NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Introduction
Cyclin-dependent kinases (CDKs) are essential regulators of the cell cycle and transcription. In the budding yeast Saccharomyces cerevisiae and the fission yeast Saccharomyces pombe, a single CDK (Cdc28p or its ortholog Cdc2, respectively) catalyzes all major cell cycle transitions.1,2 In higher eukaryotes, there has been an expansion in the number of CDKs that regulate the cell cycle. For example, in mammals, CDK4, CDK6, and CDK3 regulate the G1-S phase transition; CDK2 controls entry into S phase and DNA replication; and CDK1 (CDC2) is essential for mitosis.3–7
CDKs also regulate transcription. In budding yeast, Kin28p, Srb10p, and Ctk1p regulate mRNA synthesis by phosphorylating the carboxyl-terminal domain (CTD) of RNA polymerase II.8–11 The budding yeast CDK Sgv1p also functions to regulate transcription, but its substrates are unknown.12 In metazoa, the ortholog of Sgv1p, CDK9, is a component of the positive transcription elongation factor b (pTEFb) that promotes productive RNA Pol II transcription elongation by phosphorylating the CTD.13,14
CDK activity is highly regulated in cells. There are four major regulatory mechanisms: 1) most CDKs require binding to cyclin proteins to become active; 2) CDK activity is inhibited by the binding of cyclin-dependent kinase inhibitors (CKIs); 3) phosphorylation of conserved residues in the ATP-binding pocket of the CDK inhibits its activity; and 4) phosphorylation of a conserved residue in the T-loop of CDKs is required for the activation of most CDKs.
The activating phosphorylation of CDKs is catalyzed by a CDK-activating kinase (CAK). CAK phosphorylates a conserved serine (Ser) or threonine (Thr) site in the T-loop of the CDKs. We will refer to this site as Thr-160 (T160) based on its location in human CDK2. When not phosphorylated, the T-loop blocks the entrance of the CDK active site cleft to prevent the binding of protein substrates.15 Phosphorylation on T160 induces a conformational change in the CDK, resulting in enhanced CDK-cyclin interaction and substrate binding.16 Both cell cycle CDKs such as CDK1, CDK2, and CDK4, and transcription CDKs such as CDK7 and Kin28p have been shown to require CAK phosphorylation to be active.17–23 A loss of CAK activity leads to cell cycle arrest as well as transcription defects.22–24
The Evolution of CAKs
In this chapter, we address the evolution of CAKs in the context of the evolution of the entire CDK-family, to which known CAKs belong. The focus of the phylogenetic analyses described in this article is the assignment of orthology. Orthologous genes are direct descendents from the same ancestral gene and are only separated from each other by speciation events. Biochemical and cellular functions of orthologs are often conserved across species, and therefore orthologs are candidates for functional tests. Further, understanding orthology provides information on how central biological processes have evolved over time, e.g., whether a the CDK-activating kinase (CAK) particular cellular process is ancient and performed by orthologous genes in diverged organisms or whether different genes have evolved to perform the process. In the case of CAK evolution, it is clear that certain CAK pathways have not been conserved across all eukaryotes. The approach described in this article is to identify all CDK-family members in completed genomes and then assign orthology based on significant associations in phylogenetic trees.
Two Categories of CAKs
Two categories of CAKs have been found in eukaryotes. The first category includes two orthologous genes, budding yeast CAK1 and its fission yeast ortholog csk1. The second category contains orthologous genes from metazoa, CDK7, and fission yeast, mcs6. Members of both categories of CAKs belong to the extended CDK family (Table 9.1).25
Table 1
Extended CDK family members used in this study.
Cak1p and Csk1 are diverged CDK family members.17–19 When comparing the conserved kinase domain,26 Cak1p and Csk1 only share 20% sequence identity with Cdc28p, the prototype CDK, while most yeast CDKs are more than 35% identical to Cdc28p. Both Cak1p and Csk1 are monomeric CAKs, that is, they are constitutively active without the need for a cyclin partner.17–19 Further, they don't require phosphorylation of the T160 site to be active.17–19 In fact, neither Cak1p nor Csk1 has a Ser or Thr at the T160 position.
