NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Cytokines are important protein mediators of immunity, inflammation, cell proliferation, differentiation, and fibrosis.1 These are the major biological processes underlying autoimmunity. Hence, it is not surprising that there is now convincing evidence that the dysregulation of cytokines plays an important role in the pathogenesis of autoimmunity.2,3
Despite the existence of more than 100 cytokines, it has been shown that only very few of them, namely TNF-α, IL-1β, and recently added IL-12, IL-15 and IL-18, are consistently linked to the pathogenesis of rheumatoid arthritis (RA) and other autoimmune diseases.4 Acting alone or in concert TNF-α, IL-1β, IL-12, IL-15, IL-18 and other cytokines and chemokines induce inflammation.1,4 Based on these observations, it has been hypothesized that blocking the production or biological activities of these cytokines may help to control chronic autoimmune diseases like rheumatoid arthritis (RA).4–6 This hypothesis was tested in in vitro experiments, where neutralizing anti-TNF-α antibodies diminish the induction of other proinflammatory cytokines (e.g., IL-1β and IL-6).1–4 Further experiments showed that anti-TNF-α Abs diminish the incidence and reduce the severity of collagen-induced arthritis (CIA), an animal model of RA.7 Encouraged by these results, an open-label trial on 20 RA patients treated with chimeric (human/mouse) anti-TNF-α cA2 Ab (presently known as infliximab) was conducted in 1992.8 The results show remarkable efficacy of the treatment, further proof that blocking TNF-α may represent a new strategy to control RA.8 Based on these successful results several other biological agents aimed to neutralize TNF-α were developed. FDA recently approved etanercept and infliximab for the treatment of RA. Although both drugs could be used as a monotherapy, higher efficacy is achieved in combination with methotrexate.9–11 etanercept and infliximab treatment blocks (i) the early inflammatory autoimmunity of RA and (ii) joint degradation as measured by radiographic analysis during one year treatment period.10,11 Several excellent reviews describing both basic and clinical aspects of anti-TNF-α therapy have recently been published.12,13 Despite the success of anti-TNF-α therapy, there are some limitations. First, a group of 20–40% of RA patients does not respond to the treatment.9–11 Second, the therapy does not cure the disease; clinical symptoms of RA recurred after the cessation of the treatment.9–13
Another candidate cytokine for participation in the pathogenesis of RA is IL-1. IL-1 exerts many proinflammatory properties of TNF-α.34 While the inflammatory process of RA depends more on TNF-α,14,15 the IL-1 plays a more important role in the pathogenesis of articular cartilage degradation.14,15 Based on the results of in vitro and in vivo experiments on animal models,14,15 clinical trials using natural IL-1 receptor antagonist (IL-1Ra) were conducted. Despite the encouraging decrease of cartilage degradation of RA reported from the natural interleukin-1 receptor antagonist (IL-1Ra) clinical trial, the overall clinical improvement of RA patients was less satisfactory compared with that treated with anti-TNF-α therapy.16,17 At present, clinical trials using combined anti-TNF-α and IL-1Ra therapy are in progress.
Progress made during recent years highlights IL-15 as a key cytokine contributing to autoimmunity. In this chapter, we will focus on the biology and clinical applications of this interesting cytokine.
