NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Summary
A central problem in the study of vertebrate development is defining the cellular and molecular mechanisms responsible for the patterning of embryonic tissues and the differentiation of specific cell types. A large number of studies indicate that the Wnt family of secreted glycoproteins plays an important role in the processes leading to the development of a broad range of cell types, tissues and organs. In this chapter, the contribution of Wnt signaling to the induction and patterning of the nervous system will be reviewed. Emphasis will be specifically placed on Wnt functions during neural induction, anteroposterior and dorsoventral patterning of the spinal cord and neural crest formation.
Introduction
Intercellular communication mediated by secreted polypeptides represents one of the primary strategies used by the developing embryo to achieve proper body plan organization. These factors released in the extracellular space can promote cell growth, cell fate decisions, establish tissue boundaries and initiate differentiation programs. In recent years five major signaling pathways have emerged as key regulators of embryonic development: the transforming growth factor (TGF)β,1 fibroblast growth factor (FGF),2 platelet derived growth factor (PDGF),3 Hedgehog (hh)4 and Wnt5 families. Among these, molecules of the Wnt family of secreted glycoproteins have been shown to mediate a broad range of activities during development of a variety of tissues and organs (reviewed in Refs. 6, 7) including the nervous system (reviewed in refs. 8, 9).
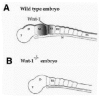
Initial evidence for the function of Wnt signaling during early development of the nervous system came from the analysis of neural defects resulting from the disruption of the Wnt-1 gene in mouse embryos.10,11 In all vertebrates Wnt-1 expression follows a restricted and unique expression pattern (Fig. 1A). Wnt-1 transcripts are detected in the most posterior domain of the midbrain and at the junction with the hindbrain, also known as the isthmus.12 Later during embryogenesis, this region of the midbrain will contribute to the anterior half of the cerebellum. The posterior half of the cerebellum is derived from the anterior portion of the hindbrain,13 which does not express Wnt-1.12 Mouse embryos homozygous for a targeted disruption of the Wnt-1 gene exhibit a severe truncation of the midbrain and the cerebellum (Fig. 1B). In the most extreme cases, the phenotype of Wnt-1 mutant mice is characterized by the total absence of cerebellum.10,11 Subsequent studies have established that at the isthmus, Wnt-1 is implicated in the maintenance of engrailed expression in the mesencephalon and in the anterior portion of the hindbrain.14 Engrailed proteins are believed to be required for the proper outgrowth of the cerebellum.14 Wnt-1 mutant embryos appear to have a normal spinal cord and hindbrain, even though Wnt-1 is also normally expressed in the posterior hindbrain and in the roof plate of the spinal cord (Fig. 1). The lack of a phenotype at other Wnt-1 expression domains is likely to be due to a functional compensation by other Wnt genes, including Wnt-3a, which is expressed in overlapping domains in the more caudal regions of the hindbrain and along the entire length of the spinal cord. 15 These results imply a prominent role of Wnt-1 in the development of specific regions of the brain, illustrating one of the numerous functions of Wnt-1 during embryogenesis.
Since the publication of this work in the early 90s,10,11 the description of the sites of expression of several Wnt family members and their frizzled receptors have been reported in much detail in a number of species.16-18 These descriptive studies indicate: (i) that the expression pattern of these molecules is very well conserved across species and (ii) that a majority of these molecules present very highly restricted expression domains throughout the nervous system. This implies that other Wnt proteins may exhibit patterning functions similar to those described for Wnt-1 at other time and locations.
Genetic studies in mouse and zebrafish have established that mutations in extracellular or intracellular components of the Wnt signaling pathway result in a broad range of defects affecting multiple aspects of neural development. As illustrated in Table 1, analysis of these mutant embryos indicate that during neurogenesis, Wnt signaling can regulate cell growth, cell determination and differentiation, tissue identity and patterning, as well as programmed cell death. In this chapter, we will review recent results illustrating Wnt signaling functions in the control of neural induction and subsequent patterning decisions including regionalization of the neuraxis and neural crest formation.
Table 1
Mutations in components of the Wnt signaling pathway resulting in neural defects.
Induction of the Neural Plate
Neural induction constitutes the initial step in the generation of neural tissue from the ectoderm. This classical embryonic induction has been best understood in amphibians. During gastrulation, signals derived from the dorsal mesoderm (organizer) induce the neural plate, making it distinct from the adjacent non-neural ectoderm. These inductive signals, including noggin, chordin and follistatin, share the same molecular property, the ability to block bone morphogenetic protein (BMP) signaling in the ectoderm, thereby eliciting a neural differentiation program (reviewed in 36–38). Thus, the model for neural induction in frogs suggests that neural fate is acquired in response to the activity of organizer-specific BMP antagonists. In zebrafish, a mutation in the chordin gene (known as dino, or chordino) causes embryos to develop a reduced neural plate 39, providing genetic evidence that BMP signaling inhibition in the ectoderm is necessary to promote neural development.
Studies in chick and mouse do not support a strict requirement for BMP antagonists activity to generate neural fate. For example, chordin/noggin double mutant mouse embryos form a neural plate. However, functional redundancy by other BMP inhibitors cannot be excluded in these studies. 40 In the chick the timing and pattern of expression of noggin, chordin and follistatin indicate that BMP antagonists are neither sufficient nor needed to generate neural fate. 41-43 Therefore, the requirement for BMPs inhibitors derived from the organizer may not be the entire basis of neural induction. In fact, BMP inhibition in the chick epiblast requires FGF signaling rather than BMP antagonists.44,45 BMP transcripts are absent from epiblast explants specified as neural plate. Exposure of the epiblast to FGF receptor antagonists prevents down-regulation of BMP transcripts and convert the epiblast to an epidermal fate. 44
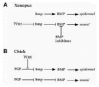
Recently, it has been found that ectopic activation of Wnt signaling pathway in Xenopus, by overexpression of various Wnt ligands, or a dominant-negative GSK3 or a constitutively active β-catenin, is sufficient to activate neural markers in presumptive ectoderm.46 In these explants Wnt neural inducing activity appears to be mediated by down-regulation of BMP mRNA expression, which in turn is sufficient to allow neural fate to develop. The mechanism by which Wnt signaling down-regulate BMP transcripts levels remains unclear. The function of Wnt signaling in neural induction in Xenopus46 appears similar to the activity proposed for FGF signaling in the chick epiblast,44,45 in that both lead to attenuation of BMP mRNA expression. Based on these observations, a revised model of neural induction in frog has been proposed.47 While BMP antagonists block the activity of BMP proteins resident in the ectoderm, Wnt signaling, by an unknown mechanism, prevents further BMP transcription resulting in development of neural fate (Fig. 2A).
