NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Transition metal ions such as copper, iron, manganese and zinc serve as essential cofac tors for a variety of biological processes including cell energetics, gene regulation and control of free radicals. However, these essential nutrients, are toxic at elevated levels. Therefore metal ion transporters play a crucial role in maintaining the vital metal-ions' homeostasis. The yeast (Saccharomyces cerevisiae) metal transporters SMFgens and their homologues in other organisms (NRAMP-related transporters) play a central role in the accumulation of metal ions in different tissues and distinct cellular organelles. The use of yeast as an eukaryotic model system facilitated the study of these metal ion transporters and served also as a heterologous expression system through complementation of various yeast null mutants with related genes. For this end, we generated mutant yeast strains with null mutations in each individual SMF gene as well as in all combinations of the three genes. The triple null mutant smf1,2,3Δ can not grow on medium with reduced divalent metal ions concentration (containing EGTA) or medium buffered at pH 7.5. The yeast Smf1p and the mammalian transporter DCT1 suppress the above phenotype and allow growth in the presence of EGTA or at pH 7.5. The Mycobacterium leprae transporter NRMIT suppresses the EGTA sensitivity of the mutant smf3Δ Both yeast and mammalian transporters were expressed in Xenopus oocytes and the uptake activity as well as the electrophysiological properties of Smf1p and DCT1 were studied. A novel clutch mechanism of slippage that operates via continuously variable stoichiometry between the driving force pathway (H) and the transport pathway (divalent metal ions) was proposed. The possible physiological advantage of proton slip through DCT1 and sodium slip through Smf1p is discussed. The mechanism of metal ion transport of those transporters is essential for understanding of certain human diseases.
Introduction
Factors controlling metal ion transport across cellular membranes, intracellular homeostasis and regulatory responses of cells to changing environmental supply of divalent metal ions, have been subjects to many studies in recent years (Ref. 1 for reviews). Of particular interest were divalent trace metals like Cu2+, Mn2+, Fe2+, Zn2+ because of their important role in cell metabolism, especially as cofactors of many enzymes. Usually their intracellular concentration is kept at a low, rather constant physiological level by a number of transporters, sensor systems, storage proteins and chaperones. These physiological levels can be disturbed in several ways, which frequently lead to severe disease phenotypes. Genetic disorders as well as malnutrition can be the cause for suboptimal concentrations and, sometimes, for accumulation of these essential ions in cells.
Toxic metals like Cd2+, Co2+ and Ni2+ can be a major threat to the health of mammals and could suppress plants growth, mostly because they interfere in various ways with transport, homeostasis or function of the essential metals. To minimize their deteriorating effects cells have developed various strategies, among which transport or sequestration into organelles and binding by thiols are most prominent.14 A specific set of transporters functions in each cellular compartment to provide a delicate balance of transport activities across their membranes.5,6 Because metal-ions are vital for several life processes, and their action also inflicts damage on DNA and proteins, their proper distribution is vital and a slight alteration in their activity could cause severe disease. For example, abnormal iron uptake has been implicated in the most common hereditary disease hemochromatosis, as well as in neurological diseases such as Parkinson's disease, Friedreich ataxia and Pica.610 Apparently any aberration in the cellular metal-ion concentrations may cause a shortage of a vital metabolic element or inflict damage that may lead to cell death.
Only in recent years has research focused on genes encoding metal ion transporters in eukaryotes and on the genetic disorders associated with them.68 As a result, a large number of candidate genes were discovered and some of the transporters have been described in detail, particularly those for Ca2+, Cu2+, Fe2+. Yet, several observations indicate that many more proteins, involved in transport and homeostasis of metal ions, await their identification.
The use of the yeast Saccharomyces cerevisiae as a model system for the identification and isolation of these proteins is particularly attractive. Yeast is an Eukaryote which has a haploid and diploid form, a known, relatively small genome with few introns, excellent rate of homologous recombination, and easy methods for genetic screens. Yeast is also an easy organism to grow under metal ion stress. Transporters identified in this model organism frequently proved to be rather well conserved among eukaroytes. Indeed the attributed function of the NRAMP family as divalent metal ion transporters was discovered through the function of homologous genes in yeast.11 Mammalian or plant homologues of yeast genes could be recognized either by screening sequences in the database or by complementation of yeast mutants with higher organisms' cDNA libraries.