IL-15 and IL-15Rα
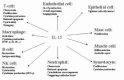
Since the cloning of interleukin (IL)-15 six years ago, there have been numerous studies examining the molecular and cellular biology of this cytokine. Owing to shared receptor (R) signaling components (IL-2Rβ and γ), the in vitro biologic activities of IL-15 are similar to those of IL-2.18–20 However, specificity for IL-15 versus IL-2 is provided by unique private a-chain receptors that complete the IL-15R and IL-2R heterimeric high-affinity receptor complexes and allow differential responsiveness depending on the ligand and high-affinity receptor expressed.21 In contrast to the IL-2Ra, which has low affinity for IL-2 (Kd approximately 10−8 M) in the absence of IL-2R;βγ,22 the IL-15Rα alone was sufficient for high-affinity (Kd greater than or equal to 10−11 M) binding of IL-15. Similar to IL-2Ra, IL-15Rα seemed play no role in signal transduction.21 Intriguingly, both IL-15 and IL-15R transcripts have a much broader tissue distribution than IL-2/IL-2R. IL-15 mRNA is produced by multiple tissues (placenta, skeletal muscle, kidney, lung, heart, monocytes/macrophages).23 IL-15Rα is expressed in activated T cells, activated NK cells, activated B cells, activated macrophages, activated vascular endothelial cells, as well as thymic and bone marrow stromal cells. IL-15Rα mRNA also has wide range of tissue expression including liver, heart, spleen, lung, and skeletal muscle.21,24 Further, multi-level complex regulatory mechanisms tightly control IL-15 expression.25 Although transcriptional control of IL-15 is important, the principal level of IL-15 regulation appears to be posttranscriptional.25 In addition, there are two IL-15 isoforms that differ only by the signal sequence.26–28 The isoform having a 48 amino acid (aa) long signal sequence is directed to the plasma membrane or may be secreted.27,28 Surface expressed IL-15 exerts biological activities: stimulates T-cell proliferation25 and induces granzyme B and perforin.30 The protease, cleaving mature protein from the surface, has not been identified yet. The other IL-15 isoform containing a 21 aa short signal sequence is found in the cell cytoplasm and nucleus. The biological role of this isoform has to be elucidated. Thus, based upon complex regulation, as well as differential patterns of IL-15 and IL-15R expression, it is consistent with evidence that in vivo functions of this receptor/ligand pair differ from those of IL-2 and IL-2R. Studies to date examining the biology of IL-15 have identified several key nonredundant roles, such as the importance roles in the development and homeostasis of natural killer (NK) cells, NK-T cells, CD8+ T cells, and intestinal intraepithelial lymphocytes (Fig. 1).
IL-15/IL-15R System Is Critical for NK Cell Development and Function
Recently generated mice with targeted disruption of the IL-15R and IL-15 (IL-15RKO and IL-15KO) provide direct evidence that the IL-15/IL-15R system is critical for murine NK cell development.31 The IL-15RKO mice contain multiple defects in innate immune effectors, including an absence of splenic NK cells and NK cytotoxic activity. It is significant that IL-15KO mice also lack any phenotypic or functional NK cells in the spleen and liver, a defect that is reversible upon administration of exogenous IL-15 for 1 week.32 Moreover, exogenous IL-15 treatment of normal mice enhances NK cell activity and increases both the percentage and absolute number of splenic NK cells.32–34 Transgenic mice that overexpress murine IL-15 manifest a striking early expansion in NK cells.35 Thus, in vivo evidence demonstrates that IL-15 is requisite for murine NK cell development, and exogenous IL-15 supports the differentiation.
IL-15 is a potent stimulus for GM-CSF production by resting human and murine NK cells.19,36 In concert with IL-12, IL-15 costimulates NK cells to produce the macrophage-activating factors IFN-γ and TNF-α.19,37 Therefore, in both mice and humans, activated macrophages and NK cells interact through a paracrine feedback loop, with macrophages producing monokines (e.g., IL-15 and IL-12) that bind to surface receptors constitutively present on NK cells, resulting in the production of macrophage-activating factors (e.g., IFN-γ). NK cell-derived macrophage-activating factors in turn feed back upon the macrophages to further augment their activation. Thus, macrophage-derived IL-15 contributes with other monokines (especially IL-12) to the proinflammatory cascade leading to IFN-γ production.
In addition, IL-15 stimulates NK cells to produce the C-C chemokines, macrophage inflammatory protein (MIP)-1, which is augmented with the addition of IL-12.36,38 Because C-C chemokines also serve as chemoattractants for NK cells,39 MIP-1 production may direct trafficking of additional NK cells to the site of inflammation. Moreover, chemokine production may have implications in the interactions between macrophages and NK cells, as MIP-1 has been shown to potentiate IFN-γ inducible secretion of inflammatory cytokines by macrophages such as IL-1.40 Therefore, IL-15 seems to play a critical role in the proinflammatory cascade. Thus blockade of the IL-15/IL-15R signal pathway may represent a novel strategy to interrupt the proinflammatory cascade of autoimmune responses.
