NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The molecular cascade of events associated with hippocampal processing of information for long-term storage is a time-limited event. Learning sets in motion neural processes that continue to evolve after training, a phenomenon known as consolidation. The consolidation process has been proposed to involve the translation of neural activity into enduring synaptic change by a cascade of sequential molecular steps involving gene induction, increased protein synthesis and synaptic growth mediated, in part, by cell recognition systems. However, evidence for dendritic remodelling being a mechanism of memory consolidation is still in its infancy. There is no clear consensus as to whether spine or synapse frequency change accompanies memory formation. This may arise from a failure to consider temporal change in synaptic dynamics during information processing. By contrast, there is significant evidence that the temporal orchestration of cell adhesion molecule function is an integral process for memory consolidation. An attractive possibility is that transmembrane cell adhesion molecule expression is modulated in an activity-dependent manner that permits rapid alteration in synapse structure and/or efficacy. Evidence exists to correlate cell adhesion molecule function with spine and synapse formation following learning but also for their increased synaptic expression in the absence of dendritic remodelling. There appears to be a need to correlate the temporal dynamics of cell adhesion molecule function to synapse structure if a morphological correlate of learning is to be established.
Introduction
An important problem in the neurobiology of memory is whether cellular mechanisms of learning and memory include the formation of new synapses or the remodelling of existing ones. To elucidate this problem, numerous studies have examined alterations in the number and structure of hippocampal dendritic spines and synapses (see Geinisman et al in this book). This brain region, along with associated cortical structures in the temporal lobe, has been implicated in holding and processing information destined for consolidation as long term memory within the neocortex.2Moreover, this consolidation process has been demonstrated to involve a molecular cascade of events within the medial temporal lobe in which enhanced neural activity activates gene transcription, protein synthesis and synaptic reorganisation.5,37,70,72 Activity-dependent synapse selection is attractive in its simplicity and most likely will provide a basis to understand neuroplastic events subservient to behavioural adaptation in the adult animal.
Most excitatory synapses in the adult brain are located on the bulbous heads of dendritic spines,45 where they occur in vast numbers, estimated to be in the order of 10 14 for the human cerebral cortex.121 Dendritic filopodia are initially involved in the generation of shaft synapses, which later develop into spines.28 This distinctive structure of dendritic spines is specialized by underlying microfilaments composed of actin that contrasts with the cytoplasmic microtubules that are dominant in the dendritic shaft.68 The actin microfilaments are in close association with the postsynaptic density, a membrane specialization thought to be important for the clustering of postsynaptic receptors and ion channels and for the assembly of the postsynaptic signalling machinery.42
Cell adhesion molecule-regulated cell-cell recognition and/or signal transduction events play an integral role in dendritic spine development and synapse elaboration.9 Cell-cell adhesion across the synaptic cleft is thought to hold the presynaptic active zone and the postsynaptic density in close register.36 As yet, there is no clear consensus on the role of cell recognition systems in dendritic growth and synapse restructuring. Such molecules may function to promote dendritic growth and synapse formation and provide mechanisms that restrict elaboration of the dendritic arbor.15 Moreover, in the adult hippocampus, region-specific cell genesis has been suggested to contribute to the fine-tuning of the neural structure throughout life by invoking a developmental replay of cell-cell recognition events.55
The challenge ahead lies in identifying the network of molecular interactions, including cell adhesion molecules (CAMs), that together produce the activity-induced morphological change, such as dendrite and synapse formation, as the greatest pitfall in research on activity-related plasticity is to overstate the relevance of single identified regulators.
Is Net Synapse Formation a Correlate of Learning?
Results from Studies Employing Long-Term Potentiation
Neural activity plays an important role in the emergence of new spines, the stabilization of existing spines, and changes in spine morphology. However, the extent to which dendritic motility depends on afferent innervation or synaptic activity is under debate. Much of this debate centres on the use of a cellular model of learning-long-term potentiation (LTP). The essence of LTP is a remarkably persistent enhancement of synaptic responses resulting from brief, repetitive activation of an excitatory afferent monosynaptic pathway by high frequency trains of electrical impulses.11 LTP has been studied most intensively at excitatory hippocampal synapses formed by Schaffer collaterals on CA1 pyramidal cells or by perforant path fibres on dentate gyrus granule cells.
Recently developed imaging techniques that permit time-lapse observations have allowed an unprecedented understanding of dendritic spine dynamics. Studies using coordinated patterns of activity, such as the tetanic stimulation required for the induction of LTP, results in the growth of CA1 dendritic protrusions that are dependent on NMDA receptor-mediated neurotransmission.27,65 Further, these dendritic spines have been shown to persist for >45 min in the absence of additional evoked activity.
Synaptic modifications are also reported to follow the induction of LTP in the perforant path. For example, following 4 daily tetanizations of the perforant path the ratio of perforated to non-perforated synapses in the mid-molecular layer of the dentate gyrus becomes increased by approximately 23% one hour after the last tetanization (Genisman; this book).38,39 These studies suggest that spine splitting may be one of the mechanisms by which LTP is maintained at the synaptic level. Studies on activated synapses, identified by the detection of calcium precipitates in the spine apparatus, support this suggestion. Activated synapses appear transiently in the 30-60min post-tetanic period, exhibit perforations and, in the ensuing 2-3h period, an enduring three-fold increase in the frequency of activated CA1 presynaptic elements with at least two dendritic spines contacting the same axon terminal.124
By contrast, however, Sorra and Harris110have failed to observe new synapse formation at 2h following tetanization of the hippocampal CA1 region. Moreover, in separate studies utilizing a single tetanization of the perforant path, no change in spine frequency was observed in the outer-molecular layer of the dentate gyrus when examined 24h after potentiation.105 These results suggest that LTP does not cause an overall formation of new synapses. Alternatively, presynaptic terminals may move in concert with dendritic spines or synapses might be broken and reformed rapidly, in a matter of seconds to minutes, so that structural changes are relatively balanced across the dendritic arbor. This phenomenon has been observed for Purkinje cells in cerebellar slices and retinal ganglion cells in culture.24,132
Results from Studies Employing Behavioural Paradigms
A number of electron microscopic studies available in the literature report that the numerical density of synapses is increased in relevant areas of the vertebrate brain as a consequence of learning.5,43Change in hippocampal CA1 synapse numerical density has been observed at 1h following acquisition of a brightness discrimination task and in the intermediate hyperstriatum ventrale following avoidance conditioning in the chick.20,130 In the hippocampal dentate gyrus, spine frequency changes occur at 6h following passive avoidance or water maze training that are transient and return to basal levels at 72h.85,86 Motor skill learning also results in an increased synapse density in the rodent cerebellar cortex10that persists for at least 4 weeks.59
However, trace eyeblink conditioning in rabbits, a hippocampus-dependent task that is acquired over 5-10 days, failed to reveal a change in total CA1 synapse number when examined at 24h following the final conditioning session.39 Moreover, extended water maze training, over a five day period, failed to elicit changes in either axospinous or axodendritic synapses in either the CA1 or dentate gyrus molecular layer at six days after the last training session.97 Similar results have been obtained with rats subjected to a one-way active avoidance procedure over 3 days as no change in total synapse number in the molecular layer of the hippocampal denate gyrus could be observed.127 Taken together, these results would suggest that the formation of long-term memory does not necessarily involve enduring synapse formation.
