NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
This review focuses on the RNAs of HDV, with emphasis on RNA structure, RNA transcription, and post-transcriptional RNA processing. Included is an evaluation of two current models of HDV RNA replication.
Introduction and Scope
Hepatitis delta virus (HDV) was first discovered in 1977 through the work of Rizzetto and coworkers.1 Around 1986 several other labs began to work on the molecular virology of this agent and over and over, HDV has provided us with intriguing and unique phenomena in molecular virology. There are still important questions that need to be resolved. However, as a problem of natural infections in humans, HDV is apparently slowly "vanishing"2 (see Chapter 2 in this book).
The molecular biology of the HDV RNAs and their mechanism of replication depend upon the production of two related virus-encoded proteins, the small and large forms of the delta antigen (δAg), referred to here as δAg-S and δAg-L, respectively. The properties of these essential proteins are the focus of Chapter 5. In the present chapter, the focus will be on the RNAs of HDV, with consideration of such features as structure, transcription, post-transcriptional processing, and stabilization. The reader might also want to consider earlier reviews of these topics.3-10
The RNAs
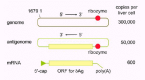
Many different complete HDV RNA sequences have now been reported.11-13 Most sequences are at or about 1,679 nucleotide (nt) in length. We will in this review use the numbering system of Kuo et al.13 The origin of this numbering is indicated in Figure 1. For the genomic RNA the numbering increases for the 5'-3' direction. For the antigenome, the numbering decreases for the 5'-3' direction.
Consider now the three HDV RNA species that get the most attention. As diagrammed in Figure 1, they are the genome, the antigenome, and the mRNA. The RNA species that is assembled into virus particles is, by definition, the genome. It is a single-stranded RNA with a circular conformation. Within cells where this genome is replicating there is also present typically 5-20 fold lower amounts of the antigenome, an exact complement of the genome. The third RNA species is of the same polarity as the antigenome. It is linear, 5'-capped14 and 3'-polyadenylated.15 Its length of about 800 nt spans the open reading frame for δAg and is considered to be its mRNA. As will be explained in more detail, not just the mRNA but all three of these RNAs have undergone one or more forms of post-transcriptional RNA processing.
Actually, during HDV RNA replication there are minor amounts of yet other processed RNAs. These include relatively low amounts of dimers and even trimers of the unit-length, for both genomic and antigenomic polarity.16 For these multimers as well as for the monomers, the majority of the RNA is in a circular conformation.
The 5'-end of the mRNA was initially mapped at position 1631 using primer extension assays.15 In later studies using 5'-RACE procedures, it was mapped to position 1630.17 This is the predominant 5'-end but there seem to be other sites that are less abundant and less specific.17 Uncertainty arises because in many of the early studies the mRNA was of low abundance, typically <2% relative to the antigenomic RNA. At the right side of Figure 1 is indicated what has been deduced for the number of the three HDV RNAs per average hepatocyte in an HDV infected liver.16
While the unit-length genomic and antigenomic RNAs are primarily in a circular conformation, there are unit-length linear forms and their nature may be complex. Some may be species whose ends have been defined by ribozyme cleavage and have yet to be circularized. Others may be circles that have been reopened by ribozyme cleavage or exonuclease action. In one study, many 5'-ends of linear genomic monomers detected within the liver of an infected animal were mapped to a specific site that was not a site of ribozyme cleavage.18 As considered later, the site is more likely to be a site of endonucleolytic opening on preformed circles than a site of initiation of transcription.
Yet another class of HDV RNAs must exist. These are the unprocessed and partially processed linear RNAs of both genomic and antigenomic polarity. Some studies have detected species of much greater than unit-length that may be examples of unprocessed nascent transcripts.19 Presumably because of their transitory nature and/or the techniques used, the unprocessed species are more difficult to detect and characterize. Such RNAs should include species containing the anticipated 5'-ends of nascent transcripts. However, at this time we have no data for the detection of 5'-ends for genomic RNAs. As explained below, the 5'-end of the mRNA species might correspond to an initiation site of antigenomic RNA.
RNA Structure
The first complete sequence of HDV genomic RNA provided not only evidence that (at least some of) this RNA was circular in conformation, but also the computer prediction that the RNA has the ability to fold on itself, into what has become known as "an un-branched rod-like structure".11 The genomic RNA is predicted to have 74% base pairing, and a negative free energy of 805 kcal.13 Other studies have used electron microcopy to detect the rod-like structure.20 Also, using electrophoresis under nondenaturing conditions, the HDV RNA behaves as if it were double-stranded.21 As will be subsequently considered (and in Chapter 6), the action of RNA-editing also supports the interpretation that at least part of the antigenomic HDV RNA can fold into the rod-like structure. Yet another piece of circumstantial evidence is derived from the observation that the circular RNA, once formed, is not recleaved. One interpretation, is that the ribozyme domain is now inactivated by being forced to adopts the rod-like folding. However, even with all this evidence in support of the potential to fold into such a structure, it must be pointed out that we do not have direct proof that this structure predominates in vivo.
Even though there is a tendency towards ascribing a single rod-like structure to HDV RNAs we must make clear that multiple structures are not only possible but probably also essential. For the case of the plant viroids, there are elegant studies supportive of the concept of metastable RNA structures. For example, with potato spindle tuber viroid (PSTVd) in addition to a predicted rod-like folding there is also a predicted hairpin structure that is not present on that rod.22 Good genetic evidence shows that this hairpin must be able to form for PSTVd replication to occur. Therefore it should come as no surprise that HDV RNAs also need more than one structure. For example, the folding of the ribozyme domains on both the genome and antigenome are in no ways like that of the rod-like structure. No doubt other examples of metastable states for HDV RNAs will be found.
In addition to the known structural features of HDV RNAs there are also features that at this time can only be inferred from the primary sequence. Specifically, there are patches of G and C that have been noted by Branch and co-workers.23 No significance for these has been shown as yet, although it has been speculated that they might facilitate some aspect of transcription, such as initiation.
One approach to understanding intra-molecular structure of HDV RNAs, genomic and antigenomic, has been to test cross-linking produced by UV irradiation. Initially, this strategy led to the definition of a genomic RNA site, referred to as an E-loop motif.24 More recent studies have found a cross-linkable site on antigenomic RNA, but it is not of the same motif.25 It should be noted that such cross-linking has been previously defined for other RNAs such as the viroid PSTVd and the host cell 5S RNA.26 However, for both HDV and the viroids, the biological relevance is not proven.
Studies have been reported of the binding to HDV RNA sequences of the protein kinase PKR. These studies were performed in vitro and with less than full-length HDV RNA sequences. It was concluded that a region of the antigenomic RNA folded into a structure other than the rod-like structure, that under certain conditions was needed for PKR binding.27 At this time, there are data both for and against the relevance of PKR activation for HDV.27
One important comment regarding HDV RNA structure and processing, is that the initial structure for the HDV RNAs might be dictated by transcription. Others have referred to this phenomenon as "co-transcriptional folding". Specifically, it is proposed that structures that can be achieved during transcription might actually be different from those detected for the folding-refolding of complete and processed RNAs.28
Experimental Systems for the Initiation of HDV Replication
The original experimental studies of HDV replication were carried out using infections of chimpanzees.29 Soon it was realized that HDV would also replicate in woodchucks, if these animals were coinfected with woodchuck hepatitis virus, WHV, which is very similar to HBV.30 Later, it was even found that after injection of natural virus, even some of the hepatocytes of a mouse could be infected.31 More recently, one study implanted human hepatocytes beneath the kidney capsule of a mouse and subsequently showed that the animal could now be infected via an i.v. injection with HDV (or HBV).32
Separate studies showed that infection could be achieved for primary cultures of hepatocytes of primate or woodchuck origin.33,34 Such primary cultures are expensive and difficult to establish. Therefore, it should not be surprising that many labs moved to more convenient systems.
