NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Radiation is a major form of cancer therapy that has been successfully utilized for many decades. Over the years, substantial improvements have been made in radiotherapy regimens, resulting in increased survival time and improved quality of life. However, normal tissues in the vicinity of the tumor invariably are also exposed during radiation therapy; as a result serious side effects sometimes supervene. Ionizing radiations, such as X-rays, γ-rays, and protons, damage cells by transferring sufficient energy to atoms and biomolecules to modify their characteristics. The generation of reactive oxygen radicals, which occurs primarily through the decomposition of cellular water, is an important consequence of this energy transfer in that secondary ionization is produced, resulting in further damaging effects to the cells' component molecules. Free oxygen radicals are also formed by phagocytic cells as an indirect consequence of the irradiation (i.e., neutrophils and cells of the monocyte-macrophage lineage); these become activated in response to radiation-injured tissues. Among the most critical antioxidants that ameliorate the effects of oxidative stress within cells are enzymes such as the superoxide dismutases (SODs). Because of their importance, the SODs have received much attention in efforts to minimize oxygen radical-induced damage to normal tissues. Since the administration of exogenous SODs themselves has often proven to be problematic, a variety of innovative approaches are currently being explored in conjunction with radiotherapy. Among these are SOD mimetics.
Introduction
The American Cancer Society estimates that there will be approximately 1,284,900 newly diagnosed cases of cancer and 555,500 cancer-related deaths in the U.S.A. in 2002.1 Although great advances in a variety of anti-cancer treatments have been made over the years, much room remains for improvement. Radiation is a well-established modality for the treatment of many cancers; approximately one-half of all patients receive radiotherapy at sometime during management of their disease.2 However, the efficacy of radiation treatment is limited by the dose that can be delivered to the tumor without significantly damaging normal tissues within the irradiated field. Radiation-associated toxicities can be seriously debilitating and sometimes are life-threatening. The nature of the toxicities varies depending upon the particle species, energy, intensity, fractionation schema and total dose. In addition, the location of the tumor, the total volume irradiated, and the patient's general health and inherent characteristics can significantly influence the radiation effects a patient experiences. Some side effects can include leukopenia, anemia, nausea, diarrhea, skin reaction, alopecia, mucositis, cognitive and other neurological deficits, pneumonitis, fibrosis and development of secondary neoplasms.3-5 Thus, there is a need for innovative strategies that can reduce the volume and severity of normal tissue damage without minimizing the beneficial anti-tumor effects of radiotherapy.
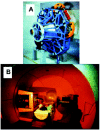
Most radiotherapy regimens involve the use of X-rays or γ-rays that consist of photons and/ or electrons. Radiotherapy with heavy charged particles, such as protons, provides a superior method of tumor targeting compared to photons by reducing volume integral dose to normal tissues. The dose distribution generated by a proton beam allows the radiation oncologist to confine the high-dose region to the targeted three-dimensional configuration and thereby reduce the dose delivered to normal tissues by a factor of 2 to 5 compared to conventional radiotherapy, thereby allowing a higher total dose to be delivered to the tumor target.6 Thus, proton beam therapy has the potential to realize higher cure rates with fewer side effects than can be expected with conventional photon beams.7,8 Loma Linda University Medical Center established the world's first hospital-based proton treatment center in 1990.8,9 More than 8,200 patients have now received proton therapy at the facility. A model of a gantry and a patient on a proton treatment couch at Loma Linda University Medical Center are shown in Figure 1. Based on a number of recent clinical studies which indicated promising results,10-12 utilization of proton treatment is increasing throughout the world. Additional strategies to improve the therapeutic ratio over conventional radiation therapy include the use of high-linear-energy transfer (LET) particles; hypofractionation; agents that preferentially increase the radiosensitivity of tumor cells such as misonidazole and halogenated pyrimidines (e.g., bromodeoxyuridine); tumor targeting with monoclonal antibodies conjugated to high energy radionuclides; biological response modifiers; and gene therapy.13-21
Mechanisms of Radiation-induced Free Radical Formation and DNA Damage
X-rays and γ-rays make up the high-energy portion of the electromagnetic radiation spectrum which also includes microwaves, ultraviolet (UV) radiation, visible light and radio waves. X-rays are generated when electrons are rapidly deaccelerated, whereas γ-rays are emitted from the nucleus of an atom that is passing from a high energy state to lower energy. X-rays and γ-rays consist of small bundles (quanta) of energy called photons that travel at the speed of light (2.988 x 108 m/s). Photons in the high energy region of the electromagnetic spectrum are known as ‘ionizing radiation’ because they have enough energy to eject an electron from its orbit, resulting in a positively charged ion. If an electron is not completely displaced, the atom or molecule nonetheless may reside in an ‘excited’ state resulting from the excess energy transferred from the photon. Photons transfer their energy by three processes: pair production, the photoelectric effect and the Compton effect. Within the energy range most commonly used in radiation therapy (100 KeV to 20 MeV), the Compton effect plays the most important role. UV radiation, such as is present in sunlight, deposits energy in certain molecules that absorb energy in the range of 3-10 eV.22 This amount of energy is not sufficient to induce ionizations, but can result in excitation of the molecule and subsequent formation of pyrimidine dimers and other linkages in DNA.23 In contrast to photons and γ-rays, which consist only of energy, other forms of ionizing radiations used for clinical radiation therapy have mass with or without a positive or negative charge. These types of radiation include α-rays, β-rays, electrons, neutrons, pions and heavy ions, in addition to the protons mentioned earlier (see Table 1). Most of the electromagnetic energy transferred to cells exposed to photons or particle forms of radiations results in primary ionization of the cell's constituents. The initial events are summarized in Figure 2. About one to two percent of the energy is deposited within the nuclei of the atoms which in turn can recoil, causing additional ionization, or can fragment, leading to various nuclear particles that add to further ionization events and thus eventually lead to biological sequelae.
Table 1
Examples of ionizing forms of radiation.
Figure 2
Model of atom with nucleus and orbiting electrons (e-) exposed to ionizing radiation.
Although the deposition of energy following irradiation can affect directly or indirectly many molecules within a cell (e.g., nucleic acids, proteins, carbohydrates and lipids), interaction with water is most likely since it is the major component of living cells (i.e., approximately 80% of an average cell consists of water).24 When water is exposed to ionizing radiation, it becomes positively charged (ionized) if an electron (e-) is ejected from its orbit around a nucleus. The e- subsequently interacts with another water molecule, which then becomes negatively charged (ionized). Additional products that are generated as a result of these interactions include H2O2, HO2, OH and H. Some of these products are highly unstable, persisting for only a small fraction of a second (10-16 s), and dissociate into free radicals such as H+, OH, H and OH-. These free radicals, while diffusing through the medium, can, in turn, interact with each other, with intact or already damaged molecules or with oxygen. This sequence of events is illustrated in Figure 3. More detailed description of these processes can be found elsewhere.24
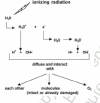
Figure 3
Schematic description of ionization events when water is exposed to ionizing radiation.
It has long been assumed that radiation-induced changes in DNA are by far the most critical in determining whether cell killing will occur. This assumption is supported by numerous studies demonstrating that irradiation of the nucleus is much more likely to result in cell death than irradiation of the cytoplasm.25,26 Double-strand breaks in the sugar-phosphate backbone of DNA are especially important, since they are frequently not repaired or are repaired inaccurately. The interaction of oxygen radicals with DNA can also lead to single-strand breaks, changes in structure or loss of bases and DNA-DNA or DNA-protein cross-linkages.24 Oxygen species can also interact with certain cell proteins, resulting in activation of a ceramide-sphingomyelin pathway and apoptosis in cell types such as lymphocytes and endothelial cells.24,25 Shut-down of DNA, RNA, and protein synthesis, as well as other metabolic processes, may also occur, but generally require relatively high doses of radiation.
