NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The association of high levels of IL-10 with autoimmune diseases suggests that this cytokine plays an important role in the disease pathogenesis. Family studies of first-degree relatives and analysis of twins indicate that heritable genetic factors underlie inter-individual differences in quantitative IL-10 production. Genome wide scans have shown an association between the chromosomal region containing the IL-10 locus and autoimmune diseases, and studies of the IL-10 gene have identified both single nucleotide polymorphisms (SNP) and short tandem repeat polymorphisms (STRP) in the promoter. Susceptibility to and manifestations of autoimmune diseases have reported associations with SNPs, SNP haplotypes and STRPs in the IL-10 promoter region, but results have varied. Since the total numbers of affecteds studied in different ethnic groups have been small, further studies with greater power will be necessary to uncover the genetic basis of dysregulated IL-10 production in autoimmune diseases.
Introduction
Autoimmune diseases are characterized by the presence of dysregulated cytokine expression that plays a role in the maintenance of autoreactive lymphocytes. Genetic polymorphisms that alter the function or expression of these cytokine genes are likely candidates as factors either predisposing to disease or modulating disease manifestations, and are the subject of intense research.
Interleukin-10 (IL-10), a cytokine that exhibits multiple modulatory effects on the immune system, has altered expression in many autoimmune diseases. The expression of IL-10 appears to be influenced by polymorphisms in its promoter, which may be associated with autoimmune disease. However, the definition of which of the many currently identified promoter polymorphisms are involved in disease pathogenesis, and whether new, as yet unidentified polymorphisms might also play a role in IL-10 dysregulation, is still a subject of continued investigation.
Interleukin-10 is produced by CD4 and CD8T cells, activated B lymphocytes, monocytes, macrophages, and keratinocytes.1,2 As an anti inflammatory cytokine IL-10 down-regulates the expression of Th1 cytokines, MHC class II and costimulatory molecules on macrophages. However, IL-10 also stimulates FcγR expression on the same cells,1,2 and has been shown to prolong B cell survival, to induce B cell differentiation, and to enhance B cell proliferation and antibody production.1-5 The effects of IL-10 on B cells, particularly on the stimulation and survival of autoreactive B cells are believed to be of great importance in autoimmune diseases. 2,5 Additionally, IL-10 may play an important role in influencing the balance of Th1 versus Th2 cytokines, which can influence the progression of autoimmune diseases.6-8
Association of IL-10 Production with Autoimmune Diseases
A number of studies of both mouse and human models of autoimmune diseases have documented altered IL-10 serum levels, suggesting a direct link between IL-10 levels and disease.1,2 Evidence from mouse models suggest that IL-10 production may play a protective role in organ-specific autoimmune disease and alter the balance between pathogenic Th1 cells and protective anti-inflammatory Th2 cells.2 However, while some studies show that administration of IL-10 could result in improvement of the disease phenotype, other studies have shown that IL-10-deficient mice fail to develop an autoimmune syndrome, and the expression of an IL-10 transgene resulted in earlier disease onset or exacerbated disease rather than in protection.2
High serum levels of IL-10 have been documented in human autoimmune diseases. In systemic lupus erythematosus (SLE) patients, high IL-10 levels have been shown to correlate with disease activity,9,10 and studies indicate that cultured PBMC from lupus patients spontaneously produce high levels of IL-10.1 Production of high levels of IL-10 has also been demonstrated in synovial T-lymphocytes of rheumatoid arthritis patients, in the serum of systemic sclerosis, Kawasaki disease, and ALPS patients, as well as in the cultured cells of polymyositis and dermatomyositis patients.2 High IL-10 levels have been found in the blister fluid of pemphigus vulgaris and bullous pemphigoid patients and in the mucosal T cells in ulcerative colitis.2,11-16 Increased expression of IL-10 mRNA by PBMCs and/or various other tissues was associated with Sjogren's syndrome, Grave's disease, myasthenia gravis, psoriasis and autoimmune