The first simplified system was to construct expression vectors with tandem dimers or trimers of unit-length cDNA clones of HDV sequences. These were then transfected into established cell lines and HDV RNA replication was achieved.35 Soon it was realized that at early times after transfection, most of the detected unit-length HDV RNA, be it genomic or antigenomic, was DNA-directed rather than RNA-directed. For this reason cDNA constructs were reduced to sizes just larger than unit-length. In this way it was possible to be sure that the accumulation of unit-length transcripts was based on RNA-directed rather than DNA-directed transcription.21
Another strategy was to transcribe HDV RNA sequences in vitro, and then transfect these into cells. This has never led to HDV replication. However, if the transfected RNA enters a cell already expressing the δAg-S, then replication can be initiated.36 Similarly, if the transfected RNA is already in a complex with δAg, then replication can be initiated. This can be a complex between in vitro transcribed RNA and recombinant δAg.37 However, it also possible to use ribonucleoprotein complexes released from natural virus, or even the intact virus itself.38
In 1998, Lai and co-workers showed that these direct sources of δAg could be replaced by an indirect source. They found that when mRNA for δAg was cotransfected along with HDV RNA of greater than unit-length, HDV replication occurred.39 In this method, δAg-S must be translated before it can contribute to HDV genome replication. This experimental strategy uses the mRNA species in amounts 100-fold more relative to antigenomic than would be expressed in a natural infection. Not surprisingly then, the HDV genome replication is faster and more extensive.
It should also be noted that it is possible to use recombinant HDV sequences to infect animals. The first example was to transfect a HDV cDNA construct into the liver of an HBV infected chimp.40 This has been repeated with woodchucks.41 Another variation has been to use a hydrodynamics-based transfection of a mouse with either cDNA or even a combination of greater than unit-length RNA and mRNA.42
While many different methods have been developed to study aspects of HDV replication, one has to be concerned that some transfection strategies may give answers very different from what happens in natural infections. One should expect that the stoichiometry of δAg molecules per molecule of RNA template might be an important parameter of HDV replication.43 It might also be that this antigen might have to be newly formed. For example, we know that this protein can accumulate with time, various levels of phosphorylation,44 which in turn could change the biological properties of the protein (as discussed in Chapter 5).
Now the virus particles that are assembled during a natural infection will contain RNA genomes that have accumulated sequence changes. Some of these changes will compromise the replication competence of RNA. For one, there is the ADAR editing (Chapter 6), which can be >50% and leads to HDV RNAs that can no longer achieve synthesis of the essential δAg-S. For another, there are mis-incorporations that arise during RNA-directed RNA replication. The frequent change at nt 1375 might be of this class.43 A serious consequence of these and other changes is that a large fraction of the assembled genomes are not fully replication competent.
This accumulation of compromising changes arises not only during natural infections but will also apply to HDV particles assembled by cotransfection of cells with plasmids to express the envelope proteins of the helper virus, HBV.
Table 1 summarizes various experimental systems that have been used to study HDV replication. Certainly the choice of experimental strategy used to initiate HDV replication can depend upon the question being asked. However, as we move to questions, such as how particles can attach and enter into susceptible cells, it will become more relevant to use replication-competent virions and naturally occurring susceptible cells.
Table 1
Partial list of experimental systems for studying HDV genome replication.
RNA-Directed Transcription
For some time it has been clear that HDV genome replication does not involve DNA intermediates, and must therefore use RNA-directed transcription.16 However, the next step of clarifying which polymerase(s) are used for this transcription has remained largely unsolved and even then, controversial. Currently there are three types of evidence for the involvement of the host RNA polymerase II.
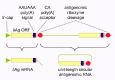
The first class of evidence, albeit circumstantial, is that one of the HDV RNAs looks like a pol II transcript. The mRNA has at least three characteristics that provide a good circumstantial argument for pol II involvement. This mRNA has a 5'-cap, a 3'-poly(A), and on the presumptive antigenomic RNA precursor, one can see essential signals for polyadenylation, namely, a AAUAAA signal, 3' of this a CA acceptor site for poly(A) addition, and further 3', a short sequence rich in G and C.15 These are all features seen for the polyadenylated mRNA of the host cell. (These features are indicated in Fig. 2).
The second class of evidence is linked up with the use of the polymerase inhibitor amanitin. At relatively low doses this inhibitor will specifically block DNA-directed RNA transcription by pol II. Higher doses are needed to block pol III and even higher doses to block pol I.45 Studies with several different experimental approaches, but in all cases involving amanitin, agree that such low doses can block the transcription and accumulation of both the HDV mRNA and of genomic RNA. (As will be discussed, different results have been reported for the inhibition of new antigenomic RNA.)
A third class of evidence is now being achieved by immuno-precipitation strategies. One lab first showed that δAg could be bound to the HDV RNA by formaldehyde treatment of intact cells in which HDV replication was occurring.46 Then, in a similar but more difficult experiment, they were able to show that pol II could be cross-linked in vivo to HDV RNAs (Niranjanakumari, Lasda, Brazas, and Garcia-Blanco, personal communication). One can expect that this approach will be used to test whether polymerases other than pol II can be found bound to HDV RNAs. It may also be able to capture other host proteins involved in the transcription and processing of HDV RNAs.
There is a fourth type of evidence that is realized to be necessary. These are in vitro studies in which attempts are made to get nuclear extracts or even better, purified polymerases, to act on added HDV RNAs. Certain positive results were reported but subsequently could not be reproduced.47 Other studies should be viewed as at best, partially positive. These are studies in which added HDV RNAs were able to act as templates but the transcription was exclusively 3'-end addition and the lengths of the added sequences were very short, typically <50 nucleotides.14,48,49 In one such study, a 3'-addition was achieved after a site-specific endonucleolytic cut on the HDV RNA produced the relevant 3'-OH site.49 In another set of studies, δAg was added to this in vitro transcription, and it was shown that longer additions were achieved.50,51 This effect is considered in more detail in Chapter 8.
However, in all these in vitro studies there has yet to be demonstrated reproducible and extensive transcription for an added HDV RNA template. Also needed is clear evidence for the initiation of transcription, if in fact this can be achieved. In one study it was claimed that initiation was achieved but the data do not clearly demonstrate this.48
Now we have to consider a serious complication to our understanding of HDV transcription. Two studies have reported that the transcription from a genomic RNA template to make antigenomic RNA involves a polymerase other than pol II.19,52 These data are based solely on what is interpreted as a resistance of the transcription to high doses of amanitin; for DNA-directed RNA transcription in animal cells this is usually an indication of transcription by pol I.45 It should be noted that such antigenomic RNAs are significantly less abundant than genomic RNA, and thus more difficult to detect. From these reports the authors have proposed that pol I is involved, and they have presented a model in which HDV replication involves the incoming genomic RNA acting first as a template for pol II to make a mRNA precursor and then for this same RNA, to act as a template for the non-pol II enzyme to transcribe multimers of antigenomic RNA (Fig. 4). This model is discussed later.
