NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The generation of superoxide is an unavoidable consequence of aerobic metabolism and all aerobes require methods to detoxify it for survival. The superoxide dismutase (SOD) enzymes are the most common way aerobes detoxify superoxide. In humans, a number of pathologies involve the overproduction of superoxide through inflammatory pathways. A large number of animal models of disease have shown that genetically engineered mice, which lack SODs are more sensitive and those that over express SODs are resistant. These findings have spurred research to develop small antioxidant compounds with SOD activity as therapeutic agents. This review focuses on the recent development of cationic metallo¬porphyrins as catalytic antioxidants with potent SOD activity and on their utility in animal models of human diseases.
Introduction
The Normal Biology of O2-
It was apparent, very soon after the demonstration that xanthine oxidase produces O2 and the discovery of superoxide dismutase,1,2 that: O2- production must be a commonplace event in aerobic biology; and that SOD must provide a needed defense. The ubiquity of SOD in aerobes and its paucity in anaerobes3 lent support to this view, as did the induction of SOD by exposure to O2 and the protection against hyperoxia provided by elevated levels of SOD4. Although originally seen in bacteria, similar observations in small rodents were soon forthcoming.5,6 The oxygen-dependent phenotypic deficits imposed by mutational deletion of SOD in Escherichia coli ultimately proved this point.7 Similar results were obtained with yeast,8,9Drosophila,10 a blue green alga,11 Neurospora crassa12 and ultimately with mice.13
At present, several families of SODs are known. SODs are highly compartmentalized in cells and tissues. Thus, there is the CuZnSOD found in eukaryotic cytosols,2 chloroplasts,14 the periplasm of gram-negative bacteria,15 and the intermembrane space of mitochondria.16 There is the MnSOD found in the cytoplasm of bacteria,17 and in the matrix of mitochondria.18,19 There is a FeSOD found in bacteria20 and in plants21 and there is a NiSOD in fungi.22 All the aforementioned SODs are intracellular enzymes but there are also SODs made specifically for export and they are referred to as extracellular superoxide dismutases or ECSODs. The mammalian ECSOD is a tetrameric, glycosylated CuZn SOD.23 Other ECSODs include those found in plant sap24 and nectar,25 and on the outer surface of bacteria.26 Presumably all SODs serve to scavenge O2- in these various compartments, thus protect vulnerable targets from this radical.
In spite of its toxicity, O2- is sometimes made on purpose to serve some physiological goal. Thus the respiratory burst of phagocytic leukocytes is due to the activation of a membrane-bound NADPH oxidase that produces O2-.27 Genetic defects in this NADPH oxidase, or in the components of the pathway causing its activation, cause the failure of the respiratory burst and a hypersensitivity to infection. The associated genetic disease is called chronic granulomatous disease.28 Its characteristics establish the NADPH oxidase and O2- production as part of the antimicrobial armamentarium of neutrophils. Another evolving area is the role of ROS as cell signaling agents involved in cell growth and differentiation pathways.29,30 However, most of the limited data to date implicates hydrogen peroxide as the main ROS signaling molecule.
O2- in Pathologies and the Use of Superoxide Dismutases as Therapeutics
The production of large amounts of O2- by activated neutrophils, and the infiltration of sites of inflammation by neutrophils, led to the suspicion that O2- might play a role in the tissue damage associated with inflammation. This led to the first attempts to use a SOD as an anti-inflammatory therapeutic agent.31 The short half life of CuZn SOD injected into circulation led to the covalent coupling of polyethylene glycol onto the enzyme in order to increase its molecular weight and thus to slow its removal from circulation by the kidneys and at the same time to decrease its antigenicity.32
A second area of O2- mediated pathology in which SODs were used as therapeutics was in conditions of direct excess O2- production, i.e., pulmonary oxygen toxicity and radiation induced injury. The known production of O2- by radiolysis of aerobic aqueous solutions led to the use of SOD as a radioprotectant with positive results.33-35 In pulmonary oxygen toxicity the initial trials using aerosolization of large quantities of CuZn SOD failed to protect against severe lung injury.36 The use of the SOD protein as a therapeutic was limited by its size, charge and rapid clearance. Subsequent trials of aerosolized MnSOD in a primate model of pulmonary oxygen toxicity were successful,37 likely due to the net positive charge of MnSOD allowing it to better distribute and bind to the lung extracellular matrix.
The traditional view of the damage that followed temporary interruption of blood flow to a tissue was the harm due to ischemia; hence the term ischemic injury. The proposal that ischemic injury could be due to events occurring during reperfusion that could include oxygen-derived free radicals38 was followed by data demonstrating the reality of this view;38-45 hence the term reperfusion injury.
Small Molecules Are Better
The efficacy of the SOD enzymes in treating models of inflammation and of reperfusion injury suggested that small molecule mimetics of SOD activity should be sought. Thus if the ultimate goal is pharmaceutical use, then small molecules would: be easier to produce in large amounts than the enzymes; be more likely to enter cells; avoid the problem of antigenicity; and might be capable of oral administration. Since SODs based upon Cu (II), Fe (III), Ni (III) and Mn (III) were known, complexes of those metals had to be considered. However free Cu, Ni or Fe are highly toxic and may create a problem if the mimetics were metabolized. The choice quickly narrowed to Mn since it is better tolerated in vivo and does not participate in Fenton chemistry.46 Free Mn(III) is a strong oxidant and can oxidize NADPH47 or diketogulonate.48 Chelation of Mn(III) by pyrophosphate decreases its oxidative activity,48 presumably nucleoside diphosphates would also stabilize Mn(III). There was another reason for selecting Mn. While studying Lactobacillus plantarum it was noted that this bacterium was devoid of SOD yet could tolerate aerobic conditions. This was a puzzle until it was found that this organism, which normally ferments Mn-rich plant materials, concentrates Mn from the medium and maintains intracellular [Mn] at 25 mM. This Mn (II) complexed to α-hydroxy acid metabolites can catalyze the dismutation of O2- and serve as a functional replacement for SOD.49-51
Good metal ligands abound in all cells; hence the Mn complexes wanted would have to be very stable to avoid ligand exchange and loss of activity (Table 1). Stability to excess EDTA was chosen as a simple screen for stability. Another important criterion in the selection of an optimal small molecular antioxidant was the redox potential at the metal center. Thus all the natural SODs exhibit redox potentials close to +300mV.52,53 This makes perfect sense for enzymes that operate at close to the diffusion limit; since it is halfway between the redox potentials of the two half reactions of the catalytic cycle. Thus:
Table 1
Comparision of the antioxidant properties of metalloporphyrins.