A possible reason for the disparity in these data sets may relate to the dynamic nature of the learning phenomenon. Stereological analyses performed at different time points during memory consolidation have suggested spine frequency changes to be transient in nature.85,86 Similar studies have also provided evidence for temporally-regulated and region-specific changes in synapse number. Following avoidance conditioning in the chick, for example, transient synapse number increases are first observed in the intermediate hyperstriatum ventrale and, subsequently, in the lobus parolfactorius at 24h following training.20,50
Do Cell Adhesion Molecules Have a Role in Learning?
CAM Structure and Function
The spine surface exhibits an array of proteins many of which span the membrane thereby permitting bidirectional communication.134 These proteins include ligand- and second messenger-gated ion channels, G-protein-coupled receptors and cell adhesion molecules that coexist in an organized but dynamic array in the postsynaptic density. Spectrin and spectrin-like proteins link these proteins to actin filaments that mingle with larger intermediate filaments in the spine head.
Analogous to the postsynaptic density, the presynaptic cytomatrix is organized into active zones that determine sites of synaptic vesicle fusion and recycling. These active zones are likely to spatially restrict proteins involved in vesicle docking and fusion, such as the SNAP (soluble N-ethylmaleimide-sensitive factor attachment protein) receptor complex and synaptotagamin. In addition, the active zone includes cytoskeletal proteins, such as members of the membrane-associated guanylate kinase (MAGUK) superfamily, as well as other multidomain proteins that are tightly associated with synaptic junctions. These are thought to function as adaptor proteins involved in localizing and assembling pre- and postsynaptic signalling complexes that also include cell adhesion molecules.36
The cell adhesion molecules (CAMs) localised to the synaptic complex include representatives from the cadherin, immunoglobulin and integrin superfamilies and, in addition, the neurexins and neuroligands.9 The cadherins mediate Ca2+-dependent cell-cell adhesion that is mainly trans homophilic. They have a single pass membrane domain that interacts with the actin cytoskeleton via a group of proteins termed the catenins. The integrins are formed from two non-covalently linked heterodimeric proteins that have single pass membrane domains that can link to actin by a variety of adaptor proteins, such as talin or vinculin. The extracellular domains of the integrins form a ligand binding site that requires divalent cations and interacts with defined matrix protein sequences (such as _Arg-Gly-Asp-).4 The immunoglobulin superfamily is characterized by the presence of Ig-like domains and fibronectin repeats in their extracellular domains.
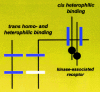
These adhesion molecules can interact in a hetero- or homophilic manner between cells (trans interactions) or in the same plane of the membrane (cis interactions) (Fig. 1). Many CAMs have single pass membrane domains, some of which interact with actin, or attach to the cell surface via a glycosylphosphatidylinositol linkage. The neurexins and neuroligands are located to the pre- and postsynaptic membranes, respectively, and mediate Ca2+-dependent cell-cell adhesion through their extracellular N-terminal domains.
Unfortunately, the precise mechanism(s) of CAM signalling still remain to be resolved. 18,41,56,67,131
CAMs and Learning-Induced Synaptic Plasticity
Across species and paradigms, numerous examples exist to support a role for CAMs in the synaptic plasticity that accompanies behavioural adaptation, however, these examples relate mainly to members of the immunoglobulin and integrin superfamilies. Targeted mutation of the volado gene product, an a-integrin subunit that is enriched in mushroom body neurons, impairs olfactory-avoidance learning in Drosophila.44 Moreover, integrin receptor antagonists block the induction of LTP in the rat hippocampal formation112 and intra-dentate injections of the integrin-associated protein impair avoidance conditioning.49 With respect to the immunoglobulin superfamily, antibody interventive studies have implicated both NCAM and L1 to be necessary for the induction of LTP and avoidance conditioning and spatial learning paradigms.1,21,64,87,92,102,103,123
Disruption of CAM function does not always impair synaptic plasticity. For example, pretreatment of hippocampal slices with antibodies raised against the extracellular domain of either N-cadherin or E-cadherin have no effect on basal synaptic properties but significantly reduce LTP.120 By contrast, deletion of cadherin-11 enhances LTP.66 The basis for these opposing effects on LTP that are obtained by blocking N-cadherin or cadherin-11 is unknown but it suggests that different cadherins may have unique roles in synaptic signalling. An alternative explanation, as offered by Sanes and Lichtman,100 is that many molecules are likely to be required to mediate the multi-step process of LTP that has been described to have at least four phases: initial, early, intermediate and late.11 This aspect is further discussed below.
The case for members of the immunoglobulin superfamily being involved in the synaptic plasticity associated with memory formation is more substantial. Mice with a targeted null mutation in the L1 gene exhibit impaired spatial learning.33 Moreover, adult mice with a targeted deletion of the NCAM gene exhibit behavioural abnormalities that include altered exploratory activity accompanied by increased anxiety and intermale aggression.113,114 Not surprisingly, mice deficient for NCAM function fail to sustain LTP and exhibit an impaired spatial learning ability.16,17 However, by crossing NCAM-deficient mice with those generated to over-express the NCAM180 isoform, the isoform enriched in synapses of postmitotic neurons,89 Schachner and colleagues could rehabilitate the behavioural abnormalities induced by lack of NCAM expression.115 This singular experiment clearly provides evidence of CAM function regulating behaviour at the level of the synapse.
Do Cell Adhesion Molecules Have a Temporal Role in Learning?
The molecular cascade of events associated with hippocampal processing of information for long-term storage is a time-limited event (Fig. 2).58,72,135 Learning sets in motion neural processes that continue to evolve after training, a phenomenon known as consolidation. The consolidation process has been proposed to involve the translation of neural activity into enduring synaptic change by a cascade of sequential molecular steps involving gene induction, increased protein synthesis and synaptic growth mediated, in part, by cell recognition systems.6 As time passes, a more permanent memory develops which is independent of the hippocampal formation and most likely located in the neocortex. Such studies have contributed to a model of memory consolidation in which the medial temporal lobe memory system serves as a temporary reservoir prior to the eventual storage of long term memory within the cortex.2
At least two temporally and mechanistically distinct processes contribute to activity-dependent synaptic plasticity, which lasts from tens of minutes to hours or more. After its induction, LTP passes through a 30-60min period during which unknown chemistries make it progressively less vulnerable to disruption or reversal. For example, hypoxia of duration just sufficient to transiently block synaptic responses completely eliminates LTP when applied within the first minutes after induction but is without effect 30min later.3 Also, LTP reversal is readily obtained with theta pattern stimulation when applied within the first minutes and becomes progressively less effective over 30min post-induction.111
Little is known about the neurochemical events responsible for consolidation. Protein and mRNA synthesis inhibitors are reported to dissipate LTP beginning several hours after induction.80 Whether this reflects a need for newly synthesised molecules in order for potentiation to enter a late stage, as opposed to sustaining already established LTP, is an unresolved issue. In any event, the effects obtained with protein synthesis inhibitors develop too slowly to be part of the stabilisation process that begins within the first minute after induction.