It is important to emphasize here that these findings contrast with several reports indicating that Wnt signaling is not sufficient to neuralize the frog ectoderm.48-52 While it is difficult to reconcile these observations, differences in the amount of inducers used could explain some of these conflicting results. However, by suggesting that induction of neural tissues may occur through a Wnt-dependent pathway, this model clearly challenges our current view on neural induction in Xenopus.
In the chick, since BMP antagonists either alone or in combination with FGF signaling are not sufficient to induce neural fate in the prospective ectoderm,44,45 additional signals might also be required for neuralization. It has been recently proposed that Wnt signaling may regulate epidermal and neural fate decisions in the chick epiblast.53 During normal development medial and lateral epiblast will develop neural and epidermal fates respectively. Wnt-3a and Wnt-8C mRNA are expressed in the lateral but not in the medial epiblast cells at the blastula stage.53 In a series of very elegant experiments Wilson et al53 have shown that blockage of Wnt signaling in the lateral epiblast, by exposure to a truncated soluble fragment of the mouse Wnt receptor Frizzled 8 (MFz-8-CRD), prevents epidermal fate and allows FGF-mediated neuralization of lateral epiblast cells to occur. If both Wnt and FGF signaling pathways are simultaneously inhibited, lateral epiblast cells will develop their normal epidermal fate. Conversely, exposure of soluble Wnt to medial epiblast cells prevents FGF-mediated neuralization and promotes epidermal differentiation. Therefore, it has been proposed that endogenous Wnt signaling in the lateral epiblast blocks FGF-mediated attenuation of BMP transcription leading to the epidermal fate.53 By contrast in the medial epiblast in which Wnt signaling is absent, FGF signaling function is to repress BMP mRNA expression required for progression to the neural fate (Fig. 2B).
There is an interesting parallel between Wnt function during neural induction in frog and chick embryos (Fig. 2). However, in each species Wnt activity leads to opposite effects on the prospective ectoderm. While in the chick Wnt signaling acts to promote epidermal fate by blocking BMP attenuation mediated by FGF, in Xenopus Wnt function is required in the ectoderm to promote neural fate by blocking accumulation of BMP transcripts. Therefore, Wnt proteins can act both as a positive (chick epiblast) and negative (frog ectoderm) regulator of BMP signaling. Neural induction in both species shares one common feature in that they both require inhibition of BMP transcription/signaling but differs in the type of molecular effectors used to achieve this function.
Anteroposterior Patterning of the Neural Plate
Shortly after neural induction, the neuroectoderm becomes subdivided along the anteroposterior axis into forebrain, midbrain, hindbrain and spinal cord. Classical experiments in amphibian embryos have implicated a two-signal model of neural induction and anteroposterior patterning of the neuroectoderm.54 According to this model, anterior neural tissue is induced by early-involuting mesendoderm during a step known as activation. Anterior neural tissue is subsequently modified to a more posterior character by a set of transforming signals emanating from the late-involuting mesoderm in a phase of transformation. During the latter step, a gradient of posteriorizing factor confers progressive posterior identity to the neural tissue.54
It is now well established that the activation step is largely mediated by BMP inhibitors secreted by the dorsal mesoderm, although FGFs and Wnts may also be implicated in this process. On the other hand, candidate-transforming molecules have remained elusive for quite some time. Proposed candidates include FGF and retinoic acid (RA) which can posteriorize anterior neural tissue with virtually no neural inducing activity on their own.55-58
The first evidence pointing in the direction of a Wnt signal involved in the posteriorization of the neural plate was established in Xenopus embryos. Expression of Xwnt-3a induces posterior and suppresses anterior neural markers in neuralized animal explants.59 Conversely, in vivo blockage of Wnt ligands by expression of a dominant negative Xwnt-8 results in loss of posterior neural fates.51,60
Genetic evidence also indicates that Wnt signaling is implicated in the posteriorization of the neuroectoderm. In the zebrafish, headless mutant embryos carrying a mutation that abolishes the repressor function of TCF-3, a transcriptional modulator of Wnt signaling, have severe anterior defects.35 Similarly, masterblind mutant embryos exhibit anterior brain defects as a result of a mutation of the scaffolding protein Axin1, a negative regulator of Wnt signaling.29 Moreover, mouse embryos with mutation in the secreted Wnt antagonist Dkk-1 lack a forebrain,19 whereas mice mutants for Wnt-3a15 and the Wnt co-receptor LRP627 show posterior truncations. Altogether these results strongly argue that Wnt signaling is required for establishing an anteroposterior pattern in the neuraxis.