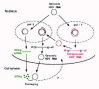
Figure 4
Macnaughton et al. model of HDV replication. See text for explanation. This is the model of Macnaughton et al and is shown here with permission.
In studies of HDV it has often been valuable to consider the analogy between HDV and the plant viroids.8 Just one aspect of the analogy is that these agents, like HDV, are totally dependent on parasitizing a host RNA polymerase to achieve transcription of their genomes. This being said, can we gain insights for HDV, by considering which host polymerases are used to transcribe viroid RNAs? Two different polymerases have been implicated. One class of viroids, of which potato spindle tuber viroid (PSTVd) is the prototype, are considered to be transcribed by the plant pol II.53 The second class, with avocado sunblotch viroid (ASBVd) as the prototype, are considered to use a nuclear-encoded RNA polymerase that normally acts in the plant chloroplast.54
In the above discussion of HDV and viroid transcription it is accepted that a host RNA polymerase that normally uses DNA as a template can be redirected to carry out transcription on an RNA template. If this is indeed true, we still need to understand what it is that makes these RNAs able to so achieve the redirection. Some authors have tried to define what might be a promoter element on the HDV RNAs.48 Most studies have accepted the possibility that the 5'-end of the mRNA could well be a unique initiation site, predominantly at position 163017 on the 1679 nt sequence of Kuo et al.13
Some comment needs to be made regarding the possible existence of a mammalian RNA polymerase that does not need to be redirected. Several years ago an RNA-directed RNA polymerase was purified from a plant.55 Soon after this polymerase was finally cloned, it was realized that all plants contain at least one copy of such a gene.56 Next it was realized that this polymerase can play an essential role in the amplification of small interfering RNAs (siRNA).57 However, there is as yet no evidence that such a polymerase is involved in viroid replication. In addition, in mammalian genomes a sequence for such a polymerase has not yet been found nor has such an activity been detected.
Template-Switching and Recombination
For some positive-strand RNA viruses it is known that during replication there can be an inter-molecular template-switching.58 This is a form of recombination. In contrast, such recombination has not been found for negative-strand viruses. HDV is essentially negative-stranded since the only open-reading frame is on the antigenome. An unsuccessful attempt was made to detect such recombination between genetically marked HDV RNAs replicating in cultured cells (Wu and JMT, unpublished observations). As an alternative approach, another group studied HDV RNAs in patients infected with more than one form (genotype) of HDV. Their evaluation of the RNA sequences present in such patients is that intermolecular recombination could have occurred.59 It is worth noting that claims have been made for intermolecular recombination between viroids, but it must be remembered that viroids are much smaller and have no open reading frame.
Another form of template-switching is intra-molecular rather than inter-molecular. Such switching is an essential part of the life cycle for many viruses, such as retroviruses and hepadnaviruses. It could be reasonably argued that such events are not needed for HDV if the only templates used are circular. That is, rolling-circle models such as those in Figures 3 and 4, would seem to obviate the need for template-switching. As an alternative opinion, it is thus relevant that a recent study in which replication was forced to initiate with HDV RNAs that were not circular but linear, was able to prove that template-switching could occur.60 Consistent with this interpretation was that for some linear RNAs that were almost exactly unit-length, there arose circular RNA transcripts that had undergone at the discontinuity site, small deletions of HDV sequences and even insertions of nonHDV sequences. In such cases, the template switching is considered to have been imprecise.
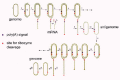
Figure 3
Taylor et al. model of HDV replication. See text for explanation. This figure represents further adaptation of a model first presented in 199998 and then adapted in 2000. It is shown here with permission.
Post-Transcriptional Processing
The three main RNAs of HDV, as represented in Figure 1, are all the products of post-transcriptional RNA processing. Both the genome and antigenome contain ribozyme domains. Thus greater than unit-length multimers of genomic and antigenomic RNA can undergo self-cleavage, followed by ligation, to produce unit-length circles. Antigenomic RNAs can undergo three additional forms of RNA-processing. The 5'-end of the mRNA can be capped. The 3'-end of the mRNA is defined by poly(A) processing. And, for some of the antigenomic RNAs, they can be targets for RNA-editing by a host adenosine deaminase.
It is thus obvious that HDV RNAs offer some very interesting cases of post-transcriptional RNA processing. In addition, such processing has to be regulated. And, after the RNAs have been processed to their mature forms, there is the chance for additional processing by host endonucleases. However, as will be explained, there seems to be no involvement of dicer, a key endonuclease involved in the generation of small interfering RNAs (siRNA).
As explained below and in Figure 2, a good example for discussion of HDV RNA processing is to consider what events can occur on a nascent antigenomic RNA. (Relative to this, discussion of genomic RNA processing has two problems. First, we have no idea of where the transcripts start and second, there is not the complication of poly(A) processing.)
Polyadenylation
For a long time we have known that during replication of HDV RNA there arises a less than full-length RNA.16 There is indirect evidence that this RNA is 5'-capped14 and there is direct evidence that it is 3'-polyadenylated.15 Puzzling features surround this mRNA.
The first concerns its abundance. In most studies the amount of this mRNA relative to the antigenomic RNA is 2% or less. In some experimental situations, the mRNA even needs to be detected by a PCR procedure rather than by poly(A)-selection and Northern analysis. There are two obvious explanations. The processing may be relatively inefficient. Alternatively, the RNA, once processed, may be relatively unstable.
It is plausible that the polyadenylation may be much less than 100% efficient. After all, the transcripts are being made from a RNA rather than a DNA template. How does this polyadenylation compare with the typical polyadenylation of DNA-directed RNA transcripts? In early experiments this question was addressed but at the time, there was a much less clear picture of what is required. Now for example, we would consider it likely that transcription will proceed much further beyond the CA acceptor site before there is (i) pausing, (ii) separation of the polymerase from the template, (iii) endonucleolytic cleavage of the paused RNA, and (iv) specific cleavage at the CA site, (v) finally followed by polyadenylation. On top of this standard picture, we have to superimpose the consequences of there being for HDV, a ribozyme located just 3' of the CA acceptor. (Some of these features are indicated in Fig. 2.) Does this interfere with transcriptional pausing? Does it reduce the efficiency of the polyadenylation? More experiments are needed.
Ribozyme Cleavage
For some time, we have known that both the genomic and antigenomic RNAs contain a domain that will act as a ribozyme.61,62 These two ribozymes can be reduced to a minimal contiguous sequence of about 85 nt.63 Many studies have been made to characterize these ribozymes both in vivo and in vitro. The cleavage reaction is a trans-esterification reaction leading to the production of ends with a 5'-OH and a 2', 3'-cyclic monophosphate.61 The two ribozymes share many sequence features although as enzymes, they have some quite different properties.64 An atomic structure has been determined for the genomic ribozyme.65 This has provided the basics for some very detailed studies of the mechanism involved in the cleavage. The HDV ribozymes are discussed in more detail in Chapter 7.
Studies have been reported to address the question of whether HDV genome replication can proceed in the absence of one or the other of these two ribozymes.66,67 The studies so far indicate no accumulation of processed RNAs when either ribozyme is inactivated. In some ways this is puzzling because for many of the plant viroids, the replication does not demand that both strands are processed to unit-length circular species; that is, it can be that just one polarity has such circular RNAs.