Response of Inflammatory Cells to Radiation-induced Damage
The radiation-induced damage to cellular DNA and other biomolecules may be amplified by recruitment of inflammatory cells to the site of injury, as shown in Figure 4. Tissue injury, regardless of the underlying cause, is almost always accompanied by release of inflammatory mediators that produce four classical signs: erythema, edema, heat and pain. Neutrophils and cells of the monocyte-macrophage lineage are bone marrow-derived cells that are largely responsible for inflammation and make up a significant portion of innate immune defense mechanisms.28-30 These cells phagocytize and degrade debris from damaged tissues, bacteria and other particulate material. Lymphocytes are also eventually called to the scene of damage and function in adaptive immunity. T helper lymphocytes secrete a variety of cytokines that regulate immune responses; T cytotoxic cells attack foreign or aberrant cells directly; B cells secrete antibodies.
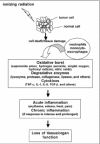
Figure 4
Sequence of radiation-induced changes in the tumor tissue microenvironment.
Neutrophils (also known as polymorphonuclear cells due to their multilobed nucleus) circulate in the blood, where they normally constitute approximately 40% to 60% of the total leukocyte population.29 They are short-lived cells that die an apoptotic or “programmed” death less than 12 hours after entering the blood-stream. When tissue injury occurs, neutrophils are rapidly recruited to the site by a variety of signals that emanate from the injured area. They respond to concentration gradients of certain substances (i.e., chemoattractants) by migrating towards their highest concentration. The beginning of neutrophil infiltration is seen as early as 30 minutes after injury and very large numbers are readily detectable within 8-12 hours. Once phagocytosis has been accomplished, the ingested material is destroyed by degradative enzymes (e.g., myeloperoxidase, lysozyme, proteinase, collagenase and elastase) and other factors such as defensins that are stored in their granules. More importantly, however, the ingested material is also exposed to potent oxidizing agents generated by multi-protein complexes that make up the NADPH-oxidase enzyme system. The complex is rapidly assembled when neutrophils become activated. The action of NADPH oxidase results in highly reactive superoxide anion (O2-) and hydrogen peroxide (H2O2) intermediates. The H2O2, in the presence of myeloperoxidase, combines with chloride ions to form hypochlorous acid (HOCl) that oxidizes nucleic acids, proteins and other molecules. Less powerful, but longer-lived, oxidizing agents such as chloramines are also generated. Nitric oxide (NO) is produced via a different series of events that involve the expression of inducible NO synthetase. Collectively, these oxidative pathways result in a tremendous increase in overall oxygen consumption by neutrophils and generation of what is sometimes referred to as an ‘oxidative burst.‘ Neutrophils are end-stage cells (i.e., they do not replicate) and many die at the site of inflammation. If the inflammatory response is intense or prolonged, nearby tissue can be seriously damaged by the release of degradative enzymes and reactive oxygen radicals.
Cells of the monocyte-macrophage lineage respond to tissue injury in much the same way as neutrophils (fig. 4). Although they also have granules or lysosomes, they are much less abundant than in neutrophils. After leaving the bone marrow, monocytes circulate in the blood for approximately one day, where they make up about 1% to 5% of the leukocytes.29 From the blood, they migrate into various tissues and undergo further maturation into macrophages. Some of these cells reside for extended periods of time in a particular tissue, whereas others migrate continuously. Some tissues and organs have macrophages as permanent or ‘fixed’ residents; examples include microglial cells in the central nervous system, Kuppfer cells in the liver and osteoclasts in bone. Macrophages continuously sample their environment by pinocytosis and have numerous receptors for inflammatory mediators. Activation is exemplified by an increased metabolic rate, motility and phagocytic activity. Superoxide anion, hydrogen peroxide, hydroxyl radicals, singlet oxygen, and nitric oxide are generated in much the same way as in neutrophils. In addition, activated macrophages secrete over 100 different products. Some of the most important ones that exacerbate inflammation and facilitate fibrosis include tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6 and transforming growth factor-β (TGF-β). Macrophage arrival at the scene of tissue damage is much slower than that of neutrophils, generally taking approximately 7-10 days before significant numbers are detected. In contrast to neutrophils, which play a major role in acute inflammatory reactions, macrophages are especially important in chronic inflammation. These cells can amplify the initial inflammatory response by facilitating cell recruitment and by increasing the abundance of reactive oxygen species.31
Pathological Consequences of Radiation-induced Damage
In patients undergoing radiotherapy, treatment-related side effects or complications have been classified into acute (occur during or within 3-6 months after treatment), subacute (occur between 3-6 months and 1 year) and late or delayed (occur after 1 year).32 Early effects typically are caused by death of rapidly proliferating cell populations and include manifestations such as inflammation. In contrast, late effects are largely characterized by gradual obliteration of small blood vessels within the irradiated field, resulting in tissue anoxia, contracture and fibrosis or ulceration. For reasons that are unclear, late effects sometimes occur many years after the irradiation event. The time interval between early and late effects has been thought to be a silent or latent period until recently. Based on data from studies of post-irradiation pulmonary fibrosis, Rubin and colleagues have postulated that there is no latent period.33 Instead, they propose that radiation exposure triggers a perpetual cascade of cytokines, leading to intercellular communication among various cell types that then determines the final outcome. In addition, Barcellos-Hoff has shown that latent TGF-ß1 can be activated by oxidative stress with consequences that include extracellular matrix remodeling and altered regulation of target cells and their redox states.34,35 These findings strongly suggest that oxidative stress from radiation exposure can set into motion a cascade of downstream events that could lead to late effects in tissues or potentiate the transformation of oxidatively damaged cells into malignant cells. Although TGF-β has been strongly implicated in late effects, other profibrogenic factors such as IL-1, TNF-α, platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) may very well also be involved. It appears possible that early intervention with an effective antioxidant may arrest the cascade of events that lead to acute, subacute and late radiation effects.
Responses of individual cells to radiation exposure are highly variable and somewhat dependent upon whether they are immature, differentiating or functional. However, the efficacy of DNA repair mechanisms within a particular cell type is undoubtedly also important. In general, mature cells that have a regulated function are more resistant than those cells that have a higher mitotic index. The greater radioresistance of functioning cells may be due at least partly to the cytoplasmic residence of the functional mechanisms. The effects of radiation on functioning tissues and organs are more complex, since they are likely to consist of a variety of cell types and often depend upon other structures, such as the vasculature, for life-support.
The bone marrow, gastrointestinal tract and lung are among the most radiosensitive parts of the body. The bone marrow is especially sensitive and some degree of injury occurs at virtually any radiation dose.36 For the sake of brevity, this discussion is limited to radiation effects on the immune system, which includes cells of the bone marrow, blood, lymph nodes and thymus. The immune system plays a pivotal role in regulating homeostasis in response to external insults, including radiation, that result in tissue injury. It is also highly radiosensitive. Significant depression in lymphocyte counts in blood has been noted after whole-body exposure to 0.25 Gy.37 Alterations in lymphocyte morphology and mobility have been reported at doses as low as 0.02 to 0.05 Gy.38 These and other similar findings suggest that immunological changes soon after radiation exposure may serve as quantitative biomarkers for radiation exposure. It is also important to note that ionizing radiation can induce the expression of many genes, including some that encode cytokines. Leukocytes are major producers of and responders to these molecules. As such, these cells communicate indirectly, as well as directly, with numerous other cell types in response to irradiation. Some cytokines recruit immunological defenses against infectious agents such as bacteria and viruses, stimulate apoptosis and modulate the promotion and progression of carcinogenesis.39-42 TGF-β1, TNF-α, IL-2 and interferon-γ (IFN-γ) are among the most important cytokines with respect to immune system modulation, radiation inducibility and control of cancer progression.34,43-46
Radiation-induced Carcinogenesis
In vitro studies of carcinogenesis following radiation exposure have demonstrated that at least two events must occur before cell transformation takes place.23 First, a radiation-induced event must affect a large fraction of exposed cells, and second, a rare event must take place in only a few of the descendants of the irradiated cells. The next event is apparently a mutation.47,48 It appears that the target in this case is likely to be larger than a single gene and smaller than a chromosome.23 This possibility is supported by the fact that radiation-induced damage to DNA is typically manifested as deletions, chromosomal translocations and inversions. The initial step, which may be epigenetic in nature or may also involve a number of different genes, results in genetic instability that can be transmitted over many cell generations.49-52 Hematopoietic cells, many of which function to regenerate mature cells of the immune system, have been extensively studied for the induction of genomic instability by ionizing radiation. The expression of genomic instability by these cells depends heavily on their phenotype.53-55 Ultimately, it appears that malignant transformation is likely to involve the activation of a proto-oncogene such as c-myc and/or inactivation of a tumor suppressor gene such as TP53.56
Recent studies suggest that increases in free radical damage may be derived from cells dying by apoptotic or necrotic mechanisms and that oxidative stress may contribute indirectly to chromosomal instability.57 Furthermore, it has been demonstrated in both the mouse and human that genetic predisposition determines the incidence of genetic instability.54,58,59 For example, persistent oxidative stress is implicated in the instability of hematopoietic stem cells in CBA mice. The instability is often manifested as translocations and deletions involving chromosome 2 in CBA mice with radiation-induced acute myelogenous leukemia (AML).60 It has been suggested that the presence of these chromosomal abnormalities may provide a biomarker that aids in identification of individuals who may be at high risk for the development of cancer during the time interval between radiation exposure and clinical manifestation of disease. Recently, an association has been established between radiation and genomic instability in human leukemogenesis. Gundestrup and Storm found a 5-fold enhancement of AML incidence in a comprehensive study of Danish airline pilots receiving a radiation dose of 9 mSv/yr, including contributions from neutrons and cosmic rays.61 Finette and colleagues examined T cells from patients undergoing chemotherapy for acute lymphocytic leukemia.62 By following lineages of cells marked by unique T cell receptor CDR3 rearrangements, this study showed that multiple late-arising nonclonal hprt mutations arose in descendents of single T cells.
