NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Native Voltage-Gated Ca Channels
Early electrophysiological recordings from neurons, muscle and endocrine cells revealed voltage-activated calcium (Ca2+) currents with distinct characteristics, suggesting the existence of two major classes of Ca2+ channels based upon the membrane potentials at which they first open (see chapter by Tsien); low-voltage activated (LVA) and high-voltage activated (HVA). The LVA (or T-type) channels, typically have a small conductance (8-12 pico Siemens (pS)), open in response to small changes from the resting membrane potential and inactivate rapidly. In contrast, the HVA currents generally possess larger conductances (15-25 pS), are activated by stronger depolarizations and display variable inactivation kinetics. To date, multiple types of HVA Ca2+ channels (L-, N-, P/Q- and R-type) have been categorized on the basis of a number of criteria including, single channel conductance, kinetics, pharmacology, and cellular distribution.1-4
High Voltage-Activated Ca2+ Channels
L-Type Channels
L-type Ca2+ channels were initially described in peripheral neurons and cardiac cells, but appear to be present in all excitable as well as many types of non-excitable cells.4 In certain cells, L-type channels have been shown to be preferentially localized to specific subcellular regions. For example, the L-type channels responsible for skeletal muscle contraction are concentrated on the transverse tubule membrane,5 while neuronal L-type channels are located primarily on cell bodies and proximal dendrites.6 The L-type channel is the primary route for Ca2+ entry into cardiac, skeletal, and smooth muscles.2 The skeletal muscle L-type channel acts as a voltage sensor for excitation-contraction (E-C) coupling in skeletal muscle, presumably linking membrane depolarization to Ca2+ release from intracellular stores. While Ca2+ entry through this channel is not required for the initiation of contraction in skeletal muscle, it may provide a source of Ca2+ to replenish internal stores.2,4,7,8 There is some evidence that L-type channels are involved in exocytotic release from endocrine cells and some neurons9-13 and the localization of L-type channels on the cell soma6 has also implicated these channels in the regulation of gene expression.14-16
Much is known about the pharmacological properties of L-type Ca2+ channels. The three main classes of organic L-type channel blockers are the phenylalkylamines (verapamil), benzothiazapines (diltiazem), and 1,4-dihydropyridines (DHPs) (e.g., nitrendipine, nifedipine, nimodipine). The DHP antagonists bind preferentially to channels in the active conformation, a state favored by depolarization (producing more potent inhibition at depolarized potentials). A number of DHP agonists have also been developed, the most highly utilized of which is (-)-Bay K 8644 which increases both the open time and the single channel conductance (see chapter by Striessnig for more detail). L-type channels are also blocked by certain native peptide toxins such as ω-agatoxin IIIA (ω-Aga IIIA), isolated from the venom of the funnel web spider Agelenopsis aperta.17,18 ω-Aga IIIA reduces the current amplitude without affecting the time course and unlike the DHPs, ω-Aga IIIA inhibition is voltage-independent and blocks L-type channels at all potentials2 (see chapter by Adams and Lewis for more detail).
L-type channels have a unitary conductance ranging from 20 and 27 pS using 110 mM barium (Ba2+) as the charge carrier. L-type channels require large departures from resting potential to become activated and typically begin to open at potentials positive to -10 mV, although they can activate at significantly more negative potentials in chromaffin cells, sensory neurons, and cardiac cells. In the presence of Ba2+ as the charge carrier, once open, L-type channels do not inactivate significantly during depolarizations of hundreds of milliseconds.2,3,19 Compared to Ba2+ currents, using Ca2+ as the charge carrier L-type currents are smaller and inactivate rapidly. This Ca2+-dependent inactivation has a number of characteristic properties and inactivation attributable to Ca2+ influx is greatest at depolarizations at which Ca2+ entry through the channel is maximal.20 While the degree of inactivation is slowed by the addition of BAPTA and other Ca2+ chelators, it is not completely abolished. Ca2+-dependent inactivation can however be eliminated by intracellular applications of trypsin, suggesting that the mechanism through which Ca2+ acts to inactivate the channel is in close proximity to, if not part of, the channel complex itself.2,3,21,22 Because the rate of Ca2+-dependent inactivation does not change with channel density, Neely et al (1994)22 proposed a “local domain” hypothesis, in which Ca2+ affects only the channel through which it enters (see chapter by Lee and Catterall for more detail). Moreover, current models view calmodulin to be constitutively bound to the C-terminus forming part of the Ca2+ sensing machinery, ultimately leading to the signal transduction of Ca2+-dependent inactivation.23
N-Type Channels
In addition to L- and T-type Ca2+ channels, recordings from chick dorsal root ganglion (DRG) cells revealed a third type of single channel Ca2+ conductance of 13 pS (in 110 mM Ba2+), intermediate between that of the T- (8 pS) and L- (25 pS) type channels.1,2,19,24,25 Although this conductance shares some general electrophysiological characteristics with currents through both T- and L-type channels, it could not be attributed to either. Consequently, the corresponding channel was designated as N (neither)-type.
Although first identified in chick DRG neurons, N-type channels have also been detected in mammalian DRG cells,26-29 mammalian and amphibian sympathetic neurons,30-33 and other cells of the peripheral and central nervous systems.34-39 N-type channels appear to be expressed only in neuronal tissues,31,40 although an N-type current has been reported in rat thyroid C-cell line.41 Electrophysiologically, N-type channels are most easily distinguished from L-type channels by their inactivation properties. Unlike L-type channels, N-type channels display time-dependent inactivation (with Ba2+ as the charge carrier). N-type currents decay with a time constant (τ) ranging from 50 to 110 ms, significantly slower than the rapid (τ = 20-50 ms) inactivation of the LVA T-type channels, but much faster than the non-inactivating L-type channels. In chick DRG neurons, the N-type current decays almost completely during a test depolarization of 140 ms, while L-type current shows little inactivation over the same period of time.1 However, N-type currents do not always inactivate rapidly. In sympathetic neurons, the decay rate of N-type currents is much slower (τ = 500 to 800 ms) and can be incomplete, even over depolarizations lasting longer than one second.2,41-42 Thus, there appears to be at least two distinct components to N-type current inactivation. These differences in inactivation kinetics could reflect different subtypes of N-type channel. Alternatively, a single N-type channel could support both currents by switching between the slow- and fast-inactivating states.40,43
In addition to the time-dependence parameter, there is also a voltage-dependent aspect to N-type channel inactivation.1,24,25,29 Holding the cell membrane at potentials between -60 and -40 mV results in significant inactivation of the N-type current, and strongly negative potentials are required to reprime the channels. N-type channels are markedly more sensitive to the effects of holding potential on inactivation than are L-type channels. At resting membrane potentials of -20 mV, N-type channels are completely inactivated while L-type channels remain available for opening.
Theoretically, the different inactivation properties of N- and L-type channels provides two parameters that can be used to dissect the relative contributions of the two channel types to the whole cell HVA current.2,24 One method takes advantage of the different inactivation rates. The component of whole cell current that decays during a prolonged depolarization can be attributed to the inactivating N-type channels, while the non-inactivating portion is identified as L-type current. The second approach exploits the different ranges over which voltage-dependent inactivation takes place. The contribution of each type to the whole cell current may be determined by analyzing the differences in whole cell currents elicited by depolarizations from resting potentials of -40 and -90 mV. Because L-type channels are relatively resistant to the effects of holding potential on inactivation while N-type channels inactivate at depolarized membrane potentials, the difference under these two conditions should reflect the contribution of N-type channels to the whole-cell current. However, neither method may be adequate to properly distinguish these currents. Some N-type channels can inactivate quite slowly and inactivation may not be complete. In addition, voltage-dependent inactivation of N-type channels can be highly variable and takes place over a wide range of holding potentials between -80 to -20 mV.24,25 If N-type channels predominate in a cell, the residual current through incompletely inactivated N-type channels may be significant. Thus definitions of N- and L-type current based solely on these criteria may not be valid.
Pharmacologically, N-type channels are sensitive to inhibition by a class of native peptide toxins called the ?-conotoxins, which are a family of small (13-29 amino acid) peptides found in the venom of predatory marine snails of the genus Conus.44, 45 All known ω-conotoxins inhibit N-type Ca2+ channels, although their specificities and blocking affinities for this particular channel vary significantly. To date, ω-conotoxin GVIA (ω-CgTx), a 27-amino acid peptide from Conus geographus46 is the most specific ω-conotoxin peptide for N-type channel inhibition. ω-CgTx produces complete and irreversible inhibition of N-type currents in DRG, hippocampal, sympathetic, and sensory neurons at concentrations of approximately 100 nM to 1 μM.47-49 At higher concentrations (5-15 μM), ω-CgTx also inhibits L- and T-type currents, although unlike N-type channels, the effects are incomplete and reversible47-52 (see chapter by Adams and Lewis for more detail).
ω-CgTx binding sites (and by extension N-type Ca2+ channels) are distributed throughout the PNS and CNS, including the cortex, hippocampus, olfactory bulb, and cerebellar cortex, and appear especially concentrated in regions of high synaptic density.2,53- 58 Although N-type channels were first identified by single channel recordings from the cell bodies of DRG neurons,1 they appear to be more abundantly localized on dendrites and axon terminals. In muscle, ω-CgTx binding occurs at the active zones of presynaptic cells in spatial register with the postsynaptic acetylcholine (ACh) receptors on the muscle. Labeling is rarely found between active zones, nor is it localized to areas of the presynaptic membrane that do not face the muscle. N-type channels have also been observed to cluster in areas of synaptic contact on hippocampal CA1 neurons.54
The presence of N-type channels on the presynaptic membrane suggests that Ca2+ entry through these channels is responsible for triggering neurotransmitter release. An early study59 demonstrated that ω-CgTx blocks electrically-induced release from the frog NMJ and numerous subsequent studies have demonstrated that the application of ω-CgTx inhibits neurotransmitter release in the central and peripheral nervous system.60-67 Furthermore, biochemical studies indicate that N-type channels are physically associated with proteins such as synaptotagmin and syntaxin which are part of the exocytotic machinery.68-70 There appear to be species- and cell-specific differences in N-type-channel-regulated neurotransmission. For example, while ω-CgTx completely abolishes neurotransmission at the avian and amphibian NMJ, it has no effect on the mammalian motor nervous system.59,65,71-73 The ability of this toxin to inhibit neurotransmission also varies depending on the type of synapse within a given species.52,65-67 For example, inhibitory synaptic transmission in hippocampal CA1 neurons is strongly reduced by the application of ω-CgTx, whereas the toxin blocks excitatory transmissions to a much lesser extent. In addition, while ω-CgTx inhibits release of ACh from both autonomic and central neurons in the rat, release from central neurons is approximately 20-fold less sensitive.65
In spite of the complete and irreversible inhibition of N-type channels produced by ω-CgTx, application of the toxin to many types of neurons only partially inhibits neurotransmitter release, suggesting that other types of Ca2+ channels contribute to neurotransmitter release from both central and peripheral neurons.58,74,75 In fact, while regulation of transmitter release from peripheral neurons appears to predominantly involve N-type channels, release in the central nervous system appears to be controlled primarily by other types of Ca2+ channels that are insensitive to both ω-CgTx and DHPs.76,77
The presence of N-type channels in regions other than the synapse indicates that these channels have other functions in addition to neurotransmitter release. N-type channels localized to dendritic branch points may be involved in integration or amplification of neural inputs. 56 N-type channels may also play a role in nervous system development as evidenced by the expression of N-type channels on postmitotic cerebellar granule cells. These cells only begin migration after the appearance of N-type channels and ω-CgTx causes a cessation of migration.78
Other HVA Ca Channels: P-, Q-, and O-Types
The original classification system of Ca channels, which was expanded from the simple LVA/ HVA dichotomy to encompass T-, L- and N-channels, was subsequently found to be too restrictive to adequately describe all types of Ca2+ conductances. The availability of blocking agents that target L- and N-type channels revealed other HVA currents that could not be defined according to this scheme.36,37,45,79-81 These novel channel types, variously named P-, Q-, O-, and R-, have primarily been defined on the basis of their distinctive pharmacological properties rather than electrophysiological characteristics.
The P-type current was originally identified as an HVA current in Purkinje cells that is insensitive to the agents typically used to inhibit L- and N-type channels.82 These channels are thought to support the Ca2+-dependent action potentials in the dendrites of cerebellar Purkinje cells, which are unaffected by DHPs and ω-CgTx, but are potently blocked by components of the venom of the funnel web spider Agelenopsis aperta.82-85
Whole cell recordings from Purkinje cells reveal an HVA current that peaks at voltages between -30 and -20 mV and inactivates slowly over the duration of the depolarization.37,52,81,86 Single channel analysis of P-type channels reveals conductances in ranges similar to those of N- and L-type channels. Multiple unitary conductance levels of 9, 14, 19 pS in 110 mM Ba have been reported for P-type channels in the Purkinje cell soma and dendrites,87 and a P-type current in hypoglossal motorneurons has a unitary conductance of 20 pS.39
The venom of the funnel web spider, like that of the cone snail, is a cocktail of toxins that target different elements of the synaptic machinery. FTX, a non-peptide component of the venom (arginine polyamine and a synthetic analog of FTX, sFTX), was initially reported to be specific blockers of P-type channels,3,82,83 but subsequently shown to produce inhibition of other Ca2+ currents in conjunction with the P-type block.3,45
ω-Aga IVA, a 48-amino acid peptide also found in the venom of A. aperta potently inhibits P-type Ca2+ channels.52,84,86 In Purkinje cells, complete inhibition is observed at concentrations below 200 nM, with half-maximal block produced at concentrations between 2 and 10 nM. Inhibition is rapid, occurring within two minutes of application, and while the inhibition is poorly reversible by wash-out, it can be removed by a series of strong depolarizations (i.e., to +70 mV). Block of P-type currents by ?-Aga IVA in other neurons is qualitatively similar to that in Purkinje cells although the kinetics vary slightly. For example, while inhibition of P-type current in spinal cord interneurons and neurons in the visual cortex occurs as rapidly as that in the Purkinje cells, the rate of block is several times slower in CA1 and CA3 hippocampal neurons.86
A peptide toxin isolated from the cone snail Conus magus has also been shown to inhibit P-type channels.88 This toxin, ω-CgTx MVIIC, blocks P-type channels with an IC50 of 1-10 μM. However, ω-CgTx MVIIC also inhibits N-type channels as well as the Q- and O-type conductances88 (see chapter by Adams and Lewis for more detail).
P-type channels do not account for all of the DHP- and ω-CgTx-resistant current in neurons since a substantial fraction of current remains even after exposure to saturating concentrations of DHPs, ω-CgTx, and ω-Aga IVA. Cultured rat cerebellar granule cells express an HVA current that is unaffected by these inhibitors at concentrations which block L-, N- and P-type channels, respectively.45,89,90 However, the channels supporting this novel current (termed Q-type) are partially blocked by ?-Aga IVA at concentrations 10 to 100 times that required for P-type inhibition and are completely blocked by ω-CgTx MVIIC (IC50= 30 to 300 nM). In addition to differing sensitivities to these toxins, Q-type channels partially recover from ω-Aga IVA-induced inhibition within minutes of toxin washout. Q-type channels also display electrophysiological properties distinct from those of P-type channels. While P-type currents in Purkinje cells and cerebellar granule cells show no inactivation over a 100 ms test depolarization, the Q-type current in granule cells decays to approximately 65% of the peak current over the same time period.
The existence of O-type channels has been inferred solely from pharmacological studies. O-type channels were identified as high affinity ω-CgTx MVIIC binding sites.91 These channels are significantly more sensitive to the toxin than are other ω-CgTx MVIIC-sensitive channels45 It is possible that P-, Q-, and O-type channels are members of the same channel family which possess slightly different pharmacological and electrophysiological properties as a result of alternative splicing of the α1 subunit gene and/or different complements of auxiliary subunits. While O-type channels have not been localized immunohistochemically, evidence from binding studies suggests that they are widely distributed in the mammalian CNS.45 Estimates of O-type bindings sites in rat brain preparations suggest that O-type channels are more prevalent than N-type channels in the CNS, and it has been proposed that O-type channels are localized exclusively to synaptic termini, which would largely prevent their detection through electrophysiological means.
Many studies implicate P-, Q, and O-type channels in neurotransmitter release.45 While N-type channels mediate release at some synapses in the mammalian CNS, the ω-Aga IVA-sensitive P- and Q-type channels appear to play a more prominent role.52,92 ω-Aga IVA potently blocks Ca2+ uptake into synaptosomes84 and partially inhibits the release of dopamine and glutamate from synaptosomes93,94 and at CA1-CA3 synapses in the hippocampus.58,74,76 In the peripheral nervous system, ω-Aga IVA has little or no effect on the autonomic nervous system,95 but P-type channels are probably responsible for neurotransmitter release at the mammalian NMJ.96,97 As ω-Aga IVA blocks both P- and Q-type channels, it is possible that both channel types are involved in neurotransmission. Wheeler and colleagues found that the pharmacological properties of the ω-Aga IVA-sensitive channels supporting neurotransmission in the hippocampus and for ω-Aga IVA-induced block of inhibitory postsynaptic potentials in the cerebellum were more similar to Q- than P-type channels.58,98 Finally, O-type channels may also mediate neurotransmission at certain synapses, as norepinephrine release in the hippocampus is inhibited by subnanomolar concentrations of ω-CgTx MVIIC.45
R-Type Channels
A component of the HVA current in cerebellar granule cells remains even after the application of nimodipine, ω-CgTx , ω-Aga VIA, and ω-CgTx MVIIC. This current, categorized as R (residual or resistant)- type,89 comprises approximately 15% of the HVA current in these cells. R-type current may not necessarily reflect a single channel type, but a family of molecularly distinct channels with similar pharmacological and electrophysiological characteristics.
