NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
General Considerations of the EMT
The epithelial to mesenchymal transition (EMT) is characterized by the loss of epithelial characteristics and the gain of mesenchymal attributes in epithelial cells. It has been associated with physiological and pathological processes requiring epithelial cell migration and invasion. Initially, EMT was observed in embryological and adult development with many well characterized examples including the conversions of epiblast to primary mesenchyme (gastrulation), somite to sclerotome, somite to dermis, myotome to migratory myoblast, dorsal neural tube to neural crest, placodal ectoderm to cranial ganglion precursor, intermediate mesoderm to nephric mesenchyme, lateral mesoderm to connective/muscular tissue, endocardium to cardiac cushion mesenchyme and trophectoderm invasion.1,2 In addition, evidence is mounting to support an important role of EMT pathways in the progression of carcinoma to metastasis providing epithelial tumour cells with the ability to migrate, invade the surrounding stroma and disseminate in secondary organs.3-5
Target Genes of the EMT
A variety of general hallmarks exist for the assignment of epithelial versus mesenchymal phenotype (fig. 1). Intermediate filament proteins provide a convenient and abundant marker, with keratins indicating epithelium and vimentin indicating a mesenchymal phenotype.6 This relationship breaks down in early development, where some cells which are clearly epithelial, as judged by junctional and basal lamina criteria, lack cytokeratins and many also possess vimentin. However, in these cases, it is the vimentin positive epithelia, such as the neuroepithelium, which often subsequently undergo dramatic reorganizations, including tissue folding and EMTs.7,8 Another commonly employed index for the epithelial state is the presence and junctional localization of the classically epithelial homotypic cell adhesion molecule E-cadherin and/or the associated catenins, forming the adherens junctions. These cell-cell adhesion-related criteria are almost entirely absent in mesenchymal cells.3 Even though keratin / vimentin and E-cadherin have been and are still the widest used markers of the EMT, a variety of other mechanisms and molecules have now been implicated in physiological and pathological EMT pathways, as reviewed by others (reviewed in refs. 2, 4-6, 9). They include a reorganization of other cell-cell contact complexes (tight junctions, desmosomes), a modification of cell-substrate adhesion complexes, the synthesis of extracellular matrix proteins normally expressed by mesenchymal cells such as fibronectin or collagens I/III, and the expression of several proteases including matrix metalloproteases (MMPs) which are also predominantly expressed by stromal cells (fig. 1). These molecular changes confer on epithelial cells the ability to scatter, migrate and degrade ECM components, all properties they do not display in a normal cohesive epithelium.
Inducers and Regulators of the EMT
Several growth factors (epidermal growth factor “EGF”, basic fibroblast growth factor “FGF-2”, hepatocyte growth factor “HGF”, transforming growth factor β1 “TGF-β1”) have been documented to trigger or at least modulate EMT phenomena.4,5,9 The reorganization of cell adhesion molecules (E-cadherin complexes, zonula occludens) has also been shown to trigger EMT changes in different cell systems.3,10 Also, even though they are rather considered as target genes of the EMT, several ECM components or even MMPs can in some cell systems serve as initiator of EMT changes.11,12
The implication of several signaling pathways in the control of the EMT is now clearly established. Thus ras, MAPK, PIP3K, rho, rac and src have been shown to control EMT events and regulate EMT target genes (reviewed in refs. 4, 5, 9).
Also, transcription factors of the Snail family (Snail, Slug) and of the ETS family, as well as the transcription factor SIP-1, have been directly implicated in the regulation of EMT target genes.13-19 This is also well established for β-catenin, which, once relocalized from the membrane E-cadherin complexes, can translocate in the nucleus where it can act as a co-transcription factor and directly regulate gene expression through its binding to TCF/LEF transcription factors.20
Many cellular and molecular aspects of EMT have been characterised, and our particular interest has been in the changes associated with a class of extracellular proteases, the Matrix Metalloproteinases (MMPs). The goal of this article is to review the literature and our own data implicating MMPs and their regulation in EMT processes, both normal and pathologic.
Matrix Metalloproteases
The MMP family currently comprises 23 human homologs.21-24 MMP protein structure is made of specific domains some of which are common to all MMPs. These conserved domains include a “pre” domain which directs the MMPs to the endoplasmic reticulum, a “pro” domain which maintains the MMP in an inactive form and a “catalytic” domain (fig. 2). Most MMPs also contain a c-terminal hemopexin-like domain, which mediates interactions with substrates and in some cases, directs substrate specificity and participates in substrate binding. This is attached to the catalytic domain by a flexible linker termed the hinge region. A specialized, fibronectin-like gelatin binding domain is found in the two gelatinases (MMP-2, MMP-9) and facilitates binding to type IV collagen. The generation of active enzymes, known as the activation process, requires the cleavage of the “pro” domain. In some cases, this is effected constitutively by furin-like enzymes which cleave a consensus sequence near the end of the prodomain. MMP activity can be regulated by specific tissue inhibitors of MMPs (TIMP-1 to -4).

Figure 2
Schematic diagram showing the grouping of MMPs based on domain structure. Drawing courtesy of Dr. Neeracha Ruangpanit.
Collectively, MMPs can degrade virtually every component of the ECM. Initially, MMPs were thought to predominantly degrade specific components of the ECM thereby providing new substrates facilitating migration and invasion. Since then, it has become clear that by degrading ECM components, MMPs can also modulate signaling pathways from the ECM and modulate the bioavailability of growth factors. Furthermore, others substrates have now been identified, the cleavage of which is also involved in increased migratory and invasive properties. Thus, cell adhesion molecules (E-cadherin, CD44, αv integrin)25-27 or growth factor receptors (FGF receptor 1, members of the EGF receptor family HER2 and HER4, c-met)22,28-30 can be processed by MMP-dependent proteolysis. Particular attention has been paid to the gelatinases MMP-2 and MMP-9 (gelatinase A and B, respectively), previously denoted type-IV collagenases (72 kDa and 92 kDa type IV collagenases, respectively), since they specifically can degrade the type IV collagen. This forms a major component of the basement membranes normally segregating epithelial tissues from surrounding mesenchyme. Loss of the basement membrane is one of the most reliable signs of poor prognosis in most carcinoma systems.31,32 Like most other MMPs,23,33 MMP-2 is secreted in a latent form, requiring activation. This is effected on the cell surface by a membrane-associated subclass of MMPs called the membrane-type MMPs (MT-MMP).34 Six MT-MMPs have been identified so far (MMP-14, 15, 16, 17, 24, 25), all of which except for MMP-17 have pro-MMP-2 activation function.34,35 MT1-MMP was the first MT-MMP identified as an activator of pro-MMP-2.36 Activation of MMP-2 by MT1-MMP is particularly well documented and involves the formation of a ternary complex between MT1-MMP, pro-MMP-2 and TIMP-2. Besides their implication in MMP-2 activation, MT-MMPs can also contribute directly to cell migration and invasion by degrading specific substrates. Thus MT1-MMP can cleave several specific substrates including collagens (I, II, III), laminin and fibronectin. Through their anchoring at the plasma membrane, MT-MMPs, and particularly MT1-MMP, have been shown to play a key role in the pericellular proteolysis associated with cell migration and invasion.21,34,37,38
MMPs in general have been implicated in many steps of malignancy, including primary tumour growth, angiogenesis, invasion of the basement membrane and stroma, and metastatic progression.22,24,33 With the notable exception of MMP 7/matrilysin,39 the consensus view is that MMP's are in general not produced by epithelial cells but rather by the surrounding stromal cells.33,40 However, we will discuss here data leading to the conclusion that expression of “stromal” MMPs is one of the major attributes that epithelial cells acquire after undergoing the EMT.