Discovery of the Yeast Smf1p as a Metal-Ion Transporter Revealed that Metal Ions Function Through NRAMP in Resistance and Sensitivity to Bacterial Infection
It has long been recognized that the pathogenicity of a broad range of intracellular parasites is dependent on the availability of transition metal ions (Ref. 12, 13 for reviews). The discovery of a macrophage protein known to confer resistance to intracellular parasites Nramp1 (Natural Resistance Associated Macrophage Protein) and its recognition as a metal ion transporter substantiated it.11,14,15 Nramp1 is identical to the Ity and the Lsh gene conferring resistance to infections by Salmonella typhimurium and Leishmania donovani, respectively.16 However the enigma was what does it transport. The discovery that the yeast homologue, Smf1p is a metal-ion transporter,11 paved the way for the advancement of our knowledge about the substrate of the mammalian NRAMP. Its function as a scavenger of metal-ions from the bacteria-containing phagosomes was then suggested with the discovery of Smf1p as the yeast Nramp homologue.11 SMF1 has originally been cloned as a high copy number suppressor of a temperature-sensitive mif1-1 mutant.17 MIF1 (MAS1) and MAS2 (MIF2) encode the processing enhancing protein and the matrix processing peptidase, respectively. The two proteins function as a heterodimer to form the active holoenzyme of mitochondrial processing peptidase, which is vital for cell growth.1820 The activity of the purified peptidase is inhibited by chelators such as EDTA or orthophenantroline and is stimulated by Mn2+, Zn2+, or Co2+.21
We picked up the SMF1 gene as a suppressor of a csp2 (cdc1-1 ) mutant, that was sensitive to the presence of EGTA in the medium.11 The Chelator Sensitive Phenotype (Csp-) of the csp2 mutant was caused by a mutation in the CDC1 gene, in which Gly149 was substituted by arginine. The cdc1-1 mutant exhibited a very similar complementation characteristics to the mas1 (or mif1-1) mutant with the exception of their growth inhibiting conditions (which are EGTA and 37oC, respectively). We re-examined the mif1-1 mutant and found that its temperature-sensitivity could be alleviated by the addition of 1 mM Mn2+ to the medium or by overexpression of Smf1p.11 Thus the temperature sensitivity of mif1-1 mutant may result from reduced stability of the mutated processing peptidase which needed higher manganese concentrations for its function in higher temperature.
In both cases, the mif1-1 and the cdc1-1, the mutation could be relieved by supplementing the media with Mn2+ or overexpressing Smf1p that stimulates the Mn2+ transport from the medium and elevates its concentration in the cytoplasm.11,22 Cdc1p may be a Mn2+-dependent cell division cycle protein that is vital for cell growth23,24 but the G149R mutation rendered it less stable and the cell sensitive to low Mn2+ concentration in the medium. Further studies indicated the Smf1p is a general metal-ion transporter and can transport not only Mn2+ but also Cu2+, Fe2+, Cd2+, Ni2+ and Co2+11,22,25,26. Yeast cells contain two additional genes of this family, SMF2 and SMF3, and indirect evidence indicates that they also exhibit broad range metal-ion specificity, which differs from Smf1p.27 It is remarkable, that prior to these studies, manganese was not considered to be an essential element for yeast growth. Only by discovering that mutations in Cdc1p and Mas1p can be complemented by the addition of Mn2+ did it become apparent that this metal-ion is vital for yeast.11,23,24
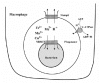
We proposed that the role of Nramp1 in macrophage defense against microbial invasion is to reduce the metal-ion concentrations inside the bacteria containing phagosomes11,25 (Fig. 1). Consequently it limits the production and function of the engulfed bacterial metalo-enzymes required for their defense against reactive oxygen and/or nitrogen toxic intermediates that are poured upon them by the phagocytes.28,29 It was proposed that Nramp1, like its yeast homologue, transports metal-ions from the phagosomal lumen into the cytoplasm. Thus the metal-ion depletion of the phagosomal lumen becomes a rate-limiting step in metalloenzymes function of the engulfed bacteria. This restricts the mycobacterial ability to produce and activate enzymes such as SOD and prevents the propagation of the ingested microorganisms (Fig. 1). Conversely, an increased concentration of metal-ions in the phagosome caused by a defective Nramp1 transporter (Bcgs ) may promote the growth of the mycobacteria and render the invaded organism sensitive to the pathogen. The discovery of Nramp -related genes (NRMIT) in several bacteria suggests that the pathogens use the same strategy in competition for the limited amounts of metal-ions inside the phagosome.11,15,30,31