CDK7 and Mcs6 are more conventional CDKs. They share 40% sequence identity with CDC28 in the kinase domain, they have Thr or Ser at their T160 sites, and they both need cyclin partners to be active.27–30 Monomeric unphosphorylated CDK7 cannot efficiently bind to its cyclin partner, cyclin H; it can only bind cyclin H after having been phosphorylated on its T160 site, or alternatively, after binding of the assembly factor MAT1.31 CDK7/Mcs6 family members function not only as CAKs but also as essential transcription factors. They are components of the general transcription factor TFIIH and function as activating CTD kinases.32 Kin28p, CDK7's budding yeast ortholog, is required for the transcription of 87% of all budding yeast genes.33
Different organisms use CAKs in different ways. In budding yeast, Cak1p is the sole known CAK and is required for the phosphorylation of both the cell cycle CDK Cdc28p and the transcription CDK Kin28p.17,18,22,23 Kin28p, which is the ortholog of CDK7 and Mcs6, does not possess CAK activity.8,34,35 In fission yeast, the monomeric CAK Csk1 phosphorylates and activates Mcs6. Csk1 and Mcs6 work redundantly to phosphorylate the cell cycle CDK Cdc2.19,36In metazoa, Drosophila Cdk7 was shown to function as a CAK for the M phase cell cycle CDK Cdc2, but not for the S phase CDK Cdk2/Cdc2c.20 No CAK1/csk1 ortholog has been found in metazoa.
Yeast CDK and CAK Orthologs
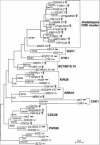
Budding yeast Cak1p and fission yeast Csk1 were initially thought to be unrelated kinases, since they share little sequence homology.19,36 However, by phylogenetic analysis, we observed that Cak1p and Csk1 group together with significant bootstrap support (Fig. 9.1).25 Both Cak1p and Csk1 have diverged considerably not only from other CDKs but also from other kinases, with both genes missing a number of key residues conserved in the eukaryotic protein kinase superfamily.17 These observations suggest that CAK1 and csk1 are rapidly evolving genes. In phylogenetic analyses, fast-evolving genes can group together solely because of their shared dissimilarity relative to slow-evolving taxa. This misleading convergence is called long-branch attraction.37 To study the true phylogenetic relationship of Cak1p and Csk1, we used four methods to circumvent long branch attraction: First, we included a variety of the closest related outgroup taxa in our analysis, as the inclusion of such taxa helps to minimize long branch attraction.38,39 Second, we used both Maximum Likelihood (ML) and Neighbor-Joining (NJ) analyses, which can better resist the effects of long branch attraction.37,39,40,41
Third, we employed gamma-corrected distances, which facilitates a more accurate phylogeny when taxa evolve at different rates.42 Fourth, based on the theoretical framework of the S-F method,43 we excluded the fastest-evolving characters from each taxa and used the RASA program38,44 to confirm that the resultant new dataset was not likely to have long-branch attraction. All four strategies indicated that the Cak1p-Csk1 grouping was real and not an artifact of long-branch attraction, suggesting that CAK1 and csk1 are orthologous genes.
Are there CAK1/csk1 orthologs in other fungi? Using Cak1p and Csk1 as queries for BLAST searches,45 we identified a Candida albicans gene, CAC05182.1. Just like Cak1p and Csk1, CAC05182.1 does not have a Ser or Thr at its T160 position. Our ML and NJ analysis places CAC05182.1 within the CAK1 clade with 99% and 100% bootstrap supports, respectively (Fig. 9.2). We also generated a JTT amino acid distance matrix for CAC05182.1 and budding yeast CDK family members (Table 9.2). A JTT distance measurement is an estimate of the average number of amino acid substitutions per site between two taxa. The shorter the distance value, the higher the similarity between the two proteins. The distance matrix shows that CAC05182.1 is more closely related to Cak1p and Csk1 than to any other yeast CDKs. In fact, it is closer to Cak1p and Csk1 than Cak1p and Csk1 are to each other. Therefore, the evidence suggests that there are at least three CAK1 orthologs in yeast: CAK1, csk1 and CAC05182.1, which we now refer to as CaCAK1.
Table 2
Distance Matrix of Candida, Arabidopsis, and Giardia CAK Orthologs vs. Yeast CDKs.