One of the major implications of this review is thus to counteract the notion that MMPs are exclusively produced by the peri-tumoural stroma. In contrast, we suggest that under the appropriate stimuli, genetic or epigenetic, certain epithelial cells will undergo EMT-like changes, and exhibit MMP production. We will summarize observations regarding MMP alterations and regulation in a number of EMT systems, both normal and neoplastic. Although our review will focus primarily on the MT-MMP/ MMP-2 axis, other MMP associations from our own work and the published literature will be summarized. The relationships which exist between the EMT and MMP-2-activation, especially those which are common to different systems, may provide insights into the implication of MMPs in EMT pathways and in their ability to modulate the migratory and invasive phenotype of epithelial cells.
MMPs in Carcinoma Model Systems for the EMT
In Vitro Observations
There are many reports of the expression of MMPs in epithelial tumour cell lines. We will only discuss here studies reporting MMP expression in epithelial cell lines in relation to well defined EMT changes. Many data have been generated by comparing different cell lines of the same origin showing different invasive potentials. Also, some cell lines have been shown to be inducible for EMT changes, either by exogenous factors or by migration opportunity in in vitro wound assays. Using these in vitro models, it has become clear that the EMT is a dynamic process and that different intermediate phenotypes can be observed. Also, whether only one EMT pathway exists is still unknown, and it can thus be considered that different EMT pathways could generate different phenotypes. Accordingly, variation may also be observed in the overall migratory/invasive behavior. For instance, based on signal transduction criteria, cell scattering can be considered to be different from, or a part of, full EMT events.41 As such, it does not necessarily correlate with increased motility and migration. Vice-versa, active cell migration does not necessary imply comprehensive cell scattering. For instance, the migration of some tumour cell types or the archetypal developmental EMT and cell migration, such as that of the neural crest (see Chapter 3), when viewed with time lapse in situ, shows that (former) epithelial cells can migrate as dynamic groups and are not always scattered individuals.42,43 The in vitro observations discussed below are summarized in Table 1.
Table 1
MMP expression and regulation in in vitro EMT models.
NBT-II Cells
Perhaps the oldest and most studied carcinoma model of the EMT is the NBT-II rat bladder carcinoma system (reviewed in ref. 9). These cells show EMT changes to a variety of specific stimuli including collagens, HGF, FGF-1 and TGFα. Responses to FGF-1 have been mapped to a splice variant of the FGFR-2,9,44 and shown to be mediated, in part at least, by the Snail family mesenchymal-inducing transcription factor Slug.15 Regarding MMP expression, one of the most rapidly detected changes in stimulated NBT-II cells is the secretion of MMP-9 and MMP-2, some of which appears in the media in the active form,45,46 which further suggests MT1-MMP expression.
Human Breast Cancer Cell Lines
Considerable circumstantial evidence exists in human breast cancer (HBC) cell lines in support of both the occurrence of EMT-like changes, and their association with a more aggressive phenotype (also reviewed in refs. 47, 48). Cell lines which are invasive in vitro, many of which also metastasize in immuno-compromised hosts, show mesenchymal tendencies. They express vimentin, show reduced cytokeratins, and lack E-cadherin, in contrast to poorly invasive HBC cell lines which lack vimentin, express keratin abundantly, and in some but not all cases express functional E-cadherin.49-53 We have also found that the vimentin-positive HBC cell lines express c-ets-1, a member of the ETS transcription factor family usually expressed by mesenchymal cells and largely implicated in MMP regulation.18 When we examined proteases shown to be regulated by c-ets-1, we saw differential expression of MMP-1, but no expression of MMP-3, as well as differential induction of the plasmin-generating axis.54 Also, invasive vimentin-positive HBC cell lines were found to express MT1-MMP (fig. 3) and activate MMP-255 in response to ConA or collagen type I which subsequently increased MT1-MMP mRNA and protein.56,57 Interestingly, all but one of the invasive HBC cell lines also expresses mRNA for MT3-MMP, while none expressed MT2-MMP mRNA, and both some non-invasive and some invasive lines expressed MT4-MMP.57 A very comprehensive survey of MMP expression by numerous HBC cell lines has been published.58,59
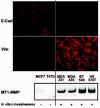
Figure 3
EMT in human breast cancer cell lines characterized by vimentin (Vim) expression, MT1-MMP expression and loss of E-cadherin (E-cad) associated with invasive properties.
Co-expression of MMPs in conjunction other mesenchymal markers in human breast cancer cell lines was also verified by independent gene array analysis by Zajchowski et al, who found that a set of 24 gene products could be used to predict the degree of invasiveness of untested cell lines.60 Higher expression of typically epithelial gene products (e.g., keratin-18, keratin-19, plakoglobin) was seen in the less invasive cells, while high expression of mesenchymal markers (e.g., integrin α3, TIMP-2, TIMP-3, BIG-H3, PAI-1, FRA-1, vimentin, osteonectin, TSP-1, collagen α1(VI) and α2(I), and thrombospondin-1) typified the highly invasive cell lines. It was notable that MT1-MMP was co-expressed with these markers in the invasive cells. Similarly, Jechlinger et al performed microarray analysis on mammary cells undergoing an EMT following ras transfection and in the list of genes upregulated, they found MMP-2, MMP-13 and MMP-12.61
We have examined the potential involvement of the EMT in two other human mammary systems that were developed to model various stages of malignancy. Transformation of the A1N4 human mammary epithelial cells with SV40 middle T antigen and v-Ha-ras, or v-mos and v-Ha-ras, but not either oncogene alone, conferred dramatically increased invasiveness and stellate Matrigel outgrowth properties on the cells. Although vimentin expression was apparent in all A1N4-derived cultures, the proportion of vimentin expression increased in the invasive transformants.62 No differences in MMP-2 or MMP-9 secretion were observed, however MT1-MMP status or collagen / ConA-induced MMP-2-activation potential was not examined. A parallel analysis was performed with various oncogene transformants derived from the MCF10A human mammary system, where again a combination of v-Ha-ras and erbB2, but not either oncogene alone, conferred a highly invasive phenotype.63 In this case, MMP-2 expression was increased, and TIMP-2 decreased, however again, the MMP-2 activation/MT1-MMP expression was not examined. Other studies also demonstrated an inducible EMT with a temporary expression of vimentin in MCF10A cells migrating to fill a monolayer wound, and indicated a functional role for vimentin in the migratory phenotype using antisense technology.64 In this system, vimentin expression also associates with a reorganization of E-cadherin/β-catenin complexes and β-catenin transactivation pathway.65 Corresponding to these EMT changes, an induction of MT1-MMP was also found exclusively in migratory cells undergoing an EMT but not in the stationary ones.66
Rodent Mammary Systems
A surprising twist to the relationship between EMT and MMP came from studies in the laboratories of Bissell and Werb, with observations that stromelysin-1 (MMP-3) could initiate EMT changes in SpC-2 mouse mammary cells.12,67 This extends considerable observations of MMP-3 expression during mammary gland morphogenesis (for review see refs. 68), and accelerated ductal development in transgenic MMTV-MMP-3 mouse mammary glands over-expressing activated MMP-3.69,70 Such over-development can progress to carcinoma in the WAP-MMP-3-transgenic mammary gland.70 Further studies have shown that MMP-3 is critical for mouse mammary cell invasion in vitro,71 and that malignant mouse mammary cells, as opposed to normal counterparts, show transcriptional regulation of MMP-3 similar to that seen in fibroblasts.72 MMP-2 and MMP-11 are also upregulated with mouse mammary gland development,69 and although stronger in humans than mouse, so is MMP-7.39,73 The capacity of MMP-3 to initiate the EMT in the SpC-2 mouse mammary system is unprecedented, and MMP-expression is usually placed downstream of the EMT in the same cell type. In the case of the mammary gland, however, it is predominantly stromal cells which produce the MMP-368,69 and this in turn could influence EMT-like changes in the neighboring mammary epithelial cells. However, MMPs, including MMP-3, also appear to be downstream targets of the EMT since endogenous MMP-3, MMP-9 and MMP-13 are upregulated following EMT induction with MMP-3.12
NMuMG cells, another mouse mammary epithelial cell line, undergoes a rapid and striking mesenchymal transdifferentiation in response to TGF-β, mediated by the TGF-β type I receptor/Alk-5 and SMAD proteins.74,75 This EMT is indicated by dramatically altered cytoarchitecture, reorganization of the actin cytoskeleton to stress fibers, and down regulation of E-cadherin and β-catenin. Although MMP-2 activation analysis has not been performed on these cells after TGF-β treatment, MT1-MMP expression is seen in these cells when they form branching tubular structures in collagen gels (Sato, unpublished data).