A recent study in Xenopus has provided evidence for the existence of an endogenous Wnt signaling gradient during gastrulation,61 which display all the characteristics of the transforming agent as defined in Nieuwkoop's model of posteriorization.54 When increasing doses of soluble Xwnt-8 are applied to neuralized animal explants, progressive posteriorization of the neural character of these explants can be generated, from forebrain to midbrain and hindbrain, respectively. Importantly this activity is completely abolished when the treatment is performed in the presence of the Wnt antagonist MFz-8-CRD.61 In vivo, at the late gastrula stage the neural plate presents an anteroposterior gradient of nuclear accumulation of β-catenin, with higher levels posteriorly and lower levels anteriorly.61 This observation clearly supports the idea of a differential activation of the Wnt signal transduction pathway in the neuroectoderm at different axial levels. These results are consistent with previous work showing that overexpression of graded amounts of the Wnt effector dishevelled in animal explants induces neural markers with increasingly posterior identity.62 Moreover, in zebrafish embryos increasing doses of Wnt-8 morpholino antisense lead to a progressive loss of posterior neural fates associated with expansion of neural tissues of anterior character.63,64
Based on these observations it has been proposed that in the more posterior regions of the frog embryo, graded amounts of Wnt signals, probably provided by multiple Wnts, are responsible for the development of a broad range of posterior structures. Whereas in the head region, a number of Wnt antagonists such as cereberus, Dkk-1, FrzBs and sFRPs,65-68 produced by the anterior neuroectoderm and mesendoderm are believed to be responsible for defining a domain free of Wnt activity required for the development of forebrain structures. The combination of both activities may therefore contribute to the establishment of a repertoire of anteroposterior positional values along the neuraxis.61
These studies also suggest that a gradient of Wnt signaling in the neuroectoderm is not sufficient to account for the entire array of anteroposterior character found in the neuraxis and suggest the requirement for additional signals. These may include RA and FGF, which also have been implicated in posteriorization of the neural plate in several species (reviewed in Ref. 36).
Dorsoventral Patterning of the Neuroepithelium
During early stages in the development of the spinal cord, three major classes of cells are generated in the ventral neural tube, floor plate cells at the midline, motor neurons at a ventrolateral position and ventral interneurons more dorsally. Cells in the dorsal neural tube give rise initially to neural crest cells and subsequently to roof plate cells at the midline and to several classes of dorsal sensory interneurons. The inductive signals that control the identity and pattern of these cell types come initially from two distinct groups of non-neural cells, the notochord ventrally and the epidermal ectoderm dorsally (Reviewed in Ref. 69). The floor plate and notochord are important organizing centers of the spinal cord, and sources of Sonic hedgehog (Shh). A gradient of Shh has been shown to be involved in establishing neuronal diversity in the ventral spinal cord (Reviewed in Ref. 69).
Wnt gene expression in the spinal cord exhibits striking restrictions along the dorsovental axis prior to differentiation of neurons. In the ventral spinal cord Wnt-4 appear restricted to the floor plate, suggesting that it may have an important role in patterning the ventral portion of the spinal cord.16,70,71 Wnt-4 deficient mice present severe kidney defects, consistent with Wnt-4 expression in the mesenchyme of the kidney and its derivatives.72 Nevertheless, these mutant embryos develop a normal pattern of neurons in the ventral spinal cord. Redundancy of expression of other Wnt genes in the ventral spinal cord, such as Wnt-7a,16 could explain the lack of an obvious phenotype in the spinal cord of Wnt-4 deficient mouse. Thus, the precise function of Wnt-4 in the floor plate remains to be elucidated.
Wnt-1 and Wnt-3a are both expressed in overlapping domains in the dorsal spinal cord, in a region extending caudal to the diencephalon.12,15,73 Mouse embryos carrying a homozygous deletion of either one of these genes have a normal spinal cord,10,11,15 due to functional redundancy. Nevertheless, compound mutant embryos lacking both Wnt-1 and Wnt-3a showed major deficiency in neural crest derivatives.22 These include partial loss of dorsal root ganglia neurons and glia, reduction of pigment cells precursors, and loss or reduction of cranial skeletal structures.22 It has been proposed that loss of Wnt-1 and Wnt-3a results in a broad deficiency in neural crest progenitors, supporting a role for Wnt-1 and Wnt-3a in expansion of neural crest population rather than in the control of specific cell fate.22 This is consistent with transgenic analysis suggesting that Wnt signaling is involved in regulating cell proliferation in the dorsal spinal cord.74,75 In mouse embryos, ectopic expression of Wnt-1 in the entire spinal cord under the control of the Hox-B4 enhancer has been shown to increase the number of dividing dorsal cells without affecting the overall polarity/fate of the spinal cord.74 Altogether these observations indicate a major function Wnt-1 and Wnt-3a in regulating cell proliferation in the dorsal spinal cord.22
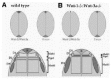
A more recent study suggests that Wnt-1 and Wnt-3a may also be required for the specification of individual population of dorsal interneurons. 23 Three subclasses of dorsal interneurons are arranged along the dorso-ventral axis of the spinal cord (Fig. 3A). They can be identified by the expression of a number of homeodomain-containing proteins. D1 interneurons express LH2, D2 interneurons express Islet1 and D3 interneurons express Pax-2. The progenitors from each population of dorsal interneurons are found in the ventricular zone of the spinal cord (Fig. 3A) and are characterized by the expression of bHLH proteins such as Math1 (D1), Ngn1 (D2) and Mash1 (D3). In Wnt-1/Wnt-3a double mutant mouse embryos, only residual expression of LH2 and Islet 1 is detected on each side of the roof plate indicating that D1 and D2 neurons are virtually absent (Fig. 3B). On the other hand Pax-2 expressing-cells were significantly increased in the dorsal half of the spinal cord, suggesting that increased D3 interneurons compensated for the loss of D1 and D2 neurons (Fig. 3 B). This loss of D1/D2 neurons correlated with a dramatic reduction of Math1 and Ngn1 progenitors in the ventricular zone, whereas Mash1 positive cells (D3 progenitors) expanded their boundary dorsally up to the roof plate (Fig. 3B). Thus, Wnt proteins are clearly required for proper development of D1 and D2 subclasses of interneurons.23
These observations were largely corroborated by in vitro analysis. Exposure of soluble Wnt-3a to chick explants of medial neural plate is sufficient to induce expression of LH2 and Islet1 and block Pax-2 expression, indicating that Wnt3a can directly induce D1 and D2 interneurons at the expense of D3 neurons.