Certain classes of adhesion receptors may be better candidates. Several of the more than 20 known integrins are expressed by hippocampal neurons and at least some of these are concentrated in the synapses91 and many of these have been implicated in the stabilization of LTP. For example, function blocking antibodies to the a5 or _ integrin subunits have no effect on initial potentiation but significantly reduce it 45min later whereas antibodies to the a(v) or a2 integrin subunits are without effect.14 Moreover, function blocking with antibodies to the a3 integrin subunit stabilize slow decay of LTP.62 Members of the immunoglobulin superfamily also appear to be involved in the maintenance of LTP as judged by the increased expression of neuroplastin-65 and NCAM180 in the late phase following the induction of LTP.104,109
There is also evidence for the temporal involvement of CAMs in the consolidation processes that follow behavioural adaptation. For example, intraventricular administration of anti-L1 effectively blocks the acquisition of avoidance conditioning when administered just prior to training and, specifically, in the 5-8h and 15-18h post-training periods.103,123 Similarly, anti-NCAM has been found to induce amnesia of avoidance conditioning and odour discrimination paradigms when administered in the 6-8h post-training period.21,96,102 More recently, NCAM has been demonstrated to play a role in the acquisition of passive avoidance learning. Intraventricular infusions of a synthetic peptide ligand of NCAM (C3) strongly inhibited recall of a passive avoidance response in adult rats when given during training or in the 6-8 hours post-training period.30 This peptide has an affinity for the IgI domain and the capability of disrupting NCAM-mediated neurite outgrowth in vitro.93
This unique amnesic action of the C3 peptide has also been related to disrupted NCAM internalization immediately following training. In the 3-4h period following training NCAM180, the synapse-associated isoform, becomes down-regulated in the dentate gyrus of the hippocampal formation. This effect is mediated by ubiquitination and prevented by C3 peptide administration during training. Thus, these findings indicate NCAM to be involved in the acquisition and in the later 6-8h post-training consolidation of a passive avoidance response in the rat. Moreover, this study provided the first in vivo evidence for CAM internalization in learning, an observation presaged by studies on ApCAM, the Aplysia NCAM homologue, that has been demonstrated to rapidly down-regulate and become internalized in an in vitro model of long-term sensitization of the gill and siphon-withdrawal reflex.7,69 Internalization of CAMs may be a general mechanism for the dynamic modulation of synaptic plasticity during memory consolidation. Both NCAM and L1 are also endocytosed by clathrin-dependent pathways54,73 and growth cone elaboration has been associated with endocytosis-dependent recycling of L1.53
Can Cell Adhesion Molecules Reveal Memory Pathway?
Studies of memory-related modifications in the mammalian brain are severely restricted by the difficulty of identifying the network in which specific memory-induced changes occur, and by the prospect that these cellular changes are too subtle to be distinguished from the background of previously acquired memories. This conundrum has, in part, been overcome by the development of a unique probe to a CAM glycosylation variant.
NCAM Polysialylation
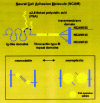
A significant post-translational modification of NCAM involves the attachment of large homopolymers of a2,8 polysialic acid (PSA), a modification that is specific to NCAM in the mammalian brain (Fig. 3).95,98 This post-translational modification of NCAM with PSA is believed to modulate NCAM-mediated homophilic adhesion and/or signal transduction events by virtue of its extensive negative charge .47,131,133 By attenuating adhesion forces and modulating overall cell surface interactions, NCAM PSA has the potential to orchestrate dynamic changes in the shape and movement of cells, as well as their processes.57,94
The development of a monoclonal antibody to extended chains of PSA found on meningococcus group B polysaccharides95 provided an unparalleled immunohistochemical tool for mapping PSA expression in the mammalian central nervous system. Moreover, a bacteriophage-derived endoneuraminidase (endo-N), that cleaves polysialosyl units associated with the K1 capsular antigen of certain Escherichia coli strains, provided the ideal control for these immunohistochemical procedures.29,128 Both anti-PSA and endo-N require a minimum recognition size of at least 10-12 residues46,71and, as a consequence, only NCAM with extended chains of polysialic acid is recognised.
Using these tools the distribution of NCAM PSA in the adult brain has been mapped and found to be associated primarily with those brain regions that undergo structural reorganization and synaptic plasticity, such as the olfactory bulb, hypothalamus and hippocampal formation and its associated structures in the medial temporal lobe.12,31,32,74,81,83,106 This convergent set of data has been used to suggest that NCAM PSA supports structural plasticity in adult nervous system.57 NCAM PSA has also been implicated in activity-dependent synaptic remodelling. The hypothalmo-neurohypophysial system expresses high levels of NCAM PSA throughout life and undergoes extensive morphological synaptic plasticity in response to physiological stimuli that is dependent on the surface expression of polysialylated NCAM.48,82 For example, microinjection of endo-N blocks cell surface expression of NCAM PSA and the synapse increase that occurs in response to lactation or osmotic stimulation.122
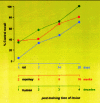
Consistent with this view is that removal of PSA with endoneuraminidase-N has been found to interfere with the induction and maintenance of hippocampal LTP and to produce spatial learning deficits in the Morris water maze paradigm.8,76 Moreover, the frequency of NCAM polysialylated hippocampal neurons at the dentate infragranular border transiently increase at 10-12h following acquisition of either spatial or conditioned avoidance tasks.22,31,77 These frequency changes are learning specific as they are not observed in animals rendered amnesic with either scopolamine or propofol.23,84 Similar coincident frequency increase of polysialylated neurons occurs in layer II of the entorhinal and perirhinal cortex following spatial learning.32,83
Regulation of NCAM Polysialylation State
Addition of PSA to NCAM takes place in the trans Golgi compartment and is entirely catalyzed by the polysialyltransferases STX (ST8SiaII ) and PST (ST8SiaIV).26,60,61,63,79 PSA expression appears to be inducible as recent studies have demonstrated inhibition of calcium-independent PKCd to be inversely related to NCAM polysialylation state both in vitro and in the hippocampal dentate gyrus during memory consolidation, an effect associated with the Golgi membrane fraction.34,35 However, the role of polysialyltransferase in other forms of synaptic plasticity is not so clear-cut. Mice with a targeted deletion of the gene encoding PST-1 exhibit an impairment of Schaffer collateral-CA1 LTP but not mossy fibre-CA3 LTP.26 These regional differences suggest that NCAM, but not PSA, is likely to be important for LTP in the hippocampal CA3 region. While PKC is known to be involved in learning and LTP, it is mainly the calcium-dependent isoforms that have been investigated and not in relation to the regulation of NCAM polysialylation.125
It would appear, however, that polysialyltransferase activity is not regulated by enzyme levels alone but is directly controlled by a cellular signalling cascade that encompasses a PKCd-dependent phosphorylation event involved in inhibition of polysialyltransferase activity.35 The cellular signalling cascade appears to be NMDA-dependent as inhibition of this receptor system prevents the rapid decrease in NCAM PSA expression observed following electrical signalling.13,78 Although cell activation seems to be an important regulatory factor in PSA synthesis other biosynthetic-independent mechanisms may also be involved. These most likely include the intracellular trafficking NCAM PSA by endocytotic cycling, however the molecular details of these mechanisms remain to be elaborated.57
The HNK1 Carbohydrate Epitope
NCAM PSA is not the only glycosylation mechanism associated with CAM function in the synaptic plasticity of memory consolidation. For example, many CAMs carry the HNK-1 carbohydrate structure, which was recognised first by a monoclonal antibody raised against human natural killer cells, hence, the acronym. The HNK-1 carbohydrate has been shown to consist of a minimal epitope of 3_-sulfated glucuronic acid attached to a lactosaminyl residue that is involved in the homophilic binding of NCAM.101 The HNK-1 epitope also appears to be involved in synaptic plasticity associated with LTP and inhibitory avoidance learning in both fish and rodents.87,99,116 However, the HNK-1 epitope lacks the advantage of NCAM PSA for defining the pathways associated with memory consolidation.
