NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
New functions are implicated for calreticulin, based on its release from cytotoxic NK and T cells. Calreticulin is the only one of six “KDEL” (Lys-Asp-Glu-Leu carboxy terminal) endoplasmic reticulum chaperone proteins present in cytotoxic granules of these lymphocytes. Calreticulin is released when the lymphocytes release their granules to kill other cells, which suggests that it is likely to have an important role. In this chapter, we briefly review the contents of cytotoxic granules and how calreticulin might interact with these proteins. Then we discuss calreticulin inactivation of lysis mediated by perforin, a pore-forming protein which is essential for granule-mediated toxicity. Calreticulin is degraded by granzymes (proteases found within the cytotoxic granules), particularly by one granzyme, Chymase 1. Chymase 1 was previously identified as required for granule-mediated lysis, which could indicate that the calreticulin function(s) may be temporally regulated. We also introduce our “inactivation of the inactivator” hypothesis (granzyme-degradation of the inactivator calreticulin) as a focus for evaluation of the present information and as a focus to identify critical information to collect in the future. Finally, we provide the reader with additional functions of calreticulin that could be important for systemic immunity.
Introduction
The topic of this chapter is “What is calreticulin doing in cytotoxic lymphocyte granules?”. The question arises because calreticulin is the only one of six “KDEL” (Lys-Asp-Glu-Leu carboxy terminal) endoplasmic reticulum (ER) chaperone proteins present in the lymphocyte granules. 1 Cytotoxic granules have been described as specialized lysosomes2 that are designed to kill cells infected with pathogens, which means that the selective retention of calreticulin begs for a functional explanation. Currently, we are pursuing the hypothesis that calreticulin provides important control of granule-dependent killing. We first introduce the cytotoxic lymphocytes and the contents of their dangerous granules, including the pore-forming granule protein, perforin,3,4 which is essential for granule-mediated death. Then we summarize information about the effects of calreticulin on perforin-mediated killing of cells. At the end, we consider other effects that could result when calreticulin is released from immune cells. At several points, we identify laboratories and their locations to emphasize that the progress in this field is truly from many countries and many disciplines.
Cytotoxic Lymphocytes and the Contents of the Granules
What are cytotoxic lymphocytes and what is the purpose of their toxic granules?
Cytotoxic lymphocytes include natural killer (NK) and T (thymically-derived) killer lymphocytes. Both types of killer lymphocytes circulate in blood and through lymphoid organs of people and other mammals all the time, whether the individuals are healthy or diseased (reviewed, ref. 5). The NK and T lymphocytes control intracellular infections by viruses (with different viral species that can infect almost all cell types) or intracellular infections by species of bacteria which selectively live inside macrophages (such as tubercle bacilli). In humans, active NK lymphocytes, which lack T cell receptors for antigen, are ˜3% of all the lymphocytes circulating in blood and constitutively contain cytotoxic granules.6 NK cells are part of the innate immune system and immediately kill other cells after detecting their infection. NK cells also kill cells that are coated with specific antibodies7 which could be to viral antigens in the plasma membranes of infected cells. In contrast, memory cytotoxic T cells lack abundant granules until granule formation is induced when individual antigen-specific T cells encounter their cognate antigens. The cytotoxic T cells and the antibody-directed killing by NK cells are part of adaptive immunity, acquired in response to specific infections. Both NK or T release (exocytose) their granules after the lymphocytes specifically bind to infected “target” cells. Granule-mediated killing affects only the target cells, while the NK or T killer cells survive without damage to move on to kill more infected cells. The target cell dies in one of two ways: direct lysis by disruption of its plasma membrane or by induced apoptosis during which its plasma membrane stays initially intact while damage begins inside the cell.
What is in the NK and T cytotoxic cell granules to make them lethal (and how would calreticulin interact with it)?