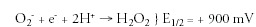
A metal-centered redox potential of ˜300mV provides equal thermodynamic driving energy to both half reactions and avoids one or the other from becoming rate-limiting.
Initially the Mn complexes of the polyhydoxamate ligands desferrioxamine B and E were examined.54,55 Although only modestly active as SOD-mimetics these complexes highlighted the importance of the entropy loss that accompanied metal complexation by linear chelating agents. Thus the Mn (III) complex with the linear desferrioxamine B was not stable to excess EDTA but the complex with the cyclic desferrioxamine E was. Henceforth our search for an optimal SOD mimetic would consider only macrocyclic ligands.
Biochemical Characterization of Meso-Substituted Metalloporphyrins
Porphyrins substituted on the methine bridge carbons (meso-positions) were selected because they would be refractory to ring opening by Heme oxygenase. Porphyrins also have very high affinities for ligated metals. Water-soluble compounds were sought to aid in testing their efficacy in biological systems. Two early prototypes developed were the anionic porphyrin Mn (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP, AEOL-10201) and the cationic porphyrin Mn(III) tetrakis (N-methylpyridinium-4-yl) porphyrin (MnTM-4-PyP, AEOL-10112). Both of these compounds allowed for aerobic growth of SOD-null E. coli.56 Although the initial goal was to develop SOD mimetics, it became clear that these early compounds could perform not only 1 electron dismutation reactions but also 2 electron dismutation reactions and thus possess modest catalase-like activities.57 It was also shown that these compounds could react rapidly with peroxynitrite, the reaction product of nitric oxide and superoxide, a powerful oxidant in biological systems.58-60
Even though these compounds could scavenge reactive oxygen species other than superoxide, development of them focused on enhancing their SOD activities and eventually focused on the cationic porphyrins because they would add the benefit of electrostatic facilitation in their interaction with the anionic O2-. The MnTM-4-PyP yielded a very stable Mn (III) complex of reasonable catalytic activity and a redox potential of + 60 mV.56 Yet the ability of these complexes to overcome the phenotypic deficits of SOD-null E. coli was better than could be accounted for by their in vitro activity. This was explained by the rapid reduction of these complexes by abundant reductants such as GSH and NADPH and by the very high rate constant for their reoxidation by O2-. Hence we surmised that these early prototypic metalloporphyrins were acting within E. coli, not as SODs but rather as superoxide reductases (SORs). Which is to say that they catalyzed the reduction of O2- by cellular reductants. Further study of the Mn (III) complex of the porphyrin with para N-methyl pyridyl substitution on the mesopositions revealed that it protected the SOD-null E. coli against the lethality of aerobic heat shock61 and against aerobic heating and stationary phase death.62 Moreover it inhibited the uptake of paraquat.63
Since the redox potential of the para N-methyl pyridyl compound was only + 60 mV we sought to shift it to higher values by bromination64 and chlorination.65 This was effective in elevating the E1/2 and the catalytic activity. Thus the octabromo compound had E1/2 = + 480 mV vs NHE, and it dismuted O2- with a rate constant of 2.2 x 108M-1s-1 at 22°C. Unfortunately shifting the E1/2 so high somewhat decreased the stability of the complexes.
An improvement was achieved by shifting the para N-methyl group to the ortho position (AEOL-10113).66 This was done for several reasons. The para compound, being planar could intercalate into DNA and was seen to lose its activity in the presence of excess DNA. The ortho N-methyl pyridyl groups, in contrast, had to be axial to the plane of the porphyrin ring, preventing this interaction with DNA. In addition, bringing the cationic quaternary nitrogens closer to the central metal raised E1/2 to +220mV and increased the catalytic rate constant to 6 x 107M-1s-1. This compound offered promise of utility in vivo.
There was still one fly in this ointment. With the steric barrier to rotation of the ortho N-alkyl pyridyl groups, and their consequent axial orientation, comes the existence of positional isomers or atropoisomers. Thus if above the plane of the porphyrin ring is denoted as A and below the plane as B, there are four atropoisomers i.e., AAAA, AAAB, AABB, and ABAB. As synthesized, the compound is a mixture of these isomers. These were separated by HPLC and the individual isomers characterized. All were found to have similar activity, but preparation of any one of the pure isomers on a large scale would be difficult. Replacing the ortho N-ethyl pyridyl groups by N, N' diethyl imidazolyl groups (AEOL-10150) obviated this problem. This compound also has the cationic quaternary nitrogens ortho to the point of attachment to the meso-positions, and the attached groups are axial to the plane of the porphyrin, but the symmetry of the attached groups eliminates the possibility of positional isomers. This compound exhibits E1/2 and catalytic activity comparable to that of the ortho N-alkyl pyridyl compound. These SOD mimetics show promising activity in several models of human pathology as will be discussed below.
Screening for Biological Activity: A Multi-Tier Approach
An early issue that arose with the development of metalloporphyrin SOD mimetics was the apparent disparity between the compound's biochemical SOD activities and biological efficacies. Early screening methods to detect biological activity involved the use of mutant E. coli lacking MnSOD (SodA) and FeSOD (SodB) that have oxygen-dependent nutritional auxotrophies that can be overcome by complementation with functional SOD genes. A number of compounds with rather modest SOD biochemical activities were found to be efficacious in this model. In fact, MnTBAP, which has only modest SOD biochemical activity, has been shown to be efficacious in an extensive number of models of oxidative stress (see review ref. 67). A possible explanation discussed earlier is that these compounds could act as superoxide reductases (SORs). However, the compounds may also be more potent in vivo by scavenging a variety of reactive oxygen species. Numerous reports in the literature show that the overexpression of both SOD and catalase or glutathione peroxidase produces better protection than either alone. It is still open to debate whether it is better to have a compound that is a selective scavenger of a particular reactive oxygen species or to have a broad spectrum of scavenging activities and how this relates to a compound's potency in models of oxidative stress. Given MnTBAP's low SOD activity, the possibility remains that it may protect for reasons unrelated to scavenging of O2-.