Function of IL-15 on TCR T Cells
The studies from IL-15RKO and IL-15KO mice reveal a pivotal role of IL-15 for the homeostasis and proliferation of memory CD8+ T-cells. IL-15RKO mice have a selective deficit in both thymic and peripheral CD8+ T-cells.31 IL-15KO mice have reduced numbers of memory-phenotype CD8+ T cells in the spleen and lymph nodes that were reversible upon provision of exogenous IL-15.32 Because IL-15KO mice had normal numbers of single-positive CD8 thymocytes, IL-15 may not be requisite for the development of CD8+ T cells but may be critical for their expansion or survival.32 The subtle differences in the thymic CD8 single-positive cells between IL-15R and IL-15 knockout mice warrant additional investigation. IL-15KO mice maintained good health when housed under specific pathogen-free conditions; however, they demonstrated a dramatic lethal sensitivity to vaccinia virus infection compared with control mice.32 Because both NK cells and CD8+ T cells are important for protection against vaccinia, failure to mount a protective host response is likely due to the deficiencies in these lymphocyte populations. IL-15 stimulates the proliferation of human memory (CD45RO+) CD4 and CD8 and naive (CD45RO) CD8+ human T cells in vitro, while having no effect on naive CD4 T lymphocytes.41 Further, in mice transgenic for the long signal peptide of IL-15 (LSP-IL-15) cDNA or with IL-2 signal peptide replacing LSP of IL-15 under the control of an MHC class I promoter, CD44hiLy-6C+CD8+ memory-phenotype T cells were increased in peripheral lymphoid tissues. Moreover, unlike IL-2, IL-15 closely mimics the effects of type I IFN in causing strong and selective stimulation of memory-phenotype CD44hi CD8+ (but not CD4+) cells in vivo; similar specificity applies to purified T cells in vitro.42
The therapeutic implication of the pivotal role of IL-15 for the homeostasis and proliferation of memory CD8+ T-cells is obvious. As T-cell costimulation blockade has proved to be a potent therapeutic strategy to blunt certain T-cell dependent autoimmunity and induce allograft tolerance,43–46 clinical trials using CTLA4/Ig fusion protein which blocks B7/CD28 costimulation pathway and anti-CD40L mAb which blocks CD40/CD40L costimulation pathway have been conducted. Despite the potent immunosuppressive effects of costimulation blockade, costimulation resistant CD8+ T cells are responsible for the failure of these therapies in certain auto- and alloimmune responses.47–49 Since IL-15 plays a pivotal role in the homeostasis and proliferation of memory CD8+ T cells, targeting IL-15/IL-15R signal pathway in concert with costimulation blockade may represent a novel strategy to prevent and treat T-cell mediated autoimmune diseases and induce allograft tolerance.
Although IL-15 and IL-2 share properties as T-cell growth factors, their effects on T-cell apoptosis are poles apart. It is notable that IL-2, IL-2Ra, and IL-2Rβ deficient mice share a similar phenotype, impaired activation induced apoptosis, lymphoproliferative disorders, and autoimmunity,50–52 indicating that IL-2 plays a unique and irreplaceable role in activation induced T-cell death (AICD). In striking contrast to IL-2, IL-15 has been shown to prevent rather than promote T-cell apoptosis.53,54 As AICD of lymphocytes is an important homeostatic mechanism in the immune system and is involved in the induction and maintenance of peripheral tolerance to auto- and alloantigens,55,56 targeting IL-15/IL-15R signal pathway may foster AICD and facilitate tolerance induction.
Moreover, Li X et al recently reported the distinct roles of IL-15 and IL-2 for primary T-cell expansion in vivo.57 IL-15 seems to be a critical growth factor in initiating T-cell division in vivo, whereas the unique role of IL-2 in vivo is to control the magnitude of clonal expansion by regulating g-c expression on cycling T cells. Thus blockade of IL-15/IL-15R signaling pathway may prevent early T-cell activation and the T-cell mediated cytopathic autoimmune process.