Several studies have addressed how sequences outside the ribozyme domains, can affect activity. In vitro, there is no question that adjacent 5' or 3' sequences can interfere with the activity of a ribozyme domain.62,68 It is interpreted that such extra sequences allow alternative foldings that are inconsistent with a fully active ribozyme.69 In vivo studies support the interpretation that alternative folding of the ribozyme domain into the predicted rod-like structure interferes with the ribozyme. Furthermore, it has been rationalized that without this inhibition, HDV RNA circles, once formed, would then self-cleave to become linears. The other side of the rod-like structure is thus considered to act as a cis-acting attenuator of the ribozyme. As further support of this model, a short RNA sequence which can 100% base pair with the attenuator can act as a trans-acting anti-attenuator, and allow circles to self-cleave.69 The anti-attenuator concept has been shown to even work during HDV RNA transcription in vitro by a phage polymerase. Specifically, a DNA oligonucleotide can function as an anti-attenuator to allow >90% in vitro cleavage during transcription (Lazinski, personal communication, with confirmation by Nie and JMT, unpublished observations). A corollary of this is that a small oligonucleotide which binds to the ribozyme, can act as an attenuator during transcription, giving >90% inhibition of self-cleavage.70 Further experiments are needed to test the effect of such trans-acting oligonucleotides on replication in vivo.
Crcularization
The genomic and antigenomic RNAs of HDV are the only examples of animal virus RNAs that are circular in conformation. There are data to support the hypothesis that such circularity provides an advantage in terms of RNA stability. In one controlled study using cell extracts, circles were 300-times more stable than the corresponding linear species.71
Of course, the circular conformation has been incorporated into models of HDV genome replication. It is considered that for both the genome and antigenome templated transcription, a double rolling-circle mechanism applies (as in Figures 3 and 4). Similar models were previously applied to explain the replication of some of the viroid RNAs.72
A puzzling issue remains as to how HDV RNAs, after cleavage by their ribozymes, can be circularized. One series of studies led to the speculation that a host factor, probably an RNA ligase, is needed for this circularization.73 Consistent with this hypothesis, no one has yet been able to achieve in vitro the combination of HDV ribozyme cleavage and circularization, all in the absence of any host protein. (There was a single report of this being achieved,74 but subsequently it was found that the interpretation of the data was incorrect.) In some ways this is puzzling because for other ribozymes, the combination of protein-free cleavage followed by ligation has been reported.75,76
Endonuclease Attack
Even though circularization does enhance the stability of the HDV RNAs, they are nevertheless susceptible to a level of endonucleolytic attack. Some studies have been interpreted as evidence that the presence of äAgs, which are RNA-binding proteins, can enhance the stabilization.21
One study reported that for genomic RNA present in the liver of an HDV infected woodchuck, it can be that many of the unit-length RNAs are opened up at a specific site.18 The mechanism of opening is not due to the HDV ribozyme but is otherwise unexplained.
Recently much attention has been drawn to a host-encoded endonuclease known as dicer.77 This activity, which is predominantly cytoplasmic, is able to convert double-stranded RNA to fragments of about 21 nt. These fragments are known as small interfering RNAs or siRNA. They are known to participate in a process known as RNA interference, RNAi, in which related mRNA species present in the cytoplasm can be specifically targeted for degradation.57 A recent study has looked without success for HDV-related siRNA during HDV genome replication.78 Also, from in vitro experiments with purified dicer, the HDV RNAs were again resistant.78 A major level of protection seems to be dependent on the long un-branched rod-like folding of the HDV RNAs.This folding is predicted to be 74% rather than 100% of all the nucleotides. These studies show that the resistance can be explained independent of the accessibility of the RNA to dicer, or the presence of δAg or any host protein.
It is possible to produce siRNA in vitro and then transfect these into mammalian cells, leading to targeted degradation. Already this strategy has been used to interfere with the replication of several different animal viruses.79-82 This strategy was applied using HDV-specific siRNAs, as transfected into cultured cells in which the HDV genome was replicating. It thus was possible to target the HDV mRNAs sequences and to achieve, indirectly, inhibition of the accumulation of HDV genomic and antigenomic RNAs.83
ADAR Attack
From the first studies of HDV it was realized that there were two main size classes of δAg that could be resolved by gel electrophoresis.84 These were given the obvious names of small (δAg-S) and large δAg (δAg-L). From nucleotide sequencing studies it was then noted that for some RNAs the amber codon used for the termination of translation of the 195 amino acid δAg-S, was changed to the codon was for tryptophan, allowing the translation of a larger form of δAg, with an extra 19 amino acids.85
Surprisingly, when a chimpanzee was transfected with an infectious clone encoding δAg-S, there appeared during the subsequent HDV replication, an increasing amount of δAg-L.40 This was soon shown to be due to the accumulation of a nucleotide change at the location corresponding to the middle of the amber termination codon.86 This is at position 1012 on the sequence of Kuo et al.13
Ultimately it was made clear that this change was achieved by the action on the antigenomic RNA of an RNA-editing enzyme, of the class now known as adenosine deaminases acting on RNA or ADAR.87 These ADAR, in addition to a deaminase activity, have the ability to bind to RNA molecules that are either 100% double-stranded or contain regions that are almost 100%. For HDV there is now clear evidence that the editing site is defined by a patch of rod-like structure surrounding position 1012 on the antigenome.43 The ADAR converts this adenosine to inosine. After a subsequent round of transcription the UAG amber codon is thus replaced with UGG, encoding tryptophan.
Recent studies have determined the minimum structural element needed for specific ADAR editing.88 Also, it has been possible to show, at least in one cultured cell line, that the editing enzyme is the gene product of what is known as ADAR-1. Furthermore, there are two main forms of the ADAR-1 protein in mammalian cells, and the HDV editing is via the form that is smaller and predominantly located in the nucleus.89
As explained in more detail in Chapter 5, both δAg-S and δAg-L are essential for HDV replication. The small form is essential for the initiation and maintenance of genome replication.90 The large form is essential for the assembly of HDV genomic RNA into particles with an outer envelope of HBV surface proteins.91 Thus, the appearance of the large form, as a consequence of ADAR editing, has to be considered as an essential event in the replication cycle.
There is evidence that other sites on the HDV RNAs, both genomic and antigenomic, can undergo ADAR editing.92,93 However, these other sites are less site-specific and less efficiently used. Additional evidence indicates that the presence of δAg can act as a negative regulator of ADAR editing of HDV RNA.92 In Chapter 6, ADAR-editing is discussed in greater detail.
RNA Assembly
In natural coinfections of hepatocytes with HBV and HDV, the surface proteins of HBV facilitate the assembly of HDV genomic RNA into particles. As discussed in Chapter 3, this assembly is mediated by δAg-L that has the dual abilities to interact with the HDV RNA and with the HBV envelope proteins. Assembly can also be achieved by transient transfection. Using cells transfected to express the envelope proteins of HBV, there is the assembly into virus-like particles of HDV genomic RNAs created by RNA-directed genome replication.91,94 Sureau and co-workers first showed that such assembled particles are able to infect cultures of primary hepatocytes.95 As for HBV, this infectivity depends on the presence of the large form of the HBV surface antigen.96
Models of Genome Replication
Figures 3 and 4 show the only two replication schemes that have been published for HDV replication. In this section we will consider some of the complications that can be ascribed to these schemes.