Metazoan CDK and CAK Orthologs
Cell cycle regulators are generally conserved among eukaryotes. However, no Cak1p/Csk1 type of monomeric CAK has been found in metazoa. On the other hand, three experimental observations suggest that there are missing CAKs in metazoa. First, mammalian CDK7's T160-equivalent site is phosphorylated in vivo,31 but the in vivo CAK for mammalian CDK7 has not been identified. Since the fission yeast ortholog of CDK7, Mcs6, is phosphorylated by Csk1,19 it is reasonable to suspect that an unidentified Cak1p/Csk1 ortholog may be phosphorylating CDK7 in metazoa. Second, when Drosophila Cdk7 activity was reduced, Cdc2 activity was impaired due to its reduced level of T160 phosphorylation. However, neither the activity nor the phosphorylation level of Cdk2 was affected, suggesting that there exists another CAK for Cdk2.20 Finally, Nagahara et al showed that in human cells there is a CAK activity for CDK2 and this CAK activity is distinct from the CDK7/Cyclin H activity.24
Could there be CAK1/csk1 orthologs in metazoa? The completely sequenced C. elegans and D. melanogaster genomes provided an opportunity for us to approach this question.46,47 If there are CAK1/csk1 orthologs in metazoa and if they still share identifiable sequence homology with the yeast CAK1/csk1 orthologs, we should be able to recognize them by phylogenetic analysis in the completed genomes. We identified all CDK family members in these organisms by BLAST and PROFILE48 searches and analyzed their phylogeny by Maximum Likelihood and Neighbor-Joining analyses.25 Most metazoan CDK family members belong to other yeast CDK clades with significant bootstrap supports, indicating that they are paralogs instead of orthologs of CAK1/csk1. We observed that no metazoan CDK-family members group with the CAK1 clade with significant bootstrap supports (Fig. 9.1).
A true metazoan CAK1 ortholog may be too divergent to group with the yeast CAK1/csk1 orthologs with high bootstrap support. There are several orphan metazoan CDK family members that don't group with any of the clades with significant bootstrap scores, making them potential CAK1/csk1 orthologs. For example, the Drosophila protein AC017707 and the C. elegans protein H01G02.2 branched at the base of the CAK1 clade, and human CCRK branched nearby (Fig. 9.1).
Sequence analyses of these proteins indicated that H01G02.2 and CCRK share one characteristic with CAK1/csk1 orthologs: they do not have Ser or Thr at their T160 sites.
However, a recently identified mouse CCRK ortholog, PNQLARE (GenBank acc. no. AAF89089), has an intact T-loop with a Thr at the T160 position. This finding suggests that the vertebrate ancestor of CCRK had a T-loop and may therefore have required activating phosphorylation.
Research in our lab suggests that C. elegans H01G02.2 is not likely to function as a CAK. First, a deletion allele of H01G02.2 that is a molecular null (obtained from the C. elegans Gene Knockout Consortium) is phenotypically wild type and fertile without obvious cell cycle defects. Second, H01G02.2 does not seem to be working redundantly with C. elegans cdk-7, as inactivating both H01G02.2 and cdk-7 by RNAi did not cause a more severe defect than inactivating cdk-7 alone. Furthermore, while csk1 can complement a cak1 mutant,19 ectopic expression of H01G02.2 failed to complement budding yeast cak1 mutants.25
Arabidopsis CDK and CAK Orthologs
Our analysis suggested that there is no identifiable CAK1/csk1 ortholog in metazoa. If the CAK1 clade is ancient, that is, if it arose very early in eukaryotic evolution, it may have been subsequently lost in certain modern eukaryotic lineages such as metazoa. If this were the case then we would expect to see it in other eukaryotic kingdoms. Vascular plants are predicted to have diverged from the major eukaryotic lineage shortly before fungi diverged.51
We therefore performed a phylogenetic analysis on the 98% finished Arabidopsis thaliana genome to search for a CAK1/csk1 ortholog. We identified 24 Arabidopsis CDK family members by BLAST searches. Phylogenetic analysis groups them into five major clades, four of which contain yeast CDK family members (Fig. 9.3).
Arabidopsis cdc2a and cdc2b genes belong to the CDC28 clade. cdc2a is involved in cell cycle regulation. When a dominant cdc2a mutant was expressed in Arabidopsis, cell division was reduced.52,53 cdc2bhas been shown to be involved in cell elongation rather than cell division in hypocotyl growth in Arabidopsis.54
Arabidopsis MBK5.8 belongs to the SRB10 clade. Although nothing is known of MBK5.8's function, its yeast ortholog Srb10 and human ortholog CDK8 both function as CTD kinases that negatively regulate transcription.55
Arabidopsis F12B7.13 and K9H21.7 both belong to the BC18H10.15 clade, which contains one fission yeast CDK family member BC18H10.15, as well as two C. elegans, two Drosophila, and two human CDK family members. Interestingly, this clade does not contain a budding yeast ortholog, suggesting it was lost (Fig. 9.3). The functions of the clade members are largely unknown, with the exceptions that the human orthologs PISSLRE and PITSLRE have been implicated in apoptosis and the G2/M cell cycle transition, respectively.56–58
One cluster of Arabidopsis CDK family members has no obvious ortholog in yeast. It branches near the SGV1 and CTK1 clades and contains 14 CDK family members: T22H22.5, T12H1.1, K16E14.2, F21B7.1, AT4G22940, F8L10.9, F26A9.10, AT4G10010, F14J9.26, F6A14.22, F1M20.1, AAF21469.1, T4P13.23, and MXK3.19. The biological functions of these genes have not yet been defined.