What about Neurogenesis in Learning?
Bromodeoxyuridine (BrdU) incorporation studies suggest that a proportion of the polysialylated neurons observed in the adult hippocampal dentate gyrus are recently generated106 and necessary for odour discrimination.40 Moreover, their depletion, following neurotoxic lesions, results in impaired trace conditioning of the eyeblink response, a hippocampal-dependent paradigm.107 However, the learning-induced transient increase in polysialylated denate neuron frequency during the 12h post-training period does not appear to be associated with increased neurogenesis.31 Indeed, the specificity of BrdU as a neurogenic marker has been recently questioned.90 In contrast to 3H-thymidine, BrdU is not a marker for cell division but rather a marker for DNA synthesis. This would explain the presence of BrdU-positive cells in layers other than those of the infragranular zone (Fig. 4).31 Moreover, the cells of the infragranular zone are remarkably heterogenous75 and, to date, there has been no attempt to relate BrdU or PSA labelling with their neurotransmitter phenotype. These polysialylated neurons receive synaptic input and are distinguished from the mature neurons, located in the superficial granule cell layer, by showing paired pulse facilitation and having a lower threshold for induction of LTP.126,129 In my view, the most likely functional significance of learning-induced transient increases in the polysialylated neurons of the dentate infragranular zone is to facilitate the dendritic elaboration of recently acquired neurons in response to the acquisition of novel behavioural repertoires.
Perspective
The function of dendritic remodelling as a mechanism for the consolidation of information is still in its infancy. There is still no clear consensus as to whether spine or synapse frequency change accompanies memory formation. Aside from the consideration of differing tissue fixation and stereological procedures, the majority of studies have failed to take account of the fleeting nature of hippocampal information processing prior to its eventual consolidation within the neocortex.117 Moreover, the diverse nature of the learning paradigms employed is also likely to result in temporal phase shifts of synaptic dynamics. For example, synapse frequency change occurs within minutes following the induction of LTP124 as compared to the changes wrought at 6-8h following the acquisition of a spatial learning paradigm.86 Moreover, 24h after the induction of LTP there is little evidence of spine frequency change or at 6 days following extensive training in the water maze.97,104 Studies on spine and synapse frequency change following behavioural adaptation must also be cognisant of the separate pathways and sub-regions of the medial temporal lobe that are involved in the processing of information. Spine frequency change has been observed in the dentate gyrus but not CA1 region of the hippocampal formation following successful acquisition of tasks dependent on this brain region.37,86
The question of cell signalling from the extracellular environment to the nucleus of the neuron is central to how the brain modifies its structure and function to learn and remember. In this regard, ideal transmembrane signalling molecules should control the connections formed between neurons and govern cytoskeletal dynamics in a manner that regulates their morphology. CAMs are cell surface macromolecules that control cell-cell interactions during development of the nervous system by regulating such processes as neuronal adhesion and migration, neurite outgrowth, fasciculation, synaptogenesis and intracellular signalling.
There is now substantial evidence that CAMs are intimately associated with the molecular cascade that underpins the synaptic plasticity of memory consolidation and, moreover, their functions follow a defined sequence of events. The temporal involvement of CAMs in memory consolidation is dramatically illustrated by interventive studies using anti-L1 in chick avoidance conditioning.103,123 In this paradigm L1 function is required at three discrete time periods over an 18h period. Again, with NCAM, and its PSA glycosylated variant, discrete periods of function are described for its role in acquisition, in a 6h post-training period that coincides with increased synapse production and, later at the 12h period.21,22,30,31,32,77,83,96,102 The fact that antibodies are ineffective in the intervening periods further supports the notion that CAMs have specific temporal actions in the molecular cascade of memory consolidation. Moreover, these experiments are consistent with the need for their extensive involvement in the processing of information within the medial temporal lobe prior to its re-distribution to multiple neocortical sites.
What is unclear about the role of CAMs in memory consolidation, however, is their suggested contribution to synapse connectivity pattern that is proposed to be generated by activity-dependent spine growth and de novo synapse formation. Indeed, the significance of de novo synapse formation as a functional correlate of memory consolidation is far from clear, as some studies have failed to equate this morphological associate with learning.37 Aside from the obvious consideration of differing tissue fixation procedures, failure to observe morphological change may relate to the temporal aspects of memory consolidation, as is exemplified by the CAM interventive studies. Although spine and synapse formation have been correlated with periods of CAM function in some studies,22,85,86,102 others have failed to find an increased spine number but, rather, an increased frequency of synapses expressing greater levels of CAM.104 In this regard, it is interesting to suggest that the increased NCAM labelling observed in the chick paradigm of avoidance conditioning, at a time sensitive to memory disruption with anti-L1 and anti-NCAM, may simply reflect selective retention of a synaptic population normally eliminated during the precocial development of this animal.102,103,108
What the future seems to hold is the need to associate increased CAM functions, such as signalling, and their role in de novo synapse formation. Too often CAM function has been extrapolated from in vitro studies and their role in the adult nervous system may be significantly different. This view is supported by the numerous models of targeted deletion of CAM function that have no significant developmental outcome but frequently exhibit learning deficits.16,33 Another example that supports a potential role for CAMs in synapse remodelling, as opposed to de novo synapse formation, comes from studies of peptide ligands with an affinity for the homophilic binding domain of NCAM.30 In vitro, such peptides induce neuritogenesis,93 a function that might predict an inherent cognition-enhancing property if the model of de novo synapse formation has relevance to memory consolidation. By contrast, these peptides produce a profound amnesia when administered during training or in the 6-8h post-training period in which anti-NCAM and spine formation is observed to occur following training.30 A more parsimonious interpretation could be that these peptide ligands disrupt pre- to postsynaptic signalling necessary for synapse strengthening.
An attractive possibility is that transmembrane CAMs transmit activity-dependent change of intracellular-signalling across the synapse hence modulating the strength of cell-cell binding and thereby rapidly altering synaptic structure and efficacy. Although the expression patterns of N-cadherin, NCAM and L1 can be regulated by distinct patterns of action potentials,51,52 their adhesive interactions across the synaptic cleft and in synaptic function has remained elusive. If expression of cell adhesion molecules can be regulated by activity, then newly synthesised molecules may participate in the formation and modification of synapses. For example, at certain synapses in the central nervous system, pre- and postsynaptic adhesion is mediated by N-cadherin that, upon depolarisation, dimerizes and becomes markedly protease resistant.119 Other in vitro studies have demonstrated activity-dependent strengthening of synapses to be dependent on integrins and/or the relative levels of post-synaptic, as opposed to pre-synaptic, NCAM.14,19
In conclusion, CAMs can no longer be viewed as the structural scaffold of the synapse but as active participants that are involved in all aspects of its plasticity, including trans-synapse signalling and structural modification. Understanding these trans-synapse signalling mechanisms will be crucial in relating extracellular adhesive interactions to gene transcription and protein synthesis that is required for long-term changes in synaptic function.