Radiation-associated carcinogenesis may also relate partly to immune depression that occurs as a result of exposure. Although the concept of immune surveillance remains controversial, there is overwhelming evidence that T lymphocytes, natural killer (NK cells), monocyte-macrophages, and neutrophils can kill neoplastic cells through cytolysis and/or apoptosis.63-65 If these cells are made incompetent, tumor incidence and progression are enhanced. Furthermore, irradiation of animals at doses ranging from 1-3.5 Gy has been used to render them susceptible to otherwise nontumorigenic immortalized cells.66 Glucocorticoid-mediated immune system suppression in CBA/Ca and other mouse strains leads to a potentiation of spontaneous and radiation-induced leukemogenesis.67 These latter findings suggest that transformed, but benign, cells are more likely to progress to a malignant state in an immunocompromised host. There are numerous examples of human immune deficiencies that are associated with an increased prevalence of cancer.68-72 It has been well documented in humans that leukemias and lymphomas are among the most common cancers associated with immunodepression, as well as exposure to radiation.73 Comparisons of animal models prone to develop AML in response to irradiation with those that are not prone to the disease is pertinent, since AML is among the most frequent neoplasms observed in humans following radiation exposure.23,73
SOD and Radiation Effects
The extent of damage due to oxygen radicals is largely determined by the activity of free radical scavengers. Antioxidants, in general, can be classified into three major categories: a) enzymes such as SOD, catalase and glutathione peroxidase; b) large molecules such as ferritin and albumin; and c) small molecules such as glutathione, ascorbic acid, β-carotene, methionine and bilirubin. When the capacity of these naturally occurring substances to maintain a redox balance is overwhelmed by an abundance of reactive oxygen species, extensive tissue damage may occur. The SODs are oxidoreductases that contain Cu, Fe or Mn at the active site and catalyze the dismutation of O2- to O2 and H2O2. They are the major intracellular enzymes that protect against oxygen toxicity. The precise mechanisms responsible for the radioprotective effects of SOD are not known, but SOD apparently can inhibit superoxide-induced DNA strand breaks.74 Indeed, increased radiosensitivity occurs when a deficiency is present in antioxidant enzymes.50,75 Interestingly, Joksic and colleagues found that proton irradiation of human lymphocytes resulted in marked reduction of MnSOD activity, whereas an increase was observed after exposure to X-rays.76 In addition, protons were more effective in inducing certain DNA aberrations, compared to X-rays. These investigators concluded that the reduced activity of MnSOD may be an important contributor to the increased biological effectiveness of protons.
Since reactive oxygen species produced during irradiation are very short-lived, it has been assumed that SOD needs to be present at the time of their generation in order to have an effect. Previous studies showing maximum mouse survival and protection of bone marrow cells when SOD was administered 30-60 minutes prior to irradiation, support this premise.77,78 However, other studies have demonstrated that SOD treatments of rodents before and after irradiation result in increased survival and cellularity of the bone marrow and reduced skin reactions (erythema, hair loss, scarring), pneumonitis and pulmonary fibrosis.27,28,79,80 Decreased incidence of leukemia in SOD-treated animals has also been reported and has been associated with relatively low doses given intravenously before and after irradiation. In the lung, pneumonitis occurs early (days to weeks), while fibrosis is a late effect. In experiments reported by Malaker and Das, 30 Gy 60Co γ-rays were delivered to the right hemithorax of rats.79 Cu/ZnSOD was administered intraperitoneally 1 hour before, 1 hour after and every 3 days post-irradiation for 4 weeks. Pneumonitis at 4 weeks post-irradiation was reduced by SOD treatment and lymph node inflammation was completely suppressed.79 When rats were irradiated with 10 Gy to the lower half of the lung, micronuclei were formed in fibroblasts of both the lower and upper lung. Administration of SOD before or after irradiation significantly reduced micronuclei in both the in-field and out-of-field cells, showing that trans-acting substances resulting in clastogenesis can be generated by radiation exposure and that their formation or actions can be blocked by SOD treatment.81 An MnSOD plasmid/liposome complex administered to mice 24 hours prior to lung irradiation (37 Gy) improved esophagitis and survival, while mRNA levels for several cytokines were substantially reduced, suggesting that SOD activity diminishes cytokine release following radiation treatment.82
To date, clinical trials have primarily used Cu/ZnSOD from bovine liver, other mammalian cells or human erythrocytes (e.g., Orgotein). SOD has been used successfully to ameliorate the side effects of radiotherapy. For example, in a double-blind placebo controlled study of patients with bladder or prostate cancer, Orgotein was administered 15-30 minutes after each radiation treatment, resulting in a significant reduction in proctitis, urinary pain and voiding difficulties.83 In similar studies with cervical carcinomas treated with doses of 60 Gy, SOD relieved cystitis, edema, fibrosis, ulceration, bleeding and functional bladder symptoms when applied post-irradiation by catheter to local areas of exposure. As mentioned earlier, inflammatory processes associated with radiation treatment are initiated by tissue damage that attracts phagocytes, which respond with an oxidative burst and thus increase inflammation. SOD treatment is apparently able to arrest the process before it is amplified.84,85 The usefulness of SOD as an anti-inflammatory agent is thought to be due to decreased release of prostaglandin, thromboxane and leukotriene from lipid peroxidation products.86 Administration of bovine CuSOD is usually by means of intramuscular injection as free, polyethylene glycol-derivatized or liposome-encapsulated enzyme, after which the enzyme distributes to a maximum plasma concentration at 48 hours. Intravenous administration results in 99% clearance in 90 minutes. The concentration versus efficacy for administered SOD follows a bell-shaped curve, indicating that optimization of concentration is important for any application.86
Potential drawbacks in SOD enzyme therapy include hypersensitivity reactions, although these occur infrequently.86-88 A second limitation is the short half-life of SOD after intravenous administration (6 minute half-life and ˜99% clearance in <1 hour);89 with subcutaneous or intramuscular injection, SOD may persist for >8 hours.90 Furthermore, the effectiveness of SOD depends upon cellular uptake.91 However, since it is a large protein, it cannot traverse cell membranes easily. In the mouse, lymphocytes exhibit the greatest uptake; erythrocytes exhibit the lowest.92 An increased uptake of exogenous SOD may be possible when the plasma membrane lipid packing order is disrupted; increased uptake may also occur through other manipulations.93 However, the natural distribution of SOD in cells and tissues leaves cell membranes and the external medium relatively unprotected. At clinically effective doses the total increase of SOD to the patient is only 0.2% above baseline levels. Therefore, it appears that activity at specific sites or cellular compartments must be responsible for its protective effects. Experiments with human fibroblasts show that 100 to 1000 molecules of SOD bind to semi-specific sites on the external surface of cells and are responsible for protection against UV-radiation damage.86 The enzyme is not taken up by most cells, but is internalized by renal tubules and phagocytes. Therefore, exogenously administered enzyme activity is largely restricted to the extracellular space.31