R-type currents begin to activate around -40 mV and reach a peak amplitude at 0 mV. The current inactivates rapidly, and the increased rate of inactivation with Ca as the charge carrier suggests that the channels supporting the R-type current inactivate in a Ca2+-dependent manner. R-type channels are equally sensitive to block by Cd2+ and Ni2+ ions. The exact nature of the channels supporting this current is currently unknown. See the below section on Class E channels for further discussion.
Cloned Calcium Channels
HVA Ca2+ Channels Are Multi-Subunit Complexes
Biochemical studies have established that high threshold voltage-gated Ca2+ channels are multi-subunit complexes. Taking advantage of the high-affinity binding of organic antagonists, several groups purified the L-type channel from skeletal muscle. Four distinct polypeptides, designated α1 (175-kDa), α2δ (170-kDa), β (52-kDa), and γ (32-kDa), co-migrate with the ligand-binding activity.99-102 A minor 212-kDa band also co-purified and was shown to represent a larger, much less abundant form of the skeletal muscle α1 subunit.103 Similar approaches have been used to isolate the cardiac L-type104 and brain N-type channels105 These complexes also consist of an a1 subunit associated with β and α2δ subunits. The β and α2-δ are highly similar, if not identical to, the subunits associated with the skeletal muscle α.106,107 However, unlike the skeletal muscle L-type channel, no γ subunits appeared as part of either complex. A novel 95-kDa polypeptide was found to comigrate with the N-type channel although it is unclear whether this represents a bona fide channel subunit or a proteolytic fragment.105
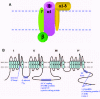
While subunit composition differs slightly depending on channel type, a general model has been proposed for HVA channels in which four to five proteins form a multisubunit complex (fig.1A). In this model, the α1 subunit forms the channel proper, comprising both the voltage-sensing mechanism and the Ca2+ selective pore, and the remaining proteins interact with the α1 subunit to modulate activity.
Primary Structure and Properties of Ca2+ Channel a1 Subunits
The first cDNAs encoding Ca2+ channel α1 subunits were isolated from rabbit skeletal muscle.5,108 The α1s L-type subunit is an 1873-residue protein that bears a high degree of amino acid similarity to the voltage-gated Na+ and potassium (K+) channels (see fig.1). The α1 subunit is predicted to consist of four homologous, mainly hydrophobic domains (designated domains I, II, III and IV). Each of the four domains is comprised of six putative membrane-spanning segments (S1-S6). The S4 segment in each domain contains positively-charged residues every third or fourth position and is believed to form part of the voltage-sensing mechanism of the channel. Between the S5 and S6 segments of each domain are two hydrophobic segments, SS1 and SS2, which are predicted to form the channel pore (fig.1B).
Based upon similarity to voltage-gated Na+ channels, Tanabe et al (1987)5 speculated that the α1 subunit may form both the Ca2+-selective pore and the voltage sensor of the channel complex. This hypothesis was supported by studies demonstrating that expression of the α1S in myotubes from dysgenic mice restored normal skeletal muscle-type E-C coupling and the slow Ca2+ current absent in these cells.7 In addition, α1S expression in dysgenic myotubes restored the charge movement observed in normal myotubes upon membrane depolarization.109 These results indicated that the skeletal muscle a1S subunit acts both as a voltage-sensor, providing a physical connection between membrane depolarization and Ca2+-release from intracellular stores for the initiation of muscle contraction, and is also part of a functional VGCC.
Using the skeletal muscle clone as a probe, cDNAs encoding homologous L-type α1 subunits have been subsequently cloned from cardiac110 and smooth muscle.111,112 Injection of the cardiac α1 subunit into dysgenic myotubes resulted in the expression of a VGCC which differed markedly in terms of activation rate, Ba2+ permeability, and E-C coupling from the current conducted through channels formed by the skeletal muscle clone.113 Expression of cardiac and smooth muscle α1 subunit clones in Xenopus oocytes110,111,114,115 resulted in large inward currents that were sensitive to the organic channel agonists and antagonists, thereby identifying them as L-type channels. Co-expression of skeletal muscle-derived α2-δ and β subunits, while affecting the amplitude and voltage dependence of the currents, was not required for channel activity or drug binding, suggesting that the α1 subunit is capable of forming a functional channel in the absence of the other subunits. However, because some VGCC subunits may be endogenously expressed by Xenopus oocytes,116,117 it is possible that the a1 protein forms a complex with these endogenous auxiliary subunits. This prompted several groups to examine the properties of the a1 subunit in cells lacking these proteins. Murine L-cells118,119 and Chinese Hamster Ovary (CHO) cells114 stably transformed with α1 subunits express voltage activated Ca2+ currents sensitive to L-type channel blockers. While Ca2+ currents in cells expressing the smooth muscle a1 subunit displayed similar drug sensitivities and kinetics to the native currents, the currents supported by α1S activated considerably more slowly than currents recorded from skeletal muscle cells.
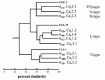
At least nine different α1 subunit genes are now known to be expressed in the mammalian nervous system (see fig.2, Table 1). Initially, four distinct classes of α1 subunits were isolated from a rat brain library on the basis of their homology to the rabbit skeletal muscle α1S subunit.120 Each cDNA hybridized to one of four distinct banding patterns on Northern blots of rat brain mRNA, allowing them to be grouped into four classes, designated α1A, α1B, α1C and α1D. Subsequently, a fifth α1 subunit (α1E) was isolated from rat brain.121 Southern blot analysis and DNA sequencing indicated that the five classes are separate members of a multigene family with the α1A, α1B, and α1E channels being more similar to one another than they are to the class C and D channels (fig.2). The class C clone is almost identical to the cardiac a1 subunit, suggesting that the class C and D clones represent members of the DHP-sensitive L-type channels, while the A, B, and E clones are DHP resistant. Four other VGCC α1 subunit genes have been identified in the mammalian genome. The α1F122,123 shares the most sequence identity with the L-type channels. The α1G, α1H, and α1I clones represent the LVA branch of the VGCC family.124-126
Table 1
Voltage-Gated Calcium Channel Subunits.
The individual α1 subunit clones share the most homology in the transmembrane domains with the majority of sequence divergence occurring in the putative cytoplasmic regions of the channels. The loop between domains II and III, and the cytoplasmic tail vary in size as well as sequence. The DHP-sensitive channels (classes C, D, F, and S) have relatively short (≈130 amino acid) sequences linking domains II and III while the analogous region in the class A and B channels are significantly larger (≈ 430 amino acid). However, despite the size similarity between the linkers, the class A and B clones show little sequence homology in this region.127 While the LVA channel clones (classes G, H, and I) share much less sequence identity with the other classes, the voltage-sensing S4 region and the loop that forms the channel pore are well conserved. Other motifs, such as the β-subunit binding site and the E-F hand, which are found in the HVA classes of VGCCs are absent in the LVA channels. Whole cell and single channel electrophysiological techniques have provided information about the functional and pharmacological properties of the cloned channels and allowed researchers to assign the individual clones to channel types (Table 1).
α1A/ Cav 2.1
Class A α1 subunits have been cloned from both rabbit (BI-1, BI-2)128 and rat (rbA-I)129 brain and Drosophila melanogaster (Dmca1A).130 Northern blot analysis identified a single RNA transcript of 9.4 kb in rabbit brain, while two transcripts of 8.3 and 8.8 kb were detected in rat brain. α1A transcripts are widely distributed throughout the nervous system, as well as being present in the heart and pituitary, but not in skeletal muscle, stomach, or kidney. In brain, the highest levels of class A transcripts were found in the cerebellum, suggesting that this clone might encode a P-type channel and initial expression studies supported this hypothesis. Studies showed that α1A clones expressed in Xenopus oocytes supported HVA currents which were insensitive to DHPs and ω-CgTx but inhibited by ω-Aga VIA.128,131,132 However, a number of discrepancies between currents elicited in oocytes expressing class A clones and native P-type currents have called this into question.131,132 The α1A currents display prominent time- and voltage-dependent inactivation, yet P-type currents show little time-dependent inactivation and are relatively insensitive to holding potential. Furthermore, the pharmacological sensitivities of α1A channels are quite different from those of P-type channels. While these currents are blocked by ω-Aga IVA, they are approximately 200-fold less sensitive to the toxin (IC50≈ 200 nM) than are P-type currents (IC50≈ 2-10 nM). In addition, α1A channels are markedly more sensitive to block by the snail toxin ω-CgTx MVIIC than P-type channels (IC50≈ 150 nM vs. 1-10 μM for P-type channels). Sather et al (1993)131 noted that the kinetic and electrophysiological features of the α1A current were more similar to the Q-type current described in cerebellar granule cells by Randall et al (1993).133 Based on these results, some researchers have suggested that the class A clones represent Q-type channels, and that P-type channels are the product of a different gene. However, Stea et al (1994)132 noted that the high correlation between the localization of α1A transcripts and P-type channel immunoreactivity implied a possible structural similarity between P- and Q-type channels and the α1A gene product. They further proposed that the functional differences between the two channel types may arise as a result of differential post-translational processing of the proteins (which could affect toxin binding), subunit composition of the channel complex, and/or alternative splicing of the α1 gene.
The auxiliary subunits of the VGCC complex are known to modulate the properties of the α1 subunit (see below). The inactivation kinetics of the α1A subunit are dramatically affected by the type of β subunit with which it is associated.132 Expression of the α1A subunit from rat brain in the absence of the β subunit results in a current that inactivates considerably (40% remained after a 400 ms test pulse). Co-expression of either the β1b or β3 subunit increased α1A current inactivation to a rate similar to that of the Q-type current. In contrast, currents recorded from oocytes expressing the α1A + β2a combination show significantly slower inactivation kinetics, such that the waveform is more similar to that of native P-type currents. The β subunit also appears to affect voltage-dependent inactivation of the α1A subunit. The β2a subunit shifted the steady-state inactivation of the α1A approximately 15 to 20 mV more depolarized, thus reducing the sensitivity of the channel to holding potential.
Multiple isoforms derived from the alternative splicing of α1A transcripts have been detected by several groups.128,134-136 Bourinet et al (1999)135 isolated an α1A variant which possessed an number of sequence differences when compared to the rbA-1 clone and examined the functional implications of these splicing events. A valine insertion in the I-II linker both slowed time-dependent inactivation and altered steady-state inactivation. α1A variants containing this valine have inactivation properties similar to P-type channels, while valine-less isoforms, such as rbA-1, appeared more Q-like. A second splice site consisted of the insertion of an asparagine-proline (N-P) pair in the IVS3-IVS4 linker. This affects the electrophysiological properties of the α1A channel by producing a depolarizing shift in the current-voltage relationship. The N-P insertion also had the effect of decreasing the affinity of the channel for ω-Aga IVA by decreasing the on-rate of the toxin and increasing the off-rate. Thus, it is likely that the α1A gene encodes both P- and Q-type channels, and the distinct channel properties reflect differences both in subunit composition and alternative splicing. P-type channels may be comprised of splice variants that contain the valine insertion in the I-II linker, but not the N-P pair in the IVS3-IVS4 loop. Conversely, Q-type currents may be produced by channels lacking the valine, but containing the N-P insertion. In addition, the association of different β subunits may also be an important determinant of the P- versus Q-type phenotype.
α1B/ Cav 2.2
Class B α1 subunits have been cloned from rat (rbB-I)137 or (α1B-1),138 human (α1B-1, α1B-2),139 and rabbit brain (BIII),140 as well as from the forebrain of the marine ray Discopyge ommata (doe-4).141 The various clones encode proteins of 2336 to 2339 amino acids with predicted molecular weights of ≈260 to 262 kDa.142 The α1B amino acid sequence is more similar to that of the α1A, with the majority of the sequence divergence occurring in the cytoplasmic loop between domains II and III and in the cytoplasmic carboxyl tail.
Initial indications that this class of α1 subunit corresponds to N-type channels came from the work of Dubel et al (1992)137 who showed that a polyclonal antibody (CNB-1) raised against the II-III loop region of the rbB-I clone immunoprecipitated almost 50% of the high-affinity ω-CgTx binding sites, but none of the DHP-binding sites from rat brain. Furthermore, Northern blot analysis of experimental cell lines showed that rbB-I expression was correlated with the presence of N-type channels in nerve tissues and cell lines that express N-type channels.137,139,140,143 Northern blotting and in situ immunohistochemistry experiments have localized the rbB-I transcripts to the cerebral cortex, hippocampus, forebrain, midbrain, cerebellum, and brainstem. At the subcellular level, rbB-I protein is found on dendrites, at presynaptic terminals and, to a lesser extent, neuronal cell bodies. The localization pattern of the α1B compares well with that observed with a monoclonal antibody against ω-CgTx,56 although the ω-CgTx antibody staining was more widely distributed.
Molecular cloning and biochemical studies have also provided evidence for the existence of multiple isoforms of the α1B subunit.138-140,143-146 At least two of these isoforms represent channels with differentially spliced carboxyl tails, and the inability of CNB-1 to immunoprecipitate all of the ω-CgTx binding sites might suggest the existence of additional isoforms with distinct II-III loop sequences. In addition, α1B clones with small insertions and deletions scattered throughout the channel have been identified, and expression studies indicate that these sequence variations have a profound influence on the properties of the channel132 (see below). These include a variant of the human N-type calcium channel that lacks the synaptic protein interaction site in the domain II-III linker145 and can therefore not associate with synaptic proteins such as syntaxin 1A and SNAP-25.
Transient expression of both human α1B-1 in HEK cells147 and rabbit brain BIII in dysgenic myotubes140 produced HVA currents that first activated between -10 and -30 mV and reached a maximum between +10 and +30 mV. Currents partially inactivated over the time course of the depolarization and were sensitive to holding potential (showing 50% current inactivation at approximately -60 mV). At a holding potential of -40 mV, the bulk of the current (90%) was inhibited. In agreement with binding studies, the α1B-induced currents were irreversibly blocked by 1 μM ω-CgTx and were insensitive to DHPs.
The properties of currents generated in Xenopus oocytes by expression of the rbB-I clone agreed well with those seen with the α1B-1 and BIII clones in terms of pharmacological sensitivities and voltage-dependence of activation. However, there were some notable discrepancies in other properties. For example, the rbB-I channel was less sensitive to holding potential, and the rates of activation and inactivation of the rbB-I clone were markedly slower, resulting in significantly different current waveforms.148 Co-expression of the β1b subunit shifted the voltage-dependence of inactivation to more negative potentials similar to those observed with the human and rabbit clones. The β subunit also increased the rate of activation such that the current attained peak magnitude in approximately 120 ms (compared to 150-250 ms for the rbB-I subunit alone), and increased the rate of inactivation of the rbB-I current. After 800 ms, current through rbB-I alone had decreased by 15-20%, whereas co-expression of the β subunit resulted in a 65-70% reduction in peak current. Despite the rate increases produced by β subunit co-expression, these parameters remained dramatically different from those displayed by the other clones. The rate of activation of rbB-I (110 ms to peak) was still significantly slower than that of a1B-1 currents (10 ms). In addition, the α1B-1 clone showed biphasic inactivation; the first, rapid phase had a τ of 46-105 ms. The τ of the slow phase ranged between 291 and 453 ms. In contrast, decay of rbB-I currents was monophasic and much slower (τ= 700 ms).
Evidence obtained by Stea et al (1999)138 from a second rat brain clone (rbB-II or α1B-II) found that the differences in channel kinetics were the result of small amino acid alterations that are most likely the product of alternative splicing and/or cDNA cloning artifacts. While α1B-II differs from α1B-I in four regions, they found that the substitution of a glycine for a glutamate in transmembrane segment IS3 was sufficient to speed the activation and inactivation kinetics. It has been noted that, while N-type channels are typically described as having fast kinetics, this is not always the case (see N-type channels, above). It may be that isoforms containing a glutamate in IS3, such as the rbB-I (α1B-I) clone may account for the slow and incomplete inactivation of N-type current that has been described in sympathetic neurons. As is the case with the α1A gene, alternative splicing and differential subunit composition may combine to produce slight modifications in channel characteristics with tissue or developmental specificities. Just recently, a newly discovered tissue specific splice isoform variant of the N-type channel has been discovered in dorsal root ganglia (DRG).149 This splice variant arising from the presence of exon 37a has the unique property of being expressed exclusively in nociceptive neurons of the DRG and ultimately may serve as a novel target for pain management.