We have recently examined another syngeneic mouse mammary cancer model system comprising a panel of cells lines clonally isolated from a naturally occurring mouse mammary tumour. These cell lines show differential metastatic potential (4T1 >> 66Cl4 >> 67NR), and further single cell cloning has yielded a variant which spontaneously metastasizes to bone from the mammary fat pad (4T1.276). We have characterized whether the EMT-like patterns accompany metastatic potential in these cells, and find that while all four cell lines express vimentin and show stellate Matrigel outgrowth, the metastatic lines show much increased Boyden chamber chemoinvasion, and selectively activate MMP-2 (4T1.2>66Cl-4). This was surprising since all of the progression variants showed similar levels of MT1-MMP expression.77 Thus, we believe that all of these cell lines have undergone some level of EMT, as indicated by their expression of vimentin and stellate outgrowth, but those which are metastatic have a more complete set of mesenchymal traits, while the non-metastatic variants either lost, or never developed, some molecular traits critical for increased MMP-2-activation and in vitro invasion. It is noteworthy that the metastatic 4T1.2 cells do not show a particularly mesenchymal appearance on plastic culture, being rather rounded.
Studying a metastatic variant of a rat carcinoma cell lines, Martorana et al also found that it has undergone an EMT and expressed higher level of MMP-3, MMP-9 and MMP-13. Furthermore, cross regulation of MMP expression was observed between the epithelial cells and the mesenchymal variants.78
Bronchial Cell Lines
An association between fibroblastoid features (vimentin expression, loss of E-cadherin) and increased invasiveness was also reported in human bronchial epithelial tumour cells. Indeed, the 16HBE14o-vimentin-negative, E-cadherin positive cell line formed cohesive clusters and was not invasive whereas BZR vimentin-positive cells displayed a high dispersion ability and were highly invasive in the Boyden chamber assay. Correspondingly, BZR cells synthesized MMP-2 and MT1-MMP in contrast to 16HBE14o-.53,79 In this system, a direct link was also made between E-cadherin/β-catenin complex reorganization and MMP regulation. Treatment of non-invasive, MMP-negative 16HBE14o- cells with a soluble fragment of E-cadherin (sE-cadherin), known to be generated by protease activity and known to enhances epithelial cell migration,25 resulted in enhanced MMP-2, MMP-9 and MT1-MMP expression.53 This also enhanced in vitro invasiveness of the cells. On the other hand, transfection of an intact E-cadherin cDNA into invasive BZR cells clearly diminished MMP-1, MMP-3, MMP-9, and MT1-MMP. Using different in vitro (cell dispersion, modified Boyden chamber) and in vivo assays (human airway epithelial xenograft), it was also shown that E-cadherin transfectants displayed decreased invasive abilities.80
Prostate Cell Lines
We recently analyzed human prostate carcinoma cell lines for the same parameters as described above for the breast cancer model systems (Williams and Thompson, unpublished observations). Indeed, we found that cell lines known to be metastatic in immunocompromised mice showed increased Boyden chamber activity and a tendency towards more extensive outgrowth in matrigel, although the differential was not as clear as seen in HBC cell lines. The invasive cells however clearly express vimentin, and show high levels of MT1-MMP mRNA and protein. Consequently, they activate MMP-2 readily when stimulated with ConA. Selective expression of MT1-MMP by the more invasive prostate cancer cell lines was also reported by others.81,82
Squamous Carcinoma Cells
EMT events (E-cadherin loss and expression of vimentin) have also been observed in several human squamous carcinoma cells. This has been shown in a model of cervical cell lines generated by transfection of human papillomavirus type 33.83 Only cell lines which expressed vimentin were highly invasive in the Boyden chamber assay, and these were found to synthesize MT1-MMP and were able to activate MMP-2 following ConA induction.84
In other established squamous cell carcinoma cell line systems, an EMT phenotype was also correlated to high levels of the transcription factor snail.85 Furthermore, transfection of snail in vimentin-negative, E-cadherin-positive A431 cells resulted in induction of vimentin and MMP-2, and loss of E-cadherin.
From all these in vitro data, it is clear that MMP expression in epithelial cells associates with EMT processes. Among MMPs, the MT1-MMP/MMP-2 axis appear as a general pathway activated in most of the in vitro systems described above.
In Vivo Observations
Studying EMT in tumour biopsies remains a major challenge. Indeed, if one considers a “full” EMT as a dynamic phenomenon resulting in the acquisition of migratory and invasive properties by epithelial tumour cells, it is very likely to be discrete and affect only a small proportion of cells in the tumour mass. Also, if the dynamic nature of the EMT is clear, the sequence of events is still not well defined and may vary. Different phenotypes might thus be observed in a tumour mass. Accordingly, the expression of EMT genes such as E-cadherin and vimentin is very heterogenous within a given tumour.
Nevertheless, the existence of EMT phenomena in tumour biopsies is now well demonstrated. For instance, considering the two well known markers of EMT (E-cadherin reorganization and vimentin expression), numerous studies have reported the reorganization of E-cadherin complexes or vimentin expression in a variety of cancer types. Regarding MMPs, a mass of data have shown the implication of several MMPs in tumour progression (reviewed in refs. 21, 22). From these data, it is clear that the peritumoural stromal cells are the major source of MMPs in most cancers. However, even though it has rarely been studied in relationship to other EMT genes, the expression of MMPs in tumour cells has been also reported in many cancers.86 This has particularly been shown for MT1-MMP in carcinomas including cervical,87 lung,88,89 prostate,90 colorectal,91 gastrointestinal,92 oesophageal,93,94 larynx,95 breast,96,97 oral,98 ovarian,99,100 head and neck,101 hepatocellular and pancreatic102 carcinomas.
Given the associations presented above from a number of in vitro model systems, it seems possible that the EMT in vivo may contribute to the expression of several MMPs in epithelial tumour cells and this results in tumours that do poorly. However, this is not proven, and the possibility remains that this is due to a more reactive stroma. It is interesting to note, however, the documentation in breast carcinomas of MMP-11 expression in metaplastic breast carcinoma cells which have undergone a degree of EMT-like changes.103 The authors speculate that this may be related to the poorer prognosis associated with such carcinomas. Similarly, Trudel et al reported the expression of MMP-2 in both stromal and epithelial tumour cells of prostate cancer.90 Importantly, MMP-2 expression by stromal cells was not associated with progression whereas MMP-2 expression by >50% of malignant epithelial cells was associated with decreased disease-free survival.90 MMP-3 is also upregulated in a model of experimental mouse skin carcinogenesis, when squamous cell carcinomas progress to spindle cell carcinomas.104
While the inherent genetic deviations and implicit uniqueness of individual cancer systems make it difficult to draw general conclusions, the fact that so many different systems show EMT like capacities which are associated with increased migration, invasion, and metastasis does offer a degree of confidence in projecting an important role for this process in carcinoma progression.
MMPs in Developmental EMT Systems
Even more supportive is the parallel observations of MMP expression in EMT systems of normal development such as kidney morphogenesis modeled with MDCK cells and avian heart development, and studies on trophoblast invasion and wounded epithelia.