Acknowledgements
The author_s work reviewed herein is currently supported by the EU 5th Framework Programme (QLRT-1999-02187) and basic research grants from Enterprise Ireland and the Health Research Board of Ireland.
References
- 1.
- Alexinsky T, Przybyslawski J, Mileusnic R. et al. Antibody to day-old chick glycoprotein produces amnesia in adult rats. Neurobiol Learn Mem. 1997;67:14–20. [PubMed: 9013496]
- 2.
- Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: A simple network model. Proc Natl Acad Sci USA. 1994;91:7041–7045. [PMC free article: PMC44334] [PubMed: 8041742]
- 3.
- Arai A, Larson J, Lynch G. Anoxia reveals a vulnerable period in the development of long-term potentiation. Brain Res. 1990;511:353–357. [PubMed: 2334854]
- 4.
- Bahr BA, Lynch G. Purification of an Arg-Gly-Asp selective matrix receptor from brain synaptic plasma membranes. Biochem J. 1992;281:137–142. [PMC free article: PMC1130651] [PubMed: 1731748]
- 5.
- Bailey CH, Kandel ER. Structural changes accompany memory storage. Annu Rev Physiol. 1993;55: 397–426. [PubMed: 8466181]
- 6.
- Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA. 1996;93:13445–13452. [PMC free article: PMC33629] [PubMed: 8942955]
- 7.
- Bailey CH, Chen M, Keller F. et al. Serotonin-mediated endocytosis of apCAM: An early step of learning-related synaptic growth in Aplysia. Science. 1992;256:645–649. [PubMed: 1585177]
- 8.
- Becker CG, Artola A, Gerardy-Schahn R. et al. The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res. 1996;45:143–152. [PubMed: 8843031]
- 9.
- Benson DL, Schnapp LM, Shapiro L. et al. Making memories: cell adhesion molecules in synaptic plasticity. Trends Cell Biol. 2000;10:473–482. [PubMed: 11050419]
- 10.
- Black JE, Isaacs KR, Anderson BJ. et al. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar motor cortex of adult rats. Proc Natl Acad Sci USA. 1990;87:5568–5572. [PMC free article: PMC54366] [PubMed: 1695380]
- 11.
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. [PubMed: 8421494]
- 12.
- Bonfanti L, Olive S, Poulain DA. et al. Mapping of the distribution of polysialylated neural cell adhesion molecule throughout the central nervous system of the adult rat: An immunohistochemical study. Neurosci. 1992;49:419–436. [PubMed: 1436474]
- 13.
- Bouzioukh F, Tell F, Jean A. et al. NMDA receptor and nitric oxide synthase activation regulate polysialylated neural cell adhesion molecule expression in adult brainstem synapses. J Neurosci. 2001;21:4721–4730. [PMC free article: PMC6762337] [PubMed: 11425899]
- 14.
- Chun D, Gall CM, Bi X. et al. Evidence that integrins contribute to multiple stages in the consolidation of long-term potentiation in rat hippocampus. Neurosci. 2001;105:815–829. [PubMed: 11530220]
- 15.
- Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol. 2001;11:118–126. [PubMed: 11179881]
- 16.
- Cremer H, Lange R, Christoph A. et al. Inactivation of the N-CAM gene in mice results size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. [PubMed: 8107803]
- 17.
- Cremer H, Chazal G, Carleton A. et al. Long-term but not short-term plasticity at mossy fiber synapses is impaired in neural cell adhesion molecule-deficient mice. Proc Natl Acad Sci USA. 1998;95:13242–13247. [PMC free article: PMC23769] [PubMed: 9789073]
- 18.
- Crossin KL, Krushel LA. Cellular signaling by neural cell adhesion molecules of the immunoglobulin superfamily. Dev Dyn. 2000;218:260–279. [PubMed: 10842356]
- 19.
- Dityatev A, Dityatev G, Schachner M. Synaptic strength as a function of post- versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron. 2000;26:207–217. [PubMed: 10798405]
- 20.
- Doubell TP, Stewart MG. Short-term changes in the numerical density of synapses in the intermediate and medial hyperstriatum ventrale following one-trial passive avoidance training in the chick. J Neurosci. 1993;13:2230–2235. [PMC free article: PMC6576578] [PubMed: 8478696]
- 21.
- Doyle E, Nolan P, Bell R. et al. Intraventricular infusions of anti-neural cell adhesion molecules in a discrete post-training period impair consolidation of a passive avoidance response in the rat. J Neurochem. 1992;59:1570–1573. [PubMed: 1402906]
- 22.
- Doyle E, Bell R, Regan CM. Hippocampal NCAM180 transiently increases sialylation during the acquisition and consolidation of a passive avoidance response in the adult rat. J Neurosci Res. 1992;31:513–523. [PubMed: 1640502]
- 23.
- Doyle E, Regan CM. Cholinergic and dopaminergic agents which inhibit a passive avoidance response attenuate paradigm-specific increases in NCAM sialylation state. J Neural Transm. 1993;92:33–49. [PubMed: 8101092]
- 24.
- Dunaevsky A, Blazeski R, Yuste R. et al. Spine motility with synaptic contact. Nature Neurosci. 2001;4:685–686. [PubMed: 11426220]
- 25.
- Eckhardt M, Muhlenhoff M, Bethe A. et al. Molecular characterisation of eukaryotic polysialyltransferase-1. Nature. 1995;373:715–718. [PubMed: 7854457]
- 26.
- Eckhardt M, Bukalo O, Chazal G. et al. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J Neurosci. 2000;20:5234–5244. [PMC free article: PMC6772332] [PubMed: 10884307]
- 27.
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. [PubMed: 10331391]
- 28.
- Fiala JC, Feinberg M, Popov V. et al. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. [PMC free article: PMC6793554] [PubMed: 9786995]
- 29.
- Finne J, Makela PH. Cleavage of polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. J Biol Chem. 1985;260:1265–1270. [PubMed: 3968060]
- 30.
- Foley AG, Hartz BP, Gallagher HC. et al. A synthetic peptide ligand of neural cell adhesion molecule (NCAM) IgI domain prevents NCAM internalisation and disrupts passive avoidance learning. J Neurochem. 2000;74:2607–2613. [PubMed: 10820224]
- 31.
- Fox GB, O_Connell AW, Murphy KJ. et al. Memory consolidation induces a transient and time-dependent increase in the frequency of neural cell adhesion molecule polysialylated cells in the adult rat hippocampus. J Neurochem. 1995;65:2796–2799. [PubMed: 7595580]
- 32.
- Fox GB, Fichera G, Barry T. et al. Consolidation of passive avoidance learning is associated with transient increases of polysialylated neurons in layer II of the rat medial temporal cortex. J Neurobiol. 2000;45:135–141. [PubMed: 11074459]
- 33.
- Fransen E, D_Hooge R, van Camp G. et al. L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploratory patterns. Hum Mol Genet. 1998;7:999–1009. [PubMed: 9580664]
- 34.
- Gallagher HC, Odumeru OA, Regan CM. Regulation of neural cell adhesion molecule polysialylation state by cell-cell contact and protein kinase C delta. J Neurosci Res. 2000;61:636–645. [PubMed: 10972960]
- 35.
- Gallagher HC, Murphy KJ, Foley AG. et al. Protein kinase C delta regulates neural cell adhesion molecule polysialylation state in the rat brain. J Neurochem. 2001;77:425–434. [PubMed: 11299305]
- 36.