α1C/ Cav 1.2
The first complete class C α1 (α1C) subunit to be cloned was isolated from cardiac muscle (CARD1).110 The cardiac and skeletal muscle L-type VGCCs arise from separate genes and are approximately 66% identical at the amino acid level. Subsequently, a1C clones were isolated from rabbit lung (pSCaL)111 and rat aorta (990: VSMα1)112 which shared 95% identity with the cardiac clone. Class C clones later isolated from rat (rbC-I, rbC-II)150 and mouse (mbC)151 brain are also more closely related to the cardiac and smooth muscle α1 subunits than to the skeletal muscle clone. The α1C clones code for proteins of 2140 to 2171 amino acids with predicted molecular masses of 235 to 239 kDa. Antibodies directed against the II-III loop of the neuronal class C channel also identify a truncated form with an approximate mass of 195 kDa.6
The high degree of similarity amongst these proteins suggest that they are products of a single gene, and this is supported by genomic Southern blotting experiments. However, there are regions of considerable diversity in these clones which are the result of alternative splicing of the primary transcript.150,152,153 pSCaL, the smooth muscle channel isolated by Biel et al (1990)111 differs from the cardiac form in the amino terminus, the IS6 and IVS3 transmembrane segments, and by a 25-amino acid insertion in the I-II linker. In contrast, the VSMα1 clone, also isolated from smooth muscle, contains a cardiac channel-like IS6 segment and a 68 residue substitution in the carboxyl tail. This sequence is also found in the neuronal clones, rbC-I and —II.150 Finally, rbC-I and rbC-II contain regions of identity with both the smooth muscle and cardiac clones, but also contain many substitutions, primarily in cytoplasmic regions of the protein and the IIIS2 transmembrane segment. Notably, many of these substitutions are localized to the cytoplasmic linker between domains II and III, and may reflect cell-specific functions of the channels. The truncated form of the neuronal protein may also be the result of alternative splicing, or may be due to post-translational processing as is the case with the skeletal muscle channel.6
As would be expected considering the diverse nature of the tissues from which class C cDNAs have been cloned, the α1C gene has a widespread pattern of expression. Transcripts of 8.9 kb have been detected in heart.110 In smooth muscle and brain, hybridizing transcripts were slightly smaller (8.6 and 8 kb, respectively).112,150 Additional transcripts of 15.5 kb (cardiac) and 12 kb (smooth muscle and brain) were also detected which were proposed to represent stable processing intermediates.112 Northern blot analysis indicate that the class C gene is expressed in heart, smooth muscle (e.g., uterine, lung, stomach, and intestine), and throughout the CNS.112,150,151 Within the brain, high expression levels are detected in the olfactory bulb, cerebellum, striatum, thalamus, hypothalamus and cortex, and at much lower levels in the pons/medulla and spinal cord.6,150 Thus far, there is no evidence for exclusive expression of α1C splice variants in specific tissues. cDNAs containing both variants of the IVS3 transmembrane segment have been isolated from heart, smooth muscle, and brain112,150,152 and the alternate carboxyl tail is expressed in both smooth muscle and neuronal tissues.112,150 However, a more detailed study of the expression pattern of class C variants in rat has revealed tissue-specific differences in expression of the rbC-I and rbC-II proteins.150 Overall, rbC-II is the more abundant form, and generally more prevalent in any given tissue, although the relative amounts of the two transcripts vary between brain regions and tissue types.
The subcellular localization of class C α1 subunits was studied using the polyclonal antibody, CNC1.6 Immunoprecipitation and Western blotting experiments indicated that class C a1 subunits comprise approximately 75% of the DHP binding sites in rat cerebral cortex and hippocampus. CNC1 immunoreactivity was distributed at low levels on cell bodies and proximal dendrites, with staining diminishing along the length of the dendrite. In addition, clusters of high levels of immunoreactivity were observed on the surface of cells (as opposed to representing a cytoplasmic pool of channels).
Expression of α1C clones in Xenopus oocytes resulted in currents with electrophysiological and pharmacological properties characteristic of L-type channels.110,111,115,154 In Ba2+, depolarizations to -10 to -30 mV elicited large inward currents that inactivated slowly, if at all, over the course of a several hundred millisecond the test pulse. The currents peaked between +10 and +30 mV and were inhibited by Cd2+ (100-200 μM) and were sensitive to DHPs. Like native L-type currents, the cloned L-type channels showed little sensitivity to holding potential. At holding potentials as high as -20 mV, half of the channels remained available for opening.
The class C channels were shown to be modulated by the auxiliary subunits in much the same manner as the class A and B channels. Co-expression of α1C with β1b and α2-δ significantly increased the magnitude of the whole cell currents. This increase appeared to be mediated primarily through interaction with the β subunit, while the addition of α2-δ had a slight synergistic effect. In addition, co-expression of rbC-II with the auxiliary subunits β1b and/or α2-δ caused a small hyperpolarizing shift in the voltage dependence of activation of the channel and altered channel kinetics.154 The rate of activation for rbC-II varied substantially among oocytes. Time constants of activation ranged between 4 and 50 ms with an average of approximately 10 ms. Coexpression with β1b and α2-δ both increased the rate of activation and reduced the degree of variability. Furthermore, the β1b and α2-δ subunits increased the rate of inactivation. Unlike the α1B rbB-I channel (see above), neither auxiliary subunit had a significant effect on the voltage dependence of inactivation. With the exception of the voltage dependence of channel activation, the modulatory effects on the kinetics and voltage dependent parameters of the channel appear to be mediated primarily through interaction with the β subunit, while the addition of α2-δ had slight synergistic effects.
Whole cell recording using Ca2+ as the charge carrier resulted in current traces with markedly different waveforms. The magnitude of the whole cell current was significantly smaller in Ca2+ than Ba2+, indicating that the channels are more permeable to Ba2+ than to Ca2+. In addition, instead of eliciting currents that are essentially non-inactivating, depolarizing pulses produce currents that decay rapidly by more than 50%.154,155 The increase in inactivation seen with Ca2+ as the permeant ion retains all the hallmarks of Ca2+-dependent inactivation (see above).
Calcium dependent inactivation (CDI) is predominantly linked to two regions of the Alpha1 subunit C-terminus. The EF hand,156 located from aa 1526 to1554, is responsible for CDI and may also regulate voltage-dependent inactivation (VDI).157 The second region (found downstream of the EF hand) is comprised of three distinct binding motifs. Peptide A; (aa 1588 to 1610) and Peptide C (1615 to 1636 aa),158 and IQ(1649 to 1669),159 all work together to form a calcium bound Calmodulin binding site. In the absence of calcium, Ca2+ free calmodulin (ie Apo Cam) is pre-associated with the channel at a site localized between the EF motif and IQ region.23,160 Calcium entering through the channel binds to calmodulin, thus inducing a conformational change that relieves an inhibitory action of the calmodulin/C-terminus complex on the voltage-dependent inactivation machinery.161
α1D/Cav1.3
A class of VGCC cDNAs sharing about 70% amino acid identity with the cardiac clones has been cloned from a variety of species including rat (RBα1;162 rCACN4A, rCACN4B163), human (α1D;51 neuroendocrine or β-cell α1 or hCACN4:164 HCa3a165), and chicken.166 A cDNA encoding an invertebrate ortholog of the class D α1 subunit has been isolated from Drosophila melanogaster (Dmca 1 D).167 These clones retained little similarity (~40% amino acid identity) with the non-DHP-sensitive class A clones,51,164 but were almost identical to the partial rat brain clone designated class D.120 In spite of the sequence divergence between cDNAs generated from class C and class D genes, the two channel types are remarkably similar in certain regions. As with all VGCC α1 subunits discussed thus far, the transmembrane regions tend to be highly conserved, while the intracellular loop sequences are much more divergent. In addition to these regions, the α1D clones are almost identical to the DHP-sensitive class C and S clones in the segments predicted to form the DHP and phenylalkylamine binding sites, suggesting that the class D α1 subunit cDNAs also encode members of the DHP-sensitive L-type family of VGCCs. The exception to this lies in the DHP-binding region in the Drosophila Dmca 1D clone which contains a number of non-conserved changes. This finding, however, is consistent with the pharmacology of phenylalkylamine and DHP binding in Drosophila head membranes,167 and provides further support for the role of these regions in drug binding.
The cloned α1D subunits range in size from the 1634-amino acid (187-kDa) rat brain isoform to the 2516-amino acid (276-kDa) Drosophila channel clone. The range in protein sizes is due primarily to the truncated carboxyl terminal ends of the RBα1, HCa3a, and rCACN4B clones.162,163,165 The rCACN4B clone is a full 535 residues smaller than its rCACN4A counterpart and is proposed to result from the use of an alternative splice acceptor site.163 In addition to the truncation of the carboxyl tail, a number of other regions have been identified in which variants have been produced through alternative splicing.51,152,162,163 These regions include insertions in the cytoplasmic linker between domains I and II, the extracellular linker connecting IVS3 and IVS4, and the transmembrane segments IS6 and IVS3. In addition, Kollmar et al (1997)166 have reported that the chicken brain and cochlear α1D proteins differ in the IIIS2 segment and IVS2-IVS3 loops, as well as in the carboxyl tail. Presumably, these splice variants impart functional differences to the channel. While it is not yet clear what these functional differences may be, Ihara et al (1995)163 note that RBa1, HCa3a and rCACN4B are all truncated at different sites. Furthermore, a number of potential PKA sites are eliminated by the truncations, which may result in the differential regulation of these isoforms by phosphorylation.
The class D channels, often termed “neuroendocrine” because of their presence in brain and pancreatic cells, have also been detected in the retina, ovaries, and cochlear hair cells, but not in heart, skeletal muscle, spleen, colon, or liver. Reports differ on whether α1D transcripts are present in kidney.164,166 Within the CNS, class D expression is found in the hippocampus, habenula, basal ganglia, and thalamus.51 The subcellular localization of the class D α1 subunit was characterized using the polyclonal antisera anti-CND1.6 Anti-CND1 was generated against a peptide homologous to the unique II-III loop of the rat brain α1D clone (Hui et al, 1991).162 Class D channels appear to be far less abundant in the rat CNS than class C channels. The sera labeled the cell bodies and proximal dendrites of both projection neurons and interneurons throughout the brain. In contrast to the punctate staining pattern seen with the class C antibody, anti-CND1 staining was evenly distributed over the cell body. The staining pattern of anti-CND1 was typical for neurons in all regions of the CNS with the notable exception of the cerebellar Purkinje cells. While the cell bodies of these neurons were labeled, there was a marked absence of staining on the Purkinje cell dendrites.
Transient expression of human α1D51 in Xenopus oocytes and stable expression of the rat CACN4A and CACN4B clones (Ihara et al, 1995)163 in CHO cells gives rise to DHP-sensitive currents, confirming the notion that class D channels are members of the L-type family. In both systems, functional expression of the α1D subunit required co-expression of the β subunit. In Xenopus oocytes, transient expression of α1D with the β and α2 subunits yielded larger currents than those produced by expression of α1D plus β alone.51 Ba2+ currents in oocytes expressing α1D+ β + α2 first activated upon depolarizations positive to -30 mV and peak current attained with depolarizations to 0 mV, thus the current-voltage relationship of the α1D is somewhat more negative than that of the α1C154 (see above). α1D channels activated rapidly and inactivated little over depolarizations lasting as long as 700 ms. α1D channels inactivate to a considerably lesser degree over long test pulses than do α1C channels.154
As indicated, class D channels fall under the heading of DHP-sensitive L-type channels. Cd2+ produces substantial block, while Ni2+ has a minimal effect on the current. The DHP agonist Bay K8644 increases current magnitude and shifts the voltage-dependence of activation by approximately -10 mV. In addition, the current is inhibited by the DHP antagonist nifedipine.51,163 However, the affinity of DHPs for α1D channels is generally lower than that observed with α1C.168,169 Moreover, unlike other DHP-sensitive channels, the cloned α1D is partially and reversibly blocked by high concentrations of ω-CgTx (10-15 μM).51
The predominance of the a1D subunit-containing VGCCs in the cochlear hair cells and in the β-cells of the pancreas suggest that these channels may be involved in tonic exocytotic release in these cells163,164,166,170,171 Kollmar et al (1997)166 suggest that the electrophysiological properties of the α1D subunit, such as its lack of inactivation during depolarizations may render it suitable for mediating tonic release. In addition, as suggested by the localization of α1D channels on the cell body and at the base of dendrites of neurons in the CNS, these channels may be involved in integrating signals impinging upon the neuron from multiple sources.6
α1E/ Cav 2.3
The class E gene encodes a VGCC α1 subunit (a1E) that does not fall neatly into either the HVA or LVA categories. α1E cDNAs have been isolated from rabbit (BII-1, BII-2)172 and rat (rbE-II)121 brain, and from Dyscopyge ommata.141 These clones code for proteins between 2178 and 2259 amino acids with predicted molecular masses of approximately 250 kDa. Splice variants of the rabbit brain channel, BII-1 and BII-2, differ from one another in their carboxyl tails, resulting in the addition of a putative PKA site.
The class E clones appear to be more closely related to the DHP-insensitive non-L-type channels (54-60% amino acid identity) than to the L-type channels (less than 45% similarity). However, class E channels are less similar to either class A or B channels than these two classes are to one another, suggesting that the class E channels are members of a novel, more distantly related subgroup of DHP-insensitive channel (fig.2).121,141,172
Northern blotting studies have identified transcripts ranging in size from 10.5 to 12 kb in the mammalian CNS.121,172 High levels of expression was identified in the cerebral cortex, hippocampus, and striatum, while lower levels were detected in the olfactory bulb, midbrain, and Purkinje and granule cell layers of the cerebellum. While α1E appears abundant in brain, none was detected in skeletal muscle, heart, stomach or kidney. At the subcellular level, α1E protein was localized nearly exclusively to the cell body of neurons throughout the CNS. Dendritic staining varies across brain regions. For example, in the cortex and hippocampal formation there is barely perceptible staining of the dendritic branches, while in Purkinje cells, α1E antibodies labeled the distal dendritic branches, but not the main dendritic trunks.173
The α1E channel was initially reported to be a novel member of the LVA family of Ca2+ channels.121 Expression of rbE-II in Xenopus oocytes produced a channel that activated rapidly at low membrane potentials (threshold≈ -50 mV) and inactivated significantly during the depolarization. Other voltage-dependent parameters of this channel (current-voltage relationship, voltage-dependence of inactivation) were also considerably more negative than those of other cloned HVA channels. The rbE-II current magnitude increased steeply with increasing depolarizations, peaking at around 10 mV, and steady state inactivation analysis indicated that the channels were inactivated near the resting membrane potential of the cell. In addition, rbE-II channels were equally permeable to Ca2+ and Ba2+, a property reported to be unique to the LVA channels. Another similarity with LVA channels was the high sensitivity of the current to block by Ni. Furthermore, the channel was found to be expressed in many of the cells that have been shown to possess T-type currents. However, Soong et al (1993)121 noted a number of discrepancies between rbE-II and native T-type currents. For example, although the voltage-dependent properties of rbE-II currents were more negative than those of the other cloned HVA Ca2+ channels, the activation and peak current potentials were not as hyperpolarized as for typical T-type channels.121 Analysis of the electrophysiological properties of other class E channels174-176 have produced some results that contradict those of Soong et al (1993).121 In these studies, the α1E clones formed HVA channels, activating at approximately -10 mV and peaking at +30 mV. The single channel conductance of α1E channels is also much larger than that of T-type channels (12-14 pS vs. ~8 pS).141,177 As α1E channels share properties with LVA as well as HVA channels, the detection of pure α1E currents in native cells may difficult.
It has been suggested that the class E channels may be one of a group of channels comprising the R-type current.89,178,179 The two currents share some electrophysiological and pharmacological characteristics, such as strong voltage-dependence of activation and insensitivity to DHPs, ω-CgTx, and ω-Aga IVA. However, the R-type current is smaller in Ca2+ than in Ba2+, whereas the α1E channels support the two currents equally.89,177 Most relevant, mice lacking the α1E gene entirely still exhibit significant R-type current.180 Thus, while class E channels may comprise a component of R-type current in cerebellar granule cells, the R-type current may actually result from incomplete block of other Ca2+ channels by applied pharmacological agents, the expression of additional splice variants of already identified Ca2+ channel subtypes, or as yet to-be-identified α1 subunits.