Consider first the model proposed by Macnaughton et al19 as shown in Figure 4. It incorporates features from an earlier study by Modahl and Lai39 from which it was proposed that HDV RNA replication and mRNA transcription occur independently and in parallel during the viral life cycle. Two additional features drive this model to be different from the other model. First, the authors try to incorporate their interpretation that while the mRNA and genome are transcribed by pol II, they consider that the antigenomic RNA is transcribed by a different polymerase, possibly pol I. This then leads the authors to indicate that the genomic RNA can serve as template for two polymerases; pol II copies it to produce mRNA then this same template is used by pol I. The authors do not discuss how it might be that the same template can be transcribed by alternative polymerases. The second feature that the model tries to incorporate is the separate finding by these authors97 of processed unit-length genomic RNAs in the cytoplasm soon after synthesis, which is considered to occur in the nucleus. The processed antigenomic RNAs do not so move to the cytoplasm and this is interpreted in the model as a contributing factor to the observed specificity with which genomic rather than antigenomic RNA is assembled into particles.
The second model, shown in Figure 3, has been passed down following early studies in my lab.98 This model does not complicate the issue by invoking more than one polymerase or by trying to account for nuclear-cytoplasmic distributions. It focuses only on the transcription and RNA-processing. However, as will now be explained it has at least 3 serious problems.
i. Throughout its 22 steps, it is presumed (for this and the other model) that the only RNAs that can act as templates for transcription are RNA circles. As discussed earlier, this may well not be true. Many experimental studies have shown that linear forms of HDV RNA, genomic and antigenomic, can act as templates to initiate replication. It may well be that the circular forms are more stable and accumulate better, but it is not yet proven that they are the only, or even that they are better templates. It is pertinent to note here that for many of the plant viroids, RNA circles are found for only one polarity; the other polarity is represented only by linear multimers. In other words, linear RNAs can be the only choice of emplate.
ii. It is presumed that transcription on the genomic RNA template is initiated at a site corresponding to the 5'-end of the mRNA. Even if we accept this, in steps 1-4 it is proposed that first there is transcribed enough antigenomic RNA to allow the RNA-processing to produce the polyadenylated mRNA. Then in steps 5-10, following elongation of what has been called "the continuing transcript", there is generated an RNA that is greater than unit-length, and contains more than one ribozyme. It is considered that ribozyme cleavage followed by ligation leads to the formation of new antigenomic RNA circles. Since this model was first proposed much has been learned about the poly(A)-processing mechanism associated with host DNA-directed pol II transcripts.99 Apparently the transcription can proceed even 2 kb beyond the poly(A) signals before transcription is terminated and complete assembly of the poly(A) machinery leads to cleavage at the CA acceptor site and a poly(A) polymerase adds the poly(A) tail. In all this, the pol II provides essential scaffolding functions via the multiple tandem repeats in the C-terminal domain of its large protein subunit. Of relevance to HDV, is that the RNA transcripts can reach sizes greater than unit-length before poly(A)-processing (or ribozyme processing) has had a chance to occur. Recent studies of DNA-directed pol II transcripts of HDV antigenomic RNA, in fact, support this interpretation for HDV (Nie, Chang, and JMT, unpublished observations). Furthermore, rather than the concept of the continuing transcript being processed in two different ways, they instead support a new model, namely that that for each antigenomic transcript one of three mutually exclusive alternative outcomes are possible. (a) Some will become poly(A)-processed. (b) Others will be ribozyme-processed, leading to ligation and the formation of unit-length circles. (c) Yet others, and this could even be the major class, will either be not processed at all, or will be processed incompletely or in alternative ways.
iii. The third major problem is that of initiation of transcription. As discussed earlier, apart from our presumption for the 5'-end of the mRNA being an initiation site, we know nothing more about the initiation of transcription. Much more needs to be done to understand initiation sites and efficiencies for HDV genomic and antigenomic RNA templates. Maybe such information will surface from in vitro experiments when we begin to use better templates, such as ribonucleoprotein complexes released from virions.
In summary, these replication schemes contain significant flaws and also fail to show some of the inherent complexity of HDV replication. Nevertheless, they serve a valid purpose if they are able to drive us to specific experimental tests and to refinements, as proven necessary.
Conclusions and Outlook
While the significance of HDV as a threat to human health is decreasing, its interest as an intriguing problem in molecular virology continues to increase. From this and other chapters, it should be clear that we have made serious advancements in our understanding the mechanism of replication of HDV but there remain some important problems yet to be solved. Among these, the two most important may be the remaining confusion about which polymerases are used and our inability to demonstrate the initiation of RNA-directed transcription in vitro.
Acknowledgments
Constructive comments on the manuscript were given by Michael Lai, William Mason, Severin Gudima, Jinhong Chang, and Chi Tarn. This work was supported by grants AI-26522 and CA-06927 from the N.I.H., and by an appropriation from the Commonwealth of Pennsylvania.
References
- 1.
- Rizzetto M, Canese MG, Arico J. et al. Immunofluorescence detection of a new antigen-antibody system associated to the hepatitis B virus in the liver and in the serum of HBsAg carriers. Gut. 1977;18:997–1003. [PMC free article: PMC1411847] [PubMed: 75123]
- 2.
- Gaeta GB, Stroffolini T, Chiaramonte M. et al. Chronic hepatitis D: A vanishing disease? An Italian multicenter study. Hepatology. 2000;32:824–827. [PubMed: 11003629]
- 3.
- Casey JL. Hepatitis delta virus: Genetics and pathogenesis. Clin Lab Med. 1996;16:451–464. [PubMed: 8792082]
- 4.
- Gerin JL, Casey JL, Purcell RH. Hepatitis Delta Virus. In: Knipe DM, Howley PM, eds. Fields_ Virology. Vol 2. Philadelphia. Lippincott Williams & Wilkins. 2001:3037–3050.
- 5.
- Lai MMC. The molecular biology of hepatitis delta virus. Ann Rev Biochem. 1995;64:259–286. [PubMed: 7574482]
- 6.
- Taylor JM. Replication of human hepatitis delta virus: Recent developments. Trends in Microbiol. 2003;11:185–190. [PubMed: 12706997]
- 7.
- Taylor JM. Hepatitis delta virus. Intervirology. 1999;42:173–178. [PubMed: 10516472]
- 8.
- Taylor JM. Replication of human hepatitis delta virus: Influence of studies on subviral plant pathogens. Adv Vir Res. 1999;54:45–60. [PubMed: 10547674]
- 9.
- Monjardino J. Molecular Biology of Human Hepatitis Viruses. London. Imperial College Press. 1998
- 10.
- Modahl LE, Lai MM. Hepatitis delta virus: The molecular basis of laboratory diagnosis. Crit Rev Clin Lab Sci. 2000;37:45–92. [PubMed: 10737440]
- 11.
- Wang K-S, Choo Q-L, Weiner AJ. et al. Structure, sequence and expression of the hepatitis delta viral genome. Nature. 1986;323:508–513. [PubMed: 3762705]
- 12.
- Makino S, Chang MF, Shieh CK. et al. Molecular cloning and sequencing of a human hepatitis delta (ä) virus RNA. Nature. 1987;329:343–346. [PubMed: 3627276]
- 13.
- Kuo MY-P, Goldberg J, Coates L. et al. Molecular cloning of hepatitis delta virus RNA from an infected woodchuck liver: Sequence, structure, and applications. J Virol. 1988;62:1855–1861 15 Structure and Replication of Hepatitis Delta Virus RNA. [PMC free article: PMC253266] [PubMed: 3367426]
- 14.