- Garner CC, Kindler S, Gundelfinger ED. Molecular determinants of presynaptic active zones. Curr Opin Neurobiol. 2000;10:321–327. [PubMed: 10851173]
- 37.
- Geinisman Y. Structural synaptic modifications associated with hippocampal LTP and behavioral learning. Cerebral Cortex. 2000;10:952–962. [PubMed: 11007546]
- 38.
- Geinisman Y, deToledo-Morrell L, Morrell F. Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with postsynaptic densities. Brain Res. 1991;566:77–88. [PubMed: 1814558]
- 39.
- Geinisman Y, Disterhoft JF, Gundersen HJG. et al. Remodelling of hippocampal synapses following hippocampus-dependent associative learning. J Comp Neurol. 2000;417:49–59. [PubMed: 10660887]
- 40.
- Gheusi G, Cremer H, McLean H. et al. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci USA. 2000;97:1823–1828. [PMC free article: PMC26520] [PubMed: 10677540]
- 41.
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. [PubMed: 10446041]
- 42.
- Grant SGN, O_Dell TJ. Multiprotein complex signaling and the plasticity problem. Curr Opin Neurobiol. 2001;11:363–368. [PubMed: 11399436]
- 43.
- Greenough WT, Bailey CH. The anatomy of memory: convergence of results across a diversity of tests. Trends Neurosci. 1988;11:142–147.
- 44.
- Groteweil MS, Beck CDO, Wu KH. et al. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. [PubMed: 9461212]
- 45.
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. [PubMed: 8210179]
- 46.
- Hayrinen J, Bitter-Suermann D, Finne J. Interaction of meningococcal group B monoclonal antibody and its Fab fragment with alpha 2-8-linked sialic acid polymers: requirement of a long oligosaccharide segment for binding. Mol Immunol. 1989;26: 523–529. [PubMed: 2505065]
- 47.
- Hoffman S, Edelman GM. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc Natl Acad Sci USA. 1983;89:5762–5766. [PMC free article: PMC384339] [PubMed: 6577452]
- 48.
- Hoyk Zs, Parducz A, Theodosis DT. The highly sialylated isoform of the neural cell adhesion molecule is required for estradiol-induced morphological plasticity in the adult arcuate nucleus. Eur J Neurosci. 2001;13:649–656. [PubMed: 11207800]
- 49.
- Huang A-M, Wang HL, Tang YP. et al. Expression of integrin-associated protein gene associated with memory formation in rats. J Neurosci. 1998;18:4305–4313. [PMC free article: PMC6792811] [PubMed: 9592107]
- 50.
- Hunter A, Stewart MG. Long-term increases in the numerical density of synapses in the chick lobus parolfactorius after passive avoidance training. Brain Res. 1993;605:251–255. [PubMed: 8481774]
- 51.
- Itoh K, Stevens B, Schachner M. et al. Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science. 1995;270:1369–1372. [PubMed: 7481827]
- 52.
- Itoh K, Ozaki M, Stevens B. et al. Activity-dependent regulation of N-cadherin in DRG neurons - differential regulation of N-cadherin, N-CAM and L1 by distinct patterns of action potentials. J Neurobiol. 1997;33:735–748. [PubMed: 9369148]
- 53.
- Kamiguchi H, Lemmon V. Recycling of the cell adhesion molecule L1 in axonal growth cones. J Neurosci. 2000;20:3676–3686. [PMC free article: PMC1237010] [PubMed: 10804209]
- 54.
- Kamiguchi H, Long KE, Pendergast M. et al. The neural cell adhesion molecule L1 intteracts with the AP-2 adaptor and is endocytosed via the clathrin-mediated pathway. J Neurosci. 1998;18:5311–5321. [PMC free article: PMC1226881] [PubMed: 9651214]
- 55.
- Kempermann G, van PraggH, Gage FH. Activity-dependent regulation of neuronal plasticity and self repair. Prog Brain Res. 2000;127:35–48. [PubMed: 11142036]
- 56.
- Kennedy MB. Signal-processing machines at the post-synaptic density. Science. 2000;290:750–754. [PubMed: 11052931]
- 57.
- Kiss JZ, Rougon G. Cell biology of sialic acid. Curr Opin Neurobiol. 1997;7:640–646. [PubMed: 9384537]
- 58.
- Kim JJ, Fanselow M. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–676. [PubMed: 1585183]
- 59.
- Kleim JA, Vij K, Ballard DH. et al. Learning-dependent synaptic modifications in the cerebellar cortex of the adult rat persist for at least four weeks. J Neurosci. 1997;17:717–721. [PMC free article: PMC6573226] [PubMed: 8987793]
- 60.
- Kojima N, Yoshida Y, Tsuji S. A developmentally regulated member of the sialyltransferase family (ST8Sia II, STX) is a polysialic acid synthase. FEBS Letts. 1995;373:119–122. [PubMed: 7589448]
- 61.
- Kojima N, Tachida Y, Yoshida Y. et al. Characterisation of mouse ST8Sia II (STX) as a neural cell adhesion molecule-specific polysialic acid synthase. J Biol Chem. 1996;271:19457–19463. [PubMed: 8702635]
- 62.
- Kramar EA, Bernard JA, Gall CM. et al. Alpha3 integrin receptors contribute to the consolidation of long-term potentiation. Neurosci. 2002;110:29–39. [PubMed: 11882370]
- 63.
- Livingston BD, Paulson JC. Polymerase chain reaction cloning of a developmentally regulated member of the sialyltransferase gene family. J Biol Chem. 1993;268:11504–11507. [PubMed: 7685014]
- 64.
- Läthi A, Laurent J-P, Figurov A. et al. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994;372:777–779. [PubMed: 7997264]
- 65.
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. [PubMed: 10082466]
- 66.
- Manabe T, Togashi H, Uchida N. et al. Loss of cadherin-11 adhesion receptor enhances plastic changes in hippocampal synapses and modifies behavioral responses. Mol Cell Neurosci. 2000;15:534–546. [PubMed: 10860580]
- 67.
- Maness PF, Beggs HE, Klinz SG. et al. Selective neural cell adhesion molecule signaling by Src family tyrosine kinases and tyrosine phosphatases. Perspect Dev Neurobiol. 1996;4:169–181. [PubMed: 9168199]
- 68.
- Matus A. Actin-based plasticity in dendritic spines. Science. 2000;290:754–758. [PubMed: 11052932]
- 69.
- Mayford M, Barzilai A, Keller F. et al. Modulation of an NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science. 1992;256:638–644. [PubMed: 1585176]
- 70.
- McGaugh JL. Memory - a century of consolidation. Science. 2000;287:248–251. [PubMed: 10634773]
- 71.
- Michon F, Brisson J-R, Jennings JH. Conformational differences between linear a(2-8)-linked homo-oligosaccharides and the epitope of the group B Meningococcal polysaccharide. Biochemistry. 1987;26:8399–8405. [PubMed: 2450562]
- 72.
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. [PubMed: 9539121]
- 73.
- Mi__ R, Duran JM, Tomas M. et al. Neural cell adhesion molecule is endocytosed via a clathrin-dependent pathway. Eur J Neurosci. 2001;13:749–756. [PubMed: 11207809]
- 74.
