;72:790–794. [PubMed: 7327378]
- 19.
- Sandoval BA, Robinson AV, Sulaiman TT. et al. Open versus laparoscopic surgery: A comparison of natural antitumoral cellular immunity in a small animal model. Am Surg. 1996;62(8):625–631. [PubMed: 8712558]
- 20.
- Colacchio TA, Yeager MP, Hildebrandt L. Perioperative immunomodulation in cancer surgery. Am J Surg. 1994;167:174–179. [PubMed: 8311130]
- 21.
- Pollock RE, Lotzová E, Stanford SD. et al. Effect of surgical stress on murine natural killer cell cytotoxicity. J Immunol. 1987;138:171–178. [PubMed: 2946775]
- 22.
- Allendorf JD, Bessler H, Horvath KD. et al. Increased tumor establishment and growth after open vs laparoscopic bowel resection in mice. Surg Endosc. 1998;12:1035–1038. [PubMed: 9685537]
- 23.
- Lee SW, Southall JC, Allendorf JD. et al. Tumor proliferative index is higher in mice undergoing laparotomy vs. CO2 pneumo-peritoneum. Dis Colon Rectum. 1999;42:477–481. [PubMed: 10215047]
- 24.
- Sacerdote P, Bianchi M, Gaspani L. et al. The effects of tramadol and morphine on immune responses after surgery in cancer patients. Anesth Analg. 2000;90(6):1411–1414. [PubMed: 10825330]
- 25.
- Salo M, Nissilä M. Cell-mediated and humoral immune responses to total hip replacement under spinal or general anaesthesia. Acta Anaesthesiol Scand. 1990;34:241–248. [PubMed: 1693030]
- 26.
- Kutza J, Gratz I, Afshar M. et al. The effects of general anesthesia and surgery on basal and interferon stimulated natural killer cell activity of humans. Anesth Analg. 1997;85(4):918–923. [PubMed: 9322480]
- 27.
- Pollock RE, Lotzová E, Stanford SD. Mechanism of surgical stress impairment of human perioperative natural killer cell cytotoxicity. Arch Surg. 1991;126:338–342. [PubMed: 1825598]
- 28.
- Faist E, Ertel W, Cohnert T. et al. Immunoprotective effects of cyclooxygenase inhibition in patients with major surgical trauma. J Trauma. 1990;30:8–18. [PubMed: 2104939]
- 29.
- Riboli EB, Terrizzi A, Arnulfo G. et al. Immunosuppressive effect of surgery evaluated by the multitest cell-mediated immunity system. Can J Surg. 1984;27(1):60–63. [PubMed: 6467104]
- 30.
- Le Cras AE, Galley HF, Webster NR. Spinal but not general anesthesia increases the ratio of T helper 1 to T helper 2 cell subsets in patients undergoing transurethral resection of the prostate. Anesth Analg. 1998;87:1421–1425. [PubMed: 9842841]
- 31.
- Decker D, Schöndorf M, Bidlingmaier F. et al. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to trauma. Surgery. 1996;119:316–325. [PubMed: 8619187]
- 32.
- Beilin B, Shavit Y, Hart J. et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesth Analg. 1996;82:492–497. [PubMed: 8623949]
- 33.
- Koltun WA, Bloomer MM, Tilberg AF. et al. Awake epidural anesthesia is associated with improved natural killer cell cytotoxicity and reduced stress response. Am J Surg. 1996;171:68–73. [PubMed: 8554154]
- 34.
- Tønnesen E, Wahlgreen C. Influence of extradural and general anaesthesia on natural killer cell activity and lymphocyte subpopulations in patients undergoing hysterectomy. Br J Anaesth. 1988;60:500–507. [PubMed: 3377925]
- 35.
- Yeager MP, Glass DD, Neff RK. et al. Epidural anesthesia and analgesia in high-risk surgical patients. Anesthesiology. 1987;66:729–736. [PubMed: 3296854]
- 36.
- Papanicolaou DA, Wilder RL, Manolagas SC. et al. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. [PubMed: 9441573]
- 37.
- Chambrier C, Chassard D, Bienvenu J. et al. Cytokine and hormonal changes after cholecystectomy. Effect of ibuprofen pretreatment. Ann Surg. 1996;224(2):178–182. [PMC free article: PMC1235339] [PubMed: 8757381]
- 38.
- Yeager MP, Lunt P, Arruda J. et al. Cerebrospinal fluid cytokine levels after surgery with spinal or general anesthesia. Reg Anesth Pain Med. 1999;24(6):557–562. [PubMed: 10588562]
- 39.
- Labib M, Palfrey S, Paniagua E. et al. The postoperative inflammatory response to injury following laparoscopic assisted vaginal hysterectomy versus abdominal hysterectomy. Ann Clin Biochem. 1997;34:543–545. [PubMed: 9293310]
- 40.
- Bellón JM, Manzano L, Larrad A. et al. Endocrine and immune response to injury after open and laparoscopic cholecystectomy. Int Surg. 1998;83:24–27. [PubMed: 9706511]
- 41.