Role of IL-15 in Autoimmune and Inflammatory Disease
Rheumatoid arthritis (RA) is a chronic degenerative condition of synovial membranes mediated in part by aberrant cytokine regulation that ultimately results in abnormally high levels of proinflammatory cytokines, such as TNF-α, within the joints. Lymphocytes and TNF-α are strongly implicated in the pathogenesis of CIA and clinical RA.4 Furthermore, the success of therapeutic strategies that neutralize TNF-α in the murine CIA model58,59 and in clinical RA60,61 underscore the crucial role played by TNF-α in disease pathogenesis. McInnes et al reported that TNF-α production is increased by a direct T cell to macrophage contact through an IL-15 dependent process that also results in activation of T cells.62 This effect was cell-contact dependent, and antibodies to CD69, lymphocyte function-associated antigen (LFA)-1, and intercellular adhesion molecule (ICAM)-1 inhibited the T-cell-induced production of TNF-α by macrophages. Moreover, IL-15, but not LPS or TNF-α, triggers IL-17 production by T-cells.63 It is noteworthy that high level of IL-15 protein was demonstrated in the synovial fluids and synovial membranes of patients with active RA. Coincidently, high levels of IL-17 are present in the synovial fluid of RA patients.63 Moreover, elevated levels of synovial fluid IL-15 and IL-17 are correlated, suggesting that IL-15 may activate inert synovial fluid T cells and trigger IL-17 production.63 In addition, the presence of IL-15 in peripheral blood of patients with RA has also been reported. Interestingly, there are no significant differences between levels of IL-15 in serum and synovial fluid from the same patients.63 This may suggest that IL-15 is produced both in the joints and in the circulating blood. On the other hand, in the joints, fibroblast-like synoviocytes of RA patients may represent a major source of IL-15.64 These cells increase IL-15 production and release in response to TNF-α and IL-1β.64 Therefore, the loop between IL-15-triggered TNF-α production and TNF-α-induced IL-15 production by synoviocytes may well contribute to the proinflammatory cytokine overproduction seen in RA. In addition, synovial fluids from patients with active RA were found to promote not only activation of T cells but also chemoattraction. These effects were partially abrogated by the addition of anti-IL-15 antibodies. In addition, injection of a single dose of IL-15 resulted in a lymphocytic inflammatory infiltrate in vivo.62 Current hypotheses suggest that abnormal T-cell trafficking to the joints may be a key early step in this process.4
IL-15/IL-15R Targeting Strategies
On the basis of work describing IL-15 as a potent T-cell attractant,65 McInnes et al suggested that IL-15 might play a primary role in the development of RA.66 Thus, the development of agents blocking IL-15 or targeting the receptor and signaling elements of IL-15 may provide a new perspective for treatment of diseases associated with expression of IL-15/IL-15R.
Soluble IL-15R Alpha
The soluble form of IL-15Rα neutralizes the biological functions of IL-15 in vitro.67 Moreover, administration of soluble IL-15Rα prevents collagen-induced arthritis in a murine model, suggesting that development of effective IL-15-blocking agents such as soluble receptors may be useful in the treatment of RA.67 However, treated mice developed acute CIA soon after the discontinuation of sIL-15Rα administration.67 These experiments further prove the important role of IL-15 in the pathogenesis of CIA and warrant the development of more effective IL-15/IL-15R targeting agents.
Mutant Cytolytic IL-15/Fc Fusion Protein
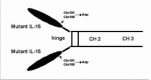
The development of agents targeting the receptor and signaling elements of IL-15 may provide a new perspective for treatment of diseases associated with expression of IL-15/IL-15R. As many other cytokines, IL-15 possesses a very high affinity for its receptor. However, its short circulating half-life and agonist activity, triggering activation of receptor-bearing target cells, limit or preclude its utility as a receptor site antagonist or a vehicle for targeting cytocidal agents to cytokine receptor bearing cells without transiently stimulating the target cells. The impetus for creating mutant IL-15/Fc fusion protein to be used as IL-15R site antagonist stems from the homologues of glutamine residues within the C terminus of the four helix structure shared by IL-2 and IL-15 (Fig. 2), as the 141 glutamine residue of IL-2 has been reported to be crucial for IL-2 binding to IL-2Rγ.68 To overcome the problem associated with the short t1/2 and agonist activity of IL-15, we designed, genetically constructed, and expressed a receptor site-specific IL-15 antagonist by mutating glutamine residues within the C terminus of IL-15 to aspartic acid and genetically linked this mutant IL-15 to murine Fcγ2a (Fig. 3).69 This immunoligand retains the properties of murine Fcγ2a fragment, i.e., prolonged circulating t1/2 and the ability to direct ADCC and CDC activities to target the cells recognized by IL-15 moiety.69,70 These mutant IL-15 proteins specifically bind to the IL-15R, competitively inhibit IL-15-triggered cell proliferation, and do not activate the STAT signaling pathway.