The above hypothesis was addressed by several studies proving that the transporter indeed transports divalent metal ions. However, in respect to the directionality of the metal-ion pumping by Nramp1 in macrophages, the researchers differ. One group proposes Nramp1 pumps metal ions into the macrophage phagosomes and facilitates accumulation of metal ions in their lumen. Addition of Fe2+ to Nramp1 expressing macrophages in tissue culture was shown to further inhibit the growth of Mycobacterium avium and that this effect could be reversed by the addition of hydroxyl radicals scavengers.32 Moreover, Nramp1 expressing macrophages grown with 55Fe accumulated four times more Fe2+ in their phagosomes than the cells lacking Nramp1 same was true for import into isolated phagosomes. There was a burst of hydroxyl radicals after infection in the Nramp1 expressing cells but not in the nonexpressing cells.33 The iron uptake was shown to be dependent on pH gradient and with its disruption by lysomotropic agents like chloroquine and ammonium chloride, the amount of iron import to isolated Nramp1 expressing phagosomes decreased. This iron uptake could be also inhibited by treatment of the phagosomes with antibody against the putative outer fourth loop.34 Another group expressed Nramp1 in Xenopus oocytes and demonstrated that it could transport Fe2+, Zn2+ and Mn2+ in exchange with H35. Although large metal-ion induced currents were recorded, the metal ion transport was extremely low. The activity of Nramp1 mRNA injected oocytes was only up to 2 fold in comparison with water-injected oocytes whereas with Nramp2 (DCT1) injected oocytes rates that are more then 1000 fold the background are frequently obtained.36 This makes one wonder what is the source of the large metal-ion induced currents (see below). These groups believe Nramp1 functions as a pH dependent antiporter, importing divalent cations into the bacteria containing phagosome. The accumulated cations in the phagosome generate highly reactive hydroxyl radicals by Haber-Weiss reaction, which contribute to the bacteriostatic effect of the macrophage on the engulfed bacteria.3235
The other group proposes that the transporter pumps out the metal ions from the phagosome into the cytoplasm. They showed that in Nramp1 expressing macrophage 28% of the iron was bound to ferritin and 60% was in a soluble fraction namely, in the cytoplasm.37 Upon induction of the macrophage, the iron in the cytoplasm was increased to 82% on the expense of iron bound to ferritin that was less then 5%. This suggests that Nramp1 is required for releasing iron from phagosomes and transporting it into the cytoplasm.37 Using calcein as a marker, it was demonstrated that Nramp1 could transport iron from vesicles to the cytoplasm in relaxed macrophage.38 Using zymosan-FF6 as a phagocytosed indicator, it was shown that phagosomes from wild type mice transported Mn2+ out of the phagosome significantly faster as compared to knock-out mice. This transport was dependent on the pH gradient generated by V-ATPase.39 In analogy, Nramp2 (DCT1) was found to associate with erythrocyte containing phagosomes suggesting a role in recycling of iron from dying erythrocytes, which are the main source of iron in our body. Nramp2 was also found in sperm containing phagosomes of Sertoli cells suggesting a role in iron recycling from degenerating spermatozoids.40 This too suggests that the transporter works by exporting metal ions from the phagosome into the cytoplasm. These groups argue that the transport of divalent cations out of the microenvironment of the bacteria (phagosome) by Nramp1, results in an enhanced bacteriostatic activity.
Regardless of the directionality of the transport, there is no dispute about the necessity of transport of divalent cations by this transporter for the defense against bacterial infection. However, as mentioned above, we favor the metal ion depletion hypothesis, where the reduction in metal ion concentration in the phagosomal lumen becomes a rate-limiting step in metalloenzymes production by the engulfed bacteria (Fig. 1). This will restrict mycobacterial ability to produce active enzymes such as SOD and prevent the propagation of the ingested microorganisms. Conversely, an increased concentration of metal-ions in the phagosome caused by a defective Nramp1 transporter (Bcgs ) may result in higher metal-ion concentration, promote the growth of the mycobacteria and render the organism sensitive to the pathogen. The discovery of Nramp homologous genes (NRMIT) in several bacteria suggests that the pathogens use the same strategy in competition for the limited amounts of metal-ions inside the phagosome.15,31
A Glimpse into the Mechanism of Metal-Ion Uptake
Characterization of Metal-Ion Transporters Expressed in Xenopus Oocytes
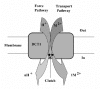
The mechanism of metal-ion transport by eukaryotic cells is largely obscure. The large number of transporters occupying the plasma membrane of a typical eukaryotic cell makes the study into the mechanism of a single transporter, very difficult. Therefore most of the information about those transporters has come from electrophysiological studies on DCT1 (Nramp2) and Smf1p that were expressed in Xenopus oocytes.26,36,41 One of the main advantages of oocytes as heterologous expression system, is their being self sufficient in terms of nutrition and therefore providing a system with very low background for foreign transporters expressed in their plasma membrane. DCT1 transports a wide range of divalent metal-ions, which is H driven. Uptake of radioactive cations such as Mn2+ and Co2+ into the oocyte is up to 1,000 fold above the background of