- Miragall F, Kadman G, Husmann M. et al. Expression of cell adhesion molecules in the olfactory system of the adult mouse: presence of the embyronic form of NCAM. Dev Biol. 1988;129:516–531. [PubMed: 3417050]
- 75.
- Mott DD, Turner DA, Okazaki MM. et al. Interneurons of the dentate-hilus border of the rat dentate gyrus: Morphological and electrophysiological heterogeneity. J Neurosci. 1997;17:3990–4005. [PMC free article: PMC6573561] [PubMed: 9151716]
- 76.
- Muller D, Wang C, Skibo G. et al. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17:413–422. [PubMed: 8816705]
- 77.
- Murphy KJ, O_Connell AW, Regan CM. Repetitive and transient increases in hippocampal neural cell adhesion molecule polysialylation state following multi-trial spatial training. J Neurochem. 1996;67:1268–1274. [PubMed: 8752135]
- 78.
- Nacher J, Rosell DR, Alonso-Llosa G. et al. NMDA receptor antagonist induces a long-lasting increase in the number of proliferating cells, PSA-NCAM-immunoreactive granule neurons and radial glia in the adult rat dentate gyrus. Eur J Neurosci. 2001;13:512–520. [PubMed: 11168558]
- 79.
- Nakayama J, Fukuda M. A human polysialyltransferase directs in vitro synthesis of polysialic acid. J Biol Chem. 1996;271:1829–1832. [PubMed: 8567623]
- 80.
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. [PubMed: 8066450]
- 81.
- Ní Dhúill CM, Fox GB, Pittock SJ. et al. Polysialylated neural cell adhesion molecule expression in the dentate gyrus of the human hippocampal formation from infancy to old age. J Neurosci Res. 1999;55:99–106. [PubMed: 9890438]
- 82.
- Nothias F, Vernier P, von Boxberg Y. et al. Modulation of NCAM polysialylation is associated with morphofunctional modifications in the hypothalamo-neurohypophysial system during lactation. Eur J Neurosci. 1997;9:1553–1565. [PubMed: 9283810]
- 83.
- O_Connell AW, Fox GB, Murphy KJ. et al. Spatial learning activates neural cell adhesion molecule polysialylation in a cortico-hippocampal pathway within the medial temporal lobe. J Neurochem. 1997;68:2538–2546. [PubMed: 9166750]
- 84.
- O_Gorman DA, O_Connell AW, Murphy KJ. et al. Nefiracetam prevents propofol-induced anterograde and retrograde amnesia in the rodent without compromising quality of anesthesia. Anesthesiology. 1998;89:699–706. [PubMed: 9743408]
- 85.
- O_Malley A, O_Connell C, Regan CM. Ultrastructural analysis reveals avoidance conditioning to induce a transient increase in hippocampal dentate spine density in the 6h post-training period of consolidation. Neuroscience. 1998;87:607–613. [PubMed: 9758227]
- 86.
- O_Malley A, O_Connell C, Murphy KJ. et al. Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompany consolidation of a spatial learning task in the rodent. Neuroscience. 2000;99:229–232. [PubMed: 10938428]
- 87.
- Pradel G, Schachner M, Schmidt R. Inhibition of memory consolidation by antibodies against cell adhesion molecules after active avoidance conditioning in zebrafish. J Neurobiol. 1999;39:197–206. [PubMed: 10235674]
- 88.
- Pradel G, Schmidt R, Schachner M. Involvement of L1.1 in memory consolidation after active avoidance conditioning in zebrafish. J Neurobiol. 2000;43:389–403. [PubMed: 10861564]
- 89.
- Pollerberg GE, Burridge K, Krebs KE. et al. The 180-kD component of the neural cell adhesion molecule N-CAM is involved in cell-cell contacts and cytoskeleton-membrane interactions. Cell Tiss Res. 1997;250:227–236. [PubMed: 3308110]
- 90.
- Rakic P. Adult neurogenesis in mammals: an identity crisis. J Neurosci. 2002;22:614–618. [PMC free article: PMC6758501] [PubMed: 11826088]
- 91.
- Rohrbough J, Groteweil MS, Davis RL. et al. Integrin-mediated regulation of synaptic morphology, transmission and plasticity. J Neurosci. 2000;20:6868–6878. [PMC free article: PMC6772806] [PubMed: 10995831]
- 92.
- Rønn LCB, Bock E, Linnemann D. et al. NCAM-antibodies modulate induction of long-term potentiation in rat hippocampal CA1. Brain Res. 1995;677:145–151. [PubMed: 7606459]
- 93.
- Rønn LCB, Olsen M, Øtergaard S. et al. Stimulation of neurite outgrowth by a synthetic NCAM ligand identified by means of combinatorial libraries. Nature Biotechnol. 1999;17:1000–1005.
- 94.
- Rougon G. Structure, metabolism and cell biology of polysialic acids. Eur J Cell Biol. 1993;61:197–207. [PubMed: 7693470]
- 95.
- Rougon G, Dubois C, Buckley N. et al. A monoclonal antibody against meningococcus group B polysaccharides distinguishes embryonic from adult N-CAM. J Cell Biol. 1986;103:2429–2437. [PMC free article: PMC2114611] [PubMed: 3536966]
- 96.
- Roullet P, Mileusnic R, Rose SPR. et al. Neural cell adhesion molecules play a role in rat memory formation in appetitive as well as aversive tasks. Neuroreport. 1997;8:1907–1911. [PubMed: 9223075]
- 97.
- Rusakov DA, Davies HA, Harrison E. et al. Ultrastructural synaptic correlates of spatial learning in rat hippocampus. Neuroscience. 1997;80:69–77. [PubMed: 9252221]
- 98.
- Sadoul R, Hirn M, Deagostini H. et al. Adult and embryonic mouse neural cell adhesion molecules have different binding properties. Nature. 1983;304:347–349. [PubMed: 6877355]
- 99.
- Saghatelyan AK, Gorissen S, Albert M. et al. The extracellular matrix molecule tenascin-R and its HNK-1 carbohydrate modulate periosomatic inhibition and long-term potentiation in the CA1 region of the hippocampus. Eur J Neurosci. 2000;12:3331–3342. [PubMed: 10998116]
- 100.
- Sanes JR, Lichtman JW. Can molecules explain long-term potentiation? Nature Neurosci 1999; 2:597-604. [PubMed: 10404178]
- 101.
- Schachner M, Martini R. Glycans and the modulation of neural-recognition molecule function. Trends Neurosci. 1995;18:183–91. [PubMed: 7539963]
- 102.
- Scholey AB, Rose SPR, Zamani MR. et al. A role for the neural cell adhesion molecule in a late, consolidating phase of glycoprotein synthesis six hours following passive avoidance training in the young chick. Neuroscience. 1993;55:499–509. [PubMed: 8377940]
- 103.
- Scholey AB, Mileusnic R, Schachner M. et al. A role for a chicken homologue of the neural cell adhesion molecule L1 in consolidation of a passive avoidance task in the chick. Learn Memory. 1995;2:17–25. [PubMed: 10467563]
- 104.
- Schuster T, Krug M, Hassan H. et al. Increase in proportion of hippocampal spine synapses expressing neural cell adhesion molecule NCAM180 following long-term potentiation. J Neurobiol. 1998;37:359–372. [PubMed: 9828042]
- 105.