To partially address these issues, compounds were screened in a lipid peroxidation assay that utilizes a rat brain homogenate and iron plus ascorbate.68 The use of this type of assay in addition to biochemical screening for SOD and catalase activities provides further insight to the possible mechanisms and biological potency not always apparent from the standard assays. In general it was found that the vast majority of compounds that have high SOD activities are also the most potent inhibitors of lipid peroxidation. However, a few compounds were found that had little SOD activity while being very potent inhibitors of lipid peroxidation. Such a compound has also been shown to be efficacious in a rat model of colitis.69 From the multi-tier screening, the N-alkyl pyridinium and N, N'-alkyl imidazolium series of manganic porphyrins emerged as lead candidates for further testing. Although compounds need to be potent and efficacious, they also need to meet a number of other important characteristics to be viable drug candidates. Some of these properties include issues related to safety, pharmacodynamics, purity, and the capability to scale up bulk synthesis.
Toxicology
The ligated metal makes a large difference in the acute toxicity of metalloporphyrins. This was evident from toxicology studies with TBAP, where the LD50 in mice was 100 mg/kg, ip for the manganese complex70 and only 30 mg/kg, ip for the iron complex (BJ Day, unpublished data). It is still not clear what accounts for this difference in toxicity since the compounds are largely excreted without loss of the metal. The manganic cationic porphyrin also had a high LD50 in mice ranging from 90 mg/kg, ip, for MnTE-2-PyP (AEOL-10113) to 300 mg/kg, ip, for MnTDM-1,3-ImP (AEOL-10150). A peculiar species-specific toxicity associated with cationic metalloporphyrins is related to their vasodilator activity in rats.71 The most potent vasodilator compounds are the N-substituted pyridinium manganic porphyrins. The N-substituted imidazolium manganic porphyrins are an order of magnitude less potent rat vasodilators. It is interesting that none of the other manganic porphyrins have been found to produce this effect in rats and that this effect is restricted to the rat and not seen in other species such as the mouse, rabbit, dog or baboon. Another toxicity of the cationic porphyrins, AEOL-10113 and AEOL-10150, was noted in i.c.v. dosed rats and mice and involved an ataxia. AEOL-10150 was found to be 15 times less potent at producing ataxia than AEOL-10113. Two-week toxicology in mice with constant infusion of AEOL-10113 (40 mg/kg/day) only revealed small elevations in serum enzymes and sporadic focal periportal lesions in the liver (BJ Day, unpublished data). Overall, the manganese containing porphyrins tested to date have been well tolerated by mice at doses up to 15 mg/kg/day.
Another potential area for toxicity with catalytic antioxidants is their ability to interfere with ROS and RNS cell signaling pathways. Although there is no data to date to suggest this is a problem with any of the 3 classes of metal-containing SOD mimetics, it may still be a possibility especially at high doses. The fact that O2- can modulate NO responses and that O2- is a specific target of many of these classes of catalytic antioxidant suggests they could modulate NO-mediated cell signaling responses. Many of the dose response curves with catalytic antioxidants exhibit biphasic patterns suggesting that at higher doses the beneficial effects are lost and this may be due to over suppression of ROS/RNS. This is reminiscent of early studies using SOD enzymes as therapeutics in a heart model of ischemia-reperfusion.72,73 Further studies need to be done to validate this hypothesis.
Pharmacodynamics
Water-soluble meso-substituted manganese porphyrins readily cross cell membranes and can distribute into the mitochondria.74 Most metalloporphyrins are greater than 800 in molecular weight, which suggest they cross membranes by yet undefined transport system(s). The plasma half-lives in mice for these compounds range from about 10 hours for MnTBAP70 to as little as 20 minutes for AEOL-10150. Tissue half-lives of metalloporphyrins are dramatically longer than their plasma half-lives and the most of these compounds accumulate in the liver and kidneys. Tissue penetration and half-life can also change with disease state as was seen with AEOL-10150 in a mouse model of stroke. AEOL-10150 normally has poor blood brain permeability but had increased penetration of the blood brain barrier after a focal ischemic event. Brain levels of AEOL-10150 were doubled in the ischemic hemisphere compared to the nonischemic hemisphere.75
Pharmacology
Early work with MnTBAP and MnTM-4-PyP has demonstrated the potential efficacy of metalloporphyrins in mammalian models of oxidative stress that involve the generation of superoxide,76-78 hydrogen peroxide57,79 and peroxynitrite.58,60 Since the publication of these papers and due to the commercial availability of these metalloporphyrins, a number of research groups have utilized them in a variety of in vitro and in vivo models of oxidative stress. In fact, over 100 articles employing mostly MnTBAP have appeared in PubMed since 1995 involving models of apoptosis, neurodegeneration, sepsis, vascular reactivity, fibrosis and diabetes (Table 2). The primary focus of this review is on the newer generation of cationic manganese porphyrins containing meso-substituted pyridinium or imidazolium groups. These compounds have been most extensively investigated in models of cerebral ischemia-reperfusion.
Table 2
Published studies showing efficacy of metalloporphyrin antioxidant mimetics.
Ischemic Brain Injury (Stroke)
Cerebral ischemia produces a sustained increase in the formation of superoxide80 that can persist as long as 3-4 days.81 Early studies with antioxidants in models of stroke were inconclusive. Interest in developing catalytic antioxidants with SOD activity was rekindled by the findings that modulation of endogenous SODs produced profound effects on the outcomes of animal models of stroke. Deletion or deficiencies of any of the three endogenous SODs dramatically worsened the outcome82-84 and overexpression of these SODs85-87 improved the outcome from focal cerebral ischemia. These studies suggested that superoxide is an important reactive oxygen species in stroke and suggests a mechanism of action for pharmacologic agents and a therapeutic window of potential clinical relevance.