water injected oocyte.36 By two-electrode recordings, presteady-state and steady-state currents induced by expressed electrogenic transporters can be measured (Ref. 42, 43 for reviews). As in other transporters, in the absence of metal ions in the medium, oocytes expressing DCT1 exhibit large presteady-state currents but only at positive potentials. In the presence of those metal-ions a steady state current is induced mainly in negative potentials, which is also dependent on chloride or other permeable anions.41 Since it seems surprising that the transport of a divalent cation such as Fe2+ will be driven solely by additional cation H, we further investigated the influence of anions on the currents generated DCT1.4,36 Substitution of chloride anions by gluconate drastically reduced the 55Fe2+ uptake into the oocytes and rendered it sensitive to membrane potential.4 It remains unknown, whether Cl- is cotransported with the metal ion and whether the steady-state current results solely from the transport of positive charges of H, or is a sum of proton and metal ion transported charges. The stoichiometry between the H and the transported metal ion varied under different experimental conditions and an analogy of clutch mechanism between the metal ion transport and gear-shift cars was drown36 (Fig. 2).
The Slip Phenomenon
It was demonstrated that DCT1 cotransports Fe2+ together with H with a stoichiometry of 1:1.41 The metal ion transport is therefore dependent on proton concentration in the external side of the membrane, as protons are cotransported with iron,44 but the nature of the driving force for transport is not apparent. At physiological membrane potentials of -90 to -30 mV, the apparent affinity constant for H was about 1 μM, suggesting that at neutral pH, proton binding is the rate-limiting step in the transport process. At low pH, DCT1 expressed in Xenopus oocytes exhibited a metal-ion induced uncoupled proton current into the oocyte and under certain conditions the transporter operates as an H uniporter.41 This phenomenon we defined as a mechanistic slip that is an integral part of the transporter's mechanism of action.45 The proton slip is influenced by the membrane potential, it increases as the imposed potentials become more negative and is absolutely dependent on the presence of metal ions in the medium.36,41 The involvement of Zn2+ in the transport process and proton slippage, deviates from all the other metal ions that were tested. Zn2+ was found to be an inhibitor of iron transport by DCT1 and manganese transport by Smf1p,11,41 therefore it is likely to bind to the same site as the other metal ions. However, Zn2+ is almost not transported by DCT1, yet induces the proton slip as much as the other metal ions and therefore under these conditions DCT1 acts as an H uniporter.4
The mechanism of metal ion transport by Smf1p is closely related to that of the mammalian DCT1 and exhibits similar affinities toward the various metal ions.27,36 Although in both H is the driving force, the most striking difference between DCT1 and Smf1p is in the uncoupled slip that was shown to be H in DCT1 and Na in Smf1p.26,45 The sodium slip in Smf1p is not dependent on the presence of metal ions and increases with elevation of the pH. Sodium is unlikely to be bound to the metal-ion transport site, because metal ions do not compete with sodium on the slip current and elevation of the metal concentration did not affect the inhibition of their transport by sodium.36 Therefore, sodium is likely to compete with protons on the proton-binding site and to generate a sodium slippage through the proton transport pathway.45 The evolutionary and physiological significance of this phenomenon could be explained in terms of a protection mechanism against overloading the cell with metal ions. Apparently the evolution of the system could not provide an alternative driving force for the proton electrochemical gradient. Considering that excess metal-ions is toxic, a protection mechanism against too much transport of these elements had to be developed in DCT1 as well as in Smf1p. Several kinds of food products are highly enriched in iron and eating too much of them can also cause heartburn. The excess acid may reach the duodenum together with high iron concentrations and this combination of very high driving force and substrate abundance may be deleterious. Uncoupling by a built-in proton slip could protect the organism from too much metal-ions intake. It was suggested that a similar protection might function in the sodium slip through the yeast Smf1p.26 In this case the yeast cells may be protected against excessive influxes of toxic metal ions by evolving a sodium slippage that competes with the metal ion uptake under conditions of increasing salt concentrations in the medium.
Expression of Heterologous Metal-Ion Transporters in Yeast Cells
The expression systems of Xenopus oocyte and mammalian cells, currently used to express metal-ion transporters, provided invaluable data necessary for understanding the mode of action of the transporters. However, those systems are not adequate for studies of other features like suppressor mutants, for instance, for which bacteria or yeast provide an ideal experimental system.