SOD Mimetics
Because of the limitations associated with SOD enzyme therapy, SOD mimics such as M40403 (MetaPhore Pharmaceuticals, Inc., St. Louis, MO) have been developed.94,95 M40403 is a promising new drug that acts to catalytically eliminate superoxide that is implicated in the development of post-irradiation pathologies. M40403 is a macrocyclic coordination compound of Mn(II) with a high rate constant (>2x107 M-1s-1) for the catalytic dismutation of superoxide anion, O2Σ-. The reaction is specific for O2Σ-; other reactive oxygen or reactive nitrogen compounds are unaffected. The compound and its inactive congener, M40404, are stable to hydrolysis at physiological pH and are cleared intact by the kidneys. They are soluble in aqueous solutions and distribute readily to all tissues including those of the CNS; some partitioning into erythrocytes has been observed.95,96 M40403 has a low molecular weight (˜484 MW), is thermodynamically stable, and remains stable for up to 10 hours in whole rat blood at 37°C.95 After intravenous injection it distributes widely into heart, lungs, brain, liver and kidneys, while retaining its chemical structure, and is excreted intact in the urine and feces. The drug has been shown to be effective in suppressing carageenan-induced paw inflammation/edema and minimizing the systemic effects of reperfusion injury after splanchnic artery occlusion in rats.95 More recently, M40403 has been used to reverse hypotension, probably through maintenance of relatively high levels of epinephrine and norepinephrine, in a rat model of septic shock.97
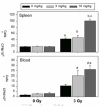
Two pilot studies with M40403 have been performed in our laboratories using C57BL/6 mice irradiated under previously described conditions.98-101 In both studies, the animals, 7-8 weeks old at arrival, were acclimatized for 1 week, weighed and randomized into groups. M40403 was prepared immediately before each of several subcutaneous injections per mouse. Whole-body γ-irradiation was delivered in a single fraction of 3 Gy at a dose rate of 1.5 Gy/min. Euthanasia was performed 4 days later, a time of severe immunodepression, and several assays were performed. Flow cytometry analyses revealed consistently higher numbers of T, Th, Tc, B and NK cells (29%, 12%, 9%, 5%, and 1% higher, respectively) in the bone marrow of irradiated mice treated with M40403, compared to irradiated animals that did not receive the drug. Spontaneous blastogenesis assays were performed on both spleen and blood cells from mice injected with M40403 (3 mg/kg or 10 mg/kg at 15 min, 24 hr, 48 hr, and 72 hr post-irradiation). This assay is used to measure the basal proliferative level of leukocytes and is a sensitive indicator of immune system activation/reconstitution in vivo. The leukocytes from irradiated groups treated with M40403 exhibited the highest level of 3H-thymidine incorporation into DNA when the results were normalized to106 cells (fig. 5), in spite of significant cell depletion in all irradiated groups. The high level of DNA synthesis in the blood is especially significant, since circulating leukocytes frequently reflect hematopoietic processes in the bone marrow. In addition, although the white blood cell counts were significantly reduced by irradiation at 4 days post-exposure, the numbers for the irradiated group receiving 10 mg/kg of M40403 were 74% higher than for the irradiated group not given the drug (Table 2). Collectively, these findings indicate that M40403 may have significant radioprotective effects. It is possible that SODm-induced increases in survivors may lead to increases in genomic instability. However, it is tempting to speculate that the consistently higher numbers of lymphocyte populations in the bone marrow and the enhanced proliferative capacity of leukocytes in blood and spleens from irradiated mice treated with M40403 were due to reduced DNA/chromosomal damage, and thus there were more survivors. If this is so, then the administration of this drug during radiation exposure may facilitate hematopoietic regeneration and result in more intact immune responses. In addition, M40403 was nontoxic at the highest concentrations used (up to 20 mg/kg body weight/injection X 3 injections/mouse), alone and in combination with radiation. It should also be noted that SOD enzyme studies have consistently shown that bone marrow protection is most obvious in the acceleration of recovery, rather than the initial die-off phase, so our M40403 tests to date were consistent with enzyme-based experiments. In vivo studies were also performed in our laboratories to determine whether M40403 suppresses radiation-induced chromosome aberrations using the model eukaryote, Caenorhabditis elegans, a free-living nematode whose biology and genetics have been extensively characterized. In larval nematodes there are a discrete number of cells that follow strictly controlled developmental programs to generate the adult self-fertilizing hermaphrodite. Four cells (Z1-Z4) generate the gonads; 20 cells generate the intestine. It has been shown previously that irradiation of the larvae will lead to reduced fertility, a measure of gonial Z cell reproductive integrity. The fertility of animals is assessed by simply counting the brood sizes of individual worms. In the intestine, 14 of the 20 int cells will undergo one round of nuclear division without cytokinesis to form binucleate cells. Irradiation of the larvae leads to the formation of stable anaphase bridges between the nuclei of binucleate, a measure of chromosomal damage. Anaphase bridges are counted in late larval worms fixed with Carnoy and stained with the fluorescent DNA dye DAPI.102-104 Synchronized populations of larvae were prepared and grown at 20°C on pH 7 agar medium seeded with a strain of E. coli (QC779) bearing mutations in both SOD genes so as not to introduce external SOD activity. They were harvested in pH 7 phosphate buffer containing 0 to 20 μg/ml of M40403 and incubated for 20 minutes prior to irradiation with graded doses of 60Co γ-rays at a dose rate of ˜1.5 Gy/min. They were then incubated an additional 30 minutes in the presence of the drug and finally plated on pH 7 agar medium with E. coli. After 2 days, worms were fixed and stained with DAPI for anaphase bridge analysis or picked individually to agar plates. The number of F1 progeny from irradiated mothers was counted manually under a dissecting microscope. A clear protection against anaphase bridge formation was evident, with maximum protection afforded at a concentration at or above 10 μg/ml (fig. 6). In the experiment with fertility, no obvious protective effect against radiation was noted, but a decrease in fertility as a function of drug concentration above 15 μg/ml was measured (data not shown). Taken together these experiments showed a clear radioprotective effect against radiation-induced chromosomal damage in vivo without significant cytotoxicity below 15 μg/ml. It is of interest to note that the life-span of C. elegans has been significantly extended through the use of SOD, as well as catalase, mimetics.105
Table 2
Number of white blood cells(WBC).