α1F/ Cav 1.4
The human class F gene (CACNA1F) was identified through genetic studies in which the X-linked visual disorder Congenital Stationary Night Blindness (CSNB) was mapped to a locus containing a putative VGCC gene.122,123 The predicted CACNA1F gene product (α1F) is between 1912 and 1966 amino acids (alternatively spliced forms have been detected) with an estimated molecular mass of 219 kDa. Sequence analysis indicates that α1F is 55-70% identical at the amino acid level to the L-type channel α1 subunits, sharing the most similarity with α1D, and 35% identical to the P- and N-type channels. In addition, the putative DHP-binding domains in IIIS6 and IVS6 appear relatively well conserved. These results suggest that the α1F is an L-type channel that diverged from the α1D subunit gene.122
α1F expression was initially reported to be restricted to the retina in situ hybridization experiments indicate high levels of α1F transcript in the two retinal layers containing the photoreceptors, and horizontal, bipolar, and amacrine cells, but not the ganglion-cell layers,122,123 however, recent reports indicate a more global distribution that includes the immune system and skeletal muscle.181 L-type Ca2+ channels have been implicated in synaptic release from photoreceptors182 and the correlation of the hereditary visual disorder CSNB with mutations in the α1F gene122,123 suggests that the α1F channel mediates neurotransmitter release at these synapses. Functional expression of α1 calcium channels reveals that these channel encode a non-inactivating L-type calcium channel that is DHP sensitive,183 and which is not regulated by ancillary subunits.181 Moreover the channel appears to lack CDI, and displays a large window current, thus making this channel ideally suited to support tonic glutamate release from photoreceptors.181
Low Voltage-Activated (T-Type) Channels
LVA (T-type) channels were first described in rat and chick sensory neurons,1,184 but also are present in other excitable tissues, including cardiac sinoatrial cells, smooth and developing skeletal muscle, neuroendocrine cells, and thalamic neurons, as well as non-excitable cells such as fibroblasts, osteoblasts, and astrocytes.2,4,185 Other cell types, such as sympathetic neurons, superior cervical ganglion cells, and adrenal chromaffin cells, appear not to express significant T-type currents.2,186 T-type Ca channels typically first activate at potentials more positive to -70 mV and whole-cell currents are usually maximal by ~ -40 mV. T-type Ca2+ channels are fully inactivated at resting potentials greater than -40 mV, inactivate rapidly in a voltage-dependent manner, and deactivate, or close, relatively slowly. Because these channels are inactivated at positive holding potentials, very negative holding potentials (-80 mV or more negative) are required for full availability of the channels. The kinetics of activation and inactivation of T-type channels also display voltage dependency; rates are slow near threshold potentials and accelerate with increasing potentials.1,24
While direct evidence linking T-type channels to specific physiological roles is limited, their electrophysiological profiles and cellular and subcellular localizations suggest a number of likely functions. For example, their expression in many cell types that display spontaneous electrical activity (sinoatrial nodal cells of the heart, neuroendocrine cells, and thalamic neurons) together with their low threshold of activation and requirement for hyperpolarized membrane potentials to overcome inactivation, suggests that T-type channels play a role in pacemaker activity and bursting behavior. T-type channels may also exert an effect by generating a resting inward current which could in turn mediate the gating of Ca2+-dependent ion channels and regulate Ca2+-dependent enzymes and gene expression. Finally, T-type currents are highly expressed in developing muscle and nervous tissue, suggesting that these channels may play a developmental role.2,187-189
The study of T-type Ca2+ channels has lagged behind that of other Ca2+ channel subtypes, in part due to the lack of cDNA clones representative of this type (see below), but primarily because of the lack of selective pharmacological agents. T-type channels are generally sensitive to the divalent cations nickel (Ni), cadmium (Cd2+), and zinc (Zn2+), with Ni2+ being the most potent. However, in some cell types, low concentrations of these cations fail to block LVA currents or also block other HVA Ca currents.190,191 A number of organic compounds inhibit T-type channels, but often at concentrations that block other Ca2+ channels. For example, octanol and the sodium (Na) channel blocker amiloride have been utilized as T-type channel antagonists, although these compounds also inhibit some components of whole cell HVA currents. 2,4,186,190,191 Ethosuximide, a drug used to treat absence epilepsy, has been shown to reduce current through T-type channels with little effect on HVA channels although the concentrations required for complete T-type block are quite high.186,190 The antihypertensive mibefridil may be the most potent T-type channel blocker identified to date (IC50 in the submicromolar range)191 although even mibefradil however has recently proven to be a relatively non-specific Ca2+ channel blocker.192
α1G, α1H and α1I
Low stringency library screening strategies such as the ones used to isolate the HVA channels discussed above proved unsuccessful for cloning the T-type Ca2+ channels. The first members of the T-type Ca2+ channels were identified by screening data banks for sequences with similarity to previously cloned Ca channels.124,193,200 Subsequently, other classes of T-type Ca2+ channels were identified by screening cDNA libraries.125,126,194
Thus far, T-type clones have been isolated from rat (α1G;124 α1G, α1H, α1I;200 α1I126), mouse (α1G193), human brain (α1G, α1I),194-196 and human heart (α1H).125 The α1G and α1H subunits are approximately 65% identical, whereas the α1I subunit shares only 53% identity with the α1H and 47% with the α1G. As expected from the failure to identify T-type channels in the low stringency hybridization screens used to isolate many of the HVA channels, the T-type channels share limited sequence homology with HVA Ca2+ channels. The highest level of sequence similarity is found in the four membrane-spanning domains. Most of the amino acid changes in these regions are conservative, thereby maintaining structural elements common to voltage-gated ion channels. The charges located in the fourth transmembrane segment of each domain are conserved, as are the pore-forming loops between the fifth and sixth transmembrane segments. In HVA channels, a glutamate residue located in each of these four loops is believed to determine the ion selectivity of the channels.197,198 All T-type channels cloned thus far contain aspartate residues instead of glutamates in the domain III and IV P-regions.124 This difference may account for the difference in the permeation properties seen between high and low voltage activated channels. The intra- and extracellular linkers joining the transmembrane domains share little homology with either HVA channels or with other T-type channels. Furthermore, the T-type channels do not seem to possess specific functional motifs that have been identified in HVA channels, including the binding site in the I-II linker or the putative EF-hand motif in the carboxyl tail.124
The three classes of T-type channel have been localized using Northern blotting, in situ hybridization, and RT-PCR techniques.124-126,193,195,200,201 The α1G subunit appears to be expressed abundantly throughout the brain and to a lesser degree in heart. Low levels have also been detected in placenta, lung, and kidney. High levels of transcript are observed in the cerebellum, hippocampus, thalamus, and olfactory bulb, with lesser amounts localized to the cerebral cortex and septal nuclei. Initially, the α1H was detected only in cardiac tissue, kidney, and liver, with very little, if any, expression in the brain.125 However, a subsequent study199 suggests that the α1H subunit may be responsible for a large proportion of the T-type current in sensory neurons, and another study indicates the expression of α1H in all areas in the rat brain.201 α1I transcripts have only been detected in brain,126,195 with one study showing specific expression in the striatum of adult rats.200
Expression of these three subunits in Xenopus oocytes124,126 and HEK-293 cells125,193,195,196,200-203 demonstrates that they support currents with most of the characteristics expected of T-type channels. Currents activated upon weak depolarizations from negative holding potentials. In one study, the three T-type channel classes had differing permeability properties.200 As has been noted for classic T-type currents, α1G channel was more permeable to Ca than to Ba. However, α1H channels were more permeable to Ba2+ than Ca2+, while α1I channels were equally permeable to the two ions. In most cases, currents were inhibited by mibefradil and Ni, although the IC50 of each T-type class varied significantly.193,200 The activation and inactivation kinetics of the T-type channels are strongly voltage-dependent. While rates of activation and inactivation are slow near threshold potentials, they accelerate as the strength of depolarization increases. Deactivation is also voltage-dependent, increasing at more hyperpolarized potentials. Steady-state inactivation analysis indicates that the majority of the channel population would be inactivated at the resting potential of most cells. However, because all the channels are not inactivated at the resting potential and the threshold of activation is so negative, a small proportion of channels are capable of opening at the resting potential, thus producing a “window” current. The window current refers to a small, but sustained influx of Ca2+ that occurs even when the cell is ostensibly at rest. This current can contribute to the overall excitability of the membrane and may contribute to the bursting and pacemaker activities attributed to the T-type channels. Finally, as anticipated for T-type channels, the three exogenously expressed channels have small single channel conductances of 5 (α1H), 7.5 (α1G), and 11(α1I) pS.126
Similar to the different classes of channels within the HVA subfamily, the biophysical properties of the three T-type channels vary considerably.126,200 The α1G and α1H possess very similar activation and inactivation potentials, while those of the α1H appear to be slightly more negative. Rates of activation and inactivation of α1G and α1H currents also are quite similar. In contrast, activation and inactivation rates for α1I currents are significantly slower. In addition, the activation threshold of α1I channels also differs from the values obtained for α1G and α1H channels. However, varying results have been reported concerning the direction of the observed voltage shift. The rat brain α1I clone studied by J.-H. Lee et al (1999)126 activated at more positive potentials than did the α1G and α1H channels, while McRory et al (1999)200 reported α1I current activation at considerably more negative potentials. That the properties of the α1I channel differ from those of the α1G and α1H is not entirely unexpected when one takes into account the degrees of similarity seen amongst the three channels. Furthermore, multiple splice variants of the α1I have been identified194,195 and may account for the contrasting results reported for α1I currents. Finally, the properties of the three LVA channel clones do not account for all of the T-type characteristics in native cells and there may be additional classes of T-type channels and/or a set of as yet unknown auxiliary subunits specific to LVA channels which further modulate the properties of the LVA α1 subunit. For example, robust alternate splicing for α1G channels has been reported and shown to result in significantly altered biophysical characteristics.203
Auxiliary Ca2+ Channel Subunits
Biochemical studies have shown that in addition to the pore-forming α1 subunit, HVA Ca2+ channel complexes include two or three other proteins: a β subunit, an α2-δ subunit, and in some cases, a γ subunit (fig.1).
β Subunits
The β subunit is the most extensively studied of the auxiliary subunits and appears to have the most profound effects on the functional properties of the α1 subunit. In mammals, there are at least four different β subunits (β1, β2, β3, and β4) which are encoded by distinct genes. The transcripts of at elast two of these genes, the β1 and β2, are alternatively spliced to give rise to β1a β1b, and β1c and β2a and β2b.
Biochemical and primary sequence analyses indicate that the β subunits are hydrophilic with no transmembrane segments or glycosylation sites.204-210 The β subunits contain potential phosphorylation sites for both protein kinase C and cAMP- dependent protein kinase. The modulatory effects of these enzymes on VGCC function may, in part, be the result of their actions on this auxiliary subunit.211
Although the specific effects of channel modulation depend upon the β subunit isoform, all β subunits appear to have the same general impact on the properties of HVA α1 subunits. Coexpression of the a1 and β subunits in both L-cells and Xenopus oocytes increased whole-cell currents and DHP binding without affecting the level of α1 message. This suggests that rather than enhancing expression of the α1 subunit, the β subunit may promote insertion of the α1 subunit into the membrane and/or stabilize a specific conformation of the protein212-215 have proposed that the β subunit potentiates coupling of the gating-charge movement caused by changes in membrane potential with the opening of the pore thereby increasing the probability of channel activation and, in turn, increasing the peak current.
In addition to increasing the magnitude of the current through the α1 subunit, co-expression of the β subunit alters channel kinetics. For most β subunits, the rate of inactivation is increased and there is a shift in the voltage-dependence of activation to more negative potentials. 148,154,207,209,216-219 The effect on kinetics of inactivation, however, varies depending upon the class of β subunit expressed. The β1 and β3 proteins increase the rate on inactivation, while the β2 subunit significantly slows inactivation.
These modulatory effects are observed regardless of which α1 and β subunits are co-expressed, suggesting that the mechanism through which the β subunit acts is common to all HVA VGCCs. The region required for β subunit modulation of the α1 subunit has been localized to a stretch of 30 amino acids at the amino-terminal side of the second of two conserved domains.220 This region, known as the BID (β subunit interaction domain) is also responsible for anchoring the β subunit to the α1. The β subunit has been shown to bind to a conserved motif of 18 amino acids in the intracellular loop between domains I and II of the α1 subunit (the AID: α1 subunit interaction domain).221 The observation that the β subunit from skeletal muscle dramatically increases the magnitude of the current through brain α1 subunits when co-expressed in Xenopus oocytes128 further supports the idea of a common mechanism of α1- β subunit interaction. The β subunits exhibit homology with the Src homology 3-guanylate kinase domain of membrane associated guanylate kinases and this region appears to regulate inactivation of HVA VGCCs.289 A more detailed account of β subunit physiology is provided in the Chapter from the Charnet lab.
α2-δ Subunits
Purification studies indicate that the VGCC α2-δ subunit consists of two distinct subunits (α2 and δ that are disulfide-bonded in the native state.8,222 The α2 and δ subunits are derived from a single gene product that is proteolytically cleaved to form 143-kDa α2 and 27-kDa δ subunits.223 There are currently four genes that code for the α2-δ family; α2-δ1, α2-δ2, α2-δ3, and α2-δ4.
Both proteins are heavily glycosylated,224 supporting the prediction that the α2-δ subunit is predominantly extracellular and a recent study has determined that no more than five residues comprise the cytoplasmic portion of the protein.225 The complex is anchored in the membrane by a single transmembrane segment formed by a portion of the δ subunit. The transmembrane domain is thought to interact with other subunit(s) in the VGCC complex while the extracellular domain is responsible for the modulatory effects.8,142,223-225
There are several splice variants of the α2-δ1 auxiliary subunit, due primarily to the alternative splicing of three specific regions. The total splicing combination of these three regions reveals 5 unique isoforms which are all expressed in a tissue specific manner.226 Skeletal muscle and brain express the isoforms α2-δ1A and α2-δ1B respectively. The heart expresses mainly α2-δ1C and α2-δ1D, and smooth muscle expresses α2-δ1D and α2-δ1E. Interestingly the cardiovascular system expresses all five isoforms.227
The α2-δ2 subunit is expressed in many different tissues including heart, brain, pancreas, testis lung, liver, kidney.227 The α2-δ2 subunit has two regions of alternative splicing. The first region is found on the a2 subunit between residues 661/663 and involves the addition of eight amino acid residues. The second is located on the δ protein and is characterized by the addition of three different residues.228 The resulting three isoforms of the α2-δ2 subunit are all expressed in hMTC (human medullary thyroid cells.) Alternately, in the human heart, only α2-δ2A is expressed. cDNA cloning of the α2-δ3 subunit did not uncover additional splice variants, and this particular α2-δ gene appears to be expressed exclusively in the brain.
In 2002, a new member of the α2-δ auxiliary subunit family was described. α2-δ4 demonstrated a unique property in that it was shown to be expressed primarily in endocrine tissues. Immunohistochemical studies revealed that the α2-δ4 subunit has limited distribution in special cell types of the pituitary, adrenal gland, colon, and fetal liver.229 Whether the a2-d4 subunit plays a physiological role in certain endocrine tissues remains to be seen. Alternative splicing of the α2-δ4 gene gives rise to four potential variants (called a-d.229
The functional effects of the α2-δ are thought to be more subtle that those of the β subunit and are highly dependent on the class of α1 subunit and the cell type used for exogenous expression. For example, Singer et al (1991)217 found that the Ca2+ current in Xenopus oocytes expressing the cardiac α1C protein was greatly enhanced by co-expression of the α2-δ subunit from skeletal muscle. In addition, the rates of activation and inactivation were increased and the voltage dependence of inactivation was shifted to more negative potentials. In contrast, Varadi et al (1991)218 did not observe these effects when they co-expressed the skeletal muscle α2 subunit with the α2-δ subunit in L cells.230 Unlike Xenopus oocytes, L cells do not express endogenous calcium channel subunits.219 Thus, it is possible that the α2-δ subunit acts synergistically with other auxiliary subunits to modulate the properties of the α1. Finally, there is some evidence that the α2-δ subunit is required for efficient expression and/or trafficking of the α1 subunit to the cell membrane,110 an idea that is supported by recent studies in tsA-201 cells showing that α2-δ subunits are key modulators of current densities of α1C, α1B and α1E channels.231
γ Subunits
γ1 (Cacng1)
The VGCC γ1 subunit protein was first identified in guinea pig skeletal muscle during the purification of 1,4-dihydrpyridine receptors. This VGCC heteromultimeric protein consisted of five different subunits. The γ1 subunit, a 28-35 kDa on SDS-Page232 proved to be one of them. Several years later Jay et al (1990)233 isolated rabbit skeletal muscle cDNA and uncovered the primary sequence the γ1 subunit. The cDNA was a 666-nucleotide clone, with a reading frame that would yield a 222 amino acid glycoprotein containing four transmembrane domains. γ1 has been shown to be expressed primarily in skeletal muscle,234 but recently has also been shown to be weakly expressed in the aorta.235 Mice lacking the γ1 subunit display altered skeletal muscle calcium current. Functional effects of the γ1 protein include a hyperpolarized shift in the steady state inactivation properties in skeletal muscleVGCC.236,237
Stargazin or γ2 (Cacng2)
For nearly a decade the presence of other γ—like subunits remained undetected. It was the discovery of the mouse stargazin gene Cacng2 by Letts et al (1998)238 that ultimately led to the description of seven new γ subunits. The stargazin gene and protein were named after the Stargazer mouse, a mouse strain that is prone to absence seizures including an upward neck tilt and prolonged gaze. These mice had acquired a transposon in a 1.5kb region on chromosome 15 and were heterozygous recessive.238,239 The stargazin gene, renamed γ2, had inherited a stop codon rendering the protein inactive and truncated. The etiological consequences of a mutated Cacng gene responsible for the absence epilepsy phenotype of the allelic stargazer (stg) and waggler (wag) mutant mice.238,240
γ2, has been classified as a γ subunit based on its structural similarity to γ1, despite having only weak protein sequence identity (25%).238 At the tissue expression level, unlike γ1, the γ2 subunits are found to be expressed in the brain. Letts et al (1998)238 found mouse γ2 mRNA to be expressed in adult mouse brain abundantly with highest expression in cerebellum, olfactory bulb, cerebral cortex, thalamus, and CA3 and dentate gyrus regions of the hippocampus.
γ3/γ4 (Cacng3/4)
Despite the low sequence homology of γ2 with its original γ1 counterpart, many of the newly discovered γ subunits demonstrate high similarity with the γ2 subunit. γ2, γ3 and γ4 are of closest similarity and make up a subfamily of neuronal γ subunits. Klugbauer et al (2000)241 used Northern blots to show that γ3 and γ4 expressions were exclusively restricted to the brain. However, Chu et al (2001)235 found γ4 to be additionally present in the atrium, aorta, and lung.
γ5 (Cacng5)
The γ5 subunit is unique in that it is expressed not only in the brain and skeletal muscle, but also in different types of endocrine tissues, primarily the liver, kidney, heart, lung and testes. There is some disagreement on the categorization of this subunit as a bona fide γ subunit, and it is also referred to as the “pr protein”. In exogenous expression experiments the mouse pr protein has been shown to the modulate properties of the α1G T-type channel.241
γ6. γ7, γ8
In 2001 Chu et al235 described three new human and rat γ—like subunits (human γ6, γ7, and γ8). γ6 was found in the atrium, ventricle, skeletal muscle and a short splice variant of its kind was found in atrium, ventricle, aorta, brain, and lung. γ7 is expressed in all tissues except aorta, kidney, liver, and spleen. γ8 was found only in brain and testis.