Molecules of the BMP family are considered as the major source of signaling in the dorsal spinal cord for the development of dorsal interneurons. 76 Interestingly, the expression of a number of these molecules (including BMP-4, BMP-6, BMP-7 and Gdf7) was unaffected in Wnt-1/Wnt-3a deficient embryos, implying that Wnt signaling directly regulates the specification of D1 and D2 interneurons (Fig. 3A,B). Altogether these observations are of great importance as they establish the first evidence for a role of Wnt signaling in the specification of neuronal fate in the dorsal spinal cord, thereby contributing to the regionalization of the spinal cord along its dorso-ventral axis.
Neural Crest Induction and Diversification
The neural crest is a unique embryonic structure composed of a migratory population of multipotent cells arising at the lateral edges of the neural plate, at the junction between the neural and the non-neural ectoderm (reviewed in Ref. 77). During neurulation, the neural crest becomes positioned at the dorsal most region of the neural tube. As the neuroepithelium closes, neural crest cells delaminate in a rostro-caudal wave and migrate throughout the embryo along specific and restricted routes. When they reach their final location, neural crest cells present the remarkable ability to differentiate into a large variety of cell types including peripheral and enteric neurons and glia, smooth muscle cells, craniofacial cartilage and bone, endocrine and pigment cells (reviewed in Ref. 77).
The molecular signals controlling neural crest specification are not fully understood (reviewed in Ref. 78). Signals emanating from the non-neural ectoderm, the paraxial mesoderm, or both are believed to be involved in the specification of the neural crest.79-81 There is evidence that molecules belonging to the BMP family, expressed by the non-neural ectoderm and the dorsal neural tube, initiate the formation of the neural crest.82,76 The neural inducers noggin and chordin act by neutralizing a BMP signal in the ectoderm83,84 and it is believed that neural crest forms in a region where BMPs and BMP antagonists reach an appropriate balance.85-87 Other classes of signaling molecules have also been proposed as potential inducers of neural crest, this is the case for members of the FGF family.88,89,50
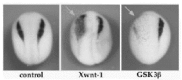
A large number of studies in the mouse,22,31,32,75 frog48-52 and zebrafish90,91 have implicated Wnt signaling in neural crest formation. In Xenopus, as illustrated in Fig. 4, ectopic expression of some Wnt family members, as well as downstream components of the pathway, enhances production of neural crest progenitors,48-52 whereas inhibition of Wnt signaling blocks neural crest formation.48,50,52
Using a functional assay in Xenopus it has been shown that signaling by XWnt-1, but not other Wnts, can be specifically enhanced by frizzled-3 (XFz-3). Consistent with this observation, XFz-3 and XWnt-1 are highly localized to dorsal neural tissues that give rise to neural crest,52 suggesting that they may participate in neural crest formation. In the embryo, XFz-3 overexpression can induce ectopic neural crest formation, similar to XWnt-1. Whereas, loss of XFz-3 function, either by depletion with a XFz-3-directed morpholino antisense or by expression of an inhibitory form of XFz-3 (NFz-3) blocks XWnt-1-mediated neural crest induction and prevents neural crest formation in whole embryos.52 These results, together with other studies,48- 51 clearly demonstrate the existence of a Wnt-dependent pathway required for neural crest formation.
Mouse embryos with targeted inactivation of the β-catenin gene, within Wnt-1 expression domain, have severe defects in the formation of cranial and dorsal root ganglia and in several craniofacial skeletal elements of neural crest origin.32 In these embryos it is believed that signaling through β-catenin is required for survival and/or differentiation of cranial neural crest cells.32 Loss of Wnt-1 and Wnt-3a results in a broad loss of neural crest derivatives and it has been proposed that Wnt signaling in the dorsal neural tube of these mutant embryos is primarily required for the expansion of neural crest progenitors.22 In Xenopus, the increased in neural crest progenitors generated upon XWnt-3a overexpression has been shown to occur independently of cell proliferation,48 suggesting that Wnt signaling may also be involved in the specification of neural crest fate.
The molecular process by which multipotent neural crest progenitors are assigned to specific lineages is believed to depend on environmental signals acting prior, during or after neural crest cell migration (reviewed in Ref. 77). In vitro studies have identified several growth factors that can direct the differentiation of neural crest precursors along distinct lineages (reviewed in refs. 92, 93).
In zebrafish embryos activation of the Wnt signaling pathway by overexpression of β-catenin in individual cranial crest progenitors promotes pigment cell formation at the expense of neurons and glia.90 Conversely, a dominant-negative Wnt favored neuronal fates at the expense of pigment cells.90 These results suggest that Wnt signaling is required to specify the melanocyte lineage thereby contributing to neural crest cell diversity. Consistent with this activity, soluble Wnt-3a increases the number of pigment cells in quail neural crest cultures while decreasing the number of neurons and glial cells, without affecting cell proliferation.94
Further evidence that Wnt signaling directly regulates pigment cell fates came from analysis of the MITF promoter (microphthalmia-associated transcription factor), a basic helix-loop-helix transcription factor required for development of melanocytes.95 The zebrafish homologue of mouse MITF, nacre, is expressed in the primordium of the retina and in premigratory and migrating neural crest cells fated to become melanocytes. nacre mutant embryos lack all neural crest-derived melanocytes throughout development.96 MITF/nacre presents in its promoter region TCF/LEF binding sites, which can mediate Wnt responsiveness.91 Binding assays and mutational analysis, have demonstrated that nacre is both necessary and sufficient for pigment cell formation in zebrafish.91 Similarly, in vitro studies indicate that soluble Wnt-3a up-regulates MITF expression in a mouse melanocyte cell line and recruits β-catenin and LEF-1 to the LEF-1 binding site of MITF promoter.97 These observations clearly indicate that Wnt signaling can not only induce and expand neural crest progenitors but also specify at least one neural crest-derived lineage by direct activation of MITF/nacre gene.