lymphoproliferative syndrome (ALPS).1,2,8,16,17 Taken together, these studies indicate that dysregulated expression of IL-10 plays a significant role in autoimmune diseases. While the disease association with IL-10 levels is clear, the degree to which IL-10 production precedes disease onset, as opposed to resulting from disease activity, is less clear. In addition, while autoimmune disease progression may play a role in altering the levels of IL-10 and other cytokines, evidence from family studies suggest that, to a large extent, heritable genetic differences determine IL-10 production and thus contribute to autoimmune disease phenotype.18,19 The association of high levels of IL-10 mRNA and protein with early stages of Grave's disease and Hashimoto's thyroiditis also suggests a role for IL-10 in the early stages of some diseases.8
IL-10 Promoter Polymorphisms and IL-10 Production
IL-10 production is regulated at the transcriptional level, and studies suggest that heritable genetic differences that associate with inter-individual differences in gene expression may differentially regulate IL-10 production.19-21 There is also evidence that suggests the existence of post-transcriptional regulation by RNA-destabilizing, AU-rich elements within the 3'-untranslated of IL-10 mRNA.22
Inter-individual differences in IL-10 production is heritable.23 First degree relatives of SLE patients produce high levels of IL-10 compared with unrelated controls,24 and first-degree relatives of nonsurvivors of fatal meningococcal disease produce significantly lower levels of IL-10 than relatives of survivors.23,25 The implication of a heritable genetic basis for IL-10 production is also supported by the concordance of IL-10 production in monozygotic twins, which suggests that genetics could account for up to 75% of IL-10 production.23 Therefore, high IL-10 production associated with autoimmune diseases may represent a risk factor for disease susceptibility or severity, and the genetic polymorphisms that regulate IL-10 production represent genetic risk factors for autoimmune diseases.1,9,10,18,19,24,26
Promoter-reporter studies have identified several positive and negative regulatory promoter sequences within the 1.3 kb region upstream of the transcription start site, and a transcription factor binding site search of the immediate 4kb promoter region has identified more than 74 potential binding sites.27,28 Three functional NF-κB and a Stat3 binding site, and four cAMP-responsive elements have been identified in the 2kb promoter region of the human IL-10 promoter.29-31 However, none of these elements appear to be polymorphic. Functional Sp1 and Sp3 transcription factor binding sites have similarly been identified in the murine IL-10 promoter.32
The human IL-10 promoter is highly polymorphic. Two (CA) short tandem repeat polymorphisms (STRP), IL-10.R and IL-10.G, have been identified at -4 kb and -1.1 kb, respectively, and three single nucleotide polymorphisms (SNP), -1082G/A, -819C/T and -597C/A, which form three predominant haplotypes (GCC, ACC, ATA), had been identified previously in the 1.0 kb promoter region.33-36 A fourth haplotype, GTA, was seen only in a Chinese population.37 The G allele at -1082 and haplotypes containing this allele have been associated with high IL-10 production, while the A allele and the ATA haplotype have been associated with low IL-10 production.35,38
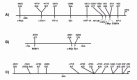
Resequencing within the 8kb promoter region by various groups has identified at least 23 additional SNPs (Fig. 1),19,39,40 and four novel SNPs have been identified recently in the 3' untranslated region ( 3' UTR).41 Significant differences in the allele frequencies of some promoter SNPs have been seen in different ethnic or geographic populations, underscoring the need for appropriate stratification in disease association studies (Table 1).42 Haplotypes defined by proximal and distal SNPs 19,35,43 and by SNPs and STRP alleles associate with IL-10 production.39,44 However, because of variable, inconsistent associations between IL-10 production and different SNP alleles, SNP haplotypes and STRP alleles, it is currently unclear which promoter polymorphisms are causal in determining differences in IL-10 production. Data from our group, which showed an association between distal SNP haplotypes and IL-10 production suggest that SNPs in the distal promoter may play a role in IL-10 production.19
Table 1
SNP allele frequencies in different ethnic group.