Kidney Morphogenesis and Disease
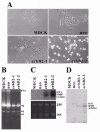
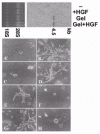
Madin Darby canine kidney (MDCK) epithelial cells are non tumourigenic cells and provide a well-accepted model for many aspects of epithelial cell biology. Under basal conditions in culture, they can polarize and differentiate. Following induction with different inducers including basic fibroblast growth factor (bFGF/FGF-2), hepatocyte growth factor (HGF), collagens and oncogenes (v-src and erbB2), they can easily undergo dynamic EMT changes (see fig. 4 for erbB2 example). This often correlates with the loosening of cell-cell contacts, cell scattering and the capacity to invade 3D-collagen gel cultures and form branching tubules, thus mimicking a physiological renal morphogenesis rather than a pathological process. When induced by hepatocyte growth factor (HGF) to form branching tubules in collagen gels, an induction of MT1-MMP is observed38,105 (see also Table 1). Furthermore, antisense RNA inhibition of the induced MT1-MMP abrogates the branching morphogenesis response to HGF. Also, TIMP-2 and BB-94, which inhibit both MMP-2 and MT1-MMP, block invasion or tubular formation in collagen gel. In contrast, TIMP-1, which inhibits MMP-2 but not MT1-MMP, fails to interfere with branching, implicating MT1-MMP rather than MMP-2 in the branching morphogenesis. Interestingly, we also see induced MT1-MMP after src transformation of MDCK cells,106 and this is accompanied by overexpression of TIMP-1.107 MT1-MMP induction is also seen after erbB2 transfection in MDCK cells (fig. 5).108 Although we have not examined vimentin expression in the MDCK cells undergoing branching morphogenesis, the src transformed cells show loss of cohesive cobblestone morphology in cell culture, and orientate as stellate single cells spread on the culture surface. They further acquire tumourigenesis in both the subcutus and kidney of athymic mice, and are metastatic to the lung when inoculated orthotopically.106
In vivo, and in organ culture systems, MMP-2 and MMP-9 have been demonstrated in conjunction with mouse renal tubulogenesis, however antibodies against MMP-9 only, and not MMP-2, blocked renal morphogenesis. 109 Although this study did not directly correlate this MMP expression with EMT, the EMT is known to be important in kidney morphogenesis. In contrast, MMP has been directly associated with the EMT changes seen in the tubular epithelium during kidney fibrosis associated with diabetic nephropathy.110,111 Here, a surprising role of MMP-2 has emerged, being necessary and sufficient to induce the EMT in renal cell models.112
Wounded Bronchial Epithelium
Considerable attention has been paid to the MMP expression in a system where confluent human surface respiratory epithelial cells are wounded, and the cells undergo EMT-like changes (vimentin expression) to migrate into the naked culture surface. In this system, we see focal expression of MMP-9,113,114 MMP-3 and MMP-11115 in the cells undergoing migration at the wound edge, and this migration can be blocked with MMP-inhibitors.114
Avian Heart Development
EMT events have been well characterized in the transformation of endothelial cells lining the atrio-ventricular canal of avian heart to form progenitors of the septa and heart valves.116 An induction of MT1-MMP and/orMMP-2 have been shown in EMT regions117-120 together with an induction of Slug and c-ets-1, transcription factors known to be involved in EMT events and to regulate MMPs.121,122
Placental Implantation
Another area in which a well described EMT has been studied with respect to MMPs is the trophoblast implantation (reviewed in refs. 123, 124). Regarding MMP expression, MT1-MMP has been observed in invading trophoblasts in vivo125 and both MMP-2 and MMP-9 in the trophoblastic columns.126 In vitro, a clear requirement of MMP-9 for Matrigel invasion of cytotrophoblasts has also been demonstrated and correlated with the abundance of MMP-9 in the first trimester.127
Neural Crest Migration
The migration of neural crest cells in vertebrate embryogenesis is initiated by EMT of the dorsal most cells of the neural epithelium, and is perhaps the most intensively studied developmental EMT and cell migration2 (see Chapter 3). This EMT is normally executed via stimulation by BMP-4 and crucially involves expression of the zinc-finger transcription factor genes Slug or Snail.128 As in the heart, c-ets-1 transcription factor is also expressed during the neural crest EMT.129 Neural crest cells just prior to and during migration express genes for a number of extracellular proteases. MMP-8 is expressed in neural crest cells during EMT in mouse embryos.130 Experiments in chicken embryos reveal the expression and importance of MMP-2 in these dynamic processes.131 It is expressed when neural crest cells detach from the neural epithelium during EMT, but switched off as the cells separate. A similar scenario is seen in the sclerotome and in the dermis, where MMP-2 expression is seen when the EMT is initiated and maintained during the migration of the cells, but down-regulated once the cells cease movement. Interestingly, MMP-2-specific knockdown with morpholino antisense oligonecleotides, and global MMP- inhibition using BB-94, confirm that MMP-2 is required for neural crest EMT, but these do not inhibit migration if applied after the EMT. In contrast, MMP-2 inhibition during mesenchyme production from the somite blocks both EMT parameters and cell migration.119 A full audit of MMP expression in relation to neural crest cell morphogenesis has not been undertaken, so the possibility of redundancy has not been addressed.
Regulation of MMPs by EMT-Associated Transcription Factors
If the data presented above clearly demonstrate that MMP expression is associated with EMT changes, several studies have also shown a direct regulation of MMPs by EMT-associated transcription factors. Thus, MMP-2 regulation by Snail has been demonstrated in squamous carcinoma cells.85 Also, even though they do not necessarily establish a link with EMT processes, the regulation of MMPs by transcription factors of the ETS family in a variety of carcinoma cells has been clearly established (review in refs. 132, 133). Accumulating data also demonstrate the implication of the β-catenin co-transcriptional activity in the regulation of MMP. Thus MMP-7,134-136 MT1-MMP,137 MMP-26138 are targets of the β-catenin/TCF pathway.
Summary and Conclusions
The EMT appears as a sequence of changes which can lead to the expression of migratory and invasive properties by epithelial cells. As shown in numerous tumoural and non tumoural in vitro systems, but also in vivo on tumour biopsies and on developmental models, the expression of MMPs (and particularly of MT1-MMP) is clearly part of EMT processes. Accordingly, the regulation of several MMPs by transcription factors (snail, ETS, β-catenin), known to regulate EMT pathways, have clearly been established. Consequently, MMPs are rather considered as target genes of EMT pathways and MMP expression as a late event of the EMT. This may be due, in part, to direct regulation of MMP gene transcription by the factors which drive EMT. Nevertheless, some MMPs (such as MMP-3) have been shown to initiate EMT changes. Also, several MMPs have been shown to be able to cleave E-cadherin, thereby inducing E-cadherin complex fragility and EMT changes. At present, the MMP field is undergoing a re-evaluation stage following the relatively poor results seen with clinical trials of MMP inhibitors. 139,140 One major factor often raised in this re-evaluation is our lack of precise knowledge regarding which specific MMPs are mediating pro-tumoural processes. Those associated with EMT are clearly contenders for this category, and may warrant specific targeting. A number of MMPs have been associated, as detailed above, with the EMT, however, MT1-MMP stands out in this regard. Although stromal cells are a major source of MMP in tumours, expression of MMPs (particularly MT1-MMP) by parenchymal cells is a clear EMT step which can ensure a pericellular proteolysis of basement membrane components but also other substrates, and thereby facilitate migration and invasion.
Acknowledgements
This work was supported by grants from the Fonds National de la Recherche Scientifique (FNRS, Belium), the Federation Belge Contre le Cancer, the Fortis Banque Assurances, the Interuniversity Attraction Poles Programme - Belgian Science Policy (Brussels, Belgium). CG is a Research Associate from the FNRS (Belgium).
