NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Dictyostelium establishes an asymmetric body axis during development that is dependent upon signaling through distinct 7-TM cAMP morphogen receptors (CARs) that regulate GSK3-mediated downstream effectors. These pathways reflect a similar dependency for metazoan cell fate decisions on Wnt/Frizzled-GSK3 signaling. In Dictyostelium, GSK3 promotes posterior (prespore) cell patterning but antagonizes anterior (prestalk) differentiation. We have shown that during pattern formation in Dictyostelium, distinct CARs alternatively activate a tyrosine kinase, ZAK1 that mediates GSK3 activation or a tyrosine phosphatase that inhibits GSK3 activity. We have identified the essential phospho-tyrosines in Dictyostelium and in mammalian GSK3 and shown that they are required for complete enzymatic activation. Thus, developmentally regulated GSK3 tyrosine phosphorylation/de-phosphorylation determines the activated/de-activated state of GSK3. We also suggest a molecular mechanism for GSK3 activation through tyrosine phosphorylation in the context of its three dimensional structure. Finally, we demonstrate that CARs and Frizzleds are phylogenetically related, providing a comparative understanding of cAMP/CAR-GSK3 and Wnt/Frizzled-GSK3 pathways.
Introduction
Protein kinase GSK3 is a central element that regulates fate patterns during multicellular development.1-9 In the metazoa, the morphogen Wnt (Wg) regulates developmental cascades by activation of the family of 7-transmembrane (TM) Frizzled receptors and effective inhibition of GSK3 activity. GSK3 also serves as a core switch for Dictyostelium cell fate determination. Although Dictyostelium proliferates as unicellular amoeba, it develops as a multicellular organism with an asymmetric body axis and distinctly differentiated compartments.5,10-11Regulation of GSK3 activity is central to processing developmental signals to direct these asymmetric cell fate decisions.
The precise molecular mechanism for GSK3 down-regulation by Wnt remains elusive, but it is argued to be more related to control of substrate accessibility than directly associated with GSK3 enzymatic activity.1-9However, we have now shown that in Dictyostelium the enzymatic activity of GSK3 is specifically and antagonistically regulated by separate 7-TM receptors, and that activated or de-activated states of GSK3 direct different developmental cell fate decisions.12-15This developmental orchestration is dependent upon the tyrosine phosphoryla- tion of GSK3.14,17,18 Antagonistic receptor signaling alternatively activates a specific tyrosine kinase, ZAK1, or a specific protein tyrosine phosphatase.14,17 We have identified essential phospho-tyrosines in GSK3, confirmed their role in activated/de-activated regulation, and related them to the predicted 3D-structure of GSK3.17,19,20 These tyrosines are conserved in all GSK3 proteins, suggesting the possibility for similar regulation in more elaborated metazoa.17,18
The cell surface 7-TM receptors of Dictyostelium do not recognize Wnt, rather they bind a distinct extracellular ligand, cAMP. However, both receptor families nonetheless share topologically organized amino acid sequence similarity through their TM and loop domains, suggesting an ancient phylogenetic relationship for regulation of GSK3. Further, downstream of GSK3, they also target structurally related β-catenin proteins.21 Thus, the capacity of Dictyostelium to differentiate into a multicellular organism may define an ancient developmental paradigm for GSK3-regulated cell fate decisions. Our molecular findings in Dictyostelium may provide novel insight into this program for all phyla. In this chapter, we will discuss novel GSK3 regulated pathways in Dictyostelium and the potential for their interface with Wnt signaling.