IL-10 Promoter Polymorphisms and Automimmune Disease
Genome wide scans have shown linkage of disease susceptibility to the region on chromosome 1 that encompasses the IL-10 locus. The IL-10 gene has been localized at 1q31-1q32 region, and several genome scans have identified regions of chromosome 1 (1q21-23, 1q31-32, 1q42-44) which may contain susceptibility loci for SLE and other autoimmune diseases.45-51
Using case-control approaches to look at the distribution of promoter SNP or STRP alleles, several studies have found an association between IL-10 promoter polymorphisms and autoimmune disease susceptibility or disease manifestations (Tables 2 and 3)19,37,38,41,43,52-65,74 In each autoimmune disease examined, some studies have failed to find associations between promoter polymorphisms and susceptibility or manifestations.37,43,61,63-73 For example, Lazarus et al (1997) have found an association between -1082G, -1082G-containing haplotypes and the presence of anti-Ro autoantibodies and nephritis in Caucasian SLE.53 A study by Crawley et al (1999) also used a Caucasian group, but failed to find a similar association, and Mok et al (1998) have found an association between the ATA haplotype and lupus nephritis in a Chinese population.37,68 Therefore, currently it is not clear which of the 26 identified SNPs, which haplotypes or which STRP alleles are relevant in the disease process. In reviewing these studies several observations are clear: 1) the total number of affecteds that have been studied is small (Table 2); 2) different genetic markers, either SNPs or STRPs, have been screened; 3) different ethnic populations have been used in different studies; and 4) different genotyping techniques have been used, often without delineation of error rates. Since there are clear differences in the allele frequencies in different ethnic and geographic groups, appropriate stratification by ethnicity will be important. In support of this, our group has found an association between the promoter SNP (-2763A/T) in African Americans with SLE 19 but not in a Dutch Caucasian cohort of 98 affecteds compared with 128 controls (-2763AA = 0.37 in affecteds vs 0.36 in controls; Gibson and Huizinga, unpublished results). Others have failed to find a similar association in an Italian SLE group.41
Table 2
Association studies of IL-10 polymorphisms and autoimmune disease susceptibility.
Table 3
Association studies of IL-10 polymorphisms and autoimmune disease manifestations.
Variable associations between IL-10 STRP alleles and autoimmune disease have also been documented. In different studies, different IL-10G STRP alleles associated with SLE in a UK Caucasian cohort, an Italian Caucasian cohort and in Mexican Americans, while IL-10R alleles associated with RA in two different UK Caucasian cohorts, and in an African American group.41,52,54,55,58 Data from our group did not show an association between the IL-10R STRP and SLE in three ethnic groups (Caucasians, African Americans or Mexican Americans). However, we found significant association between SLE and IL-10.G8 and IL-10.G9 in Caucasians, and IL-10.G8, IL-10.G9 and IL-10.G11 in African Americans (Table 4). We have not replicated the association in Mexican Americans observed by Mehrian and colleagues, but our cohort numbers were not adequately powered to provide a definitive result (Table 4).
Table 4
IL-10G allele frequencies in African American, Caucasian and Mexican American SLE populations.
Of particular interest is a recent study that examined distal SNPs in the -8kb promoter region, and extended haplotypes in the 4kb region in an Italian SLE cohort.41 While the authors found no association between SLE and distal SNPs or SNPs in the 3'UTR, a meta-analysis of four IL-10.G STRP association studies found significant association with STRPs containing larger numbers of (CA) repeats. IL-10.G alleles containing 21 (CA) repeats or more (IL-10.G9 and up), and extended SNP-STRP haplotypes that included these longer repeat alleles were associated significantly with SLE.41 This study suggests that the disease association might not be with a particular STRP allele, but with a larger stretch of (CA) repeats. The biologic basis for this observation is not clear at present.