Part of this work was also supported by grants from the US Department of Defence, Congressionally Directed Medical Research Program, Breast Cancer Research Section, grant number DAMD17-03-1-0416; the Victoriam Breast Cancer Research Consortium and an NIH Par 99-128: Insight Award to Stamp Out Breast Cancer.
References
- 1.
- Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis. 1995;26(4):678–690. [PubMed: 7573028]
- 2.
- Duband JL, Monier F, Delannet M. et al. Epithelium-mesenchyme transition during neural crest development. Acta Anat. 1995;154(1):63–78. [PubMed: 8714290]
- 3.
- Birchmeier C, Birchmeier W, Brand-Saberi B. Epithelial-mesenchymal transitions in cancer progression. Acta Anat. 1996;156(3):217–226. [PubMed: 9124038]
- 4.
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15(6):740–746. [PubMed: 14644200]
- 5.
- Gotzmann J, Mikula M, Eger A. et al. Molecular aspects of epithelial cell plasticity: implications for local tumour invasion and metastasis. Mutat Res. 2004;566(1):9–20. [PubMed: 14706509]
- 6.
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995;154(1):8–20. [PubMed: 8714286]
- 7.
- Viebahn C, Lane EB, Ramaekers FC. Keratin and vimentin expression in early organogenesis of the rabbit embryo. Cell Tissue Res. 1988;253(3):553–562. [PubMed: 2460241]
- 8.
- Erickson CA, Tucker RP, Edwards BF. Changes in the distribution of intermediate-filament types in Japanese quail embryos during morphogenesis. Differentiation. 1987;34(2):88–97. [PubMed: 2442055]
- 9.
- Boyer B, Valles AM, Edme N. Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol. 2000;60(8):1091–1099. [PubMed: 11007946]
- 10.
- Reichert M, Muller T, Hunziker W. The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells. Evidence for a role of beta-catenin/ Tcf/Lef signaling. J Biol Chem. 2000;275(13):9492–9500. [PubMed: 10734097]
- 11.
- Tucker GC, Boyer B, Gavrilovic J. et al. Collagen-mediated dispersion of NBT-II rat bladder carcinoma cells. Cancer Res. 1990;50(1):129–137. [PubMed: 2293546]
- 12.
- Lochter A, Galosy S, Muschler J. et al. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. [PMC free article: PMC2132651] [PubMed: 9412478]
- 13.
- Batlle E, Sancho E, Franci C. et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2(2):84–89. [PubMed: 10655587]
- 14.
- Cano A, Perez-Moreno MA, Rodrigo I. et al. The transcription factor snail controls epithelialmesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. [PubMed: 10655586]
- 15.
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137(6):1403–1419. [PMC free article: PMC2132541] [PubMed: 9182671]
- 16.
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23(10):912–923. [PubMed: 11598958]
- 17.
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3(3):155–166. [PubMed: 11994736]
- 18.
- Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. [PubMed: 12559563]
- 19.
- Comijn J, Berx G, Vermassen P. et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7(6):1267–1278. [PubMed: 11430829]
- 20.
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653(1):1–24. [PubMed: 12781368]
- 21.
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. [PMC free article: PMC2792593] [PubMed: 11687497]
- 22.
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. [PubMed: 11990853]
- 23.
- Benaud C, Dickson RB, Thompson EW. Roles of the matrix metalloproteinases in mammary gland development and cancer. Breast Cancer Res Treat. 1998;50(2):97–116. [PubMed: 9822215]
- 24.
- Lafleur MA, Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in angiogenesis. Expert Rev Mol Med. 2003;5:1–39. [PubMed: 14585170]
- 25.
- Noe V, Fingleton B, Jacobs K. et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114(Pt 1):111–118. [PubMed: 11112695]
- 26.
- Kajita M, Itoh Y, Chiba T. et al. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153(5):893–904. [PMC free article: PMC2174329] [PubMed: 11381077]
- 27.
- Deryugina EI, Ratnikov B, Monosov E. et al. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263(2):209–223. [PubMed: 11161720]
- 28.
- Codony-Servat J, Albanell J, Lopez-Talavera JC. et al. Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res. 1999;59(6):1196–1201. [PubMed: 10096547]
- 29.
- Vecchi M, Rudolph-Owen LA, Brown CL. et al. Tyrosine phosphorylation and proteolysis. Pervanadate-induced, metalloprotease-dependent cleavage of the ErbB-4 receptor and amphiregulin. J Biol Chem. 1998;273(32):20589–20595. [PubMed: 9685416]
- 30.
- Nath D, Williamson NJ, Jarvis R. et al. Shedding of c-Met is regulated by crosstalk between a G-protein coupled receptor and the EGF receptor and is mediated by a TIMP-3 sensitive metalloproteinase. J Cell Sci. (Pt 6). 2001;114:1213–1220. [PubMed: 11228164]
- 31.
- Barsky SH, Siegal GP, Jannotta F. et al. Loss of basement membrane components by invasive tumours but not by their benign counterparts. Lab Invest. (2). 1983;49:140–147. [PubMed: 6348406]
- 32.
- Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80(8 Suppl):1529–1537. [PubMed: 9362419]
- 33.
- Nelson AR, Fingleton B, Rothenberg ML. et al. Matrix Metalloproteinases: Biologic Activity and Clinical Implications. J Clin Oncol. 2000;18(5):1135. [PubMed: 10694567]
- 34.
- Zucker S, Pei D, Cao J. et al. Membrane type-matrix metalloproteinases (MT-MMP). Curr Top Dev Biol. 2003;54:1–74. [PubMed: 12696745]
- 35.
- English WR, Puente XS, Freije JM. et al. Membrane type 4 matrix metalloproteinase (MMP17) has tumour necrosis factor-alpha convertase activity but does not activate pro-MMP2. J Biol Chem. 2000;275(19):14046–14055. [PubMed: 10799478]
- 36.
- Sato H, Takino T, Okada Y. et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370(6484):61–65. [PubMed: 8015608]
- 37.
- Seiki M, Mori H, Kajita M. et al. Membrane-type 1 matrix metalloproteinase and cell migration. Biochem Soc Symp. 2003;(70):253–262. [PubMed: 14587298]
- 38.
- Hotary K, Allen E, Punturieri A. et al. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149(6):1309–1323. [PMC free article: PMC2175112] [PubMed: 10851027]
- 39.
- Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol. 1996;28(2):123–136. [PubMed: 8729000]
- 40.
- Almholt K, Johnsen M. Stromal cell involvement in cancer. Recent Results Cancer Res. 2003;162:31–42. [PubMed: 12790319]
- 41.
- Janda E, Lehmann K, Killisch I. et al. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156(2):299–313. [PMC free article: PMC2199233] [PubMed: 11790801]
- 42.
- Nabeshima K, Inoue T, Shimao Y. et al. Matrix metalloproteinases in tumour invasion: role for cell migration. Pathol Int. 2002;52(4):255–264. [PubMed: 12031080]
- 43.
- Kulesa P, Ellies DL, Trainor PA. Comparative analysis of neural crest cell death, migration, and function during vertebrate embryogenesis. Dev Dyn. 2004;229(1):14–29. [PubMed: 14699574]
- 44.
- Savagner P, Valles AM, Jouanneau J. et al. Alternative splicing in fibroblast growth factor receptor 2 is associated with induced epithelial-mesenchymal transition in rat bladder carcinoma cells. Mol Biol Cell. 1994;5(8):851–862. [PMC free article: PMC301106] [PubMed: 7803853]
- 45.
- Gavrilovic J, Moens G, Thiery JP. et al. Expression of transfected transforming growth factor alpha induces a motile fibroblast-like phenotype with extracellular matrix-degrading potential in a rat bladder carcinoma cell line. Cell Regul. 1990;1(13):1003–1014. [PMC free article: PMC361698] [PubMed: 2134746]
- 46.