We utilized the SMF triple mutant (smf1,2,3Δ) generated in our laboratory that fails to grow on buffered YPD at pH 7.5 or on EGTA, to complement the growth deficiency by the different SMF family members from yeast mammals and other sources.27 The work of Pinner et al.,46 demonstrated that Nramp2 can complement the lack of growth of the double knockout yeast mutant (smf1,2Δ) in the presence of EGTA. This showed that yeast null mutants lacking the SMF metal ion transporters could be used not only for studying yeast transporters but also their mammalian homologues. All the expressed transporters we tagged by appropriate amino acid sequences to enable immunological detection.27 Using this property, the assembly of the transporters into the plasma membrane could be followed. Each gene that successfully complemented one of the triple mutant deficiencies, was subjected to site directed mutagenesis. The cDNAs of those that yielded an inactive transporter that reached the plasma membrane, were subjected to random mutagenesis to obtain suppressor mutants as previously described.47 The suppressor mutants were expressed in Xenopus oocytes, where their transport mechanism was studied. That way we identified that the first outer loop is connected to the substrate and proton binding sites of DCT1 and were able to change the metal-ion specificity of the transporter.48
Studies with yeast mutants expressing bacterial metal ion transporters (NRMIT) may provide an amenable system for discovering new drugs targeted specifically against the bacterial transporters. If indeed Nramp1 and the bacterial NRMIT compete on the metal ions inside the macrophage phagosomes a screen for drugs that inhibit NRMIT but not Nramp1 using their functional expression in yeast as an assay, could be developed. We expressed the gene encoding metal-ion transporter (NRMIT) from mycobacterium leprae in the yeast mutant smf3Δ.49 The expressed bacterial gene complemented the EGTA sensitivity of the mutant and enabled its growth in the presence of the chelating agent. Attempts to express NRMIT (MntH) from Mycobacterium tuberculosis did not yield complementation of any of the yeast SMF disruptant mutants. Next we expressed Nramp1 in smf1,2,3Δ mutant, and were able to grow the transformed yeast on YPD pH 7.5 (Fig. 3). Thus the system was ready for drug screen that might have yielded substances that specifically inhibit the bacterial MIT but not the mammalian transporter. As many good intentions this was doomed by a single good experiment that showed that null mutant in NRMIT of M. tuberculosis infected mammalian cells and propagated as usual.50,51 May be we barked on the wrong bush. There must be an additional back-up transporter in the bacteria, that has the same function as NRMIT. Indeed in Salmonella enterica Serovar Typhimurium, where recently Mn+2 was shown to be important to the virulence of the above mentioned Salmonella in Nramp1 (-) mice, the major factor for the uptake of Mn+2 into the bacteria was shown to be the Mn+2/Fe+2 ABC transporter sitABCD.52 The double mutant sitABCD/MntH (the bacterial Nramp1 homologue) showed minimal Mn+2 uptake and was sensitive to H2O2 and to the divalent metal chelator 2,2- dipyridyl and defective in proliferation in the macrophage. MntH was found to transport Mn+2 rather then Fe+2 and over expression of MntH on the background of the double mutant sitABCD/MntH suppressed the H2O2 sensitivity and improved the survival in the Nramp1 (-) macrophage. Once again Mn+2 appears to be very important player in the sensitivity or resistance to infections.
The Involvement of NRAMP in Diseases
The subject of the involvement of the Nramp family of genes in diseases was pioneered by Philippe Gros and his colleagues,14 and it will be extensively reviewed in other chapters. However, by serendipity, we have made some contribution to this area as well, that might shed light on the understanding of a disease called Pica.53 While introducing the concept of NRAMP-related proteins as metal-ion transporters, we came across a paper showing that a null mutant in MALVOLIO (NRAMP-related protein from Drosophila melanogaster) had a behavioral effect that stemmed from the loss of taste to sugar.54 This observation connected the Drosophila's metal-ion transporter to neuronal responses, which brought us to the idea to complement the mutant with a dietary supplement of manganese that indeed complemented the taste behavior.55 As we published the results, Nancy Andrews pointed out to us the relevance of those findings to the Pica disease, where the patients are relieved from their unexplained compulsory symptoms by addition of iron to their diet.
References
- 1.
- Radisky D, Kaplan J. Regulation of transition metal transport across the yeast plasma membrane. J Biol Chem. 1999;274(8):4481–4484. [PubMed: 9988676]
- 2.