- Kristiansson M, Saraste L, Soop M. et al. Diminished interleukin-6 and C-reactive protein responses to laparoscopic versus open cholecystectomy. Acta Anaesthesiol Scand. 1999;43:146–152. [PubMed: 10027020]
- 42.
- Portanova JP, Zhang Y, Anderson GD. et al. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–891. [PMC free article: PMC2192784] [PubMed: 9064348]
- 43.
- Ellis NK, Duffie GP, Young MR. et al. The effects of 16,16-dimethyl PGE2 and phosphodiesterase inhibitors on con A blastogenic responses and NK cytotoxic activity of mouse spleen cells. J Leukoc Biol. 1990;47:371–377. [PubMed: 2156950]
- 44.
- Lala PK, Parhar RS, Singh P. Indomethacin therapy abrogates the prostaglandinmediated suppression of natural killer activity in tumorbearing mice and prevents tumor metastasis. Cell Immunol. 1986;99:108–118. [PubMed: 2944621]
- 45.
- Anderson GD, Hauser SD, McGarity KL. et al. Selective inhibition of cyclooxygenase (COX)2 reverses inflammation and expression of COX2 and interleukin-6 in rat adjuvant arthritis. J Clin Invest. 1996;97(11):2672–2679. [PMC free article: PMC507355] [PubMed: 8647962]
- 46.
- Ben-Eliyahu S, Shakhar G, Page GG. et al. Suppression of NK cell activity and of resistance to metastasis by stress: A role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8:154–164. [PubMed: 11124582]
- 47.
- Shakhar G, BenEliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160(7):3251–3258. [PubMed: 9531281]
- 48.
- Gatti G, Cavallo R, Sartori ML. et al. Cortisol at physiological concentrations and prostaglandin E2 are additive inhibitors of human natural killer cell activity. Immunopharmacology. 1986;11(2):119–128. [PubMed: 2423476]
- 49.
- Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–1768. [PubMed: 10846153]
- 50.
- Barlozzari T, Leonhardt J, Wiltrout RH. et al. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. J Immunol. 1985;134:2783–2789. [PubMed: 3871818]
- 51.
- Page GG, BenEliyahu S, Yirmiya R. et al. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993;54:21–28. [PubMed: 8378099]
- 52.
- Page GG, BenEliyahu S, Liebeskind JC. The roll of LGL/NK cells in surgeryinduced promotion of metastasis and its attenuation by morphine. Brain Behav Immun. 1994;8:241–250. [PubMed: 7865895]
- 53.
- Page GG, McDonald JS, BenEliyahu S. Preoperative versus postoperative administration of morphine: Impact on the neuroendocrine, behavioural, and metastaticenhancing effects of surgery. Br J Anaesth. 1998;81:216–223. [PubMed: 9813526]
- 54.
- Page GG, Blakely WP, Ben-Eliyahu S. Evidence that postoperative pain is a mediator of the tumorpromoting effects of surgery in rats. Pain. 2001;90:191–199. [PubMed: 11166986]
- 55.
- BarYosef S, Melamed R, Page GG. et al. Attenuation of the tumorpromoting effect of surgery by spinal blockade in rats. Anesthesiology. 2001;94:1066–1073. [PubMed: 11465599]
- 56.
- Page GG, Blakely WP, Duong T H T. Evidence that prostanoids contribute to surgeryinduced NK suppression and tumor promotion. Soc Neurosci Abstr. 2000:26.
- Introduction
- Animal Studies of Pain and Immune Suppression
- Pain and Immune Function in Humans
- Animal Studies of Pain, Metastasis and Immune Suppression
- The Beneficial Effects of Morphine on Surgery-Induced Decreases in Host Resistance Against Metastasis
- Pre- and Postoperative Fentanyl Attenuates Surgery-Induced Increases in Metastatic Susceptibility
- The Beneficial Effects of Preoperative Intrathecal Bupivacaine Plus Morphine on Surgery-Induced Decreases in Host Resistance Against Metastasis
- Postoperative Indomethacin Administration Attenuates Surgery-Induced Increases in Host Susceptibility to Metastasis
- Discussion and Conclusions
- References
- The Immune-Suppressive Effects of Pain - Madame Curie Bioscience DatabaseThe Immune-Suppressive Effects of Pain - Madame Curie Bioscience Database
- β-Secretase: Progress and Open Questions - Madame Curie Bioscience Databaseβ-Secretase: Progress and Open Questions - Madame Curie Bioscience Database
- The Sarcoglycans - Madame Curie Bioscience DatabaseThe Sarcoglycans - Madame Curie Bioscience Database
- Nucleic Acids Are Not Boring Long Polymers of Only Four Types of Nucleotides: A ...Nucleic Acids Are Not Boring Long Polymers of Only Four Types of Nucleotides: A Guided Tour - Madame Curie Bioscience Database
- Surgical Stress Response and Cancer Metastasis: The Potential Benefit of Periope...Surgical Stress Response and Cancer Metastasis: The Potential Benefit of Perioperative Beta Blockade - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...