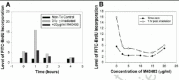
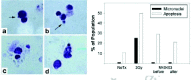
Our preliminary in vitro experiments with M40403 employed the V79-4 Chinese hamster lung fibroblast cell line, which has been extensively used in radiation studies.106 The cells were exposed to γ-rays (3 Gy, 1.33 Gy/min) and various concentrations of M40403 were assessed for DNA damage measured by fluorescein isothiocyanate (FITC)-BrdU incorporation and chromosome damage measured by the micronucleus assay, as previously described.107 V79-4 cells on glass cover slips were treated with 2.5, 5, 10, 15 or 20 μg/ml of M40403 30 minutes prior to irradiation. Samples were harvested at time zero (˜5 min) and 1, 2, and 4 hours later. The cells were fixed, incubated with FITC-conjugated BrdUTP and terminal-deoxy-transferase, counter-stained with propidium iodide and analyzed by laser scanning cytometry, with >10,000 cells scanned/sample (fig. 7). At 1 hr post-irradiation, there was a 16% increase in 3' end-breaks which returned to <5% at 2 hr, indicating that 3' ends had been repaired (fig. 7A). At this same time, with M40403 there was a suppression of radiation-induced damage (DNA cleavage) at all concentrations tested (fig. 7B). Presence of micronuclei was used as a measure of chromosomal damage induced by radiation. Micronuclei can be formed by acentric fragments, chromatid detachment from the spindle apparatus and/or nondisjunction events. The size of micronuclei reflects the type of chromosome damage, with relatively smaller micronuclei being of the chromatid type and larger structures being whole chromosomes that have been displaced.108 Cytochalasin B generates binucleated cells by inhibiting actin-mediated cytokinesis.109 Scoring micronuclei in binucleated cells (minimum of 300/slide) insured that the sample was normalized to cells that had undergone division after being irradiated (fig. 8).110 The spontaneous rate of micronuclei formation was 8%. Following 3 Gy, micronuclei were present in ˜28% of binucleated cells. The frequency in irradiated cells pretreated with 20 μg/ml M40403 was reduced to 9%. Thus, pretreatment reduced chromosome damage by >60%. The cultures pretreated with M40403 also did not have apoptotic bodies like those seen in nontreated, irradiated cultures. These data showed that pretreatment of V79-4 cells with M40403 reduced both DNA and chromosomal damage.
Several other radioprotectant/SOD mimetic compounds have been examined and many are effective in radioprotection. However, they are either less stable, have lower rate constants or lead to side effects that have not been seen with M40403. For example, the Cu and Fe coordination compounds CuDIPS, FeIIITMpyP and FeIIITPPS have high reaction constants (up to 3x107 M-1s-1), but are eventually destroyed by superoxide. The aminothiols (e.g., WR2721 and WR1065) provide the greatest radioprotection in mice of compounds tested thus far. Such compounds may scavenge hydroxyl radicals, donate hydrogen atoms to DNA or modulate DNA conformation like polyamines. Aminothiol experiments have shown that radioprotectors can prevent cell transformation and can be anti-mutagenic, suggesting that they can break the sequence of events leading from genomic damage to carcinogenesis.111,112 Using C57BL/6J and BALB/c mice exposed to 0.1 to 2 Gy of Co-60 γ-rays and neutrons, Grdina et al demonstrated that WR2721 protected against the development of numerous tumors, especially lymphoreticular tumors.113 However, aminothiols lead to nausea and vomiting in humans and behavioral toxicity in test animals, thus limiting practical use. They are also rapidly cleared with little protective activity after 2.5-3 hours.114
Stable free radicals of the nitroxide family have also been tested for radioprotection and can act as SOD mimetics. Their rate constants are significantly less than M40403 (the most active species Tempo and Tempol have values of < 1.3x106 M-1s-1 versus >2x107 M-1s-1 for M40403). The nitroxides do protect V79 cells against superoxide, hydrogen peroxide and radiation and increase the LD50(30) for C3H mice with a dose modification factor (DMF) of 1.3. Though active, the compounds are unstable and Tempol is rapidly reduced to the inactive hydroxylamine Tempol-H. They also can lead to side effects such as hypotension and, at high concentrations, may evoke seizures.115 Furthermore, since M40403 is inactivated at an acidic pH, similar to the conditions present in many tumors,116x it is possible that this drug could preferentially spare normal, but not tumor, tissues. When considering the numerous products that have been tested thus far, and given their limitations in terms of toxicity, short half-lives and inactivity, M40403 appears the most promising with the fewest mitigating factors.
Conclusion
Over the past several decades, radiation has become an increasing valuable, and often indispensable, modality for the treatment of many cancers. Recent advances in equipment, computer software and treatment plans have significantly contributed to increased survival time and quality of life for millions of patients. Better understanding of the biological effects of radiation on normal, as well as tumor, cells has also contributed to improved disease management. Attempts to further increase therapeutic efficacy and reduce the side effects of radiotherapy have stimulated investigations of the mechanisms of radiation-induced normal tissue damage. The critical role of the superoxide anion in translating initial radiation exposure into pathological consequences has now been validated in many systems. The countermeasure concept is to scavenge or block formation of the biochemical species, thus preventing or ameliorating cytopathological effects. Studies with a number of SOD mimetics such as M40403, aminothiols (WR2721 and WR1065) and members of the stable nitroxide family (Tempol) have shown promising results. The future hold great promise for the development of superior products that will serve to ameliorate the damaging effects of radiation on normal cells and tissues. Although the emphasis here has been on cancer patients and radiotherapy, there are a number of other situations involving radiation exposure in which SOD mimetics may prove to be very useful. For example, individuals in occupational settings (e.g., health care workers, airline personnel, astronauts and nuclear power plant employees) are sometimes exposed to radiation levels higher than what is deemed to be safe. Irradiation of a large number of people due to terrorist attacks is also a possibility. Thus, it is clear that an effective SOD mimetic could have a broad range of applicability.
References
- 1.
- Jemal A, Tiwari RC, Murray T. et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. [PubMed: 14974761]
- 2.
- Perez CA, Brady LW. Preface In: Principles and Practice of Radiation OncologyPhiladelphia: JB Lippincott Co.,1992 .
- 3.
- Green DM, D'Angelo GJ. Second malignant neoplasmsIn: Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, eds.Clinical Oncology2nd ed. New York: Churchill Livingstone Division of Harcourt Brace & Company20001082–1100.
- 4.
- Lichtner AS. Radiation therapyIn: Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, eds.Clinical Oncology2nd ed. New York: Churchill Livingstone Division of Harcourt Brace & Company2000423–470.
- 5.
- Strojan P, Popovic M, Jereb B. Secondary intracranial meningiomas after high-dose cranial irradiation: report of five cases and review of the literature. Int J Radiat Oncol Biol Phys. 2000;48:65–73. [PubMed: 10924973]
- 6.
- Archambeau JO, Slater JM, Coutrakon GB. et al. Proton-beam irradiation for the cancer patient: An approach to optimal therapy and normal-tissue sparing. Adv Radiat Biol. 1994;18:53–89.
- 7.
- Archambeau JO, Slater JD, Slater JM. et al. Role for proton beam irradiation in treatment of pediatric CNS malignancies. Int J Radiat Oncol Biol Phys. 1991;22:287–294. [PubMed: 1310964]
- 8.
- Slater JM, Archambeau JO, Miller DW. et al. The proton treatment center at Loma Linda University Medical Center: Rationale for and description of its development. Int J Radiat Oncol Biol Phys. 1991;22:383–389. [PubMed: 1740396]
- 9.
- Slater JM, Miller DW, Archambeau JO. Development of a hospital-based proton beam treatment center. Int J Radiat Oncol Biol Phys. 1988;14:761–775. [PubMed: 2832357]
- 10.
- McAllister B, Archambeau JO, Nguyen MC. et al. Proton therapy for pediatric cranial tumors: Preliminary report on treatment and disease-related morbidities. Int J Radiat Oncol Biol Phys. 1997;39:455–460. [PubMed: 9308950]
- 11.
- Yonemoto LT, Slater JD, Friedrichsen EJ. et al. Phase I/II study of proton beam irradiation for the treatment of subfoveal choroidal neovascularization in age-related macular degeneration: Treatment techniques and preliminary results. Int J Radiat Oncol Biol Phys. 1996;36:387–371. [PubMed: 8960515]
- 12.
- Yonemoto LT, Slater JD, Rossi CJ. et al. Combined proton and photon conformal radiation therapy for locally advanced carcinoma of the prostate: Preliminary results of a phase I/II study. Int J Radiat Oncol Biol Phys. 1997;37:21–29. [PubMed: 9054873]
- 13.
- Flynn AA, Pedley RB, Green AJ. et al. Antibody and radionuclide characteristics and the enhancement of the effectiveness of radioimmunothrapy by selective dose delivery to radiosensitive areas of tumour. Int J Radiat Biol. 2002;78:407–415. [PubMed: 12020430]
- 14.
- Gridley DS, Baer JR, Cao JD. et al. TNF- α gene and proton radiotherapy in an orthotopic brain tumor model. Int J Oncol. 2002;21:251–259. [PubMed: 12118318]
- 15.