In summary, the eight γ subunits are technically considered to be part of the Claudin family of proteins, and are differentially expressed among a variety of different tissues. They all display four membrane-spanning regions with both their C and N termini located intracellularly, while all extracellular regions display N-glycosylation sites. All the Cacng genes have four exons (with the exception of Canga5 and 7 which have five exons),242 and exon1 is predominately the largest. The carboxyl terminus is of particular interest given that γ2, γ3, γ4 and γ8 have a PDZ binding motif in this region.243, 244 The C-termini of the γ7 and γ5 are longer and lack this consensus motif, however it is interesting to note that they have inherited an SS/TSPC site, probably designated for protein interactions.235 Of particular note, stargazin and other member of the γ subunit family (γ3, γ4 and γ8) have recently been shown to define a novel family of transmembrane AMPA receptor regulatory proteins (TARPs).290 The TARPs appear to regulate two distinct roles in AMPA receptor signaling: trafficking of AMPA receptors to the plasma membrane and an agonist-mediated dynamic interaction that may contribute to synaptic plasticity. 291
Effects of γ Subunits on Channel Biophysics
Over the years, many different combinations of all of the hetermultimer subunits with different accompanying γ subunits have been tested. Wei et al (1991)219 co-expressed rabbit Cardiac α1C with γ1, and observed increased peak currents. Letts et al, (1998)238 demonstrated the modulatory effects of neuronal γ2, by co-expressing the subunit with α1A-β1-α2-δ. The effects of γ2 included a hyperpolarizing shift of the steady state inactivation properties of the channel. Similar results were obtained by Klugbauer et al (2002)245 with α1G and α1C. The same hyperpolarizing effects on channel SSI properties were observed with γ4, but were not with γ5. It is interesting to note that γ2 shifted the activation potential to a more depolarized level, when co-transfected with α1A-β1-α2-d heteromultimers, while γ3, γ4, and γ5 did not.
Rousset el al (2001)246 studied the electrophysiological properties of α1A- α2-δ channels expressed in Xenopus oocytes in the presence and absence of various γ subunits (i.e., γ1,2,3, or 4), and found that γ2 and γ3 induced a small negative shift of the inactivation curve and an acceleration of inactivation kinetics. Green et al (2001)247 studied the functional effects of γ2, γ3, and γ4 on α1I T-type channels in HEK293 cells. Their results revealed a significant slowing of deactivation with γ2 and a slight but insignificant increase in peak calcium current. γ2 was further explored by Kang et al (2001).248 These authors reported that γ2 decreased current amplitude of α1B and α1A calcium channels when co-expressed with β3-α2-δ subunits. These inhibitory effects were dependent upon the presence of the α2-δ subunit. Both γ1 and γ2 slowed the activation kinetics of α1B.
Recently, Moss et al (2002 — EMBO J)249 showed that when α7 was co-expressed transiently in either Xenopus oocytes or COS-7 cells, N-type current was completely abolished. This effect appears to be mediated by blocking expression rather than interfering with trafficking or the biophysical properties of the channel.
Summary
From the initial identification of native Ca2+ channel subtypes, tremendous progress has been made in our understanding of the molecular biology and physiology of voltage-gated Ca2+ channels, including the cloning and expression of representatives of all known Ca2+ channel types, the elucidation of their tissue distribution, and an in depth understanding of their structure and function. Moreover, we have come to understand the pathophysiology of Ca2+ channels through naturally occurring channelopathies in humans and mice (see Table 2), and through targeted gene deletions (see Table 3). The ensuing chapters in this book will provide additional details of our understanding of voltage-gated Ca2+ channels.
Table 2
Spontaneous mutations in calcium channel subunit genes.
Table 3
Induced mutations in calcium channel subunit genes.
References
- 1.
- Nowycky MC, Fow AP, Tsien RW. Three types of neuronal calcium channels with different calcium agonist sensitivity. Nature. 1985;340:233–236.
- 2.
- Bean BP. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. [PubMed: 2540697]
- 3.
- Scott RH, Pearson HA, Dolphin AC. Aspects on vertebrate neuronal voltage-activated calcium currents and their regulation. Progress Neurobiol. 1991;36:485–520. [PubMed: 1658850]
- 4.
- Tsien RW, Ellinor PT, Horne WA. Molecular diversity of voltage-dependent Ca2+ channels. TIPS. 1991;12:349–354. [PubMed: 1659003]
- 5.
- Tanabe T, Takeshima H, Flockerzi V. et al. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. [PubMed: 3037387]
- 6.
- Hell JW, Westenbroek RE, Warner C. et al. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. J Cell Biol. 1993;123(4):949–962. [PMC free article: PMC2200142] [PubMed: 8227151]
- 7.
- Tanabe TK, Beam G, Powell JA. et al. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336:134–139. [PubMed: 2903448]
- 8.
- Miller RJ. Voltage-sensitive Ca2+ channels. J Biol Chem. 1992;267(3):1403–1406. [PubMed: 1309781]
- 9.
- Perney TM, Hirning LD, Leeman SE. et al. Multiple calcium channels mediate neurotransmitter release from peripheral neurons. Proc Natl Acacd Sci USA. 1986;83(17):6656–6659. [PMC free article: PMC386563] [PubMed: 2428039]
- 10.
- Cazalis M, Dayanithi G, Nordmann JJ. Hormone release from isolated nerve endings of the rat neurohypophysis. J Physiol (London). 1987;390:55–70. [PMC free article: PMC1192166] [PubMed: 2450999]
- 11.
- Rane SG, Holz GG 4th, Dunlap K. Dihydropyridine inhibition of neuronal calcium current and substance P release. Pflugers Arch. 1987;409(4-5):361–366. [PMC free article: PMC2962864] [PubMed: 2442705]
- 12.
- Lemos JR, Nowycky MC. Two types of calcium channels co-exist in peptide releasing vertebrate nerve terminals. Neuron. 1989;2(5):1419–1426. [PubMed: 2560641]
- 13.
- Wang X, Wang G, Lemos R. et al. Ethanol directly modulates gating of a dihydropyridine-sensitive Ca2+ channel in neurohypophysial terminals. J Neurosci. 1994;14(9):5453–5460. [PMC free article: PMC6577079] [PubMed: 7521910]
- 14.
- Murphy TH, Worley PF, Baraban JM. L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron. 1991;7:625–635. [PubMed: 1657056]
- 15.
- Sutton KG, McRory JE, Guthrie H. et al. P/Q-type channels mediate the activity-dependent feedback of syntaxin-1A. Nature. 1999;401:800–804. [PubMed: 10548106]
- 16.
- Dolmetsch RE, Pajvani U, Fife K. et al. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. [PubMed: 11598293]
- 17.
- Mintz IM, Venema VJ, Adams ME. et al. Inhibition of N- and L-type Ca2+ channels by the spider venom toxin ω-Aga-IIIA. Proc Natl Acad Sci USA. 1991;88:6628–6631. [PMC free article: PMC52141] [PubMed: 1713686]
- 18.
- Cohen CJ, Ertel EA, Smith MM. et al. High affinity block of myocardial L-type calcium channels by the spider toxin ω-Aga-toxin IIIA: Advantages over 1,4-dihydropyridines. Mol Pharmacol. 1992;42:947–951. [PubMed: 1480135]
- 19.
- Tsien RW, Lipscombe D, Madison DV. et al. Multiple types of neuronal calcium channels and their selective modulation. TINS. 1988;11(10):431–438. [PubMed: 2469160]
- 20.
- Imready JP, Yue DT. Mechanism of Ca2+-sensitive inactivation of L-type channels. Neuron. 1994;12:1301–1318. [PubMed: 8011340]
- 21.
- Charnet P, Bourinet E, Dubel SJ. et al. Calcium currents recorded from a neuronal α1C L-type channel in Xenopus oocytes. FEBS Letts. 1994;344:87–90. [PubMed: 7514140]
- 22.
- Neely A, Olcese R, Wei S. et al. Ca2+-dependent inactivation of a cloned cardiac Ca2+ channel α1 subunit (α1C) expressed in Xenopus oocytes. Biophys J. 1994;66:1895–1903. [PMC free article: PMC1275915] [PubMed: 8075326]
- 23.
- Erickson MG, Alseikhan BA, Peterson BZ. et al. Preassociation of calmodulin with voltage-gated Ca(2+) channels revealed by FRET in single living cells. Neuron. 2001;31(6):973–985. [PubMed: 11580897]
- 24.
- Fox AP, Nowycky MC, Tsien RW. Kinetics and pharmacological properties distinguishing three types of calcium currents in chick sensory neurons. J Physiol (Lond). 1987;394:149–172. [PMC free article: PMC1191955] [PubMed: 2451016]
- 25.
- Fox AP, Nowycky MC, Tsien RW. Single-channel recordings of three types of calcium channels in chic sensory neurons. J Physiol (Lond). 1987;394:173–200. [PMC free article: PMC1191956] [PubMed: 2451017]
- 26.
- Gross RA, Macdonald RL. Dynorphin A selectively reduces a large transient (N-type) calcium current of mouse dorsal root ganglion neurons in cell culture. Proc Natl Acad Sci USA. 1987;84(15):5469–5473. [PMC free article: PMC298879] [PubMed: 2440050]
- 27.
- Green KA, Cottrell GA. Actions of baclofen on components of the Ca-current in rat and mouse DRG neurons in culture. Br J Pharmacol. 1988;94(1):235–245. [PMC free article: PMC1853938] [PubMed: 2456810]
- 28.
- Petersen M, Wagner G, Pierau FK. Modulation of calcium currents by capsaicin in a subpopulation of sensory neurones of guinea pig. Nauyn Schmiedebergs Arch Pharmacol. 1989;339(1-2):184–191. [PubMed: 2542804]
- 29.
- McCarthy RT, TanPiengco PE. Multiple types of high-threshold calcium channels in rabbit sensory neurons: high-affinity block of neuronal L-type by nimodipine. J Neurosci. 1992;12(6):2225–2234. [PMC free article: PMC6575939] [PubMed: 1318957]
- 30.
- Wanke E, Ferroni A, Malgaroli A. Activation of a muscarinic receptor selectively inhibits a rapidly inactivated Ca2+ current in rat sympathetic neurons. Proc Natl Acad Sci USA. 1987;84(12):4313–4317. [PMC free article: PMC305075] [PubMed: 2438697]
- 31.
- Plummer MR, Logothetis DE, Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989;2(5):1453–1463. [PubMed: 2560643]
- 32.
- Jones SW, Marks TN. Calcium currents in bullfrog sympathetic neurons. I. activation kinetics and pharmacology. J Gen Physiol. 1989;94(1):151–167. [PMC free article: PMC2228934] [PubMed: 2478659]
- 33.
- Carrier GO, Ikeda SR. TTX-sensitive Na+ channels and Ca2+ channels of the L- and N-type underlie the inward current in acutely dispersed coeliac-mesenteric ganglia neurons of adult rats. Pflugers Arch. 1992;42(1):7–16. [PubMed: 1321408]
- 34.
- Doerner D, Pitler TA, Alger BE. Protein kinase C activators block specific calcium and potassium current components in isolated hippocampal neurons. J Neurosci. 1988;8(11):4069–4078. [PMC free article: PMC6569483] [PubMed: 2846795]
- 35.
- Williams PJ, MacVivar BA, Pittman QJ. Electrophysiological properties of neuroendocrine cells of the intact rat pars intermedia: multiple calcium currents. J Neurosci. 1990;10(3):748–756. [PMC free article: PMC6570131] [PubMed: 2319302]
- 36.
- Mogul DJ, Fox AP. Evidence for multiple types of Ca2+ channels in acutely isolated hippocampal CA1 neurones of the guinea-pig. J Physiol (Lond). 1991;433:259–281. [PMC free article: PMC1181370] [PubMed: 1668752]
- 37.
- Regan LJ, Sah DWY, Bean BP. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and ω-conotoxin. Neuron. 1991;6:269–280. [PubMed: 1847065]
- 38.
- Baux G, Fossier P, Trudeau LE. et al. Presynaptic receptors for FMRFamide, histamine and buccalin regulate acetylcholine release at a neuro-neuronal synapse of Aplysia by modulating N-type Ca2+ channels. J Physiol (Paris). 1992;86(1-3):3–13. [PubMed: 1343594]
- 39.
- Umemiya M, Berger AJ. Single channel properties of four calcium channel types in rat motoneurons. J Neurosci. 1995;15(3):2218–2224. [PMC free article: PMC6578168] [PubMed: 7534346]
- 40.
- Plummer MR, Hess P. Reversible uncoupling of inactivation in N-type calcium channels. Nature. 1991;351:657–659. [PubMed: 1646965]
- 41.
- Biagi BA, Enyeart JJ. Multiple calcium currents in a thyroid C-cell line: biophysical properties and pharmacology. Am J Physiol. 1991;260:C1253–C1263. [PubMed: 1647663]
- 42.
- Jones SW, Elmslie KS. Separation and modulation of calcium currents in bullfrog sympathetic neurons. Can J Physiol Pharmacol. 1992;70(Suppl):S56–S63. [PubMed: 1338298]
- 43.
- Wang X, Treistman SN, Lemos JR. Single channel recordings of Nt- and L-type Ca2+ currents in rat neurohypophsial terminals. J Neurophysiol. 1993;70(4):1617–1628. [PubMed: 8283218]
- 44.
- Olivera BM, Gray WR, Zeikus R. et al. Peptide neurotoxins from fish-hunting cone snails. Science. 1985;230:1338–1343. [PubMed: 4071055]
- 45.
- Olivera BM, Miljanich GP, Ramachandran J. et al. Calcium channel diversity and neurotransmitter release: The ω-conotoxins and ω-agatoxins. Annu Rev Biochem. 1994;63:823–867. [PubMed: 7979255]
- 46.
- Olivera BM, McIntosh LJM, Cruz J. et al. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984;23(22):5087–5090. [PubMed: 6509012]
- 47.
- Kasai H, Aosaki T, Fukuda J. Presynaptic Ca-antagonist omega-conotoxin irreversibly blocks N-type Ca-channels in chick sensory neurons. Neurosci Res. 1987;4(3):228–235. [PubMed: 2437502]
- 48.
- McCleskey EW, Fox AP, Feldman DH. et al. Omega-conotoxin; direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci USA. 1987;84(12):4327–4331. [PMC free article: PMC305078] [PubMed: 2438698]
- 49.
- Aosaki T, Kasai H. Characterization of two kinds of high-voltage-activated Ca-channel currents in chick sensory neurons. Differential sensitivity to dihydropyridines and omega-conotoxin GVIA. Pflugers Arch. 1989;414(2):150–156. [PubMed: 2547195]
- 50.
- Wang X, Treistman SN, Lemos JR. Two types of high threshold calcium currents inhibited by omega-conotoxin in nerve terminals of rat neurohypophysis. J Physiol (Lond). 1992;445:181–199. [PMC free article: PMC1179977] [PubMed: 1323666]
- 51.
- Williams ME, Feldman DH, McCue AF. et al. Structure and functional expression of a novel human neuronal calcium channel subtype. Neuron. 1992;8(1):71–84. [PubMed: 1309651]
- 52.
- Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. TINS. 1995;18(2):89–98. [PubMed: 7537420]
- 53.
- Wagner JA, Snowman AM, Biswas A. et al. Omega-conotoxin GVIA binding to a high-affinity receptor in brain: characterization, calcium sensitivity, and solubilization. J Neurosci. 1988;8(9):3354–3359. [PMC free article: PMC6569423] [PubMed: 2845019]
- 54.
- Jones OT, Kunze DL, Angelides KJ. Localization and mobility of ω-conotoxin-sensitive Ca2+ channels in hippocampal CA1 neurons. Science. 1989;244:1189–1193. [PubMed: 2543080]
- 55.
- Takemura M, Kiyama H, Fukui H. et al. Distribution of the omega-conotoxin receptor in rat brain. An autoradiographic mapping. Neuroscience. 1989;32(2):405–416. [PubMed: 2555740]
- 56.
- Fortier LP, Trembley JP, Rafrafi J. et al. A monoclonal antibody to conotoxin reveals the distribution of a subset of calcium channels in the rat cerebellar cortex. Brain Res Mol Brain Res. 1991;9(3):209–215. [PubMed: 1851523]
- 57.
- Catterall WA, De Jongh K, Rotman E. et al. Molecular properties of calcium channels in skeletal muscle and neurons. Ann NY Acad Sci. 1993;681:342–355. [PubMed: 8395149]
- 58.
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type calcium channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. [PubMed: 7832825]
- 59.
- Kerr LM, Yoshikami D. A venom peptide with a novel presynaptic blocking action. Nature. 1984;308:282–284. [PubMed: 6608056]
- 60.
- Dooley DJ, Lupp A, Hertting G. et al. Omega-conotoxin GVIA and pharmacological modulation of hippocampal noradrenaline release. Eur J Pharmacol. 1988;148(2):261–267. [PubMed: 3378575]
- 61.
- Lundy PM, Frew R. Evidence of omega conotoxin GVIA-sensitive Ca2+ channels in mammalian peripheral nerve terminals. Eur J Pharmacol. 1988;156(3):325–330. [PubMed: 2850931]
- 62.