Antagonistic Regulation of Cell Fate Determination in Dictyosteliumby the 7-Transmembrane cAMP Receptors CAR3 and CAR4
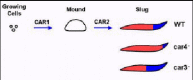
Extracellular cAMP elicits multiple, even antagonistic, responses during Dictyostelium development.5,10,11,17,22cAMP initially serves as a chemoattractant to recruit individual, undifferentiated cells to form multicellular structures, aggregation mounds (see Fig. 1). This chemotactic response is directed by CAR1, a high affinity, G protein-coupled, 7-transmembrane (TM) cell surface receptor for cAMP. CAR1 signaling also mediates induction of the cAMP-dependent gene activation pathways required for early development, thus defining the complexity of pathways regulated downstream of receptor activation. Three functionally distinct cAMP receptors, CARs 2, 3, and 4, become sequentially expressed as development proceeds.23 Their temporally and spatially restricted expressions are required for the differentiation of the various cell types and proper patterning. Following aggregate formation, the prestalk and prespore progenitor cells begin to differentiate. They eventually become spatially separated from each other, with the prestalk cells localized primarily at the tip of the aggregation mound, while the prespore cells form the base of the aggregate. The multicellular mound undergoes morphogenesis, elongating into a pseudoplasmodium, the migrating slug, that is organized along a prestalk/anterior and prespore/posterior horizontal axis (see Fig. 1). This pattern formation precedes developmental culmination and terminal differentiation of the progenitor prestalk and prespore cells into the stalk and spores of the mature fruiting body.5,10,11
There are essential and distinct roles for each of the four cAMP receptors during Dictyostelium development (see Fig. 1). The primary defects observed with car1- and car2-nulls involve developmental arrest. CAR1 is required for multi-cell formation, progression through the earliest stages of development, and even induction of CARs 2, 3, and 4. car2-null cells arrest at the next major stage, establishment of the patterned aggregate prior to slug morphogenesis (Fig. 1). Both CAR1 and CAR2 regulate patterns of cell movement, but also, gene expression during development.24
CAR3 is the major cAMP receptor of the posterior prespore cells, while CAR4 is enriched in the anterior prestalk population.15,25car3- cells have decreased prespore gene expression, but up-regulated prestalk gene expression (Fig. 1). Conversely, car4- cells have enhanced prespore gene expression patterns, with a concomitant inhibition of prespore differentiation (Fig. 1).5,12,17
Thus, a single morphogen, cAMP, can elicit antagonistic responses for cell fate determination through distinct expression patterns of CAR3 and CAR4 and the differential action on their downstream signaling components.
GSK3, a Developmental Switch Regulating Anterior/Posterior Axis Formation
GSK3, a serine/threonine protein kinase, was originally identified as a regulator of glycogen metabolism, but it has recently witnessed an experimental renaissance with the discovery of its role as an effective switch regulating diverse developmental fate choices.1-9 GSK3 has basal kinase activity, and inactive or activated GSK3 will antagonistically regulate dorsal/ventral patterns in vertebrates, posterior/anterior segment polarity in Drosophila, mesoderm/endoderm cell fates in C. elegans, and anterior/posterior cell fates in Dictyostelium, among others.1,4-8,12-17,26-29
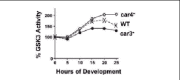
Genetic studies on canonical Wnt signaling suggested an inhibition of GSK3 activity through Frizzled receptor-dependent pathways, while in Dictyostelium, GSK3 is regulated by both inhibiting, as well as, activating pathways.5,12-17During development the specific kinase activity of GSK3 undergoes cAMP-dependent up-regulation, followed by an inhibitory, de-activating response (Fig. 2). The activation process is mediated by CAR3 and the major cell fate defects observed in car3-null cells are phenocopied in cells that lack GSK3. Indeed, GSK3 activation is blocked in car3- cells (Fig. 2). Thus, CAR3 activation of GSK3 is required to establish prespore/spore fate patterns, but is inhibitory to prestalk/stalk development. Conversely, GSK3 specific activity is antagonized by CAR4. The car4-null phenotype can be rescued by expression of a kinase-inactive form of GSK3. The dominant-negative nature of this inactive enzyme, places GSK3 as a negative target, downstream of CAR4.14,17Furthermore, repression of GSK3 specific activity is compromised in cells that lack CAR4 (Fig. 2). Given that, activated GSK3 will inhibit prestalk patterns, it is not surprising that car4-nulls exhibit significant repression of prestalk-specific gene expression.
cAMP will both stimulate and repress GSK3 through the antagonistic actions of the CAR3 and CAR4 receptors during Dictyostelium development. A CAR3-mediated GSK3 stimulating signal would prevail in the prespore cells, whereas the CAR4-dependent inhibitory pathway would dominate in the prestalk cells.