- Gridley DS, Li J, Kajioka EH. et al. Combination of pGL1-TNF- a gene and radiation (proton and γ-ray) therapy against brain tumor. Anticancer Res. 2000;20:4195–4204. [PubMed: 11205248]
- 16.
- Kim DW, Andres ML, Li J. et al. Liposome-encapsulated TNF-α enhances the effects of radiation against human colon tumor xenografts. J Interferon Cyt Res. 2001;21:885–897. [PubMed: 11747620]
- 17.
- Poggi MM, Coleman CN, Mitchell JR. Sensitizers and protectors of radiation and chemotherapy (review). Curr Probl Cancer. 2001;25:334–411. [PubMed: 11740469]
- 18.
- Sorokin P. New agents and future directions in biotherapy. Clin J Oncol Nurs. 2002;6:19–24. [PubMed: 11842484]
- 19.
- Wong GH. Protective roles of cytokines against radiation: Induction of mitochondrial MnSOD. Biochim Biophys Acta. 1995;1271:205–209. [PubMed: 7599209]
- 20.
- Wong CS, Hill RP. Experimental radiotherapyIn: Tannock IF, Hill RP, eds.The Basic Science of Oncology3rd ed. New York: McGraw-Hill,1998322–349.
- 21.
- Worthington J, Robson T, O'Keeffe M. et al. Tumor cell radiosensitization using constitutive (CMV) and radiation inducible (WAF1) promoters to drive the iNOS gene: A novel suicide gene therapy. Gene Ther. 2002;9:263–269. [PubMed: 11896465]
- 22.
- Rauth AM. The induction and repair of ultraviolet light damage in mammalian cellsIn: Burns FJ, Upton AC, Silini G, eds.Radiation Carcinogenesis and DNA AlterationsNew York: Plenum Press,1986212–226.
- 23.
- Okey AB, Harper PA, Grant DM. et al. Chemical and radiation carcinogenesisIn: Tannock IF, Hill RP, eds.The Basic Science of Oncology3rd ed. New York: McGraw-Hill,1998166–196.
- 24.
- Bristow RG, Hill RP. Molecular and cellular basis of radiotherapyIn: Tannock IF, Hill RP, eds.The Basic Science of Oncology3rd ed. New York: McGraw-Hill,199895–321.
- 25.
- Fuks Z, Haimovitz-Friedman A, Kolesnick RN. The role of the sphingomyelin pathway and protein kinase C in radiation-induced cell kill. Imp Adv Oncol. 1995:19–31. [PubMed: 7672806]
- 26.
- Nunez M, McMillan TJ, Valenzuela M. et al. Relationshiop between DNA damage, rejoining and cell killing by radiation in mammalian cells. Radiother Oncol. 1996;39:155–1165. [PubMed: 8735483]
- 27.
- Schwartz JL, Mustafi R, Beckett MA. et al. DNA double-strand break rejoining rates, inherent radiosensitivity and human tumour response to radiotherapy. Br J Cancer. 1996;74:37–42. [PMC free article: PMC2074601] [PubMed: 8679455]
- 28.
- Das RM. Radioprotection by superoxide dismutaseIn: Bump EA, Malaker K, eds.Radioprotectors: Chemical, Biological and Clinical PerspectivesNew York: CRC Press,1997127–150.
- 29.
- Parslow TG, Bainton DF. Innate immunityIn: Parslow TG, Stites DP, Terr AI, Imboden JB, eds.Medical Immunology10th ed. New York, NY: Lange Medical Books/McGraw-Hill,200119–39.
- 30.
- Rosenberg HF, Gallin JI. InflammationIn: Paul WE, ed.Fundamental Immunology4th ed. Philadelphia: Lippincott-Raven Publishers,19991051–1066.
- 31.
- Flohè L. Superoxide dismutase for therapeutic use: Clinical experience dead ends and hopes. Mol Cell Biochem. 1988;84:123–131. [PubMed: 3068519]
- 32.
- Loeffler RK, Konski AA, Dobelbower RR. et al. Radiation therapy in cancer management: Radiation reactions and complicationsIn: Moossa AR, Schimpff SC, Robson MC, eds.Comprehensive Textbook of Oncology2nd ed. Baltimore: Williams & Wilkins,1991493–501.
- 33.
- Rubin P, Johnston CJ, Williams JP. et al. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99–109. [PubMed: 7642437]
- 34.
- Barcellos-Hoff MH. How do tissues respond to damage at the cellular level? The role of cytokines in irradiated tissues. Radiat Res. 1998;150(Suppl):S109–S120. [PubMed: 9806614]
- 35.
- Barcellos-Hoff MH, Derynck R, Tsang ML. et al. Transforming growth factor-β activation in irradiated murine mammary gland. J Clin Invest. 1994;93:892–899. [PMC free article: PMC293960] [PubMed: 8113421]
- 36.
- Mauch P, Constine L, Greenberger J. et al. Hematopoietic stem cell compartment: Acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–1339. [PubMed: 7713791]
- 37.
- Anderson RE, Warner NL. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. [PubMed: 14486]
- 38.
- Stefani S, Schrek R. Cytotoxic effect of 2 and 5 roentgens on human lymphocytes irradiated in vitro. Radiat Res. 1964;22:126–130. [PubMed: 14157352]
- 39.
- Duvall E, Wyllie AH. Death and the cell. Immunol Today. 1986;7:115–119. [PubMed: 25289803]
- 40.
- Evan G, Littlewood T. A matter of life and cell death (review). Science. 1998;281:1317–1322. [PubMed: 9721090]
- 41.
- Fujiki H, Suganuma M. Tumor necrosis factor-alpha, a new tumor promoter, engendered by biochemical studies of okadaic acid. J Biochem (Tokyo). 1994;115:1–5. [PubMed: 8188614]
- 42.
- Oppenheim JJ. Summary of the fifth conference on the molecular mechanisms and physiological activities of cytokines. J Leukoc Biol. 1994;56:687–691. [PubMed: 7996044]
- 43.
- Furmanski P, Neilson EG, Bhorjee JS. The role of cytokines in the regulation of cell function and in the pathogenesis of disease: A pathology A/Pathology B Study Section Workshop. Working report from the Division of Research Grants, National Institutes of Health. Cancer Res. 1992;52:6219–6133. [PubMed: 1394240]
- 44.
- Hallahan DE, Haimovitz-Friedman A, Kufe DW. et al. The role of cytokines in radiation oncologyIn: DeVita VT, Hellman S, Rosenberg SA, eds.Important Advances in OncologyPhiladelphia: JB Lippincott Co.,199271–80. [PubMed: 8505057]
- 45.
- Musiani P, Modesti A, Giovarelli M. et al. Cytokines, tumor-cell death and immunogenicity: A question of choice. Immunol Today. 1997;18:32–36. [PubMed: 9018972]
- 46.
- Yamauchi N, Karizana H, Watanabe N. et al. Intracellular hydroxyl radical production induced by recombinant human tumor necrosis factor. Cancer Res. 1989;49:1671–1675. [PubMed: 2538230]
- 47.
- Kamiya K, Yasukawa-Barnes J, Mitchen JM. et al. Evidence that carcinogenesis involves imbalance between epigenetic high frequency initiation and suppression of promotion. Proc Natl Acad Sci USA. 1995;92:1332–1336. [PMC free article: PMC42513] [PubMed: 7877977]
- 48.
- Little JB. Changing views of cellular radiosensitivity. Radiat Res. 1994;140:299–311. [PubMed: 7972681]
- 49.
- Caron RM, Nagasawa H, Yu Y. et al. Evidence for a role for genomic instability in radition-induced mutagenesis. Radiat Oncol Invest. 1997;5:119–123. [PubMed: 9303068]
- 50.
- Murnane JP. Role of induced genetic instability in the mutagenic effects of chemicals and radiation. Mutat Res. 1996;367:11–23. [PubMed: 8596542]
- 51.
- Morgan WF, Day JP, Kaplan MI. et al. Genomic instability induced by ionizing radiation. Radiat Res. 1996;146:247–258. [PubMed: 8752302]
- 52.