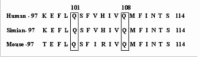
Figure 2
Homology between C-terminal alpha-helix of the human, simian and mouse IL-15. (Mutation residues in the human are boxed.)
Because the receptor site-specific antagonist IL-15 mutant/Fcγ2a fusion proteins had a prolonged t1/2 in vivo and the potential for destruction of IL-15R+ leukocytes, we examined the immunosuppressive activity of this agent in mice with methylated BSA-induced delayed type hypersensitivity (DTH) and collagen-induced arthritis (CIA), a murine model for RA. In a DTH model treatment with this unique IL-15 mutant/Fcγ2a fusion protein markedly attenuated DTH responses and decreased leukocyte infiltration within DTH sites.69 In a CIA model treatment of mutant/Fcγ2a fusion protein markedly decreased the incidence and severity of arthritis in DBA/1 mice. Treatment was associated with a dramatic decrease in intra-articular gene and protein expression of the proinflammatory cytokines, IL-1β, TNF-α and IL-17 in mice with CIA (Ferrari-Lacraz S, in press). As high affinity IL-15Rα are present on activated, but not resting, mononuclear leukocytes, it is notable that marked reductions in T-cell receptor transcripts and frequency of proliferating CD4+ T cells occur in mutant IL-15/Fc treated hosts. Histologic analysis confirms that this treatment is remarkably effective in protecting the joint from CD4+, CD8+ and CD11+ cellular infiltration. Moreover, after cessation of treatment, remission of the arthritis persists throughout the observation period. Mutant IL-15/Fc treatment was also effective in blocking the progression of ongoing arthritis, as a significant therapeutic effect was observed with the treatment initiated after the onset of arthritis in this murine CIA model.
In summary, the IL-15/IL-15R system plays an important role in the development and maintenance of immunity. The dysregulation of IL-15/IL-15R system results in autoimmunity, zealous lymphocyte proliferation and IL-15 dependent inflammation observed in the RA. Targeting IL-15/IL-15R may represent a novel and effective strategy to control these pathogenesis processes.
References
- 1.
- Oppenheim JJ, Feldmann M. The Cytokine ReferenceA Comprehensive Guide to the Role of Cytokine in Health and Disease London: Academic Press,2000. [PMC free article: PMC16904]
- 2.
- Brennan FM, Feldmann M. Cytokines in autoimmunity. Curr Opin Immunol. 1996;8:872–877. [PubMed: 8994869]
- 3.
- Feldmann M. The cytokine network in rheumatoid arthritis: Definition of TNF alpha as a therapeutic target. J R Coll Physicians Lond. 1996;30:560–570. [PMC free article: PMC5401498] [PubMed: 8961212]
- 4.
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. [PubMed: 8717520]
- 5.
- Brennan FM, Chantry D, Jackson A. et al. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;2:244–247. [PubMed: 2569055]
- 6.
- Maini RN, Elliott MJ, Brennan FM. et al. Monoclonal anti-TNF alpha antibody as a probe of pathogenesis and therapy of rheumatoid disease. Immunol Rev. 1995;144:195–223. [PubMed: 7590814]
- 7.
- Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 1992;89:9784–9788. [PMC free article: PMC50217] [PubMed: 1409699]
- 8.
- Elliott MJ, Maini RN, Feldmann M. et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993;36:1681–1690. [PubMed: 8250987]
- 9.