The N-ethyl substituted pyridinium-2-yl manganese porphyrin (AEOL-10113) was initially evaluated in a standard rat model of focal ischemia.88 Pretreatment of AEOL-10113, delivered by i.c.v. injection, attenuated focal ischemia subcortical and cortical infarct volumes and improved neurologic outcome in these rats. AEOL-10113 was also found to rescue tissues and neurological function up to 6 hours post-ischemia without affecting body temperature. These protective effects correlated with decreased indices of oxidative damage as measured by reduction in cortical DNA and protein oxidation.88 Intravenous (i.v.) therapy could not be evaluated in rats due to systemic blood pressure effects of AEOL-10113 restricted to this species. Therefore, a mouse model of focal ischemia was used to evaluate i.v. efficacy of AEOL-10113.88 AEOL-10113 was found to attenuate focal ischemia infarct volumes and improve neurological outcome in mice given bolus i.v. doses shortly after reperfusion. Given the poor blood brain permeability of AEOL-10113, these data suggest that the ischemic events change the blood brain permeability and open a window for systemic AEOL-10113 therapy. However, there were significant drug development issues (related to its nonsymmetrical structure and the presence of multiple atropoisomers of the compound) with AEOL-10113 hampered its development as a therapeutic agent (Fig. 1). In response to this N,N-diethyl imidazolyl substituted manganese porphyrin (AEOL-10150), which has a better safety and chemistry profile, was assessed for efficacy in stroke.
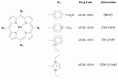
Figure 1
Structures of metalloporphyrins.
The main advantages of AEOL-10150 over AEOL-10113 are that AEOL-10150 does not exist as a positional isomer, is relatively easy to synthesize, is less toxic, and can be readily analyzed in tissues.89 AEOL-10150 was compared to AEOL-10113 in a standard rat model of focal ischemia.75 AEOL-10113 was found to produce neurotoxicity at twice its neuroprotective dose while AEOL-10150 required a 15-fold increase from its neuroprotective dose before any neurotoxicity was observed. Both compounds similarly attenuated cortical infarct volumes and improved neurologic outcome in these rats. AEOL-10150 also proved effective in reducing infarct volume 6 hours after MCAO. The brain tissue half-life for AEOL-10150 (300 ng, i.c.v.) was 10 hours. A mouse model of focal ischemia was used to evaluate i.v. efficacy of AEOL-10150.75 AEOL-10150 was found to attenuate focal ischemia infarct volumes (25%) and improved neurological outcome in mice. These data suggest that AEOL-10150 has similar potency to AEOL-10113 but with improved safety and an easier formulation as a neuroprotective agent in the treatment of stroke.
Based on the success of the cationic manganese porphyrins containing meso-substituted pyridinium or imidazolium groups in cerebral ischemia-reperfusion, they have been recently tested in a wide variety of other animal models having oxidative stress as a part of the pathogenesis. These compounds have been shown to protect lung from acute and chronic radiation-induced injury,90 protect airways from tobacco smoke-induced metaplasia,91 reduce airway inflammation in models of asthma,92 protect the premature neonatal lung from the formation of bronchopulmonary dysplasia in response to hyperoxia,93 and protect pancreatic islet cells from streptozotocin or autoimmune attack (type I diabetes).94 This class of antioxidant mimetics thus has the potential to become an effective new therapeutic in a number of disease states.
Acknowledgements
Drs. Crapo, Day and Fridovich founded Aeolus Pharmaceuticals, Inc., to develop substituted metalloporphyrins as antioxidant therapies. Dr. Crapo is Chief Executive Officer of Aelous Pharmaceuticals and Dr.s Day and Fridovich are consultants and hold equity in Aelous Pharmaceuticals.
References
- 1.
- McCord JM, Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968;243(21):5753–60. [PubMed: 4972775]
- 2.
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244(22):6049–55. [PubMed: 5389100]
- 3.
- McCord JM, Keele JrBB, Fridovich I. An enzyme-based theory of obligate anaerobiosis: The physiological function of superoxide dismutase. Proc Natl Acad Sci USA. 1971;68(5):1024–7. [PMC free article: PMC389105] [PubMed: 4995818]
- 4.
- Gregory EM, Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973;114(2):543–8. [PMC free article: PMC251807] [PubMed: 4196244]
- 5.
- Crapo JD, Tierney DF. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol. 1974;226(6):1401–7. [PubMed: 4833996]
- 6.
- Frank L, Yam J, Roberts RJ. The role of endotoxin in protection of adult rats from oxygen-induced lung toxicity. J Clin Invest. 1978;61(2):269–75. [PMC free article: PMC372536] [PubMed: 621274]
- 7.
- Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: Is superoxide dismutase necessary for aerobic life? EMBO J. 1986;5(3):623–30. [PMC free article: PMC1166808] [PubMed: 3011417]
- 8.
- Bilinski T, Krawiec Z, Liczmanski A. et al. Is hydroxyl radical generated by the fenton reaction in vivo? Biochem Biophys Res Commun. 1985;130(2):533–39. [PubMed: 2992473]
- 9.
- van LoonAP, Pesold-Hurt B, Schatz G. A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci USA. 1986;83(11):3820–4. [PMC free article: PMC323615] [PubMed: 3520557]
- 10.
- Campbell SD, Hilliker AJ, Phillips JP. Cytogenetic analysis of the cSOD microregion in Drosophila melanogaster. Genetics. 1986;112(2):205–15. [PMC free article: PMC1202696] [PubMed: 11933981]
- 11.
- Herbert SK, Samson G, Fork DC. et al. Characterization of damage to photosystems I and II in a cyanobacterium lacking detectable iron superoxide dismutase activity. Proc Natl Acad Sci USA. 1992;89(18):8716–20. [PMC free article: PMC49991] [PubMed: 1528884]
- 12.
- Chary P, Dillon D, Schroeder AL. et al. Superoxide dismutase (sod-1) null mutants of neurospora crassa: Oxidative stress sensitivity, spontaneous mutation rate and response to mutagens. Genetics. 1994;137(3):723–30. [PMC free article: PMC1206032] [PubMed: 8088518]
- 13.
- Li Y, Huang TT, Carlson EJ. et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11(4):376–81. [PubMed: 7493016]
- 14.
- Elstner EF. Oxygen activation and superoxide dismutase in chloroplasts. Encycl Plant Physiol New Ser. 1979;6:410–15.
- 15.
- Benov LT, Fridovich I. Escherichia coli expresses a copper- and zinc-containing superoxide dismutase. J Biol Chem. 1994;269(41):25310–14. [PubMed: 7929223]
- 16.
- Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem. 2001;276(42):38388–93. [PubMed: 11507097]
- 17.
- Keele JrBB, McCord JM, Fridovich I. Superoxide dismutase from Escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970;245(22):6176–81. [PubMed: 4921969]
- 18.
- Weisiger RA, Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973;248(10):3582–92. [PubMed: 4702877]
- 19.
- Weisiger RA, Fridovich I. Mitochondrial superoxide dismutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973;248(13):4793–6. [PubMed: 4578091]
- 20.
- Yost JrFJ, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973;248(14):4905–8. [PubMed: 4352182]
- 21.
- Salin ML, Bridges SM. Isolation and characterization of an iron-containing superoxide dismutase from a eucaryote, Brassica campestris. Arch Biochem Biophys. 1980;201(2):369–74. [PubMed: 7396513]
- 22.
- Youn HD, Youn H, Lee JW. et al. Unique isozymes of superoxide dismutase in Streptomyces griseus. Arch Biochem Biophys. 1996;334(2):341–8. [PubMed: 8900409]
- 23.
- Marklund SL, Holme E, Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982;126(1):41–51. [PubMed: 7172448]
- 24.
- McEwen AR, Hill HAO. Superoxide, hydrogen, peroxide, and the gelling of phloem sap from cucurbita pepo. Planta. 1982;154:295–97. [PubMed: 24276155]
- 25.
- Carter C, Thornburg RW. Tobacco nectarin I. Purification and characterization as a germin-like, manganese superoxide dismutase implicated in the defense of floral reproductive tissues. J Biol Chem. 2000;275(47):36726–33. [PubMed: 10952990]
- 26.
- Beaman BL, Scates SM, Moring SE. et al. Purification and properties of a unique superoxide dismutase from Nocardia asteroides. J Biol Chem. 1983;258(1):91–96. [PubMed: 6336758]
- 27.
- Babior BM, Curnutte JT, McMurrich BJ. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976;58(4):989–96. [PMC free article: PMC333263] [PubMed: 9426]
- 28.
- Curnutte JT, Whitten DM, Babior BM. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974;290(11):593–7. [PubMed: 4359964]
- 29.
- Burdon RH. Control of cell proliferation by reactive oxygen species. Biochem Soc Trans. 1996;24:1028–32. [PubMed: 8968506]
- 30.
- Timblin CR, Janssen YW, Mossman BT. Transcriptional activation of the proto-oncogene c-jun by asbestos and H2O2 is directly related to increased proliferation and transformation of tracheal epithelial cells. Cancer Res. 1995;55:2723–26. [PubMed: 7796393]
- 31.
- McCord JM. Free radicals and inflammation: Protection of synovial fluid by superoxide dismutase. Science. 1974;185(150):529–31. [PubMed: 4841157]
- 32.
- Hershfield MS, Chaffee S, Koro-Johnson L. et al. Use of site-directed mutagenesis to enhance the epitope-shielding effect of covalent modification of proteins with polyethylene glycol. Proc Natl Acad Sci USA. 1991;88(16):7185–9. [PMC free article: PMC52258] [PubMed: 1714590]
- 33.
- Petkau A, Chelack WS, Pleskach SD. Protection of post-irradiated mice by superoxide dismutase. Int J Radiat Biol. 1976;29(3):297–99. [PubMed: 1083851]
- 34.
- Malaker K, Das RM. Effect of superoxide dismutase on early radiation injury of lungs in the rat. Mol Cell Biochem. 1988;84(2):141–5. [PubMed: 3231220]
- 35.
- St.Clair DK, Wan XS, Oberley TD. et al. Suppression of radiation-induced neoplastic transformation by overexpression of mitochondrial superoxide dismutase. Mol Carcinog. 1992;6(4):238–42. [PubMed: 1485915]
- 36.
- Crapo JD, DeLong DM, Sjostrom K. et al. The failure of aerosolized superoxide dismutase to modify pulmonary oxygen toxicity. Am Rev Respir Dis. 1977;115(6):1027–33. [PubMed: 262097]
- 37.
- Welty-Wolf KE, Simonson SG, Huang YC. et al. Aerosolized manganese SOD decreases hyperoxic pulmonary injury in primates. II. Morphometric analysis. J Appl Physiol. 1997;83(2):559–68. [PubMed: 9262453]
- 38.
- Fridovich I. Hypoxia and oxygen toxicity. Adv Neurol. 1979;26:255–9. [PubMed: 517297]
- 39.
- Granger DN, Rutili G, McCord JM. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81(1):22–9. [PubMed: 6263743]
- 40.
- Manson PN, Anthenelli RM, Im MJ. et al. The role of oxygen-free radicals in ischemic tissue injury in island skin flaps. Ann Surg. 1983;198(1):87–90. [PMC free article: PMC1352938] [PubMed: 6859996]
- 41.
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74(4):1156–64. [PMC free article: PMC425281] [PubMed: 6434591]
- 42.
- Werns SW, Shea MJ, Lucchesi BR. Free radicals in ischemic myocardial injury. J Free Radic Biol Med. 1985;1(2):103–10. [PubMed: 3939137]
- 43.
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312(3):159–63. [PubMed: 2981404]
- 44.
- Bulkley GB. Free radical-mediated reperfusion injury: A selective review. Br J Cancer. 1987;8(Suppl):66–73. [PMC free article: PMC2149484] [PubMed: 3307876]
- 45.
- Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263(3):1353–7. [PubMed: 2826476]
- 46.
- Cheton PL, Archibald FS. Manganese complexes and the generation and scavenging of hydroxyl free radicals. Free Radic Biol Med. 1988;5:325–33. [PubMed: 2855733]
- 47.
- McPhail LC, DeChatelet LR, Shirley PS. Further characterization of NADPH oxidase activity of human polymorphonuclear leukocytes. J Clin Invest. 1976;58:775–80. [PMC free article: PMC333238] [PubMed: 965484]
- 48.
- Homann PH. Manganese-catalyzed oxidations of 23-diketogulonate. Biochemistry. 1965;4:1902–11.
- 49.
- Archibald FS, Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981;145(1):442–51. [PMC free article: PMC217292] [PubMed: 6257639]
- 50.