- Jouanneau J, Gavrilovic J, Caruelle D. et al. Secreted or nonsecreted forms of acidic fibroblast growth factor produced by transfected epithelial cells influence cell morphology, motility, and invasive potential. Proc Natl Acad Sci USA. 1991;88(7):2893–2897. [PMC free article: PMC51346] [PubMed: 1707175]
- 47.
- Bae SN, Arand G, Azzam H. et al. Molecular and cellular analysis of basement membrane invasion by human breast cancer cells in Matrigel-based in vitro assays. Breast Cancer Res Treat. 1993;24(3):241–255. [PubMed: 8435479]
- 48.
- Gilles CT. The epithelial to mesenchymal transition and metastatic progression in carcinoma. The Breast J. 1996;2(2):83–96.
- 49.
- Sommers CL, Thompson EW, Torri JA. et al. Cell adhesion molecule uvomorulin expression in human breast cancer cell lines: relationship to morphology and invasive capacities. Cell Growth Differ. 1991;2(8):365–372. [PubMed: 1793731]
- 50.
- Sommers CL, Heckford SE, Skerker JM. et al. Loss of epithelial markers and acquisition of vimentin expression in adriamycin- and vinblastine-resistant human breast cancer cell lines. Cancer Res. 1992;52(19):5190–5197. [PubMed: 1382837]
- 51.
- Thompson EW, Paik S, Brunner N. et al. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992;150(3):534–544. [PubMed: 1537883]
- 52.
- Sommers CL, Byers SW, Thompson EW. et al. Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat. 1994;31(2-3):325–335. [PubMed: 7881109]
- 53.
- Nawrocki RabyB, Polette M, Gilles C. et al. Quantitative cell dispersion analysis: new test to measure tumour cell aggressiveness. Int J Cancer. 2001;93(5):644–652. [PubMed: 11477573]
- 54.
- Gilles C, Polette M, Birembaut P. et al. Expression of c-ets-1 mRNA is associated with an invasive, EMT-derived phenotype in breast carcinoma cell lines. Clin Exp Metastasis. 1997;15(5):519–526. [PubMed: 9247254]
- 55.
- Azzam HS, Arand G, Lippman ME. et al. Association of MMP-2 activation potential with metastatic progression in human breast cancer cell lines independent of MMP-2 production. J Natl Cancer Inst. 1993;85(21):1758–1764. [PubMed: 8411260]
- 56.
- Pulyaeva H, Bueno J, Polette M. et al. MT1-MMP correlates with MMP-2 activation potential seen after epithelial to mesenchymal transition in human breast carcinoma cells [published erratum appears in Clin Exp Metastasis 1997; 15(3):338] Clin Exp Metastasis. 1997;15(2):111–120. [PubMed: 9062387]
- 57.
- Gilles C, Polette M, Seiki M. et al. Implication of collagen type I-induced membrane-type 1-matrix metalloproteinase expression and matrix metalloproteinase-2 activation in the metastatic progression of breast carcinoma. Lab Invest. 1997;76:651–660. [PubMed: 9166284]
- 58.
- Giambernardi TA, Grant GM, Taylor GP. et al. Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol. 1998;16(8):483–496. [PubMed: 9550265]
- 59.
- Grant GM, Giambernardi TA, Grant AM. et al. Overview of expression of matrix metalloproteinases (MMP-17, MMP-18, and MMP-20) in cultured human cells. Matrix Biol. 1999;18(2):145–148. [PubMed: 10372554]
- 60.
- Zajchowski DA, Bartholdi MF, Gong Y. et al. Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res. 2001;61(13):5168–5178. [PubMed: 11431356]
- 61.
- Jechlinger M, Grunert S, Tamir IH. et al. Expression profiling of epithelial plasticity in tumour progression. Oncogene. 2003;22(46):7155–7169. [PubMed: 14562044]
- 62.
- Thompson EW, Torri J, Sabol M. et al. Oncogene-induced basement membrane invasiveness in human mammary epithelial cells. Clin Exp Metastasis. 1994;12(3):181–194. [PubMed: 8194193]
- 63.
- Giunciuglio D, Culty M, Fassina G. et al. Invasive phenotype of MCF10A cells overexpressing c-Ha-ras and c-erbB-2 oncogenes. Int J Cancer. 1995;63(6):815–822. [PubMed: 8847140]
- 64.
- Gilles C, Polette M, Zahm J. et al. Vimentin contributes to human mammary epithelial cell migration [In Process Citation] J Cell Sci. 1999;112(Pt 24):4615–4625. [PubMed: 10574710]
- 65.
- Gilles C, Polette M, Mestdagt M. et al. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res. 2003;63(10):2658–2664. [PubMed: 12750294]
- 66.
- Gilles C, Polette M, Coraux C. et al. Contribution of MT1-MMP and of human laminin-5 gamma2 chain degradation to mammary epithelial cell migration. J Cell Sci. 2001;114(Pt 16):2967–2976. [PMC free article: PMC2966877] [PubMed: 11686300]
- 67.
- Sternlicht MD, Lochter A, Sympson CJ. et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98(2):137–146. [PMC free article: PMC2853255] [PubMed: 10428026]
- 68.
- Uria JA, Werb Z. Matrix metalloproteinases and their expression in mammary gland. Cell Res. 1998;8(3):187–194. [PubMed: 9791732]
- 69.
- Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell. 1995;6(10):1287–1303. [PMC free article: PMC301288] [PubMed: 8573787]
- 70.
- Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumour promoter. Oncogene. 2000;19(8):1102–1113. [PMC free article: PMC2933206] [PubMed: 10713697]
- 71.
- Lochter A, Srebrow A, Sympson CJ. et al. Misregulation of stromelysin-1 expression in mouse mammary tumour cells accompanies acquisition of stromelysin-1-dependent invasive properties. J Biol Chem. 1997;272(8):5007–5015. [PubMed: 9030563]
- 72.
- Lochter A, Werb Z, Bissell MJ. Transcriptional regulation of stromelysin-1 gene expression is altered during progression of mouse mammary epithelial cells from functionally normal to malignant. Matrix Biol. 1999;18(5):455–467. [PMC free article: PMC2933197] [PubMed: 10601733]
- 73.
- Heppner KJ, Matrisian LM, Jensen RA. et al. Expression of most matrix metalloproteinase family members in breast cancer represents a tumour-induced host response. Am J Pathol. 1996;149(1):273–282. [PMC free article: PMC1865221] [PubMed: 8686751]
- 74.
- Miettinen PJ, Ebner R, Lopez AR. et al. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127(6 Pt 2):2021–2036. [PMC free article: PMC2120317] [PubMed: 7806579]
- 75.
- Piek E, Moustakas A, Kurisaki A. et al. TGF-β type I receptor/ALK-5 and smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells [In Process Citation] J Cell Sci. 1999;112(Pt 24):4557–4568. [PubMed: 10574705]
- 76.
- Lelekakis M, Moseley JM, Martin TJ. et al. A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis. 1999;17(2):163–170. [PubMed: 10411109]
- 77.
- Tester AM, Ruangpanit N, Anderson RL. et al. MMP-9 secretion and MMP-2 activation distinguish invasive and metastatic sublines of a mouse mammary carcinoma system showing epithelial-mesenchymal transition traits. Clin Exp Metastasis. 2000;18(7):553–560. [PubMed: 11688960]
- 78.
- Martorana AM, Zheng G, Crowe TC. et al. Epithelial cells up-regulate matrix metalloproteinases in cells within the same mammary carcinoma that have undergone an epithelial-mesenchymal transition. Cancer Res. 1998;58(21):4970–4979. [PubMed: 9810007]
- 79.
- Polette M, Gilles C, de Bentzmann S. et al. Association of fibroblastoid features with the invasive phenotype in human bronchial cancer cell lines. Clin Exp Metastasis. 1998;16(2):105–112. [PubMed: 9514091]
- 80.