Toxicity is associated with proteins (rather than lipid mediators) found within the granules8 Important granule contents are summarized in Table 14.1. Membrane damage is produced by two of the granule proteins, perforin and a saponin-like protein termed granulysin in humans.9,10 Each of these proteins has direct cellular toxicity independent of the other; however, granulysin requires perforin to gain access to intracellular bacteria. Perforin, in extremely low quantities (estimated to be below 10−9 M and fewer than 500 molecules per cell), makes large pores in cells. The perforin pores cause rapid lysis (immediate necrosis) if they are stable in cell membranes. Even if they are cleared by the target cells, the pores can facilitate entry of granzymes into the targets. Once inside the target cells, several of the granzymes can mediate intracellular damage by cleaving pro-apoptotic protein substrates. In contrast, higher concentrations (˜1 μM) of granulysin are needed to lyse cells or kill bacteria.11 Granzymes, serine-dependent proteases of lymphocytes, are implicated in the control of perforin-dependent lysis.12,13 Three granzymes, Gr A, Gr B and Gr K, are capable of inducing apoptosis after gaining intracellular access to target cell substrates. The granzymes are designated by letters which correspond to their genes and have different proteolytic activities (see the footnotes of Table 14.1). Granzymes alone lack toxicity as do granules isolated from perforin-/- mice (J. Holder and D. Hudig, unpublished results). The calreticulin that is found inside cytotoxic granules is also without toxicity as would be anticipated based on its known chaperone activities. Almost all of the granule proteins are NBterminally glycosylated and the initial, high mannose carbohydrates of the glycosylation could bind to calreticulin for transport14 (which would be an early function of calreticulin). However, the chaperone functions fail to explain why calreticulin would be retained in the granules after the proteins are transported.
Table 1
Proteins of T and NK cytotoxic lymphocyte granules.
It is very important for these studies to firmly establish that calreticulin is really inside the cytotoxic granules
This consideration is warranted because ER vesicles contaminate the granules during their isolation by density centrifugation.15 The presence of calreticulin in granules was first noted by Jurg Tschopp's laboratory in Switzerland. They discovered that calreticulin is a major contaminant when perforin is purified from granules by anion exchange chromatography.16 Subsequently, Marek Michalak and Chris Bleackley and their colleagues in Canada reported that mRNA encoding calreticulin was expressed at ˜ 10 fold higher levels in activated cytotoxic T lymphocytes than in other lymphocytes.17 Since only cytotoxic lymphocytes have dense granules, the expression of message is consistent with sequestration of substantial amounts of calreticulin in the unique granules. The localization of calreticulin to cytotoxic granules was indicated by confocal microscopy1 using antibodies to calreticulin and to granzyme B which indicated that these proteins were in common compartments. Calreticulin appeared in cytotoxic granules even in perforin-/- mice.1 Finally, the extracellular release of calreticulin, after stimulation of cytotoxic T cells with antibodies to T cell antigen receptor-associated proteins,1 confirms that calreticulin really is in secretory granules. The definitive localization of calreticulin encouraged us to search for a role for calreticulin in lysis.
The Role of Calreticulin in Perforin-Dependent Lysis
What information is available and how does it support our hypothesis that calreticulin controls perforin-dependent killing?
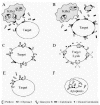
Our hypothesis is that calreticulin inactivates perforin lysis and that selected granzymes cleave calreticulin to “inactivate the inactivator” and let perforin be lytic (see Fig. 14.1). This hypothesis provides an intra-granule substrate for the granzymes in addition to the intra-target cell substrates for the granzymes A, B and K.