- Archibald FS, Fridovich I. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J Bacteriol. 1981;146(3):928–36. [PMC free article: PMC216946] [PubMed: 6263860]
- 51.
- Archibald FS, Fridovich I. The scavenging of superoxide radical by manganous complexes: In vitro. Arch Biochem Biophys. 1982;214(2):452–63. [PubMed: 6284026]
- 52.
- Vance CK, Miller AF. Novel insights into the basis for Escherichia coli superoxide dismutase's metal ion specificity from Mn-substituted FeSOD and its very high E(m). Biochemistry. 2001;40(43):13079–87. [PubMed: 11669646]
- 53.
- Barrette JrWC, Sawyer DT, Fee JA. et al. Potentiometric titrations and oxidation—reduction potentials of several iron superoxide dismutases. Biochemistry. 1983;22(3):624–27. [PubMed: 6340720]
- 54.
- Darr D, Zarilla KA, Fridovich I. A mimic of superoxide dismutase activity based upon desferrioxamine B and manganese(IV). Arch Biochem Biophys. 1987;258(2):351–5. [PubMed: 2823713]
- 55.
- Faulkner KM, Stevens RD, Fridovich I. Characterization of Mn(III) complexes of linear and cyclic desferrioxamines as mimics of superoxide dismutase activity. Arch Biochem Biophys. 1994;310(2):341–6. [PubMed: 8179317]
- 56.
- Faulkner KM, Liochev SI, Fridovich I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J Biol Chem. 1994;269(38):23471–6. [PubMed: 8089112]
- 57.
- Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys. 1997;347(2):256–62. [PubMed: 9367533]
- 58.
- Szabo C, Day BJ, Salzman AL. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. FEBS Lett. 1996;381(1-2):82–6. [PubMed: 8641445]
- 59.
- Hunt JA, Lee J, Groves JT. Amphiphilic peroxynitrite decomposition catalysts in liposomal assemblies. Chem Biol. 1997;4(11):845–58. [PubMed: 9384531]
- 60.
- Ferrer-Sueta G, Ruiz-Ramirez L, Radi R. Ternary copper complexes and manganese (III) tetrakis(4-benzoic acid) porphyrin catalyze peroxynitrite-dependent nitration of aromatics. Chem Res Toxicol. 1997;10(12):1338–44. [PubMed: 9437523]
- 61.
- Benov L, Fridovich I. Superoxide dismutase protects against aerobic heat shock in Escherichia coli. J Bacteriol. 1995;177(11):3344–46. [PMC free article: PMC177032] [PubMed: 7768839]
- 62.
- Benov L, Fridovich I. A superoxide dismutase mimic protects sodA sodB Escherichia coli against aerobic heating and stationary-phase death. Arch Biochem Biophys. 1995;322(1):291–94. [PubMed: 7574689]
- 63.
- Liochev SI, Fridovich I. A cationic manganic porphyrin inhibits uptake of paraquat by Escherichia coli. Arch Biochem Biophys. 1995;321(1):271–5. [PubMed: 7639531]
- 64.
- Batinic-Haberle I, Liochev SI, Spasojevic I. et al. A potent superoxide dismutase mimic: Manganese beta-octabromo-meso- tetrakis-(N-methylpyridinium-4-yl) porphyrin. Arch Biochem Biophys. 1997;343(2):225–33. [PubMed: 9224734]
- 65.
- Kachadurian R, Batinic-Haberle I, Fridovich I. Syntheses and superoxide dismuting activities of partially(1-4)beta-chlorinated derivatives of manganese(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin. Inorg Chem. 1999;38(2):391–96.
- 66.
- Batinic-Haberle I, Benov L, Spasojevic I. et al. The ortho effect makes manganese(III)-meso-tetrakis-(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J Biol Chem. 1998;273(38):24521–28. [PubMed: 9733746]
- 67.
- Patel M, Day BJ. Metalloporphyrin class of therapeutic catalytic antioxidants. Trends Pharmacol Sci. 1999;20(9):359–64. [PubMed: 10462758]
- 68.
- Day BJ, Batinic-Haberle I, Crapo JD. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic Biol Med. 1999;26(5-6):730–6. [PubMed: 10218663]
- 69.
- Choudhary S, Keshavarzian A, Yong S. et al. Novel antioxidants zolimid and AEOL11201 ameliorate colitis in rats. Dig Dis Sci. 2001;46(10):2222–30. [PubMed: 11680601]
- 70.
- Oury TD, Thakker K, Menache M. et al. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol. 2001;25(2):164–9. [PubMed: 11509325]
- 71.
- Ross AD, Warner DS, Sheng H. et al. Species-specific hemodynamic effects of metalloporphyrin SOD mimetics. Free Radic Biol Med. 2001;31:S97. [PubMed: 12488134]
- 72.
- Omar BA, Gad NM, Jordan MC. et al. Cardioprotection by Cu,Zn-superoxide dismutase is lost at high doses in the reoxygenated heart. Free Radic Biol Med. 1990;9:465–71. [PubMed: 1964145]
- 73.
- Omar BA, McCord JM. The cardioprotective effect of Mn-superoxide dismutase is lost at high doses in the postischemic isolated rabbit heart. Free Radic Biol Med. 1990;9:473–78. [PubMed: 2079227]
- 74.
- Li QY, Pedersen C, Day BJ. et al. Dependence of excitotoxic neurodegeneration on mitochondrial aconitase inactivation. J Neurochem. 2001;78(4):746–55. [PubMed: 11520895]
- 75.
- Sheng H, Enghild J, Bowler R. et al. Effects of metalloporphyrin catalytic antioxidants on murine brain ischemia. Free Radic Biol Med. [PubMed: 12361805]
- 76.
- Day BJ, Shawen S, Liochev SI. et al. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced endothelial cell injury, in vitro. J Pharmacol Exp Ther. 1995;275(3):1227–32. [PubMed: 8531085]
- 77.
- Patel M, Day BJ, Crapo JD. et al. Requirement for superoxide in excitotoxic cell death. Neuron. 1996;16(2):345–55. [PubMed: 8789949]
- 78.
- Day BJ, Crapo JD. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced lung injury in vivo. Toxicol Appl Pharmacol. 1996;140(1):94–100. [PubMed: 8806874]
- 79.