- Weinblatt ME, Kremer JM, Bankhurst AD. et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate [see comments] N Engl J Med. 1999;340:253–259. [PubMed: 9920948]
- 10.
- Maini R, St Clair EW, Breedveld F. et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A randomised phase III trial ATTRACT Study Group.Lancet 19993541932–1939. [PubMed: 10622295]
- 11.
- Lipsky PE, van der Heijde DM, St Clair EW. et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602. [PubMed: 11096166]
- 12.
- Markham A, Lamb HM. Infliximab: A review of its use in the management of rheumatoid arthritis. Drugs. 2000;59:1341–1359. [PubMed: 10882166]
- 13.
- Editors G, Breedveld FC, Kalden JR. et al. Advances in targeted therapies II. Ann Rheum Dis. 2000;59 Suppl 1:I. [PubMed: 19995739]
- 14.
- van den Berg WB, Joosten LA, Kollias G. et al. Role of tumour necrosis factor alpha in experimental arthritis: separate activity of interleukin 1beta in chronicity and cartilage destruction. Ann Rheum Dis. 1999;58 Suppl 1:I40–I48. [PMC free article: PMC1766568] [PubMed: 10577972]
- 15.
- Joosten LA, Helsen MM, van de Loo FA. et al. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNF alpha, anti-IL-1 alpha/beta, and IL-1Ra. Arthritis Rheum. 1996;39:797–809. [PubMed: 8639177]
- 16.
- Bresnihan B, Alvaro-Gracia JM, Cobby M. et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist [see comments] Arthritis Rheum. 1998;41:2196–2204. [PubMed: 9870876]
- 17.
- Jiang Y, Genant HK, Watt I. et al. A multicenter, double-blind, dose-ranging, randomized, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: Radiologic progression and correlation of Genant and Larsen scores. Arthritis Rheum. 2000;43:1001–1009. [PubMed: 10817552]
- 18.
- Bamford RN, Grant AJ, Burton JD. et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4940–4944. [PMC free article: PMC43905] [PubMed: 8197161]
- 19.
- Carson WE. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of IL-2 receptor. J Exp Med. 1994;180:1395–1403. [PMC free article: PMC2191697] [PubMed: 7523571]
- 20.
- Giri JG. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–2830. [PMC free article: PMC395163] [PubMed: 8026467]
- 21.
- Giri JG, Kumaki S, Ahdieh M. et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. [PMC free article: PMC394440] [PubMed: 7641685]
- 22.
- Smith KA. Interleukin-2: Inception, impact, and implications. Science. 1988;240:1169–1176. [PubMed: 3131876]
- 23.
- Grabstein KH, Eisenman J, Shanebeck K. et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. [PubMed: 8178155]
- 24.
- Anderson DM, Kumaki S, Ahdieh M. et al. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15Rα and IL2RA genes. J Biol Chem. 1995;270:29862–29869. [PubMed: 8530383]
- 25.
- Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. [PubMed: 10358752]
- 26.
- Meazza R, Gaggero A, Neglia F. et al. Expression of two interleukin-15 mRNA isoforms in human tumors does not correlate with secretion: role of different signal peptides. Eur J Immunol. 1997;27:1049–1054. [PubMed: 9174591]
- 27.
- Onu A, Pohl T, Krause H. et al. Regulation of IL-15 secretion via the leader peptide of two IL-15 isoforms. J Immunol. 1997;158:255–262. [PubMed: 8977197]
- 28.
- Tagaya Y, Kurys G, Thies TA. et al. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc Natl Acad Sci USA. 1997;94:14444–14449. [PMC free article: PMC25016] [PubMed: 9405632]
- 29.
- Musso T, Calosso L, Zucca M. et al. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 1999;93:3531–3539. [PubMed: 10233906]
- 30.
- Chae DW, Nosaka Y, Strom TB. et al. Distribution of IL-15 receptor alpha-chains on human peripheral blood mononuclear cells and effect of immunosuppressive drugs on receptor expression. J Immunol. 1996;157:2813–2819. [PubMed: 8816384]
- 31.