Although the CAR and Frizzled (Fz) 7-TM receptors are activated by distinct ligands, cAMP and Wnt, respectively, and the Fz members have been considered a strictly metazoan receptor, the resemblance of the CAR and Fz signaling pathways converging at GSK3 invited a more rigorous phylogenetic analysis. Fz family members are defined by the Fz structural domain (pfam01392) within transmembrane domains 2-6. This is especially highlighted by sequence comparison the Fz domain with the Hedgehog co-receptor Smoothened (Smo), a divergent member of the Fz family.30Two recent reports indicate that Hedgehog signaling from Smoothened requires GSK3 and emphasize the molecular connection between Fz domain-containing receptors and GSK3.31,32This relationship is further underscored by sequence comparison of CAR3 and CAR4 with the consensus Fz transmembrane domain (Fig. 3). Significant alignments (9e-04) of the CARs and Fz receptors using ClustalW and PROTPARS (in PHILYP 3.5) confirm topological, spatial, and sequence preservation throughout the entire Fz domain, and spanning both transmembrane and loop domains 2-6 of the CAR and Fz families (Fig. 3). The phylogenetic kinship among the Fz and CAR receptors, in concert with their common functional linkage to GSK3 and β-catenin, underlies the conservation of a Wnt-like pathway for regulating cell fate decisions and patterning among these diverse developmental systems.21 This suggests an ancient origin for this essential pathway.
ZAK1 and PTPase Regulate Tyrosine Phosphorylation and Activity of GSK3
We had previously identified the novel tyrosine kinase ZAK1 in Dictyostelium that is essential for normal prespore/spore differentiation.14 The developmental defects of zak1-nulls are very similar to those observed for car3- and gsk3-null cells. We have additionally shown that cAMP activates ZAK1 tyrosine kinase activity in wild-type cells, but not in cells lacking CAR3. These data indicate that ZAK1 is a downstream effector of CAR3. Furthermore, ZAK1 is required for cAMP/CAR3-mediated activation of GSK3. We, therfore, suggested that GSK3 may be a direct substrate of ZAK1 and that GSK3 activation during Dictyostelium development may be regulated by tyrosine phosphorylation.14,17
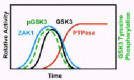
We have, in fact, demonstrated that GSK3 is tyrosine phosphorylated in response to cAMP and that phosphorylation anticipates GSK3 activation (Fig. 4). These responses are not permanent; with time, GSK3 is both de-phosphorylated and de-activated.17 We further showed that phosphorylation and activation of GSK3 is absent in car3-nulls, while both are persistent in car4-nulls. We have also demonstrated that ZAK1 can both phosphorylate and activate Dictyostelium, as well as, mammalian GSK3β (see below) in vitro.14 Finally, we showed that activation of GSK3 during Dictyostelium development is regulated in vivo by the cAMP/CAR3/ZAK1-mediated pathway (Fig. 4), while inhibition of GSK3 is mediated by a de-phosphorylation mechanism regulated by CAR4.14,17
Potentially, CAR4 could inhibit the CAR3-mediated activation of ZAK1 and in the absence of an activated ZAK1, GSK3 would be de-phosphoryated by a constitutively active PTPase and, hence, repressed. Alternatively, CAR4 may regulate directly the PTPase activity responsible for de-phosphorylating and de-activating GSK3. We measured the cAMP-mediated activation of ZAK1 in both wild-type and car4- cells and found identical results.17 In both stains, ZAK1 is transiently activated, with temporal kinetics that parallel that of GSK3 tyrosine phosphorylation in vivo (Fig. 4). Furthermore, using a tyrosine phosphorylated GSK3 substrate, we were able to detect cAMP-mediated activation of PTPase in wild-type cells, whose activation was undetectable in car4- cells (Fig. 4). These data strongly suggest that CAR4 regulates a PTPase that specifically de-activates GSK3.17
The amino acid sequences of mammalian and Dictyostelium GSK3 are highly conserved within their catalytic domains; 14 tyrosine residues are identically conserved (Fig. 5). The ability to phosphorylate GSK3 in vitro, has permitted us to map the phosphorylated tyrosines and to show that they are essential for activation by ZAK1. Figure 5 indicates the two tyrosine residues within the activation loop of GSK3 that are substrates for phosphorylation by ZAK1. Mutation of either tyrosine to phenylalanine reduces basal activity of GSK3, and expression of either Y/F mutant form in vivo phenocopies expression of kinase-inactive, dominant-negative GSK3 and mimics that of car3-, zak1-, or gsk3-nulls.17
Recent resolution of the crystal structure of unphosphorylated GSK3 provides valuable insights on the role of the tyrosine phosphorylation on GSK3.19,20The activation loops of inactive kinases, such as unphosphorylated ERK, adopt a closed conformation that limits substrate access. In contrast, the activation loop of phospho-ERK has an open conformation. Here, the phosphate group in the activation loop forms stable ionic interactions with neighboring arginine residues, which re-orients the position of the phopho-tyrosine, thus stabilizing the open structure and increasing substrate access. It is interesting that the activation loop of unphosphorylated, basally active GSK3 is already structurally open. However, the un-phosphorylated tyrosine residues of the activation loop of GSK3 are unable to interact with the neighboring arginines but instead are free to sterically restrict substrate access to the active site. Upon phosphorylation by ZAK1, these tyrosine residues may form stable ionic interactions with the positively charged arginine neighbors (see Fig. 5) which would permit full substrate access and hyperactivation.