- Milano J, Day BJ. A catalytic antioxidant metalloporphyrin blocks hydrogen peroxide- induced mitochondrial DNA damage. Nucleic Acids Res. 2000;28(4):968–73. [PMC free article: PMC102572] [PubMed: 10648790]
- 80.
- Fabian RH, DeWitt DS, Kent TA. In vivo detection of superoxide anion production by the brain using a cytochrome c electrode. J Cereb Blood Flow Metab. 1995;15(2):242–7. [PubMed: 7860658]
- 81.
- Yamaguchi S, Ogata H, Hamaguchi S. et al. Superoxide radical generation and histopathological changes in hippocampal CA1 after ischaemia/reperfusion in gerbils. Can J Anaesth. 1998;45(3):226–32. [PubMed: 9579260]
- 82.
- Kondo T, Reaume AG, Huang TT. et al. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17(11):4180–9. [PMC free article: PMC6573543] [PubMed: 9151735]
- 83.
- Murakami K, Kondo T, Kawase M. et al. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18(1):205–13. [PMC free article: PMC6793388] [PubMed: 9412501]
- 84.
- Sheng H, Brady TC, Pearlstein RD. et al. Extracellular superoxide dismutase deficiency worsens outcome from focal cerebral ischemia in the mouse. Neurosci Lett. 1999;267(1):13–6. [PubMed: 10400237]
- 85.
- Kinouchi H, Epstein CJ, Mizui T. et al. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA. 1991;88(24):11158–62. [PMC free article: PMC53093] [PubMed: 1763030]
- 86.
- Keller JN, Kindy MS, Holtsberg FW. et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: Suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18(2):687–97. [PMC free article: PMC6792529] [PubMed: 9425011]
- 87.
- Sheng H, Bart RD, Oury TD. et al. Mice overexpressing extracellular superoxide dismutase have increased resistance to focal cerebral ischemia. Neuroscience. 1999;88(1):185–91. [PubMed: 10051199]
- 88.
- Mackensen GB, Patel M, Sheng H. et al. Neuroprotection from delayed postischemic administration of a metalloporphyrin catalytic antioxidant. J Neurosci. 2001;21(13):4582–92. [PMC free article: PMC6762378] [PubMed: 11425886]
- 89.
- Kachadourian R, Menzeleev R, Agha B. et al. High-performance liquid chromatography with spectrophotometric and electrochemical detection of a series of manganese(III) cationic porphyrins. J Chromatogr B Biomed Sci Appl. 2002;767(1):61–7. [PubMed: 11863296]
- 90.
- Vujaskovic Z, Batinic-Haberle I, Rabbani ZN. et al. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33:857–63. [PubMed: 12208373]
- 91.
- Smith KR, Uyeminami DL, Kodavanti UP. et al. Inhibition of tobacco smoke-induced lung inflammation by a catalytic antioxidant. Free Radic Biol Med. 2003;33:1106–14. [PubMed: 12374622]
- 92.
- Chang LY, Crapo JD. Inhibition of airway inflammation and hyperreactivity by an antioxidant mimetic. Free Radic Biol Med. 2002;33:379–86. [PubMed: 12126760]
- 93.
- Chang LY, Subramaniam M, Yoder BA. et al. A catalytic antioxidant attenuates alveolar structural remodeling in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2003;167:57–64. [PubMed: 12502477]
- 94.
- Piganelli JD, Flores SC, Cruz C. et al. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes. 2002;51(2):347–55. [PubMed: 11812741]
- 95.
- Gardner PR, Nguyen DD, White CW. Superoxide scavenging by Mn(II/III) tetrakis (1-methyl- 4-pyridyl) porphyrin in mammalian cells. Arch Biochem Biophys. 1996;325(1):20–8. [PubMed: 8554339]
- 96.
- Konorev EA, Kennedy MC, Kalyanaraman B. Cell-permeable superoxide dismutase and glutathione peroxidase mimetics afford superior protection against doxorubicin-induced cardiotoxicity: The role of reactive oxygen and nitrogen intermediates. Arch Biochem Biophys. 1999;368:421–28. [PubMed: 10441396]
- 97.
- Misko TP, Highkin MK, Veenhuizen AW. et al. Characterization of the cytoprotective action of peroxynitrite decomposition catalysts. J Biol Chem. 1998;273(25):15646–53. [PubMed: 9624158]
- 98.
- Kang JL, Lee HS, Pack IS. et al. Iron tetrakis (N-methyl-4'-pyridyl) porphyrinato (FeTMPyP) is a potent scavenging antioxidant and an inhibitor of stimulant-induced NF-kappaB activation of raw 264.7 macrophages. J Toxicol Environ Health A. 2001;64(4):291–310. [PubMed: 11693489]
- 99.
- Zingarelli B, Day BJ, Crapo JD. et al. The potential role of peroxynitrite in the vascular contractile and cellular energetic failure in endotoxic shock. Br J Pharmacol. 1997;120(2):259–67. [PMC free article: PMC1564360] [PubMed: 9117118]
- 100.
- Ohse T, Nagaoka S, Arakawa Y. et al. Cell death by reactive oxygen species generated from water-soluble cationic metalloporphyrins as superoxide dismutase mimics. J Inorg Biochem. 2001;85(2-3):201–8. [PubMed: 11410240]
- 101.
- Cemerski S, Cantagrel A, van Meerwijk JP. et al. Reactive oxygen species differentially affect T cell receptor signaling pathways. J Biol Chem. 2002;26:26. [PubMed: 11916964]
- 102.
- Patel M. Inhibition of neuronal apoptosis by a metalloporphyrin superoxide dismutase mimic. J Neurochem. 1998;71(3):1068–74. [PubMed: 9721731]
- 103.
- Estevez AG, Spear N, Manuel SM. et al. Role of endogenous nitric oxide and peroxynitrite formation in the survival and death of motor neurons in culture. Prog Brain Res. 1998;118:269–80. [PubMed: 9932448]
- 104.
- Estevez AG, Crow JP, Sampson JB. et al. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science. 1999;286(5449):2498–500. [PubMed: 10617463]
- 105.