- Gudima S, Wu S-Y, Chiang C-M. et al. Origin of the hepatitis delta virus mRNA. J Virol. 2000;74:7204–7210. [PMC free article: PMC112241] [PubMed: 10906174]
- 15.
- Hsieh S-Y, Chao M, Coates L. et al. Hepatitis delta virus genome replication: A polyadenylated mRNA for delta antigen. J Virol. 1990;64:3192–3198. [PMC free article: PMC249524] [PubMed: 1693700]
- 16.
- Chen P-J, Kalpana G, Goldberg J. et al. Structure and replication of the genome of hepatitis ä Proc Natl Acad Sci USA. 1986;virus83:8774–8778. [PMC free article: PMC387014] [PubMed: 2430299]
- 17.
- Gudima S, Dingle K, Wu T-T. et al. Characterization of the 5'-ends for polyadenylated RNAs synthesized during the replication of hepatitis delta virus. J Virol. 1999;73:6533–6539. [PMC free article: PMC112736] [PubMed: 10400749]
- 18.
- Chang J, Moraleda G, Gudima S. Efficient site-specific nonribozyme opening of hepatitis delta virus genomic RNA in infected liver. J Virol. 2000. pp. 9889–9894. [PMC free article: PMC102025] [PubMed: 11024115]
- 19.
- Macnaughton TB, Shi ST, Modahl LE. et al. Rolling circle replication of hepatitis delta virus RNA is carried out by two different cellular RNA polymerases. J Virol. 2002;76:3920–3927. [PMC free article: PMC136092] [PubMed: 11907231]
- 20.
- Kos A, Dijkema R, Arnberg AC. et al. The hepatitis delta (ä) virus possesses a circular RNA. Nature. 1986;323:558–560. [PubMed: 2429192]
- 21.
- Lazinski DW, Taylor JM. Expression of hepatitis delta virus RNA deletions: cs and trans requirements for self-cleavage, ligation, and RNA packaging. J Virol. 1994;68:2879–2888. [PMC free article: PMC236776] [PubMed: 8151758]
- 22.
- Loss P, Schmitz M, Steger G. et al. Formation of a thermodynamically metastable structure containing hairpin II is critical for infectivity of potato spindle tuber viroid RNA. EMBO J. 1991;10:719–727. [PMC free article: PMC452707] [PubMed: 2001685]
- 23.
- Branch AD, Lee SE, Neel OD. et al. Prominent polypurine and polypyrimidine tracts in plant viroids and in the RNA of the human hepatitis delta agent. Nucl Acids Res. 1993;21:3529–3535. [PMC free article: PMC331455] [PubMed: 7688455]
- 24.
- Branch AD, Benenfeld BJ, Baroudy BM. et al. An ultraviolet-sensitive RNA structural element in a viroid-like domain of the hepatitis delta virus. Science. 1989;243:649–652. [PubMed: 2492676]
- 25.
- Circle DA, Lyons AJ, Neel OD. et al. Recurring features of local tertiary structure elements in RNA molecules exemplified by hepatitis D virus RNA. RNA. 2003. 9:280–286. [PMC free article: PMC1370394] [PubMed: 12592001]
- 26.
- Branch AD, Benenfeld BJ, Robertson HD. Ultraviolet light-induced crosslinking reveals a unique region of local tertiary structure in potato spindle tuber viroid and HeLa 5S RNA. Proc Natl Acad Sci USA Oct. 1985;82:6590–6594. [PMC free article: PMC391255] [PubMed: 3863116]
- 27.
- Robertson HD, Manche L, Mathews MB. Paradoxical interactions between human hepatitis delta agent RNA and the cellular protein kinase PKR. J Virol. 1996;70:5611–5617. [PMC free article: PMC190521] [PubMed: 8764075]
- 28.
- Heilman-Miller SL, Woodson SA. Effect of transcription on folding of the Tetrahymena ribozyme. RNA. 2003;9:722–733. [PMC free article: PMC1370439] [PubMed: 12756330]
- 29.
- Rizzetto M, Canese MG, Gerin JL. et al. Transmission of the hepatitis B virus-associated delta antigen to chimpanzees. J Infect Dis. 1980;141:590–602. [PubMed: 6989929]
- 30.
- Ponzetto A, Cote PJ, Popper H. et al. Transmission of the hepatitis B virus-associated ä agent to the eastern woodchuck. Proc Natl Acad Sci USA. 1984;81:2208–2212. [PMC free article: PMC345467] [PubMed: 6585793]
- 31.
- Netter HJ, Kajino K, Taylor J. Experimental transmission of human hepatitis delta virus to the laboratory mouse. J Virol. 1993;67:3357–3362. [PMC free article: PMC237679] [PubMed: 8497056]
- 32.
- Ohashi K, Marion PL, Nakai H. et al. Sustained survival of human hepatocytes in mice: A model for in vivo infection with human hepatitis B and hepatitis delta viruses. Nature Medicine. 2000;6:327–331. [PubMed: 10700236]
- 33.
- Taylor J, Mason W, Summers J. et al. Replication of human hepatitis delta virus in primary cultures of woodchuck hepatocytes. J Virol. 1987;61:2891–2895. [PMC free article: PMC255813] [PubMed: 3612956]
- 34.
- Sureau C, Jacob JR, Eichberg JW. et al. Tissue culture system for infection with human hepatitis delta virus. J Virol. 1991;65:3443–3450. [PMC free article: PMC241326] [PubMed: 2041075]
- 35.
- Kuo MY-P, Chao M, Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: Role of delta antigen. J Virol. 1989;63:1945–1950. [PMC free article: PMC250607] [PubMed: 2649689]
- 36.
- Glenn JS, Taylor JM, White JM. In vitro-synthesized hepatitis delta virus RNA initiates genome replication in cultured cells. J. Virol. 1990;64:3104–3107. [PMC free article: PMC249503] [PubMed: 2335830]
- 37.
- Dingle K, Bichko V, Zuccola H. et al. Initiation of hepatitis delta virus genome replication. J Virol. 1998;72:4783–4788. [PMC free article: PMC110015] [PubMed: 9573243]
- 38.
- Bichko V, Netter HJ, Taylor J. Introduction of hepatitis delta virus into animal cell lines via cationic liposomes. J Viro. 1994;68:5247–5252. [PMC free article: PMC236469] [PubMed: 8035521]
- 39.
- Modahl LE, Lai MMC. Transcription of hepatitis delta antigen mRNA continues throughout hepatitis delta virus (HDV) replication: A new model of HDV RNA transcription and regulation. J Virol. 1998;72:5449–5456. [PMC free article: PMC110180] [PubMed: 9621000]
- 40.
- Sureau C, Taylor J, Chao M. et al. A cloned DNA copy of hepatitis delta virus is infectious in the chimpanzee. J Virol. 1989;63:4292–4297. [PMC free article: PMC251044] [PubMed: 2778877]
- 41.
- Rapicetta M, Ciccaglione AR, D_Urso N. et al. Chronic infection in woodchucks infected by a cloned hepatitis delta virus. In: Hadziyannis SJ Taylor JM Bonino F eds. Hepatitis Delta Virus Molecular Biology Pathogenesis and Clinical Aspects New York Wiley-Liss, 1993451.
- 42.