- Dutar P, Rascol O, Lamour Y. Omega-conotoxin GVIA blocks synaptic transmission on the CA1 field of the hippocampus. Eur J Pharmacol. 1989;174(2-3):261–266. [PubMed: 2560980]
- 63.
- Herdon H, Nahorski SR. Investigations of the roles of dihydropyridine and omega-conotoxinsensitive calcium channels in mediating depolarization-evoked endogenous dopamine release from striatal slices. Naunyn Schmiedebergs Arch Pharmacol. 1989;340(1):36–40. [PubMed: 2552331]
- 64.
- Takemura M, Kishino J, Yamatodani A. et al. Inhibition of histamine release from rat hypothalamic slices by omega-conotoxin GVIA, but not by nilvadipine, a dihydropyridine derivative. Brain Res. 1989;496(1-2):351–356. [PubMed: 2553205]
- 65.
- Wessler I, Dooley DJ, Werhand J. et al. Differential effects of calcium channel antagonists (omega-conotoxin GVIA, nifedipine, verapamil) on the electrically-evoked release of [3H] acetylcholine from the myenteric plexus, phrenic nerve and neocortex. Naunyn Schmiedebergs Arch Pharmacol. 1990;341(4):288–294. [PubMed: 2333100]
- 66.
- Horne AL, Kemp JA. The effect of omega-conotoxin GVIA on synaptic transmission within the nucleus accumbens and hippocampus of the rat in vitro. Br J Pharmacol. 1991;103(3):1733–1739. [PMC free article: PMC1907806] [PubMed: 1657265]
- 67.
- Potier B, Dutar P, Lamour Y. Different effects of omega-conotoxin GVIA at excitatory and inhibitory synapses in rat CA1 hippocampal neurons. Brain Res. 1993;616(1-2):236–241. [PubMed: 8102938]
- 68.
- Leveque C, Hoshino T, David P. et al. The synaptic vesicle protein synaptotagmin associates with calcium channels and is a putative Lambert-Eaton myasthenic syndrome antigen. Proc Natl Acad Sci USA. 1992;89(8):3625–3629. [PMC free article: PMC48921] [PubMed: 1314395]
- 69.
- Leveque C, el FarO, Martin-Moutot N. et al. Purification of the N-type calcium channel associated with syntaxin and synaptotagmin. J Biol Chem. 1994;269(9):6306–6312. [PubMed: 8119979]
- 70.
- Sheng Z-H, Rettig J, Takahashi M. et al. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 1994;13:1303–1313. [PubMed: 7993624]
- 71.
- Sano K, Enomoto K, Maeno T. Effects of synthetic omega-conotoxin, a new type of Ca2+ antagonist, on frog and mouse neuromuscular transmission. Eur J Pharmacol. 1987;141(2):235–241. [PubMed: 2824217]
- 72.
- Protti DA, Szczupak L, Scornik FS. et al. Effects of omega-conotoxin GVIA on neurotransmitter release at the mouse neuromuscular junction. Brain Res. 1991;557(1-2):336–339. [PubMed: 1684129]
- 73.
- Adams ME, Olivera BM. Neurotoxins: Overview of an emerging research technology. TINS. 1994;17(4):151–155. [PubMed: 7517594]
- 74.
- Wu LG, Saggau P. Pharmacological identification of two types of presynaptic voltage-dependent calcium channels at CA3-CA1 synapse of the hippocampus. J Neurosci. 1994;14(9):5613–5622. [PMC free article: PMC6577093] [PubMed: 8083757]
- 75.
- Turner TJ, Lampe RA, Dunlap K. Characterization of presynaptic calcium channels with omega-conotoxin MVIIC and omega-grammotoxin SIA: Role for a resistant calcium channel type in neurosecretion. Mol Pharmacol. 1995;47(2):348–353. [PubMed: 7870043]
- 76.
- Burke SP, Adams ME, Taylor CP. Inhibition of endogenous glutamate release from hippocampal tissue by Ca2+ channel toxins. Eur J Pharmacol. 1993;238(2-3):383–386. [PubMed: 8104812]
- 77.
- Luebke JI, Dunlap K, Turner TJ. Multiple calcium channel types control glutaminergic synaptic transmission in the hippocampus. Neuron. 1993;11(5):895–902. [PubMed: 7902110]
- 78.
- Komuro H, Rakic P. Selective role of N-type calcium channels in neuronal migration. Science. 1992;257:809–806. [PubMed: 1323145]
- 79.
- Bean BP. Pharmacology of calcium channels in cardiac muscle, vascular muscle, and neurons. Am. J Hypertens. 1991;4:406S–411S. [PubMed: 1654934]
- 80.
- Randall RD, Raabe W. A non-T-, N-, or L-type calcium channel mediates release of transmitter from cerebellar granule cells in tissue culture. Abstract presented at the 22nd meeting of the Society for Neuroscience. 1992;18:429.
- 81.
- Regan LJ. Voltage-dependent calcium currents in Purkinje cells from rat cerebellar vermis. J Neurosci. 1991;11(7):2259–2269. [PMC free article: PMC6575481] [PubMed: 1712382]
- 82.
- Llinas R, Sugimori M, Lin JW. et al. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci USA. 1989;86(5):1689–1693. [PMC free article: PMC286766] [PubMed: 2537980]
- 83.
- Lin J-W, Rudy B, Llinas R. Funnel-web spider venom and a toxin fraction block calcium current expressed from rat brain mRNA in Xenopus oocytes. Proc Natl Acad Sci USA. 1990;87:4538–4542. [PMC free article: PMC54151] [PubMed: 2162047]
- 84.
- Mintz IM, Venema VJ, Swiderek KM. et al. P-type calcium channels blocked by the spider toxin ω-Aga-VIA. Nature. 1992;355:827–829. [PubMed: 1311418]
- 85.
- Bindokas VP, Brorson JR, Miller RJ. Characteristics of voltage sensitive calcium channels in dendrites of cultured rat cerebellar neurons. Neuropharmacology. 1993;32(11):1213–1220. [PubMed: 8107975]
- 86.
- Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. [PubMed: 1321648]
- 87.
- Usowicz MM, Sugimori M, Cherskey B. et al. P-type calcium channels in the somata and dendrites of adult cerebellar Purkinje cells. Neuron. 1992;9(6):1185–1199. [PubMed: 1281419]
- 88.
- Hillyard DR, Monje VD, Mintz IM. et al. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992;9:69–77. [PubMed: 1352986]
- 89.
- Zhang JF, Randall AD, Ellinor PT. et al. Distinctive pharmacology and kinetics of cloned neuronal calcium channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32(11):1075–1088. [PubMed: 8107963]
- 90.
- Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J.Neurosci. 1995;15(4):2995–3012. [PMC free article: PMC6577783] [PubMed: 7722641]
- 91.
- Adams ME, Myers RA, Imperial JS. et al. Toxityping rat brain calcium channels with ω-toxins from spider and cone snail venoms. Biochem. 1993;32:12566–12570. [PubMed: 8251474]
- 92.
- Luebke JI, Dunlap K, Turner TJ. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993;11(5):895–902. [PubMed: 7902110]
- 93.
- Turner TJ, Adams ME, Dunlap K. Calcium channels coupled to glutamate release identified by omega-Aga-IVA. Science. 1992;258:310–313. [PubMed: 1357749]
- 94.
- Turner TJ, Adams ME, Dunlap K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc Natl Acad Sci USA. 1993;90(20):9518–9522. [PMC free article: PMC47600] [PubMed: 8415733]
- 95.
- Lundy PM, Frew R. Effect f omega-agatoxin-IVA on autonomic neurotransmission. Eur J Pharmacol. 1994;261(1-2): 79–84. [PubMed: 8001657]
- 96.
- Uchitel OD, Protti DA, Sanchez V. et al. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci USA. 1992;89:3330–3333. [PMC free article: PMC48860] [PubMed: 1348859]
- 97.
- Bowersox S, Ko C-P, Sugiura Y. et al. Omega-conopeptide SNX-230 (MVIIC) blocks calcium channels in mouse neuromuscular junction nerve terminals. Abstract presented at the 23rd annual meeting of the Society for Neuroscience. 1993;Vol19:p1478.
- 98.
- Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. [PubMed: 7901765]
- 99.
- Curtis BM, Catterall WA. Solubilization of the calcium antagonist receptor from rat brain. J Biol Chem. 1983;256(12):7280–7283. [PubMed: 6305932]
- 100.
- Flockerzi V, Oeken H-J, Hofmann F. et al. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986;323: 66–68. [PubMed: 2427959]
- 101.
- Takahashi M, Seagar MJ, Jones JF. et al. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci USA. 1987;84:5478–5482. [PMC free article: PMC298881] [PubMed: 2440051]
- 102.
- De WaardM, Gurnett CA, Campbell KP. Structural and functional diversity of voltage-gated calcium channels In: T Narahashi, ed.Ion Channels.Vol4 New York Plenum Press1996 .
- 103.
- De JonghKS, Merrick DK, Catterall WA. Subunits of purified calcium channels: a 212-kDa form of alpha 1 and partial amino acid sequence of a phosphorylation site of an independent beta subunit. Proc Natl Acad Sci USA. 1989;86(21):8585–8589. [PMC free article: PMC298327] [PubMed: 2554320]
- 104.
- Norman RI, Leech RN. Subunit structure and phosphorylation of the cardiac L-type channel. Biochem Soc Transact. 1994;22:492–496. [PubMed: 7958352]
- 105.
- Witcher DR, De WaardM, Sakamoto J. et al. Subunit identification and reconstitution of the N-type Ca2+ channel complex purified from brain. Science. 1993;261:486–489. [PubMed: 8392754]
- 106.
- Ahlijanian MK, Striessnig J, Catterall WA. Phosphorylation of an alpha 1-like subunit of an omega-conotoxin-sensitive brain calcium channel by cAMP-dependent protein kinase and protein kinase. J Biol Chem. 1991;266(30):20192–20197. [PubMed: 1657916]
- 107.
- Sakamoto J, Campbell KP. A monoclonal antibody to the beta subunit of the skeletal muscle dihydropyridine receptor immunoprecipitates the brain omega-conotoxin GIVA receptor. J Biol Chem. 1991;266(28):18914–18919. [PubMed: 1655767]
- 108.
- Ellis SB, Williams ME, Ways NR. et al. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. [PubMed: 2458626]
- 109.
- Adams BA, Tanabe T, Mikami A. et al. Intragenic charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Science. 1990;346:569–572. [PubMed: 2165571]
- 110.
- Mikami A, Imoto K, Tanabe T. et al. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–233. [PubMed: 2474130]
- 111.
- Biel M, Ruth P, Bosse E. et al. Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung. FEBS. 1990;269(2):409–412. [PubMed: 2169433]
- 112.
- Koch WJ, Ellinor PT, Schwartz A.. cDNA cloning of a dihydropyridine-sensitive calcium channel from rat aorta. J Biol Chem. 1990;265(29):17786–17791. [PubMed: 2170396]
- 113.
- Tanabe T, Mikami A, Beam KG. et al. Cardiac-type excitation-contraction coupling in dysgenic skeletal muscle injected with cardiac dihydropyridine receptor cDNA. Nature. 1990;344:451–453. [PubMed: 2157159]
- 114.
- Bosse E, Bottlender R, Kleppisch T. et al. Stable and functional expression of the calcium channel α1 subunit from smooth muscle in somatic cell lines. EMBO J. 1992;11(6):2033–2038. [PMC free article: PMC556668] [PubMed: 1376244]
- 115.
- Itagaki K, Koch WJ, Bodi L. et al. Native-type DHP-sensitive calcium channel currents are produced by clone rat aortic smooth muscle and cardiac a1 subunits expressed in Xenopus laevis oocytes and are regulated by α2- and β-subunits. FEBS. 1992;297(3):221–225. [PubMed: 1371969]
- 116.
- Singer-Lahat D, Lotan I, Itagaki K. et al. Evidence for the existence of RNA of Ca2+-channel α2δ subunit in Xenopus oocytes. Biochim Biophys Acta. 1992;1137:39–44. [PubMed: 1382608]
- 117.
- Tareilus E, Roux M, Qin N. et al. A Xenopus oocyte β subunit: Evidence for a role in the assembly/ expression of voltage-gated calcium channels that is separate from its role as a regulatory subunit. Proc Natl Acad Sci USA. 1997;94:1703–1708. [PMC free article: PMC19980] [PubMed: 9050842]
- 118.
- Perez-Reyes E, Kim HS, Lacerda AE. et al. Induction of calcium currents by the expression of the α1 subunit of the dihydropyridine receptor from skeletal muscle. Nature. 1989;340:233–236. [PubMed: 2474131]
- 119.
- Kim HS, Wei X, Ruth P. et al. Studies on the structural requirements for the activity of the skeletal muscle dihydropyridine receptor/ slow calcium channel. J Biol Chem. 1990;265(20):11858–11863. [PubMed: 2164019]
- 120.
- Snutch TP, Leonard JP, Gilbert M. et al. Rat brain expresses a heterogeneous family of calcium channels. Proc Natl Acad Sci USA. 1990;87:3391–3395. [PMC free article: PMC53906] [PubMed: 1692134]
- 121.
- Soong TW, Stea A, Hodson CD. et al. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993;260:1133–1136. [PubMed: 8388125]
- 122.
- Bech-Hansen NT, Naylor MJ, Maybaum TA. et al. Loss-of-function mutations in a calcium-channel α1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:264–267. [PubMed: 9662400]
- 123.
- Strom TM, Nyakatura G, Apfelstedt-Sylla E. et al. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:260–263. [PubMed: 9662399]
- 124.
- Perez-Reyes E, Cribbs LL, Daud A. et al. Molecular characterization of a neuronal low-voltage activated T-type calcium channel. Nature. 1998;391:896–899. [PubMed: 9495342]
- 125.
- Cribbs L, Lee J-H, Yang J. et al. Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res. 1998;83:103–109. [PubMed: 9670923]
- 126.
- Lee J-H, Daud AN, Cribbs L. et al. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci. 1999;19(6):1912–1921. [PMC free article: PMC6782566] [PubMed: 10066244]
- 127.
- Snutch TP, Reiner PB. Calcium channels: diversity of form and function. Curr Opin Neurobiol. 1992;2:247–253. [PubMed: 1322749]
- 128.
- Mori Y, Friedrich T, Kim M-S. et al. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. [PubMed: 1849233]
- 129.
- Starr TVB, Prystay W, Snutch TP. Primary structure of a calcium channel that is highly expressed in the rat cerebellum. Proc Natl Acad Sci USA. 1991;88:5621–5625. [PMC free article: PMC51929] [PubMed: 1648226]
- 130.
- Smith LA, Wang X, Piexoto AA. et al. A Drosophila calcium channel alpha1 A subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci. 1996;16(24):7868–7879. [PMC free article: PMC6579206] [PubMed: 8987815]
- 131.
- Sather WA, Tanabe T, Zhang J-F. et al. Distinctive biophysical and pharmacological properties of class A (BI) calcium channel α1 subunits. Neuron. 1993;11:291–303. [PubMed: 8394721]
- 132.
- Stea A, Tomlinson J, Soong TW. et al. Localization and functional properties of a rat brain α1A calcium channel reflects similarities to neuronal Q- and P-type channels. Proc Natl Acad Sci USA. 1994;91:10576–10580. [PMC free article: PMC45064] [PubMed: 7524096]
- 133.
- Randall AD, Wendland B, Schweizer F. et al. Five pharmacologically distinct high voltage-activated Ca2+ channels in cerebellar granule cells. Abstract presented at the 23rd meeting of the Society for Neuroscience. 1993;Vol19:p1478.
- 134.
- Zhuchenko O, Bailey J, Bonnen P. et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the α1A-voltage-dependent calcium channel. Nature Genetics. 1997;15:62–69. [PubMed: 8988170]
- 135.
- Bourinet E, Soong TW, Sutton K. et al. Splicing of α1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci. 1999;2(5):407–415. [PubMed: 10321243]
- 136.
- Soong TW, DeMaria CD, Alvania RS. et al. Systematic identification of splice variants in human P/Q-type channel alpha1(2.1) subunits: implications for current density and Ca2+-dependent inactivation. J Neurosci. 2002 1;22(23):10142–52. [PMC free article: PMC6758771] [PubMed: 12451115]
- 137.
- Dubel SJ, Starr TVB, Hell J. et al. Molecular cloning of the a-1 subunit of an ω-conotoxin-sensitive calcium channel. Proc Natl Acad Sci USA. 1992;89:5058–5062. [PMC free article: PMC49228] [PubMed: 1317580]
- 138.
- Stea A, Dubel SJ, Snutch TP. Alpha 1B N-type calcium channel isoforms with distinct biophysical properties. Ann NY Acad Sci. 1999;868:118–130. [PubMed: 10414290]
- 139.
- Williams ME, Brust PF, Feldman DH. et al. Structure and functional expression of an ω-conotoxin-sensitive human N-type calcium channel. Science. 1992a;257:389–395. [PubMed: 1321501]
- 140.
- Fujita Y, Mynlieff M, Dirksen RT. et al. Primary structure and functional expression of the ω-conotoxin-sensitive N-type calcium channel from rabbit brain. Neuron. 1993;10:585–598. [PubMed: 8386525]
- 141.
- Horne WA, Ellinor PT, Inman I. et al. Molecular diversity of Ca2+ channel α1 subunits from the marine ray Discopyge ommata. Proc Natl Acad Sci USA. 1993;90:3787–3791. [PMC free article: PMC46390] [PubMed: 7683405]
- 142.