The KIN28 clade contains Arabidopsis F25P22.11 and T10F20.5 genes. F25P22.11 and T10F20.5 share more than 85% DNA sequence identity with each other, suggesting that they are derived from a recent gene duplication within plants.
Another Arabidopsis gene cak1At is located at the base of the KIN28 clade, although with insignificant bootstrap support (43% and 84% for NJ and ML analyses, respectively). A JTT distance matrix comparing Cak1At to the budding yeast CDK-family members indicates that Cak1At is most similar to Kin28p and is least similar to Cak1p (Table 9.2). Umeda et al showed that cak1At can complement both a budding yeast cak1 mutant and a fission yeast mcs6 mutant, indicating that it has in vivo CAK activity.59 They also showed that Cak1At has in vitro CAK activity towards Cdk2.59 The authors classified Cak1At as a novel type of CAK, different from both Cak1p and CDK7, as Cak1At has a divergent sequence.59 However, based on the JTT distance data and the 84% bootstrap support in our ML tree, we think that cak1At is more likely to be a divergent KIN28/mcs6/CDK7 ortholog.
There are no Arabidopsis CDK-family members that group with the CAK1 clade. Also, the only Arabidopsis orphan CDK family members, cak1At and members of the “Arabidopsis CDK cluster”, are unlikely to be CAK1/csk1 orthologs, because both BLAST searches and the distance matrix showed that they are very dissimilar to CAK1 clade members. Therefore, with 98% of the genome sequenced, we have not observed a CAK1/csk1 ortholog in Arabidopsis.
Giardia lamblia CAK Orthologs
Giardia lamblia represents one of the most ancient eukaryotic lineages.60 We searched the 4× sequenced Giardia lamblia genome by BLAST, PROFILE, and HMM searches61 for a CAK1/csk1 ortholog and failed to find one. In contrast, a KIN28 ortholog in Giardia lamblia (AC052571) was readily identifiable by BLAST search. The affiliation of AC052571 with the KIN28 clade is supported by both ML and NJ analyses with 95% and 81% bootstrap supports, respectively (data not shown), and is reflected in a JTT distance matrix (Table 9.2).
Three Hypotheses on CAK1 Evolution
Our analyses have failed to identify a recognizable CAK1 clade member in the plant Arabidopsis, or in the metazoa C. elegans, Drosophila, and humans. Assuming that a CAK1/csk1 ortholog is not in the remaining unsequenced 2% of the Arabidopsis genome, three possibilities for CAK1/csk1 evolution remain:
- The CAK1 clade is ancient with members present in the major eukaryotic kingdoms; however, the clade members in distantly related phyla have diverged to the extent that identification by phylogeny is not possible.
- The CAK1 clade is ancient and could be identified by phylogeny; however, its orthologs have been lost independently in both plants and animals.
- The CAK1 clade originated in fungi and does not exist in any other eukaryotic kingdoms.
The CDK-Activating Kinase (CAK)
The first possibility suggests that CAK1/csk1 orthologs exist in metazoa and plants, but they are too divergent to be identified. One way to address this possibility is to assign all of the CDK-family members in a plant or metazoan species to known ancestral CDK clades and demonstrate either that CAK1/csk1 orthologs are present or are missing. We have been able to assign ancestry to the majority of metazoan CDK-family members with the exceptions of the orphan genes (Fig. 9.1).25 The metazoan orphan genes are quite divergent from each other and do not have counterparts in Arabidopsis, suggesting that they are undergoing rapid evolution that is obscuring their relationships to ancestral clades. The sequencing of “missing-link” species may allow assignment of the ancestry of the metazoan orphan CDK family members, the members of the “Arabidopsis CDK cluster”, as well as provide a definitive assignment for cak1At.