- Hediger MA. Membrane permeability. The diversity of transmembrane transport processes. Curr Opin Cell Biol. 1997;9(4):543–546. [PubMed: 9261063]
- 3.
- Eide DJ. The molecular biology of metal ion transport in Saccharomyces cerevisiae. Annu Rev Nutr. 1998;18:441–469. [PubMed: 9706232]
- 4.
- Nelson N. Metal ion transporters and homeostasis. EMBO J. 1999;18(16):4361–4371. [PMC free article: PMC1171511] [PubMed: 10449402]
- 5.
- Eide D. Molecular biology of iron and zinc uptake in eukaryotes. Curr Opin Cell Biol. 1997;9(4):573–577. [PubMed: 9263657]
- 6.
- Askwith C, Kaplan J. Iron and copper transport in yeast and its relevance to human disease. Trends Biochem Sci. 1998;23(4):135–138. [PubMed: 9584616]
- 7.
- Babcock M, de Silva D, Oaks R. et al. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science. 1997;276(5319):1709–1712. [PubMed: 9180083]
- 8.
- Andrews NC, Levy JE. Iron is hot: An update on the pathophysiology of hemochromatosis. Blood. 1998;92(6):1845–1851. [PubMed: 9731040]
- 9.
- Fleming MD, Trenor CC III, Su MA. et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16(4):383–386. [PubMed: 9241278]
- 10.
- Fleming RE, Migas MC, Zhou X. et al. Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: increased duodenal expression of the iron transporter DMT1. Proc Natl Acad Sci USA. 1999;96(6):3143–3148. [PMC free article: PMC15909] [PubMed: 10077651]
- 11.
- Supek F, Supekova L, Nelson H. et al. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc Natl Acad Sci USA. 1996;93(10):5105–5110. [PMC free article: PMC39414] [PubMed: 8643535]
- 12.
- Bullen JJ. The significance of iron in infection. Rev Infect Dis. 1981;3(6):1127–1138. [PubMed: 7043704]
- 13.
- Payne SM. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1993;1(2):66–69. [PubMed: 8044465]
- 14.
- Vidal SM, Malo D, Vogan K. et al. Natural resistance to infection with intracellular parasites: Isolation of a candidate for Bcg. Cell. 1993;73(3):469–485. [PubMed: 8490962]
- 15.
- Agranoff DD, Krishna S. Metal ion homeostasis and intracellular parasitism. Mol Microbiol. 1998;28(3):403–412. [PubMed: 9632246]
- 16.
- Plant JE, Blackwell JM, O'Brien AD. et al. Are the Lsh and Ity disease resistance genes at one locus on mouse chromosome 1? Nature. 1982;297(5866):510–511. [PubMed: 7045676]
- 17.
- West AH, Clark DJ, Martin J. et al. Two related genes encoding extremely hydrophobic proteins suppress a lethal mutation in the yeast mitochondrial processing enhancing protein. J Biol Chem. 1992;267(34):24625–24633. [PubMed: 1447206]
- 18.
- Pollock RA, Hartl FU, Cheng MY. et al. The processing peptidase of yeast mitochondria: The two cooperating components MPP and PEP are structurally related. EMBO J. 1988;7(11):3493–3500. [PMC free article: PMC454850] [PubMed: 3061797]
- 19.
- Yang M, Jensen RE, Yaffe MP. et al. Import of proteins into yeast mitochondria: The purified matrix processing protease contains two subunits which are encoded by the nuclear MAS1 and MAS2 genes. EMBO J. 1988;7(12):3857–3862. [PMC free article: PMC454964] [PubMed: 2905264]
- 20.
- Witte C, Jensen RE, Yaffe Mp. et al. MAS1, a gene essential for yeast mitochondrial assembly, encodes a subunit of the mitochondrial processing protease. EMBO J. 1988;7(5):1439–1447. [PMC free article: PMC458394] [PubMed: 3044780]
- 21.
- Hawlitschek G, Schneider H, Schmidt B. et al. Mitochondrial protein import: identification of processing peptidase and of PEP, a processing enhancing protein. Cell. 1988;53(5):795–806. [PubMed: 2967109]
- 22.
- Liu XF, Supek F, Nelson N. et al. Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J Biol Chem. 1997;272(18):11763–11769. [PubMed: 9115231]
- 23.
- Paidhungat M, Garrett S. Cdc1 is required for growth and Mn2+ regulation in Saccharomyces cerevisiae. Genetics. 1998;148(4):1777–1786. [PMC free article: PMC1460114] [PubMed: 9560392]
- 24.