Neural Crest Apoptosis
In the hindbrain, neural crest cells originate from segmental units, known as rhombomeres, and migrate into individual streams that populate the branchial arches. This migration into segregated streams is of great importance to allow uninterrupted transfer of patterning information from the neuraxis to the branchial arches (reviewed in 98). In the chick and mouse embryos, rhombomere 3 (R3) and 5 (R5) are depleted of neural crest cells by programmed cell death, thereby defining two neural crest-free zones that contribute to the segregation of neural crest streams. It is now well established that apoptosis in R3 and R5 depends on signal(s) derived from adjacent even-numbered rhombomeres and requires Msx2 induction mediated by BMP-4 (reviewed in ref. 98). Consistent with this role, BMP-4 and Msx2 expression pattern in the hindbrain is concurrent with the pattern of neural crest apoptosis.99
Experiments in the chick embryo suggest that Wnt signaling may act as a regulator of neural crest apoptosis in the hindbrain.100 The secreted frizzled related proteins (sFRP) act as Wnt antagonists. Over-expression of one member of this family (sFRP-2) prevents BMP-4 expression in R3 and R5 leading to ectopic production of neural crest cells from both rhombomeres. Conversely, loss of sFRP-2 or Wnt-1 overexpression results in elevated levels of Msx2 transcripts and ectopic apoptosis in R4. Interestingly, early on sFRP-2 is expressed throughout the hindbrain but is down-regulated in odd-numbered rhombomeres by the time cell death is initiated in R3 and R5. 100 These results indicate that while a number of Wnt molecules are broadly expressed throughout the hindbrain their activity is tightly regulated by sFRP-2 in even-numbered rhombomeres. Therefore, it has been proposed that Wnt signaling in R3 and R5 promote neural crest apoptosis by up-regulating BMP-4/Msx2 expression while in adjacent rhombomeres sFRP-2 blocks Wnt signaling allowing, by default, production of neural crest cells.
Additional evidence for a role of Wnt signaling in cranial neural crest apoptosis has also been reported in transgenic mouse embryos. 31 Adenomatous polyposis coli (APC) is a negative regulator of Wnt signaling through down-regulation of β-catenin. Loss of APC causes accumulation of β-catenin resulting in the activation of specific downstream target genes. The function of APC during neural crest development was analyzed through neural crest specific disruption of APC using the Cre-loxP recombination system. Mice carrying the APC gene flanked by loxP sites were mated with Protein 0 (P0)-Cre transgenic mice. P0-Cre transgenic animals expressed Cre in a wide range of cranial and trunk neural crest derivatives including, dorsal root ganglia, sympathetic and enteric nervous system, craniofacial mesenchyme and septum of the outflow tract of the heart.101 P0-Cre/APC mutant mice display severe craniofacial and cardiac defects.31 These defects are always associated with an early loss of neural crest progenitors by apoptosis. The sites of apoptosis in affected neural crest derivatives are found within regions of accumulation of β-catenin consistent with activation of Wnt signaling pathway. 31 Neural crest derivatives from the trunk region (peripheral nerves and melanocytes) appeared unaffected in these transgenic animals indicating that neural crest progenitors from different axial levels may differ in their ability to respond to Wnt signals. These studies indicate that activation of Wnt signaling in the neural crest-forming region promotes apoptosis in the cranial neural crest, and suggests that inhibition of Wnt signals may be required to promote cranial neural crest formation in the developing embryo.
These observations supporting an active role for Wnt signaling in programmed cell death in the rhombencephalic neural crest 100,31 contrast significantly with previous studies in mouse, frog and zebrafish that have established a positive role for Wnt signals in the generation of neural crest cells.22,31,32,48-52,75,90,91 While it is yet difficult to reconcile both observations, it may indicate dose-dependence activities of Wnt proteins and/or differences in temporal-responsiveness to Wnt signals at the multiple stages of the neural crest development.
Conclusions
During the last decade an impressive amount of novel information has been generated that furthered our understanding of the roles of Wnt proteins during vertebrate embryogenesis. In this chapter, only a portion of some of these activities has been reviewed in the context of the developing nervous system. As illustrated in Table 1, numerous other aspects of neural development are also regulated by Wnt signaling. These include, eye development,26,102 axonal remodeling,24,103 formation of the hippocampus21,33,34,104 and social behavior.28 One striking feature is that Wnt signaling as the ability within the same tissue and at different times to regulate activities as diverse as cell proliferation, cell fate specification and programmed cell death. This observation establishes the extremely versatile nature of this class of signaling molecules. Nevertheless, the molecular mechanisms underlying most of these activities are still poorly understood. A greater understanding of the role of the Wnt signaling during formation of the nervous system will certainly benefit from the more systematic development of temporal- and tissue-specific loss of function of various Wnt and frizzled family members.
Acknowledgements
The author is extremely grateful to Trish Labosky for critical reading of the manuscript and to Natasha Saint-Germain for helping with bibliography. Research in the author's laboratory is supported by the National Institutes of Health, March of Dimes, Deafness Research Foundation, Johnson & Johnson and Whitehall Foundation.
References
- 1.
- Massague J, Chen Y. Controlling TGF-β signaling. Genes Dev. 2000;14:627–644. [PubMed: 10733523]
- 2.
- Szebenyi G, Fallon J. Fibroblast growth factors as multifunctional signaling factors. Int Rev Cytol. 1999;185:45–106. [PubMed: 9750265]
- 3.
- Betsholtz C, Karlsson L, Lindhal P. Developmental roles of platelet-derived growth factors. BioEssays. 2001;23:494–507. [PubMed: 11385629]
- 4.
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. [PubMed: 11731473]
- 5.
- Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. [PubMed: 1617723]
- 6.
- Parr BA, McMahon AP. Wnt genes and Vertebrate development. Curr Biol. 1994;4:523–528. [PubMed: 7950319]
- 7.