- Schuster T, Krug M, Stalder M. et al. Immunoelectron microscopic localization of the neural recognition molecules L1, NCAM, and its isoform NCAM180, the NCAM-associated polysialic acid, beta1 integrin and the extracellular molecule tenascin-R in synapses of the adult rat hippocampus. J Neurobiol. 2001;49:142–158. [PubMed: 11598921]
- 106.
- Seki T, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci. 1993;13:2351–2358. [PMC free article: PMC6576495] [PubMed: 7684771]
- 107.
- Shors TJ, Miesegaes G, Beylin A. et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. [PubMed: 11268214]
- 108.
- Skibo GC, Davies HA, Rusakov DA. et al. Increased immunogold labelling of neural cell adhesion molecule isoforms in synaptic active zones of the chick striatum 5-6 hours after one-trial passive avoidance training. Neuroscience. 1998;82:1–5. [PubMed: 9483498]
- 109.
- Smalla KH, Matthies H, Langnase K. et al. The synaptic glycoprotein neuroplastin is involved in long-term potentiation at hippocampal CA1 synapses. Proc Natl Acad Sci USA. 2000;97:4327–32. [PMC free article: PMC18241] [PubMed: 10759566]
- 110.
- Sorra KE, Harris KM. Stability in synapse number and size at 2hr after long-term potentiation in hippocampal area CA1. J Neurosci. 1998;18:658–671. [PMC free article: PMC6792539] [PubMed: 9425008]
- 111.
- Staubli U, Chun D. Factors regulating the reversibility of long term potentiation. J Neurosci. 1996;16:853–860. [PMC free article: PMC6578635] [PubMed: 8551365]
- 112.
- Staubli U, Vanderklish P, Lynch G. An inhibitor of integrin receptors blocks long-term potentiation. Behav Neur Biol. 1990;53:1–5. [PubMed: 2154174]
- 113.
- Stork O, Welzl H, Cremer H. et al. Increased intermale aggression and neuroendocrine response in mice deficient for the neural cell adhesion molecule (NCAM). Eur J Neurosci. 1997;9:1117–1125. [PubMed: 9215693]
- 114.
- Stork O, Welzl H, Wotjak CT. Anxiety and increased 5HT1A receptor response in NCAM null mice. J Neurobiol. 1999;40:343–355. [PubMed: 10440734]
- 115.
- Stork O, Welzl H, Wolfer D. et al. Recovery of emotional behaviour in neural cell adhesion molecule (NCAM) mutant mice through transgenic expression of NCAM180. Eur J Neurosci. 2000;12:3291–3306. [PubMed: 10998113]
- 116.
- Strekalova T, Wotjak CT, Schachner M. Intrahippocampal administration of an antibody against the HNK-1 carbohydrate impairs memory consolidation in an inhibitory learning task in mice. Mol Cell Neurosci. 2001;17:1102–1113. [PubMed: 11414798]
- 117.
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 2001;99:195–231. [PubMed: 1594723]
- 118.
- Squire LR, Barondes SH. Variable decay of memory and its recovery in cycloheximide-treated mice. Proc Natl Acad Sci USA. 1972;69:1416–1420. [PMC free article: PMC426715] [PubMed: 4504352]
- 119.
- Tanaka H, Shan W, Phillips GR. et al. Molecular modification of N-cadherin in response to synaptic activity. Neuron. 2000;25:93–107. [PubMed: 10707975]
- 120.
- Tang L, Hung CP, Schuman EM. A role for the cadherin family of cell adhesion molecules in hippocampal long-termpotentiation. Neuron. 1998;20:1165–1175. [PubMed: 9655504]
- 121.
- Tang Y, Nyengaard JR, deGroot DMG. et al. Total regional and global number of synapses in the human brain neocortex. Synapse. 2001;41:258–273. [PubMed: 11418939]
- 122.
- Theodosis DT, Bonhomme R, Vitiello S. et al. Cell surface expression of polysialic acid on NCAM is a prerequisite for activity-dependent morphological neuronal and glial plasticity. J Neurosci. 1999;19:10228–10236. [PMC free article: PMC6782420] [PubMed: 10575020]
- 123.
- Tiunova A, Anokhin KV, Rose SPR. Three time windows for amnestic effect of antibodies to cell adhesion molecule L1 in chicks. Neuroreport. 1998;9:1645–1648. [PubMed: 9631480]
- 124.
- Toni N, Buchs P-A, Nikonenko I. et al. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. [PubMed: 10586883]
- 125.
- van der ZeeEA, Douma BRK. Historical review of research on protein kinase C in learning and memory. Prog Neuropsychopharm Biol Psychiat. 1997;21:379–406. [PubMed: 9153065]
- 126.
- van PraggH, Schinder AF, Christie BR. et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. [PMC free article: PMC9284568] [PubMed: 11875571]
- 127.
- van ReemptsJ, Dikova M, Werbrouck L. et al. Synaptic plasticity in rat hippocampus associated with learning. Behav Brain Res. 1992;51:179–83. [PubMed: 1334671]
- 128.
- Vimr ER, McCoy RD, Vollger HF. et al. Use of prokaryotic-derived probes to identify poly(sialic acid) in neonatal neuronal membranes. Proc Natl Acad Sci USA. 1984;81:1971–1975. [PMC free article: PMC345418] [PubMed: 6371806]
- 129.
- Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed: 10640331]
- 130.
- Wenzel J, Kammerer E, Kirsche W. et al. Electron microscopic and morphometric studies on synaptic plasticity in the hippocampus of the rat following conditioning. J Hirnforsch. 1980;21:647–54. [PubMed: 7229348]
- 131.
- Williams EJ, Furness J, Walsh FS. et al. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. [PubMed: 7917292]
- 132.
- Wong WT, Faulkner-Jones BE, Sanes JR. et al. Rapid dendritic remodeling in the developing retina: dependence on neurotransmission and reciprocal regulation by Rac and Rho. J Neurosci. 2000;20:5024–5036. [PMC free article: PMC6772263] [PubMed: 10864960]
- 133.
- Yang P, Major D, Rutishauser U. Role of charge and hydration in effects of polysialic acid on molecular interactions on and between cell membranes. J Biol Chem. 1994;269:23039–23044. [PubMed: 8083205]
- 134.
- Zhang W, Benson DL. Development and molecular organization of dendritic spines and their synapses. Hippocampus. 2000;10:512–526. [PubMed: 11075822]
- 135.
- Zola-Morgan S, Squire LR. The primate hippocampal formation. Evidence for a time-limited role in memory storage. Science. 1990;250:288–290. [PubMed: 2218534]
- Cell Adhesion Molecules - Madame Curie Bioscience DatabaseCell Adhesion Molecules - Madame Curie Bioscience Database
- Coronin Structure and Implications - Madame Curie Bioscience DatabaseCoronin Structure and Implications - Madame Curie Bioscience Database
- Matrix Metalloproteases and Epithelial-to-Mesenchymal Transition: Implications f...Matrix Metalloproteases and Epithelial-to-Mesenchymal Transition: Implications for Carcinoma Metastasis - Madame Curie Bioscience Database
- Possible Roles of DNA Supercoiling in Transcription - Madame Curie Bioscience Da...Possible Roles of DNA Supercoiling in Transcription - Madame Curie Bioscience Database
- Proteases and Their Cognate Inhibitors of the Serine and Metalloprotease Subclas...Proteases and Their Cognate Inhibitors of the Serine and Metalloprotease Subclasses, in Testicular Physiology - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...