The second hypothesis predicts that the CAK1 clade is ancient but was lost independently in metazoa and plants. We have not found a CAK1/csk1 ortholog in the diplomonad Giardia, indicating that the CAK1 clade may not have existed in early eukaryotic lineages. However, it is still possible that the CAK1 clade is ancient but arose after the divergence of diplomonads from the main eukaryotic lineage. The test of this hypothesis will be determining whether there are CAK1/csk1 orthologs in taxa that diverged shortly before or after fungi did, such as the choanozoa.62
The third hypothesis, that the CAK1 clade originated in fungi, is consistent with our inability to detect CAK1/csk1 orthologs in other species. This hypothesis generates a prediction that can be tested. If the CAK1 clade arose in fungi, then it would have come from the duplication of a fungal CDK. Finding this gene duplication event would strongly indicate that the CAK1 clade arose in fungi. Ideally, if we identified a fungal CAK1/csk1 ortholog that shares significant sequence homology with one of the other fungal CDK genes, then this would indicate that the CAK1 clade derived from the ancestor of this CDK gene. A successful example of this type of analysis was the study of bicoid's evolution.63 bicoid is an essential homeobox gene and had been identified only in the closest relatives of the schizophoran fly Drosophila. Stauber et al cloned bicoid from a basal cyclorrhaohan fly, Megaselia abdita, and showed by phylogenetic analysis that the gene originated from a duplication of the Megaselia gene zerknullt, which is also conserved in vertebrates. Such an approach may be possible for the study of CAK1 evolution as well.
Summary
There are two categories of CAKs in eukaryotes encompassing the orthologous genes CAK1/csk1/CaCAK1 and mcs6/CDK7. mcs6/CDK7 orthologs are highly conserved from Giardia to metazoa, indicating that this is an extremely ancient eukaryotic CDK clade, whose members are under functional constraints that limit their evolution. Members of this clade, including the budding yeast KIN28, function as central regulators of transcription.32 The conservation of this clade may therefore be linked to its essential role in transcription rather than to its CAK activity. CAK activity for Mcs6/CDK7 orthologs has only been observed in fission yeast and in metazoa. It is an open question whether CAK activity for Mcs6/CDK7 orthologs is evolutionarily conserved beyond certain fungi and metazoa.
CAK1/csk1 orthologs have not been identified in species other than yeast. The members of the clade appear to be evolving under relaxed evolutionary constraints as they are very divergent even within yeast. Currently it is not known whether the CAK1/csk1 genes are specific for fungi or whether their metazoan and plant orthologs have diverged to an extent that precludes their current identification.
Acknowledgments
We would like to thank R. P. Fisher for sharing information about the mouse CDK PNQALRE sequence; and G.L. Moulder and R. J. Barstead for providing H01G02.2 knock-out animals. E.T.K. is supported by grants from NIH (R01 GM55297) and the Human Frontiers Science Program Organization (RG-229).
References
- 1.
- Nasmyth KA, Reed SI. Isolation of genes by complementation in yeast: molecular cloning of a cell-cycle gene. Proc Natl Acad Sci USA. 1980;77(4):2119–2123. [PMC free article: PMC348663] [PubMed: 6246523]
- 2.
- Beach D, Durkacz B, Nurse P. Functionally homologous cell cycle control genes in budding and fission yeast. Nature. 1982;300(5894):706–709. [PubMed: 6757758]
- 3.
- van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. [PubMed: 8266103]
- 4.
- King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994;79:563–571. [PubMed: 7954823]
- 5.
- Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. [PubMed: 7954821]
- 6.
- Stillman B. Cell Cycle Control of DNA Replication. Science. 1996;274:1659–1664. [PubMed: 8939847]
- 7.
- Morgan DO. Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. [PubMed: 9442875]
- 8.
- Valay JG, Simon M, Dubois MF, Bensaude O, Facca C. et al. The KIN28 gene is required both for RNA polymerase II mediated transcription and phosphorylation of the Rpb1p CTD. J Mol Biol. 1995;249(3):535–544. [PubMed: 7783209]
- 9.
- Liao SM, Zhang J, Jeffery DA, Koleske AJ, Thompson CM. et al. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature. 1995;374(6518):193–196. [PubMed: 7877695]
- 10.
- Lee J, Greenleaf AL. CTD kinase large subunit is encoded by CTK1, a gene required for normal growth of Saccharomyces cerevisiae. Gene Expr. 1991;1(2):149–167. [PMC free article: PMC5952209] [PubMed: 1820212]
- 11.