Inhibitory homodimers of GSK3 have also been reported, with the activation loop as a major interaction surface. We speculate that the phosphorylation of tyrosine residues within the activation loop may disrupt the dimer formation, potentially providing a secondary mechanism for GSK3 activation through tyrosine phosphorylation.
Downstream Targets of GSK3 Aar (β-catenin)
β- catenin is a multifunctional protein with transcriptional co-factor properties and is a principal target immediately downstream of GSK3. In the canonical Wnt pathway, GSK3 phosphorylated β-catenin is subject to rapid degradation by a ubiquitin/proteosome pathway involving APC, βTrCP, among others;1-9 Wnt-stimulated inhibition of GSK3, thus, stabilizes β-catenin and activates transcriptional pathways further downstream. In Dictyostelium, the armadillo-repeat protein Aardvark, Aar, which is structurally related to β-catenin, also appears to function downstream of GSK3. As with β-catenin, Aar has multiple, consensus GSK3 phosphorylation sites in its amino-terminal region (Fig. 6), although Aar lacks the C-terminal transactivating domain found in β-catenin.
While β-catenin serves as a negative target of GSK3 in the canonical pathway, cAMP-induced prespore gene expression appears dependent upon Aar, placing it in a pathway positively regulated by GSK3 signaling. This has parallel with the positive regulation of the β-catenin-like Wrm-1 by GSK3 during C. elegans endoderm differentiation (see Fig. 7).29 In addition, while GSK3 acts as a developmental switch regulating both prestalk and prespore pathways, disruption of Aar only alters prespore differentiation without inducing the concomitant increase in prestalk specific gene expression that is observed in car3-, zak1- and gsk3-nulls.33 These data indicate that Dictyostelium requires a prestalk-specific, GSK3-sensitive transcriptional factor that is distinct from Aar. This may suggest the presence of an additional, novel β-catenin-like protein in Dictyostelium.
Perspectives—Integrating Tyrosine Phosphorylation with Other Signaling Components
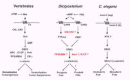
Multicellular organisms share a common dependency on a GSK3/β-catenin signaling cascade to regulate cell fate patterns (Fig. 7). In vertebrates, β-catenin acts as a transcriptional co-factor to positively regulate a series of developmental events, including, dorsal/ventral or tumorigenic pathways, among many others.1,4-9,34-36 GSK3 phosphorylation of β-catenin pro- motes an APC/βTrCP-dependent path for degradation; Axin scaffolds GSK3, APC and β-catenin, while CKIα serves as a priming kinase for GSK3 phosphorylation.37,38 PP2A/B56 may also facilitate degradative events (Fig. 7). The morphogen Wnt initiates an inhibitory signal cascade that requires dishevelled (dsh) to functionally down-regulate GSK3, potentially by decreasing GSK3 accessibility to β-catenin. Secreted Wnt may be specifically antagonized by a variety of extracellular molecules, including sFRP. Caesin kinases I and II have been implicated in the inhibitory pathway, but their actions have not been explicitly defined; additional components, including the LRP co-receptors are not shown. In the presence of Wnt, unphosphorylated β-catenin can accumulate; in complex with LEF/TCF, it can potentiate transcriptional activation.