- Chang J, Sigal LJ, Lerro A. et al. Replication of the human hepatitis delta virus genome is initiated in mouse hepatocytes following intravenous injection of naked DNA or RNA sequences. J Virol. 2001;75:3469–3473. [PMC free article: PMC114140] [PubMed: 11238873]
- 43.
- Gudima SO, Chang J, Moraleda G. et al. Parameters of human hepatitis delta virus replication: The quantity, quality, and intracellular distribution of viral proteins and RNA. J Virol. 2002;76:3709–3719. [PMC free article: PMC136113] [PubMed: 11907210]
- 44.
- Mu JJ, Chen DS, Chen PJ. The conserved serine 177 in the delta antigen of hepatitis delta virus is one putative phosphorylation site and is required for efficient viral RNA replication. J Virol. 2001;75:9087–9095. [PMC free article: PMC114477] [PubMed: 11533172]
- 45.
- Darnell J, Lodish H, Baltimore D. Molecular Cell Biology. New York. Scientific American Books Ltd. 1986
- 46.
- Niranjanakumari S, Lasda E, Brazas R. et al. Reversible cross-linking combined with immunoprecipitation to study RNA-protein interactions in vivo. Methods. 2002;26:182–190. [PubMed: 12054895]
- 47.
- Fu T-B, Taylor J. The RNAs of hepatitis delta virus are copied by RNA polymerase II in nuclear homogenates. J Virol. 1993;67:6965–6972. [PMC free article: PMC238155] [PubMed: 8230419]
- 48.
- Beard MR, Macnaughton TB, Gowans EJ. Identification and characterization of a hepatitis delta virus RNA transcriptional promoter. J Virol. 1996;70:4986–4995. [PMC free article: PMC190452] [PubMed: 8764005]
- 49.
- Filipovska J, Konarska MM. Specific HDV RNA-templated transcription by pol II in vitro. RNA. 2000;6:41–54. [PMC free article: PMC1369892] [PubMed: 10668797]
- 50.
- Yamaguchi Y, Delehouzee S, Handa H. HIV and hepatitis delta virus: Evolution takes different paths to relieve blocks in transcriptional elongation. Microbes Infect. 2002;4:1169–1175. [PubMed: 12361917]
- 51.
- Yamaguchi Y, Filipovska J, Yano K. et al. Stimulation of RNA polymerase II elongation by hepatitis delta antigen. Science. 2001;293:124–127. [PubMed: 11387440]
- 52.
- Modahl LE, Macnaughton TB, Zhu N. et al. RNA-dependent replication and transcription of hepatitis delta virus RNA involve distinct cellular RNA polymerases. Mol Cell Biol. 2000;20:6030–6039. [PMC free article: PMC86079] [PubMed: 10913185]
- 53.
- Schindler I-M, Muhlbach H-P. Involvement of nuclear DNA-dependent RNA polymerases in potato spindle tuber viroid replication: A reevaluation. Plant Sci. 1992;84:221–229.
- 54.
- Navarro J-A, Vera A, Flores R. A chloroplastic RNA polymerase resistant to targetitoxin is involved in replication of avocado sunblotch virod. Virology. 2000;268:218–225. [PubMed: 10683343]
- 55.
- Schiebel W, Haas B, Marinkovik S. et al. RNA-directed RNA polymerase from tomato leaves. I purification and physical properties. J Biol Chem. 1993;268:11851–11857. [PubMed: 7685022]
- 56.
- Schiebel W, Pelissier T, Riedel L. et al. Isolation of an RNA-directed RNA polymerase-specific cDNA from tomato. Plant Cell. 1998;10:2087–2101. [PMC free article: PMC143969] [PubMed: 9836747]
- 57.
- Hannon GJ. RNA interference. Nature. 2002;418:244–251. [PubMed: 12110901]
- 58.
- Lai MMC. Genetic recombination in RNA viruses. Curr Topics Microbiol Immunol. 1992;176:21–32. [PubMed: 1600753]
- 59.
- Wu JC, Chiang TY, Shiue WK. et al. Recombination of hepatitis D virus RNA sequences and its implications. Mol Biol Evol. 1999;16:1622–1632. [PubMed: 10555293]
- 60.
- Chang J, Taylor J. In vivo RNA-directed transcription, with template switching, by a mammalian RNA polymerase. EMBO J. 2002;21:157–164. [PMC free article: PMC125818] [PubMed: 11782435]
- 61.
- Sharmeen L, Kuo MY, Dinter-Gottlieb G. et al. The antigenomic RNA of human hepatitis delta virus can undergo self-cleavage. J Virol. 1988;62:2674–2679. [PMC free article: PMC253699] [PubMed: 2455816]
- 62.
- Kuo MYP, Sharmeen L, Dinter-Gottlieb G. et al. Characterization of self-cleaving RNA sequences on the genome and antigenome of human hepatitis delta virus. J Virol. 1988;62:4439–4444. [PMC free article: PMC253552] [PubMed: 3184270]
- 63.
- Perrotta AT, Been MD. A pseudoknot-like structure required for efficient self-cleavage of hepatitis delta virus RNA. Nature. 1991;350:436–436. [PubMed: 2011192]
- 64.
- Shih IH, Been MD. Catalytic strategies of the hepatitis delta virus ribozymes. Ann Rev Biochem. 2002;71:887–917. [PubMed: 12045114]
- 65.
- FerreD_Amare AR, Zhou K, Doudna JA. Crystal structure of a hepatitis delta virus ribozyme. Nature. 1998;395:567–574. [PubMed: 9783582]
- 66.
- Jeng K-S, Daniel A, Lai MMC. A pseudoknot structure is active in vivo and required for hepatitis delta virus RNA replication. J Virol. 1996;70:2403–2410. [PMC free article: PMC190083] [PubMed: 8642668]
- 67.
- Macnaughton TB, Wang Y-J, Lai MMC. Replication of hepatitis delta virus RNA: Effect of mutations of the autocatalytic cleavage sites. J Virol. 1993;67:2228–2234. [PMC free article: PMC240348] [PubMed: 8445730]
- 68.
- Diegelman-Parente A, Bevilacqua PC. A mechanistic framework for cotranscriptional folding of the HDV genomic ribozyme in the presence of downstream sequence. J Mol Biol. 2002;324:1–16. [PubMed: 12421555]
- 69.
- Lazinski DW, Taylor JM. Regulation of the hepatitis delta virus ribozymes: To cleave or not to cleave. RNA. 1995;1:225–233. [PMC free article: PMC1369076] [PubMed: 7489495]
- 70.
- Mao Q, Wang S, Li Q. [Inhibition of genomic HDV ribozyme activity by antisense oligodeoxynucleotides] Zhonghua Yi Xue Za Zhi. 1996;76:30–33. [PubMed: 8758460]
- 71.
- Puttaraju M, Been M. Generation of nuclease resistant circular RNA decoys for HIV-tat and HIV-rev by autocatalytic splicing. Nucl Acids Res. 1995;33:49–51. [PubMed: 8643395]
- 72.
- Branch AD, Robertson HD. A replication cycle for viroids and small infectious RNAs. Science. 1984;223:450–455. [PubMed: 6197756]
- 73.
- Reid CE, Lazinski DW. A host-specific function is required for ligation of a wide variety of ribozyme-processed RNAs. Proc Natl Acad Sci USA. 2000;97:424–429. [PMC free article: PMC26679] [PubMed: 10618434]
- 74.