In summary, associations between IL-10 promoter polymorphisms, IL-10 production and autoimmune diseases have been documented. However, while association studies have yielded inconsistent results, the total number of affecteds from each of the various ethnic groups studied has not been large. Additionally, adequate numbers of studies have not been done in all ethnic groups. Recent resequencing efforts have uncovered a more complex promoter structure, and additional SNPs are being discovered in the promoter and in the 3'UTR41,75 (and Gibson, unpublished results). This raises the possibility that other, more complex SNP associations with disease phenotypes might be uncovered in future studies.
References
- 1.
- Lalani I, Bhol K, Ahmed AR. Interleukin-10: Biology, role in inflammation and autoimmunity. Ann Allergy Asthma Immunol. 1997;79:469–483. [PubMed: 9433360]
- 2.
- Moore KW, de Waal Malefy tR, Coffman RL. et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. [PubMed: 11244051]
- 3.
- te VeldeAA, de Waal Malefijt R, Huijbens RJ. et al. IL-10 stimulates monocyte Fc gamma R surface expression and cytotoxic activity. Distinct regulation of antibody-dependent cellular cytotoxicity by IFN-gamma, IL-4, and IL-10. J Immunol. 1992;149:4048–4052. [PubMed: 1460289]
- 4.
- Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994;93:424–428. [PMC free article: PMC293803] [PubMed: 8282815]
- 5.
- Llorente L, Zou W, Levy Y. et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–844. [PMC free article: PMC2191898] [PubMed: 7869046]
- 6.
- Katsikis PD, Cohen SB, Londei M. et al. Are CD4+ Th1 cells pro-inflammatory or anti-inflammatory? The ratio of IL-10 to IFN-gamma or IL-2 determines their function. Int Immunol. 1995;7:1287–1294. [PubMed: 7495735]
- 7.
- Hagenbaugh A, Sharma S, Dubinett SM. et al. Altered immune responses in interleukin 10 transgenic mice. J Exp Med. 1997;185:2101–2110. [PMC free article: PMC2196349] [PubMed: 9182682]
- 8.
- Mirakian R, Hammond LJ, Bottazzo GF. TH1 and TH2 cytokine control of thyrocyte survival in thyroid autoimmunity. Nat Immunol. 2001;2:371. [PubMed: 11323682]
- 9.
- Mongan AE, Ramdahin S, Warrington RJ. Interleukin-10 response abnormalities in systemic lupus erythematosus. Scand J Immunol. 1997;46:406–412. [PubMed: 9350293]
- 10.
- Houssiau FA, Lefebvre C, Vanden Berghe M. et al. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4:393–395. [PubMed: 8563734]
- 11.
- Llorente L, Richaud-Patin Y, Fior R. et al. In vivo production of interleukin-10 by nonT cells in rheumatoid arthritis, Sjogren's syndrome, and systemic lupus erythematosus. A potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum. 1994;37:1647–1655. [PubMed: 7980676]
- 12.
- Cush JJ, Splawski JB, Thomas R. et al. Elevated interleukin-10 levels in patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:96–104. [PubMed: 7818579]
- 13.
- Cohen SB, Katsikis PD, Chu CQ. et al. High level of interleukin-10 production by the activated T cell population within the rheumatoid synovial membrane. Arthritis Rheum. 1995;38:946–952. [PubMed: 7612044]
- 14.
- Isomaki P, Luukkainen R, Saario R. et al. Interleukin-10 functions as an antiinflammatory cytokine in rheumatoid synovium. Arthritis Rheum. 1996;39:386–395. [PubMed: 8607887]
- 15.
- Furuzawa-Carballeda J, Alcocer-Varela J. Interleukin-8, interleukin-10, intercellular adhesion molecule- 1 and vascular cell adhesion molecule-1 expression levels are higher in synovial tissue from patients with rheumatoid arthritis than in osteoarthritis. Scand J Immunol. 1999;50:215–222. [PubMed: 10447928]
- 16.