- Sterner DE, Lee JM, Hardin SE, Greenleaf AL. The yeast carboxyl-terminal repeat domain kinase CTDK-I is a divergent cyclin-cyclin-dependent kinase complex. Mol Cell Biol. 1995;15(10):5716–5724. [PMC free article: PMC230822] [PubMed: 7565723]
- 12.
- Prelich G, Winston F. Mutations thatt supress the deletion of an upstream activating sequence in yeast: involvement of a protein kinase and histone H3 in repressing transcription in vivo. Genetics. 1993;135(3):665–676. [PMC free article: PMC1205711] [PubMed: 8293972]
- 13.
- Zhu Y, Pe'ery T, Peng J, Ramanathan Y, Marshall N, Marshall T. et al. Transcription elongation factor P-TEFb is required for HIV-1 tattransactivation in vitro. Genes Dev. 1997;11(20):2622–2632. [PMC free article: PMC316609] [PubMed: 9334325]
- 14.
- Reines D, Conaway RC, Conaway JW. Mechanism and regulation of transcriptional elongation by RNA polymerase II. Curr Opin Cell Biol. 1999;11(3):342–346. [PMC free article: PMC3371606] [PubMed: 10395562]
- 15.
- De Bondt HL, Rosenblatt J, Jancarik J, Jones HD, Morgan DO. et al. Crystal structure of cyclin-dependent kinase 2. Nature. 1993;363(6430):595–602. [PubMed: 8510751]
- 16.
- Russo AA, Jeffrey PD, Pavletich NP. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat Struct Biol. 1996;3(8):696–700. [PubMed: 8756328]
- 17.
- Kaldis P, Sutton A, Solomon MJ. The Cdk-activating kinase (CAK) from budding yeast. Cell. 1996;86:553–564. [PubMed: 8752210]
- 18.
- Thuret JY, Valay JG, Faye G, Mann C. Civ1 (CAK in vivo), a novel Cdk-activating kinase. Cell. 1996;86(4):565–576. [PubMed: 8752211]
- 19.
- Hermand D, Pihlak A, Westerling T, Damagnez V, Vandenhaute J. et al. Fission yeast Csk1 is a CAK-activating kinase (CAKAK). Embo J. 1998;17(24):7230–7238. [PMC free article: PMC1171069] [PubMed: 9857180]
- 20.
- Larochelle S, Pandur J, Fisher RP, Salz HK, Suter B. Cdk7 is essential for mitosis and for in vivo Cdk-activating kinase activity. Genes Dev. 1998;12:370–381. [PMC free article: PMC316490] [PubMed: 9450931]
- 21.
- Kato JY, Matsuoka M, Strom DK, Sherr CJ. Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase Mol Cell Biol 199414(4):2713–2721.The CDK-Activating Kinase (CAK) [PMC free article: PMC358637] [PubMed: 8139570]
- 22.
- Espinoza FH, Farrell A, Nourse JL, Chamberlin HM, Gileadi O. et al. Cak1 is required for Kin28 phosphorylation and activation in vivo. Mol Cell Biol. 1998;18(11):6365–6373. [PMC free article: PMC109222] [PubMed: 9774652]
- 23.
- Kimmelman J, Kaldis P, Hengartner CJ, Laff GM, Koh SS. et al. Activating phosphorylation of the Kin28p subunit of yeast TFIIH by Cak1p. Mol Cell Biol. 1999;19(7):4774–4787. [PMC free article: PMC84276] [PubMed: 10373527]
- 24.
- Nagahara H, Ezhevsky SA, Vocero-Akbani AM, Kaldis P, Solomon MJ. et al. Transforming growth factor beta targeted inactivation of cyclin E:cyclin-dependent kinase 2 (Cdk2) complexes by inhibition of Cdk2 activating kinase activity. Proc Natl Acad Sci USA. 1999;96(26):14961–14966. [PMC free article: PMC24755] [PubMed: 10611320]
- 25.
- Liu J, Kipreos ET. Evolution of cyclin-dependent kinases (CDKs) and CDK-activating kinases (CAKs): Differential conservation of CAKs in yeast and metazoa. Mol Biol Evol. 2000;17(7):1061–1074. [PubMed: 10889219]
- 26.
- Hanks SK, Hunter T. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed: 7768349]
- 27.
- Poon R Y C, Yamashita K, Adamczewski JP, Hunt T, Shuttleworth J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 1993;12:3123–3132. [PMC free article: PMC413578] [PubMed: 8393783]
- 28.