- Choi WS, Yoon SY, Oh TH. et al. Two distinct mechanisms are involved in 6-hydroxydopamineand MPP+- induced dopaminergic neuronal cell death: Role of caspases, ROS, and JNK. J Neurosci Res. 1999;57(1):86–94. [PubMed: 10397638]
- 106.
- Arstall MA, Sawyer DB, Fukazawa R. et al. Cytokine-mediated apoptosis in cardiac myocytes: The role of inducible nitric oxide synthase induction and peroxynitrite generation. Circ Res. 1999;85(9):829–40. [PubMed: 10532951]
- 107.
- Hildeman DA, Mitchell T, Teague TK. et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10(6):735–44. [PubMed: 10403648]
- 108.
- Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165(2):779–85. [PubMed: 10878351]
- 109.
- Petersen A, Castilho RF, Hansson O. et al. Oxidative stress, mitochondrial permeability transition and activation of caspases in calcium ionophore A23187-induced death of cultured striatal neurons. Brain Res. 2000;857(1-2):20–9. [PubMed: 10700549]
- 110.
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptotic cell death in dopaminergic cells. Free Radic Biol Med. 2001;31:1473–85. [PubMed: 11728820]
- 111.
- Stull ND, Polan DP, Iacovitti L. Antioxidant compounds protect dopamine neurons from death due to oxidative stress in vitro. Brain Res. 2002;931:181–85. [PubMed: 11897104]
- 112.
- MacCarthy PA, Grieve DJ, Li JM. et al. Impaired endothelial regulation of ventricular relaxation in cardiac hypertrophy: Role of reactive oxygen species and NADPH oxidase. Circulation. 2001;104(24):2967–74. [PubMed: 11739314]
- 113.
- Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol. 1998;80(1):452–7. [PubMed: 9658063]
- 114.
- Mok JS, Paisley K, Martin W. Inhibition of nitrergic neurotransmission in the bovine retractor penis muscle by an oxidant stress: Effects of superoxide dismutase mimetics. Br J Pharmacol. 1998;124(1):111–8. [PMC free article: PMC1565368] [PubMed: 9630350]
- 115.
- d'Uscio LV, Baker TA, Mantilla CB. et al. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21(6):1017–22. [PubMed: 11397713]
- 116.
- d'Uscio LV, Smith LA, Katusic ZS. Hypercholesterolemia impairs endothelium-dependent relaxations in common carotid arteries of apolipoprotein e-deficient mice. Stroke. 2001;32(11):2658–64. [PubMed: 11692031]
- 117.
- Pfeiffer S, Lass A, Schmidt K. et al. Protein tyrosine nitration in mouse peritoneal macrophages activated in vitro and in vivo: Evidence against an essential role of peroxynitrite. FASEB J. 2001;15(13):2355–64. [PubMed: 11689461]
- 118.
- Cuzzocrea S, Zingarelli B, Costantino G. et al. Beneficial effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in carrageenan-induced pleurisy. Free Radic Biol Med. 1999;26(1-2):25–33. [PubMed: 9890637]
- 119.
- Imam SZ, Crow JP, Newport GD. et al. Methamphetamine generates peroxynitrite and produces dopaminergic neurotoxicity in mice: Protective effects of peroxynitrite decomposition catalyst. Brain Res. 1999;837(1-2):15–21. [PubMed: 10433983]
- 120.
- Liang LP, Ho YS, Patel M. Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience. 2000;101(3):563–70. [PubMed: 11113305]
- 121.
- Niwa K, Carlson GA, Iadecola C. Exogenous a beta1-40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000;20(12):1659–68. [PubMed: 11129782]
- 122.
- Niwa K, Porter VA, Kazama K. et al. A beta-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol Heart Circ Physiol. 2001;281(6):H2417–24. [PubMed: 11709407]
- 123.
- Leski ML, Bao F, Wu L. et al. Protein and DNA oxidation in spinal injury: Neurofilaments—an oxidation target. Free Radic Biol Med. 2001;30(6):613–24. [PubMed: 11295359]
- 124.
- Melov S, Schneider JA, Day BJ. et al. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat Genet. 1998;18(2):159–63. [PubMed: 9462746]
- 125.
- Ferret PJ, Hammoud R, Tulliez M. et al. Detoxification of reactive oxygen species by a nonpeptidyl mimic of superoxide dismutase cures acetaminophen-induced acute liver failure in the mouse. Hepatology. 2001;33(5):1173–80. [PubMed: 11343246]
- 126.
- Malassagne B, Ferret PJ, Hammoud R. et al. The superoxide dismutase mimetic MnTBAP prevents fas-induced acute liver failure in the mouse. Gastroenterology. 2001;121(6):1451–9. [PubMed: 11729124]
- 127.
- Cuzzocrea S, Costantino G, Mazzon E. et al. Beneficial effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in zymosan-induced shock. Br J Pharmacol. 1999;128(6):1241–51. [PMC free article: PMC1571737] [PubMed: 10578138]
- 128.
- Frame MD, Fox RJ, Kim D. et al. Diminished arteriolar responses in nitrate tolerance involve ROS and angiotensin II. Am J Physiol Heart Circ Physiol. 2002;282:H2377–H85. [PubMed: 12003849]
- 129.
- Cuzzocrea S, Costantino G, Mazzon E. et al. Protective effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in paw oedema induced by carrageenan in the rat. Biochem Pharmacol. 1999;58(1):171–6. [PubMed: 10403531]
- 130.
- Wang W, Jittikanont S, Falk SA. et al. Interaction among nitric oxide, reactive oxygen species, and antioxidants during endotoxemia-related acute renal failure. Am J Physiol Renal Physiol. 2003;284:F532–F37. [PubMed: 12556364]
- Development of Manganic Porphyrin Mimetics of Superoxide Dismutase Activity - Ma...Development of Manganic Porphyrin Mimetics of Superoxide Dismutase Activity - Madame Curie Bioscience Database
- The Role of Maspin in Tumor Progression and Normal Development - Madame Curie Bi...The Role of Maspin in Tumor Progression and Normal Development - Madame Curie Bioscience Database
- Rapid Evolution of Sex-related Genes: Sexual Conflict or Sex-specific Adaptation...Rapid Evolution of Sex-related Genes: Sexual Conflict or Sex-specific Adaptations? - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...