Thus, Wnt signaling in the vertebrates promotes dorsal fate patterning, for example, in vertebrates, but antagonizes ventral pattern formation (Fig. 7). In Drosophila, Wg signaling promotes naked cuticle formation and posterior segment polarity, but antagonizes denticle formation and anterior segment polarity.
In the endoderm/mesoderm fate pathway of C. elegans, Wnt-signaling does not inhibit GSK3 (Fig. 7). The Wnt homologue mom-2 initiates a positive signal cascade from the Fz homologue mom-5 to GSK3. Active GSK3 is required for endoderm formation, while loss of GSK3 function will lead to a mesodermal fate.29 Additional molecular intermediates in this pathway have not been identified.
By contrast, Dictyostelium exhibits both positive and negative signaling cascades downstream of their morphogen cAMP receptors to regulate the common target GSK3. Stimulation of CAR3 activates GSK3 and promotes prespore fate patterns, whereas CAR4 stimulation will inhibit GSK3 and promote prestalk differentiation. CAR3 activation of GSK3 is mediated by the tyrosine kinase ZAK1, while CAR4 inhibits GSK3 by a tyrosine phosphatase (PTPase)-dependent pathway. Thus, tyrosine phosphorylation/de-phosphorylation of GSK3 mediates antagonistic receptor regulation for GSK3 activation/de-activation and cell fate determination. Both pathways are downregulated by secreted cAMP phosphodiesterase (PDE), which will remove the activating extracellular cAMP signal. We have recently turned our focus to the potential roles of CKI, CKII, and PP2A/B56 in Dictyostelium.39,40 Their genes have been isolated and their developmental functions are being assessed. Further, since expression of kinase-inactive GSK3 in Dictyostelium functions as a dominant-negative mutant to phenocopy the gsk3-null, we argue that enzymatic activity alone can not explain the entire regulatory pathway.41 We, therefore, suggest that a scaffolding protein functionally equivalent to Axin, must also be present in Dictyostelium. In addition, pathways dependent on the function of βTrCP-related F-box proteins are also essential for Dictyostelium development.42,43
It remains an open question if tyrosine phosphorylation of GSK3 can regulate cell fate decisions in other systems. Potentially, tyrosine phosphorylation and, hence, hyper-activation of GSK3 could directly antagonize Wnt signaling to provide additional regulation for differentiation and tumor suppression; furthermore, an activated PTPase may also be functionally equivalent to Wnt signaling to promote tumorigenesis.
Acknowledgements
We are indebted to Drs. J. Brzostowski, T. Khurana, L. Kreppel, X. Liu, and D. Rosel for their enthusiasm and discussion. We also appreciate the efforts of Jingchun Liu and Kate Tifft during the course of these studies and continued collaboration with our colleague Dr. Adrian Harwood.
References
- 1.
- Kuhl M. Wnt Signalling indevelopment Landes Biosciences Texas, USA.
- 2.
- Ali A, Hoeflich KP, Woodgett J.R. Glycogen synthase kinase-3: properties, functions, and regulation. Chem Rev. 2001;101:2527–2540. [PubMed: 11749387]
- 3.
- Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. [PubMed: 11584304]
- 4.
- Ferkey DM, Kimelman D. GSK-3: new thoughts on an old enzyme. Dev Biol. 2000;225:471–479. [PubMed: 10985864]
- 5.
- Kim L, Kimmel AR. GSK3, a Master switch regulating cell fate specification and tumorigenesis. Curr Opin Genet Dev. 2000;10:508–514. [PubMed: 10980428]
- 6.
- McEwen DG, Peifer M. Wnt signaling: Moving in a new direction. Curr Biol. 2000;10:R562–564. [PubMed: 10959830]
- 7.
- Moon RT, Bowerman B, Boutros M. et al. The promise and perils of Wnt signaling through beta-catenin. Science. 2002:1644–1646. [PubMed: 12040179]
- 8.
- Wodarz A, Nusse R. Mechanism of Wnt signaling in Development Annu Rev Cell Dev Biol 19981459–88. (See also: www.stanford.edu/~rnusse/wntwindow.html) [PubMed: 9891778]
- 9.
- Woodgett J.R. Judging a protein by more than its name: GSK-3. Sci STKE. 2001;100:RE12. [PubMed: 11579232]
- 10.