- Melgar S, Yeung MM, Bas A. et al. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol. 2003;134:127–137. [PMC free article: PMC1808826] [PubMed: 12974765]
- 17.
- Lopatin U, Yao X, Williams RK. et al. Increases in circulating and lymphoid tissue interleukin-10 in autoimmune lymphoproliferative syndrome are associated with disease expression. Blood 15. 2001;97(10):3161–3170. [PubMed: 11342444]
- 18.
- Harley JB, Moser KL, Gaffney PM. et al. The genetics of human systemic lupus erythematosus. Curr Opin Immunol. 1998;10:690–696. [PubMed: 9914226]
- 19.
- Gibson AW, Edberg JC, Wu J. et al. Novel single nucleotide polymorphisms in the distal IL-10 promoter affect IL-10 production and enhance the risk of systemic lupus erythematosus. J Immunol. 2001;166:3915–3922. [PubMed: 11238636]
- 20.
- Le T, Leung L, Carroll WL. et al. Regulation of interleukin-10 gene expression: Possible mechanisms accounting for its upregulation and for maturational differences in its expression by blood mononuclear cells. Blood. 1997;89:4112–4119. [PubMed: 9166853]
- 21.
- Knolle PA, Uhrig A, Protzer U. et al. Interleukin-10 expression is autoregulated at the transcriptional level in human and murine Kupffer cells. Hepatology. 1998;27:93–99. [PubMed: 9425923]
- 22.
- Powell MJ, Thompson SA, Tone Y. et al. Posttranscriptional regulation of IL-10 gene expression through sequences in the 3'-untranslated region. J Immunol. 2000;165:292–296. [PubMed: 10861064]
- 23.
- Westendorp RG, Langermans JA, Huizinga TW. et al. Genetic influence on cytokine production in meningococcal disease. Lancet. 1997;349:1912–1913. [PubMed: 9217780]
- 24.
- Llorente L, Richaud-Patin Y, Couderc J. et al. Dysregulation of interleukin-10 production in relatives of patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:1429–1435. [PubMed: 9259422]
- 25.
- van DisselJT, van LangeveldeP, Westendorp RG. et al. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351:950–953. [PubMed: 9734942]
- 26.
- Hagiwara E, Gourley MF, Lee S. et al. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10:interferon-gamma-secreting cells in the peripheral blood. Arthritis Rheum. 1996;39:379–385. [PubMed: 8607886]
- 27.
- Kube D, Platzer C, von Knethen A. et al. Isolation of the human interleukin 10 promoter. Characterization of the promoter activity in Burkitt's lymphoma cell lines. Cytokine. 1995;7:1–7. [PubMed: 7749063]
- 28.
- Eskdale J, Kube D, Tesch H. et al. Mapping of the human IL10 gene and further characterization of the 5' flanking sequence. Immunogenetics. 1997;46:120–128. [PubMed: 9162098]
- 29.
- Mori N, Prager D. Activation of the interleukin-10 gene in the human T lymphoma line HuT 78: Identification and characterization of NF-kappa B binding sites in the regulatory region of the interleukin-10 gene. Eur J Haematol. 1997;59:162–170. [PubMed: 9310124]
- 30.
- Platzer C, Fritsch E, Elsner T. et al. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur J Immunol. 1999;29:3098–3104. [PubMed: 10540320]
- 31.
- Benkhart EM, Siedlar M, Wedel A. et al. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol. 2000;165:1612–1617. [PubMed: 10903771]
- 32.
- Tone M, Powell MJ, Tone Y. et al. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J Immunol. 2000;165:286–291. [PubMed: 10861063]
- 33.
- Eskdale J, Gallagher G. A polymorphic dinucleotide repeat in the human IL-10 promoter. Immunogenetics. 1995;42:444–445. [PubMed: 7590988]
- 34.