We found that calreticulin will inactivate perforin-mediated lysis.18,19 Perforin is routinely assayed by hemolysis (hemoglobin release) of red blood cells (rbc's) which are very sensitive to perforin. Alternately, perforin is assayed by lysis of other cells indicated by release of radioactive 51Cr as a tracer. The radioactive method requires about 30-fold more perforin for cell lysis but represents the cells that are the physiological targets of the killer lymphocytes. Calreticulin blocks perforin lysis in both assays (for rbc's18,19 and unpublished results for K562 cell targets). Calreticulin blocks perforin-mediated lysis of rbc's at 2 × 10−7 M concentrations of native calreticulin. Slightly greater (9 × 10−7 M) concentrations of recombinant calreticulin also block perforin lysis. When the three major domains of calreticulin are compared, the C-domain prevented lysis while the N and P (proline-rich) domains were lacking effects. The recombinant C domain was nearly as effective as whole recombinant calreticulin.19 The inactivation of perforin persists for over five hours. This observation is consistent with either stable inactivation and/or with the unrelated loss of activity of perforin during the assay. It is striking that while calreticulin inactivates partially purified perforin it rarely inactivates lysis mediated by unfractionated granules.19
It was this observation that led us to discover that granzymes degrade calreticulin. First, we noted that the granzymes of unfractionated granules completely digest 125I-labeled calreticulin (Hudig and Elliott, unpublished results). If we allow the assumption that the labeled and the endogenous calreticulin are equivalent, then the intra-granule calreticulin is also rapidly degraded by the endogenous granzymes. Later, we found that only one of four chymases (granzymes which cleave after aromatic amino acid residues, as does chymotrypsin) degrades calreticulin: chymase 1, which appears essential for lysis,13,20 cleaves calreticulin at two sites within the P domain (Hudig and Schegg, unpublished results). The proteolytic sites probably reflect the specificity of chymase 1, particularly since the other granzyme chymases had no effect on calreticulin. The P domain cleavage sites are unusual since the C region of calreticulin is the most susceptible region to proteolysis.21,22 A tryptase other than Gr A also cleaves calreticulin, at an as yet unidentified site. Furthermore, based on the ability of the C domain alone to block lysis, it may be that the tryptase cleavage is in the C domain and its activity participates in inactivation of calreticulin.
How could calreticulin inactivate perforin lysis?
We have considered and discounted several explanations. Calreticulin is unlikely to form soluble complexes with perforin that are able to prevent perforin from binding to cells even though the two proteins can interact in solution. Soluble perforin will bind to calreticulin in the absence of calcium.1 Perforin has the amino acid sequence KVFF (residues 439–442) which matches the first four amino acids of the KXFF[K/R]R sequence that supports calreticulin binding to the cytoplasmic domain of α-subunit of integrins23 and to several steroid receptors.24,25 However, the 0.1 mM or higher calcium concentrations that support perforin-dependent lysis dissociate soluble perforin-calreticulin complexes.1 In other words, under lytic conditions, perforin and calreticulin are dissociated in solution; however, it should be noted that membrane-associated complexes in the presence of calcium are still possible. Competition of calreticulin with perforin for binding sites on membranes also seems unlikely. Perforin binds to phosphorylcholine26 and calreticulin does not. Furthermore, there is insufficient calreticulin bound to red blood cells (at inactivating concentrations of calreticulin) to obscure substantial areas of membrane.18 Calreticulin is also unlikely to prevent lysis by binding free calcium. The concentration of calreticulin that prevents lysis is insufficient to bind enough calcium to interfere with perforin lysis. Specifically, micro molar concentrations of calreticulin inactivate lysis but are insufficient to reduce 1 mM calcium to below 0.1 mM calcium, levels at which the lytic activity of perforin decreases. Our calculations have taken into account the 25–30 moles of calcium that can be bound per mole of calreticulin. Calreticulin also fails to inactivate the protease activities of the chymases and other granzymes that function in lysis.19
After consideration of the possibilities listed above, we have formed a hypothesis
We propose that when calreticulin binds to target cell membranes, it acquires an altered conformation. In the new conformation we believe that it forms complexes with perforin to prevent perforin from entering into the membrane (Fig. 14.1A and B). In this model, Chymase 1 and/or another granzyme must degrade calreticulin to free perforin from the complex and allow it to enter the target cell membrane (Fig. 14.1C). Lysis will occur only after sufficient calreticulin has been cleaved (Fig. 14.1D) and/or the unbound calreticulin in equilibrium with the membrane has diffused away under in vivo conditions. Similarly, cleavage of calreticulin would be needed for perforin to form channels to allow granzyme B (or Gr A or Gr K) to enter cells and then cleave intracellular substrates to initiate apoptosis (Fig. 14.1E and F).