- Lodolce JP, Boone DL, Chai S. et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. [PubMed: 9846488]
- 32.
- Kennedy MK, Glaccum M, Brown SN. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice [see comments] J Exp Med. 2000;191:771–780. [PMC free article: PMC2195858] [PubMed: 10704459]
- 33.
- Munger W, DeJoy SQ, Jeyaseelan R. et al. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: Comparison with interleukin-2. Cell Immunol. 1995;165:289–293. [PubMed: 7553894]
- 34.
- Evans R, Fuller JA, Christianson G. et al. IL-15 mediates anti-tumor effects after cyclophosphamide injection of tumor-bearing mice and enhances adoptive immunotherapy: The potential role of NK cell subpopulations. Cell Immunol. 1997;179:66–73. [PubMed: 9259773]
- 35.
- Fehniger TA, Suzuki K, Ponnappan A. Fetal leukemia in interleukin-15 transgenic mice follows early expansion in NK and memory phenotype CD8+ T cells. J Exp Med. 2001;193:219–231. [PMC free article: PMC2193336] [PubMed: 11208862]
- 36.
- Fehniger TA, Shah MH, Turner MJ. et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: Implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed: 10201989]
- 37.
- Ross ME, Caligiuri MA. Cytokine-induced apoptosis of human natural killer cells identifies a novel mechanism to regulate the innate immune response. Blood. 1997;89:910–918. [PubMed: 9028322]
- 38.
- Bluman EM, Bartynski KJ, Avalos BR. et al. Human natural killer cells produce abundant macrophage inflammatory protein-1 alpha in response to monocyte-derived cytokines. J Clin Invest. 1996;97:2722–2727. [PMC free article: PMC507364] [PubMed: 8675682]
- 39.
- Maghazachi AA, al-Aoukaty A, Schall TJ. C-C chemokines induce the chemotaxis of NK and IL-2-activated NK cells. Role for G proteins. J Immunol. 1994;153:4969–4977. [PubMed: 7525719]
- 40.
- Fahey TJD, Tracey KJ, Tekamp-Olson P. et al. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol. 1992;148:2764–2769. [PubMed: 1573267]
- 41.
- Kanegane H, Tosato G. Activation of naive and memory T cells by interleukin-15. Blood. 1996;88:230–235. [PubMed: 8704178]
- 42.
- Zhang X, Sun S, Hwang I. et al. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. [PubMed: 9620680]
- 43.
- Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225–1227. [PubMed: 7520604]
- 44.
- Lenschow DJ, Zeng Y, Thistlethwaite JR. et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789–792. [PubMed: 1323143]
- 45.
- Buhlmann JE, Noelle RJ. Therapeutic potential for blockade of the CD40 ligand, gp39. J Clin Immunol. 1996;16:83–89. [PubMed: 8690776]
- 46.
- Mohan C, Shi Y, Laman JD. et al. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J Immunol. 1995;154:1470–1480. [PubMed: 7529804]
- 47.
- Jones ND, Van Maurik A, Hara M. et al. CD40-CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J Immunol. 2000;165:1111–1118. [PubMed: 10878390]
- 48.
- Newell KA, He G, Guo Z. et al. CTLA4Ig fails to prevent intestinal allograft rejection due to an inability to inhibit CD8 T cell responses. Transplantation. 1999;67:s45.
- 49.
- Trambley J, Bingaman AW, Lin A. et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–1722. [PMC free article: PMC409885] [PubMed: 10606625]
- 50.
- Schorle H, Holtschke T, Hunig T. et al. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. [PubMed: 1830926]
- 51.
- Willerford DM, Chen J, Ferry JA. et al. Interleukin-2 receptor alpha chain regulates the size and content of the perpheral lymphoid compartment immunity. Immunity. 1995;3:521–530. [PubMed: 7584142]
- 52.
- Suzuki H, Kundig TM, Furlonger C. et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. [PubMed: 7770771]
- 53.
- Dooms H, Desmedt M, Vancaeneghem S. et al. Quiescence-inducing and antiapoptotic activities of IL-15 enhance secondary CD4+ T cell responsiveness to antigen. J Immunol. 1998;161:2141–2150. [PubMed: 9725205]
- 54.