- Paidhungat M, Garrett S. Cdc1 and the vacuole coordinately regulate Mn2+ homeostasis in the yeast Saccharomyces cerevisiae. Genetics. 1998;148(4):1787–1798. [PMC free article: PMC1460059] [PubMed: 9560393]
- 25.
- Supek F, Supekova L, Nelson H. et al. Function of metal-ion homeostasis in the cell division cycle, mitochondrial protein processing, sensitivity to mycobacterial infection and brain function. J Exp Biol. 1997;200(Pt 2):321–330. [PubMed: 9050240]
- 26.
- Chen XZ, Peng JB, Cohen A. et al. Yeast SMF1 mediates H(+)-coupled iron uptake with concomitant uncoupled cation currents. J Biol Chem. 1999;274(49):35089–35094. [PubMed: 10574989]
- 27.
- Cohen A, Nelson H, Nelson N. The family of SMF metal ion transporters in yeast cells. J Biol Chem. 2000;275(43):33388–33394. [PubMed: 10930410]
- 28.
- Segal AW, Abo A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci. 1993;18(2):43–47. [PubMed: 8488557]
- 29.
- Chan J, Xing Y, Magliozzo RS. et al. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175(4):1111–1122. [PMC free article: PMC2119182] [PubMed: 1552282]
- 30.
- Cellier M, Prive G, Belouchi A. et al. Nramp defines a family of membrane proteins. Proc Natl Acad Sci USA. 1995;92(22):10089–10093. [PMC free article: PMC40741] [PubMed: 7479731]
- 31.
- Makui H, Roig E, Cole ST. et al. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol. 2000;35(5):1065–1078. [PubMed: 10712688]
- 32.
- Zwilling BS, Kuhn DE, Wikoff L. et al. Role of iron in Nramp1-mediated inhibition of mycobacterial growth. Infect Immun. 1999;67(3):1386–1392. [PMC free article: PMC96472] [PubMed: 10024586]
- 33.
- Kuhn DE, Baker BD, Lafuse WP. et al. Differential iron transport into phagosomes isolated from the RAW264.7 macrophage cell lines transfected with Nramp1Gly169 or Nramp1Asp169. J Leukoc Biol. 1999;66(1):113–119. [PubMed: 10410998]
- 34.
- Kuhn DE, Lafuse WP, Zwilling BS. Iron transport into mycobacterium avium-containing phagosomes from an Nramp1(Gly169)-transfected RAW264.7 macrophage cell line. J Leukoc Biol. 2001;69(1):43–49. [PubMed: 11200066]
- 35.
- Goswami T, Bhattacharjee A, Babal P. et al. Natural-resistance-associated macrophage protein 1 is an H/bivalent cation antiporter. Biochem J. 2001;354(Pt 3):511–519. [PMC free article: PMC1221682] [PubMed: 11237855]
- 36.
- Sacher A, Cohen A, Nelson N. Properties of the mammalian and yeast metal-ion transporters DCT1 and Smf1p expressed in Xenopus laevis oocytes. J Exp Biol. 2001;204(Pt 6):1053–1061. [PubMed: 11222124]
- 37.
- Mulero V, Searle S, Blackwell JM. et al. Solute carrier 11a1 (Slc11a1; formerly Nramp1) regulates metabolism and release of iron acquired by phagocytic, but not transferrin-receptor-mediated, iron uptake. Biochem J. 2002;363(Pt 1):89–94. [PMC free article: PMC1222455] [PubMed: 11903051]
- 38.
- Atkinson PG, Barton CH. High level expression of Nramp1G169 in RAW264.7 cell transfectants: Analysis of intracellular iron transport. Immunology. 1999;96(4):656–662. [PMC free article: PMC2326790] [PubMed: 10233755]
- 39.
- Jabado N, Jankowski A, Dougaparsad S. et al. Natural resistance to intracellular infections: Natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med. 2000;192(9):1237–1248. [PMC free article: PMC2193348] [PubMed: 11067873]
- 40.
- Jabado N, Canonne-Hergaux F, Gruenheid S. et al. Iron transporter Nramp2/DMT-1 is associated with the membrane of phagosomes in macrophages and Sertoli cells. Blood. 2002;100(7):2617–2622. [PubMed: 12239176]
- 41.
- Gunshin H, Mackenzie B, Berger UV. et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388(6641):482–488. [PubMed: 9242408]
- 42.
- Wright EM, Loo DD, Panayotova-Heiermann M. et al. ‘Active’ sugar transport in eukaryotes. J Exp Biol. 1994;196:197–212. [PubMed: 7823022]
- 43.