What on membranes binds calreticulin, to then promote localized calreticulin-perforin complex formation? Calreticulin has different affinities for the plasma membranes of different cells (and the concentrations that inactivate lysis are above these affinities). We have found that in the presence of calcium, calreticulin has a Kd of 2.7 × 10−7 M for rbc's at unknown binding site(s).18 Kuwabara et al., also in the USA, found a Kd of 7.4 × 10−9 M for calreticulin binding to endothelial cells, also at unknown binding site(s).27 In addition, the cell surface protein heterodimer CD91 binds calreticulin,28 as well as alpha-2 macroglobulin. Thus membrane interactions of calreticulin provide an alternate site for calreticulin-perforin interactions.
We don't know why some cells, such as RBCs, are much more susceptible to perforin than others, but it may correlate with the type and density of the calreticulin receptors. Other mechanisms may contribute to regulation of perforin damage. It should be added for perspective, that exocytic clearance of perforin lesions from resistant cells has compelling experimental support.29,30 This mechanism may be completely independent of calreticulin.
What would be the biological advantages of “ inactivation of the inactivator”?, you may ask
The rates of pore formation would be slower, which would provide time for the killer cell to patch and release its perforin (indicated by the killer cell bleb with perforin and calreticulin in Fig. 14.1B). The scenario offers systemic protection: membranes of neighboring cells will remain protected from lysis by calreticulin if the chymase is inactivated (by plasma protease inhibitors). Furthermore, the presence of calreticulin on the cell surface of activated T lymphocytes, 31 as observed by Arosa et al. in Portugal, indicates that its location could also help protect the killer cells from the toxic agents they release.
Other Functions for Calreticulin in Immunity
What other effects could calreticulin have after it is released by immune cells?
When calreticulin binds to CD91 on macrophages, CD91 and calreticulin form a complex to promote uptake and clearance of apoptotic vesicles. For this occurrence, the apoptotic fragments must also be tagged with collectins such as complement C1q or mannose binding protein (see reference 32 and the chapter in this book by P.M. Henson and colleagues). It is the collectins that associate with calreticulin33 in the CD91/CRT complexes and then the interaction triggers the cellular uptake of the collectin-coated vesicles. In a lymphocyte-mediated scenario, calreticulin would be present after induction of granzyme-induced apoptosis and be able to produce calreticulin-CD91 complexes on neighboring macrophages to promote clearance of the apoptotic fragments.
Another intriguing possibility is opened by the discoveries of Srivastava et al. in the USA. They found that extracellular complexes of calreticulin with foreign proteins can be ingested by cells to result in the intracellular degradation of the complexed protein. After degradation, the peptide fragments of the foreign protein are bound to MHC I (major histocompatibility complex) proteins. The MHC I proteins with the peptides are sent to the plasma membrane for antigen presentation to CD8 T cells,34 providing a novel way to load peptides from extracellular proteins onto MHC I. The novel pathway allows MHC I (rather than MHC II) presentation of antigens that were synthesized outside the cytoplasm of the antigen-presenting cell. By this mechanism, viral and bacterial antigens from cells that died from NK cell-mediated perforin lytic disruption (rather than granzyme-induced apoptosis) could be salvaged. The salvaged antigens could then be recycled for presentation to CD8 T cells. This scenario would permit the immediate elimination of infections, mediated by the innate immunity of NK cells, to stimulate the long-lasting adaptive immunity of CD8 T cells that recognize antigens in the context of MHC I and not MHC II.