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes & Dev. 1997;11:3286–3305. [PubMed: 9407023]
- 8.
- Patapoutian A, Reichardt L. Roles of Wnt proteins in neural development and maintenance. Curr Opinion Neurobiol. 2000;10:392–399. [PMC free article: PMC4943213] [PubMed: 10851180]
- 9.
- Wilson SW, Rubenstein JL. Induction and dorsoventral patterning of the telencephalon. Neuron. 2000;28:641–651. [PubMed: 11163256]
- 10.
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. [PubMed: 2205396]
- 11.
- Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990;346:847–850. [PubMed: 2202907]
- 12.
- Wilkinson DG, Bailes JA, McMahon AP. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987;50:79–88. [PubMed: 3594565]
- 13.
- Hollonet MER, Teillet MA, Le Douarin NM. A new approach to the development of the cerebellum provided by the quail-chick marker system. Development. 1990;108:19–31. [PubMed: 2351063]
- 14.
- Joyner AL. Engrailed, Wnt and Pax genes regulate midbrain-hindbrain development. Trends Genet. 1996;12:15–20. [PubMed: 8741855]
- 15.
- Takada S, Stark KL, Shea MJ. et al. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1997;48:174–189. [PubMed: 8299937]
- 16.
- Parr BA, Shea MJ, Vassileva G. et al. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb bud. Development. 1993;119:247–261. [PubMed: 8275860]
- 17.
- Hollyday M, McMahon JA, McMahon AP. Wnt expression patterns in chick embryo nervous system. Mech Dev. 1995;52:9–25. [PubMed: 7577679]
- 18.
- Gradl D, Kuhl M, Weldich D. Keeping a close eye on Wnt-1/wg signaling in Xenopus. Mech Dev. 1999;86:3–15. [PubMed: 10446261]
- 19.
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C. et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–434. [PubMed: 11702953]
- 20.
- Yoshikawa Y, Fujimori T, McMahon AP. et al. Evidence that absence of Wnt3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol. 1997;183:234–242. [PubMed: 9126297]
- 21.
- Lee SMK, Tole S, Grove E. et al. A local Wnt-3a signal is required for development of mammalian hippocampus. Development. 2000;127:457–467. [PubMed: 10631167]
- 22.
- Ikeya M, Lee SMK, Johnson JE. et al. Wnt signaling is required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. [PubMed: 9353119]
- 23.
- Muroyama Y, Fujihara M, Ikeya M. et al. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16:548–553. [PMC free article: PMC155351] [PubMed: 11877374]
- 24.
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by Wnt-7a signaling. Cell. 2000;100:525–535. [PubMed: 10721990]
- 25.
- Wang Y, Huso D, Cahill H. et al. Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. J Neurosci. 2001;21:4761–4771. [PMC free article: PMC6762346] [PubMed: 11425903]
- 26.
- Gong Y, Slee RB, Fukai N. et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. [PubMed: 11719191]
- 27.
- Pinson KI, Brennan J, Monkley S. et al. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. [PubMed: 11029008]
- 28.
- Lijam N, Paylor R, McDonald MP. et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. [PubMed: 9298901]
- 29.
- Heisenberg CP, Houart C, Take-Uchi M. et al. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev. 2001;15:1427–1434. [PMC free article: PMC312705] [PubMed: 11390362]
- 30.
- Zeng l, Fagotto F, Zhang T. et al. The mouse fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. [PubMed: 9230313]
- 31.
- Hasegawa S, Sato T, Akazawa H. et al. Apoptosis in neural crest cells by functional loss of APC tumor suppressor gene. Proc Natl Acad Sci USA. 2002;99:297–302. [PMC free article: PMC117555] [PubMed: 11756652]
- 32.
- Brault V, Moore R, Kutsch S. et al. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of cranifacial development. Development. 2001;128:1253–1264. [PubMed: 11262227]
- 33.
- Galceran J, Miyashita-Lin EM, Devaney E. et al. Hippocampus, development and generation of dentate gyrus granule cells is regulated by LEF-1. Development. 2000; 127:469–482. [PubMed: 10631168]
- 34.
- Galceran J, Farinas I, Depew MJ. et al. Wnt3a-/- -like phenotype and limb deficiency in LEF-1(-/ -)TCF-1(-/-) mice. Genes Dev. 1999;13:709–717. [PMC free article: PMC316557] [PubMed: 10090727]
- 35.
- Kim CH, Oda T, Itoh M. et al. Repressor activity of Headless/TCF-3 is essential for vertebrate head formation. Nature. 2000;407:913–916. [PMC free article: PMC4018833] [PubMed: 11057671]
- 36.
- Sasai Y, De Robertis EM. Ectodermal patterning in vertebrate embryos. Dev Biol. 1997;182:5–20. [PubMed: 9073437]
- 37.
- Weinstein DC, Hemmati-Brivanlou A. Neural Induction. Ann Rev Cell Dev Biol. 1999;15:411–433. [PubMed: 10611968]
- 38.
- Wilson SI, Edlund T. Neural induction: toward a unifying mechanism. Nature (Neuro. Supp.). 2001;4:1161–1168.
- 39.
- Hammerschmidt M, Pelegri F, Mullins MC. et al. dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development. 1996;123:95–102. [PubMed: 9007232]
- 40.
- Bachiller D, Klingensmith J, Kemp C. et al. The organizer factors, Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. [PubMed: 10688202]
- 41.
- Streit A, Lee KJ, Woo I. et al. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 1998;125:507–519. [PubMed: 9425145]
- 42.
- Storey KG, Crossley JM, De Robertis EM. et al. Neural induction and regionalization in the chick embryo. Development. 1992;114:729–741. [PubMed: 1618139]
- 43.
- Levin M. The roles of activin and follistatin signaling in chick gastrulation. Int J Dev Biol. 1997;42:553–559. [PubMed: 9694626]
- 44.