- Diegelman AM, Kool ET. Mimicry of the hepatitis delta virus replication cycle mediated by synthetic circular oligodeoxynucleotides. Chem Biol. 1999;6:569–576. [PubMed: 10421762]
- 75.
- Buzayun J, Gerlach W, Bruening G. Nonenzymatic cleavage and ligation of RNAs complementary to a plant virus satellite RNA. Nature. 1986. 323:349–353.
- 76.
- Saville B, Collins R. RNA-mediated ligation of self-cleavage products of a Neurospora mitochondrial plasmid transcript. Proc Natl Acad Sci USA. 1991;88:8826–8830. [PMC free article: PMC52603] [PubMed: 1833766]
- 77.
- Bernstein E, Caudy AA, Hammond SM. et al. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. [PubMed: 11201747]
- 78.
- Chang J, Provost P, Taylor JM. Resistance of human hepatitis delta virus RNAs to dicer activity. J Virol. 2003;77:11910–11917. [PMC free article: PMC254274] [PubMed: 14581527]
- 79.
- Bitko V, Barik S. Phenotypic silencing of cytoplasmic genes using sequence-specific double-stranded short interfering RNA and its application in the reverse genetics of wild type negative-strand RNA viruses. BMC Microbiol. 2001;1(1):34. [PMC free article: PMC64570] [PubMed: 11801185]
- 80.
- Coburn GA, Cullen BR. Potent and specific inhibition of human immunodeficiency virus type 1 replication by RNA interference. J Virol. 2002;76:9225–9231. [PMC free article: PMC136455] [PubMed: 12186906]
- 81.
- Ge Q, McManus MT, Nguyen T. et al. RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. Proc Natl Acad Sci USA. 2003;100:2718–2723. [PMC free article: PMC151407] [PubMed: 12594334]
- 82.
- Gitlin L, Karelsky S, Andino R. Short interfering RNA confers intracellular antiviral immunity in human cells. Nature. 2002;418:430–434. [PubMed: 12087357]
- 83.
- Chang J, Taylor JM. Susceptibility of human hepatitis delta virus RNAs to small interfering RNA action. J Virol. 2003;77:9728–9731. [PMC free article: PMC187427] [PubMed: 12915586]
- 84.
- Bergmann KF, Gerin JL. Antigens of hepatitis delta virus in the liver and serum of humans and animals. J Infect Dis. 1986;154:702–706. [PubMed: 3745977]
- 85.
- Weiner AJ, Choo Q-L, Wang K-S. et al. A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 and p27. J Virol. 1988;62:594–599. [PMC free article: PMC250573] [PubMed: 2447291]
- 86.
- Luo G, Chao M, Hsieh S-Y. et al. A specific base transition occurs on replicating hepatitis delta virus RNA. J Virol. 1990;64:1021–1027. [PMC free article: PMC249212] [PubMed: 2304136]
- 87.
- Casey JL, Gerin JL. Hepatitis D virus RNA editing: Specific modification of adenosine in the antigenomic RNA. J Virol. 1995;69:7593–7700. [PMC free article: PMC189698] [PubMed: 7494266]
- 88.
- Sato S, Wong SK, Lazinski DW. Hepatitis delta virus minimal substrates competent for editing by ADAR1 and ADAR2. J Virol. 2001;75:8547–8555. [PMC free article: PMC115100] [PubMed: 11507200]
- 89.
- Wong SK, Lazinski DW. Replicating hepatitis delta virus RNA is edited in the nucleus by the small form of ADAR1. Proc Natl Acad Sci USA. 2002;99:15118–15123 Hepatitis Delta Virus 18. [PMC free article: PMC137553] [PubMed: 12399548]
- 90.
- Chao M, Hsieh S-Y, Taylor J. The antigen of hepatitis delta virus: Examination of in vitro RNA-binding specificity. J Virol. 1991;65:4057–4062. [PMC free article: PMC248837] [PubMed: 1906549]
- 91.
- Chang FL, Chen PJ, Tu SJ. et al. The large form of hepatitis ä antigen is crucial for the assembly of hepatitis ä virus. Proc Natl Acad Sci USA. 1991;88:8490–8494. [PMC free article: PMC52534] [PubMed: 1924308]
- 92.
- Polson AG, Ley, III HL, Bass BL. et al. Hepatitis delta virus RNA editing is highly specific for the amber/W site and is suppressed by the delta antigen. Mol Cell Biol. 1998;18:1919–1926. [PMC free article: PMC121421] [PubMed: 9528763]
- 93.
- Netter HJ, Wu T-T, Bockol M. et al. Nucleotide sequence stability of the genome of hepatitis delta virus. J Virol. 1995;69:1687–1692. [PMC free article: PMC188769] [PubMed: 7853505]
- 94.
- Ryu W-S, Bayer M, Taylor J. Assembly of hepatitis delta virus particles. J Virol. 1992;66:2310–2315. [PMC free article: PMC289026] [PubMed: 1548764]
- 95.
- Sureau C, Moriarty AM, Thornton GB. et al. Production of infectious hepatitis delta virus in vitro and neutralization with antibodies directed against hepatitis B virus preS antigens. J Virol. 1992;66:1241–1245. [PMC free article: PMC240836] [PubMed: 1309901]
- 96.
- Sureau C, Guerra B, Lanford RE. Role of the large hepatitis B virus envelope protein in infectivity of the hepatitis delta virion. J Virol. 1993;67:366–372. [PMC free article: PMC237372] [PubMed: 8416375]
- 97.
- Macnaughton TB, Lai MM. Genomic but not antigenomic hepatitis delta virus RNA is preferentially exported from the nucleus immediately after synthesis and processing. J Virol. 2002;76:3928–3935. [PMC free article: PMC136093] [PubMed: 11907232]
- 98.
- Taylor JM. Human hepatitis delta virus: Structure and replication of the genome. Curr Top Microbiol Immunol. 1999;239:108–122. [PubMed: 9893371]
- 99.
- Dye MJ, Proudfoot NJ. Multiple transcript cleavage precedes polymerase release in termination by RNA polymerase II. Cell. 2001;105:669–681. [PubMed: 11389836]
- 100.
- Polo JM, Lim B, Govindarajan S. et al. Replication of hepatitis delta virus RNA in mice after intramuscular injection of plasmid DNA. J Virol. 1995. 69:5203–5207. [PMC free article: PMC189347] [PubMed: 7609095]
- 101.
- Flint SJ, Enquist LW, Krug RM. et al. Principles of Virology. Washington. ASM Press. 2000
- Structure and Replication of Hepatitis Delta Virus RNA - Madame Curie Bioscience...Structure and Replication of Hepatitis Delta Virus RNA - Madame Curie Bioscience Database
- Neural Crest and the Development of the Enteric Nervous System - Madame Curie Bi...Neural Crest and the Development of the Enteric Nervous System - Madame Curie Bioscience Database
- Calcium Signaling During Phagocytosis - Madame Curie Bioscience DatabaseCalcium Signaling During Phagocytosis - Madame Curie Bioscience Database
- Regulation of Cell Adhesion Responses by Abl Family Kinases - Madame Curie Biosc...Regulation of Cell Adhesion Responses by Abl Family Kinases - Madame Curie Bioscience Database
- X Chromosome Inactivation and Embryonic Stem Cells - Madame Curie Bioscience Dat...X Chromosome Inactivation and Embryonic Stem Cells - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...