- Stea A, Soong TW, Snutch TP. Voltage-gated calcium channels In: RA North, ed.Handbook of Receptors and Channels: Ligand- and Voltage-gated Ion Channels Boca Raton: CRC Press1995113–150.
- 143.
- Westenbroek RE, Hell JW, Warner C. et al. Biochemical properties and subcellular distribution of an N-type calcium channel α1 subunit. Neuron. 1992;(9):1099–1115. [PubMed: 1334419]
- 144.
- Pan JQ, Lipscombe D. Alternative splicing in the cytoplasmic II-III loop of the N-type Ca channel alpha 1B subunit: functional differences are beta subunit-specific. J Neurosci. 2000 1;20(13):4769–75. [PMC free article: PMC6772276] [PubMed: 10864934]
- 145.
- Kaneko S, Cooper CB, Nishioka N. et al. Identification and characterization of novel human Ca(v)2.2 (alpha 1B) calcium channel variants lacking the synaptic protein interaction site. J Neurosci. 2002;122(1):82–92. [PMC free article: PMC6757606] [PubMed: 11756491]
- 146.
- Bell TJ, Thaler C, Castiglioni AJ. et al. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 2004;841(1):127–38. [PubMed: 14715140]
- 147.
- Williams BD, Schrank B, Huynh C. et al. A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics. 1992;131(3):609–624. [PMC free article: PMC1205034] [PubMed: 1321065]
- 148.
- Stea A, Dubel SJ, Pragnell M. et al. A β-subunit normalizes the electrophysiological properties of a cloned N-type Ca2+ channel α1 subunit. Neuropharmacol. 1993;32(11):1103–1116. [PubMed: 8107965]
- 149.
- Bell TJ, Thaler C, Castiglioni AJ. et al. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 2004 8;41(1):127–38. [PubMed: 14715140]
- 150.
- Snutch TP, Tomlinson WJ, Leonard JP. et al. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron. 1991;7:45–57. [PubMed: 1648941]
- 151.
- Ma W-J, Holz RW, Uhler MD. Expression of a cDNA for a neuronal calcium channel α1 subunit enhances secretion from adrenal chromaffin cells. J Biol Chem. 1992;267(32):22728–22732. [PubMed: 1385406]
- 152.
- Perez-Reyes E, Wei X, Castellano A. et al. Molecular diversity of L-type calcium channels. J Biol Chem. 1990;265(33):20430–20436. [PubMed: 2173707]
- 153.
- Diebold RJ, Koch WJ, Ellinor PT. et al. Mutually exclusive exon splicing of the cardiac calcium channel α1 subunit gene generates developmentally regulated isoforms in the rat heart. Proc Natl Acad Sci USA. 1992;89:1497–1501. [PMC free article: PMC48478] [PubMed: 1311102]
- 154.
- Tomlinson J, Stea A, Bourinet E. et al. Functional properties of a neuronal class C L-type calcium channel. Neuropharmacology. 1993;32(11):1117–1126. [PubMed: 8107966]
- 155.
- Bourinet E, Charnet P, Tomlinson WJ. et al. Voltage-dependent facilitation of a neuronal alpha 1C L-type calcium channel. EMBO J. 1994;13(21):5032–5039. [PMC free article: PMC395449] [PubMed: 7957069]
- 156.
- Babitch J. Channel hands. Nature. 1990;346:321–322. [PubMed: 2165218]
- 157.
- Bernatchez G, Talwar D, Parent L. Mutations in the EF-hand motif impair the inactivation of barium currents of the cardiac alpha1C channel. Biophys J. 1998;75(4):1727–1739. [PMC free article: PMC1299844] [PubMed: 9746514]
- 158.
- Pitt GS, Zuhlke RD, Hudmon A. et al. Molecular basis of calmodulin ethering and Ca2+ dependent inactivation of L-type Ca2+ channels. J Biol Chem. 2001;276:30794–30802. [PubMed: 11408490]
- 159.
- Zühlke RD, Pitt GS, Deisseroth K. et al. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. [PubMed: 10335846]
- 160.
- Mouton J, Ronjat M, Jona I. et al. Skeletal and cardiac ryanodine receptors bind to the Ca(2+)-sensor region of dihydropyridine receptor alpha(1C) subunit. FEBS Lett. 2001;505(3):441–444. [PubMed: 11576544]
- 161.
- Kim J, Ghosh S, Nunziato DA. et al. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron. 2004;41(5):745–754. [PubMed: 15003174]
- 162.
- Hui A, Ellinor PT, Kiizanova O. et al. Molecular cloning of multiple subtypes of a novel rat brain isoform of the α1 subunit of the voltage-dependent calcium channel. Neuron. 1991;7:35–44. [PubMed: 1648940]
- 163.
- Ihara Y, Yamada Y, Fujii Y. et al. Molecular diversity and functional characterization of voltage-dependent calcium channels (CACN4) expressed in pancreatic β-cells. Mol Endocrinol. 1995;9(1):121–130. [PubMed: 7760845]
- 164.
- Seino S, Chen L, Seino M. Proc Natl Acad Sci USA. 1992. pp. 584–588. [PMC free article: PMC48283] [PubMed: 1309948]
- 165.
- Yaney GC, Wheeler MB, Wei X. et al. Cloning of a novel alpha 1-subunit of the voltage-dependent calcium channel from the beta-cell. Mol Endocrinol. 1992;6(12):2143–2152. [PubMed: 1337146]
- 166.
- Kollmar R, Montgomery LG, Fak J. et al. Predominance of the α1D subunit in L-type voltage-gated Ca2+ channels of hair cells in the chicken's cochlea. Proc Natl Acad Sci USA. 1997;94:14883–14888. [PMC free article: PMC25132] [PubMed: 9405708]
- 167.
- Zheng WG, Feng D, Ren D. et al. Cloning and characterization of a calcium channel α1 subunit from Drosophila melanogaster with similarity to the rat brain type D isoform. J Neurosci. 1995;15(2):1132–1143. [PMC free article: PMC6577841] [PubMed: 7869089]
- 168.
- Koschak A, Reimer D, Huber I. et al. alpha 1D (Cav1.3) subunits can form l-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276(25):22100–22106. [PubMed: 11285265]
- 169.
- Xu W, Lipscombe D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21(16):5944–5951. [PMC free article: PMC6763157] [PubMed: 11487617]
- 170.
- Platzer J, Engel J, Schrott-Fischer A. et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102(1):89–97. [PubMed: 10929716]
- 171.
- Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23(34):10832–10840. [PMC free article: PMC6740966] [PubMed: 14645476]
- 172.
- Niidome T, Kim M-S, Friedrich T. et al. Molecular cloning and characterization of a novel calcium channel from rabbit brain. FEBS Lett. 1992;308(1): 7–13. [PubMed: 1379552]
- 173.
- Yokoyama CT, Westenbroek RE, Hell JW. et al. Biochemical properties and subcellular distribution of the neuronal class E calcium channel α1 subunit. J Neurosci. 1995;15(10):6419–6432. [PMC free article: PMC6577977] [PubMed: 7472405]
- 174.
- Ellinor PT, Zhang J-F, Randall AD. et al. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993;363:455–458. [PubMed: 8389006]
- 175.
- Wakamori M, Niidome T, Furutama D. et al. Distinctive functional properties of the neuronal BII (class E) calcium channel. Recept Channels. 1994;2:303–314. [PubMed: 7719708]
- 176.
- Williams ME, Marubio LM, Deal R. et al. Structure and functional characterization of neuronal α1E calcium channel subtypes. J Biol Chem. 1994;269(35):22347–22357. [PubMed: 8071363]
- 177.
- Bourinet E, Zamponi GW, Stea A. et al. The α1E calcium channel exhibits permeation properties similar to low-voltage-activated calcium channels. J Neurosci. 1996;16(6):4983–4993. [PMC free article: PMC6579290] [PubMed: 8756429]
- 178.
- Smith SM, Piedras-Renteria ES, Namkung Y. et al. Neuronal voltage-activated calcium channels: On the roles of the α1E and β3 subunits. Ann NY Acad Sci. 1999;868:175–198. [PubMed: 10414294]
- 179.
- Tottene A, Volsen S, Pietrobon D. alpha(1E) subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. J Neurosci. 2000;20(1):171–178. [PMC free article: PMC6774111] [PubMed: 10627594]
- 180.
- Wilson SM, Toth PT, Oh SB. et al. The status of voltage-dependent calcium channels in alpha 1E knock-out mice. J Neurosci. 2000;20(23):8566–8571. [PMC free article: PMC6773068] [PubMed: 11102459]
- 181.
- McRory JE, Hamid J, Doering CJ. et al. The CACNA1F gene encodes an L-type calcium channel with unique biophysical properties and tissue distribution. J Neurosci. 2004;24(7):1707–1718. [PMC free article: PMC6730460] [PubMed: 14973233]
- 182.
- Tachibana M, Okada T, Arimura T. et al. Dihydropyridine-sensitive calcium current mediates neurotransmitter release from bipolar cells of the goldfish retina. J Neurosci. 1993;13:2898–2090. [PMC free article: PMC6576667] [PubMed: 7687280]
- 183.
- Koschak A, Reimer D, Walter D. et al. Cav1.4alpha1 subunits can form slowly inactivating dihydropyridine-sensitive L-type Ca2+ channels lacking Ca2+-dependent inactivation. J Neurosci. 2003;23(14):6041–6049. [PMC free article: PMC6740341] [PubMed: 12853422]
- 184.
- Carbone E, Lux HD. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984;46(3):413–418. [PMC free article: PMC1434947] [PubMed: 6487739]
- 185.
- Miller RJ. Multiple calcium channels and neuronal function. Science. 1987;235:46–52. [PubMed: 2432656]
- 186.
- Bean BP, McDonough SI. Two for T. Neuron. 1998;20:825–828. [PubMed: 9620687]
- 187.
- Beam KG, Knudson CM. Calcium currents in embryonic and neonatal mammalian skeletal muscle. J Gen Physiol. 1998;91(6):781–798. [PMC free article: PMC2217630] [PubMed: 2458429]
- 188.
- Beam KG, Knudson CM. Effect of postnatal development on calcium currents and slow charge movement in mammalian skeletal muscle. J Gen Physiol. 1988;91(6):799–815. [PMC free article: PMC2217626] [PubMed: 2458430]
- 189.
- Kostyuk PG. Diversity of calcium ion channels in cellular membranes. Neuroscience. 1989;28(2):253–261. [PubMed: 2537937]
- 190.
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. [PubMed: 8815798]
- 191.
- Zamponi GW, Bourinet E, Snutch TP. Nickel block of a family of neuronal calcium channels: Subtype- and subunit-dependent action at multiple sites. J Membrane Biol. 1996;151:77–90. [PubMed: 8661496]
- 192.
- Jimenez C, Bourinet E, Leuranguer V. et al. Determinants of voltage-dependent inactivation affect Mibefradil block of calcium channels. Neuropharmacology. 2000;39(1):1–10. [PubMed: 10665814]
- 193.
- Klugbauer N, Marais E, Lacinová L. et al. A T-type channel from mouse brain. Eur. J. Physiol1999;437:710–715. [PubMed: 10087148]
- 194.
- Mittman SJ, Guo M, Emerick C. et al. Structure and alternative splicing of the gene encoding α1I, a human brain T calcium channel α1 subunit. Neurosci Letts. 1999;269:121–124. [PubMed: 10454147]
- 195.
- Monteil A, Chemin J, Leuranguer V. et al. Specific properties of T-type calcium channels generated by the human alpha 1I subunit. J Biol Chem. 2000;275(22):16530–16535. [PubMed: 10749850]
- 196.
- Beedle AM, Hamid J, Zamponi GW. Inhibition of transiently expressed low- and high-voltage-activated calcium channels by trivalent metal cations. J Membr Biol. 2002;187(3):225–38. [PubMed: 12163980]
- 197.
- Heinemann SH, Terlau H, Stühmer W. et al. Calcium channel characteristics conferred of the sodium channel by single mutations. Nature. 1992;356:441–443. [PubMed: 1313551]
- 198.
- Yang J, Ellinor PT, Sather WA. et al. Molecular determinants of calcium selectivity and ion permeation in L-type calcium channels. Nature. 1993;366:158–161. [PubMed: 8232554]
- 199.
- Lambert RC, McKenna F, Maulet Y. et al. Low-voltage-activated Ca2+ currents are generated by members of CavT subunit family (α1G/H) in primary sensory neurons. J Neurosci. 1998;18(21):8605–8613. [PMC free article: PMC6793515] [PubMed: 9786968]
- 200.
- McRory JE, Santi CM, Hamming KSC. et al. Molecular and functional characterization of family of rat brain T-type calcium channels. J Biol Chem. 2001;276(6):3999–4011. [PubMed: 11073957]
- 201.
- Talley EM, Cribbs LL, Lee J-H. et al. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19(6):1895–1911. [PMC free article: PMC6782581] [PubMed: 10066243]
- 202.
- Monteil A, Chemin J, Bourinet E. et al. Molecular and functional properties of the human alpha(1G) subunit that forms T-type calcium channels. J Biol Chem. 2000;275(9):6090–6100. [PubMed: 10692398]
- 203.
- Chemin J, Monteil A, Bourinet E. et al. Alternatively spliced alpha (1G) (Ca(V)3.1) intracellular loops promote specific T-type Ca(2+) channel gating properties. Biophys J. 2001;80(3):1238–1250. [PMC free article: PMC1301319] [PubMed: 11222288]
- 204.
- Ruth P, Röhrkasten A, Biel M. et al. Primary structure of the β subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989;245:1115–1118. [PubMed: 2549640]
- 205.
- Pragnell M, Sakamoto J, Jay SD. et al. Cloning and tissue-specific expression of the brain calcium channel β-subunit. FEBS Lett. 1991;291(2):253–258. [PubMed: 1657644]
- 206.
- Hullin R, Singer-Lahat D, Freichel M. et al. Calcium channel β subunit heterogeneity: functional expression of cloned cDNA from heart, aorta, and brain. EMBO J. 1992;11(3):885–890. [PMC free article: PMC556528] [PubMed: 1312465]
- 207.
- Perez-Reyes E, Castellano A, Kim HS. et al. Cloning and expression of a cardiac/brain β subunit of the L-type calcium channel. J Biol Chem. 1992;267(3):1792–1797. [PubMed: 1370480]
- 208.
- Powers PA, Liu S, Hogan K. et al. Skeletal muscle and brain isoforms of a β-subunit of human voltage-dependent calcium channels are encoded by a single gene. J Biol Chem. 1992;267(32):22967–22972. [PubMed: 1385409]
- 209.
- Castellano A, Wei X, Birnbaumer L. et al. Cloning and expression of a third calcium channel β subunit. J Biol Chem. 1993;268(5):3450–3455. [PubMed: 7679112]
- 210.
- Castellano A, Wei X, Birnbaumer L. et al. Cloning and expression of a neuronal calcium channel β subunit. J Biol Chem. 1993;268(17):12359–12366. [PubMed: 7685340]
- 211.
- Isom LL, De Jongh KS, Catterall WA. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994;12:1183–1194. [PubMed: 7516685]
- 212.
- Nishimura S, Takeshima H, Hofmann F. et al. Requirement of the calcium channel β subunit for functional conformation. FEBS Lett. 1993;324(3):283–286. [PubMed: 8405367]
- 213.
- Lory P, Varadi G, Slish DF. et al. Characterization of beta subunit modulation of a rabbit cardiac L-type Ca2+ channel alpha 1 subunit as expressed in mouse L cells. FEBS Lett. 1993;315(2):167–172. [PubMed: 8380271]
- 214.
- Chien AJ, Zhao X, Shirokov RE. et al. Roles of membrane-localized β subunit in the formation and targeting of functional L-type Ca2+ channels. J Biol Chem. 1995;270(50):30036–30044. [PubMed: 8530407]
- 215.
- Neely A, Wei X, Olcese R. et al. Potentiation by the beta subunit of the ratio of the ionic current to the charge movement in the cardiac calcium channel. Science. 1993;262(5133):575–578. [PubMed: 8211185]
- 216.
- Lacerda AE, Kim HS, Ruth P. et al. Normalization of current kinetics by interaction between the α1 and β subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature. 1991;352:527–530. [PubMed: 1650913]
- 217.
- Singer D, Biel M, Lotan I. et al. The roles of the subunits in the function of the calcium channels. Science. 1991;253:1553–1557. [PubMed: 1716787]
- 218.
- Varadi G, Lory P, Schultz D. et al. Acceleration of activation and inactivation by the β subunit of the skeletal muscle calcium channel. Nature. 1991;352:159–162. [PubMed: 1712427]
- 219.
- Wei X, Perez-Reyes E, Lacerda AE. et al. Heterologous regulation of the cardiac Ca2+ channel α1 subunit by skeletal muscle β and γ subunits. J Biol Chem. 1991;266(32):21943–21947. [PubMed: 1718988]
- 220.
- De WaardM, Pragnell M, Campbell KP. Ca2+ channel regulation by a conserved β subunit domain. Neuron. 1994;13:495–503. [PubMed: 8060623]
- 221.
- Pragnell M, De WaardM, Mori Y. et al. Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1 subunit. Nature. 1994;368:67–70. [PubMed: 7509046]
- 222.
- Kim HL, Kim H, Lee P. et al. Rat brain expresses an alternatively spliced form of the dihydropyridine-sensitive L-type calcium channel alpha 2 subunit. Proc Natl Acad Sci USA. 1992;89(8):3251–3255. [PMC free article: PMC48844] [PubMed: 1314383]
- 223.