- Solomon MJ, Harper JW, Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993;12:3133–3142. [PMC free article: PMC413579] [PubMed: 8344252]
- 29.
- Buck V, Russell P, Millar JB. Identification of a cdk-activating kinase in fission yeast. EMBO J. 1995;14(24):6173–6183. [PMC free article: PMC394742] [PubMed: 8557037]
- 30.
- Damagnez V, Makela TP, Cottarel G. Schizosaccharomyces pombe Mop1-Mcs2 is related to mammalian CAK. EMBO J. 1995;14(24):6164–6172. [PMC free article: PMC394741] [PubMed: 8557036]
- 31.
- Fisher RP, Jin P, Chamberlin HM, Morgan DO. Alternative mechanisms of CAK assembly require an assembly factor or an activating kinase. Cell. 1995;83(1):47–57. [PubMed: 7553872]
- 32.
- Orphanides G, Lagrange T, Reinberg D. The general transcription factors of RNA polymerase II. Genes Dev. 1996;10(21):2657–2683. [PubMed: 8946909]
- 33.
- Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR. et al. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95(5):717–728. [PubMed: 9845373]
- 34.
- Feaver WJ, Svejstrup JQ, Henry NL, Kornberg RD. Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell. 1994;79(6):1103–1109. [PubMed: 8001136]
- 35.
- Cismowski MJ, Laff GM, Solomon MJ, Reed SI. KIN28 encodes a C-terminal domain kinase that controls mRNA transcription in Saccharomyces cerevisiae but lacks cyclin-dependent kinase-activating kinase (CAK) activity. Mol Cell Biol. 1995;15(6):2983–2992. [PMC free article: PMC230529] [PubMed: 7760796]
- 36.
- Lee KM, Saiz JE, Barton WA, Fisher RP. Cdc2 activation in fission yeast depends on Mcs6 and Csk1, two partially redundant Cdk-activating kinases (CAKs). Curr Biol. 1999;9(8):441–444. [PubMed: 10226032]
- 37.
- Felsenstein J. Cases in which parsimony and compatibility methods will be positively misleading. Syst. Zool. 1978;24:401–410.
- 38.
- Lyons-Weiler J, Hoelzer GA. Escaping from the Felsenstein zone by detecting long branches in phylogenetic data. Mol Phylogenet Evol. 1997;8(3):375–384. [PubMed: 9417895]
- 39.
- Saitou N, Imanishi T. Relative efficiencies of the Fitch-Margoliash, maximum parsimony, maximum likelihood, minimum evolution, and neighbor-joining methods of phylogenetic tree construction in obtaining the correct tree. Mol Biol Evol. 1989;6(5):514–528.
- 40.
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376. [PubMed: 7288891]
- 41.
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. [PubMed: 3447015]
- 42.
- Yang Z. Among site rate variation and its impact on phylogenetic analyses. Trends Ecol. Evol. 1996;11:367–372. [PubMed: 21237881]
- 43.
- Brinkmann H, Philippe H. Archaea sister group of bacteria? Indications from tree reconstruction artifacts in ancient phylogenies. Mol Biol Evol. 1999;16(6):817–825. [PubMed: 10368959]
- 44.
- Lyons-Weiler J, Hoelzer GA, Tausch RJ. Relative apparent synapomorphy analysis (RASA). I: The statistical measurement of phylogenetic signal. Mol Biol Evol. 1996;13(6):749–757. [PubMed: 8754211]
- 45.
- Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z. et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. [PMC free article: PMC146917] [PubMed: 9254694]
- 46.
- Consortium TC. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science. 1998;282:2012–2018. [PubMed: 9851916]
- 47.
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD. et al. The genome sequence of Drosophila melanogaster. Science. 2000;287(5461):2185–2195. [PubMed: 10731132]
- 48.
- Gribskov M, Luthy R, Eisenberg D. Profile analysis. Methods Enzymol. 1990;183:146–159. [PubMed: 2314273]
- 49.
- Johnson LN, Noble ME, Owen DJ. Active and inactive protein kinases: Structural basis for regulation. Cell. 1996;85(2):149–158. [PubMed: 8612268]
- 50.
- Martinez AM, Afshar M, Martin F, Cavadore JC, Labbe JC. et al. Dual phosphorylation of the T-loop in cdk7: its role in controlling cyclin H binding and CAK activity. Embo J. 1997;16(2):343–354. [PMC free article: PMC1169640] [PubMed: 9029154]
- 51.