- Eskdale J, Kube D, Gallagher G. A second polymorphic dinucleotide repeat in the 5' flanking region of the human IL10 gene. Immunogenetics. 1996;45:82–83. [PubMed: 8881045]
- 35.
- Turner DM, Williams DM, Sankaran D. et al. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1–8. [PubMed: 9043871]
- 36.
- Rieth H, Assohou AC, Mormann M. et al. A new allelic variation within the 5'-flanking region of the interleukin-10 gene. Eur J Immunogenet. 2003;30:191–193. [PubMed: 12786996]
- 37.
- Mok CC, Lanchbury JS, Chan DW. et al. Interleukin-10 promoter polymorphisms in Southern Chinese patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1090–1095. [PubMed: 9627019]
- 38.
- Crawley E, Kay R, Sillibourne J. et al. Polymorphic haplotypes of the interleukin-10 5' flanking region determine variable interleukin-10 transcription and are associated with particular phenotypes of juvenile rheumatoid arthritis. Arthritis Rheum. 1999;42:1101–1108. [PubMed: 10366102]
- 39.
- Eskdale J, Keijsers V, Huizinga T. et al. Microsatellite alleles and single nucleotide polymorphisms (SNP) combine to form four major haplotype families at the human interleukin-10 (IL-10) locus. Genes Immun. 1999;1:151–155. [PubMed: 11196662]
- 40.
- D'Alfonso S, Rampi M, Rolando V. et al. New polymorphisms in the IL-10 promoter region. Genes Immun. 2000;1:231–233. [PubMed: 11196718]
- 41.
- D'Alfonso S, Giordano M, Mellai M. et al. Association tests with systemic lupus erythematosus (SLE) of IL10 markers indicate a direct involvement of a CA repeat in the 5' regulatory region. Genes Immun. 2002;3:454–463. [PubMed: 12486603]
- 42.
- Moraes MO, Santos AR, Schonkeren JJ. et al. Interleukin-10 promoter haplotypes are differently distributed in the Brazilian versus the Dutch population. Immunogenetics. 2003;54:896–899. [PubMed: 12671741]
- 43.
- Hajeer AH, Lazarus M, Turner D. et al. IL-10 gene promoter polymorphisms in rheumatoid arthritis. Scand J Rheumatol. 1998;27:142–145. [PubMed: 9572641]
- 44.
- Eskdale J, Gallagher G, Verweij CL. et al. Interleukin 10 secretion in relation to human IL-10 locus haplotypes. Proc Natl Acad Sci USA. 1998;95:9465–9470. [PMC free article: PMC21361] [PubMed: 9689103]
- 45.
- Tsao BP, Cantor RM, Kalunian KC. et al. Evidence for linkage of a candidate chromosome 1 region to human systemic lupus erythematosus. J Clin Invest. 1997;99:725–731. [PMC free article: PMC507856] [PubMed: 9045876]
- 46.
- Cornelis F, Faure S, Martinez M. et al. New susceptibility locus for rheumatoid arthritis suggested by a genome-wide linkage study. Proc Natl Acad Sci USA. 1998;95:10746–10750. [PMC free article: PMC27966] [PubMed: 9724775]
- 47.
- Gaffney PM, Kearns GM, Shark KB. et al. A genome-wide search for susceptibility genes in human systemic lupus erythematosus sib-pair families. Proc Natl Acad Sci USA. 1998;95:14875–14879. [PMC free article: PMC24543] [PubMed: 9843983]
- 48.
- Moser KL, Neas BR, Salmon JE. et al. Genome scan of human systemic lupus erythematosus: Evidence for linkage on chromosome 1q in African-American pedigrees. Proc Natl Acad Sci USA. 1998;95:14869–14874. [PMC free article: PMC24542] [PubMed: 9843982]
- 49.
- Shai R, Quismorio JrFP, Li L. et al. Genome-wide screen for systemic lupus erythematosus susceptibility genes in multiplex families. Hum Mol Genet. 1999;8:639–644. [PubMed: 10072432]
- 50.