- Nawrocki-Raby B, Gilles C, Polette M. et al. E-Cadherin mediates MMP down-regulation in highly invasive bronchial tumour cells. Am J Pathol. 2003;163(2):653–661. [PMC free article: PMC1868220] [PubMed: 12875984]
- 81.
- Jung M, Romer A, Keyszer G. et al. mRNA expression of the five membrane-type matrix metalloproteinases MT1-MT5 in human prostatic cell lines and their down-regulation in human malignant prostatic tissue. Prostate. 2003;55(2):89–98. [PubMed: 12661033]
- 82.
- Daja MM, Niu X, Zhao Z. et al. Characterization of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2003;6(1):15–26. [PubMed: 12664060]
- 83.
- Gilles C, Piette J, Peter W. et al. Differentiation ability and oncogenic potential of HPV-33- and HPV-33 + ras-transfected keratinocytes. Int J Cancer. 1994;58(6):847–854. [PubMed: 7927877]
- 84.
- Gilles C, Polette M, Piette J. et al. Epithelial-to-mesenchymal transition in HPV-33-transfected cervical keratinocytes is associated with increased invasiveness and expression of gelatinase A. Int J Cancer. 1994;59(5):661–666. [PubMed: 7960239]
- 85.
- Yokoyama K, Kamata N, Fujimoto R. et al. Increased invasion and matrix metalloproteinase-2 expression by Snail-induced mesenchymal transition in squamous cell carcinomas. Int J Oncol. 2003;22(4):891–898. [PubMed: 12632084]
- 86.
- Polette M, Nawrocki-Raby B, Gilles C. Tumour Invasion and Matrix Metalloproteases. 2004 In press . [PMC free article: PMC521084] [PubMed: 15036258]
- 87.
- Gilles C, Polette M, Piette J. et al. High level of MT-MMP expression is associated with invasiveness of cervical cancer cells. Int J Cancer. 1996;65(2):209–213. [PubMed: 8567119]
- 88.
- Polette M, Nawrocki B, Gilles C. et al. MT-MMP expression and localisation in human lung and breast cancers. Virchows Arch. 1996;428(1):29–35. [PubMed: 8646366]
- 89.
- Yamamura T, Nakanishi K, Hiroi S. et al. Expression of membrane-type-1-matrix metalloproteinase and metalloproteinase-2 in nonsmall cell lung carcinomas. Lung Cancer. 2002;35(3):249–255. [PubMed: 11844598]
- 90.
- Trudel D, Fradet Y, Meyer F. et al. Significance of MMP-2 expression in prostate cancer: an immunohistochemical study. Cancer Res. 2003;63(23):8511–8515. [PubMed: 14679018]
- 91.
- Kikuchi R, Noguchi T, Takeno S. et al. Immunohistochemical detection of membrane-type-1-matrix metalloproteinase in colorectal carcinoma. Br J Cancer. 2000;83(2):215–218. [PMC free article: PMC2363481] [PubMed: 10901373]
- 92.
- Ohtani H, Motohashi H, Sato H. et al. Dual over-expression pattern of membrane-type metalloproteinase-1 in cancer and stromal cells in human gastrointestinal carcinoma revealed by in situ hybridization and immunoelectron microscopy. Int J Cancer. 1996;68(5):565–570. [PubMed: 8938135]
- 93.
- Etoh T, Inoue H, Yoshikawa Y. et al. Increased expression of collagenase-3 (MMP-13) and MT1-MMP in oesophageal cancer is related to cancer aggressiveness. Gut. 2000;47(1):50–56. [PMC free article: PMC1727967] [PubMed: 10861264]
- 94.
- Ohashi K, Nemoto T, Nakamura K. et al. Increased expression of matrix metalloproteinase 7 and 9 and membrane type 1-matrix metalloproteinase in esophageal squamous cell carcinomas. Cancer. 2000;88(10):2201–2209. [PubMed: 10820340]
- 95.
- Cazorla M, Hernandez L, Nadal A. et al. Collagenase-3 expression is associated with advanced local invasion in human squamous cell carcinomas of the larynx. J Pathol. 1998;186(2):144–150. [PubMed: 9924429]
- 96.
- Ueno H, Nakamura H, Inoue M. et al. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res. 1997;57(10):2055–2060. [PubMed: 9158005]
- 97.
- Ishigaki S, Toi M, Ueno T. et al. Significance of membrane type 1 matrix metalloproteinase expression in breast cancer. Jpn J Cancer Res. 1999;90(5):516–522. [PMC free article: PMC5926108] [PubMed: 10391091]
- 98.
- Nakamura H, Ueno H, Yamashita K. et al. Enhanced production and activation of progelatinase A mediated by membrane-type 1 matrix metalloproteinase in human papillary thyroid carcinomas. Cancer Res. 1999;59(2):467–473. [PubMed: 9927064]
- 99.
- Davidson B, Goldberg I, Gotlieb WH. et al. High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clin Exp Metastasis. 1999;17(10):799–808. [PubMed: 11089877]
- 100.
- Davidson B, Goldberg I, Berner A. et al. Expression of membrane-type 1, 2, and 3 matrix metalloproteinases messenger RNA in ovarian carcinoma cells in serous effusions. Am J Clin Pathol. 2001;115(4):517–524. [PubMed: 11293899]
- 101.
- Imanishi Y, Fujii M, Tokumaru Y. et al. Clinical significance of expression of membrane type 1 matrix metalloproteinase and matrix metalloproteinase-2 in human head and neck squamous cell carcinoma. Hum Pathol. 2000;31(8):895–904. [PubMed: 10987249]
- 102.
- Maatta M, Soini Y, Liakka A. et al. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in hepatocellular and pancreatic adenocarcinoma: implications for tumour progression and clinical prognosis. Clin Cancer Res. 2000;6(7):2726–2734. [PubMed: 10914717]
- 103.
- Ahmad A, Hanby A, Dublin E. et al. Stromelysin 3: an independent prognostic factor for relapse-free survival in node-positive breast cancer and demonstration of novel breast carcinoma cell expression. Am J Pathol. 1998;152(3):721–728. [PMC free article: PMC1858384] [PubMed: 9502414]
- 104.
- Wright JH, McDonnell S, Portella G. et al. A switch from stromal to tumour cell expression of stromelysin-1 mrna associated with the conversion of squamous to spindle carcinomas during mouse skin tumour progression. Mol Carcinog. 1994;10(4):207–215. [PubMed: 8068181]
- 105.
- Kadono Y, Shibahara K, Namiki M. et al. Membrane type 1-matrix metalloproteinase is involved in the formation of hepatocyte growth factor/scatter factor-induced branching tubules in madin-darby canine kidney epithelial cells. Biochem Biophys Res Commun. 1998;251(3):681–687. [PubMed: 9790969]
- 106.
- Kadono Y, Okada Y, Namiki M. et al. Transformation of epithelial Madin-Darby canine kidney cells with p60(v-src) induces expression of membrane-type 1 matrix metalloproteinase and invasiveness. Cancer Res. 1998;58(10):2240–2244. [PubMed: 9605772]
- 107.
- Noritake H, Miyamori H, Goto C. et al. Overexpression of tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) in metastatic MDCK cells transformed by v-src. Clin Exp Metastasis. 1999;17(2):105–110. [PubMed: 10411101]
- 108.
- Miyamori H, Hasegawa K, Kim KR. et al. Expression of metastasis-associated mts1 gene is co-induced with membrane type-1 matrix metalloproteinase (MT1-MMP) during oncogenic transformation and tubular formation of Madin Darby canine kidney (MDCK) epithelial cells. Clin Exp Metastasis. 2000;18(1):51–56. [PubMed: 11206838]
- 109.