- Bulfone-Paus S, Ungureanu D, Pohl T. et al. Interleukin-15 protects from lethal apoptosis in vivo. Nat Med. 1997;3:1124–1128. [PubMed: 9334724]
- 55.
- Wells AD, Li XC, Li Y. et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. [PubMed: 10545998]
- 56.
- Li Y, Li XC, Zheng XX. et al. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. [PubMed: 10545997]
- 57.
- Li XC, Demirci G, Ferrari-Lacraz S. et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7:114–118. [PubMed: 11135625]
- 58.
- Wooley PH, Dutcher J, Widmer MB. et al. Influence of a recombinant human soluble tumor necrosis factor receptor FC fusion protein on type II collagen-induced arthritis in mice. J Immunol. 1993;151:6602–6607. [PubMed: 8245488]
- 59.
- Williams RO, Ghrayeb J, Feldmann M. et al. Successful therapy of collagen-induced arthritis with TNF receptor-IgG fusion protein and combination with anti-CD4. Immunology. 1995;84:433–439. [PMC free article: PMC1415129] [PubMed: 7751027]
- 60.
- Elliott MJ, Maini RN, Feldmann M. et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–1110. [PubMed: 7934491]
- 61.
- Moreland LW, Baumgartner SW, Schiff MH. et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein [see comments] N Engl J Med. 1997;337:141–147. [PubMed: 9219699]
- 62.
- McInnes IB, Leung BP, Sturrock RD. et al. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–195. [PubMed: 9018238]
- 63.
- Ziolkowska M, Koc A, Luszczykiewicz G. et al. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164:2832–2838. [PubMed: 10679127]
- 64.
- Harada S, Yamamura M, Okamoto H. et al. Production of interleukin-7 and interleukin-15 by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:1508–1516. [PubMed: 10403280]
- 65.
- Wilkinson PC, Liew FY. Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med. 1995;181:1255–1259. [PMC free article: PMC2191928] [PubMed: 7869044]
- 66.
- McInnes IB, al-Mughales J, Field M. et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis [see comments] Nat Med. 1996;2:175–182. [PubMed: 8574962]
- 67.
- Ruchatz H, Leung BP, Wei XQ. et al. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: A role for IL-15 in development of antigen-induced immunopathology. J Immunol. 1998;160:5654–5660. [PubMed: 9605172]
- 68.
- Zurawski SM, Zurawski G. Receptor antagonist and selective agonist derivatives of mouse interleukin-2. EMBO J. 1992;11:3905–3910. [PMC free article: PMC556900] [PubMed: 1396584]
- 69.
- Kim YS, Maslinski W, Zheng XX. et al. Targeting the IL-15 receptor with an antagonist IL-15 mutant/Fc gamma2a protein blocks delayed-type hypersensitivity. J Immunol. 1998;160:5742–5748. [PMC free article: PMC3806316] [PubMed: 9637483]
- 70.
- Zheng XX, Steele AW, Hancock WW. et al. IL-2 receptor-targeted cytolytic IL-2/Fc fusion protein treatment blocks diabetogenic autoimmunity in nonobese diabetic mice. J Immunol. 1999;163:4041–4048. [PubMed: 10491008]
- Cytokines in the Treatment and Prevention of Autoimmune Responses - Madame Curie...Cytokines in the Treatment and Prevention of Autoimmune Responses - Madame Curie Bioscience Database
- Metal-ion Transporters— From Yeast to Human Diseases - Madame Curie Bioscience D...Metal-ion Transporters— From Yeast to Human Diseases - Madame Curie Bioscience Database
- Class II Fusion Proteins - Madame Curie Bioscience DatabaseClass II Fusion Proteins - Madame Curie Bioscience Database
- Expression of Hox Genes in the Nervous System of Vertebrates - Madame Curie Bios...Expression of Hox Genes in the Nervous System of Vertebrates - Madame Curie Bioscience Database
- The Function of Toll-Like Receptors - Madame Curie Bioscience DatabaseThe Function of Toll-Like Receptors - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...