- Capon F, Novelli G, Semprini S. et al. Searching for psoriasis susceptibility genes in Italy: Genome scan and evidence for a new locus on chromosome 1. J Invest Dermatol. 1999;112:32–35. [PubMed: 9886260]
- 51.
- Johanneson B, Lima G, von Salome J. et al. A major susceptibility locus for systemic lupus erythemathosus maps to chromosome 1q31. Am J Hum Genet. 2002;71:1060–1071. [PMC free article: PMC385085] [PubMed: 12373647]
- 52.
- Eskdale J, Wordsworth P, Bowman S. et al. Association between polymorphisms at the human IL-10 locus and systemic lupus erythematosus. Tissue Antigens. 1997;49:635–639. [PubMed: 9234486]
- 53.
- Lazarus M, Hajeer AH, Turner D. et al. Genetic variation in the interleukin 10 gene promoter and systemic lupus erythematosus. J Rheumatol. 1997;24:2314–2317. [PubMed: 9415634]
- 54.
- Eskdale J, McNicholl J, Wordsworth P. et al. Interleukin-10 microsatellite polymorphisms and IL-10 locus alleles in rheumatoid arthritis susceptibility. Lancet. 1998;352:1282–1283. [PubMed: 9788463]
- 55.
- Mehrian R, Quismorio JrFP, Strassmann G. et al. Synergistic effect between IL-10 and bcl-2 genotypes in determining susceptibility to systemic lupus erythematosus. Arthritis Rheum. 1998;41:596–602. [PubMed: 9550468]
- 56.
- Rood MJ, Keijsers V, van der Linden MW. et al. Neuropsychiatric systemic lupus erythematosus is associated with imbalance in interleukin 10 promoter haplotypes. Ann Rheum Dis. 1999;58:85–89. [PMC free article: PMC1752835] [PubMed: 10343522]
- 57.
- Asadullah K, Eskdale J, Wiese A. et al. Interleukin-10 promoter polymorphism in psoriasis. J Invest Dermatol. 2001;116:975–978. [PubMed: 11407990]
- 58.
- D'Alfonso S, Rampi M, Bocchio D. et al. Systemic lupus erythematosus candidate genes in the Italian population: Evidence for a significant association with interleukin-10. Arthritis Rheum. 2000;43:120–128. [PubMed: 10643707]
- 59.
- de JongBA, Westendorp RG, Eskdale J. et al. Frequency of functional interleukin-10 promoter polymorphism is different between relapse-onset and primary progressive multiple sclerosis. Hum Immunol. 2002;63(4):281–285. [PubMed: 12039409]
- 60.
- Martinez DoncelA, Rubio A, Arroyo R. et al. Interleukin-10 polymorphisms in Spanish multiple sclerosis patients. J Neuroimmunol. 2002;131:168–172. [PubMed: 12458048]
- 61.
- Goedecke V, Crane AM, Jaakkola E. et al. Interleukin 10 polymorphisms in ankylosing spondylitis. Genes Immun. 2003;4:74–76. [PubMed: 12595905]
- 62.
- Hensen P, Asadullah K, Windemuth C. et al. Interleukin-10 promoter polymorphism IL10.G and familial early onset psoriasis. Br J Dermatol. 2003;149:381–385. [PubMed: 12932247]
- 63.
- Kingo K, Koks S, Silm H. et al. IL-10 promoter polymorphisms influence disease severity and course in psoriasis. Genes Immun. 2003;4:455–457. [PubMed: 12944983]
- 64.
- Lard LR, van GaalenFA, Schonkeren JJ. et al. Association of the -2849 interleukin-10 promoter polymorphism with autoantibody production and joint destruction in rheumatoid arthritis. Arthritis Rheum. 2003;48:1841–1848. [PubMed: 12847677]
- 65.