Neutrophils also release calreticulin,35 as reported by Paul Eggleton and colleagues in the U.K., which indicates that there are additional sources of calreticulin for these immune functions which are likely to be unrelated to control of perforin.
Conclusions
There is a wide gap between experimentally reproducible data and physiologically valid conclusions. Descriptive information is needed to test our hypothesis, such as the amounts of calreticulin, calreticulin-degrading granzymes and perforin in granules. Critical in vitro experiments are also needed, using isolated perforin and calreticulin with differing concentrations of the granzyme calreticulinases. We could learn much from cellular experiments with lymphocytes lacking calreticulin (from CRT-/- mice) or expressing only calreticulin variants that would be refractory to granzyme degradation (from “knock-in calreticulin variants” of CRT-/- mice) . We hope to close this gap concerning the role of calreticulin in lysis, by our own discoveries and by integrating the rapid progress made by other researchers who are also addressing the roles of extracellular calreticulin.
Acknowledgments
This publication was supported in part by the NIH (USA) grant R01 CA38942. We thank Ms. Viki Elliott, Myra Godfrey, and Dr. W.H. Welch at the University of Nevada, Reno, for helpful criticism of the manuscript.
References
- 1.
- Andrin C, Pinkoski MJ, Burns K. et al. Interaction between a Ca2+-binding protein calreticulin and perforin, a component of the cytotoxic T-cell granules. Biochemistry. 1998;37:10386–10394. [PubMed: 9671507]
- 2.
- Page LJ, Darmon AJ, Uellner R. et al. L is for lytic granules: lysosomes that kill. Biochim Biophys Acta. 1998;1401:146–156. [PubMed: 9531970]
- 3.
- Liu C-C, Walsh CM, Young JDE. Perforin: structure and function. Immunol Today. 1995;16:194–201. [PubMed: 7734048]
- 4.
- Tschopp J, Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Ann Rev Immunol. 1990;8:279–302. [PubMed: 2188665]
- 5.
- Henkart PA. Cytotoxic T lymphocytesIn: Paul WE Jr, ed.Fundamental ImmunologyPhiladelphia: Lippincott-Raven,19991021–1050.
- 6.
- Bonavida B, Bradley TP, Grimm EA. The single cell assay in cell-mediated cytotoxicity. Im Today. 1983;4:196–198. [PubMed: 25289827]
- 7.
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. [PMC free article: PMC7131425] [PubMed: 2683611]
- 8.
- Smyth MJ, Kelly JM, Sutton VR. et al. Unlocking the secrets of cytotoxic granule proteins. J Leukoc Biol. 2001;70:18–29. [PubMed: 11435481]
- 9.
- Pena SV, Hanson DA, Carr BA. et al. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small lytic granule proteins. J Immunol. 1997;158:2680–2688. [PubMed: 9058801]
- 10.
- Pena SV, Krensky AM. Granulysin, a new human cytolytic granule-associated protein with possible involvement in cell-mediated cytotoxicity. Semin Immunol. 1997;9:117–125. [PubMed: 9194222]
- 11.
- Stenger S, Hanson DA, Teitelbaum R. et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. [PubMed: 9756476]
- 12.
- Hudig D, Ewoldt GR, Woodard SL. Proteases and lymphocyte cytotoxic killing mechanisms. Curr Opin Immunol. 1993;5:90–96. [PubMed: 8452680]
- 13.
- Woodard SL, Fraser SA, Winkler U. et al. Purification and characterization of lymphocyte Chymase I, a granzyme implicated in perforin-mediated lysis. J Immunol. 1998;160:4988–4993. [PubMed: 9590247]
- 14.
- Vassilakos A, Michalak M, Lehrman MA. et al. Oligosaccharide binding characteristics of the molecular chaperones calnexin and calreticulin. Biochemistry. 1998;37:3480–3490. [PubMed: 9521669]
- 15.