- Watson GE, Lorimore SA, Wright EG. Long-term in vivo transmission of alpha-particle-induced chromosomal instability in murine haemopoietic cells. Int J Radiat Biol. 1996;69(2):175–182. [PubMed: 8609453]
- 53.
- Kadhim MA, Macdonald DA, Goodhead DT. et al. Transmission of chromosomal instability after plutonium alpha-particle irradiation. Nature. 1992;355:738–740. [PubMed: 1741061]
- 54.
- Watson GE, Lorimore SA, Clutton SM. et al. Genetic factors influencing alpha-particle-induced chromosomal instability. Int J Radiat Biol. 1997;71(5):497–503. [PubMed: 9191894]
- 55.
- Wright EG. Radiation-induced genomic instability in haemopoietic cells: Implications for radiation pathology. Radiat Oncol Invest. 1997;5:115–118. [PubMed: 9303067]
- 56.
- Cox R. Molecular mechanisms of radiation oncogenesis. Int J Radiat Biol. 1994;65:57–64. [PubMed: 7905910]
- 57.
- Limoli CL, Hartmann A, Shephard L. et al. Apoptosis, reproductive failure, and oxidative stress in Chinese hamster ovary cells with compromised genomic integrity. Cancer Res. 1998;58:3712–3718. [PubMed: 9721883]
- 58.
- Kadhim MA, Marsden SJ, Wright EG. Radiation-induced chromosomal instability in human fibroblasts: Temporal effects and the influence of radiation quality. Int J Radiat Biol. 1998;73:143–148. [PubMed: 9489560]
- 59.
- Harper K, Lorimore SA, Wright EG. Delayed appearance of radiation-induced mutations at the Hprt locus in murine hemopoietic cells. Exp Hematol. 1997;25:263–269. [PubMed: 9091304]
- 60.
- Rithidech KN, Cronkite EP, Bond VP. Advantages of the CBA mouse in leukemogenesis research. Blood Cells Mol Dis. 1999;25:38–45. [PubMed: 10349512]
- 61.
- Gundestrup M, Storm HH. Radiation-induced acute myeloid leukaemia and other cancers in commercial jet cockpit crew: A population-based cohort study. Lancet. 1999;354:2029–2031. [PubMed: 10636367]
- 62.
- Finette BA, Homans AC, Albertini RJ. Emergence of genetic instability in children treated for leukemia. Science. 2000;288:514–517. [PubMed: 10775110]
- 63.
- Flesher E, Gonen P, Keisari Y. Oxidative burst-dependent tumoricidal and tumoristatic activities of paraffin oil-elicited mouse macrophages. J Natl Cancer Inst. 1984;72:1341–1347. [PubMed: 6587155]
- 64.
- Lichtenstein A. Spontaneous tumor lysis mediated by inflammatory neutrophils: Dependence upon divalent cations and reduced oxygen intermediates. Blood. 1986;62:657–661. [PubMed: 3004619]
- 65.
- Melder RJ, Brownell AL, Shoup TM. et al. Imaging of activated natural killer cells in mice by positron emission tomography: Preferential uptake in tumors. Cancer Res. 1993;53:5867–5871. [PubMed: 8261395]
- 66.
- Duhrsen U, Metcalf D. A model system for leukemic transformation of immortalized hemopoietic cells in irradiated recipient mice. Leukemia. 1988;2:329–333. [PubMed: 3287020]
- 67.
- Seki M, Yoshida K, Nishimura M. et al. Radiation-induced myeloid leukemia in C3H/He mice and the effect of prednisolone acetate on leukemogenesis. Radiat Res. 1991;127:146–149. [PubMed: 1946997]
- 68.
- Cotelingham JD, Witebsky FG, Hsu SM. et al. Malignant lymphoma in patients with the Wiskott-Aldrich syndrome. Cancer Invest. 1985;3:515–522. [PubMed: 3910193]
- 69.
- Garrido F, Ruiz-Cabello F, Cabrera T. et al. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997;18:89–95. [PubMed: 9057360]
- 70.
- Karp JE, Broder S. Acquired immunodeficiency and non-Hodgkin's lymphomas. Cancer Res. 1991;51:4743–4756. [PubMed: 1893369]
- 71.
- Mintz B, Silvers WK. Accelerated growth of melanoma after specific immune destruction of tumor stroma in a mouse model. Cancer Res. 1996;56:463–466. [PubMed: 8564953]
- 72.
- Waldmann TA, Misiti J, Nelson DL. et al. Ataxia-telangiectasia: A multisystem hereditary disease with immunodeficiency, impaired organ maturation, X-ray hypersensitivity, and a high incidence of neoplasia. Ann Intern Med. 1983;99:367–379. [PubMed: 6193747]
- 73.
- Ichimaru M, Ishimaru T, Belsky JL. Incidence of leukemia in atomic bomb survivors belonging to a fixed cohort in Hiroshima and Nagasaki, 1950—71. Radiation dose, years after exposure, age at exposure, and type of leukemia. J Radiat Res (Tokyo). 1978;19:262–282. [PubMed: 739446]
- 74.
- Birnboim HC. DNA strand breaks in human leukocytes induced by superoxide anion, hydrogen peroxide and tumor promoters are repaired slowly compared to breaks induced by ionizing radiation. Carcinogenesis. 1986;7:1511–1517. [PubMed: 3017600]
- 75.
- Ward JF, Milligan JR, Jones GDD. Biological consequences of nonhomogenous energy deposition by ionizing radiation. Radiat Protect Dosimet. 1994;52:271–276.
- 76.
- Joksic G, Pajovic SB, Stankovic M. et al. Chromosome aberrations, micronuclei, and activity of superoxide dismutases in human lymphocytes after irradiation in vitro. Cell Mol Life Sci. 2000;57:842–850. [PubMed: 10892348]
- 77.
- Petkau A, Chelack WS. Radioprotection by superoxide dismutase of macrophage progenitor cells from mouse bone marrow. Biochem Biophys Res Commun. 1984;119:1089–1095. [PubMed: 6712667]
- 78.
- Petkau A, Kelly K, Chelack WS. et al. Radioprotection of bone marrow stem cells by superoxide dismutase. Biochem Biophys Res Commun. 1975;67:1167–1174. [PubMed: 1201065]
- 79.
- Malaker K, Das RM. The effect of superoxide dismutase on the pathogenesis of radiation-induced pulmonary damage in the rat. Pharmacol Ther. 1988;39:327–330. [PubMed: 3200892]
- 80.
- Petkau A. Radiation protection by superoxide dismutase. Photochem Photobiol. 1978;28:765–774. [PubMed: 366641]
- 81.
- Khan MA, VanDyk J, Hill RP. Partial volume lung irradiation: Investigation of an out-of-field effect. Albuquerque: 47th Annual Meeting of the Radiation Research Society. 2000
- 82.
- Epperly MW, Gretton J, Defilippi S. et al. Prevention of irradiation-induced esophagitis by intraesophageal injection of MnSOD-plasmid/liposome complex. Albuquerque: 47th Annual Meeting of the Radiation Research Society. 2000
- 83.
- Edsmyr F. Superoxide dismutase efficacy in ameliorating side effects of radiation therapy: Double-blind, placebo-controlled trials in patients with bladder and prostate tumorsIn: Autor AM ed.Pathology of OxygenNew York: Academic,1982215.
- 84.
- Edsmyr F, Huber W, Menander KB. Orgotein efficacy in ameliorating side effects due to radiation therapy. I. Double-blind, placebo-controlled trial in patients with bladder tumors. Curr Ther Res Clin Exp. 1976;19:198–211. [PubMed: 813958]
- 85.
- Marberger H, Bartsch G, Huber W. et al. Orgotein: A new drug for the treatment of radiation cystitis. Curr Ther Res Clin Exp. 1975;18:466–475. [PubMed: 810307]
- 86.
- Jardot G, Vaille A, Maldonado J. et al. Clinical pharmacokinetics and delivery of bovine superoxide dismutase. Clin Pharmacokinet. 1995;28:17–25. [PubMed: 7712659]
- 87.