- Aubry L, Firtel R. Integration of signaling networks that regulate Dictyostelium differentiation. Annu Rev Cell Dev. 1999;15:469–517. [PubMed: 10611970]
- 11.
- Kay RR. Development at the edge of multi-cellularity: Dictyostelium discoideum. Int J Dev Biol. 2000;44:35–38. [PubMed: 10761844]
- 12.
- Ginsburg GT, Kimmel AR. Autonomous and non-autonomous regulation of axis formation by antagonistic signalling via 7-span cAMP receptors and GSK3 of Dictyostelium. Genes Dev. 1997;11:2112–2123. [PMC free article: PMC316452] [PubMed: 9284050]
- 13.
- Harwood AJ, Plyte SE, Woodgett J, Strutt H, Kay RR. Glycogen synthase kinase 3 regulates cell fate in Dictyostelium. Cell. 1995;80:139–148. [PubMed: 7813009]
- 14.
- Kim L, Liu J, Kimmel AR. The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell. 1999;99:399–408. [PubMed: 10571182]
- 15.
- Louis JM, Ginsburg GT, Kimmel AR. The cAMP receptor CAR4 regulates axial patterning and cellular differentiation during late development of Dictyostelium. Genes Dev. 1994;8:2086–2096. [PubMed: 7958880]
- 16.
- Plyte SE, O'Donovan E, Woodgett JR. et al. Glycogen synthase kinase-3 (GSK-3) is regulated during Dictyostelium development via the serpentine receptor cAR3. Development. 1999;126:325–333. [PubMed: 9847246]
- 17.
- Kim L, Harwood A, Kimmel AR. Receptor-dependent and tyrosine phosphatase-mediated inhibition of GSK3 regulates cell fate choice. Dev Cell. 2002;3:523–532. [PubMed: 12408804]
- 18.
- Hughes K, Nikolakaki E, Plyte SE, et al. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993;12:803–808. [PMC free article: PMC413270] [PubMed: 8382613]
- 19.
- Dajani R, Fraser E, Mark SM, et al. Crystal structure of glycogen synthase kinase 3β:structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–732. [PubMed: 11440715]
- 20.
- ter HaarE, Coll JT, Austen DA, et al. Structure of GSK3β reveals a primed phosphorylation mechanism. Nat Struct Biol. 2001;8:593–596. [PubMed: 11427888]
- 21.
- Grimson MJ, Coates JC, Reynolds JP. et al. Adherens junctions and beta-catenin-mediated cell signalling in a non-metazoan organism. Nature. 2000;408:27–731. [PubMed: 11130075]
- 22.
- Berks M, Kay RR. Combinatorial control of cell differentiation by cAMP and DIF-1 during development of Dictyostelium discoideum. Development. 1990;110:977–984. [PubMed: 1965164]
- 23.
- Saxe CL, Johnson RL, Devreotes PN, et al. Expression of a cAMP receptor gene of Dictyostelium and evidence for a multigene family. Genes Develop. 1991;5:1–8. [PubMed: 1989903]
- 24.
- Saxe CL, Ginsburg GT, Louis JL, et al. CAR2, a prestalk cAMP receptor required for tip formation and late development in Dictyostelium. Genes Develop. 1993;7:262–272. [PubMed: 8436297]
- 25.
- Gollop R, Kimmel AR. Control of cell-type specific gene expression in Dictyostelium by the general transcription factor GBF. Development. 1997;124:3395–3405. [PubMed: 9310334]
- 26.
- Dominguez I, Itoh K, Sokol SY. Role of glycogen synthase kinase 3 beta as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc. Natl. Acad. Sci. 1995;92:8498–8502. [PMC free article: PMC41184] [PubMed: 7667318]
- 27.
- He X, Saint-Jeannet JP, Woodgett JR. et al. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature. 1995;374:617–22. [PubMed: 7715701]
- 28.
- Yost C, Torres M. Miller JR, et al. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. [PubMed: 8666229]
- 29.
- Schlesinger A, Shelton CA, Maloof JN. et al. Wnt pathway components orient a mitotic spindle in the early Caenorhabditis elegans embryo without requiring gene transcription in the responding cell. Genes Dev. 1999;13:2028–2038. [PMC free article: PMC316921] [PubMed: 10444600]
- 30.