- Podack ER, Konigsberg PJ.1984.Cytolytic T cell granules: Isolation, structural, biochemical, and functional characterization J Exp Med 1984160695–710. [PMC free article: PMC2187410] [PubMed: 6332169]
- 16.
- Dupuis M, Schaerer E, Krause K-H. et al. The calcium-binding protein calreticulin is a major constituent of lytic granules in cytolytic T lymphocytes. J Exp Med. 1993;177:1–7. [PMC free article: PMC2190868] [PubMed: 8418194]
- 17.
- Burns K, Helgason CD, Bleackley RC. et al. Calreticulin in T-lymphocytes. Identification of calreticulin in T-lymphocytes and demonstration that activation of T cells correlates with increased levels of calreticulin mRNA and protein. J Biol Chem. 1992;267:19039–19042. [PubMed: 1527030]
- 18.
- Fraser SA, Michalak M, Welch WH. et al. Calreticulin, a component of the endoplasmic reticulum and of cytotoxic lymphocyte granules, regulates perforin-mediated lysis in the hemolytic model system. Biochem Cell Biol. 1998;76:881–887. [PubMed: 10353724]
- 19.
- Fraser SA, Karimi R, Michalak M. et al. Perforin lytic activity is controlled by calreticulin. J Immunol. 2000;164:4150–4155. [PubMed: 10754310]
- 20.
- Woodard SL, Jackson DS, Abuelyaman AS. et al. Chymase-directed serine protease inhibitor that reacts with a single 30 kDa granzyme and blocks NK-mediated cytotoxicity. J Immunol. 1994;153:5016–5025. [PubMed: 7963562]
- 21.
- Corbett EF, Michalak KM, Oikawa K. et al. The conformation of calreticulin is influenced by the endoplasmic reticulum luminal environment. J Biol Chem. 2000;275:27177–27185. [PubMed: 10842171]
- 22.
- Bouvier M, Stafford WF. Probing the three-dimensional structure of human calreticulin. Biochemistry. 2000;39:14950–14959. [PubMed: 11101311]
- 23.
- Coppolino MG, Woodside MJ, Demaurex N. et al. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997;386:843–847. [PubMed: 9126744]
- 24.
- Burns K, Duggan B, Atkinson EA. et al. Modulation of gene expression by calreticulin binding to the glucocorticoid receptor. Nature. 1994;367:476–480. [PubMed: 8107808]
- 25.
- Dedhar S, Rennie PS, Shago M. et al. Inhibition of nuclear hormone receptor activity by calreticulin. Nature. 1994;367:480–483. [PubMed: 8107809]
- 26.
- Tschopp J, Sch:afer S, Masson D. et al. Phosphorylcholine acts as a Ca2+-dependent receptor molecule for lymphocyte perforin. Nature. 1989;337:272–274. [PubMed: 2783478]
- 27.
- Kuwabara K, Pinsky DJ, Schmidt A. et al. Calreticulin, an Antithrombotic Agent Which Binds to Vitamin K-dependent Coagulation Factors, Stimulates Endothelial Nitric oxide Production, and Limits Thrombosis in Canine Coronary Arteries. J Biol Chem. 1995;270:8179–8187. [PubMed: 7713923]
- 28.
- Basu S, Binder RJ, Ramalingam T. et al. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. [PubMed: 11290339]
- 29.
- Jones J, Morgan BP. Killing of cells by perforin. Resistance to killing is not due to diminished binding of perforin to cell membrane. Biochem J. 1991;280:199–204. [PMC free article: PMC1130620] [PubMed: 1741748]
- 30.
- Jones J, Hallett MB, Morgan BP. Reversible cell damage by T-cell perforins. Calcium influx and propidium iodide uptake into K562 cells in the absence of lysis. Biochem J. 1990;267:303–307. [PMC free article: PMC1131287] [PubMed: 2334394]
- 31.