- Petkau A. Scientific basis for the clinical use of superoxide dismutase. Cancer Treat Rev. 1986;13:17–44. [PubMed: 3521852]
- 88.
- Corominas M, Bas J, Romeu A. et al. Hypersensitivity reaction after orgotein (superoxide dismutase) administration. Allergol Immunopathol (Madr). 1990;18:297–299. [PubMed: 2151501]
- 89.
- McCord JM. Superoxide dismutase: Rationale for use in reperfusion injury and inflammation. J Free Radic Biol Med. 1988;2:307–310. [PubMed: 3598062]
- 90.
- Huber W. Orgotein - (bovine Cu-Zn superoxide dismutase), an anti-inflammatory protein drug: Discovery, toxicology and pharmacology. Eur J Rheumatol Inflamm. 1981;4:173–182. [PubMed: 7343319]
- 91.
- Zepp RA, Chelack WS, Petkau A. Bovine superoxide dismutase preparations: Comparison of their biochemical and biological characteristicsIn: Bannister JV, Hill HAO, eds.Chemical and Biochemical Aspects of Superoxide and Superoxide DismutaseNew York: Elsevier/North Holland,1980201.
- 92.
- Kelly K, Barefoot C, Sehon A. et al. Bovine superoxide dismutase: A radioimmunoassay. Arch Biochem Biophys. 1978;190:531–538. [PubMed: 102252]
- 93.
- Lepock JR, Arnold LD, Petkau A. et al. Interaction of superoxide dismutase with phospholipid liposomes. An uptake, spin label and calorimetric study. Biochim Biophys Acta. 1981;649:45–57. [PubMed: 6272859]
- 94.
- Salvemini D, Wang Z-Q, Stern MK. et al. Peroxynitrite decomposition catalysts: Therapeutics for peroxynitrite-mediated pathology. Proc Natl Acad Sci USA. 1998;95:2659–2663. [PMC free article: PMC19452] [PubMed: 9482943]
- 95.
- Salvemini D, Wang AQ, Zweier JL. et al. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. [PubMed: 10514375]
- 96.
- Riley DP, Lennon PJ, Neumann WL. et al. Toward the rational design of superoxide dismutase mimics: Mechanistic studies for the elucidation of substituent effects on the catalytic activity of macrocyclic manganese(II) complexes. J Am Chem Soc. 1997;119:6522–6528.
- 97.
- Macarthur H, Westfall TC, Riley DP. et al. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc Natl Acad Sci USA. 2000;97:9753–9758. [PMC free article: PMC16937] [PubMed: 10944234]
- 98.
- Gridley DS, Pecaut MJ, Miller GM. et al. Dose and dose rate effects of whole-body ?-irradiation: II. Hematological variables and cytokines. In Vivo. 2001;15:209–216. [PubMed: 11491015]
- 99.
- Kajioka EH, Andres ML, Li J. et al. Acute effects of whole-body proton irradiation on the immune system of the mouse. Radiat Res. 2000;153:587–594. [PubMed: 10790280]
- 100.
- Miller GM, Kajioka EH, Andres ML. et al. Dose and timing of total-body irradiation mediate tumor progression and immunomodulation. Oncol Res. 2002;13:9–18. [PubMed: 12201676]
- 101.
- Pecaut MJ, Nelson GA, Gridley DS. Dose and dose rate effects of whole-body γ-irradiation. I. Lymphocytes and lymphoid organs. In Vivo. 2001;15:195–208. [PubMed: 11491014]
- 102.
- Nelson G, Schubert W, Marshall T. Radiobiological studies with the nematode Caenorhabditis elegans. Genetic and developmental effects of high LET radiation. Nucl Tracks Radiat Meas. 1992;20:227–232. [PubMed: 11537531]
- 103.
- Nelson G, Schubert W, Marshall T. et al. Radiation effects in Caenorhabditis elegans: Mutagenesis by high and low LET ionizing radiation. Mutat Res. 1989;212:181–192. [PubMed: 2733713]
- 104.
- Nelson G, Schubert W, Kazarians G. Heavy ion radiobiology of the nematode Caenorhabditis elegansIn: Dewey W, Edinton M, Fry R, Hall E, Whitmore G, eds.Radiation Research. A Twentieth Century PerspectiveSan Diego: Academic Press Inc.,1992493–497.
- 105.
- Melov S, Ravenscroft J, Malik S. et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. [PubMed: 10968795]
- 106.
- Sankaranarayanan K. Ionizing radiation and genetic risks. III. Nature of spontaneous and radiation-induced mutations in mammalian in vitro systems and mechanisms of induction of mutations by radiation. Mutat Res. 1991;258:75–97. [PubMed: 2023601]
- 107.
- Green LM, Murray DK, Tran DT. et al. Response of thyroid follicular cells to gamma versus proton irradiation: I. Initial characterization of DNA damage, micronuclei formation, apoptosis, survival and cell cycle phase redistribution. Radiat Res. 2001;155:32–42. [PubMed: 11121213]
- 108.
- Bocker W, Muller WU, Streffer C. Image processing algorithms for the automated micronucleus assay in binucleated human lymphocytes. Cytometry. 1995;19:283–294. [PubMed: 7796693]
- 109.
- Fenech M, Morley A. Solutions to the kinetic problem in the micronucleus assay. Cytobios. 1985;43:233–246. [PubMed: 4075848]
- 110.
- Manti L, Jamali M, Prise KM. et al. Genomic instability in Chinese hamster cells after exposure to X rays or alpha particles of different mean linear energy transfer. Radiat Res. 1997;147:22–28. [PubMed: 8989365]
- 111.
- Balcer-Kubiczek EK, Harrison GH, Hill CK. et al. Effects of WR-1065 and WR-151326 on survival and neoplastic transformation in C3H/10T1/2 cells exposed to TRIGA or JANUS fission neutrons. Int J Radiat Biol. 1993;63:37–42. [PubMed: 8093466]
- 112.
- Hill CK, Nagy B, Peraino C. etal.2-[(Aminopropyl)amino]ethanethiol (WR-1065) is antineoplastic and anti-mutagenic when given during 60Coγ-irradiation. Carcinogenesis. 1986;7:665–671. [PubMed: 3698198]
- 113.
- Grdina DJ, Carnes BA, Grahn D. et al. Protection against late effects of radiation by S-2-(3-aminopropylamino)ethylphosphorothioic acid. Cancer Res. 1991;51:4125–4130. [PubMed: 1651155]
- 114.
- Kumar KS, Vaishnav YN, Weiss JF. Radioprotection by antioxidant enzymes and enzyme mimetics. Pharmac Ther. 1988;39:301–309. [PubMed: 3059373]
- 115.
- Hahn SM, Krishna CM, Mitchell JB. Stable free radicals as radiation protectorsIn: Bump EA, Malaker K, eds.Radioprotectors: Chemical, Biological, and Clinical PerspectivesNew York: CRC Press,1998111–126.
- 116.
- Martin GR, Jain RK. Noninvasive measurement of interstitial pH profiles in normal and neoplastic tissue using fluorescence ratio imaging microscopy. Cancer Res. 1994;54:5670–5674. [PubMed: 7923215]
- Therapeutic Utilities of SOD Mimetics: Cancer, Radiotherapy and SOD Mimetics - M...Therapeutic Utilities of SOD Mimetics: Cancer, Radiotherapy and SOD Mimetics - Madame Curie Bioscience Database
- TRAFs in RANK Signaling - Madame Curie Bioscience DatabaseTRAFs in RANK Signaling - Madame Curie Bioscience Database
- Keratins As Targets in and Modulators of Liver Diseases - Madame Curie Bioscienc...Keratins As Targets in and Modulators of Liver Diseases - Madame Curie Bioscience Database
- Embryonic Salivary Gland Branching Morphogenesis - Madame Curie Bioscience Datab...Embryonic Salivary Gland Branching Morphogenesis - Madame Curie Bioscience Database
- Earthworm Immunity - Madame Curie Bioscience DatabaseEarthworm Immunity - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...