- Myhr KM, Vagnes KS, Maroy TH. et al. Interleukin-10 promoter polymorphisms in patients with Guillain-Barre syndrome. J Neuroimmunol. 2003;139:81–83. [PubMed: 12799024]
- 66.
- Coakley G, Mok CC, Hajeer AH. et al. Interleukin-10 promoter polymorphisms in rheumatoid arthritis and Felty's syndrome. Br J Rheumatol. 1998;37:988–991. [PubMed: 9783765]
- 67.
- Alarcon-Riquelme ME, Lindqvist AK, Jonasson I. et al. Genetic analysis of the contribution of IL10 to systemic lupus erythematosus. J Rheumatol. 1999;26:2148–2152. [PubMed: 10529131]
- 68.
- Crawley E, Woo P, Isenberg DA. Single nucleotide polymorphic haplotypes of the interleukin-10 5' flanking region are not associated with renal disease or serology in Caucasian patients with systemic lupus erythematosus. Arthritis Rheum. 1999;42:2017–2018. [PubMed: 10513824]
- 69.
- Cantagrel A, Navaux F, Loubet-Lescoulie P. et al. Interleukin-1beta, interleukin-1 receptor antagonist, interleukin-4, and interleukin-10 gene polymorphisms: Relationship to occurrence and severity of rheumatoid arthritis. Arthritis Rheum. 1999;42:1093–1100. [PubMed: 10366101]
- 70.
- Pickard C, Mann C, Sinnott P. et al. Interleukin-10 (IL10) promoter polymorphisms and multiple sclerosis. J Neuroimmunol. 1999;101:207–210. [PubMed: 10580805]
- 71.
- Maurer M, Kruse N, Giess R. et al. Genetic variation at position -1082 of the interleukin 10 (IL10) promotor and the outcome of multiple sclerosis. J Neuroimmunol. 2000;104:98–100. [PubMed: 10683520]
- 72.
- Dijstelbloem HM, Hepkema BG, Kallenberg CG. et al. The R-H polymorphism of Fcgamma receptor IIa as a risk factor for systemic lupus erythematosus is independent of single-nucleotide polymorphisms in the interleukin-10 gene promoter. Arthritis Rheum. 2002;46:1125–1126. [PubMed: 11953994]
- 73.
- MacKay K, Milicic A, Lee D. et al. Rheumatoid arthritis susceptibility and interleukin 10: A study of two ethnically diverse populations. Rheumatology (Oxford). 2003;42:149–153. [PubMed: 12509628]
- 74.
- Lin M, Storer B, Martin P. et al. Relation of an interleukin-10 promoter polymorphism to Graftversus- Host Disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349:2201–2210. [PubMed: 14657427]
- 75.
- Kube D, Rieth H, Eskdale J. et al. Structural characterisation of the distal 5' flanking region of the human interleukin-10 gene. Genes Immun. 2001;2:181–190. [PubMed: 11477472]
- The Role of IL-10 in Autoimmune Pathology - Madame Curie Bioscience DatabaseThe Role of IL-10 in Autoimmune Pathology - Madame Curie Bioscience Database
- Mating Cell-Cell Channels in Conjugating Bacteria - Madame Curie Bioscience Data...Mating Cell-Cell Channels in Conjugating Bacteria - Madame Curie Bioscience Database
- FROM STRUCTURE TO FUNCTION OF RNA BINDING DOMAINS - Madame Curie Bioscience Data...FROM STRUCTURE TO FUNCTION OF RNA BINDING DOMAINS - Madame Curie Bioscience Database
- Regulation and Coordination of Intracellular Trafficking: An Overview - Madame C...Regulation and Coordination of Intracellular Trafficking: An Overview - Madame Curie Bioscience Database
- Pre-Ribosomal RNA Processing in Multicellular Organisms - Madame Curie Bioscienc...Pre-Ribosomal RNA Processing in Multicellular Organisms - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...