- Wilson SI, Graziano E, Harland RM. et al. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol. 2000;10:421–429. [PubMed: 10801412]
- 45.
- Streit A, Berliner AJ, Papanayotou C. et al. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. [PubMed: 10894544]
- 46.
- Baker JC, Beddington RSP, Harland RM. Wnt signaling in Xenopus embryos inhibits Bmp4 expression and activates neural development. Genes Dev. 1999;13:3149–3159. [PMC free article: PMC317181] [PubMed: 10601040]
- 47.
- Harland RM. Neural induction. Curr Opin Genet Dev. 2000;10:357–362. [PubMed: 10889069]
- 48.
- Saint-Jeannet J-P, He X, Varmus HE. et al. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc Natl Acad Sci USA. 1997;94:13713–13718. [PMC free article: PMC28371] [PubMed: 9391091]
- 49.
- Chang C, Hemmati-Brivanlou A. Neural crest induction by Xwnt7B in Xenopus. Dev Biol. 1998;194:129–134. [PubMed: 9473337]
- 50.
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signals model. Development. 1998;125:2403–2414. [PubMed: 9609823]
- 51.
- Bang AG, Papalopulu N, Goulding MD. et al. Expression of Pax-3 in the lateral neural plate is dependent on a Wnt-mediated signal from the posterior non-axial mesoderm. Dev Biol. 1999;212:366–380. [PubMed: 10433827]
- 52.
- Deardorff MA, Tan C, Saint-Jeannet J-P. et al. A role for frizzled-3 in neural crest development. Development. 2001;128:3655–3663. [PubMed: 11585792]
- 53.
- Wilson SI, Rydström A, Trimborn T. et al. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. [PubMed: 11357137]
- 54.
- Nieuwkoop PD. Activation and organization of the central nervous system in amphibians. J Exp Zool. 1952;120:1–108.
- 55.
- Doniach T. Basic FGF as an inducer of anteroposterior neural pattern. Cell. 1995;83:1067–1070. [PubMed: 8548794]
- 56.
- Holowacz T, Sokol S. FGF is required for posterior neural patterning but not for neural induction. Dev Biol. 1999;205:296–308. [PubMed: 9917365]
- 57.
- Taira M, Saint-Jeannet J-P, Dawid I. Role of Xlim-1 and Xbra genes in anteroposterior patterning of the neural tissue by the head and trunk organizer. Proc Natl Acad Sci USA. 1997;94:895–900. [PMC free article: PMC19610] [PubMed: 9023353]
- 58.
- Kolm PJ, Apekin V, Sive H. Xenopus hindbrain patterning requires retinoic signaling. Dev Biol. 1997;192:1–16. [PubMed: 9405093]
- 59.
- McGrew LL, Lai CC, Moon RT. Specification of the anteroposterior neural axis through synergistic interaction of the Wnt signaling cascade with noggin and follistatin. Dev Biol. 1995;172:337–342. [PubMed: 7589812]
- 60.
- McGrew LL, Hoppler S, Moon RT. Wnt and FGF pathways cooperatively patterns anteroposterior neural ectoderm in Xenopus. Mech Dev. 1997;69:105–114. [PubMed: 9486534]
- 61.
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. [PubMed: 11684656]
- 62.
- Itoh K, Sokol SY. Graded amounts of Xenopus dishevelled specify discrete anteroposterior cell fates in prospective ectoderm. Mech Dev. 1997;61:113–125. [PubMed: 9076682]
- 63.
- Erter CE, Wilm TP, Basler N. et al. Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development. 2001;128:3571–3583. [PubMed: 11566861]
- 64.
- Lekven AC, Thorpe CJ, Waxman JS. et al. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell. 2001;1:103–114. [PubMed: 11703928]
- 65.
- Piccolo S, Agius E, Leyns L. et al. The head uinducerr Cerberus is a multifunctional antagonist of nodal, BMP and Wnt signals. Nature. 1999;397:707–710. [PMC free article: PMC2323273] [PubMed: 10067895]
- 66.
- Kazanskaya O, Glinka A, Niehrs C. The role of dickkopf1 in prechordal plate specification and neural patterning. Development. 2000;27:4981–4992. [PubMed: 11044411]
- 67.
- Bradley L, Sun B, Collins-Rice L. et al. Different activities of the frizzled-related proteins frzb2 and sizzled2 during Xenopus anteroposterior patterning. Dev Biol. 2000;227:118–132. [PubMed: 11076681]
- 68.
- Pera EM, De Robertis EM. A direct screen for secreted proteins in Xenopus embryos identifies distinct activities for the Wnt antagonist crescent and Frzb-1. Mech Dev. 1999;96:183–195. [PubMed: 10960783]
- 69.
- Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274:1115–1123. [PubMed: 8895454]
- 70.
- McGrew LL, Otte AP, Moon RT. Analysis of Xwnt-4 in embryos of Xenopus laevis: a Wnt family member expressed in the brain and the floor plate. Development. 1992;115:463–473. [PubMed: 1425335]
- 71.
- Ungar AR, Kelly GM, Moon RT. Wnt4 affects morphogenesis when misexpressed in the zebrafish embryo. Mech Dev. 1995;52:153–164. [PubMed: 8541205]
- 72.
- Stark K, Vainio S, Vassileva G. et al. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. [PubMed: 7990960]
- 73.
- Wolda SL, Moody CJ, Moon RT. Overlapping expression of Xwnt-3A and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev Biol. 1993;155:46–57. [PubMed: 8416844]
- 74.
- Dickinson ME, Krumlauf R, McMahon AP. Evidence for a mitogenic effect of Wnt-1 in the developing mammalian central nervous system. Development. 1994;120:1453–1471. [PubMed: 8050356]
- 75.
- Dunn KJ, Williams BO, Li Y. et al. Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development. Proc Natl Acad Sci USA. 2000;97:10050–10055. [PMC free article: PMC34553] [PubMed: 10963668]
- 76.