- Alcedo J, Ayzenzon M, Von OhlenT, Noll M, Hooper JE. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell. 1996 Jul. 2686(2)221-32 [PubMed: 8706127]
- 31.
- Jia J, Amanai K, Wang G, et al. Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature. 2002416548-552
- 32.
- Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell. 2002108823-35 [PubMed: 11955435]
- 33.
- Coates JC, Grimson MJ, Williams RS, Bergman W, Blanton RL, Harwood AJ.Loss. of the beta-catenin homologue aardvark causes ectopic stalk formation in Dictyostelium. Mech Dev. 2002;116:117–27. [PubMed: 12128211]
- 34.
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. [PubMed: 11057903]
- 35.
- Manoukian AS, Woodgett JR. ole of glycogen synthase kinase-3 in cancer: regulation by wnts and other signaling pathways. Adv. Cancer Res. 2002;84:203–229. [PubMed: 11883528]
- 36.
- Polakis P. Wnt Signaling and Cancer. Genes Dev. 2000;14: 1837–1851. [PubMed: 10921899]
- 37.
- Amit S, Hatzubai A, Birman Y. et al. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. [PMC free article: PMC186245] [PubMed: 12000790]
- 38.
- Liu C, Li Y, Semenov M, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. [PubMed: 11955436]
- 39.
- Kikkawa U, Mann SK, Firtel RA. et al. Molecular cloning of casein kinase II alpha subunit from Dictyostelium discoideum and its expression in the life cycle. Mol Cell Biol. 1992;12:5711–23. [PMC free article: PMC360511] [PubMed: 1448100]
- 40.
- Moreno-Bueno G, Cales C, Behrens MM, et al. Isolation and characterization of casein kinase I from Dictyostelium discoideum. Biochem J. 2000;349:527–37. [PMC free article: PMC1221176] [PubMed: 10880352]
- 41.
- Fraser E, Young N, Dajani R. et al. Identification of the Axin and FRAT/GBP binding region of Glycogen Synthase Kinase-3. J Biol Chem. 2002;277:2176–2185. [PubMed: 11707456]
- 42.
- Ennis HL, Dao DN, Pukatzki SU. et al. Dictyostelium amoebae lacking an F-box protein form spores rather than stalk in chimeras with wild type. Proc Natl Acad Sci U S A. 2000;97:3292–3297. [PMC free article: PMC16232] [PubMed: 10725352]
- 43.
- Nelson MK, Clark A, Abe T. et al. An F-Box/WD40 repeat-containing protein important for Dictyostelium cell-type proportioning, slug behaviour, and culmination. Dev Biol. 2000;224:42–59. [PubMed: 10898960]
- Introduction
- Antagonistic Regulation of Cell Fate Determination in Dictyosteliumby the 7-Transmembrane cAMP Receptors CAR3 and CAR4
- GSK3, a Developmental Switch Regulating Anterior/Posterior Axis Formation
- ZAK1 and PTPase Regulate Tyrosine Phosphorylation and Activity of GSK3
- Downstream Targets of GSK3 Aar (β-catenin)
- Perspectives—Integrating Tyrosine Phosphorylation with Other Signaling Components
- Acknowledgements
- References
- GSK3-Signal Regulation of Pattern Formation in Dictyostelium: Wnt-Like Pathways ...GSK3-Signal Regulation of Pattern Formation in Dictyostelium: Wnt-Like Pathways During Non-Canonical Multicellular Development - Madame Curie Bioscience Database
- A New Member of the CtBP/BARS Family from Plants: Angustifolia - Madame Curie Bi...A New Member of the CtBP/BARS Family from Plants: Angustifolia - Madame Curie Bioscience Database
- Shh Expression in Pulmonary Injury and Disease - Madame Curie Bioscience Databas...Shh Expression in Pulmonary Injury and Disease - Madame Curie Bioscience Database
- Preimplantation Genetic Diagnosis (PGD): Screening for Aneuploidy in Human Oocyt...Preimplantation Genetic Diagnosis (PGD): Screening for Aneuploidy in Human Oocytes and Polar Bodies - Madame Curie Bioscience Database
- Experimental Evidence for Immunomodulatory Effects of Opioids - Madame Curie Bio...Experimental Evidence for Immunomodulatory Effects of Opioids - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...