- Lelongt B, Trugnan G, Murphy G. et al. Matrix metalloproteinases MMP2 and MMP9 are produced in early stages of kidney morphogenesis but only MMP9 is required for renal organogenesis in vitro. J Cell Biol. 1997;136(6):1363–1373. [PMC free article: PMC2132511] [PubMed: 9087449]
- 110.
- Li Y, Yang J, Dai C. et al. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112(4):503–516. [PMC free article: PMC171389] [PubMed: 12925691]
- 111.
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–1784. [PMC free article: PMC297008] [PubMed: 14679171]
- 112.
- Cheng S, Lovett DH. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol. 2003;162(6):1937–1949. [PMC free article: PMC1868144] [PubMed: 12759250]
- 113.
- Buisson AC, Zahm JM, Polette M. et al. Gelatinase B is involved in the in vitro wound repair of human respiratory epithelium. J Cell Physiol. 1996;166(2):413–426. [PubMed: 8592002]
- 114.
- Legrand C, Gilles C, Zahm JM. et al. Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodeling. J Cell Biol. 1999;146(2):517–529. [PMC free article: PMC3206576] [PubMed: 10427102]
- 115.
- Buisson AC, Gilles C, Polette M. et al. Wound repair-induced expression of a stromelysins is associated with the acquisition of a mesenchymal phenotype in human respiratory epithelial cells. Lab Invest. 1996;74(3):658–669. [PubMed: 8600317]
- 116.
- Markwald R, Eisenberg C, Eisenberg L. et al. Epithelial-mesenchymal transformations in early avian heart development. Acta Anat. 1996;156(3):173–186. [PubMed: 9124035]
- 117.
- Alexander SM, Jackson KJ, Bushnell KM. et al. Spatial and temporal expression of the 72-kDa type IV collagenase (MMP-2) correlates with development and differentiation of valves in the embryonic avian heart. Dev Dyn. 1997;209(3):261–268. [PubMed: 9215641]
- 118.
- Cai DH, Vollberg Sr. TM, Hahn-Dantona E. et al. MMP-2 expression during early avian cardiac and neural crest morphogenesis. Anat Rec. 2000;259(2):168–179. [PubMed: 10820319]
- 119.
- Duong TD, Erickson CA. MMP-2 plays an essential role in producing epithelial-mesenchymal transformations in the avian embryo. Dev Dyn. 2004;229(1):42–53. [PubMed: 14699576]
- 120.
- Song W, Jackson K, McGuire PG. Degradation of type IV collagen by matrix metalloproteinases is an important step in the epithelial-mesenchymal transformation of the endocardial cushions. Dev Biol. 2000;227(2):606–617. [PubMed: 11071778]
- 121.
- Macias D, Perez-Pomares JM, Garcia-Garrido L. et al. Immunoreactivity of the ets-1 transcription factor correlates with areas of epithelial-mesenchymal transition in the developing avian heart. Anat Embryol (Berl). 1998;198(4):307–315. [PubMed: 9764544]
- 122.
- Carmona R, Gonzalez-Iriarte M, Macias D. et al. Immunolocalization of the transcription factor Slug in the developing avian heart. Anat Embryol (Berl). 2000;201(2):103–109. [PubMed: 10672362]
- 123.
- Vicovac L, Aplin JD. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat. 1996;156(3):202–216. [PubMed: 9124037]
- 124.
- Nawrocki BPM, Maquoi E, Birembaut P. Expression of matrix metalloproteinases and their inhibitors during human placental development. Trophoblast Research. 1997;10:97–113.
- 125.
- Nawrocki B, Polette M, Marchand V. et al. Membrane-type matrix metalloproteinase-1 expression at the site of human placentation. Placenta. 1996;17(8):565–572. [PubMed: 8916204]
- 126.
- Polette M, Nawrocki B, Pintiaux A. et al. Expression of gelatinases A and B and their tissue inhibitors by cells of early and term human placenta and gestational endometrium. Lab Invest. 1994;71(6):838–846. [PubMed: 7807965]
- 127.
- Librach CL, Werb Z, Fitzgerald ML. et al. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113(2):437–449. [PMC free article: PMC2288933] [PubMed: 1849141]
- 128.
- del BarrioMG, Nieto MA. Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development. 2002;129(7):1583–1593. [PubMed: 11923196]
- 129.
- Fafeur V, Tulasne D, Queva C. et al. The ETS1 transcription factor is expressed during epithelial-mesenchymal transitions in the chick embryo and is activated in scatter factor-stimulated MDCK epithelial cells. Cell Growth Differ. 1997;8(6):655–665. [PubMed: 9185999]
- 130.
- Giambernardi TA, Sakaguchi AY, Gluhak J. et al. Neutrophil collagenase (MMP-8) is expressed during early development in neural crest cells as well as in adult melanoma cells. Matrix Biol. 2001;20(8):577–587. [PubMed: 11731274]
- 131.
- Cai DH, Brauer PR. Synthetic matrix metalloproteinase inhibitor decreases early cardiac neural crest migration in chicken embryos. Dev Dyn. 2002;224(4):441–449. [PubMed: 12203736]
- 132.
- Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumour invasion. Faseb J May. 1999;13(8):781–792. [PubMed: 10224222]
- 133.
- Singh S, Barrett J, Sakata K. et al. ETS proteins and MMPs: partners in invasion and metastasis. Curr Drug Targets. 2002;3(5):359–367. [PubMed: 12182227]
- 134.
- Brabletz T, Jung A, Dag S. et al. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155(4):1033–1038. [PMC free article: PMC1867011] [PubMed: 10514384]
- 135.
- Crawford HC, Fingleton BM, Rudolph-Owen LA. et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumours. Oncogene. 1999;18(18):2883–2891. [PubMed: 10362259]
- 136.
- Crawford HC, Fingleton B, Gustavson MD. et al. The PEA3 subfamily of Ets transcription factors synergizes with beta-catenin-LEF-1 to activate matrilysin transcription in intestinal tumours. Mol Cell Biol. 2001;21(4):1370–1383. [PMC free article: PMC99589] [PubMed: 11158322]
- 137.
- Takahashi M, Tsunoda T, Seiki M. et al. Identification of membrane-type matrix metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in human colorectal cancers. Oncogene. 2002;21(38):5861–5867. [PubMed: 12185585]
- 138.
- Marchenko GN, Marchenko ND, Leng J. et al. Promoter characterization of the novel human matrix metalloproteinase-26 gene: regulation by the T-cell factor-4 implies specific expression of the gene in cancer cells of epithelial origin. Biochem J. 2002;363(Pt 2):253–262. [PMC free article: PMC1222473] [PubMed: 11931652]
- 139.
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. [PubMed: 11923519]
- 140.
- Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2(9):657–672. [PubMed: 12209155]
- Matrix Metalloproteases and Epithelial-to-Mesenchymal Transition: Implications f...Matrix Metalloproteases and Epithelial-to-Mesenchymal Transition: Implications for Carcinoma Metastasis - Madame Curie Bioscience Database
- Possible Roles of DNA Supercoiling in Transcription - Madame Curie Bioscience Da...Possible Roles of DNA Supercoiling in Transcription - Madame Curie Bioscience Database
- Proteases and Their Cognate Inhibitors of the Serine and Metalloprotease Subclas...Proteases and Their Cognate Inhibitors of the Serine and Metalloprotease Subclasses, in Testicular Physiology - Madame Curie Bioscience Database
- Roles of Poly(ADP-Ribose) Metabolism in the Regulation of Centrosome Duplication...Roles of Poly(ADP-Ribose) Metabolism in the Regulation of Centrosome Duplication and in the Maintenance of Neuronal Integrity - Madame Curie Bioscience Database
- Enzyme-RNA Substrate Recognition in RNA-Modifying Enzymes - Madame Curie Bioscie...Enzyme-RNA Substrate Recognition in RNA-Modifying Enzymes - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...