- Arosa FA, De Jesus O, Porto G. et al. Calreticulin is expressed on the cell surface of activated human peripheral blood T lymphocytes in association with major histocompatibility complex class I molecules. J Biol Chem. 1999;274:16917–16922. [PubMed: 10358038]
- 32.
- Ogden CA, deCathelineau A, Hoffmann PR. et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–795. [PMC free article: PMC2195958] [PubMed: 11560994]
- 33.
- Sim RB, Moestrup SK, Stuart GR. et al. Interaction of C1q and the collectins with the potential receptors calreticulin (cC1qR/collectin receptor) and megalin. Immunobiology. 1998;199:208–224. [PubMed: 9777407]
- 34.
- Basu S, Srivastava PK. Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J Exp Med. 1999;189:797–802. [PMC free article: PMC2192945] [PubMed: 10049943]
- 35.
- Kishore U, Sontheimer RD, Sastry KN. et al. Release of calreticulin from neutrophils may alter C1q-mediated immune functions. Biochem.J. 1997;322:543–550. [PMC free article: PMC1218224] [PubMed: 9065775]
- 36.
- Beresford PJ, Xia Z, Greenberg AH. et al. Granzyme A loading induces rapid cytolysis and a novel form of DNA damage independently of caspase activation. Immunity. 1999;10:585–594. [PubMed: 10367904]
- 37.
- Zhang D, Pasternack MS, Beresford PJ. et al. Induction of rapid histone degradation by the cytotoxic T lymphocyte protease Granzyme A. J Biol Chem. 2001;276:3683–3690. [PubMed: 11060286]
- 38.
- Kam C-M, Hudig D, Powers JC. Granzymes (lymphocyte serine proteases): characterization with natural and synthetic substrates and inhibitors. Biochim Biophys Acta. 2000;1477:307–323. [PubMed: 10708866]
- 39.
- Sharif-Askari E, Alam A, Rheaume E. et al. Direct cleavage of the human DNA fragmentation factor-45 by granzyme B induces caspase-activated DNase release and DNA fragmentation. EMBO J. 2001;20:3101–3113. [PMC free article: PMC150191] [PubMed: 11406587]
- 40.
- Shi L, Kam C-M, Powers JC. et al. Purification of three lymphocyte granule serine proteases that induce apoptosis through distinct substrate and target interactions. J Exp Med. 1992;176:1521–1529. [PMC free article: PMC2119451] [PubMed: 1460416]
- 41.
- Smyth MJ, Sayers TJ, Wiltrout T. et al. Met-ase: cloning and distinct chromosomal location of a serine protease preferentially expressed in human natural killer cells. J Immunol. 1993;151:6195–6205. [PubMed: 8245461]
- 42.
- Griffiths GM, Isaaz S. Granzymes A and B are targeted to the lytic granules of lymphocytes by the mannose-6-phosphate receptor. J Cell Biol. 1993;120:885–896. [PMC free article: PMC2200067] [PubMed: 8432729]
- Calreticulin in Cytotoxic Lymphocyte-Mediated Cytotoxicity - Madame Curie Biosci...Calreticulin in Cytotoxic Lymphocyte-Mediated Cytotoxicity - Madame Curie Bioscience Database
- Predicting the Spread of a Transgene in African Malaria Vector Populations: Curr...Predicting the Spread of a Transgene in African Malaria Vector Populations: Current Knowledge and Limitations - Madame Curie Bioscience Database
- Coronin 1 in Innate Immunity - Madame Curie Bioscience DatabaseCoronin 1 in Innate Immunity - Madame Curie Bioscience Database
- CTLA-4 in Type 1 Diabetes Mellitus - Madame Curie Bioscience DatabaseCTLA-4 in Type 1 Diabetes Mellitus - Madame Curie Bioscience Database
- Cell Adhesion Molecules - Madame Curie Bioscience DatabaseCell Adhesion Molecules - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...