- Loo DD, Hirayama BA, Gallardo EM. et al. Conformational changes couple Na and glucose transport. Proc Natl Acad Sci USA. 1998;95(13):7789–7794. [PMC free article: PMC22758] [PubMed: 9636229]
- 44.
- Tandy S, Williams M, Leggett A. et al. Nramp2 expression is associated with pH-dependent iron uptake across the apical membrane of human intestinal Caco2 cells. J Biol Chem. 2000;275(2):1023–1029. [PubMed: 10625641]
- 45.
- Nelson N, Sacher A, Nelson H. The significance of molecular slips in transport systems. Nat Rev Mol Cell Biol. 2002;3(11):876–881. [PubMed: 12415305]
- 46.
- Pinner E, Gruenheid S, Raymond M. et al. Functional complementation of the yeast divalent cation transporter family SMF by NRAMP2, a member of the mammalian natural resistance-associated macrophage protein family. J Biol Chem. 1997;272(46):28933–28938. [PubMed: 9360964]
- 47.
- Supek F, Supekova L, Nelson N. Features of vacuolar H(+)-ATPase revealed by yeast suppressor mutants. J Biol Chem. 1994;269(42):26479–26485. [PubMed: 7929369]
- 48.
- Cohen A, Nevo Y, Nelson N. The first external loop of the metal-ion transporter DCT1 is involved in metal-ion binding and specificity. Proc Natl Acad Sci USA. 2003;100:10694–10699. [PMC free article: PMC196866] [PubMed: 12954986]
- 49.
- Reeve I, Hummel D, Nelson N. et al. Overexpression, purification, and site-directed spin labeling of the Nramp metal transporter from Mycobacterium leprae. Proc Natl Acad Sci USA. 2002;99(13):8608–8613. [PMC free article: PMC124330] [PubMed: 12077319]
- 50.
- Domenech P, Pym AS, Cellier M. et al. Inactivation of the Mycobacterium tuberculosis Nramp orthologue (mntH) does not affect virulence in a mouse model of tuberculosis. FEMS Microbiol Lett. 2002;207(1):81–86. [PubMed: 11886755]
- 51.
- Boechat N, Lagier-Roger B, Petit S. et al. Disruption of the gene homologous to mammalian Nramp1 in Mycobacterium tuberculosis does not affect virulence in mice. Infect Immun. 2002;70(8):4124–4131. [PMC free article: PMC128187] [PubMed: 12117920]
- 52.
- Boyer E, Bergevin I, Malo D. et al. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2002;70(11):6032–6042. [PMC free article: PMC130432] [PubMed: 12379679]
- 53.
- Moore DF Jr, Sears DA. Pica, iron deficiency, and the medical history. Am J Med. 1994;97(4):390–393. [PubMed: 7942944]
- 54.
- Rodrigues V, Cheah PY, Ray K. et al. Malvolio, the Drosophila homologue of mouse NRAMP-1 (Bcg), is expressed in macrophages and in the nervous system and is required for normal taste behaviour. EMBO J. 1995;14(13):3007–3020. [PMC free article: PMC394361] [PubMed: 7621816]
- 55.
- Orgad S, Nelson H, Segal D. et al. Metal ions suppress the abnormal taste behavior of the Drosophila mutant malvolio. J Exp Biol. 1998;201(Pt 1):115–120. [PubMed: 9390942]
- Introduction
- Discovery of the Yeast Smf1p as a Metal-Ion Transporter Revealed that Metal Ions Function Through NRAMP in Resistance and Sensitivity to Bacterial Infection
- A Glimpse into the Mechanism of Metal-Ion Uptake
- Expression of Heterologous Metal-Ion Transporters in Yeast Cells
- The Involvement of NRAMP in Diseases
- References
- Metal-ion Transporters— From Yeast to Human Diseases - Madame Curie Bioscience D...Metal-ion Transporters— From Yeast to Human Diseases - Madame Curie Bioscience Database
- Class II Fusion Proteins - Madame Curie Bioscience DatabaseClass II Fusion Proteins - Madame Curie Bioscience Database
- Expression of Hox Genes in the Nervous System of Vertebrates - Madame Curie Bios...Expression of Hox Genes in the Nervous System of Vertebrates - Madame Curie Bioscience Database
- The Function of Toll-Like Receptors - Madame Curie Bioscience DatabaseThe Function of Toll-Like Receptors - Madame Curie Bioscience Database
- Gastroenterologic and Hepatic Diseases - Madame Curie Bioscience DatabaseGastroenterologic and Hepatic Diseases - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...