- Schlegel M. Molecular phylogeny of eukaryotes. Trends Ecol Evol. 1994;9(9):330–335. [PubMed: 21236876]
- 52.
- Hemerly AS, Ferreira PC, Van Montagu M, Engler G, Inze D. Cell division events are essential for embryo patterning and morphogenesis: Studies on dominant-negative cdc2aAt mutants of Arabidopsis. Plant J. 2000;23(1):123–130. [PubMed: 10929107]
- 53.
- Hemerly A, Engler J D A, Bergounioux C, Van Montagu M, Engler G. et al. Dominant negative mutants of the Cdc2 kinase uncouple cell division from iterative plant development. Embo J. 1995;14(16):3925–3936. [PMC free article: PMC394471] [PubMed: 7664733]
- 54.
- Yoshizumi T, Nagata N, Shimada H, Matsui M. An Arabidopsis cell cycle-dependent kinase-related gene, CDC2b, plays a role in regulating seedling growth in darkness. Plant Cell. 1999;11(10):1883–1896. [PMC free article: PMC144097] [PubMed: 10521519]
- 55.
- Hampsey M, Reinberg D. RNA polymerase II as a control panel for multiple co-activator complexes. Curr Opin Genet Dev. 1999;9(2):132–139. [PubMed: 10322136]
- 56.
- Lahti JM, Xiang J, Heath LS, Campana D, Kidd VJ. PITSLRE protein kinase activity is associated with apoptosis. Mol Cell Biol. 1995;15(1):1–11. [PMC free article: PMC231901] [PubMed: 7528324]
- 57.
- Ariza ME, Broome-Powell M, Lahti JM, Kidd VJ, Nelson MA. Fas-induced apoptosis in human malignant melanoma cell lines is associated with the activation of the p34(cdc2)-related PITSLRE protein kinases. J Biol Chem. 1999;274(40):28505–28513. [PubMed: 10497214]
- 58.
- Li S, MacLachlan TK, De Luca A, Claudio PP, Condorelli G. et al. The cdc-2-related kinase, PISSLRE, is essential for cell growth and acts in G2 phase of the cell cycle. Cancer Res. 1995;55(18):3992–3995. [PubMed: 7664269]
- 59.
- Umeda M, Bhalerao RP, Schell J, Uchimiya H, Koncz C. A distinct cyclin-dependent kinase-activating kinase of Arabidopsis thaliana. Proc Natl Acad Sci USA. 1998;95(9):5021–5026. [PMC free article: PMC20206] [PubMed: 9560221]
- 60.
- Smith MW, Aley SB, Sogin M, Gillin FD, Evans GA. Sequence survey of the Giardia lamblia genome. Mol Biochem Parasitol. 1998;95(2):267–280. [PubMed: 9803418]
- 61.
- Eddy SR. Hidden Markov models. Curr Opin Struct Biol. 1996;6(3):361–365. [PubMed: 8804822]
- 62.
- Embley TM, Hirt RP. Early branching eukaryotes? Curr Opin Genet Dev. 1998;8(6):624–629. [PubMed: 9914207]
- 63.
- Stauber M, Jackle H, Schmidt-Ott U. The anterior determinant bicoid of Drosophila is a derived Hox class 3 gene. Proc Natl Acad Sci USA. 1999;96(7):3786–3789. [PMC free article: PMC22372] [PubMed: 10097115]
- 64.
- Adachi J, Hasegawa M. MOLPHY version 2.3: Programs for molecular phylogenetics based on maximum likelihood Computer Science Monographs, No. 28. Institute of Statistical Mathematics, Tokyo1996.
- The Evolution of CDK-Activating Kinases - Madame Curie Bioscience DatabaseThe Evolution of CDK-Activating Kinases - Madame Curie Bioscience Database
- Cell Cycle Reactivation in Skeletal Muscle and Other Terminally Differentiated C...Cell Cycle Reactivation in Skeletal Muscle and Other Terminally Differentiated Cells - Madame Curie Bioscience Database
- A Conceptual Framework - Madame Curie Bioscience DatabaseA Conceptual Framework - Madame Curie Bioscience Database
- Biocompatibility of Polyurethanes - Madame Curie Bioscience DatabaseBiocompatibility of Polyurethanes - Madame Curie Bioscience Database
- Ceramide in the Regulation of Neuronal Development: Two Faces of a Lipid - Madam...Ceramide in the Regulation of Neuronal Development: Two Faces of a Lipid - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...