- De JonghK, Warner SC, Catterall WA. Subunits of purified calcium channels. J Biol Chem. 1990;265(25):14738–14741. [PubMed: 2168391]
- 224.
- Gurnett CA, Campbell KP. Transmembrane auxiliary subunits of voltage-dependent ion channels. J Biol Chem. 1996;271(45):27975–27978. [PubMed: 8910401]
- 225.
- Gurnett CA, De WaardM, Campbell KP. Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. [PubMed: 8789958]
- 226.
- Angelotti T, Hofmann F. Tissue-specific expression of splice variants of the mouse voltage-gated calcium channel alpha2/delta subunit. FEBS Lett. 1996;397(2-3):331–337. [PubMed: 8955374]
- 227.
- Klugbauer N, Marais E, Hofmann F. Calcium channel alpha2delta subunits: differential expression, function and drug binding. J Bioenerg Biomemr. 2003;35(6):639–647. [PubMed: 15000524]
- 228.
- Hobom M, Dai S, Marais E. et al. Neuronal distribution and functional characterization of the calcium channel alpha2delta-2 subunit. Eur J Neurosci. 2000;12(4):1217–1226. [PubMed: 10762351]
- 229.
- Qin N, Yagel S, Momplaisir ML. et al. Molecular cloning and characterization of the human voltage-gated calcium channel alpha(2)delta-4 subunit. Mol Pharmacol. 2002;62(3):485–496. [PubMed: 12181424]
- 230.
- Catterall WA. Functional subunit structure of voltage-gated ion channels. Science. 1991;253:1499–1500. [PubMed: 1654596]
- 231.
- Yasuda T, Lewis RJ, Adams DJ. Overexpressed Cav{beta}3 Inhibits N-type (Cav2.2) Calcium Channel Currents through a Hyperpolarizing Shift of “Ultra-slow” and “Closed-state” Inactivation. J Gen Physiol. 2004;123(4):401–416. [PMC free article: PMC2217459] [PubMed: 15024042]
- 232.
- Glossmann H, Striessnig J, Hymel L. et al. Purified L-type calcium channels: only one single polypeptide (alpha 1-subunit) carries the drug receptor domains and is regulated by protein kinases. Biomed Biochim Acta. 1987;46(8-9):S351–356. [PubMed: 2449181]
- 233.
- Jay SD, Ellis SB, McCue AF. et al. Primary structure of the γ subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1990;248:490–492. [PubMed: 2158672]
- 234.
- Powers PA, Liu S, Hogan K. et al. Molecular characterization of the gene encoding the gamma subunit of the human skeletal muscle 1,4-dihydropyridine-sensitive Ca2+ channel (CACNLG), cDNA sequence, gene structure, and chromosomal location. J Biol Chem. 1993;268(13):9275–9279. [PubMed: 8387489]
- 235.
- Chu PH, Bardwell WM, Gu Y. et al. FHL2 (SLIM3) is not essential for cardiac development and function. Mol Cell Biol. 2000;20(20):7460–7462. [PMC free article: PMC86299] [PubMed: 11003643]
- 236.
- Freise D, Held B, Wissenbach U. et al. Absence of the gamma subunit of the skeletal muscle dihydropyridine receptor increases L-type Ca2+ currents and alters channel inactivation properties. J Biol Chem. 2000;275(19):14476–14481. [PubMed: 10799530]
- 237.
- Ahern CA, Powers PA, Biddlecome GH. et al. Modulation of L-type Ca2+ current but not activation of Ca2+ release by the gamma1 subunit of the dihydropyridine receptor of skeletal muscle. BMC Physiol. 2001;1(1):8. [PMC free article: PMC37314] [PubMed: 11495636]
- 238.
- Letts VA, Felix R, Biddlecome GH. et al. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet. 1998;19(4):340–347. [PubMed: 9697694]
- 239.
- Letts VA, Valenzuela A, Kirley JP. et al. Genetic and physical maps of the stargazer locus on mouse chromosome 15. Genomics. 1997;43(1):62–68. [PubMed: 9226373]
- 240.
- Chen L, Bao S, Qiao X. et al. Impaired cerebellar synapse maturation in waggler, a mutant mouse with a disrupted neuronal calcium channel gamma subunit. Proc Natl Acad Sci USA. 1999;96(21):12132–7. [PMC free article: PMC18424] [PubMed: 10518588]
- 241.
- Klugbauer N, Dai S, Specht V. et al. A family of gamma-like calcium channel subunits. FEBS Lett. 2000;24470(2):189–97. [PubMed: 10734232]
- 242.
- Moss FJ, Viard P, Davies A. et al. The novel product of a five-exon stargazing-related gene abolishes Ca(V)2.2 calcium channel expression. EMBO J. 2002;21(7):1514–1523. [PMC free article: PMC125363] [PubMed: 11927536]
- 243.
- Kornau HC, Seeburg PH, Kennedy MB. Interaction of ion channels and receptors with PDZ domain proteins. Curr Opin Neurobiol. 1997;7(3):368–373. [PubMed: 9232802]
- 244.
- Tomita S, Nicoll RA, Bredt DS. PDZ protein interactions regulating glutamate receptor function and plasticity. J Cell Biol. 2001;153(5):F19–24. [PMC free article: PMC2174328] [PubMed: 11381098]
- 245.
- Klugbauer N, Welling A, Specht V. et al. L-type Ca(2+) channels of the embryonic mouse heart. Eur J Pharmacol. 2002;447(2-3):279–284. [PubMed: 12151019]
- 246.
- Rousset M, Cens T, Restituito S. et al. Functional roles of gamma2, gamma3 and gamma4, three new Ca2+ channel subunits, in P/Q-type Ca2+ channel expressed in Xenopus oocytes. J Physiol. 2001;532(Pt 3):583–593. [PMC free article: PMC2278567] [PubMed: 11313431]
- 247.
- Green PJ, Warre R, Hayes PD. et al. Kinetic modification of the alpha(1I) subunit-mediated T-type Ca(2+) channel by a human neuronal Ca(2+) channel gamma subunit. J Physiol. 2001;533(Pt 2):467–478. [PMC free article: PMC2278624] [PubMed: 11389205]
- 248.
- Kang MG, Chen CC, Felix R. et al. Biochemical and biophysical evidence for gamma 2 subunit association with neuronal voltage-activated Ca2+ channels. J Biol Chem. 2001;276(35):32917–32924. [PubMed: 11441000]
- 249.
- Moss FJ, Viard P, Davies A. et al. The novel product of a five-exon stargazing-related gene abolishes Ca(V)2.2 calcium channel expression. EMBO J. 2002;21(7):1514–1523. [PMC free article: PMC125363] [PubMed: 11927536]
- 250.
- Ophoff RA, Terwindt GM, Vergouwe MN. et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87(3):543–52. [PubMed: 8898206]
- 251.
- Matsuyama Z, Wakamori M, Mori Y. et al. Direct alteration of the P/Q-type Ca2+ channel property by polyglutamine expansion in spinocerebellar ataxia 6. J Neurosci. 1999;19(12):RC14. [PMC free article: PMC6782654] [PubMed: 10366652]
- 252.
- Fletcher CF, Lutz CM, O'Sullivan TN. et al. Fletcher CF, et al; Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87(4):607–617. [PubMed: 8929530]
- 253.
- Mori Y, Wakamori M, Oda S. et al. Reduced voltage sensitivity of activation of P/Q-type Ca2+ channels is associated with the ataxic mouse mutation rolling Nagoya (tg(rol)). J Neurosci. 2000;20(15):5654–5662. [PMC free article: PMC6772543] [PubMed: 10908603]
- 254.
- Lorenzon NM, Lutz CM, Frankel WN. et al. Altered calcium channel currents in Purkinje cells of the neurological mutant mouse leaner. J Neurosci. 1998;18(12):4482–4489. [PMC free article: PMC6792698] [PubMed: 9614225]
- 255.
- Kraus RL, Sinnegger MJ, Koschak A. et al. Three new familial hemiplegic migraine mutants affect P/Q-type Ca(2+) channel kinetics. J Biol Chem. 2000;275(13):9239–9243. [PubMed: 10734061]
- 256.
- Kraus RL, Sinnegger MJ, Glossmann H. et al. Familial hemiplegic migraine mutations change alpha1A Ca2+ channel kinetics. J Biol Chem. 1998;273(10):5586–5590. [PubMed: 9488686]
- 257.
- Wappl E, Koschak A, Poteser M. et al. Functional consequences of P/Q-type Ca2+ channel Cav 2.1 missense mutations associated with episodic ataxia type 2 and progressive ataxia. J Biol Chem. 2002;277(9):6960–6966. [PubMed: 11742003]
- 258.
- Ptacek LJ, Tawil R, Griggs RC. et al. Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell. 1994;77(6):863–868. [PubMed: 8004673]
- 259.
- Morrill JA, Brown RHJr, Cannon SC. Gating of the L-type Ca channel in human skeletal myotubes: an activation defect caused by the hypokalemic periodic paralysis mutation R528H. J Neurosci. 1998;18(24):10320–10334. [PMC free article: PMC6793372] [PubMed: 9852570]
- 260.
- Knudson CM, Chaudhari N, Sharp AH. et al. Specific absence of the alpha 1 subunit of the dihydropyridine receptor in mice with muscular dysgenesis. J Biol Chem. 1989;264(3):1345–1348. [PubMed: 2536362]
- 261.
- Chen Y, Lu J, Pan H. et al. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann Neurol. 2003;54(2):239–243. [PubMed: 12891677]
- 262.
- Khosravani H, Altier C, Simms B. et al. Gating effects of mutations in the Cav 3.2 T-type calcium channel associated with childhood absence epilepsy. J Biol Chem. 2004;279(11):9681–9684. [PubMed: 14729682]
- 263.
- Brill J, Klocke R, Paul D. et al. entla, a novel epileptic and ataxic Cacna2d2 mutant of the mouse. J Biol Chem. 2004;279(8):7322–7330. [PubMed: 14660671]
- 264.
- Barclay J, Balaguero N, Mione M. et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21(16):6095–104. [PMC free article: PMC6763162] [PubMed: 11487633]
- 265.
- Brodbeck J, Davies A, Courtney JM. et al. The ducky mutation in Cacna2d2 results in altered Purkinje cell morphology and is associated with the expression of a truncated alpha 2 delta-2 protein with abnormal function. J Biol Chem. 2002;277(10):7684–7693. [PubMed: 11756448]
- 266.
- Burgess DL, Jones JM, Meisler MH. et al. Mutation of the Ca2+ channel beta subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell. 1997;88(3):385–392. [PubMed: 9039265]
- 267.
- Letts VA, Felix R, Biddlecome GH. et al. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet. 1998;19(4):340–347. [PubMed: 9697694]
- 268.
- Jun K, Piedras-Renteria ES, Smith SM. et al. Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc Natl Acad Sci USA. 1999;96(26):15245–15250. [PMC free article: PMC24805] [PubMed: 10611370]
- 269.
- Kim C, Jun K, Lee T. et al. Altered nociceptive response in mice deficient in the alpha(1B) subunit of the voltage-dependent calcium channel. Mol Cell Neurosci. 2001;(2):235–245. [PubMed: 11520183]
- 270.
- Hatakeyama S, Wakamori M, Ino M. et al. Differential nociceptive responses in mice lacking the alpha(1B) subunit of N-type Ca(2+) channels. Neuroreport. 2001;12(11):2423–2427. [PubMed: 11496122]
- 271.
- Ino M, Yoshinaga T, Wakamori M. et al. Functional disorders of the sympathetic nervous system in mice lacking the alpha 1B subunit (Cav 2.2) of N-type calcium channels. Proc Natl Acad Sci USA. 2001;98(9):5323–5328. [PMC free article: PMC33208] [PubMed: 11296258]
- 272.
- Saegusa H, Kurihara T, Zong S. et al. Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J. 2001;20(10):2349–2356. [PMC free article: PMC125247] [PubMed: 11350923]
- 273.
- Saegusa H, Kurihara T, Zong S. et al. Altered pain responses in mice lacking alpha 1E subunit of the voltage-dependent Ca2+ channel. Proc Natl Acad Sci USA. 2000;97(11):6132–6137. [PMC free article: PMC18570] [PubMed: 10801976]
- 274.
- Vessey JP, Lalonde MR, Mansergh F. et al. Mutation of the Ca channel gene Cacna1F disrupts calcium signaling and synaptic transmission in mouse retina. 33rd Annual Meeting Society for neuroscience meeting; Prog# 817.4.
- 275.
- Kim D, Park D, Choi S. et al. Thalamic control of visceral nociception mediated by T-type Ca2+ channels. Science. 2003;302:117–119. [PubMed: 14526084]
- 276.
- Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron. 2001;31(1):35–45. [PubMed: 11498049]
- 277.
- Chen CC, Lamping KG, Nuno DW. et al. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science. 2003;302(5649):1416–1418. [PubMed: 14631046]
- 278.
- Seisenberger C, Specht V, Welling A. et al. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav 1.2) calcium channel gene in the mouse. J Biol Chem. 2000;275(50):39193–39199. [PubMed: 10973973]
- 279.
- Strube C, Beurg M, Powers PA. et al. Reduced Ca2+ current, charge movement, and absence of Ca2+ transients in skeletal muscle deficient in dihydropyridine receptor beta 1 subunit. Biophys J. 1996;71(5):2531–2543. [PMC free article: PMC1233741] [PubMed: 8913592]
- 280.
- Gregg RG, Messing A, Strube C. et al. Absence of the beta subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the alpha 1 subunit and eliminates excitation-contraction coupling. Proc Natl Acad Sci USA. 1996;93(24):13961–13966. [PMC free article: PMC19477] [PubMed: 8943043]
- 281.
- Strube C, Beurg M, Powers PA. et al. Reduced Ca2+ current, charge movement, and absence of Ca2+ transients in skeletal muscle deficient in dihydropyridine receptor beta 1 subunit. Biophys J. 1996;71(5):2531–2543. [PMC free article: PMC1233741] [PubMed: 8913592]
- 282.
- Ball SL, Powers PA, Shin HS. et al. Role of the beta(2) subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci. 2002;43(5):1595–1603. [PubMed: 11980879]
- 283.
- Namkung Y, Smith SM, Lee SB. et al. Targeted disruption of the Ca2+ channel beta3 subunit reduces N- and L-type Ca2+ channel activity and alters the voltage-dependent activation of P/ Q-type Ca2+ channels in neurons. Proc Natl Acad Sci USA. 1998;20:12010–12015. [PMC free article: PMC21756] [PubMed: 9751781]
- 284.
- Cullinane AB, Coca-Prados M, Harvey BJ. Chloride dependent intracellular pH effects of external ATP in cultured human non-pigmented ciliary body epithelium. Curr Eye Res. 2001;23(6):443–447. [PubMed: 12045894]
- 285.
- Mathews EA, Garcia E, Santi CM. et al. Critical residues of the Caenorhabditis elegans unc-2 voltage-gated calcium channel that affect behavioral and physiological properties. J Neurosci. 2003;23(16):6537–6545. [PMC free article: PMC6740628] [PubMed: 12878695]
- 286.
- Lee RY, Lobel L, Hengartner M. et al. Mutations in the alpha1 subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J. 1997;16(20):6066–6076. [PMC free article: PMC1326290] [PubMed: 9321386]
- 287.
- Schafer WR, Sanchez BM, Kenyon CJ. Genes affecting sensitivity to serotonin in Caenorhabditis elegans. Genetics. 1996;143(3):1219–1230. [PMC free article: PMC1207392] [PubMed: 8807295]
- 288.
- Heron Se, Phillips HA, Mulley JC. et al. Genetic variation of CACNA1H in idiopathic generalized epilepsy. Annals Neurol. 2004;55(4):595–596. [PubMed: 15048902]
- 289.
- McGee AW, Nunziato DA, Maltez JM. et al. Calcium channel function regulated by the SH3-GK module in beta subunits. Neuron. 2004;42(1):89–99. [PubMed: 15066267]
- 290.
- Tomita S, Chen L, Kawasaki Y. et al. Functional studies and distribution define a family of transmembrane APMA receptor regulatory proteins. J Cell Biol. 2003;161(4):805–816. [PMC free article: PMC2199354] [PubMed: 12771129]
- 291.
- Tomita S, Fukata M, Nicoll et al. Dynamic interaction of stargazing-like TARPs with cycling APMA receptors at synapses. Science. 2004;303:1508–1511. [PubMed: 15001777]
- Molecular Properties of Voltage-Gated Calcium Channels - Madame Curie Bioscience...Molecular Properties of Voltage-Gated Calcium Channels - Madame Curie Bioscience Database
- Matrix Metalloproteinases, Tissue Inhibitors of Metalloproteinase and Matrix Tur...Matrix Metalloproteinases, Tissue Inhibitors of Metalloproteinase and Matrix Turnover and the Fate of Hepatic Stellate Cells - Madame Curie Bioscience Database
- MSK1 and Nuclear Receptors Signaling - Madame Curie Bioscience DatabaseMSK1 and Nuclear Receptors Signaling - Madame Curie Bioscience Database
- Sonic Hedgehog Signaling in the Developing and Regenerating Fins of Zebrafish - ...Sonic Hedgehog Signaling in the Developing and Regenerating Fins of Zebrafish - Madame Curie Bioscience Database
- Muscle Morphogenesis: The Process of Embryonic Myoblast Fusion - Madame Curie Bi...Muscle Morphogenesis: The Process of Embryonic Myoblast Fusion - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...