- Liem KF, Tremml G, Jessell TM. A role for roof plate and its resident TGF-β-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. [PubMed: 9335341]
- 77.
- LeDouarin NM, Kalcheim C. The neural crest 1999. S3econd Edition, London: Cambridge University Press. [PMC free article: PMC140126]
- 78.
- LaBonne C, Bronner-Fraser M. Molecular mechanisms of neural crest formation. Annu Rev Cell Dev Biol. 1999;15:81–112. [PubMed: 10611958]
- 79.
- Selleck MA, Bronner-Fraser M. Origins of the avian neural crest: The role of neural plate-epidermal interactions. Development. 1995;121:525–538. [PubMed: 7768190]
- 80.
- Mancilla A, Mayor R. Neural crest formation in Xenopus laevis: mechanism of Xslug induction. Dev Biol. 1996;177:580–589. [PubMed: 8806833]
- 81.
- Bonstein L, Elias S, Frank D. Paraxial-fated mesoderm is required for neural crest induction in Xenopus embryos. Dev Biol. 1998;193:156–168. [PubMed: 9473321]
- 82.
- Liem KF, Tremml G, Roelink H. et al. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. [PubMed: 7553857]
- 83.
- Zimmerman LB, de Jesus-Escobar JM, Harland R. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. [PubMed: 8752214]
- 84.
- Piccolo S, Sasai Y, Lu B. et al. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. [PMC free article: PMC3070603] [PubMed: 8752213]
- 85.
- Morgan R, Sargent MG. The role in neural patterning of translation initiation factor eIF4AII; 3induction of neural fold genes. Development. 1997;124:2751–2760. [PubMed: 9226446]
- 86.
- Merchant L, Linker C, Ruiz P. et al. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev Biol. 1998;198:319–329. [PubMed: 9659936]
- 87.
- Nguyen VH, Schmid B, Trout J. et al. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol. 1998;199:93–110. [PubMed: 9676195]
- 88.
- Kengaku M, Okamoto H. Basic fibroblast growth factor induces differentiation of neural tube and neural crest lineages of cultured ectoderm cells from Xenopus gastrula. Development. 1993;119:1067–1078. [PubMed: 8306875]
- 89.
- Mayor R, Guerrero N, Martinez C. Role of FGF and noggin in neural crest induction. Dev Biol. 1997;189:1–12. [PubMed: 9281332]
- 90.
- Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396:370–373. [PubMed: 9845073]
- 91.
- Dorsky RI, Raible DW, Moon RT. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev. 2000;14:158–162. [PMC free article: PMC316348] [PubMed: 10652270]
- 92.
- Anderson DJ. Cellular and molecular biology of neural crest cell lineage determination. Trends Genet. 1997;13:337–345. [PubMed: 9242050]
- 93.
- Dorsky RI, Moon RT, Raible DW. Environmental signals and cell fate specification in premigratory neural crest. BioEssays. 2000;22:708–716. [PubMed: 10918301]
- 94.
- Jin E-J, Erickson CA, Takada S. et al. Wnt and BMP signaling govern lineage segregation of melanocytes in the avian embryo. Dev Biol. 2001;233:22–37. [PubMed: 11319855]
- 95.
- Golding CR. Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev. 2000;14:1712–1728. [PubMed: 10898786]
- 96.
- Lister JA, Robertson CP, Lepage T. et al. Nacre ecodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development. 1999;126:3757–3767. [PubMed: 10433906]
- 97.
- Takeda K, Yasumoto K, Takada R. et al. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J Biol Chem. 2000;19:14013–14016. [PubMed: 10747853]
- 98.
- Graham A, Koentges G, Lumsden A. Neural crest apoptosis and the establishment of craniofacial pattern: an honorable death. Mol Cell Neurosci. 1996;8:36–83.
- 99.
- Graham A, Francis-West P, Brickell P. et al. The signaling molecule BMP4 mediates apoptosis in the rhombencephalis neural crest. Nature. 1994;372:684–686. [PubMed: 7990961]
- 100.
- Ellies DL, Church V, Francis-West P. et al. The Wnt antagonist cSFRP-2 modulates programmed cell death in the developing hindbrain. Development. 2000;127:5285–5295. [PubMed: 11076751]
- 101.
- Yamauchi Y, Abe K, Mantani A. et al. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev Biol. 1999;212:191–203. [PubMed: 10419695]
- 102.
- Rasmussen JT, Deardorff MA, Tan C. et al. Regulation of eye development by frizzled signaling in Xenopus. Proc Natl Acad Sci USA. 2001;98:3861–3866. [PMC free article: PMC31143] [PubMed: 11274406]
- 103.
- Salinas PC. Wnt factors in axonal remodelling and synaptogenesis. Biochem Soc Symp. 1999;65:101–109. [PubMed: 10320935]
- 104.
- Roelink H. Hippocampus formation: an intriguing collaboration. Curr Biol. 2000;10:R279–R281. [PubMed: 10753739]
- Patterning the Vertebrate Neural Plate by Wnt Signaling - Madame Curie Bioscienc...Patterning the Vertebrate Neural Plate by Wnt Signaling - Madame Curie Bioscience Database
- Phylogenomics and Molecular Evolution of Polyomaviruses - Madame Curie Bioscienc...Phylogenomics and Molecular Evolution of Polyomaviruses - Madame Curie Bioscience Database
- Rho Family GTPases and Cellular Transformation - Madame Curie Bioscience Databas...Rho Family GTPases and Cellular Transformation - Madame Curie Bioscience Database
- Deciphering the Complex Enzymatic Pathway for Biosynthesis of Wyosine Derivative...Deciphering the Complex Enzymatic Pathway for Biosynthesis of Wyosine Derivatives in Anticodon of tRNAPhe - Madame Curie Bioscience Database
- Tests of a Stereochemical Genetic Code - Madame Curie Bioscience DatabaseTests of a Stereochemical Genetic Code - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...